- 1Department of Rehabilitation Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 2Department of Orthopedic and Trauma Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 3Department of Cardiology, The Affiliated Cardiovascular Hospital of Kunming Medical University 6 (Fuwai Yunnan Cardiovascular Hospital), Kunming, Yunnan, China
Spinal cord injury (SCI), a debilitating neurological disorder with complex pathophysiology, involves primary mechanical trauma followed by multifactorial cascades of secondary inflammation, oxidative stress, and apoptosis. Hormones have emerged as a research focus in SCI therapeutics due to their neuroprotective properties. As pivotal regulators of cellular signaling, hormones exhibit dual roles in either exacerbating or mitigating secondary damage. This review synthesizes three decades of research, highlighting that hormones such as corticosteroids, melatonin, and estrogen demonstrate significant therapeutic potential in animal models and clinical studies, though controversies persist regarding their efficacy and safety profiles. Key findings include: (1) Glucocorticoids, exemplified by methylprednisolone (MP), suppress inflammation and reduce tissue damage but face skepticism over long-term benefits, with high-dose regimens correlating with significant adverse effects such as gastrointestinal bleeding, hyperglycemia, and metabolic complications; (2) Melatonin exerts multi-target neuroprotection by modulating autophagy, inhibiting apoptosis, and suppressing inflammasome activation; (3) Sex hormones (e.g., testosterone, progesterone) improve functional recovery through metabolic balance regulation and neural regeneration, while estrogen enhances angiogenesis and motor function via the synergistic involvement of multiple receptor-mediated genomic (ERα/ERβ) and non-genomic (GPER) signaling pathways. The non-genomic actions rapidly activate kinase cascades, such as PI3K/Akt-CREB and ERK, which in turn regulate both immediate cellular functions and gene expression profiles, contributing to the overall neuroprotective effects; (4) Combinatorial therapies (e.g., MP with neurotrophic factors) and novel delivery systems (e.g., nanoparticle-based drug carriers) represent promising strategies to optimize therapeutic outcomes. These advances elucidate the multidimensional mechanisms of hormonal interventions while revealing critical challenges, including dose-dependent adverse effects, antagonistic effects in polypharmacy, and unresolved long-term safety concerns. Overall, hormonal therapies for SCI present a “dual-edged sword” of efficacy versus risks, necessitating future innovations in precision regulation and mechanistic exploration to bridge translational gaps.
1 Introduction
Spinal cord injury (SCI) initiates with primary mechanical trauma, followed by secondary inflammatory and degenerative cascades involving neuroinflammation, blood-spinal cord barrier (BSCB) disruption, and axonal degeneration. Current treatments, including corticosteroids and immunosuppressants, exhibit limited efficacy and significant adverse effects. Glucocorticoids such as MP were once considered standard acute-phase therapeutics; however, due to an unfavorable risk-benefit profile, they have been downgraded to non-recommended options in major guidelines (1). Hormones, with their pleiotropic roles in inflammation and tissue repair, harbor untapped therapeutic potential. This review systematically examines the roles of hormones in SCI, encompassing molecular mechanisms, clinical evidence, risk-benefit analyses, and cutting-edge technological applications, aiming to inform optimized therapeutic strategies.
2 Pathophysiological mechanisms and therapeutic controversies of classical hormones
MP, a representative glucocorticoid, was once regarded as a standard therapy for acute SCI due to its inhibition of lipid peroxidation and inflammatory mediator release. The NASCIS-II protocol recommended a loading dose of 30 mg/kg within 8 hours post-injury, followed by a 24-hour maintenance infusion at 5.4 mg/kg/h, which reportedly improved motor scores (2). However, subsequent studies found no significant motor function improvement in acute traumatic SCI (TSCI) patients under this regimen, alongside markedly increased complication risks (3).
Multiple studies confirm that MP reduces apoptotic cell death post-SCI (4–6). It lowers levels of malondialdehyde, a lipid peroxidation biomarker (7), and exerts neuroprotection by mitigating axonal damage, enhancing blood flow, reducing calcium influx, and suppressing microglial/macrophage aggregation and inflammatory mediator expression (8). Mechanistically, MP inhibits lipid peroxidation (9) and downregulates pro-inflammatory cytokines (e.g., TNF-α, IL-6) (10), while blocking oxidative stress cascades (11) and suppressing oligodendrocyte apoptosis via the STAT5 pathway (12) (Figure 1).
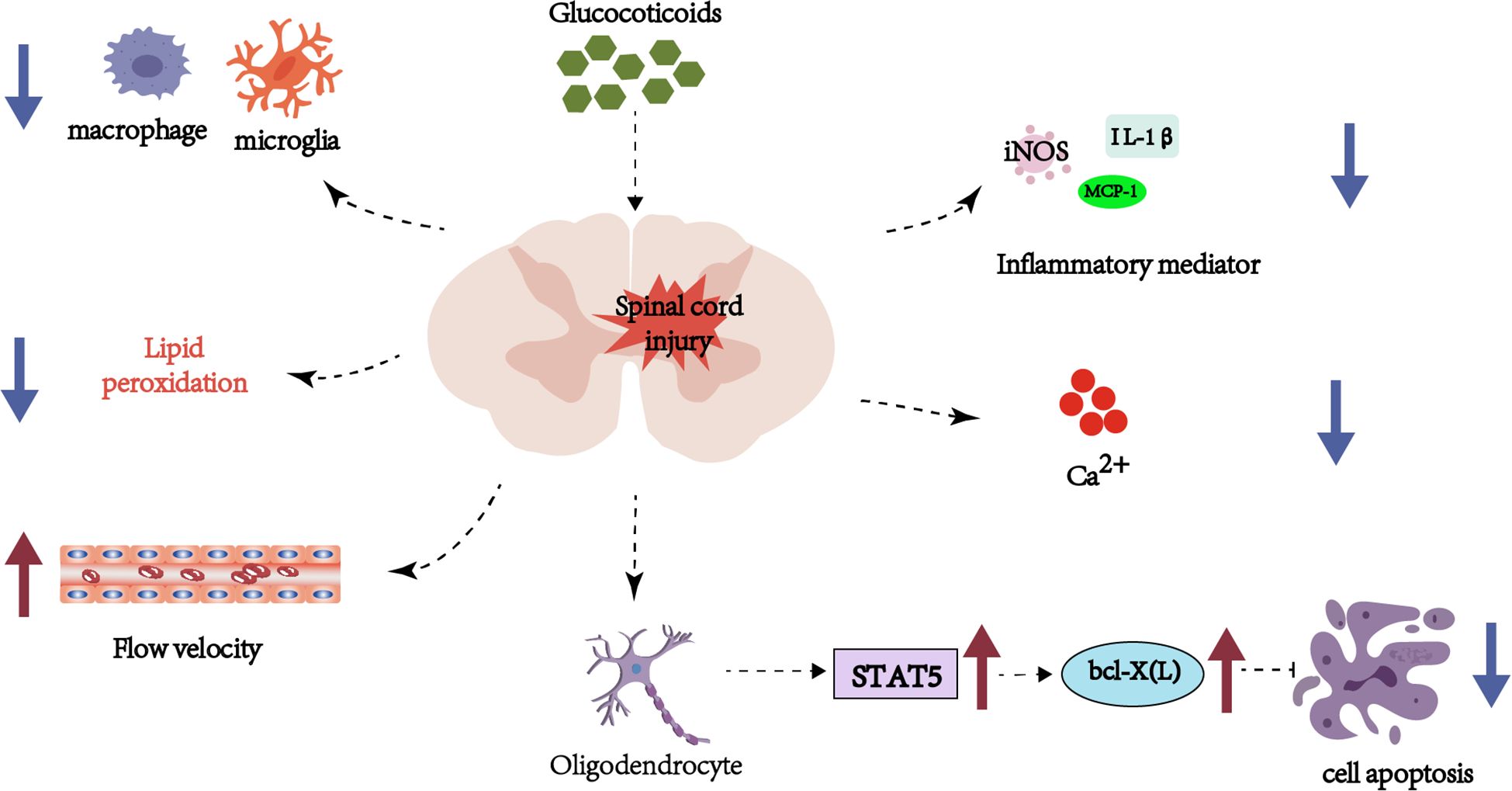
Figure 1. Schematic representation of hormonal and inflammatory pathways in spinal cord injury, emphasizing glucocorticoid involvement, cellular apoptosis, and immune cell interactions.
