- Division of Pediatrics, Department of Health Sciences, University of Piemonte Orientale, Novara, Italy
The incidence and prevalence of type 2 diabetes (T2D) are increasing worldwide, affecting both adults and a growing number of children and adolescents. Notably, youth-onset T2D differs in its pathogenesis from later-onset T2D and progresses more rapidly. It is associated with a higher incidence of complications, and these occur earlier. Over the past few decades, numerous therapeutic agents from various drug classes have been approved for use in adults with T2D. In contrast, there is a scarcity of approved treatments for T2D in children. Sodium-glucose cotransporter-2 (SGLT2) inhibitors represent one of the newest classes of oral hypoglycemic agents, which lower blood glucose concentrations by increasing urinary glucose excretion. They have significantly influenced the management of T2D in adults. Promising results have also been reported in the pediatric population. In particular, empagliflozin, a potent and highly selective SGLT2 inhibitor, is an effective and generally well-tolerated anti-hyperglycemic agent widely approved for the treatment of adults with T2D. It has been recently approved for the management of T2D in children aged 10 years and older, in combination with diet and exercise. Here, we report a case of empagliflozin use in a 14-year-old girl with poorly controlled T2D. Furthermore, we review existing data on the use of SGLT2 inhibitors in the pediatric population.
1 Introduction
Type 2 diabetes (T2D) has become a significant global health concern, affecting both adults and an increasing number of children and adolescents, with relevant implications for public health systems.
According to the International Diabetes Federation (IDF), more than 537 million adults aged 20 – 79 were affected by T2D globally in 2021, with an estimated global healthcare expenditure of USD 966 billion, representing a staggering 316% increase over the past 15 years. Projections indicate that this number could rise to 783 million by 2045 if current trends continue (1–3).
In the pediatric population, the incidence of T2D has also shown a disturbing increase. Historically, T2D was considered rare in children and adolescents but over the past two decades the prevalence has surged, especially in populations with high rates of obesity. The SEARCH for Diabetes in Youth study revealed an annual increase of 4.8% in the incidence of T2D among U.S. children from 2002 to 2012 (4). Additionally, some authors highlighted that the prevalence of youth-onset T2D during adolescence (typically between the ages of 10 and 18), has risen 2 to 3 times compared to 30 years ago, with an estimated 41.600 new cases worldwide in 2021, especially in China, India, and the United States (5).
The mechanisms behind the rise of T2D in both adults and children are multifactorial. Genetic predisposition, environmental factors and the increasing prevalence of obesity have all been implicated. However, the pathophysiology of T2D in youth differs from that in adults with a more rapid decline in pancreatic β-cell function (about 20 - 35% per year in youth versus about 7 - 11% per year in adults), suggesting that T2D in youth is a more aggressive and severe condition (6, 7). In particular, obesity and insulin resistance play a relevant role in the pediatric population, where rapid weight gain during puberty exacerbates metabolic dysfunction. Peripheral insulin resistance is a key feature that occurs early in the disease course, and initially is compensated by increased insulin secretion reflected in hyperinsulinemia. Sustained hyperglycemia over time results in beta cell exhaustion and declining insulin secretion (glucose toxicity). This places children at greater risk of early onset complications such as nephropathy, retinopathy and cardiovascular disease, which are associated with long-term diabetes in adults.
Figure 1 displays different targets and mechanisms of action of the many therapeutic agents and drug classes that have been approved for use in adults with T2D (8, 9). By contrast, there is a paucity of treatments for T2D in youth. Up to 2019, metformin and insulin were the only approved treatment options in children and adolescents with T2D. However, an increasing number of clinical trials of agents in youth-onset T2D have been completed or are nearing completion (7).
