- 1Department of Obstetrics, Guangdong Women and Children Hospital, Guangzhou, China
- 2Department of Health Care, Guangdong Women and Children Hospital, Guangzhou, China
Objective: To examine the associations between well-controlled gestational diabetes mellitus (GDM) and early neurodevelopmental trajectories in offspring.
Methods: This retrospective cohort study included 2810 mother–infant pairs from Guangdong Women and Children Hospital (2016–2022). GDM was diagnosed via a 75 g oral glucose tolerance test at 24–28 gestational weeks, and women with well-controlled GDM were those who maintained blood glucose levels defined as a third-trimester HbA1c < 6% without requiring medication. Neurodevelopment was assessed via the Children’s Neuropsychological and Behavioral Scale-Revision 2016 at 6 and 12 months of age.
Results: Among 2810 mother–infant pairs, 451 (16.05%) were diagnosed with GDM. Compared with non-GDM mothers, mothers with GDM had a greater median age (31.00 vs. 29.00 years; P < 0.001) and prepregnancy BMI (21.26 vs. 20.20 kg/m²; P < 0.001). No significant differences were observed in neonatal sex, birth weight or low birth weight (<2500 g) proportions. Neurodevelopmental assessments at 6 and 12 months revealed no significant differences in gross motor, fine motor, or adaptive behavior; language; or personal–social scores (all P > 0.05). Adjusted multivariate analyses revealed no associations between GDM and neurodevelopmental delay (≥2 subdomains below the threshold) at 6 months (OR = 0.92, 95% CI: 0.57–1.48; P = 0.739) or 12 months (OR = 0.87, 95% CI: 0.58–1.29; P = 0.479).
Conclusions: Well-controlled GDM was not associated with adverse neurodevelopmental outcomes in early infancy, suggesting that optimized perinatal management may mitigate risks.
Introduction
Gestational diabetes mellitus (GDM), the most prevalent metabolic disorder during pregnancy globally, affects 14.8% of pregnancies in mainland China (global range: 7.1%-27.6%) (1, 2). Its impact on maternal and neonatal health has expanded beyond traditional perinatal complications to encompass long-term neurodevelopmental effects in offspring (3, 4). Animal studies and epidemiological evidence suggest that GDM may disrupt fetal neurodevelopment through mechanisms involving intrauterine oxidative stress and chronic low-grade inflammation (5). Maternal hyperglycemia increases the production of reactive oxygen species, primarily via mitochondrial pathways, leading to membrane damage, activation of pro-apoptotic proteins, and excessive apoptosis, which can result in abnormal development of the fetal central nervous system (6). In addition, maternal hyperglycemia may elevate levels of pro-inflammatory cytokines, contributing to responses that could further impair fetal neurodevelopment, and potentially increase the risk of cognitive impairment, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and motor dysfunction (5–10). A meta-analysis encompassing 56 million mother–child pairs (202 observational studies) confirmed significant positive associations between maternal diabetes and offspring neurodevelopmental disorders, with particular correlations for ASD and ADHD (11). These findings underscore the critical importance of early detection and management of maternal diabetes to improve childhood health outcomes.
However, the dynamic trajectory of GDM-related neurodevelopmental impacts remains controversial (12). Longitudinal cohort studies have demonstrated that male offspring of mothers with GDM exhibit persistent neurodevelopmental delays, particularly in problem-solving abilities, fine motor coordination, and personal–social functioning, with these deficits being detectable as early as 6 months and remaining clinically significant through 4 years of age (13). Critical modifiers include exposure duration, diagnostic timing, and disease severity: early diagnosis (≤26 weeks) correlates with elevated ASD/ADHD risk versus late diagnosis (>26 weeks), and pharmacologically managed GDM confers greater neurodevelopmental risk than does diet-controlled cases (11). Current clinical protocols enable glycemic target attainment in >80% of gestational diabetes cases via structured dietary interventions (carbohydrate-controlled meal plans with regulated meal timing), leading to marked reductions in obstetric complications, including fetal macrosomia and neonatal glucose dysregulation (14, 15). Nevertheless, robust evidence of the neurodevelopmental benefits associated with optimized maternal glycemic regulation in modern care paradigms is lacking. This retrospective cohort study systematically evaluated neurodevelopmental trajectories (motor, language, and social domains) in infants of mothers with well-controlled GDM, aiming to clarify neurodevelopmental patterns under current management paradigms and optimize perinatal interventions.
