- 1Department of Surgery, Ajou University School of Medicine, Suwon, Republic of Korea
- 2Department of Radiology, Ajou University School of Medicine, Suwon, Republic of Korea
Background: The American Thyroid Association guidelines recommend maintaining thyroid-stimulating hormone (TSH) levels < 2 mIU/L postoperatively in low-risk patients. Patients with low-risk differentiated thyroid cancer, defined as intrathyroidal tumors without vascular invasion, aggressive histology, or metastasis according to ATA criteria, were included. Many patients who undergo hemithyroidectomy often maintain normal TSH levels, i.e., a euthyroid status, without taking levothyroxine after surgery. However, some patients continue to receive levothyroxine supplementation post-surgery. In this study, we analyzed the risk factors and predictors of levothyroxine withdrawal.
Methods: The medical records of 132 patients who underwent hemithyroidectomy for thyroid cancer at Ajou University Hospital between February 2016 and February 2018 were reviewed. The medical records included data on demographics, type of operation, pathological findings, pre- and postoperative changes in TSH levels, levothyroxine dosage and discontinuation timing, and pre- to postoperative changes in thyroid gland volume. All patients were started on a fixed dose of levothyroxine immediately after surgery, which was subsequently tapered and withdrawn based on the TSH levels.
Results: Among 132 patients who underwent hemithyroidectomy, 67 (51%) eventually withdrew from postoperative levothyroxine. Of the many dependent variables, multivariate analysis revealed the statistical significance of preoperative TSH levels (P=0.014), preoperative thyroid volume measured by 3-dimensional (3D) CT, and the ratio of preoperative-to-postoperative residual thyroid volume (P=0.026 and P=0.012, respectively). In the subgroup analysis of the group that resumed levothyroxine administration after levothyroxine withdrawal, only the ratio of the preoperative to postoperative residual thyroid volume was statistically significant (P<0.043).
Conclusion: Preoperative TSH level and thyroid volume were the most important predictors of successful postoperative levothyroxine withdrawal. The pre- to postoperative thyroid volume ratio may be affected by surgery and a ratio of <33% was significantly correlated with the ability to discontinue levothyroxine.
1 Introduction
Thyroid lobectomy is a widely accepted treatment for patients with benign thyroid disease and may even be curative in patients with early-stage differentiated thyroid carcinoma (DTC) (1). Theoretically, a single thyroid lobe should contain an adequate number of functioning thyrocytes to maintain a euthyroid state. Therefore, many surgeons and patients prefer unilateral thyroid resection, anticipating that postoperative thyroid hormone replacement therapy can be avoided. However, patients with preoperative suboptimal thyroid function may not retain sufficient functional thyroid tissue after lobectomy (2). Therefore, the potential need for lifelong thyroid hormone supplementation remains a critical factor in determining the extent of surgery, particularly in cases where the indications for total thyroidectomy and lobectomy are closely balanced. According to the literature, approximately 10–50% of patients require levothyroxine supplementation after thyroid lobectomy (3–7).
In cases of early DTC, a more cautious approach is warranted when considering levothyroxine therapy. As widely recognized, the 2015 ATA guidelines recommended maintaining a postoperative TSH level of <2 mIU/L in low-risk DTC patients (8). However, such targets should be individualized, taking into account the patient’s comorbidities, particularly their potential impact on bone health, cardiac rhythm, and overall quality of life. In this context, the latest ATA guidelines recommend for a risk-adapted strategy (8). Reflecting a broader shift toward treatment de-escalation—including limited surgical approaches (e.g., lobectomy), active surveillance in selected cases, and more judicious use of radioactive iodine—the guidelines emphasize the importance of carefully weighing the potential benefits of TSH-suppressive levothyroxine therapy against its potential adverse effects (9).
Recent studies have aimed to identify the preoperative predictors of postoperative thyroid hormone replacement. Nonetheless, a consensus on the standardized indications for levothyroxine supplementation has yet to be reached across institutions. Given this variability, surgeons must establish personalized criteria for postoperative thyroid hormone replacement and thoroughly inform patients during preoperative consultations about the likelihood of requiring levothyroxine supplementation following surgery. To date, several factors have been proposed as influencing the need for levothyroxine supplementation following thyroid lobectomy. These include preoperative TSH levels, the presence of microsomal antibodies, and autoimmune-related biomarkers such as thyroid peroxidase antibody (anti-TPO Ab) and thyroglobulin antibody (4, 6, 7, 10–12). However, the significance of these variables has not been consistently demonstrated across studies. Additionally, although several studies have assessed postoperative thyroid volume, studies directly correlating residual thyroid volume with the requirement for levothyroxine therapy remain limited.
This study analyzed the relationship between preoperative clinical factors and the eventual need for levothyroxine supplementation after thyroid lobectomy. Our goal was to provide predictive insights that could support clinicians in offering accurate and personalized preoperative counseling to patients.