Nevertheless, high-dose MP (>5000 mg), though effective in reducing spinal edema, significantly elevates complication risks (13). In experimental models with a single dose equivalent to that used in humans, the effect found of MP after 24h was an increase in the amount of water due to a decrease in the expression of AQP4, as well as greater damage to the BSCB. Moreover, MP increased the extravasation of plasma components after SCI and enhanced tissue swelling and edema. Notably, MP’s neuroprotection exhibits a critical time window: Two-photon microscopy reveals that administration within 30 minutes post-injury attenuates progressive axonal injury, neuronal death, and microglial/macrophage activation, whereas delayed treatment (>8 hours) drastically diminishes efficacy (14). This narrow timeframe for intervention is further corroborated by time-series transcriptomic analysis, which identifies 8–12 hours post-injury as the optimal window for MP to exert its core immunomodulatory effects (15). These dose- and time-dependent contradictions underscore the necessity for individualized risk-benefit assessments in clinical practice.
Weaver et al. compared the efficacy of MP with anti-CD11d monoclonal antibodies, revealing that while MP preserves spinal cord tissue, it fails to improve neurological function and may antagonize the effects of other immunotherapies (16). Wu et al. demonstrated that 24-hour MP treatment in rats reduced skeletal muscle mass and upregulated atrophy-related genes (FOXO1, MAFbx, MuRF1, and REDD1) (17). High-dose MP significantly elevates risks of infections (e.g., pneumonia), hyperglycemia, and gastrointestinal hemorrhage (18), with higher complication rates observed in pediatric patients (19). Long-term use further correlates with muscle atrophy (17) and diminished ovarian reserve (20). Multicenter studies (3)and clinical guidelines (21) indicate that MP fails to confer significant neurological improvement, challenging its historical status as a “gold standard.” These conflicting outcomes underscore the necessity for rigorous risk-benefit evaluations in MP clinical applications, with future research prioritizing low-dose short-term regimens or combinatorial therapies to mitigate adverse effects.
3 Multidimensional regulation of sex hormones: from metabolic modulation to neural regeneration
3.1 Testosterone: dual roles in metabolic homeostasis and axonal repair
SCI disrupts hypothalamic-pituitary-gonadal (HPG) axis function, leading to hypogonadism in approximately 60% of male patients (22). Clinical studies demonstrate that testosterone replacement therapy (TRT) significantly increases lean body mass, reduces visceral adiposity, and enhances resting energy expenditure in hypogonadal males with SCI (23, 24). Spinal motor neurons highly express androgen receptor (AR) with predominantly nuclear localization, without significant sexual dimorphism, providing a target for testosterone action (25). Testosterone exerts neuroprotective effects via an AR-dependent mechanism, significantly reducing motor neuron death after injury (26). Clinical studies further confirm that TRT significantly improves motor function in male patients with incomplete SCI (with ASIA Scale scores increasing by 10.9–11.8 points in grades C/D), but shows no efficacy in complete injuries (27), highlighting the reparative potential of AR-mediated testosterone therapy on residual motor pathways. In animal models, testosterone treatment partially reverses MP-induced muscle atrophy and mitigates reductions in muscle fiber cross-sectional area (CSA) (28). Although the precise molecular mechanisms remain unclear, experimental evidence suggests that testosterone regulates the balance between muscle protein synthesis and degradation (29). Furthermore, combining testosterone with resistance training amplifies muscle mass recovery, increasing quadriceps CSA by 9% (30). In neural repair, testosterone administration in adult female rats with spinal contusion preserved dendritic length of motor neurons and maintained muscle mass and fiber CSA, despite no significant effects on lesion volume or motor neuron count (31).
3.2 Progesterone: anti-inflammatory pathways and molecular mechanisms of myelin regeneration
Progesterone exerts therapeutic effects in SCI through multifaceted mechanisms. It suppresses pro-inflammatory mediators (e.g., TNF-α, iNOS, COX-2) and glial activation (32, 33), attenuates neuroinflammation via NF-κB pathway inhibition, and achieves neuroprotection by upregulating BDNF expression (34) and preserving neuronal ultrastructure (35). Progesterone promotes multi-mechanistic repair after SCI via classical progesterone receptors. This leads to reduced release of pro-inflammatory cytokines (36). Progesterone enhances remyelination by increasing oligodendrocyte numbers. Treatment groups showed 35% oligodendrocyte presence compared to 7.5% in controls. It also upregulates myelin basic protein expression (37). It supports white matter preservation. Lesion volume is reduced, and white matter sparing increases to 16%, versus 7% in controls. Motor coordination and gait parameters also improve (38). Long-term treatment over 60 days is crucial for functional recovery. It does not induce hyperalgesia. Its clinical safety profile is better than MP. These findings support its translational potential (39). Progesterone also promotes oligodendrocyte differentiation and remyelination (40), alleviates neuropathic pain by inhibiting NMDA receptor hyperactivation (41), and modulates neuropeptide systems (42). Preclinical studies confirm its efficacy in enhancing motor function and axonal survival (43, 44), while a clinical trial combining progesterone with vitamin D demonstrated improved functional recovery in acute SCI (45).
3.3 Estrogen: multi-mechanistic neuroprotective effects of estrogen
Among known hormones, estrogens possess some of the most potent neuroprotective effects. Three types of receptors primarily mediate the effects of estrogen: the classical nuclear receptors ERα and ERβ, and the G protein-coupled estrogen receptor (GPER). ERα and ERβ are localized within the nuclei of neurons, particularly in regions associated with reproductive functions such as the dorsal root ganglia, pelvic ganglia, and spinal dorsal horn. Among these, ERα predominates in superficial sensory layers, while ERβ is more abundant in deep autonomic regions, regulating neuropeptide expression and neural plasticity through genomic effects (46). GPER, on the other hand, is an endogenous estrogen receptor located on the endoplasmic reticulum/nuclear membrane. Upon binding with estrogen, it activates nuclear PI3K, leading to the in situ synthesis of PIP3. The accumulation of nuclear PIP3 subsequently recruits and activates signaling molecules such as Akt, rapidly regulating transcription factors and gene expression. GPER is an intracellular receptor primarily localized to the endoplasmic reticulum and Golgi apparatus, capable of binding estrogen with high affinity and triggering non-genomic signaling pathways via the Gi/o-EGFR axis to achieve rapid physiological regulation (47). GPER exhibits tissue-specific functions: it promotes proliferation of ER-negative cells in cancer, and plays active roles in cardiovascular protection and metabolic diseases (48). Its ligand promiscuity (e.g., activation by Tamoxifen (TMX)) provides a rationale for developing targeted therapies to circumvent the side effects of traditional estrogen drugs. These receptors together form an integrated nuclear-membrane-intracellular signaling network that precisely regulates the diverse functions of estrogen.
Estrogen exerts neuroprotective effects in SCI through mechanisms including promoting remyelination, anti-apoptosis, pro-angiogenesis, and modulation of microglial and glial cell activity. Estrogen directly promotes remyelination, including inhibiting demyelination processes (49, 50) and enhancing Schwann cell differentiation via the ERβ-ERK1/2 pathway (51). Simultaneously, it reduces oligodendrocyte apoptosis by suppressing the RhoA-JNK3-cJun axis (52), maintaining white matter integrity. Estrogen synergizes with cell therapies by improving the remyelination capacity of stem cells (53) and Schwann cells (54, 55).
Estrogen upregulates the anti-apoptotic protein Bcl-2 via the PI3K/Akt-CREB pathway (56) and inhibits pro-inflammatory cytokines (e.g., TNF-α, IL-1β) and inflammasome activity (57), reducing neuronal apoptosis. Specifically, GPER-mediated non-canonical signaling plays an indispensable role in estrogen’s anti-apoptotic effects, as demonstrated by the findings that the specific GPER agonist G-1 mimics estrogen’s protection against SCI-induced apoptosis while GPER knockdown abolishes this effect, independent of classical nuclear ER pathways (56, 58). In terms of vascular protection, estrogen maintains BSCB integrity by suppressing MMP-9 (59) and stimulates angiogenesis (60). Angiogenesis not only provides nutritional support but also acts synergistically with neural repair processes; for example, the localized nanoparticle-based estrogen delivery platform can reduce glial scar formation and promote axonal regeneration (61), highlighting the interplay of multiple mechanisms.
Estrogen modulates microglial phenotypic polarization through ERα, ERβ, and GPER: suppressing the pro-inflammatory M1 phenotype (reducing expression of CD86, iNOS, and IL-1β) (62) and promoting the anti-inflammatory M2 phenotype (increasing expression of Arg-1 and CD206) (63). The mechanisms involve inhibition of the NF-κB/MAPK pathway (64) and NLRP3 inflammasome activation, as well as delaying the activation of disease-associated microglia through metabolic reprogramming (65). These effects can delay disease progression in experimental autoimmune encephalomyelitis models (66).