Methods
Study population
A retrospective cohort study involving pregnant women who received antenatal care and delivered at our facility between 2016 and 2022 was conducted at Guangdong Women and Children Hospital. The study protocol involved two phases of data collection: initial extraction of GDM screening records from electronic medical databases for women undergoing 75 g oral glucose tolerance test (OGTT) at 24–28 weeks gestation, followed by comprehensive manual review of complete medical records by investigators to verify data accuracy, including infant neurodevelopmental assessments at 6 and 12 months postpartum. The eligibility criterion was singleton pregnancies with term deliveries (≥37 weeks gestation). The exclusion criteria included multiple gestations, fetal demise, pregestational diabetes (type 1 or 2), insulin therapy, maternal chronic conditions (chronic hypertension or gestational hypertensive disorders, cardiopulmonary/hepatic/renal diseases), fetal chromosomal abnormalities, congenital malformations, and cases with incomplete documentation of critical information (OGTT results, neurodevelopmental evaluation outcomes, or essential pregnancy parameters).
Ethical approval
The study protocol received ethical clearance from the Institutional Review Board of Guangdong Women and Children Hospital (Approval ID: 202201203). As this retrospective analysis utilized anonymized clinical data extracted from the institution’s electronic health records system, the ethics committee formally exempted the requirement for informed consent in compliance with national regulations governing deidentified medical data research.
GDM assessment
The 75-g OGTT was administered to pregnant individuals between 24 and 28 gestational weeks. Diagnostic thresholds were defined as fasting glucose ≥5.1 mmol/L, 1-hour postload glucose ≥10.0 mmol/L, or 2-hour postload glucose ≥8.5 mmol/L. GDM diagnosis requires a single abnormal value meeting or exceeding these cutoffs (16). Third-trimester HbA1c measurements were obtained to evaluate glycemic management. Women with well-controlled GDM refers specifically to pregnant women who maintained good glycemic control throughout pregnancy (as defined by third-trimester HbA1c < 6% and no need for medication). In our retrospective cohort, only a very small proportion (about 1.1%, n=5) of GDM cases had poor glycemic control (third-trimester HbA1c > 6% or required pharmacological intervention), and these cases were excluded from our analysis.
Neurodevelopmental assessment
The Children’s Neuropsychological and Behavioral Scale-Revision 2016 (CNBS-R2016) was employed to assess neurodevelopmental progression in infants at 6 and 12 months of age. This psychometrically validated instrument, developed through standardized protocols by the Capital Institute of Pediatrics (China), provides age-normed developmental benchmarks for children from 1 month through 72 months (17). As a clinician-administered diagnostic tool, it quantifies neuropsychological maturation through a composite developmental quotient (DQ) and five functional subdomains: gross motor, fine motor, language, personal-social, and adaptive behaviors. DQs are calculated via the following established formula: DQ = (Mental Age [months]/Chronological Age [months]) × 100. These metrics yield both domain-specific and composite scores, with clinical interpretation thresholds defined as follows: scores ≥80 reflect age-appropriate development, scores between 70–79 indicate mild developmental delay, and scores <70 signify clinically significant developmental delay.
Statistical analysis
Analyses were performed via SAS 9.4 (SAS Institute) and R version 4.4.3 (R Core Team). The figures were plotted via R version 4.5.0 (R Core Team) and the ggplot2 package. Maternal and neonatal characteristics are presented as mean with standard deviation (SD) for continuous variables and as numbers (%) for categorical variables. Independent samples Satterthwaite t tests were conducted to examine differences in the means of continuous variables. Chi-square tests were used to compare differences in the proportions of categorical variables. The development delay rates with 95% CIs of the non-GDM and GDM groups at 6 months and 12 months were calculated via a binomial distribution exact method. Adjusted associations between development delay (≥2 subdomains) and GDM at 6 months and 12 months were examined separately via a multivariate logistic regression model adjusted for maternal age, BMI at conception, parity, delivery mode, infant sex, and birthweight. The LS mean and LS mean differences between the Non-GDM and GDM groups in terms of gross motor, fine motor, language, personal-social, and adaptive behaviors at 6 months and 12 months were calculated via a mixed model and adjusted for maternal age; BMI at conception; parity; delivery mode; infant sex; and birthweight. The confidence level was set at 95%, and P <0.05 was considered statistically significant.
