- 1Patanjali Herbal Research Department, Patanjali Research Foundation, Haridwar, India
- 2Institute of Pharmaceutical Research, GLA University, Mathura, India
- 3Department of Pharmaceutical Sciences, Gurukul Kangri (Deemed to be University), Haridwar, India
- 4Department of Drug Discovery and Development, Harrison College of Pharmacy, Auburn University, Auburn, AL, United States
- 5Department of Yoga Science, University of Patanjali, Haridwar, India
- 6Department of Biotechnology and Bioinformatics, Academy of Higher Education and Research, Mysore, India
- 7School of Pharmaceutical Sciences, MVN University, Palwal, India
Polycystic Ovarian Syndrome (PCOS) is a complex endocrine and metabolic disorder affecting women of reproductive age, characterized by hyperandrogenism, insulin resistance, chronic inflammation, and ovulatory dysfunction. Conventional therapies, such as oral contraceptives, insulin sensitizers, and anti-androgens, primarily offer symptomatic relief and are often associated with chronic adverse effects, underscoring the need for safer and more holistic alternatives. Naturally occurring bioactives have emerged as promising adjunct or alternative therapeutic agents in this context. This review critically examines the therapeutic potential of two phytochemicals or natural bioactives, apigenin and ellagic acid, in the integrative management of PCOS and its associated metabolic disturbances and comorbidities. Apigenin, a flavonoid abundantly present in parsley, chamomile, and citrus fruits, and ellagic acid, a polyphenol found in pomegranates and berries, both demonstrate significant anti-inflammatory, antioxidant, insulin-sensitizing, and anti-androgenic activities. Mechanistic studies reveal their ability to regulate ovarian steroidogenesis, suppress pro-inflammatory cytokines, improve insulin sensitivity via the PI3K/Akt signaling pathway, and reduce hyperandrogenism by inhibiting 5α-reductase. Preclinical and preliminary clinical studies support the efficacy of these treatments in restoring ovarian morphology, normalizing hormonal profiles, and ameliorating metabolic dysfunctions in PCOS models. Although limited by poor bioavailability, both compounds exhibit favorable safety and metabolic profiles, and emerging formulation approaches such as nano-delivery systems, phytosomes, and liposomes offer promising strategies to enhance their clinical applicability. This review advocates incorporating apigenin and ellagic acid into integrative PCOS treatment strategies. It highlights the need for well-designed clinical trials to validate efficacy, establish standardized dosing, and develop advanced delivery systems.
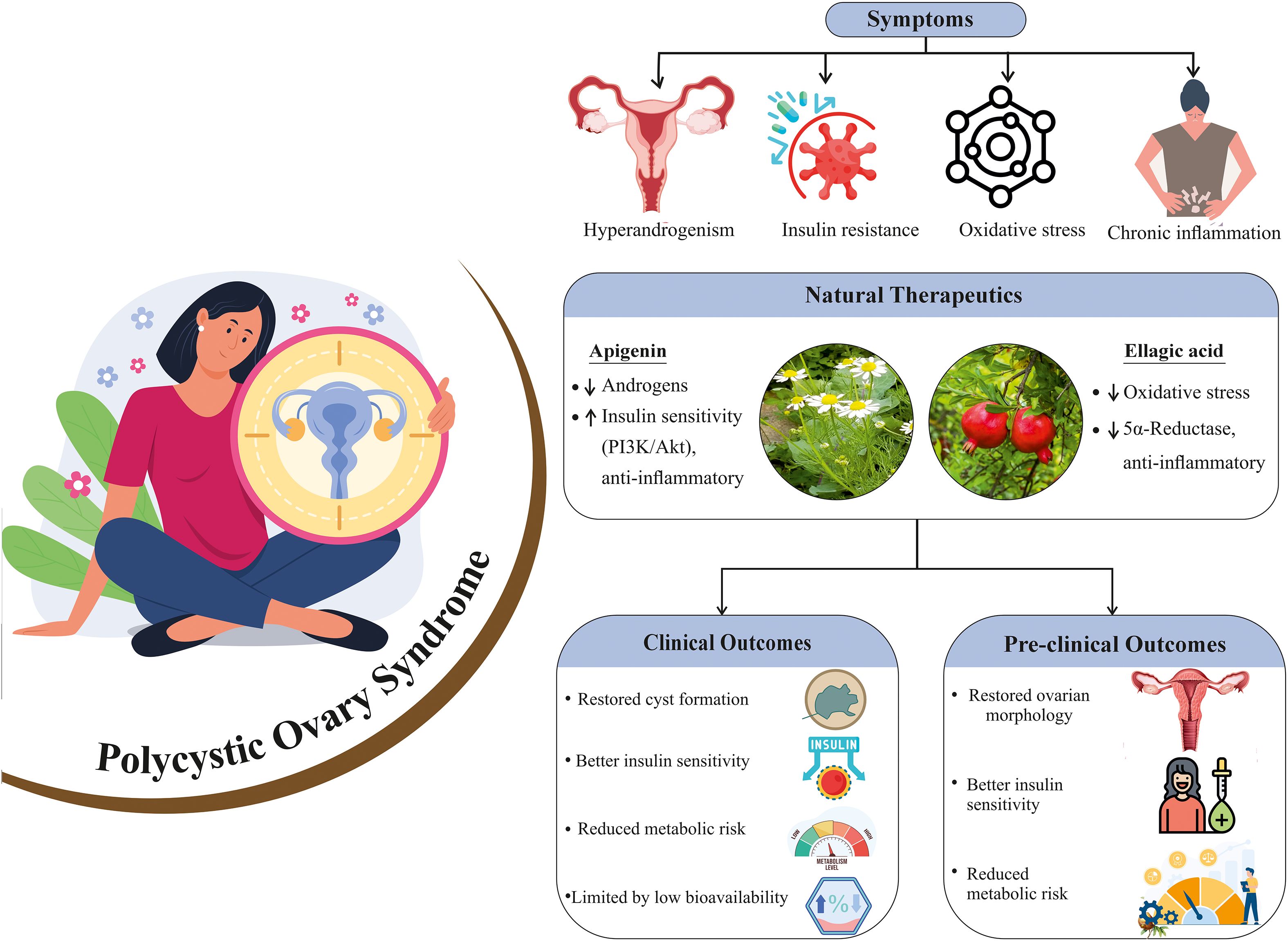
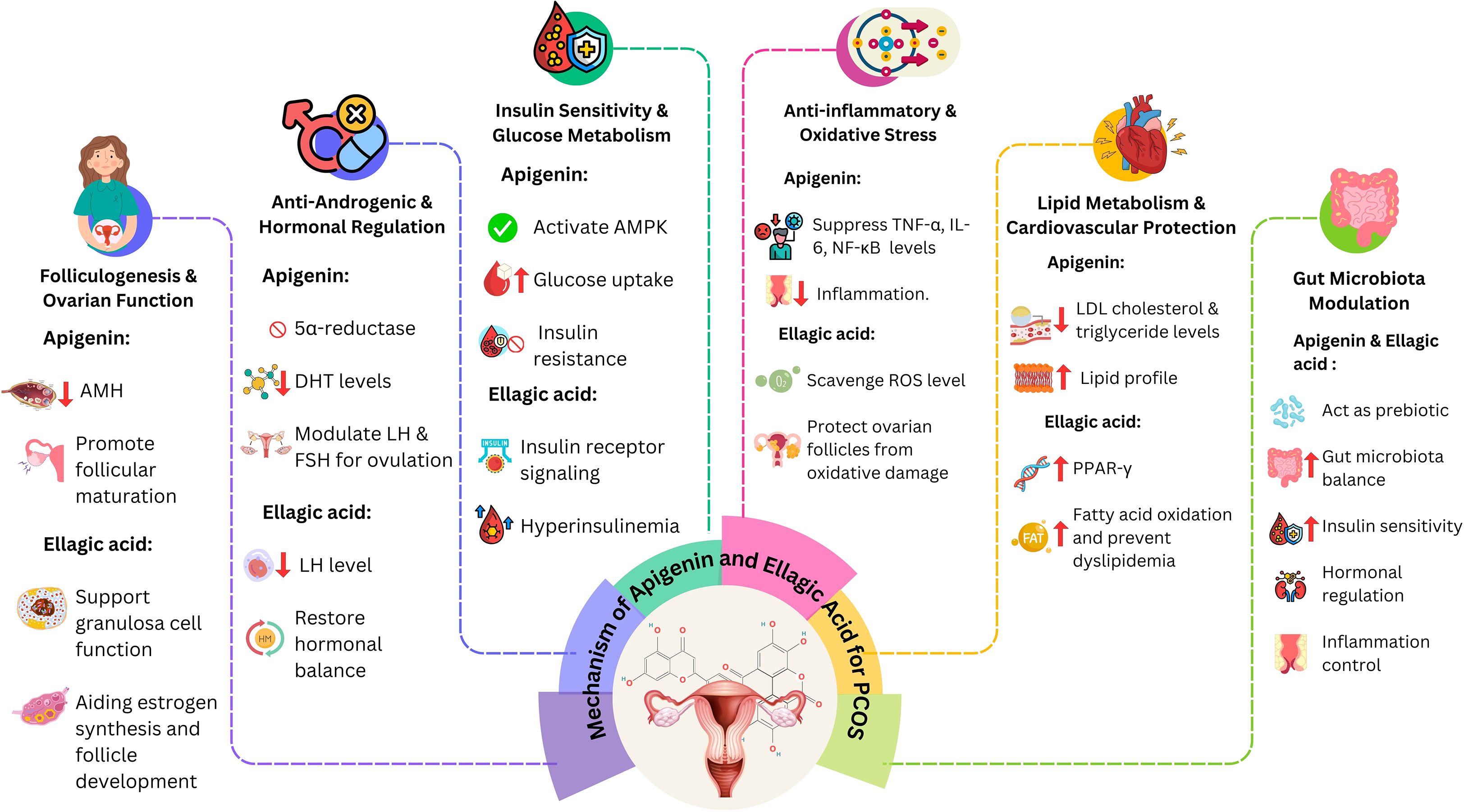
Graphical Abstract. This graphical abstract represents an overview of PCOS pathology and the therapeutic potential of natural compounds apigenin and ellagic acid, highlighting their effects on androgen levels, insulin sensitivity, oxidative stress, and inflammation, with associated clinical and pre-clinical outcomes.
1 Background
Polycystic ovary syndrome (PCOS) is a prevalent endocrine and metabolic disorder affecting 4–20% of women of reproductive age worldwide, depending on diagnostic criteria (1). It is a complex condition marked by hormonal imbalance, insulin resistance, chronic low-grade inflammation, and reproductive dysfunction (2). These mechanisms underlie its clinical manifestations, which include menstrual irregularities, hyperandrogenism (hirsutism, acne, alopecia), anovulation, infertility, obesity, dyslipidemia, and type 2 diabetes (3). Due to its heterogeneous nature, PCOS presents diagnostic challenges and requires multidimensional management. Conventional treatments—oral contraceptives, metformin, and anti-androgens—remain first-line options but primarily manage symptoms without addressing root causes. Their use is further limited by adverse effects, hypersensitivity, and long-term dependency risks (4). For instance, spironolactone and metformin improve insulin resistance but cause side effects (5), while metformin may induce ovarian histological alterations (6). A meta-analysis also linked standard therapies to gastrointestinal intolerance, menstrual irregularities, and hepatotoxicity (7). These limitations have driven interest in safer, holistic alternatives, particularly natural bioactives with antioxidant, anti-inflammatory, and hormone-modulating properties (8). Evidence supports the efficacy of phytochemicals and medicinal plants (9), such as Mentha spicata, which improves hormonal balance and folliculogenesis (10). Among natural compounds, apigenin and ellagic acid have emerged as promising candidates (11). Apigenin, a flavonoid abundant in parsley, chamomile, celery, and citrus fruits, shows antioxidant, anti-inflammatory, anti-androgenic, and insulin-sensitizing properties (12). Preclinical studies report its ability to modulate ovarian steroidogenesis, enhance insulin sensitivity, and restore reproductive-metabolic balance (13). Similarly, ellagic acid, found in pomegranates, berries, nuts, and grapes, exerts insulin-sensitizing, anti-inflammatory, and anti-hyperandrogenic effects, while also improving ovarian function (14, 15). Both compounds reduce oxidative stress and key metabolic disturbances, potentially lowering long-term risks such as cardiovascular disease, hyperglycemia, and infertility. Beyond health implications, PCOS imposes a significant socioeconomic burden. Women often face reduced quality of life due to infertility, hirsutism, acne, obesity, and associated psychological distress, including anxiety and depression (16). These challenges increase healthcare utilization and treatment costs for metabolic comorbidities and infertility. In India, high prevalence is compounded by underdiagnosis, delayed care, and limited specialized infrastructure (17, 18). Globally, undiagnosed cases further magnify the burden (19). Given these concerns, exploring safe, effective, and targeted therapies is imperative. Natural bioactives such as apigenin and ellagic acid offer promising complementary or adjunct strategies to conventional treatments. This review evaluates their pharmacological properties, preclinical and clinical evidence, and integration potential into holistic treatment approaches, highlighting their role in improving reproductive, metabolic, and overall health outcomes in PCOS.
