- 1Liaoning University of Traditional Chinese Medicine, Liaoning, China
- 2Department of Endocrinology, General Hospital of Northern Theater Command, Liaoning, China
- 3Department of Dermatology, General Hospital of Northern Theater Command, Liaoning, China
- 4Department of Clinical Epidemiology and Center of Evidence-Based Medicine, The First Hospital of China Medical University, Shenyang, China
Background: As the global population ages, promoting healthy aging has become a critical public health priority. Emerging evidence suggests that environmental stressors—particularly residential noise—may influence endocrine health by disrupting behaviors essential to physiological homeostasis, such as sleep and physical activity.
Methods: In this cross-sectional study of 2,483 community-dwelling adults aged ≥60 years, we examined the associations between behavioral and environmental risk factors and the presence of thyroid nodules, assessed via standardized ultrasonography. Residential noise exposure was estimated using geospatial monitoring data. Sleep duration and quality, as well as physical activity levels, were collected through validated questionnaires. Poor sleep was defined as self-reported sleep duration of ≤6 hours and/or symptoms of disturbed sleep. Multivariable logistic regression models were used to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
Results: Poor sleep (adjusted OR = 3.24; 95% CI: 2.70–3.90), low physical activity (adjusted OR = 2.51; 95% CI: 2.08–3.02), and high residential noise exposure (adjusted OR = 4.46; 95% CI: 3.70–5.39) were each significantly associated with the presence of thyroid nodules. Increasing age was also independently associated with higher risk. Mediation analyses indicated that sleep quality and physical activity jointly accounted for approximately 15–20% of the effect of noise exposure on thyroid nodule risk.
Conclusions: Behavioral and environmental stressors, particularly poor sleep, physical inactivity, and noise exposure, may contribute to thyroid nodule formation in older adults. These findings highlight the importance of addressing modifiable behavioral pathways when evaluating environmental impacts on endocrine health.
1 Introduction
The rapid acceleration of global population aging has heightened interest in strategies that foster “healthy aging” (1, 2). As individuals grow older, they experience greater burdens of chronic diseases (3), progressive functional decline (4), and reduced quality of life, underscoring the need to identify modifiable determinants that can delay pathological aging and sustain late-life health. Beyond conventional lifestyle factors, environmental exposures have gained prominence as important—but still under-appreciated—drivers of age-related health outcomes (5).
Accumulating evidence indicates that air pollution (6), extreme heat (7), and persistent ambient noise (8) can undermine key health-related behaviors, notably sleep and physical activity. Elevated concentrations of fine particulate matter (PM2.5) and chronic noise exposure are linked to poorer sleep quality, shorter sleep duration, and lower activity levels (9). These behavioral perturbations carry significant clinical relevance: inadequate sleep precipitates immune and inflammatory dysregulation—for example, through PGD2-mediated cytokine surges that can result in multi-organ injury (10)—whereas regular physical activity enhances cardiovascular function, metabolic control, and overall survival (11).
Despite expanding research on environmental influences over behavioral health, their implications for thyroid function remain inadequately explored, particularly in older adults. Thyroid nodules are prevalent in late life (12, 13) and can signal underlying dysfunction or malignancy. While factors such as iodine status (14), radiation exposure (15), and metabolic abnormalities (16) have been implicated in nodule formation, the potential contribution of environmental stressors—and the behavioral pathways through which they act—has not been clearly delineated.
Accordingly, the present study investigates associations between environmental exposures (specifically PM2.5 and ambient noise) and the prevalence of thyroid nodules in an older adult cohort. We further examine whether sleep characteristics and physical activity mediate these relationships. By elucidating these environmental-behavioral-endocrine pathways, our findings aim to inform integrated prevention strategies that target both individual behaviors and environmental conditions to improve thyroid health in aging populations.
2 Materials and methods
2.1 Study population
This study is based on a cohort of community-dwelling older adults recruited through the District Health Surveillance Programme in Chinese hospitals between 2021 and 2023. Participants were eligible for inclusion if they were aged 60 years or older and underwent standardized thyroid ultrasonography during their health examination.
This age threshold was selected to align with national definitions of older adults in China and to focus on a population at elevated risk for thyroid dysfunction. Individuals aged ≥60 years tend to exhibit greater biological vulnerability to environmental stressors due to age-related changes in immune function, endocrine regulation, and cumulative exposure load. Moreover, behavioral disturbances such as poor sleep and reduced physical activity are more prevalent and clinically relevant in this demographic, providing a meaningful context for exploring modifiable pathways to endocrine outcomes.
Individuals were excluded if they lacked data on key variables, including environmental exposures (PM2.5 or ambient noise), self-reported sleep patterns, physical activity, or essential covariates. After applying these criteria, a total of 2,483 participants were retained for the final analysis. All study procedures were conducted in accordance with the Declaration of Helsinki and approved by the institutional ethics committee. Written informed consent was obtained from all participants prior to data collection (Figure 1).
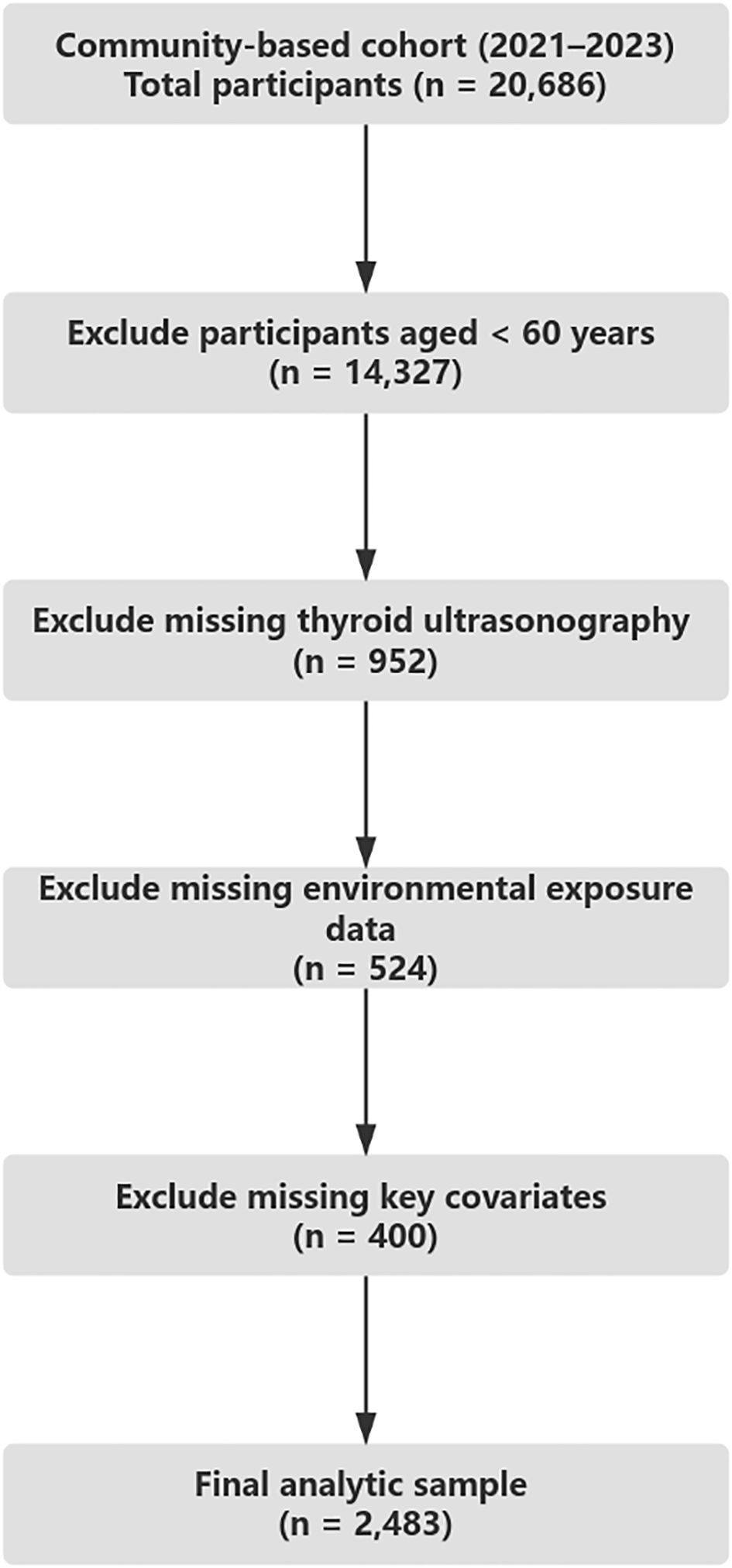
Figure 1. Sample selection flowchart. Flowchart outlining participant inclusion and exclusion criteria based on age, data completeness, and eligibility for final analysis in a community-based cohort (2021–2023).
