Abstract
The sphenoid sinus is a complex part of the skull base that has a high degree of anatomical variation, the most interesting of which occurs with hyperpneumatization, in which pneumatized air cells extend beyond their normal limits into the clivus, pterygoid processes, and sphenoidal wings. These hard to note hyperpneumatized imaging variants are disregarded in routine imaging but have potential to grossly alter important neurovascular landmarks, which is a challenge for the precision and safety of transsphenoidal surgical approaches. In this review, we provide an exten- sive, state-of-the-art investigation of sphenoid sinus hyperpneumatization, synthesizing novel pri- mary research discoveries with primordial radiological, anatomical, and clinical intrepidity. Our exploration to unravel the embryological basis for sinus development elicits an intricate balancing act between osteoclastic activity and the myriads of molecular actors such as RANKL/OPG, SHH, and BMP signaling pathways that delineate pneumatization in the skull base system. We demon- strate via in-depth radiological analysis how high-resolution CT (HRCT), dual-energy CT (DECT), and 7T MRI furnish unparalleled visualization of these variants, allowing identification of involved thinned bony walls, dehiscent canals, and high-risk zones for neurovascular insults. Clinically hy- perpneumatization is not just an anatomical curiosity, it may foreshadow operative complications and neurological symptoms. We discuss how it complicates endoscopic transsphenoidal ap- proaches and may increase the risk of internal carotid artery (ICA) injury, optic nerve impingement, and cerebrospinal fluid (CSF) leak. Surgical advances such as AR/VR-assisted neuronavigation and hydroxyapatite-based skull base reinforcement techniques are explored for their potential to de-risk these procedures and improve outcomes. Proactively, we propose that the future of sphenoid sinus hyperpneumatization research be one that adopts AI-driven morphometric analyses, clinically standardized classification systems, and longitudinal clinical studies to dissect its pathophysiolog- ical mysteries. This paper aims to develop an understanding of this omitted but clinically important anatomical variant by integrating basic anatomical principles with technology in order to provide clinicians, researchers, and surgical teams with a more nuanced, applicable exploration of the topic.
1 Introduction
1.1 Background and rationale
The sphenoid sinus is an air-filled space encased within the sphenoid bone in the skull base, located at the sella turcica and pituitary gland inferiorly, anteriorly to the clivus, and laterally to the carotid sinuses (1). Due to its close anatomical relationship to critical neurovascular structures including the internal carotid arteries (ICAs), optic chiasm, and cranial nerves that run through the cavernous sinus, the sphenoid sinus is of extreme importance in the transsphenoidal neurosurgical approach but can inherently pose risk in some individuals due to anatomical variants with large amounts of pneumatization (2).
Sinuses begin forming during the fourth month of gestation when epithelium invades the presphenoid cartilage. This process is ongoing into childhood, and into early adulthood, extending posterolaterally and inferiorly (3). Most sinus areas extend to the sella turcica in adulthood, while some develop into adjacent structures such as the clivus, pterygoid processes, or greater wings, forming large irregular cavities with an area of air space. In these scenarios, the sinus may be associated with thinned walls, or even completely atrophied and possessing no bony wall, increasing the risk to surrounding structures with instrumentation (4). Moreover, septal asymmetries are frequent, and in some circumstances, the intersinus septa can deviate off the midline and insert onto the optic or carotid canals, creating another level of operative complexity, notwithstanding the majority being asymptomatic (5).
Pneumatized patterns of the sphenoid sinus can be described as conchal, presellar, sellar or postsellar dependent on the relationship of the sinus to the sella turcica. While conchal variants feature compact bony coverage, sellar and postsellar variants progressively expose neighboring structures as wall attenuates. Overdeveloped sinuses often demonstrate complicated and confusing septal anatomy with variable septal presence resulting in decreased confidence for orientation during the endoscopic approach—and increased risk of iatrogenic injury (6).
On imaging, these spaces may resemble pathological processes like tumor erosion, ostelytic lesions, or chronic sinusitis with associated bone remodeling. Although high-resolution computed tomography (CT) is the primary imaging modality for visualizing cortical anatomy, septal insertions, and dehiscence; magnetic resonance imaging (MRI) provides pertinent information about adjacent soft tissues, especially the optic apparatus and pituitary complex (7, 8). Discerning physiological hyperexpansion from pathological remodeling requires careful review of cortical thickness, bony margins, and adjacent soft tissue findings.
From a neurosurgical perspective, hyperexpanded sinuses may provide increased surgical access; however, they leave the anatomy more unpredictable. Thinner bony walls, displaced septa, and changes to normal surgical landmarks may diminish orientation or directly expose sensitive structures (9). Therefore, endoscopic neuronavigation and intraoperative imaging have become important adjuncts to help maintain surgical orientation and minimize complications (10). In the clinical setting, hyper-expansive sinuses have also been linked to retro-orbital discomfort, barotrauma, and spontaneous cerebrospinal fluid (CSF) leaks—albeit a precise pathophysiological explanation is still being elucidated (11).
This review not only aims to integrate the anatomical, radiological, and operative viewpoint but also offers a new conceptual approach to understanding sphenoid sinus hyper-expansion—not just in geometrical extent but fragility, neurovascular exposure, and intraoperative uncertainty. Although based on existing morphologic classification, our approach intends to emphasize clinically relevant actionable risk types (1). The rationale for this work is detailed in the final section of the review where we propose a functional-surgical model to advance the preoperative individual assessment and procedure planning approach.
1.2 Aim of the review
This review aims to present a evidence-based synthesis of the existing knowledge of sphenoid sinus hyperpneumatization with a particular focus on its developmental basis, anatomical variability, radiological appearances, and neurosurgical relevance. We hope to provide clinical information from imaging studies, anatomical studies, and operative reports that define the clinical characteristics of this frequently under-appreciated CT variation.
To do so, we first evaluate the embryological and post-natal processes of sphenoid sinus pneumatization, illustrate the various morphologic subtypes representing excessive expansion, and evaluate the implications of the morphologies for both standard and complicated transsphenoidal neurosurgical approaches. We examined various classification schemes that have defined hyperpneumatized sinuses, examining their clinical utility, as well as their limitations.
We highlight the radiological features of particular concern that can complicate diagnosis or create surgical dilemmas, including septal anomolies, dehiscent walls and important neurovascular relationships, provide a summary of noted complications available in the literature, and identify possible anatomical predictors of unfavourable surgical outcomes.
Finally, we present future relevant areas of research - such as creating consensus radiologic criteria, studying the biomechanical properties of expanded sinus bone, and augmenting virtual reality applications for surgical planning. We intend to provide a translational construct of radiologic and surgical planning and anatomical science to provide a clinically relevant perspective to both clinicians and researchers.
2 Anatomical and embryological overview
2.1 Development of the sphenoid sinus
The sphenoid sinus is a paranasal air cell and is located in the body of the sphenoid bone, at the base of the skull in a midline position and is clinically and structurally important because of its location to significant neurovascular structures (12). The sphenoid sinus develops through changes in the sphenoid bone, as an embryologic outpouching becoming a multicompartmentalized chamber in the presence of pneumatisation and septation; sought of an outcome interplay of genetic predispositions, molecular signaling pathways, and environmental influences (13). Generally, the timing of development is fairly consistent, however hyperpneumatized variants are clinically important due to differences in size, bone quality, and morphology, all of which create challenges in respect to differential diagnostics and surgical risk (14). Embryologically, the sphenoid sinus develops from posterior ethmoidal air cells that evaginate out of the sphenoethmoidal recess by way of nasal epithelial invaginations causing a mesenchymal stem cell transition to osteoclast-like activity to resorb cartilage at the presphenoid, collectively from epithelial-mesenchymal signaling pathways (15). For example, the sonic hedgehog (SHH) cascade up-regulates epithelial proliferation due to PTCH1-SMO activation, while bone morphogenic proteins (BMPs) stimulate SMAD-governed bone resorption and bio-organized directional growth of the sinus cavity (16).
The Wnt/β-catenin signaling can also stimulate osteoblast differentiation and relevant ossification of bone, by way of LRP5/LRP6 activation (17). A reduced Wnt/β-catenin signaling cascade is associated with mutations in RNA binding motif (Rbm) proteins (RUNX2) and abnormal cranial bone development. Likewise, fibroblast growth factors (FGF) 2 and 10 provide incidental support to continue proliferation of the mucosa, and developing blood vessels importantly needed for sinus cavity expansion (18). Also, mutations in fibroblast growth factor receptors (FGFR1/2) are involved with sphenoid morphology changes through syndromic craniosynostoses (19). Histologically, the sinus walls are transformed from immature woven bone to mature lamellar bone by endochondral ossification; and at various times screens even from hyperpneumatization variants, demonstrate the increased RANKL expression in mucosa to increase osteoclastogenesis leading to softened bone, exquisitely thin over the overlying ICAs and optic nerves, increasing the risk for intracranial bleeding during surgery (20). Postnatally, the expansion occurs in two phases. Phase one is approximately 2-4 years of age, presumably suggests an increased nasal airflow and mucosal proliferation. Studies using computational fluid dynamics (CFD) have reported the high-velocity turbulent flow in the sphenoethmoidal recess creates levels of shear stress in the cavity that as a result of the activation of Piezo1 ion channels in osteocytes to recruit osteoclasts for bone-resorbing (21, 22) Phase two corresponds with puberty and adolescence, when sex hormones, GH and IGF-1, ignite synergistic further expansion posterior to the clivus, leading to probable poor quality bone metabolism and bone turnover (23).
In summary, the overall development leads to four patterns of pneumatization, conchal, presellar, sellar and postsellar (24). Conchal would be believed to be extremely rare with notable thickness of bone and would frequently occur with craniofacial dysmorphism. Sellar and postsellar variations have been reported to occasionally distally expand posteriorly with postsellar typically reported to extend posteriorly to the clivus, dorsum sellae or pterygoid processes. There have been reports of sphenoid sinus extending to anterior margin of cranial base (foramen magnum) demonstrate raises questions on what that means for stability & structural integrity of the cranial base (13).
Biomechanically, it has been postulated that in vivo pneumatised cavities could modulate stresses during mastication or mechanism trauma. With the idiosyncratic dissemination of stresses, such a variation would predispose the cranial base to: stress corrosion, microfractures, spontaneous CSF leaks into the middle ear cavity, and spontaneous pneumocephalus (25). With those considerations a question could be posed from a developmental evolutionary tint because comparative literature demonstrated non-human primate species with smaller floatable and non-pneumatized variations of the sphenoid sinus cavity. In contrast, comparative evidence proposes humans advantageously with greater spatial expansion (pneumatization) of the sphenoid sinuses promotes increased respiration efficiency; and a posteriorly rotated base of the skull to suggest our encephalization process is indeed inverting (26, 27).
2.2 Relevant neurovascular relationships
Sphenoid sinus disease is clinically complex due in part to an anatomical relationship with important neurovascular structures. The sphenoid sinus posterior wall is in direct contact with the clivus and dorsum sellae; the superior wall, vagary the sphenoid sinus, forms the floor of the sella. A transsphenoidal corridor exposes the pituitary gland with cavernous sinuses on either side containing the ICAs, optic nerves, cranial nerves III, IV, VI, and V1 and V2. Extreme hyperpneumatization can change the size and configuration of these compartments leading to pre-operative variations that can have implications upon any surgical approach to the sphenoid (28).
The most concerning elements are the ICAs and particularly so if the normally present thin bony lamella separating the aircraft noses from the sphenoid sinus has flattened or been absorbed altogether. Bony dehiscence of the ICA canal has been demonstrated to exist in almost 25% of hyperpneumatized cases on high-resolution CT angiography greatly increasing the opportunity for vascular injury when attempting to access the sella turcica or pituitary fossa utilizing a transsphenoidal approach (29). Additionally, in some patients, there may be a fenestration of the ICA, as a normal structure when the vessel divides and rejoins intraluminally, that may coexist with hyperpneumatized coverage and thereby obstruct the surgical pathway and could significantly obscure landmark structures with irregularities in the wall and traditional endoscopic visualization. Similarly, optic nerves are also at risk. There is rupture at the optic canal in 15-20% of hyperpneumatized instances of sphenoids, and when this occurs the nerve is positioned directly within the air cavity. The likelihood of trauma to the surgical instruments as well as additional risk for compressive trauma from inflammatory changes within the sinus. These normal anatomical references such as the opticocarotid recess (OCR)—the place where the optic and carotid canals meet—may be distorted in these variants as previously defined by severity making surgical navigation less intuitive (30).
The cavernous sinus located laterally to the sphenoid consists at the least a complex venous plexus along with multiple cranial nerves. It is plausible that hyperpneumatization may create an overabundance of air cells in the lateral wall that may push or restrict neural components. Rarely, the abducens nerve may protrude into the sinus cavity which not only provides a surgical quandary but may also be a finding recognized as incidental (31). The internal constituents of the sinus also rely on many of the overall endoscopic pictures, still somewhat difficult to depict successfully in the last series of hyperpneumatized examples. Typically, the intersinus septa arise from the midline; however, in the hyperpneumatized examples from the presentations, they are often asymmetrical either to the ventral surface of the ICA or optic nerve. The concern in the course of an endoscopic procedure is that the operating surgeon may violate and disrupt a midline septa: leading to a range of bad outcomes, possibly even lacerating the ICA. There are examples of septal avulsion injuries in the literature that reiterate the necessity to have some awareness of the anatomy being encountered (32). Recent technology has captured new imaging technologies to not only become aware and identify variant sinus morphology, but to also maintain operational awareness. Radiomic analysis may allow the surgeon to quantify a limited number of textural and geometric features from CT datasets and thereby provide further information on the morphology or integrity of bony walls. Artificial intelligence (AI) algorithms trained on multiple craniofacial imaging datasets can now also algorithmically predict hyperpneumatizations based on skull base and craniofacial geometries. When these technologies are coupled with augmented rationality (AR) platforms, the opportunity to create real-time patient specific anatomical overlays provides a superior level of surgical action and consequentially facilitates a tailored patient pre-operative risk assessment (33).
In conclusion, the sphenoid sinus acts like a crossroads among developmental biology, craniofacial biomechanics, and evolutionary adaptation. Hyperpneumatized anatomical variants often remain silent; however, they naturally present variability that comes to an end in clinical/operative challenges and difficulties intraoperatively. Appreciation of developmental/vertricular sequence, development morphology incorporated to significant relationships with neurovascular structures is necessary information for limiting potential iatrogenic harm and obtaining successful treatment outcomes for this complex anatomical area (34).
3 Definition and classification of hyperpneumatization
3.1 Criteria for Hyperpneumatization
Hyperpneumatization of the sphenoid sinus is defined as an excessive, anatomically significant protrusion of air-filled cavities beyond physiological limits, frequently passing through adjunct structures like the clivus, pterygoid (PP), dorsum sellae, and in extreme presentations even the basioccipital (35).Although it stilloccurs normally via usual pathways of pneumatization, this excessive development can rupture neurovascular barriers, thin or eat away various courses of bony segments and result in a different direction of approach to access these areas during surgery on the skull base or management of the pathologically affected sphenoid sinus (36). Current assessment is predominantly through radiological relationships to known anatomical markers. Usually, pneumatization of the sphenoid sinus is limited anteriorly of the clivus with posterior limits being at the level of the dorsum sellae. In hyperpneumatized variants however, there are air cavities marginally beyond the dorsum sellae or occasionally extending backwards to the level of the anterior occipital bone foramen magnum (37). A common criterion cited in CT studies is one where air cells extend into areas > 10 mm posterior to the posterior edge of the sella turcica. There is frequently a concomitant volumetric expansion of greater than 15 cm³ in adults following population morphometric analyses broken down by age, sex, and existence of craniofacial structure (38, 39). Hyperpneumatization can also be identified by involvement of adjacent bony markers - e.g. posterior extension into the clivus, lateral pneumatization of the pterygoid processes and sphenoid body thinning. Bone densitometry in these above areas frequently shows cortical bone thinning of < 0.5 mm based on high definition CT images (40). In severe instances, there may be no lamellae that protect the optic nerve canals or ICA, resulting in direct exposure of these structures. This exposure increases risk for surgical complication through neurovascular injury and CSF leaks (41).