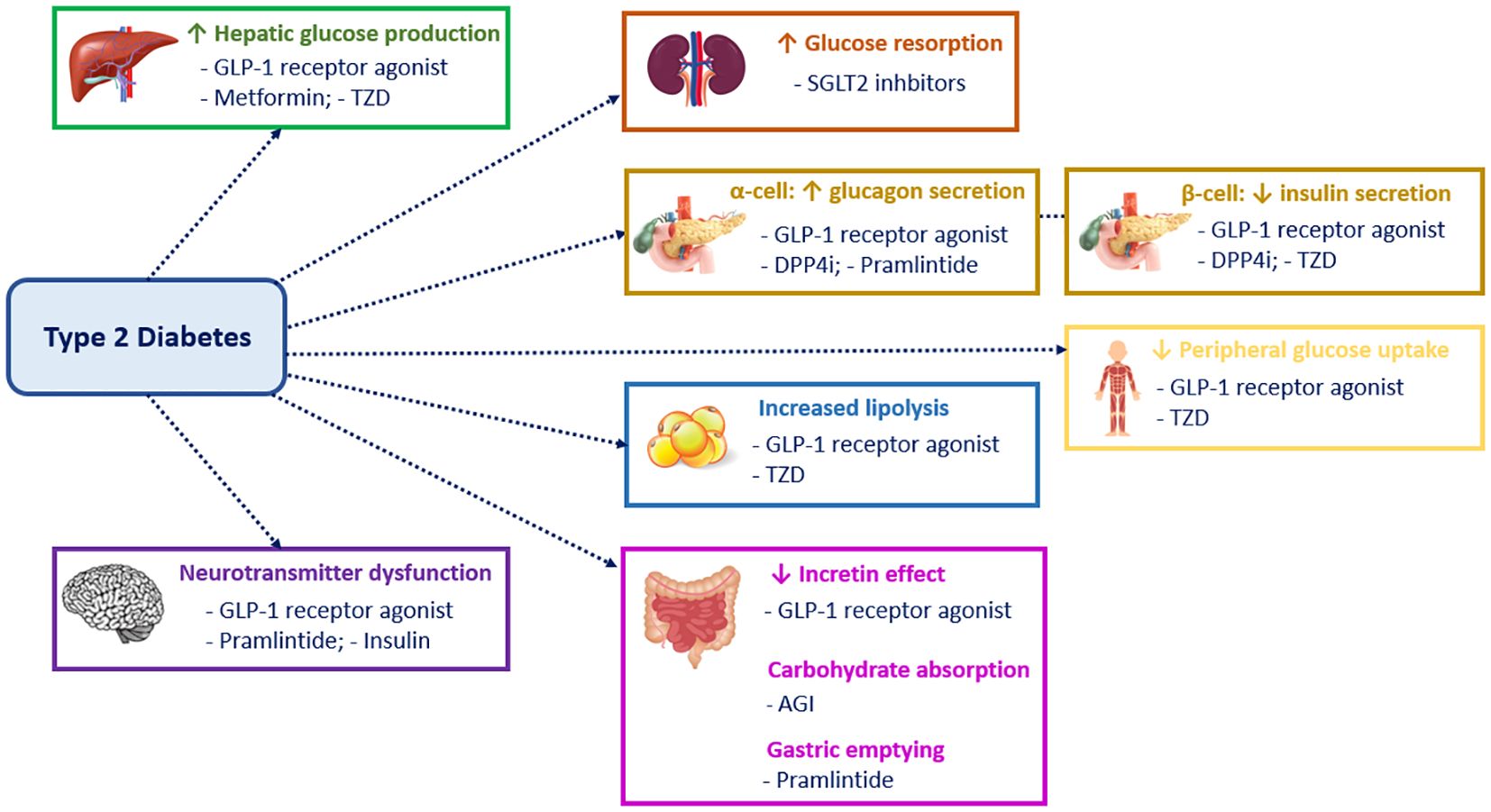
Figure 1. Mechanism of action of the different classes of type 2 diabetes drugs. GLP1, Glucagon-like peptide 1; TZD, Thiazolidinediones; SGLT2, Sodium-glucose cotransporter-2; DPP4i, Dipeptidyl peptidase-4 inhibitors; AGI, Alpha-glucosidase inhibitors.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are one of the newest classes of oral hypoglycemic agents which lower blood glucose concentrations by increasing urinary glucose excretion. They have significantly influenced the management of T2D in adults; some of these medications, like dapagliflozin and empagliflozin, have recently been approved for the treatment of T2D in children and adolescents with promising results. As further evidence we present a clinical case of a 14-year-old female patient with T2D, who was successfully treated with empagliflozin therapy.
2 Case presentation
A 14-year-old female patient presented to our center with poorly controlled T2D, requiring multiple daily insulin injections (MDI) since diagnosis. Her previous medical history was uneventful; she was born full term after a pregnancy complicated by gestational diabetes. She was diagnosed with T2D at the age of 10, when she presented with episodes of hyperglycemia accompanied by sweating, polyuria, polydipsia, and fatigue. Blood tests showed negative type 1 diabetes autoantibodies and negative genetic testing for monogenic diabetes. An abdominal ultrasound showed a mildly thinned and heterogeneous-appearing pancreas; fecal elastase, lipase and pancreatic amylase were normal. Her family history was notable for T2D in maternal grandparents, and mother was affected by thyroid cancer.
At her first visit to our center, the patient appeared to be in good general condition. She presented with obesity (BMI 30 kg/m²). Her examination shows the presence of acanthosis nigricans on her neck and armpits. Her daily MDI therapy consisted of 9 units (U) of insulin glargine and 12 U of insulin lispro before meals (0.25 U/kg/day); her mean glycate hemoglobin (HbA1c) was 8.5%. Given the results of her previous tests, we decided to slightly reduce the total daily insulin dose and to start metformin therapy. Given the initial improvement, we gradually stopped insulin therapy and increased metformin dose to 2 g per day, together with a hypocaloric diet after consultations with dietitians and 10 hours per week of physical activity. After 12 months, this therapy resulted in weight improvement, with a weight loss of 10 kg and a BMI reduction from 30 to 26.4 kg/m², and better glycemic control, as demonstrated by a reduction in HbA1c levels (7.4%).