Whether the neuroprotective effects of estrogen are sex-specific remains debatable, but most evidence supports the influence of sex differences. For instance, remyelination in female animals does not depend on CXCR4, whereas it depends on the testosterone-CXCR4 axis in males (67, 68); the intrinsic ER expression in microglia is independent of circulating estrogen levels (69), but estrogen loss after menopause exacerbates neuroinflammation (70, 71). Selective estrogen receptor modulators (SERMs) like TMX can mimic the protective effects (72), but supraphysiological doses may exacerbate damage via the ERα/NF-κB pathway (73). These findings provide a basis for developing sex-specific neuroprotective strategies targeting microglia (74). Although some studies suggest limited sex specificity (75), the overall consensus supports considering sex factors in treatment strategies. It is worth noting that the efficacy of TMX is not limited to male animals. Studies by Colón et al. in female rat models demonstrated that TMX administration immediately or 24 hours after SCI similarly improves locomotor recovery and reduces secondary damage (76).
Basic research reveals that 17β-estradiol can significantly reduce immune cell infiltration (e.g., monocytes/macrophages/neutrophils) during the acute phase of SCI, but long-term high-dose use carries carcinogenic risks. TMX can mimic the neuroprotective effects of estradiol in SCI models (e.g., suppressing inflammation, antioxidant, and anti-apoptotic effects) and alleviates tissue damage by downregulating the expression of the Ccr2 and Mmp12 genes in microglia. Ccr2, primarily expressed on microglia and infiltrating macrophages, mediates the recruitment of inflammatory cells to the injury site. In the early phase of SCI (within 24 hours), activated microglia highly express Ccr2, exacerbating neuroinflammation and secondary damage. By downregulating Ccr2, TMX significantly reduces inflammatory cell infiltration, thereby containing the inflammatory response and preserving neuronal and axonal integrity, which aligns with its broad anti-inflammatory action. Mmp12, mainly produced by macrophages/microglia, is involved in extracellular matrix degradation, BSCB disruption, and pro-inflammatory factor activation. In SCI, Mmp12 overexpression aggravates tissue destruction, axonal demyelination, and cell death. By inhibiting Mmp12, TMX helps maintain extracellular matrix stability, reduces tissue damage, and promotes a reparative microenvironment. These mechanisms collectively demonstrate that TMX exerts its neuroprotective effects in the acute phase of SCI (≤24 hours) through multi-targeted interventions in both inflammatory and matrix degradation pathways (76, 77). Administration within 24 hours post-injury can still improve motor function, offering a wider therapeutic window than MP (78). Clinically, although there are no large-scale SCI trials, studies on estrogen use for menopausal syndrome or cardiovascular diseases indirectly support its neuroprotective potential. Animal models show that estrogen treatment improves functional scores, increases axon number and diameter, and enhances motor evoked potentials [with significantly shorter latency (17-fold reduction) and higher amplitude (7-fold increase)]. Future work needs to integrate novel delivery systems (e.g., nano-platforms) and receptor-specific modulation to optimize clinical translation (79).
3.4 Gonadotropin-releasing hormone: a potential target for neuroplasticity
GnRH improves SCI outcomes through multi-target mechanisms involving neuroprotection, urological repair, and endocrine regulation. Calderón-Vallejo et al. (80) demonstrated that GnRH enhances motor function in ovariectomized rats by upregulating neurofilament expression and promoting axonal regeneration. Additionally, GnRH exhibits urological protective effects (81), restoring voluntary urination in 68% of SCI rats by reducing bladder wall thickening and renal fibrosis. However, combinatorial therapies may induce complex effects (82). They found that co-administration of GnRH with growth hormone (GH) suppresses motor recovery, underscoring the need for cautious design of multi-hormone regimens. GnRH also modulates the HPG axis: Bauman et al. (83) reported enhanced follicle-stimulating hormone (FSH) responses to GnRH stimulation in SCI males, while Sullivan et al. (50) emphasized that HPG axis central inhibition requires testosterone replacement to improve metabolic health comprehensively.
4 Mechanistic roles of metabolic-immune crosstalk hormones
4.1 Leptin: bridging metabolic dysregulation and neuroinflammation
Leptin is an energy homeostatic regulatory peptide secreted by white adipocytes. It suppresses appetite and increases energy expenditure by binding to receptors in the hypothalamus. Within the nervous system, the leptin receptor (LepRb) is widely expressed in the hippocampus, cortex, and spinal cord, where it contributes to the regulation of synaptic plasticity (84). Leptin protects neuronal function by inhibiting ATP-induced astrocyte damage, reducing arachidonic acid and prostaglandin E2 release, and activating the JAK2/Stat3 pathway to upregulate caveolin-1 expression (85). Acute leptin treatment decreases caspase-3 activity, suppresses pro-inflammatory molecules, and improves sensory-motor recovery post-SCI (86). Leptin can exacerbate pain by activating microglia (87), whereas blocking leptin signaling suppresses microglial proliferation and alleviates pain. Leptin exhibits a dual role in SCI. Endogenously elevated leptin levels are associated with metabolic syndrome (88), abdominal obesity (89), and neuropathic pain mediated via microglial activation (87). In contrast, acute exogenous leptin administration upregulates caveolin-1 through the JAK2/Stat3 pathway, suppresses ATP-induced inflammatory responses in astrocytes (85), reduces caspase-3 activity and pro-inflammatory cytokine release, promotes oligodendrocyte survival and white matter preservation, and ultimately improves motor functional recovery (86). Its efficacy depends on injury type (protective in complete SCI vs. promotive of pain in root injury) and timing (effective in acute phase) (90). However, long-term leptin treatment may be limited due to exacerbation of lean mass loss (91) or dysregulated bone metabolism (92). Elevated leptin levels in SCI patients correlate with central obesity, metabolic syndrome, and cardiovascular risks (88, 89). This elevation likely stems from sympathetic dysfunction and altered fat distribution, with higher leptin concentrations observed in individuals with more rostral injury levels (93). Notably, leptin positively correlates with lean mass but associates solely with fat mass in sarcopenic obesity, reflecting its metabolic remodeling post-SCI (91). Leptin also enhances bone healing, with increased expression linked to improved callus formation in fracture-SCI models (92).
4.2 Melatonin: circadian rhythm-integrated neuroprotection and motor synergy leptin: bridging metabolic dysregulation and neuroinflammation
Melatonin, an indoleamine hormone secreted by the pineal gland, plays essential roles in regulating circadian rhythms—by synchronizing the biological clock via receptors in the suprachiasmatic nucleus to promote sleep—, antioxidant defense through direct scavenging of free radicals (e.g., •OH/ONOO−), and activation of enzymes such as SOD and GPx, and immune modulation by suppressing pro-inflammatory cytokines (e.g., TNF-α, IL-6) and enhancing lymphocyte activity. In the context of SCI, these innate functions extend to multi-target neuroprotective mechanisms, including inhibition of inflammasome activation, restoration of mitochondrial dysfunction, and stabilization of the BSCB. Melatonin has emerged as a therapeutic focus in SCI. Its mechanisms include mitochondrial protection, autophagy-apoptosis balance regulation, and inflammasome modulation. Melatonin suppresses NLRP3 inflammasome activity via the Nrf2/ARE pathway, reducing oxidative stress and pro-inflammatory cytokines (e.g., IL-1β, TNF-α) (92), while improving mitochondrial dysfunction and neuronal apoptosis through SIRT1/Drp1 signaling (94). It balances autophagy and apoptosis via PI3K/AKT/mTOR and Wnt/β-catenin pathways, upregulating Beclin-1 and LC3B to enhance cellular clearance (95), and promotes motor neuron survival (96). At the tissue level, melatonin stabilizes the BSCB by inhibiting MMP3/AQP4-mediated microvascular permeability (97) and reduces neuroinflammation via microglial M2 polarization (98).
5 Neuroregeneration-oriented hormones and growth factors
5.1 Erythropoietin: dual pathways in angiogenesis and anti-apoptosis
EPO, with its anti-apoptotic and anti-inflammatory properties, is a promising therapeutic candidate for SCI. Preclinical studies show EPO inhibits inflammation (reducing myeloperoxidase activity) and apoptosis (lowering caspase-3 activity), while attenuating pathological damage via downregulation of TSP-1 and TGF-β (99). It promotes neural regeneration by upregulating PDGF-β and GFAP expression (64). Clinically, EPO combined with MP improves neurological function and daily living activities in long-term follow-ups (100), with animal studies confirming its superiority over MP monotherapy (101). A clinical trial reported higher primary endpoint achievement in the EPO group versus MP alone (102), and early application (within 6 hours) may enhance efficacy (103), though other trials found no significant differences (104).
