- 1Division of Thyroid Surgery, Department of General Surgery; Laboratory of Thyroid and Parathyroid Diseases, Frontiers Science Center for Disease-Related Molecular Network, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Respiratory and Critical Care Medicine, Frontiers Science Center for Disease-related Molecular Network, Center of Precision Medicine, Precision Medicine Key Laboratory of Sichuan Province, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Operating Room, West China Hospital, West China School of Nursing, Sichuan University, Chengdu, Sichuan, China
Objective: Current preoperative diagnostics inadequately differentiate benign from malignant thyroid follicular neoplasms. This study evaluated the diagnostic utility of thyroid function markers and contrast-enhanced ultrasound (CEUS) features in differentiating follicular thyroid adenoma (FTA) from follicular thyroid carcinoma (FTC), focusing on a novel parameter: the thyroglobulin-to-tumor volume ratio (Tg/Vol ratio).
Methods: We retrospectively analyzed 432 resected thyroid follicular neoplasms. A comprehensive comparison was performed regarding baseline characteristics, thyroid function profiles, and CEUS features between FTA and FTC groups through univariate and multivariate binary logistic regression. Diagnostic performance was determined via receiver operating characteristic (ROC) curve analysis. The prevalence of FTC across serum marker subgroups was assessed, followed by the development of a multivariate diagnostic model integrating the Tg/Vol ratio with CEUS characteristics.
Results: Among 432 patients (352 females, 81.5%) with a median age of 47 years, multivariate logistic regression analysis revealed three independent predictors of FTC: capsular involvement (odds ratio [OR] = 9.958, 95% confidence interval [CI]: 2.453 – 40.424, p = 0.001), Tg/Vol ratio >7.412 (OR = 3.508, 95% CI: 1.388 – 8.868, p = 0.008), and male gender (OR = 3.474, CI: 1.751 – 6.891, p < 0.001). Subgroup analyses revealed higher FTC prevalence in patients with Tg > 409.18 μg/L (20.41%, p = 0.002) and Tg/Vol ratio > 20.68 (20.41%, p = 0.009). The combined diagnostic model incorporating Tg/Vol ratio and CEUS features demonstrated 69.4% sensitivity, 77.0% specificity, and the area under the curve(AUC) of 0.769.
Conclusion: While elevated preoperative Tg correlates with malignant potential, but the Tg/Vol ratio emerges as a more robust preoperative discriminator. The combined diagnostic model incorporating Tg/Vol ratio and CEUS features significantly improves FTC detection accuracy.
Introduction
Thyroid follicular neoplasms, the second most prevalent thyroid tumors, are histologically classified into follicular thyroid adenoma (FTA) and follicular thyroid carcinoma (FTC) (1). The diagnostic gold standard—identification of capsular and/or vascular invasion—can only be confirmed postoperatively through pathological examination, posing a critical clinical dilemma in preoperative decision-making (2).
While ultrasonography serves as the primary screening modality for thyroid nodules, its diagnostic accuracy for follicular neoplasms remains suboptimal (3, 4). The European Thyroid Association guidelines noted that FTC may more frequently exhibit irregular margins and microcalcifications (5); however, a recent study involving 705 patients revealed significant limitations in conventional Thyroid Imaging Reporting and Data System in differentiating FTC from FTA (6). Unlike papillary thyroid carcinoma (PTC), fine-needle aspiration biopsy demonstrates limited diagnostic value for FTC due to its inability to assess capsular or vascular invasion, the pathological hallmarks of malignancy (7).
In contrast to medullary thyroid carcinoma (MTC), where calcitonin serves as a reliable serum marker (8), no validated preoperative biomarker currently exists for FTC (9). Emerging evidence suggests that serum thyroglobulin (Tg) levels correlate with both the secretory activity and malignant potential of thyroid tumors, with FTC typically exhibiting elevated Tg secretion (10, 11). This positions Tg as a potential discriminator between benign and malignant follicular neoplasms.
However, serum Tg concentration alone suffers from limited specificity due to confounding factors, including thyroid volume and inflammatory status (12, 13). While Tg levels exhibit nonspecific elevation due to thyroid tissue volume, the nonlinear correlation between tumor size and neoplastic Tg production further compromises diagnostic accuracy. (14). To address these limitations, the serum thyroglobulin-to-tumor volume ratio (Tg/Vol ratio), a novel parameter correcting for tumor dimension, may better reflect the functional-morphological disparities between FTA and FTC by normalizing secretory output to lesion size.
Therefore, this study aimed to evaluate the diagnostic utility of preoperative thyroid function profiles and CEUS features in distinguishing FTA from FTC; and develop a composite diagnostic model to enhance preoperative FTC detection; Additionally, this represents the first large-scale study to systematically evaluate the diagnostic efficacy of the noninvasive Tg/Vol ratio as a novel discriminator for thyroid follicular neoplasms.
Materials and methods
Study population
This retrospective study analyzed consecutive patients who underwent initial surgical resection for thyroid follicular neoplasms at West China Hospital between January 2012 and December 2024. Exclusion criteria were as follows: (1) Insufficient preoperative data (serum thyroid markers or CEUS examinations); (2) Final pathological diagnosis of follicular tumor of uncertain malignant potential (FT-UMP) or noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP); (3) Concurrent acute or chronic thyroiditis (Patients with clinical manifestations of neck pain, swelling, or tenderness, positive thyroid autoantibodies, and characteristic ultrasonographic features such as heterogeneous parenchymal echotexture or increased vascularity); (4) Predominantly cystic lesions or significant thyroid gland enlargement. The study was assessed and approved by the ethics committees of the hospitals (No.20242251). The requirement for written informed consent was waived due to the study’s retrospective design and no patients were excluded based on consent status.
Serological analysis
Serum thyroglobulin (Tg; reference range: 3.50 – 77.00 μg/L), thyroglobulin antibodies (TgAb; 0 – 115.00 IU/mL), thyroid peroxidase antibodies (TPOAb; 0 – 34.00 IU/mL), and thyroid-stimulating hormone receptor antibodies (TRAb; 0 – 1.75 IU/mL) were quantified via electrochemiluminescence immunoassay. Thyroid-stimulating hormone (TSH; 0.27 – 4.2 mIU/L), free triiodothyronine (FT3; 3.60 – 7.50 pmol/L), and free thyroxine (FT4; 12.0 – 22.0 pmol/L) levels were measured using chemiluminescent immunoassay.
