- 1Department of Orthopedics, The Affiliated Hospital Southwest Medical University, Luzhou, Sichuan, China
- 2Trauma Center, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Objective: Traditional pharmacological treatments for osteoporosis face challenges due to various limitations, including long-term safety concerns and limited bone anabolic effects. Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have emerged as a promising cell-free alternative therapy. However, their preclinical efficacy and the factors driving heterogeneity still require systematic evaluation.
Methods: A systematic search was conducted in PubMed, EMBASE, Cochrane Library, and Web of Science (from inception to February 2025). Two independent authors performed literature screening, data extraction, and risk of bias assessment. A random-effects model was used to pool and analyze bone mineral density (BMD), bone volume fraction (BV/TV), trabecular/cortical structural parameters, and biomechanical test results. Publication bias was assessed using funnel plots and Egger’s test, while leave-one-out sensitivity analysis was performed to evaluate the stability of the results. Subgroup analyses were conducted based on animal type, EVs source, synthesis method, engineering approach, intervention route, frequency, and treatment duration.
Results: A total of 17 studies were included. The results demonstrated that, compared to the control group, MSC-EVs significantly increased BMD, BV/TV, trabecular number (Tb.N), trabecular thickness (Tb.Th), cortical thickness (Ct.Th), mineral apposition rate (MAR), and the ultimate load-bearing capacity of the femur, while reducing trabecular separation (Tb.Sp). Significant heterogeneity and publication bias were observed in all analyses. Sensitivity analysis confirmed the robustness of all results.
Conclusions: MSC-EVs demonstrate significant improvements in preclinical osteoporosis models, highlighting its potential for clinical translation. However, further standardized studies are needed to evaluate the long-term efficacy and safety of MSC-EVs.
Introduction
Osteoporosis is a progressive systemic skeletal disease caused by an imbalance between bone formation and bone resorption, characterized by decreased bone mass and disruption of bone microstructure, which significantly increases the risk of fractures (1, 2). Globally, this disease affects approximately 200 million middle-aged and elderly individuals, with the risk of osteoporotic fractures increasing annually among those over 60 years old (3). Epidemiological data indicate that the annual cumulative number of osteoporotic fractures exceeds 8.9 million cases (4). The disease burden is particularly severe in older populations, with a prevalence of 77.1% in women over 80 years old and 46.3% in men of the same age group (5). Hip fractures, the most severe complication, result in approximately 20% of patients dying within one year after surgery, drawing widespread attention in the medical field (6, 7).
The current clinical treatment for osteoporosis primarily relies on bisphosphonate drugs, which inhibit bone resorption (8, 9). However, long-term use of these drugs may lead to severe adverse effects, such as osteonecrosis of the jaw and atypical femoral fractures (10). Although new anti-osteoporosis drugs, such as cathepsin K inhibitors and parathyroid hormone analogs, have been introduced in recent years, challenges remain, including high treatment costs, complex administration methods, and uncertain long-term efficacy (11, 12). These treatment limitations have driven researchers to explore novel, safe, and effective alternative therapies.
MSC-based cell therapy has garnered attention due to its regenerative and differentiation capabilities, demonstrating effectiveness in autoimmune diseases, graft-versus-host disease, and articular cartilage injuries (13, 14). The therapeutic potential of MSCs in osteoporosis relies on three mechanisms: migration and homing, induction of angiogenesis, and immunomodulation (15, 16). However, MSC-mediated cell therapy faces challenges, particularly in maintaining cell viability and efficacy throughout the treatment process (17). To address these limitations, extracellular vesicles secreted by mesenchymal stem cells (MSC-EVs) have emerged as a key mediator of paracrine effects and a research hotspot in regenerative medicine due to their unique nano-carrier properties. Compared to traditional stem cell transplantation, MSC-EVs can stably deliver functional miRNAs, cytokines, and signaling proteins while avoiding issues such as low cell survival rates, tumorigenic risks, and immune rejection (18, 19). In the field of osteoporosis treatment, Wang et al. (20) utilized “click chemistry” to conjugate MSC-EVs with alendronate, demonstrating high affinity for hydroxyapatite. This approach significantly promoted osteoblast differentiation in vitro and exhibited anti-osteoporotic effects and safety in osteoporotic mice. Another study found that miR-27a carried by MSC-EVs improved osteoporosis by inhibiting DKK2 expression, thereby activating the Wnt/β-catenin signaling pathway (21). Additionally, MSC-EVs can regulate vascular endothelial growth factor (VEGF) secretion to enhance local microvascular formation, which is crucial for providing nutritional support for bone regeneration (22, 23).
Since 2020, preclinical studies on MSC-EVs for osteoporosis treatment have increased; however, a comprehensive and up-to-date meta-analysis on their efficacy remains lacking, which is crucial for clinical translation. Notably, existing studies exhibit significant heterogeneity in EV preparation methods (such as isolation techniques and engineering strategies), administration protocols (including dosage, frequency, and delivery routes), and osteoporosis modeling approaches (such as ovariectomy-induced and drug-induced models). These variations may influence the analytical outcomes. Therefore, in addition to evaluating the potential benefits of MSC-EVs in improving osteoporosis models, we conducted a subgroup analysis to explore the impact of these influencing factors on therapeutic efficacy. This meta-analysis aims to provide evidence supporting the clinical translation of MSC-EVs for osteoporosis treatment.
Materials and methods
Systematic review
This study was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (24). The present study was registered in the International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/prospero/, CRD420251047216).
Search strategy
Two researchers independently searched four major databases, including PubMed, EMBASE, Web of Science, and Cochrane Library, from their inception to January 1, 2025. The search strategy combined Medical Subject Headings (MeSH) and free-text terms, focusing on intervention-related terms (e.g., mesenchymal stem cell-derived extracellular vesicles, exosomes, or microvesicles) and disease models (e.g., animal osteoporosis or bone loss). The detailed search strategy is provided in Supplementary Table 1. Discrepancies in search results were resolved through discussion with a third researcher. Additionally, the references of studies meeting the inclusion criteria were reviewed to identify potentially relevant studies.
Inclusion and exclusion criteria
Inclusion criteria
1. Controlled study design with MSC-EVs intervention in the experimental group, with no restrictions on engineering details or intervention methods;
2. Any osteoporosis animal model, including rats and mice, with no restrictions on induction methods (e.g., ovariectomy-induced osteoporosis models);
3. Studies including a control group receiving placebo or no treatment;
4. Reporting at least one bone-related quantitative outcome, such as bone mineral density (BMD), bone volume/total volume (BV/TV), trabecular number (Tb.N), trabecular separation (Tb.Sp), trabecular thickness (Tb.Th).
