- 1Department of Pharmacognosy, Pharmacology and Botany, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine
- 2Department of Pharmaceutical, Organic and Bioorganic Chemistry, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine
- 3Department of Clinical Pharmacy, Pharmacotherapy, Pharmacognosy and Pharmaceutical Chemistry, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine
- 4Department of Biological Chemistry, Zaporizhzhia State Medical and Pharmaceutical University, Zaporizhzhia, Ukraine
- 5Department of Composite Materials, Chemistry and Technologies, National University “Zaporizhzhia Polytechnic”, Zaporizhzhia, Ukraine
- 6Research Institute of Chemistry and Geology, Oles Honchar Dnipro National University, Dnipro, Ukraine
- 7Department of Biochemistry and Pharmacology, Uzhhorod National University, Uzhhorod, Ukraine
- 8Department of Microbiology, Virology, and Immunology, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Type 2 diabetes mellitus (T2DM) remains a significant and multifaceted challenge for modern healthcare. This issue becomes even more pressing during times of armed conflict and the subsequent recovery period, as research indicates an increased incidence of T2DM among combat veterans, largely due to post-traumatic stress disorder. Although numerous antidiabetic drugs are currently available, achieving optimal control of hyperglycemia continues to be problematic. In this context, and as part of a focused search for biologically active substances within the class of substituted and condensed [1,2,4]triazino[2,3-c]quinazolines, we explored the hypoglycemic effects of a newly synthesized series of such compounds. The study involved 21 synthesized compounds bearing the [1,2,4]triazino[2,3-c]quinazoline core. Experiments were conducted using white Wistar rats weighing between 260 and 280 grams. Prescreening of hypoglycemic activity was evaluated based on changes in blood glucose levels before and after compound administration by rats with normoglycemia. Compounds that demonstrated the most pronounced activity were selected for extended pharmacological evaluation using oral glucose tolerance test, adrenaline test, and rapid insulin tests in rats with dexamethasone-induced insulin resistance. Initial pharmacological screening under normoglycemic conditions showed that seven studied compounds significantly lowered blood glucose levels. Follow-up investigations validated the high hypoglycemic effect of 1,2,2-trimethyl-3-(3-methyl-2-oxo-2H- [1,2,4]triazino[2,3-c]quinazolin-6-yl)cyclopentane-1-carboxylic acid. Among the tested substances, compound 3-phenyl-6-(phenylamino)-2H-[1,2,4]triazino[2,3-c]quinazolin-2-one was the only one to exhibit moderate activity in the adrenaline tolerance test. None of the compounds enhanced insulin sensitivity in the liver or peripheral tissues. The findings suggest that substituted [1,2,4]triazino[2,3-c]quinazolines constitute a promising scaffold for the development of new hypoglycemic agents. 11β-Hydroxysteroid dehydrogenase is the most likely molecular target for lead-compound 1,2,2-trimethyl-3-(3-methyl-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazolin-6-yl)cyclopentane-1-carboxylic acid.
1 Introduction
The issue of type 2 diabetes mellitus (T2DM) represents one of the most complex challenges for modern medical science (1–4). The issue of diabetes becomes particularly urgent during wartime and in the postwar period (5–8), as it has been shown that combat veterans are at increased risk of developing type 2 diabetes due to the consequences of post-traumatic stress disorder (9–12). Despite the continuous improvement of prevention strategies and pharmacological interventions, the incidence of this pathology demonstrates a persistent upward trend (13–16). Diabetes mellitus, as both a social and economic problem, is primarily associated with substantial societal expenditures—not only for the management of hyperglycemic conditions (17–19), but also for the treatment of a wide range of comorbidities (20–24). Furthermore, the high rate of disability associated with diabetes significantly increases the financial burden related to the support of individuals who have lost their ability to work (25–27). The current therapeutic paradigm for T2DM involves the early use of hypoglycemic agents in order to prevent disease progression (13, 28–30). The arsenal of drugs used to manage hyperglycemic states in patients with T2DM is fairly extensive and includes sulfonylurea derivatives, thiazolidinediones, and biguanides, among others (13, 31). Based on their mechanisms of action, additional agents include α-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and prandial glucose regulators (13, 32–34).
Despite the availability of numerous agents in this therapeutic group, the problem of effectively treating hyperglycemia remains unresolved (35–38). This is due to the diversity of pathological manifestations of hyperglycemia, combined with the individual physiological characteristics of patients, which necessitates the development of drugs with improved pharmacotherapeutic profiles (39–41). The search for novel hypoglycemic agents is being actively pursued among various classes of organic compounds (42), including quinazoline and triazine derivatives (43–46).
Particular attention in modern approaches to the treatment of T2DM is directed toward metformin—a biguanide that has long remained the “gold standard” of first-line pharmacotherapy (47–50). Its high efficacy in reducing blood glucose levels is complemented by a favorable safety profile, a low risk of hypoglycemia, and beneficial effects on body weight (51–54). The primary mechanism of action of metformin involves the suppression of hepatic glucose production, enhancement of peripheral insulin sensitivity, and improvement of glucose uptake (55–57). In addition to its antihyperglycemic properties, metformin exhibits several pleiotropic effects, including anti-inflammatory, cardioprotective, and potentially neuroprotective actions (58–61). Recent studies also suggest its potential role in reducing the risk of certain malignancies (62–65). In the context of wartime and postwar periods, when a significant proportion of patients present with comorbid psycho-emotional and somatic disorders, metformin may play a pivotal role in comprehensive therapy (66–69). Its capacity to modulate metabolic and inflammatory pathways positions metformin as a promising agent not only in the management of hyperglycemia but also in addressing systemic consequences of stress and chronic inflammation (70–73).
In this context, special interest is being directed toward novel heterocyclic compounds, particularly [1,2,4]triazino[2,3-c]quinazoline derivatives (74–77). These molecules exhibit promising hypoglycemic and anti-inflammatory properties, making them attractive candidates for further investigation as multi-target agents in the treatment of T2DM, especially in patients with stress-related metabolic disturbances (78–81).
Aim. In view of the above, and within the framework of a targeted search for biologically active agents among substituted [1,2,4]triazino[2,3-c]quinazolines, we investigated the hypoglycemic activity of a series of compounds that are derivatives of abovementioned heterocyclic core.
2 Materials and methods
2.1 Studied compounds
A total of 21 compounds (Figure 1) containing the [1,2,4]triazino[2,3-c]quinazoline fragment were selected for the investigation of hypoglycemic activity. These compounds were obtained using previously described methods (77, 82–85) and exhibited identical spectral and physical characteristics. Selected compounds differ in the substituents at positions 3 and 6, the degree of saturation of the pyrimidine fragment, and the presence or absence of a fused or spiro-fused moiety. Such structural diversity provides a rational basis for identifying promising classes of compounds for further investigation.
2.2 Pharmacological studies
247 White male Wistar rats, each weighing between 260 and 280 g and aged 3.5 months, were employed in the experimental investigations. These animals were procured from the “Biomodelservis” nursery and maintained on a standard diet under a regular light–dark cycle, with unrestricted access to food and water. All experimental procedures were conducted in strict accordance with the “Regulations on the Use of Animals in Biomedical Research” (86). After a quarantine period, the individually identified animals were randomly allocated into groups of six male rats, ensuring uniformity in body weight (within a ±15% range) and the absence of external disease indicators.
2.2.1 Preliminary screening
Prior to the commencement of the experiments, the rats were fasted overnight, and each animal was weighed. The test substances were administered intragastrically in either an aqueous solution or as a finely dispersed suspension stabilized with Tween 80, at a dosage of 50 mg/kg. The hypoglycemic potential of the synthesized compounds was determined by assessing the alterations in blood glucose levels before and following administration. For each compound, glucose levels were measured in six rats at 2-, 4-, 6-, and 8-hours post-administration. The evaluation of the potential hypoglycemic activity was based on the observed change in blood glucose concentration after a single oral dose, with measurements obtained via a “One Touch Select” blood glucose meter. A dynamic area under the curve (AUC) was calculated, where the time intervals (0, 2, 4, 6, and 8 hours) served as the z-coordinate and the percentage decrease in glucose levels as the y-coordinate.
2.2.2 Induction of primary insulin resistance
Primary insulin resistance was induced by administering daily intramuscular injections of dexamethasone at a dosage of 0.125 mg/kg over a period of 13 days (87, 88). The studied compounds in dose 10 mg/kg were administrated daily simultaneously with injection of dexamethasone. Control groups of animals were also administered dexamethasone at the same dose and period, but instead of the suspension of the studied substance, they were administered an equivalent volume of water. The glucose homeostasis was then assessed by evaluating basal glycemia and carbohydrate tolerance. This assessment was performed using an oral glucose tolerance test, as well as adrenaline tests and rapid insulin test (87, 88). As reference standards, “Metformin” (administered at doses of 50 mg/kg) and “Gliclazide” (administered at 50 mg/kg) were used.
2.2.3 Oral glucose tolerance test
Glucose was administered intragastrically at a dosage of 3 g/kg using a noninvasive probe. Blood samples for glucose determination were collected immediately before administration and subsequently at 15, 30, 60, and 120 minutes thereafter. A dynamic area under the curve (AUC) was calculated, where the time intervals (0, 0.25, 0.5, 1, 1.5 hours) served as the z-coordinate and the percentage increase in glucose levels as the y-coordinate.
2.2.4 Adrenaline test
Rats received an intragastric dose of a 0.18% adrenaline solution at 0.5 mg/kg. Blood samples were collected for glucose analysis immediately before the administration, and at 30 and 90 minutes after the dose.
2.2.5 Rapid Insulin test
Insulin was administered intraperitoneally at a dosage of 1 unit/kg. Glucose levels were measured immediately before and 30 minutes after injection.
2.2.6 Statistical analysis
Data were processed using standard statistical software packages, specifically “Microsoft Office Excel 2003” and “STATISTICA® for Windows 6.0” (StatSoft Inc., № AXXR712D833214FAN5). For each parameter, the arithmetic mean (M) and the standard error of the mean (± m) were calculated. The Mann-Whitney test was performed to prove the difference between studied groups of animals. The null hypothesis was rejected when the statistical criterion yielded a value of p < 0.05.