However, one year later, despite these initial improvements, her glycemic control remained suboptimal, with a time in range (TIR) of 61%. Considering the patient’s persistent hyperglycemia and the maternal history of thyroid cancer, which made Glucagon-Like Peptide-1 receptor agonists (GLP1-RA) less suitable, empagliflozin therapy was initiated at a dose of 10 mg daily, while maintaining metformin therapy. After six months of empagliflozin treatment, the patient’s glycemic control significantly improved: HbA1c levels decreased from 7.4% to 6.2% and TIR increased from 61% to 91%. She maintained a stable weight of 75 kg with a BMI of 26.2 kg/m².
No adverse events, such as diabetic ketoacidosis (DKA) or genital infections, were reported during the follow-up period. Table 1 shows her progresses over time.
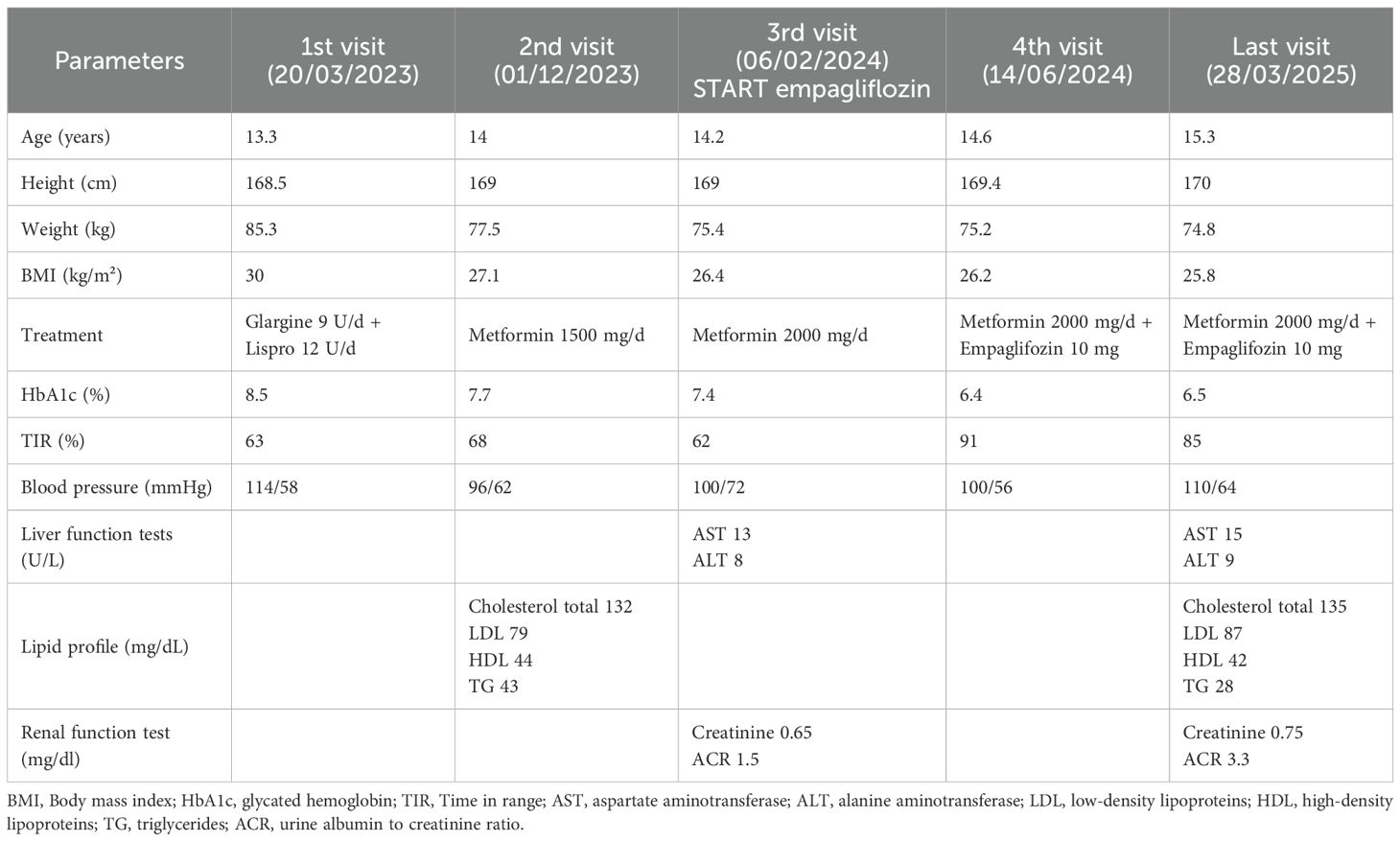
Table 1. Anthropometric measurements and data on glycemic control and treatments of the patient at first visit and during follow-up.