MRI contributes complementary evidence to the clinical picture for the assessment of anatomical considerations of borderline bony dehiscences or soft tissue changes. An example of this is assessment of mucosal thickening with T2 sequences, seeing this with inflammatory or barobasic modifications within hyperpneumatized sinuses (42). Diffusion-weighted imaging (DWI) and magnetic resonance angiography (MRA) can provide additional information about the adjacent neurovascular structures, that can be interpreted with CT data (43). Ultra-high-field 7T MRI adds further value by assessing the microtrabecular architecture of the bone and could distinguish between non-cynical (benign) hyperpneumatization, versus osteolytic or neoplastic processes (44). Pathophysiologically, hyperpneumatization is postulated to occur from deregulated balance between osteoblastic-osteoclastic factors. Enhanced airflow through the nares and local changes in mucosal inflammation, along with a background of abnormal craniofacial biomechanics are believed to denote osteoclastic activity during increased sinus expansion (45). CFD studies of turbulent flow and anatomy of the sphenoethmoidal recess demonstrate that the shear stress of air turbulence in this area activates the Piezo1 channels in osteocytes potentially stimulating bone remodelling (46). Inflammatory mediators cytokines e.g. IL-6 and TNF-a have been implicated in modulation of the mucosal environment and reconstruction. Polymorphisms in RUNX2 and FGFR2 genes are implicated in predisposing excessive development of the sinus, especially in patients with craniofacial syndromes or abnormal bone metabolism status (47).
3.2 Patterns of pneumatization
Traditionally, the pneumatization of the sphenoid sinus is categorized based on relative location of the sinus cavity and the sella turcica: conchal, presellar, sellar, and postsellar. However, imaging studies have revealed a larger morphological cohort that includes accessory air cells and unusual expansions that bypass classical classification. Recognizing these anatomical variations is important, especially in the neurosurgical setting where sinus architecture is involved in designing preoperative approaches and intraoperative hazards (48, 49). The conchal type is the most perplexing. It consists of a small, shallow air space anterior to the sella turcica, limited to the presphenoid. It is often observed in those with craniofacial deformities such as craniosynostosis or mid-face hypoplasia which are associated with faulty SHH signaling during development. Owing to the thick bony walls and lack of internal sinus landmarks the conchal pattern is the most challenging surgical access, even with the aid of CT (50–52). The presellar type has air cells extending to the anterior border of the sella but not invading the sellar floor and represents a transitional stage of pneumatization that is typically seen in the younger population or individuals with delayed skull base maturation. This also has the potential to distort the anticipated anatomical configurations based on the unpredictability of variable presence or absence of septal insertions. Based on population studies it has a reported frequency of between 20-30%, with an average volume of 6-9 cm³ (53). The sellar type has air cells expanding beneath the pituitary fossa, under the sella turcica. This variant occurs in roughly 60-70% of adults and has the same benefits for surgical access, but typically results in some thinning (probably also dehiscence) of the sellar floor with a subsequent risk of CSF leak, as well as change in landmarks close by, including the OCR (35).
The postsellar pattern, accompanied by hyperpneumatization, can extend into the clivus, dorsum sellae, or pterygoid processed (the pterygoid recess). The postsellar pattern is present in 15-20% of adults, accompanied by thinning at the critical wall—clival recess, wall just lateral to the cavernous sinus, etc. Septal insertions onto ICA canals are particularly problematic during transsphenoidal resection (54). In addition to the canonical types, many additional accessory concepts of pneumatization variability have been identified using high-resolution imaging. The pterygoid recess will displace the vidian nerve and occupy space in the pterygopalatine fossa during a postsellar pattern of pneumaticity. The clival recess extension can include the anterior occipital bone; and rare reports of lesser-wing pneumatization associated with retro-orbital symptoms suggest the possibility of obstructed access to the anterior skull base (55, 56). Biomechanical modeling, especially finite element analysis (FEA), models suggest that extensive pneumatization has an impact model on cranial base biomechanics and loading (pneumatization redistributes cranial base stress). These sinuses may act as stress amplifiers upon impact loading, and in the context of cranial base stress, may be involved in spontaneous CSF leaks or even microfractures at sites of cortical thinning. The clinical findings presented subsequently are based on the supposition that there is a biomechanical component described here; most support the notion that hyperpneumatized variants are associated with significant complication rates (57, 58).
An evolutionary perspective may offer some insight into the variant morphology of sphenoid sinus expansion with cranial base flexion and different model of encephalizating Homo sapiens. Whereas non-human primates (gorillas, chimpanzees) have a very low-end ethmoid-sinus system, the anatomy of the sphenoid complex indicates that humans have a more complex, larger, and variable set of sphenoidal complexes (and cavities). The anatomical shifts in these soft-tissue structures were likely functional adaptations permitting greater oxygenation and price approximation of the brain in a bipedal species (59, 60). From a clinical standpoint, being able to tell between patterns will be important for risk stratification, when planning the procedure: pneumatisiational variants of postsellar or accessory type will require further imaging expectations and even a plan to proceed navigating intraoperatively. As imaging technology has improved, the number of studies showing efficacy coupling 3D CT reconstruction with AR -assisted navigation - or relying on radiomics algorithms - have greatly aided in pre-operatively classifying configurations at risk. Future studies should focus on genetics and molecular behavior underlying genetic and singular corporativisms of incidental sinus. Understanding the relationship between genetic make-up of the biophysicical bio-chemical, structural properties of variance will lead to individualized surgical methods based on risk stratification of critical configurations, and possibly, to early identification as a high-risk patient group (61, 62).
4 Radiological assessment
4.1 Diagnostic imaging modalities
Investigation of sphenoid sinus hyperpneumatization is most useful with a multi- modal imaging assessment that can accurately distinguish normal from abnormal ana- tomical variations. The need for advanced imaging techniques to improve diagnostic accu- racy and surgical planning stems from the proximity of the sphenoid sinus with neuro- vascular structures (e.g., optic nerves, ICAs and cavernous sinuses) and variability in structures due to hyperpneumatization (2, 63). Advancements in technologies in CT and MRI modality in the last few decades have succeeded in visualizing such anatomical var- iations precisely and reliably (64).
For evaluation of normal sphenoid sinus anatomy, high-resolution CT continues to be the gold standard owing to its unique ability to image fine bony structures at submil- limeter resolution. State-of-the-art multidetector CT (MDCT) machines, particularly 64- slice or higher types, provide isotropic voxel acquisition with slice thicknesses of as low as 0.5 mm and offer well-defined cross-sectional images of the sinus cavity (65). Imaging protocols are generally performed at 120 to 140 kVp tube voltages and tube currents of about 150–250 mAs, for optimized cranial base imaging that balances tissue contrast vs radiation exposure (66). The overlapped datasets are reshaped by MEEs with state-of-the- art algorithms such as adaptive statistical iterative reconstruction (ASIR) and model-based iterative reconstruction (MBIR), to preserve details while reducing noise and CT radiation artifacts (67, 68).
The recent focus on dual-energy CT (DECT) in skull base im-aging is likely due to its capability to render bone, air and soft tissues distinguishable based on differences in X- ray attenuation at two energy levels. 80 kVp and 140 kVp DECT protocols enable a better evaluation of hyperpneumatized sphenoid walls, especially for the differentiation be- tween physiological thin cortical bone (which accompanies the pneumatization of the sphenoid) versus pathological osteolysis (69). This is a vital distinction as hyperpneuma- tization could mimic pathologic processes such as chordoma related bone destruction or invasive pituitary adenomas invading into the sphenoid sinus. Radiodensity measure- ments in hyperpneumatized areas typically range from 250 to 400 Hounsfield units (HU) and are markedly different from the osteolytic lesions, which show values below 100 HU due to trabecular bone loss (70–72).
Cone-beam CT (CBCT) has become a valuable adjunctive tool, most notably in an intraoperative setting. CBCT systems were developed for endoscopic skull base surgery to image in real-time at high resolution and with less radiation exposure than conven- tional CT (73). These systems have served a special role in terms of guiding transsphe- noidal procedures, through hyperpneumatized sphenoid sinuses with altered anatomy mitigating standard neuronavigation systems (74). Intraoperative CBCT fused with AR overlays allows for real-time recognition of sinus septations, neurovascular relations, and sites of potential bony dehiscence (75).
Although not effective in characterizing bony anatomy, MRI also is critical in as- sessing the soft tissue structures near hyperpneumatized sphenoid sinuses. High-contrast resolution to evaluate the pituitary gland, cavernous sinuses, and optic nerves is obtained with standard T1- and T2-weighted sequences (76).
In recent years, several innovative methods of constructive interference in steady- state (CISS) or fast imaging employing steady-state acquisition (FIESTA) images have demonstrated advantages in visualization of thin mucosal membranes and the complex path of cranial nerves in the sinus walls (77). DWI has been particularly helpful in detect- ing subtle soft-tissue lesions that may look like hyperpneumatized air cells. Signal heter- ogeneity in mucosal tissues affected by chronic barotrauma, a condition possibly result- ing from the altered aeration patterns in hyperpneumatized sinuses, has been demon- strated in studies evaluating diffusion kurtosis imaging (DKI) (78).
Additional functional MRI features, including dynamic contrast-enhanced MRI (DCE-MRI) and arterial spin labeling (ASL) (Figure 1), have specifically added new infor- mation related to the vascular dynamics that are expected with hyperpneumatized sinus walls. In fact, data from DCE-MRI studies showed that most patients with hypervascular mucosal patterns, which are usually identified with chronic inflammation, observed less frequently in cases of hyperpneumatized variants (79). This result suggests that the in- creased pneumatization is influenced by balanced osteoclastic activity rather than being a chronic inflammatory process. By providing a measure of tissue perfusion in the absence of exogenous contrast agents, ASL appears to be a valuable method for studying sinus wall vascularization patterns, of particular interest in the evaluation of cases suspected of vascular involvement (80, 81).
Figure 1

Schematic representation of arterial spin labeling workflow. This figure illustrates the core principles of the ASL technique, a non-invasive MRI-based method for quantifying cerebral blood flow (CBF). The upper portion depicts a sagittal brain view, highlighting the labeling plane (striped region) where arterial blood water is magnetically tagged. This tagging process occurs prox- imal to the cerebral circulation, ensuring that the labeled blood serves as an endogenous tracer for perfusion measurement. The lower section presents a cross-sectional schematic of the ASL perfusion map, emphasizing the transition of tagged arterial blood (red) through the microvascular network into venous circulation (blue). The yellow-highlighted region indicates the imaging plane where signal differences between the label and control images are measured. The resulting perfusion map reflects regional CBF, providing critical insights into vascular function without the need for exoge- nous contrast agents. ASL’s capacity to capture dynamic perfusion patterns has proven particularly valuable in neuroimaging applications, including the evaluation of cerebrovascular reactivity, is- chemic lesions, and neurodegenerative disorders.
4.2 Key radiological features
The diagnosis of hyperpneumatized sphenoid sinuses necessitates the awareness of certain radiological features that separates these variants from normal anatomical and pathological processes. The extension of air cells outside normal anatomic boundaries is one of the most diagnostically important features. In normal adult anatomy, the sphenoid sinus has posteriorly extended to the sella turcica but is uncommon to invade the clivus or pterygoid processes (82). However, posterior extension into the clival region on CT im- aging, often with pneumatization reaching the anterior margin of the foramen magnum, is seen in hyperpneumatized variants. Such pneumatization has the appearance of com- plex air-cell constructs with different cortex remodeling (4, 83).
Hyperpneumatized sphenoid sinuses also have another characteristic appearance: bony thinning or dehiscence. Cortical thinning exceeding 50% compared to non-hyperp- neumatized siblings is frequently observed on high resolution CT scans. The thinning of this structure is especially prone to be seen at the sellar floor, OCR, and lateral sinus walls and is secondary to its anatomical configuration and their proximity to the airflow motion within the sinus cavity (84). For hyperpneumatized sinuses, measurements of cortical thickness in these regions commonly are <0.4 mm and in some cases, the optic nerve ca- nals are directly exposed to the sinus cavity, as in complete dehiscence of the region, or the ICAs lie directly adjacent to the sinus cavity (85).
Formation of accessory air-cells is one of the unique imaging features of hyperpneu- matization. Standard pneumatization would generally lead to symmetrical allocation of air-cells around midline, while hyperpneumatized variants may show asymmetric air- cell development (86). The pterygoid recess, for instance, can be pneumatized, pushing air cells laterally into the pterygoid processes, often displacing the vidian nerve, or com- pressing contiguous vascular structures. Clival recess pneumatization is characterized by posterior air cell extension onto the clivus, often associated with cortical thinning adjacent to the basioccipital synchondrosis. Less frequent is lesser-wing pneumatization, which has been correlated with retro-orbital pain due to pressure on the orbital apex (87).
The differentiation of hyperpneumatization from pathological bone erosion contin- ues to pose a significant diagnostic conundrum. Neoplasm or chronic inflammatory con- dition-associated osteolytic lesions can also cause similar cortical thinning when seen along hyperpneumatized sinuses. Hyperpneumatized bone has a smooth, corticated margin, in contract, erosive bone has irregular, shaggy edges with overlying soft tissue infiltration. To differentiate trabecular bone from osteolytic tis-sue according to the en- ergy-dependent attenuation patterns, dual-energy CT has been especially valuable in these situations (88, 89). Both neural and vascular involvement are a radiologically con- siderable risk in hyperpneumatized sinuses. In cases where air cells extend laterally into the sinus wall, cranial nerves III, IV, V1, V2, and VI may be partially or completely ex- posed to the sinus cavity (28). Diffusion tensor imaging (DTI)-based MRI tractography has shown aberrant trajectories of the optic nerve and maxillary nerve pathways in hyperp- neumatized variants. Particularly, the internasal air cells may be hyperpneumatized and extend into the cavernous portion of the internal carotid artery, raising the potential for vascular injury during endoscopy (90).
4.3 Preoperative considerations
Preoperative evaluation of sphenoid sinus hyperpneumatization requires undergo meticulous image analysis, as it can greatly influence not only surgical risk profiles, but also procedural approaches. High-resolution CT with isotropic voxel acquisition is pre- ferred for any imaging protocols, thus enabling accurate three-dimensional reconstruc- tions (91, 92). Preoperative CT data should be scrutinized in the coronal, axial, and sagit- tal planes to assess the full extent of the sinus cavity, the integrity of its walls, and the relationships of adjacent neurovascular structures. Multiplanar reconstructions using thin-slice protocols (0.3–0.5 mm slices) are crucial for the identification of subtle bony de- hiscences or atypical septal attachments in the case of suspected hyperpneumatization (93).
Three-dimensional (3D) modeling is gaining importance in the preoperative plan- ning especially for cases with complex hyperpneumatization patterns. Software-based segmentation algorithms can produce patient-specific 3D reconstructions that better il- lustrate spatial anatomy of sinus architecture (94). AR applications can be incorporated into navigation systems in the OR, allowing real-time overlying of these models on the surgical site during endoscopy (95). Studies have suggested a 25–30% decrease in neuro- vascular complications only when AR-assisted navigation is integrated in surgeries with hyperpneumatized sphenoid sinuses (96). Re-operative conversations within the multi- disciplinary team (neurosurgeons, otolaryngologists, and radiologists) should include an- atomical obstacles and surgical techniques. These conversations should account for hy- perpneumatization location and extent, neurovascular dehiscence presence, and surgical corridor exit angle & trajectory (97). For example, hyperpneumatized sinuses with lateral pneumatization into the pterygoid recess may require modified approaches to prevent vidian nerve injury. Likewise, cases with optic nerve canal dehiscence will need to be approached with special care so as to avoid accidental nerve injury during instrumenta- tion (55).
AI-powered tools in preoperative planning: understanding the future for sinus anat- omy. Convolutional neural networks (CNN) and support vector machine (SVM) can per- form radiomics-based analysis and extract quantitative features from CT datasets that may help predict hyperpneumatization related complications (98). Such algorithms ex- amine texture patterns, volumes of the sinuses, and bone density metrics, generating pre- dictive clues regarding anatomical variants that could complicate the surgical approach by computational analysis. AI-augmented preoperative planning shows a 20–30% im- provement in anatomical landmark identification compared to human assessment alone in pilots studies (99). This needs to be a collaborative, technologically driven synthesis of advanced imaging techniques and deep knowledge of sinus anatomy and potential for surgery. Advances in imaging modalities, as well as the wider use of integrating AI and 3D modeling technologies, will likely lead to improved diagnostic accuracy, reduced in- traoperative risk and, consequently, better outcomes in skull base surgery with respect to hyperpneumatized sphenoid sinuses. Realization of these im-aging, anatomical, and sur- gical planning benefits has been followed by an expanding body of primary literature that examines the detailed intricacies of sphenoid sinus hyperpneumatization (100). A wide range of studies have characterized these aspects, including anatomical differences, mo- lecular pathways, imaging technologies and clinical results (101). Key findings from re- cent studies regarding the anatomical, radiological, and surgical concerns associated with sphenoid sinus hyperpneumatization, as well as their clinical significance and potential avenues for future research, are summarized in Table 1.