2.3 Docking study
Docking was carried out using the CB-Dock service (89, 90), which applies a protein-surface-curvature-based cavity detection approach to guide molecular docking with AutoDock Vina. α-Amylase (PDB ID: 1HNY), glucokinase (PDB ID: 1V4S), 11β-hydroxysteroid dehydrogenase (PDB ID: 2BEL), α-glucosidase (PDB ID: 3WY1), maltase-glucoamylase (PDB ID: 3TOP), fructose-1,6-bisphosphatase (PDB ID: 2JJK), and PPAR-γ (PDB ID: 2PRG) models were used as possible molecular targets.
3 Results
3.1 Pharmacological studies
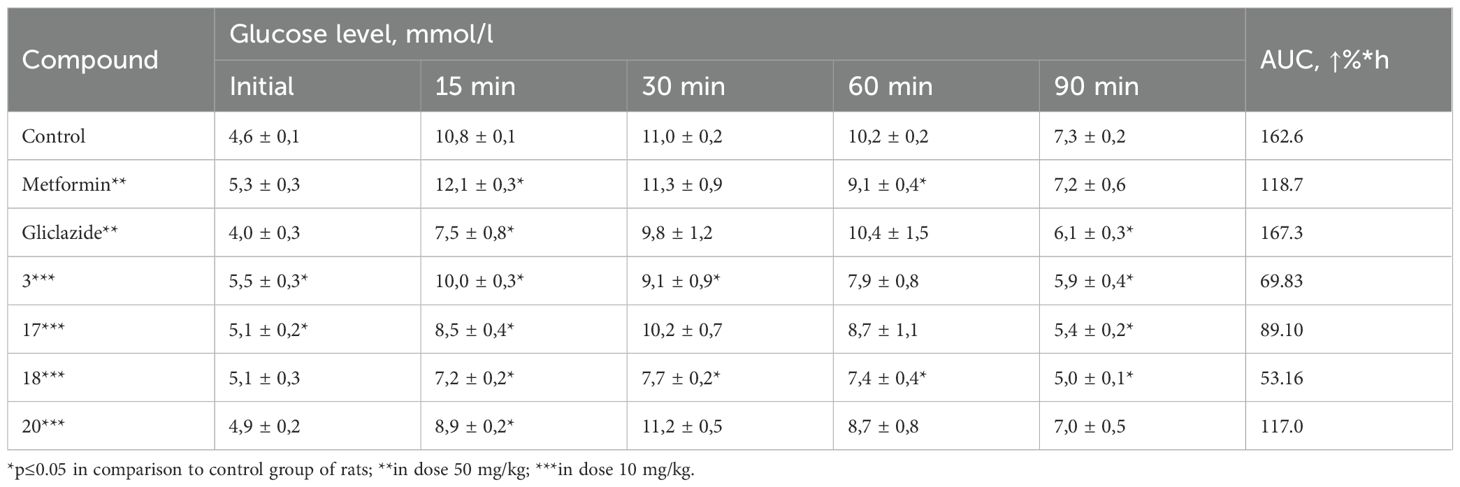
At the initial stage, in order to identify promising candidates for further in-depth investigation of hypoglycemic activity, the blood glucose-lowering effect of a series of synthesized compounds was assessed using a normoglycemic model. The selection of doses for the experimental compounds was based on the efficacy of structurally related drugs or those with similar mechanisms of hypoglycemic action, as recommended by established guidelines. Based on these considerations, a dose of 50 mg/kg was chosen. Each compound was tested on a group of six rats during the screening phase. To evaluate the glucose-lowering effect over time, the percentage reduction in blood glucose was measured every two hours following oral administration of the test compound. Based on these data, the area under the curve (AUC) was calculated for the mean percentage decrease in blood glucose over time. The results of the hypoglycemic activity assessment in the normoglycemic model (Table 1) indicated that compounds 3, 12, and 17–21 demonstrated ability to reduce blood glucose levels in conditions of normoglycemic test.
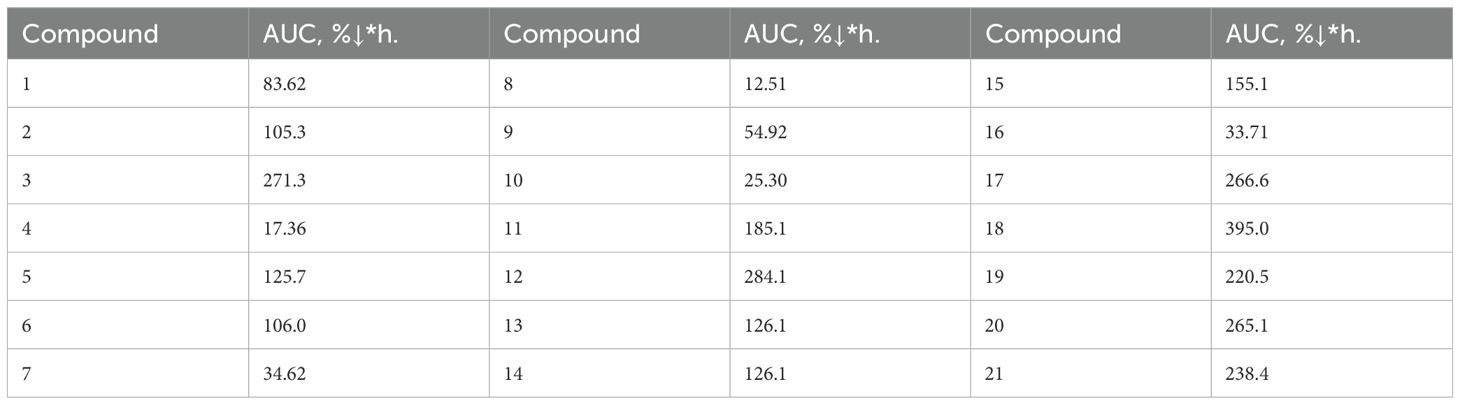
Table 1. Effect of the tested compounds on blood glucose levels in rats under normoglycemic conditions.
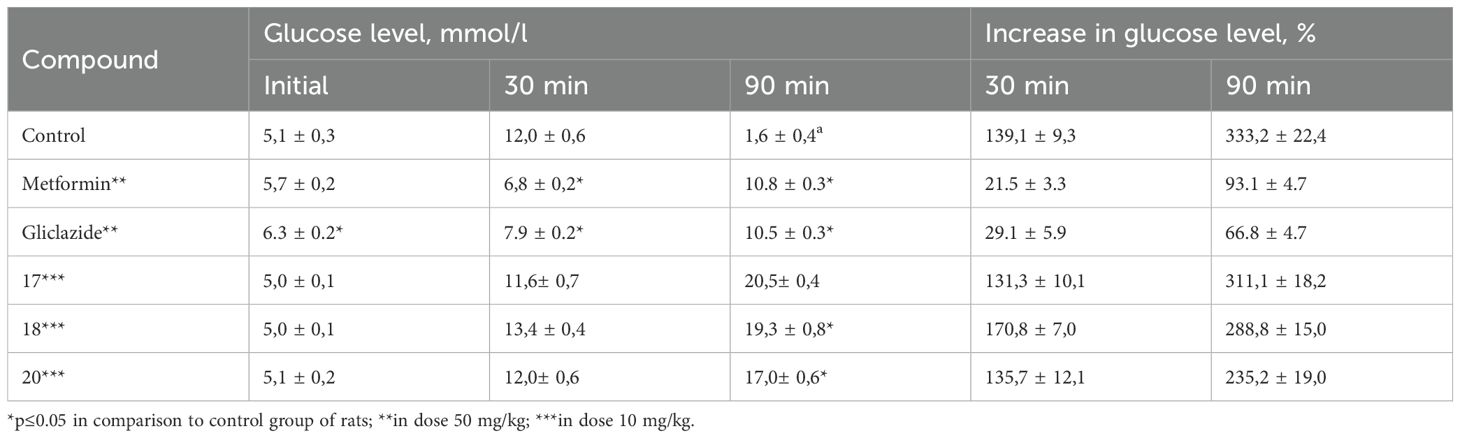
Based on the results of the primary screening of hypoglycemic activity, compounds 3, 17, 18, and 20 were selected for further in-depth investigation using a dexamethasone-induced diabetes model. The selection of compounds was based on their pronounced glucose-lowering activity under normoglycemic conditions, as well as their belonging to different classes of triazino[2,3-c]quinazoline derivatives. Specifically, compound 17 contains the simplest substituent at 6th position, compound 3 incorporates pharmacophoric sulfo-group, compound 18 bears a camphoric acid moiety, and compound 20 can be considered a structural analogue of biguanides. This model reproduces key pathological features such as impaired secretory function of pancreatic β-cells, development of insulin resistance, reduced carbohydrate tolerance, and decreased sensitivity of peripheral tissues to insulin action. Glucose homeostasis parameters were assessed using the oral glucose tolerance test (OGTT), the adrenaline test, and the short insulin test. These tests allow for the evaluation of basal glycemia, insulinemia, and carbohydrate tolerance. The results of the studies are presented in0 Tables 2–4.
As can be seen, the obtained results confirmed the data from the primary pharmacological screening regarding the pronounced hypoglycemic activity of compound 18. In the group of animals treated with compound 18, the average increase in blood glucose levels at 15, 30, 60, and 90 minutes after glucose loading increased to 42.8%, 52.4%, 46.4%, and –1.7%, respectively. Moreover, the area under the glucose-time curve (AUC) was the lowest among all tested groups, reaching 53.16%*h (Table 2). Hypoglycemic activity was also observed for compound 3. As shown by the results (Table 3), compound 20 was the only one among the tested substances to exhibit moderate hypoglycemic activity under the conditions of this model.