3 Discussion
This study describes the innovative and effective use of empagliflozin in a pediatric patient with T2D. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend SGLT2 inhibitors as a cornerstone of T2D treatment in adults (10). Currently, SGLT2 inhibitors such as dapagliflozin and empagliflozin are the only additional oral agents to have received regulatory approval from the European Medicines Agency (EMA) for use in children aged 10 years or older (11), addressing a previous scarcity of treatment options beyond metformin and insulin in this population. Pharmacodynamic and pharmacokinetic studies have elucidated that approximately 180g of glucose per day is excreted through glomerular filtration into the primary urine. This glucose is then almost completely reabsorbed by SGLT1 and SGLT2 in the proximal tubule via sodium-glucose cotransport across apical cell membranes (12, 13). In humans, six different SGLT isoforms have been identified (14). SGLT2 is highly expressed in the early segments (S1-S2) of the proximal renal tubule and reabsorbs approximately 90% of filtered glucose, while SGLT1 normally reabsorbs the remaining glucose in the S3 segment. In patients with hyperglycemia, the maximum reabsorption capacity is amplified; this amplification, coupled with an increased concentration of systemic glucose, results in persistent hyperglycemia and glucotoxicity, leading to β-cell dysfunction (15).
SGLT2 inhibitors reduce the maximum reabsorption of glucose, thereby increasing urinary glucose excretion and lowering blood glucose concentration in an insulin-independent manner, as depicted in Figure 2, according to previous studies (16–18). Consequently, they improve β-cell function by alleviating glucotoxicity and potentially reducing β-cell workload (19). Furthermore, the excretion of glucose leads to energy loss from the body, promoting weight loss; conversely, glucagon secretion increases after the administration of SGLT2 inhibitors, thus contributing to the promotion of lipolysis and the reduction of liver fat and visceral adiposity (20). The improvement of overweight and obesity promotes a reduction in insulin resistance and other metabolic parameters, such as blood pressure, lipid profile, and uric acid levels (17, 20).
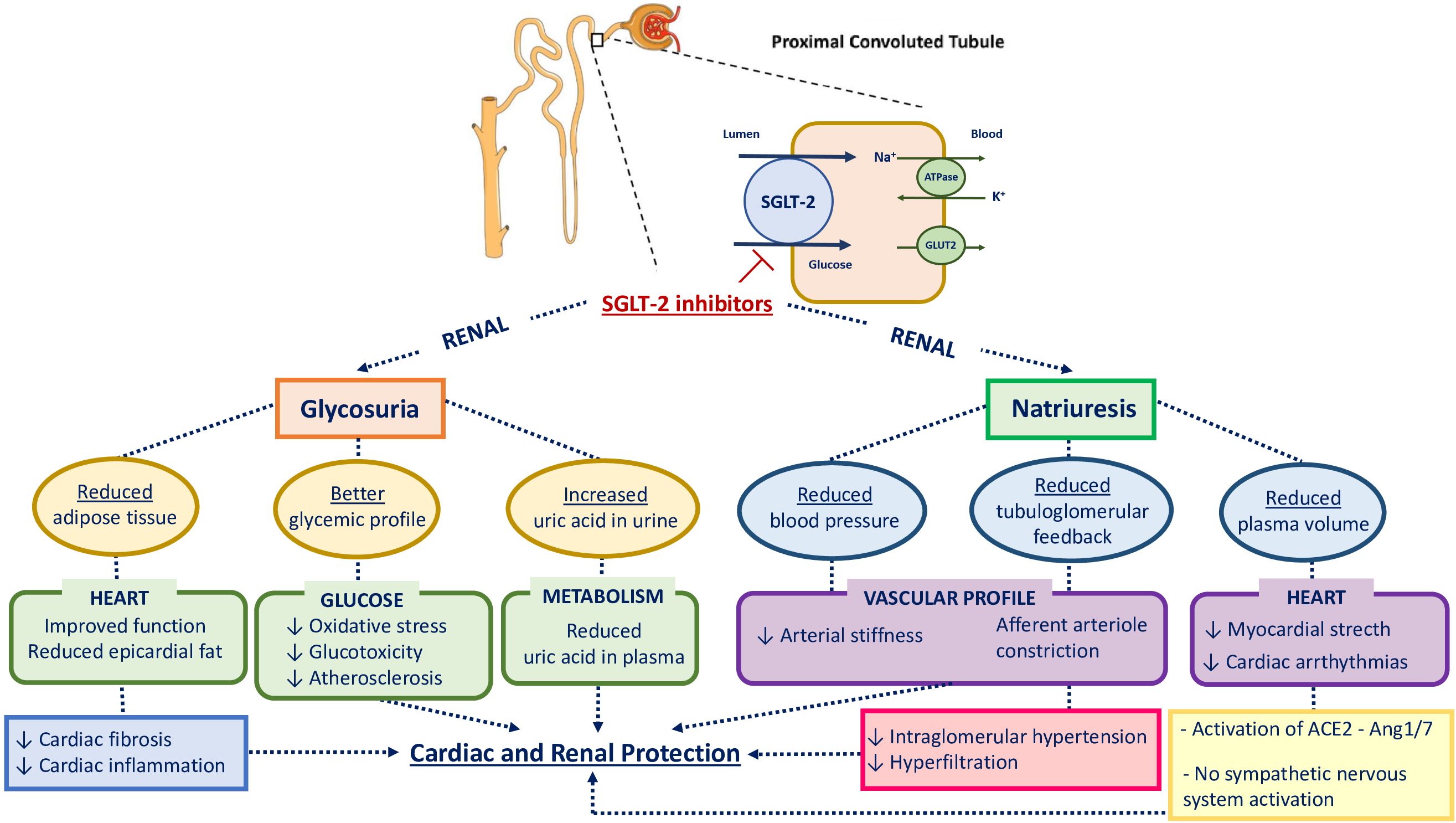
Figure 2. Physiological mechanisms involved in cardiovascular and renal protection with SGLT2 inhibition. HbA1c, glycated hemoglobin; SGLT2, sodium-glucose cotransporter-2; GLUT2, glucose transporter type 2; ACE2, angiotensin-converting enzyme 2; Ang 1 - 7, Angiotensin 1 - 7.
