- 1Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Henan Key Laboratory of Reproduction and Genetics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Henan Provincial Obstetrical and Gynecological Diseases, Reproductive Medicine, Clinical Research Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Objective: To evaluate the impact of endometrial thickness (EMT) variations on clinical outcomes in two distinct ovarian stimulation protocols: the early-follicular long-acting GnRH agonist protocol and the midluteal short-acting GnRH agonist long protocol.
Methods: This retrospective cohort study analyzed 21,290 first-time IVF/ICSI fresh embryo transfer cycles conducted at the Reproductive Center of the First Affiliated Hospital of Zhengzhou University between January 2013 and December 2020. Restricted cubic spline (RCS) analysis was employed to assess the relationship between EMT and clinical pregnancy outcomes.
Results: In the early-follicular long-acting GnRH agonist protocol group, both clinical pregnancy and live birth rates increased with EMT up to 10.6 mm, beyond which the rates plateaued. Conversely, in the midluteal short-acting GnRH agonist long protocol group, a continuous positive correlation was observed between EMT and both clinical pregnancy and live birth rates. Overall, the early-follicular long-acting protocol demonstrated superior pregnancy outcomes compared to the midluteal short-acting protocol when EMT was less than 15 mm. However, when EMT was ≥15 mm, both protocols yielded comparable clinical pregnancy and live birth rates.
Conclusion: The study indicates that in the early-follicular long-acting GnRH agonist protocol, increasing EMT up to 10.6 mm is associated with improved clinical pregnancy and live birth rates, with no further benefits observed beyond this threshold. In contrast, the midluteal short-acting GnRH agonist long protocol exhibits a continuous positive relationship between EMT and pregnancy outcomes. Overall, the early-follicular long-acting protocol offers better clinical outcomes for patients with EMT less than 15 mm, while both protocols perform similarly when EMT is ≥15 mm.
1 Background
Successful pregnancy depends on both high-quality embryos and optimal endometrial receptivity. Research indicates that approximately two-thirds of implantation failures are attributed to inadequate endometrial receptivity and defective embryo–endometrium interactions (1). Endometrial thickness on the day of human chorionic gonadotropin (hCG) administration has been studied as a potential prognostic factor for assisted reproductive technology (ART) outcomes and is considered one of the markers of endometrial receptivity (1–4). Multiple studies have reported that thinner endometrial thickness is associated with lower clinical pregnancy rates, higher miscarriage rates, and increased risks of small-for-gestational-age (SGA) infants. Conversely, thicker endometrial thickness (>7–8 mm) is correlated with improved outcomes in in vitro fertilization (IVF) (5–10). Gonadotropin-releasing hormone (GnRH) agonists have been utilized for years to control ovarian stimulation, resulting in lower spontaneous ovulation rates and higher pregnancy rates. There are two primary protocols: the early-follicular long-acting GnRH agonist protocol (EFLL) and the midluteal short-acting GnRH agonist long protocol (MLSL). Studies have shown that the EFLL protocol yields higher clinical pregnancy and live birth rates compared to the MLSL protocol (11, 12). However, it remains unclear whether this advantage persists as endometrial thickness increases on the day of human chorionic gonadotropin (hCG) administration, and whether the trends in the relationship between endometrial thickness and pregnancy outcomes differ between these two protocols.
This retrospective analysis utilized clinical data from 21,290 patients who underwent their first IVF/ICSI fresh embryo transfer cycles at the Reproductive Center of the First Affiliated Hospital of Zhengzhou University between January 2013 and December 2020. The study employed Restricted Cubic Spline (RCS) analysis to examine the dynamic relationship between endometrial thickness on the day of hCG administration and pregnancy outcomes under two ovarian stimulation protocols: the Early-Follicular Long-Acting GnRH Agonist Protocol (EFLL) and the Midluteal Short-Acting GnRH Agonist Long Protocol (MLSL). The analysis aimed to identify the optimal endometrial thickness thresholds for achieving the best pregnancy outcomes and to assess potential interaction effects between endometrial thickness and stimulation protocols. This large-sample retrospective study provides valuable insights into the dynamic association between endometrial thickness and pregnancy outcomes under different stimulation protocols, offering clinical guidance for protocol selection.
2 Materials and methods
2.1 Study design
A retrospective analysis was conducted on the clinical data of 21,290 patients who underwent their first IVF/ICSI fresh embryo transfer cycles at the Reproductive Center of the First Affiliated Hospital of Zhengzhou University between January 2013 and December 2020. After propensity score matching, 6,974 patients were assigned to the early-follicular long-acting GnRH agonist protocol (EFLL) group, and 2,324 patients to the midluteal short-acting GnRH agonist long protocol (MLSL) group.
Inclusion Criteria: Patients who underwent their first IVF/ICSI cycle with fresh embryo transfer at the Reproductive Center of the First Affiliated Hospital of Zhengzhou University between January 2013 and December 2020.
Exclusion Criteria: Patients with untreated hydrosalpinx, hyperprolactinemia, diabetes mellitus, or other endocrine disorders; uterine malformations; preimplantation genetic diagnosis or screening; uterine fibroids >3 cm in diameter compressing the endometrium; endometriosis or adenomyosis; intrauterine adhesions; cervical insufficiency; history of tuberculosis; or unoperated endometrial polyps.
Patients were categorized into groups based on their endometrial thickness on their human chorionic gonadotropin day; thicknesses of <7 mm, >16 mm, and 1-mm increments for the remaining measurements were noted, resulting in a total of 11 groups.
This study was approved by the Research and Clinical Trials Ethics Committee of the First Affiliated Hospital of Zhengzhou University (project number 2024-KY-0412-001). In accordance with national legislation, institutional requirements, and the principles of the Declaration of Helsinki, written informed consent was not required for this study.
2.2 Controlled superovulation protocols
2.2.1 Early follicular long-acting GnRH agonist protocol
Patients receive a 3.75 mg dose of the long-acting GnRH agonist triptorelin (Pfizer Pharmaceutical Co., Ltd., Germany) on days 2–3 of their menstrual cycle, following natural menstruation or oral dydrogesterone administration. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progesterone (P) are measured 28 days after injection. Follicular development is monitored via transvaginal ultrasound.
2.2.2 Midluteal short-acting GnRH agonist long protocol
For patients with regular menstrual cycles, the protocol begins on days 21–22 of the natural cycle. For those with irregular cycles, oral contraceptive pills containing drospirenone and ethinylestradiol (Bayer Pharmaceuticals, Germany) are administered for 21 days, starting on day 5 of menstruation. From day 16 of oral contraceptive use, subcutaneous injections of short-acting GnRH agonist triptorelin (IPSEN Biotechnology, France) are given at a daily dose of 0.1 mg for 14–16 days. During this period, oral contraceptive pills are continued at a daily dose of 1 tablet. After injection, serum levels of FSH, LH, E2, and P are measured, and follicular development is monitored via transvaginal ultrasound.
When pituitary downregulation criteria are met (FSH < 5 U/L, LH < 3 U/L, E2 < 50 pg/mL, antral follicle diameter approximately 3–5 mm, no ovarian cysts >10 mm), controlled ovarian stimulation (COS) is initiated with gonadotropins (Gn). Gn starting doses are determined based on the patient’s body mass index (BMI). Gn doses are adjusted according to follicular growth and serum E2 levels. When two or more follicles reach 18 mm in diameter, or over two-thirds of follicles reach 16 mm, recombinant human chorionic gonadotropin (hCG, Merck Serono, Italy) is administered to trigger ovulation. Oocyte retrieval is performed 36–37 hours after hCG administration. Luteal phase support was initiated on the day of oocyte retrieval using intramuscular progesterone injections (60 mg/day; Zhejiang Xianju Pharmaceutical Co., Ltd., China) and continued until pregnancy test results were confirmed. This regimen was implemented according to our center’s routine clinical practice and relevant guideline recommendations. In vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is used for fertilization.