5.2 Growth hormone: BSCB repair and insulin-like growth factor-1 synergistic mechanisms
GH exerts neuroprotective and reparative effects in SCI through multiple pathways. GH mitigates BSCB disruption and edema formation by reducing tracer extravasation (e.g., Evans blue) and preserving spinal cord evoked potential amplitudes, thus protecting neural conduction (105, 106). Its mechanisms likely involve suppression of vascular hyperpermeability and cellular injury, indicating direct neuroprotective potential in acute phases. GH also indirectly promotes repair by elevating IGF-1 levels, with TiO2 nanowire-loaded GH enhancing this effect while reducing BSCB damage and neuronal loss (107). TiO2 nanowires are one-dimensional nanomaterials composed of titanium dioxide, with diameters ranging from 1 to 100 nm. They exhibit high specific surface area, good biocompatibility, and photo-responsive properties. Surface functionalization—such as amino modification—confers a positive charge on TiO2 nanowires, enabling electrostatic adsorption with negatively charged GH molecules. Alternatively, covalent conjugation strategies like glutaraldehyde cross-linking can be employed to achieve GH immobilization. Preclinical studies suggest GH may improve hemodynamics; for instance, octreotide (a somatostatin analog) increases clitoral and vaginal blood flow post-SCI, though its mechanism differs from GH (108). Notably, SCI patients frequently exhibit GH-IGF-I axis hypoactivity, characterized by blunted GH responses and metabolic abnormalities (e.g., increased adiposity), which GH supplementation may ameliorate (109).
5.3 Thyrotropin-releasing hormone: ion homeostasis and neural repair
As a neuropeptide, TRH promotes SCI recovery by regulating monoamine neurotransmitters and ion channel activity. TRH exerts neuroprotective and repair effects through multiple mechanisms in SCI. Basic studies have shown that TRH maintains ion homeostasis, such as reducing cellular edema by enhancing Na+-K+-ATPase activity (110), and regulates the metabolic balance of TRH and 5-HT in the injured spinal cord to minimize distal neurotransmitter depletion (111). Its anti-inflammatory effects include inhibiting vasogenic edema (112) and downregulating microglial activation to alleviate central pain (113). Additionally, TRH analogs (e.g., CG3703) exhibit therapeutic potential due to their high affinity for spinal TRH receptors, though their efficacy is closely linked to molecular modifications (114). Clinical studies demonstrate that TRH significantly improves motor and sensory functions in patients with incomplete SCI (115). Notably, TRH receptor expression decreases shortly after injury but gradually normalizes with the recovery of endogenous TRH (116), supporting the need for exogenous TRH supplementation. However, the long-term effects of TRH on neuronal excitability remain unclear (117).
6 Cross-regulation between the HPA and HPG axes
Following acute SCI, the hypothalamic-pituitary-adrenal (HPA) axis induces excessive glucocorticoid release (e.g., cortisol) via the sympathoadrenal reflex, directly or indirectly inhibiting hypothalamic GnRH secretion and causing central hypogonadism (118). For example, Prüss et al. observed increased cortisol and decreased norepinephrine in mice after acute SCI, suggesting that excessive HPA axis activation disrupts HPG axis function through neuroendocrine mechanisms (118). HPG axis dysfunction exacerbates metabolic disorders: HPG axis inhibition (e.g., low testosterone) is closely associated with increased body fat and metabolic syndrome in SCI patients, further promoting sustained HPA axis activation. Sullivan et al. found that low testosterone in young male SCI patients is primarily due to hypothalamic-pituitary drive deficiency and correlates significantly with increased body fat (50). Bauman et al.confirmed that the pituitary gland in SCI patients shows enhanced FSH response but insufficient luteinizing hormone response to GnRH, indicating that hypothalamic regulation impairment may be related to HPA axis negative feedback (49). Through GnRH dose-response experiments, Bauman et al. identified specific differences in pituitary responses among SCI patients, highlighting the need for optimized interventions (e.g., glucocorticoid antagonism or testosterone supplementation) to break the vicious cycle (119).
In summary, interactions between the HPA and HPG axes after SCI form a closed-loop of “increased glucocorticoids-gonadal inhibition-metabolic abnormalities,” necessitating combined targeted therapies to improve outcomes.
7 Combination therapies and novel delivery strategies: overcoming therapeutic efficacy limitations
7.1 Combination therapies
The limitations of single-agent hormone therapies have driven researchers to explore combination regimens and innovative delivery technologies. Combination therapies can overcome the efficacy ceiling of monotherapy. MP, a classic anti-inflammatory agent, exhibits marked variability in its combinatorial effects. For instance, Gorio et al. demonstrated that MP antagonizes the neuroprotective effects of EPO by suppressing EPO receptor upregulation or interfering with its anti-apoptotic pathways (120). In contrast, a clinical study by Xiong et al. revealed that co-administration of EPO and MP significantly improved neurological function and daily living capacity in ischemia-reperfusion injury (100). This paradox suggests that MP’s immunosuppressive properties may exert dual-phase effects depending on pathological stages—suppressing inflammation acutely while potentially impeding regenerative signaling pathways.
7.2 Combination strategies targeting antioxidant and immunomodulatory pathways show greater synergistic potential
Carnosine is an endogenous dipeptide (β-alanyl-L-histidine) widely present in muscular and neural tissues, known for its antioxidant, anti-glycation (inhibiting AGEs formation), and pH-buffering capacities. Irisin is an exercise-induced myokine released through the cleavage of FNDC5 protein, primarily involved in regulating energy metabolism (promoting browning of white adipose tissue), neuroprotection (upregulating BDNF), and anti-inflammatory pathways (suppressing NF-κB). In the context of SCI, the combination of MP and carnosine alleviates neural damage by elevating irisin levels, likely through synergistically enhancing antioxidant defenses and promoting the secretion of neurotrophic factors. This pathway has been further confirmed by Albayrak et al (121). Teixeira et al. further demonstrated that MP combined with granulocyte colony-stimulating factor (G-CSF) significantly enhanced motor function and reduced inflammatory cell infiltration, indicating complementary mechanisms between G-CSF’s neuroregenerative effects and MP’s anti-inflammatory actions (122). Similarly, Genovese et al. confirmed that melatonin combined with dexamethasone synergistically suppressed neutrophil infiltration and apoptosis, mitigating secondary injury (123). In summary, successful combination therapies require meticulous optimization of temporal coordination, target complementarity, and dosage regimens.
8 Clinical translation potential of targeted delivery systems
To circumvent systemic side effects of conventional drug administration, MP-loaded nanoparticles (e.g., PLGA-MP) and in situ gels (e.g., fibrin/chitosan composites) have emerged as research priorities. Cox et al. utilized estrogen-loaded nanoparticle patches targeting injury sites, reducing glial scar formation and promoting axonal regeneration while avoiding systemic toxicity from high-dose estrogen (74). Similarly, Karabey-Akyurek et al. developed an MP-nano-fibrin gel for localized delivery, achieving efficacy comparable to high-dose systemic MP with significantly reduced adverse effects (124). Qin et al. designed Nano-MP, a prodrug-based system selectively targeting injured areas, which not only enhanced neuroprotection in rat models but also avoided glucocorticoid-induced muscle atrophy and osteoporosis (125). Wang et al. reported carrier-free nanoparticle MP(2)-TK@RU NPs integrated with the antioxidant rutin, enabling ROS-responsive MP release to simultaneously suppress inflammation, oxidative damage, and promote functional recovery (126). Chvatal et al. achieved deep MP penetration using PLGA nanoparticles combined with hydrogels (127), while Zhai et al. developed a microneedle-CD-MOF system to breach the dura barrier for precise controlled release (128). Despite diverse strategies, these studies collectively demonstrate that targeted delivery systems can overcome the limitations of conventional therapies. Future efforts should prioritize validating long-term safety and clinical applicability to advance nanodelivery technologies from experimental to clinical stages.
9 Conclusion and prospect
Hormonal therapies exhibit multi-layered mechanisms in SCI repair, ranging from anti-inflammatory actions to neuroregeneration. However, their clinical application remains challenged by inconsistent efficacy and significant side effects, necessitating precise strategies to harness their therapeutic potential. Research on hormonal interventions for SCI has evolved from single-molecule approaches to complex regulatory networks. Beyond classical glucocorticoids, it is now well described that sex hormones like estrogen or its SERMs, like TMX, as well as other hormone (e.g., melatonin, GH, and leptin) contribute to neuroprotection and repair via multi-target mechanisms. Future studies should focus on: 1. Elucidating hormone-cytokine-epigenetic crosstalk to develop multi-pathway synergistic agents; 2. Leveraging nanotechnology and gene editing to overcome delivery barriers and advance novel delivery platforms; 3. Deciphering receptor-specific signaling pathways for targeted agonists/antagonists using OMICs and bioinformatics approaches; 4. Optimizing combination strategies, such as integrating hormones with stem cells or biomaterials.
Author contributions
SZ: Supervision, Writing – original draft. WG: Data curation, Writing – original draft, Writing – review & editing. YW: Data curation, Writing – original draft, Writing – review & editing. JX: Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was granted by the National Natural Science Foundation of China (82360451); Guangxi Science and Technology Department (AB25069038, 2022GXNSFBA035545); Guangxi Health Commission (S2023062).