2 Materials and methods
2.1 Study design
In this retrospective study, we reviewed the electronic medical records of 132 patients who underwent hemithyroidectomy for thyroid cancer at Ajou University Hospital between February 2016 and February 2018. To ensure minimum statistical validity, at least 50 patients per group were required for both the control and experimental groups. Considering the potential risk of dropout, a total of 132 patients were ultimately enrolled in the study. To minimize bias related to surgical technique, all procedures were performed by a single surgeon (HK). Cases of thyroidectomy after lobectomy due to contralateral lobe or lateral neck node metastasis, pregnancy within five years of lobectomy, pediatric patients under the age of 19 years, those who were lost to follow-up, and patients who were pregnant during the study period were excluded.
Patient demographics, surgical details, pathological findings, pre- and postoperative thyroid-stimulating hormone (TSH) levels, levothyroxine dosage and withdrawal timing, and pre- to postoperative changes in the volume of the thyroid gland were extracted from the patients’ medical records and reviewed retrospectively.
2.2 Patients and treatment
All 132 patients underwent thyroid lobectomy and were diagnosed with papillary thyroid carcinoma. After lobectomy, all patients were started on a fixed dose of levothyroxine from POD 1 (100 µg for men and 75 µg for women). All patients underwent prophylactic ipsilateral central neck node dissection and experienced no postoperative complications. All included patients underwent regular follow-up for more than 5 years at Ajou University Hospital.
2.3 Perioperative studies and withdrawal of thyroid hormone supplementation
Preoperative thyroid function tests (free T4 and thyroid-stimulating hormone [TSH] levels) were performed. Postoperative free T4, TSH, thyroglobulin, and thyroglobulin antibodies were measured after 2 weeks, 3 months, 6 months, and 12 months, and annually at the outpatient clinic. The total thyroid volume was measured on preoperative 3-dimensional CT (3D CT), and the residual thyroid volume was measured one year postoperatively to determine the percentage of remaining thyroid tissue.