Exclusion criteria
1. Non-controlled studies or studies with combined interventions (e.g., EVs co-administered with drugs);
2. Reviews, meta-analyses, conference abstracts, or commentaries lacking original data;
3. Non-osteoporosis models (e.g., fracture healing or bone tumor models);
4. Studies not published in English;
5. Studies with unavailable or unextractable data.
Study selection
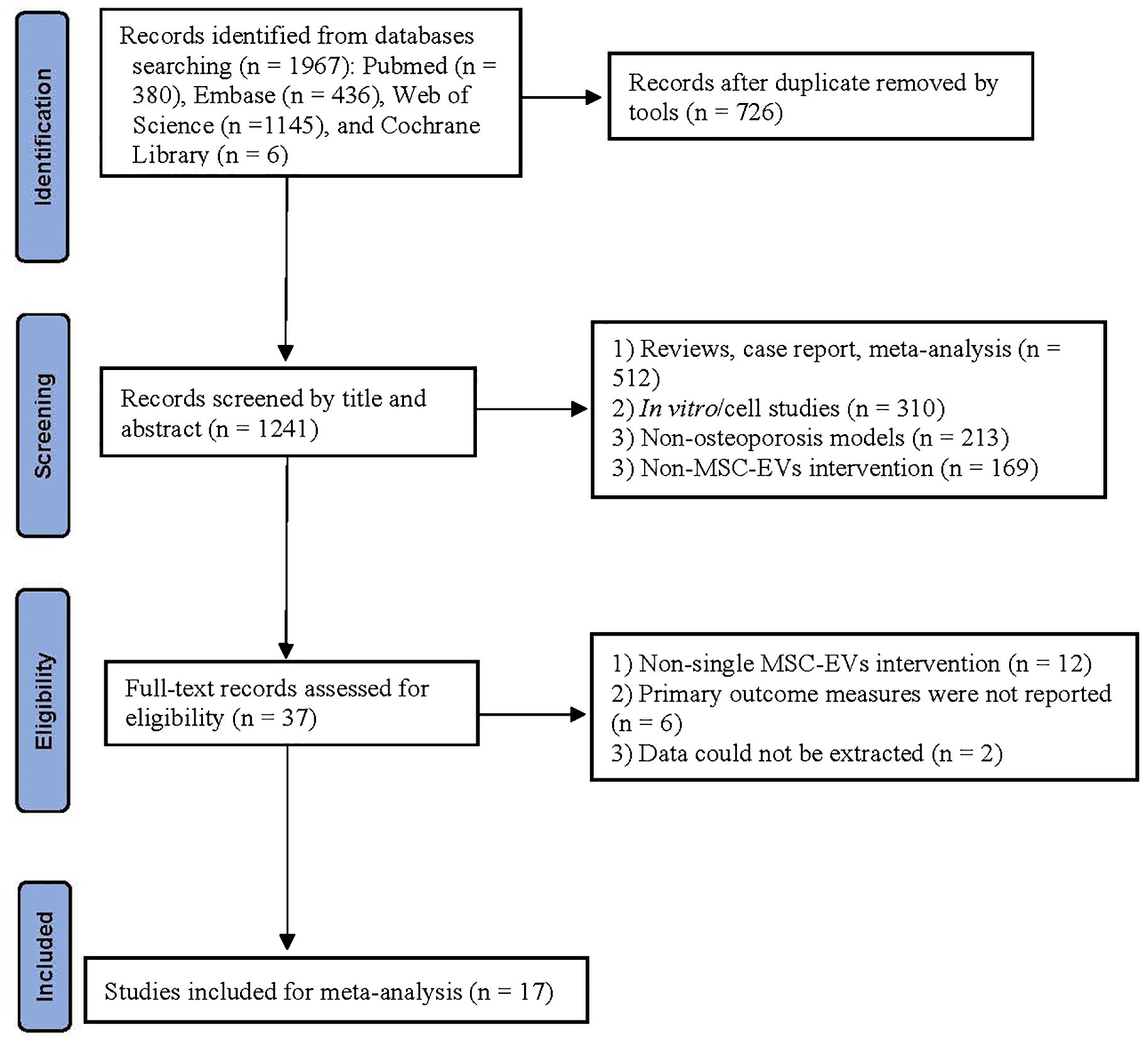
Initially, all retrieved records were compiled, and duplicate entries were automatically removed using EndNote X20. Subsequently, preliminary screening was conducted based on titles and abstracts to exclude irrelevant studies. Finally, full-text articles were reviewed according to the inclusion and exclusion criteria to identify eligible studies for meta-analysis. The screening process was independently performed by two researchers, and discrepancies were resolved through discussion with a third researcher. The selection process strictly followed the PRISMA flowchart, with detailed documentation of the number of excluded studies and reasons at each stage.
Data extraction
Two researchers independently extracted data using a standardized Excel template, including: (1) study characteristics (author, year, animal species, gender, weight, modeling method); (2) EVs properties (source, engineering method); (3) intervention protocols (frequency, route, duration); (4) outcome data (BMD, BV/TV, Tb.N, Tb.Sp, Tb.Th, Ct.Th). Graphical data were extracted using Origin software (2021 version), and quantitative data were presented as mean ± standard deviation (mean ± SD). Discrepancies in data extraction were resolved through discussion with a third researcher. For data not directly available, attempts were made to contact the corresponding authors for further information.
Primary and secondary outcomes
Primary outcomes were obtained through microCT analysis, including BMD, BV/TV, and trabecular bone structural parameters (Tb.Th, Tb.N, and Tb.Sp). Secondary outcomes primarily included Ct.Th, mineral apposition rate (MAR, observed through double fluorescent labeling), and the ultimate load-bearing capacity of the femur (determined by three-point bending test). All parameters were reported as mean ± standard deviation (mean ± SD).
Risk of bias assessment
The methodological quality of animal studies was assessed using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool. This tool includes 10 criteria: sequence generation, allocation concealment, baseline characteristics, random housing, blinding of participants, random outcome assessment, blinding of outcome detection, incomplete data, selective reporting, and other biases. Two reviewers independently scored each study based on the criteria, with results categorized as “low risk,” “high risk,” or “unclear risk.” Discrepancies in assessment results were resolved through discussion with a third reviewer. The summarized results were visualized using Review Manager (RevMan) 5.3.