Acknowledgments
We thank the public databases PubMed for its assistance in our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. (2015) 76 Suppl 1:S71–83. doi: 10.1227/01.neu.0000462080.04196.f7
2. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. (1990) 322:1405–11. doi: 10.1056/NEJM199005173222001
3. Evaniew N, Noonan VK, Fallah N, Kwon BK, Rivers CS, Ahn H, et al. Methylprednisolone for the treatment of patients with acute spinal cord injuries: A propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J Neurotrauma. (2015) 32:1674–83. doi: 10.1089/neu.2015.3963
4. Vaquero J, Zurita M, Oya S, Aguayo C, and Bonilla C. Early administration of methylprednisolone decreases apoptotic cell death after spinal cord injury. Histol Histopathol. (2006) 21:1091–102. doi: 10.14670/HH-21.1091
5. Mallei A, Aden SA, Bachis A, Brandoli C, Ongini E, and Mocchetti I. The nitrosteroid NCX 1015, a prednisolone derivative, improves recovery of function in rats after spinal cord injury. Brain Res. (2005) 1062:16–25. doi: 10.1016/j.brainres.2005.08.057
6. Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, et al. Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci: Off J Soc Neurosci. (2008) 28:3141–9. doi: 10.1523/JNEUROSCI.5547-07.2008
7. Christie SD, Comeau B, Myers T, Sadi D, Purdy M, and Mendez I. Duration of lipid peroxidation after acute spinal cord injury in rats and the effect of methylprednisolone. Neurosurg Focus. (2008) 25:E5. doi: 10.3171/FOC.2008.25.11.E5
8. Tang P, Zhang Y, Chen C, Ji X, Ju F, Liu X, et al. In vivo two-photon imaging of axonal dieback, blood flow, and calcium influx with methylprednisolone therapy after spinal cord injury. Sci Rep. (2015) 5:9691. doi: 10.1038/srep09691
9. Koc RK, Akdemir H, Kurtsoy A, Pasaoglu H, Kavuncu I, Pasaoglu A, et al. Lipid peroxidation in experimental spinal cord injury. Comparison of treatment with Ginkgo biloba, TRH and methylprednisolone. Res Exp Med Z für die Gesamte Experimentelle Med Einschließlich Experimenteller Chirurgie. (1995) 195:117–23. doi: 10.1007/BF02576781
10. Ahmadi F, Zargari M, Nasiry D, and Khalatbary AR. Synergistic neuroprotective effects of hyperbaric oxygen and methylprednisolone following contusive spinal cord injury in rats. J Spinal Cord Med. (2022) 45:930–9. doi: 10.1080/10790268.2021.1896275
11. Hall ED. Neuroprotective actions of glucocorticoid and nonglucocorticoid steroids in acute neuronal injury. Cell Mol Neurobiol. (1993) 13:415–32. doi: 10.1007/BF00711581
12. Xu J, Chen S, Chen H, Xiao Q, Hsu CY, Michael D, et al. STAT5 mediates antiapoptotic effects of methylprednisolone on oligodendrocytes. J Neurosci: Off J Soc Neurosci. (2009) 29:2022–6. doi: 10.1523/JNEUROSCI.2621-08.2009
13. Chikuda H, Yasunaga H, Takeshita K, Horiguchi H, Kawaguchi H, Ohe K, et al. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emergency Med J: EMJ. (2014) 31:201–6. doi: 10.1136/emermed-2012-202058
14. Zhang Y, Zhang L, Ji X, Pang M, Ju F, Zhang J, et al. Two-photon microscopy as a tool to investigate the therapeutic time window of methylprednisolone in a mouse spinal cord injury model. Restorative Neurol Neurosci. (2015) 33:291–300. doi: 10.3233/RNN-140463
15. Yang LY, Tsai MY, Juan SH, Chang SF, Yu CR, Lin JC, et al. Exerting the appropriate application of methylprednisolone in acute spinal cord injury based on time course transcriptomics analysis. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms222313024
16. Weaver LC, Gris D, Saville LR, Oatway MA, Chen Y, Marsh DR, et al. Methylprednisolone causes minimal improvement after spinal cord injury in rats, contrasting with benefits of an anti-integrin treatment. J Neurotrauma. (2005) 22:1375–87. doi: 10.1089/neu.2005.22.1375
17. Wu Y, Hou J, Collier L, Pan J, Hou L, Qin W, et al. The administration of high-dose methylprednisolone for 24 h reduced muscle size and increased atrophy-related gene expression in spinal cord-injured rats. Spinal Cord. (2011) 49:867–73. doi: 10.1038/sc.2011.28
18. Suberviola B, González-Castro A, Llorca J, Ortiz-Melón F, and Miñambres E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. (2008) 39:748–52. doi: 10.1016/j.injury.2007.12.005
19. Caruso MC, Daugherty MC, Moody SM, Falcone RA, Bierbrauer KS, and Geis GL. Lessons learned from administration of high-dose methylprednisolone sodium succinate for acute pediatric spinal cord injuries. J Neurosurg Pediatr. (2017) 20:567–74. doi: 10.3171/2017.7.PEDS1756
20. Osmanagaoglu MA, Usul H, Yulug E, Kesim M, and Karahan SC. Hormonal and histological changes in the ovaries with high-doses of methylprednisolone administration for acute spinal cord injury: an experimental study. J Obstet Gynaecol: J Institute Obstet Gynaecol. (2013) 33:585–90. doi: 10.3109/01443615.2013.789833
21. Hejrati N, Aarabi B, Neal CJ, Ugiliweneza B, Kurpad SN, Shaffrey CI, et al. Trends in the use of corticosteroids in the management of acute spinal cord injury in North American clinical trials network sites. J Neurotrauma. (2023) 40:1938–47. doi: 10.1089/neu.2022.0409
22. Clark MJ, Schopp LH, Mazurek MO, Zaniletti I, Lammy AB, Martin TA, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. (2008) 87:758–67. doi: 10.1097/PHM.0b013e3181837f4f
23. Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones Metab. (2011) 43:574–9. doi: 10.1055/s-0031-1280797
24. Gorgey AS, Khalil RE, Gill R, Gater DR, Lavis TD, Cardozo CP, et al. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma. (2019) 36:2631–45. doi: 10.1089/neu.2018.6136
25. Lumbroso S, Sandillon F, Georget V, Lobaccaro JM, Brinkmann AO, Privat A, et al. Immunohistochemical localization and immunoblotting of androgen receptor in spinal neurons of male and female rats. Eur J Endocrinol. (1996) 134:626–32. doi: 10.1530/eje.0.1340626
26. Tehranipour M and Moghimi A. Neuroprotective effects of testosterone on regenerating spinal cord motoneurons in rats. J Motor Behav. (2010) 42:151–5. doi: 10.1080/00222891003697921
27. Clark MJ, Petroski GF, Mazurek MO, Hagglund KJ, Sherman AK, Lammy AB, et al. Testosterone replacement therapy and motor function in men with spinal cord injury: a retrospective analysis. Am J Phys Med Rehabil. (2008) 87:281–4. doi: 10.1097/PHM.0b013e318168bbec
28. Wu Y, Collier L, Pan J, Qin W, Bauman WA, and Cardozo CP. Testosterone reduced methylprednisolone-induced muscle atrophy in spinal cord-injured rats. Spinal Cord. (2012) 50:57–62. doi: 10.1038/sc.2011.91
29. Gregory CM, Vandenborne K, Huang HF, Ottenweller JE, and Dudley GA. Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord. (2003) 41:23–8. doi: 10.1038/sj.sc.3101370
30. Gorgey AS, Graham ZA, Chen Q, Rivers J, Adler RA, Lesnefsky EJ, et al. Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J Appl Physiol (Bethesda Md: 1985). (2020) 128:1487–96. doi: 10.1152/japplphysiol.00865.2019
31. Byers JS, Huguenard AL, Kuruppu D, Liu NK, Xu XM, and Sengelaub DR. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J Comp Neurol. (2012) 520:2683–96. doi: 10.1002/cne.23066
32. Coronel MF, Labombarda F, De Nicola AF, and González SL. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur J Pain (London England). (2014) 18:348–59. doi: 10.1002/j.1532-2149.2013.00376.x
33. Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, and Wright DW. Progesterone’s role in neuroprotection, a review of the evidence. Brain Res. (2013) 1530:82–105. doi: 10.1016/j.brainres.2013.07.014
34. González SL, Labombarda F, González Deniselle MC, Guennoun R, Schumacher M, and De Nicola AF. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience. (2004) 125:605–14. doi: 10.1016/j.neuroscience.2004.02.024
35. González SL, López-Costa JJ, Labombarda F, González Deniselle MC, Guennoun R, Schumacher M, et al. Progesterone effects on neuronal ultrastructure and expression of microtubule-associated protein 2 (MAP2) in rats with acute spinal cord injury. Cell Mol Neurobiol. (2009) 29:27–39. doi: 10.1007/s10571-008-9291-0