Results
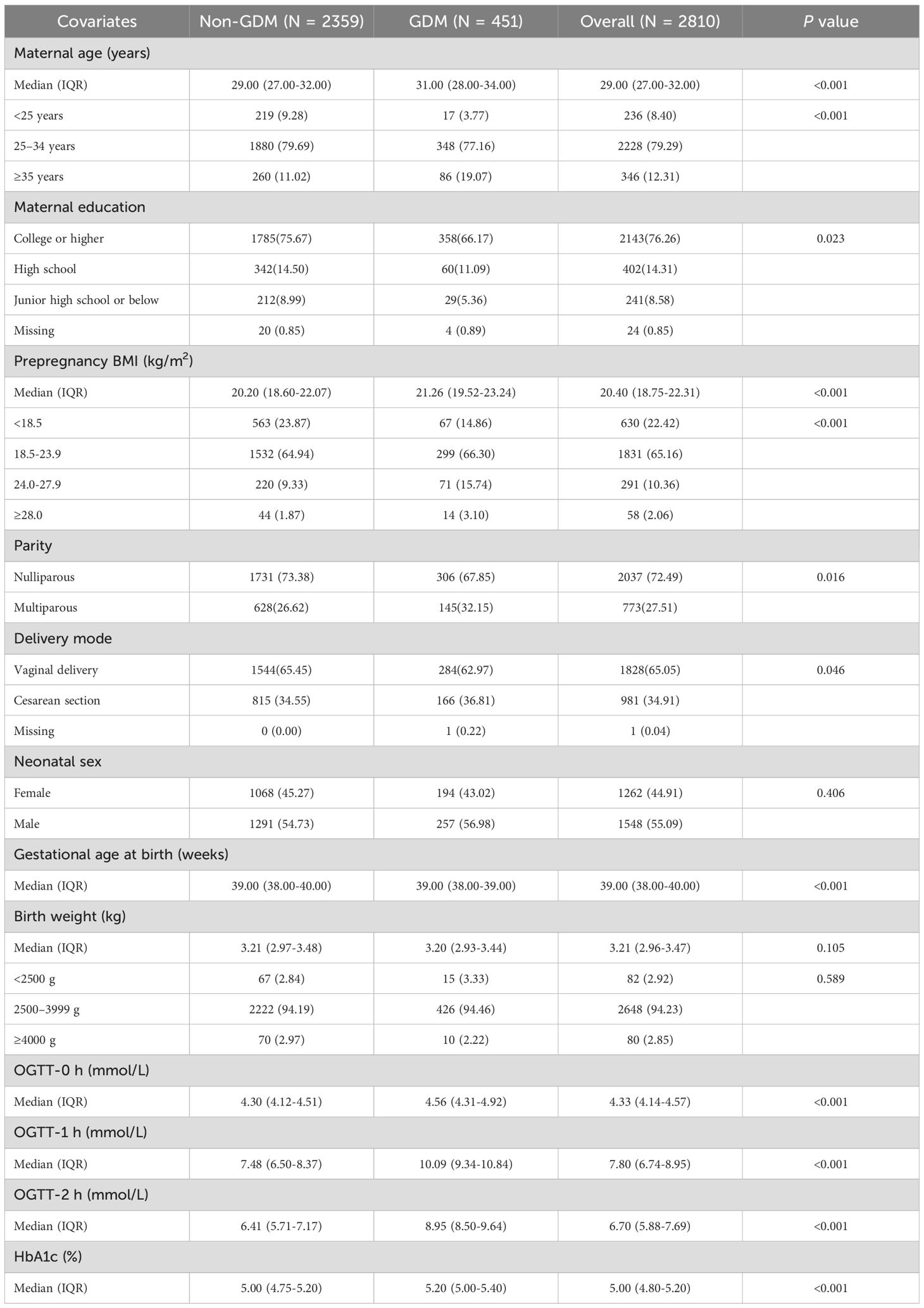
This study included 2810 mother–infant pairs, 451 (16.05%) of which were diagnosed with GDM. As shown in Table 1, GDM mothers were more likely to be older, have a higher prepregnancy BMI, and have lower educational attainment. The GDM group had a significantly greater median maternal age (31.00 vs. 29.00 years; P < 0.001) and a greater proportion of women aged ≥35 years (19.07% vs. 11.02%). The proportion of college-educated individuals was significantly lower in the GDM group (66.17% vs. 75.67%; P = 0.023). The median prepregnancy BMI was greater in the GDM group (21.26 vs. 20.20 kg/m²; P < 0.001), with a significantly increased proportion of women with a BMI ≥24.0 (18.84% vs. 11.20%; P < 0.001). Nulliparity was less common (67.85% vs. 73.38%; P = 0.016), and cesarean delivery rates were higher in the GDM group (36.81% vs. 34.55%; P = 0.046). No significant differences were observed in neonatal sex, birth weight or low birth weight (<2500 g) proportions.
