- 1Division of Medical Research, SRM Medical College Hospital and Research Centre, SRM Institute of Science and Technology, Chengalpattu, Tamil Nadu, India
- 2Department of Obstetrics and Gynecology, SRM Medical College Hospital and Research Centre, SRM Institute of Science and Technology, Chengalpattu, Tamil Nadu, India
Background: Gestational Diabetes Mellitus (GDM) is a transient diabetogenic state that often leads to adverse maternal and fetal outcomes. The rising burden of exposure to endocrine-disrupting chemicals like phthalates essentially disrupts the tightly regulated endocrine system, thereby modulating the insulin signaling pathways, leading to GDM.
Objective: In the present work, a systematic review was performed to examine the probable relation between maternal exposure to phthalates, as endocrine-disrupting compounds, and GDM.
Methods: Relevant studies from their inception to April 2025 were identified by searching PubMed, Embase, Scopus, and Science Direct. The data were screened using the Rayyan tool, and the risk of bias was assessed using the New Castle Ottawa Scale selection tool.
Results: We identified 13 studies that showed a significant presence of phthalates in the urine samples of GDM patients. 5 phthalate secondary metabolites, Monoethyl Phthalate, Monobutyl phthalate, Mono-Isobutyl Phthalate, and Monobenzyl Phthalate and the primary phthalate Di(2-ethylhexyl) Phthalate were found to be most commonly present in the urine samples of the GDM patients.
Conclusion: Urinary phthalate levels can be used as a non-invasive biomarker for GDM, thereby also reducing the risk of associated adverse pregnancy outcomes.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251023656.
1 Introduction
Endocrine-disrupting chemicals (EDCs) are essentially exogenous compounds that are capable of interfering with the normal functioning of the hormonal system (1). They have been known to mimic, block, and disrupt endogenous hormonal signals, affecting homeostasis, development, reproduction, and metabolism. EDCs are omnipresent and are found in our everyday lives, present in cleaning products, industrial chemicals, and personal and home care products (2). Phthalate, which is a group of synthetic chemicals used as plasticizers in polyvinyl chloride, are one of the most widely studied classes of EDCs (3).
Phthalates are essentially synthetic diesters of phthalic acid. They are mainly used as plasticizers to increase the flexibility of polyvinyl chloride (PVC) and other polymers (4). Phthalates are primarily classified into high molecular weight (HMW) and low molecular weight (LMW) phthalates, with each having distinct chemical structures and applications (5). Common HMW phthalates include di(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBzP), and diisononyl phthalate (DINP), which are commonly found in medical devices, flooring, food packaging, and automotive products (6). LMW phthalates, such as diethyl phthalate (DEP), dimethyl phthalate (DMP), and dibutyl phthalate (DBP), are extensively used in daily personal care products, including perfumes, lotions, and cosmetics.
Human exposure to phthalates is pervasive and primarily occurs through inhalation, ingestion, and dermal absorption. Research has found that one of the most significant sources of exposure to phthalates is dietary intake through contaminated food and beverages stored in plastic containers or wrapped in plastic films (7, 8). Additionally, human exposure to phthalates via personal care and cosmetic products, which contain phthalates as solvents and fragrance stabilizers, is also a matter of great concern (9). High concentrations of phthalates in airborne particles and settled indoor dust, particularly in environments with high PVC content, are also known to pose potential harm to humans (10). Studies have also shown that even medical devices contain phthalate-based plasticizers, particularly those made with DEHP-containing tubing, such as IV bags and catheters (11, 12).
Research on phthalates has been gaining traction due to their potential role in disrupting reproductive and metabolic health, especially among vulnerable populations such as pregnant women. Phthalates can cross the placental barrier and disrupt maternal-fetal metabolic signaling, and the vulnerability of pregnant women to phthalate exposure is of special concern.
Gestational diabetes mellitus (GDM), defined as glucose intolerance first recognized during pregnancy, poses substantial health risks for both mother and fetus (13), including Hypertensive Disorders of Pregnancy (HDP), and future risk of type 2 diabetes in mothers. GDM can also lead to macrosomia, obesity, or metabolic dysfunction in the offspring (14). GDM can broadly be classified into GDMA1 and GDMA2 (15). GDMA1 is diet-controlled, meaning blood sugar is managed through nutrition and exercise alone, posing lower risks (15). GDMA2 requires medication (insulin or oral agents) due to uncontrolled glucose levels, increasing risks of fetal macrosomia, neonatal hypoglycemia, and maternal type 2 diabetes post-pregnancy (15). Early diagnosis and strict monitoring are crucial for both types to ensure healthy outcomes for mother and baby. The exposure pattern to these EDCs can be dietary, behavioral, or residential (16, 17). The most common risk factors for GDM include age, obesity, and family history. New evidence from contemporary studies emphasizes the role of environmental exposures, particularly EDCs such as phthalates, to be increasingly relevant contributors (17). It was found that around 50% of GDM patients appear to be prone to lifestyle stressors such as endocrine-disrupting chemicals (17, 18). Additionally, various studies have found that phthalate exposure can interfere with glucose and lipid metabolism, pancreatic β-cell function, and insulin sensitivity (19, 20). Studies have shown that phthalates have been shown to activate peroxisome proliferator-activated receptors (PPARs), which play a crucial role in lipid metabolism and adipogenesis, which in turn influences insulin resistance (21).
While extensive research has examined the relationship between phthalate exposure and GDM, there is a lack of systematic synthesis of findings across different geographic, socioeconomic, and clinical settings. Additionally, previous researchers have rarely considered contextual factors such as specific phthalate metabolites measured. Moreover, they have not considered the regional disparities in the prevalence of GDM and phthalate exposure (Supplementary Table S1). It is widely known that both GDM prevalence and phthalate exposure vary considerably across the globe, with considerable differences being observed in high-income countries (HICs), low and middle-income countries (LMICs), and low-income countries (LICs) (22).
Thus, this study aims to address these gaps by collating and analyzing the existing evidence on phthalate concentrations in women diagnosed with GDM across diverse populations, focusing specifically on biomarker-based studies. Additionally, this study seeks to assess whether phthalate exposure is consistently elevated in GDM cases compared to controls and to evaluate the magnitude and direction of associations across different contexts.
Furthermore, the study contributes to the broader field of environmental reproductive epidemiology by advancing our understanding of how ubiquitous environmental pollutants may intersect with maternal and fetal health outcomes. Thus, the main objective of this review is to systematically evaluate and examine the probable relation between maternal exposure to phthalates, as endocrine-disrupting compounds, and GDM. The findings of the study could be useful to provide evidence that can inform future research priorities, clinical guidelines, and environmental health policies aimed at safeguarding maternal metabolic health.
2 Methods
2.1 Registration
The systematic review was performed as outlined a priori in the registered protocols (PROSPERO registration ID CRD420251023656). Ethical approval was not required for the systematic review as these were secondary studies using published data.
2.2 Eligibility criteria
We included original reports that analyzed the phthalate levels in the urine samples of GDM patients (cases) and non-diabetic subjects (controls) in human trials. All case-control, cross-sectional and cohort studies were included in the study. Studies utilizing animal models and cell lines were excluded. The studies focused on the estimation of phthalates derived only from urine samples were included in our analysis. Study population, intervention, comparator, outcome, and study design (PICOS) parameters were predefined for objective and reproducible analysis.