2 Methods
2.1 Search strategy and databases
A structured literature search was conducted across PubMed and Google Scholar to identify studies examining the therapeutic role of apigenin and ellagic acid in polycystic ovarian syndrome (PCOS). The search covered the period January 1999 to August 2025, corresponding to the timeframe of submission of this manuscript. The start year 1999 was selected as it marks the period following the widespread adoption of the Rotterdam and NIH criteria for PCOS diagnosis, which provided greater consistency in defining study populations. This timeframe also captures the rise of phytochemical and polyphenol research in endocrine and metabolic disorders, ensuring the inclusion of both foundational and contemporary evidence.
2.2 Search terms
We used controlled vocabulary and free-text terms in various combinations, including: “PCOS,” “polycystic ovary syndrome,” “apigenin,” “ellagic acid,” “flavonoids,” “polyphenols,” “natural bioactives,” “antioxidant,” “anti-inflammatory,” and “insulin sensitizer.” Boolean operators (AND/OR) were applied to refine the search.
2.3 Eligibility criteria
Inclusion: Preclinical (in vitro and in vivo) and clinical studies evaluating apigenin and/or ellagic acid in PCOS or related metabolic/endocrine dysfunctions; studies published in English; peer-reviewed full-text articles. Exclusion: Duplicates, conference abstracts without full data, editorials, commentaries, and studies not directly related to PCOS.
2.4 Study selection
The initial search (January 1999–August 2025) yielded 18,200 citations; after duplicate removal, 17,000 unique records remained. Titles and abstracts were screened independently by two reviewers, leading to 780 full-text articles retrieved for detailed evaluation. After applying eligibility criteria and excluding studies with methodological limitations or insufficient relevance, 107 articles were included in this review.
2.5 Quality appraisal
Although this is primarily a narrative review, the included studies were evaluated for methodological quality. Preclinical studies were assessed for reproducibility of models, dosing regimens, and outcome measures, while clinical studies were examined for study design (randomized, controlled, blinded), sample size, and risk of bias. The review was conducted under the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, where applicable, to ensure transparency and reproducibility. The search cutoff was August 2025, corresponding to the manuscript submission date, to ensure inclusion of the most up-to-date available evidence.
3 Global burdens of PCOS
The global prevalence of PCOS varies considerably by region, influenced by genetic predispositions, lifestyle and environmental factors, and the efficiency of healthcare delivery systems. A 2019 global survey highlighted marked geographic disparities, with the highest prevalence reported in countries such as Italy, Japan, and New Zealand, and the lowest in Bosnia, Albania, and North Macedonia (20). In South Asia, particularly in the Indian subcontinent, PCOS has emerged as a major public health challenge, with reported prevalence rates ranging from 9% to 22% among women of reproductive age (21). The burden is particularly pronounced in urban and semi-urban regions, where rapid transitions in lifestyle, characterized by poor dietary patterns, physical inactivity, obesity, and chronic psychosocial stress, are prevalent. Current estimates suggest that up to 20% of Indian women may be affected by PCOS, although underdiagnosis remains widespread due to low awareness, cultural stigma, and limited access to specialized care.
PCOS is among the most prevalent endocrine disorders in women, affecting approximately 8–13% of women of reproductive age worldwide (22). Alarmingly, up to 70% of cases remain undiagnosed, largely due to the lack of uniform diagnostic criteria, limited public awareness, and disparities in access to healthcare services (19). The choice of diagnostic criteria—NIH (1990), Rotterdam (2003), or AES (2006)—substantially influences reported prevalence. The NIH criteria are more restrictive, requiring both hyperandrogenism and oligo-anovulation, typically yielding lower prevalence rates (around 6–9%). The Rotterdam criteria, which require any two of three features (hyperandrogenism, oligo-anovulation, or polycystic ovarian morphology), generate higher prevalence estimates (up to 20%). The AES criteria, which prioritize hyperandrogenism, fall between these two approaches. This diagnostic variability complicates comparisons across populations and highlights the importance of standardized definitions for epidemiological surveillance. PCOS significantly impacts both reproductive and metabolic health across a broad age spectrum. Reproductive manifestations typically include infertility, menstrual irregularities, and hirsutism, while long-term complications often involve type 2 diabetes mellitus, cardiovascular disease, and an increased risk of endometrial cancer (23). In adolescents, early signs such as acne, irregular menstruation, and weight gain are frequently overlooked or misattributed, contributing to delays in diagnosis and intervention. The psychological burden of PCOS is also considerable. In sociocultural contexts such as India—where fertility and physical appearance are closely tied to identity—many women experience psychological distress, including anxiety, depression, low self-esteem, and body image dissatisfaction (16). Beyond health and psychological consequences, PCOS also imposes substantial economic costs. In the United States, the annual healthcare expenditure related to PCOS, including diagnosis, management of infertility, and treatment of comorbidities, has been estimated at more than USD 4 billion (24). Indirect costs such as reduced productivity, absenteeism, and long-term management of diabetes and cardiovascular disease further add to this economic burden. Emerging evidence also implicates environmental stressors, particularly endocrine-disrupting chemicals (EDCs) found in industrial pollutants, plastics, and pesticides, in exacerbating hormonal imbalances and ovarian dysfunction. These effects are especially pronounced in urban and industrialized settings (25). The use of varying diagnostic criteria, including those proposed by the Rotterdam consensus, the National Institutes of Health (NIH), and the Androgen Excess and PCOS Society (AES), further complicates efforts to standardize prevalence estimates across and within populations. This diagnostic variability hinders epidemiological surveillance and delays the formulation of targeted healthcare strategies. Addressing the multifaceted burden of PCOS necessitates a comprehensive and integrative approach. Standardized diagnostic protocols, enhanced public education, and equitable access to reproductive and metabolic healthcare, particularly in underserved rural regions, are critical priorities (18). Lifestyle interventions focusing on balanced nutrition, physical activity, and stress management remain cornerstone strategies in PCOS prevention and management (26). Integrating PCOS screening, education, and awareness campaigns into primary healthcare systems and school-based health programs could significantly improve early detection and long-term outcomes. Moreover, large-scale, population-based epidemiological studies are essential to refine global and regional prevalence estimates and to inform evidence-based policy development aimed at improving the health and quality of life of women with PCOS worldwide (17).
4 Pathobiology of PCOS
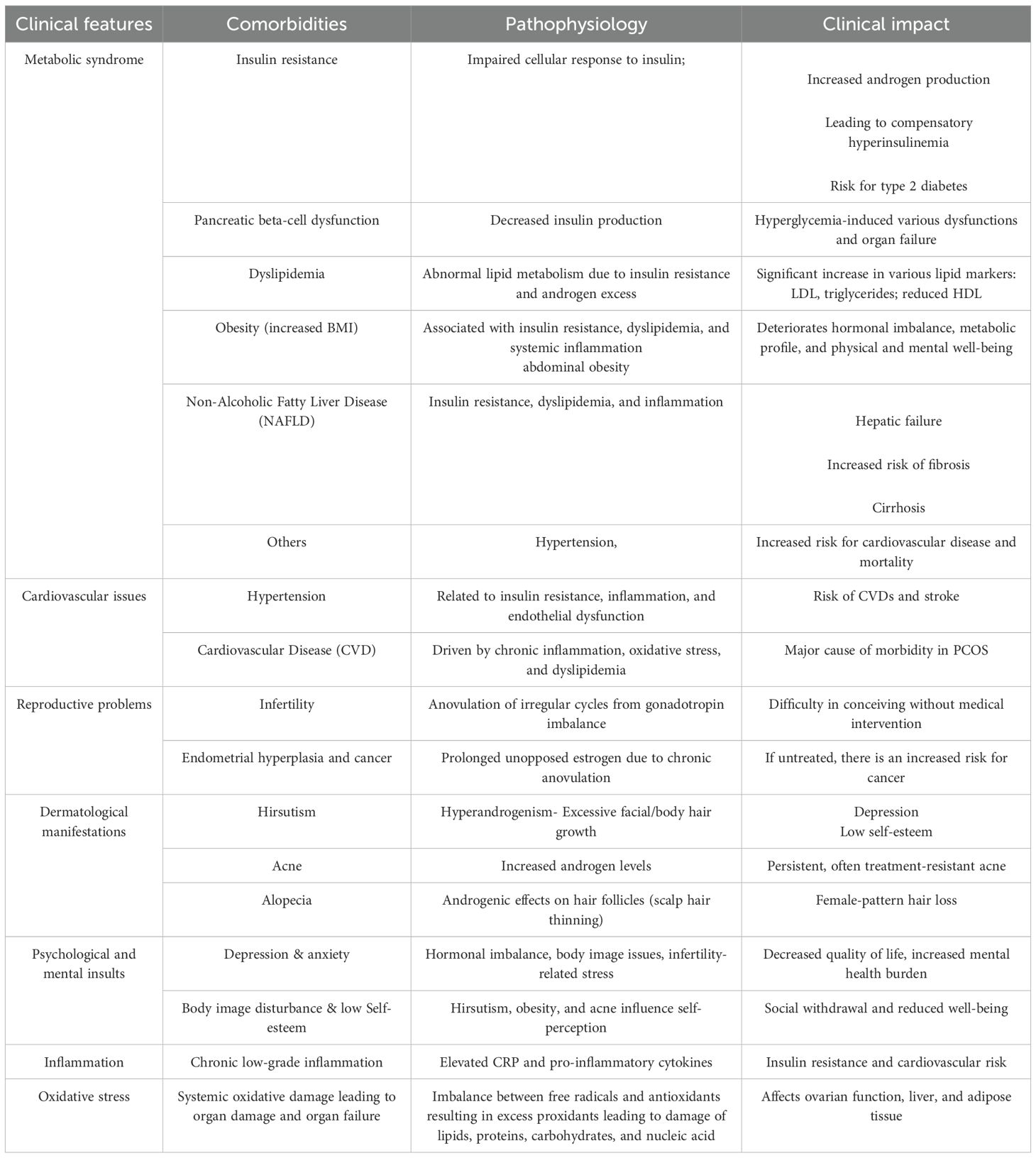
PCOS is a complex, multifactorial endocrine disorder in women, arising from the interplay of genetic susceptibility, environmental exposures, and modifiable lifestyle factors such as diet and physical activity. However, the precise etiology remains elusive; both hereditary influences and lifestyle-related contributors, including obesity and physical inactivity, are recognized as key determinants in the onset and progression of the syndrome (3). The pathophysiology of PCOS is principally marked by hormonal and metabolic dysregulation. Hyperandrogenism, a clinical hallmark feature of PCOS, is characterized by elevated circulating levels of androgens, which contribute to clinical manifestations such as hirsutism, acne, and androgenic alopecia (27), as given in Table 1. Another pivotal endocrine abnormality involves altered gonadotropin secretion, particularly an increased luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio. This imbalance disrupts normal folliculogenesis, resulting in anovulation and menstrual irregularities. It also contributes to the formation of multiple small, fluid-filled follicles or “cysts” within the ovaries, leading to the characteristic polycystic ovarian morphology observed on ultrasonography. Insulin resistance is another critical component of PCOS pathogenesis (28). In affected individuals, peripheral tissues exhibit diminished sensitivity to insulin, prompting compensatory hyperinsulinemia. Elevated insulin levels exacerbate hyperandrogenism by stimulating ovarian theca cells to produce excess androgens, thereby intensifying endocrine dysfunction (29). Diagnostic criteria for PCOS—including those from the NIH (1990), Rotterdam consensus (2003), and the Androgen Excess and PCOS Society (2006)—differ in their requirements and thereby influence prevalence estimates. A detailed discussion of how these criteria affect global prevalence is provided in Section 3. Moreover, insulin resistance significantly elevates the risk of metabolic sequelae, including impaired glucose tolerance and type 2 diabetes mellitus. Obesity, particularly central or abdominal obesity, is frequently coexistent with PCOS and further amplifies insulin resistance and its associated reproductive and metabolic derangements. Clinically, PCOS presents with a broad spectrum of signs and symptoms, encompassing reproductive (e.g., anovulation, menstrual irregularity, infertility), dermatological (e.g., hirsutism, acne, alopecia), and metabolic (e.g., weight gain, dyslipidemia, insulin resistance) disturbances. Chronic health implications include an increased risk of type 2 diabetes, cardiovascular disease, and endometrial hyperplasia or cancer (30). In addition to hormonal and metabolic abnormalities, chronic low-grade inflammation and oxidative stress have been increasingly recognized as central to the pathophysiology of PCOS. Elevated levels of inflammatory markers, such as C-reactive protein (CRP), suggest a persistent pro-inflammatory state, which is thought to further impair insulin signaling and elevate cardiovascular risk (31). Oxidative stress is an imbalance between reactive oxygen species and antioxidant defenses, exacerbates ovarian dysfunction, and may contribute to systemic metabolic impairment, including effects on hepatic and adipose tissue function (32). The convergence of hormonal, metabolic, inflammatory, and oxidative mechanisms creates a self-reinforcing cycle that complicates disease progression and therapeutic management. Consequently, the effective management of PCOS necessitates a comprehensive and individualized approach. This typically involves lifestyle interventions such as dietary modification, physical activity, and weight management, pharmacologic therapies, and, where appropriate, complementary and non-pharmacologic treatments. Psychological support is also essential, given the emotional and mental health challenges frequently experienced by women with PCOS. Thus, due to its heterogeneous presentation and chronic course, PCOS often requires coordinated, multidisciplinary care tailored to the unique needs and goals of each patient (3).