In Table 2 and Figure 1, neurodevelopmental levels at 6 and 12 months were compared between infants of mothers with and without GDM. No statistically significant differences were observed in gross motor, fine motor, adaptive, language, or personal–social development scores (all P > 0.05). Table 3 presents adjusted associations between GDM and neurodevelopmental delay (≥2 subdomains below the threshold) at 6 and 12 months of age, with odds ratios of 0.92 (95% CI: 0.57–1.48; P = 0.739) and 0.87 (0.58–1.29; P = 0.479), respectively, indicating no statistically significant associations.
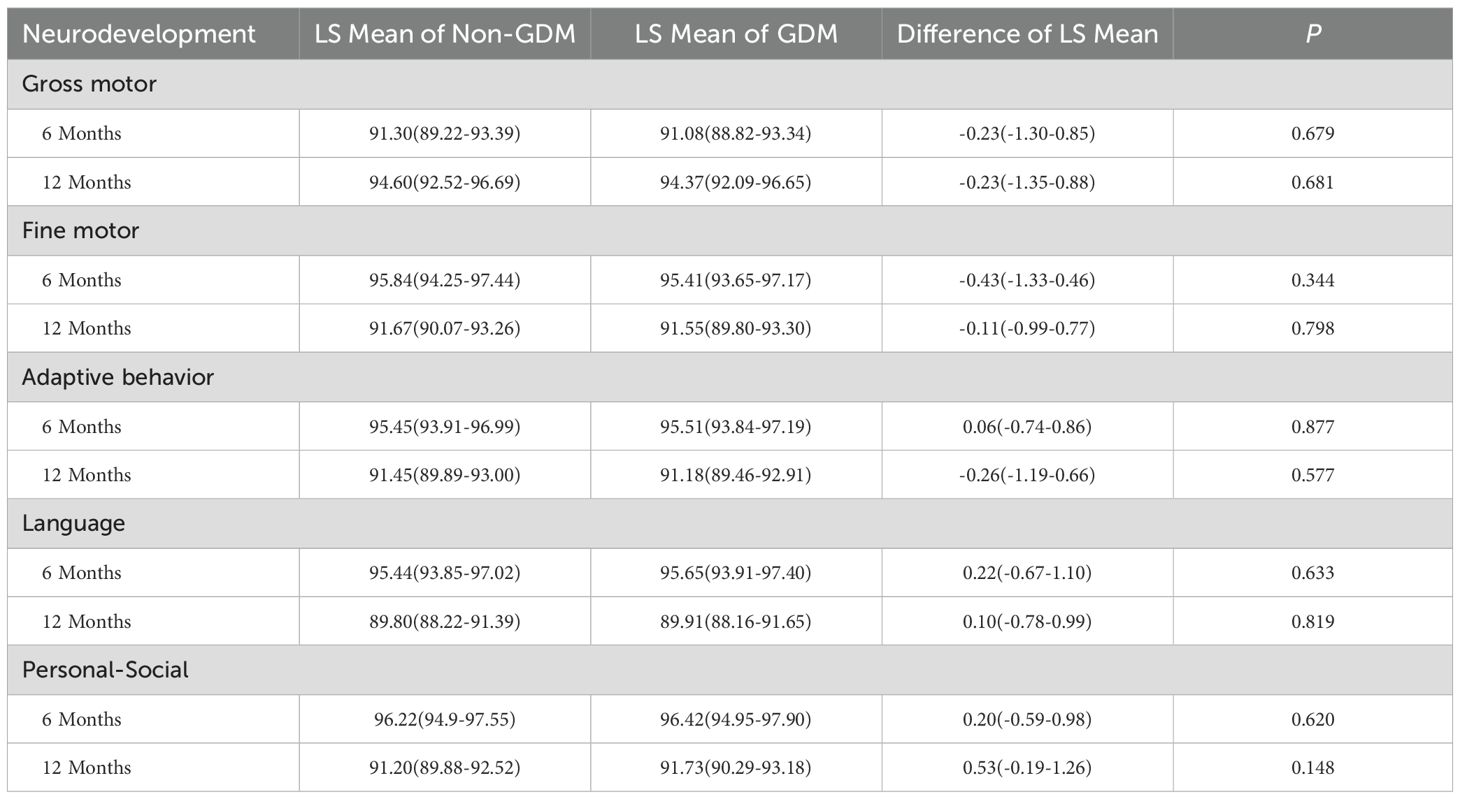
Table 2. Neurodevelopmental levels in infants of mothers with GDM vs non-GDM mothers at 6 and 12 months.