Statistical analysis
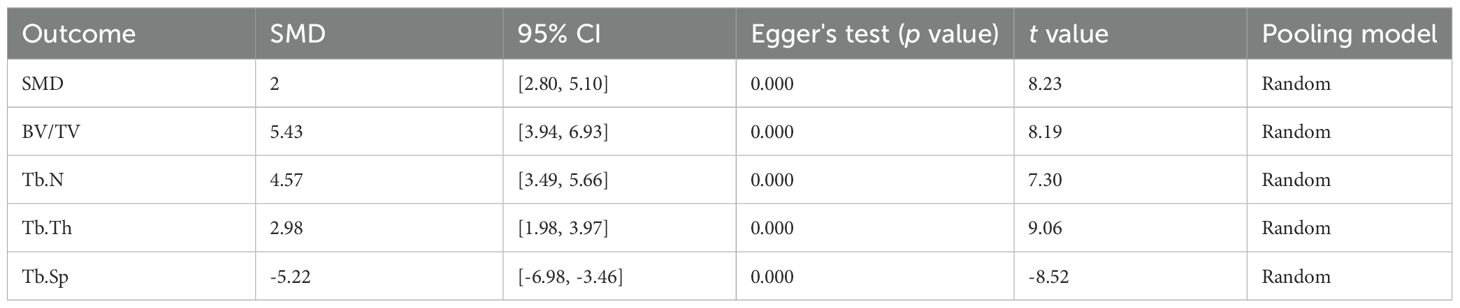
Due to methodological heterogeneity, a random-effects model was applied for the meta-analysis of continuous data, with results presented as standardized mean differences (SMDs) with 95% confidence intervals (CIs). Heterogeneity was evaluated using the I² statistic, where I² ≥ 50% indicated significant heterogeneity. Subgroup analyses were conducted when at least 10 studies reported the relevant indicators, based on predefined categories, including animal species, age, EV source, size, isolation method, purification method, intervention route, dose, frequency, and duration. Sensitivity analysis was performed to assess the robustness of the pooled results. Publication bias was evaluated using funnel plots and Egger’s regression test. A P-value < 0.05 was considered statistically significant. All analyses were conducted using RevMan 5.3 and Stata SE 16.0 software.
Results
Literature selection
A total of 1,967 records were identified through database searches: PubMed (380), Embase (436), Web of Science (1,145), and Cochrane Library (6). After removing 726 duplicates, 1,241 articles underwent title/abstract screening. Exclusions at this stage included reviews/case reports (512), in vitro studies (310), non-osteoporosis models (213), and non-MSC-EV interventions (169). Subsequently, full-text assessment of 37 articles led to the exclusion of 20 studies, with 17 studies (20, 21, 25–39) meeting the inclusion criteria. The literature selection process is detailed in the PRISMA flowchart (Figure 1).
Study characteristics
Between 2016 and 2024, all 17 studies were conducted in China. Notably, 16 studies were published in 2020 or later, indicating increasing attention to MSC-EVs in osteoporosis treatment in recent years. The animal models primarily used female animals (15/17 studies), with Sprague-Dawley rats (n = 6) and C57BL/6 mice (n = 9) as the main species. Except for one study using a hindlimb unloading (HU)-induced osteoporosis model, the remaining studies (n = 15) employed ovariectomy-induced osteoporosis models. Table 1 details the main characteristics of the animal models.
Additionally, Table 2 presents the characteristics of EVs and intervention details in the included studies. Specifically, EVs were primarily derived from bone marrow mesenchymal stem cells (BMSCs, n = 10) and human umbilical cord mesenchymal stem cells (n = 2). The diameter of EVs, reported in 14 studies, ranged from 30 to 5000 nm. The most common methods for isolating and purifying EVs are ultracentrifugation (n = 14) and filtration (n = 11), respectively. Regarding MSC-EVs intervention details, 12 studies administered EVs via intravenous injection, 2 studies via intraperitoneal injection, 1 study via scaffold loading, and 1 study via femoral periosteal injection. Injection frequencies included once a week (n = 8), twice a week (n = 5), thrice a week (n = 1), every 3 days (n = 1), once a day (n = 1), and once (n = 1). Treatment durations included 1 week (n = 1), 2 weeks (n = 1), 4 weeks (n = 5), 6 weeks (n = 1), 2 months (n = 7), and 3 months (n = 2).
Risk of bias assessment
The included studies did not clearly specify whether sequence generation methods were used for animal grouping, nor did they provide detailed descriptions of allocation concealment. Unclear risks of bias were identified in the areas of blinding of participants, blinding of outcome assessment, and randomization of outcome evaluation. Eight studies reported baseline characteristics of the included animals in detail, and seven studies described random housing of animals, which were considered to have a low risk of bias. Additionally, the included studies exhibited a low risk of bias in selective reporting. Overall, the included studies generally had unclear risks of bias, though some studies showed low risks of bias in specific domains. The detailed results of the risk of bias assessment are presented in Figures 2A, B.

Figure 2. Risk of bias assessment results for 17 studies based on SYRCLE’s ROB tool. (A) Risk of bias graph; (B) Risk of bias summary.
Meta-analysis results
MSC-EVs intervention significantly increases bone mineral density and bone volume in osteoporosis models
Fourteen studies reported the effects of MSC-EVs on BMD in osteoporosis models. Meta-analysis results showed that MSC-EVs intervention significantly increased BMD in animal models (SMD = 3.95; 95% CI: 2.80 to 5.10; P < 0.00001) (Figure 3). Due to significant heterogeneity (I² = 72%, P < 0.00001), subgroup analyses were further conducted. Subgroups were categorized based on stem cell source (BMSC or non-BMSC), animal ages (immature or adult), gender (male or female), isolation method (ultracentrifugation), purification technique (filtered through a filter), EV size (small or large EVs), intervention routes (intravenous injection), frequency (once or twice a week), dose (≤ 100 μl/μg or > 100 μl/μg) and duration (<2 months or ≥2 months). Results indicated that all subgroups significantly increased BMD in osteoporosis models, but none were significant sources of heterogeneity (Supplementary Figures 1–10).

Figure 3. Forest plot showing the effect of MSC-EVs on BMD in osteoporosis models. Data are presented as standardized mean differences (SMD) with 95% confidence intervals (CI).
Additionally, to explore the effects of MSC-EVs on bone volume in osteoporosis models, 14 studies reporting BV/TV were pooled. Results showed that MSC-EVs intervention significantly increased bone volume compared to the control group (SMD = 5.43; 95% CI: 3.94 to 6.93; P < 0.00001; I² = 76%, P < 0.00001) (Figure 4). Further subgroup analyses revealed that, except for the “> 100 μl/μg” subgroup, all other subgroups improved bone volume in osteoporosis models. However, none were significant sources of heterogeneity (Supplementary Figures 11–19). These results demonstrate that MSC-EVs intervention significantly increases BMD and bone volume in models, thereby ameliorating osteoporosis-induced bone loss.