The diagnosis of PCOS poses inherent challenges due to its diverse clinical manifestations and the overlap of its symptoms with other prevalent endocrine disorders. Over the past few decades, various diagnostic frameworks have been established, with the most widely adopted being the 1990 National Institutes of Health (NIH) criteria, the 2003 Rotterdam Consensus, and the 2006 Androgen Excess and PCOS Society (AES) criteria. The discrepancies among these guidelines have contributed to considerable variations in reported prevalence and clinical diagnoses across different populations and research studies. According to the NIH criteria, a PCOS diagnosis necessitates the presence of both chronic anovulation and clinical or biochemical evidence of hyperandrogenism, after excluding other underlying causes. The Rotterdam criteria, supported by the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM), broadened the diagnostic scope by requiring any two of the following three features: oligo- or anovulation, clinical or biochemical signs of hyperandrogenism, and polycystic ovarian morphology observed via ultrasonography, again following the exclusion of alternative diagnoses. Conversely, the AES criteria prioritize hyperandrogenism as a fundamental diagnostic criterion, requiring either oligo-anovulation or polycystic ovarian morphology in addition. Scientifically, Polycystic ovarian morphology is typically characterized by the presence of 12 or more small follicles (2–9 mm in diameter) in each ovary or an increased ovarian volume exceeding 10 cm³. However, recent advancements in high-resolution ultrasound technology suggest refining these thresholds to 20 or more follicles per ovary (27). It’s crucial to recognize that ovarian morphology alone is not definitively indicative of PCOS and can be observed in up to one-third of healthy women without any clinical symptoms, particularly in adolescent and young adult populations.
Laboratory assessment in suspected PCOS involves measuring total and free testosterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), and 17-hydroxyprogesterone to evaluate for hyperandrogenism and rule out late-onset congenital adrenal hyperplasia. Serum LH and FSH levels may reveal an elevated LH/FSH ratio, although this is not a mandatory diagnostic marker. Further evaluations, such as fasting glucose and insulin levels, lipid profile, and oral glucose tolerance testing, may be indicated, especially in individuals who are overweight or obese, to screen for associated metabolic abnormalities (3). Caution is warranted when diagnosing PCOS in adolescents, as physiological anovulation and acne are common during this developmental stage. Current recommendations for this age group emphasize the need for both persistent hyperandrogenism and menstrual irregularities extending beyond two years post-menarche for a presumptive diagnosis, with less emphasis placed on ultrasonography due to the frequent occurrence of multicystic ovaries in this population (28).
5 Integrative potential of natural bioactives in complementary therapeutics
Conventional medical treatments for PCOS primarily aim at symptom management rather than targeting the underlying pathophysiology of the disorder. In recent years, natural bioactives derived from plants, microbes, and animals have gained increasing attention as complementary and alternative therapeutic options due to their ability to influence hormonal, metabolic, and inflammatory pathways central to PCOS (33). Among these, polyphenols—particularly flavonoids—have emerged as promising therapeutic agents because of their antioxidant, anti-inflammatory, and hormone-regulating properties (34). These compounds contribute to ameliorating metabolic disturbances and chronic inflammation, thereby supporting hormonal equilibrium and addressing comorbid conditions such as type 2 diabetes mellitus, obesity, and cardiovascular disease (35). Plant-derived flavonoids are of special importance because they regulate hormonal activity, improve insulin sensitivity, and attenuate systemic inflammation, all of which are crucial in PCOS management (36). Several herbal sources rich in polyphenols and other natural bioactives have been investigated for their pharmacological effects in PCOS, as summarized in Table 2. Chamomile (Chamomilla matricariae L.), which contains apigenin, gallic acid, and tannins, has been reported to stimulate ovulation and alleviate oligomenorrhea and hirsutism while exerting anti-inflammatory and antioxidant effects (37, 38). Cinnamon (Cinnamomum cassia (L.) J. Presl), rich in terpenoids and glycosides, improves insulin sensitivity, regulates menstrual cycles, and modulates glucose metabolism (39, 40). Barberry (Berberis aristata Lindl.) provides berberine, which lowers leptin levels, improves insulin resistance, reduces oxidative stress, and supports lipid metabolism (41). Chaste tree (Vitex agnus-castus L.) contains agnuside and flavonoids that restore menstrual regularity and enhance fertility outcomes by modulating prolactin and estrogen balance (42, 43). Turmeric (Curcuma longa L.), which contains curcumin, luteolin, and apigenin, lowers androgen levels, increases estrogen, and provides strong antioxidant and anti-inflammatory effects (44, 45). Similarly, stinging nettle (Urtica dioica L.) supplies flavonoids, polyphenols, and sterols that reduce hirsutism, regulate inflammatory markers, and exert antioxidant activity (42). Other botanicals, such as spearmint (Mentha spicata L.), fenugreek (Trigonella foenum-graecum L.), parsley (Petroselinum crispum (Mill.) Nym.), green tea (Camellia sinensis (L.) Kuntze), ginger (Zingiber officinale Roscoe), licorice (Glycyrrhiza glabra L.), black seed (Nigella sativa L.), pomegranate (Punica granatum L.), and raspberry (Rubus idaeus L.) have also been documented to exert diverse benefits including anti-androgenic effects, improved ovarian function, enhanced insulin sensitivity, reduced ovarian volume, lipid metabolism regulation, and restoration of endocrine balance (44, 46–49). Flavonoids, in particular, play a central role in PCOS by alleviating oxidative stress, which is a key contributor to ovarian dysfunction. Through neutralization of free radicals, they help preserve cellular integrity and ovarian function (50). Additionally, they regulate inflammatory signaling and lipid metabolism, thereby addressing insulin resistance and hyperlipidemia, both common metabolic disturbances in PCOS (51). Flavonoids also influence steroidogenesis, which is critical for maintaining androgen levels and supporting regular ovulation (52). Among these, apigenin and ellagic acid have attracted particular interest for their therapeutic relevance. Apigenin, found abundantly in chamomile, parsley, celery, and citrus fruits, possesses a unique chemical structure enabling strong antioxidant and anti-inflammatory actions. It has been reported to modulate ovarian function, reduce hyperandrogenism, and improve ovulatory outcomes (34, 38). Ellagic acid, naturally present in pomegranates, berries, and nuts, regulates lipid metabolism, reduces inflammation, lowers androgen levels, and enhances insulin sensitivity, while its potent antioxidant capacity counteracts oxidative stress and metabolic dysfunction (44, 53).
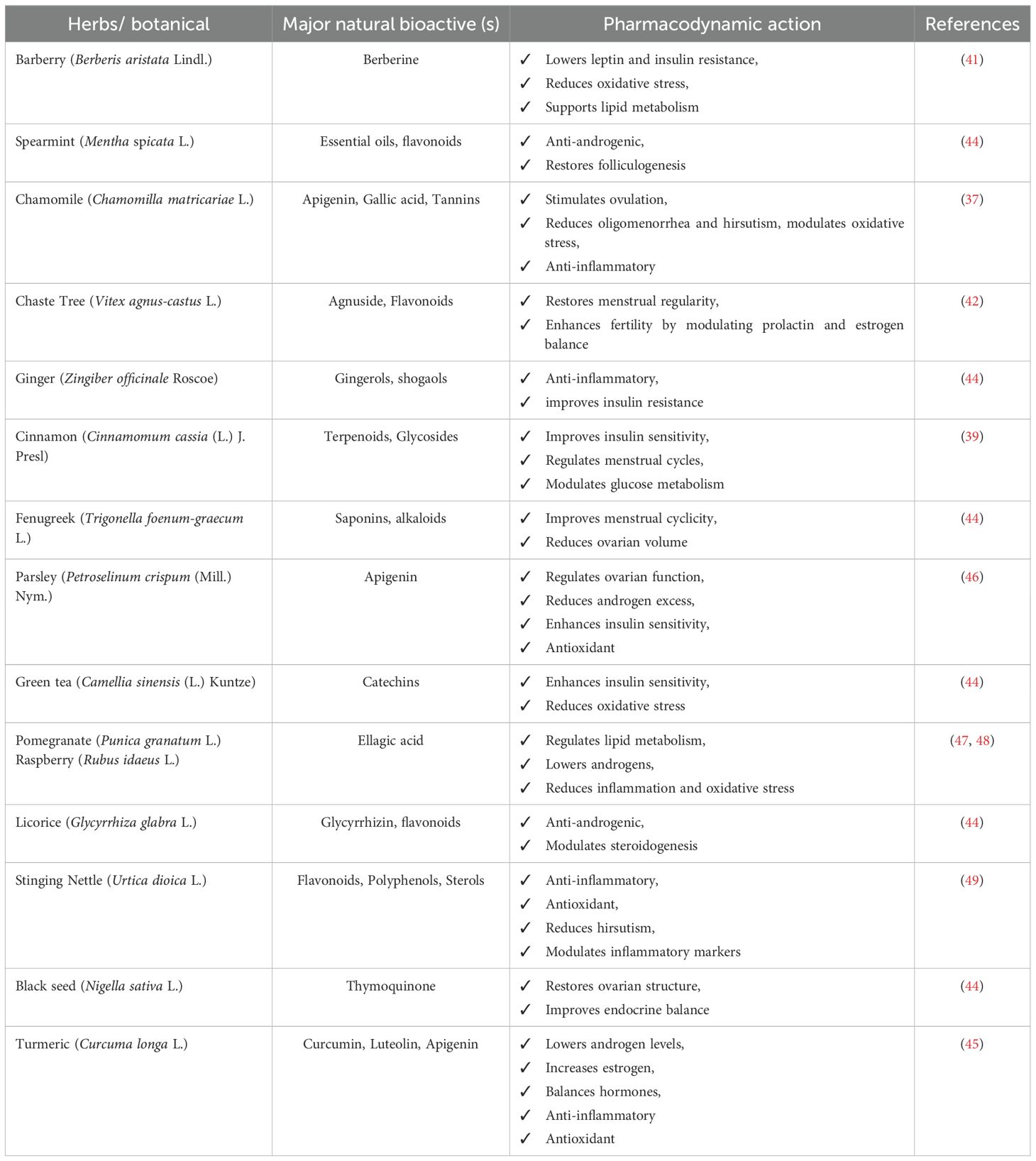
Table 2. Herbs/botanicals, natural bio-actives, and their pharmacodynamic action in PCOS management.
Taken together, these findings highlight that natural bioactives, particularly flavonoids such as apigenin and ellagic acid, provide multifaceted benefits in PCOS by targeting hormonal imbalance, metabolic dysfunction, and chronic inflammation. Their integrative use alongside conventional therapies represents a promising approach for more holistic and personalized management of PCOS (35).