TgAb positivity was defined as ≥ 115.00 IU/mL. Given potential TgAb interference, Tg analysis was restricted to TgAb-negative patients (< 115.00 IU/mL). For Tg values exceeding the upper detection limit (5,000 μg/L), results were censored at 5,000 μg/L. All measurements were conducted in this center according to unified standard protocols to ensure reproducibility.
Tumor volume calculation
Tumor volume (cm³) was calculated using the prolate ellipsoid formula, which is currently one of the standard methods for ultrasonographic tumor volume measurement and has been validated in prior thyroid studies (15). Dimensions were obtained from CEUS reports, with data validity confirmed through rigorous reliability testing.
Image analysis
A linear array probe (5–12 MHz) was used. Patients assumed a supine position with full neck exposure. Contrast-enhanced ultrasound dynamically visualized multiple thyroid sections. Two experienced sonographers independently analyzed all image parameters; discrepancies were resolved by a third physician’s consultation for consensus.
Statistical analysis
All statistical analyses were performed using SPSS 27.0 (IBM Corp., Armonk, NY, USA), with a two-tailed significance level set at α = 0.05. Continuous variables with non-normal distributions were expressed as median (range) and compared using the Mann-Whitney U test. Categorical variables were presented as frequencies (percentages) and analyzed by χ² test or Fisher’s exact test, as appropriate.
To identify risk factors for FTC, both univariate and multivariate binary logistic regression analyses were conducted. The diagnostic performance of significant predictors was evaluated using receiver operating characteristic (ROC) curve analysis, with sensitivity and specificity reported. For statistically significant indicators, the area under the ROC curve (AUC) was compared using DeLong’s test to assess predictive value and determine optimal cutoff thresholds. A combined diagnostic model was subsequently developed through binary logistic regression.
Additionally, Spearman’s rank correlation analysis was employed to examine the association between FTC prevalence and various patient subgroups, with correlation coefficients (r) reported.
Results
Clinical characteristics
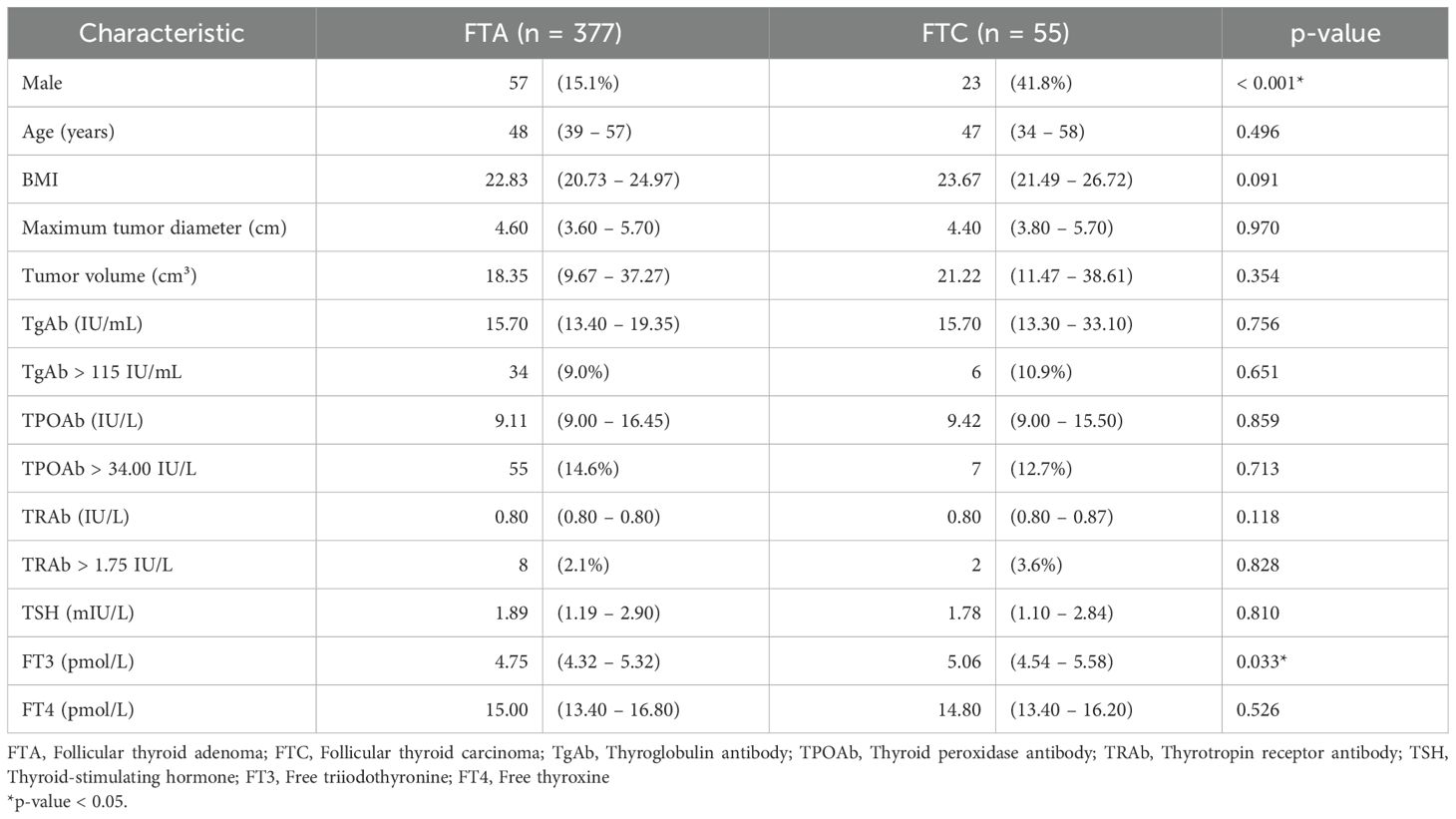
The study cohort comprised 432 patients with thyroid follicular neoplasms, including 377 cases of FTA and 55 cases of FTC. The cohort included 352 females (81.5%) and 80 males (18.5%), with an age range of 3–80 years (mean ± standard deviation [SD], 47.4 ± 13.6 years).