Table 1
| Research Focus | Key Findings | Original Literature Insights | Clinical Implications | References |
|---|---|---|---|---|
| Anatomical Var- iability | Pneumatization varies in direction and extent, frequently extending into clival and pterygoid regions. Air-cell patterns correlate with craniofacial morphology and airflow dynamics. |
CFD modeling suggests airflow- driven osteoclastic activation influ- ences air-cell extension. | Understanding anatomical variability aids in preopera- tive planning and reduces sur- gical uncertainty. | (50, 102, 103) |
| Neurovascular Risks | ICA dehiscence occurs in 15–25% of hy- perpneumatized sinuses; optic nerve canal dehiscence in 10–15%. Lateral pneumatization linked to higher cav- ernous sinus exposure. |
Histopathological studies show in- creased RANKL/OPG expression in dehiscent ICA walls; nerve displace- ment confirmed by MRI tractog- raphy. |
Preoperative identification of vascular dehiscence reduces the risk of hemorrhage and nerve injury. | (5, 104, 105) |
| Radiological As- sessment | High-resolution CT remains the stand- ard; DECT and PCCT improve bone- soft tissue contrast. 7T MRI reveals sub- tle mucosal remodeling and sinus wall irregularities. |
AI-enhanced CT protocols demon- strate improved detection of thin- walled sinus regions, with 90% accu- racy in identifying dehiscent ICA ca- nals. |
Refined imaging protocols en- hance surgical planning and reduce misdiagnosis of hy- perpneumatization as patho- logical erosion. |
(40, 106, 107) |
| Molecular Mechanisms | Pneumatization driven by osteoclastic activity; RANKL/OPG dysregulation increases trabecular bone resorption. SHH/BMP signaling influences cranio- facial growth patterns. |
Genomic analysis identifies RUNX2 polymorphisms associated with ex- cessive sinus pneumatization in de- velopmental syndromes. | Early genetic screening may identify patients at risk for hy- perpneumatization and re- lated complications. | (14, 15, 108) |
| Surgical Impli- cations | Hyperpneumatized sinuses complicate transsphenoidal approaches, distorting landmarks and increasing CSF leak risk. Lateral pneumatization affects vid- ian nerve trajectory. |
Neuronavigation advancements (AR/VR overlays) reduce operative time by 15%; PATS protocol demon- strates improved orientation in hy- perpneumatized cavities. |
Procedural adaptation and in- traoperative navigation re- duce complications in ana- tomically complex cases. | (109–112) |
| CSF Leak Dy- namics | Thinned skull base increases CSF leak risk by 3×; spontaneous leaks associated with posterior clival pneumatization. | Biomechanical studies reveal in- creased tensile stress at mucosa- bone interface in hyperpneumatized sinuses, correlating with leak inci- dence. |
Preventive use of collagen- based grafts and hydroxyap- atite reinforcements mitigates leak risks. | (11, 113) |
| Symptomatic Presentations | Retro-orbital pain, facial numbness, and atypical headaches common; associated with nerve displacement and sinus aer- odynamics. |
DTI studies demonstrate trigeminal nerve compression in pneumatized pterygoid recesses, correlating with facial pain severity. |
Early identification and sinus decompression can alleviate nerve irritation and related symptoms. |
(28, 114, 115) |
| Innovations in Sinus Surgery | AR/VR tools enhance sinus navigation; flexible endoscopic instruments im- prove visualization in irregular sinus cavities. |
Clinical trials show 30% complica- tion reduction when AR-guided neuronavigation used in hyperpneu- matized sinus surgeries. |
Adoption of AR-assisted tech- niques improves surgical pre- cision and reduces learning curve. |
(116, 117) |
| Long-Term Im- plications | Unclear long-term outcomes; potential links to CSF pressure irregularities and craniofacial pain syndromes. | Pilot studies suggest pneumatiza- tion patterns may correlate with al- tered intracranial compliance and headache incidence. |
Long-term follow-up with ad- vanced imaging may uncover predictive markers for chronic complications. |
(22, 118–120) |
This table presents a curated summary of recent primary research studies investigat- ing sphenoid sinus hyperpneumatization.
It highlights critical findings across anatomical, radiolog- ical, molecular, and surgical domains, providing a detailed overview of the structural variations, clinical implications, and technological advancements associated with this anatomical variant. The insights summarized here offer a foundation for understanding the challenges posed by hyperp- neumatized sphenoid sinuses and underscore the importance of continued research in this evolving field.
5 Clinical significance in neurosurgical practice
The sphenoid sinus has a uniquely strategic location at the skull base serving as an important anatomical corridor for transsphenoidal surgical approaches to the sellar and parasellar compartments. Hyperpneumatization of this sinus adds considerable variabil- ity to sinus anatomy with clinical significance beyond anatomical interest. Sphenoid hy- perpneumatization, defined by expansion of the sphenoid sinus into surrounding struc- tures, including the clivus, pterygoid processes and anterior occipital bone, positively im- pacts surgical access, procedural safety and outcomes (83). The importance of sphenoid sinus hyperpneumatization to clinical practice results not only from its ability to change the position of anticipated anatomical landmarks, but also from the environments it gen- erates to provide both opportunities and risks of surgery. These anatomical variants re- quire more sophisticated surgical plans, refined preoperative imaging protocols, and dil- igence in understanding the pathophysiological underpinnings of sinus pneumatization (121).
The increasing use of EEAs has highlighted the importance of understanding sphe- noid sinus anatomy in detail, as hyperpneumatized patterns can be an asset but also pos- ing potential difficulties in surgical procedures involving this area. Excessive pneumati- zation can also facilitate an extended surgical corridor into the sellar region, potentially reducing the amount of bony removal necessary during surgical resection (122). Often, these benefits are compromised by the structural vulnerabilities engendered by thin or dehiscent bony walls, especially when such walls protect neurovascular structures (optic nerves, ICAs, and cavernous sinus). The unpredictable anatomical variations that accom- pany hyperpneumatization highlight the important role for multidetector high-resolution imaging, sophisticated neuronavigation systems, and meticulous surgical techniques ad- dressing the altered biomechanical matrix of the sinus (5, 123).
Research indicates that the clinical ramifications of hyperpneumatized sphenoid si- nuses go beyond surgical considerations alone. More recently, the association of exces- sive pneumatization with some craniofacial pain syndromes and potential changes of cer- ebrospinal fluid dynamics and biomechanics of the pituitary gland have been reported (25). Despite being early in their investigation, these hypotheses highlight the wider im- plications of sphenoid sinus anatomy for both clinical and research interpretation (124). Therefore, elucidating the role of hyperpneumatization in neurosurgical practices neces- sitates a multi-tiered inquiry including molecular pathways, biomechanical processes, clinical implications, and surgical innovation (50).
5.1 Impact on transsphenoidal approaches
The transsphenoidal approach, performed employing either microscopic or endo- scopic techniques, relies upon the consistency of certain anatomical markers within the sphenoid sinus. For normal anatomy, the midline sphenoid septum, sellar floor and OCR function as landmarks that orientate the surgeon during the operation (11, 125). But in hyperpneumatized sinuses, these landmarks can deviate from their normal location, re- gress, or cease to exist altogether. These changes disrupt the surgical workflow and intro- duce navigational uncertainty, particularly when approaching deep-seated sellar or par- asellar lesions (2, 36).
The intersinus septum, which normally provides a stable midline reference, is sig- nificantly variable in hyperpneumatized variants. In nearly 30% of hyperpneumatized sphenoid sinuses, the main septum is laterally displaced or eccentrically inserted into the bony walls of the carotid canal or optic nerve sheath (126). Such an atypical septal anat- omy can confuse surgeons who depend on conventional midline septal orientation and can increase the risk of iatrogenic injury. Furthermore, the hyperpneumatization process often produces accessory septa that do not have the normal structural integrity and are thin, fragile bony plates that easily fracture when handled. This fragility complicates transsphenoidal approaches, because fractured septal fragments can inadvertently trau- matize surrounding neurovascular structures (127). The OCR, an important anatomical landmark and demarcation of transition from the sphenoid sinus to the cavernous sinus, is noted to undergo major structural alterations in hyperpneumatized sinuses. In many instances, the recess looks elongated, laterally displaced, or distorted by irregular air-cell extensions (128). MRI tractography analyses have demonstrated that hyperpneumatiza- tion can shift the relative positions of the optic nerves and ICAs, sometimes resulting in the protrusion of these structures into the sinus cavity with little or no bony separation. Such protrusions lead to dangerous arenas where even slight instrument malpositioning can have disastrous consequences (129).
Hyperpneumatization can lead to thinning of the sellar floor and even dehiscence. Morphometric CT studies have shown that hyperpneumatization of the sellar floor is as- sociated with its average thickness being reduced more than 60% compared to that of nor- mally pneumatized sellar floors (130). This thinning not only raises the risk of CSF leaks but also deprives the pituitary gland of its mechanical support. Imaging follow-up post- operatively in these patients with significant sellar floor thinning has also documented descent of the pituitary gland into the sphenoid cavity, raising concern of related dysfunc- tion of the gland in these patients (131).
Recent molecular biological advances have started to enhance our understanding of the mechanisms behind sphenoid sinus hyperpneumatization. In hyperpneumatized var- iants, the regulated interaction between osteoblastic and osteoclastic activity, which con- trols paranasal sinus development, seems to be disturbed. Overexpression of RANKL alongside lower levels of osteoprotegerin (OPG) has been found in sinus mucosa from patients undergoing transsphenoidal surgeries (132). This increased osteoclastic action hastens the resorption of trabecular bone and leads to the hyperpneumatized cavities seen in hyperpneumatized sinuses (133). Studies have also implicated mutations in genes in- cluding RUNX2, which regulates osteoblast differentiation, and FGFR2, a major mediator of cranio-facial morphogenesis, in the pathogenesis of hyperpneumatization. Certainly, these findings implicate sphenoid sinus hyperpneumatization as potentially, in some cases, manifesting an individual genetic predisposition toward aberrant cranial base de- velopment (134). Sphenoid sinus hyperpneumatization becomes relevant for the transsphenoidal approach; thus, the biomechanical effect of sphenoid sinus hyperpneu- matization is significant. Finite element modeling (FEM) studies showed that thinning of the sphenoid sinus walls changes the distribution of mechanical loads forced through the cranial base (135). In hyperpneumatized variants, load-bearing areas are transferred to the posterior skull base, this leads to increasing mechanical stress within the clival region. This redistribution may explain the increased incidence of stress fractures and spontane- ous CSF leaks in patients with extensive pneumatization of the sinuses (136).
In addition, modified mechanical properties of the sinus walls may affect the stabil- ity of surgical reconstructions used to treat iatrogenic CSF leaks. The reduced adherence of graft materials when applied onto hyperpneumatized sellar floors has been corrobo- rated by experimental investigations, implying that in such situations, conventional au- tologous fat grafts and collagen patches may require adapted application techniques. The possible relationship between patterns of sinus pneumatization and pituitary gland bio- mechanics, by virtue of the direct anatomical relationship to the sellar floor, is yet another area of potential exploration (41). The anatomical variability associated with hyperpneu- matization warrants aquisition of modern image guided neuronavigation within tran- sphemoidal operations (110). These systems take preoperative CT and MRI datasets to generate 3D models of the sinus cavity and can provide real-time feedback on the posi- tion of an instrument during surgery. Neuronavigation is especially advantageous in cases of hyperpneumatized sinuses, where classic ananatomic landmarks may be unreli- able or missing (137).
Current neuronavigation systems utilize AR interfaces, projecting virtual reconstruc- tions of the sinuses onto the endoscopic display. Researchers examining AR-assisted neu- ronavigation for skull base surgery in a clinical study scribed a 27% decrease in intraoper- ative complications versus conventional guide methods. The decreased use of the soft- ware was attributed to better visualization of dehiscent bone areas and ectopically posi- tioned septa, in hyperpneumatized sinuses. Instead, intraoperative CT devices with neu- ronavigation systems provide for real-time reorientation due to anatomical displacements produced by tumor removal or CSF loss (95).
Hyperpneumatized sphenoid sinuses can be detrimental and have led to new surgi- cal techniques to maximize procedural outcomes. One of these innovations is as follows, by utilizing pneumatization-adapted transsphenoidal surgery (PATS) which is a protocol that adapted the path of surgical trajectories based on preoperative imaging features. PATS protocols integrate sinus morphometry via the navigation algorithm to account for anatomical distortions during planning. Initial findings indicate that PATS leads to re- duced operative durations as a result of fewer intraoperative recalibrations and improved anatomical predictability (9, 138). A novel method includes the usage of hydroxyapatite-reinforced bone grafts for the reconstruction of thinned sinus walls after tumor removal. These grafts offer greater me- chanical stability than conventional fat or fascia grafts and have shown long-term struc- tural stability in hyperpneumatized sinuses. Nor has the concept of using biodegradable collagen matrices for mucosal regeneration been ignored, and, most recently, surgeons have attempted to employ this material in order to avoid postoperative collapse of the enhancing mucosal flap, especially in extremes of pneumatization (139). Hyperpneumati- zation of the sphenoid sinus has previously been considered a purely anomalous anatom- ical variant, however new studies have suggested that extensive pneumatisation may be associated with some craniofacial pain syndrome. Synonyms since pain is often sug- gested in cases of persistent facial pain and headache, trigeminal nerve compression, caused by expansion of the sinus cavity into the recess of the jawbone. Changes in posi- tion of the pituitary gland that occur with thinning of the sellar floor have also raised the possibility of endocrine sequelae, but evidence remains limited (140). The evolution of new neurosurgical techniques and imaging technologies will certainly improve our knowledge of sphenoid sinus hyperpneumatization and its clinical significance. Future research should prioritize the development of predictive models that estimate hyperpneu- matization severity from craniofacial morphometry data, as well as the genetic basis that causes such anatomical variation. Furthermore, machine learning algorithms applied to radiomic data could offer new biomarkers for the preoperative identification of high-risk variants (141). The future of neurosurgical management of hyperpneumatized sphenoid sinuses will truly be paved with a combination of these anatomical, molecular, biome- chanical and clinical insights. With greater understanding, surgical algorithms will likely utilize ever increasing constructs of patient anatomical data to augment procedure safety and efficacy (142).
5.2 Risks and complications
Hyperpneumatization of the sphenoid sinus, while generally considered a benign anatomical variant, presents neurosurgical concerns that arise from the challenge to skull base integrity. Any aberrant pneumatization of the clivus, pterygoid processes, or greater wings of the sphenoid has the potential to alter the biomechanical stability of the cranial base, cause thinning or dehiscence of protective bony walls, and predispose the surgical approach to increased risk of CSF leaks or neurovascular injury. If this were the extent of the potential adverse effects, the implications would be timely corrective surgical measures. However, these structural shifts or anomalies can correlate with various symptomatic presentations like headache and facial pain. The extensive nature of these complications clearly warrants a thorough examination of their potential pathophysiological mechanisms, clinical significance, and existing approaches to diagnosis and management (143).
Neurovascular injury is perhaps the most significant complication associated with hyperpneumatization, specifically in relation to injury to the ICA during surgery. ICA canal dehiscence is present in 15–25% of cases of the hyperpneumatized sphenoid sinus, with complete bony exposure in 3–7% of cases (6, 30). The anatomical reason for this vulnerability relates to excessive osteoclastic activity through which the expression of RANKL is upregulated and OPG is suppressed in the sinus mucosa (144, 145). RUNX2 polymorphisms could negatively impact bone integrity of the sphenoid area (146). The association of intra-operative ICA injuries experienced when the anterior septal attachments extend to the carotid canal reinforce the importance of high-resolution CTA preoperatively to characterize at-risk configurations (147, 148). Optic nerve canal dehiscence, which can occur in 10–15% of cases, also increases the risk of iatrogenic optic nerve injury. Bony attenuation at areas like the opticocarotid recess can reduce wall thickness to below 0.2 mm and place the nerve at risk for sustained mechanical injury or thermal injury. DTI research has shown that altered fiber pathways will be found in these scenarios that correlate with a chronic compressive stress (149). Involvements into lateral or pterygoid recesses may add pressure to cranial nerves such as III, IV, V1, V2, VI, or the vidian nerve in an occupying manner that presents with chronic orbits facial pain, and/or chronic orality with or without autonomics. Trigeminal neuralgia has even been described with pneumatization to the foramen rotundum (16).
The only route for prevention is detailed preoperative imaging. Dual energy CT and high-angle CTA will require thin slice (0.3 mm) CT so that critical neurovascular components can be adequately mapped (150). In the case of hyperpneumatization, dual energy CT (DECT) will be helpful to help delineate thinned hyperpneumatized bone, and pathologic erosion. The integration of AR integrated neuronavigation is capable of providing improved intraoperative orientation even with the distortion of anatomical landmarks (151). In situations where dehiscence is noted, it may be possible to employ low-speed drilling instruments (e.g., diamond burrs) in situations of thermal injury and the Doppler ultrasonography can preserve intraoperative vascular mapping (152). An MRA may be helpful postoperatively to report on vascular integrity or the presence of pseudoaneurysm (153).
