- Department of Pediatrics, Endocrinology, and Diabetology with Cardiology Division, Medical University of Bialystok, Białystok, Poland
Type 1 diabetes (T1D) in children is a serious, chronic, incurable disease associated with the frequent and early occurrence of additional, well-known cardiovascular risk factors and exacerbation of the risk of future cardiovascular diseases (CVD). Lately, accumulating evidence suggests that exosomal miRNAs play a major role not only in the pathophysiology of T1D but also in its late complications. Since premature CVD is one of the leading causes of morbidity and mortality in diabetes, considerable efforts have been made to define the molecular and pathological features and to develop new diagnostic and therapeutic strategies. Dysregulation of the expression or function of various miRNAs may affect angiogenesis, vascular inflammation, or cardiac remodeling, which play key roles in the development and progression of cardiovascular complications. While CVD usually appear in adulthood, pathology and early markers may appear during adolescence, emphasizing the need for careful monitoring and prevention in this age group. In this narrative review, we aimed to summarize the latest findings on miRNAs and their role as biomarkers of cardiovascular risk factors and subsequent complications in children with T1D, presenting promising candidates for clinical applications.
1 Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease, increasingly common in the pediatric population, characterized by a progressive destruction of insulin-secreting β-cells of the pancreas (1, 2). As a result of loss of their function, patients are completely dependent on exogenously administered insulin to maintain normal blood glucose levels. It is common knowledge that long-term excessively high glucose concentrations eventually lead to various complications such as cardiovascular disease, diabetic retinopathy, neuropathy, renal failure, hearing impairment or more (3). Therefore, it is important to maintain glycemic control, appropriate for children and adolescents, within the recommended limits of glycated hemoglobin (HbA1C) <7% (<53 mmol/mol) and to spend more than 70% of the time in range (TIR) (70 – 180 mg/dL [3.9 – 10.0 mmol/L]) as measured by continuous glucose monitoring (CGM) to lower the hazard and incidence of macrovascular and microvascular complications (4). For selected individuals, an even more stringent HbA1C target of <6.5% (<48 mmol/mol) is suggested. Although the above principles help to reduce the risk or delay the occurrence of later diabetic macrovascular complications, unfortunately they cannot always be prevented. Diabetic angiopathy refers to a group of vascular diseases that develop as a consequence of diabetes and include damage to blood vessels, both small (microangiopathy) and large (macroangiopathy), caused by high blood sugar levels. We can distinguish an early, preclinical phase, in which patients often exhibit no clinical symptoms, followed by the development of measurable retinal, renal, or nerve damage, under the influence of persistent hyperglycemia, as well as endothelial dysfunction (ED), thickening of the carotid intima media, and atherosclerosis, ultimately associated with an increased risk of heart attack, stroke, or lower limb amputation. Childhood T1D is associated with an increased incidence of additional risk factors already at a young age, such as excess body weight, further aggravated by insulin therapy, lipid profile disorders or hypertension, which, cumulatively, may lead to a type of metabolic syndrome (5). In addition, early vascular changes in the form of endothelial dysfunction, arterial stiffness or increased carotid intima-media thickness (cIMT) have been demonstrated in children with type 1 diabetes (6, 7). All of the above-mentioned causes may contribute to further macrovascular complications, including peripheral artery disease (PAD), coronary artery disease (CAD) leading to myocardial infarction or cerebrovascular disease. Currently, known risk factors and previously studied biomarkers do not fully explain the pathophysiology, frequency of vascular changes, and reduced life expectancy in type 1 diabetes. Knowledge of the molecular mechanisms underlying the pathogenesis of diabetic micro- and macroangiopathy is extremely important for prevention, delaying the onset and, in particular, identifying new targets for the treatment of cardiovascular complications of diabetes. In the pursuit of precision medicine, new biomarkers are sought, which emerge to be miRNAs.
