- 1Department of Acupuncture, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
- 2Department of Epidemiology and Health Statistics, School of Public Health, Capital Medical University, Beijing, China
- 3Department of Reproductive Medicine, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
- 4Department of Good Clinical Practice, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
- 5Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
Objective: To evaluate the efficacy and safety of the Peiyu Granules (PYG) compared with placebo on early miscarriage rates among women undergoing embryo transfer.
Methods: A double-blind, parallel-group randomized clinical trial between February 15, 2017, and June 17, 2019, within 10 months of pregnancy follow-up until March 2020. This clinical trial was conducted at Beijing Obstetrics and Gynecology Hospital, Capital Medical University. A total of 886 women were included in this study. The intervention group (n = 443) received PYG on the night of Embryo Transfer (ET) until the day of the hCG test. If it was negative, the patient stopped taking medicine. In contrast, the treatment continued until 70 days after ET. The women in the control group (n = 443) consumed the same amount of placebo as the intervention group. All women enrolled were subject to the same follow-up protocols. The primary outcome was early miscarriage rate. The secondary outcomes were clinical intrauterine pregnancy rate and live birth rate.
Results: Among the 886 randomized women (mean [SD] age, 32.8 [3.6] years), 854 women (96.4%) underwent ET and followed the treatment of random grouping. Early miscarriage occurred among 17 of 133 women (12.8%) receiving PYG compared with 35 of 156 women (22.4%) receiving placebo (relative risk[RR], 0.51 [95% CI, 0.27 to 0.95], P = 0.02). Clinical intrauterine pregnancy rates were 30.0% (133 of 443) in the intervention group and 35.2% (156 of 443) in the control group (relative risk[RR], 0.79 [95% CI, 0.60 to 1.05], P = 0.10). Live-birth rates were 25.3% (112 of 443) in the intervention group and 25.7% (114 of 443) in the control group (relative risk[RR], 0.98 [95% CI, 0.72 to 1.32], P = 0.88). Live birth rates in the clinical pregnant population were 84.2% (112/133) in the intervention group and 73.7% (115/156) in the control group (relative risk [RR], 1.14 [95% CI, 1.01 to 1.29], P = 0.03).
Conclusion: The findings suggested that PYG reduced early miscarriage rates among women undergoing embryo transfer. However, there were no significant improvement in clinical pregnancy rates and live birth rates.
Clinical trial registration: https://www.chictr.org.cn/showproj.html?proj=12343 identifier, ChiCTR-inr-16010087.
Introduction
Infertility is a serious disease (1, 2) and a public health problem (3) that is increasingly recognized and considered. Assisted reproductive technology (ART), as one of the important methods to treat infertility, has unsatisfactory success rates, still hovering between 30%-40% (4–6). At present, it is difficult to make a breakthrough in improving the pregnancy outcome of ART with Western medicine alone, so an increasing number of researchers are gradually concerned with traditional Chinese medicine, which is considered a complementary or alternative medicine for infertility treatment (7–10).
Growing evidence has revealed that Chinese herbal medicines (CHMs) can improve pregnancy outcomes among infertile couples undergoing ART treatment (8, 11–14). However, most of them focus on using CHMs before or during in vitro fertilization (IVF) to optimize egg quality (15, 16), ovarian function or endometrial receptivity (17, 18). Until we began this study, only a few studies had focused on the use of CHMs after embryo transfer to reduce the miscarriage rate (13). Infertile couples have a higher frequency of spontaneous abortions than the general population (19). Besides, spontaneous abortion rate of frozen thawed embryo transfer cycle is also high (20, 21). With the implementation of China’s two-child and three-child policies, the age of infertile women in demand for ART is increasing (22, 23). However, early miscarriage rates are high and fertility rates are low in old age group (24, 25). Meanwihle, a high early miscarriage rate seriously threatens the live birth rate of ART. For thousands of years, CHMs used to prevent and treat miscarriage have been popular in Asian countries (26, 27).
Peiyu Granules are a TCM compound granule preparation composed of 19 herbals (Supplementary Table S1). It was created by Professor Zhao Quansong, a highly regarded doctor nationwide. Moreover, PYG has showed great clinical efficacy and safety to prevent and treat miscarriage for decades. Previous clinical trial has demonstrated that PYG significantly upregulates luteinizing hormone and progesterone in patients with threatened miscarriage (28). Further foundational study has revealed that PYG enhances invasion and proliferation capability of human trophoblast cell (29). Grounded on initial clinical findings and experimental evidence, we hypothesize that PYG could decrease the early miscarriage rates among women undergoing embryo transfer, and conducted a large-scale, double-blind, randomized, placebo-controlled trial to evaluate its efficacy and safety.
Methods
Study design
This single-center, randomized, double-blind, placebo-controlled superiority trial was conducted in Beijing Obstetrics and Gynecology Hospital, Capital Medical University. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) Extension for Chinese Herbal Medicine Formulas 2017 reporting guideline.
Ethical approval
The trial was approved by the ethics committee of Beijing Obstetrics and Gynecology Hospital (IEC-C-29-V01.1) under the ethics approval number 2016-KY-082-01. The study protocol was approved by all 12 review committee members who attended the meeting and voted, including 12 independent members and 2 with TCM-related professional backgrounds. All eligible women signed written informed consent.
Participants
We recruited participants who intended to undergo ET from the reproductive center of Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Participants were enrolled from February 15, 2017 to June 17, 2019. Follow-up of live births were completed in April 2020.
The inclusion criteria were 1) women with infertility aged between 22 and 40, and 2) body mass index (calculated as weight in kilograms divided by height in meters squared) of 35 or less.
The exclusion criteria were 1) Women with three or more spontaneous miscarriages; 2) severe endometriosis, including adenomyosis of the uterus and ovarian “chocolate” cysts; 3) anatomical cacogenesis of uterus (eg, uterus unicornis, arcuate uterus, septate uterus, etc); 4) untreated bilateral hydrosalpinx; 5) untreated endometrial diseases (eg, endometritis, endometrial polyp); or 6) diseases unsuitable for ART or pregnancy.
Randomization and masking
The block randomization method was used. The length of the block was 4 and SAS9.4 (SAS Institute Inc., Cary, NC, United States) was used to generate a randomization sequence for 884 subjects (intervention group, control group) according to a 1:1 ratio and list the treatment allocation corresponding to serial numbers 001–884 (that is, a random coding table). The last two patients were assigned to intervention group, control group using a simple randomization method, with the randomization sequence generated by SAS software. After being introduced to the trial procedure, eligible women were randomly divided into either the intervention group or the placebo control group. The randomization sequence was independently generated by a statistician using SAS. To ensure allocation concealment, the subject number, random number and corresponding drug number were obtained only by inputting the eligible women’s birth date into the Central Randomization System for Clinical Research (Web Edition). Investigators, women enrolled, clinical trial coordinators and analysts were blinded to group allocation. The allocation was individually sealed by a statistician in an opaque envelope. Blinding was maintained until statistical analysis was completed. In the event of a medical emergency, unblinding was permitted. The reason and time for unblinding was well documented and initialed by the supervising physician.