2.3 Research indicators
We measured serum β-human chorionic gonadotropin levels 14 days after embryo transfer. Clinical pregnancy was confirmed by the presence of a gestational sac on ultrasonography, which was performed 35 days after transfer. High-quality embryo rate = number of high-quality embryos/number of 2PN cleavages*100%; biochemical pregnancy rate = number of HCG-positive cycles/number of transfer cycles*100%; implantation rate = number of visible gestational sacs under ultrasound/number of transferred embryos*100%; clinical pregnancy rate = number of clinical pregnancy cycles/total number of transfer cycles*100%; live birth rate = number of live birth cycles/total number of transfer cycles*100%; abortion rate = number of abortion cycles/number of clinical pregnancy cycles*100%; ectopic pregnancy rate = number of ectopic pregnancy cycles/number of clinical pregnancy cycles*100%; premature birth rate = number of premature birth cycles/number of clinical pregnancy cycles*100%.
2.4 Statistical analysis
Due to the significantly larger sample size in the early-follicular long-acting GnRH agonist protocol group compared to the midluteal short-acting GnRH agonist long protocol group, propensity score matching (PSM) was employed to ensure comparability between the two groups. A 1:3 matching ratio was applied based on covariates including age, BMI, infertility type, antral follicle count, FSH, LH, E2, testosterone, fertilization method, and number of embryos transferred. The matching process was performed using the nearest neighbor matching algorithm with a caliper width set at 0.02 and without replacement. After matching, the balance between groups was evaluated using the standardized mean difference (SMD), with all covariates showing SMD values <0.1, indicating good matching quality.
In the original cohort, there were 2,361 cases in the midluteal short-acting GnRH agonist long protocol group and 18,929 cases in the early-follicular long-acting GnRH agonist protocol group. After matching, 2,324 cases from the midluteal short-acting GnRH agonist long protocol group and 6,974 cases from the early-follicular long-acting GnRH agonist protocol group were included. The matching ratio did not strictly achieve 1:3, as 37 cases from the midluteal short-acting GnRH agonist long protocol group were excluded due to propensity scores falling outside the caliper range. Among the remaining 2,324 cases, some were matched to 2–4 cases from the early-follicular long-acting GnRH agonist protocol group, resulting in an average matching ratio close to 3:1 (6,974/2,324 ≈ 3.00), with negligible differences.
Continuous variables were assessed for normality using the Shapiro-Wilk test. Since all continuous variables were normally distributed (P > 0.05), t-tests were performed for comparisons between groups. Categorical variables were expressed as percentages and compared using chi-square tests. Multivariate logistic regression was conducted to adjust for potential confounders, including age, BMI, infertility type, number of embryos transferred, embryo or blastocyst transfer, initial Gn dosage, total Gn dosage, antral follicle count, number of oocytes retrieved, and stimulation protocol. Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) were calculated to examine the association between endometrial thickness and pregnancy outcomes. Interaction effects between protocol type and endometrial thickness were evaluated by introducing an interaction term (“protocol × endometrial thickness”) into the model and testing its significance using the likelihood ratio test.
Restricted cubic spline (RCS) analysis was used to examine the relationship between endometrial thickness and clinical pregnancy rate, live birth rate, miscarriage rate, and preterm birth rate. All models were adjusted for age, body mass index (BMI), type of infertility, number of transferred embryos, embryo or blastocyst stage at transfer, initial gonadotropin dose, total gonadotropin dose, antral follicle count (AFC), number of retrieved oocytes, and ovarian stimulation protocol.
To balance model complexity and the risk of overfitting, the RCS model was specified with four knots placed at the 5th, 35th, 65th, and 95th percentiles of endometrial thickness, with three degrees of freedom. The likelihood ratio test and Wald test were used to assess the significance of nonlinearity (P for nonlinearity < 0.05 indicating a significant nonlinear relationship). If a nonlinear relationship was detected, piecewise logistic regression models were constructed based on the identified inflection points.
All statistical analyses were performed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) and R version 4.2.1. Two-sided P-values < 0.05 were considered statistically significant.
3 Results
3.1 General characteristics and clinical outcomes of patients undergoing different ovarian stimulation protocols
Table 1 shows that after propensity score matching, there were no significant differences between the two groups in terms of age, BMI, infertility type, antral follicle count, FSH, LH, E2, T, and embryo transfer type (all P > 0.05). Compared to the midluteal short-acting GnRH agonist group, the early-follicular long-acting GnRH agonist group had a higher number of oocytes retrieved, greater endometrial thickness on the hCG day, higher biochemical pregnancy rate, clinical pregnancy rate, and live birth rate, and lower ectopic pregnancy rate (all P < 0.05). However, there were no significant differences between the two groups in miscarriage rate and preterm birth rate (all P > 0.05). Overall, the midluteal short-acting GnRH agonist protocol was associated with a lower incidence of pregnancy-related complications, specifically a lower rate of gestational diabetes mellitus (P < 0.001), while there were no significant differences between the two groups in terms of pregnancy-induced hypertension, premature rupture of membranes, and intrauterine distress (all P > 0.05, Table 1).
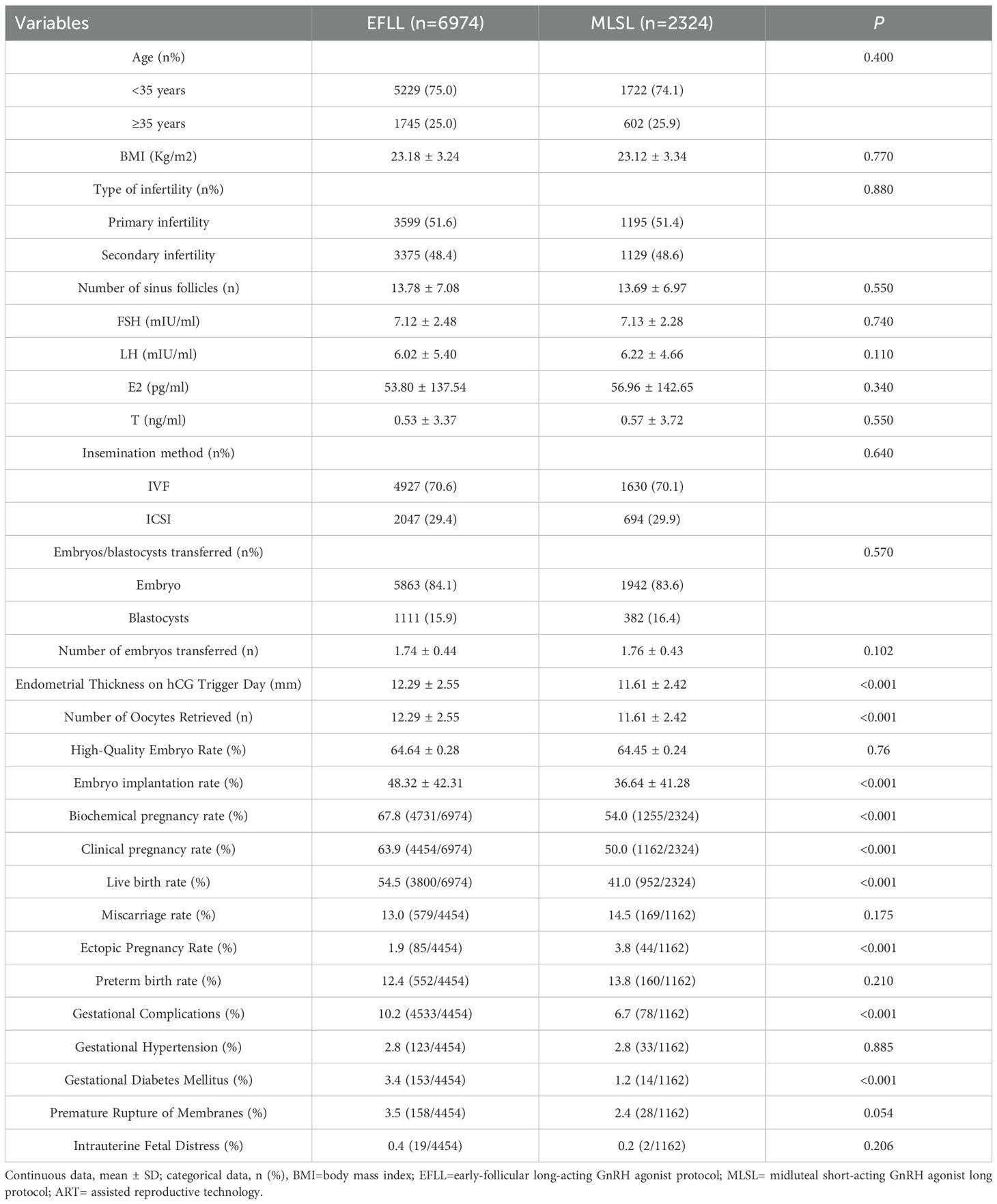
Table 1. General characteristics and clinical outcomes of patients undergoing different ovulation induction protocols.