![Forest plot showing the standardized mean differences (SMD) with 95% confidence intervals (CI) for various studies comparing MSC-EVs and control groups. Individual study results are displayed with green squares and lines representing the CIs. The overall effect size is illustrated by a diamond at the bottom, indicating a significant effect favoring MSC-EVs, with an overall SMD of 5.43 [3.94, 6.93]. Heterogeneity statistics include Tau² = 5.38, Chi² = 55.29 with 13 degrees of freedom (P < 0.00001), and I² = 76%.](https://www.frontiersin.org/files/Articles/1625969/fendo-16-1625969-HTML/image_m/fendo-16-1625969-g004.jpg)
Figure 4. Forest plot depicting the effect of MSC-EVs on BV/TV in osteoporosis models. Data are presented as standardized mean differences (SMD) with 95% confidence intervals (CI).
MSC-EVs intervention significantly improves bone structural parameters in osteoporosis models
Trabecular bone structural parameters (Tb.N, Tb.Th, and Tb.Sp) are key indicators for assessing the spatial morphology of trabecular bone. Sixteen studies reported Tb.N parameters before and after MSC-EVs intervention. Meta-analysis results showed that MSC-EVs intervention significantly increased Tb.N in animal models (SMD = 4.57; 95% CI: 3.49 to 5.66; P < 0.00001; I² = 68%, P < 0.0001) (Figure 5A). Subgroup analyses revealed that, except for the “> 100 μl/μg” subgroup in intervention dose, all other subgroups significantly increased Tb.N in osteoporosis models, but none were significant sources of heterogeneity (Supplementary Figures 20–29). Pooled analysis of 14 studies showed that MSC-EVs intervention significantly increased Tb.Th in animal models (SMD = 2.98; 95% CI: 1.98 to 3.97; P < 0.00001) (Figure 5B). Due to significant heterogeneity (I² = 76%, P < 0.00001), further subgroup analyses indicated that, except for the “other frequencies” and “other routes” subgroups, all subgroups significantly increased Tb.Th in osteoporosis models, but none were significant sources of heterogeneity (Supplementary Figures 30–39). Next, pooled analysis of 13 studies on Tb.Sp before and after MSC-EVs intervention showed that MSC-EVs intervention significantly reduced Tb.Sp in osteoporosis models (SMD = -5.22; 95% CI: -6.98 to -3.46; P < 0.00001; I² = 83%, P < 0.0001) (Figure 5C). Further subgroup analyses revealed that, except for the “> 100 μl/μg” subgroup in intervention dose, all other subgroups reduced Tb.Sp (Supplementary Figures 40–49). However, none of the subgroups were significant sources of heterogeneity.
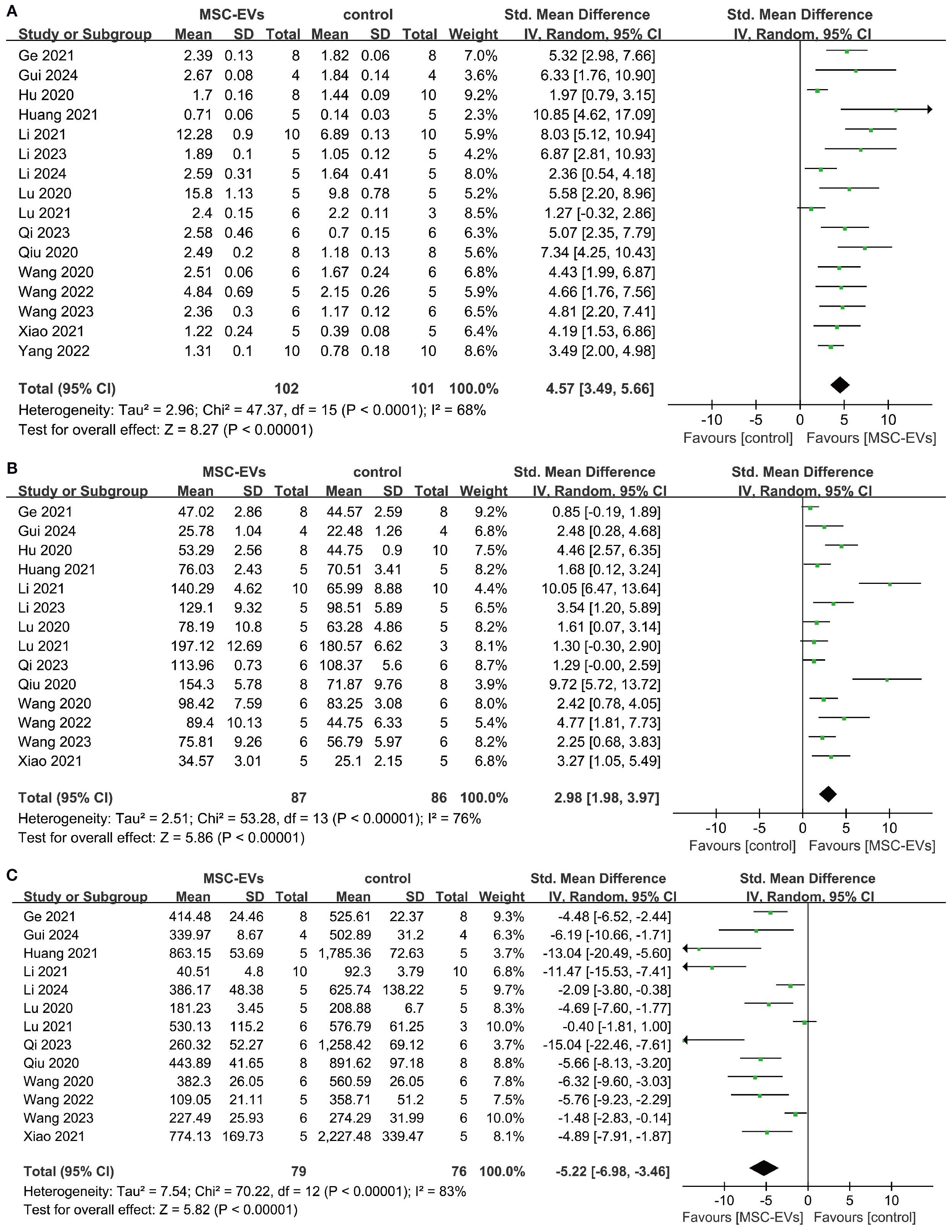
Figure 5. Forest plot showing the effect of MSC-EVs on trabecular structural parameters in osteoporosis models. (A) trabecular number (Tb. N); (B) trabecular thickness (Tb. Th); (C) trabecular separation/marrow thickness (Tb. Sp). Data are presented as standardized mean differences (SMD) with 95% confidence intervals (CI).