2.2 Assessment of environmental exposures
Exposure to ambient fine particulate matter (PM2.5) was estimated based on the participants’ residential addresses, matched to annual average PM2.5 concentrations retrieved from local ecological and environmental monitoring stations operating under the regional air quality surveillance system. All measurements adhered to the technical specifications issued by the Chinese Ministry of Ecology and Environment. PM2.5 levels were categorized into population-based quintiles for descriptive analysis and dichotomized (high exposure defined as top two quintiles) for subgroup and sensitivity evaluations.
Neighborhood noise exposure was estimated using standardized geographic information system (GIS)-based models. These models incorporated residential distance to major traffic roads, industrial facilities, and commercial noise zones. Participants were classified into high or low noise exposure groups based on validated spatial noise distribution thresholds.
2.3 Assessment of health-related behaviors
Sleep quality and duration were assessed using structured questionnaires administered during in-person interviews. Poor sleep was defined as an average sleep duration ≤6 hours per night, or the presence of frequent sleep complaints such as insomnia, difficulty maintaining sleep, or early awakening. Physical activity was evaluated using a validated Chinese version of the Global Physical Activity Questionnaire (GPAQ), capturing self-reported frequency and duration of moderate to vigorous physical activity (MVPA) during a typical week. Total weekly physical activity was converted into metabolic equivalent task (MET) hours. Participants were categorized as having low (<3 MET-hours/week) or adequate (≥3 MET-hours/week) physical activity levels.
2.4 Outcome ascertainment
The primary outcome was the presence of thyroid nodules, identified through high-resolution B-mode ultrasonography performed by certified sonographers using uniform diagnostic protocols across all participating sites. Nodule presence was defined based on standard morphological criteria, including hypoechogenicity, irregular margins, calcifications, and nodule size ≥5 mm. All scans were subject to centralized quality control by an experienced endocrinologist panel. To reduce inter-observer variability, all sonographers received centralized training and adhered to a unified imaging protocol across sites.
2.5 Covariates
A range of demographic, lifestyle, and health-related variables were included as potential confounders, selected based on existing literature and biological plausibility. These included: age, sex, body mass index (BMI), educational attainment (low, middle, high), household income level, smoking status (never, former, current), alcohol consumption (yes/no), presence of depressive symptoms (assessed using a validated Chinese screening tool), level of social engagement (frequency of contact with friends or relatives), and history of residential relocation (within the past five years). All data were collected through standardized questionnaires and verified by trained health workers at each clinical site.
2.6 Statistical analysis
Descriptive analyses were conducted to summarize participant characteristics across quintiles of PM2.5 exposure. Continuous variables were presented as medians with interquartile ranges (IQR), and categorical variables as counts with percentages. Between-group differences were assessed using Kruskal–Wallis tests for continuous variables and chi-square tests for categorical variables.
Multivariable logistic regression models were employed to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between environmental exposures (PM2.5 and noise) and the presence of thyroid nodules, controlling for all listed covariates. Model performance was evaluated using the area under the receiver operating characteristic (ROC) curve, as well as the Hosmer–Lemeshow test and calibration plots to assess goodness-of-fit.
To investigate potential behavioral mediation pathways, we applied the product-of-coefficients approach, quantifying the indirect effects of sleep quality, physical activity, and depression on the association between environmental exposures and thyroid nodules. Bootstrapping with 5,000 replications was used to derive 95% confidence intervals for indirect effects. This mediation framework was chosen for its suitability in estimating indirect effects in observational epidemiology, where exposure–mediator–outcome relationships may not follow normal distributions. Bootstrapping with 5,000 resamples was employed to generate empirical confidence intervals, thereby improving accuracy and inferential validity.
Subgroup analyses were performed to explore effect modification by sex, BMI category, depressive symptoms, educational level, and social engagement. Interaction terms were included in regression models to assess heterogeneity by sleep duration, residential urbanicity, and income level. P values for interaction were obtained using likelihood ratio tests. All statistical analyses were conducted using R (version 4.3.3). A two-sided p value <0.05 was considered statistically significant.
3 Results
3.1 Participant characteristics
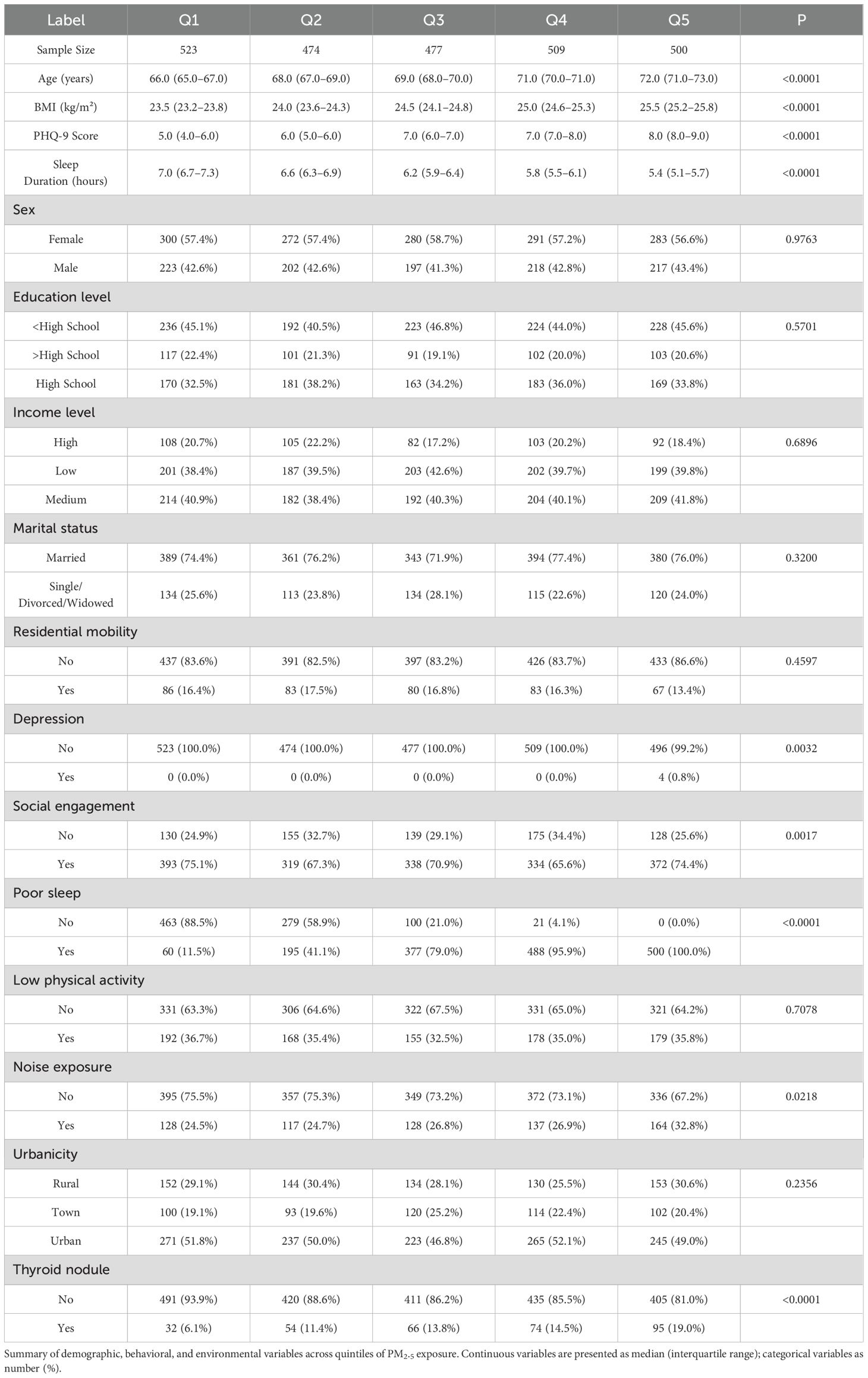
A total of 2,483 community-dwelling adults aged ≥60 years were included in the final analysis. The median age of the study population was 69 years (interquartile range: 66–71), and 56.6% were female. Baseline characteristics stratified by quintiles of PM2.5 exposure are presented in Table 1.
