- 1Department of Fundamental Pathology, Endocrinology Research Centre, Moscow, Russia
- 2Institute of Clinical Morphology and Digital Pathology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
Introduction: Papillary thyroid microcarcinomas (PTMCs), defined as papillary thyroid carcinomas measuring ≤1 cm, are frequently diagnosed incidentally and are generally associated with favorable outcomes. However, a significant subset of patients develops regional lymph node metastases. This study aimed to identify clinical and morphological features associated with metastatic spread in PTMCs.
Methods: A total of 100 cases were retrospectively analyzed, including 50 with confirmed lymph node metastases and 50 without. A detailed histological assessment included evaluation of tumor subtype, nuclear features, presence of psammoma bodies, tumor “buds,” fibrosis, and other parameters. Clinical variables such as age, sex, body mass index, and presence of the BRAF V600E mutation were also considered.
Results: Logistic regression revealed that younger age, classical histological subtype, tumor “budding,” and presence of psammoma bodies were independently associated with an increased risk of regional metastases, while concomitant multinodular hyperplasia was negatively associated.
Discussion: The combination of these features may enhance risk stratification and guide the clinical management of PTMC patients, including decisions on surgical extent and follow-up strategies. Our findings support the relevance of comprehensive histological and clinical evaluation in predicting the metastatic potential of PTMCs.
1 Introduction
Thyroid cancer has been and remains the most common malignant neoplasm of the endocrine system (1, 2). Approximately 80% of malignant thyroid tumors are differentiated forms of cancer, the most frequent of which is papillary carcinoma (1). This histological type accounts for 50% to 80% of all malignant thyroid tumors, according to various data (3, 4). In many countries, recent years have seen an increase in the incidence of thyroid cancer, primarily due to the increased diagnosis of papillary carcinoma (5). However, this rise in thyroid carcinoma incidence has not been accompanied by an increase in tumor-specific mortality. This fact is mainly due to the more frequent diagnosis of papillary microcarcinomas, incidentally detected either during pathological examination of thyroid tissue removed for non-tumor pathology or during ultrasound examinations performed for reasons unrelated to thyroid pathology (6). Such tumors account for about 28% of diagnosed papillary thyroid carcinomas (4). Papillary thyroid microcarcinomas are defined as papillary carcinomas measuring no more than 1 cm, regardless of growth patterns (3, 7). Previously, terms such as “occult papillary carcinoma,” “latent papillary carcinoma,” “small papillary carcinoma,” “nonencapsulated thyroid tumor,” and “occult sclerosing carcinoma” were used for these tumors (8, 9). According to various sources, papillary thyroid microcarcinomas, which are clinically silent, may be found in 4–35.6% of cases during total histological examination of thyroid glands (4, 10, 11). The frequency of their incidental discovery in thyroid glands operated on for other diseases is directly proportional to the thoroughness of the histological examination (12). Researchers agree that the prognosis for patients operated on for papillary thyroid microcarcinomas is very good, with high recurrence-free and tumor-specific survival rates (12–14). Nevertheless, data from several studies indicate that the frequency of regional lymph node metastases in papillary thyroid microcarcinomas ranges from 17.1% to 49% (4, 14–16). It is known that a higher risk of metastasis is observed in tall cell, diffuse sclerosing, solid/trabecular, columnar cell, and hobnail subtypes of papillary carcinoma (17–21). Additionally, regional metastases are more frequently detected in pediatric patients (22). A higher risk of lymph node metastasis is also characteristic of patients with tumor invasion into lymphatic vessel lumens (23) and those with the BRAF V600E mutation in tumor tissue (24, 25). However, in practical work, situations often arise where metastatic involvement of regional lymph nodes is detected in patients who do not belong to known risk groups. Thus, identifying predictors of regional metastasis will allow for future optimization of clinical management protocols for such patients, refinement of the diagnostic criteria for papillary thyroid microcarcinoma, and consideration of reclassifying a portion of these tumors from malignant to neoplasms with limited malignant potential. This, in turn, would justify the implementation of active surveillance strategies and the incorporation of such an approach into clinical algorithms and guidelines.
The aim of the present study was to identify clinical and morphological predictors of regional metastasis in papillary thyroid microcarcinomas.
2 Materials and methods
2.1 Patients and samples
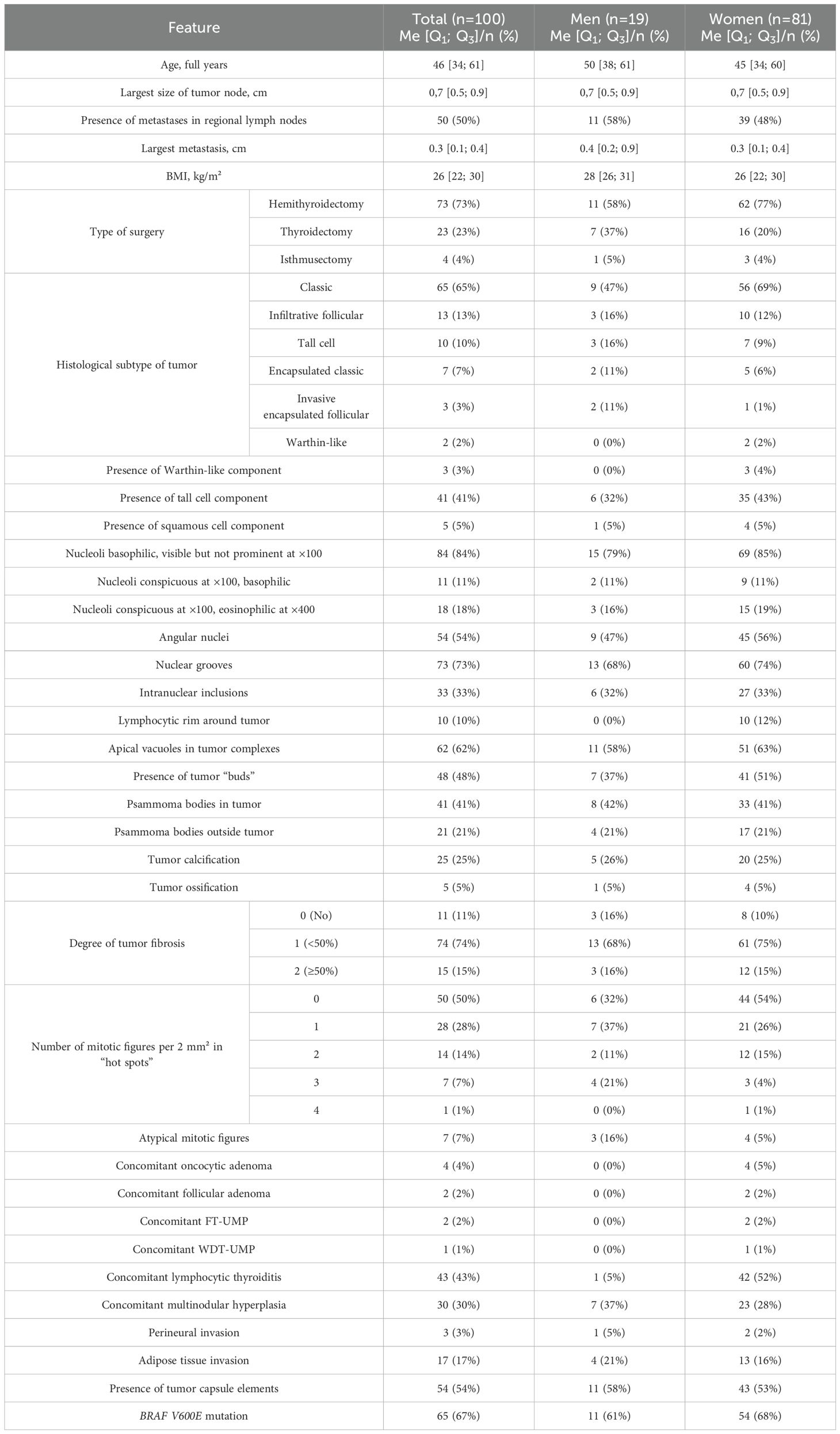
The study was conducted on surgical material from 100 patients aged 24 to 72 years (Me = 46 [34;61] years) operated on at the Endocrinology Research Centre (Moscow, Russia), for thyroid diseases between January 1, 2022, and September 1, 2023, with morphologically verified papillary thyroid carcinomas measuring ≤1 cm in greatest dimension (median = 0.7 cm; Q1 = 0.5 cm; Q3 = 0.9 cm). Exclusion criteria included age younger than 19 years and multifocal tumor growth. The study group comprised 50 patients with histologically verified synchronous metastases in regional lymph nodes (11 men, 39 women, aged 24 to 65 years). Among them, 45 patients had metastases only in the central compartment lymph nodes (pN1a according to TNM 8), and 5 patients had metastases in both the central and lateral compartments (pN1b according to TNM 8). The control group consisted of 50 patients without clinical or morphological evidence of metastatic involvement of regional lymph nodes, aged 25 to 72 years, including 8 men and 42 women. For the control group, there was also no evidence of regional metastasis development within 183 days after surgery.
Prophylactic lymph node dissection was performed only in patients presenting with clinical signs of lymph node involvement. In cases where the tumor was localized in one lobe of the thyroid gland and regional lymph node metastases were suspected in the central compartment (level VI), ipsilateral central lymph node dissection was carried out. Involvement of lateral neck lymph nodes was observed in four patients, who subsequently underwent lymphadenectomy of levels II to V; one patient underwent dissection of level III lymph nodes only.
In our study, none of the four patients with tumors located in the thyroid isthmus showed clinical evidence of regional lymph node involvement; therefore, intentional lymph node dissection was not performed. Nevertheless, in three of these patients, between two and four level VI lymph nodes, which were intimately adjacent to the isthmus, were unintentionally removed. In two of them, metastatic involvement of one lymph node was identified.
2.2 Morphological examination
The surgical material was fixed in 10% neutral buffered formalin (pH 7.4) for 18–20 hours, followed by sectioning, processing in a vacuum histoprocessor using a standard protocol, and embedding in paraffin blocks. For histological preparations, sections 3–4 µm thick were made. Then the slides were stained with hematoxylin and eosin according to the standard procedure.
The groups were compared based on a number of tumor morphological features. The following were evaluated: the largest tumor node (Figure 1) and metastatic focus size (Figure 2), the histological subtype of papillary carcinoma according to the WHO classification (26), the presence of tall cell (Figure 3), Warthin-like, and squamous cell components in the tumor, foci of calcification and ossification in the tumor, a lymphocytic rim around the tumor node in the thyroid lobe tissue, apical vacuoles in tumor complexes, psammoma bodies in the tumor tissue (Figure 4) and in the thyroid tissue outside the tumor, the presence of a tumor capsule or its fragments, tumor “buds” (Figure 5), invasion of adipose tissue, perineural invasion, concomitant nodular thyroid diseases (tumor and non-tumor), and thyroiditis. Additionally, the following nuclear features of tumor cells were noted: angular contours, nuclear grooves, and intranuclear pseudoinclusions (Figure 6). Nucleoli in tumor cell nuclei were assessed using criteria partly adopted from the modified WHO/ISUP classification by S.A. Fuhrman for grading renal cell carcinomas (27), namely: the presence of nucleoli basophilic, visible but not prominent at ×100, or nucleoli conspicuous at ×100, basophilic, or nucleoli conspicuous at ×100, eosinophilic at ×400. Furthermore, the number of mitotic figures per 2 mm² of tumor tissue in “hot spots” and the presence of atypical mitotic figures were assessed. The degree of tumor fibrosis was visually graded as 0 (no fibrosis), 1 (fibrosis in less than 50% of the tumor node), or 2 (fibrosis in 50% or more of the tumor node) (Figure 7). A tumor “bud” was defined as a tumor complex no larger than 0.1 cm in diameter, located no more than 0.1 cm far from the tumor node, with one or more non-neoplastic thyroid follicles between them, which could not be reliably differentiated during routine histological examination between a tumor embolus in a vessel lumen (blood or lymphatic), an additional independent microfocus of tumor growth, or a tangential section of a tumor invasion area into adjacent thyroid tissue.