We herein describe the progresses in treatment of pediatric T2D in a real-life setting. Metformin and insulin were the established treatments for T2D in youth until the recent approval by the Food and Drug Administration (FDA) of two SGLT2 inhibitors (dapagliflozin and empagliflozin) for children and adolescents above 10 years of age with T2D, for use in combination with diet and exercise. This approval marked a significant advancement in the therapeutic landscape for this vulnerable population. Table 2 lists previous studies about the effects of these treatments in the pediatric population.
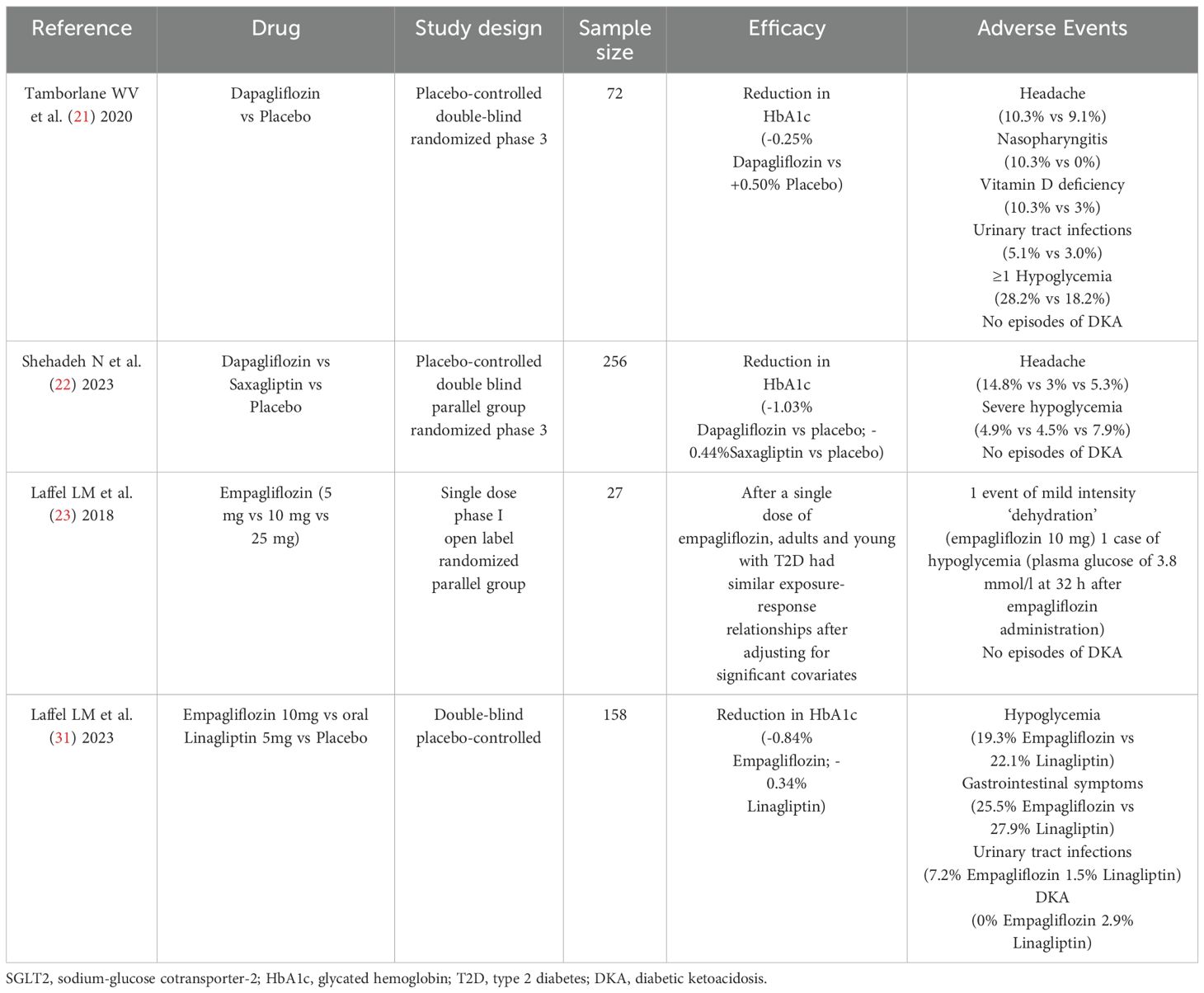
Specifically, dapagliflozin was the second oral treatment following metformin to be approved by the EMA in children 10 years of age or older, following a phase 3 trial (11, 21). This multicenter, randomized, parallel-group study evaluated the efficacy and safety of dapagliflozin versus placebo in young people aged 10 – 24 years with T2D (HbA1c 6.5%-11%). Seventy-two participants were randomized to dapagliflozin 10 mg or placebo during a 24-week double-blind period, followed by a 28-week open-label safety extension where all participants received dapagliflozin.