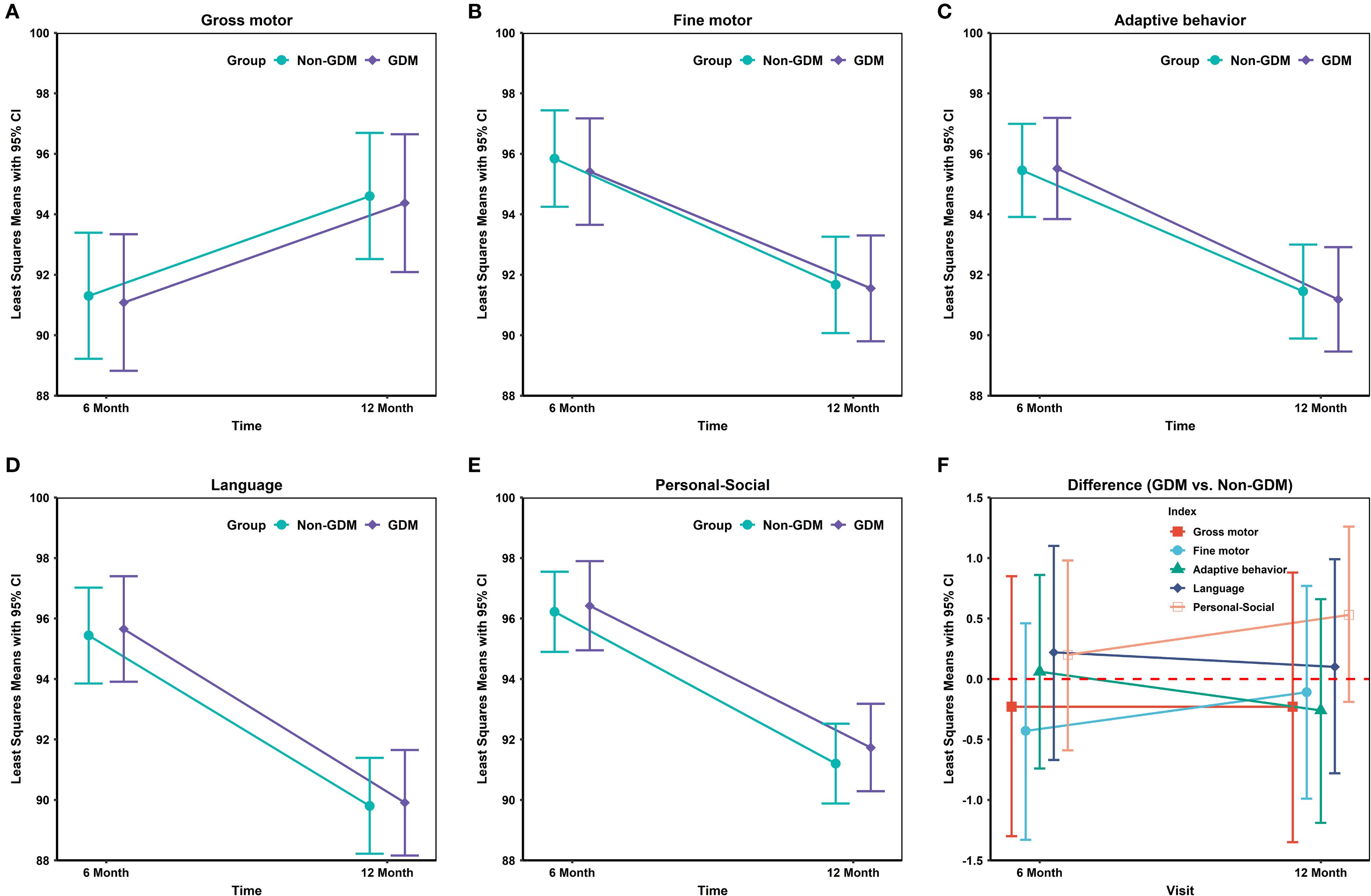
Figure 1. Differences in neurodevelopmental domain scores between GDM- and non-GDM-exposed infants at 6 and 12 months.