3.2 Clinical outcomes associated with different endometrial thicknesses
As shown in Table 2 and Figure 1, when the endometrial thickness is less than 15 mm, the clinical pregnancy rate and live birth rate in the follicular-phase long protocol are consistently higher than those in the luteal-phase short protocol, with statistical significance (all P < 0.05, Table 2). However, when the endometrial thickness is 15 mm or greater, there is no significant difference in clinical pregnancy rate and live birth rate between the two groups, both exhibiting similar pregnancy outcomes (all P > 0.05, Table 2).
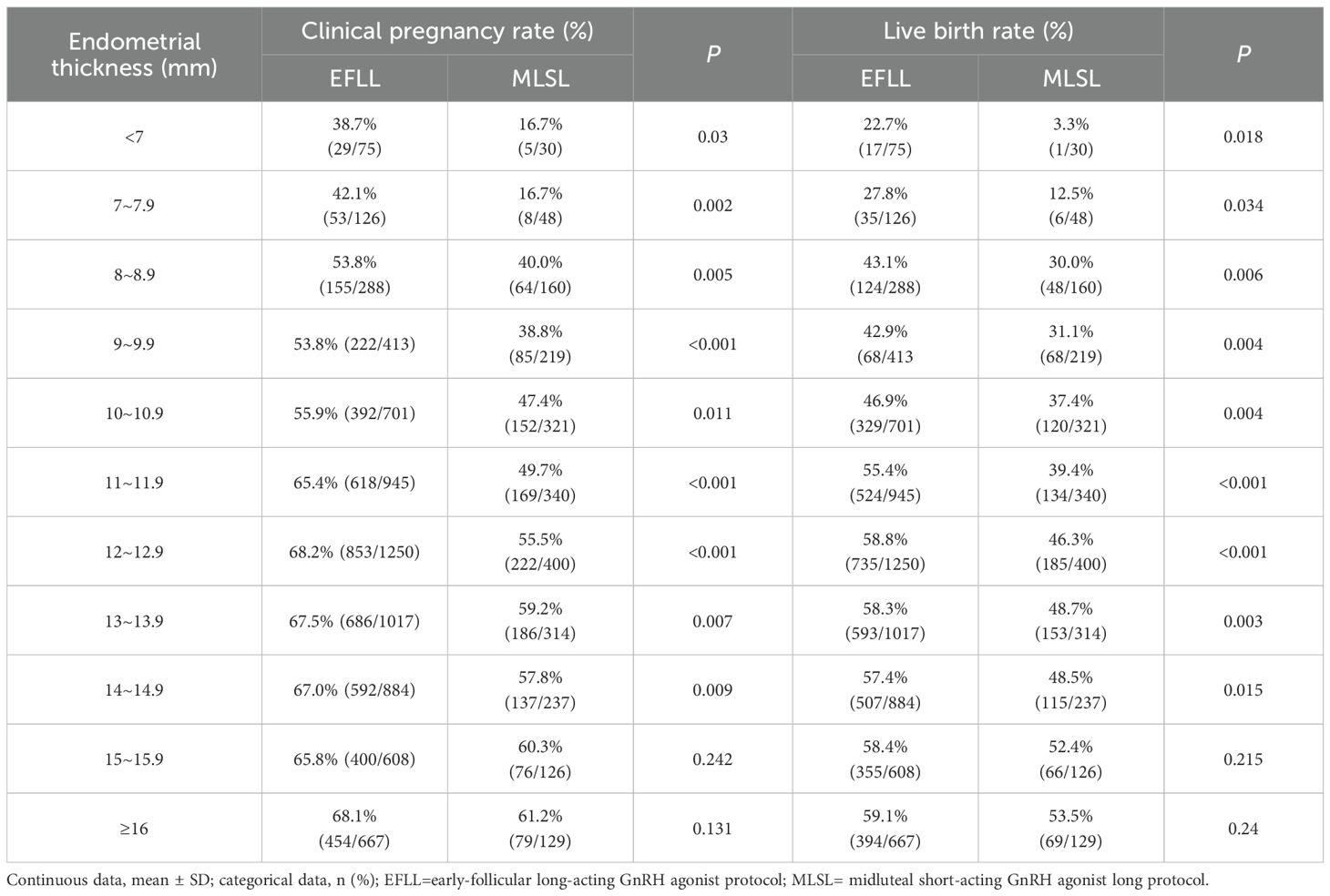
Table 2. Clinical outcomes of different endometrial thicknesses in different controlled ovulation induction protocols.
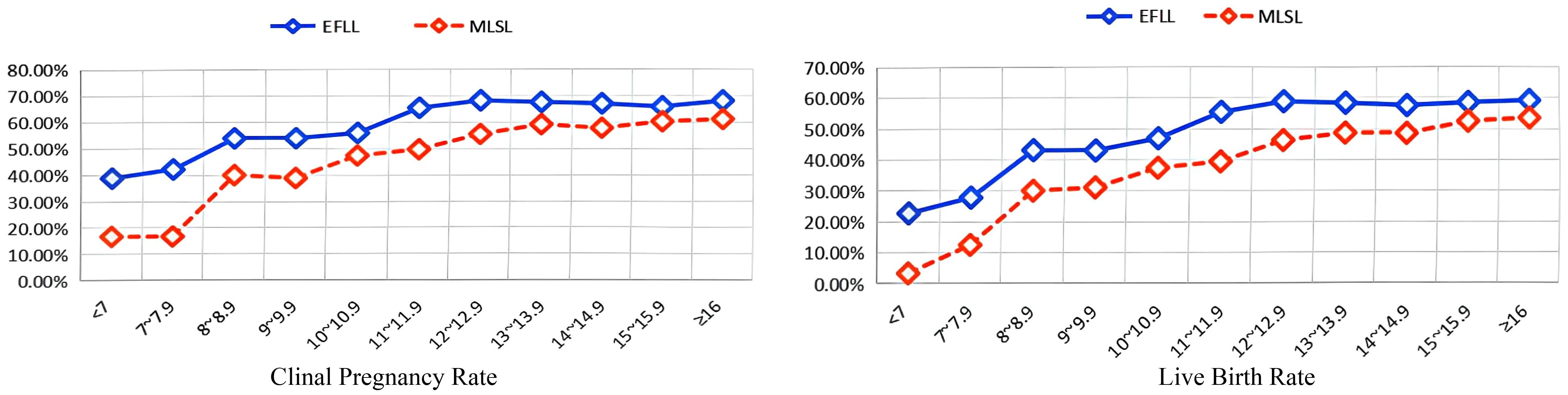
Figure 1. Relationship between endometrial thickness and clinical outcomes in different controlled ovulation induction protocols. EFLL, early-follicular long-acting GnRH agonist protocol; MLSL, midluteal short-acting GnRH agonist long protocol.
3.3 Interaction between ovulation induction protocols and endometrial thickness
As depicted in Figure 2, when endometrial thickness is less than 15 mm, a significant interaction exists between the ovulation induction protocol and endometrial thickness (all P < 0.05, Figure 2). However, when the endometrial thickness is 15 mm or greater, this interaction is no longer observed (P > 0.05, Figure 2).