Levothyroxine was started postoperatively with the aim of gradual reduction and eventual discontinuation, taking into account the TSH and free T4 levels and the patient’s condition. The goal of thyroid hormone supplementation is to maintain euthyroid status. Levothyroxine was discontinued if the TSH level was < 7.0 mIU/L (13, 14) and the patient was receiving a low maintenance dose of 50 µg or 25 µg, provided they agreed to discontinue the medication. If levothyroxine was discontinued during the follow-up period, TSH levels were measured three months later to determine if reinstitution was necessary.
2.4 Statistical analysis
Data analysis was performed using IBM SPSS statistical software (IBM Corp., Armonk, NY, USA). Fisher’s exact test or chi-square test was used to compare categorical variables. Student’s t-test was used to compare continuous variables, which are presented as means and standard deviations.
3 Results
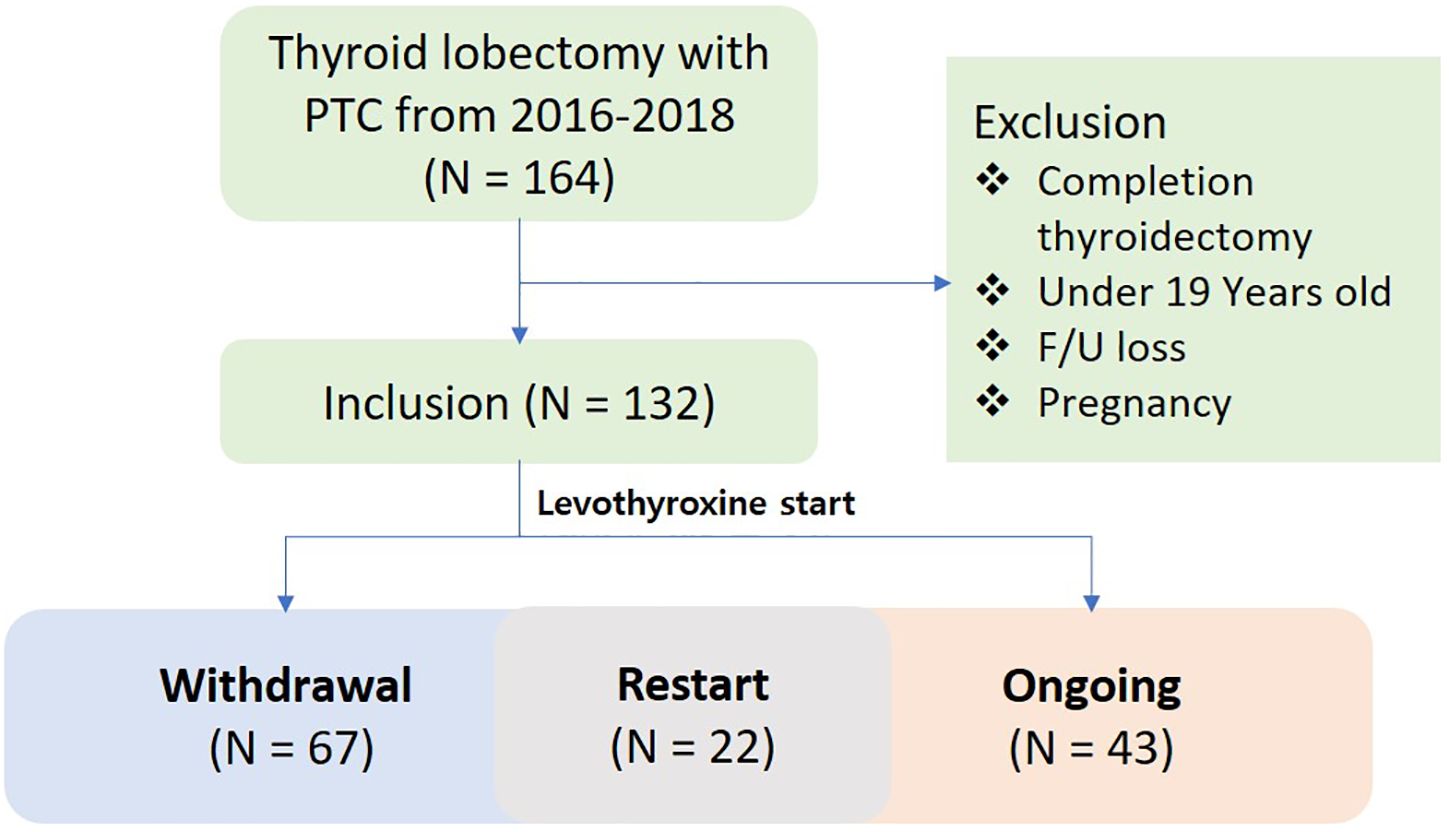
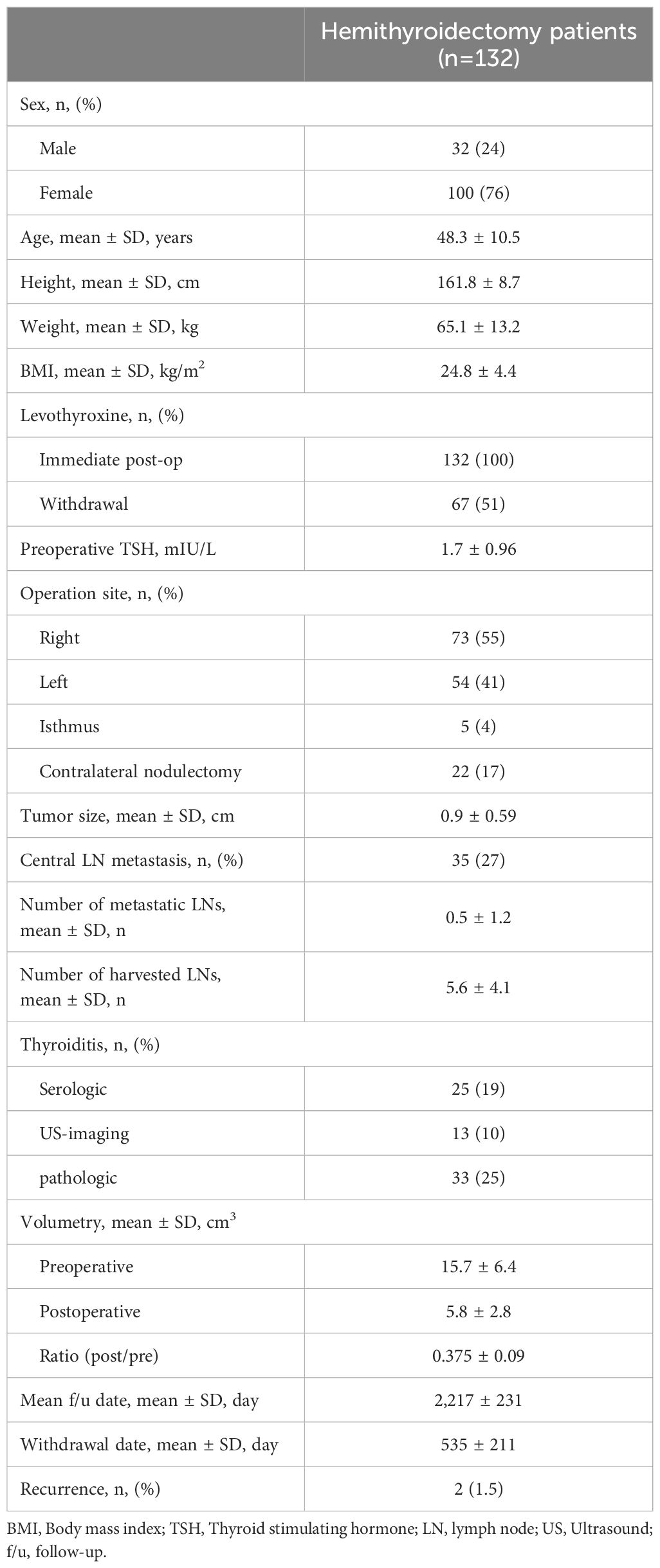
Data from a total of 164 patients with thyroid cancer who underwent lobectomy over a two-year period from February 2016 to February 2018 were reviewed. Of these, 132 patients were included in the final analysis after excluding those who satisfied the exclusion criteria. In the study population, 43 patients never discontinued levothyroxine, 67 patients ultimately withdrew, and 22 patients resumed levothyroxine treatment after withdrawal (Figure 1). The demographic data of the entire patient cohort, including sex, age, height, weight, and BMI, showed no significant differences compared to previously reported populations (5, 10, 11, 15–17). The preoperative TSH level was 1.7 mIU/L. The evaluation for thyroiditis included cases with positive preoperative serum thyroglobulin antibodies, cases suspected on ultrasound, and cases confirmed through final histopathological findings, with the numbers summarized in Table 1. The average preoperative thyroid volume, as measured by 3D CT, was 15.7 cm3, which reduced to an average postoperative volume of 5.8 cm3, resulting in a mean post-/preoperative volume ratio of 37.5%. The mean levothyroxine withdrawal period was 535 days (Table 1).