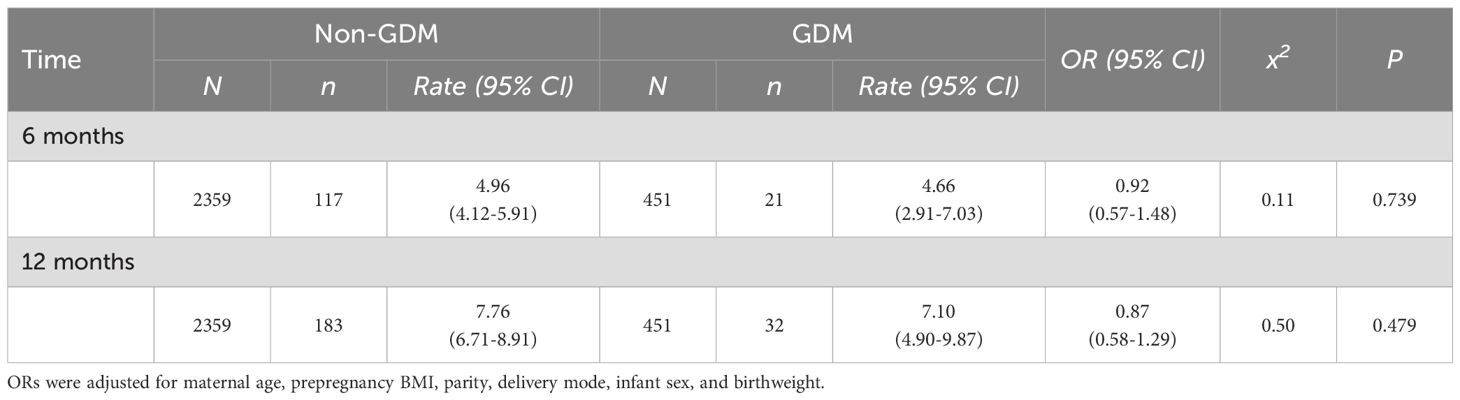
Table 3. Associations between GDM and neurodevelopmental delay (≥2 subdomains below threshold) at 6 and 12 months of age.
Discussion
This study evaluated neurodevelopmental outcomes at 6 and 12 months of age in the offspring of mothers with GDM and non-GDM controls. No significant differences were observed across five domains—gross motor, fine motor, language, personal-social, and adaptive behavior—suggesting that GDM has no clear adverse effect on early neurodevelopment. These findings align with those of several prior studies but contrast with those of most reports suggesting adverse neurodevelopmental effects of GDM.
Recent large-scale cohort studies and meta-analyses indicate a potential association between GDM and offspring neurodevelopmental abnormalities (11, 13). The underlying mechanisms may involve the chronic immune dysregulation characteristic of GDM, manifested through a cytokine imbalance and altered immune cell profiles that are similar to the immunological features observed in neurodevelopmental disorders (18, 19). Additionally, maternal hyperglycemia induces neonatal hypoglycemia, which may cause irreversible neurological damage if severe or prolonged (20). These early metabolic disturbances may subsequently contribute to impairments in multiple neurodevelopmental domains, including cognitive function, reading ability, and both gross and fine motor skills (21–23).
Research also provides conflicting evidence regarding GDM and offspring neurodevelopment. A Norwegian study reported no link between GDM risk and adverse neurodevelopmental outcomes (24), whereas an Indian study reported no reduction in cognitive ability among GDM offspring—with some scores even higher than those of controls (25). Our results partially support these observations, possibly because our cohort was derived from a single-center, well-managed prenatal care population. In current practice, most GDM patients receive standardized dietary guidance, weight management, and glucose monitoring, with only a minority requiring drug intervention. Systematic management may mitigate potential risks. The evidence suggests that optimal glycemic control during pregnancy normalizes neurodevelopmental outcomes in offspring (26, 27). Our study demonstrated that offspring of well-managed GDM mothers with controlled glucose levels exhibit no significant neurodevelopmental delays in infancy. These findings underscore the clinical importance of contemporary GDM screening and management strategies in preventing early neurodevelopmental impairment.
In this study, neurodevelopmental assessment was conducted using the CNBS-R2016, a clinician-administered instrument validated for children aged 1–72 months in the Chinese population. This scale offers several advantages, including comprehensive coverage of developmental domains and robust applicability to the Chinese pediatric population. However, it may underestimate subtle or higher-order cognitive deficits, is susceptible to inter-rater variability, and limits cross-study comparability with cohorts assessed by international tools such as the Bayley Scales of Infant Development or Griffiths Scales. In addition, although this retrospective cohort study evaluated neurodevelopmental trajectories at 6 and 12 months through multidomain assessments, several limitations should be considered. The sample size may be underpowered to detect small effect sizes, especially since most GDM pregnancies demonstrated adequate glycemic control and severity-stratified analyses were not performed. Furthermore, the absence of extended follow-up beyond infancy restricts the detection of late-onset neurodevelopmental abnormalities, such as executive dysfunction and learning difficulties, which often emerge at school age. Lastly, important confounding variables, including infant feeding practices (particularly breastfeeding duration) and family environmental factors, which are well-established determinants of neurodevelopment (28), were not systematically collected, limiting our ability to adjust for these covariates.