6 Therapeutic potential and mechanistic insights
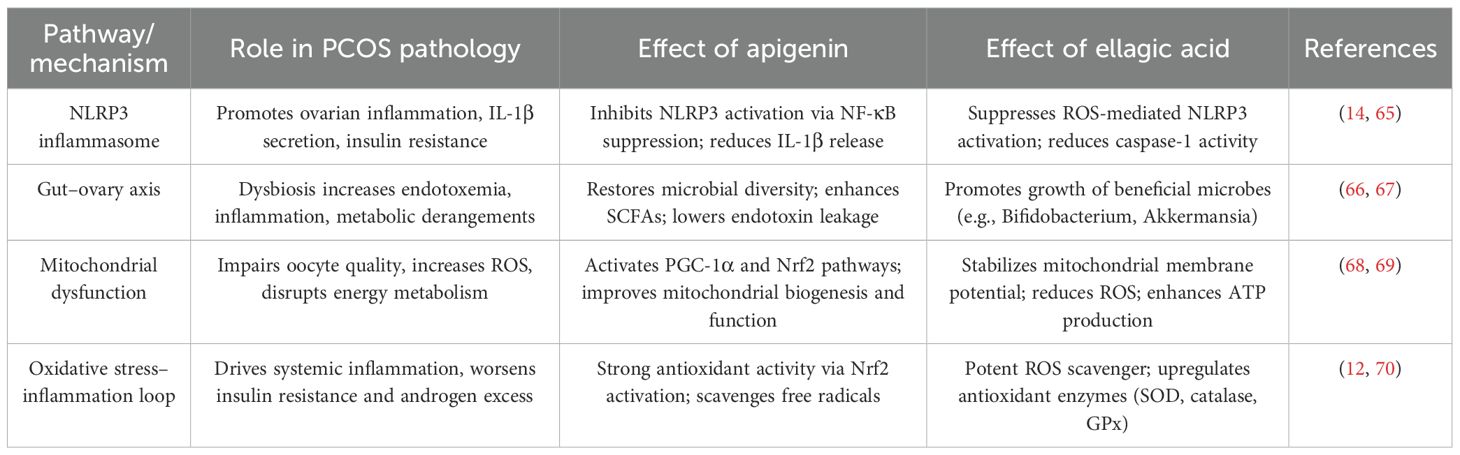
Apigenin and ellagic acid are naturally occurring polyphenols with significant therapeutic potential in polycystic ovary syndrome (PCOS) (15, 54). Both act through multi-targeted mechanisms—anti-inflammatory, antioxidant, anti-androgenic, and insulin-sensitizing effects—thereby addressing the endocrine, metabolic, and inflammatory disturbances characteristic of the disorder (55). Apigenin improves ovarian steroidogenesis and insulin sensitivity via PI3K/Akt signaling (56), while ellagic acid reduces oxidative stress and inflammation through ROS scavenging, NF-κB/TNF-α suppression, and 5α-reductase inhibition, in parallel regulating glucose- and lipid-metabolizing enzymes to restore metabolic balance (57, 58). Beyond these established actions, both compounds target emerging PCOS-related pathways. They alleviate mitochondrial dysfunction (apigenin via PGC-1α/Nrf2-mediated biogenesis; ellagic acid through stabilization of mitochondrial membrane potential and ATP enhancement), inhibit NLRP3 inflammasome activation, and restore gut microbial diversity, including butyrate-producing taxa, thereby reducing endotoxin-driven inflammation and improving the gut–ovary axis (59). While usually studied independently, apigenin and ellagic acid exhibit complementary and potentially synergistic effects by enhancing antioxidant defenses, improving insulin sensitivity, regulating ovarian function, and suppressing hyperandrogenism. Collectively, these actions underscore their promise as natural adjunctive agents in PCOS management, supporting future clinical trials of combinatorial and personalized interventions (see Table 3).
7 Comparative effectiveness of apigenin and ellagic acid vs. standard drugs
7.1 Apigenin vs. standard drugs
Natural flavonoids like apigenin have shown anti-inflammatory, hormone-modulating, and antioxidant benefits that are pertinent to PCOS (60). Common PCOS medications, such as metformin and clomiphene citrate, mainly target ovulation induction and insulin resistance, respectively (61). Apigenin may improve the hormonal balance in PCOS by dramatically lowering estrogen and testosterone levels while raising progesterone and FSH levels, according to research conducted in animal models (55). Standard hormonal therapies, such as oral contraceptives, manage hormone levels, although they may have distinct processes and adverse effect profiles (62). Similar to metformin, apigenin has demonstrated potential in enhancing antioxidant status and lipid profiles in PCOS rat models. Metformin is a well-known medication for treating insulin resistance and related metabolic issues in PCOS (60, 63). Apigenin has been shown to increase superoxide dismutase activity and overall antioxidant capacity while decreasing pro-inflammatory cytokines (TNF-α, IL-6) and total oxidative state. PCOS is frequently associated with oxidative stress and persistent low-grade inflammation, both of which may be treated by conventional medications, but sometimes in different ways (55, 64). Apigenin may have fewer negative effects than synthetic medications due to its natural composition. However, this must be extensively studied in clinical studies.
7.2 Ellagic acid vs. standard drugs
A polyphenolic substance found in large quantities in pomegranates, berries, and nuts, ellagic acid has significant anti-inflammatory and antioxidant properties, which makes it a promising therapy adjunct for PCOS. There are similarities and differences when compared to common medications like letrozole, clomiphene citrate, and metformin. Similar to metformin, ellagic acid has a positive modulation of lipid profiles, lowers fasting blood glucose, and dramatically increases insulin sensitivity. However, ellagic acid also has direct anti-androgenic effects by restricting 5α-reductase, decreasing circulating testosterone levels. This trait is not shared by metformin, which primarily targets insulin resistance. In contrast to ovulation-inducing drugs like letrozole and clomiphene, ellagic acid restores follicular dynamics via reducing oxidative stress and restoring normal ovarian morphology, rather than directly altering estrogen receptors. This mechanism suggests a possible function for ellagic acid as an adjuvant to conventional ovulation medications, as it enhances rather than replicates the action of selective estrogen receptor modulators. Evidence from clinical trials supports these conclusions more thoroughly. In a randomized, double-blind, placebo-controlled study, women with PCOS were given 200 mg of ellagic acid daily for eight weeks (14; n = 60). Improvements in metabolic and hormonal parameters were statistically significant when compared to a placebo. In particular, ellagic acid decreased serum total testosterone (−0.6 ± 0.2 ng/mL; p < 0.05), fasting insulin (−3.5 ± 1.1 μIU/mL; p < 0.05), fasting blood sugar (−11.2 ± 3.6 mg/dL; p < 0.01), and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR index) (−1.3 ± 0.4; p < 0.01). While HDL showed an apparent but nonsignificant rise, lipid markers such as total cholesterol (−15.4 ± 4.2 mg/dL; p < 0.05) and LDL cholesterol (−12.7 ± 3.9 mg/dL; p < 0.05) also improved dramatically. The levels of pro-inflammatory cytokines, such as TNF-α and IL-1β, were decreased (p < 0.01). According to these findings, ellagic acid has distinct anti-inflammatory and androgen-lowering qualities in addition to similarities with metformin’s metabolic advantages, offering a wider range of therapeutic options for the treatment of PCOS (65–70).
Standard pharmacological treatments such as oral contraceptive pills (OCPs), metformin, clomiphene citrate, and letrozole remain first-line options for PCOS (71); however, these primarily target symptomatic relief rather than addressing the underlying pathophysiological mechanisms, and their long-term use may be associated with adverse effects. In contrast, apigenin and ellagic acid demonstrate multi-targeted actions by simultaneously modulating insulin resistance, hyperandrogenism, oxidative stress, inflammation, and broader metabolic dysfunctions. Unlike OCPs or selective estrogen receptor modulators, which focus mainly on reproductive hormone regulation, these natural bioactives also exert antioxidant, mitochondrial-protective, and gut–ovary axis–modulating effects, thereby offering wider systemic benefits. Although their clinical evidence base is less extensive than that of standard drugs, their favorable safety profiles, broad mechanistic actions, and potential for synergistic use with conventional therapies highlight their promise as integrative or adjunctive agents in PCOS management (72). A comparative summary (Table 4) underscores the distinctions between apigenin, ellagic acid, and standard therapies in terms of molecular targets, therapeutic effects, evidence base, and side-effect profiles. Notably, while ellagic acid is less extensively studied than apigenin, emerging data suggest it may be particularly effective in reducing inflammation and hyperandrogenism while achieving comparable metabolic regulation to standard medications. Nonetheless, conclusive comparative effectiveness, especially against ovulation-inducing drugs and metformin, requires rigorously designed head-to-head clinical trials.
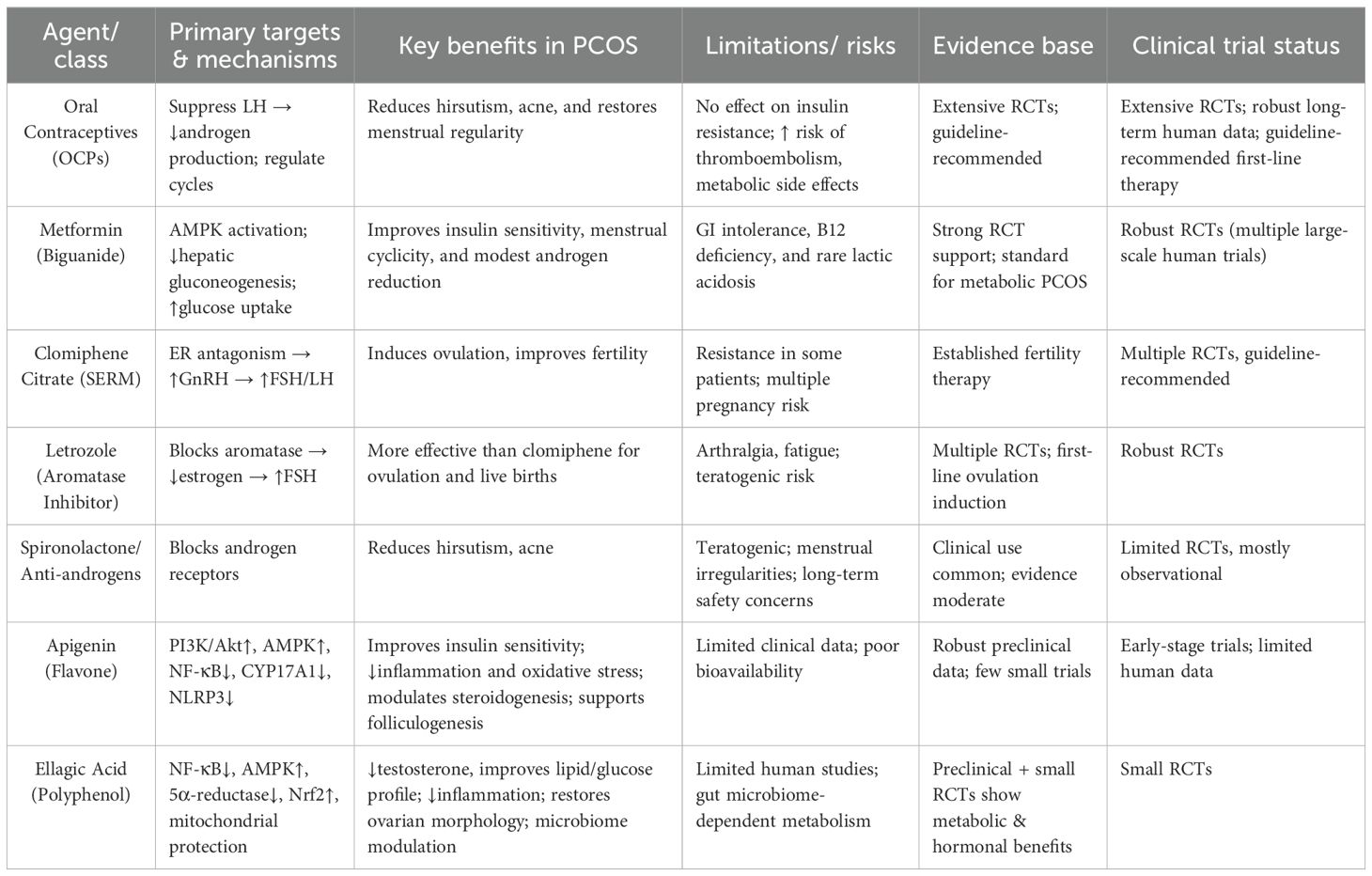
Table 4. Comparative evaluation of apigenin and ellagic acid versus standard therapies in PCOS management.