2.3 Population
Any human study of GDM was included. Studies involving in vitro, ex vivo, or pre-clinical animal models of GDM were excluded.
2.4 Intervention
Studies included in our analysis did not deal with administering insulin dosages or any other interventions on human subjects.
2.5 Comparator
The main comparators were the association between urinary phthalate concentrations and GDM and related complications. Non-comparative studies were excluded.
2.6 Outcome
The main outcome of the study was the correlation of urinary phthalate concentrations with GDM. The secondary adverse pregnancy outcomes associated with GDM were also observed.
2.7 Study design
All English-language, full-text, clinical studies comparing urinary phthalate levels between pregnant women with and without GDM were included. Review articles, non-comparative studies, commentaries, editorials, case reports, case series, and other study types were excluded. Studies investigating the concentration of phthalates from urine samples in GDM patients were included. Studies dealing with other EDCs (heavy metals, parabens, bisphenols, triclosan, PFAS, organophosphates) were also excluded from the study. In Vitro studies, animal model studies or studies dealing with type 2 diabetes mellitus were also excluded from the study.
2.8 Search strategy
This study was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (PRISMA 2020 statement) (http://www.prisma-statement.org/). A literature search strategy was developed according to the four different parameters of the study question (participants, intervention, comparison, and outcome) and the study design. PubMed (pubmed.ncbi.nlm.nih.gov), Scopus (www.scopus.com), Embase (www.embase.com), and Science Direct (www.sciencedirect.com) electronic databases were used to identify eligible original articles published up to April 5, 2025. The published articles in these electronic databases from last 10 years were included. The search terms that were used in these databases are “((Phthalate) OR (Phthalates) OR (Phthalate esters) OR (Phthalate metabolites)) AND ((Gestational Diabetes Mellitus) OR (Gestational Diabetes)) AND (Urine) AND (Human)”.
2.9 Data analysis
The data screening was done with the help of the Rayyan tool. The risk of bias was analyzed using the New Castle Ottawa Scale selection tool.
2.10 Comparison of phthalate concentration
The concentration of the phthalates that was most common among the included studies were compared using a heat map. All the values were converted to μg/L before comparison. The values of extreme variations in phthalate concentrations across studies were adjusted using a log 10 scale. This transformation ensures all values are visually interpretable, while still preserving relative differences.
3 Results
3.1 Study selection
Figure 1 illustrates the study selection process. We retrieved 748 records and, after removing the duplicates and irrelevant articles, examined the titles and abstracts of the remaining 597 papers. Finally, after reviewing the full text of the remaining 33 papers, we identified 13 studies suitable for a systematic review (23–35). Age, Body Mass Index (BMI), parity, smoking status, and dietary patters were adjusted among all the pregnant women in the 13 studies.
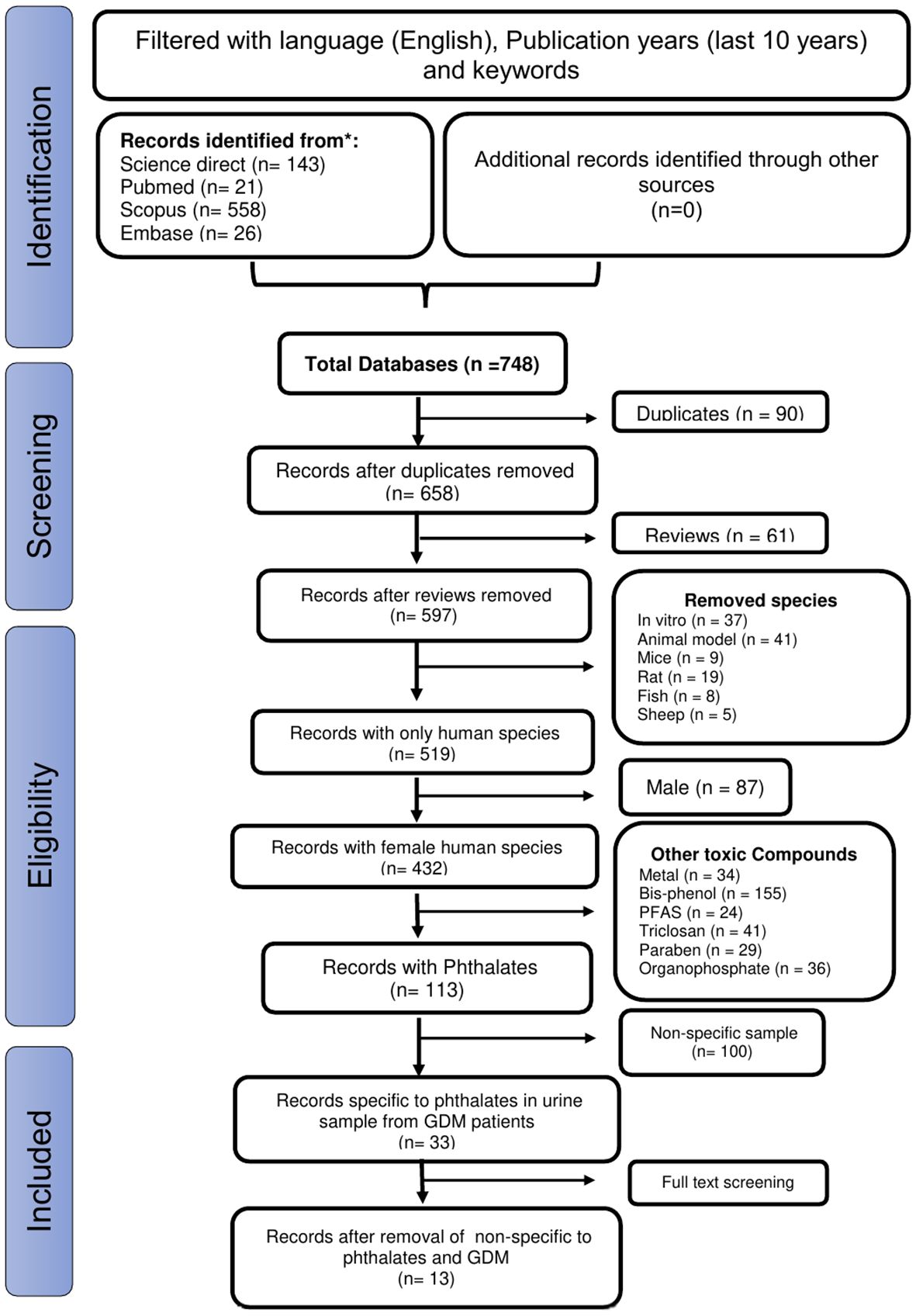
Figure 1. The PRISMA diagram shows the procedure used to select articles based on the predetermined inclusion and exclusion criteria.