Additionally, three studies reported Ct.Th in the models. Pooled analysis showed that MSC-EVs intervention significantly increased Ct.Th (SMD = 1.82; 95% CI: 1.00 to 2.64; P < 0.0001; I² = 0%, P = 0.40) (Figure 6). Two studies reported the bone remodeling parameter mineral apposition rate (MAR). Meta-analysis showed that MSC-EVs intervention accelerated bone mineralization, possibly indicating increased osteoblast activity (SMD = 8.88; 95% CI: 2.23 to 15.53; P = 0.009; I² = 74%, P = 0.05) (Figure 7). Overall, compared to the control group, MSC-EVs intervention significantly improved trabecular and cortical bone structural parameters in osteoporosis models and promoted bone mineralization.
![Forest plot showing the standard mean difference in studies comparing MSC-EVs to control. Three studies (Hu 2020, Lu 2020, Xiao 2021) are included. The plot indicates positive effects favoring MSC-EVs, with an overall effect size of 1.82 (95% CI [1.00, 2.64]). Heterogeneity is low, with I² at 0%.](https://www.frontiersin.org/files/Articles/1625969/fendo-16-1625969-HTML/image_m/fendo-16-1625969-g006.jpg)
Figure 6. Forest plot depicting the effect of MSC-EVs on Ct.Th in osteoporosis models. Data are presented as standardized mean differences (SMD) with 95% confidence intervals (CI).

Figure 7. Forest plot showing the effect of MSC-EVs on mineral apposition rate (MAR) in the osteoporosis model. Data are presented as standardized mean differences (SMD) with 95% confidence intervals (CI).
Ultimate load-bearing capacity of femur
Three studies also evaluated the biomechanical properties of the femur in animal models before and after MSC-EVs intervention. Meta-analysis results showed that MSC-EVs intervention significantly increased the ultimate load-bearing capacity of the femur (SMD = 2.38; 95% CI: 1.03 to 3.72; P = 0.0005; I² = 50%, P = 0.14) (Figure 8).
![Forest plot comparing MSC-EVs to control in three studies: Hu 2020, Wang 2023, and Yang 2022. Each study shows mean differences with 95% confidence intervals. Combined effect size is 2.38 with a confidence interval of [1.03, 3.72]. Test for overall effect shows Z = 3.46 (P = 0.0005), indicating statistical significance. Heterogeneity is moderate with I² = 50%. Solid squares and a diamond represent individual and total study effects, respectively.](https://www.frontiersin.org/files/Articles/1625969/fendo-16-1625969-HTML/image_m/fendo-16-1625969-g008.jpg)
Figure 8. Forest plot depicting the effect of MSC-EVs on ultimate load-bearing capacity of the femur in osteoporosis model. Data are presented as standardized mean differences (SMD) with 95% confidence intervals (CI).
Sensitivity analysis and publication bias
To evaluate the robustness of the results, sensitivity analyses were conducted for BMD, BV/TV, Tb.N, Tb.Th, and Tb.Sp. Results showed that the outcomes remained consistent after excluding each individual study (Figures 9A–E), demonstrating the reliability and stability of the results. Further assessment of publication bias revealed asymmetry in the funnel plots, indicating the presence of publication bias (Supplementary Figure 50), which was confirmed by Egger’s test (Table 3). Trim-and-fill analysis for BMD, BV/TV, Tb.N, Tb.Th, and Tb.Sp showed no significant changes in heterogeneity, suggesting robust outcomes (Supplementary Figure 51).

Figure 9. Sensitivity analysis results for primary outcomes. (A) BMD; (B) BV/TV; (C) Tb. N; (D) Tb. Th; (E) Tb. Sp.
Discussion
To our knowledge, this is the first meta-analysis assessing the preclinical therapeutic efficacy of MSC-EVs for osteoporosis, providing a certain degree of reference value for further mechanistic exploration and clinical translation. This meta-analysis included 17 preclinical studies involving 625 animals. The pooled analysis results indicated that MSC-EVs intervention improved BMD, bone mass, structural parameters, bone remodeling parameters (MAR), and bone biomechanical properties in osteoporosis. Specifically, it increased BMD, BV/TV, Tb.N, Tb.Th, Ct.Th, MAR, and the ultimate load-bearing capacity of the femur while reducing Tb.Sp. These improvements suggest that MSC-EVs may contribute to the overall structural repair of osteoporotic bone, demonstrating promising potential for osteoporosis treatment in animal models. However, considering the limitations of study heterogeneity and the number of studies, further research is still needed to support the beneficial effects of MSC-EVs in osteoporosis models.
BMD, as an indicator of bone strength, is a key factor in the clinical diagnosis and treatment of osteoporosis as well as in the assessment of fracture risk (40). Specifically, an increase in BMD indicates that bone formation exceeds bone loss, resulting in increased bone mass. BV/TV represents the ratio of bone volume to tissue volume, directly reflecting changes in bone mass and playing a crucial role in evaluating the efficacy of osteoporosis treatments (41, 42). Among the included studies, 14 reported pre- and post-intervention measurements of BMD and BV/TV, highlighting their potential reference value and clinical significance. Based on the meta-analysis results, MSC-EVs increased BMD levels in the osteoporosis model compared to the control group, demonstrating a beneficial effect on bone strength and bone mass. However, given the significant heterogeneity observed in the pooled results for both indicators, these findings should be interpreted with caution. Although subgroup analysis showed that improvements in BMD and BV/TV were observed across various subgroups classified by EVs source, engineering methods, targets, intervention pathways, frequency, duration, and animal model types, none of these factors were identified as significant contributors to the observed heterogeneity.