To analyze the risk factors or predictors in the group unable to discontinue levothyroxine, univariate and multivariate analyses were conducted, and the results were compared with those in the withdrawal group (Table 2). In the univariate analysis, preoperative TSH level, number of harvested lymph nodes, pre- and postoperative thyroid volume, and the ratio of post-/preoperative thyroid volume were statistically significant. Multivariate analysis confirmed that preoperative TSH, preoperative thyroid volume, and the ratio of post-/preoperative thyroid volume were statistically significant (preoperative TSH, p=0.014, OR=0.581; preoperative thyroid volume, p=0.026, OR=1.469; the ratio of post-/preoperative thyroid volume, p=0.012, OR=2.539).
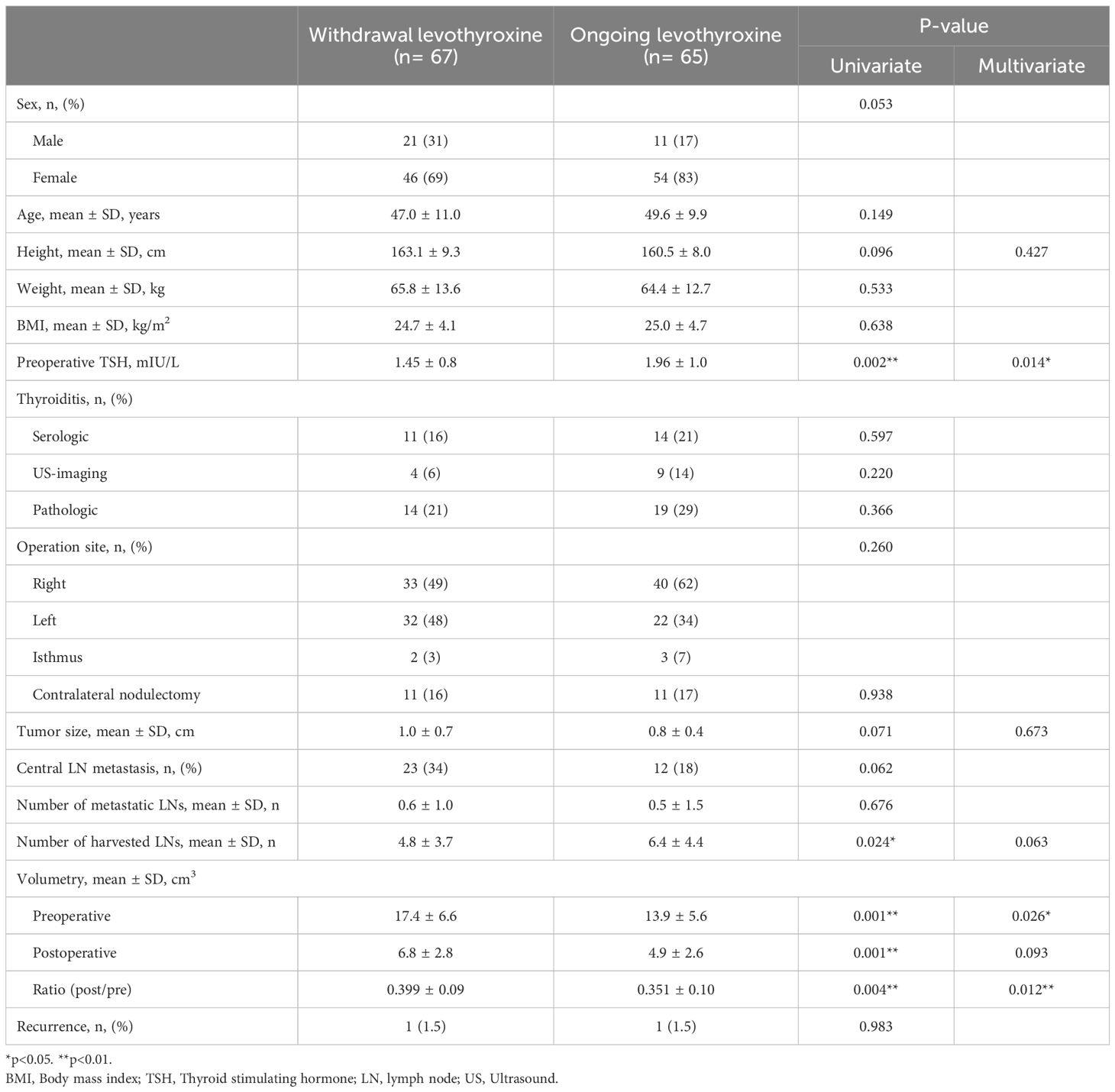
Table 2. Univariate and multivariate analysis between levothyroxine withdrawal patients and ongoing patients.