CSF leaks are a more serious consideration. These leaks will typically occur at the area of the skull base to the location of the skull base where the cortex, at the sellar floor and/or clival recess area, thins out (154). Further, risk for CSF leaks is at least doubled (2-3 fold) with hyperpneumatized anatomy versus what considered normal sinus anatomy (155). Finite element models demonstrate significant stress concentrating due to an intracranial pulley from the thinning of the cortex to below 0.5 mm thickness (58, 156). These risks are only going to be magnified by any form of inflammatory process. CFD-documented alterations in air flow patterns increase shear stress in the sphenoethmoidal recess stimulate mucosal remodeling and have been shown to upregulate matrix metalloproteinases (MMPs) and decrease collagen deposition weakening the dura (157, 158). The symptoms of CSF rhinorrhea can present very subtly and mimic allergic rhinitis. Associated symptoms can include orthostatic headache, a metallic taste, or visual disturbances (159).
High-resolution CT cisternography and intrathecal gadolinium-enhanced MRI imaging provide sensitive diagnostic modalities to localize the leaks (160). The standard for repair is autologous fascia lata or collagen grafts. For larger defects, vascularized nasoseptal flaps will provide a solid closure and integration into the mucosa (161). Hydrogel sealing systems are now marketed and experimental stem cell filled scaffolding are being utilized but not yet formally studied for dural regeneration (162). The symptomatic syndromes will also include chronic headaches, facial pain, and visual disturbances. Headaches are usually deep, retro-orbital, or occipital which can closely resemble primary headache syndromes. Such headaches could be the result of trigeminal nerve impingement, or mucosal inflammation or compression of the optic nerve. DTI studies are showing microstructural changes in the affected nerve in imaging of symptomatic patients (163–165). Facial pain is common if the pterygoid recess is involved, especially if the vidian nerve is displaced. In some cases, decompressing this area helped alleviate symptoms (166, 167). Visual symptoms including diplopia or optic neuritis have been reported if the optic nerve where compressed in patients with canal dehiscence, likely as a result of ischemia or direct mechanical insult to the nerve (168).
In conclusion, sphenoid sinus hyperpneumatization may no longer be hypocritical as simply an incidental anatomical variation. It deserves a much deeper understanding of the pathogenesis, radiological findings, and clinical impacts as it relates to skull base neurosurgery. Further research into the unique genetic and molecular regulators of pneumatization signals such as FGF and SHH signalling pathway could be invaluable to developing knowledge on disease processes. Future directions that utilize AI morphometric and radiomic assessments for earlier risk stratification, and biomechanical assessments of 3D printed sinuses, could help direct surgical developments. Greater understanding of these processes in turn could improve safety and care for those undergoing neurosurgical intervention (1, 169).
6 Management strategies
The management of hyperpneumatization of the sphenoid sinus presents a particu- larneurosurgical challenge due to the anatomical unpredictability and surgical vulnera- bilities evoked by excessive pneumatizations. The large air cells, which at times extend to the clivus, pterygoid processes, or greater wings of sphenoid, conjoin to form a surgical terrain unique in distorted spatial relations and obliterated or missing bony partitions ad- jacent to neurovascular elements of paramount significance. This anatomical deviations demand an individualized evidence-based strategy of management with respect to the preoperative, intraoperative and postoperative phases (170). As evident from this paper, optimal results will be reliant on complex imaging protocols, straightforward surgical modifications, and close postoperative vigilance, all with directive assistance from a re- search and technology-intensive multiprofessional milieu (171).
6.1 Preoperative planning
Consequently, preoperative planning of hyperpneumatized sphenoid sinuses in CLG must follow a multi-pronged strategy, ensuring anatomical fidelity yet preserving with facets of procedural insight. In hyperpneumatized cases, dynamic alteration of sinus morphology should guide individual assessment, as well as the consideration of detailed imaging analysis, anatomical risk stratification, and a multidisciplinary approach for planning (172).
Radiological investigation of the hyperpneumatized sphenoid sinuses which pre- sents a complexity in evaluation because; unlike ordinary protocols, there are subtle struc- tural changes it being a rare entity, thus demanding detailed investigation of the same. High-resolution multidetector computed tomography (MDCT) is the anatomical gold standard, with modern CT scanners capable of achieving isotropic voxel sizes of 0.2–0.3 mm to create three-dimensional reconstructions with exquisite spatial resolution (71). However, hyperpneumatized variants are intricate and supplementary methods are needed. New spectral pho-ton-counting CT (PCCT) utilizes energy-de-pendent attenua- tion coefficients within the area of interest to map in vivo trabecular micro-architecture at submillimeter resolution, ultimately providing enhanced sensitivity in differentiating a hyperpneumatized bone from pathological osteolysis (173). Risk stratification is being transformed with the use of algorithms based on bone texture analysis derived from pre- operative imaging. Such analyses depend on algorithms for which machine learning models are trained using very large-scale sinus CT datasets to evaluate bone homogeneity, cortical porosity, and fractal dimensions to describe sites at high risk for intraoperative fractures or dural dehiscence (174). Early clinical studies demonstrated that applying these machine learning techniques to bone texture analysis within the pre-operative work- flow improved the prediction of surgically significant anatomical variants by 20–25% and such information offers a rationale for actionable insights which could help inform surgi- cal planning (175, 176).
MRI is key to understanding adjacent neurovascular anatomy adj neurovascular structures and the integrity of the surrounding soft tissues. Black-blood MRI techniques, which attenuate signals from vascular lumens and accentuate walls of vessels, proved particularly useful in the identification of CA dehiscensce and early vessel wall pathology (177). There has also been recent application of diffusion-weighted magnetic resonance spectroscopy (DW-MRS) further characterizing sinus mucosal changes by quantifying re- spective molecular signatures abroad of chronic mucosal stress and dysregulated dynam- ics of airway pneumatization (178).
In summary, hyperpneumatization presents with some anatomical complexity that should be discussed in detail in a multidisciplinary team preoperatively. Planning and collaborative discussion of the imaging findings would facilitate anticipation of what challenges should be anticipated procedurally, and contingency strategies amongst the neurosurgeons, otolaryngologists, neuroradiologist, and anesthesiologists (179). Discus- sions should ideally address the presence of anatomical variances (i.e. extensions of the clival recess, aberrant septal attachments, pneumatization of the pterygoid process) that- may complicate surgical navigation. In order to make this collaborative process easier, innovative computational tools such as personalized anatomical atlases have been devel- oped These are produced from patient-specific imaging datasets and allow interactive ma- nipulation in a 3D sinus model, simulating appeals and predicting anatomical shifts due to tumor resection or CSF leak repair for a surgical team (180).
Patients should be able to specify in preoperative counseling visit whether they have hyperpneumatized sinus anatomy and to what extent and should understand (in evi- dence based terms) the risk this anatomy poses. In general practice, such discussions should arise not only in the context of the risk of standard surgery but also with respect to longer operative times, elevated risks of CSF leak and subsequent risk for transient postoperative neurologic symptoms like altered facial sensation or headache. The benefit of using patient-specific 3D models is that during these sessions, the patients realized bet- ter understanding of the surgical procedure and develop less perioperative anxiety (41).
In support of this patient-centered and technology-integrated approach, Figure 2 intends to outline a structured preoperative planning workflow that synthesizes imaging precision, risk modeling, and multidisciplinary coordination to enhance both surgical preparedness and patient understanding.
Figure 2
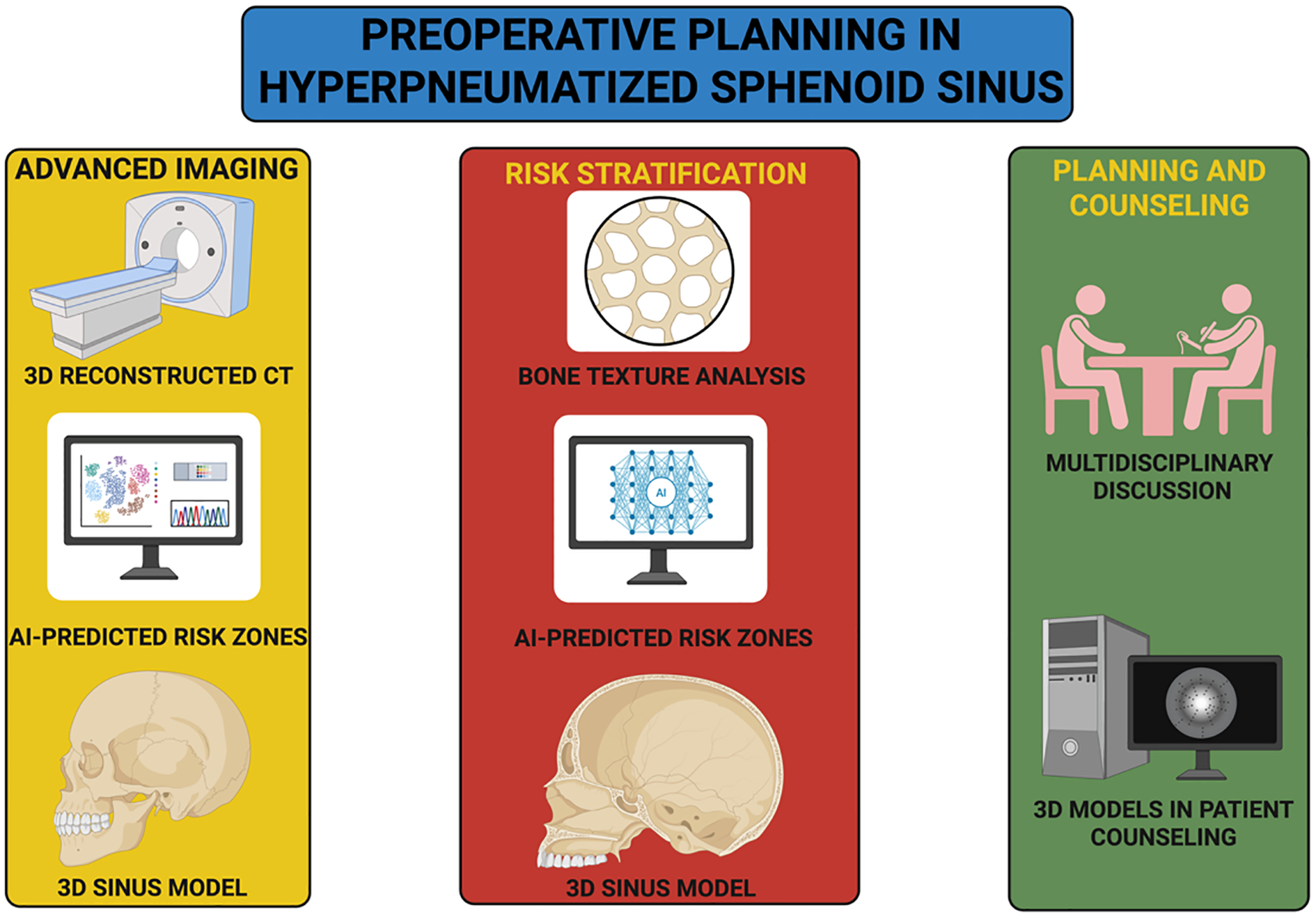
Structured preoperative planning workflow for hyperpneumatized sphenoid sinus.
The diagram illustrates an integrated clinical pathway for managing anatomically complex sphenoid sinus pneumatization. The process begins with advanced imaging techniques, including high-resolution CT and AI-augmented analysis to generate personalized 3D sinus reconstructions and identify high-risk anatomical zones. These data inform risk stratification through bone texture analysis and machine learning–based prediction of structural vulnerabilities, such as dural dehiscence or ICA exposure. The workflow culminates in multidisciplinary discussion involving neurosurgeons, otolaryngologists, neuroradiologists, and anesthesiologists, accompanied by patient counseling aided by interactive 3D models. This structured approach aims to enhance surgical planning, support shared decision-making, and reduce perioperative uncertainty.
6.2 Surgical techniques
Hyperpneumatized sphenoid sinuses in Publications should be addressed surgically with methods, specifically adapted to the particular constraints of such anatomic malfor- mations, malleable bony covers andremodelled neurovascular interactions and the need for greater interdisciplinary communication and collaboration. The erratic course of such pneumatized air cells call for surgical flexibility, vigilance inspite of surgical progresses, and the application of innovative surgical technologies (181). Hyperpneumatization cre- ates and imperfect space which the Atlas and inverse forms of the aforementioned tech- niques must be adapted to. The primary modification is to recalibrate the anatomy re- peatably in the operative navigation systems. In cases of hyperpneumatized sinuses, standard landmarks such as the sphenoid septum and opticocarotid recess may be located in their normal locations, but visualization can be difficult (182). In these situations, ma- chine-learning–augmented neuronavigation platforms have greatly improved an-atomi- cal localization. These systems combine real-time imaging data analysis on a continuous basis and identify and visualize the anatomical transformations that occur during bonet thinning associated with pneumatization (183). When selecting access corridors in hy- perpneumatized sinuses, consideration should be given to the distribution of air cells and the location of critical neurovascular structures. A new approach ‘lateralized sphenoid corridor technique’ serves as a way to remove lesions present in highly pneumatized si- nuses with extreme lateralized pneumatization. Intraoperative Doppler ultrasound has since been used to identify the vidian canal and sphenopalatine artery as landmarks in approaching pneumatized pterygoid recesses, however this technique was carried out prior to these ultrasound-derived landmarks being described (10).
Given the fragile nature of hyperpneumatized sphenoid walls, we can expect to per- form bone removal with fine instruments and fine-controlled forces. An approach that can prevent accidental penetration through thinned osseous partitions is sensorized re- sistance-based adaptive microdrills. These drills tab auto-rotate torque modulate when they encounter the resistance of low-density bone and reduce the risk of a dural perfora- tion. Tools specifically designed for cranial sinuses, such as angle endoscopes with stere- otactic overlay systems have also been employed within complex pneumatizedconfigura- tions. To facilitate safer dissections around important landmarks (i.e., cavernous sinus and optic canal) (184).
In hyperpneumatized sphenoid surgeries, preserving mucosal integrity is crucial in order to reduce postoperative complications. Surgical techniques which aim to preserve architecture such as mucosal-sparing dissection contemporaneous with blunt dissection and hydro dissection agents have demonstrated smooth sinus mucosal architecture in the most pneumatized of cavities. To a comparative reduction of postoperative sinus dysfunc- tion and more rapid reepithelialization of the mucosa in-situ (185).
6.3 Postoperative care and follow-up
The management of patients with hyperpneumatized sphenoid sinuses in the post- operative period must be adapted to the vulnerabilities that excessive pneumatization provides. The major goals in this period are the early identification of complications, preservation of sinus function, and anatomical and functional assessment (105).
Hyperpneumatized skull bases are structurally deficient whereby patients are sus- ceptible to postoperative CSF leaks that can be associated with significant morbidity. Sur- veillance protocols must contain serial nasal endoscopic examinations to evaluate surgical disruption sites and early indicators of CSF leak as chronic rhinorrhea or enhanced nasal output. Of these, transnasal intrathecal fluorescein testing has demonstrated the highest sensitivity for occult leaks, with sensitivities to detect a leak of >95%, when high-resolu- tion MRI cisternography is utilized in conjunction (186). Dynamic nasal airflow monitor- ing is a new noninvasive assessment of postoperative sinus function. This method uses micropressure sensors to measure air-pressure differentials in the sphenoethmoidal recess and provides indirect evidence of CSF leaks by detecting abnormal airflow patterns with- inunsealed dural defects (187). Mucociliary clearance dynamics (altered) with increased mucosal (surface) area for infection—hyperpneumatized sphenoid sinuses are predis- posed to development of postoperativeinfections. Preventive strategies should focus on optimal sinus aeration and regulating mucosal health. Nasal irrigations that disrupt bio- films that include xylitol-based solutions are shown to reduce postoperative sinus infec- tions by destroying bacterial biofilms and preventing bacterialcolonization of exposed mucosal surfaces (188). Compared to conventional non-contrast enhanced sequences, con- trast-enhanced T1-weighted MRI sequences should be integrated into the postoperative imaging armamentarium to evaluate for infectious complication, possibly involving the out-of-sinus cavity. Infections of the pneumatised clival or pterygoid recess can be intra- cranially spreading and should be treated expeditiously (antibiotic spectrum guided by intra-operative cultures when available) (189).