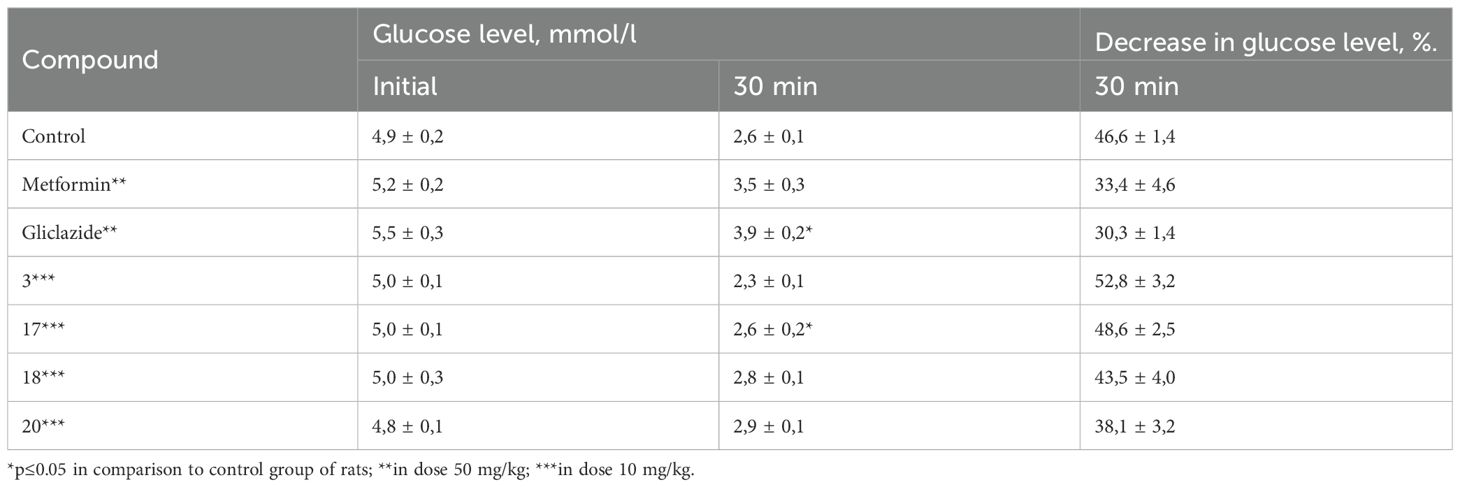
The short insulin tolerance test was performed to evaluate the sensitivity of both the liver and peripheral tissues to insulin. Analysis of the obtained results demonstrated that none of the tested compounds were capable of enhancing liver or peripheral tissue sensitivity to insulin. The average reduction in blood glucose levels 30 minutes after insulin administration in all groups treated with the test compounds showed only minor differences compared to the control group (Table 4).
3.2 Docking studies
Considering that biological studies have identified a promising hypoglycemic agent—namely, 1,2,2-trimethyl-3-(3-methyl-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazolin-6-yl)cyclopentane-1-carboxylic acid (compound 18)—a investigation was initiated to determine the compound’s potential molecular mechanism of action. To achieve this goal, preliminary molecular docking studies were performed for compound 18 against the most common molecular targets of hypoglycemic drugs: α-amylase (PDB ID: 1HNY), glucokinase (PDB ID: 1V4S), 11β-hydroxysteroid dehydrogenase (PDB ID: 2BEL), α-glucosidase (PDB ID: 3WY1), maltase-glucoamylase (PDB ID: 3TOP), fructose-1,6-bisphosphatase (PDB ID: 2JJK), and PPAR-γ (PDB ID: 2PRG).
Docking was carried out using the CB-Dock service (89, 90), which applies a protein-surface-curvature-based cavity detection approach to guide molecular docking with AutoDock Vina. For each target, five distinct binding cavities were identified, and AutoDock Vina affinity scores were calculated for each site. Table 5 summarizes the characteristics of the ligand–target complexes with the highest predicted binding affinities.
The results indicate that compound 18 exhibits a notable predicted affinity for all the tested molecular targets. The highest affinity was observed for 11β-hydroxysteroid dehydrogenase (PDB ID: 2BEL), with an AutoDock Vina score of –10.6 kcal/mol. To better understand the interaction patterns between compound 18 and the molecular targets, docking results were visualized, allowing identification of the specific amino acid residues involved in ligand binding and the nature of these interactions (Table 6).
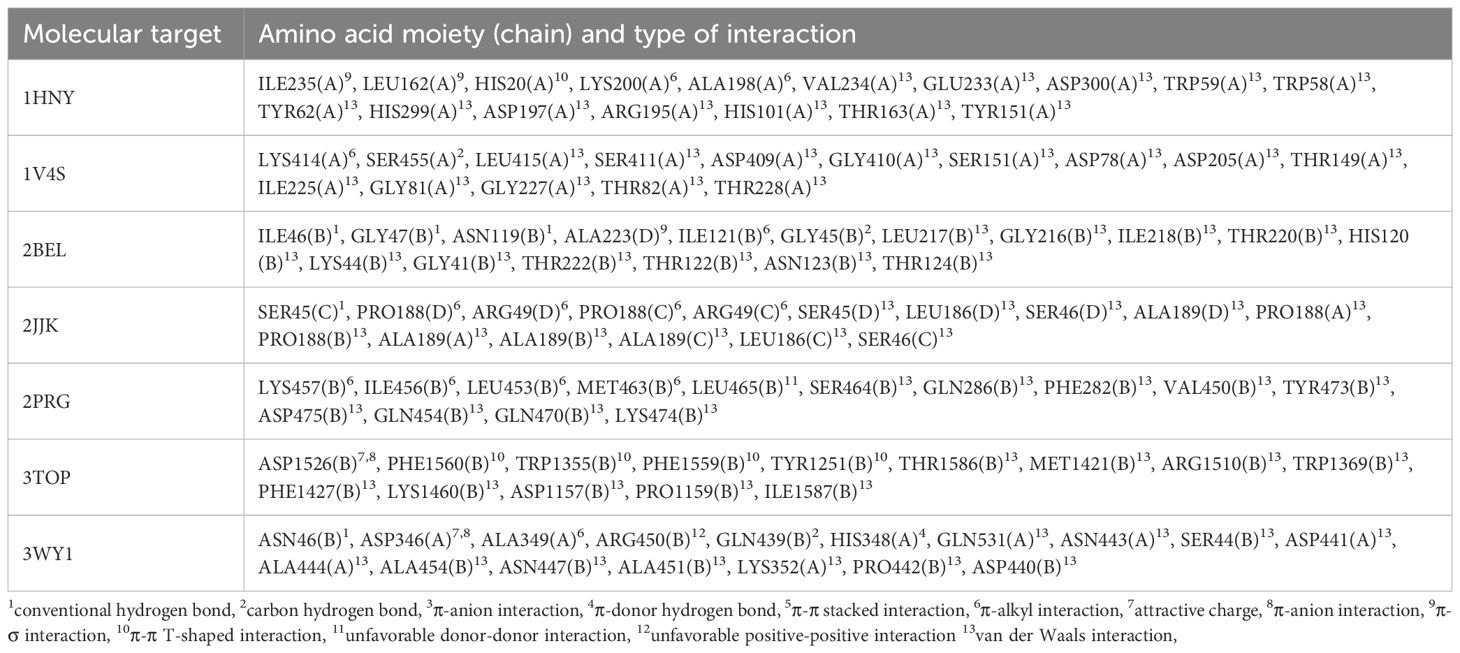
Table 6. The nature of amino acid moieties involved in formation of molecular target-ligand complex and nature of interactions.
As the data show, conventional hydrogen bonds were observed in only three complexes: 2BEL, 2JJK, and 3WY1. This type of interaction is known to contribute significantly to the strength and stability of ligand binding.
Among these, the complex formed between compound 18 and 11β-hydroxysteroid dehydrogenase (PDB ID: 2BEL) stands out. It features three conventional hydrogen bonds involving the carboxyl group of the ligand and amino acid residues ILE46(B), GLY47(B), and ASN119(B), suggesting a particularly strong and specific interaction (Figure 2). In addition, the formation of a carbon–hydrogen bond with residue GLY45(B), a π–alkyl interaction with ILE121(B) and ALA223(B), and a π–σ interaction with ALA223(B) is also predicted, further contributing to the stability of the ligand–target complex. These findings are consistent with the highest calculated affinity of compound 18 toward 11β-hydroxysteroid dehydrogenase in comparison with other molecular targets. Thus, the obtained results suggest that 11β-hydroxysteroid dehydrogenase is the most likely molecular target for the lead compound 18. At the same time, it cannot be ruled out that the obtained compounds may exert hypoglycemic effects through interactions with multiple molecular targets. Considering the fact that multi-target drug development for the treatment of metabolic disorders is currently a trending area of research (91), this hypothesis underscores the relevance of experimentally elucidating the mechanism of the glucose-lowering action of the synthesized compounds.
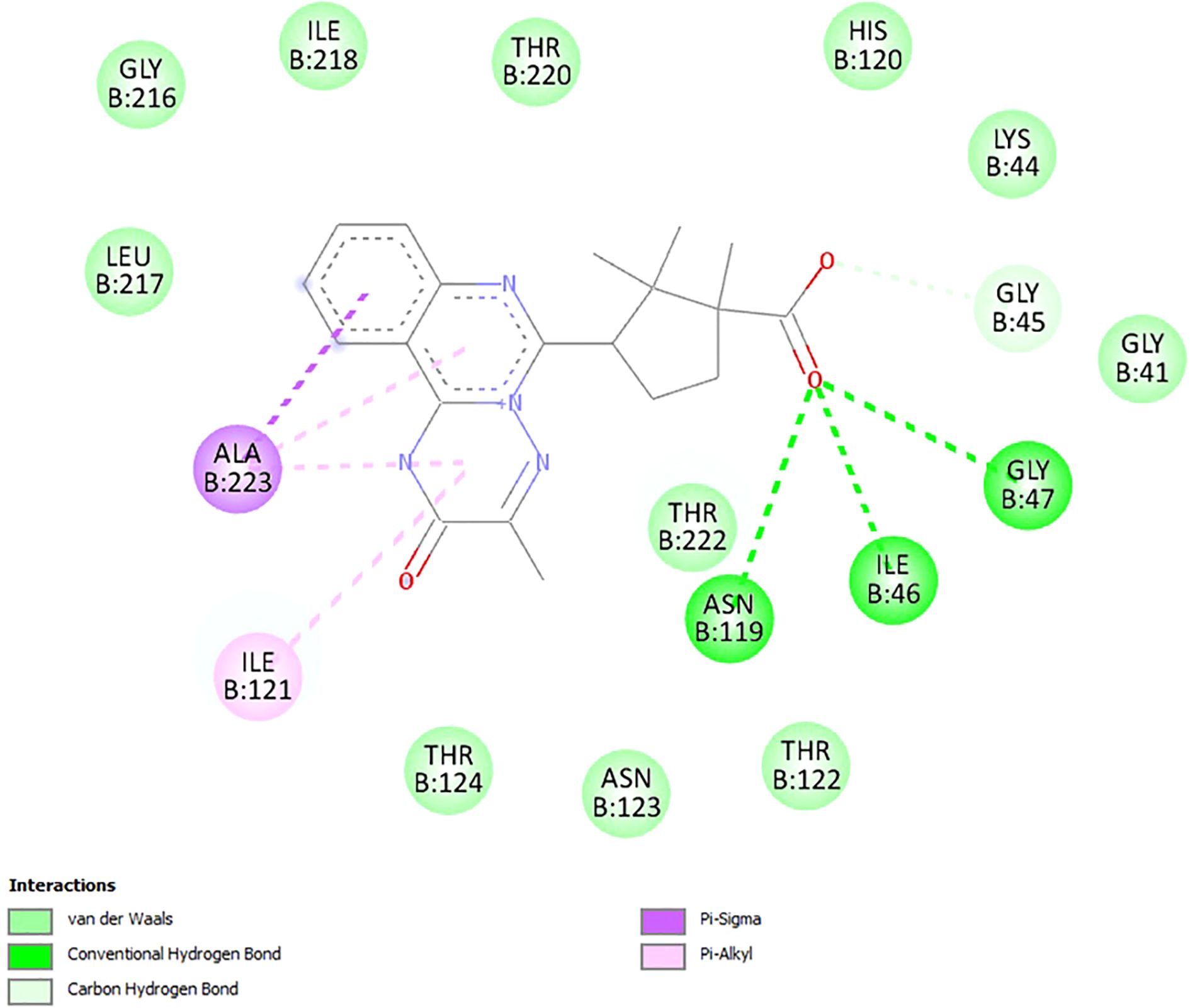
Figure 2. Visualization of docking study of compound 18 toward 11β-hydroxysteroid dehydrogenase (PDB ID: 2BEL).