Intervention
Women were recruited on the day of ET and randomly assigned in a 1:1 ratio to receive traditional Chinese medicine PYG [a dose of 3 bags (10.1 g/bag), 2 times a day] or matching placebo [a dose of 3 bags (10.1 g/bag), 2 times a day]. PYG and placebo were provided by Jiangyin Tianjiang Medicine Co. Ltd. (China), and their preparations are provided in Supplementary Table S1. The packaging and granules of placebo and PYG are identical in appearance, smelling and taste. Following randomization, women with special prescription for this study were taken to Good Clinical Practice (GCP) pharmacy for PYG or placebo by clinical trial coordinator. The two persons in charge gave the drugs and diary cards to women after checking their basic information and prescription, concurrently informing them of the drugs taking method, matters needing attention and next follow-up time.
The first treatment course was from the night of ET to the day of the hCG test (generally 12–14 days after ET). If it was positive (hCG > 10 miu/ml), the second treatment course started and remained the same until the day of transvaginal ultrasound. Medication was continued until the 70th day after ET if women achieved clinical pregnancy. When a woman was not pregnant after ET, drug treatment and follow-up were terminated. Adherence to medication was monitored by requiring women to return any unused drugs, packages of the drugs and diary cards that recorded the details of daily medications. A trained researcher recorded pregnancy outcomes at the 12th week of gestational age and after birth by follow-up telephone.
Eligible women in both groups received the same western medicine luteal support protocol. Oral progesterone capsules (Zhejiang Xianju Pharmaceutical Co. Ltd., Chinese medicine approval: H20041902, 100 mg each time, twice a day) combined with vaginal progesterone soft capsules (Besins Healthcare Benelux, Imported Drug Registration No. H20160265, Spain, 0.2 g each time, three times a day) from the day of ET until the 10th week of pregnancy.
Sample size calculation
A survey showed that the risk of pregnancy loss among women under 33 years using their own oocytes and freshly fertilized embryos was 22% (30). Combined with the pre-experimental results, it was assumed that Peiyu Granules intervention could reduce the risk of pregnancy loss by half. Pregnancy loss rate decreased from 22% to 11%, requiring 175 clinical pregnancies per group (α error, 0.05; β error,0.2). The clinical pregnancy rate at the Center of Reproductive Medicine of Beijing Obstetrics and Gynecology Hospital, Capital Medical University, was 41.5%. And assuming a 5% dropout rate, the total number of women was 443 for each group.
Outcomes
The primary outcome was early miscarriage, defined as the loss of a viable intrauterine pregnancy up to and including gestational week 12 + 0. Secondary outcomes included clinical intrauterine pregnancy, late miscarriage and live births. Ultrasonography showing an intrauterine sac with or without a fetal heart could be diagnosed as clinical intrauterine pregnancy (31). Late miscarriage was defined as pregnancy loss between 13 and 28 weeks of gestation (32). Live births were defined as the delivery of at least one living infant (33). In addition, women with persistent pregnancy were followed up until delivery. The delivery mode, gestational age and birth weight were all recorded. The adverse events of the intervention were detailed from the beginning to the end of the study.
Statistical analysis