3.2 Study characteristics
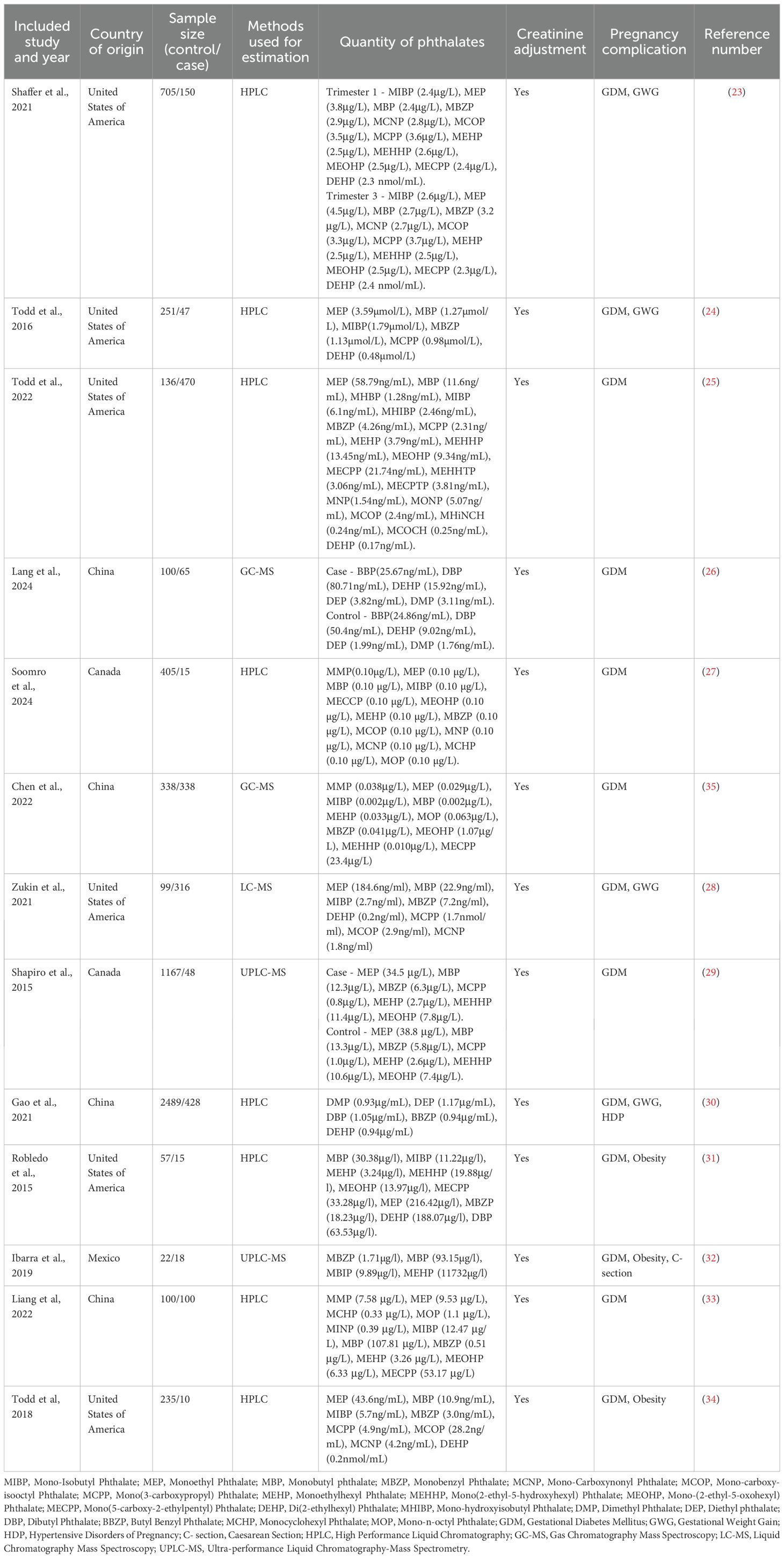
The 13 included studies, published between 2015 and 2025, focused specifically on human research. Research settings ranged from university laboratories to multicentered collaborations and private practices, with studies conducted in the United States of America (n=6), Canada (n=2), China (n=4), and Mexico (n=1). The procedure that was used to quantify the concentration of urinary phthalates was High Performance Liquid Chromatography (n=8), Gas Chromatography Mass Spectroscopy (n=2), Liquid Chromatography Mass Spectroscopy (n=1), Ultra-performance Liquid Chromatography-Mass Spectrometry (n=2) (Table 1).
3.3 Association of urinary phthalates with GDM
All 13 included studies showed an increase in the concentration of urinary phthalate concentrations among pregnant women with GDM compared to those of pregnancies without GDM (Figure 1). Our findings from the included studies showed not only the presence of secondary phthalate metabolites like Mono-Isobutyl Phthalate (MIBP), Monoethyl Phthalate (MEP), Monobutyl Phthalate (MBP), Monobenzyl Phthalate (MBZP), Mono-Carboxynonyl Phthalate, Mono-carboxy-isooctyl Phthalate, Mono(3-carboxypropyl) Phthalate, Monoethylhexyl Phthalate, Mono(2-ethyl-5-hydroxyhexyl) Phthalate, Mono-(2-ethyl-5-oxohexyl) Phthalate, Mono(5-carboxy-2-ethylpentyl) Phthalate, Monocyclohexyl Phthalate, and Mono-n-octyl Phthalate the urine sample of GDM patients but also showed a significant increase in the levels of primary phthalate metabolites like Di(2-ethylhexyl) Phthalate (DEHP), Dimethyl Phthalate, Diethyl phthalate, and Dibutyl Phthalate in the urine sample of GDM patients (Table 1) (Supplementary Table S2). The values of the most common phthalates among the 13 studies were converted to μg/L, and log10 scale values are also provided in the Supplementary Table S2 to maintain homogeneity among the concentrations in these 13 studies.
Most of the studies inferred not only a significant rise in the concentrations of the secondary metabolites MEP, MBP, MIBP, and MBZP but also the primary metabolite DEHP in the urine samples of GDM pregnancies.
The comparison in the concentration of the phthalates that were most common among the 13 studies is shown using a heat map of the log10 phthalate concentration values in μg/L (Figure 2). 6 studies showed an elevated level of MEP (24, 25, 28, 29, 31, 34), 6 studies showed an elevated concentration of MBP (25, 28, 31–34), 2 studies showed an elevated concentration of MIBP (25, 34), 2 studies showed an elevated concentration of MBZP (25, 34), and 5 studies showed an elevated concentration of DEHP (23, 25, 30–32).