Comparative analysis between FTA and FTC groups revealed statistically significant differences in male predominance (15.1% vs. 41.8%, p < 0.001) and preoperative serum FT3 levels (median [interquartile range, IQR]: 4.75 [4.32 – 5.32] vs. 5.06 [4.54 – 5.58] pmol/L, p = 0.033). No significant differences were observed in age, body mass index (BMI), maximal tumor diameter, tumor volume, or levels of TgAb, TPOAb, TRAb, TSH, or FT4. Additionally, the proportions of patients with elevated TgAb, TPOAb, or TRAb did not differ significantly between groups (Table 1).
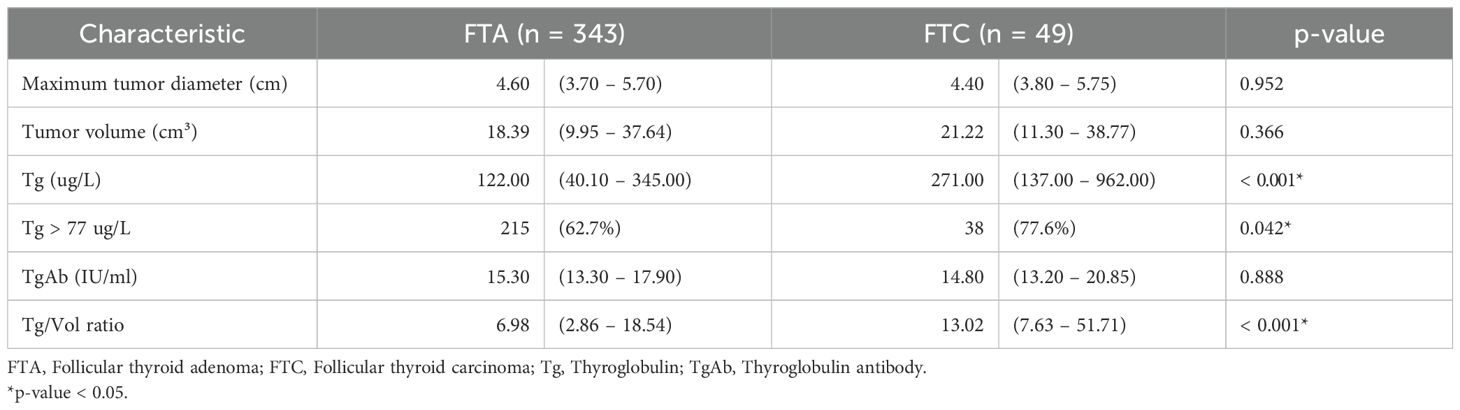
To minimize interference from elevated TgAb, analyses involving Tg and Tg/Vol ratio were restricted to TgAb-negative patients (< 115.00 IU/mL). Among 392 TgAb-negative patients (343 FTA, 49 FTC), significant intergroup differences were noted in preoperative serum Tg levels (122.00 [40.10 – 345.00] vs. 271.00 [137.00 – 962.00] μg/L, p < 0.001), Tg elevation prevalence (62.7% vs. 77.6%, p = 0.042), and Tg/Vol ratio (6.98 [2.86 – 18.54] vs. 13.02 [7.63 – 51.71], p < 0.001). No differences were detected in maximal tumor diameter, tumor volume, or TgAb levels (Table 2).
Ultrasonographic features
Comparative analysis of 377 FTA and 55 FTC cases demonstrated significant differences in tumor morphology (p = 0.003), margin clarity (p = 0.020), calcification patterns (p < 0.001), and capsular involvement (p < 0.001). No significant variations were observed in tumor location (p = 0.158), aspect ratio (p = 1.000), cystic-solid composition (p = 0.172), or Adler blood flow grading (p = 0.773) (Supplementary Table 1).
Odds ratios of clinical and ultrasonographic characteristics
Univariate analysis revealed statistically significant differences between FTA and FTC patients regarding gender, FT3 levels, Tg levels, Tg/Vol ratio, tumor morphology, margin clarity, presence and type of calcifications, and capsular involvement (all p < 0.05).
ROC curves were constructed for FT3, Tg, and Tg/Vol ratio in FTC diagnosis (Figure 1). Optimal cutoff values determined by Youden’s index were: FT3 > 5.045 pmol/L (AUC 0.647), Tg > 217.5 μg/L (AUC 0.608), and Tg/Vol ratio > 7.412 (AUC 0.631) (Supplementary Table 2).