4 Discussion
The findings of this study support the growing body of evidence that emphasizes the need for novel hypoglycemic agents with multi-target pharmacological properties (92–94). The promising glucose-lowering effects demonstrated by several [1,2,4]triazino[2,3-c]quinazoline derivatives, especially compound 18, underscore the therapeutic potential of this heterocyclic scaffold in the management of T2DM. The pronounced activity of these compounds in both normoglycemic and insulin-resistant models confirms their relevance for further pharmacological development. Notably, the docking results suggest that 11β-hydroxysteroid dehydrogenase may represent a key molecular target, which highlights the dual potential of these compounds in modulating both glucose metabolism and stress-related hormonal pathways.
Particular attention should be paid to metformin, a biguanide that remains the cornerstone of T2DM pharmacotherapy (95–97). Its efficacy, safety profile, and beneficial metabolic effects have made it the first-line treatment for decades (98–100). Beyond its antihyperglycemic action through hepatic glucose suppression and increased insulin sensitivity, metformin exerts pleiotropic effects—including anti-inflammatory, cardioprotective, and neuroprotective actions—which are especially valuable in patients with multiple comorbidities (101, 102). These properties highlight the need for new agents to retain or even expand upon these systemic effects, particularly in the postwar context, where stress-related metabolic disorders are prevalent (103–105).
An emerging area of interest in diabetes research is the role of gut microbiota (106, 107). Disruption of microbial balance—known as dysbiosis—has been implicated in the pathogenesis of insulin resistance and chronic inflammation (108–111). Recent studies suggest that the glucose-lowering effect of metformin is partly mediated through alterations in gut microbiota composition (112–114). Therefore, future investigation of [1,2,4]triazino[2,3-c]quinazoline derivatives should include an evaluation of their effects on the gut microbial ecosystem, particularly in stress-related and antibiotic-associated dysbiosis models.
Furthermore, the COVID-19 pandemic has highlighted the vulnerability of patients with T2DM to infectious diseases (115–117). The intersection between metabolic dysregulation and impaired immune responses creates a high-risk scenario for severe outcomes in viral infections such as SARS-CoV-2 (118–121). In this regard, the anti-inflammatory properties of several triazinoquinazoline derivatives may offer added value, potentially reducing cytokine-mediated complications during viral infections (122–125). Multifunctional agents that exert both metabolic and immunomodulatory actions could significantly improve outcomes in patients with dual metabolic and infectious burdens (126–128).
Genetic variability also plays a substantial role in the heterogeneity of T2DM presentation and treatment response (129–131). Single nucleotide polymorphisms (SNPs) in genes encoding insulin receptors, glucose transporters, inflammatory mediators, and drug-metabolizing enzymes can influence both disease progression and pharmacological outcomes (132–137). Understanding these genetic factors may enable the development of personalized therapeutic strategies that optimize efficacy and minimize adverse effects in individuals with T2DM (138–140), particularly those with comorbid conditions and increased susceptibility to infectious diseases (141–143). Integration of pharmacogenetic approaches may optimize the therapeutic application of newly developed agents, including the lead [1,2,4]triazino[2,3-c]quinazoline analogs, by aligning treatment choices with individual genetic profiles.
Importantly, T2DM rarely occurs in isolation and is often accompanied by multiple comorbidities such as obesity, cardiovascular disease, non-alcoholic fatty liver disease, depression, and post-traumatic stress disorder (144–146). These coexisting conditions complicate glycemic control and increase the risk of treatment failure (147–150). Therefore, the development of agents with broad systemic effects—including antioxidant, anti-inflammatory, and potentially psychotropic actions—is essential. The observed effects of some [1,2,4]triazino[2,3-c]quinazoline derivatives in this study suggest that these compounds may hold such potential and should be further examined in preclinical models of comorbidity.
Taken together, our results suggest that [1,2,4]triazino[2,3-c]quinazoline derivatives represent a promising chemical class for the development of novel hypoglycemic drugs. However, the complexity of T2DM requires an interdisciplinary approach that includes not only pharmacology, but also microbiology, genetics, immunology, and psychosomatic medicine (151–153). Further investigation is needed to elucidate the precise molecular targets and signaling pathways modulated by these derivatives (154–156). Given the involvement of chronic low-grade inflammation in T2DM and its comorbidities, special attention should be paid to the immunomodulatory properties of these compounds (157–160). Preliminary in vitro findings should be validated using in vivo models that accurately reflect the multifactorial nature of T2DM (161–163). Additionally, it is crucial to assess the safety profile, potential drug–drug interactions, and pharmacokinetic characteristics of these agents. Integration of omics technologies may help identify biomarkers predictive of response and toxicity (164–167). The inclusion of behavioral and cognitive endpoints in preclinical trials may also yield important insights into their psychotropic potential (168–170). Ultimately, translational research efforts will be essential to determine whether [1,2,4]triazino[2,3-c]quinazoline derivatives can address the unmet therapeutic needs of patients with T2DM and complex comorbid profiles.
5 Conclusions
Substituted and condensed [1,2,4]triazino[2,3-c]quinazolines have been demonstrated to represent a promising class of hypoglycemic agents. Preliminary screening under normoglycemic conditions revealed that 7 out of the 21 tested compounds exhibited a pronounced glucose-lowering effect. Selected compounds were further evaluated for their hypoglycemic activity using a model of primary insulin resistance. The oral glucose tolerance test indicated that 1,2,2-trimethyl-3-(3-methyl-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazolin-6-yl)cyclopentane-1-carboxylic acid (compound 18) is a highly effective glucose-lowering agent. Conversely, in the adrenaline-induced hyperglycemia test, only 3-phenyl-6-(phenylamino)-2H-[1,2,4]triazino[2,3-c]quinazolin-2-one (compound 20) demonstrated a moderate hypoglycemic effect. None of the studied compounds enhanced insulin sensitivity in hepatic or peripheral tissues. Conducted docking study revealed that 11β-hydroxysteroid dehydrogenase is the most likely molecular target for lead-compound 1,2,2-trimethyl-3-(3-methyl-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazolin-6-yl)cyclopentane-1-carboxylic acid (compound 18). These findings highlight the high potential of [1,2,4]triazino[2,3-c]quinazolin-2-one derivatives as a structural class for the development of novel hypoglycemic agents.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study protocol was approved by the Ethics Committee of Zaporizhzhia State Medical and Pharmaceutical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ST: Supervision, Methodology, Data curation, Writing – review & editing, Conceptualization. IN: Formal Analysis, Resources, Writing – review & editing, Investigation. AK: Resources, Writing – review & editing, Formal Analysis, Investigation. DS: Formal Analysis, Investigation, Resources, Writing – review & editing. HB: Writing – review & editing, Investigation, Formal Analysis, Resources. VS: Writing – review & editing, Data curation, Formal Analysis, Methodology. OV: Writing – original draft, Visualization, Validation. SK: Writing – review & editing, Conceptualization, Supervision. PP: Writing – review & editing. OK: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1638013/full#supplementary-material
References
1. Garg P and Duggal N. Type 2 diabetes mellitus, its impact on quality of life and how the disease can be managed-a review. Obes Med. (2022) 35:100459. doi: 10.1016/j.obmed.2022.100459
2. Nameghi SM. Exploring the recent advancements and future prospects of personalized medicine in type 2 diabetes. Endocrine Metab Sci. (2024) 16:100193. doi: 10.1016/j.endmts.2024.100193
3. Khartabil N and Avoundjian A. Gene therapy and diabetes: A narrative review of recent advances and the role of multidisciplinary healthcare teams. Genes. (2025) 16:107. doi: 10.3390/genes16010107
4. Guan H, Zhao S, Li J, Wang Y, Niu P, Zhang Y, et al. Exploring the design of clinical research studies on the efficacy mechanisms in type 2 diabetes mellitus. Front endocrinology. (2024) 15:1363877.