Participants in the highest exposure quintile had a greater proportion of individuals with lower educational attainment and were more likely to reside in urban neighborhoods. The prevalence of poor sleep increased substantially across exposure levels, rising from 11.5% in the lowest quintile (Q1) to 100.0% in the highest (Q5). Physical inactivity showed minor fluctuations, with proportions ranging from 32.5% to 36.7% across quintiles. The overall prevalence of thyroid nodules was 13.1%, increasing progressively from 6.1% in Q1 to 19.0% in Q5 (p for trend < 0.001).
3.2 Model performance
The final multivariable logistic regression model, which included poor sleep, low physical activity, noise exposure, and age as predictors, demonstrated good discrimination, with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.77 (Figure 2). Model calibration also indicated strong agreement between predicted and observed risks across deciles of estimated probability, suggesting the model had adequate fit (Figure 3).
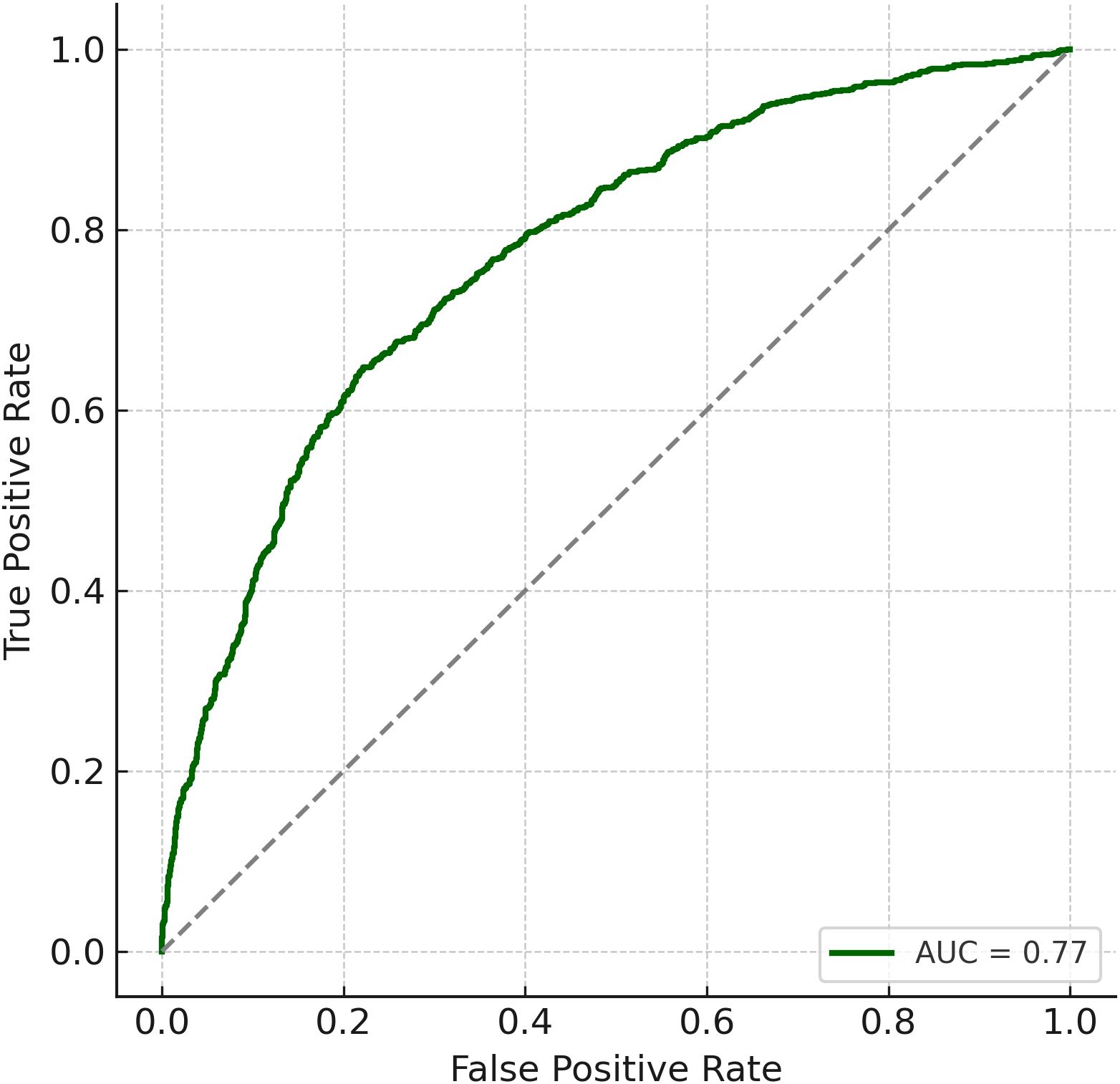
Figure 2. Receiver operating characteristic (ROC) curve. The prediction model demonstrated strong discrimination with an AUC of 0.77.