Double-entry is applied by using the ClinResearch Electronic Data Capture (V4.0). Analyses are performed after the data was checked by inspectors. The main outcomes are compared between two groups using chi-square test and risk differences and relative risks with associated 95% Confidence Intervals. Comparison between groups was performed with independent sample t test or Mann-Whitney test, or Pearson chi-square test or fisher’s exact test as appropriate.
The intention-to-treat (ITT) and per-protocol methods were used to analyze primary and secondary end points, as well as safety indexes. ITT analysis was defined as participants who were randomly allocated to intervention and control group, regardless of whether they actually received the treatment or not. Participants who were lost to follow up were counted as no clinical pregnancy in intention-to-treat analysis. Best-case and worst-case sensitivity analysis were conducted on early miscarriage, clinical intrauterine pregnancies and live birth if participants were lost to follow-up. In best-case analysis, unknown events were defined as most favorable assumptions for PYG and least favorable assumptions for placebo. In worst-case analysis, unknown events were defined as least favorable assumptions for PYG and most favorable assumptions for placebo. All Statistical analyses were conducted using the statistical package SPSS, version 26.0 (IBM Corp). Statistical significance was defined as P < 0.05 with two tails.
Results
Enrollment and baseline characteristics of participants
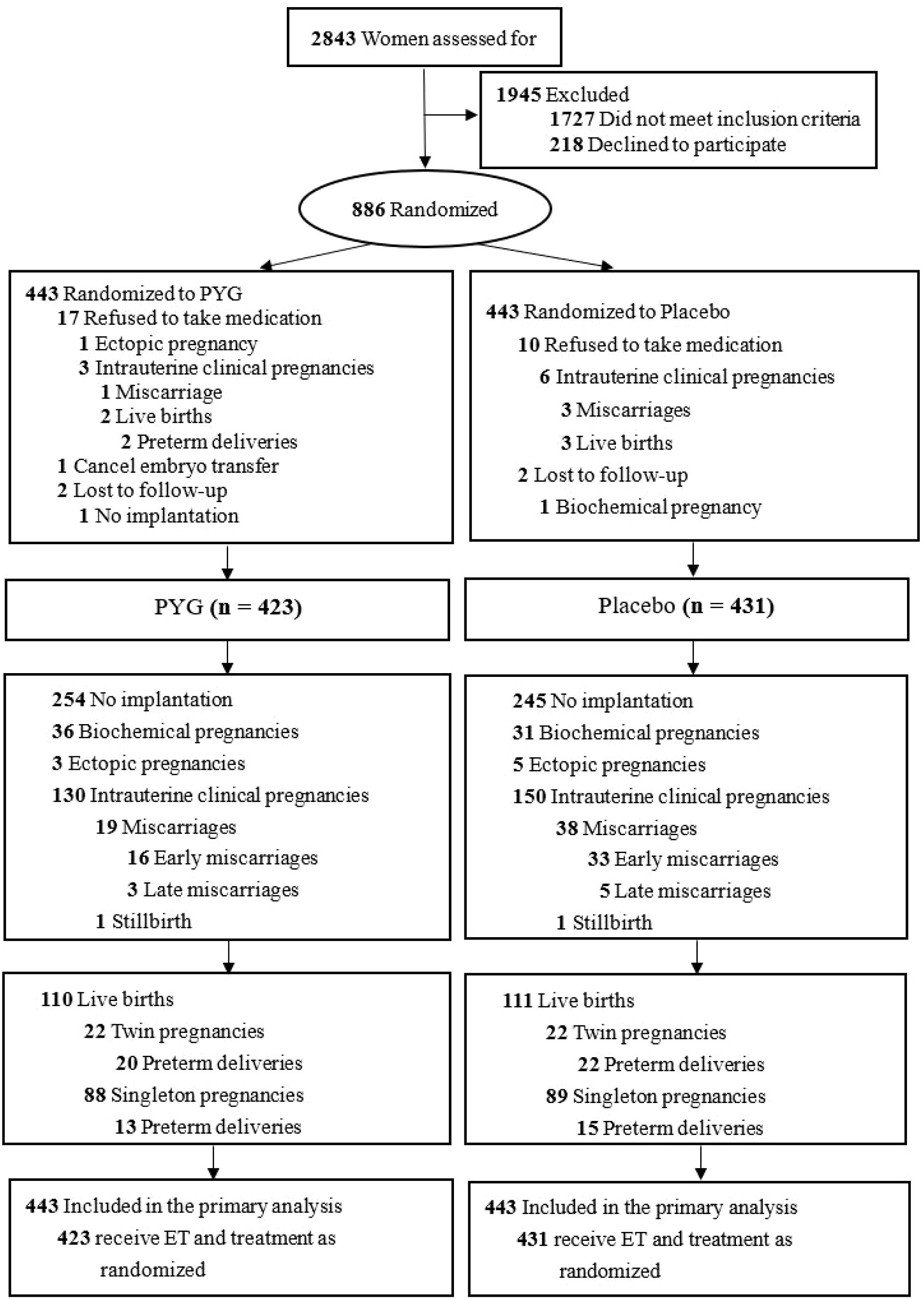
Between February 15, 2017, and June 17, 2019, a total 886 of 2843 eligible women were enrolled and randomly divided into intervention group (n=443) and control group (n=433). The flowchart was showed in Figure 1. After randomization, 32 participants withdrew (20 in intervention group and 12 in control group). Among the 32 withdrawers, 27 participants refused to take medication (17 in intervention group and 10 in control group). 4 participants lost to follow up (2 in intervention group and 2 in control group). 1 participant in intervention group canceled embryo transfer. We collected efficacy and safety outcomes of drop-out participants through medical record reviews or telephone follow-ups. 2 participants (1 in intervention group and 1 in control group) lost to follow-up. Participants who lost to follow up were counted as no clinical pregnancy in intention-to-treat analysis. Finally, 886 participants (433 in intervention group and 433 in control group) comprised the ITT analysis for subsequent analysis (per-protocol set, Supplementary Table S2).
As presented in Table 1, intervention group and control group have similiar baseline characteristics in age, BMI, primary infertility, duration of infertility, previous spontaneous miscarriages, occupation, education background, causes of infertility, follicle-stimulating hormone, luteinizing hormone, estradiol, testosterone, prolactin, progesterone and thyroid-stimulating hormone.
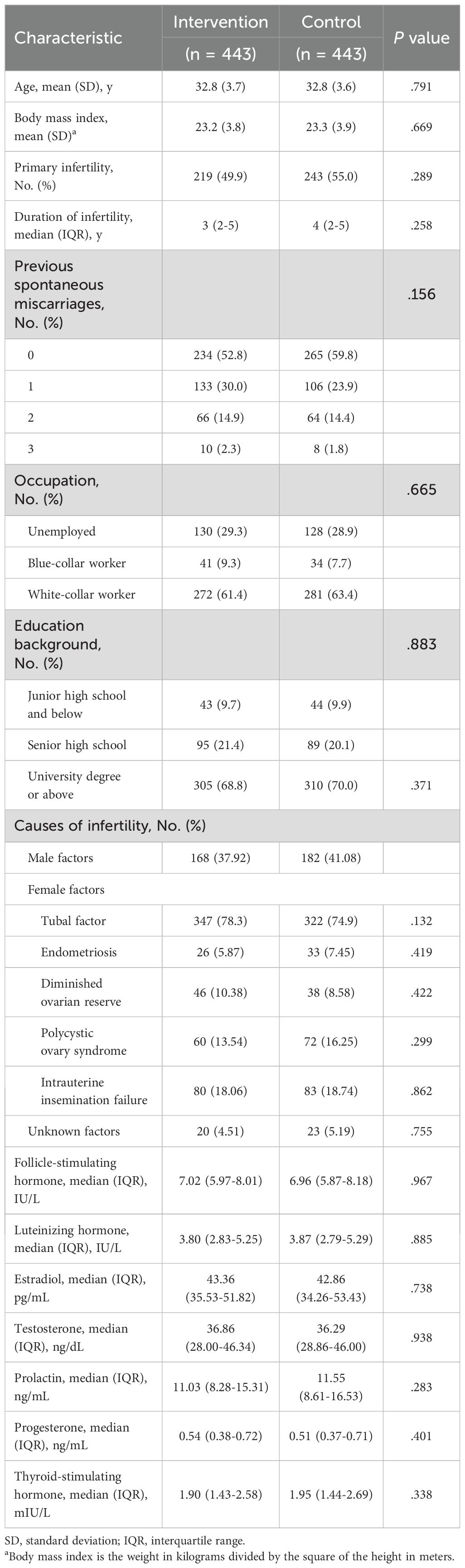
Table 1. Baseline characteristics of the participants in intervention and control groups (intention-to-treat analysis).
Protocols and IVF-ET data in participants
The percentage of fresh and frozen thawed embryo transfer, distribution of protocols for controlled ovarian hyperstimulation, times of ET, stage of embryos transferred and number of transferred embryos did not significantly differ between groups (Table 2, Supplementary Table S3).