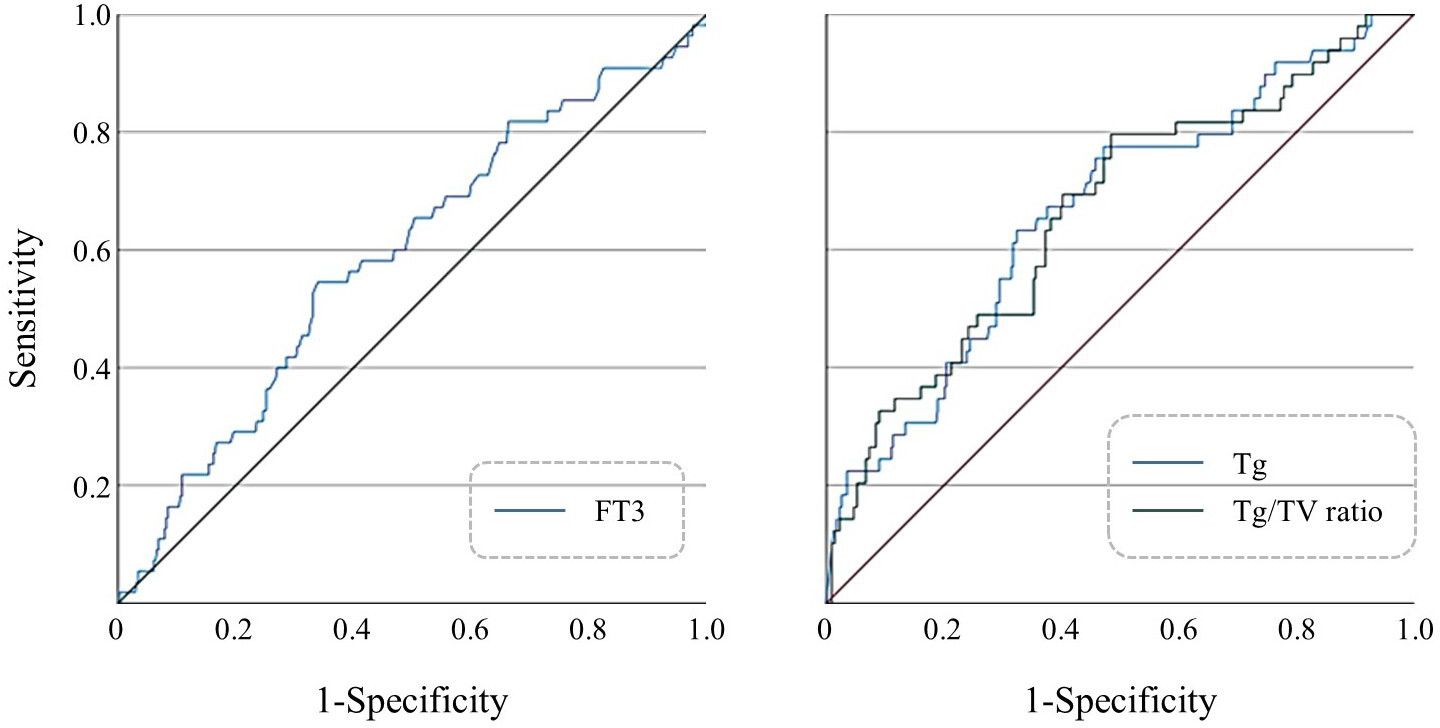
Figure 1. (Left) ROC curve of preoperative FT3 levels for predicting FTC; (Right) ROC curves of preoperative serum Tg levels and Tg/Vol ratio for predicting FTC.
Diagnostic logistic regression analysis
Variables demonstrating statistical significance in univariate analysis (male gender, FT3 > 5.045 pmol/L, irregular tumor shape, irregular margins, microcalcifications, and capsular involvement) were subsequently incorporated into multivariate logistic regression modeling (Supplementary Table 3). The analysis revealed male gender (p < 0.001) and capsular involvement (p = 0.001) as statistically significant independent risk factors, while FT3 > 5.045 pmol/L (p = 0.105), irregular tumor morphology (p = 0.377), irregular margins (p = 0.681), and microcalcifications (p = 0.297) failed to demonstrate independent predictive value.
To mitigate potential confounding effects from thyroglobulin antibodies (TgAb), analyses incorporating Tg and Tg/Vol ratio were exclusively performed in TgAb-negative patients (<115.00 IU/mL, n=392). Multivariate evaluation of significant univariate predictors (Tg > 217.5 μg/L and Tg/Vol ratio > 7.412) identified only Tg/Vol ratio > 7.412 (p = 0.008) as maintaining independent predictive significance, whereas Tg > 217.5 μg/L (p = 0.507) showed no statistically meaningful association (Table 3).
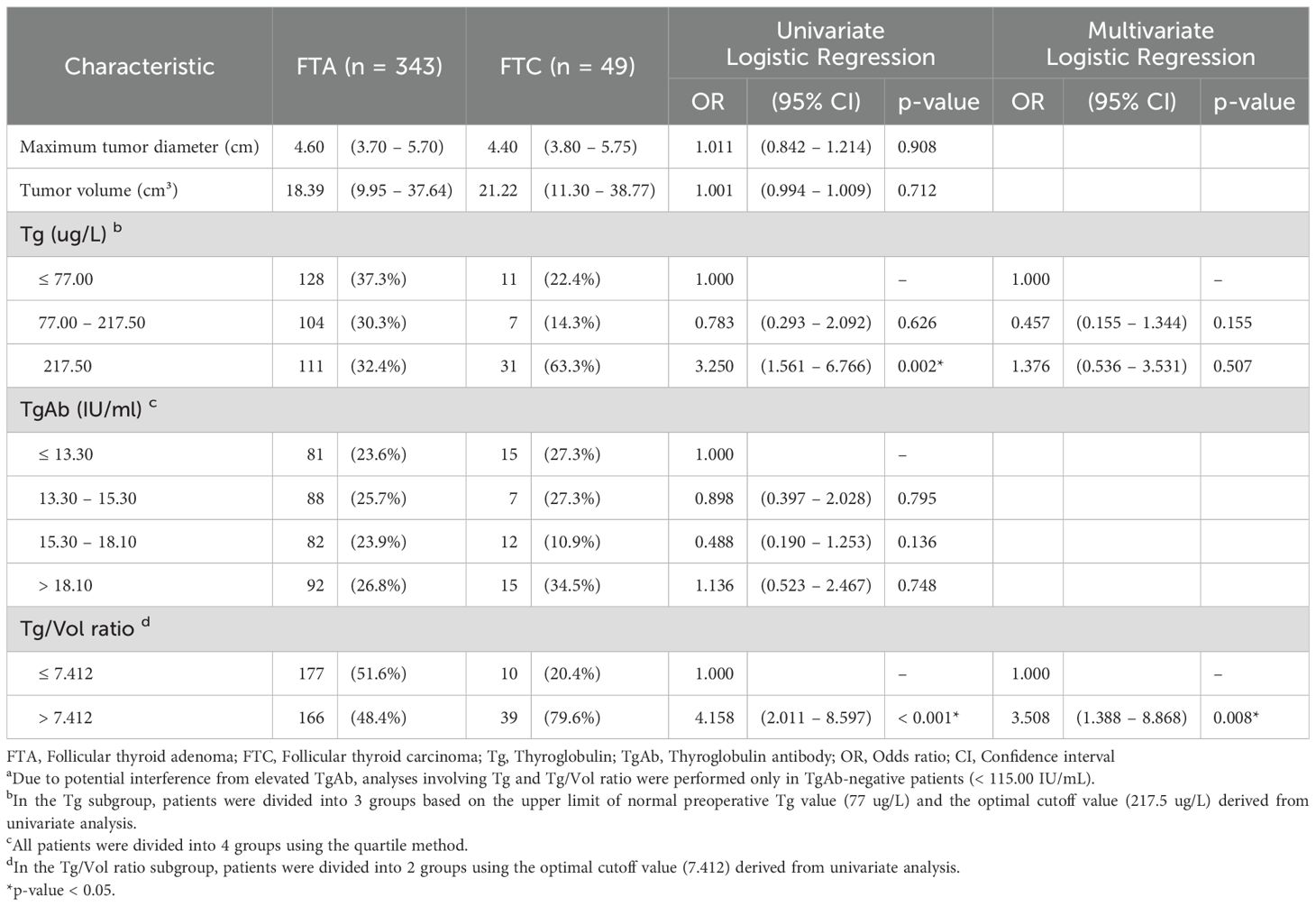
Table 3. Binary logistic regression analysis of clinical characteristics and CEUS features in predicting FTC in TgAb-negative patients a.