Beyond clinical outcomes, cost is an important factor in PCOS management. Conventional therapies such as metformin and letrozole are inexpensive generic drugs but require long-term use and monitoring for side effects, which adds to healthcare costs. In contrast, natural bioactives like apigenin and ellagic acid are derived from dietary sources (parsley, chamomile, pomegranates, berries), making them relatively accessible and affordable as nutraceuticals. Although standardized formulations may increase costs initially, their favorable safety profiles and potential to reduce polypharmacy could enhance long-term cost-effectiveness in integrative approaches. However, formal pharmacoeconomic analyses comparing these agents to standard drugs are currently lacking and should be prioritized in future research.
8 Molecular targets and signaling pathways
While Section 6 outlined the broad systemic mechanisms, this section focuses on the specific molecular targets regulated by apigenin and ellagic acid. Key intracellular cascades implicated in PCOS pathogenesis include PI3K/Akt, NF-κB, AMPK, CYP17A1, and Nrf2. By modulating these pathways, apigenin and ellagic acid address core features of the disorder such as insulin resistance, hyperandrogenism, oxidative stress, and chronic inflammation. Dysregulation of PI3K/Akt signaling underlies insulin resistance in PCOS. In preclinical models, apigenin restores insulin sensitivity and ovarian function by inhibiting PI3K/Akt and downstream mTOR activation, thereby reducing excessive follicular growth and anovulation (73). Chronic inflammation in PCOS is driven by NF-κB–mediated transcription of pro-inflammatory cytokines such as TNF-α and IL-6; both compounds suppress NF-κB activation, lowering systemic and ovarian inflammation (65). Ellagic acid further improves metabolic homeostasis by stimulating AMPK, which enhances glucose uptake, lipid oxidation, and energy balance (73). Apigenin also inhibits CYP17A1, a key enzyme in androgen biosynthesis, thereby attenuating hyperandrogenism and lowering circulating testosterone levels (74). Through coordinated modulation of these interlinked pathways, apigenin and ellagic acid exert multi-targeted therapeutic effects, reinforcing their potential as natural agents for managing both the endocrine and metabolic dimensions of PCOS.
9 Toxicological and safety data
Experimental research indicates that apigenin is neither mutagenic nor genotoxic and is widely regarded as safe, given its broad occurrence in dietary sources such as parsley, chamomile, and celery (75). Similarly, ellagic acid, found in pomegranates, berries, and nuts, is considered safe at dietary levels. Most available safety data, however, are derived from animal studies. High doses of ellagic acid (≥200 mg/kg) have been associated with renal and cardiac toxicity in rats, while reported LD50 values vary across models (76). Apigenin also demonstrates a favorable safety profile in rodents, with several studies reporting no observed adverse effects at nutritionally relevant doses (77). Importantly, NOAEL values for apigenin and ellagic acid have not yet been established in human clinical studies. Current human trials have primarily evaluated short-term supplementation and have not systematically assessed dose–toxicity relationships. This gap highlights the need for carefully designed safety studies in humans before therapeutic dosing ranges can be standardized for PCOS management. Another practical consideration is the potential for herb–drug interactions. Both apigenin and ellagic acid undergo extensive metabolism via cytochrome P450 (CYP450) enzymes and phase II conjugation pathways. Apigenin, for example, has been reported to inhibit CYP3A4 and CYP2C9, raising the possibility of altered pharmacokinetics when co-administered with oral contraceptives, statins, or antidiabetic drugs (78). Ellagic acid is subject to gut microbiota–mediated metabolism to urolithins, which may interact with drugs affecting intestinal absorption or hepatic clearance. While clinically significant interactions have not been reported in PCOS trials, caution is warranted in integrative practice, and future studies should specifically evaluate safety in the context of polypharmacy.
10 Microbiome and gut-ovary axis
The pathophysiology of PCOS is now understood to be significantly influenced by the complex interaction between the gut microbiota and the ovaries, known as the gut-ovary axis (66). Women with PCOS have often been shown to have dysbiosis, which is defined as an imbalance in the gut microbiota’s composition and activity (79). Altered gut microbiota may increase intestinal permeability, allowing metabolites like LPS into circulation, triggering chronic inflammation and insulin resistance—key features of PCOS (80). For example, in animal models, ellagic acid, a polyphenol included in fruits and nuts, has been shown to decrease the prevalence of pro-inflammatory bacteria while increasing the development of beneficial bacteria such as Bifidobacterium species and Akkermansia muciniphila (67). Likewise, it has been demonstrated that apigenin, a flavonoid found in many plants, has a beneficial effect on the variety and composition of gut microbes, which may lessen metabolic abnormalities linked to dysbiosis (81). More study is needed to completely understand the particular processes by which ellagic acid and apigenin modify the gut microbiota and how these changes translate into enhancements in ovarian function and metabolic health in women with PCOS.
11 Discussion on nano delivery or formulation strategies
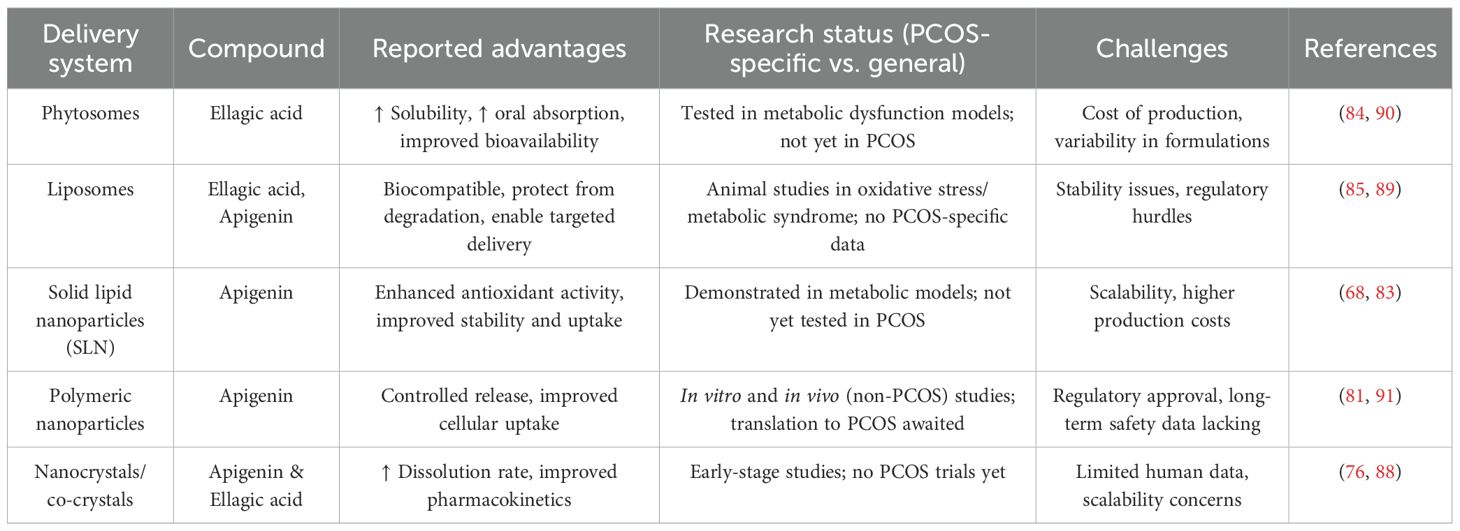
The therapeutic use of apigenin and ellagic acid is limited by poor oral bioavailability due to low solubility, rapid metabolism, and restricted permeability (82). Nanotechnology-based carriers such as nanoparticles, liposomes, and phytosomes have been developed to address these barriers by improving solubility, stability, and targeted delivery (83–87). Preclinical studies on related polyphenols demonstrate their potential: quercetin nanoparticles showed enhanced antioxidant activity (88), liposomal silymarin improved hepatoprotective efficacy, and phytosomal curcumin exhibited greater absorption (89). For apigenin, nanoformulations such as solid lipid and polymeric nanoparticles have enhanced stability, uptake, and antioxidant effects in metabolic models (88), while ellagic acid phytosome and nano-liposome formulations improved systemic exposure and insulin sensitivity in metabolic dysfunction models (90, 91). However, PCOS-specific evidence remains scarce, and translation to clinical application is hindered by high production costs, formulation variability, and the need for rigorous toxicological evaluation. Even so, these delivery platforms offer a promising strategy to enhance the therapeutic efficacy of apigenin and ellagic acid in PCOS and related comorbidities. Future research should prioritize PCOS-specific nanoformulation trials and clinical translation to fully realize the therapeutic potential of these compounds. To summarize these considerations, Table 5 outlines the main delivery systems investigated for apigenin and ellagic acid, highlighting their potential benefits and current research status.
12 Preclinical and clinical studies
Numerous 0preclinical experimental (animal) and clinical (human) studies have investigated the pharmacological and toxicological effects of natural bioactives in the management of PCOS. Berk et al. (92) reported that apigenin exerts protective effects against PCOS in female Wistar albino rats by reducing oxidative stress, body weight, and the levels of progesterone, FSH, LH, as well as the LH/FSH ratio. Additionally, apigenin suppresses inflammatory mediators, including IL-1β, IL-13, and IL-18. Similarly, Darabi et al. demonstrated that apigenin significantly decreased levels of androgenic hormones (including estrogen, testosterone, LH, and the LH/FSH ratio) and inflammatory cytokines (TNF-α and IL-6) in a PCOS-induced Wistar rat model (55). This was accompanied by an increase in total antioxidant levels and superoxide dismutase (SOD) activity, leading to a reduction in ovarian cysts and theca layer thickness, while improving the corpora lutea and granulosa layer thickness. These effects were associated with the downregulation of NF-κB transcriptional activity. Peng et al. further supported these findings in a study using Sprague Dawley rats, where apigenin treatment improved lipid profiles, enhanced antioxidant levels, and elevated estradiol concentrations, while suppressing TNF-α, IL-6, ovarian cyst diameter, and the thickness of granulosa and theca layers. (60) In addition to flavonoids like apigenin, plant-based therapies have also shown promise. Tetracera potatoria, a plant known for its antioxidant, anti-inflammatory, and hormone-balancing properties, demonstrated beneficial effects against PCOS in female Wistar rats (93). Galati et al. conducted a study on Sprague Dawley rats using a PCOS model and observed that treatment led to reduced body weight, improved lipid profiles, decreased cyst diameter, and restoration of gonadotropin hormones such as estradiol and testosterone (94). The treatment also supported follicular health by suppressing TNF-α and IL-6 levels. Another promising natural bioactive is ellagic acid (EA), which possesses both antioxidant and anti-inflammatory properties. In a PCOS model induced by estradiol valerate in mice, EA reversed elevated LH levels, normalized miRNA-21 expression, and restored the number of primordial and Graafian follicles (95, 96). Moreover, EA treatment improved ovarian morphology, reduced theca layer thickness, enhanced the oocyte layer, and improved the quality of antral and preovulatory follicles (Figure 1). Additionally, a placebo-controlled, randomized, double-blind clinical trial was conducted to assess the therapeutic efficacy of EA in patients with PCOS (14). The study involved 60 participants, who were randomly assigned to receive 200 mg/day of EA for 8 weeks. Throughout the trial, daily blood samples were collected at night, and 95% patient compliance was reported. After the study, no significant change in body weight was observed. However, patients who received EA demonstrated notable reductions in fasting blood sugar (FBS), insulin levels, lipid profile markers, including total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL), as well as inflammatory mediators, specifically TNF-α and IL-1β, and insulin resistance indices. Moreover, EA supplementation resulted in a significant increase in the persistence of beneficial gut bacteria and a reduction in oxidative stress. Hormonally, EA-treated patients showed decreased levels of total testosterone, prolactin (PRL), and anti-Müllerian hormone (AMH), while levels of FSH and LH remained unchanged compared to baseline values. In another clinical trial, EA supplementation was examined in 12 female patients with metabolic syndrome led to a significant reduction in waist circumference (from 102.2 ± 4.2 cm to 99.5 ± 3.2 cm, p < 0.05). Other improvements, such as lower blood pressure, triglycerides, fasting glucose, insulin, and enhanced insulin sensitivity, were observed in the overall cohort (97). These findings underscore the potential role of ellagic acid, along with apigenin, as effective complementary agents in the management of PCOS. Further preclinical and clinical studies are warranted to elucidate their mechanisms of action and confirm their therapeutic value in human populations affected by PCOS.