Additionally, trabecular and cortical bone structural parameters are equally important for evaluating the therapeutic effects of osteoporosis treatment (43). Trabecular bone forms a porous lattice structure through interconnections and is arranged according to stress distribution patterns, which helps enhance the mechanical strength of bone tissue (44). As key indicators of trabecular spatial morphology, Tb.N, Tb.Th, and Tb.Sp were analyzed in this meta-analysis. The results showed that, compared to the control group, MSC-EVs treatment increased Tb.N and Tb.Th while reducing Tb.Sp, indicating that bone formation exceeded bone resorption, leading to significant structural improvements in the osteoporotic model. Compared to trabecular parameters, fewer studies have measured cortical bone parameters, as cortical bone changes often occur later than trabecular bone alterations. Among the included studies, three reported Ct.Th measurements, showing that MSC-EVs increased Ct.Th, which may suggest that MSC-EVs also hold considerable therapeutic potential in the later stages of bone formation. However, further studies with longer treatment durations are necessary to validate these findings.
Clinical drugs primarily improve bone strength and increase bone mass by inhibiting bone resorption and promoting bone formation, thereby regulating bone metabolism. Similar to clinical drugs, the therapeutic strategy of MSC-EVs also focuses on bone metabolism regulation (45). Mechanistically, multiple signaling pathways are involved in the bone remodeling process mediated by MSC-EVs in osteoporosis models, including the RANKL/RANK/OPG, WNT/β-catenin, Hippo, and PI3K/Akt pathways. Zhao et al. (46) found that BMSC-EVs promote osteoblast proliferation and differentiation in vitro via the MAPK pathway. Another study reported that BMSC-EVs reduce intracellular oxidative stress, promote DNA damage repair, and mitigate bone loss by activating the Wnt/β-catenin signaling pathway (47). Additionally, Li et al. (29) demonstrated that EVs derived from BMSCs facilitate bone repair in osteoporotic rats by delivering miR-186 through the Hippo pathway. Similar to these findings, this meta-analysis included seven studies investigating the potential mechanisms by which MSC-EVs improve osteoporosis, involving signaling pathways such as MAPK (25, 31), Wnt/β-catenin (21, 28, 37), PI3K/Akt (33), and NF-κB (38) (Figure 10). Given that the precise mechanisms underlying MSC-EVs treatment for osteoporosis remain unclear, further research is needed to supplement and refine current knowledge.
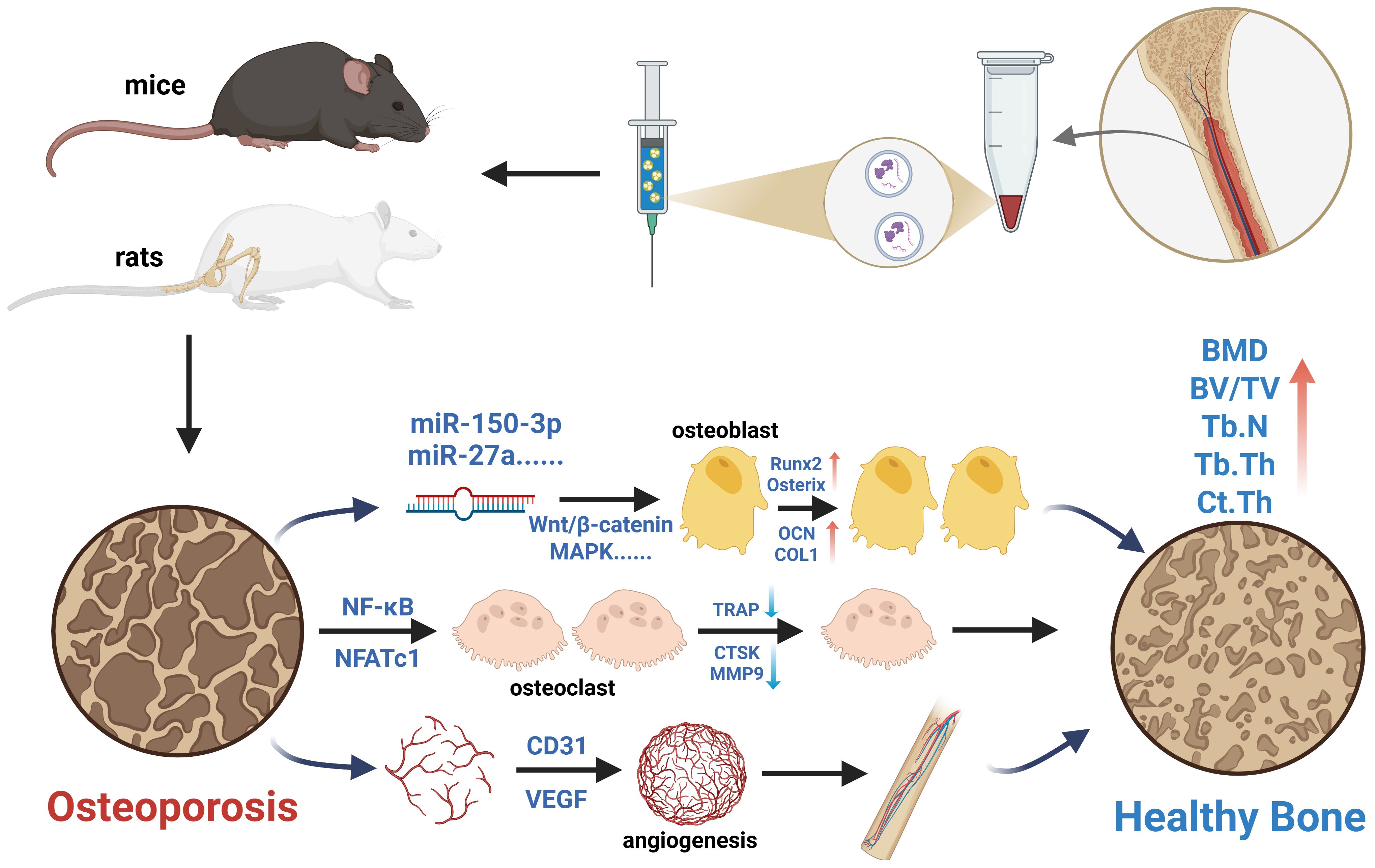
Figure 10. Schematic illustration of the potential mechanisms of MSC-EVs in the treatment of osteoporosis models. Created in https://BioRender.com.
Currently, research on MSC-EVs intervention in osteoporotic animal models primarily focuses on the efficacy comparison of bone structural parameters while overlooking the therapeutic mechanisms and potential microscopic effects of MSC-EVs. These include the activity and function of osteoblasts, osteoclasts, human umbilical vein endothelial cells, and immune cells. Therefore, beyond bone metabolism regulation, future studies should place greater emphasis on exploring the angiogenic and immunomodulatory effects of MSC-EVs to further elucidate their therapeutic potential.