The final multivariate predictive model established three independent risk factors for follicular thyroid carcinoma, ranked in descending order of predictive strength: capsular involvement (adjusted odds ratio [OR] = 9.958; 95% CI: 2.453-40.424), Tg/Vol ratio >7.412 (adjusted OR = 3.508; 95% CI: 1.388-8.868), and male gender (adjusted OR = 3.474; 95% CI: 1.751-6.891).
Prevalence of malignancy
Among all thyroglobulin antibody (TgAb)-negative patients, the overall prevalence of FTC was 12.5% (49/392). A statistically significant positive correlation was observed between malignancy rates and serum Tg concentrations (Pearson’s r = 0.176, p < 0.001). Notably, the subgroup with Tg levels exceeding 409.18 μg/L manifested a substantially elevated FTC prevalence (20/98 20.41%, p = 0.002) when compared to patients with lower Tg concentrations (Supplementary Figure 1).
Similarly, a proportional relationship was revealed between FTC prevalence and the Tg/Vol ratio (Pearson’s r = 0.155, p = 0.002). Patients exhibiting a Tg/Vol ratio surpassing 20.68 similarly demonstrated a significantly higher malignancy rate (20/98 20.41%, p = 0.009) relative to other ratio subgroups (Supplementary Figure 2).
Diagnostic performance of multivariate model
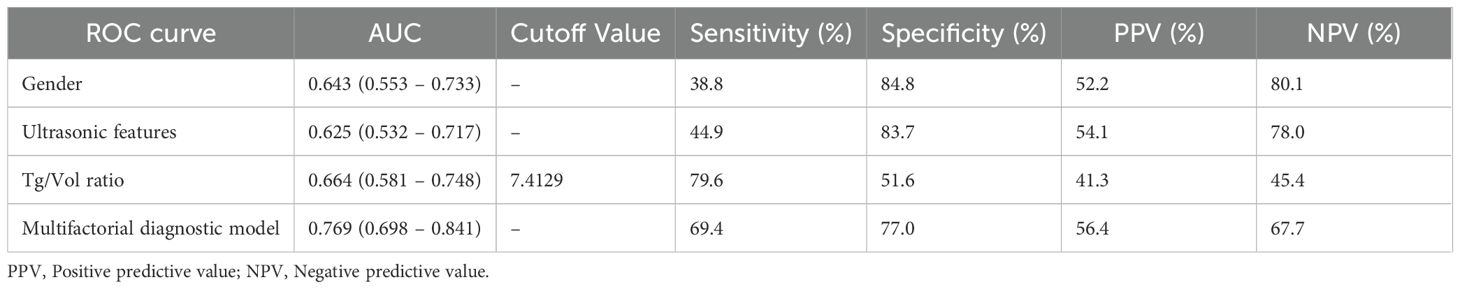
ROC curves were constructed to evaluate the diagnostic performance of individual parameters (gender, CEUS features, Tg/Vol ratio) and their combined multifactorial model for distinguishing FTC (Figure 2). Comparative analysis of the AUC revealed differential diagnostic efficacy among these approaches. The diagnostic value of CEUS features alone was marginally inferior to that of the Tg/Vol ratio alone. Notably, the multifactorial diagnostic model integrating Tg/Vol ratio with gender and CEUS features demonstrated superior performance (AUC 0.769) compared to any single-parameter approach, achieving 69.4% sensitivity and 77.0% specificity (Table 4). Decision curve analysis revealed the combined model conferred clinical utility in low-threshold ranges with maximum net benefit of 0.116, whereas the calibration curve demonstrated excellent probabilistic calibration (Brier=0.096). Notably, score distributions verified the model’s efficacy in segregating typical benign cases (Supplementary Figures 3-5).