13 Pharmacokinetics, bioavailability, and endocrine modulation
EA, a natural bioactive present in ellagitannin-rich foods (98), undergoes digestion beginning in the stomach and absorption in the jejunum and ileum (99). Its metabolism is largely gut microbiota–dependent, producing bioactive urolithins such as urolithin A, dihydroxy urolithin A, and urolithin B (90, 100). These metabolites undergo hepatic phase I (hydroxylation) and phase II transformations (methylation, sulfonation, glucuronidation), enhancing solubility and promoting urinary excretion (101, 102). EA and its derivatives distribute across muscle, adipose tissue, heart, and lungs (103), where they persist for 24–48 hours due to enterohepatic recycling (104, 105). While apigenin, a flavone abundant in parsley, chamomile, and celery, also demonstrates low oral bioavailability, attributed to poor solubility and extensive first-pass metabolism (78). It is absorbed along the gastrointestinal tract via active transport in the duodenum/jejunum and passive diffusion in the ileum/colon (106), undergoing hydrolysis and glucuronidation (91, 107, 108). Once in circulation, apigenin binds serum transferrin and distributes to the liver, kidney, and intestines (109). It undergoes phase I metabolism via CYP450 and FMO enzymes, and phase II via sulfonation and glucuronidation, producing β-monoglucuronides (94, 110, 111). Ultimately, it is excreted through urine, with residual elimination via feces, partly influenced by gut microbiota (112).
Both apigenin and EA face the challenge of poor oral bioavailability, but advanced formulation strategies are showing promise. Nanoparticles, solid lipid nanoparticles, nanocrystals, liposomes, and phytosomes improve stability, solubility, and targeted delivery (76, 84, 85, 88). Innovative approaches such as prodrug design and co-crystal technology further enhance pharmacokinetics (83). Moreover, modulation of gut microbiota, which plays a key role in EA metabolism to urolithins, represents an emerging strategy to boost efficacy (67, 90, 100). Beyond pharmacokinetics, both compounds exhibit endocrine modulation relevant to PCOS. Apigenin restores LH/FSH balance, inhibits CYP17A1 to reduce androgen excess, and enhances progesterone and FSH levels, thereby supporting folliculogenesis and ovulation (55, 68, 113). Ellagic acid lowers testosterone and dihydrotestosterone (DHT) via 5α-reductase inhibition and antioxidant activity, while indirectly modulating estrogen–progesterone feedback loops (14, 114). Collectively, these mechanisms normalize gonadotropin signaling, alleviate hyperandrogenism, and restore menstrual cyclicity. Unlike OCPs, which suppress the hypothalamic–pituitary–ovarian (HPO) axis pharmacologically, apigenin and ellagic acid modulate endocrine function physiologically, suggesting potential for safer long-term reproductive outcomes (62). Taken together, advances in formulation strategies and a deeper understanding of endocrine modulation highlight the potential of apigenin and ellagic acid as clinically viable therapeutics. Future research should integrate nanodelivery systems, prodrugs, and microbiota-targeted approaches with rigorous endocrine profiling in clinical trials to optimize their role in PCOS management (82, 86).
14 Constraints of existing evidence
Despite promising preclinical and early clinical findings, several limitations constrain the translation of apigenin and ellagic acid into mainstream PCOS therapy. A major hurdle lies in regulatory classification. In the United States, the FDA regulates polyphenols marketed as supplements under the Dietary Supplement Health and Education Act (DSHEA, 1994), which restricts claims to general “structure–function” benefits (e.g., “supports metabolic health”). Any disease-specific indication, such as PCOS, would require progression through the Investigational New Drug (IND) and New Drug Application (NDA) pathways, with robust randomized controlled trials. In Europe, the European Food Safety Authority (EFSA) requires formal evaluation and approval of health claims under Regulation (EC) No 1924/2006, while nanoformulations or novel delivery systems often fall under the Novel Food Regulation (EU 2015/2283), requiring comprehensive safety and toxicological data. These frameworks mean that standardized nanoformulations of apigenin or ellagic acid would need substantially more data than conventional plant extracts to meet regulatory approval. Another key limitation is bioavailability. Both compounds undergo rapid metabolism and poor absorption, limiting systemic exposure. While nano-delivery systems (e.g., phytosomes, liposomes, solid lipid nanoparticles) have shown improved pharmacokinetics in metabolic and oxidative stress models, no PCOS-specific nanoformulation trials have yet been reported. From a practical perspective, widespread clinical use depends on the balance between benefit and cost. Nanoformulations are considerably more expensive to manufacture and scale compared to conventional extracts, with added challenges of stability and regulatory toxicology (115). Nevertheless, if such formulations can demonstrably enhance exposure two- to three-fold, reduce dosing frequency, or minimize adverse effects relative to standard therapy, they may ultimately prove cost-effective by lowering reliance on multiple drugs and reducing downstream healthcare costs. Rigorous pharmacoeconomic studies will therefore be essential to establish their real-world feasibility.
15 Research gaps and roadmap for clinical translation
Although apigenin and ellagic acid demonstrate strong promise in preclinical and limited clinical settings, critical research gaps remain before they can be integrated into evidence-based management of PCOS. Future trials should incorporate validated metabolic and endocrine biomarkers to better define treatment responses. Key candidates include HOMA-IR to assess insulin resistance, serum testosterone and sex hormone–binding globulin (SHBG) for androgen excess, anti-Müllerian hormone (AMH) to reflect ovarian reserve and follicular activity, and inflammatory cytokines such as TNF-α and IL-6. Integrating these biomarkers with traditional clinical measures such as menstrual cyclicity and ovulation rates would provide deeper mechanistic insights and allow phenotype-specific efficacy assessments. Another important research direction is the evaluation of synergistic or add-on therapeutic strategies. Since apigenin and ellagic acid modulate complementary pathways including AMPK activation, NF-κB inhibition, and CYP17A1 or 5α-reductase suppression, their combined use may provide broader benefits than either compound alone. Moreover, combining these bioactives with standard therapies such as metformin or letrozole has the potential to enhance outcomes while reducing required drug doses, thereby minimizing adverse effects. Beyond biochemical and mechanistic endpoints, future clinical trials should align more closely with patient priorities by incorporating quality-of-life measures, psychological well-being assessments, and fertility-related outcomes such as ovulation, conception, and live birth rates. These endpoints capture the multidimensional burden of PCOS and ensure that therapeutic benefits are meaningful in real-world contexts. Collectively, the roadmap toward clinical translation should include systematic human safety and pharmacokinetic studies, biomarker-driven and phenotype-stratified randomized controlled trials, rigorous exploration of combination regimens, and incorporation of patient-centered outcomes. Such an approach would not only strengthen the clinical evidence base but also facilitate regulatory acceptance and support cost-effective integration of apigenin and ellagic acid into PCOS management.
16 Conclusions
Apigenin and ellagic acid represent promising adjuncts for the integrative management of PCOS, with evidence suggesting benefits across metabolic, endocrine, and inflammatory pathways. Moving forward, emphasis should shift from reiterating preclinical challenges toward designing rigorous, biomarker-driven randomized controlled trials that can define their true clinical value. Such trials should consider phenotype-stratified populations—for example, insulin-resistant versus lean hyperandrogenic subgroups—to determine whether responses differ across the heterogeneous PCOS spectrum. Combination strategies also warrant exploration, including co-administration of apigenin and ellagic acid or their use alongside standard therapies such as metformin or letrozole to enhance efficacy and reduce drug-related side effects. Importantly, future research should adopt a personalized medicine framework, integrating molecular biomarkers, patient-centered outcomes, and cost-effectiveness analyses. This precision approach would not only advance the clinical translation of these bioactives but also ensure that therapies are tailored to the diverse presentations and priorities of women living with PCOS.
Author contributions
AB: Visualization, Writing – review & editing. ManR: Writing – original draft. SM: Writing – review & editing. RA: Writing – original draft. SKR: Conceptualization, Writing – review & editing. MD: Supervision, Writing – review & editing. MamR: Writing – original draft. VA: Conceptualization, Writing – review & editing. RR: Supervision, Writing – review & editing. AU: Supervision, Writing – review & editing. SS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Ministry of AYUSH, Government of India under the AYURSWASTHYA Yojana-3988, and facilitated by Patanjali Ayurved Hospital for CoE Hospital (GSTIN-05AAATD1114EIZL).
Acknowledgments
The authors are thankful to the Ministry of AYUSH under Grant-in-Aid for the Establishment of the Centre of Excellence of Renovation and Upgradation of Patanjali Ayurveda Hospital, Haridwar, India. Further, the authors also thank the National Mission for Clean Ganga, Ministry of Jal Shakti for the effective execution of the project under the Namami Gange Mission-II. Sunil Kumar and Vaibhav Sharma, designers at Patanjali Research Foundation, assisted the authors with the infographics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fahs D, Salloum D, Nasrallah M, and Ghazeeri G. Polycystic Ovary Syndrome: Pathophysiology and controversies in diagnosis. Diagnostics. (2023) 13:1559. doi: 10.3390/diagnostics13091559
2. Chaudhuri A. Polycystic ovary syndrome: Causes, symptoms, pathophysiology, and remedies. Obes Med. (2023) 39:100480. doi: 10.1016/j.obmed.2023.100480
3. Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, et al. Polycystic ovary syndrome: Etiology, current management, and future therapeutics. J Clin Med. (2023) 12:1454. doi: 10.3390/jcm12041454
5. Long T, Zhang Y, Zeng C, Zheng S, Zhou L, and Liu H. Effects of low-dose spironolactone combined with metformin or either drug alone on insulin resistance in patients with polycystic ovary syndrome: A pilot study. Int J Endocrinol. (2022) 2022:9927240. doi: 10.1155/2022/9927240
6. Lohrasbi P, Karbalay-Doust S, Tabei SMB, Azarpira N, Alaee S, Rafiee B, et al. The effects of melatonin and metformin on histological characteristics of the ovary and uterus in letrozole-induced polycystic ovarian syndrome mice: A stereological study. Int J Reprod Biomed. (2022) 20:973. doi: 10.18502/ijrm.v20i11.12365
7. Domecq JP, Prutsky G, Mullan RJ, Sundaresh V, Wang AT, Erwin PJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2013) 98:4646–54. doi: 10.1210/jc.2013-2374
8. Jia LY, Feng JX, Li JL, Liu FY, Xie LZ, Luo SJ, et al. The complementary and alternative medicine for polycystic ovary syndrome: A review of clinical application and mechanism. Evidence-Based Complement Altern Med. (2021) 2021:5555315. doi: 10.1155/2021/5555315
9. Goudarzi K, Kamalpour J, Zekri S, Aringazina RA, Safarzoda RS, Tamadon A, et al. Polycystic ovary syndrome herbal treatments and medicinal plants: A comprehensive review. J Infertil Reprod Biol. (2025) 13:4–26. doi: 10.18502/jirb.v13i1.18766
10. Alaee S, Bagheri MJ, Ataabadi MS, and Koohpeyma F. Capacity of Mentha spicata (spearmint) extracts in alleviating hormonal and folliculogenesis disturbances in a polycystic ovarian syndrome rat model. World’s Vet J. (2020) 10:451–6. doi: 10.36380/scil.2020.wvj56
11. Jang JY, Kim D, Im E, and Kim ND. Therapeutic potential of pomegranate extract for women’s reproductive health and breast cancer. Life. (2024) 14:1264. doi: 10.3390/life14101264
12. Mushtaq Z, Sadeer NB, Hussain M, Mahwish, Alsagaby SA, Imran M, et al. Therapeutical properties of apigenin: a review on the experimental evidence and basic mechanisms. Int J Food Properties. (2023) 26:1914–39. doi: 10.1080/10942912.2023.2236329
13. Allemailem KS, Almatroudi A, Alharbi HOA, AlSuhaymi N, Alsugoor MH, Aldakheel FM, et al. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines. (2024) 12:1353. doi: 10.3390/biomedicines12061353
14. Kazemi M, Lalooha F, Nooshabadi MR, Dashti F, Kavianpour M, and Haghighian HK. Randomized double blind clinical trial evaluating the Ellagic acid effects on insulin resistance, oxidative stress and sex hormones levels in women with polycystic ovarian syndrome. J Ovarian Res. (2021) 14:1–12. doi: 10.1186/s13048-021-00849-2
15. Sharifi-Rad J, Quispe C, Castillo CMS, Caroca R, Lazo-Vélez MA, Antonyak H, et al. Retracted] ellagic acid: A review on its natural sources, chemical stability, and therapeutic potential. Oxid Med Cell Longevity. (2022) 2022:3848084. doi: 10.1155/2022/3848084
16. Dewani D, Karwade P, and Mahajan KS. The invisible struggle: The psychosocial aspects of polycystic ovary syndrome. Cureus. (2023) 15:1–8. doi: 10.7759/cureus.51321
17. Bharali MD, Rajendran R, Goswami J, Singal K, and Rajendran V. Prevalence of polycystic ovarian syndrome in India: A systematic review and meta-analysis. Cureus. (2022) 14:1–9. doi: 10.7759/cureus.32789
18. Singh N, Hooja N, Yadav A, Bairwa P, and Jaiswal A. Comparison of the various diagnostic criteria used in polycystic ovary syndrome. Int J Reproduct Contraception Obstet Gynecol. (2022) 11:2180–3. doi: 10.18203/2320-1770.ijrcog20221999
19. World Health Organization. Polycystic ovary syndrome (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/polycystic-ovary-syndrome (Accessed February 7, 2025).