A subgroup analysis was conducted on 22 patients who required levothyroxine reinstitution after withdrawal and presented with hypothyroidism and elevated TSH levels. Among these patients, multivariate analysis revealed that only the ratio of post-/preoperative thyroid volume was statistically significant (p=0.043) (Table 3). Although the preoperative thyroid volume did not reach statistical significance (p=0.051), it showed a tendency toward variation. The mean TSH level at the time of levothyroxine discontinuation was approximately 2 mIU/L in both groups, with no significant difference. The period from surgery to levothyroxine discontinuation was approximately 500 days in both groups, while the average period from discontinuation to resumption was 314 days.
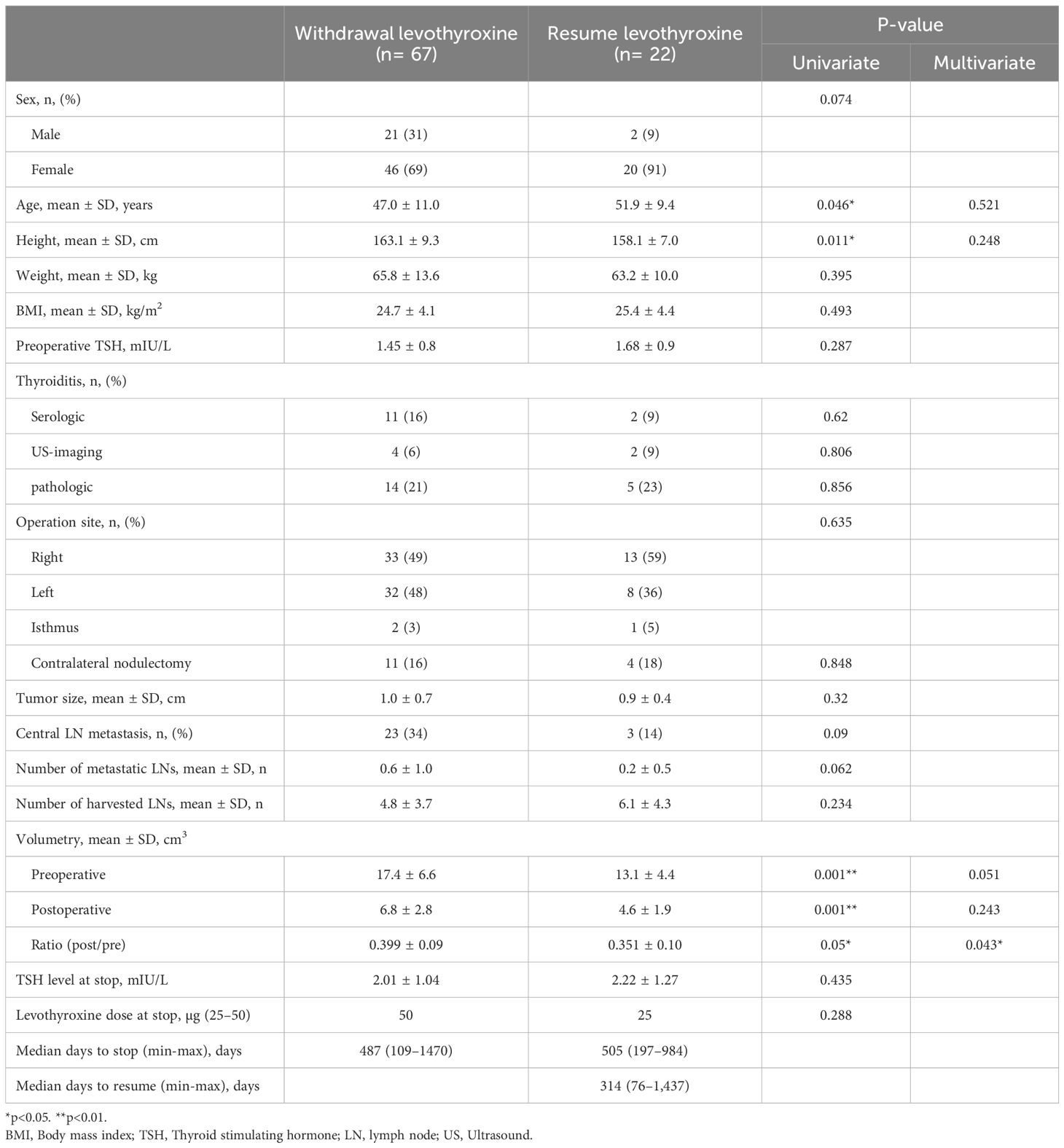
Table 3. Univariate and multivariate analysis between levothyroxine withdrawal patients and resume patients.