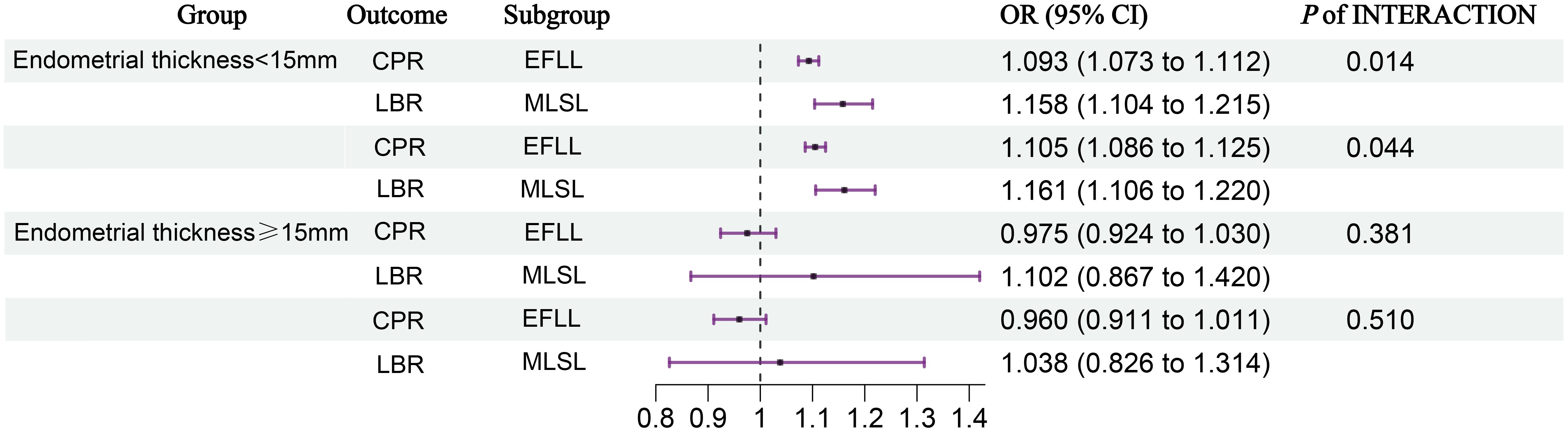
Figure 2. Interaction analysis between ovulation induction protocols and endometrial thickness. We adjusted for age, BMI, infertility type, number of embryos transferred, type of embryos transferred, Gn initiation, total Gn, number of sinus follicles, number of follicle retrievals, and controlled ovulation induction protocol. CI, confidence interval; EFLL, early-follicular long-acting GnRH agonist protocol; MLSL, midluteal short-acting GnRH agonist long protocol; CPR, clinical pregnancy rate; LBR, live birth rate.
3.4 Trends in clinical pregnancy and live birth rates across endometrial thickness in different ovulation induction protocols
As illustrated in Figure 3, among patients undergoing the early-follicular long-acting GnRH agonist protocol, endometrial thickness exhibited a nonlinear relationship with both clinical pregnancy and live birth rates (P for nonlinear < 0.05). In contrast, for those following the midluteal short-acting GnRH-a long protocol, a linear association was observed (P for nonlinear > 0.05).
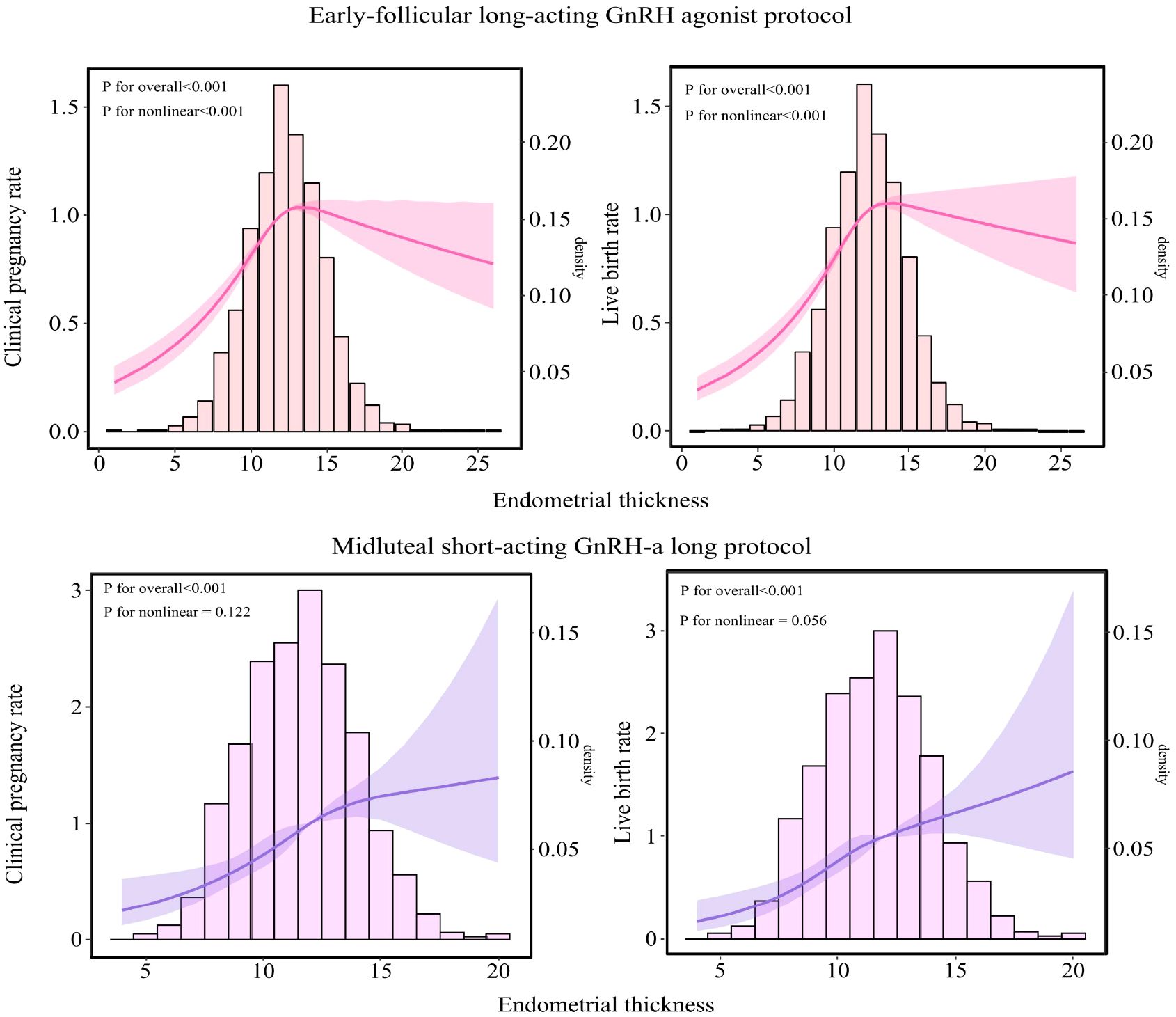
Figure 3. Relationship between endometrial thickness and pregnancy outcomes in populations undergoing different ovarian stimulation protocols. The RCS was used to calculate the estimated changes (95% CIs) adjusted for age, BMI, infertility type, number of embryos transferred, Gn initiation, total Gn, number of sinus follicles, number of follicle retrievals, and controlled ovulation induction protocol. CI, confidence interval; BMI, body mass index; Gn, gonadotropin.
Specifically, in the early-follicular long-acting protocol group, when endometrial thickness was less than 10.6 mm, increases in thickness were associated with significant improvements in clinical pregnancy and live birth rates (aOR = 1.239; 95% CI: 1.183–1.297 and aOR = 1.229; 95% CI: 1.181–1.279, respectively; Table 3). However, when endometrial thickness reached or exceeded 10.6 mm, these rates plateaued, showing no further significant changes (all P > 0.05).