5. Sulaieva O, Yerokhovych V, Zemskov S, Komisarenko I, Gurianov V, Pankiv V, et al. The impact of war on people with type 2 diabetes in Ukraine: a survey study. eClinicalMedicine. (2025) 79:103008. doi: 10.1016/j.eclinm.2024.103008
6. Fradkin JE. Confronting the urgent challenge of diabetes: an overview. Health affairs (Project Hope). (2012) 31:12–9.
7. Hossain MJ, Al-Mamun M, and Islam MR. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci Rep. (2024) 7:e2004. doi: 10.1002/hsr2.2004
8. Jawad M, Millett C, Sullivan R, Alturki F, Roberts B, and Vamos EP. The impact of armed conflict on cancer among civilian populations in low- and middle-income countries: a systematic review. Ecancermedicalscience. (2020) 14:1039. doi: 10.3332/ecancer.2020.1039
9. Bergman BP, Mackay D, and Pell JP. Type 2 diabetes in Scottish military veterans: a retrospective cohort study. BMJ Open. (2022) 12:e057431. doi: 10.1136/bmjopen-2021-057431
10. Nikpour S, Mehrdad N, Sanjari M, Aalaa M, Heshmat R, Khabaz Mafinejad M, et al. Challenges of type 2 diabetes mellitus management from the perspective of patients: conventional content analysis. Interactive J Med Res. (2022) 11:e41933. doi: 10.2196/41933
11. Vaccarino V, Goldberg J, Magruder KM, Forsberg CW, Friedman MJ, Litz BT, et al. Posttraumatic stress disorder and incidence of type-2 diabetes: a prospective twin study. J Psychiatr Res. (2014) 56:158–64. doi: 10.1016/j.jpsychires.2014.05.019
12. Agyemang C, Goosen S, Anujuo K, and Ogedegbe G. Relationship between post-traumatic stress disorder and diabetes among 105,180 asylum seekers in the Netherlands. Eur J Public Health. (2012) 22:658–62. doi: 10.1093/eurpub/ckr138
13. Reed J, Bain S, and Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab syndrome obesity: Targets Ther. (2021) 14:3567–602. doi: 10.2147/DMSO.S319895
14. Młynarska E, Czarnik W, Dzieża N, Jędraszak W, Majchrowicz G, Prusinowski F, et al. Type 2 diabetes mellitus: new pathogenetic mechanisms, treatment and the most important complications. Int J Mol Sci. (2025) 26:1094. doi: 10.3390/ijms26031094
15. Carrarini C, Russo M, Dono F, Barbone F, Rispoli MG, Ferri L, et al. Agitation and dementia: prevention and treatment strategies in acute and chronic conditions. Front neurology. (2021) 12:644317.
16. Cross AJ, Elliott RA, Petrie K, Kuruvilla L, and George J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database systematic Rev. (2020) 5:Cd012419.
17. Hidayat B, Ramadani RV, Rudijanto A, Soewondo P, Suastika K, and Siu Ng JY. Direct medical cost of type 2 diabetes mellitus and its associated complications in Indonesia. Value Health Regional Issues. (2022) 28:82–9.
18. Seidu S, Cos X, Brunton S, Harris SB, Jansson SPO, Mata-Cases M, et al. 2022 update to the position statement by Primary Care Diabetes Europe: a disease state approach to the pharmacological management of type 2 diabetes in primary care. Primary Care Diabetes. (2022) 16:223–44. doi: 10.1016/j.pcd.2022.02.002
19. Boadu AA, Yeboah-Manu M, Osei-Wusu S, and Yeboah-Manu D. Tuberculosis and diabetes mellitus: The complexity of the comorbid interactions. Int J Infect Diseases. (2024) 146:107140.
20. Butt M, Ong S, Ong A, Rafiq M, Kalam A, Sajjad M, et al. A systematic review of the economic burden of diabetes mellitus: contrasting perspectives from high and low middle-income countries. J Pharm Policy Pract. (2024) 17. doi: 10.1080/20523211.2024.2322107
21. Mangoulia P, Milionis C, Vlachou E, and Ilias I. The interrelationship between diabetes mellitus and emotional well-being: current concepts and future prospects. Healthcare. (2024) 12:1457. doi: 10.3390/healthcare12141457
22. Topol I and Kamyshny A. Study of expression of TLR2, TLR4 and transckription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian Med news. (2013) 225):115–22.
23. Dzhuryak V, Sydorchuk L, Andrii S, Kamyshnyi O, Kshanovska A, Levytska S, et al. The cytochrome 11B2 aldosterone synthase gene CYP11B2 (RS1799998) polymorphism associates with chronic kidney disease in hypertensive patients. Biointerface Res Appl Chem. (2020) 10:5406–11. doi: 10.33263/BRIAC
24. Kamyshna II, Pavlovych LB, Maslyanko VA, and Kamyshnyi AM. Analysis of the transcriptional activity of genes of neuropeptides and their receptors in the blood of patients with thyroid pathology. J Med Life. (2021) 14:243–9. doi: 10.25122/jml-2020-0183
25. Oyewole OO, Ale AO, Ogunlana MO, and Gurayah T. Burden of disability in type 2 diabetes mellitus and the moderating effects of physical activity. World J Clin cases. (2023) 11:3128–39.
26. Rodríguez-Escaja C, ÁN C, González-Diéguez L, Cadahía V, Varela M, de Jorge M, et al. Diabetes is not associated with an increased risk of hepatocellular carcinoma in patients with alcoholic or hepatitis C virus cirrhosis. Rev espanola enfermedades digestivas. (2021) 113:505–11.
27. Bilous II, Pavlovych LL, and Kamyshnyi AM. Primary hypothyroidism and autoimmune thyroiditis alter the transcriptional activity of genes regulating neurogenesis in the blood of patients. Endocrine regulations. (2021) 55:5–15.
28. Borse SP, Chhipa AS, Sharma V, Singh DP, and Nivsarkar M. Management of type 2 diabetes: current strategies, unfocussed aspects, challenges, and alternatives. Med principles practice: Int J Kuwait University Health Sci Centre. (2021) 30:109–21.
29. Padhi S, Nayak AK, and Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine Pharmacotherapy. (2020) 131:110708.
30. Aloke C, Egwu CO, Aja PM, Obasi NA, Chukwu J, Akumadu BO, et al. Current advances in the management of diabetes mellitus. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10102436
31. Gieroba B, Kryska A, and Sroka-Bartnicka A. Type 2 diabetes mellitus - conventional therapies and future perspectives in innovative treatment. Biochem biophysics Rep. (2025) 42:102037. doi: 10.1016/j.bbrep.2025.102037
32. Halabitska I, Babinets L, Oksenych V, and Kamyshnyi O. Diabetes and osteoarthritis: exploring the interactions and therapeutic implications of insulin, metformin, and GLP-1-based interventions. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12081630
33. Kshirsagar AD, Aggarwal AS, Harle UN, and Deshpande AD. DPP IV inhibitors: Successes, failures and future prospects. Diabetes Metab Syndrome: Clin Res Rev. (2011) 5:105–12. doi: 10.1016/j.dsx.2012.02.017
34. DeMarsilis A, Reddy N, Boutari C, Filippaios A, Sternthal E, Katsiki N, et al. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism: Clin experimental. (2022) 137:155332.
35. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2012) 35:1364–79. doi: 10.2337/dc12-0413
36. Su J, Luo Y, Hu S, Tang L, and Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int J Mol Sci. (2023) 24:13381. doi: 10.3390/ijms241713381
37. Menon S, Rossi R, Dusabimana A, Zdraveska N, Bhattacharyya S, and Francis J. The epidemiology of tuberculosis-associated hyperglycemia in individuals newly screened for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Infect diseases. (2020) 20:937. doi: 10.1186/s12879-020-05512-7
38. Halabitska I, Oksenych V, and Kamyshnyi O. Exploring the efficacy of alpha-lipoic acid in comorbid osteoarthritis and type 2 diabetes mellitus. (2024) Basel, Switzerland: MDPI.
39. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2022) 65:1925–66. doi: 10.1007/s00125-022-05787-2
40. Antar SA, Ashour NA, Sharaky M, Khattab M, Ashour NA, Zaid RT, et al. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomedicine Pharmacotherapy. (2023) 168:115734. doi: 10.1016/j.biopha.2023.115734
41. Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng G, et al. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal transduction targeted Ther. (2024) 9:262. doi: 10.1038/s41392-024-01951-9
42. Dowarah J and Singh VP. Anti-diabetic drugs recent approaches and advancements. Bioorganic medicinal Chem. (2020) 28:115263. doi: 10.1016/j.bmc.2019.115263
43. Peytam F, Hosseini FS, Fathimolladehi R, Nayeri MJD, Moghadam MS, Bayati B, et al. Design, synthesis, and evaluation of novel substituted imidazo[1,2-c]quinazoline derivatives as potential α-glucosidase inhibitors with bioactivity and molecular docking insights. Sci Rep. (2024) 14:27507. doi: 10.1038/s41598-024-78878-2
44. Ghoneim MM, Abdelgawad MA, Elkanzi NAA, Parambi DGT, Alsalahat I, Farouk A, et al. A literature review on pharmacological aspects, docking studies, and synthetic approaches of quinazoline and quinazolinone derivatives. Archiv der Pharmazie. (2024) 357:e2400057. doi: 10.1002/ardp.202400057
45. Kabil MF, Lababidi JM, and Azzazy HME-S. Chapter Three - Linagliptin: A comprehensive profile. In: Al-Majed A, editor. Profiles of drug substances, excipients and related methodology, vol. 50. New York, USA: Elsevier Inc. (2025). p. 97–123.
46. Valipour M, Zakeri Khatir Z, Kiadaliry K, Mojtabavi S, Faramarzi MA, Sayyad MS, et al. Design, synthesis, α-glucosidase inhibition and hypoglycemic activity of 3-aceto(benzo)hydrazide-1,2,4-triazines as potential anti-diabetic agents. Eur J Medicinal Chem Rep. (2024) 12:100207. doi: 10.1016/j.ejmcr.2024.100207
47. Nemeth DV, Iannelli L, Gangitano E, D’Andrea V, and Bellini MI. Energy metabolism and metformin: effects on ischemia-reperfusion injury in kidney transplantation. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12071534
48. Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, et al. Metformin: Is it a drug for all reasons and diseases? Metabolism: Clin Exp. (2022) 133:155223.
49. Zajda A, Sikora J, Huttunen KM, and Markowicz-Piasecka M. Structural comparison of sulfonamide-based derivatives that can improve anti-coagulation properties of metformin. Int J Mol Sci. (2022) 23:4132. doi: 10.3390/ijms23084132
50. Petakh P, Griga V, Mohammed IB, Loshak K, Poliak I, and Kamyshnyiy A. Effects of metformin, insulin on hematological parameters of COVID-19 patients with type 2 diabetes. Med Arch (Sarajevo Bosnia Herzegovina). (2022) 76:329–32.
51. Halimi S, Schweizer A, Minic B, Foley J, and Dejager S. Combination treatment in the management of type 2 diabetes: focus on vildagliptin and metformin as a single ta blet. Vasc Health Risk management. (2008) 4:481–92.
52. Xie W, Su F, Wang G, Peng Z, Xu Y, Zhang Y, et al. Glucose-lowering effect of berberine on type 2 diabetes: A systematic review and meta-analysis. Front Pharmacol. (2022) 13:1015045. doi: 10.3389/fphar.2022.1015045
53. Giri B, Dey S, Das T, Sarkar M, Banerjee J, and Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2018) 107:306–28.