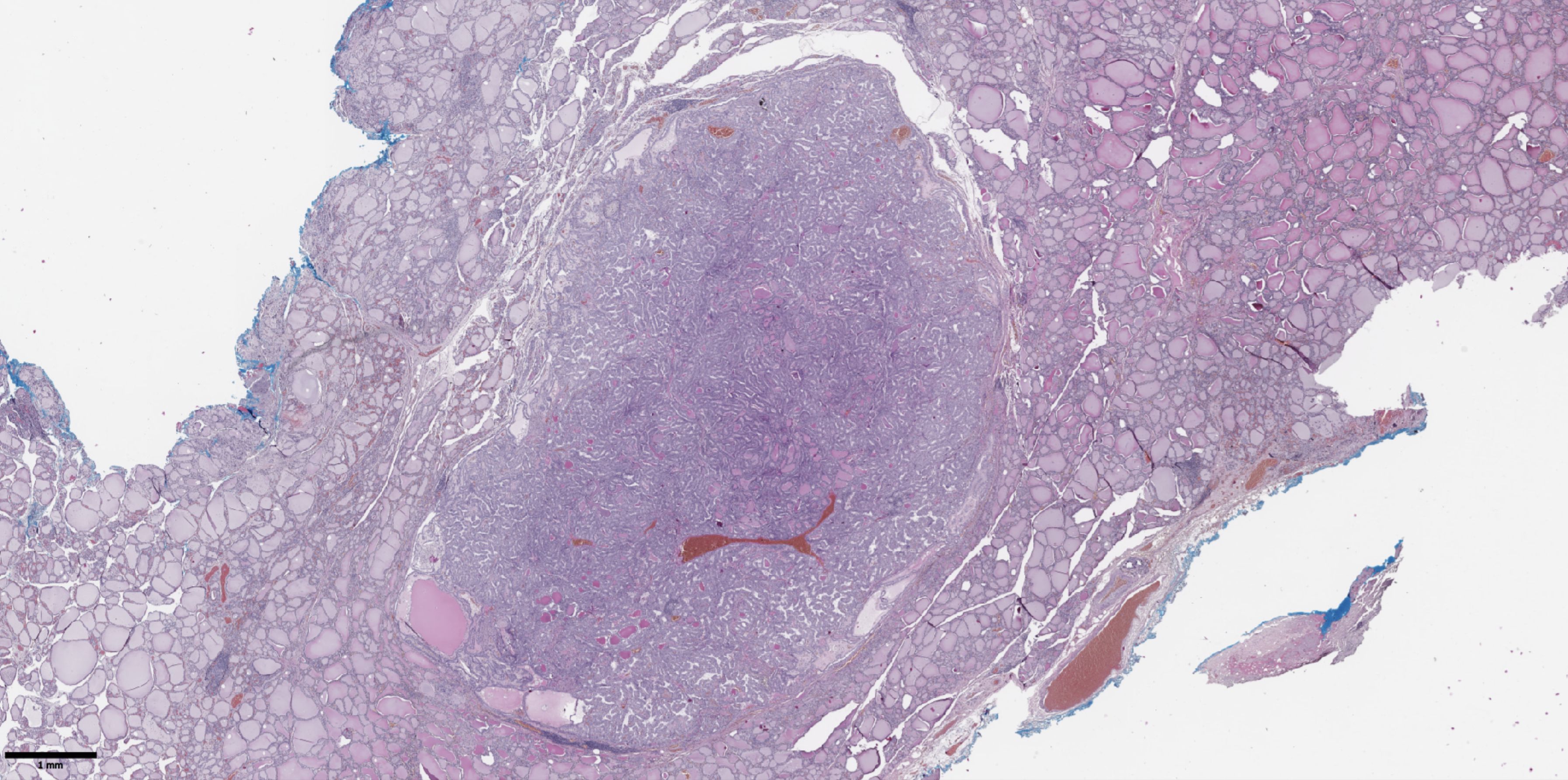
Figure 1. Histological image of papillary thyroid microcarcinoma. The tumor node is represented by papillary and follicular structures formed by cells with papillary nuclear features. Hematoxylin–eosin, х20.
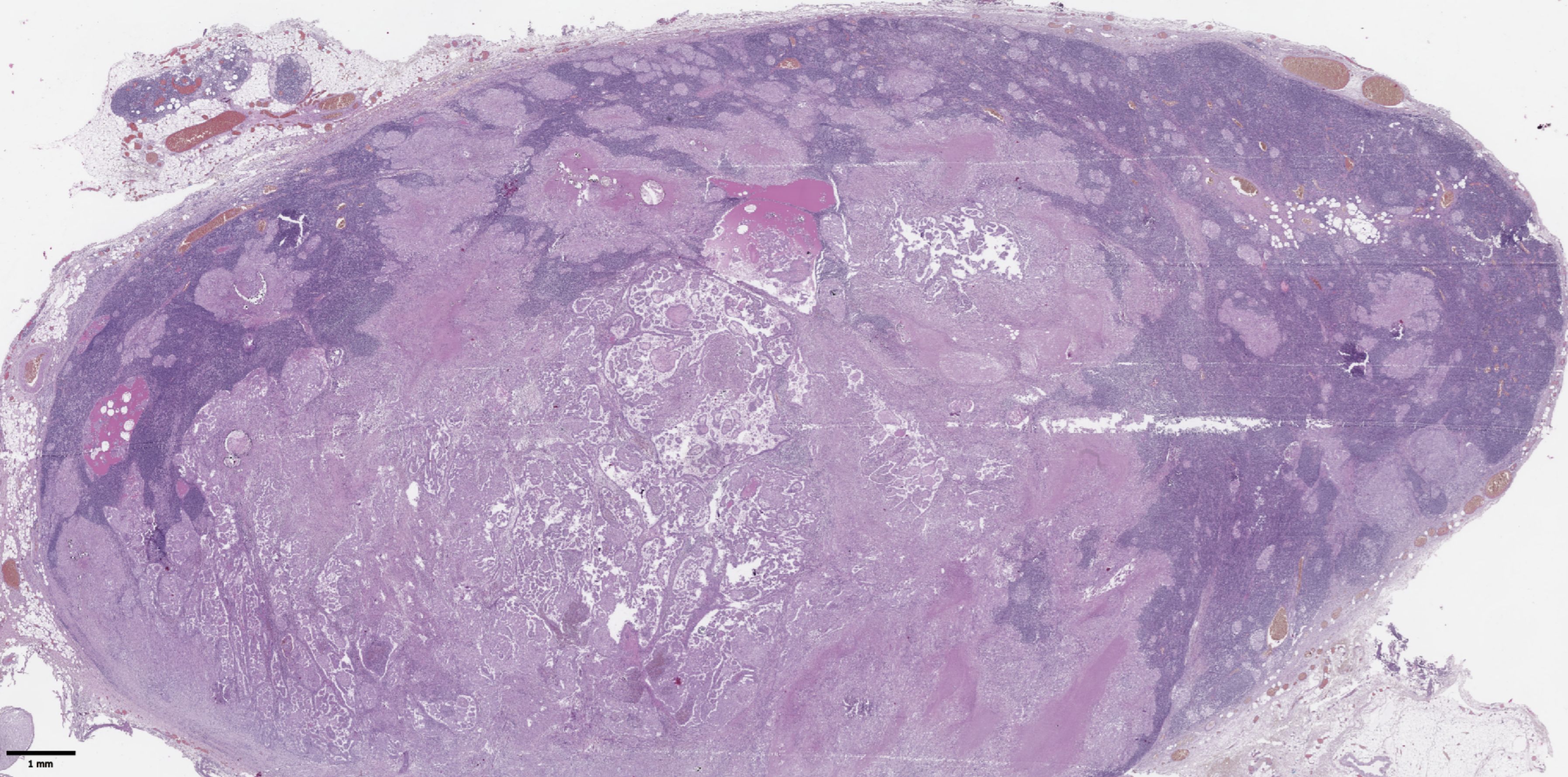
Figure 2. Histological images of metastases in regional lymph nodes of papillary thyroid microcarcinoma. Most of the lymph node tissue is replaced by a tumor. Hematoxylin–eosin, х30.
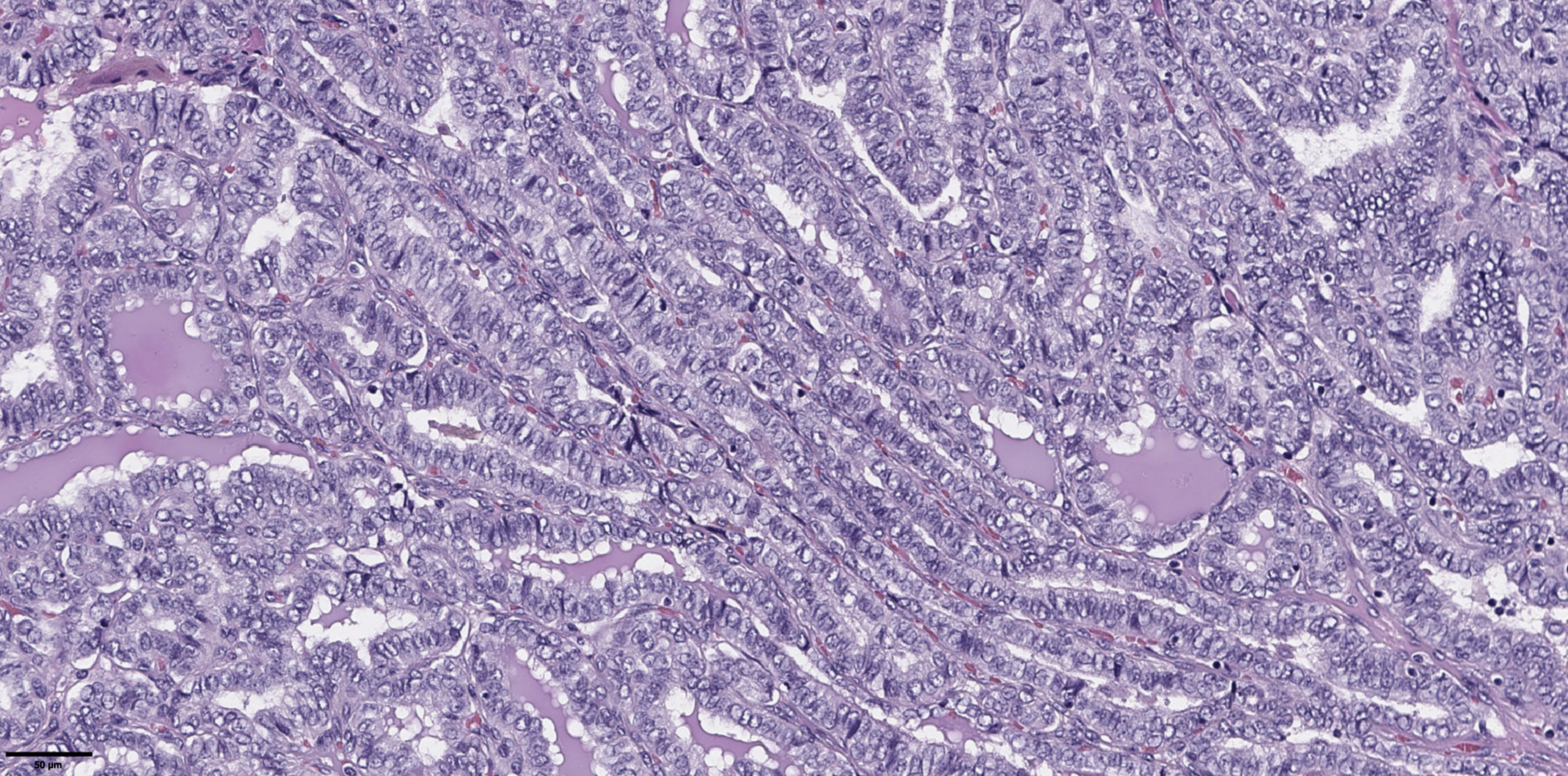
Figure 3. Histological images of papillary thyroid microcarcinoma, tall cell subtype. The tumor is composed of complex papillary and elongated trabecular formations that have a «tram-track» appearance. They are lined by crowded cells that have a height-to-width ratio of ≥ 3:1. Hematoxylin–eosin, х100.