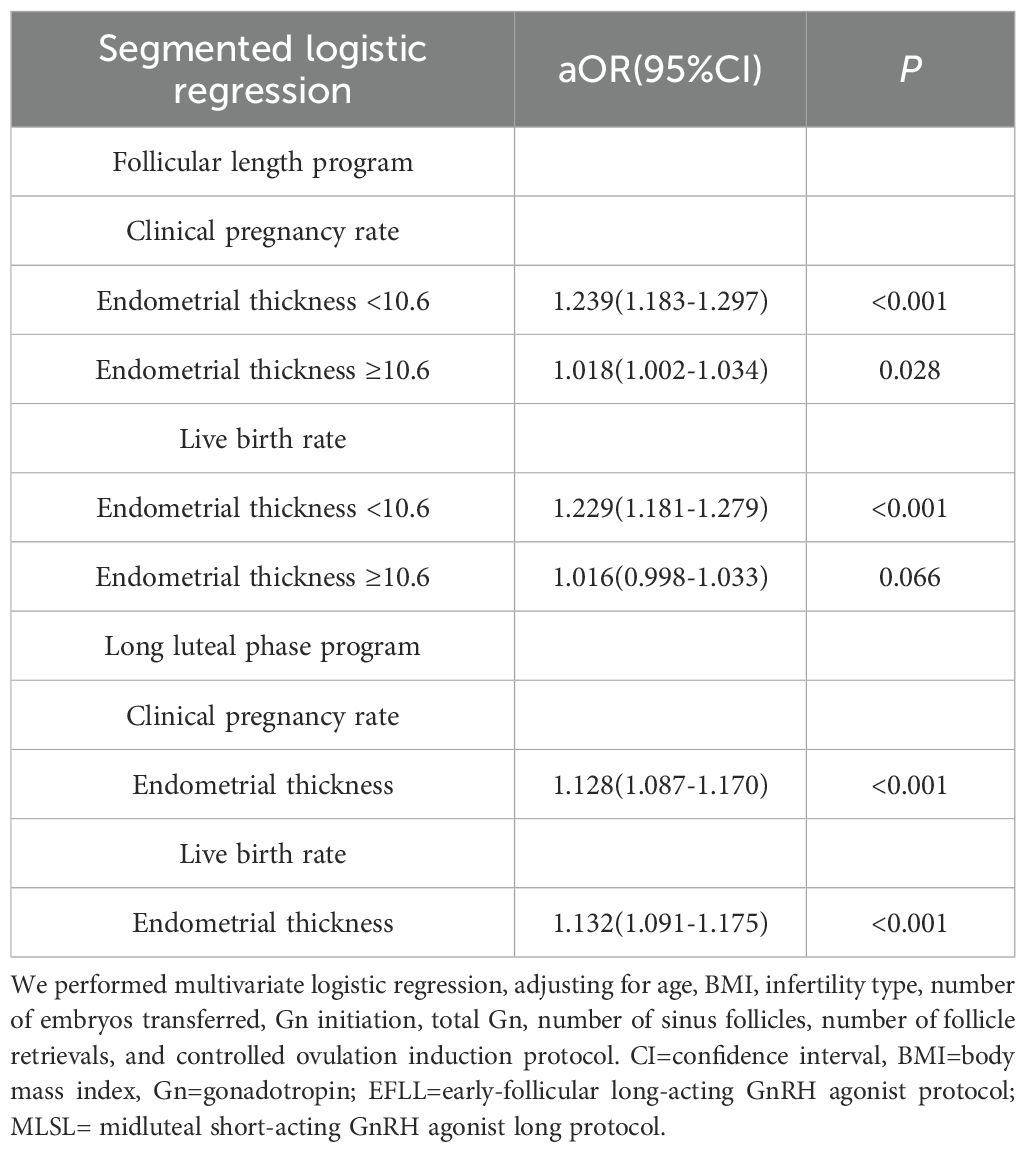
Table 3. Segmented logistic regression analysis of endometrial thickness on clinical pregnancy and live birth rates across different ovulation induction protocols.
Conversely, in the midluteal short-acting protocol group, both clinical pregnancy and live birth rates increased steadily with endometrial thickness across the entire range studied (aOR = 1.128; 95% CI: 1.087–1.170 and aOR = 1.132; 95% CI: 1.091–1.175, respectively; Table 3).
3.5 Trends in miscarriage and preterm birth rates across endometrial thickness in different ovulation induction protocols
As illustrated in Figure 4, among patients undergoing the early-follicular long-acting GnRH agonist protocol, endometrial thickness exhibited a nonlinear relationship with miscarriage rates (P for nonlinear < 0.05). In contrast, for those following the midluteal short-acting GnRH-a long protocol, a linear association was observed (P for nonlinear > 0.05).
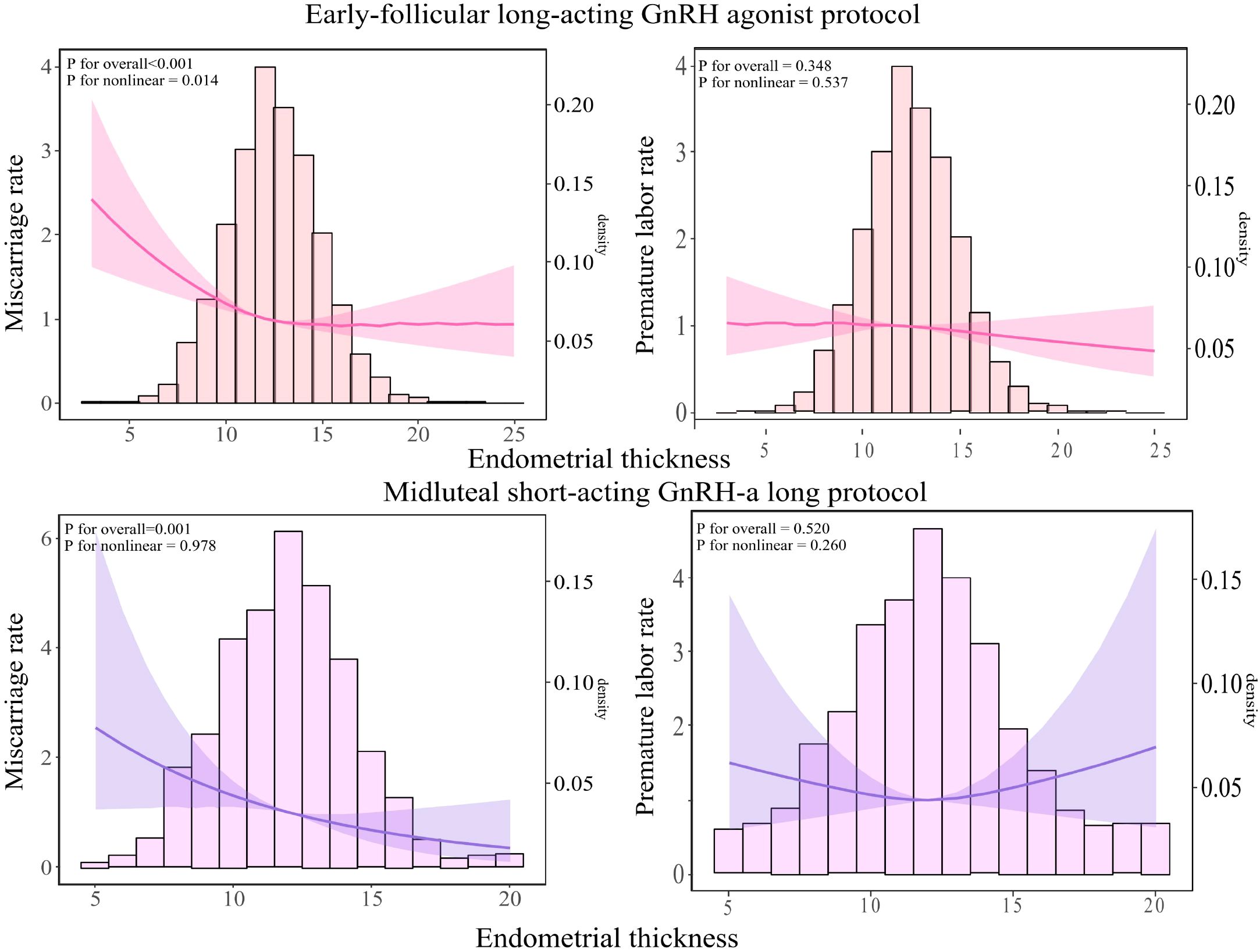
Figure 4. Relationship between endometrial thickness and miscarriage and live birth rates across different ovulation induction protocols. The RCS was used to calculate the estimated changes (95% CIs) adjusted for age, BMI, infertility type, number of embryos transferred, Gn initiation, total Gn, number of sinus follicles, number of follicle retrievals, and controlled ovulation induction protocol. CI, confidence interval; BMI, body mass index, Gn, gonadotropin.
Specifically, in the early-follicular long-acting protocol group, when endometrial thickness was less than 15 mm, increases in thickness were associated with a significant decrease in miscarriage rates (aOR = 0.93; 95% CI: 0.905–0.956; Table 4). However, when endometrial thickness reached or exceeded 15 mm, miscarriage rates plateaued, showing no further significant changes (P = 0.087).