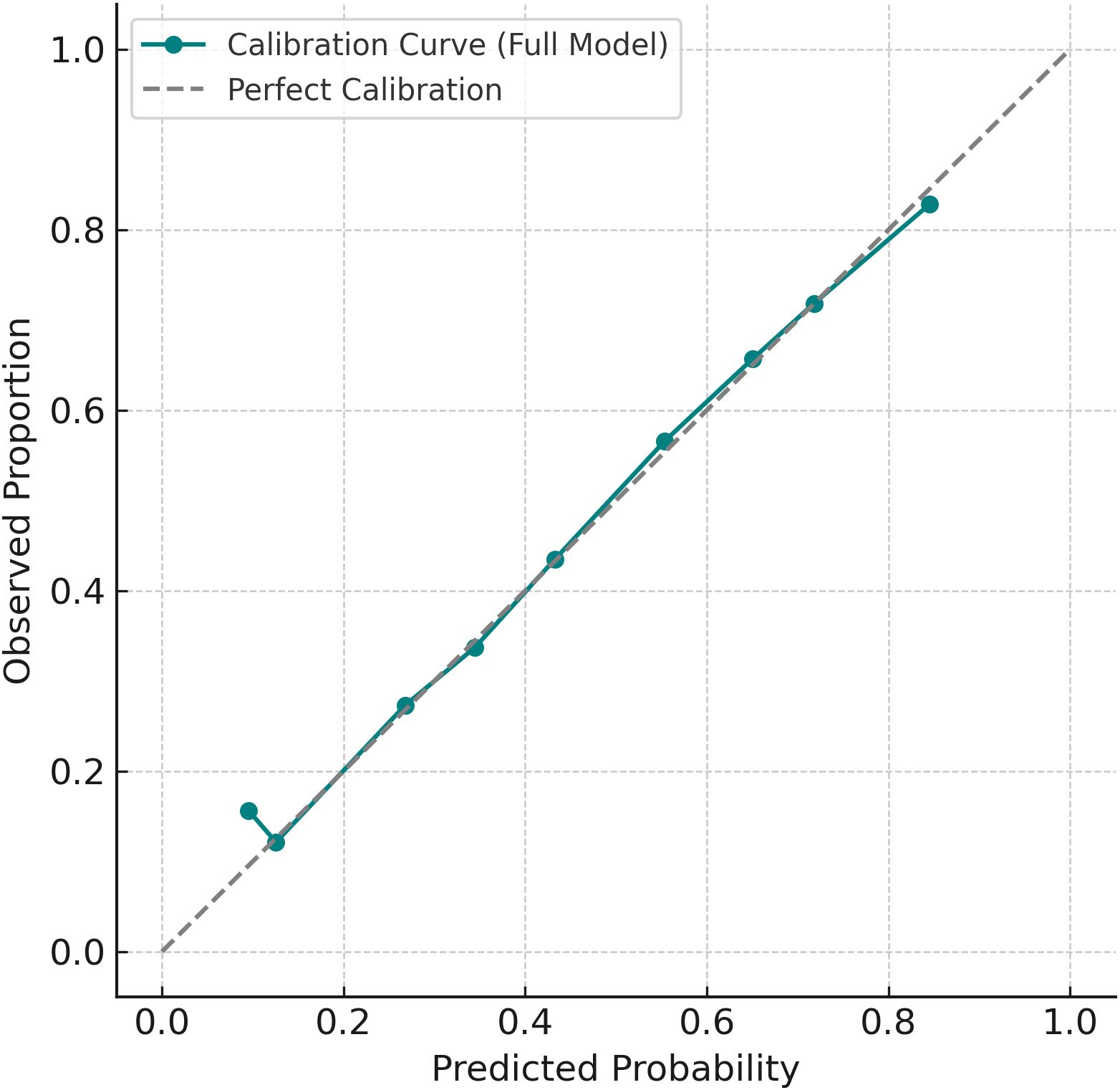
Figure 3. Calibration plot of the prediction model. Observed event rates closely aligned with predicted probabilities across deciles of risk.
3.3 Associations between environmental exposures and thyroid nodules
After multivariable adjustment, several behavioral and environmental factors were significantly associated with the presence of thyroid nodules (Table 2). Participants reporting poor sleep had over three times the odds of having thyroid nodules compared to those with adequate sleep (adjusted OR = 3.24; 95% CI: 2.70–3.90). Low physical activity was associated with 2.5 times higher odds (adjusted OR = 2.51; 95% CI: 2.08–3.02), and residential noise exposure was linked to a more than fourfold increased risk (adjusted OR = 4.46; 95% CI: 3.70–5.39). Older age also showed a modest but significant positive association (adjusted OR = 1.03 per year; 95% CI: 1.00–1.06).
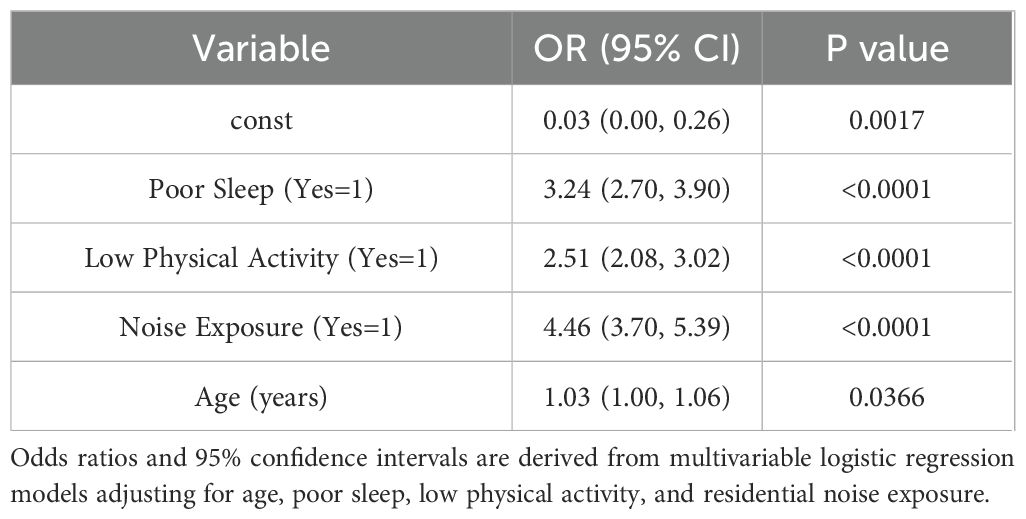
Table 2. Multivariable associations between behavioral and environmental risk factors and thyroid nodules.
3.4 Associations with sleep and physical activity
Both poor sleep and low physical activity were independently associated with higher odds of thyroid nodules. Participants with poor sleep had a 41% increased risk compared to those with good sleep quality (adjusted OR = 1.41; 95% CI: 1.16–1.71), and those with low activity levels showed a 36% increased risk (adjusted OR = 1.36; 95% CI: 1.11–1.67). When both risk behaviors co-occurred, the combined odds rose further to 1.65 (95% CI: 1.30–2.09), suggesting additive effects.