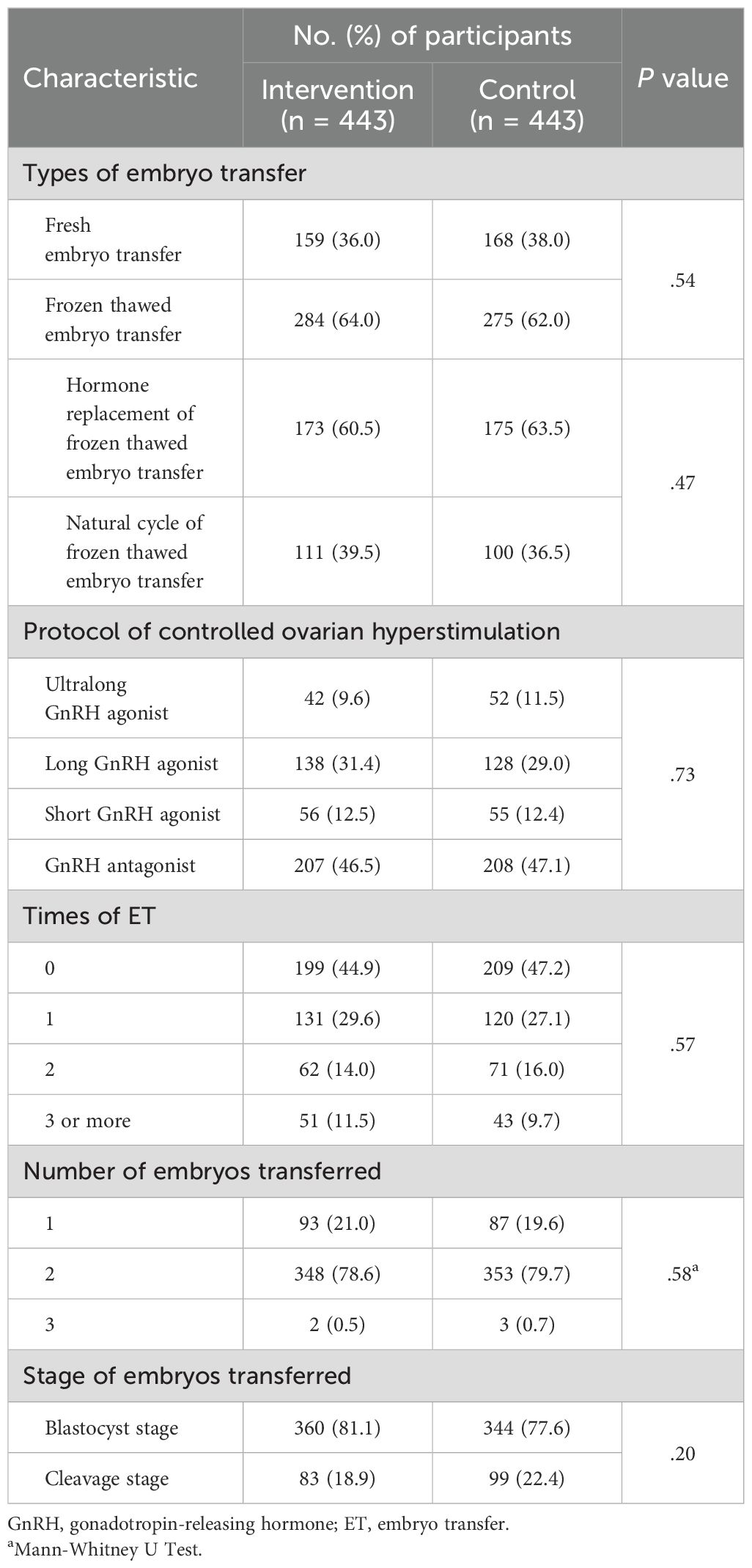
Table 2. Protocols of controlled ovarian hyperstimulation and data of in vitro fertilization and embryo transfer (intention-to-treat analysis).
Primary outcome
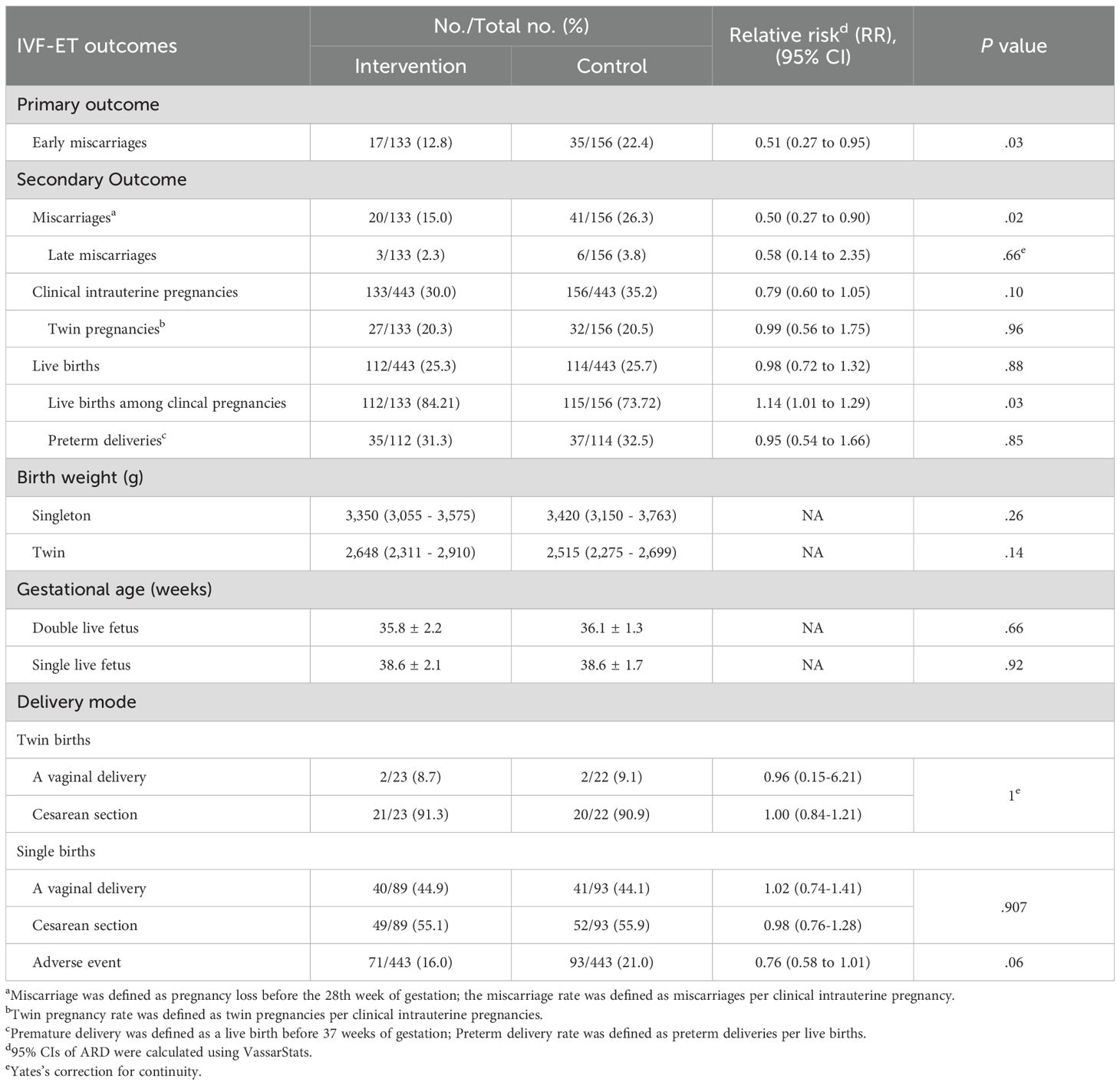
There was significant difference in the early miscarriage between intervention group and control group. As shown in Table 3, the early miscarriage rate was 12.8% (17/133) in intervention group compared with 22.4% (35/156) in the control group according to the intention-to-treat analysis (rate ratio[RR], 0.51[95%CI,0.27 to 0.95]; P=0.03).