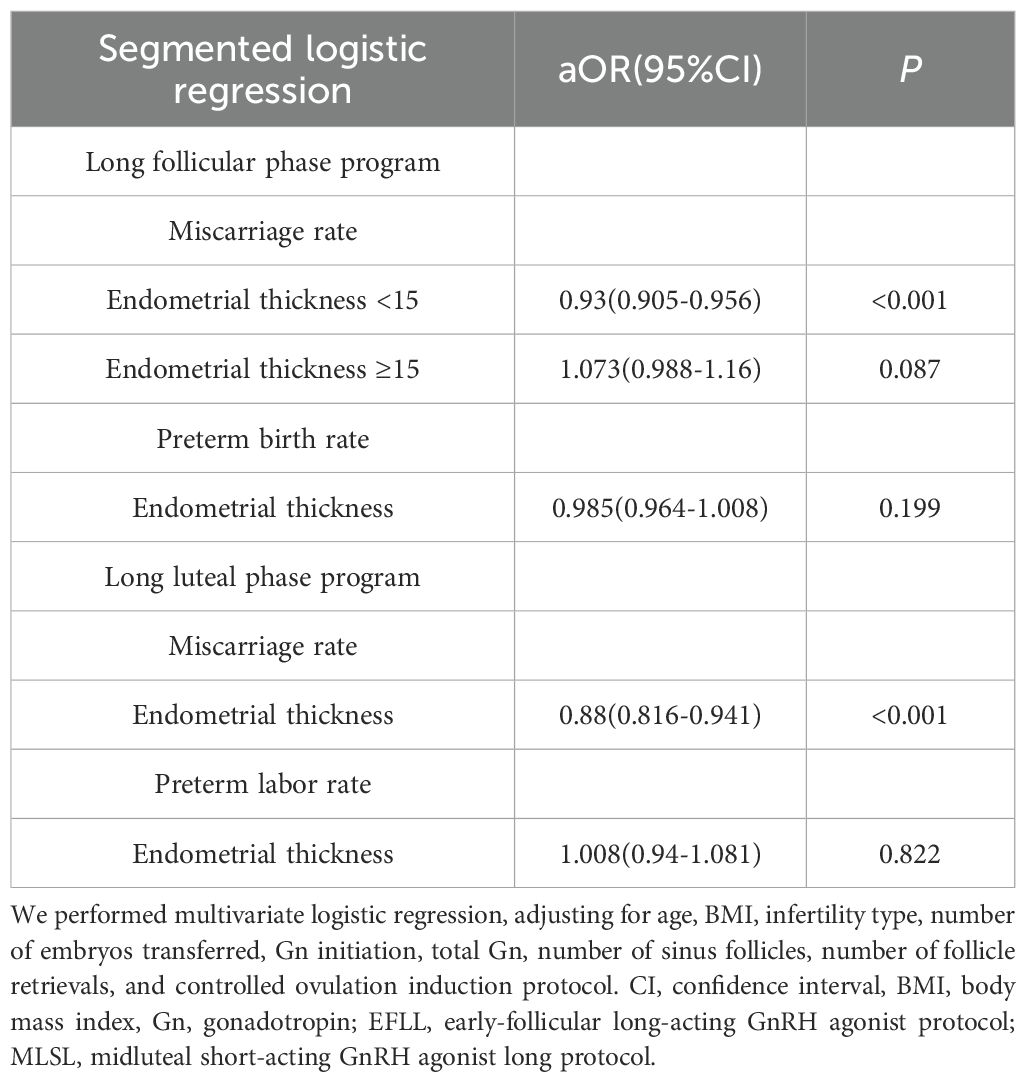
Table 4. Segmented logistic regression analysis of endometrial thickness on miscarriage and preterm birth rates across different ovulation induction protocols.
Conversely, in the midluteal short-acting protocol group, miscarriage rates decreased steadily with increasing endometrial thickness across the entire range studied (aOR = 0.88; 95% CI: 0.816–0.941; Table 4).
Notably, in both protocols, endometrial thickness was not significantly associated with preterm birth rates (all P > 0.05).
4 Discussion
Multiple studies have explored the relationship between endometrial thickness and pregnancy outcomes, as well as the optimal threshold (13–16). The association between endometrial thickness and pregnancy outcomes in assisted reproductive technology (ART) cycles is nonlinear. Large-scale retrospective studies have shown that as endometrial thickness increases from extremely thin to an optimal range (approximately 10–12 mm), clinical pregnancy rates and live birth rates improve significantly. For example, Xu et al., analyzing data from 42,132 fresh embryo transfer cycles, found that for every 1 mm increase in endometrial thickness below 12 mm, the clinical pregnancy rate (CPR) and live birth rate (LBR) increased by approximately 9–10% (16). However, when endometrial thickness reached 12–15 mm, the pregnancy rates tended to plateau. In contrast, when the endometrium is too thin (<7 mm), implantation success rates are significantly reduced due to insufficient glandular development, inadequate vascularization, and hormonal support, and this is also associated with a higher miscarriage rate (17). To date, no studies have compared the trends of endometrial thickness and pregnancy outcomes, as well as clinical pregnancy and perinatal outcomes at different endometrial thickness levels, between the early-follicular long-acting GnRH agonist protocol and the midluteal short-acting GnRH-a long protocol. Therefore, this study investigated the impact of changes in endometrial thickness on clinical outcomes under these two ovarian stimulation protocols. The results showed that in the population undergoing the early-follicular long-acting protocol, endometrial thickness exhibited a pattern of initially increasing and then plateauing in relation to clinical pregnancy rate and live birth rate, while miscarriage rate showed an initial decrease followed by stabilization. When endometrial thickness was less than 10.6 mm, pregnancy rates increased significantly with increasing thickness; however, beyond 10.6 mm, pregnancy rates no longer improved with further increases in endometrial thickness. This is consistent with the findings of Mahutte N et al. in 2022 and Gallos ID et al. in 2018 (6, 14). In contrast, among patients receiving the midluteal short-acting GnRH-a long protocol, endometrial thickness was positively correlated with clinical pregnancy rate and live birth rate, and negatively correlated with miscarriage rate. These findings are consistent with those reported by Zhang J et al (18).
There is still controversy regarding whether an excessively thick endometrium adversely affects pregnancy outcomes. Xu J et al. found that when endometrial thickness is ≥15 mm, both clinical pregnancy rate and live birth rate decrease (16). Most studies have shown that an excessively thick endometrium does not adversely affect pregnancy outcomes (13, 14, 19, 20). Recent meta-analyses indicate that a thick endometrium (>14 mm) overall does not reduce the chance of pregnancy (17), with only a thin endometrium significantly lowering pregnancy success rates. Therefore, the current consensus is that the optimal endometrial thickness (EMT) is approximately 8–12 mm; an extremely thin endometrium is clearly detrimental, while the effects of very high thickness remain inconclusive (17), which aligns with the findings of this study. Although some studies have suggested that an excessively thick endometrium may negatively affect pregnancy outcomes, our findings—together with existing evidence on endometrial blood perfusion and receptivity marker expression—indicate that endometrial thickening is not necessarily detrimental. Our results demonstrate that an endometrial thickness ≥15 mm does not negatively impact pregnancy outcomes. On the contrary, in the midluteal short-acting GnRH-a long protocol group, pregnancy rates continued to increase as endometrial thickness exceeded 15 mm.
Previous studies have shown that the expression levels of endometrial receptivity markers HOXA10, MEIS1, and LIF (21–25) are higher in patients undergoing the early-follicular long-acting GnRH agonist protocol compared with those receiving the midluteal short-acting long protocol (11), suggesting that the follicular-phase long protocol confers better endometrial receptivity (26). This indicates that long-acting GnRHa administration may improve the implantation environment by enhancing the expression of genes associated with endometrial receptivity, which could explain why the follicular-phase long protocol achieves a relatively high pregnancy rate even within the moderate endometrial thickness range.