Due to the close proximity of hyperpneumatized sphenoid sinuses to important neu- rovascular structures, we recommend that postoperative neurological assessment extend beyond standard visual and cranial nerve exams. In follow-up assessments, multimodal evoked potentials (MEPs) are able to detect subclinical deficits in cranial nerve function, most notably in-patients with optic nerve canal pneumatization (190). Studies showed that the detection of early-stage optic neuropathy was sensitive and non-invasive in individu- als with predominated sinus pneumatization using pattern-reversal visual evoked poten- tials (PR-VEPs) (191). Hyperpneumatization may have implications for the stability of the skullbase in long-term follow-up. High-tech complementing imaging with4D flow MRI revealed deranged cerebrospinal fluid dynamics in patients of hyperpneumatized si- nuses that could pave the way for idiopathic intracranial hypertension or spontaneous CSF leaks’years down line (192). Reassuring intradural ICP episodes and periodical im- aging should be reserved to those with prominent posterior or clival pneumatization (193).
Over time, imaging, surgical instrumentation, and personalized medicine will grad- ually improve and revolutionize the non-invasive management strategies of sphenoid si- nus hyper-pneumatization. It would be helpful to further explore the different molecular mechanisms governing this process of sinus pneumatization, and in particular whether hypoxia-inducible factors (HIFs) affect the activity of osteoclasts at the sinus walls (194, 195). Furthermore, there could potentially be individualized risk-based surgical al- gorithms introduced in preoperative planning software combined with AI-based ana- tomic mapping algorithms allowing materialized predictive tracts (196).
With further studies, the development of clinical, anatomical, and biomechanical workup will be essential in order to optimize surgical techniques, improve patient out- comes and define sphenoid sinus hyperpneumatization as an anatomical variant of im- portance (124).
7 Future directions and conclusions
The sphenoid sinus was once considered an inert anatomical space of limited clinical significance. Pneumatization patterns, particularly when excessive, afford them surgical topographies counter to traditional guidelines, necessitating a problem-solving mentality, adaptability, and multimodal strategy. However, the literature review indicated that our understanding of sphenoid sinus hyperpneumatization remains fraught with a number of questions. This review illustrates the incapability of current approaches with the modernimaging modalities, molecular biology, computational modeling, and advanced surgical techniques that are leading us to a future in which these questions may be an- swered (40). It does so by subsequently providing areas of continued exploration to focus on, emerging technologies likely to strongly influence their clinical application, and mus- ings on the enduring scientific interest in this remarkable organ.
7.1 Research gaps and unanswered questions
To date, anatomical counterparts of sphenoid sinus hyperpneumatization have been documented with increasing frequency but remain unclassified using a uniform diagnos- tic taxonomy. However, similar terminology denominative conchal, presellar, sellar, and postsellar patterns—current in writing but does not study the morphometric characteris- tics that differentiate distinctly one of each other. This non-standardized characterization limits the ability to associate variants with clinical results, making more challenging deci- sions during the surgery and also comparative data from institution to institution. Such performance based grading system should be developed universally based on volumetric, morphometric, and biomechanical parameters. A modern variation of sucha system would likely be capable of not only assigning an LOC stage to the patient based on the multislice CT images, but also of stratifying severity with complex radiomic analyses, and metrics like ratios of sinus volume to bone-thickness and the extent of pneumatization into contiguous skull base structures (197).
In courage, wherever classification fails, the long run consequences of sphenoid si- nus hyperfect-one-ness are still yet to be completely gripped. While short-term perioper- ative complications such as CSF leaks and neurovascular injury have been extensively characterized, long-term sequelae have remained relatively understudied (198). Does hy- perpneu-matization affects the compliancy of cranial base leading to spontaneous intra- cranial hypotension as risk factor? Does chronic mechanical stress on the neurovascu- larunits explain late-onset cranial nerve complications? Further prospectivelongitudinal studies with imaging and neurocognitive assessments at established intervals may eluci- date these possible associations, and inform preventive interventions (199).
Otherwise, molecular basis of hyperpneumatization Also remain to be the underex- plored frontier. Introduction: Pneumatization is a multifactorial process regulated by sig- naling from the epithelium, osteoclastic activity and cranial base growth patterns. Pre- clinical data indicate that dysregulation of RANKL/OPG signaling pathways may play a role to be responsible for exaggerated boneresorption in regions of hyperpneumatization (200). The regulatory networks downstream of this process, and above all how theyare modulated by hypoxia-inducible factors (HIFs) and fibroblast growth factor 10 (FGF10), remain poorly characterized. Investigation of these pathways utilizing high-resolution single cell RNAsequencing and CRISPR-based gene editing models may unveil molecu- lar signatures that serve as predictive markers for the development of a pathological sinus (201).
An avenue that establishes the correlation of hyperpneumatization and sinonasal physiologythis needs exploration. Several studies mentioned earlier based on CFD indicate that excess pneumatization alters airflow dynamics within the sphenoethmoidal re- cess area which might play a role in inducing the mucosal inflammation, barosinusitis or headache syndromes (202). But those simulations are still theoretical, with no clinical data to support them. Such theory was distributed at modeling efforts, pro-spective studies with in vivo (real-time)nasal-airflow monitoring may help find if such aerodynamic dis- turbances are clinically relevant (203).
7.2 Technological advancements and future innovations
The field of skull base surgery is evolving into a dynamic portal in the sky, where technological developments are rapidly changing the diagnostic and treatment para- digms. Novel technologies like imaging, navigation and intraoperative monitoring can significantly enhance the handling of hyperpneumatized sphenoid sinuses (204).
7.2.1 Augmented reality and virtual reality in surgical planning
AR and VR platforms capable of integration in the preoperative planning have moved from proof-of-concept to practical application. AR systems including marker- based tracking, or surface registration algorithms are already delivering some value for intraoperative orientation and augmenting anatomical landmarks in the operative field (205). We are at the tipping point between future AR systems and holographic sinus re- constructions that are projected directly into the endoscopic viewport. The holograms were generated from high-resolution CT datasets and could potentially be updated dy- namically to match the position of a instrument in real time, if this were done in reality, thus providing a more intuitive, spatially accurate presentation of the3D architecture of the sinus cavity (206).
While virtual reality is mainly used for surgical education at this time, it may soon prove to be essential within the patient-centered area of pre-operative simulations. VR platforms that use physics based anatomical modeling could enable surgeons to practice navigating hyperpneumatized cavities in silico, practicing potential complications such as intraoperative hemorrhage or septal dislocation. Pilot studies have demonstrated that VR-based rehearsal sessions are able to decrease operative time byas much as 18%, high- lighting the more tangible applications of this type of technology in this domain (207).
7.2.2 AI-driven imaging and predictive analytics
AI applications in sinus imaging represent one of the most promising frontiers in Its brightest future for skull base surgery may lie in applications for imaging ofthe sinus. Radiomic analysis, as a novel technique based on quantification of patterns of texture, shapes, and intensity on imaging datasets, has become a new tool to assess and predict potential hyperpneumatization degree and associated surgeons’ risk. These may in turn be followed by deep learning CNNs trained on large-scale imaging repositories to detect anatomical signatures of high risk variants — such as carotid artery dehiscence or sellar floor thinning (208).
But not on anatomical characterization, the concept of predictive analytics also ex- tends. An area of recent research has focused on integrative (prognostic) models to predict long-term outcomes by integrating imaging features with traditional patient de- mographics, genetic markers, and clinical history into a unified model. Such models, if confirmed in heterogeneous patient cohorts, may provide individually tailored risk fac- tors to surgeons, and guide decision making for choice of proc-l strategy (209, 210).
Endoscopic instrumentation is evolving with new imaging and navigation technol- ogy. Through novel adaptive optical systems, next-generation endoscopes are now able to dynamically adjust both focus length and image resolution in real continuum to best visualize patency in anatomically non-conforming cavities. Creating scalpel-formatted multi-lumen endoscopes containing intraluminal, embedded probes for nerve stimulation also offers an additional layer of safety when resecting adjacent hyperpneumatized lat- eral walls containing cranial nerves (211).
7.3 Expanding clinical perspectives
The implications of sphenoid sinus hyperpneumatization are not restricted to skull base surgery. The functional consequences of changed sinus anatomy may be widespread, spanning specialties from neurology, to oto-laryngology, to pain medicine (212).
For instance, the suspected linkage of hyperpneumatization to headache syndromes has not yet been definitively established. The persistence of retro-orbital discomfort in some patients with pneumatized clival recesses is contained in retrospective studies that corroborate potential trigeminal nerve irritation. Perhaps studies combining neurologists and headache specialists might clarify these associations and further the role of sinus anatomy in the physiopathology of craniofacial pain (213). Moreover, hyperpneumatiza- tion may also contribute to alterations in biomechanics, which may affect alterations in cerebrospinal fluid dynamics that may play a role in diseases such as idiopathic intracra- nialhypertension and spontaneous intracranial hypotension. Studies using 4D flow MRI to clarify CSF flow patterns through different sinus morphologies may offer some clues towards cranial base related subsystems that contribute to overall regulation of ICP (214). One area of growing importance in sinus management is regenerative medicine. Tissue-engineering approaches, including a bioengineered mucosal graft with patient-de- rived stem cells, could conceivably be employed to fortify thinned sphenoid walls or re- store injured mucosa in the future (215). Pilot studies have as yet validated the feasibility of this approach for frontal sinus repair with case for future expansion to defects related tosphenoid hyperpneumatization (9).
7.4 Conclusions
The sphenoid sinus is one of the most enigmatic paranasal sinuses and a microcosm of anatomical surgical and scientific interest. What initially appeared to be a passive ana- tomic variant, hyperpneumatization, has subsequently emerged as a dynamic, clinically relevant event that challenges many of the tenets of established wisdom about cranial base morphology and skull base surgery. We have navigated these hallowed lumens of sphe- noidsinus, however meticulous, understanding the anatomical details, radiological ex- pression, surgical ramifications of sphenoid sinus hyperpneumatization like never be- fore. These observations highlight how important it is to take a cooperative, innovative approach to such anatomical variation, which reduces morbidity. Now, with the advance- ment in imaging modes, well-honed surgical techniques and the possibilities, computa- tional modes are helping us immensely in tuning hyperpneumatization of sinus and in treating them better. But even with those advances, we still don’t know enough. Mecha- nisms to date explaining pneumatization, biomechanical effects of sinus cavity expansion, and long-term effects of reduced or absent cranial nerve function for the remainder of an individual’s life have differed, and one correlate of this lack of adaptive ease may be the multitude of molecular pathways augmented by biomephysical and biomechanical fac- tors that remain richly fertile domains of future research.
In many ways, the anatomy of the sphenoid sinus is a microcosm for the larger pur- suit of anatomical knowledge. It serves as a reminder that small ana-tom-ical curiosities can have mighty clinical consequences, overturning well established assumptions and leading to new revelations. The sphenoid sinus remains at the end of this paper what it has always been: the hidden chamber at the heart of the skull base, breathing soft whis- pers of secrets to those who will listen. And while there have been broader mysteries and more to come, the multitude of layers of billions of singular economies sending their tem- porary gifts out is too much to overcome, but as the spectres of future higher minds await the riddle of the dead and how immediate, as if we knew exactly what human tissues lay impounded inside the irony of the anatomical histories.
Statements
Author contributions
AB: Conceptualization, Writing – original draft, Validation, Data curation. FF: Writing – review & editing, Conceptualization, Validation, Formal Analysis. CT: Methodology, Supervision, Validation, Conceptualization, Writing – original draft, Visualization, Resources. RC: Data curation, Investigation, Writing – original draft, Writing – review & editing, Visualization. OM: Writing – original draft, Resources, Supervision. MS: Methodology, Investigation, Writing – review & editing, Supervision, Resources, Formal Analysis, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
12 January 2026 A correction has been made to this article. Details can be found at: 10.3389/fendo.2026.1781516.
16 January 2026 A correction has been made to this article. Details can be found at: 10.3389/fendo.2026.1781516.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Sagar S Jahan S Kashyap SK . Prevalence of anatomical variations of sphenoid sinus and its adjacent structures pneumatization and its significance: A CT scan study. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. (2023) 75:2979–89. doi: 10.1007/s12070-023-03879-y
2
Jain Y Shinde V Babu M . A computed tomography (CT)-based observational study of anatomical var- iations in the sphenoid sinus: implications for surgical planning and patient outcomes. Cureus. (2024) 16:e66410. doi: 10.7759/cureus.66410
3
Ominde BS Ikubor J Igbigbi PS . Pneumatization patterns of the sphenoid sinus in adult Nigerians and their clinical implications. Ethiop J Health Sci. (2021) 31:1295–302. doi: 10.4314/ejhs.v31i6.26
4