Conclusions
On the basis of current evidence and our findings, GDM with prenatal care and glycemic control does not appear to pose significant risks for infant neurodevelopmental outcomes. These results support the effectiveness of contemporary GDM management, but long-term follow-up studies are warranted to comprehensively evaluate the potential long-term effects of GDM on offspring neurodevelopment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Guangdong Women and Children Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because As this retrospective analysis utilized anonymized clinical data extracted from the institution’s electronic health records system, the ethics committee formally exempted the requirement for informed consent in compliance with national regulations governing deidentified medical data research.
Author contributions
JP: Data curation, Investigation, Writing – original draft. HM: Formal Analysis, Methodology, Writing – review & editing. LZ: Investigation, Writing – review & editing, Conceptualization. JJ: Data curation, Writing – review & editing, Investigation. LH: Investigation, Writing – review & editing. DX: Investigation, Writing – review & editing. YG: Writing – original draft, Formal Analysis, Writing – review & editing, Data curation, Conceptualization. GL: Project administration, Validation, Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported in part by the Guangzhou Municipal Science and Technology Bureau (202102080493).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gao C, Sun X, Lu L, Liu F, and Yuan J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig. (2019) 10:154–62. doi: 10.1111/jdi.12854
2. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. Idf diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
3. Ye W, Luo C, Huang J, Li C, Liu Z, and Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
4. Ornoy A, Becker M, Weinstein-Fudim L, and Ergaz Z. Diabetes during pregnancy: A maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int J Mol Sci. (2021) 22:2965. doi: 10.3390/ijms22062965
5. Chan AYL, Gao L, Hsieh MH, Kjerpeseth LJ, Avelar R, Banaschewski T, et al. Maternal diabetes and risk of attention-deficit/hyperactivity disorder in offspring in a multinational cohort of 3.6 million mother-child pairs. Nat Med. (2024) 30:1416–23. doi: 10.1038/s41591-024-02917-8
6. Ornoy A, Reece EA, Pavlinkova G, Kappen C, and Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today. (2015) 105:53–72. doi: 10.1002/bdrc.21090
7. Damtie Y, Dachew BA, Ayano G, Tadesse AW, Betts K, and Alati R. The association between maternal diabetes and the risk of attention deficit hyperactivity disorder in offspring: an updated systematic review and meta-analysis. Eur Child Adolesc Psychiatry. (2025) 34:2417–29. doi: 10.1007/s00787-025-02645-5
8. Rowland J and Wilson CA. The association between gestational diabetes and asd and adhd: A systematic review and meta-analysis. Sci Rep. (2021) 11:5136. doi: 10.1038/s41598-021-84573-3
9. Desoye G and Carter AM. Fetoplacental oxygen homeostasis in pregnancies with maternal diabetes mellitus and obesity. Nat Rev Endocrinol. (2022) 18:593–607. doi: 10.1038/s41574-022-00717-z
10. Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, and Klungsoyr K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry. (2017) 81:452–9. doi: 10.1016/j.biopsych.2015.11.024
11. Ye W, Luo C, Zhou J, Liang X, Wen J, Huang J, et al. Association between maternal diabetes and neurodevelopmental outcomes in children: A systematic review and meta-analysis of 202 observational studies comprising 56.1 million pregnancies. Lancet Diabetes Endocrinol. (2025) 13:494–504. doi: 10.1016/S2213-8587(25)00036-1
12. Li C, Zhou P, Cai Y, Peng B, Liu Y, Yang T, et al. Associations between gestational diabetes mellitus and the neurodevelopment of offspring from 1 month to 72 months: study protocol for a cohort study. BMJ Open. (2020) 10:e040305. doi: 10.1136/bmjopen-2020-040305
13. Saito Y, Kobayashi S, Ito S, Miyashita C, Umazume T, Cho K, et al. Neurodevelopmental delay up to the age of 4 years in infants born to women with gestational diabetes mellitus: the Japan environment and children’s study. J Diabetes Investig. (2022) 13:2054–62. doi: 10.1111/jdi.13907