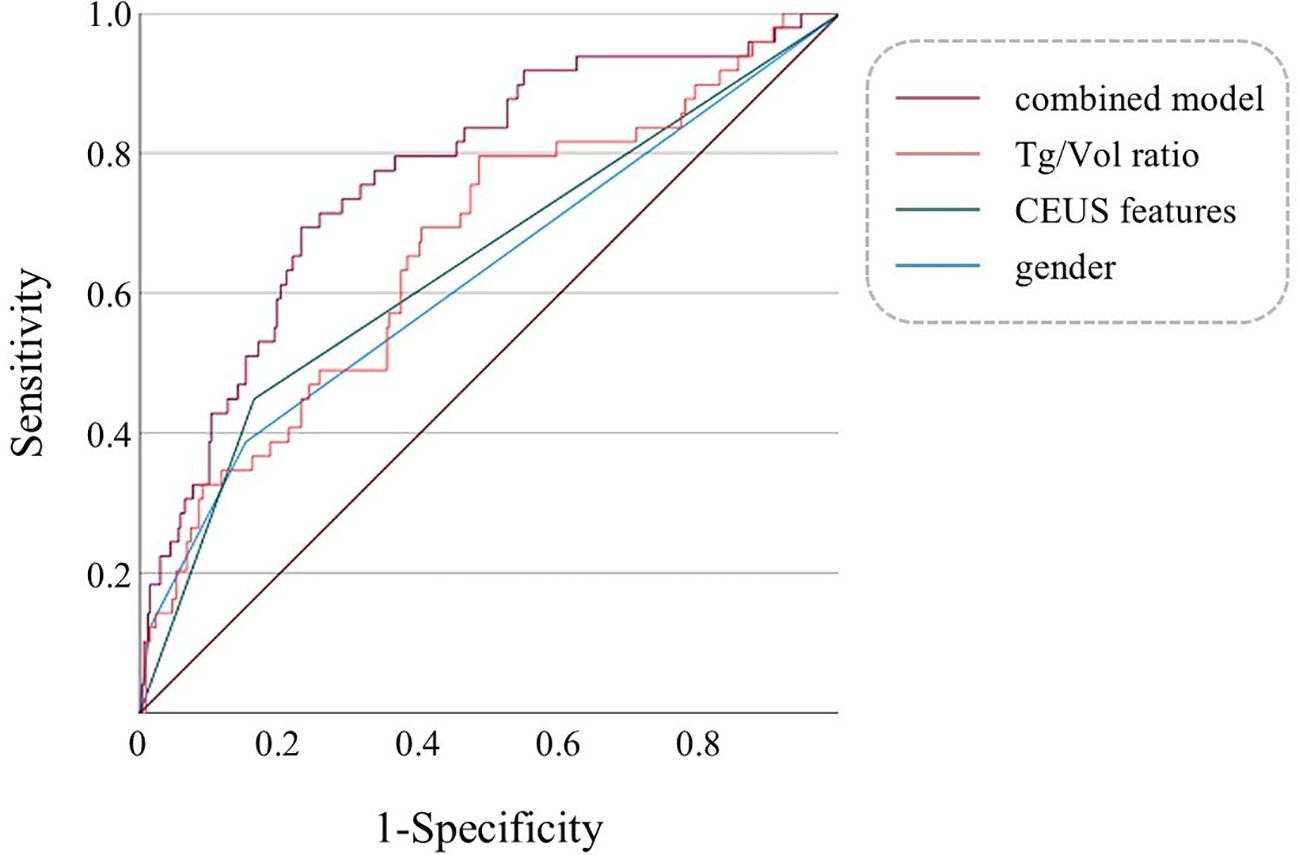
Figure 2. Multivariable ROC curves of gender, CEUS features, Tg/Vol ratio, and the combined model for diagnosing FTC. The combined model refers to a multifactorial diagnostic model incorporating gender, CEUS features, and the Tg/Vol ratio.
Discussion
To our knowledge, our study represented the first large-scale investigation to establish the clinical validity of the Tg/tumor volume ratio as a novel, noninvasive biomarker for preoperative discrimination between FTA and FTC. This readily calculable parameter addressed a critical unmet need in thyroid oncology by providing both diagnostic and prognostic information prior to surgical intervention.
The clinical management of follicular thyroid neoplasms remains challenging due to persistent diagnostic uncertainties in the preoperative phase (16, 17). Current practice patterns revealed two concerning trends: excessive surgical intervention for benign disease (18), as Ra et al. reported that the rate of unnecessary surgeries for resected follicular tumors was approximately 26% (19); and delayed diagnosis of malignant cases, with substantial proportion of misclassified FTC cases presenting with distant metastases at definitive diagnosis (20, 21). This diagnostic dilemma underscores the imperative for improved preoperative risk stratification tools.
The diagnostic value of serum Tg in preoperative differentiation of FTC remains controversial. While studies such as Chen et al. demonstrated correlations between serum Tg and FTC risk (10, 22, 23), inherent limitations persist. Recent studies reported that the existence of TgAb interferes with Tg measurement, compounded by the absence of standardized Tg thresholds (20 – 30% variability across immunoassay methods) (24, 25). These discrepancies suggest that although Tg reflects tumor secretory activity, its standalone diagnostic reliability remains suboptimal.
In TgAb-negative patients, our analysis revealed significantly higher preoperative serum Tg levels in FTC compared to FTA. However, multivariate analysis showed that Tg > 217.5 μg/L lost statistical significance when adjusted for the Tg/Vol ratio. This implies that large FTA may elevate Tg levels via mass effect leading to false positives, while small FTC could exhibit high Tg/Vol ratio despite modest absolute Tg concentrations due to early invasive potential. Consequently, the Tg/Vol ratio emerges as a more stable biomarker.
The optimal Tg/Vol ratio cutoff was 7.412 (AUC = 0.664, sensitivity 79.6%, specificity 51.6%), with multivariate validation confirming its independent predictive value (OR = 3.508, p = 0.008). Notably, FTC prevalence increased significantly when the Tg/Vol ratio exceeded 8.07 (p = 0.0092). These findings advocate incorporating preoperative Tg/Vol ratio into surgical decision-making and surveillance protocols.
While the Tg/Vol ratio is not an independent diagnostic tool, it serves as a valuable triage tool that significantly enhances the accuracy of risk stratification and refines the diagnostic evaluation of thyroid follicular neoplasms. For lesions with indeterminate fine-needle aspiration (FNA) results, a low Tg/Vol ratio may obviate unnecessary diagnostic resection surgery. Conversely, an elevated Tg/Vol ratio suggests increased malignant potential, necessitating prompt surgical consultation. Furthermore, given the limited sensitivity of intraoperative frozen section analysis for detecting thyroid follicular carcinoma, cases exhibiting elevated Tg/Vol ratios in conjunction with suspicious malignant ultrasound features warrant consideration for extended surgical intervention. Postoperatively, serial monitoring of the Tg/Vol ratio also aids in the early detection of tumor recurrence in patients who have undergone partial thyroidectomy.
Serum Tg levels are influenced by multifactorial physiological, pathological, and technical determinants. Emerging evidence highlights iodine nutritional status as a pivotal modulator of Tg, with both deficiency and excess inducing elevation. Additional influencers include TSH, thyroiditis, thyroid medications, and assay variations, all of which may perturb Tg levels and thereby impact Tg/Vol ratios. Thus, interpretation of Tg/Vol values requires consideration of these confounders, with trend monitoring offering greater diagnostic utility than single measurements.