Secondary outcomes
As shown in Table 3, there was no significant differece in late miscarriage, clinical intrauterine pregnancy, and live births. The clinical intrauterine pregnancies rate is 30% (133/443) in the intervention group vs 35.2% (156/443) in the control group (rate ratio[RR], 0.79[95%CI,0.60 to 1.05]; P=0.10). The late miscarriage rate is 2.3% (3/133) in the intervention group vs 3.8% (6/156) in the control group (rate ratio[RR], 0.58[95%CI,0.14 to 2.35]; P=0.51). The total live births rate is 25.3% (112/433) in the intervention group vs 25.7% (114/443) in the control group (rate ratio[RR], 0.98[95%CI,0.72 to 1.32]; P=0.88). Live birth rates in the clinical pregnant population were 84.2% (112/133) in the intervention group and 73.7% (115/156) in the control group (relative risk[RR], 1.14 [95% CI, 1.01 to 1.29], P = 0.03). As shown in Table 3 and Supplementary Table S7, a total of 164 women suffered from adverse events, with no significant difference between groups (16.0% vs 21.0%; rate ratio[RR], 0.76 [95% CI, 0.58 to 1.01]).
Post hoc outcomes
The post hoc per-protocol analysis remained consistent with the intention-to-treat analysis (Supplementary Table S4). Meanwhile, not only the best-case sensitivity analysis but also the worst-case sensitivity analysis exhibited uniformity in both intention-to-treat and per-protocol sets (Table 4, Supplementary Table S5).
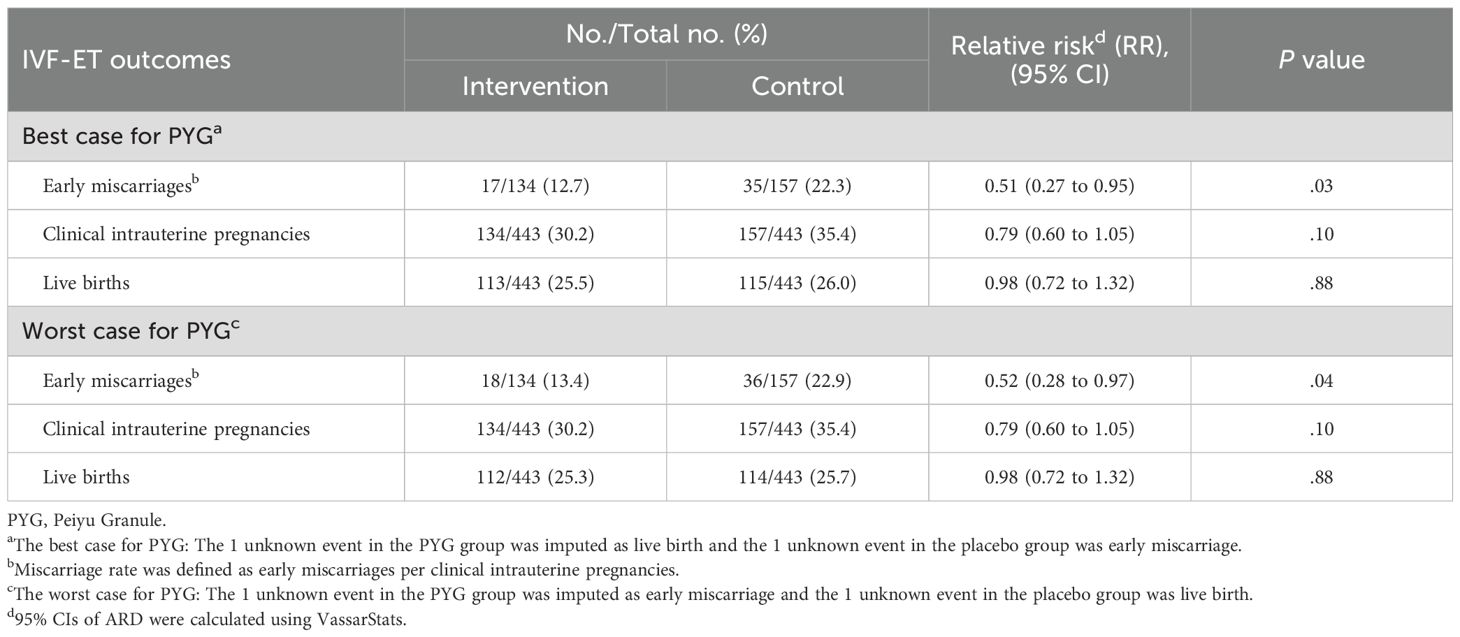
Table 4. Post hoc sensitivity analysis for pregnancy outcomes of the participants (intention-to-treat analysis).
Discussion
In vitro fertilization and embryo transfer (IVF-ET) has developed rapidly since its inception 40 years ago. Recently, it has become one of the most commonly used treatments in infertility. However, the success rate of IVF is still 19% to 22% (34, 35). In order to improve success rate, a growing list of adjuvant treatments are used (36, 37). Previous research suggested that prednisone significantly reduced the biochemical pregnancy loss among women with recurrent implantation failure (38).
To our knowledge, this study was the first large-scale, double-blind, placebo-controlled, randomized clinical trial to investigate CHM as a add-on therapy to reduce early miscarriages among women undergoing IVF-ET. Our findings revealed that PYG significantly reduced the early miscarriage rate compared with placebo. 2 participants (1 in intervention group and 1 in control group) lost to follow-up, and we failed to collect their early miscarriage outcome. To minimize the impact of missing data, best-case and worst-case sensitivity analysis were conducted. In best-case analysis, the 1 unknown event in the PYG group was imputed as live birth and the 1 unknown event in the placebo group was early miscarriage. In worst-case analysis, the 1 unknown event in the PYG group was imputed as early miscarriage and the 1 unknown event in the placebo group was live birth. Best-case and worst-case analysis showed similar results to ITT analysis, suggesting stability and incredible of the result.