36. Lee TH, Liu HL, Yang ST, Yang JT, Yeh MY, and Lin JR. Effects of aging and hypertension on cerebral ischemic susceptibility: evidenced by MR diffusion-perfusion study in rat. Exp Neurol. (2011) 227:314–21. doi: 10.1016/j.expneurol.2010.12.003
37. Miller AM and Stella N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantification of migration by a novel near-infrared method. Glia. (2009) 57:875–83. doi: 10.1002/glia.20813
38. Schroeter ML, Mueller K, Arelin K, Sacher J, Holiga S, Kratzsch J, et al. Serum neuron-specific enolase is related to cerebellar connectivity: A resting-state functional magnetic resonance imaging pilot study. J Neurotrauma. (2015) 32:1380–4. doi: 10.1089/neu.2013.3163
39. Labombarda F and Garcia-Ovejero D. Give progesterone a chance. Neural Regeneration Res. (2014) 9:1422–4. doi: 10.4103/1673-5374.139456
40. Labombarda F, González SL, Lima A, Roig P, Guennoun R, Schumacher M, et al. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. (2009) 57:884–97. doi: 10.1002/glia.20814
41. Coronel MF, Labombarda F, Villar MJ, De Nicola AF, and González SL. Progesterone prevents allodynia after experimental spinal cord injury. J Pain. (2011) 12:71–83. doi: 10.1016/j.jpain.2010.04.013
42. Coronel MF, Villar MJ, Brumovsky PR, and Gonzalez SL. Spinal neuropeptide expression and neuropathic behavior in the acute and chronic phases after spinal cord injury: Effects of progesterone administration. Peptides. (2017) 88:189–95. doi: 10.1016/j.peptides.2017.01.001
43. Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, and Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine (Phila Pa 1976). (1999) 24:2134–8. doi: 10.1097/00007632-199910150-00013
44. Yang Z, Xie W, Ju F, Khan A, and Zhang S. In vivo two-photon imaging reveals a role of progesterone in reducing axonal dieback after spinal cord injury in mice. Neuropharmacology. (2017) 116:30–7. doi: 10.1016/j.neuropharm.2016.12.007
45. Aminmansour B, Asnaashari A, Rezvani M, Ghaffarpasand F, Amin Noorian SM, Saboori M, et al. Effects of progesterone and vitamin D on outcome of patients with acute traumatic spinal cord injury; a randomized, double-blind, placebo controlled study. J Spinal Cord Med. (2016) 39:272–80. doi: 10.1080/10790268.2015.1114224
46. Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, et al. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. (2001) 304:193–214. doi: 10.1007/s004410100363
47. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, and Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. (2005) 307:1625–30. doi: 10.1126/science.1106943
48. Prossnitz ER and Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. (2011) 7:715–26. doi: 10.1038/nrendo.2011.122
49. Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. (2009) 57:807–14. doi: 10.1002/glia.20806
50. Taylor LC, Puranam K, Gilmore W, Ting JP, and Matsushima GK. 17beta-estradiol protects male mice from cuprizone-induced demyelination and oligodendrocyte loss. Neurobiol Dis. (2010) 39:127–37. doi: 10.1016/j.nbd.2010.03.016
51. Gu Y, Wu Y, Su W, Xing L, Shen Y, He X, et al. 17β-estradiol enhances Schwann cell differentiation via the ERβ-ERK1/2 signaling pathway and promotes remyelination in injured sciatic nerves. Front Pharmacol. (2018) 9:1026. doi: 10.3389/fphar.2018.01026
52. Lee JY, Choi SY, Oh TH, and Yune TY. 17β-Estradiol inhibits apoptotic cell death of oligodendrocytes by inhibiting RhoA-JNK3 activation after spinal cord injury. Endocrinology. (2012) 153:3815–27. doi: 10.1210/en.2012-1068
53. Ragerdi Kashani I, Hedayatpour A, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, et al. 17β-Estradiol enhances the efficacy of adipose-derived mesenchymal stem cells on remyelination in mouse model of multiple sclerosis. Acta Med Iranica. (2012) 50:789–97.
54. Namjoo Z, Mortezaee K, Joghataei MT, Moradi F, Piryaei A, Abbasi Y, et al. Targeting axonal degeneration and demyelination using combination administration of 17β-estradiol and Schwann cells in the rat model of spinal cord injury. J Cell Biochem. (2018) 119:10195–203. doi: 10.1002/jcb.27361
55. Luo Y, Xiao Q, Chao F, He Q, Lv F, Zhang L, et al. 17β-estradiol replacement therapy protects myelin sheaths in the white matter of middle-aged female ovariectomized rats: a stereological study. Neurobiol Aging. (2016) 47:139–48. doi: 10.1016/j.neurobiolaging.2016.07.023
56. Hu R, Sun H, Zhang Q, Chen J, Wu N, Meng H, et al. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit Care Med. (2012) 40:3230–7. doi: 10.1097/CCM.0b013e3182657560
57. Zendedel A, Mönnink F, Hassanzadeh G, Zaminy A, Ansar MM, Habib P, et al. Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol Neurobiol. (2018) 55:1364–75. doi: 10.1007/s12035-017-0400-2
58. Cheng Q, Meng J, Wang XS, Kang WB, Tian Z, Zhang K, et al. G-1 exerts neuroprotective effects through G protein-coupled estrogen receptor 1 following spinal cord injury in mice. Biosci Rep. (2016) 36. doi: 10.1042/BSR20160134
59. Lee JY, Choi HY, Na WH, Ju BG, and Yune TY. 17β-estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats. Endocrinology. (2015) 156:1838–50. doi: 10.1210/en.2014-1832
60. Ni S, Cao Y, Jiang L, Luo Z, Lu H, Hu J, et al. Synchrotron radiation imaging reveals the role of estrogen in promoting angiogenesis after acute spinal cord injury in rats. Spine (Phila Pa 1976). (2018) 43:1241–9. doi: 10.1097/BRS.0000000000002629
61. Cox A, Capone M, Matzelle D, Vertegel A, Bredikhin M, Varma A, et al. Nanoparticle-based estrogen delivery to spinal cord injury site reduces local parenchymal destruction and improves functional recovery. J Neurotrauma. (2021) 38:342–52. doi: 10.1089/neu.2020.7047
62. Aryanpour R, Zibara K, Pasbakhsh P, Jame’ei SB, Namjoo Z, Ghanbari A, et al. 17β-estradiol reduces demyelination in cuprizone-fed mice by promoting M2 microglia polarity and regulating NLRP3 inflammasome. Neuroscience. (2021) 463:116–27. doi: 10.1016/j.neuroscience.2021.03.025
63. Thakkar R, Wang R, Wang J, Vadlamudi RK, and Brann DW. 17β-estradiol regulates microglia activation and polarization in the hippocampus following global cerebral ischemia. Oxid Med Cell Longev. (2018) 2018:4248526. doi: 10.1155/2018/4248526
64. Du ZR, Feng XQ, Li N, Qu JX, Feng L, Chen L, et al. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine. (2018) 43:11–20. doi: 10.1016/j.phymed.2018.03.039
65. Cleland NRW, Potter GJ, Buck C, Quang D, Oldham D, Neal M, et al. Altered metabolism and DAM-signatures in female brains and microglia with aging. bioRxiv: preprint Server Biol. (2023). doi: 10.1101/2023.11.28.569104
66. Jansson L, Olsson T, and Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol. (1994) 53:203–7. doi: 10.1016/0165-5728(94)90030-2
67. Bardy-Lagarde M, Asbelaoui N, Schumacher M, and Ghoumari AM. Estradiol promotes myelin repair in the spinal cord of female mice in a CXCR4 chemokine receptor-independent manner. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26104752
68. Patel R, Moore S, Crawford DK, Hannsun G, Sasidhar MV, Tan K, et al. Attenuation of corpus callosum axon myelination and remyelination in the absence of circulating sex hormones. Brain Pathol (Zurich Switzerland). (2013) 23:462–75. doi: 10.1111/bpa.12029
69. Garrido-Gil P, Pedrosa MA, Garcia-Garrote M, Pequeño-Valtierra A, Rodríguez-Castro J, García-Souto D, et al. Microglial angiotensin type 2 receptors mediate sex-specific expression of inflammatory cytokines independently of circulating estrogen. Glia. (2022) 70:2348–60. doi: 10.1002/glia.24255
70. Sanchez K, Wu SL, Kakkar R, Darling JS, Harper CS, and Fonken LK. Ovariectomy in mice primes hippocampal microglia to exacerbate behavioral sickness responses. Brain Behav Immun Health. (2023) 30:100638. doi: 10.1016/j.bbih.2023.100638