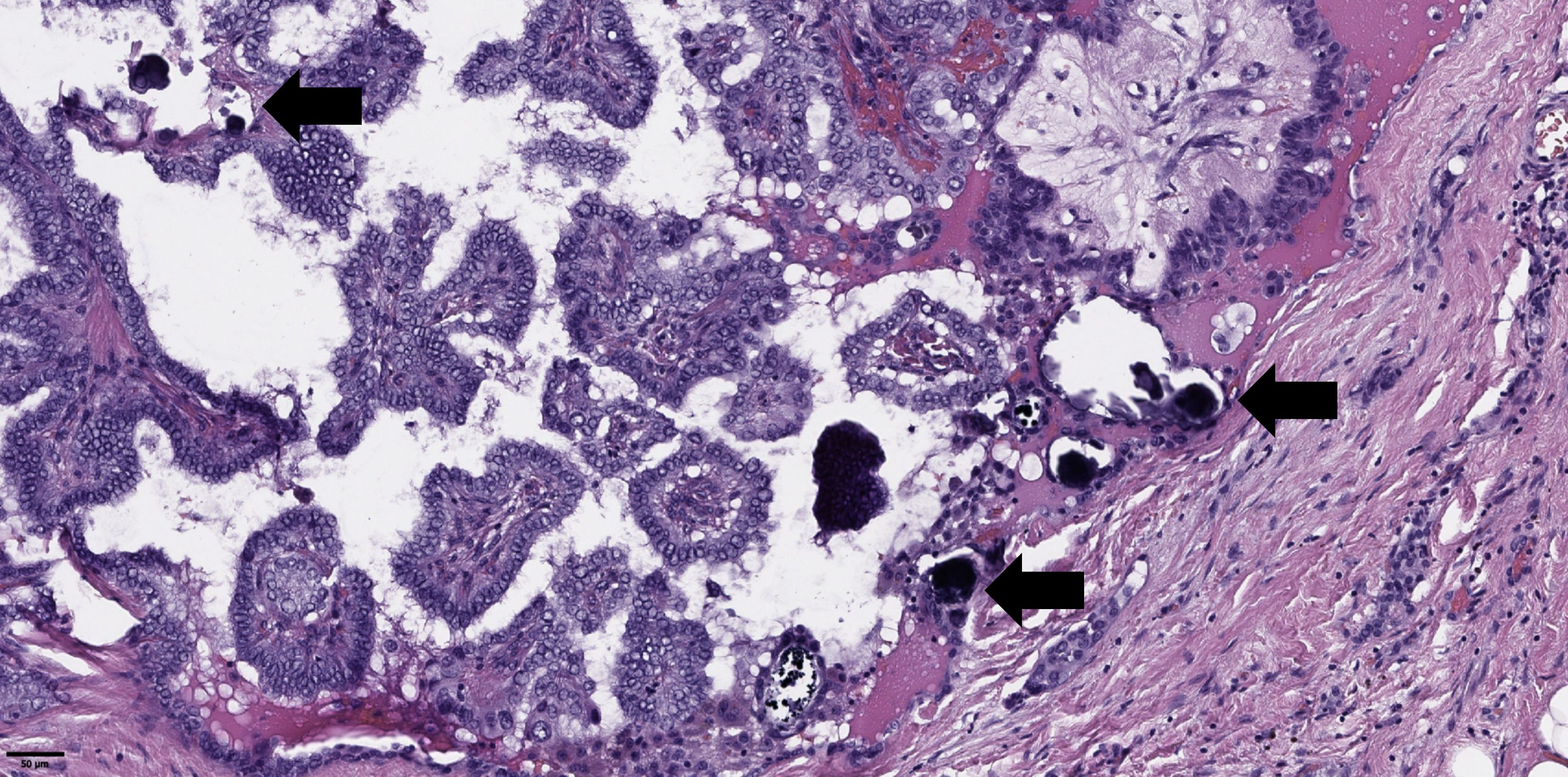
Figure 4. Histological images of psammoma bodies in tumor of papillary thyroid microcarcinoma (black arrows). Hematoxylin–eosin, х100.
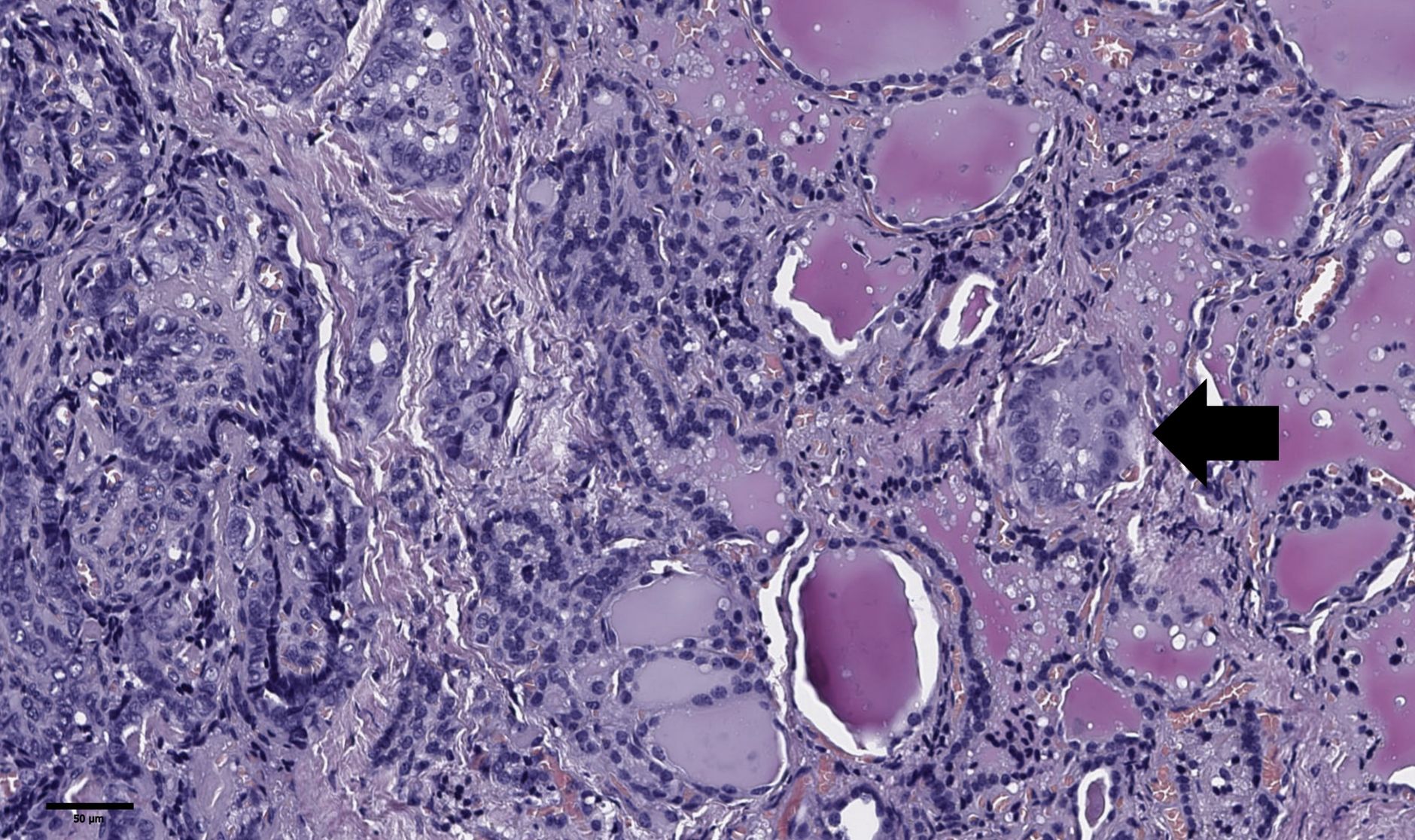
Figure 5. Histological images of tumor “bud” of papillary thyroid microcarcinoma (black arrow). A tumor “bud” was defined as a tumor complex no larger than 0.1 cm in diameter, located no more than 0.1 cm far from the tumor node. Hematoxylin–eosin, х100.
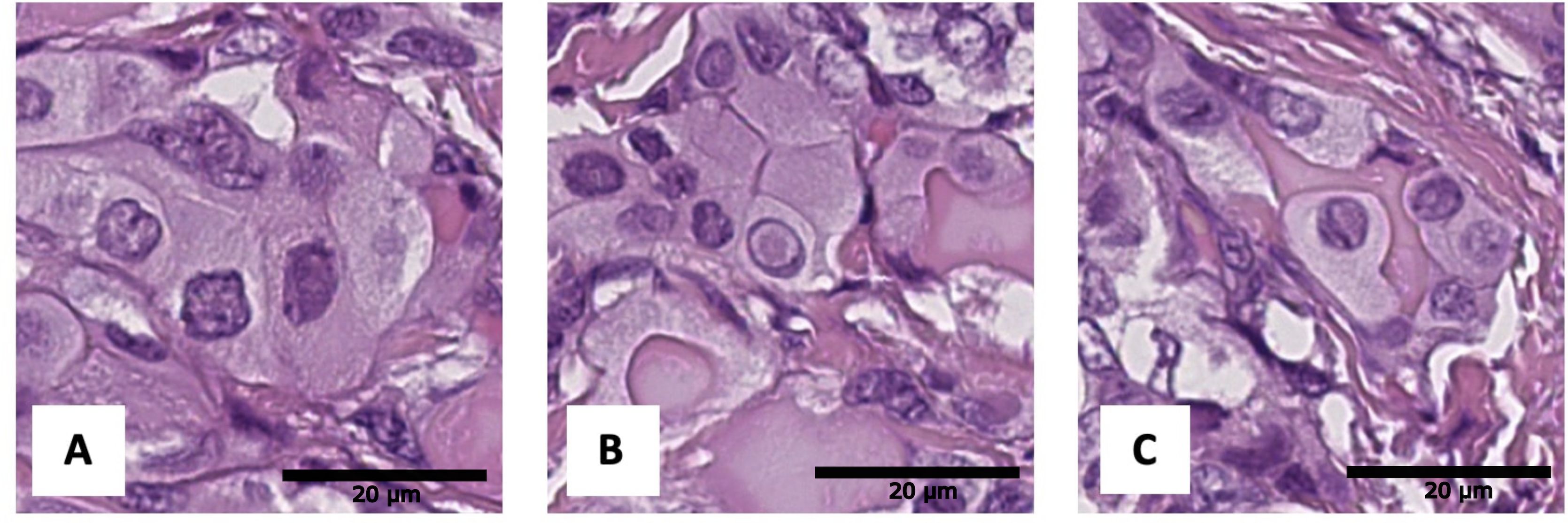
Figure 6. Histological images of tumor nuclear features: (A) angular contours, (B) intranuclear pseudoinclusions, (C) nuclear grooves. Hematoxylin–eosin, х600.
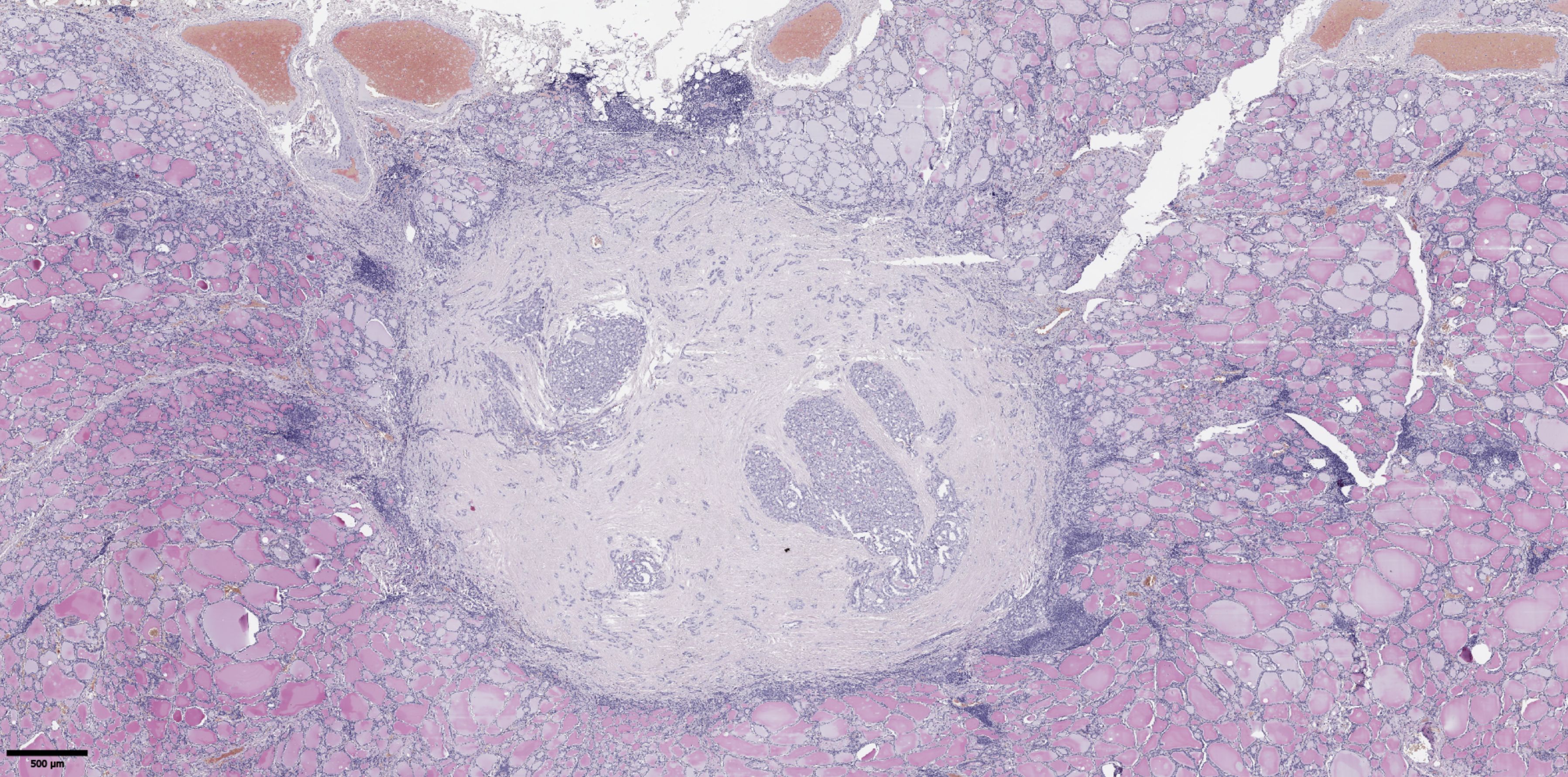
Figure 7. Histological images of papillary thyroid microcarcinoma with fibrosis in 50% or more of the tumor node. Hematoxylin–eosin, х20.