However, there was no significant between-group difference in clinical pregnancy and live birth. Despite successful randomization, the placebo group exhibited a higher rate of clinical pregnancies (35.2% vs. 30.0%, absolute difference: 5.2%). As the sample size was large enough, the pearson chi-square test was used to compare between-group difference. Given that RR value included one and P vaule was greater than 0.05, this difference did not reach statistical significance (RR 0.79, 95% CI 0.60-1.05; p=0.10). Additionally, several factors contributing to this difference should be further considered. PYG group had a higher previous miscarriage rate (47.18% vs. 40.18%, absolute difference: 7%), although no significant difference was observed. Recurrent pregnancy loss can be caused by endometrial dysfunction, which might lead to poor clinical pregnancy outcome (39). Additionally, embryo quality is another potential factor associated with clinical pregnancy (40). Mean grade of transferred embryos has proven to be a well-established, independent predictor of clinical pregnancy rate (40). However, variables such as endometrial receptivity, embryo quality were not detected in baseline assessment. Further exploration of this question is planned in our subsequent studies. Multiple previous studies supported our findings that adjuvant treatments (endometrial scratching, acupuncture, etc.) had no significant effect on clinical pregnancy rate and live birth (33, 37, 41).
Another interesting finding was that PYG reduced early miscarriage but failed to improve clinical pregnancy rates. This might be attributed to the consequence that PYG mainly reduce the risk of early miscarriage by improving the endometrial environment and supporting embryonic stability after implantation. However, as it may not directly influence embryo quality or the implantation process itself, its effect on improving the clinical pregnancy rate remains limited. This is further supported by our previous findings that PYG could improve placental function, enhance the invasion and proliferation of villous trophoblast cells to prevent spontaneous abortion in early pregnancy (29). A retrospective study revealed that wenyang huazhuo compound had significant effect on clinical pregnancy, but no significant effect on early miscarriage in women undergoing ET (42). Another clinical trial demonstrated that self-made ovary-nourishing compound increased high-quality embryo rates, but not early miscarriage rates (43). The differences in therapeutic efficacy may be attributed to the fact that different TCM compounds have distinct mechanisms of action. IVF-ET is a complex and multifaceted procedure that requires targeted TCM compounds at different stages to improve the overall success rate.
Increasing the success rate of IVF-ET is a complicated process that has not yet been thoroughly investigated. Early miscarriage is an important factors affecting the outcome of the procedure (24, 34, 35). It is reported the early miscarriage is negatively associated with live birth (44). Comprehensive basic research has demonstrated multiple regulatory effects of PYG on IVF-ET process. Pharmacological studies show that the flavonoid components contained in Semen Cuscutae have an estrogen-like function, which can improve the reproductive endocrine function (45). The flavonoid glycosides in Herba Taxilli have progesterone-like effects and can supplement the endocrine function of patients (46).
We noticed that more participants refused to take the medicine in PYG group than in placebo group. We re-checked the visual, taste and olfactory indexes of placebo, and all three indexed met our requirements (Supplementary 3). Therefore, other contingent factors might result in this difference. Although the dropout rate is low (3.8% vs. 2.3%), related details should be paid more attention to make results more credible and robust.
Although PYG was mainly designed to treat patients with threatened abortion of spleen and kidney deficiency syndrome, its sophisticated formula, which is composed of 19 different herbs, allows them to address a variety of other syndromes. For example, E’jiao and Shanyao possess the function of tonifying Qi and blood; Shudihuang and Heshouwu have the effect of nourishing the liver and kidneys; Qianshi and Sharen are known for their actions in strengthening the spleen and removing dampness. Previous study demonstrated that PYG could enhance invasion and proliferation abilities of human trophoblast cell. Human trophoblast cell plays an important role in early pregnancy (29). Therefore, PYG has its potential effect on conditions beyond spleen-kidney deficiency. Additionally, there is no unified standard for the diagnosis of syndrome types in the population undergoing embryo transfer. RCTs indeed require meticulous design including proper diagnosis. Considering reasons mentioned above, we didn’t add syndrome differentiation into inclusion criteria. However, syndrome differentiation is fundamental principle of TCM practice. Clinical practice guideline or expert consensus for the classification and diagnosis of syndrome differentiation in embryo transfer populations are urgently needed.
Our study has several strengths. First, the IVF-ET protocols are balanced between intervention and control groups, thus excluding potential bias that affected our results Second, sensitivity analysis showed the similar results to the overall findings, indicating the robustness and credibility of each outcome. Third, the trial had a long follow-up duration and period and a high follow-up rate, which allowed for the acquisition of live birth information. Forth, the treatment protocol was formulated by consensus of clinical experts based on optimal practice.
Limitations
First, this study was a single-center randomized clinical study, and generalization of this finding to other patient populations should be used with caution. However, conducting the study at a single center allowed for greater consistency and control over the quality of laboratory procedures and IVF-ET techniques. To provide more robust evidence for the future clinical application of PYG, a multi-center randomized clinical trial will be necessary. Second, all eligible women who underwent ET received the same TCM formula (PYG, applied to treat patients with threatened abortion of spleen and kidney deficiency syndrome), and we did not take the TCM pattern diagnosis using tools such as pulse, tongue, general physical and emotional wellbeing, etc. However, this is indeed a contradiction that RCTs usually utilize a fixed protocol, while proper diagnosis and individualized protocols are fundamental to the basic principles of TCM. This is a challenge, as some form of uniform IVF plan is required. Third, women in the first trimester had difficulty adhering to medication for the special smell and taste of CHM, sometimes even aggravating the pregnancy reaction. Therefore, future research should focus on how to improve CHM dosage forms to ensure patient adherence to medication. Forth, all patients included in this study received a standardized luteal phase support regimen, which is a commonly used protocol in clinical practice to support early pregnancy (47). While the primary aim of our study was to evaluate the independent effects of PYG on improving reproductive outcomes, the potential interaction between PYG and progesterone warrants further investigation. We recognize that the lack of a control group without progesterone supplementation limited our ability to fully compare the independent effects of PYG versus progesterone, or to assess interactions between the two. This was a limitation of the current study design, primarily due to ethical and clinical considerations, as not providing luteal phase support in women undergoing ET would not align with standard care practices. Fifth, the sample size was calculated based on achieving 175 clinical pregnancies per group. However, actual clinical pregnancies were substantially lower (133 in PYG vs. 156 in placebo), which might influence the reliability of primary outcome. According to the published article (48) and pre-experimental results, we assumed that the early miscarriage was 27.27% among women undergoing ET, and 132 clinical pregnancies per group were required. In our study, there were 133 clinical pregnancies in PYG group and 156 clinical pregnancies in placebo group, suggesting that the sample size is enough for primary outcome. Considering long research period and the development of ET, miscarriage rates of ET might decrease as the trial proceeded. Therefore, we chose a conservative miscarriage rate (22%) for sample size calculation.