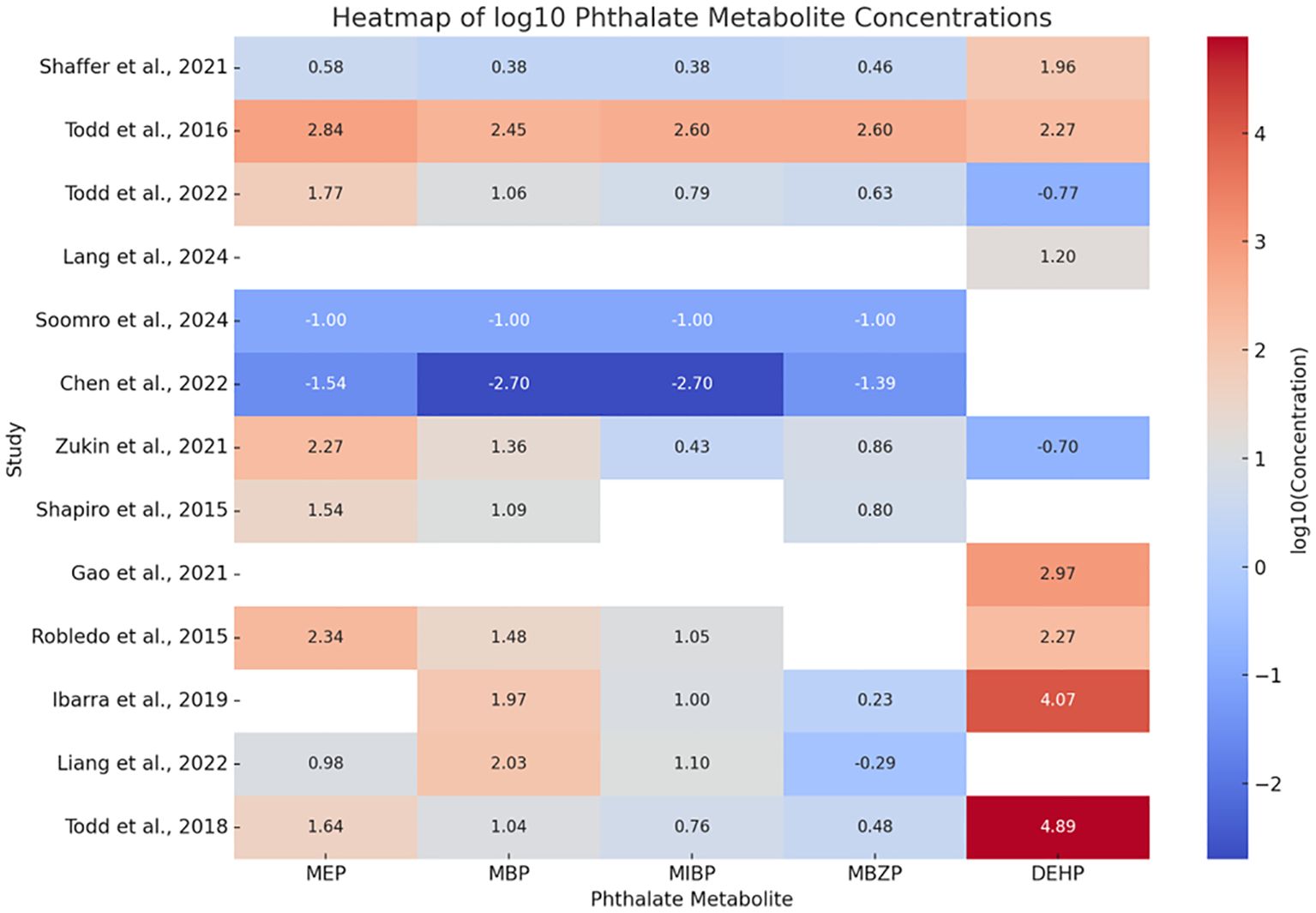
Figure 2. Comparison of the concentration of common phthalates between all 13 studies using a heat map that the authors have analyzed. The values from all 13 selected studies have been converted to µg/L to maintain homogeneity of values, followed by conversion to a log10 scale to eliminate existing extreme differences in the values. The red color in the log10 scale represents higher concentrations, while the blue color represents lower concentrations.
3.4 Pregnancy outcomes
Pregnancy outcomes following the diagnosis of the pregnant women from all included thirteen studies were broadly divided into primary and secondary outcomes.
The primary outcome of the study refers to the most common metabolic dysfunction affecting both the mother and the fetus in the studies included in the systematic review for data analysis. The primary pregnancy outcome that was seen commonly among the study participants recruited in the included studies is impaired glucose tolerance due to GDM (23–35). These studies show that women have greater fasting plasma glucose and post-prandial glucose concentrations than normal subjects because of higher amounts of systemic insulin resistance.
The secondary outcomes analyzed as part of this study focused on additional severe maternal and fetal outcomes of pregnancy that help to interpret the results of the primary outcome of GDM. The secondary outcomes included obesity, gestational weight gain, and hypertensive disorders of pregnancy (30, 32).
3.5 Risk of bias analysis
The current systematic review included 13 cohort studies, initially assessed for methodological quality using the Newcastle-Ottawa Quality Assessment Scale (NOS), which scores studies across three domains: selection, comparability, and outcome. Based on the NOS scores, further outcome-specific evaluations were carried out using the Risk of Bias 2 (RoB 2) tool. Risk of bias judgments were presented in tabular form (Supplementary Table S3) and visualized through traffic light plots to reflect the level of bias across studies. These assessments informed the interpretation of findings and the overall grading of evidence quality, identifying one study (33) as having a high risk of bias (Figure 3).
3.6 Additional findings
Other EDCs were also found to be significantly associated with GDM. Many studies also suggested associating urinary heavy metals levels like arsenic, lead, cadmium, manganese, mercury, antimony, copper, magnesium, molybdenum, selenium and zinc in GDM patients (27). The elevated levels of flame retardants like tris (2-butoxyethyl) phosphate, tributyl phosphate, tris (2-chloroethyl) phosphate, tris (1,3-dichloro-2-propyl) phosphate, tri-ortho-cresyl phosphate, and triphenyl phosphate were also seen to be associated with GDM (26). Studies also showed a significant increase in urinary bisphenols and perfluoroalkyl acid levels in GDM patients (27, 29, 32). A study also found that di(isononyl) cyclohexane 1,2-dicarboxylate was significantly associated (24).
In a study by Ibarra et al., they showed that elevated levels of phthalates are related to the overexpression of micro ribonucleic acids (miRs) in the serum samples of the patients, including miR-9-5p, miR-16-5p, miR-29-3p, and miR-330-3p (32).
4 Discussion
The studies included in this review highlight clear differences in both the types and concentrations of phthalate metabolites detected among pregnant women with and without GDM. This study is an alternative perspective on the impact of phthalates in gestational diabetes mellitus (GDM), emphasizing their role as endocrine-disrupting chemicals (EDCs) that interfere with metabolic and hormonal regulation during pregnancy (36). EDCs may interact differently with nuclear hormone receptors (e.g. PPARγ and estrogen receptors) in GDMA2 compared to GDMA1 due to hormonal imbalances and altered adipokine profiles. GDMA2 individuals tend to have greater degrees of insulin resistance and dysregulated glucose metabolism. EDCs including phthalates, BPA, and parabens have been linked to impaired β-cell function, disruption of insulin signaling, and increased oxidative stress (37). These effects may be more prominent in GDMA2, exacerbating the already existing metabolic abnormalities and potentially resulting to poor maternal and neonatal outcomes.
4.1 Effect of phthalates on metabolic pathways
Phthalates can bind to nuclear receptors such as peroxisome proliferator-activated receptors (PPARs), particularly PPARγ, disrupting adipogenesis, lipid metabolism, and insulin sensitivity, key pathways involved in the pathophysiology of GDM (36). This disruption may impair glucose uptake and exacerbate insulin resistance, especially during the second and third trimesters when insulin resistance naturally increases (36).
Moreover, the placenta is a critical mediator in fetal-maternal metabolic exchange and is highly sensitive to environmental toxicants (38). Studies suggest that phthalate exposure can lead to placental inflammation, oxidative stress, and changes in gene expression, all of which are linked to impaired glucose metabolism (38, 39). Epigenetic alterations, such as changes in DNA methylation or miRNA regulation, have also been implicated, potentially influencing both maternal glycemic control and fetal metabolic programming (40, 41). Furthermore, in vivo rat studies show gestational DEHP exposure impairs offspring glucose tolerance, while DBP worsens hyperglycemia and glucose handling (17). DBP also disrupts FOXM1, reduces β-cell viability, and impairs STAT1 signaling in vitro (17).