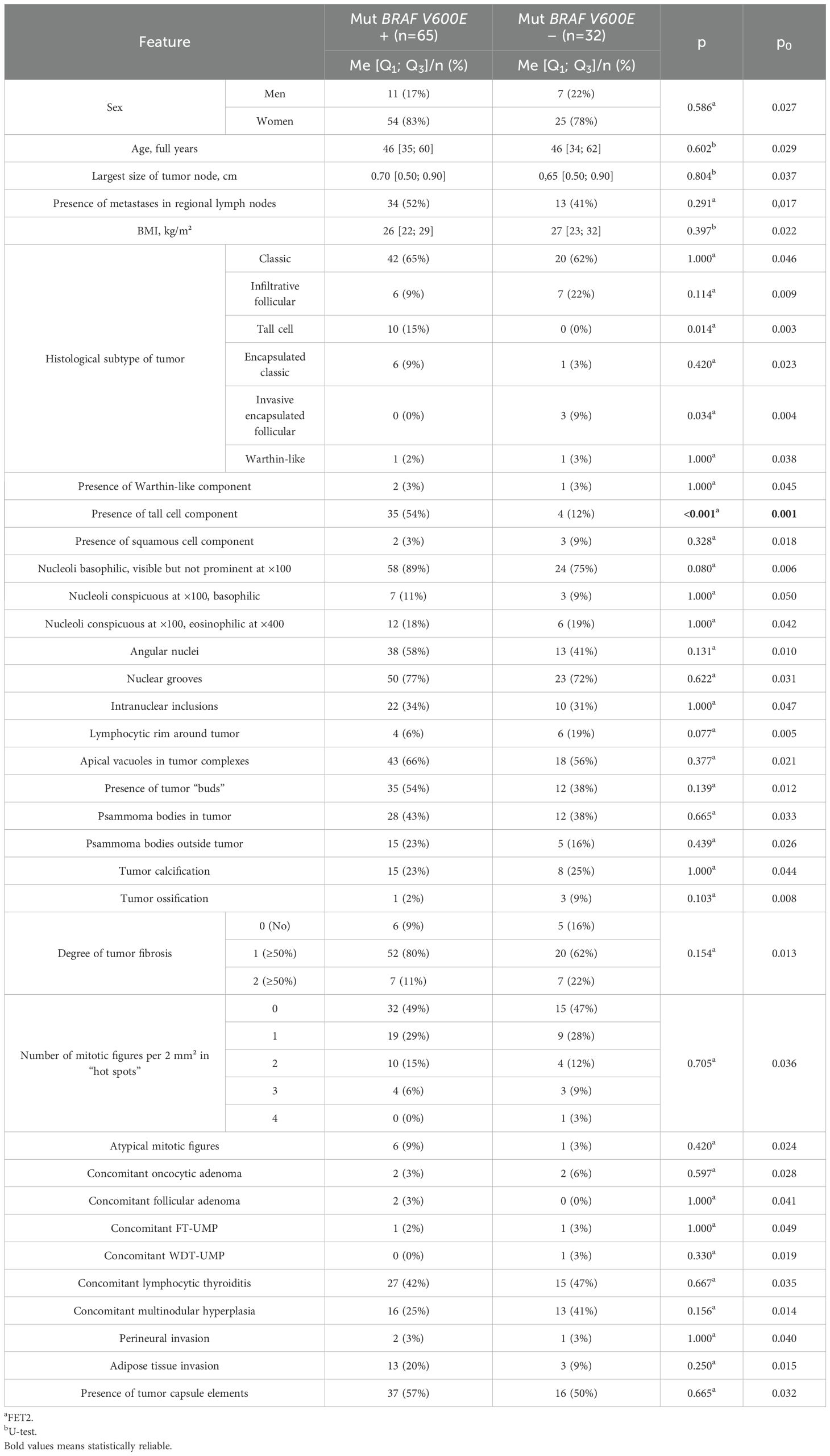
Scanning of histological slides was performed using a Leica Aperio AT2 histoscanner (Leica Biosystems) at ×200 magnification. Measurements of tumor “bud” size and their distance from the main tumor node were performed on scanned histological slides using the built-in measurement tool in Aperio eSlide Manager software (version 12.4.3.5008, Leica Biosystems). Histological slides and their digital scans were independently evaluated by three specialists from the Department of Fundamental Pathomorphology of the Endocrinology Research Center (Moscow, Russia). No significant discrepancies in the interpretation of the observed changes were noted; in rare cases involving specific items, decisions were made collectively. No patients were excluded from the study due to a lack of consensus among the experts. The groups were also compared based on the presence of the BRAF V600E mutation in tumor tissue, sex, age, and body mass index (BMI) of the patients. BMI was calculated using the standard formula as the ratio of the patient’s weight (in kilograms) to the square of their height (in meters). The BRAF V600E mutation was determined by Real-Time PCR using the “Test-BRAF-tissue-24” system (TestGen). The study was performed in 97 out of 100 cases—material from 1 patient in the group without lymph node involvement and 2 patients in the group with lymph node metastases was excluded from the analysis due to severe calcification and “harsh” decalcification of the material. The main characteristics of the overall patient group are presented in Table 1.
2.3 Statistical analysis
Statistical analysis was performed using the Python 3.11 programming language. Descriptive statistics for quantitative features are presented as medians, first and third quartiles in the format Me [Q1; Q3], and categorical features as absolute and relative frequencies in the format n (%). Comparative analysis of two independent groups for quantitative features was performed using the Mann-Whitney U test, and for categorical features using the two-sided Fisher’s exact test (FET2). The critical level of statistical significance was set at 0.05. For multiple comparisons, the Benjamini-Hochberg correction (p0) was applied. Cutoff points for individual parameters were determined using ROC analysis. For cutoff points, odds ratios (OR) with 95% confidence intervals (CI) were calculated. To identify a combination of factors statistically significantly associated with the presence of metastases, logistic regression analysis was performed.
3 Results
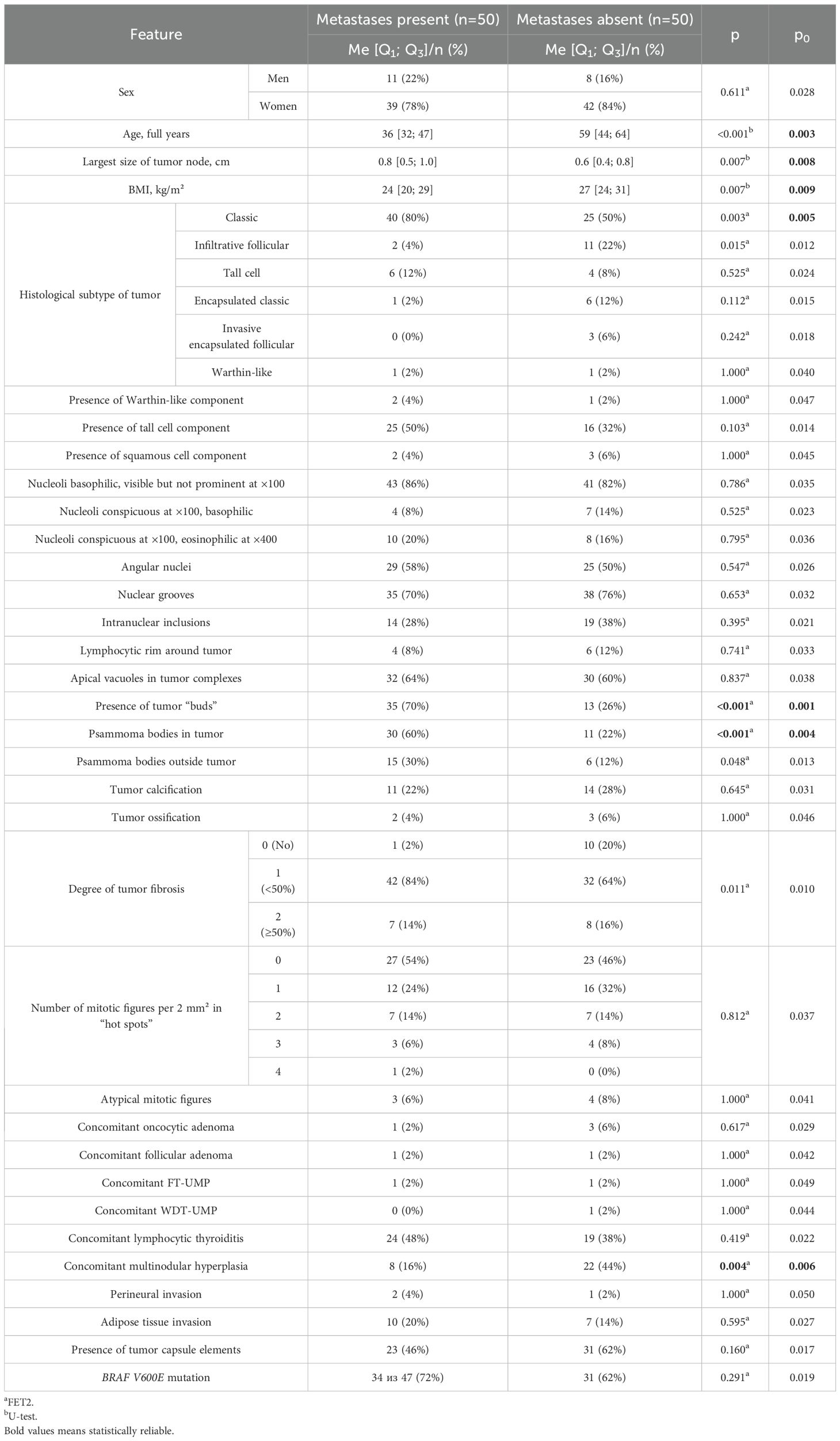
A comparative analysis of patients with and without metastases in regional lymph nodes is presented in Table 2.
The comparative analysis revealed that patients with metastases were younger (p<0.001; p0 = 0.003), had a lower BMI (p=0.007; p0 = 0.009), and a larger tumor node size (p=0.007; p0 = 0.008). The classic subtype of carcinoma (p=0.003; p0 = 0.005), psammoma bodies in tumor tissue (p<0.001; p0 = 0.004), and tumor “buds” (p<0.001; p0 = 0.001) were more frequently identified in these patients. No metastases were found in patients with tumor node sizes <0.3 cm. Concomitant multinodular hyperplasia was less common in patients with metastases compared to those without (p=0.004; p0 = 0.006). Patients with metastases also showed a statistical trend toward more pronounced tumor fibrosis (p=0.011; p0 = 0.010) and more frequent detection of psammoma bodies in thyroid tissue outside the tumor (p=0.048; p0 = 0.013). In patients without metastases, a statistical trend toward more frequent detection of the infiltrative follicular tumor subtype was observed (p=0.015; p0 = 0.012).