14. Pittyanont S, Suriya N, Sirilert S, and Tongsong T. Comparisons of the rates of large-for-gestational-age newborns between women with diet-controlled gestational diabetes mellitus and those with non-gestational diabetes mellitus. Clin Pract. (2024) 14:536–45. doi: 10.3390/clinpract14020041
15. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S19–40. doi: 10.2337/dc23-S002
16. Yang HX. Diagnostic criteria for gestational diabetes mellitus (Ws 331-2011). Chin Med J (Engl). (2012) 125:1212–3. doi: 10.3760/cma.j.issn.0366.6999.2012.07.00
17. Li HH, Feng JY, Wang B, Zhang Y, Wang CX, and Jia FY. Comparison of the children neuropsychological and behavior scale and the griffiths mental development scales when assessing the development of children with autism. Psychol Res Behav Manag. (2019) 12:973–81. doi: 10.2147/PRBM.S225904
18. Han VX, Patel S, Jones HF, and Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. (2021) 17:564–79. doi: 10.1038/s41582-021-00530-8
19. Kim E, Huh JR, and Choi GB. Prenatal and postnatal neuroimmune interactions in neurodevelopmental disorders. Nat Immunol. (2024) 25:598–606. doi: 10.1038/s41590-024-01797-x
20. Lee J, Lee NK, and Moon JH. Gestational diabetes mellitus: mechanisms underlying maternal and fetal complications. Endocrinol Metab (Seoul). (2025) 40:10–25. doi: 10.3803/EnM.2024.2264
21. Alsweiler JM, Harris DL, Harding JE, and McKinlay CJD. Strategies to improve neurodevelopmental outcomes in babies at risk of neonatal hypoglycaemia. Lancet Child Adolesc Health. (2021) 5:513–23. doi: 10.1016/S2352-4642(20)30387-4
22. Wickstrom R, Skiold B, Petersson G, Stephansson O, and Altman M. Moderate neonatal hypoglycemia and adverse neurological development at 2–6 years of age. Eur J Epidemiol. (2018) 33:1011–20. doi: 10.1007/s10654-018-0425-5
23. Kaiser JR, Bai S, Gibson N, Holland G, Lin TM, Swearingen CJ, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: A population-based study. JAMA Pediatr. (2015) 169:913–21. doi: 10.1001/jamapediatrics.2015.1631
24. Kolseth AJ, Kulseth S, Stafne SN, Morkved S, Salvesen KA, and Evensen KAI. Physical health and neurodevelopmental outcome in 7-year-old children whose mothers were at risk of gestational diabetes mellitus: A follow-up of a randomized controlled trial. Acta Obstet Gynecol Scand. (2023) 102:1193–202. doi: 10.1111/aogs.14593
25. Veena SR, Krishnaveni GV, Srinivasan K, Kurpad AV, Muthayya S, Hill JC, et al. Childhood cognitive ability: relationship to gestational diabetes mellitus in India. Diabetologia. (2010) 53:2134–8. doi: 10.1007/s00125-010-1847-0
26. Yamashita Y, Kawano Y, Kuriya N, Murakami Y, Matsuishi T, Yoshimatsu K, et al. Intellectual development of offspring of diabetic mothers. Acta Paediatr. (1996) 85:1192–6. doi: 10.1111/j.1651-2227.1996.tb18227.x
27. Griffith RJ, Harding JE, McKinlay CJD, Wouldes TA, Harris DL, Alsweiler JM, et al. Maternal glycemic control in diabetic pregnancies and neurodevelopmental outcomes in preschool aged children. A prospective cohort study. Early Hum Dev. (2019) 130:101–8. doi: 10.1016/j.earlhumdev.2019.01.010
Keywords: gestational diabetes mellitus (GDM), development, neurodevelopment, infant, blood glucose control
Citation: Peng J, Miao H, Zhang L, Jin J, He L, Xue D, Guo Y and Liu G (2025) Neurodevelopmental trajectories in well-controlled gestational diabetes mellitus offspring: No differences were found at the 6- and 12-month assessments. Front. Endocrinol. 16:1624334. doi: 10.3389/fendo.2025.1624334
Received: 07 May 2025; Accepted: 26 August 2025;
Published: 10 September 2025.
Edited by:
Carlos Alonso Escudero, University of the Bío Bío, ChileReviewed by:
Enrique Guzmán-Gutiérrez, University of Concepcion, ChileMichael J. Wolyniak, Hampden–Sydney College, United States
Copyright © 2025 Peng, Miao, Zhang, Jin, He, Xue, Guo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Guo, Z2V5b25nMDg0QDE2My5jb20=; Guocheng Liu, aG5saXVndW9jaGVuZ0AxMjYuY29t
†These authors have contributed equally to this work
 Jing Peng1†
Jing Peng1† Huazhang Miao
Huazhang Miao Jing Jin
Jing Jin Yong Guo
Yong Guo