After 24 weeks, there was a statistically significant decrease in HbA1c with dapagliflozin compared to placebo. Over the entire 52 weeks of the study, AEs occurred in 74.4% of participants randomized to dapagliflozin, the most common were headache, nasopharyngitis, and vitamin D deficiency. No episodes of DKA were observed, and the only AE that led to treatment discontinuation was a genital infection. Hypoglycemia occurred in one-third of participants with dapagliflozin (almost all of whom were also receiving insulin), mainly asymptomatically; no hypoglycemia events led to discontinuation of treatment. There was no notable effect of treatment on body weight, BMI, or blood pressure (21).
More recently, the T2NOW trial compared dapagliflozin and saxagliptin (a dipeptidyl peptidase-4 inhibitor (DPP - 4i)) separately versus a single placebo group. This was a 52-week trial with a 26-week extension among children (10 – 17 years of age) with uncontrolled T2D (HbA1c 6.5 - 10.5%) receiving metformin, insulin, or both. Participants were randomly assigned to 5 mg of dapagliflozin, 2.5 mg of saxagliptin, or placebo. With dapagliflozin, a significant decrease in HbA1c and fasting plasma glucose was observed early in treatment and sustained over 52 weeks, statistically significant versus placebo. The most common AE was headache, followed by hypoglycemia, which occurred in almost one-third of patients; most events were mild, and none was considered serious or resulted in discontinuation. No new safety signals were observed (22). Compared with the previous phase 3 trial of dapagliflozin (21), in which most patients were either White or Black/African American, this trial was more representative of the ethnically diverse population affected by T2D globally (22), potentially enhancing the generalizability of its findings.
The second SGLT2 inhibitor, empagliflozin, was tested in a randomized trial assessing its pharmacokinetic and pharmacodynamic profile and identifying the appropriate doses in young people with T2D aged 10 to 17 years. The most important finding of this study was that the pharmacokinetic characteristics of the 10 and 25 mg doses of empagliflozin approved for use in adults were generally similar in young people with T2D. As observed in adult patients, the pharmacodynamic actions of empagliflozin showed dose-dependent increases in urinary glucose excretion and corresponding decreases in fasting plasma glucose and mean daily glucose levels. Protocol-specified AEs of specialinterest (hepatic injury, decreased renal function, and DKA) were not reported. No meaningful changes in systolic and diastolic blood pressure were observed (23), as confirmed in our patient. This contrasts with studies in adults with T2D, that described the cardiovascular benefits of this drug in comparison to GLP - 1 Ras and placebo (24, 25), although the reason for this difference remains unclear (26).
Our patient showed normal renal function at baseline and during treatment with empagliflozin. SGLT2 inhibitors have also consistently shown renoprotective effects in adults (27–30), but these effects have not been explored in the pediatric population.
The most recent randomized, double-blind, parallel-group, Phase 3 DINAMO trial assessed the efficacy and safety of empagliflozin versus placebo on glycemic control in children and adolescents with T2D who had been previously treated with metformin or insulin. One hundred fifty-eight participants aged 10 – 17 years (HbA1c 6.5 - 10.5%) were randomly assigned to oral empagliflozin 10 mg, oral linagliptin 5 mg (DPP - 4i), or placebo. Participants in the empagliflozin group who did not achieve an HbA1c below 7% by week 12 underwent a second randomization, either remaining on 10 mg or increasing to 25 mg. Participants in the placebo group were randomly reassigned at week 26 to linagliptin 5 mg or one of the empagliflozin doses. The study demonstrated that empagliflozin provided clinically relevant placebo-corrected reductions in HbA1c, whereas linagliptin did not. The safety profile of empagliflozin was comparable to that observed in adult patients: hypoglycemia was the most frequently reported adverse event, with higher rates for those on active drug treatment compared with placebo. No severe hypoglycemia nor DKA were reported. The occurrence of urinary tract infections were slightly higher in the empagliflozin group than in the placebo group (31). Our patient did not show any AE over more than one year of treatment with empagliflozin.