On the other hand, the duration and dosage of GnRHa pretreatment can also affect the expression of endometrial cytokines. For instance, studies have reported that GnRHa treatment upregulates IL-11 and integrin αvβ3 expression in the endometrium, thereby promoting trophoblast invasion and supporting early pregnancy maintenance (27). In addition, adequate endometrial blood supply and vascular development are essential for maintaining normal endometrial function and receptivity. Endometrial proliferation and differentiation depend on sufficient blood flow to deliver oxygen, nutrients, and hormones while removing metabolic waste products. Vascular endothelial growth factor (VEGF) is abundantly expressed during the proliferative phase to stimulate microvascular dilation and neovascularization, ensuring rich perfusion during the implantation window. In contrast, excessively thin endometrium is often associated with poor perfusion and sparse vasculature, which can be reflected by an elevated resistance index (RI) of the uterine radial artery (RA-RI) on Doppler ultrasound (28). A study focusing on “thin” endometrium demonstrated that, compared with women with normal endometrial thickness, patients with thin endometrium showed significantly higher uterine radial artery resistance throughout the cycle, along with reduced glandular development, decreased vascular density, and markedly lower VEGF expression during the mid- to late-proliferative phases (28). These findings suggest that inadequate vascularization and limited blood flow in a thin endometrium lead to poor nutrient and oxygen supply, thereby compromising embryo survival and implantation potential. This also explains why endometrial thickness below 7 mm is often associated with low pregnancy rates.
Clinical intervention studies have further shown that improving uterine blood flow—through supplementation with vitamin E, L-arginine, or sildenafil citrate, among others—can reduce radial artery resistance and increase endometrial thickness (29). Enhanced local perfusion provides a more oxygenated and nutrient-rich endometrial microenvironment that better supports embryo implantation. When endometrial thickness reaches approximately 10–12 mm, an optimal range, the vascular bed is typically well established: spiral arteries have undergone remodeling, microvascular density is increased, and vascular resistance is reduced. At this stage, the endometrium receives adequate perfusion throughout all layers, creating a stable environment for embryo implantation and exchange of nutrients and gases. Further thickening beyond this point does not significantly improve blood flow, as perfusion is no longer the limiting factor.
Our study also showed that when endometrial thickness reaches or exceeds 15 mm, both protocols achieve similar pregnancy rates. This may be because increased endometrial thickness is associated with reduced resistance in uterine radial artery blood flow and improved vascular development (29, 30), thereby significantly enhancing endometrial receptivity and resulting in comparable pregnancy outcomes between the two protocols. Overall, the pregnancy rate advantage of the early-follicular long-acting protocol may be related to the upregulation of endometrial receptivity markers such as HOXA10 and LIF, while the midluteal short-acting protocol may achieve similar outcomes in cases of thicker endometrium by improving uterine blood flow.
Compared to the midluteal short-acting GnRH-a long protocol, the early-follicular long-acting GnRH agonist protocol is associated with a higher incidence of gestational diabetes mellitus (3.4% vs. 1.2%), which is consistent with previous findings by Du L and Wang D (31, 32). This may be related to the use of long-acting GnRH agonists potentially causing impaired glucose tolerance and increased insulin resistance (33). Additionally, studies have indicated that the dose of gonadotropins is a risk factor for the development of gestational diabetes mellitus (34), and the early-follicular long-acting protocol involves higher and longer gonadotropin usage during ovarian stimulation.
The relationship between endometrial thickness and preterm birth rate remains controversial. This study found no association between endometrial thickness and preterm birth rate in either protocol, which is consistent with several previously published studies (17, 35, 36). However, a 2023 study by Wu J et al. reported a negative correlation between endometrial thickness and preterm birth rate (37). This discrepancy may reflect differences in study design, sample selection, as well as regional and population variations. Furthermore, the mechanisms by which endometrial thickness might influence preterm birth remain unclear and may involve complex endocrine and uterine environmental factors. Therefore, prospective studies are needed in the future to clarify the relationship between endometrial thickness and preterm birth rate and to explore the underlying biological mechanisms.
This study is the first to use restricted cubic spline (RCS) analysis to investigate the differing trends between endometrial thickness and clinical pregnancy rates in IVF/ICSI patients undergoing the early-follicular long-acting GnRH agonist protocol and the midluteal short-acting GnRH-a long protocol. This study has several strengths. First, by using RCS to build nonlinear models, it provides a more precise characterization of how pregnancy rates vary with endometrial thickness. Second, the strict inclusion and exclusion criteria ensured that the study population closely represents the general patient population. Lastly, with a large sample size of 21,290 clinical records, the modeling results are more reliable. However, this study still has certain limitations. First, all cases were derived from a single reproductive medicine center. While this ensured consistency in treatment protocols and laboratory procedures, it may limit the external generalizability of the results. Second, as a retrospective study, it remains subject to potential selection and information biases, although we attempted to control measurable confounders through propensity score matching (PSM) and multivariate logistic regression. Nevertheless, due to limitations in the original clinical dataset, several potential confounding factors—such as lifestyle (diet, daily routine, psychological stress), environmental exposures, previous medication use, and hormonal imbalances—were not systematically collected and thus could not be incorporated into the model analysis. These unmeasured variables might have influenced the pregnancy outcomes to some extent.
In addition, because of the restricted study period and incomplete follow-up data, neonatal and long-term maternal outcomes were not available, making it difficult to assess the long-term health effects of different stimulation protocols. Future research should combine long-term follow-up cohorts to systematically evaluate the long-term safety and reproductive outcomes of various ovarian stimulation protocols. Moreover, prospective, multicenter studies with more comprehensive data collection are needed to further enhance the reliability and generalizability of the conclusions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research and Clinical Trials Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CW: Methodology, Writing – original draft, Data curation, Writing – review & editing. CY: Writing – review & editing. WD: Data curation, Writing – review & editing. LH: Writing – review & editing, Methodology. HK: Writing – review & editing, Investigation. GY: Writing – review & editing, Investigation. XW: Methodology, Writing – review & editing. ZB: Writing – review & editing, Investigation. YP: Methodology, Writing – review & editing. JZ: Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant No. 82071649), the Key Scientific Research Projects of Higher Education Institutions in Henan Province (Grant No. 22A320025) and the Henan Provincial Science and Technology Project (Grant No. 252300421263).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Craciunas L, Gallos I, Chu J, Bourne Tom, Quenby Siobhan, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:202–23. doi: 10.1093/humupd/dmy044
2. Kasius A, Smit JG, Torrance HL, Eijkemans MJC, Willem MB, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. (2014) 20:530–41. doi: 10.1093/humupd/dmu011
3. Ribeiro VC, Santos-Ribeiro S, De Munck N, and Drakopoulos P. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online. (2018) 36:416–26. doi: 10.1016/j.rbmo.2017.12.016
4. Zhao J, Zhang Q, Wang Y, and Li Y. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod Biomed Online. (2014) 29:291–8. doi: 10.1016/j.rbmo.2014.05.011