54. Petakh P, Kamyshna I, Oksenych V, Kainov D, and Kamyshnyi A. Metformin therapy changes gut microbiota alpha-diversity in COVID-19 patients with type 2 diabetes: the role of SARS-coV-2 variants and antibiotic treatment. Pharm (Basel Switzerland). (2023) 16. doi: 10.3390/ph16060904
55. Rena G, Hardie DG, and Pearson ER. The mechanisms of action of metformin. Diabetologia. (2017) 60:1577–85.
56. Foretz M, Guigas B, Bertrand L, Pollak M, and Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. (2014) 20:953–66. doi: 10.1016/j.cmet.2014.09.018
57. Horakova O, Kroupova P, Bardova K, Buresova J, Janovska P, Kopecky J, et al. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci Rep. (2019) 9:6156. doi: 10.1038/s41598-019-42531-0
58. Putilin DA, Evchenko SY, Fedoniuk LY, Tokarskyy OS, Kamyshny OM, Migenko LM, et al. The influence of metformin to the transcriptional activity of the mTOR and FOX3 genes in parapancreatic adipose tissue of streptozotocin-induced diabetic rats. J Med Life. (2020) 13:50–5. doi: 10.25122/jml-2020-0029
59. Halabitska I, Petakh P, Lushchak O, Kamyshna I, Oksenych V, and Kamyshnyi O. Metformin in antiviral therapy: evidence and perspectives. Viruses. (2024) 16:1938. doi: 10.3390/v16121938
60. Dutta S, Shah RB, Singhal S, Dutta SB, Bansal S, Sinha S, et al. Metformin: A review of potential mechanism and therapeutic utility beyond diabetes. Drug design Dev Ther. (2023) 17:1907–32. doi: 10.2147/DDDT.S409373
61. Buczyńska A, Sidorkiewicz I, Krętowski AJ, and Adamska A. Examining the clinical relevance of metformin as an antioxidant intervention. Front Pharmacol. (2024) 15:1330797. doi: 10.3389/fphar.2024.1330797
62. Kasznicki J, Sliwinska A, and Drzewoski J. Metformin in cancer prevention and therapy. Ann Trans Med. (2014) 2:57.
63. Pavlo P, Kamyshna I, and Kamyshnyi A. Effects of metformin on the gut microbiota: A systematic review. Mol Metab. (2023) 77:101805.
64. Kamyshnyi O, Matskevych V, Lenchuk T, Strilbytska O, Storey K, and Lushchak O. Metformin to decrease COVID-19 severity and mortality: Molecular mechanisms and therapeutic potential. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2021) 144:112230.
65. Saraei P, Asadi I, Kakar MA, and Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manage Res. (2019) 11:3295–313. doi: 10.2147/CMAR.S200059
66. Petakh P, Oksenych V, and Kamyshnyi A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2023) 163:114892.
67. Halabitska I, Petakh P, and Kamyshnyi O. Metformin as a disease-modifying therapy in osteoarthritis: bridging metabolism and joint health. Front Pharmacol. (2025) 16. doi: 10.3389/fphar.2025.1567544
68. Löwe B, Toussaint A, Rosmalen JGM, Huang W-L, Burton C, Weigel A, et al. Persistent physical symptoms: definition, genesis, and management. Lancet. (2024) 403:2649–62. doi: 10.1016/S0140-6736(24)00623-8
69. Ibarra-Patrón D, Medina-Vidales G, and Garza-Guerrero C. Case report: Diagnostic reconceptualization in the DSM-V on somatoform disorders. Med Universitaria. (2015) 17:102–7.
70. Belenichev I, Popazova O, Bukhtiyarova N, Savchenko D, Oksenych V, and Kamyshnyi O. Modulating nitric oxide: implications for cytotoxicity and cytoprotection. Antioxidants. (2024) 13:504. doi: 10.3390/antiox13050504
71. Zherebiatiev A and Kamyshnyi A. Expression levels of proinflammatory cytokines and NLRP3 inflammasome in an experimental model of oxazolone-induced colitis. Iranian J allergy asthma Immunol. (2016) 15:39–45.
72. Kruczkowska W, Gałęziewska J, Buczek P, Płuciennik E, Kciuk M, and Śliwińska A. Overview of metformin and neurodegeneration: A comprehensive review. Pharmaceuticals. (2025) 18:486.
73. Foretz M, Guigas B, and Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinology. (2023) 19:460–76.
74. Bilyi AK, Antypenko LM, Ivchuk VV, Kamyshnyi OM, Polishchuk NM, and Kovalenko SI. 2-heteroaryl-[1,2,4]triazolo[1,5-c]quinazoline-5(6 H)-thiones and their S-substituted derivatives: synthesis, spectroscopic data, and biological activity. ChemPlusChem. (2015) 80:980–9. doi: 10.1002/cplu.201500051
75. Nosulenko IS, Voskoboynik OY, Berest GG, Safronyuk SL, Kovalenko SI, Kamyshnyi OM, et al. Synthesis and antimicrobial activity of 6-thioxo-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]-quinazolin-2-one derivatives. Scientia pharmaceutica. (2014) 82:483–500.
76. Kumar G, Kumar P, Soni A, Sharma V, and Nemiwal M. Efficient synthesis and molecular docking analysis of quinazoline and azole hybrid derivatives as promising agents for anti-cancer and anti-tuberculosis activities. J Mol Struct. (2024) 1310:138289. doi: 10.1016/j.molstruc.2024.138289
77. Grytsak O, Shabelnyk K, Severina H, Ryzhenko V, Voskoboinik O, Belenichev I, et al. Bioisosteric replacement in the search for biologically active compounds: design, synthesis and anti-inflammatory activity of novel [1,2,4]triazino[2,3-c]quinazolines. Pharmaceuticals. (2024) 17:1437. doi: 10.3390/ph17111437
78. Salehi B, Ata A N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. (2019) 9(10):551. doi: 10.3390/biom9100551
79. Saikia R, Pathak K, Pramanik P, Islam MA, Karmakar S, Gogoi S, et al. Exploring the therapeutic potential of xanthones in diabetes management: Current insights and future directions. Eur J Medicinal Chem Rep. (2024) 12:100189. doi: 10.1016/j.ejmcr.2024.100189
80. Saini K, Sharma S, and Khan Y. DPP-4 inhibitors for treating T2DM - hype or hope? an analysis based on the current literature. Front Mol biosciences. (2023) 10:1130625.
81. Hu W, Yan G, Ding Q, Cai J, Zhang Z, Zhao Z, et al. Update of Indoles: Promising molecules for ameliorating metabolic diseases. Biomedicine Pharmacotherapy. (2022) 150:112957.
82. Voskoboynik OY, Kolomoets OS, Kovalenko SI, and Shishkina SV. 1,2,4]Triazino[2,3-с]quinazolines 1. Methods for the preparation and spectral characteristics of substituted 3-R1-6-R3-6,7-dihydro-2H-[1,2,4]triazino[2,3-с]quinazolin-2-ones. Chem Heterocyclic Compounds. (2017) 53:892–904.
83. Voskoboynik OY, Kolomoets OS, Palchikov VA, Kovalenko SI, Belenichev IF, and Shishkina SV. 1,2,4]Triazino[2,3-с]quinazolines 2*. Synthesis, structure, and anticonvulsant activity of new 3′-R1-spiro[(aza/oxa/thia)cycloalkyl-1(3, 4),6′-[1,2,4]triazino[2,3-c]quinazolin]-2′(7′H)-ones. Chem Heterocyclic Compounds. (2017) 53:1134–47.
84. Stavytskyi V, Voskoboinik O, Antypenko O, Krasovska N, Shabelnyk K, Konovalova I, et al. Tandem heterocyclization of 2-(azolyl-(azinyl-))anilines as an efficient method for preparation of substituted pyrrolo[1,2-a]azolo-(azino-)[c]quinazolines. J Heterocyclic Chem. (2020) 57:1249–60. doi: 10.1002/jhet.3862
85. Voskoboynik OY, Skorina DY, Shishkina SV, Shishkin OV, Kovalenko SI, and Ivchuk VV. Peculiarites of interaction between 3-(2-aminophenyl)-6-R-1,2,4-triazin-5(2H)-ones and cyclic anhydrides of non-symmetric dicarboxylic acids. J Organic Pharm Chem. (2015) 13:25–31. doi: 10.24959/ophcj.15.817
86. European convention for the protection of vertebrate animals used for experimental and other scientific purposes Vol. 1986. Strasbourg: Council of Europe (1986) p. 03–18.
87. Akinmokun A, Selby PL, Ramaiya K, and Alberti KG. The short insulin tolerance test for determination of insulin sensitivity: a comparison with the euglycaemic clamp. Diabetic medicine: J Br Diabetic Assoc. (1992) 9:432–7. doi: 10.1111/j.1464-5491.1992.tb01813.x
88. Weinstein SP, Wilson CM, Pritsker A, and Cushman SW. Dexamethasone inhibits insulin-stimulated recruitment of GLUt4 to the cell surface in rat skeletal muscle. Metab - Clin Experimental. (1998) 47:3–6.
89. Liu Y, Yang X, Gan J, Chen S, Xiao ZX, and Cao Y. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. (2022) 50:W159–w64. doi: 10.1093/nar/gkac394
90. Yang X, Liu Y, Gan J, Xiao ZX, and Cao Y. FitDock: protein-ligand docking by template fitting. Briefings Bioinf. (2022) 23. doi: 10.1093/bib/bbac087
91. Lillich FF, Imig JD, and Proschak E. Multi-target approaches in metabolic syndrome. Front Pharmacol. (2020) 11:554961. doi: 10.3389/fphar.2020.554961
92. Jadhav S, Yeram P, Alavala RR, and Vora A. Advances in the design of novel antidiabetic agents using in-silico approaches. In: Rao G and Alavala RR, editors. Applications of computational tools in drug design and development. Springer Nature Singapore, Singapore (2025). p. 763–800.
93. Chee YJ and Dalan R. Novel Therapeutics for Type 2 Diabetes Mellitus—A Look at the Past Decade and a Glimpse into the Future. Biomedicines. (2024) 12:1386.
94. Banday MZ, Sameer AS, and Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. (2020) 10:174–88.
95. Baker C, Retzik-Stahr C, Singh V, Plomondon R, Anderson V, and Rasouli N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther Adv Endocrinol Metab. (2021) 12:2042018820980225.