71. Wu D, Bi X, and Chow KH. Identification of female-enriched and disease-associated microglia (FDAMic) contributes to sexual dimorphism in late-onset Alzheimer’s disease. J Neuroinflamm. (2024) 21:1. doi: 10.1186/s12974-023-02987-4
72. Barreto GE, Santos-Galindo M, and Garcia-Segura LM. Selective estrogen receptor modulators regulate reactive microglia after penetrating brain injury. Front Aging Neurosci. (2014) 6:132. doi: 10.3389/fnagi.2014.00132
73. Li M, Zhang J, Chen W, Liu S, Liu X, Ning Y, et al. Supraphysiologic doses of 17beta-estradiol aggravate depression-like behaviors in ovariectomized mice possibly via regulating microglial responses and brain glycerophospholipid metabolism. J Neuroinflamm. (2023) 20:204. doi: 10.1186/s12974-023-02889-5
74. Traiffort E, Kassoussi A, and Zahaf A. Revisiting the role of sexual hormones in the demyelinated central nervous system. Front Neuroendocrinol. (2025) 76:101172. doi: 10.1016/j.yfrne.2024.101172
75. Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, et al. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. (2007) 24:473–80. doi: 10.1089/neu.2006.0167
76. Colon JM, Torrado AI, Cajigas A, Santiago JM, Salgado IK, Arroyo Y, et al. Tamoxifen administration immediately or 24 hours after spinal cord injury improves locomotor recovery and reduces secondary damage in female rats. J Neurotrauma. (2016) 33:1696–708. doi: 10.1089/neu.2015.4111
77. Colon JM and Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regeneration Res. (2016) 11:1208–11. doi: 10.4103/1673-5374.189164
78. Cabrera-Aldana EE, Balderas-Martinez YI, Velazquez-Cruz R, Tovar YRLB, Sevilla-Montoya R, Martinez-Cruz A, et al. Administration of tamoxifen can regulate changes in gene expression during the acute phase of traumatic spinal cord injury. Curr Issues Mol Biol. (2023) 45:7476–91. doi: 10.3390/cimb45090472
79. Shvetcov A, Ruitenberg MJ, Delerue F, Gold WA, Brown DA, and Finney CA. The neuroprotective effects of estrogen and estrogenic compounds in spinal cord injury. Neurosci Biobehav Rev. (2023) 146:105074. doi: 10.1016/j.neubiorev.2023.105074
80. Calderón-Vallejo D and Quintanar JL. Gonadotropin-releasing hormone treatment improves locomotor activity, urinary function and neurofilament protein expression after spinal cord injury in ovariectomized rats. Neurosci Lett. (2012) 515:187–90. doi: 10.1016/j.neulet.2012.03.052
81. Calderón-Vallejo D, Hernández-Jasso I, Martínez-Moreno CG, Arámburo C, Munoz A, Martínez-Saldaña MC, et al. Preventive effects of gonadotropin-releasing hormone treatment on urinary bladder and kidney damage in spinal cord injured rats. Neurourol Urodynamics. (2025) 44:220–8. doi: 10.1002/nau.25591
82. Martinez-Moreno CG, Calderon-Vallejo D, Diaz-Galindo C, Hernandez-Jasso I, Olivares-Hernandez JD, Avila-Mendoza J, et al. Neurotrophic and synaptic effects of GnRH and/or GH upon motor function after spinal cord injury in rats. Sci Rep. (2024) 14:26420. doi: 10.1038/s41598-024-78073-3
83. Sullivan SD, Nash MS, Tefera E, Tinsley E, Blackman MR, and Groah S. Prevalence and etiology of hypogonadism in young men with chronic spinal cord injury: A cross-sectional analysis from two university-based rehabilitation centers. PM R: J Injury Funct Rehabil. (2017) 9:751–60. doi: 10.1016/j.pmrj.2016.11.005
84. Zhang Y and Chua S Jr. Leptin function and regulation. Compr Physiol. (2017) 8:351–69. doi: 10.1002/j.2040-4603.2018.tb00002.x
85. Li B, Qi S, Sun G, Yang L, Han J, Zhu Y, et al. Leptin suppresses adenosine triphosphate-induced impairment of spinal cord astrocytes. J Neurosci Res. (2016) 94:924–35. doi: 10.1002/jnr.23795
86. Fernandez-Martos CM, Gonzalez P, and Rodriguez FJ. Acute leptin treatment enhances functional recovery after spinal cord injury. PloS One. (2012) 7:e35594. doi: 10.1371/journal.pone.0035594
87. Chang KT, Lin YL, Lin CT, Hong CJ, Tsai MJ, Huang WC, et al. Leptin is essential for microglial activation and neuropathic pain after preganglionic cervical root avulsion. Life Sci. (2017) 187:31–41. doi: 10.1016/j.lfs.2017.08.016
88. Latifi S, Koushki D, Norouzi Javidan A, Matin M, and Sabour H. Changes of leptin concentration in plasma in patients with spinal cord injury: a meta-analysis. Spinal Cord. (2013) 51:728–31. doi: 10.1038/sc.2013.82
89. Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, et al. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. (2008) 46:494–9. doi: 10.1038/sj.sc.3102171
90. Gezici AR, Ergun R, Karakas A, and Gunduz B. Serum leptin levels following acute experimental spinal cord injury. J Spinal Cord Med. (2009) 32:416–21. doi: 10.1080/10790268.2009.11753205
91. Park AJ, Battaglino RA, Nguyen NMH, and Morse LR. Associations between lean mass and leptin in men with chronic spinal cord injury: Results from the FRASCI-muscle study. PloS One. (2018) 13:e0198969. doi: 10.1371/journal.pone.0198969
92. Wang L, Tang X, Zhang H, Yuan J, Ding H, and Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J Spinal Cord Med. (2011) 34:501–9. doi: 10.1179/2045772311Y.0000000034
93. Huang TS, Wang YH, and Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehabil. (2000) 81:1582–6. doi: 10.1053/apmr.2000.9173
94. Zhong G, Yang Y, Feng D, Wei K, Chen J, Chen J, et al. Melatonin protects injured spinal cord neurons from apoptosis by inhibiting mitochondrial damage via the SIRT1/Drp1 signaling pathway. Neuroscience. (2023) 534:54–65. doi: 10.1016/j.neuroscience.2023.10.010
95. Li Y, Guo Y, Fan Y, Tian H, Li K, and Mei X. Melatonin enhances autophagy and reduces apoptosis to promote locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR signaling pathway. Neurochem Res. (2019) 44:2007–19. doi: 10.1007/s11064-019-02838-w
96. Shen Z, Zhou Z, Gao S, Guo Y, Gao K, Wang H, et al. Melatonin inhibits neural cell apoptosis and promotes locomotor recovery via activation of the Wnt/β-catenin signaling pathway after spinal cord injury. Neurochem Res. (2017) 42:2336–43. doi: 10.1007/s11064-017-2251-7
97. Wu Q, Jing Y, Yuan X, Zhang X, Li B, Liu M, et al. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J Mol Neurosci: MN. (2014) 54:714–22. doi: 10.1007/s12031-014-0430-4
98. Zhang Y, Liu Z, Zhang W, Wu Q, Zhang Y, Liu Y, et al. Melatonin improves functional recovery in female rats after acute spinal cord injury by modulating polarization of spinal microglial/macrophages. J Neurosci Res. (2019) 97:733–43. doi: 10.1002/jnr.24409
99. Okutan O, Solaroglu I, Beskonakli E, and Taskin Y. Recombinant human erythropoietin decreases myeloperoxidase and caspase-3 activity and improves early functional results after spinal cord injury in rats. J Clin Neurosci: Off J Neurosurg Soc Australasia. (2007) 14:364–8. doi: 10.1016/j.jocn.2006.01.022
100. Xiong M, Chen S, Yu H, Liu Z, Zeng Y, and Li F. Neuroprotection of erythropoietin and methylprednisolone against spinal cord ischemia-reperfusion injury. J Huazhong Univ Sci Technol Med Sci = Hua zhong ke ji da xue xue bao Yi xue Ying wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. (2011) 31:652. doi: 10.1007/s11596-011-0576-z
101. Boran BO, Colak A, and Kutlay M. Erythropoietin enhances neurological recovery after experimental spinal cord injury. Restorative Neurol Neurosci. (2005) 23:341–5. doi: 10.3233/RNN-2005-00320
102. Costa DD, Beghi E, Carignano P, Pagliacci C, Faccioli F, Pupillo E, et al. Tolerability and efficacy of erythropoietin (EPO) treatment in traumatic spinal cord injury: a preliminary randomized comparative trial vs. methylprednisolone (MP). Neurol Sci: Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. (2015) 36:1567–74. doi: 10.1007/s10072-015-2182-5
103. Alibai E, Zand F, Rahimi A, and Rezaianzadeh A. Erythropoietin plus methylprednisolone or methylprednisolone in the treatment of acute spinal cord injury: a preliminary report. Acta Med Iranica. (2014) 52:275–9.