Based on our findings, we suggest the following cut-off as potential predictors of successful levothyroxine withdrawal, either pre- or postoperatively. The probability of discontinuing levothyroxine was significantly higher under the following conditions: if the preoperative TSH level was less than 2.0 mIU/L (p<0.001) if the preoperative thyroid volume exceeds 15.3 cm3 (p<0.001), and if the ratio of post-/preoperative thyroid volume was greater than 33% (p=0.002).
4 Discussion
In the present study, we found that preoperative TSH levels, particularly below 2.0 mIU/L, were associated with an increased likelihood of levothyroxine withdrawal. In a previous study investigating the risk factors for levothyroxine use after thyroid lobectomy for benign thyroid nodules, preoperative TSH levels of ≥ 2.0 mIU/L were reported to be significantly associated with the initiation of levothyroxine, while levels of ≥ 3.0 mIU/L were significantly correlated with its continued administration (12).
These findings offer clinically relevant insights into individualized postoperative management, especially considering the variable trajectory of hypothyroidism in patients undergoing hemithyroidectomy for benign or low-risk malignant thyroid disease.
Furthermore, the same study reported that older patients were more likely to start or continue levothyroxine therapy. Another study found that preoperative TSH levels >2.5 mIU/L, as well as the presence of a positive microsomal antibody, were significantly correlated with levothyroxine supplementation (10, 18). Additionally, patients who test positive for thyroid autoimmunity-associated markers such as anti-TPO Ab or thyroglobulin antibody have a higher incidence of hypothyroidism following thyroid lobectomy (10). In the current study, we examined various modalities and markers that provide evidence of thyroiditis, including serological markers (e.g., thyroglobulin antibody), ultrasound features suggestive of diffuse thyroid disease, and thyroiditis findings on postoperative pathology. In contrast, our data suggest that patients with lower baseline TSH levels (<2.0 mIU/L)—even in the presence of equivocal markers of thyroiditis—may possess sufficient residual thyroid function to allow for successful levothyroxine withdrawal. These findings may clarify conflicting evidence regarding predictors of remnant thyroid gland function and highlight a potential threshold that could inform clinical decision-making.
Studies aimed at accurately measuring thyroid volume and postoperative residual thyroid volume have been conducted using ultrasonography and various CT modalities (16, 18–21). In the present study, 3D CT was used to measure preoperative and postoperative thyroid lobe volumes. We found that both preoperative thyroid volume and the ratio of preoperative to postoperative thyroid volume were significant predictors of levothyroxine withdrawal. Research assessing changes in the volume of the thyroid lobe after lobectomy in patients with thyroid cancer is rare and underscores the clinical significance of this study. Several previous studies have demonstrated that even when approximately half of the thyroid gland is preserved during surgery, a postoperative reduction in the remnant thyroid volume is observed (16, 22). Moreover, a study examining changes in remnant thyroid volume following surgery in patients with Graves’ disease reported that young age and a lower residual volume ratio were the two most significant factors affecting changes in remnant thyroid volume (16). Although the remnant thyroid volume or the ratio of pre- to postoperative thyroid volumes measured in our study did not provide unequivocal guidance on the optimal volume to be preserved during surgery, these findings may indirectly support the observations from studies suggesting that isthmus-preserving thyroid lobectomy is associated with a lower incidence of postoperative hypothyroidism (23, 24).
These findings are clinically relevant as they provide evidence that volumetric factors—rather than solely biochemical or serologic markers—may more accurately inform postoperative hormone requirements. Although routine use of 3D volumetry is currently limited by technical and logistical constraints, its growing integration in surgical planning workflows may enable its broader clinical application in the future.
The American Thyroid Association guidelines recommend maintaining TSH levels <2 mIU/L postoperatively in low-risk patients (8). In this study, levothyroxine withdrawal was attempted even in patients with postoperative TSH levels >2 mIU/L, provided that the patients were at a very low‐risk, i.e., showing no symptoms and maintaining normal TSH levels (in most cases ≤ 4.5 mIU/L, with only two cases <7 mIU/L). Since the publication of the 2015 ATA guidelines, ongoing advances in surgical techniques and various treatment modalities have fueled debate regarding the prognosis of patients with thyroid cancer with TSH levels >2 mIU/L, and a large-scale multicenter study is currently underway (25).