4 Conclusion
T2D is becoming increasingly prevalent not only in adults but also in the pediatric population; it represents one of the most significant risk factors for both microvascular and macrovascular complications. Recent advances have been made in the treatment of T2D. In particular, SGLT2 inhibitors have revolutionized the management of diabetes, offering not only effective glycemic control but also significant cardiovascular and renal benefits demonstrated in adult studies. While numerous studies have evaluated their efficacy and safety in adults, fewer studies have focused on the pediatric population. The recent approval of empagliflozin for children and adolescents with T2D marks a crucial step forward, with initial studies reporting promising results. In the clinical case we presented, the use of empagliflozin enabled us to achieve excellent glycemic control in our 14-year-old patient, a result that had never been reached before with metformin therapy and lifestyle intervention, highlighting its potential in managing this challenging condition. The reported data are consistent with existing literature and further support the applicability of SGLT2 inhibitors in real-world clinical practice. Youth-onset T2D presents a significant clinical challenge due to its rapid progression and increased risk of early complications, underscoring the need for novel and effective therapeutic strategies. This new therapeutic approach with SGLT2 inhibitors could potentially change the course of the disease in young individuals. Further studies are warranted to establish the long-term safety and efficacy of SGLT2 inhibitors in the pediatric age group and to optimize their use in this specific population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
VC: Writing – original draft, Writing – review & editing. EC: Writing – original draft. SS: Conceptualization, Methodology, Writing – review & editing. EP: Conceptualization, Methodology, Writing – review & editing. CPa: Writing – original draft. CPi: Conceptualization, Resources, Writing – review & editing. SD: Resources, Data curation, Writing – original draft. MD: Resources, Data curation, Writing – original draft. FM: Resources, Data curation, Writing – original draft. IR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SGLT2, Sodium-glucose cotransporter-2; T2D, Type 2 diabetes; IDF, International Diabetes Federation; GLP1, Glucagon-like peptide 1; TZD, Thiazolidinediones; DPP4i, Dipeptidyl peptidase-4 inhibitors; AGI, Alpha-glucosidase inhibitors; ADA, American Diabetes Association; EASD, European Association of the Study for Diabetes; EMA, European Medicines Agency; HbA1c, Glycated hemoglobin; GLUT2, Glucose transporter type 2; ACE, Angiotensin-converting enzyme; Ang 1-7, Angiotensin 1-7; DKA, Diabetic ketoacidosis; FDA, Food and Drug Administration; AE, Adverse event; MDI, Multiple daily injections; U, Units; BMI, Body Mass Index
References
1. Magliano DJ, Boyko EJ IDF, and Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS. In: (IDF diabetes atlas), 10th ed. International Diabetes Federation, Brussels (2021). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK581934/.
2. American Diabetes Association Professional Practice Committee, ElSayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes–2024. Diabetes Care. (2024) 47:S145–57. doi: 10.2337/dc24-S008
3. Crawford AL and Laiteerapong N. Type 2 diabetes. Ann Intern Med. (2024) 177:ITC81–96. doi: 10.7326/AITC202406180
4. Hamman RF, Bell RA, Dabelea D, D’Agostino RB, Dolan L, Imperatore G, et al. The SEARCH for diabetes in youth study: rationale, findings, and future directions. Diabetes Care. (2014) 37:3336–44. doi: 10.2337/dc14-0574
5. Titmuss A, Korula S, Wicklow B, and Nadeau KJ. Youth-onset type 2 diabetes: an overview of pathophysiology, prognosis, prevention and management. Curr Diabetes Rep. (2024) 24:183–95. doi: 10.1007/s11892-024-01546-2
6. Taylor R. Understanding the cause of type 2 diabetes. Lancet Diabetes Endocrinology. (2024) 12:664–73. doi: 10.1016/S2213-8587(24)00157-8
7. Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. (2022) 23:872–902. doi: 10.1111/pedi.13409
8. DeMarsilis A, Reddy N, Boutari C, Filippaios A, Sternthal E, Katsiki N, et al. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism. (2022) 137:155332. doi: 10.1016/j.metabol.2022.155332
9. American Diabetes Association Professional Practice Committee, ElSayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2024. Diabetes Care. (2024) :S158–78. doi: 10.2337/dc24-S009
10. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2019) 63:221–8. doi: 10.1007/s00125-019-05039-w
11. Anon. European Medicine Agency. Available online at: https://www.ema.europa.eu/en/homepagehttps://www.ema.europa.eu/en/homepage (Accessed October 9, 2024).