Contrary to studies linking thyroid cancer with elevated TSH, FT3, or FT4 levels, our cohort (excluding patients with acute or chronic thyroiditis) showed no independent predictive value for these markers (26–28). Regarding imaging, conventional CEUS features (irregular morphology, irregular margins, microcalcifications, and capsular invasion) correlated with FTC. The 2015 American Thyroid Association (ATA) management guidelines corroborate our finding that capsular involvement demonstrated the highest predictive strength (OR = 9.958, specificity 83.7%), aligning with intraoperative frozen-section accuracy and underscoring its potential as a diagnostic gold standard (29).
The combined model integrating the Tg/Vol ratio with CEUS features achieved superior diagnostic balance (AUC = 0.769, representing a 12.1% improvement over individual metrics), with sensitivity 69.4% and specificity 77.0%. Suboptimal positive and negative predictive values may reflect our study’s relatively low FTC prevalence. Additionally, decision curve and calibration curve analyses showed the model excelled in predictive probability (Brier=0.096), low-threshold clinical value with the max net benefit of 11.6%, and typical benign case identification. Its main limitation was conservative malignant predictions, failing to reliably identify high-risk patients—attributed to dataset imbalance from insufficient positive cases. This highlights the need for future studies to collect more malignant cases for dataset balancing and develop risk stratification strategies accordingly.
Several limitations in our study should be mentioned. First, the single-center retrospective design precluded longitudinal assessment of thyroid functiondata. Second, while TgAb exclusion strengthened internal validity, it introduced selection bias with implications for real-world applicability. Additionally, volumetric estimations derived from CEUS-acquired linear dimensions may not fully capture true tumor geometry, particularly for subcentimeter nodules.
To further validate the clinical utility, future studies should employ three-dimensional CEUS reconstruction technology to improve volumetric measurement accuracy (30, 31), complemented with artificial intelligence (AI) -assisted ultrasound to characterize FTC-specific vascular patterns (32–34). Propose specific alternative biomarkers or adjusted algorithms should be proposed to determine true Tg levels in TgAb-positive cases. Furthermore, prospective multicenter studies are planned to further refine the diagnostic model, thereby contributing robust evidence toward the precision diagnostic criteria for thyroid follicular neoplasms.
Conclusion
In summary, our study suggested that the Tg/Vol ratio > 7.412 as a clinically useful preoperative discriminator for FTC. The combined diagnostic model incorporating CEUS characteristics significantly improves diagnostic accuracy, mitigating risks of misdiagnosis in thyroid follicular neoplasms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board and Ethics Committee of West China Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This is a retrospective study, and written consent could not be obtained from all patients.
Author contributions
XZ: Data curation, Writing – original draft. FL: Data curation, Writing – original draft. JL: Writing – review & editing, Conceptualization, Funding acquisition. ZL: Resources, Supervision, Writing – review & editing. YM: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is supported by National Natural Science Foundation of China (82403761).
Acknowledgments
We thank the participants of the study and all the study staff for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1626766/full#supplementary-material
Abbreviations
AUC, Area under the curve; CEUS, Contrast-enhanced ultrasound; CI, Confidence interval; FTA, Follicular thyroid adenoma; FTC, Follicular thyroid carcinoma; FT-UMP, Follicular tumor of uncertain malignant potential; FT3, Free triiodothyronine; FT4, Free thyroxine; MTC, Medullary thyroid carcinoma; NIFTP, Noninvasive follicular thyroid neoplasm with papillary-like nuclear features; OR, Odds ratio; PTC, Papillary thyroid carcinoma; ROC, Receiver operating characteristic; Tg, Thyroglobulin; TgAb, Thyroglobulin antibodies; Tg/Vol ratio, Serum thyroglobulin-to-tumor volume ratio; TPOAb, Thyroid peroxidase antibodies; TRAb, Thyroid-stimulating hormone receptor antibodies; TSH, Thyroid-stimulating hormone.
References
1. Sobrinho-Simões M, Eloy C, Magalhães J, Lobo C, and Amaro T. Follicular thyroid carcinoma. Mod Pathol. (2011) 24 Suppl 2:S10–8. doi: 10.1038/modpathol.2010.133
2. Gharib H and Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. (1993) 118:282–9. doi: 10.7326/0003-4819-118-4-199302150-00007
3. Kuo TC, Wu MH, Chen KY, Hsieh MS, Chen A, and Chen CN. Ultrasonographic features for differentiating follicular thyroid carcinoma and follicular adenoma. Asian J Surg. (2020) 43:339–46. doi: 10.1016/j.asjsur.2019.04.016
4. Grani G, Lamartina L, Durante C, Filetti S, and Cooper DS. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. (2018) 6:500–14. doi: 10.1016/s2213-8587(17)30325-x
5. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, and Leenhardt L. European thyroid association guidelines for ultrasound Malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. (2017) 6:225–37. doi: 10.1159/000478927
6. Li J, Li C, Zhou X, Huang J, Yang P, Cang Y, et al. US risk stratification system for follicular thyroid neoplasms. Radiology. (2023) 309:e230949. doi: 10.1148/radiol.230949
7. Feldkamp J, Führer D, Luster M, Musholt TJ, Spitzweg C, and Schott M. Fine needle aspiration in the investigation of thyroid nodules. Dtsch Arztebl Int. (2016) 113:353–9. doi: 10.3238/arztebl.2016.0353
8. Trimboli P, Mian C, Piccardo A, and Treglia G. Diagnostic tests for medullary thyroid carcinoma: an umbrella review. Endocrine. (2023) 81:183–93. doi: 10.1007/s12020-023-03326-6