Postoperative hypothyroidism is a well-recognized concern after thyroid lobectomy. While a previous study (17) demonstrated that delaying thyroid hormone supplementation until about one-month post-surgery, in which the remnant thyroid function is evaluated, reduces the time to levothyroxine withdrawal and improves the success rate, many patients undergoing lobectomy do not receive immediate postoperative levothyroxine. In contrast, all patients included in our study were administered a predetermined dose of levothyroxine postoperatively. Despite the low incidence of immediate symptomatic hypothyroidism, this proactive approach was adopted to minimize the potential one-month period of symptoms like fatigue, cold intolerance, depression, and mood changes before treatment initiation.
Though our results are promising, this study has several limitations. First, its retrospective, single-center design may limit the generalizability of our findings. Second, the volumetric measurements required specialized 3D CT software, which may not be universally accessible. Further multicenter, prospective validation is essential to establish robust clinical thresholds and predictive models that integrate biochemical, radiological, and pathological factors.
Despite these limitations, our study highlights the importance of comprehensive preoperative assessment—including evaluation of TSH, thyroid autoimmunity, and gland volume—as a practical strategy to individualize postoperative levothyroxine therapy. If validated in larger populations, these findings could lead to improved patient counseling, enhance shared decision-making, and ultimately reduce levothyroxine overtreatment in select lobectomy patients.
5 Conclusion
TSH levels and thyroid volume were the most important preoperative predictors of postoperative levothyroxine withdrawal following hemithyroidectomy. Furthermore, the ratio of pre- to postoperative thyroid volume, a factor influenced by the extent of surgical resection, demonstrates that a reduction to < 33% of the original volume is significantly associated with levothyroxine withdrawal. These findings suggest that preoperative assessment of TSH and thyroid volume, along with the anticipated residual thyroid volume, can aid in predicting the likelihood of long-term levothyroxine independence.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of Ajou University Hospital, Ajou University College of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Due to the retrospective nature of this study, neither patient approval nor informed consent was required.
Author contributions
JL: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. EH: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. HJ: Data curation, Investigation, Validation, Visualization, Writing – review & editing. SK: Data curation, Investigation, Validation, Visualization, Writing – review & editing. HK: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors thank the reviewers for their constructive comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
2. Farkas EA, King TA, Bolton JS, and Fuhrman GM. A comparison of total thyroidectomy and lobectomy in the treatment of dominant thyroid nodules. Am Surg. (2002) 68:678–82; discussion 82-3. doi: 10.1177/000313480206800805
3. Seiberling KA, Dutra JC, and Bajaramovic S. Hypothyroidism following hemithyroidectomy for benign nontoxic thyroid disease. Ear Nose Throat J. (2007) 86:295–9. doi: 10.1177/014556130708600517
4. Su SY, Grodski S, and Serpell JW. Hypothyroidism following hemithyroidectomy: a retrospective review. Ann Surg. (2009) 250:991–4. doi: 10.1097/SLA.0b013e3181ae5426
5. Stoll SJ, Pitt SC, Liu J, Schaefer S, Sippel RS, and Chen H. Thyroid hormone replacement after thyroid lobectomy. Surgery. (2009) 146:554–8; discussion 8-60. doi: 10.1016/j.surg.2009.06.026
6. Miller FR, Paulson D, Prihoda TJ, and Otto RA. Risk factors for the development of hypothyroidism after hemithyroidectomy. Arch Otolaryngol Head Neck Surg. (2006) 132:36–8. doi: 10.1001/archotol.132.1.36
7. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, and Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. (2012) 97:2243–55. doi: 10.1210/jc.2012-1063
8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
9. Rossi L, Paternoster M, Cammarata M, Bakkar S, and Miccoli P. Levothyroxine therapy in thyroidectomized patients: ongoing challenges and controversies. Front Endocrinol (Lausanne). (2025) 16:1582734. doi: 10.3389/fendo.2025.1582734
10. Lee DY, Seok J, Jeong WJ, and Ahn SH. Prediction of thyroid hormone supplementation after thyroid lobectomy. J Surg Res. (2015) 193:273–8. doi: 10.1016/j.jss.2014.07.003