Another significant consideration is that phthalate exposure is often not uniform across populations. Social and environmental determinants, including dietary habits (e.g., processed food consumption), use of personal care products, and occupational exposures, disproportionately affect certain groups, contributing to environmental health disparities (42).
4.2 Effect of phthalates on miRNA expression levels
The current systematic review aimed to assess the impact of phthalate exposure on the development of GDM. Evidence from epidemiological studies and mechanistic research indicates a positive association between phthalate exposure during pregnancy and impaired glucose regulation, increasing the risk of GDM. Numerous studies demonstrate that elevated urinary phthalate metabolites correlate with abnormal glucose metabolism in pregnant women (23). Higher phthalate exposure has also been linked with a greater incidence of GDM (25, 33, 35). Data from prospective cohorts further confirm these associations and highlight that trimester-specific exposure patterns may modulate the degree of risk (27, 29). Longitudinal evidence supports a link between phthalate exposure and disturbances in glucose levels and weight gain during pregnancy (30, 31). These associations are particularly evident in high-risk populations, emphasizing the disproportionate exposure burden among certain demographic groups (28). Epigenetic studies also suggest a mechanistic role through altered miRNA expression in pregnant women with GDM (32). Specifically, significant changes in miRNA patterns have been observed in GDM-affected pregnancies with high phthalate exposure, implicating pathways related to glucose metabolism and insulin signaling (17, 32). These findings support the integration of environmental exposure assessments into prenatal care protocols. Regulatory attention is warranted to limit phthalate exposure, particularly among reproductive-age women, to reduce GDM prevalence (1). Although this review synthesizes evidence from both human and experimental models, heterogeneity in study designs, exposure timing, phthalate types measured, and diagnostic criteria for GDM complicate direct comparisons across studies. Additional confounding factors may influence the observed associations, including diet, body mass index, and socioeconomic status. Moreover, many mechanistic insights are derived from animal models, which may not fully represent human metabolic responses (25, 26, 34). The impact of phthalates on GDM extends beyond direct metabolic disruption and involves complex interactions with hormonal pathways, placental function, and social determinants of health. Understanding these multidimensional effects is crucial for developing effective interventions and regulatory policies that protect maternal and fetal health.
4.3 Regional difference in phthalate profiles
The studies included in this review highlight clear regional differences in phthalate levels, with most studies originating from HICs such as the United States and Canada. In contrast, others were conducted in MICs regions like China and Mexico. Phthalate profiles and concentration levels varied remarkably by geographical regions. Possible reasons for wide variations could include differences in industrial use, consumer product regulations, and lifestyle factors such as food packaging, usage patterns of personal care products, and housing and living conditions. Additionally, the usage of different detection methods across studies resulted in methodological diversity. These varying regional trends in phthalate exposure underscore the importance of interpreting associations with GDM within local environmental and regulatory contexts, with a focus on regional variability in exposure sources when developing preventive strategies.