12. Garcia-Ropero A, Badimon JJ, and Santos-Gallego CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicology. (2018) 14:1287–302. doi: 10.1080/17425255.2018.1551877
13. Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. (2015) 54:691–708. doi: 10.1007/s40262-015-0264-4
14. Poulsen SB, Fenton RA, and Rieg T. Sodium-glucose cotransport: current opinion in nephrology and hypertension. Curr Opin Nephrol Hypertens. (2015) 24:463–9. doi: 10.1097/MNH.0000000000000152
15. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. (2015) 66:255–70. doi: 10.1146/annurev-med-051013-110046
16. Nagahisa T and Saisho Y. Cardiorenal protection: potential of SGLT2Inhibitors and GLP-1 receptor agonists in the treatment of type 2Diabetes. Diabetes Ther. (2019) 10:1733–52. doi: 10.1007/s13300-019-00680-5
17. Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium– glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med. (2013) 159:262. doi: 10.7326/0003-4819-159-4-201308200-00007
18. Heerspink HJL, Perkins BA, Fitchett DH, Husain M, and Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. (2016) 134:752–72. doi: 10.1161/CIRCULATIONAHA.116.021887
19. Saisho Y. Changing the concept of type 2 diabetes: beta cell workload hypothesis revisited. EMIDDT. (2019) 19:121–7. doi: 10.2174/1871530318666180821161825
20. Saisho Y. SGLT2 inhibitors: the star in the treatment of type 2 diabetes? Diseases. (2020) 8:14. doi: 10.3390/diseases8020014
21. Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinology. (2022) 10:341–50. doi: 10.1016/S2213-8587(22)00052-3
22. Shehadeh N, Barrett T, Galassetti P, Karlsson C, Monyak J, Iqbal N, et al. Dapagliflozin or saxagliptin in pediatric type 2 diabetes. NEJM Evid. (2023) 2(12):EVIDoa230021. doi: 10.1056/EVIDoa2300210
23. Laffel LMB, Tamborlane WV, Yver A, Simons G, Wu J, Nock V, et al. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: a randomized trial. Diabetes Med. (2018) 35:1096–104. doi: 10.1111/dme.13629
24. McGuire DK, Zinman B, Inzucchi SE, Wanner C, Fitchett D, Anker SD, et al. Effects of empagliflozin on first and recurrent clinical events in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a secondary analysis of the EMPA-REG OUTCOME trial. Lancet Diabetes Endocrinology. (2020) 8:949–59. doi: 10.1016/S2213-8587(20)30344-2
25. Htoo PT, Tesfaye H, Schneeweiss S, Wexler DJ, Everett BM, Glynn RJ, et al. Cardiorenal effectiveness of empagliflozin vs. glucagon-like peptide-1 receptor agonists: final-year results from the EMPRISE study. Cardiovasc Diabetol. (2024) 23:57. doi: 10.1186/s12933-024-02150-0
26. Dos Santos Borges R, Conegundes AF, Haikal de Paula L, Lara Santos R, Alves SN, Amaral Machado R, et al. Efficacy and safety of SGLT2 inhibitors in pediatric patients and young adults: A systematic review and meta-analysis of randomized controlled trials. Pediatr Diabetes. (2024) 22:6295345. doi: 10.1155/2024/6295345
27. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
28. Zheng C, Lin M, Chen Y, Xu H, Yan L, and Dai H. Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. Cardiovasc Diabetol. (2021) 20:83. doi: 10.1186/s12933-021-01272-z
29. Kani R, Watanabe A, Miyamoto Y, Ejiri K, Iwagami M, Takagi H, et al. Comparison of effectiveness among different sodium-glucose cotransoporter-2 inhibitors according to underlying conditions: A network meta-analysis of randomized controlled trials. JAHA. (2024) 13:e031805. doi: 10.1161/JAHA.123.031805
30. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
31. Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, et al. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinology. (2023) 11:169–81. doi: 10.1016/S2213-8587(22)00387-4
Keywords: type 2 diabetes, children, SGLT2 inhibitors, empagliflozin, hyperglycemia
Citation: Castorani V, Chiarle E, Savastio S, Pozzi E, Partenope C, Pignatiello C, Dimarakis S, Demichelis M, Medina F and Rabbone I (2025) Established and emerging use of sodium-glucose cotransporter-2 inhibitors in pediatric population with type 2 diabetes: a case report and literature review. Front. Endocrinol. 16:1629488. doi: 10.3389/fendo.2025.1629488
Received: 15 June 2025; Accepted: 14 August 2025;
Published: 10 September 2025.
Edited by:
Federico Bertuzzi, Niguarda Ca’ Granda Hospital, ItalyReviewed by:
Pratikeswar Panda, Siksha O Anusandhan University, IndiaBasilio Pintaudi, ASST Grande Ospedale Metropolitano Niguarda, Italy
Copyright © 2025 Castorani, Chiarle, Savastio, Pozzi, Partenope, Pignatiello, Dimarakis, Demichelis, Medina and Rabbone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Castorani, Y2FzdG9yYW5pdkBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Valeria Castorani
Valeria Castorani Eleonora Chiarle†
Eleonora Chiarle† Silvia Savastio
Silvia Savastio Cristina Partenope
Cristina Partenope Ivana Rabbone
Ivana Rabbone