de Mendonca̧ DS Newman NI Leite JER Ribeiro EC Gurgel ML Júnior CMC et al . Surgical-related morphological characteristics of sphenoid sinuses: A comprehensive CT-based analysis. J Clin Exp Dent. (2024) 16:e1445–53. doi: 10.4317/jced.62099
5
Karpishchenko S . Isolated sphenoid sinusitis: anatomical features for choosing a method of treatment, a case-control study. Diagnostics. (2022) 12. doi: 10.3390/diagnostics12051284
6
Fadda GL Petrelli A Urbanelli A Castelnuovo P Bignami M Crosetti E et al . Risky anatomical variations of sphenoid sinus and surrounding structures in endoscopic sinus surgery. Head Face Med. (2022) 18:29. doi: 10.1186/s13005-022-00336-z
7
Hsu C-P Lin C-F Yang C-C Jeng J-Y Huang C-H . Gender differences in ethmoid sinus morphology_ 3D reconstruction of computed tomographic images. BMC Med Imaging. (2024) 24:139. doi: 10.1186/s12880-024-01319-z
8
Abarca-Olivas J Bärtschi P Monjas-Cánovas I González-López P García-Garrigós E Sánchez-Payá J et al . Three-dimensional reconstruction of the sphenoid sinus anatomy for presurgical plan- ning with free OSIRIX software. J Neurol Surg Part B Skull Base. (2021) 83:e244–52. doi: 10.1055/s-0041-1725169
9
Ramos HF Pinheiro-Neto CD Possatti LL . Pneumatization of the sphenoidal sinus may affect endo- nasal cranial base reconstruction. J Craniofac Surg. (2022) 33:e808–10. doi: 10.1097/SCS.0000000000008690
10
Treviño-Gonzalez JL Santos-Santillana KM Maldonado-Chapa F Morales-Del Angel JA . Neurovas- cular structures in the lateral recess of the sphenoid sinus. A computed tomography evaluation. Neurocir Engl Ed. (2023) 34:105–11. doi: 10.1016/j.neucie.2022.11.011
11
Poojary SR Kini DV Kapilamoorthy TR Chittaragi KB Gurumurthy B . Spontaneous cerebrospinal fluid fistula secondary to hyper-pneumatized paranasal sinuses and skull base: two case reports. Egypt J Radiol Nucl Med. (2023) 54:9. doi: 10.1186/s43055-023-00955-9
12
Utsunomiya N Katsube M Yamaguchi Y Yoneyama A Morimoto N Yamada S . The first 3D analysis of the sphenoid morphogenesis during the human embryonic period. Sci Rep. (2022) 12:5259. doi: 10.1038/s41598-022-08972-w
13
Açar G Gökşan AS Aydoğdu D . Computed tomography based evaluation of the association between sphenoid sinus pneumatization patterns and variations of adjacent bony structures in relation to age and gender. Neu- rosurg Rev. (2024) 47:349. doi: 10.1007/s10143-024-02594-8
14
Higashino M Abe S Sawada M Yamada H Ayani Y Haginomori S-I et al . Development of the sphenoid sinus in Japanese children: A retrospective longitudinal study using three-dimensional computed tomography. J Clin Med. (2022) 11. doi: 10.3390/jcm11216311
15
Aksakal C Aktı S Çeker ME Subaşı Aksakal B Sapmaz E Gökçe E . Development of the sphenoid sinus from newborn to age 18: A computed tomography imaging analysis. Int J Pediatr Otorhinolaryngol. (2022) 162:111327. doi: 10.1016/j.ijporl.2022.111327
16
Whyte MP Weinstein RS Phillips PH McAlister WH Ramakrishnaiah RH Schaefer GB et al . Transmembrane protein 53 craniotubular dysplasia (OMIM 619727): The skeletal disease and consequent blindness of this new disorder. Bone. (2024) 188:117218. doi: 10.1016/j.bone.2024.117218
17
Li C Li Z Zhang Y Fathy AH Zhou M . The role of the Wnt/β-catenin signaling pathway in the prolif- eration of gold nanoparticle-treated human periodontal ligament stem cells. Stem Cell Res Ther. (2018) 9:214. doi: 10.1186/s13287-018-0954-6
18
Kawane T . Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci Rep. (2018) 8:13551. doi: 10.1038/s41598-018-31853-0
19
Farooq M Khan AW Kim MS Choi S . The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells. (2021) 10:3242. doi: 10.3390/cells10113242
20
Paronett EM Bryan CA Maynard ME Goroff JA Meechan DW LaMantia AS et al . Ranbp1 modulates morphogenesis of the craniofacial midline in mouse models of 22q11.2 deletion syndrome. Hum Mol Genet. (2023) 32:1959–74. doi: 10.1093/hmg/ddad030
21
Ueharu H Pan H Liu X Ishii M Pongetti J Kulkarni AK et al . Augmentation of BMP Signaling in Cranial Neural Crest Cells Leads to Premature Cranial Su- tures Fusion through Endochondral Ossification in Mice. JBMR Plus. (2023) 7:e10716. doi: 10.1002/jbm4.10716
22
Li Q Wang Z Wang C Wang H-L . Characterizing the respiratory-induced mechanical stimulation at the maxillary sinus floor following sinus augmentation by computational fluid dynamics. Front Bioeng Biotechnol. (2022) 10:885130. doi: 10.3389/fbioe.2022.885130
23
Thabet RA Sherif EM ElAal AOA Mahmoud RA . Insulin-like growth factor 1 and sex hormones for assessment of anthropometric and pubertal growth of Egyptian children and adolescents with type 1 diabetes melli- tus (single center study). BMC Endocr Disord. (2024) 24:62. doi: 10.1186/s12902-024-01596-3
24
Wang M Mao F-F Jin X-H Huang J-P Dai A Ding P-H . The influence of different extraction indica- tions on the morphological changes in the maxillary sinus: A retrospective cohort study. J Periodontal Res. (2024). doi: 10.1111/jre.13348
25
Fatihoglu E Aydin S Karavas E Kantarci M . The pneumatization of the sphenoid sinus, its variations and relations with surrounding neurovascular anatomic structures: A computerized tomography study. Am J Otolaryngol. (2021) 42:102958. doi: 10.1016/j.amjoto.2021.102958
26
Gâta A Toader C Trombitaș VE Ilyes A Albu S . Endoscopic skull base repair strategy for CSF leaks associated with pneumocephalus. J Clin Med. (2021) 10:1. doi: 10.3390/jcm10010046
27
Smith TD Zinreich SJ Márquez S King SEE Evans S DeLeon VB . Growth and microanatomy of the paranasal sinuses in two species of New World monkeys. Anat Rec. (2024) 307:49–65. doi: 10.1002/ar.25222
28
Pensak ML . The cavernous sinus: An anatomic study with clinical implication. Laryngoscope Investig Otolaryn- gol. (2024) 9:e1226. doi: 10.1002/lio2.1226
29
Mishra R Konar SK Shukla DP . Internal carotid artery injury during the endoscopic transsphenoidal surgery of pituitary adenoma: case illustration, introspection, and systematic review. Acta Neurochir Suppl. (2025) 133:89–97. doi: 10.1007/978-3-031-61601-3_15
30
Kanotra S Bashir S Sharma P Purbi S Manzoor M Gupta K . Anatomical variations of the optic nerve in the sphenoid sinus: do ethnic variations matter? Indian J Otolaryngol Head Neck Surg. (2023) 75:1943–9. doi: 10.1007/s12070-023-03798-y
31
Tesfaye S Hamba N Gerbi A Negeri Z . Radio-anatomic variability in sphenoid sinus pneumatization with its relationship to adjacent anatomical structures and their impact upon reduction of complications following en- donasal transsphenoidal surgeries. Transl Res Anat. (2021) 24:100126. doi: 10.1016/j.tria.2021.100126
32
Arkoulis N Kearns C Deeny M Telfer J . The interdigitating Y-plasty procedure for the correction of transverse vaginal septa. BJOG Int J Obstet Gynaecol. (2017) 124:331–5. doi: 10.1111/1471-0528.14228
33
Payman AA El-Sayed I Rubio RR . Exploring the combination of computer vision and surgical neu- roanatomy: A workflow involving artificial intelligence for the identification of skull base foramina. World Neuro- Surg. (2024) 191:e403–10. doi: 10.1016/j.wneu.2024.08.137
34
Papadopoulou A-M Bakogiannis N Skrapari I Bakoyiannis C . Anatomical variations of the sinonasal area and their clinical impact on sinus pathology: A systematic review. Int Arch Otorhinolaryngol. (2022) 26:e491–8. doi: 10.1055/s-0042-1742327
35
Famurewa OC Ibitoye BO Ameye SA Asaleye CM Ayoola OO Onigbinde OS . Sphenoid sinus pneumatization, septation, and the internal carotid artery: A computed tomography study. Niger Med J J Niger Med Assoc. (2018) 59:7–13. doi: 10.4103/nmj.NMJ_138_18
36
Al Habsi T Al-Ajmi E Al Washahi M Al Lawati M Al Maawali S Mahajan A et al . Does frontal recess cell variation associate with the development of frontal sinusitis? A narrative review. Diagnostics. (2024) 14:1. doi: 10.3390/diagnostics14010103
37
Ilków W Waligóra M Kunc M Kucharzewski M . Pneumatization of the sphenoid sinus, dorsum sellae and posterior clinoid processes in computed tomography. Pol J Radiol. (2018) 83:e366–71. doi: 10.5114/pjr.2018.78322
38
Ghavate VR et al . Retrospective evaluation of the morphometric properties of intact maxillary sinus using cone- beam computed tomography for sex estimation in an Indian population. PeerJ. (2024) 12:e16991. doi: 10.7717/peerj.16991
39
Adachi T et al . Improved delineation of CT virtual bronchoscopy by ultrahigh-resolution CT: comparison among different reconstruction parameters. Jpn J Radiol. (2020) 38:884–9. doi: 10.1007/s11604-020-00972-y
40
Parameshwar Keerthi BH Savagave SG Sakalecha AK Reddy V L YU . The evaluation of variations in patterns of sphenoid sinus pneumatization using computed tomography in a south Indian population. Cureus. (2022) 14:e23174. doi: 10.7759/cureus.23174
41
Vinciguerra A et al . Iatrogenic cerebrospinal fluid leak in endoscopic sinus surgery: topographical map and influence of skull base asymmetry. J Pers Med. (2024) 14:226. doi: 10.3390/jpm14030226
42
R V Hegde G Botchu R . MRI imaging of soft tissues tumours and tumour like lesions-SLAM approach. J Clin Orthop Trauma. (2022) 28:101872. doi: 10.1016/j.jcot.2022.101872
43
Drake M et al . Diffusion-weighted imaging, MR angiography, and baseline data in a systematic multicenter analysis of 3,301 MRI scans of ischemic stroke patients—Neuroradiological review within the MRI-GENIE study. Front Neurol. (2020), 577. doi: 10.3389/fneur.2020.00577
44
Okada T et al . Neuroimaging at 7 Tesla: a pictorial narrative review. Quant Imaging Med Surg. (2022) 12:3406–35. doi: 10.21037/qims-21-969
45
Lo Bue E et al . Anatomical differences in sphenoid sinus during endoscopic transsphenoidal surgery: com- parison between nonfunctioning pituitary neuroendocrine tumor (PiTNET) and growth hormone-secreting piTNET. World Neurosurg. (2024) 190:e701–6. doi: 10.1016/j.wneu.2024.07.209
46
Du Y Xu B Li Q Peng C Yang K . The role of mechanically sensitive ion channel Piezo1 in bone remod- eling. Front Bioeng Biotechnol. (2024) 12:1342149. doi: 10.3389/fbioe.2024.1342149
47
Kwon MJ et al . Genetic association between inflammatory-related polymorphism in STAT3, IL-1β, IL-6, TNF- α and idiopathic recurrent implantation failure. Genes. (2023) 14:8. doi: 10.3390/genes14081588
48
Tavakoli M Jafari-Pozve N Aryanezhad SS . Sphenoid sinus pneumatization types and correlation with adjacent neurovascular structures using cone-beam computed tomography. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. (2023) 75:2245–50. doi: 10.1007/s12070-023-03796-0
49
Cornejo J et al . Anatomical engineering and 3D printing for surgery and medical devices: international review and future exponential innovations. BioMed Res Int. (2022) 2022:6797745. doi: 10.1155/2022/6797745
50
Hassan NA Ah Mahdi M Irhyyim NS . Correlation between sphenoid sinus pneumatization and sella turcica dimensions using computed tomography. J Int Med Res. (2024) 52:3000605241287021. doi: 10.1177/03000605241287021
51
Xu J Iyyanar PPR Lan Y Jiang R . Sonic hedgehog signaling in craniofacial development. Differ Res Biol Divers. (2023) 133:60–76. doi: 10.1016/j.diff.2023.07.002
52
Liu J Ji C . Endoscopic transsphenoidal resection of a non-functioning ectopic pituitary adenoma located in the clivus: a case report and literature review. Egypt J Neurosurg. (2023) 38:74. doi: 10.1186/s41984-023-00261-6
53
Gandhi K Patil ST Kumar B Patel M Chawre P Ahmad M et al . Morphometry and intracranial relations of the sphenoid sinus in context to endoscopic trans- nasal transsphenoidal surgery. Cureus. (2023) 15:e40187. doi: 10.7759/cureus.40187
54
Buyukozsoy AK Karatay E Toz HT . Evaluation of sphenoid sinus pneumatization subtypes in postero- anterior and lateral directions on CT imaging. J Kermanshah Univ Med Sci. (2024) 28:2. doi: 10.5812/jkums-131911
55
Li L London NR Prevedello DM Carrau RL . Endonasal exposure of lateral recess of the sphenoid sinus: significance of pterygoid process pneumatization. Am J Rhinol Allergy. (2023) 37:291–7. doi: 10.1177/19458924221139019
56
Nardi C Maraghelli D Pietragalla M Scola E Locatello LG Maggiore G et al . A practical overview of CT and MRI features of developmental, inflammatory, and neoplastic lesions of the sphenoid body and clivus. Neuroradiology. (2022) 64:1483–509. doi: 10.1007/s00234-022-02986-x
57
Akbulut A Demirel O Orhan K . Investigation of the prevalence and main features of skull-base anomalies and characteristics of the sphenoid sinus using cone-beam computed tomography. J Korean Assoc Oral Maxillofac Surg. (2022) 48:207–18. doi: 10.5125/jkaoms.2022.48.4.207
58
Tomaszewska M Brożek-Mądry E Krzeski A . Spontaneous sphenoid sinus cerebrospinal fluid leak and meningoencephalocele – are they due to patent Sternberg’s canal? Videosurgery Miniinvasive Tech. (2015) 10:347–58. doi: 10.5114/wiitm.2014.47097
59
Balzeau A et al . Frontal sinuses and human evolution. Sci Adv. (2022) 8:eabp9767. doi: 10.1126/sci-adv.abp9767
60
Lea AJ Peng J Ayroles JF . Diverse environmental perturbations reveal the evolution and context-de- pendency of genetic effects on gene expression levels. Genome Res. (2022) 32:1826–39. doi: 10.1101/gr.276430.121
61
Yavas G Caliskan KE Cagli MS . Three-dimensional-printed marker-based augmented reality neuronav- igation: a new neuronavigation technique. Neurosurg Focus. (2021) 51:E20. doi: 10.3171/2021.5.FO-CUS21206
62
Chen-Yoshikawa TF . Evolution of three-dimensional computed tomography imaging in thoracic surgery. Cancers. (2024) 16:2161. doi: 10.3390/cancers16112161
63
De Groote S et al . A regions of interest voxel-based morphometry study of the human brain during high- frequency spinal cord stimulation in patients with failed back surgery syndrome. Pain Pract Off J World Inst Pain. (2020) 20:878–88. doi: 10.1111/papr.12922
64
Foti G et al . Dual-energy CT in oncologic imaging. Tomography. (2024) 10:3. doi: 10.3390/tomography10030024
65
Movahhedian N Paknahad M Abbasinia F Khojatepour L . Cone beam computed tomography analysis of sphenoid sinus pneumatization and relationship with neurovascular structures. J Maxillofac Oral Surg. (2021) 20:105–14. doi: 10.1007/s12663-020-01326-x
66
Klempka A Ackermann E Clausen S Groden C . Photon counting computed tomography for accurate cribriform plate (Lamina cribrosa) imaging in adult patients. Tomography. (2024) 10:400–14. doi: 10.3390/tomography10030031
67
Li Y Liu X Zhuang X-H Wang M-J Song X-F . Assessment of low-dose paranasal sinus CT imaging using a new deep learning image reconstruction technique in children compared to adaptive statistical iterative recon- struction V (ASiR-V). BMC Med Imaging. (2022) 22:106. doi: 10.1186/s12880-022-00834-1
68
Mayer P et al . Diffusion kurtosis imaging—A superior approach to assess tumor–stroma ratio in pancreatic ductal adenocarcinoma. Cancers. (2020) 12:6. doi: 10.3390/cancers12061656
69
Ahmed J et al . A comparative analysis of sphenoid and frontal sinuses using cone beam computed tomography for sex determination. J Oral Biol Craniofacial Res. (2024) 14:478–83. doi: 10.1016/j.jobcr.2024.05.004
70
Karpishchenko S Arustamyan I Stancheva O Sharko K Kaplun D Bogachev MI . Intraoperative sphe- noid sinus volume measurement as an alternative technique to intraoperative computer tomography. Diagnostics. (2020) 10:6. doi: 10.3390/diagnostics10060350
71
Jaworek-Troć J Walocha JA Skrzat J Iwanaga J Tubbs RS Mazur M et al . A computed tomography comprehensive evaluation of the ostium of the sphenoid sinus and its clinical significance. Folia Morphol. (2022) 81:3. doi: 10.5603/FM.a2021.0063
72
Tunç O Yazıcı A Aytaç İ. Tümüklü K Akşamoğlu M . Value of hounsfield units in the evaluation of isolated sphenoid sinus lesions. Allergy Rhinol. (2021) 12:21526567211032560. doi: 10.1177/21526567211032560
73
Song H-S Yoon H-S Lee S Hong C-K Yi B-J . Surgical navigation system for transsphenoidal pituitary surgery applying U-net-based automatic segmentation and bendable devices. Appl Sci. (2019) 9:24. doi: 10.3390/app9245540