5. Bu Z and Sun Y. The impact of endometrial thickness on the day of human chorionic gonadotrophin (hCG) administration on ongoing pregnancy rate in patients with different ovarian response. PloS One. (2015) 10:e0145703. doi: 10.1371/journal.pone.0145703
6. Gallos ID, Khairy M, Chu J, Rajkhowa M, Tobias A, Campbell A, et al. Optimal endometrial thickness to maximize live births and minimize pregnancy losses: Analysis of 25,767 fresh embryo transfers. Reprod Biomed Online. (2018) 37:542–8. doi: 10.1016/j.rbmo.2018.08.025
7. Gao G, Cui X, Li S, Ding P, and Zhang Y. Endometrial thickness and IVF cycle outcomes: a meta-analysis. Reprod Biomed Online. (2020) 40:124–33. doi: 10.1016/j.rbmo.2019.09.005
8. Chen SL, Wu FR, Luo C, Chen X, Shi XY, Zheng HY, et al. Association between endometrial thickness and birth weight in fresh IVF/ICSI embryo transfers: a retrospective cohort study of 9273 singleton births. Reprod Biomed Online. (2021) 43:1087–94. doi: 10.1016/j.rbmo.2021.08.021
9. Hong L, Xiuzhu L, Jiangbo D, Xiufeng L, Feiyang D, Qun L, et al. Effect of endometrial thickness and embryo quality on live-birth rate of fresh IVF/ICSI cycles: a retrospective cohort study. Reprod Biol Eendocrinol: RB&E. (2020) 18:89. doi: 10.1186/s12958-020-00636-6
10. Jones GM, Trounson AO, Lolatgis N, and Wood C. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertility Sterility. (2007) 87:53–9. doi: 10.1016/j.fertnstert.2006.05.064
11. Xu B, Geerts D, Hu S, Yue J, Li Z, Zhu G, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod (Oxford England). (2020) 35:1306–18. doi: 10.1093/humrep/deaa086
12. Zhang Y, Zhao W, Han Y, Chen X, Xu S, Hu Y, et al. The follicular-phase depot GnRH agonist protocol results in a higher live birth rate without discernible differences in luteal function and child health versus the daily mid-luteal GnRH agonist protocol: a single-centre, retrospective, propensity score matched cohort study. Reprod Biol Eendocrinol: RB&E. (2022) 20:140. doi: 10.1186/s12958-022-01014-0
13. Dietterich C, Check JH, Choe JK, Nazari A, and Lurie D. Increased endometrial thickness on the day of human chorionic gonadotropin injection does not adversely affect pregnancy or implantation rates following in vitro fertilization-embryo transfer. Fertility Sterility. (2002) 77:781–6. doi: 10.1016/S0015-0282(01)03276-9
14. Mahutte N, Hartman M, Meng L, Lanes A, Luo ZC, and Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertility Sterility. (2022) 117:792–800. doi: 10.1016/j.fertnstert.2021.12.025
15. Weissman A, Gotlieb L, and Casper RF. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertility Sterility. (1999) 71:147–9. doi: 10.1016/S0015-0282(98)00413-0
16. Xu J, Zhang S, Jin L, Mao Y, Shi J, Huang R, et al. The effects of endometrial thickness on pregnancy outcomes of fresh IVF/ICSI embryo transfer cycles: an analysis of over 40,000 cycles among five reproductive centers in China. Front Endocrinol. (2021) 12:788706. doi: 10.3389/fendo.2021.788706
17. Liao Z, Liu C, Cai L, Shen L, Sui C, Zhang H, et al. The effect of endometrial thickness on pregnancy, maternal, and perinatal outcomes of women in fresh cycles after IVF/ICSI: A systematic review and meta-analysis. Front Endocrinol. (2021) 12:814648. doi: 10.3389/fendo.2021.814648
18. Zhang J, Sun YF, Xu YM, Shi BJ, Han Y, Luo ZY, et al. Effect of endometrium thickness on clinical outcomes in luteal phase short-acting gnRH-a long protocol and gnRH-ant protocol. Front Endocrinol. (2021) 12:578783. doi: 10.3389/fendo.2021.578783
19. Fang R, Cai L, Xiong F, Chen J, Yang W, and Zhao X. The effect of endometrial thickness on the day of hCG administration on pregnancy outcome in the first fresh IVF/ICSI cycle. Gynecol Endocrinol. (2016) 32:473–6. doi: 10.3109/09513590.2015.1132304
20. Zhang X, Chen CH, Confino E, Barnes R, Milad M, and Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertility Sterility. (2005) 83:336–40. doi: 10.1016/j.fertnstert.2004.09.020
21. Aghajanova L. Update on the role of leukemia inhibitory factor in assisted reproduction. Curr Opin Obstet Gynecol. (2010) 22:213–9. doi: 10.1097/GCO.0b013e32833848e5
22. Hu L, Li H, Huang CL, Chen H, Zhu G, and Qian K. Regulation of myeloid ecotropic viral integration site 1 and its expression in normal and abnormal endometrium. Fertility Sterility. (2014) 102:856–63.e2. doi: 10.1016/j.fertnstert.2014.05.036
23. Sarno JL, Kliman HJ, and Taylor HS. AHOXA10, Pbx2, and Meis1 protein expression in the human endometrium: formation of multimeric complexes on HOXA10 target genes. J Clin Endocrinol Metab. (2005) 90:522–8. doi: 10.1210/jc.2004-0817
24. Satokata I, Benson G, and Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. (1995) 374:460–3. doi: 10.1038/374460a0
25. Daftary GS and Taylor HS. Implantation in the human: the role of HOX genes. Semin Reprod Med. (2000) 18:311–20. doi: 10.1055/s-2000-12568
26. Song J, Sun X, and Qian K. Endometrial but not Ovarian Response is Associated With Clinical Outcomes and can be Improved by Prolonged Pituitary Downregulation in Patients With Thin and Medium Endometrium. Reprod Sci (Thousand Oaks Calif). (2019) 26:1409–16. doi: 10.1177/1933719118816835
27. Li L, Liu L, Kou Z, Huo M, An J, and Zhang X. GnRH agonist treatment regulates IL-6 and IL-11 expression in endometrial stromal cells for patients with HRT regiment in frozen embryo transfer cycles. Reprod Biol. (2022) 22:100608. doi: 10.1016/j.repbio.2022.100608
28. Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, and Sugino N. Pathophysiologic features of “thin” endometrium. Fertility Sterility. (2009) 91:998–1004. doi: 10.1016/j.fertnstert.2008.01.029
29. Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, and Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertility Sterility. (2010) 93:1851–8. doi: 10.1016/j.fertnstert.2008.12.062
30. Mitao H, Yoshizato T, Fujita T, Fukagawa M, Nakashima A, Horinouchi T, et al. Novel application to evaluate endometrial blood flow using transvaginal superb microvascular imaging: A preliminary study describing physiological changes from ovulation to mid-luteal phase. Acta Obstet Gynecol Scandinavica. (2023) 102:914–20. doi: 10.1111/aogs.14585
31. Du L, Song J, Fan W, Ye T, and Kong H. Safety profiles of offspring born from early-follicular long-acting GnRH agonist protocol and daily mid-luteal GnRH agonist protocol: a retrospective study. BMC Pregnancy Childbirth. (2024) 24:393. doi: 10.1186/s12884-024-06589-7
32. Wang D, Chu T, Yu T, and Zhai J. Is early-follicular long-acting GnRH agonist protocol an alternative for patients with polycystic ovary syndrome undergoing in vitro fertilization? Reprod Biol Eendocrinol: RB&E. (2022) 20:137. doi: 10.1186/s12958-022-01007-z
33. Taşcilar ME, Bilir P, Akinci A, Köse K, Akçora D, Inceoğlu D, et al. The effect of gonadotropin-releasing hormone analog treatment (leuprolide) on body fat distribution in idiopathic central precocious puberty. Turkish J Pediatr. (2011) 53:27–33.
34. Jie Z, Yiling D, and Ling Y. Association of assisted reproductive technology with adverse pregnancy outcomes. Iranian J Reprod Med. (2015) 13:169–80.
35. Guo Z, Xu X, Zhang L, Zhang L, Yan L, and Ma J. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. Fertility Sterility. (2020) 113:745–52. doi: 10.1016/j.fertnstert.2019.12.014
36. Huang J, Lin J, Lu X, Gao H, Song N, Cai R, et al. Association between endometrial thickness and neonatal outcomes in intrauterine insemination cycles: a retrospective analysis of 1,016 live-born singletons. Reprod Biol Eendocrinol: RB&E. (2020) 18:48. doi: 10.1186/s12958-020-00597-w
Keywords: endometrial thickness, early-follicular long-acting GnRH agonist protocol, midluteal short-acting GnRH agonist long protocol, endometrial receptivity, restricted cubic spline (RCS)
Citation: Wang C, Yang C, Dai W, Han L, Kong H, Yao G, Wang X, Bu Z, Peng Y and Zhai J (2025) The influence of endometrial thickness on clinical pregnancy outcomes in early-follicular long-acting and midluteal short-acting GnRH agonist long protocols: a large retrospective study. Front. Endocrinol. 16:1637587. doi: 10.3389/fendo.2025.1637587
Received: 29 May 2025; Accepted: 04 November 2025; Revised: 25 October 2025;
Published: 26 November 2025.
Edited by:
Li Xiao, Sichuan University, ChinaReviewed by:
Meng Dong, China Medical University, ChinaYanli Fan, Second Hospital of Hebei Medical University, China
Copyright © 2025 Wang, Yang, Dai, Han, Kong, Yao, Wang, Bu, Peng and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhai, YmVzdHpoYWkyMDA1QDE2My5jb20=
 Chen Wang1,2,3
Chen Wang1,2,3 Wei Dai
Wei Dai Liping Han
Liping Han Huijuan Kong
Huijuan Kong Guidong Yao
Guidong Yao Xiao Wang
Xiao Wang Zhiqin Bu
Zhiqin Bu Jun Zhai
Jun Zhai