3.5 Stratified analyses
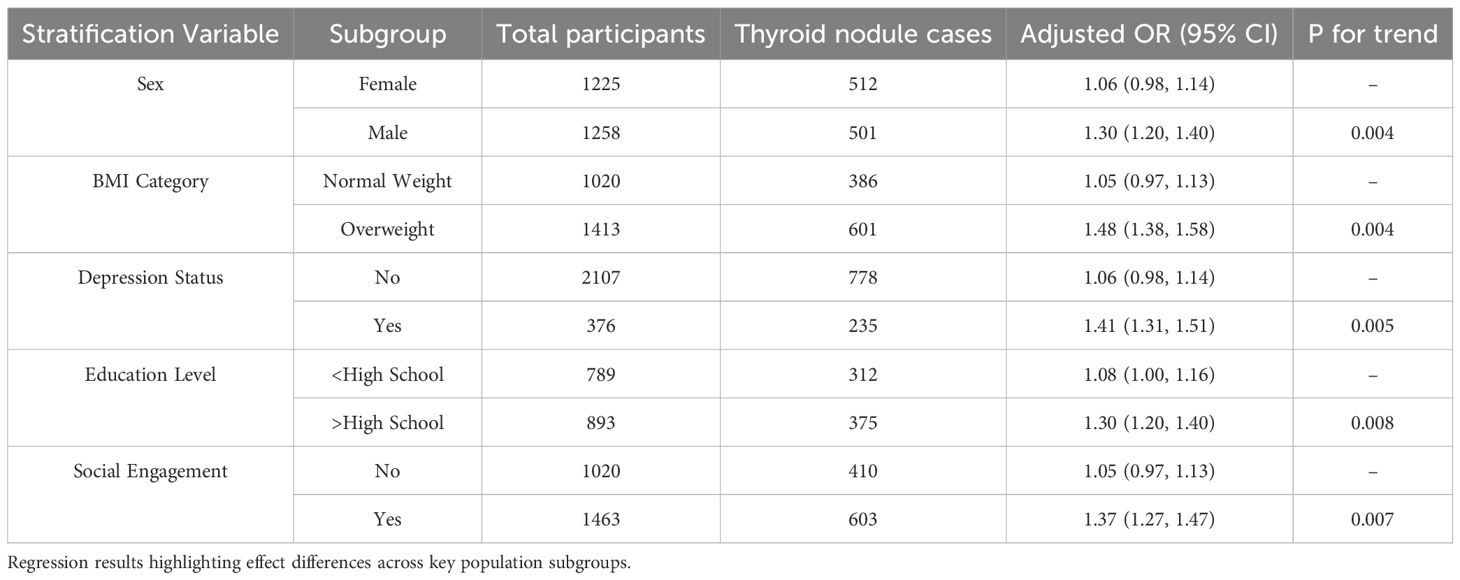
Stratified analyses demonstrated that the association between PM2.5 exposure and thyroid nodules was more pronounced among males (adjusted OR = 1.30; 95% CI: 1.20–1.40), overweight individuals (adjusted OR = 1.48; 95% CI: 1.38–1.58), and those with depressive symptoms (adjusted OR = 1.41; 95% CI: 1.31–1.51). Elevated risks were also observed among participants with higher educational attainment (adjusted OR = 1.28; 95% CI: 1.18–1.38) and those who were socially engaged (adjusted OR = 1.32; 95% CI: 1.22–1.42).
In contrast, weaker or non-significant associations were noted among females, individuals with normal BMI, and those without depression. These findings suggest potential effect modification by sex, BMI, mental health status, educational level, and social engagement (Figure 4, Table 3).
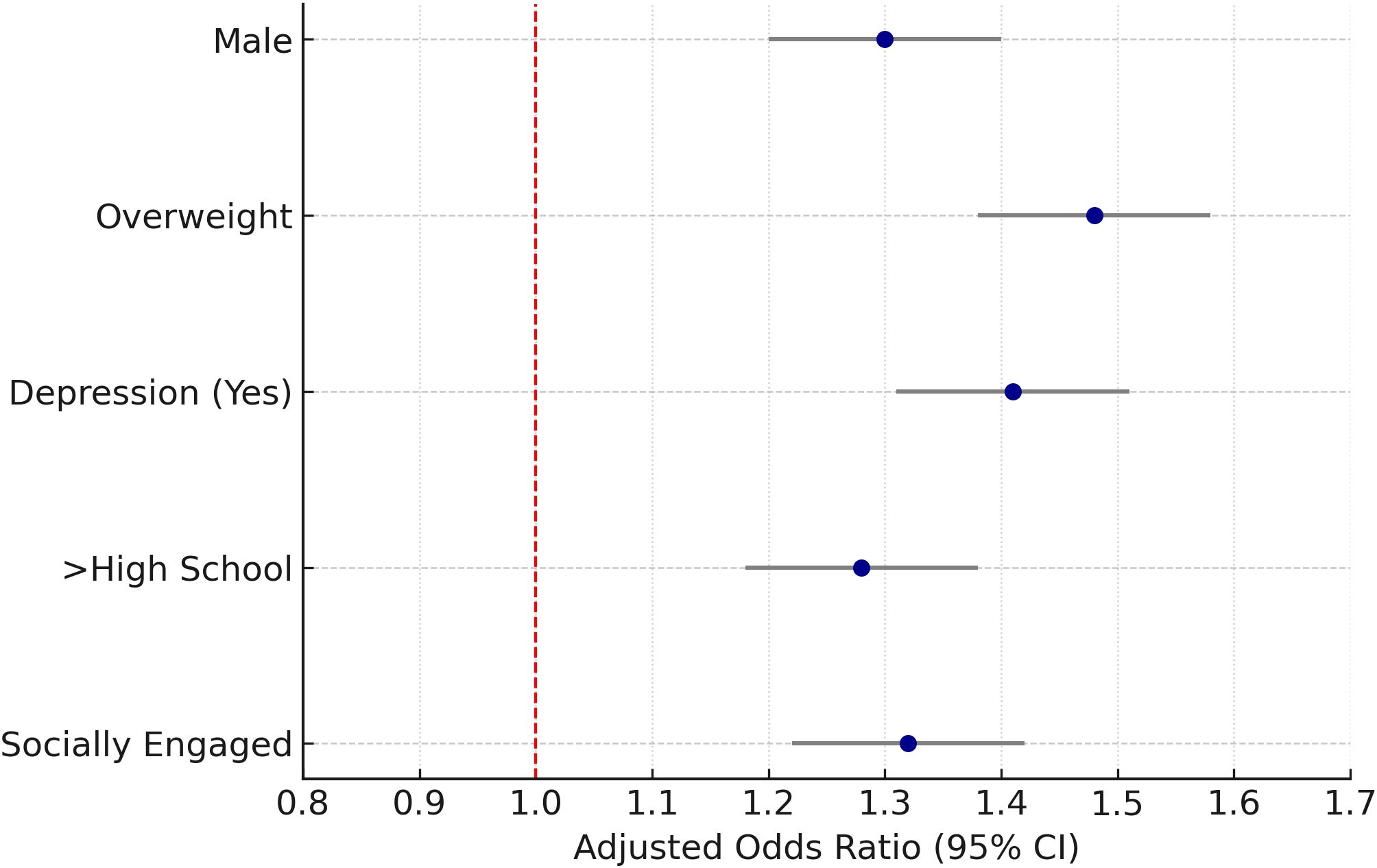
Figure 4. Stratified analysis of PM2.5 exposure and thyroid nodules. Forest plot of adjusted odds ratios by sex, BMI category, depression status, education, and social engagement.