74
Chung E-J et al . Effectiveness of cone-beam computed tomography-generated cephalograms using artificial intelligence cephalometric analysis. Sci Rep. (2022) 12:20585. doi: 10.1038/s41598-022-25215-0
75
Remschmidt B Rieder M Gsaxner C Gaessler J Payer M Wallner J . Augmented reality-guided apicoectomy based on maxillofacial CBCT scans. Diagnostics. (2023) 13:19. doi: 10.3390/diag-nostics13193037
76
Jannatdoust P Valizadeh P Razaghi M Rouzbahani M Abbasi A Arian A . Role of abbreviated non- contrast-enhanced MR-enterography in the evaluation of Crohn’s disease activity and complications as an alternative for full protocol contrast-enhanced study: A systematic review and meta-analysis. Res Diagn Interv Imaging. (2023) 6:100030. doi: 10.1016/j.redii.2023.100030
77
Bingbing G et al . Diffusion kurtosis imaging of microstructural changes in gray matter nucleus in Parkinson disease. Front Neurol. (2020) 11:252. doi: 10.3389/fneur.2020.00252
78
Ciurea M-V Ștefan Florian I Lenghel M Petea-Balea D-R Roman A Albu S . Magnetic resonance im- aging features of the sphenoid sinus in patients with non-functioning pituitary adenoma. Medicina (Mex.). (2024) 60:5. doi: 10.3390/medicina60050708
79
Li J Luo R Gao W . Diagnostic value of nasal sinus computed tomography/magnetic resonance imaging examination and serum cytokines in unilateral benign sphenoid sinus lesions. J Radiat Res Appl Sci. (2025) 18:101187. doi: 10.1016/j.jrras.2024.101187
80
Liu J et al . Arterial spin labeling of nasopharyngeal carcinoma shows early therapy response. Insights Imaging. (2022) 13:114. doi: 10.1186/s13244-022-01248-x
81
Schipke JD Cleveland S Drees M . Sphenoid sinus barotrauma in diving: case series and review of the literature. Res Sports Med Print. (2018) 26:124–37. doi: 10.1080/15438627.2017.1365292
82
Khan FQ Deshmukh PT Gaurkar SS . Pneumatization pattern and status of the mastoid antrum in chronic otitis media: A review. Cureus. (2022) 14:e27068. doi: 10.7759/cureus.27068
83
Dogan ME Kotanlı S Yavuz Y Wahjuningrum DA Pawar AM . Computed tomography-based assess- ment of sphenoid sinus and sella turcica pneumatization analysis: a retrospective study. PeerJ. (2023) 11:e16623. doi: 10.7717/peerj.16623
84
Refaat R Basha MAA . The impact of sphenoid sinus pneumatization type on the protrusion and dehis- cence of the adjacent neurovascular structures: A prospective MDCT imaging study. Acad Radiol. (2020) 27:e132–9. doi: 10.1016/j.acra.2019.09.005
85
Kizhisseri M Gharaie S Schluter J . An analytical method informed by clinical imaging data for estimating outlet boundary conditions in computational fluid dynamics analysis of carotid artery blood flow. Sci Rep. (2023) 13:14973. doi: 10.1038/s41598-023-42004-5
86
Jaworek-Troć J Ochwat K Walocha JA Zamojska I Lipski M Żytkowski A et al . Prevalence of the Onodi cell in the Polish adult population: an anatomical computed to- mography study. Folia Morphol. (2023) 82:4. doi: 10.5603/FM.a2023.0001
87
Battal B Zamora C . Imaging of skull base tumors. Tomography. (2023) 9. doi: 10.3390/tomography9040097
88
Hagen F Fritz J Mair A Horger M Bongers MN . Dual-energy computed tomography-based quanti- tative bone marrow imaging in non-hematooncological subjects: associations with age, gender and other variables. J Clin Med. (2022) 11:4094. doi: 10.3390/jcm11144094
89
Aksakal C Beyhan M Gökçe E . Evaluation of the association between paranasal sinus osteomas and anatomic variations using computed tomography. Turk Arch Otorhinolaryngol. (2021) 59:54–64. doi: 10.4274/tao.2020.5811
90
Khaladkar SM Julakanti S Pandey A Paidlewar S Sharma O . Vascular and neurological complications in sphenoid sinusitis. Cureus. (2024) 16:e66181. doi: 10.7759/cureus.66181
91
Lee J-H Park J-T . Three-dimensional measurements of sphenoid sinus size by sex in a Korean population: an exploratory study. Diagnostics. (2024) 14:24. doi: 10.3390/diagnostics14242888
92
Raseman J et al . Preoperative computed tomography imaging of the sphenoid sinus: striving towards safe transsphenoidal surgery. J Neurol Surg Part B Skull Base. (2020) 81:251–62. doi: 10.1055/s-0039-1691831
93
Ali AHA et al . Incidental detection of paranasal sinuses abnormalities on CT imaging of the head in Saudi adult population. PloS One. (2022) 17:e0270764. doi: 10.1371/journal.pone.0270764
94
Molinari G et al . Assessment of a novel patient-specific 3D printed multi-material simulator for endoscopic sinus surgery. Front Bioeng Biotechnol. (2022) 10:974021. doi: 10.3389/fbioe.2022.974021
95
Hey G Guyot M Carter A Lucke-Wold B . Augmented reality in neurosurgery: A new paradigm for training. Medicina (Mex.). (2023) 59:10. doi: 10.3390/medicina59101721
96
Ruparelia J Manjunath N Nachiappan DS Raheja A Suri A . Virtual reality in preoperative planning of complex cranial surgery. World Neurosurg. (2023) 180:e11–8. doi: 10.1016/j.wneu.2023.06.014
97
Upreti G . Advancements in skull base surgery: navigating complex challenges with artificial intelligence. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. (2024), 2184–90. doi: 10.1007/s12070-023-04415-8
98
Morgan N Van Gerven A Smolders A de Faria Vasconcelos K Willems H Jacobs R . Convolutional neural network for automatic maxillary sinus segmentation on cone-beam computed tomographic images. Sci Rep. (2022) 12:7523. doi: 10.1038/s41598-022-11483-3
99
Taylor A Habib A-R Kumar A Wong E Hasan Z Singh N . An artificial intelligence algorithm for the classification of sphenoid sinus pneumatisation on sinus computed tomography scans. Clin Otolaryngol Off J ENT- UK Off J Neth Soc Oto-Rhino-Laryngol. Cervico-Facial Surg. (2023) 48:888–94. doi: 10.1111/coa.14088
100
Hiremath SB Gautam AA Sheeja K Benjamin G . Assessment of variations in sphenoid sinus pneuma- tization in Indian population: A multidetector computed tomography study. Indian J Radiol Imaging. (2018) 28:273–9. doi: 10.4103/ijri.IJRI_70_18
101
Bocanegra-Becerra JE Neves Ferreira JS Simoni G Hong A Rios-Garcia W Mirahmadi Eraghi MM et al . Machine learning algorithms for neurosurgical preoperative planning: A scoping review. World Neurosurg. (2025) 194:123465. doi: 10.1016/j.wneu.2024.11.048
102
Tao F Feng Y Sun B Wang J Chen X Gong J . Septoplasty effect on the enhancement of airflow distri- bution and particle deposition in nasal cavity: A numerical study. Healthcare. (2022) 10:9. doi: 10.3390/healthcare10091702
103
Bourke JM Witmer LM . Soft tissues influence nasal airflow in diapsids: Implications for dinosaurs. J Morphol. (2023) 284:e21619. doi: 10.1002/jmor.21619
104
Sethi KS Choudhary S Ganesan PK Sood N Ramalingum WBS Basil R et al . Sphenoid sinus anatomical variants and pathologies: pictorial essay. Neuroradiology. (2023), 1–17. doi: 10.1007/s00234-023-03163-4
105
Şimşek S İşlek A . Pneumatization of the sphenoid sinus is the major factor determining the variations of adjacent vital structures. Egypt J Otolaryngol. (2024) 40:2. doi: 10.1186/s43163-023-00560-7
106
Hackenbroch C Strobel JRB Lorenz KJ Beer M Schüle S . Dose development in sinonasal imaging over the last decade – a retrospective patient study. Head Face Med. (2023) 19:28. doi: 10.1186/s13005-023-00378-x
107
Kaatsch HL et al . Ultra-low-dose photon-counting CT of paranasal sinus: an in vivo comparison of radiation dose and image quality to cone-beam CT. Dento Maxillo Facial Radiol. (2024) 53:103–8. doi: 10.1093/dmfr/twad010
108
Walton IS et al . Reassessing the association: Evaluation of a polyalanine deletion variant of RUNX2 in non- syndromic sagittal and metopic craniosynostosis. J Anat. (2024) 245:874–8. doi: 10.1111/joa.14052
109
Sahovaler A et al . Augmented reality and intraoperative navigation in sinonasal Malignancies: A preclinical study. Front Oncol. (2021) 11:723509. doi: 10.3389/fonc.2021.723509
110
Bopp MHA et al . Use of neuronavigation and augmented reality in transsphenoidal pituitary adenoma surgery. J Clin Med. (2022) 11:19. doi: 10.3390/jcm11195590
111
Saad M Taha AN Serag S Shata H Zakaria WK . Giant invasive pituitary adenomas: surgical approach selection paradigm and its influence on the outcome—case series. Egypt J Neurosurg. (2023) 38:35. doi: 10.1186/s41984-023-00214-z
112
Yataco-Wilcas CA Diaz-Llanes BE Coasaca-Tito YS Lengua-Vega LA Salazar-Campos CE . Mor- phometric analysis of transsphenoidal surgery in Peruvian population. Surg Neurol Int. (2024) 15:156. doi: 10.25259/SNI_239_2024
113
Ouattassi N Laamarti H Cheikhhamoud Z Chafai H Zaki Z El Alami MNEA . Solitary sphenoid sinus benign lesions: management and prognostic values as retrospective audit of seven case series. Egypt J Otolaryn- gol. (2023) 39:5. doi: 10.1186/s43163-022-00365-0
114
Lovász K et al . A radioanatomical study of 3rd segment terminal branches of the maxillary artery in the ptery- gopalatine fossa. Sci Rep. (2023) 13:3401. doi: 10.1038/s41598-023-29975-1
115
Ziegeler C Beikler T Gosau M May A . Idiopathic facial pain syndromes. Dtsch Ärztebl Int. (2021) 118:81–7. doi: 10.3238/arztebl.m2021.0006
116
Dennler C et al . Augmented reality in the operating room: a clinical feasibility study. BMC Musculoskelet Dis- ord. (2021) 22:451. doi: 10.1186/s12891-021-04339-w
117
Ito T et al . Integration of augmented reality in temporal bone and skull base surgeries. Sensors. (2024) 24:21. doi: 10.3390/s24217063
118
Degaga TK Zenebe AM Wirtu AT Woldehawariat TD Dellie ST Gemechu JM . Anatomographic variants of sphenoid sinus in Ethiopian population. Diagnostics. (2020) 10:11. doi: 10.3390/di-agnostics10110970
119
Urbančič J Bošnjak R Vozel D . Transglabellar butterfly incision for anterior cranial vault access: case report. Curr Oncol. (2024) 31:9. doi: 10.3390/curroncol31090387
120
Barcia Castilla V et al . Evaluation of postoperative intracranial pressure in patients with radiological diagnosis of idiopathic intracranial hypertension. Am J Otolaryngol. (2025) 46:104542. doi: 10.1016/j.am-joto.2024.104542
121
Kuan EC Kaufman AC Lerner D Kohanski MA Tong CCL Tajudeen BA et al . Lack of sphenoid pneumatization does not affect endoscopic endonasal pediatric skull base surgery outcomes. Laryngoscope. (2019) 129:832–6. doi: 10.1002/lary.27600
122
Safarian M Sadeghi M Saedi B . Endoscopic sphenoid sinus anatomic considerations: A study on 60 cadavers. Iran. J Otorhinolaryngol. (2021) 33:237–42. doi: 10.22038/ijorl.2021.47273.2554
123
Valencia-Sanchez BA Kim JD Zhou S Chen S Levy ML Roxbury C et al . Special considerations in pediatric endoscopic skull base surgery. J Clin Med. (2024) 13:7. doi: 10.3390/jcm13071924
124
Periyasamy V Sumana R Doddappaiah A Mythilikrishnan R . Anatomical variation in the sphenoidal sinuses in patients with chronic rhinosinusitis: A CT scan study. J Taibah Univ Med Sci. (2024) 19:114–21. doi: 10.1016/j.jtumed.2023.09.005
125
Van Zele T Pauwels B Dewaele F Gevaert P Bachert C . Prospective study on the outcome of the sphe- noid drill out procedure. Rhinology. (2018) 56:178–82. doi: 10.4193/Rhin17.078
126
Tomita Y Yagi M Seki F Komaki Y Matsumoto M Nakamura M . Cerebrospinal fluid dynamics anal- ysis using time-spatial labeling inversion pulse (Time-SLIP) magnetic resonance imaging in mice. J Clin Med. (2024) 13:15. doi: 10.3390/jcm13154550
127
Chahboun B Kaba O El Aidouni G Bkiyar H Housni B . Pneumosinus dilatans of the frontal and ethmoidal sinuses revealed by conscience disorder and respiratory distress: case report and literature review. Cureus. (2024) 16. doi: 10.7759/cureus.53539
128
Rondão MBA Hsu B. R. R. H. S. Centeno RS de Aguiar PHP . Dyke-Davidoff-Masson Syndrome: Main clinical and radiological findings- systematic literature review. Seizure Eur J Epilepsy. (2023) 110:58–68. doi: 10.1016/j.seizure.2023.04.020
129
ELKammash TH Enaba MM Awadalla AM . Variability in sphenoid sinus pneumatization and its impact upon reduction of complications following sellar region surgeries. Egypt J Radiol Nucl Med. (2014) 45:705–14. doi: 10.1016/j.ejrnm.2014.04.020
130
Nyquist GG . Surgical management of rhinosinusitis in endoscopic-endonasal skull-base surgery. Int Forum Allergy Rhinol. (2015) 5:339–43. doi: 10.1002/alr.21476
131
Baban MIA Castelnuovo P Hadi M Karligkiotis A Battaglia P Shawkat A . Surgical instructions in revision endoscopic sinus surgery: pearls and pitfalls. Indian J Otolaryngol Head Neck Surg. (2022) 74:813–20. doi: 10.1007/s12070-020-01861-6
132
Oyer SL Mulligan JK Psaltis AJ Henriquez OA Schlosser RJ . Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. (2013) 123:E72–78. doi: 10.1002/lary.24305
133
Baumert U Golan I Redlich M Aknin J-J Muessig D . Cleidocranial dysplasia: Molecular genetic analysis and phenotypic-based description of a Middle European patient group. Am J Med Genet A. (2005) 139A:78–85. doi: 10.1002/ajmg.a.30927
134
Jiang Q Mei L Zou Y Ding Q Cannon RD Chen H et al . Genetic polymorphisms in FGFR2 underlie skeletal malocclusion. J Dent Res. (2019) 98:1340–7. doi: 10.1177/0022034519872951
135
Malde O Libby J Moazen M . An overview of modelling craniosynostosis using the finite element method. Mol Syndromol. (2019) 10:74–82. doi: 10.1159/000490833
136
Komori T . Molecular mechanism of runx2-dependent bone development. Mol Cells. (2020) 43:168–75. doi: 10.14348/molcells.2019.0244
137
Schmale IL Vandelaar LJ Luong AU Citardi MJ Yao WC . Image-guided surgery and intraopera- tive imaging in rhinology: clinical update and current state of the art. Ear Nose Throat J. (2021) 100:NP475–86. doi: 10.1177/0145561320928202
138
Ojo OA Onyia CU Kanu OO . Proposal of a novel anatomic guide to the sphenoid sinus. World Neurosurg. (2023) 171:124–31. doi: 10.1016/j.wneu.2022.12.131
139
Martinez-Perez R Kunigelis KE Ward RC Ung TH Arnone GD Cass SP et al . Hydroxyapatite cement cranioplasty for reconstruction of translabyrinthine approach: aesthetic results, long-term satisfaction, quality of life, and complications. Acta Neurochir (Wien). (2022) 164:669–77. doi: 10.1007/s00701-021-05024-6
140
Yan Y Guo F Liu J Yu M Huang Y . Anatomical variants, pneumatization classification, and volumetric studies of the sphenoid sinus with high-resolution computed tomography. J Craniofac Surg. (2021) 32:2542–5. doi: 10.1097/SCS.0000000000007570
141
Toader C Eva L Tataru C-I . Frontiers of cranial base surgery: integrating technique, technology, and teamwork for the future of neurosurgery. Brain Sci. (2023) 13:10. doi: 10.3390/brainsci13101495
142
Cellina M et al . Sphenoid sinuses: pneumatisation and anatomical variants-what the radiologist needs to know and report to avoid intraoperative complications. Surg Radiol Anat SRA. (2020) 42:1013–24. doi: 10.1007/s00276-020-02490-y
143
Wareham LK Liddelow SA Temple S Benowitz LI Di Polo A Wellington D-I et al . Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. (2022) 17:23. doi: 10.1186/s13024-022-00524-0
144
Rochette L Meloux A Rigal E Zeller M Cottin Y Vergely C . The role of osteoprotegerin and its ligands in vascular function. Int J Mol Sci. (2019) 20:705. doi: 10.3390/ijms20030705
145
Weitzmann MN . The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal inter- face in physiological bone turnover and osteoporosis. Scientifica. (2013) 2013:125705. doi: 10.1155/2013/125705
146
Komori T . Roles of runx2 in skeletal development. Adv Exp Med Biol. (2017) 962:83–93. doi: 10.1007/978-981-10-3233-2_6
147
Alzhrani G Sivakumar W Park MS Taussky P Couldwell WT . Delayed complications after transsphenoidal surgery for pituitary adenomas. World Neurosurg. (2018) 109:233–41. doi: 10.1016/j.wneu.2017.09.192
148
London NR AlQahtani A Barbosa S Castelnuovo P Locatelli D Stamm A et al . Characterization of outcomes and practices utilized in the management of internal carotid artery injury not requiring definitive endovascular management. Laryngoscope Investig Otolaryngol. (2021) 6:634–40. doi: 10.1002/lio2.621
149