Next, odds ratios (OR) were calculated for features that showed statistically significant differences. The classification matrix for patients with and without metastases based on these features is presented in Table 3.
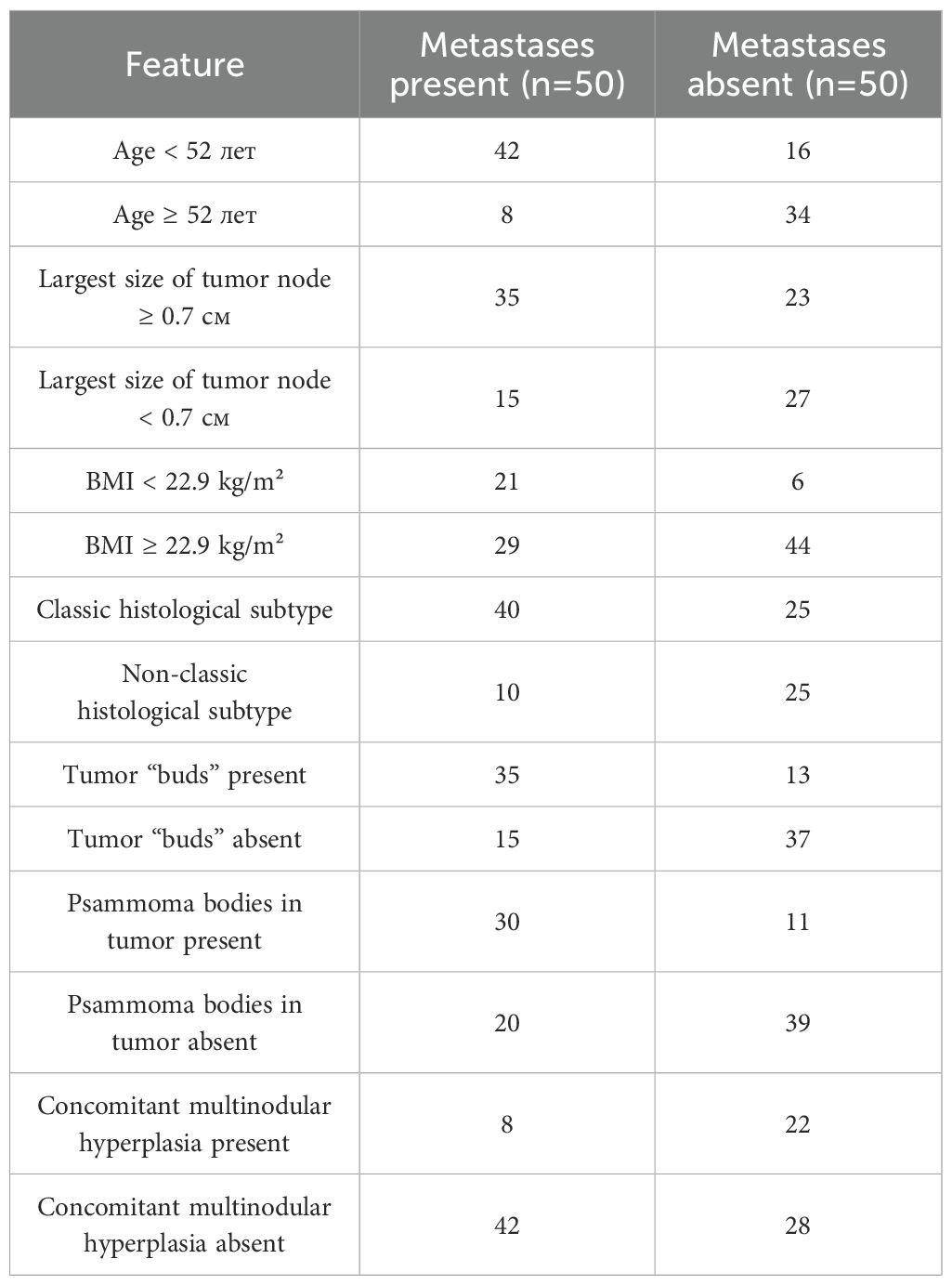
Table 3. Classification matrix for patients with and without metastases based on features showing statistically significant differences.
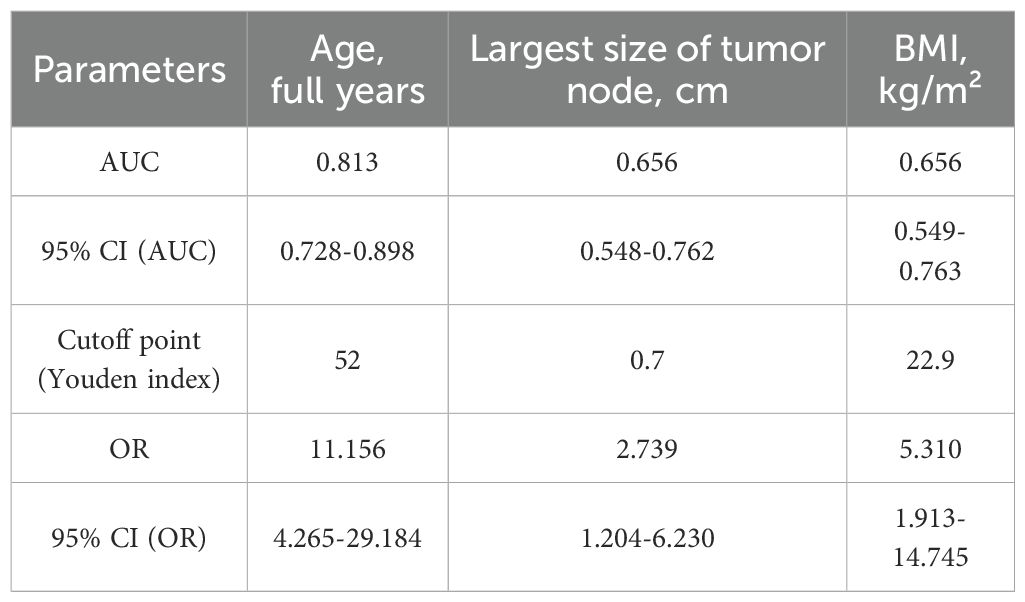