96. Ferrannini E. The target of metformin in type 2 diabetes. New Engl J Med. (2014) 371:1547–8. doi: 10.1056/NEJMcibr1409796
97. Solini A and Tricò D. Clinical efficacy and cost-effectiveness of metformin in different patient populations: A narrative review of real-world evidence. Diabetes Obes Metab. (2024) 26 Suppl 3:20–30. doi: 10.1111/dom.15729
98. Andraos J, Smith SR, Tran A, and Pham DQ. Narrative review of data supporting alternate first-line therapies over metformin in type 2 diabetes. J Diabetes Metab Disord. (2024) 23:385–94. doi: 10.1007/s40200-024-01406-6
99. Top WMC, Kooy A, and Stehouwer CDA. Metformin: A narrative review of its potential benefits for cardiovascular disease, cancer and dementia. Pharm (Basel Switzerland). (2022) 15. doi: 10.3390/ph15030312
100. Ray KK, Nicholls SJ, Li N, Louie MJ, Brennan D, Lincoff AM, et al. Efficacy and safety of bempedoic acid among patients with and without diabetes: prespecified analysis of the CLEAR Outcomes randomised trial. Lancet Diabetes Endocrinology. (2024) 12:19–28.
101. Goel S, Singh R, Singh V, Singh H, Kumari P, Chopra H, et al. Metformin: Activation of 5’ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front Genet. (2022) 13:1022739. doi: 10.3389/fgene.2022.1022739
102. Froldi G. View on metformin: antidiabetic and pleiotropic effects, pharmacokinetics, side effects, and sex-related differences. Pharmaceuticals. (2024) 17. doi: 10.3390/ph17040478
103. Kivimäki M, Bartolomucci A, and Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinology. (2023) 19:10–27.
104. Du Y, Zhu Y-J, Zhou Y-X, Ding J, and Liu J-Y. Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study. Mol biomedicine. (2022) 3:41. doi: 10.1186/s43556-022-00108-w
105. Bai B and Chen H. Metformin: A novel weapon against inflammation. Front Pharmacol. (2021) 12:622262. doi: 10.3389/fphar.2021.622262
106. Chen J, Chen Z, Khan BA, and Hou K. Editorial: Role of gut microbiota in diabetes mellitus and tumor immunity. Front Immunol. (2023) 14:1185080. doi: 10.3389/fimmu.2023.1185080
107. Sadagopan A, Mahmoud A, Begg M, Tarhuni M, Fotso M, Gonzalez NA, et al. Understanding the role of the gut microbiome in diabetes and therapeutics targeting leaky gut: A systematic review. Cureus. (2023) 15:e41559. doi: 10.7759/cureus.41559
108. Shen Y, Fan N, Ma SX, Cheng X, Yang X, and Wang G. Gut microbiota dysbiosis: pathogenesis, diseases, prevention, and therapy. MedComm. (2025) 6:e70168. doi: 10.1002/mco2.70168
109. Hrncir T. Gut microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. (2022) 10. doi: 10.3390/microorganisms10030578
110. Acevedo-Román A, Pagán-Zayas N, Velázquez-Rivera LI, Torres-Ventura AC, and Godoy-Vitorino F. Insights into gut dysbiosis: inflammatory diseases, obesity, and restoration approaches. Int J Mol Sci. (2024) 25:9715. doi: 10.3390/ijms25179715
111. Halabitska I, Petakh P, Kamyshna I, Oksenych V, Kainov DE, and Kamyshnyi O. The interplay of gut microbiota, obesity, and depression: insights and interventions. Cell Mol Life sciences: CMLS. (2024) 81:443.
112. Szymczak-Pajor I, Drzewoski J, Kozłowska M, Krekora J, and Śliwińska A. The gut microbiota-related antihyperglycemic effect of metformin. Pharmaceuticals. (2025) 18:55.
113. Wang D, Liu J, Zhou L, Zhang Q, Li M, and Xiao X. Effects of oral glucose-lowering agents on gut microbiota and microbial metabolites. Front endocrinology. (2022) 13:905171.
114. Tariq HMA, Khan NY, Manzoor H, and Kayani MUR. Exploring the impact of type 2 diabetes and glucose-lowering drugs on gut microbiome dynamics. Discover Med. (2025) 2:47. doi: 10.1007/s44337-025-00241-9
115. Tong ZWM, Grant E, Gras S, Wu M, Smith C, Barrett HL, et al. The role of T-cell immunity in COVID-19 severity amongst people living with type II diabetes. FEBS J. (2021) 288:5042–54. doi: 10.1111/febs.16105
116. Fatoke B, Hui AL, Saqib M, Vashisth M, Aremu SO, Aremu DO, et al. Type 2 diabetes mellitus as a predictor of severe outcomes in COVID-19 - a systematic review and meta-analyses. BMC Infect diseases. (2025) 25:719.
117. Cheng Y, Yue L, Wang Z, Zhang J, and Xiang G. Hyperglycemia associated with lymphopenia and disease severity of COVID-19 in type 2 diabetes mellitus. J Diabetes its complications. (2021) 35:107809.
118. Khwatenge CN, Pate M, Miller LC, and Sang Y. Immunometabolic dysregulation at the intersection of obesity and COVID-19. Front Immunol. (2021) 12:732913. doi: 10.3389/fimmu.2021.732913
119. DeWolf S, Laracy JC, Perales MA, Kamboj M, van den Brink MRM, and Vardhana S. SARS-CoV-2 in immunocompromised individuals. Immunity. (2022) 55:1779–98.
120. Hamad RS, Al-kuraishy HM, Alexiou A, Papadakis M, Ahmed EA, Saad HM, et al. SARS-CoV-2 infection and dysregulation of nuclear factor erythroid-2-related factor 2 (Nrf2) pathway. Cell Stress Chaperones. (2023) 28:657–73.
121. Petakh P, Isevych V, Kamyshnyi A, and Oksenych V. Weil’s disease-immunopathogenesis, multiple organ failure, and potential role of gut microbiota. Biomolecules. (2022) 12. doi: 10.3390/biom12121830
122. Lan Y, Wang H, Wu J, and Meng X. Cytokine storm-calming property of the isoquinoline alkaloids in Coptis chinensis Franch. Front Pharmacol. (2022) 13:973587. doi: 10.3389/fphar.2022.973587
123. Mallick R, Basak S, Chowdhury P, Bhowmik P, Das RK, Banerjee A, et al. Targeting cytokine-mediated inflammation in brain disorders: developing new treatment strategies. Pharmaceuticals. (2025) 18:104. doi: 10.3390/ph18010104
124. Al-Qahtani AA, Alhamlan FS, and Al-Qahtani AA. Pro-inflammatory and anti-inflammatory interleukins in infectious diseases: A comprehensive review. Trop Med Infect Dis. (2024) 9. doi: 10.3390/tropicalmed9010013
125. Buchynskyi M, Oksenych V, Kamyshna I, Vorobets I, Halabitska I, and Kamyshnyi O. Modulatory roles of AHR, FFAR2, FXR, and TGR5 gene expression in metabolic-associated fatty liver disease and COVID-19 outcomes. Viruses. (2024) 16. doi: 10.3390/v16060985
126. Parveen S, Shen J, Lun S, Zhao L, Alt J, Koleske B, et al. Glutamine metabolism inhibition has dual immunomodulatory and antibacterial activities against Mycobacterium tuberculosis. Nat Commun. (2023) 14:7427. doi: 10.1038/s41467-023-43304-0
127. Wang Y, Li Z, Yu R, Chen Y, Wang D, Zhao W, et al. Metal-phenolic network biointerface-mediated cell regulation for bone tissue regeneration. Materials Today Bio. (2025) 30:101400.
128. Xiong Y, Knoedler S, Alfertshofer M, Kim B-S, Jiang D, Liu G, et al. Mechanisms and therapeutic opportunities in metabolic aberrations of diabetic wounds: a narrative review. Cell Death disease. (2025) 16:341.
129. Suzuki K, Hatzikotoulas K, Southam L, Taylor HJ, Yin X, Lorenz KM, et al. Genetic drivers of heterogeneity in type 2 diabetes pathophysiology. Nature. (2024) 627:347–57. doi: 10.1038/s41586-024-07019-6
130. Bazzazzadehgan S, Shariat-Madar Z, and Mahdi F. Distinct roles of common genetic variants and their contributions to diabetes: MODY and uncontrolled T2DM. Biomolecules. (2025) 15. doi: 10.3390/biom15030414
131. Cefalu WT, Andersen DK, Arreaza-Rubín G, Pin CL, Sato S, Verchere CB, et al. Heterogeneity of diabetes: β-cells, phenotypes, and precision medicine: proceedings of an international symposium of the canadian institutes of health research’s institute of nutrition, metabolism and diabetes and the U.S. National institutes of health’s national institute of diabetes and digestive and kidney diseases. Diabetes Care. (2022) 45:3–22. doi: 10.2337/dci21-0051
132. Singh S, Usman K, and Banerjee M. Pharmacogenetic studies update in type 2 diabetes mellitus. World J diabetes. (2016) 7:302–15.
133. Ercegović V, Džimbeg M, and Gelemanović A. Genetic susceptibility of type 2 diabetes and metabolic syndrome. Diabetology. (2025) 6:11.
134. Povel CM, Boer JM, Onland-Moret NC, Dollé ME, Feskens EJ, and van der Schouw YT. Single nucleotide polymorphisms (SNPs) involved in insulin resistance, weight regulation, lipid metabolism and inflammation in relation to metabolic syndrome: an epidemiological study. Cardiovasc diabetology. (2012) 11:133. doi: 10.1186/1475-2840-11-133
135. Ray GW, Zeng Q, Kusi P, Zhang H, Shao T, Yang T, et al. Genetic and inflammatory factors underlying gestational diabetes mellitus: a review. Front endocrinology. (2024) 15:1399694.
136. Buchynskyi M, Oksenych V, Kamyshna I, Budarna O, Halabitska I, Petakh P, et al. Genomic insight into COVID-19 severity in MAFLD patients: a single-center prospective cohort study. Front Genet. (2024) 15:1460318. doi: 10.3389/fgene.2024.1460318
137. Lyubomirskaya ES, Kamyshnyi AM, Krut YY, Smiianov VA, Fedoniuk LY, Romanyuk LB, et al. SNPs and transcriptional activity of genes of innate and adaptive immunity at the maternal-fetal interface in woman with preterm labour, associated with preterm premature rupture of membranes. Wiadomosci lekarskie (Warsaw Poland: 1960). (2020) 73:25–30.