Zhang Y Vakhtin AA Jennings JS Massaband P Wintermark M Craig PL et al . Diffusion tensor tractography of brainstem fibers and its application in pain. PloS One. (2020) 15:e0213952. doi: 10.1371/journal.pone.0213952
150
Güldner C Pistorius SM Diogo I Bien S Sesterhenn A Werner JA . Analysis of pneumatization and neurovascular structures of the sphenoid sinus using cone-beam tomography (CBT). Acta Radiol Stockh Swed 1987. (2012) 53:214–9. doi: 10.1258/ar.2011.110381
151
Grote A Neumann F Menzler K Carl B Nimsky C Bopp MHA . Augmented reality in extratemporal lobe epilepsy surgery. J Clin Med. (2024) 13:19. doi: 10.3390/jcm13195692
152
Spektor S Dotan S Mizrahi CJ . Safety of drilling for clinoidectomy and optic canal unroofing in anterior skull base surgery. Acta Neurochir (Wien). (2013) 155:1017–24. doi: 10.1007/s00701-013-1704-2
153
O’Leary C et al . Use of intraoperative doppler ultrasonography in predicting life-threatening vascular com- plications after adult deceased donor liver transplantation. Cureus. (2024) 16:e73588. doi: 10.7759/cu-reus.73588
154
Illing E Schlosser RJ Palmer JN Curé J Fox N Woodworth BA . Spontaneous sphenoid lateral recess cerebrospinal fluid leaks arise from intracranial hypertension, not Sternberg’s canal. Int Forum Allergy Rhinol. (2014) 4:246–50. doi: 10.1002/alr.21262
155
Sumislawski P Piotrowska M Regelsberger J Flitsch J Rotermund R . Sphenoid sinus mucosal flap after transsphenoidal surgery—A systematic review. Medicina (Mex.). (2024) 60:2. doi: 10.3390/me-dicina60020282
156
Cirovic A Jadzic J Djukic D Djonic D Zivkovic V Nikolic S et al . Increased cortical porosity, reduced cortical thickness, and reduced trabecular and cortical microhardness of the superolateral femoral neck confer the increased hip fracture risk in individuals with type 2 diabetes. Calcif Tissue Int. (2022) 111:457–65. doi: 10.1007/s00223-022-01007-6
157
Lo Giudice A et al . Changes in upper airway airflow after rapid maxillary expansion considering normal crani- ofacial development as a factor: a retrospective study using computer fluid dynamics. Eur J Orthod. (2024) 47:cjae077. doi: 10.1093/ejo/cjae077
158
de Souza Xavier Costa N et al . COVID-19 induces more pronounced extracellular matrix deposition than other causes of ARDS. Respir Res. (2023) 24:281. doi: 10.1186/s12931-023-02555-7
159
Ali M Elgassim MA Faisal HM Saied ASS Elgassim M . Spontaneous cerebrospinal fluid rhinor- rhea secondary to idiopathic intracranial hypertension. Cureus. (2023) 15:e42353. doi: 10.7759/cureus.42353
160
Nacar Dogan S Kizilkilic O Kocak B Isler C Islak C Kocer N . Intrathecal gadolinium-enhanced MR cisternography in patients with otorhinorrhea: 10-year experience of a tertiary referral center. Neuroradiology. (2018) 60:471–7. doi: 10.1007/s00234-018-2014-4
161
Khan DZ et al . Skull base repair following endonasal pituitary and skull base tumour resection: a systematic review. Pituitary. (2021) 24:698–713. doi: 10.1007/s11102-021-01145-4
162
Talamonti G Horaczek J-A Torres RT Deppo LD Carter MJ . PEG hydrogel sealant versus fibrin glue in posterior fossa surgery: an economic comparison across five European countries. J Comp Eff Res. (2024) 13:e230047. doi: 10.57264/cer-2023-0047
163
Han SB Kim JM Park EG Han JY Lee J . Clinical significance of isolated sphenoid sinusitis identified in pediatric patients presenting with headache. Medicina (Mex.). (2024) 60:10. doi: 10.3390/me-dicina60101625
164
Mungoven TJ Meylakh N Marciszewski KK Macefield VG Macey PM Henderson LA . Micro- structural changes in the trigeminal nerve of patients with episodic migraine assessed using magnetic resonance imag- ing. J Headache Pain. (2020) 21:59. doi: 10.1186/s10194-020-01126-1
165
Qi X He Y Wang Q Ren S Yao H Cao W et al . Diffusion tensor and kurtosis imaging reveal microstructural changes in the trigeminal nerves of patients with trigeminal neuralgia. Eur Radiol. (2023) 33:8046–54. doi: 10.1007/s00330-023-09762-2
166
Elkholy SF Gadalla AAEH Mohammad ME Mahmoud BE . Diffusion tensor imaging in trigeminal neuralgia: beyond the normal morphology. Egypt J Radiol Nucl Med. (2023) 54:154. doi: 10.1186/s43055-023-01102-0
167
Eide JG et al . Craniofacial pain locations and outcomes after endoscopic sinus surgery for unilateral sphe- noid sinusitis: A multi-institutional study. Laryngoscope. (2025). doi: 10.1002/lary.31985
168
Elsaid N Ahmed O Belal T Razek A Azab A . Pathogenesis and evaluation of the effects of idiopathic intracranial hypertension on the optic nerves. Neuro-Ophthalmol. (2020) 44:281–9. doi: 10.1080/01658107.2020.1751859
169
Cobzeanu BM Baldea V Costan VV Cobzeanu MD Palade OD Gheorghe L et al . Anatomical variants of internal carotid artery—Results from a retrospective study. Medicina (Mex.). (2023) 59:6. doi: 10.3390/medicina59061057
170
Mohebbi A Rajaeih S Safdarian M Omidian P . The sphenoid sinus, foramen rotundum and vidian canal: a radiological study of anatomical relationships. Braz J Otorhinolaryngol. (2016) 83:381–7. doi: 10.1016/j.bjorl.2016.04.013
171
Almeida LE Zammuto S Lopez DF . Evaluating surgical approaches for hemimandibular hyperplasia associated with osteochondroma: A systematic literature review. J Clin Med. (2024) 13:6988. doi: 10.3390/jcm13226988
172
Trimarchi G et al . Charting the unseen: how non-invasive imaging could redefine cardiovascular prevention. J Cardiovasc Dev Dis. (2024) 11:8. doi: 10.3390/jcdd11080245
173
Meloni A Maffei E Clemente A De Gori C Occhipinti M Positano V et al . Spectral photon-counting computed tomography: technical principles and applications in the assessment of cardiovascular diseases. J Clin Med. (2024) 13:2359. doi: 10.3390/jcm13082359
174
Ritter D et al . Machine learning models can define clinically relevant bone density subgroups based on patient- specific calibrated computed tomography scans in patients undergoing reverse shoulder arthroplasty. J Shoulder Elbow Surg. (2024), S1058–2746. doi: 10.1016/j.jse.2024.07.006
175
Burgos MA et al . Reducing variability in nasal surgery outcomes through computational fluid dynamics and advanced 3D virtual surgery techniques. Heliyon. (2024) 10:e26855. doi: 10.1016/j.heliyon.2024.e26855
176
Lee Y-J Oh J-H Kim S-G . Virtual surgical plan with custom surgical guide for orthognathic surgery: systematic review and meta-analysis. Maxillofac Plast Reconstr Surg. (2024) 46:39. doi: 10.1186/s40902-024-00449-2
177
Yadav KL Gupta A Swarnkar CP Mannan N . Role of vessel wall magnetic resonance imaging in the diagnosis of intracranial vasculopathies. Cureus. (2024) 16:e73714. doi: 10.7759/cureus.73714
178
De Marco R et al . Diffusion-weighted MR spectroscopy (DW-MRS) is sensitive to LPS-induced changes in hu- man glial morphometry: A preliminary study. Brain Behav Immun. (2022) 99:256–65. doi: 10.1016/j.bbi.2021.10.005
179
Komasawa N . Advancements in respiratory surgery anesthesia: A collaborative approach to perioperative management and recovery. Anesth Res. (2024) 1:3. doi: 10.3390/anesthres1030019
180
Lin Z Lei C Yang L . Modern image-guided surgery: A narrative review of medical image processing and visualization. Sensors. (2023) 23:24. doi: 10.3390/s23249872
181
Zhou J Shi Q Jiang L Xie Y Deng B Zhan Y . Association study of the pneumatization degree of mastoid air cells and postoperative complications after microvascular decompression in hemifacial spasm. Acta Neurochir (Wien). (2022) 164:1543–50. doi: 10.1007/s00701-022-05155-4
182
Peris-Celda M Kucukyuruk B Monroy-Sosa A Funaki T Valentine R Rhoton AL . The recesses of the sellar wall of the sphenoid sinus and their intracranial relationships. Neurosurgery. (2013) 73:ons117–131; discussion ons131. doi: 10.1227/NEU.0000000000000184
183
Carbone M et al . Targeting accuracy of neuronavigation: a comparative evaluation of an innovative wearable AR platform vs. traditional EM navigation. Front Digit Health. (2025) 6:1500677. doi: 10.3389/fdgth.2024.1500677
184
Doi K et al . Usefulness of a novel density measurement drill for evaluating cancellous bone density: correlation between CT value and drilling torque value in bovine ribs. Int J Implant Dent. (2025) 11:7. doi: 10.1186/s40729-025-00596-9
185
Amano K Hori T Kawamata T Okada Y . Repair and prevention of cerebrospinal fluid leakage in transsphenoidal surgery: a sphenoid sinus mucosa technique. Neurosurg Rev. (2016) 39:123–131; discussion 131. doi: 10.1007/s10143-015-0667-6
186
Alanazi KM et al . Risk factors associated with postoperative cerebrospinal fluid leak after endoscopic endo- nasal skull base surgery: Two-center retrospective cohort study. Surg Neurol Int. (2024) 15:272. doi: 10.25259/SNI_331_2024
187
Urner LM Kohler M Bloch KE . Automatic processing of nasal pressure recordings to derive continu- ous side-selective nasal airflow and conductance. Front Physiol. (2019) 9:1814. doi: 10.3389/fphys.2018.01814
188
Chegini Z Noei M Hemmati J Arabestani MR Shariati A . The destruction of mucosal barriers, epithelial remodeling, and impaired mucociliary clearance: possible pathogenic mechanisms of Pseudomonas aeruginosa and Staphylococcus aureus in chronic rhinosinusitis. Cell Commun Signal CCS. (2023) 21:306. doi: 10.1186/s12964-023-01347-2
189
Nurminen J et al . Pictorial review of MRI findings in acute neck infections in children. Children. (2023) 10:6. doi: 10.3390/children10060967
190
Miura G . Visual evoked potentials for the detection of diabetic retinal neuropathy. Int J Mol Sci. (2023) 24:8. doi: 10.3390/ijms24087361
191
Liu J Jin M Zhang M Wang Y Sun S . Multimodal evoked potentials are useful for the diagnosis of pediatric acute disseminated encephalomyelitis. BMC Pediatr. (2024) 24:92. doi: 10.1186/s12887-024-04576-7
192
Yamada S Otani T Ii S Ito H Iseki C Tanikawa M et al . Modeling cerebrospinal fluid dynamics across the entire intracranial space through integration of four-dimensional flow and intravoxel incoherent motion magnetic resonance imaging. Fluids Barriers CNS. (2024) 21:47. doi: 10.1186/s12987-024-00552-6
193
Kuriakose S Ak N Revankar S Shetty B Shetty S . Morphology, pneumatization, septation, and protru- sion of nearby structures into the sphenoid sinus: A retrospective radiological study. Bull Med Biol Res. (2024) 3. doi: 10.61751/bmbr/3.2024.25
194
Bilgir E Bayrakdar İŞ . A new classification proposal for sphenoid sinus pneumatization: a retrospective radio-anatomic study. Oral Radiol. (2021) 37:118–24. doi: 10.1007/s11282-020-00467-6
195
Lotsios NS et al . Expression and regulation of hypoxia-inducible factor signalling in acute lung inflamma- tion. Cells. (2024) 14:29. doi: 10.3390/cells14010029
196
Olawade DB Marinze S Qureshi N Weerasinghe K Teke J . The impact of artificial intelligence and machine learning in organ retrieval and transplantation: A comprehensive review. Curr Res Transl Med. (2025) 73:103493. doi: 10.1016/j.retram.2025.103493
197
Gitto S et al . MRI radiomics-based machine learning classification of atypical cartilaginous tumour and grade II chondrosarcoma of long bones. EBioMedicine. (2022) 75:103757. doi: 10.1016/j.ebiom.2021.103757
198
Schievink WI Maya M Moser F Nuño M . Long-term risks of persistent ventral spinal CSF leaks in SIH: superficial siderosis and bibrachial amyotrophy. Neurology. (2021) 97:e1964–70. doi: 10.1212/WNL.0000000000012786
199
De Looze C Feeney J Seeher KM Amuthavalli Thiyagarajan J Diaz T Kenny RA . Assessing cognitive function in longitudinal studies of ageing worldwide: some practical considerations. Age Ageing. (2023) 52:iv13–25. doi: 10.1093/ageing/afad122
200
Udagawa N et al . Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner. Metab. (2021) 39:19–26. doi: 10.1007/s00774-020-01162-6
201
Habuta M et al . Fgf10-CRISPR mosaic mutants demonstrate the gene dose-related loss of the accessory lobe and decrease in the number of alveolar type 2 epithelial cells in mouse lung. PLoS One. (2020) 15:e0240333. doi: 10.1371/journal.pone.0240333
202
Wang DY Lee HP Gordon BR . Impacts of fluid dynamics simulation in study of nasal airflow phys- iology and pathophysiology in realistic human three-dimensional nose models. Clin Exp Otorhinolaryngol. (2012) 5:181–7. doi: 10.3342/ceo.2012.5.4.181
203
Johnsen SG . Computational rhinology: unraveling discrepancies between in silico and in vivo nasal airflow assessments for enhanced clinical decision support. Bioengineering. (2024) 11:3. doi: 10.3390/bi-oengineering11030239
204
Mao JZ et al . Technologic evolution of navigation and robotics in spine surgery: A historical perspective. World Neurosurg. (2021) 145:159–67. doi: 10.1016/j.wneu.2020.08.224
205
Shen Y Wang S Shen Y Hu J . The application of augmented reality technology in perioperative visual guidance: technological advances and innovation challenges. Sensors. (2024) 24:22. doi: 10.3390/s24227363
206
Yang X Zhang H Wang Q-H . A fast computer-generated holographic method for VR and AR near- eye 3D display. Appl Sci. (2019) 9:19. doi: 10.3390/app9194164
207
Shahrezaei A Sohani M Taherkhani S Zarghami SY . The impact of surgical simulation and training technologies on general surgery education. BMC Med Educ. (2024) 24:1297. doi: 10.1186/s12909-024-06299-w
208
Suero Molina E Di Ieva A . Artificial intelligence, radiomics, and computational modeling in skull base surgery. Adv Exp Med Biol. (2024) 1462:265–83. doi: 10.1007/978-3-031-64892-2_16
209
Maleki Varnosfaderani S Forouzanfar M . The role of AI in hospitals and clinics: transforming healthcare in the 21st century. Bioengineering. (2024) 11:337. doi: 10.3390/bioengineering11040337
210
Krones F Marikkar U Parsons G Szmul A Mahdi A . Review of multimodal machine learning approaches in healthcare. Inf Fusion. (2025) 114:102690. doi: 10.1016/j.inffus.2024.102690
211
Richter A Steinmann T Rosenthal J-C Rupitsch SJ . Advances in real-time 3D reconstruction for med- ical endoscopy. J Imaging. (2024) 10:5. doi: 10.3390/jimaging10050120
212
Vaezi A Cardenas E Pinheiro-Neto C Paluzzi A Branstetter BF Gardner PA et al . Classification of sphenoid sinus pneumatization: relevance for endoscopic skull base surgery. Laryngoscope. (2015) 125:577–81. doi: 10.1002/lary.24989
213
Li S Liao C Yang X Zhang W . Association of concomitant continuous pain in trigeminal neuralgia with a narrow foramen ovale. Front Neurol. (2023) 14:1277654. doi: 10.3389/fneur.2023.1277654
214
Maeda S Otani T Yamada S Watanabe Y Ilik SY Wada S . Biomechanical effects of hyper-dynamic cerebrospinal fluid flow through the cerebral aqueduct in idiopathic normal pressure hydrocephalus patients. J Bio- Mech. (2023) 156:111671. doi: 10.1016/j.jbiomech.2023.111671
215
Dantas LR Ortis GB Suss PH Tuon FF . Advances in regenerative and reconstructive medicine in the prevention and treatment of bone infections. Biology. (2024) 13:8. doi: 10.3390/biol-ogy13080605
Summary
Keywords
hyperpneumatization, skull base anatomy, transsphenoidal surgery, cerebrospinal fluid leakage, neurovascular injury, advanced imaging, artificial intelligence
Citation
Baloiu AI, Filipoiu F, Toader C, Covache-Busuioc R-A, Munteanu O and Serban M (2025) Sphenoid sinus hyperpneumatization: anatomical variants, molecular blueprints, and AI-augmented roadmaps for skull base surgery. Front. Endocrinol. 16:1634206. doi: 10.3389/fendo.2025.1634206
Received
23 May 2025
Accepted
04 August 2025
Published
18 September 2025
Corrected
16 January 2026
Volume
16 - 2025
Edited by
Alfio Spina, San Raffaele Hospital (IRCCS), Italy
Reviewed by
Daisuke Wajima, Okinawa Prefectural Nanbu Medical Center, Japan
Ünal Özüm, Cumhuriyet University, Türkiye
Updates
Copyright
© 2025 Baloiu, Filipoiu, Toader, Covache-Busuioc, Munteanu and Serban.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florin Filipoiu, florin.filipoiu@umfcd.ro; Corneliu Toader, corneliu.toader@umfcd.ro; Matei Serban, matei.serban2021@stud.umfcd.ro
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.