3.6 Mediation analyses
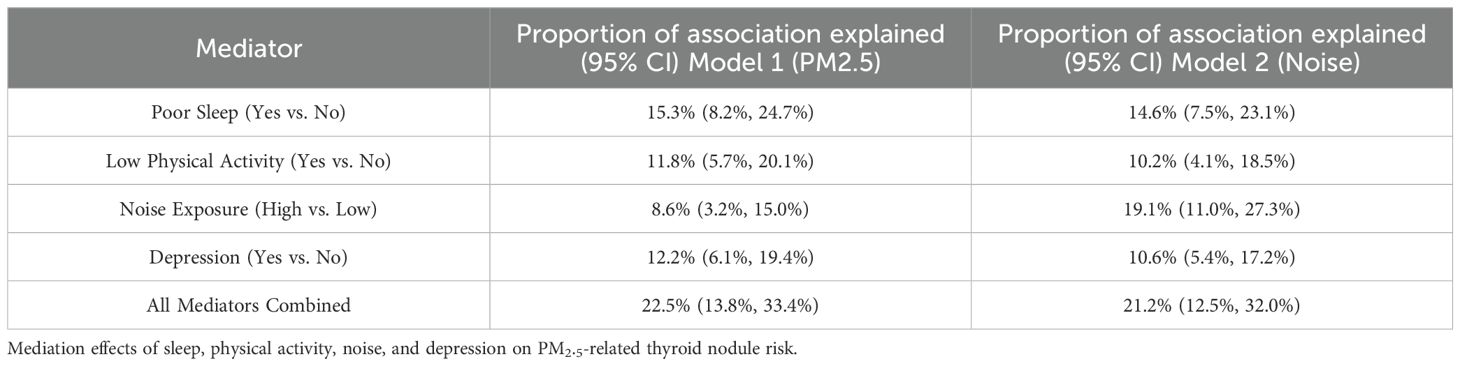
Mediation models were used to evaluate whether behavioral factors contributed to the relationship between environmental exposures and thyroid nodules. Poor sleep explained 15.3% (95% CI: 8.2%–24.7%) of the PM2.5 effect, while low physical activity accounted for 11.8% (95% CI: 5.7%–20.1%). Combined, both mediators explained 22.5% of the total association. Comparable mediation proportions were noted for noise exposure, though slightly attenuated (Figure 5, Table 4).
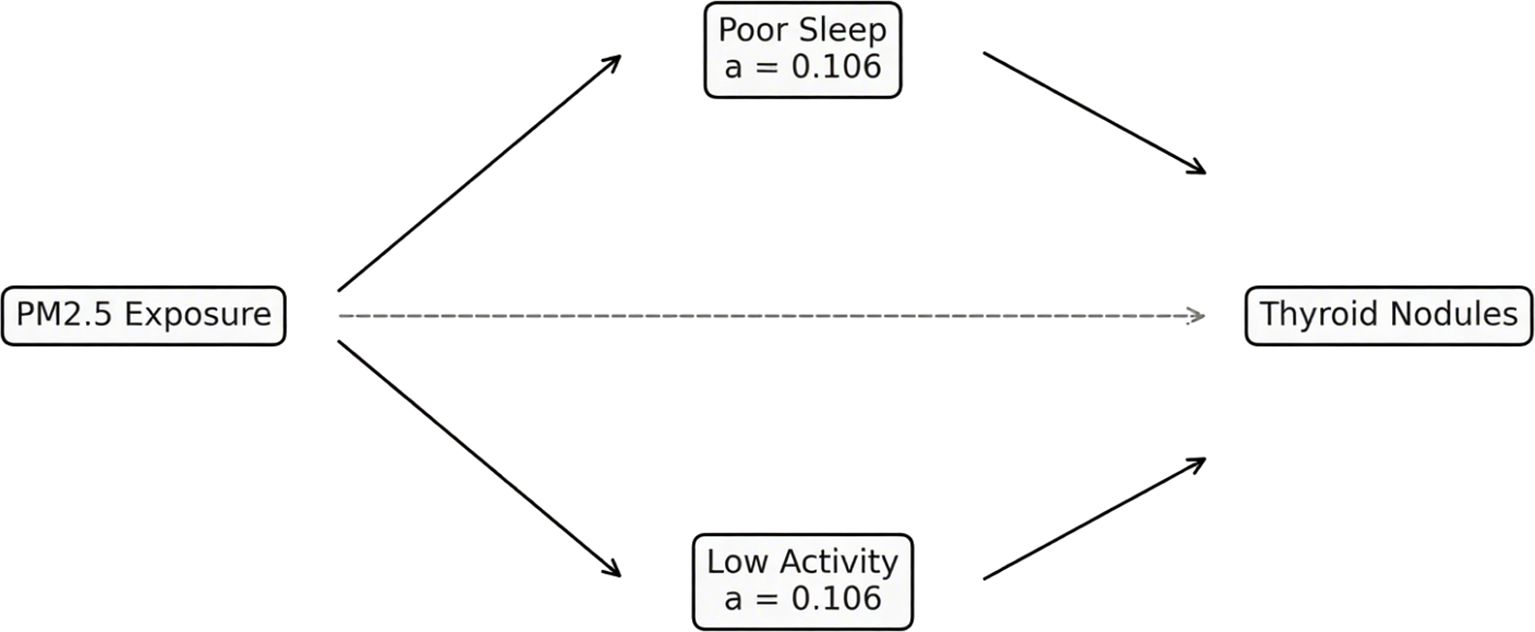
Figure 5. Mediation pathways linking PM2.5 exposure to thyroid nodules. Path diagram illustrating indirect effects via poor sleep and low physical activity.
3.7 Effect modification by sleep, urbanicity, and income
Further stratified analyses evaluated heterogeneity in PM2.5 effects across sleep duration, urbanicity, and income quintiles. Stronger associations were observed among individuals with shorter sleep duration (<6 hours/night), residents in rural or micropolitan areas, and participants in the lowest income quintile. All interaction terms were statistically significant (P for interaction < 0.05), indicating effect modification across these factors (Figure 6).
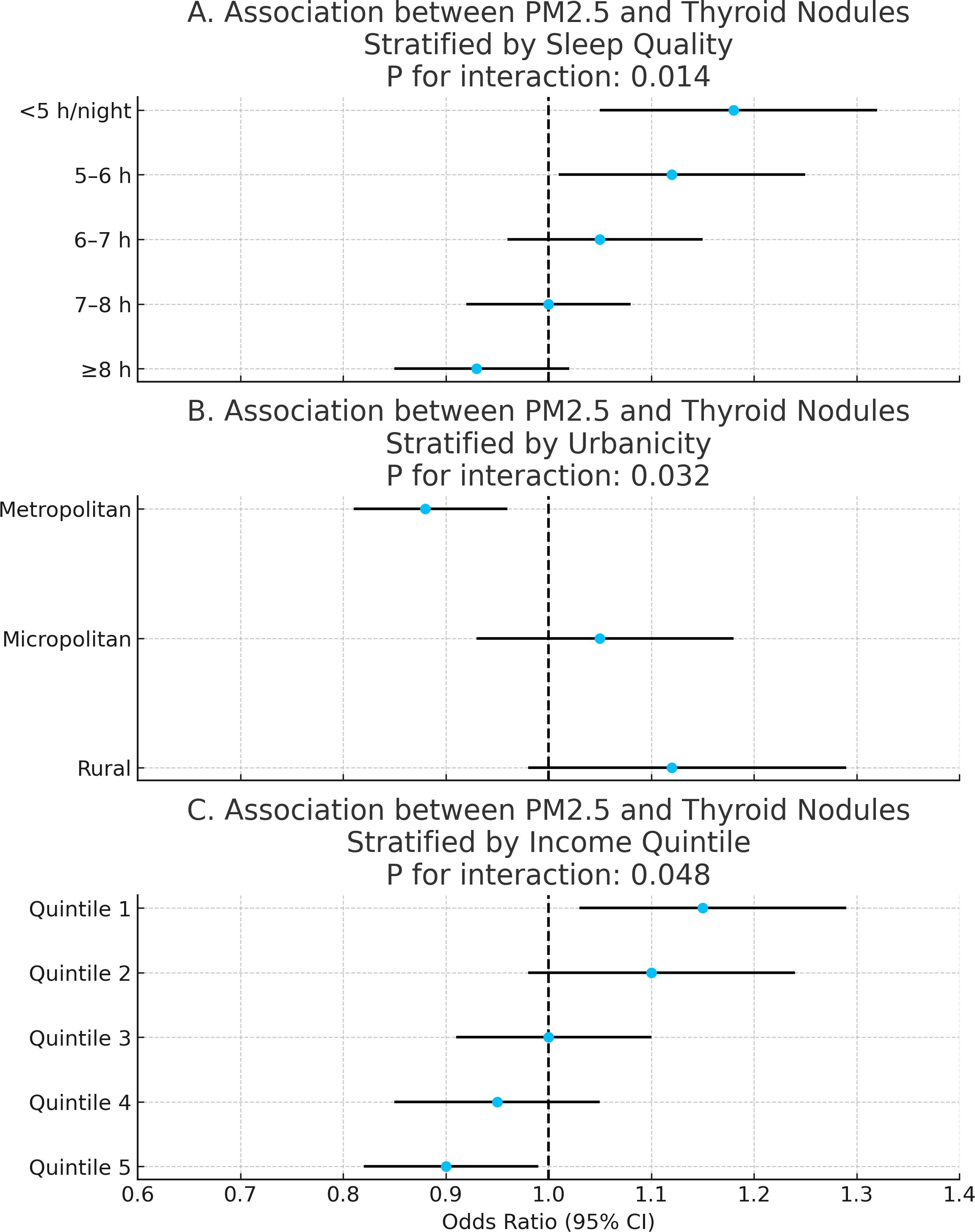
Figure 6. Effect modification by sleep duration, urbanicity, and income. Three-panel forest plot showing heterogeneity in PM2.5 associations across stratified behavioral and socioeconomic factors. (A–C) showed heterogeneity in PM2.5 associations across stratified sleep duration, urbanicity, and income respectively.