104. Ganjeifar B, Rezaee H, Keykhosravi E, Tavallaii A, Bahadorkhan G, Nakhaei M, et al. The effect of combination therapy with erythropoietin and methylprednisolone in patients with traumatic cervical spinal cord injury: a pilot randomized controlled trial. Spinal Cord. (2021) 59:347–53. doi: 10.1038/s41393-020-00604-2
105. Nyberg F and Sharma HS. Repeated topical application of growth hormone attenuates blood-spinal cord barrier permeability and edema formation following spinal cord injury: an experimental study in the rat using Evans blue, ([125])I-sodium and lanthanum tracers. Amino Acids. (2002) 23:231–9. doi: 10.1007/s00726-001-0134-2
106. Winkler T, Sharma HS, Stålberg E, Badgaiyan RD, Westman J, and Nyberg F. Growth hormone attenuates alterations in spinal cord evoked potentials and cell injury following trauma to the rat spinal cord. An experimental study using topical application of rat growth hormone. Amino Acids. (2000) 19:363–71. doi: 10.1007/s007260070067
107. Muresanu DF, Sharma A, Lafuente JV, Patnaik R, Tian ZR, Nyberg F, et al. Nanowired delivery of growth hormone attenuates pathophysiology of spinal cord injury and enhances insulin-like growth factor-1 concentration in the plasma and the spinal cord. Mol Neurobiol. (2015) 52:837–45. doi: 10.1007/s12035-015-9298-8
108. Abdel-Karim AM and Carrier S. Possible improvement of the clitoral and vaginal blood flow using a somatostatin analog in chronic spinalized Sprague-Dawley rats. J Sex Marital Ther. (2002) 28 Suppl 1:1–9. doi: 10.1080/00926230252851140
109. Bauman WA, Zhang RL, and Spungen AM. Provocative stimulation of growth hormone: a monozygotic twin study discordant for spinal cord injury. J Spinal Cord Med. (2007) 30:467–72. doi: 10.1080/10790268.2007.11754579
110. Baykal S, Ceylan S, Usul H, Akturk F, and Deger O. Effect of thyrotropin-releasing hormone on Na(+)-K(+)-Adenosine triphosphatase activity following experimental spinal cord trauma. Neurol Medico Chirurgica. (1996) 36:296–9. doi: 10.2176/nmc.36.296
111. Shapiro S, Kubek M, Siemers E, Daly E, Callahan J, and Putty T. Quantification of thyrotropin-releasing hormone changes and serotonin content changes following graded spinal cord injury. J Surg Res. (1995) 59:393–8. doi: 10.1006/jsre.1995.1181
112. Salzman SK, Hirofugi E, Knight PB, Llados-Eckman C, Beckman AL, and Winokur A. Treatment of experimental spinal trauma with thyrotropin-releasing hormone: central serotonergic and vascular mechanisms of action. Cent Nervous Syst Trauma: J Am Paralysis Assoc. (1987) 4:181–96. doi: 10.1089/cns.1987.4.181
113. Saghaei E, Abbaszadeh F, Naseri K, Ghorbanpoor S, Afhami M, Haeri A, et al. Estradiol attenuates spinal cord injury-induced pain by suppressing microglial activation in thalamic VPL nuclei of rats. Neurosci Res. (2013) 75:316–23. doi: 10.1016/j.neures.2013.01.010
114. Sharif NA, To Z, Michel AD, and Whiting RL. Differential affinities of TRH analogs at the mammalian spinal cord TRH receptor: implications for therapy in spinal injuries. Neurosci Lett. (1989) 104:183–8. doi: 10.1016/0304-3940(89)90352-2
115. Pitts LH, Ross A, Chase GA, and Faden AI. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotrauma. (1995) 12:235–43. doi: 10.1089/neu.1995.12.235
116. Vita G, Haun CK, Hawkins EF, and Engel WK. Down-regulation of thyrotropin-releasing hormone (TRH) receptors in spinal cord after transection as revealed by quantitative autoradiography. Exp Brain Res. (1991) 83:381–4. doi: 10.1007/BF00231162
117. White SR, Crane GK, and Jackson DA. Thyrotropin-releasing hormone (TRH) effects on spinal cord neuronal excitability. Ann New York Acad Sci. (1989) 553:337–50. doi: 10.1111/j.1749-6632.1989.tb46655.x
118. Prüss H, Tedeschi A, Thiriot A, Lynch L, Loughhead SM, Stutte S, et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat Neurosci. (2017) 20:1549–59. doi: 10.1038/nn.4643
119. Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, and Spungen AM. Administration of increasing doses of gonadotropin-releasing hormone in men with spinal cord injury to investigate dysfunction of the hypothalamic-pituitary-gonadal axis. Spinal Cord. (2018) 56:247–58. doi: 10.1038/s41393-017-0002-x
120. Gorio A, Madaschi L, Di Stefano B, Carelli S, Di Giulio AM, De Biasi S, et al. Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci U S A. (2005) 102:16379–84. doi: 10.1073/pnas.0508479102
121. Albayrak S, Atci İB, Kalayci M, Yilmaz M, Kuloglu T, Aydin S, et al. Effect of carnosine, methylprednisolone and their combined application on irisin levels in the plasma and brain of rats with acute spinal cord injury. Neuropeptides. (2015) 52:47–54. doi: 10.1016/j.npep.2015.06.004
122. Teixeira WGJ, Cristante AF, Marcon RM, Bispo G, Ferreira R, and de Barros-Filho TEP. Granulocyte colony-stimulating factor combined with methylprednisolone improves functional outcomes in rats with experimental acute spinal cord injury. Clinics (Sao Paulo Brazil). (2018) 73:e235. doi: 10.6061/clinics/2018/e235
123. Genovese T, Mazzon E, Crisafulli C, Esposito E, Di Paola R, Muià C, et al. Effects of combination of melatonin and dexamethasone on secondary injury in an experimental mice model of spinal cord trauma. J Pineal Res. (2007) 43:140–53. doi: 10.1111/j.1600-079X.2007.00454.x
124. Karabey-Akyurek Y, Gurcay AG, Gurcan O, Turkoglu OF, Yabanoglu-Ciftci S, Eroglu H, et al. Localized delivery of methylprednisolone sodium succinate with polymeric nanoparticles in experimental injured spinal cord model. Pharm Dev Technol. (2017) 22:972–81. doi: 10.3109/10837450.2016.1143002
125. Zhao W, Jia Z, Bauman WA, Qin Y, Peng Y, Chen Z, et al. Targeted-delivery of nanomedicine-enabled methylprednisolone to injured spinal cord promotes neuroprotection and functional recovery after acute spinal cord injury in rats. Nanomed: Nanotechnol Biol Med. (2024) 60:102761. doi: 10.1016/j.nano.2024.102761
126. Wang H, Lin F, Wu Y, Guo W, Chen X, Xiao C, et al. Carrier-free nanodrug based on co-assembly of methylprednisolone dimer and rutin for combined treatment of spinal cord injury. ACS Nano. (2023) 17:12176–87. doi: 10.1021/acsnano.3c00360
127. Chvatal SA, Kim YT, Bratt-Leal AM, Lee H, and Bellamkonda RV. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. (2008) 29:1967–75. doi: 10.1016/j.biomaterials.2008.01.002
Keywords: spinal cord injury, hormonal signaling, glucocorticoids, neuroinflammation, hormone therapy, bioinformatics
Citation: Guo W, Wu Y, Zhao S and Xu J (2025) Hormonal regulatory networks in spinal cord injury: mechanistic insights, crosstalk, and therapeutic innovations. Front. Endocrinol. 16:1627414. doi: 10.3389/fendo.2025.1627414
Received: 20 May 2025; Accepted: 06 October 2025;
Published: 17 October 2025.
Edited by:
Guillem Paniagua Soriano, Principe Felipe Research Center (CIPF), SpainReviewed by:
Eibar Ernesto Cabrera-Aldana, National Autonomous University of Mexico, MexicoCopyright © 2025 Guo, Wu, Zhao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianwen Xu, eHVqaWFud2VuQGd4bXUuZWR1LmNu; Shijian Zhao, emhhb3NoaWppYW4xMDI1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Wenliang Guo
Wenliang Guo Yinteng Wu
Yinteng Wu Shijian Zhao
Shijian Zhao Jianwen Xu
Jianwen Xu