4.4 Co-exposure to other endocrine disruptors
Co-exposure to multiple environmental toxicants such as bisphenols, heavy metals, perfluoroalkyl acids, and flame retardants poses complex health risks due to potential additive or synergistic effects. Bisphenols disrupt endocrine pathways, while heavy metals like lead, mercury, and cadmium impair neurodevelopment and metabolic health (17, 43). PFAs are highly persistent, bioaccumulative, and linked to immune, hepatic, and reproductive dysfunctions (17, 44). Flame retardants, particularly polybrominated diphenyl ethers (PBDEs), interfere with thyroid regulation and neurodevelopment. Emerging evidence suggests that combined exposure may exacerbate oxidative stress, metabolic disorders, and developmental toxicity beyond individual chemical effects (17, 45). Hence, co-exposure assessment is vital for realistic risk evaluation in environmental health.
5 Conclusion
GDM, a transient hyperglycemic stage with higher than usual plasma glucose levels during pregnancy, has been linked to pregnancy issues such as hypertension, macrosomia, preterm delivery, preeclampsia, and stillbirths (46). GDM prevalence has grown dramatically, with reported frequencies ranging from 15% to 25% (47). Some data suggest that environmental contaminants may impair glucose homeostasis and glucose tolerance in healthy women. The risk of GDM and maternal exposure to phthalates were systematically reviewed in this study. This study’s findings revealed a link between phthalate exposure during pregnancy and the likelihood of GDM.
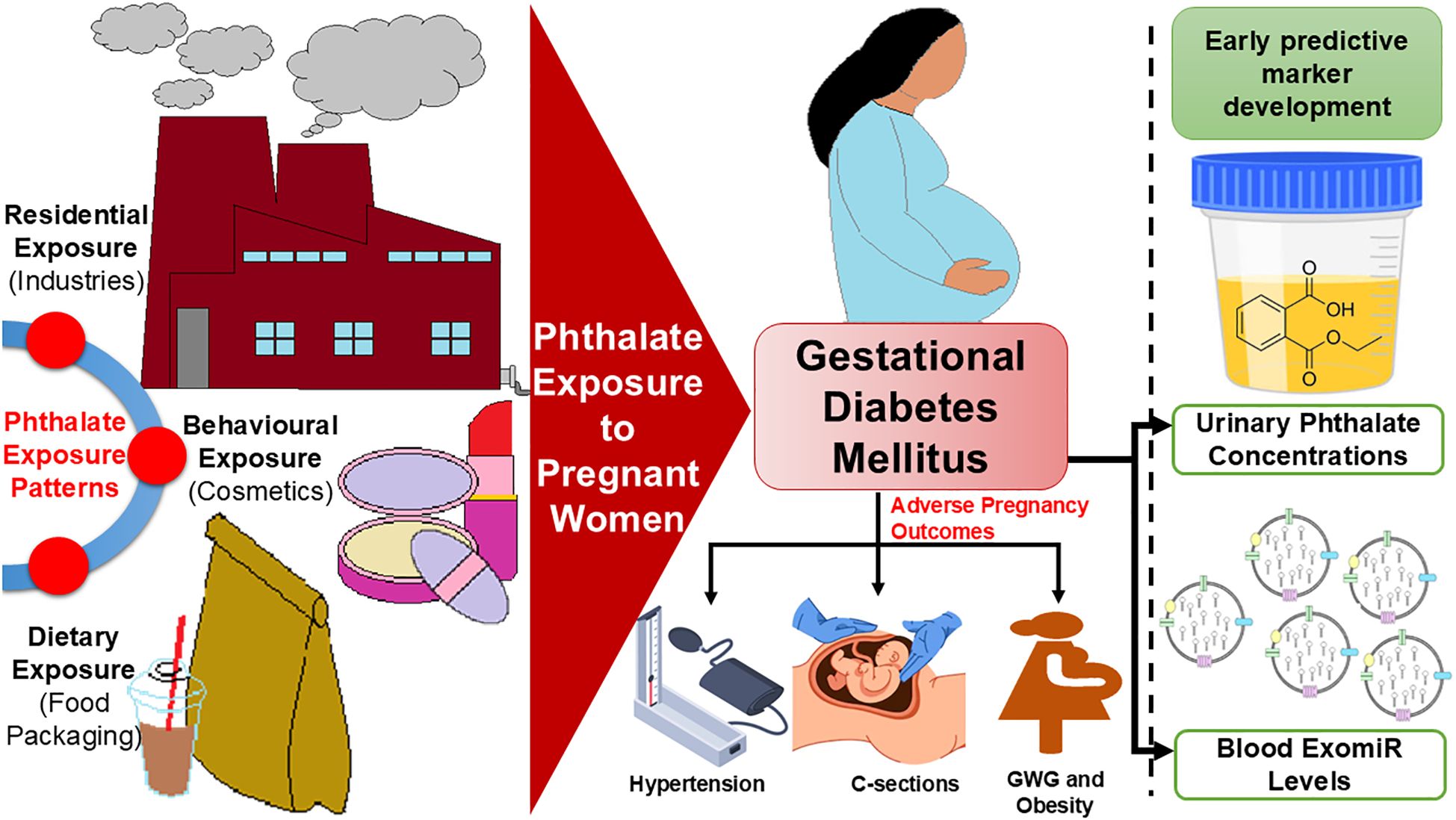
Our systematic review details the associations between phthalates and GDM pathophysiology, suggesting that urinary phthalates may serve as a predictor of GDM and associated complications. Phthalates significantly contribute to the development of insulin resistance, which often results in impaired glucose tolerance. These findings further underscore the importance of these plasticizers in the incidence of GDM. Additionally, phthalates are linked to pre-pregnancy BMI, which increases the likelihood of adverse pregnancy outcomes, such as HDP, GWG, and cesarean sections. Our findings in GDM patients align with previous research on phthalates concerning adverse maternal complications like HDP and gestational anemia (48), as well as neonatal complications, including various cardiovascular and neurological anomalies (13, 48–50). Furthermore, utilizing EDC-associated ExomiRs from patients’ blood can facilitate the early detection of GDM (13), enabling patients to be triaged based on escalating risk factors of the clinicopathologic illness (Figure 4). Single-point measurements of short-half-life phthalates like MEP and MBP may lack reliability as early GDM markers due to rapid metabolism and high intra-individual variability. While some studies link phthalate exposure to insulin resistance, longitudinal or repeated measurements are likely needed for robust prediction (51).
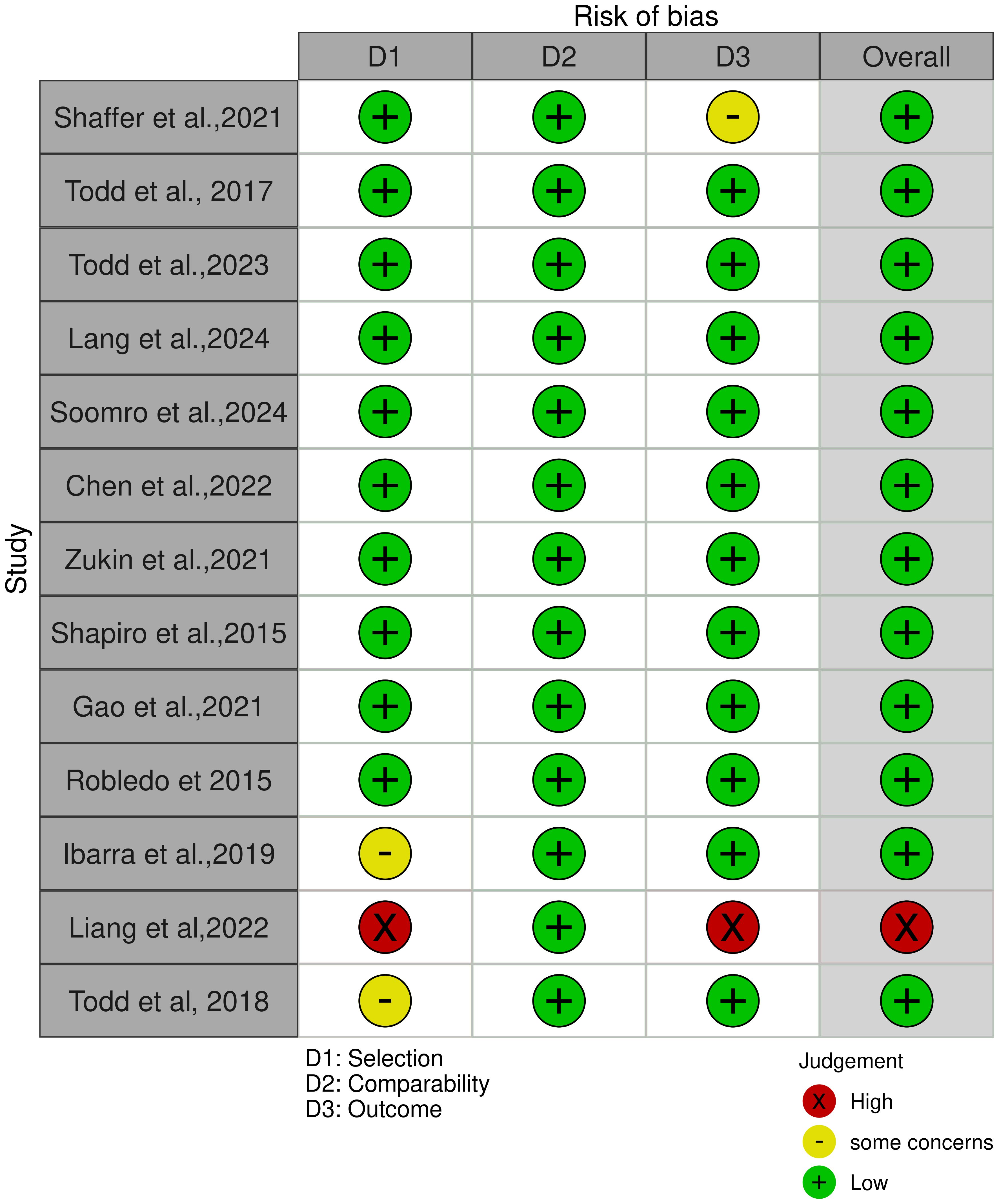
Figure 4. Depicts the exposure pattern of pregnant women to phthalates resulting in GDM and associated complications.
However, due to the scarcity of publications on this subject, it appears that the true impact of phthalate exposure remains unknown. As a result, more well-designed studies with a bigger sample size and longitudinal design are strongly suggested so that urinary phthalate levels can be strongly used as a non-invasive early predictive tool for GDM.