20. Jiang B. The global burden of polycystic ovary syndrome in women of reproductive age: findings from the GBD 2019 study. Int J Women’s Health. (2025) 17:153–65. doi: 10.2147/IJWH.S490836
21. Joshi B, Mukherjee S, Patil A, Purandare A, Chauhan S, and Vaidya R. A cross-sectional study of polycystic ovarian syndrome among adolescent and young girls in Mumbai, India. Indian J Endocrinol Metab. (2014) 18:317–24. doi: 10.4103/2230-8210.131162
22. Yasmin A, Roychoudhury S, Paul Choudhury A, Ahmed AF, Dutta S, Mottola F, et al. Polycystic ovary syndrome: An updated overview foregrounding impacts of ethnicities and geographic variations. Life. (2022) 12:1974. doi: 10.3390/life12121974
23. Cooney LG and Dokras A. Beyond fertility: Polycystic ovary syndrome and long-term health. Fertil Steril. (2018) 110:794–809. doi: 10.1016/j.fertnstert.2018.08.002
24. Azziz R, Marin C, Hoq L, Badamgarav E, and Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. (2005) 90:4650–8. doi: 10.1210/jc.2005-0628
25. Yildiz BO, Bozdag G, Yapici Z, Esinler I, and Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. (2012) 27:3067–73. doi: 10.1093/humrep/des232
26. Alaee S, Ekramzadeh M, Samare-Najaf M, Jahromi BN, Shokri S, Ghomashi F, et al. Nutritional intake and lifestyle in infertile women with and without polycystic ovary syndrome: A case-control study. J Infertil Reprod Biol. (2024) 12:15–30. doi: 10.18502/jirb.v12i4.17975
27. Dong J and Rees DA. Polycystic ovary syndrome: pathophysiology and therapeutic opportunities. BMJ Med. (2023) 2:e000548. doi: 10.1136/bmjmed-2022-000548
28. Akpang N, Kwiatkowski J, Zaborowska L, and Ludwin A. Autoantibodies targeting the hypothalamic-pituitary-ovarian axis in polycystic ovary syndrome: emerging key players in pathogenesis? Int J Mol Sci. (2025) 26:4121. doi: 10.3390/ijms26094121
29. Ding H, Zhang J, Zhang F, Zhang S, Chen X, Liang W, et al. Resistance to the insulin and elevated level of androgen: A major cause of polycystic ovary syndrome. Front Endocrinol. (2021) 12:741764. doi: 10.3389/fendo.2021.741764
30. Barber TM, Hanson P, Weickert MO, and Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights: Reprod Health. (2019) 13:1179558119874042. doi: 10.1177/1179558119874042
31. Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HED, and Amer S. The role of chronic inflammation in polycystic ovarian syndrome—a systematic review and meta-analysis. Int J Mol Sci. (2021) 22:2734. doi: 10.3390/ijms22052734
32. Yan H, Wang L, Zhang G, Li N, Zhao Y, Liu J, et al. Oxidative stress and energy metabolism abnormalities in polycystic ovary syndrome: From mechanisms to therapeutic strategies. Reprod Biol Endocrinol. (2024) 22:159. doi: 10.1186/s12958-024-01337-0
33. Manouchehri A, Abbaszadeh S, Ahmadi M, Nejad FK, Bahmani M, and Dastyar N. Polycystic ovaries and herbal remedies: A systematic review. JBRA Assisted Reprod. (2023) 27:85. doi: 10.5935/1518-0557.20220024
34. Rani R, Hajam YA, Kumar R, Bhat RA, Rai S, and Rather MA. A landscape analysis of the potential role of polyphenols for the treatment of Polycystic Ovarian Syndrome (PCOS). Phytomed Plus. (2022) 2:100161. doi: 10.1016/j.phyplu.2021.100161
35. Jabarpour M, Amidi F, Aleyasin A, Nashtaei MS, and Marghmaleki MS. Randomized clinical trial of astaxanthin supplement on serum inflammatory markers and ER stress-apoptosis gene expression in PBMCs of women with PCOS. J Cell Mol Med. (2024) 28:e18464. doi: 10.1111/jcmm.18464
36. Akbaribazm M, Goodarzi N, and Rahimi M. Female infertility and herbal medicine: An overview of the new findings. Food Sci Nutr. (2021) 9:5869–82. doi: 10.1002/fsn3.2523
37. Mollabashi EN, Ziaie T, Bekhradi R, and Khalesi ZB. Do Chamomile effect on duration, amount of bleeding, and interval of menstrual cycles? J Pharmacopuncture. (2020) 23:25. doi: 10.3831/KPI.2020.23.004
38. Afiat M, Lor AA, Najafi MN, Ghazanfarpour M, and Jafarabadi M. Examining the effect of chamomile on clinical symptoms and hormonal parameters among patients with polycystic ovarian syndrome. J Family Reprod Health. (2022) 16:248. doi: 10.18502/jfrh.v16i4.11355
39. Liu Y, Liu F, Xing D, Wang W, Yang Q, Liao S, et al. Effects of cinnamon powder on glucose metabolism in diabetic mice and the molecular mechanisms. Foods. (2023) 12:3852. doi: 10.3390/foods12203852
40. Dastgheib M, Barati-Boldaji R, Bahrampour N, Taheri R, Borghei M, Amooee S, et al. A comparison of the effects of cinnamon, ginger, and metformin consumption on metabolic health, anthropometric indices, and sexual hormone levels in women with poly cystic ovary syndrome: A randomized double-blinded placebo-controlled clinical trial. Front Nutr. (2022) 9:1071515. doi: 10.3389/fnut.2022.1071515
41. Mushtaq F, Akhtar MF, Saleem A, Sharif A, Akhtar B, Askary AE, et al. Berberis aristata DC extract counteracts the high fat diet-induced reproductive toxicity in female Wistar rats via modulating oxidative stress and resistance to leptin and insulin. Endocrine Metab Immune Disorders-Drug Targets. (2022) 22:1390–402. doi: 10.2174/1871530322666220429125241
42. Wuttke W, Jarry H, Christoffel V, Spengler B, and Seidlova-Wuttke D. Chaste tree (Vitex agnus-castus)–pharmacology and clinical indications. Phytomedicine. (2003) 10:348–57. doi: 10.1078/0944-7113-00220
43. Feyzollahi Z, Kouchesfehani HM, Jalali H, Eslimi-Esfahani D, and Hosseini AS. Effect of Vitex agnus-castus ethanolic extract on hypothalamic KISS-1 gene expression in a rat model of polycystic ovary syndrome. Avicenna J Phytomed. (2021) 11:292. doi: 10.22038/ajp.2020.17046
44. Malik S, Saeed S, Saleem A, Khan MI, Khan A, and Akhtar MF. Alternative treatment of polycystic ovary syndrome: pre-clinical and clinical basis for using plant-based drugs. Front Endocrinol. (2024) 14:1294406. doi: 10.3389/fendo.2023.1294406
45. Kamal DAM, Salamt N, Yusuf ANM, Kashim MIAM, and Mokhtar MH. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients. (2021) 13:3126. doi: 10.3390/nu13093126
46. Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandström B, et al. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr. (1999) 81:447–55. doi: 10.1017/S000711459900080X
47. Zafrilla P, Ferreres F, and Tomás-Barberán FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem. (2001) 49:3651–5. doi: 10.1021/jf010192x
48. Eghbali S, Askari SF, Avan R, and Sahebkar A. Therapeutic effects of Punica granatum (pomegranate): An updated review of clinical trials. J Nutr Metab. (2021) 2021:5297162. doi: 10.1155/2021/5297162
49. Bhusal KK, Magar SK, Thapa R, Lamsal A, Bhandari S, Maharjan R, et al. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review. Heliyon. (2022) 8:1–8. doi: 10.1016/j.heliyon.2022.e09620
50. Mihanfar A, Nouri M, Roshangar L, and Khadem-Ansari MH. Polyphenols: Natural compounds with promising potential in treating polycystic ovary syndrome. Reprod Biol. (2021) 21:100500. doi: 10.1016/j.repbio.2021.100500
51. Peng MF, Tian S, Song YG, Li CX, Miao MS, Ren Z, et al. Effects of total flavonoids from Eucommia ulmoides Oliv. leaves on polycystic ovary syndrome with insulin resistance model rats induced by letrozole combined with a high-fat diet. J Ethnopharmacol. (2021) 273:113947. doi: 10.1016/j.jep.2021.113947
52. Hoeger KM, Dokras A, and Piltonen T. Update on PCOS: Consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. (2021) 106:e1071–83. doi: 10.1210/clinem/dgaa859
53. Novakovic S, Jakovljevic V, Jovic N, Andric K, Milinkovic M, Anicic T, et al. Exploring the antioxidative effects of ginger and cinnamon: A Comprehensive review of evidence and molecular mechanisms involved in polycystic ovary syndrome (PCOS) and other oxidative stress-related disorders. Antioxidants. (2024) 13:392. doi: 10.3390/antiox13040392
54. Irmak E, Sanlier NT, and Sanlier N. Could polyphenols be an effective treatment in the management of polycystic ovary syndrome? Int J Vitamin Nutr Res. (2024) 94:422–33. doi: 10.1024/0300-9831/a000791
55. Darabi P, Khazali H, and Mehrabani Natanzi M. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: Via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecol Endocrinol. (2020) 36:582–7. doi: 10.1080/09513590.2019.1706084
56. Yoon JH, Kim MY, and Cho JY. Apigenin: A therapeutic agent for treatment of skin inflammatory diseases and cancer. Int J Mol Sci. (2023) 24:1498. doi: 10.3390/ijms24021498
57. Luo ED, Jiang HM, Chen W, Wang Y, Tang M, Guo WM, et al. Advancements in lead therapeutic phytochemicals polycystic ovary syndrome: A review. Front Pharmacol. (2023) 13:1065243. doi: 10.3389/fphar.2022.1065243
58. Xu Q, Li S, Tang W, Yan J, Wei X, Zhou M, et al. The effect of ellagic acid on hepatic lipid metabolism and antioxidant activity in mice. Front Physiol. (2021) 12:751501. doi: 10.3389/fphys.2021.751501
59. Rogerio AP, Fontanari C, Borducchi É., Keller AC, Russo M, Soares EG, et al. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur J Pharmacol. (2008) 580:262–70. doi: 10.1016/j.ejphar.2007.11.033
60. Peng F, Hu Y, Peng S, Zeng N, and Shi L. Apigenin exerts protective effect and restores ovarian function in dehydroepiandrosterone induced polycystic ovary syndrome rats: A biochemical and histological analysis. Ann Med. (2022) 54:578–87. doi: 10.1080/07853890.2022.2034933
61. Xiao J, Chen S, Zhang C, and Chang S. The effectiveness of metformin ovulation induction treatment in patients with PCOS: A systematic review and meta-analysis. Gynecol Endocrinol. (2012) 28:956–60. doi: 10.3109/09513590.2012.705368
62. De Leo V, Musacchio MC, Cappelli V, Piomboni P, and Morgante G. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. (2016) 22:634–46. doi: 10.1093/humupd/dmw016
63. Li M, Yang M, Zhou X, Fang X, Hu W, Zhu W, et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2015) 100:1485–93. doi: 10.1210/jc.2014-2544
64. Mancini A, Bruno C, Vergani E, d’Abate C, Giacchi E, and Silvestrini A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: Controversies and new insights. Int J Mol Sci. (2021) 22:1667. doi: 10.3390/ijms22041667
65. Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li M, et al. Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J Clin Endocrinol Metab. (2015) 100:201–11. doi: 10.1210/jc.2014-2419
66. Dong Y, Yang S, Zhang S, Zhao Y, Li X, Han M, et al. Modulatory impact of Bifidobacterium longum subsp. longum BL21 on the gut–brain–ovary axis in polycystic ovary syndrome: Insights into metabolic regulation, inflammation mitigation, and neuroprotection. mSphere. (2025) 10:1–19. doi: 10.1128/msphere.00887-24
67. Anghel AC, Țăranu I, Orțan A, Marcu Spinu S, Dragoi Cudalbeanu M, Rosu PM, et al. Polyphenols and microbiota modulation: Insights from swine and other animal models for human therapeutic strategies. Molecules. (2024) 29:6026. doi: 10.3390/molecules29246026
68. Alam W, Rocca C, Khan H, Hussain Y, Aschner M, De Bartolo A, et al. Current status and future perspectives on therapeutic potential of apigenin: Focus on metabolic-syndrome-dependent organ dysfunction. Antioxidants. (2021) 10:1643. doi: 10.3390/antiox10101643
69. Liu Y, Li X, Zhu Y, Liu J, and Liu S. Subclinical hypothyroidism contributes to poor glycemic control in patients with type 2 diabetes mellitus, and ellagic acid attenuates methimazole-induced abnormal glucose metabolism in mice model. J Food Biochem. (2021) 45:e13753. doi: 10.1111/jfbc.13753
70. Devipriya N, Srinivasan M, Sudheer AR, and Menon VP. Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance: A drug dose dependent study. Singapore Med J. (2007) 48:311.