11. Johner A, Griffith OL, Walker B, Wood L, Piper H, Wilkins G, et al. Detection and management of hypothyroidism following thyroid lobectomy: evaluation of a clinical algorithm. Ann Surg Oncol. (2011) 18:2548–54. doi: 10.1245/s10434-011-1627-1
12. Song HS, Kim CJ, Lee S, Bae JS, Jung CK, and Jang J. Risk factors that predict levothyroxine medication after thyroid lobectomy. Acta Endocrinol (Buchar). (2020) 16:454–61. doi: 10.4183/aeb.2020.454
13. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. Jama. (2010) 304:1365–74. doi: 10.1001/jama.2010.1361
14. Cojić M and Cvejanov-Kezunović L. Subclinical hypothyroidism - whether and when to start treatment? Open Access Maced J Med Sci. (2017) 5:1042–6. doi: 10.3889/oamjms.2017.195
15. Cox C, Bosley M, Southerland LB, Ahmadi S, Perkins J, Roman S, et al. Lobectomy for treatment of differentiated thyroid cancer: can patients avoid postoperative thyroid hormone supplementation and be compliant with the American Thyroid Association guidelines? Surgery. (2018) 163:75–80. doi: 10.1016/j.surg.2017.04.039
16. Chen PY, Chao CM, Wu TJ, and Huang SM. Volume changes in remnant thyroid tissue after thyroidectomy in Graves disease. J Formos Med Assoc. (2014) 113:629–33. doi: 10.1016/j.jfma.2012.08.013
17. Park S, Jeon MJ, Song E, Oh HS, Kim M, Kwon H, et al. Clinical features of early and late postoperative hypothyroidism after lobectomy. J Clin Endocrinol Metab. (2017) 102:1317–24. doi: 10.1210/jc.2016-3597
18. Morris LF, Iupe IM, Edeiken-Monroe BS, Warneke CL, Hansen MO, Evans DB, et al. Pre-operative ultrasound identification of thyroiditis helps predict the need for thyroid hormone replacement after thyroid lobectomy. Endocr Pract. (2013) 19:1015–20. doi: 10.4158/EP12334.OR
19. Freesmeyer M, Wiegand S, Schierz JH, Winkens T, and Licht K. Multimodal evaluation of 2-D and 3-D ultrasound, computed tomography and magnetic resonance imaging in measurements of the thyroid volume using universally applicable cross-sectional imaging software: a phantom study. Ultrasound Med Biol. (2014) 40:1453–62. doi: 10.1016/j.ultrasmedbio.2014.02.013
20. Lee SJ, Chong S, Kang KH, Hur J, Hong BW, Kim HJ, et al. Semiautomated thyroid volumetry using 3D CT: prospective comparison with measurements obtained using 2D ultrasound, 2D CT, and water displacement method of specimen. AJR Am J Roentgenol. (2014) 203:W525–32. doi: 10.2214/AJR.13.12206
21. Freesmeyer M, Kühnel C, and Westphal JG. Time efficient 124I-PET volumetry in benign thyroid disorders by automatic isocontour procedures: mathematic adjustment using manual contoured measurements in low-dose CT. Ann Nucl Med. (2015) 29:8–14. doi: 10.1007/s12149-014-0903-0
22. Daly AP, Romanelli-Gobbi M, Miller JL, Rosen D, Cognetti DM, and Pribitkin EA. Using ultrasonic preoperative thyroid volume to determine incision length for minimally invasive thyroid surgery. Ear Nose Throat J. (2015) 94:346–52.
23. Cho MJ, Yu HW, Kim W, Kim YK, Choi SI, Kim SJ, et al. Comparison of the incidence of postoperative hypothyroidism in patients undergoing conventional thyroid lobectomy and pyramid- and isthmus-preserving lobectomy. Int J Endocrinol. (2021) 2021:8162307. doi: 10.1155/2021/8162307
24. Salih AM. Prevalence of hypothyroidism among patients with isthmus-preserved thyroid lobectomy. J Int Med Res. (2018) 46:3819–23. doi: 10.1177/0300060518781228
25. Lee EK, Kang YE, Park YJ, Koo BS, Chung KW, Ku EJ, et al. A multicenter, randomized, controlled trial for assessing the usefulness of suppressing thyroid stimulating hormone target levels after thyroid lobectomy in low to intermediate risk thyroid cancer patients (MASTER): A study protocol. Endocrinol Metab (Seoul). (2021) 36:574–81. doi: 10.3803/EnM.2020.943
Keywords: thyroid carcinoma, hemithyroidectomy, levothyroxine, hypothyroidism, thyroid volumetry
Citation: Lee JS, Ha EJ, Jeong HJ, Kim SY and Kim HK (2025) Levothyroxine supplementation after hemithyroidectomy in patients with low-risk differentiated thyroid cancer: risk factors and withdrawal strategy. Front. Endocrinol. 16:1627721. doi: 10.3389/fendo.2025.1627721
Received: 13 May 2025; Accepted: 29 August 2025;
Published: 22 September 2025.
Edited by:
Rosa Maria Paragliola, Saint Camillus International University of Health and Medical Sciences, ItalyReviewed by:
Flávia Dornelas Kurkowski, Pontifical Catholic University of Rio Grande do Sul, BrazilMostafa Vaghari-Tabari, Tabriz University of Medical Sciences, Iran
Diego Bromfman Pianta, Pontifical Catholic University of Rio Grande do Sul, Brazil
Helena Marcelino, University of Beira Interior, Portugal
Copyright © 2025 Lee, Ha, Jeong, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyeung Kyoo Kim, a2ltaGtAYXVtYy5hYy5rcg==
 Jin Seok Lee
Jin Seok Lee Eun Ju Ha2
Eun Ju Ha2 Hyeung Kyoo Kim
Hyeung Kyoo Kim