6 Limitations of the study
Some of the constraints encountered during this investigation are described below. First, the research design criteria may have resulted in a bias (selection bias) in the included studies. Future studies can circumvent this limitation by implementing and reporting on randomization, blinding, and the a priori technique. Second, only a few studies have been selected for subgroup analysis, which may limit the ability to detect the influence of xenobiotics, such as phthalates, on GDM. Moreover, the GDM patients included in the research population in all 13 investigations were not separated between GDMA1 and GDMA2, indicating a possible mechanistic difference. Furthermore, the authors didn’t separate the research population into early and late GDM. Patients who acquire GDM early in their pregnancy have a higher chance of developing gestational anemia. Finally, any dietary and lifestyle changes offered to patients during pregnancy may have a major impact on the concentration of urine phthalates, which must not be ruled out. Despite the variation across trials, similar effects of phthalates on individuals with GDM were discovered, justifying clinical translation efforts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
TM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. AA: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. RT: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. SS: Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing. SK: Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing. RJ: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors gratefully acknowledge the financial support by SRM Medical College & Research Centre for bearing the defrayed costs of publishing this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1638655/full#supplementary-material
References
1. Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, and Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. (2020) 8:719–30. doi: 10.1016/S2213-8587(20)30128-5
2. Encarnação T, Pais AA, Campos MG, and Burrows HD. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment (2019). doi: 10.1177/0036850419826802
3. Rosenfeld CS. Bisphenol A. and phthalate endocrine disruption of parental and social behaviors. Front Neurosci. (2015) 9:57. doi: 10.3389/fnins.2015.00057
4. Howick CJ. Plasticizers. In: Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Ltd (2021). p. 1–36. doi: 10.1002/0471238961.1612011903010415.a01.pub2
5. Pagoni A, Arvaniti OS, and Kalantzi OI. Exposure to phthalates from personal care products: Urinary levels and predictors of exposure. Environ Res. (2022) 212:113194. doi: 10.1016/j.envres.2022.113194
6. Deng M, Han X, Ge J, Liang X, Du B, Li J, et al. Prevalence of phthalate alternatives and monoesters alongside traditional phthalates in indoor dust from a typical e-waste recycling area: Source elucidation and co-exposure risk. J Hazardous Materials. (2021) 413:125322. doi: 10.1016/j.jhazmat.2021.125322
7. da Costa JM, Kato LS, Galvan D, Lelis CA, Saraiva T, and Conte-Junior CA. Occurrence of phthalates in different food matrices: A systematic review of the main sources of contamination and potential risks. Compr Rev Food Sci Food Safety. (2023) 22:2043–80. doi: 10.1111/1541-4337.13140
8. Perestrelo R, Silva CL, Algarra M, and Câmara JS. Evaluation of the occurrence of phthalates in plastic materials used in food packaging. Appl Sci. (2021) 11:2130. doi: 10.3390/app11052130
9. Koniecki D, Wang R, Moody RP, and Zhu J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ Res. (2011) 111:329–36. doi: 10.1016/j.envres.2011.01.013
10. Bu S, Wang Y, Wang H, Wang F, and Tan Y. Analysis of global commonly-used phthalates and non-dietary exposure assessment in indoor environment. Building Environment. (2020) 177:106853. doi: 10.1016/j.buildenv.2020.106853
11. Veiga M, Bohrer D, Nascimento PC, Ramirez AG, Carvalho LM, and Binotto R. Migration of phthalate-based plasticizers from PVC and non-PVC containers and medical devices. J Braz Chem Soc. (2012) 23:72–7. doi: 10.1590/S0103-50532012000100011
12. Saab Y, Oueis E, Mehanna S, Nakad Z, Stephan R, and Khnayzer RS. Risk assessment of phthalates and their metabolites in hospitalized patients: A focus on di- and mono-(2-ethylhexyl) phthalates exposure from intravenous plastic bags. Toxics. (2022) 10:357. doi: 10.3390/toxics10070357
13. Mitra T, Gulati R, Uppal A, Kumari SR, Tripathy S, Ranjan P, et al. Prospecting of exosomal-miRNA signatures as prognostic marker for gestational diabetes mellitus and other adverse pregnancy outcomes. Front Endocrinol. (2023) 14:1097337. doi: 10.3389/fendo.2023.1097337
14. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. (2017) 13:161–73. doi: 10.1038/nrendo.2016.186
15. Zamstein O, Sheiner E, Wainstock T, Landau D, and Walfisch A. Maternal gestational diabetes and long-term respiratory related hospitalizations of the offspring. Diabetes Res Clin Practice. (2018) 140:200–7. doi: 10.1016/j.diabres.2018.03.050
16. Martin L, Zhang Y, First O, Mustieles V, Dodson R, Rosa G, et al. Lifestyle interventions to reduce endocrine-disrupting phthalate and phenol exposures among reproductive age men and women: A review and future steps. Environ Int. (2022) 170:107576. doi: 10.1016/j.envint.2022.107576
17. Mitra T, Gulati R, Ramachandran K, Rajiv R, Enninga EA, Pierret CK, et al. Endocrine disrupting chemicals: gestational diabetes and beyond. Diabetol Metab Syndr. (2024) 16:95. doi: 10.1186/s13098-024-01317-9
18. Bellavia A, Mínguez-Alarcón L, Ford JB, Keller M, Petrozza J, Williams PL, et al. Association of self-reported personal care product use with blood glucose levels measured during pregnancy among women from a fertility clinic. Sci Total Environ. (2019) 695:133855. doi: 10.1016/j.scitotenv.2019.133855
19. Li L, Huang L, Lei R, Zhang P, Yang Y, Liu H, et al. common phthalates, induce glucose metabolism disorders in rats via oxidative damage of PI3K/Akt/GLUT4 signaling. Environ Pollution. (2024) 341:122948. doi: 10.1016/j.envpol.2023.122948
20. Martínez-Pinna J, Sempere-Navarro R, Medina-Gali RM, Fuentes E, Quesada I, Sargis RM, et al. Endocrine disruptors in plastics alter β-cell physiology and increase the risk of diabetes mellitus. Am J Physiology-Endocrinology Metab. (2023) 324:E488–505. doi: 10.1152/ajpendo.00068.2023
21. Liang X, Liang J, Zhang S, Yan H, and Luan T. Di-2-ethylhexyl phthalate disrupts hepatic lipid metabolism in obese mice by activating the LXR/SREBP-1c and PPAR-α signaling pathways. Sci Total Environment. (2024) 914:169919. doi: 10.1016/j.scitotenv.2024.169919
22. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Practice. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
23. Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int. (2019) 123:588–96. doi: 10.1016/j.envint.2018.12.021
24. James-Todd T, Ponzano M, Bellavia A, Williams PL, Cantonwine DE, Calafat AM, et al. Urinary phthalate and DINCH metabolite concentrations and gradations of maternal glucose intolerance. Environ Int. (2022) 161:107099. doi: 10.1016/j.envint.2022.107099