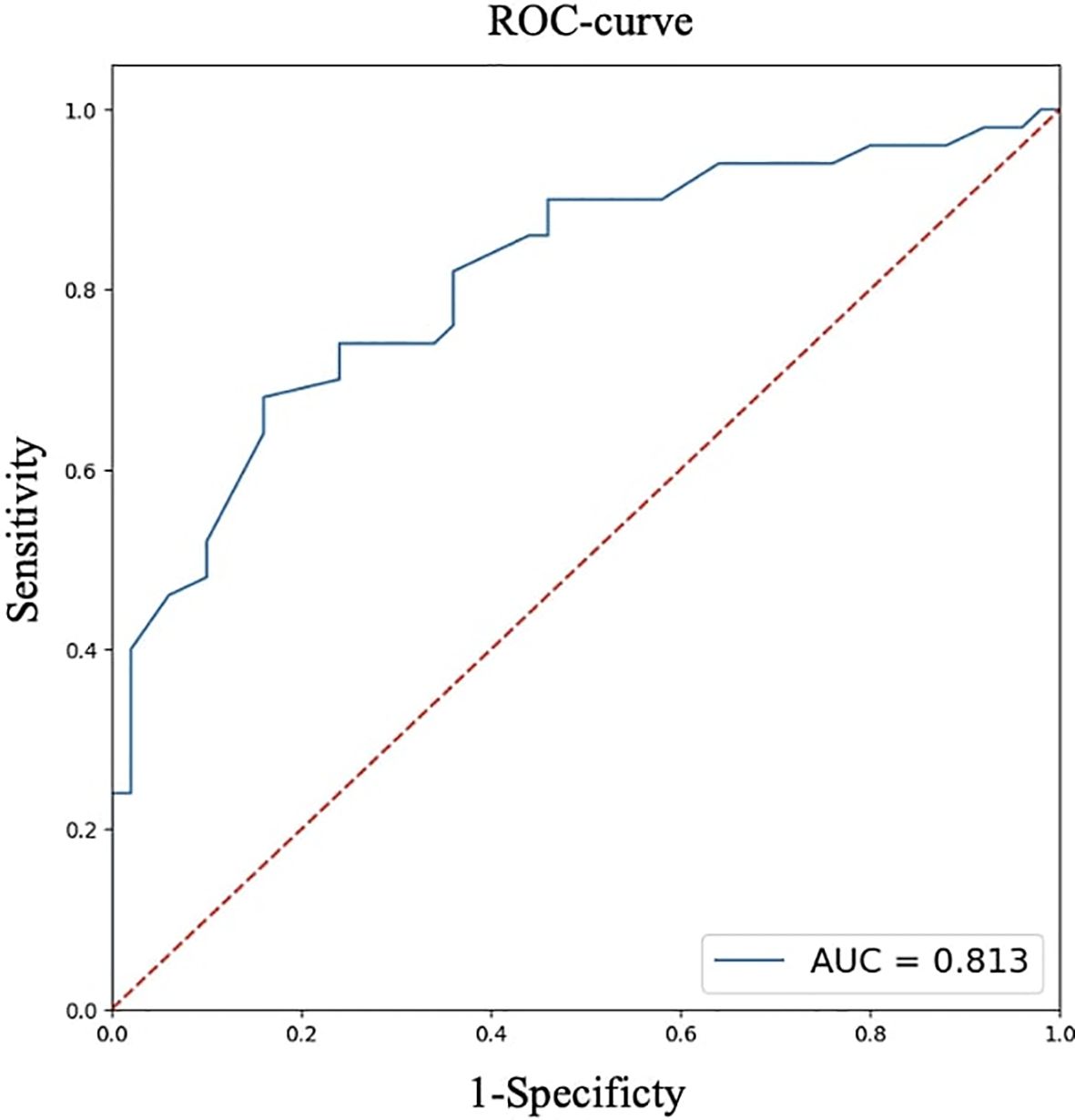
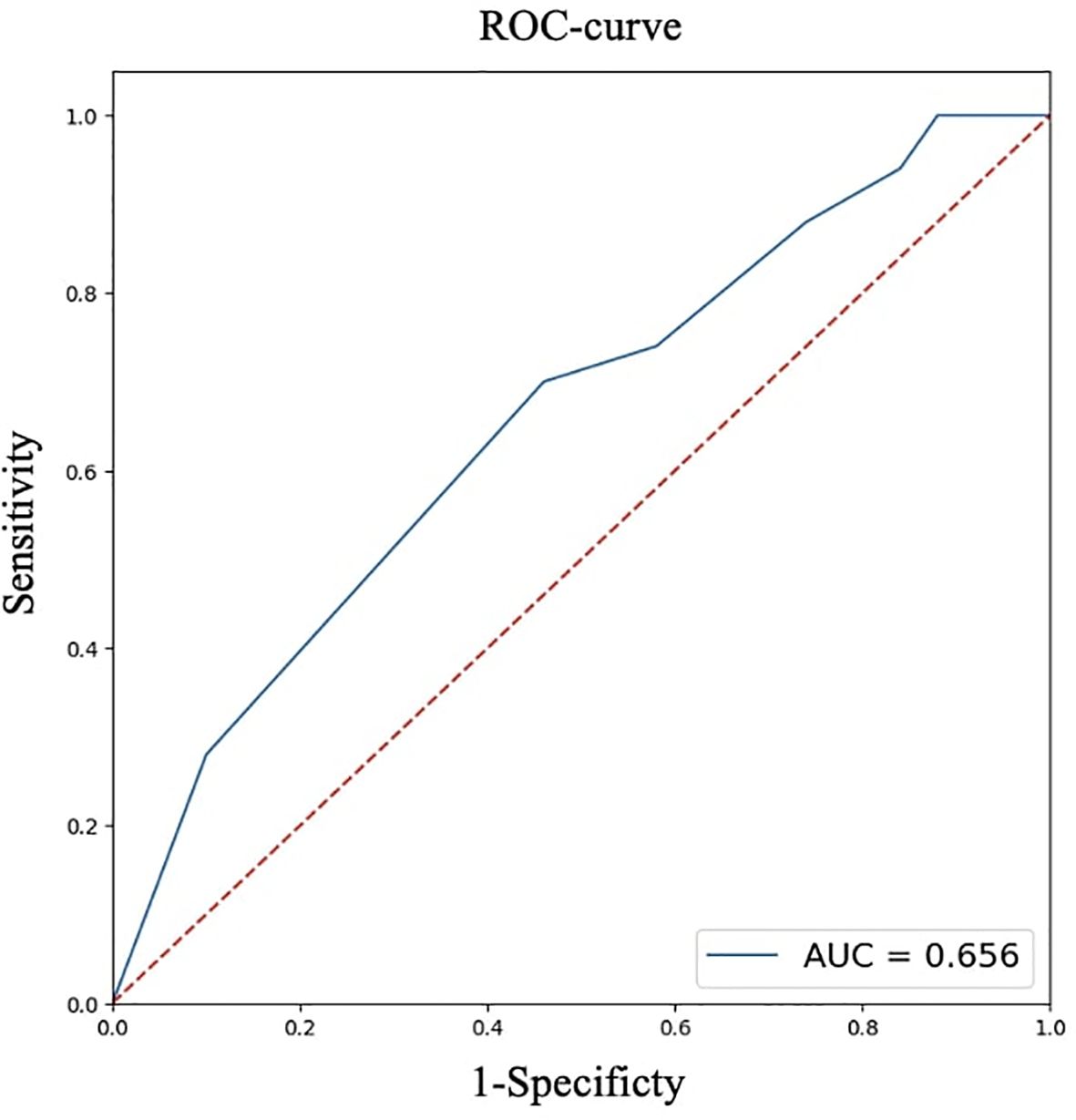
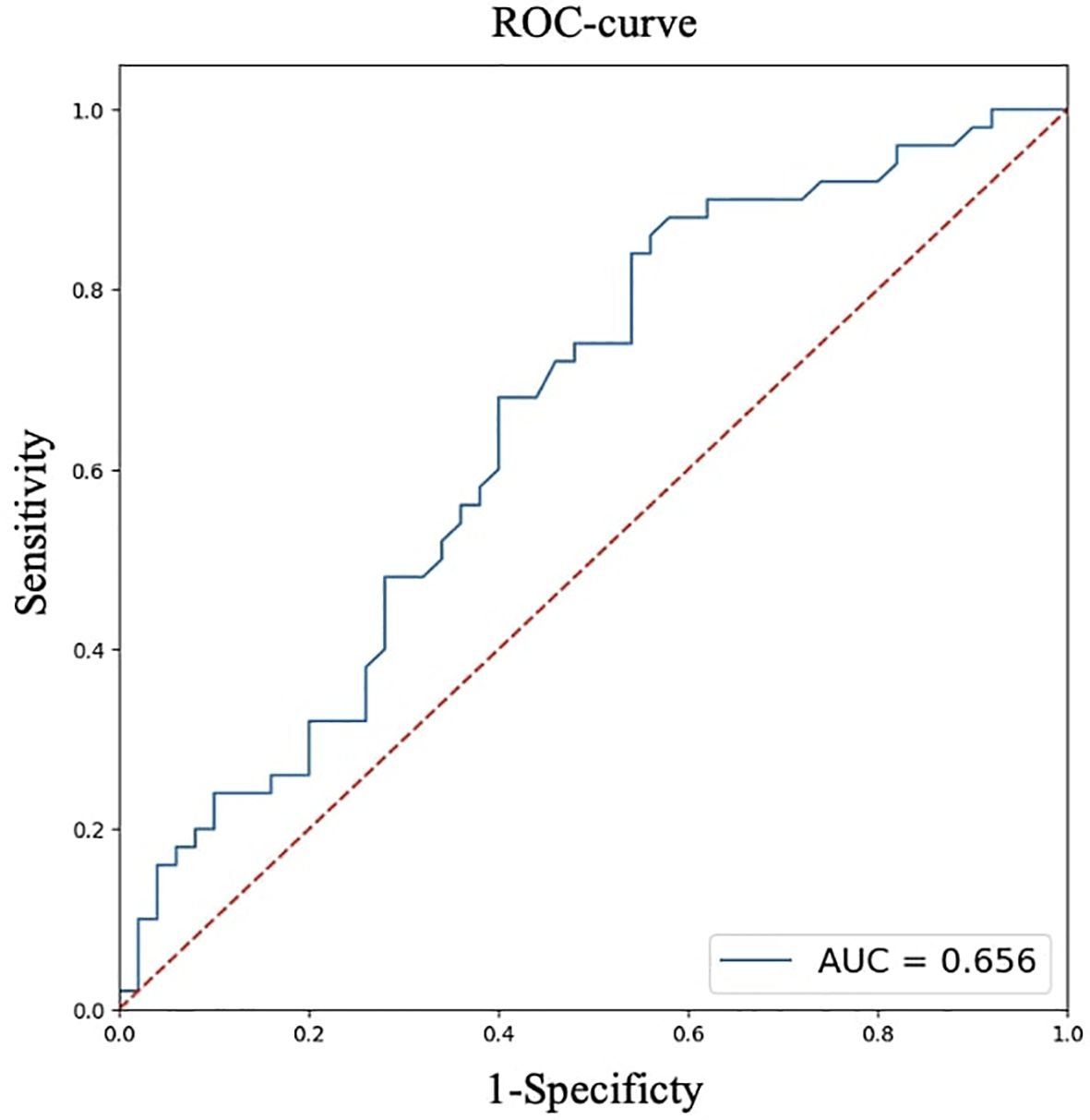
For quantitative features, ROC analysis was preliminarily performed (Table 4, Figures 8–12).
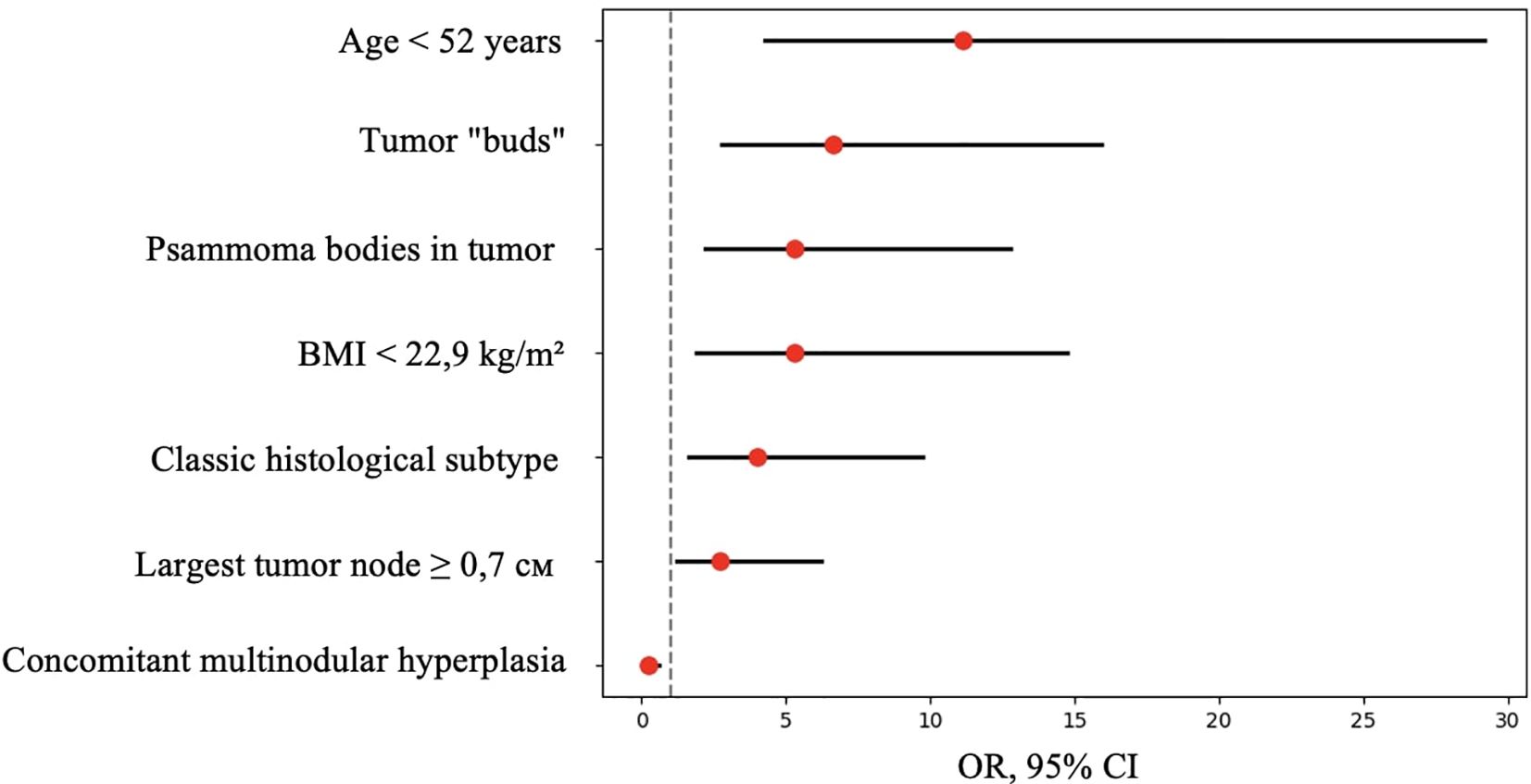
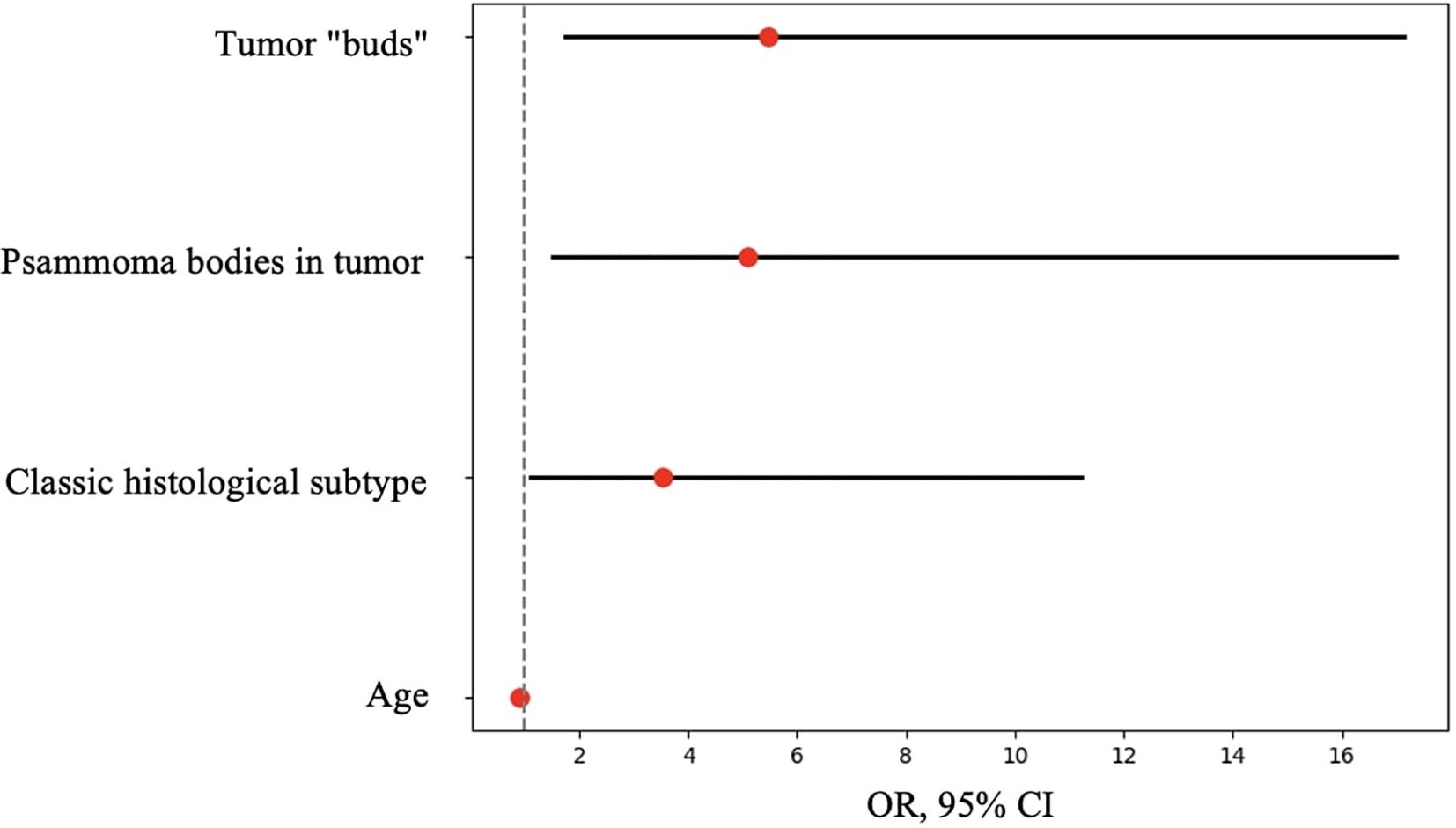
Figure 11. OR, 95% CI for features showing statistically significant differences between patients with and without metastases.