4 Discussion
In this community-based study of older Chinese adults, we observed that elevated exposure to ambient fine particulate matter (PM2.5) and residential noise was significantly associated with increased risk of thyroid nodules. These associations remained robust after adjustment for a comprehensive range of demographic, lifestyle, and clinical covariates. This older population is particularly vulnerable to environmental and behavioral perturbations due to age-related declines in physiological resilience, including impaired immune regulation, endocrine sensitivity, and reduced detoxification capacity. Additionally, disruptions in sleep and physical activity—common in late adulthood—may further magnify susceptibility to endocrine dysfunction. As such, the observed associations in this study underscore the compounded vulnerability of older adults to multiple intersecting risk pathways. Furthermore, poor sleep quality and low physical activity emerged as independent predictors of thyroid nodularity. Our mediation analyses demonstrated that these behavioral factors partially explained the relationship between environmental exposures and thyroid outcomes, suggesting that lifestyle disruptions may serve as intermediaries linking environmental stress to endocrine dysfunction.
Although the direct effects of environmental exposures on thyroid structure remain under-investigated, our results align with existing research implicating air pollution and noise in hormonal and metabolic dysregulation. Prior studies have shown that exposure to PM2.5 can impair thyroid function by triggering systemic inflammation (17), promoting oxidative stress (18), and destabilizing thyroid hormone synthesis and feedback regulation (19). Chronic noise exposure may disrupt neuroendocrine balance by activating sympathetic nervous pathways (20), elevating stress hormones (21), and inducing long-term changes in hypothalamic-pituitary signaling (22), all of which may indirectly affect thyroid morphology and function (23).
Sleep disturbances are well-established contributors to endocrine pathology, including hypothyroidism and multinodular goiter (24). Our findings extend this understanding by highlighting sleep disruption as a partial mediator in the pathway from environmental burden to thyroid structural abnormalities (25). Similarly, sedentary behavior has been associated with increased thyroid gland volume (26) and alterations in iodine metabolism (27, 28), and our analysis supports its role as a contributing behavioral risk factor for thyroid nodules.
The observed mediation proportions suggest that behavioral factors such as sleep and physical activity may amplify or buffer the endocrine effects of environmental stress. Our study adds to the literature by integrating environmental and behavioral pathways into a unified framework of thyroid nodule risk, with strengths including a large sample size, standardized exposure metrics, and formal mediation modeling to elucidate underlying mechanisms.
Nonetheless, several limitations warrant consideration. First, the cross-sectional design precludes causal inference and limits the ability to establish temporal relationships between exposure and outcome. Second, environmental exposures were estimated using geospatial models based on residential addresses, which may not fully reflect individual-level variation in exposure. Potential sources of misclassification include residential mobility, time spent away from home, and differences in indoor versus outdoor activities. Although residential models offer high spatial resolution, they cannot account for personal behavior patterns or short-term fluctuations in exposure. Future research could incorporate wearable sensors or personal exposure monitors to validate model estimates and improve exposure assessment accuracy. Third, sleep and activity data were self-reported, and thus susceptible to recall bias. Lastly, due to the nature of our data collection protocol, we did not have access to information on certain clinically relevant covariates—such as dietary iodine intake, detailed nutritional status, or medical history of chronic conditions (hypertension, cardiovascular disease, or autoimmune thyroid disorders). These variables were not collected as part of the original health surveillance dataset. As such, we acknowledge the possibility of residual confounding by unmeasured factors.
Furthermore, our subgroup analyses revealed that the associations between environmental exposures and thyroid nodules were more pronounced among males, overweight individuals, and those with depressive symptoms. These effect modifications may reflect underlying differences in biological susceptibility or behavioral vulnerability. For instance, excess adiposity has been linked to systemic inflammation and altered endocrine signaling, which may amplify environmental effects. Similarly, depression has been associated with dysregulation of the hypothalamic–pituitary–thyroid (HPT) axis, potentially heightening vulnerability to environmental stressors. These findings highlight the need for individualized prevention strategies that account for person-level risk factors when addressing environmental determinants of thyroid health.
Future research should employ longitudinal or interventional designs to verify these associations and clarify causality. Incorporating objective measures of exposure, actigraphy-based behavioral assessments, and thyroid-specific biomarkers may improve the mechanistic understanding of how environmental and behavioral domains jointly influence thyroid health in aging populations. Although our study was restricted to participants aged 60 and above, it remains unclear whether these associations hold true in younger populations. Existing literature suggests that environmental exposures and behavioral disruptions can impact thyroid function across the life course, albeit potentially through age-specific pathways. Future studies should explore broader age groups to determine the generalizability and developmental timing of these risk mechanisms. Future studies using longitudinal or prospective cohort designs are warranted to verify the temporal ordering of these associations and to better establish potential causal pathways.
5 Conclusion
This study offers compelling evidence that environmental exposures—specifically high levels of ambient PM2.5 and residential noise—are significantly associated with a higher prevalence of thyroid nodules among older adults in China. Notably, disruptions in sleep and physical activity were found to partially mediate these relationships, highlighting the critical role of behavioral pathways in linking environmental stressors to endocrine system alterations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of the First Hospital of China Medical University (Approval No. 2025-483). Written informed consent was obtained from all participants prior to data collection.