Conclusion
This trial demonstrated that the PYG reduced the early miscarriage rates among women who underwent ET. However, no beneficial effects were observed in clinical pregnancy and live birth. PYG were shown to be effective and safe, offering a promising intervention for women undergoing IVF-ET to improve success rates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing Obstetrics and Gynecology Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TL: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. DH: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft. XW: Formal analysis, Methodology, Writing – review & editing. YoL: Data curation, Investigation, Resources, Writing – original draft. CJ: Data curation, Formal analysis, Supervision, Writing – original draft. LZ: Conceptualization, Data curation, Investigation, Writing – original draft. YuL: Data curation, Resources, Writing – original draft. YiL: Data curation, Investigation, Writing – original draft. YD: Data curation, Writing – original draft. WY: Formal analysis, Writing – review & editing. RL: Investigation, Writing – original draft. ZL: Data curation, Writing – original draft. CM: Formal analysis, Writing – original draft. DL: Formal analysis, Writing – original draft. YW: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing – review & editing. CY: Conceptualization, Funding acquisition, Investigation, Validation, Writing – review & editing. YM: Data curation, Writing – original draft. XY: Data curation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201511).
Acknowledgments
We would like to thank all the participants enrolled in this trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1631313/full#supplementary-material
References
1. The Practice Committee of the American Society for Reproductive Medicine. Definition of “infertility. Fertil Steril. (2006) 86:S228. doi: 10.1016/j.fertnstert.2006.08.051
2. Practice Committee of the American Society for Reproductive Medicine. Electronic address aao. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. (2020) 113:533–5. doi: 10.1016/j.fertnstert.2019.11.025
3. Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, et al. A public health focus on infertility prevention, detection and management: executive summary. Fertility And Sterility. (2010) 93:16–20. doi: 10.1016/j.fertnstert.2008.09.046
4. Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, and Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. (2015) 313:255–63. doi: 10.1001/jama.2014.17985
5. Toner JP, Coddington CC, Doody K, Van Voorhis B, Seifer DB, Ball GD, et al. Society for Assisted Reproductive Technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril. (2016) 106:541–6. doi: 10.1016/j.fertnstert.2016.05.026
6. Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, et al. ART in Europe, 2017: results generated from European registries by ESHRE. Hum Reprod Open. (2021) 3:hoab026. doi: 10.1093/hropen/hoab026
7. Ried K and Alfred A. Quality of life, coping strategies and support needs of women seeking Traditional Chinese Medicine for infertility and viable pregnancy in Australia: a mixed methods approach. BMC Womens Health. (2013) 13:17. doi: 10.1186/1472-6874-13-17
8. Xia JF, Inagaki Y, Zhang JF, Wang L, and Song PP. Chinese medicine as complementary therapy for female infertility. Chin J Of Integr Med. (2017) 23:245–52. doi: 10.1007/s11655-016-2510-5
9. Lans C, Taylor-Swanson L, and Westfall R. Herbal fertility treatments used in North America from colonial times to 1900, and their potential for improving the success rate of assisted reproductive technology. Reprod biomedicine Soc Online. (2018) 5:60–81. doi: 10.1016/j.rbms.2018.03.001
10. Casale M. Improving the health and treatment success rates of in vitro fertilization patients with traditional Chinese medicine: Need for more robust evidence and innovative approaches. J Integr Med. (2022) 20:187–92. doi: 10.1016/j.joim.2022.02.004
11. Jiang D, Li L, and Zeng BY. Treatment of Chinese herbal medicine for female infertility. Int Rev Neurobiol. (2017) 135:233–47. doi: 10.1016/bs.irn.2017.02.011
12. Hullender Rubin LE, Opsahl MS, Wiemer KE, Mist SD, and Caughey AB. Impact of whole systems traditional Chinese medicine on in-vitro fertilization outcomes. Reprod biomedicine Online. (2015) 30:602–12. doi: 10.1016/j.rbmo.2015.02.005
13. Cao H, Han M, Ng EH, Wu X, Flower A, Lewith G, et al. Can Chinese herbal medicine improve outcomes of in vitro fertilization? A systematic review and meta-analysis of randomized controlled trials. PLoS One. (2013) 8:e81650. doi: 10.1371/journal.pone.0081650
14. Smith JF, Eisenberg ML, Millstein SG, Nachtigall RD, Shindel AW, Wing H, et al. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertil Steril. (2010) 93:2169–74. doi: 10.1016/j.fertnstert.2010.02.054
15. Guo J, Li D, Liu C, Ji X, Li R, and Du X. Effects of Chinese herbs combined with in vitro fertilization and embryo transplantation on infertility: a clinical randomized controlled trial. J traditional Chin Med = Chung i tsa chih ying wen pan. (2014) 34:267–73. doi: 10.1016/s0254-6272(14)60089-3
16. Sun J, Song JY, Dong Y, Xiang S, and Guo Q. Erzhi tiangui granules improve in vitro fertilization outcomes in infertile women with advanced age. Evidence-Based complementary Altern medicine: eCAM. (2021) 2021:9951491. doi: 10.1155/2021/9951491
17. Guo J, Wang LN, and Li D. Exploring the effects of Chinese medicine in improving uterine endometrial blood flow for increasing the successful rate of in vitro fertilization and embryo transfer. Zhong xi yi jie he xue bao = J Chin Integr Med. (2011) 9:1301–6. doi: 10.3736/jcim20111204
18. Li L, Jiang H, Wei X, Geng D, He M, and Du H. Bu Shen Zhu Yun decoction improves endometrial receptivity via VEGFR-2-mediated angiogenesis. Evidence-Based complementary Altern medicine: eCAM. (2019) 2019:3949824. doi: 10.1155/2019/3949824
19. Coulam CB. Association between infertility and spontaneous-abortion. Am J Of Reprod Immunol. (1992) 1927:128–9. doi: 10.1111/j.1600-0897.1992.tb00739.x
20. Pezeshki K, Feldman J, Stein DE, Lobel SM, and Grazi RV. Bleeding and spontaneous abortion after therapy for infertility. Fertility And Sterility. (2000) 74:504–8. doi: 10.1016/s0015-0282(00)00707-x
21. Schieve LA, Tatham L, Peterson HB, Toner J, and Jeng G. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstetrics And Gynecology. (2003) 101:959–67. doi: 10.1016/s0029-7844(03)00121-2
22. Yang S, Jiang Q, and Sánchez-Barricarte JJ. China’s fertility change: an analysis with multiple measures. Population Health metrics. (2022) 20:12. doi: 10.1186/s12963-022-00290-7