Thus, patients younger than 52 years had a 4.3–29.2 times higher chance of having metastases compared to older patients. For tumor node sizes ≥0.7 cm, the chance of metastases was 1.2–6.2 times higher than for smaller tumors. Patients with a BMI <22.9 kg/m² had a 1.9–14.7 times higher chance of metastases compared to those with a higher BMI.
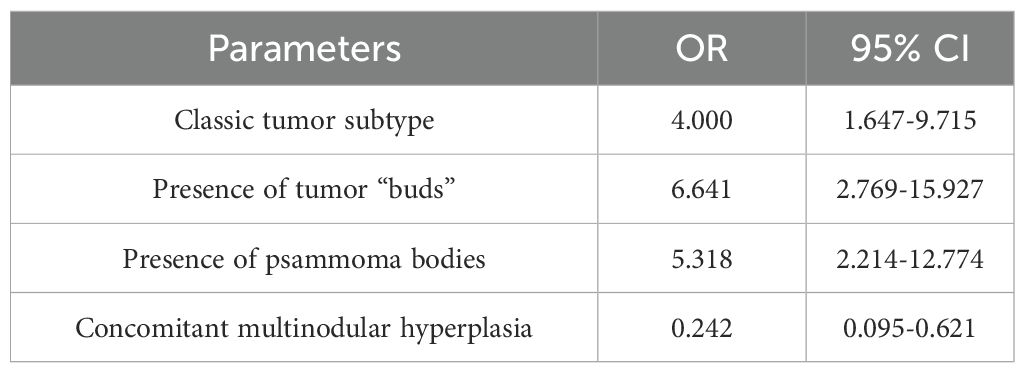
Based on the obtained data (Table 5), patients with the classic tumor subtype had a 1.6–9.7 times higher chance of metastases compared to other subtypes. The presence of tumor “buds” increased the chance of detecting metastases by 2.7–15.9 times, and patients with psammoma bodies in tumor tissue had a 2.2–12.8 times higher chance of metastases compared to those without. Conversely, patients with concomitant multinodular hyperplasia had a 1.6–10.5 times lower chance of metastases compared to those without it.
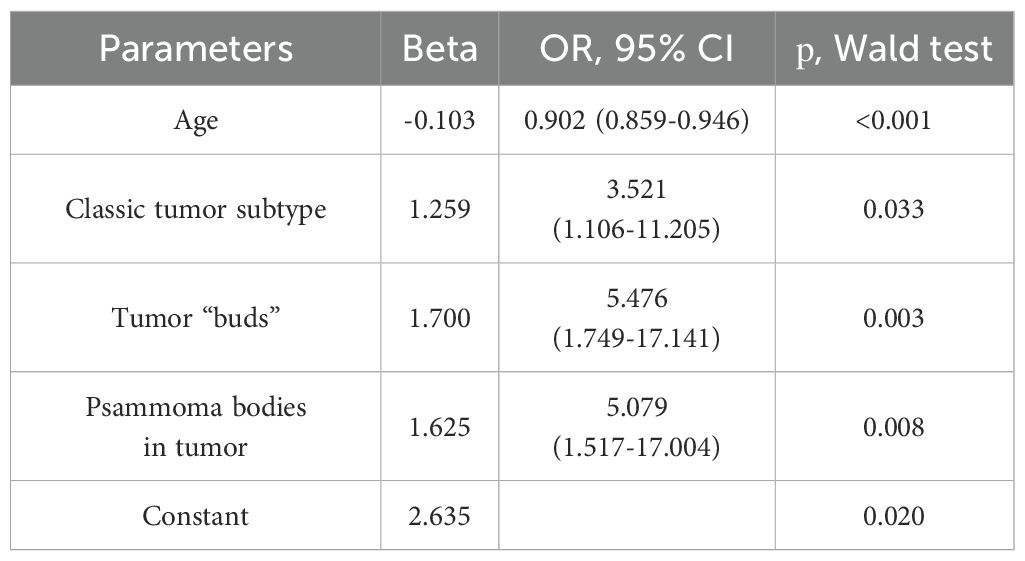
Next, logistic regression analysis was performed to identify a combination of factors associated with the presence of metastases. All parameters that showed statistical significance in the comparative analysis were analyzed as predictors:
● Age, years
● BMI, kg/m²
● Largest size of tumor node, cm
● Classic tumor subtype (yes/no)
● Tumor “buds” (yes/no)
● Psammoma bodies in tumor tissue (yes/no)
● Concomitant multinodular hyperplasia (yes/no)
The response variable was the presence of metastases (yes/no). The results identified a combination of parameters statistically significantly associated with the presence of metastases (Table 6).
Thus, the presence of a classic tumor subtype, tumor “buds,” and psammoma bodies in tumor tissue increased the chance of metastases by 3.5, 5.5, and 5 times, respectively. Each additional year of age reduced the chance of metastases by 10% of the current value.
A comparative analysis of patients with and without the BRAF V600E mutation is presented in Table 7.
Analysis of the BRAF V600E mutation revealed its presence in 65 out of 97 patients in the overall group (11 men, 54 women). Patients with the BRAF V600E mutation had a significantly higher frequency of tall cell tumor components (p<0.001; p0 = 0.001). There was also a statistical trend toward a higher frequency of the tall cell tumor subtype (p=0.014; p0 = 0.003) and a lower frequency of the invasive encapsulated follicular subtype (p=0.034; p0 = 0.004) compared to patients without the mutation.
4 Discussion and conclusions
Thus, according to the univariate analysis of the overall patient group, an increased risk of synchronous regional lymph node metastases in unifocal papillary thyroid microcarcinoma was observed in patients with the classic tumor subtype, the presence of tumor “buds,” psammoma bodies in tumor tissue, and the absence of concomitant multinodular hyperplasia. Additionally, risk factors included a tumor node size ≥0.7 cm, BMI <22.9 kg/m², and age <52 years. However, in the multivariate analysis, only the presence of tumor “buds,” psammoma bodies in tumor tissue, and patient age showed significant associations with the risk of synchronous regional lymph node metastases. Therefore, in our definition tumor “buds” may indeed be a sign of more aggressive invasive tumor growth, intraorgan dissemination, or lymphatic vessel invasion. The possibility of de novo development of additional tumor foci cannot be entirely ruled out. Despite the identified morphological and clinical predictors of metastasis, the significance of these findings in the context of disease prognosis remains debatable, as overall and recurrence-free survival rates for papillary thyroid microcarcinoma remain extremely high (28, 29). In this study, we did not aim to determine the nature of tumor “buds” in each specific case. Further research using 3D visualization, immunohistochemical markers (e.g., CD31, D2-40), molecular profiling, and sequencing could clarify their nature—whether they are emboli, microfoci, tangential sections, or invasion areas. However, this process may be labor-intensive, costly, and not always successful due to the small size of such complexes (≤0.1 cm). Nevertheless, the term “tumor bud” seems appropriate as a risk factor detectable during routine histological examination. It should be noted that the term “tumor budding” is currently not used in the context of thyroid carcinomas. In histological evaluation of colorectal adenocarcinomas, it refers to single cells or small clusters composed of fewer than five cells located at the invasive front of the tumor (30). Therefore, the definition of tumor “budding” adopted in our study is novel and differs from the definition used in studies of colorectal carcinomas.
Regarding the higher frequency of psammoma bodies in tumor tissue and the statistical trend toward more frequent detection of psammoma bodies outside the tumor in patients with metastases, similar findings have been reported in large studies by Bai et al. and Pyo et al. (31, 32). Unfortunately, Bai et al. did not specify whether psammoma bodies were detected in tumor tissue or in thyroid tissue outside the tumor. Additionally, the study group in that work consisted of patients with tumor nodes ≥1 cm (mostly ≥2 cm). Pyo et al. included patients with papillary microcarcinomas as well as larger tumors, and approximately 30% of the overall group had multifocal tumor growth. In that study, the presence of psammoma bodies in both tumor tissue and thyroid tissue outside the tumor showed statistical significance for regional metastasis detection. Unfortunately, in our study, the detection of psammoma bodies outside the tumor showed only a statistical trend toward more frequent synchronous metastasis detection. Moreover, there are differences in statistical methods. However, the association between psammoma bodies in tumor tissue and regional lymph node metastases was confirmed in both univariate and multivariate analyses. Furthermore, our findings align well with the results of J.V. Johannessen and M. Sobrinho-Simões, who suggested that psammoma bodies may represent calcified papillary tumor structures or tumor emboli in lymphatic vessels (33). Additionally, Liu et al. (34) noted that intra- and extratumoral microcalcifications detected during ultrasound examinations of patients with papillary thyroid cancer are risk factors for cervical lymph node metastases. The association between patient age and the risk of synchronous regional lymph node metastases identified in our study also correlates well with the results of Liu et al.: we found an increased risk of metastases in patients younger than 52 years, while Liu et al. reported an increased risk in patients younger than 55 years.
Tumor size is a criterion in most staging systems for differentiated thyroid cancer (35), including AJCC TNM (36). In our study, a tumor node size ≥0.7 cm emerged as a risk factor, but only in univariate analysis. It is noteworthy that no metastatic lymph node involvement was observed in patients with tumor sizes <0.3 cm. The identified association between patient BMI and the risk of regional lymph node metastasis is of particular interest. Recently, excess body weight has been considered a risk factor for papillary thyroid cancer development (1, 37). According to the large MASTER study published in 2023, obesity was associated with the frequency of extrathyroidal extension and the development of intermediate-risk papillary thyroid carcinomas in women (38). There is also evidence of a correlation between increased BMI and the development of papillary thyroid carcinomas >4 cm in size (39). Meanwhile, J.M. Kim reported a possible association between overweight/obesity and multifocal papillary thyroid cancer growth and recurrence but did not note a link with tumor size, extrathyroidal extension, or regional lymph node metastasis development (40). Our study found a significant association between lower BMI and an increased risk of regional lymph node metastasis, but only in univariate analysis.
In this study, we attempted to use nucleolar features of tumor cells—similar to those employed in the modified WHO/ISUP classification by S.A. Fuhrman—as a potential predictor of metastasis. It is known, for instance, that the original S.A. Fuhrman criteria have also been applied in the L.M. Weiss classification developed for adrenocortical tumors (41). The criteria we developed were conceptually similar to the original classification: we also assessed the basophilia and eosinophilia of nucleoli within the nuclei of tumor cells at ×100 and ×400 magnification. However, significant differences existed in the specific details of the criteria. This is due to difficulties we encountered in directly applying the original criteria from the modified S.A. Fuhrman classification, which is widely used for grading renal cell carcinoma. These difficulties arose from the predominance of basophilic nucleoli and the absence of well-defined eosinophilic nucleoli at ×100 magnification in cells of papillary thyroid microcarcinoma.
Unfortunately, this study did not identify any correlation between the structural features of nucleoli in tumor cells and the presence of regional lymph node metastases, nor with the BRAF V600E mutation. Besides, the presence of the BRAF V600E mutation in tumor tissue showed no association with the frequency of regional metastasis detection. However, patients with this mutation had a higher frequency of tall cell tumor components and a statistical trend toward a higher frequency of the tall cell tumor subtype and a lower frequency of the invasive encapsulated follicular subtype—consistent with current data on molecular alterations in various papillary carcinoma subtypes. It is known that the high-cell subtype of papillary thyroid microcarcinoma has a >92% frequency of the BRAF V600E mutation, while the invasive encapsulated follicular subtype is primarily associated with mutations in genes such as RAS and PAX8/PPARγ (42).