9. Yamazaki H, Sugino K, Katoh R, Matsuzu K, Kitagawa W, Nagahama M, et al. Management of follicular thyroid carcinoma. Eur Thyroid J. (2024) 13(5):e240146. doi: 10.1530/etj-24-0146
10. Chen Z, Lin Y, Lai S, Wang P, Li J, Wang L, et al. The utility of serum anti-thyroglobulin antibody and thyroglobulin in the preoperative differential diagnosis of thyroid follicular neoplasms. Endocrine. (2022) 76:369–76. doi: 10.1007/s12020-022-02993-1
11. Kim HJ, Mok JO, Kim CH, Kim YJ, Kim SJ, Park HK, et al. Preoperative serum thyroglobulin and changes in serum thyroglobulin during TSH suppression independently predict follicular thyroid carcinoma in thyroid nodules with a cytological diagnosis of follicular lesion. Endocr Res. (2017) 42:154–62. doi: 10.1080/07435800.2016.1262395
12. Berlińska A and Świątkowska-Stodulska R. Clinical use of thyroglobulin: not only thyroid cancer. Endocrine. (2024) 84:786–99. doi: 10.1007/s12020-023-03658-3
13. Nishihara E, Hobo Y, Miyauchi A, Ito Y, Higuchi M, Hirokawa M, et al. Serum thyroglobulin evaluation on LC-MS/MS and immunoassay in TgAb-positive patients with papillary thyroid carcinoma. Eur Thyroid J. (2022) 11(1):e210041. doi: 10.1530/etj-21-0041
14. Giovanella L, D'Aurizio F, Algeciras-Schimnich A, Görges R, Petranovic Ovcaricek P, Tuttle RM, et al. Thyroglobulin and thyroglobulin antibody: an updated clinical and laboratory expert consensus. Eur J Endocrinol. (2023) 189:R11–r27. doi: 10.1093/ejendo/lvad109
15. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. (2017) 143:1015–20. doi: 10.1001/jamaoto.2017.1442
16. Alexander EK and Cibas ES. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol. (2022) 10:533–9. doi: 10.1016/s2213-8587(22)00101-2
17. Nabhan F, Dedhia PH, and Ringel MD. Thyroid cancer, recent advances in diagnosis and therapy. Int J Cancer. (2021) 149(5):984–992. doi: 10.1002/ijc.33690
18. Conzo G, Calò PG, Gambardella C, Tartaglia E, Mauriello C, Della Pietra C, et al. Controversies in the surgical management of thyroid follicular neoplasms. Retrospective analysis of 721 patients. Int J Surg. (2014) 12 Suppl 1:S29–34. doi: 10.1016/j.ijsu.2014.05.013
19. Yoon RG, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid. (2014) 24:1612–7. doi: 10.1089/thy.2014.0140
20. Xu B and Ghossein R. Encapsulated thyroid carcinoma of follicular cell origin. Endocr Pathol. (2015) 26:191–9. doi: 10.1007/s12022-015-9376-5
21. Bischoff LA, Ganly I, Fugazzola L, Buczek E, Faquin WC, Haugen BR, et al. Molecular alterations and comprehensive clinical management of oncocytic thyroid carcinoma: A review and multidisciplinary 2023 update. JAMA Otolaryngol Head Neck Surg. (2024) 150:265–72. doi: 10.1001/jamaoto.2023.4323
22. Li S, Ren C, Gong Y, Ye F, Tang Y, Xu J, et al. The role of thyroglobulin in preoperative and postoperative evaluation of patients with differentiated thyroid cancer. Front Endocrinol (Lausanne). (2022) 13:872527. doi: 10.3389/fendo.2022.872527
23. Kim H, Park SY, Choe JH, Kim JS, Hahn SY, Kim SW, et al. Preoperative serum thyroglobulin and its correlation with the burden and extent of differentiated thyroid cancer. Cancers (Basel). (2020) 12(3):625. doi: 10.3390/cancers12030625
24. Hoofnagle AN and Roth MY. Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab. (2013) 98:1343–52. doi: 10.1210/jc.2012-4172
25. Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN, et al. Thyroglobulin (Tg) testing revisited: tg assays, tgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab. (2015) 100:E1074–83. doi: 10.1210/jc.2015-1967
26. Fiore E and Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab. (2012) 97:1134–45. doi: 10.1210/jc.2011-2735
27. Kim TH, Lee MY, Jin SM, and Lee SH. The association between serum concentration of thyroid hormones and thyroid cancer: a cohort study. Endocr Relat Cancer. (2022) 29:635–44. doi: 10.1530/erc-22-0094
28. Zhang X, Tian L, Teng D, and Teng W. The relationship between thyrotropin serum concentrations and thyroid carcinoma. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15205017
29. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
30. Huang K, Bai Z, Bian D, Yang P, Li X, and Liu Y. Diagnostic accuracy of contrast-enhanced ultrasonography in papillary thyroid microcarcinoma stratified by size. Ultrasound Med Biol. (2020) 46:269–74. doi: 10.1016/j.ultrasmedbio.2019.10.001
31. Yao J, Wang Y, Lei Z, Wang K, Feng N, Dong F, et al. Multimodal GPT model for assisting thyroid nodule diagnosis and management. NPJ Digit Med. (2025) 8:245. doi: 10.1038/s41746-025-01652-9
32. Peng S, Liu Y, Lv W, Liu L, Zhou Q, Yang H, et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicenter diagnostic study. Lancet Digit Health. (2021) 3:e250–9. doi: 10.1016/s2589-7500(21)00041-8
33. Yao J, Zhang Y, Shen J, Lei Z, Xiong J, Feng B, et al. AI diagnosis of Bethesda category IV thyroid nodules. iScience. (2023) 26:108114. doi: 10.1016/j.isci.2023.108114
Keywords: thyroid follicular neoplasm, follicular thyroid carcinoma, the serum thyroglobulin-to-tumor volume ratio, thyroglobulin, ultrasonography
Citation: Zhu X, Liu F, Liu J, Li Z and Ma Y (2025) Thyroglobulin-to-tumor volume ratio combined with ultrasound features for diagnosing thyroid follicular neoplasms. Front. Endocrinol. 16:1626766. doi: 10.3389/fendo.2025.1626766
Received: 11 May 2025; Accepted: 23 June 2025;
Published: 10 July 2025.
Edited by:
Umberto Malapelle, University of Naples Federico II, ItalyReviewed by:
Jincao Yao, University of Chinese Academy of Sciences, ChinaShahram Taeb, Gilan University of Medical Sciences, Iran
Copyright © 2025 Zhu, Liu, Liu, Li and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Ma, bWF5dWp6eEB3Y2hzY3UuY24=
†These authors have contributed equally to this work and shared the first authorship
 Xixi Zhu1,2†
Xixi Zhu1,2† Jiaye Liu
Jiaye Liu Yu Ma
Yu Ma