23. Tatum M. China’s fertility treatment boom. Lancet (London England). (2020) 396:1622–3. doi: 10.1016/s0140-6736(20)32475-2
24. Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, and Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ (Clinical Res ed). (2000) 320:1708–12. doi: 10.1136/bmj.320.7251.1708
25. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, and Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ (Clinical Res ed). (2019) 364:l869. doi: 10.1136/bmj.l869
26. Yang M, Luo J, Yang Q, and Xu L. Research on the medication rules of Chinese herbal formulas on treatment of threatened abortion. Complementary therapies Clin Pract. (2021) 43:101371. doi: 10.1016/j.ctcp.2021.101371
27. Zeng P, Zhou H, Guo P, Xia W, Huang J, and Zeng Q. Efficacy and safety of traditional Chinese herbal medicine in the treatment of threatened abortion: A protocol for systematic review and meta-analysis. Medicine. (2021) 100:e23288. doi: 10.1097/md.0000000000023288
28. Zhang Y. Effect of Peiyu granules on serum levels of chorionic gonadotropin, progesterone and urinary luteinizing hormone in patients with early threatened miscarriage. Chin J Basic Med Traditional Chin Med. (2010) 16:129–30. doi: 10.19945/j.cnki.issn.1006-3250.2010.02.018
29. Wang JY, Sun LP, Zhao PW, and Liang XY. Influence of the Peiyu particles on invasion and proliferation of human trophoblast cell. Jilin J Traditional Chin Med. (2015) 35:719–22. doi: 10.13463/j.cnki.jlzyy.2015.07.022
30. Farr SL, Schieve LA, and Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol. (2007) 165:1380–8. doi: 10.1093/aje/kwm035
31. Çakar E, Tasan HA, Kumru P, Cogendez E, Usal NT, Kutlu HT, et al. Combined use of oestradiol and progesterone to support luteal phase in antagonist intracytoplasmic sperm injection cycles of normoresponder women: a case-control study. J obstetrics gynaecology. (2020) 40:264–9. doi: 10.1080/01443615.2019.1631765
32. Bromwich P. Late abortion. Br Med J (Clinical Res ed). (1987) 294:527–8. doi: 10.1136/bmj.294.6571.527
33. Smith CA, de Lacey S, Chapman M, Ratcliffe J, Norman RJ, Johnson NP, et al. Effect of acupuncture vs sham acupuncture on live births among women undergoing in vitro fertilization: A randomized clinical trial. JAMA. (2018) 319:1990–8. doi: 10.1001/jama.2018.5336
34. De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum reproduction. (2018) 33:1586–601. doi: 10.1093/humrep/dey242
35. Adamson GD, de Mouzon J, Chambers GM, Hochschild FZ, Mansour R, Ishihara O, et al. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril. (2018) 110:1067–80. doi: 10.1016/j.fertnstert.2018.06.039
36. Kamath MS, Mascarenhas M, Franik S, Liu E, and Sunkara SK. Clinical adjuncts in in vitro fertilization: a growing list. Fertil Steril. (2019) 112:978–86. doi: 10.1016/j.fertnstert.2019.09.019
37. Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: A randomized clinical trial. JAMA. (2017) 318:2190–8. doi: 10.1001/jama.2017.18249
38. Sun Y, Cui L, Lu Y, Tang J, Dong X, Ni T, et al. Prednisone vs placebo and live birth in patients with recurrent implantation failure undergoing in vitro fertilization: A randomized clinical trial. JAMA. (2023) 329:1460–8. doi: 10.1001/jama.2023.5302
39. Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, and Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. (2020) 6:98. doi: 10.1038/s41572-020-00228-z
40. Berger DS, Zapantis A, Merhi Z, Younger J, Polotsky AJ, and Jindal SK. Embryo quality but not pronuclear score is associated with clinical pregnancy following IVF. J Assist Reprod Genet. (2014) 31:279–83. doi: 10.1007/s10815-013-0162-3
41. Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med. (2019) 380:325–34. doi: 10.1056/NEJMoa1808737
42. Hu Z, Zhang Y, Song J, and Xia T. Effect of Wenyang Huazhuo Compound on pregnancy outcomes in frozen-thawed embryo transfer cycle of patients with repeated implantation failure. Chin J Integrated Traditional Western Med. (2020) 40:1038–41. doi: 10.7661/j.cjim.20200119.138
43. Yao Y, Li J, Wang X, and Zhang L. Clinical observation of the effect of self-formulated ovary-nourishing prescription on IVF-ET outcomes in patients with diminished ovarian reserve and kidney deficiency and blood stasis syndrome. Hunan J Traditional Chin Med. (2021) 37:12–5.
44. Savukoski SM and Niinimäki M. Choice of treatment to manage early miscarriage does not affect future fertility. Fertil Steril. (2021) 115:76–7. doi: 10.1016/j.fertnstert.2020.09.137
45. Meng N, Xu S, Lyu W, Bian W, Wang Q, and Lin X. Research progress on pharmacological mechanism of flavonoids in Cuscutae Semen in the treatment of infertility. Global Traditional Chin Med. (2025) 18:857–62. doi: 10.3969/j.issn.1674-1749.2025.04.035
46. Liu YJ, Huang GY, Yang MW, Gong P, and Lu F. Effect of jiantai liquid on expression of estrogen and progesterone receptors in uterus of mice with embryo implantation dysfunction induced by mifepristone. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chin J integrated traditional Western Med. (2004) 24:816–9.
47. Zaat TR, Kostova EB, Korsen P, Showell MG, Mol F, and van Wely M. Obstetric and neonatal outcomes after natural versus artificial cycle frozen embryo transfer and the role of luteal phase support: a systematic review and meta-analysis. Hum Reprod Update. (2023) 29:634–54. doi: 10.1093/humupd/dmad011
Keywords: Peiyu granules, early miscarriage, embryo transfer, RCT - randomized controlled trial, in vitro fertilization (IVF)
Citation: He D, Lyu T, Wang X, Ma Y, Lan Y, Yang X, Jia C, Zhou L, Liang Y, Li Y, Dai Y, Yue W, Liu R, Liu Z, Ma C, Liu D, Wu Y and Yin C (2025) Effect of the Peiyu granules on early miscarriage among women undergoing embryo transfer: a randomized, double-blind, placebo-controlled trial. Front. Endocrinol. 16:1631313. doi: 10.3389/fendo.2025.1631313
Received: 19 May 2025; Accepted: 12 August 2025;
Published: 09 September 2025.
Edited by:
Lihua Yang, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Chenglian Feng, Chinese Research Academy of Environmental Sciences, ChinaHongxiu Liu, Huazhong University of Science and Technology, China
Copyright © 2025 He, Lyu, Wang, Ma, Lan, Yang, Jia, Zhou, Liang, Li, Dai, Yue, Liu, Liu, Ma, Liu, Wu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenghong Yin, eWluY2hoQGNjbXUuZWR1LmNu; Ying Wu, d3V5aW5nMTUwNUBjY211LmVkdS5jbg==
†These authors have contributed equally to this work
 Dandan He
Dandan He Tianyi Lyu
Tianyi Lyu Xiaonan Wang2†
Xiaonan Wang2† Wentao Yue
Wentao Yue Ruixia Liu
Ruixia Liu Dan Liu
Dan Liu Ying Wu
Ying Wu Chenghong Yin
Chenghong Yin