Interestingly, in 2023, our colleagues from a hospital in Shandong Province (China) published a study focused on risk factors for metastasis in papillary thyroid carcinoma, the results of which partially contradict our own findings (43). Among other factors, they identified age ≥45 years, BMI ≥25, and the presence of the BRAF V600E mutation as risk factors. Several notable differences between our studies may account for the observed discrepancies in results. From a research standpoint, the strengths of the Chinese study include a significantly larger sample size (400 patients) and the routine performance of central lymph node dissection in accordance with Chinese clinical guidelines for the diagnosis and treatment of differentiated thyroid carcinoma. This allowed for pathologic assessment of lymph node metastases in all patients. Despite this, the proportion of patients with lymph node metastases was lower (35%). Their cohort also included patients with multifocal tumor growth patterns and more than 20% of patients had tumors measuring ≥1 cm. Papillary thyroid carcinoma was not subtyped in that study.
In our study, the control group included patients without clinical evidence of lymph node metastases in the neck, and, in accordance with Russian clinical guidelines for the treatment of differentiated thyroid carcinoma, central lymph node dissection was not routinely performed. However, individual lymph nodes closely adjacent to the thyroid tissue may have been removed during surgery. One must also take into account the substantial ethnic differences between the patient populations studied.
Among the results we have obtained, the higher frequency of the classic tumor subtype among patients with metastases is noteworthy, as, according to current understanding, patients with this subtype are not typically classified as high-risk. It is possible that histological subtyping is more subjective for papillary microcarcinomas than for larger tumors, and the challenges increase with smaller tumor sizes. Therefore, it may be worthwhile to conduct a study assessing the degree of interobserver agreement in histological subtyping of papillary thyroid microcarcinomas. It cannot be ruled out that some features distinguishing non-classic subtypes are minimally represented in small tumors, raising questions about the necessity of precise subtyping for such cases. Additionally, the prognostic significance of gene expression profiles in tumor tissue may be more relevant for papillary microcarcinomas than for larger tumors, but this requires separate investigation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Endocrinology Research Center (protocol code 10, 26.05.2020). The patients/participants provided their written informed consent to participate in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AV: Methodology, Writing – review & editing, Writing – original draft. AE: Writing – review & editing, Data curation. AM: Writing – review & editing, Data curation. NP: Writing – review & editing, Writing – original draft. AD: Writing – review & editing. KS: Writing – review & editing. PN: Writing – review & editing. DB: Writing – review & editing. VV: Writing – review & editing. LU: Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The research was carried out within the state assignment of Ministry of Health of the Russian Federation (theme No. 124020600011-5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kitahara CM and Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
2. McConahey WM, Hay ID, Woolner LB, van Heerden JA, and Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. (1986) 61:978–96. doi: 10.1016/s0025-6196(12)62641-x
3. Rosai J, Carcangiu ML, and DeLellis RA. Tumors of the thyroid gland. In: Atlas of tumor pathology, Third Series, Fascicle 5. Washington, DC: Armed Forces Institute of Pathology. (1992). p. 96–100.
4. Kuenstner W, Alzedaneen Y, and Burman KD. Update in papillary thyroid cancer. Endocrinol Metab Clin North Am. (2025) 54:329–40. doi: 10.1016/j.ecl.2025.03.007
5. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. (2021) 9:225–34. doi: 10.1016/S2213-8587(21)00027-9
6. Aliyev E, Ladra-González MJ, Sánchez-Ares M, Abdulkader-Nallib I, Piso-Neira M, Rodríguez-Carnero G, et al. Thyroid papillary microtumor: validation of the (Updated) porto proposal assessing sex hormone receptor expression and mutational BRAF gene status. Am J Surg Pathol. (2020) 44:1161–72. doi: 10.1097/PAS.0000000000001522
7. Forma A, Klodnicka K, Pająk W, Flieger J, Teresińska B, Januszewski J, et al. Thyroid cancer: epidemiology, classification, risk factors, diagnostic and prognostic markers, and current treatment strategies. Int J Mol Sci. (2025) 26:5173. doi: 10.3390/ijms26115173
8. Hazard HB, Crile G Jr, and Dempsey WS. Nonencapsulated sclerosing tumors of the thyroid. J Clin Endocrinol Metab. (1949) 9:1216–31. doi: 10.1210/jcem-9-11-1216
9. Klinck GH and Winship T. Occult sclerosing carcinoma of the thyroid. Cancer. (1955) 8:701–6. doi: 10.1002/1097-0142(1955)8:4<701::aid-cncr2820080412>3.0.co;2-v
10. Harach HR, Franssila KO, and Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. (1985) 56:531–8. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3
11. Carnazza M, Quaranto D, DeSouza N, Moscatello AL, Garber D, Hemmerdinger S, et al. The current understanding of the molecular pathogenesis of papillary thyroid cancer. Int J Mol Sci. (2025) 26:4646. doi: 10.3390/ijms26104646
12. Neuhold N, Schultheis A, Hermann M, Krotla G, Koperek O, and Birner P. Incidental papillary microcarcinoma of the thyroid - further evidence of a very low Malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol. (2011) 18:3430–6. doi: 10.1245/s10434-011-1663-x
13. Robenshtok E, Bachar G, and Ritter A. Approach to the patient with thyroid cancer: selection and management of candidates for lobectomy. J Clin Endocrinol Metab. (2025) 110:e2327–37. doi: 10.1210/clinem/dgae903
14. Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. (2008) 144:980–8. doi: 10.1016/j.surg.2008.08.035
15. Qubain SW, Nakano S, Baba M, Takao S, and Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. (2002) 131:249–56. doi: 10.1067/msy.2002.120657
16. Wu Z, Han L, Li W, Wang W, Chen L, Yao Y, et al. Which is preferred for initial treatment of papillary thyroid cancer, total thyroidectomy or lobotomy? Cancer Med. (2021) 10:1614–22. doi: 10.1002/cam4.3743
17. Ohashi R. Solid variant of papillary thyroid carcinoma: an under-recognized entity. Endocr J. (2020) 67:241–8. doi: 10.1507/endocrj.EJ19-0414
18. Ghossein R and Livolsi VA. Papillary thyroid carcinoma tall cell variant. Thyroid. (2008) 18:1179–81. doi: 10.1089/thy.2008.0164
19. Malandrino P, Russo M, Regalbuto C, Pellegriti G, Moleti M, Caff A, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: A meta-analysis. Thyroid. (2016) 26:1285–92. doi: 10.1089/thy.2016.0168
20. Wang S, Xiong Y, Zhao Q, Song H, Yi P, and Liu C. Columnar cell papillary thyroid carcinoma prognosis: findings from the SEER database using propensity score matching analysis. Am J Transl Res. (2019) 11:6262–70.
21. Asioli S, Erickson LA, Righi A, and Lloyd RV. Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Hum Pathol. (2013) 44:320–8. doi: 10.1016/j.humpath.2012.06.003
22. Hay ID, Johnson TR, Kaggal S, Reinalda MS, Iniguez-Ariza NM, Grant CS, et al. Papillary thyroid carcinoma (PTC) in children and adults: comparison of initial presentation and long-term postoperative outcome in 4432 patients consecutively treated at the mayo clinic during eight decades (1936-2015). World J Surg. (2018) 42:329–42. doi: 10.1007/s00268-017-4279-x
23. Machens A, Lorenz K, and Dralle H. Metastatic risk profile of microscopic lymphatic and venous invasion in papillary thyroid cancer. Clin Otolaryngol. (2022) 47:440–6. doi: 10.1111/coa.13919
24. Patel KN, Yip L, Lubitz CC, Grubbs EG, Miller BS, Shen W, et al. The american association of endocrine surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. (2020) 271:e21–93. doi: 10.1097/SLA.0000000000003580
25. Liu Y, Xiang L, Liu FY, Yahya N, Chai JN, Hamid HA, et al. Accuracy of radiomics in the identification of extrathyroidal extension and BRAFV600E mutations in papillary thyroid carcinoma: A systematic review and meta-analysis. Acad Radiol. (2025) 32:1385–97. doi: 10.1016/j.acra.2024.11.014
26. WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours. Lyon (France: International Agency for Research on Cancer (2022). Available online at: https://tumourclassification.iarc.who.int/chapters/53. WHO classification of tumours series, 5th ed.; vol. 10 (Accessed June 22, 2025).
27. Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. (2013) 37:1490–504. doi: 10.1097/PAS.0b013e318299f0fb
28. Wen X, Jin Q, Cen X, Qiu M, and Wu Z. Clinicopathologic predictors of central lymph node metastases in clinical node-negative papillary thyroid microcarcinoma: a systematic review and meta-analysis. World J Surg Oncol. (2022) 20:106. doi: 10.1186/s12957-022-02573-7
29. Roman BR, Brito JP, Saucke MC, Lohia S, Jensen CB, Zaborek N, et al. National survey of endocrinologists and surgeons regarding active surveillance for low-risk papillary thyroid cancer. Endocr Pract. (2021) 27:1–7. doi: 10.1016/j.eprac.2020.11.003
30. Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, and Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. (2009) 52:438–45. doi: 10.1007/DCR.0b013e318197e37f
31. Bai Y, Zhou G, Nakamura M, Ozaki T, Mori I, Taniguchi E, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol. (2009) 22:887–94. doi: 10.1038/modpathol.2009.38
32. Pyo JS, Kang G, Kim DH, Park C, Kim JH, and Sohn JH. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J Surg. (2013) 37:2330–5. doi: 10.1007/s00268-013-2107-5
33. Johannessen JV and Sobrinho-Simões M. The origin and significance of thyroid psammoma bodies. Lab Invest. (1980) 43:287–96.
34. Liu J, Jia X, Gu Y, Chen X, Guan L, Yan J, et al. Thyroid parenchyma microcalcifications on ultrasound for predicting lymph node metastasis in papillary thyroid carcinoma: A prospective multicenter study in China. Front Oncol. (2021) 11:609075. doi: 10.3389/fonc.2021.609075
35. Momesso DP and Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. (2014) 43:401–21. doi: 10.1016/j.ecl.2014.02.010
36. Brierley JD, Gospodarowicz MK, and Wittekind Ch. eds. TNM classification of Malignant tumours. 8th ed. New York: Wiley-Blackwell (2017). p. 272.
37. Renehan AG, Tyson M, Egger M, Heller RF, and Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
38. Kim M, Kang YE, Park YJ, Koo BS, Ku EJ, Choi JY, et al. Potential impact of obesity on the aggressiveness of low- to intermediate-risk papillary thyroid carcinoma: results from a MASTER cohort study. Endocrine. (2023) 82:134–42. doi: 10.1007/s12020-023-03416-5
39. Kitahara CM, Pfeiffer RM, Sosa JA, and Shiels MS. Impact of overweight and obesity on US papillary thyroid cancer incidence trends (1995-2015). J Natl Cancer Inst. (2020) 112:810–7. doi: 10.1093/jnci/djz202
40. Kim JM. The clinical importance of overweight or obesity on tumor recurrence in papillary thyroid carcinoma. Gland Surg. (2022) 11:35–41. doi: 10.21037/gs-21-695
41. Weiss LM, Medeiros LJ, and Vickery AL. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. (1989) 13:202–6. doi: 10.1097/00000478-198903000-00004
42. Soares P, Póvoa AA, Melo M, Vinagre J, Máximo V, Eloy C, et al. Molecular pathology of non-familial follicular epithelial-derived thyroid cancer in adults: from RAS/BRAF-like tumor designations to molecular risk stratification. Endocr Pathol. (2021) 32:44–62. doi: 10.1007/s12022-021-09666-1
Keywords: papillary thyroid microcarcinoma, lymph node metastasis, histopathology, risk factors, psammoma bodies, tumor budding
Citation: Varlamov A, Elfimova A, Matrosova A, Pachuashvili N, Dzodzaeva A, Slashchuk K, Nikiforovich P, Beltsevich D, Vanushko V and Urusova L (2025) Clinical and morphological predictors of metastasis in papillary thyroid microcarcinoma. Front. Endocrinol. 16:1637850. doi: 10.3389/fendo.2025.1637850
Received: 29 May 2025; Accepted: 18 August 2025;
Published: 10 October 2025.
Edited by:
Erivelto Martinho Volpi, Hospital Alemão Oswaldo Cruz, BrazilReviewed by:
Ke Lv, Peking Union Medical College Hospital (CAMS), ChinaAtsumi Tamura, Endocrinology Section Cancer Innovation Laboratory Center for Cancer Research National Cancer Institute, United States
Copyright © 2025 Varlamov, Elfimova, Matrosova, Pachuashvili, Dzodzaeva, Slashchuk, Nikiforovich, Beltsevich, Vanushko and Urusova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nano Pachuashvili, bnBhY2h1YXNodmlsaUBiay5ydQ==
 Andrey Varlamov1
Andrey Varlamov1 Nano Pachuashvili
Nano Pachuashvili