Limitations
However, several study limitations must be considered. Firstly, significant differences in baseline characteristics among the included studies may have influenced the meta-analysis results, including variations in animal models, EVs preparation, and intervention characteristics (such as administration route, frequency, and treatment duration). Although subgroup analysis indicated that these factors were not significant contributors to heterogeneity, the interpretation of results should still be approached with caution. Future research should emphasize efficacy evaluation and comparison under standardized conditions based on animal models and EVs characteristics. Secondly, publication bias was present in all analytical results, which affected the quality of evidence in the meta-analysis. Future studies with larger sample sizes and standardized methodologies are needed to address this limitation. Thirdly, although sensitivity analysis confirmed the stability of the results, the absence of randomization and blinding procedures may have led to an overestimation of the therapeutic effects of MSC-EVs. Moreover, the analysis revealed varying degrees of heterogeneity and publication bias. Future studies should carefully consider negative or null findings to ensure the objectivity and robustness of the conclusions. Finally, most studies lacked safety data on MSC-EVs treatment, including toxicity and immunogenicity. Future research should prioritize the long-term monitoring of safety parameters to ensure the clinical applicability of MSC-EVs.
Conclusions
In conclusion, this meta-analysis highlights the potential therapeutic value of MSC-EVs in osteoporotic animal models by assessing bone strength, bone mass, structural parameters, remodeling parameters, and biomechanical properties. The pooled analysis results provide evidence supporting the efficacy of MSC-EVs therapy in preclinical osteoporosis models. However, due to significant heterogeneity and publication bias, the findings should be interpreted with caution. Additionally, further studies are needed to establish standardized protocols and evaluate the safety of MSC-EVs interventions in more animal models and clinical trials.
Author contributions
YZ: Formal analysis, Methodology, Resources, Writing – original draft, Investigation, Software. YL: Methodology, Resources, Writing – original draft, Investigation, Software. SW: Data curation, Supervision, Visualization, Funding acquisition, Writing – review & editing. CW: Project administration, Validation, Data curation, Supervision, Conceptualization, Visualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1625969/full#supplementary-material
Abbreviations
BMD, Bone Mineral Density; BMSCs, Bone Marrow Mesenchymal Stem Cells; BV/TV, Bone Volume/Total Volume; CI, Confidence Interval; Ct.Th, Cortical Thickness; EVs, Extracellular Vesicles; HU, Hindlimb Unloading; MAR, Mineral Apposition Rate; MAPK, Mitogen-Activated Protein Kinase; MSCs, Mesenchymal Stem Cells; MSC-EVs, Mesenchymal Stem Cell-Derived Extracellular Vesicles; NF-κB, Nuclear Factor-kappa B; OPG, Osteoprotegerin; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RANKL, Receptor Activator of Nuclear Factor-κB Ligand; SD, Standard Deviation; SMD, Standardized Mean Difference; SYRCLE, Systematic Review Centre for Laboratory Animal Experimentation; Tb.N, Trabecular Number; Tb.Sp, Trabecular Separation; Tb.Th, Trabecular Thickness; VEGF, Vascular Endothelial Growth Factor.
References
1. Johnston CB and Dagar M. Osteoporosis in older adults. Med Clin North Am. (2020) 104:873–84. doi: 10.1016/j.mcna.2020.06.004
2. Lewiecki EM and Watts NB. New guidelines for the prevention and treatment of osteoporosis. South Med J. (2009) 102:175–9. doi: 10.1097/SMJ.0b013e31818be99b
3. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. (2014) 29:2520–6. doi: 10.1002/jbmr.2269
4. Johnell O and Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. (2006) 17:1726–33. doi: 10.1007/s00198-006-0172-4
5. Wright NC, Saag KG, Dawson-Hughes B, Khosla S, and Siris ES. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos Int. (2017) 28:1225–32. doi: 10.1007/s00198-016-3865-3
6. Sözen T, Özışık L, and Başaran N. An overview and management of osteoporosis. Eur J Rheumatol. (2017) 4:46–56. doi: 10.5152/eurjrheum.2016.048
7. Kannegaard PN, van der Mark S, Eiken P, and Abrahamsen B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. (2010) 39:203–9. doi: 10.1093/ageing/afp221
8. Ukon Y, Makino T, Kodama J, Tsukazaki H, Tateiwa D, Yoshikawa H, et al. Molecular-based treatment strategies for osteoporosis: A literature review. Int J Mol Sci. (2019) 20:2557. doi: 10.3390/ijms20102557
9. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, and Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab. (2020) 105:dgaa048. doi: 10.1210/clinem/dgaa048
10. Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. Jama. (2010) 304:1480–4. doi: 10.1001/jama.2010.1360
11. Martin TJ and Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. (2005) 11:76–81. doi: 10.1016/j.molmed.2004.12.004
12. Mullard A. Merck &Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. (2016) 15:669. doi: 10.1038/nrd.2016.207
13. Krampera M and Le Blanc K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell. (2021) 28:1708–25. doi: 10.1016/j.stem.2021.09.006
14. Galipeau J and Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. (2018) 22:824–33. doi: 10.1016/j.stem.2018.05.004
15. Chen FM, Wu LA, Zhang M, Zhang R, and Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials. (2011) 32:3189–209. doi: 10.1016/j.biomaterials.2010.12.032
16. Manieri NA, Mack MR, Himmelrich MD, Worthley DL, Hanson EM, Eckmann L, et al. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Invest. (2015) 125:3606–18. doi: 10.1172/JCI81423
17. Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. (2011) 108:1340–7. doi: 10.1161/CIRCRESAHA.110.239848
18. Keshtkar S, Azarpira N, and Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. (2018) 9:63. doi: 10.1186/s13287-018-0791-7
19. Boulestreau J, Maumus M, Jorgensen C, and Noël D. Extracellular vesicles from mesenchymal stromal cells: Therapeutic perspectives for targeting senescence in osteoarthritis. Adv Drug Delivery Rev. (2021) 175:113836. doi: 10.1016/j.addr.2021.113836