71. Yurtdaş G and Akdevelioğlu Y. A new approach to polycystic ovary syndrome: the gut microbiota. J Am Coll Nutr. (2020) 39:371–82. doi: 10.1080/07315724.2019.1657515
72. Zhou P, Feng P, Liao B, Fu L, Shan H, Cao C, et al. Role of polyphenols in remodeling the host gut microbiota in polycystic ovary syndrome. J Ovarian Res. (2024) 17:69. doi: 10.1186/s13048-024-01354-y
73. Yuan J, Li Z, Yu Y, Wang X, and Zhao Y. Natural compounds in the management of polycystic ovary syndrome: a comprehensive review of hormonal regulation and therapeutic potential. Front Nutr. (2025) 12:1520695. doi: 10.3389/fnut.2025.1520695
74. Abaneme D. The impact of hunteria umbellata aqueous extract on the morphology of reproductive and metabolic organs in rats with polycystic ovary syndrome. (2024).
75. Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. Int J Mol Sci. (2019) 20:1305. doi: 10.3390/ijms20061305
76. Hurtado-Nuñez GE, Cortés-Rojo C, Sánchez-Ceja SG, Martínez-Flores HE, Salgado-Garciglia R, Bartolomé-Camacho MC, et al. Gallic, ellagic acids and their oral combined administration induce kidney, lung, and heart injury after acute exposure in Wistar rats. Food Chem Toxicol. (2022) 170:113492. doi: 10.1016/j.fct.2022.113492
77. Zhou X, Wang F, Zhou R, Song X, and Xie M. Apigenin: A current review on its beneficial biological activities. J Food Biochem. (2017) 41:e12376. doi: 10.1111/jfbc.12376
78. Meyer H, Bolarinwa A, Wolfram G, and Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metab. (2006) 50:167–72. doi: 10.1159/000090736
79. Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. (2017) 8:324. doi: 10.3389/fmicb.2017.00324
80. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, and Hyland NP. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. (2015) 9:392. doi: 10.3389/fncel.2015.00392
81. Magadán-Corpas P, Pérez-Valero Á, Ye S, Sordon S, Huszcza E, Popłoński J, et al. Gut microbiota and inflammation modulation in a rat model for ulcerative colitis after the intraperitoneal administration of apigenin, luteolin, and xanthohumol. Int J Mol Sci. (2024) 25:3236. doi: 10.3390/ijms25063236
82. Singh A, Singh J, Parween G, Khator R, and Monga V. A comprehensive review of apigenin a dietary flavonoid: Biological sources, nutraceutical prospects, chemistry and pharmacological insights and health benefits. Crit Rev Food Sci Nutr. (2024) 65:1–37. doi: 10.1080/10408398.2024.2390550
83. Bhatia S and Bhatia S. Nanoparticle types, classification, characterization, fabrication methods and drug delivery applications. Natural Polymer Drug Deliv Systems: Nanoparticles Plants Algae. (2016), 33–93. doi: 10.1007/978-3-319-41129-3_2
84. Lu M, Qiu Q, Luo X, Liu X, Sun J, Wang C, et al. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J Pharm Sci. (2019) 14:265–74. doi: 10.1016/j.ajps.2018.05.011
85. De Leo V, Milano F, Agostiano A, and Catucci L. Recent advancements in polymer/liposome assembly for drug delivery: From surface modifications to hybrid vesicles. Polymers. (2021) 13:1027. doi: 10.3390/polym13071027
86. Shi M, Li X, Xing L, Li Z, Zhou S, Wang Z, et al. Polycystic ovary syndrome and the potential for nanomaterial-based drug delivery in therapy of this disease. Pharmaceutics. (2024) 16:1556. doi: 10.3390/pharmaceutics16121556
87. Alharbi WS, Almughem FA, Almehmady AM, Jarallah SJ, Alsharif WK, Alzahrani NM, et al. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics. (2021) 13:1475. doi: 10.3390/pharmaceutics13091475
88. Zverev YF and Rykunova AY. Modern nanocarriers as a factor in increasing the bioavailability and pharmacological activity of flavonoids. Appl Biochem Microbiol. (2022) 58:1002–20. doi: 10.1134/S0003683822090149
89. Shriram RG, Moin A, Alotaibi HF, Khafagy ES, Al Saqr A, Abu Lila AS, et al. Phytosomes as a plausible nano-delivery system for enhanced oral bioavailability and improved hepatoprotective activity of silymarin. Pharmaceuticals. (2022) 15:790. doi: 10.3390/ph15070790
90. González-Sarrías A, Giménez-Bastida JA, García-Conesa MT, Gómez-Sánchez MB, García-Talavera NV, Gil-Izquierdo A, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. (2010) 54:311–22. doi: 10.1002/mnfr.200900152
91. Hanske L, Loh G, Sczesny S, Blaut M, and Braune A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J Nutr. (2009) 139:1095–102. doi: 10.3945/jn.108.102814
92. Berk B, İlhan N, Susam S, Tedik F, and Tektemur NK. The ameliorating effects of apigenin and chrysin alone and in combination on polycystic ovary syndrome induced by dehydroepiandrosterone in rats. Marmara Med J. (2024) 37:198–207. doi: 10.5472/marumj.1479311
93. Ogunlakin AD, Sonibare MA, Jabeen A, Shaheen F, and Shah SF. Antiproliferative and ameliorative effects of Tetracera potatoria and its constituent. Adv Traditional Med. (2021) 21:815–24. doi: 10.1007/s13596-020-00511-0
94. Galati G, Moridani MY, Chan TS, and O’Brien PJ. Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: Glutathione oxidation and conjugation. Free Radical Biol Med. (2001) 30:370–82. doi: 10.1016/s0891-5849(00)00481-0
95. Dos Santos AG. Treatments for Polycystic Ovarian Syndrome (PCOS)-Associated Insulin Resistance (Doctoral dissertation, Washington College). (2022).
96. Khoshvaghti A and Rahbari R. The effect of ellagic acid on sex hormones and miRNA-21 expression in rats with polycystic ovary syndrome. Naunyn-Schmiedeberg’s Arch Pharmacol. (2024) 397:4263–73. doi: 10.1007/s00210-023-02895-7
97. Hidalgo-Lozada GM, Villarruel-López A, Martínez-Abundis E, Vázquez-Paulino O, González-Ortiz M, and Pérez-Rubio KG. Ellagic acid effect on the components of metabolic syndrome, insulin sensitivity and insulin secretion: a randomized, double-blind, placebo-controlled clinical trial. J Clin Med. (2022) 11:5741. doi: 10.3390/jcm11195741
98. Ito H, Iguchi A, and Hatano T. Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J Agric Food Chem. (2008) 56:393–400. doi: 10.1021/jf0726942
99. Seeram NP, Zhang Y, McKeever R, Henning SM, Lee RP, Suchard MA, et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Medicinal Food. (2008) 11:390–4. doi: 10.1089/jmf.2007.650
100. Cerdá B, Periago P, Espín JC, and Tomás-Barberán FA. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. (2005) 53:5571–6. doi: 10.1021/jf050384i
101. Cerdá B, Llorach R, Cerón JJ, Espín JC, and Tomás-Barberán FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. (2003) 42:18–28. doi: 10.1007/s00394-003-0396-4
102. Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, and Espín JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. (2006) 54:1611–20. doi: 10.1021/jf0527403
103. Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. (2007) 55:7732–7. doi: 10.1021/jf071303g
104. Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, and Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. (2006) 136:2481–5. doi: 10.1093/jn/136.10.2481
105. Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, and Tomás-Barberán FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. (2007) 55:10476–85. doi: 10.1021/jf0723864
106. Chen T, Li LP, Lu XY, Jiang HD, and Zeng S. Absorption and excretion of luteolin and apigenin in rats after oral administration of Chrysanthemum morifolium extract. J Agric Food Chem. (2007) 55:273–7. doi: 10.1021/jf062088r
107. Chen J, Lin H, and Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. (2003) 304:1228–35. doi: 10.1124/jpet.102.046409
108. Tang L, Zhou J, Yang CH, Xia BJ, Hu M, and Liu ZQ. Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences. J Agric Food Chem. (2012) 60:3223–33. doi: 10.1021/jf201987k
109. Cai H, Boocock DJ, Steward WP, and Gescher AJ. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin, flavones with putative cancer chemopreventive properties. Cancer Chemotherapy Pharmacol. (2007) 60:257–66. doi: 10.1007/s00280-006-0368-5
110. Švehlíková V, Wang S, Jakubíková J, Williamson G, Mithen R, and Bao Y. Interactions between sulforaphane and apigenin in the induction of UGT1A1 and GSTA1 in CaCo-2 cells. Carcinogenesis. (2004) 25:1629–37. doi: 10.1093/carcin/bgh169
111. Liu Y and Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a Perused rat intestinal model. Drug Metab Disposition. (2002) 30:370–7. doi: 10.1124/dmd.30.4.370
112. Gradolatto A, Basly JP, Berges R, Teyssier C, Chagnon MC, Siess MH, et al. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Disposition. (2005) 33:49–54. doi: 10.1124/dmd.104.000893
113. Sirotkin AV and Harrath AH. Apigenin as a promising agent for enhancing female reproductive function and treating associated disorders. Biomedicines. (2024) 12:2405. doi: 10.3390/biomedicines12102405
114. Fu H, Li W, Liu J, Tang Q, Weng Z, Zhu L, et al. Ellagic acid inhibits dihydrotestosterone-induced ferroptosis and promotes hair regeneration by activating the wnt/β-catenin signaling pathway. J Ethnopharmacol. (2024) 330:118227. doi: 10.1016/j.jep.2024.118227
Keywords: PCOS, flavonoids, apigenin, ellagic acid, natural bio-actives, pharmacokinetics
Citation: Balkrishna A, Rana M, Mishra S, Agrawal R, Rajput SK, Dhanasekaran M, Rana M, Arya V, Ramu R, Upadhayay A and Singh S (2025) Exploring the therapeutic potential of phytochemicals apigenin and ellagic acid in managing polycystic ovarian syndrome and its comorbidities: a comprehensive review. Front. Endocrinol. 16:1633377. doi: 10.3389/fendo.2025.1633377
Received: 22 May 2025; Accepted: 17 September 2025;
Published: 05 November 2025.
Edited by:
Senthilkumar Palaniappan, Karpagam Academy of Higher Education, IndiaReviewed by:
Sudhanshu Kumar Bharti, Patna University, IndiaSanaz Alaee, Shiraz University of Medical Sciences, Iran
Copyright © 2025 Balkrishna, Rana, Mishra, Agrawal, Rajput, Dhanasekaran, Rana, Arya, Ramu, Upadhayay and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shalini Singh, c2hhbGluaS5zaW5naEBwcmZ0LmNvLmlu; Vedpriya Arya, dmVkcHJpeWEuYXJ5YUBwYXRhbmphbGkucmVzLmlu
†ORCID: Vedpriya Arya, orcid.org/0000-0001-7562-1731
Shalini Singh, orcid.org/0000-0002-3743-8888
 Acharya Balkrishna
Acharya Balkrishna Maneesha Rana1
Maneesha Rana1 Satyendra Kumar Rajput
Satyendra Kumar Rajput Muralikrishnan Dhanasekaran
Muralikrishnan Dhanasekaran Ramith Ramu
Ramith Ramu Shalini Singh
Shalini Singh