25. James-Todd T, Meeker J, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int. (2016) 96:118–26. doi: 10.1016/j.envint.2016.09.009
26. Lang Q, Qin X, Yu X, Wei S, Wei J, Zhang M, et al. Association of joint exposure to organophosphorus flame retardants and phthalate acid esters with gestational diabetes mellitus: a nested case-control study. BMC Pregnancy Childbirth. (2024) 24:736. doi: 10.1186/s12884-024-06925-x
27. Soomro MH, England-Mason G, Reardon AJF, Liu J, MacDonald AM, Kinniburgh DW, et al. Maternal exposure to bisphenols, phthalates, perfluoroalkyl acids, and trace elements and their associations with gestational diabetes mellitus in the APrON cohort. Reprod Toxicology. (2024) 127:108612. doi: 10.1016/j.reprotox.2024.108612
28. Zukin H, Eskenazi B, Holland N, and Harley KG. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ Res. (2021) 194:110712. doi: 10.1016/j.envres.2021.110712
29. Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int. (2015) 83:63–71. doi: 10.1016/j.envint.2015.05.016
30. Gao H, Zhu Bb, Huang K, Zhu YD, Yan SQ, Wu XY, et al. Effects of single and combined gestational phthalate exposure on blood pressure, blood glucose and gestational weight gain: A longitudinal analysis. Environ Int. (2021) 155:106677. doi: 10.1016/j.envint.2021.106677
31. Robledo CA, Peck JD, Stoner J, Calafat AM, Carabin H, Cowan L, et al. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int J Hyg Environ Health. (2015) 218:324–30. doi: 10.1016/j.ijheh.2015.01.005
32. Martínez-Ibarra A, Martínez-Razo LD, Vázquez-Martínez ER, Martínez-Cruz N, Flores-Ramírez R, García-Gómez E, et al. Unhealthy levels of phthalates and bisphenol A in mexican pregnant women with gestational diabetes and its association to altered expression of miRNAs involved with metabolic disease. Int J Mol Sci. (2019) 20:3343. doi: 10.3390/ijms20133343
33. Liang QX, Lin Y, Fang XM, Gao YH, and Li F. Association between phthalate exposure in pregnancy and gestational diabetes: A chinese cross-sectional study. Int J Gen Med. (2022) 15:179–89. doi: 10.2147/IJGM.S335895
34. EARTH Study Team, James-Todd TM, Chiu YH, Mínguez-Alarcón L, Ford JB, Keller M, et al. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health. (2018) 17:55. doi: 10.1186/s12940-018-0399-5
35. Chen W, He C, Liu X, An S, Wang X, Tao L, et al. Effects of exposure to phthalate during early pregnancy on gestational diabetes mellitus: a nested case–control study with propensity score matching. Environ Sci pollut Res. (2023) 30:33555–66. doi: 10.1007/s11356-022-24454-y
36. Makhubela SD, Kgopa AH, Mokgotho MP, and Shai LJ. Di(2-ethylhexyl)phthalate and type 2 diabetes. Environ Sci: Adv. (2024) 3:1679–97. doi: 10.1039/D4VA00121D
37. Aayush MV, Nikthesh G, Rajmohan D, Ravindran CP, Vasantharekha R, Thangavelu S, et al. Redefining the pathogenesis of Gestational Diabetes Mellitus: The cumulative impact of endocrine disrupting environmental chemicals in key metabolic pathways. Med Hypotheses. (2025) 201:111693. doi: 10.1016/j.mehy.2025.111693
38. Vornic I, Buciu V, Furau CG, Gaje PN, Ceausu RA, Dumitru CS, et al. Oxidative stress and placental pathogenesis: A contemporary overview of potential biomarkers and emerging therapeutics. Int J Mol Sci. (2024) 25:12195. doi: 10.3390/ijms252212195
39. Dharod JM, Labban JD, Tadese H, Flax VL, Black MM, and Ammerman AS. Cyclic formula feeding among infants participating in the special supplemental nutrition program for women, infants, and children. J Nutr. (2024) 154:2284–9. doi: 10.1016/j.tjnut.2024.05.004
40. Zhu W, Shen Y, Liu J, Fei X, Zhang Z, Li M, et al. Epigenetic alternations of microRNAs and DNA methylation contribute to gestational diabetes mellitus. J Cell Mol Med. (2020) 24:13899–912. doi: 10.1111/jcmm.15984
41. Linares-Pineda T, Peña-Montero N, Fragoso-Bargas N, Gutiérrez-Repiso C, Lima-Rubio F, Suarez-Arana M, et al. Epigenetic marks associated with gestational diabetes mellitus across two time points during pregnancy. Clin Epigenetics. (2023) 15:110. doi: 10.1186/s13148-023-01523-8
42. Chan M, Mita C, Bellavia A, Parker M, and James-Todd T. Racial/ethnic disparities in pregnancy and prenatal exposure to endocrine-disrupting chemicals commonly used in personal care products. Curr Envir Health Rpt. (2021) 8:98–112. doi: 10.1007/s40572-021-00317-5
43. Mundstock Dias GR, Freitas Ferreira AC, Miranda-Alves L, Graceli JB, and Pires de Carvalho D. Endocrine disruptors chemicals: impacts of bisphenol A, tributyltin and lead on thyroid function. Mol Cell Endocrinology. (2025) 599:112467. doi: 10.1016/j.mce.2025.112467
44. Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. (2021) 40:606–30. doi: 10.1002/etc.4890
45. Goodyer CG, Poon S, Aleksa K, Hou L, Atehortua V, Carnevale A, et al. A case–control study of maternal polybrominated diphenyl ether (PBDE) exposure and cryptorchidism in canadian populations. Environ Health Perspectives. (2017) 125:057004. doi: 10.1289/EHP522
46. Gestational diabetes mellitus. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4404472/ (Accessed May 21, 2025).
47. Choudhury AA and Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomedicine Pharmacotherapy. (2021) 143:112183. doi: 10.1016/j.biopha.2021.112183
48. Kumari RS, Agrawal P, Yadav VD, Mitra T, Sahu I, and Janardhanan R. Potential role of SLC40A1 and HMOX1 in establishing relation between Gestational Anemia and Gestational Diabetes Mellitus. Indian Heart J. (2024) 76:S60. doi: 10.1016/j.ihj.2024.11.120
49. Mitra T, Yadav VD, Agrawal P, V S, Chandrasekaran A, Kumari RS, et al. Aberrations in the vascular tone among infants born to mothers with gestational diabetes mellitus. Indian Heart J. (2024) 76:S54. doi: 10.1016/j.ihj.2024.11.106
50. Márquez-Valadez B, Valle-Bautista R, García-López G, Díaz NF, and Molina-Hernández A. Maternal diabetes and fetal programming toward neurological diseases: beyond neural tube defects. Front Endocrinol. (2018) 9. doi: 10.3389/fendo.2018.00664
Keywords: gestational diabetes mellitus, phthalates, urine, pregnancy outcomes, systematic review
Citation: Mitra T, Anil Kumar AH Sruthi, Thangaraju R, S. M. SM, Kumari R. S and Janardhanan R (2025) Impact of phthalate exposure on gestational diabetes mellitus: a systematic review. Front. Endocrinol. 16:1638655. doi: 10.3389/fendo.2025.1638655
Received: 31 May 2025; Accepted: 04 September 2025;
Published: 22 September 2025.
Edited by:
Min Nian, Shanghai Jiao Tong University, ChinaReviewed by:
Mojtaba Akbari, Isfahan University of Medical Sciences, IranSlawomir Gonkowski, University of Warmia and Mazury in Olsztyn, Poland
Ruxianguli Aimuzi, Xinjiang Medical University, China
Copyright © 2025 Mitra, Anil Kumar, Thangaraju, S. M., Kumari R. and Janardhanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajiv Janardhanan, cmFqaXZqQHNybWlzdC5lZHUuaW4=
 Tridip Mitra
Tridip Mitra A. H. Anil Kumar Sruthi
A. H. Anil Kumar Sruthi Rithika Thangaraju1
Rithika Thangaraju1 Shakthi Mani S. M.
Shakthi Mani S. M. Rajiv Janardhanan
Rajiv Janardhanan