20. Wang Y, Yao J, Cai L, Liu T, Wang X, Zhang Y, et al. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int J Nanomed. (2020) 15:7967–77. doi: 10.2147/IJN.S263756
21. Wang Y, Zhou X, and Wang D. Mesenchymal stem cell-derived extracellular vesicles inhibit osteoporosis via microRNA-27a-induced inhibition of DKK2-mediated wnt/β-catenin pathway. Inflammation. (2022) 45:780–99. doi: 10.1007/s10753-021-01583-z
22. Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. (2017) 18:1450. doi: 10.3390/ijms18071450
23. Gangadaran P, Rajendran RL, Lee HW, Kalimuthu S, Hong CM, Jeong SY, et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release. (2017) 264:112–26. doi: 10.1016/j.jconrel.2017.08.022
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Yahao G and Xinjia W. The role and mechanism of exosomes from umbilical cord mesenchymal stem cells in inducing osteogenesis and preventing osteoporosis. Cell Transplant. (2021) 30:9636897211057465. doi: 10.1177/09636897211057465
26. Gui L, Ye Q, Yu L, Dou G, Zhou Y, Liu Y, et al. Bone-targeting peptide and RNF146 modified apoptotic extracellular vesicles alleviate osteoporosis. Int J Nanomed. (2024) 19:471–88. doi: 10.2147/IJN.S433511
27. Hu Y, Zhang Y, Ni CY, Chen CY, Rao SS, Yin H, et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics. (2020) 10:2293–308. doi: 10.7150/thno.39238
28. Huang B, Su Y, Shen E, Song M, Liu D, and Qi H. Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model. Life Sci. (2021) 272:119208. doi: 10.1016/j.lfs.2021.119208
29. Li L, Zhou X, Zhang JT, Liu AF, Zhang C, Han JC, et al. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J Orthop Surg Res. (2021) 16:23. doi: 10.1186/s13018-020-02160-0
30. Li F, Zhao X, Zhang Y, Zhuang Q, Wang S, Fang X, et al. Exosomal circFAM63Bsuppresses bone regeneration of postmenopausal osteoporosis via regulating miR-578/HMGA2 axis. J Orthop Res. (2024) 42:1244–53. doi: 10.1002/jor.25776
31. Li M, Tang Q, Liao C, Wang Z, Zhang S, Liang Q, et al. Extracellular vesicles from apoptotic BMSCs ameliorate osteoporosis via transporting regenerative signals. Theranostics. (2024) 14:3583–602. doi: 10.7150/thno.96174
32. Lu GD, Cheng P, Liu T, and Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol. (2020) 8:608521. doi: 10.3389/fcell.2020.608521
33. Lu CH, Chen YA, Ke CC, Chiu SJ, Jeng FS, Chen CC, et al. Multiplexed molecular imaging strategy integrated with RNA sequencing in the assessment of the therapeutic effect of wharton’s jelly mesenchymal stem cell-derived extracellular vesicles for osteoporosis. Int J Nanomed. (2021) 16:7813–30. doi: 10.2147/IJN.S335757
34. Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. (2016) 12:836–49. doi: 10.7150/ijbs.14809
35. Qi H, Shen E, Shu X, Liu D, and Wu C. ERK-estrogen receptor α signaling plays a role in the process of bone marrow mesenchymal stem cell-derived exosomes protecting against ovariectomy-induced bone loss. J Orthop Surg Res. (2023) 18:250. doi: 10.1186/s13018-023-03660-5
36. Qiu M, Zhai S, Fu Q, and Liu D. Bone marrow mesenchymal stem cells-derived exosomal microRNA-150-3p promotes osteoblast proliferation and differentiation in osteoporosis. Hum Gene Ther. (2021) 32:717–29. doi: 10.1089/hum.2020.005
37. Wang X, Zou C, Hou C, Bian Z, Jiang W, Li M, et al. Extracellular vesicles from bone marrow mesenchymal stem cells alleviate osteoporosis in mice through USP7-mediated YAP1 protein stability and the Wnt/β-catenin pathway. Biochem Pharmacol. (2023) 217:115829. doi: 10.1016/j.bcp.2023.115829
38. Xiao F, Zuo B, Tao B, Wang C, Li Y, Peng J, et al. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann Transl Med. (2021) 9:798. doi: 10.21037/atm-21-1838
39. Yang Z, Liu X, Zhao F, Yao M, Lin Z, Yang Z, et al. Bioactive glass nanoparticles inhibit osteoclast differentiation and osteoporotic bone loss by activating lncRNA NRON expression in the extracellular vesicles derived from bone marrow mesenchymal stem cells. Biomaterials. (2022) 283:121438. doi: 10.1016/j.biomaterials.2022.121438
40. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS-2020 UPDATE. Endocr Pract. (2020) 26:1–46. doi: 10.4158/GL-2020-0524SUPPL
41. Tamimi I, Cortes ARG, Sánchez-Siles JM, Ackerman JL, González-Quevedo D, García Á, et al. Composition and characteristics of trabecular bone in osteoporosis and osteoarthritis. Bone. (2020) 140:115558. doi: 10.1016/j.bone.2020.115558
42. Stadelmann VA, Gerossier E, Kettenberger U, and Pioletti DP. Combining systemic and local osteoporosis treatments: A longitudinal in vivo microCT study in ovariectomized rats. Bone. (2025) 192:117373. doi: 10.1016/j.bone.2024.117373
43. Ito M, Nishida A, Koga A, Ikeda S, Shiraishi A, Uetani M, et al. Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone. (2002) 31:351–8. doi: 10.1016/S8756-3282(02)00830-X
44. Wehrli FW. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J Magn Reson Imaging. (2007) 25:390–409. doi: 10.1002/jmri.20807
45. Lu CH, Chen YA, Ke CC, and Liu RS. Mesenchymal stem cell-derived extracellular vesicle: A promising alternative therapy for osteoporosis. Int J Mol Sci. (2021) 22:12750. doi: 10.3390/ijms222312750
46. Zhao P, Xiao L, Peng J, Qian YQ, and Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. (2018) 22:3962–70. doi: 10.26355/eurrev_201806_15280
Keywords: extracellular vesicle, osteoporosis, mesenchymal stem cell, bone mineral density, meta-analysis
Citation: Zhang Y, Liu Y, Wang S and Wang C (2025) Therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in osteoporosis models: a systematic review and meta-analysis of preclinical studies. Front. Endocrinol. 16:1625969. doi: 10.3389/fendo.2025.1625969
Received: 09 May 2025; Accepted: 29 August 2025;
Published: 16 September 2025.
Edited by:
Bingdong Sui, Air Force Medical University, ChinaReviewed by:
Wencai Liu, Shanghai Jiao Tong University, ChinaHongbo Tan, The 920 Affiliated Hospital of Kunming Medical University, China
Copyright © 2025 Zhang, Liu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Wang, MzE0Mzc5Njk1QHFxLmNvbQ==; Shaoyun Wang, Mjg1NTc4NDQwQHFxLmNvbQ==
†These authors have contributed equally to this work
 Ying Zhang
Ying Zhang Yi Liu2†
Yi Liu2†