138. Joya F, Zafar A, Sikander S, and Akhlaq H. Understanding the genetic basis of type 2 diabetes: implications for precision medicine and novel therapeutic approaches. Indus J Bioscience Res. (2025) 3:559–73.
139. Kapellou A, Salata E, Vrachnos DM, Papailia S, and Vittas S. Gene–diet interactions in diabetes mellitus: current insights and the potential of personalized nutrition. Genes. (2025) 16:578. doi: 10.3390/genes16050578
140. Pei X, Huang D, and Li Z. Genetic insights and emerging therapeutics in diabetic retinopathy: from molecular pathways to personalized medicine. Front Genet. (2024) 15:1416924. doi: 10.3389/fgene.2024.1416924
141. Kamyshnyi A, Koval H, Kobevko O, Buchynskyi M, Oksenych V, Kainov D, et al. Therapeutic effectiveness of interferon-α2b against COVID-19 with community-acquired pneumonia: the ukrainian experience. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24086887
142. Petakh P, Behzadi P, Oksenych V, and Kamyshnyi O. Current treatment options for leptospirosis: a mini-review. Front Microbiol. (2024) 15:1403765. doi: 10.3389/fmicb.2024.1403765
143. Topol IA, Kamyshny AM, Abramov AV, and Kolesnik YM. Expression of XBP1 in lymphocytes of the small intestine in rats under chronic social stress and modulation of intestinal microflora composition. Fiziolohichnyi zhurnal (Kiev Ukraine: 1994). (2014) 60:38–44.
144. Frankowski R, Kobierecki M, Wittczak A, Różycka-Kosmalska M, Pietras T, Sipowicz K, et al. Type 2 diabetes mellitus, non-alcoholic fatty liver disease, and metabolic repercussions: the vicious cycle and its interplay with inflammation. Int J Mol Sci. (2023) 24:9677. doi: 10.3390/ijms24119677
145. Vadakkiniath IJ. Prevalence and correlates of stress, anxiety, and depression in patients with chronic diseases: a cross-sectional study. Middle East Curr Psychiatry. (2023) 30:66. doi: 10.1186/s43045-023-00340-2
146. Michalopoulou E, Thymis J, Lampsas S, Pavlidis G, Katogiannis K, Vlachomitros D, et al. The triad of risk: linking MASLD, cardiovascular disease and type 2 diabetes; from pathophysiology to treatment. J Clin Med. (2025) 14:428. doi: 10.3390/jcm14020428
147. Dinavari MF, Sanaie S, Rasouli K, Faramarzi E, and Molani-Gol R. Glycemic control and associated factors among type 2 diabetes mellitus patients: a cross-sectional study of Azar cohort population. BMC endocrine Disord. (2023) 23:273. doi: 10.1186/s12902-023-01515-y
148. Fanelli G, Raschi E, Hafez G, Matura S, Schiweck C, Poluzzi E, et al. The interface of depression and diabetes: treatment considerations. Trans Psychiatry. (2025) 15:22. doi: 10.1038/s41398-025-03234-5
149. Suprapti B, Izzah Z, Anjani AG, Andarsari MR, Nilamsari WP, and Nugroho CW. Prevalence of medication adherence and glycemic control among patients with type 2 diabetes and influencing factors: A cross-sectional study. Global Epidemiol. (2023) 5:100113. doi: 10.1016/j.gloepi.2023.100113
150. Ali SN, Dang-Tan T, Valentine WJ, and Hansen BB. Evaluation of the clinical and economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the United States. Adv Ther. (2020) 37:869–82. doi: 10.1007/s12325-019-01199-8
151. McGill M, Blonde L, Chan JCN, Khunti K, Lavalle FJ, and Bailey CJ. The interdisciplinary team in type 2 diabetes management: Challenges and best practice solutions from real-world scenarios. J Clin Trans endocrinology. (2017) 7:21–7.
152. Tao M, Ye W, Wu Y, Chang W, Liu F, and Zhu Y. Identification and validation of five novel protein targets for type 2 diabetes mellitus. Sci Rep. (2025) 15:12127. doi: 10.1038/s41598-025-97416-2
153. Chen Y, Wen Q, Yang B, Feng L, and Jia X. Active constituent of HQS in T2DM intervention: efficacy and mechanistic insights. Int J Mol Sci. (2025) 26:4578. doi: 10.3390/ijms26104578
154. Xie X, Macknight HP, Lu AL, and Chalfant CE. RNA splicing variants of the novel long non-coding RNA, CyKILR, possess divergent biological functions in non-small cell lung cancer. Mol Ther Nucleic Acids. (2025) 36:102412. doi: 10.1016/j.omtn.2024.102412
155. Shi K, Peng X, Xu T, Lin Z, Sun M, Li Y, et al. Precise electromagnetic modulation of the cell cycle and its applications in cancer therapy. Int J Mol Sci. (2025) 26:4445. doi: 10.3390/ijms26094445
156. Li M, Wang M, Wen Y, Zhang H, Zhao GN, and Gao Q. Signaling pathways in macrophages: molecular mechanisms and therapeutic targets. MedComm. (2023) 4:e349. doi: 10.1002/mco2.349
157. Okdahl T, Wegeberg AM, Pociot F, Brock B, Størling J, and Brock C. Low-grade inflammation in type 2 diabetes: a cross-sectional study from a Danish diabetes outpatient clinic. BMJ Open. (2022) 12:e062188. doi: 10.1136/bmjopen-2022-062188
158. Napiórkowska-Baran K, Treichel P, Czarnowska M, Drozd M, Koperska K, Węglarz A, et al. Immunomodulation through nutrition should be a key trend in type 2 diabetes treatment. Int J Mol Sci. (2024) 25:3769. doi: 10.3390/ijms25073769
159. Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. (2019) 14:50–9. doi: 10.15420/ecr
160. Saad B. Prevention and treatment of obesity-related inflammatory diseases by edible and medicinal plants and their active compounds. Immuno. (2022) 2:609–29.
161. Ojurongbe TA, Afolabi HA, Oyekale A, Bashiru KA, Ayelagbe O, Ojurongbe O, et al. Predictive model for early detection of type 2 diabetes using patients’ clinical symptoms, demographic features, and knowledge of diabetes. Health Sci Rep. (2024) 7:e1834. doi: 10.1002/hsr2.1834
162. Farrugia F, Aquilina A, Vassallo J, and Pace NP. Bisphenol A and type 2 diabetes mellitus: A review of epidemiologic, functional, and early life factors. Int J Environ Res Public Health. (2021) 18:716. doi: 10.3390/ijerph18020716
163. Reed MJ and Scribner KA. In-vivo and in-vitro models of type 2 diabetes in pharmaceutical drug discovery. Diabetes Obes Metab. (1999) 1:75–86. doi: 10.1046/j.1463-1326.1999.00014.x
164. Chen J, Lin A, and Luo P. Advancing pharmaceutical research: A comprehensive review of cutting-edge tools and technologies. Curr Pharm Analysis. (2024) 21:1–19.
165. Rasooly D, Pereira AC, and Joseph J. Drug discovery and development for heart failure using multi-omics approaches. Int J Mol Sci. (2025) 26:2703. doi: 10.3390/ijms26062703
166. Qiu S, Cai Y, Yao H, Lin C, Xie Y, Tang S, et al. Small molecule metabolites: discovery of biomarkers and therapeutic targets. Signal transduction targeted Ther. (2023) 8:132. doi: 10.1038/s41392-023-01399-3
167. Singh AV, Chandrasekar V, Paudel N, Laux P, Luch A, Gemmati D, et al. Integrative toxicogenomics: Advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomedicine Pharmacotherapy. (2023) 163:114784. doi: 10.1016/j.biopha.2023.114784
168. Gkintoni E, Vantarakis A, and Gourzis P. Neuroimaging insights into the public health burden of neuropsychiatric disorders: A systematic review of electroencephalography-based cognitive biomarkers. Medicina. (2025) 61:1003. doi: 10.3390/medicina61061003
169. Omidian H and Omidian A. The emergence of psilocybin in psychiatry and neuroscience. Pharm (Basel Switzerland). (2025) 18. doi: 10.3390/ph18040555
170. Sauder C, Allen LA, Baker E, Miller AC, Paul SM, and Brannan SK. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study. Transl Psychiatry. (2022) 12:491. doi: 10.1038/s41398-022-02254-9
Keywords: [1,2,4]triazino[2,3-c]quinazolines, hypoglycemic activity, diabetes mellitus, insulin resistance, molecular docking
Citation: Trzhetsynskyi S, Nosulenko I, Kinichenko A, Skoryna D, Berest H, Shvets V, Voskoboinik O, Kovalenko S, Petakh P and Kamyshnyi O (2025) Future horizons in diabetes treatment: hypoglycemic activity of [1,2,4]triazino[2,3-c]quinazoline derivatives. Front. Endocrinol. 16:1638013. doi: 10.3389/fendo.2025.1638013
Received: 30 May 2025; Accepted: 05 September 2025;
Published: 18 September 2025.
Edited by:
Nazarii Kobyliak, Bogomolets National Medical University, UkraineReviewed by:
Kay Hau Aaron Choy, Barwon Health, AustraliaVolodymyr Pankiv, Ukrainian Scientific and Practical Center of Endocrine Surgery, Ukraine
Ganna Zaychenko, Bogomolets National Medical University, Ukraine
Nirvana Ali, Modern University for Information and Technology, Egypt
Copyright © 2025 Trzhetsynskyi, Nosulenko, Kinichenko, Skoryna, Berest, Shvets, Voskoboinik, Kovalenko, Petakh and Kamyshnyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlo Petakh, cGF2bG8ucGV0YWtoQHV6aG51LmVkdS51YQ==; Oleksandr Kamyshnyi, a2FteXNobnlpX29tQHRkbXUuZWR1LnVh
 Serhii Trzhetsynskyi1
Serhii Trzhetsynskyi1 Volodymyr Shvets
Volodymyr Shvets Oleksii Voskoboinik
Oleksii Voskoboinik Serhii Kovalenko
Serhii Kovalenko Pavlo Petakh
Pavlo Petakh Oleksandr Kamyshnyi
Oleksandr Kamyshnyi