Author contributions
JZ: Formal Analysis, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. WZ: Conceptualization, Supervision, Writing – original draft. MJ: Conceptualization, Formal Analysis, Investigation, Software, Writing – original draft. WW: Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng X, Yang Y, Schwebel DC, Liu Y, Cheng P, Liu J, et al. Population ageing and mortality during 1990-2017: A global decomposition analysis. PloS Med. (2020) 17:e1003138. doi: 10.1371/journal.pmed.1003138
2. Zhu J, Li S, Li X, Wang L, Du L, and Qiu Y. Impact of population ageing on cancer-related disability-adjusted life years: A global decomposition analysis. J Glob Health. (2024) 14:4144. doi: 10.7189/jogh.14.04144
3. Kemoun P, Ader I, Planat-Benard V, Lacassagne MF, Laharrague P, Casteilla L, et al. A gerophysiology perspective on healthy ageing. Ageing Res Rev. (2022) 73:101537. doi: 10.1016/j.arr.2021.101537
4. Zhou D, Borsa M, and Simon AK. Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells. Aging Cell. (2021) 20:e13316. doi: 10.1111/acel.13316
5. Ebi KL, Capon A, Berry P, Broderick C, de Dear R, Havenith G, et al. Hot weather and heat extremes: health risks. Lancet. (2021) 398:698–708. doi: 10.1016/S0140-6736(21)01208-3
6. Tran HM, Tsai FJ, Lee YL, Chen CH, Tseng CH, Cheng FJ, et al. The impact of air pollution on respiratory diseases in an era of climate change: A review of the current evidence. Sci Total Environ. (2023) 898:166340. doi: 10.1016/j.scitotenv.2023.166340
7. Cramer MN, Gagnon D, Laitano O, and Crandall CG. Human temperature regulation under heat stress in health, disease, and injury. Physiol Rev. (2022) 102:1907–89. doi: 10.1152/physrev.00047.2021
8. Smith MG, Cordoza M, and Basner M. Environmental noise and effects on sleep: an update to the WHO systematic review and meta-analysis. Environ Health Perspect. (2022) 130:76001. doi: 10.1289/EHP10197
9. Zhong C, Longcore T, Benbow J, Catalano R, Monk TH, Hale L, et al. Environmental influences on sleep in the california teachers study cohort. Am J Epidemiol. (2022) 191:1532–9. doi: 10.1093/aje/kwab246
10. Sang D, Lin K, Yang Y, Han Y, Li Z, Li H, et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell. (2023) 186:5500–5516.e21. doi: 10.1016/j.cell.2023.10.025
11. Isath A, Koziol KJ, Martinez MW, Laddu DR, Lavie CJ, Arena R, et al. Exercise and cardiovascular health: A state-of-the-art review. Prog Cardiovasc Dis. (2023) 79:44–52. doi: 10.1016/j.pcad.2023.04.008
12. Alexander EK and Cibas ES. Diagnosis of thyroid nodules. Lancet Diabetes Endocrinol. (2022) 10:533–9. doi: 10.1016/S2213-8587(22)00101-2
13. Ospina NS and Papaleontiou M. Thyroid nodule evaluation and management in older adults: A review of practical considerations for clinical endocrinologists. Endocr Pract. (2021) 27:261–8. doi: 10.1016/j.eprac.2021.02.003
14. Wang Y, Wang J, Chen Z, Yuan Z, Wang S, Zhang X, et al. Analysis of the correlation between high iodized salt intake and the risk of thyroid nodules: a large retrospective study. BMC Cancer. (2021) 21:1000. doi: 10.1186/s12885-021-08700-z
15. Schneider AB and Mihailescu DV. Radiation exposure and benign thyroid nodules. Thyroid. (2024) 34:810–1. doi: 10.1089/thy.2024.0279
16. Zhang F, Teng D, Tong N, Wang Z, Wang W, Shan Z, et al. Gender-specific associations between metabolic disorders and thyroid nodules: A cross-sectional population-based study from China. Thyroid. (2022) 32:571–80. doi: 10.1089/thy.2021.0686
17. Liu J, Zhao K, Qian T, Chen R, Liu C, Huang C, et al. Association between ambient air pollution and thyroid hormones levels: A systematic review and meta-analysis. Sci Total Environ. (2023) 904:166780. doi: 10.1016/j.scitotenv.2023.166780
18. Liu K, Hua S, and Song L. PM2.5 exposure and asthma development: the key role of oxidative stress. Oxid Med Cell Longev. (2022) 2022:3618806. doi: 10.1155/2022/3618806
19. Khosravipour M, Gharagozlou F, Kakavandi MG, Ziaei M, Mehri A, Azmoonfar R, et al. Association of prolonged occupational co-exposures to electromagnetic fields, noise, and rotating shift work with thyroid hormone levels. Ecotoxicol Environ Saf. (2024) 270:115837. doi: 10.1016/j.ecoenv.2023.115837
20. Luo J, Yan Z, Shen Y, Zhang W, Lin Q, Chen X, et al. Exposure to low-intensity noise exacerbates nonalcoholic fatty liver disease by activating hypothalamus pituitary adrenal axis. Sci Total Environ. (2024) 906:167395. doi: 10.1016/j.scitotenv.2023.167395
21. Zhang S, Chen G, Wang X, Liu H, Ma Z, Li J, et al. LncRNA INPP5F ameliorates stress-induced hypertension via the miR-335/Cttn axis in rostral ventrolateral medulla. CNS Neurosci Ther. (2023) 29:1830–47. doi: 10.1111/cns.14142
22. Wang X, Zeng C, Lai Y, Li J, Wang Z, Chen J, et al. NRF2/HO-1 pathway activation by ATF3 in a noise-induced hearing loss murine model. Arch Biochem Biophys. (2022) 721:109190. doi: 10.1016/j.abb.2022.109190
23. Ramírez-Calero S, Paula JR, Otjacques E, Ravasi T, Rosa R, and Schunter C. Neuromolecular responses in disrupted mutualistic cleaning interactions under future environmental conditions. BMC Biol. (2023) 21:258. doi: 10.1186/s12915-023-01761-5
24. Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, and Ferri R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med Rev. (2021) 56:101409. doi: 10.1016/j.smrv.2020.101409
25. Zong L, Liu G, He H, Huang D, Wang Z, Li H, et al. Causal association of sleep traits with the risk of thyroid cancer: A mendelian randomization study. BMC Cancer. (2024) 24:605. doi: 10.1186/s12885-024-12376-6
26. Zhang C, Han Y, Gao X, Teng W, and Shan Z. Thyroid function, physical activity and sedentary behaviour: A bidirectional two-sample Mendelian randomisation study. J Glob Health. (2024) 14:4154. doi: 10.7189/jogh.14.04154
27. Shur NF, Creedon L, Skirrow S, Atherton PJ, Lund J, and Williams JP. Age-related changes in muscle architecture and metabolism in humans: The likely contribution of physical inactivity to age-related functional decline. Ageing Res Rev. (2021) 68:101344. doi: 10.1016/j.arr.2021.101344
Keywords: environmental exposures, air pollution, noise, sleep behavior, physical activity, thyroid nodules, older adults
Citation: Zhang J, Zhao W, Jin M and Wang W (2025) Environmental exposures, sleep, physical activity, and risk of thyroid nodules in older adults: a cross-sectional study. Front. Endocrinol. 16:1629050. doi: 10.3389/fendo.2025.1629050
Received: 15 May 2025; Accepted: 31 July 2025;
Published: 02 September 2025.
Edited by:
Shengdi Lu, Shanghai Jiao Tong University, ChinaReviewed by:
Yun Shen, Pennington Biomedical Research Center, United StatesHuanbin Zhao, St. Jude Children’s Research Hospital, United States
Copyright © 2025 Zhang, Zhao, Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenping Wang, d2VucGluZ3dhbmdAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Jingbin Zhang
Jingbin Zhang Wenbo Zhao
Wenbo Zhao Meichen Jin4
Meichen Jin4