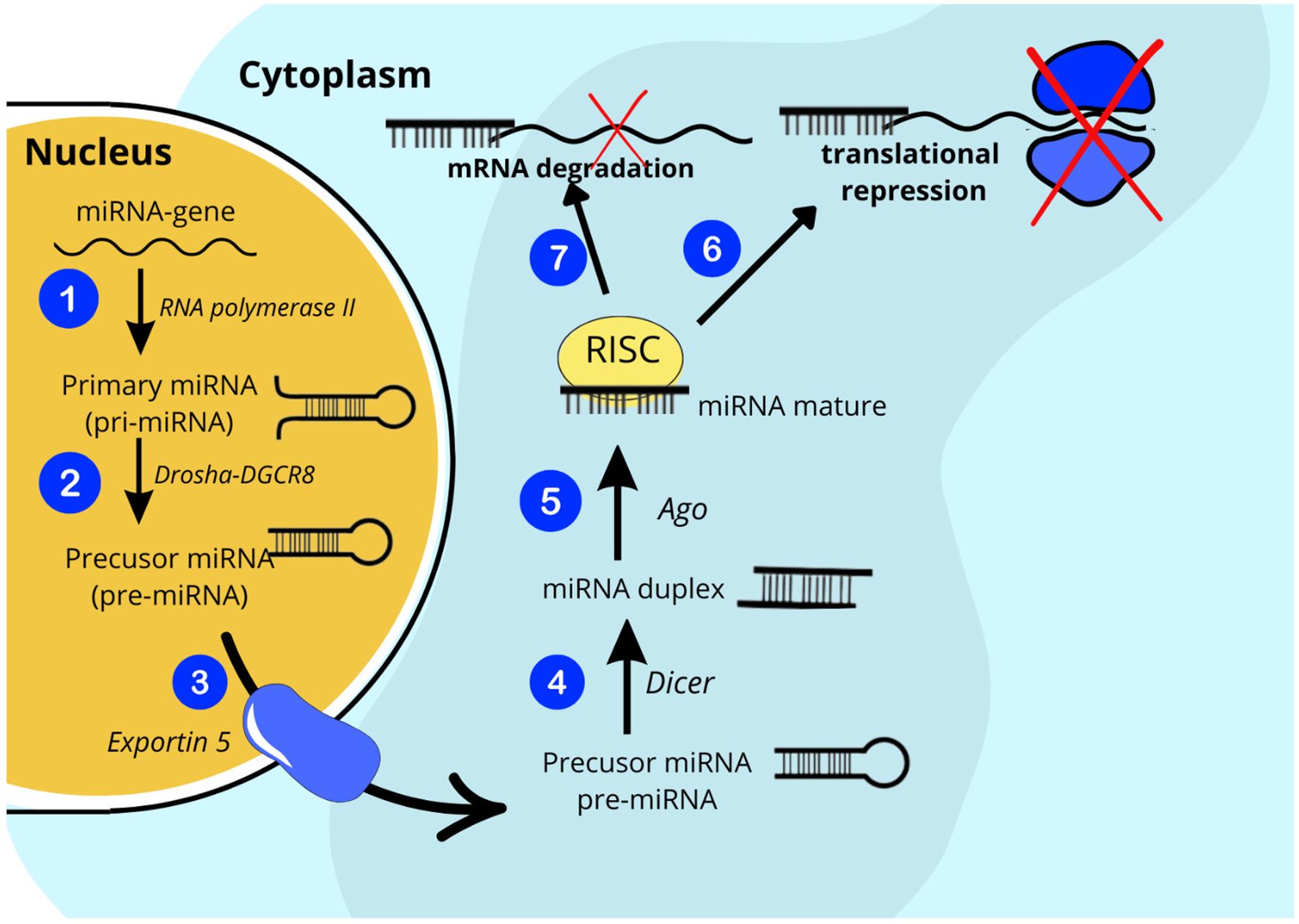
Recently, accumulating evidence suggests that exosomal miRNA plays a tremendous role not only in the pathophysiology of T1D but also in its late complications (8, 9). Exosomes are nanometer-sized bilayer extracellular vesicles (EVs) (30 – 200 nm in diameter) that can be secreted by virtually all cell types in response to internal or external stimuli (10). Once secreted, EVs gain access to the interstitial space and ultimately to the circulation, where they exert local paracrine or distal systemic effects. The number and molecular cargo of exosomes are tightly regulated by physiological and pathological factors. For these reasons, EVs are important components involved in intercellular and interorgan communication via their contents, such as proteins, lipids, and RNAs, including microRNAs, which are attributed to the greatest share of biological effects of exosomes (11). MiRNAs are a class of small (~ 22 nucleotides in length) single-stranded non-coding RNAs that act as regulators of protein-coding genes. By binding to target messenger ribonucleid acids (mRNAs), they have the ability to modulate gene expression at the posttranscriptional level by facilitating sequence-specific RNA interference, either by inhibiting translation or by promoting mRNA degradation (Figure 1) (12, 13). To date, more than 2,600 different mature human miRNAs have been reported in the miRBase database (14). Surprisingly, together they may have the capacity to regulate even about 30% of all genes in the human genome. Bioinformatic predictions indicate that one miRNA can influence changes in more than a hundred mRNAs and that a single mRNA can be regulated by many different miRNAs. It has been presented that miRNA expression levels are tissue-specific, stable in body fluids even long after sample collection and freeze-thaw cycles, and can be collected minimally invasively from readily accessible biofluids, making miRNAs promising candidates for biomarkers enabling accurate diagnosis and prognosis of the progression of a wide range of diseases (15–18). On the other hand, the reproducibility of miRNA biomarker assays is often limited due to the combined influence of biological and technical factors, including limited understanding of the normal range of miRNA expression in different body fluids and the lack of disease specificity of some miRNAs, inconsistent sample preparation, which may affect molecular stability or measurement techniques, or the lack of standardized data normalization across studies, which makes it difficult to translate research results into clinical practice (19).
Cardiovascular disease (CVD) is considered to be one of the leading causes of morbidity and mortality in young and older adults with type 1 diabetes and is more common in this population compared to healthy individuals (20), which is why extraordinary efforts have been made to define the molecular and pathological features of the diseased heart and vasculature as well as to develop novel diagnostic and therapeutic strategies. MiRNAs have been associated with nearly all cardiovascular diseases in which they have been studied, including heart failure, cardiac hypertrophy, post-myocardial infarction remodeling, arrhythmia, atherosclerosis, atrial fibrillation, and peripheral artery disease. Dysregulation of the expression or function of various miRNAs may affect angiogenesis, vascular inflammation, or cardiac remodeling, which play a key role in the development and progression of cardiovascular complications (21).
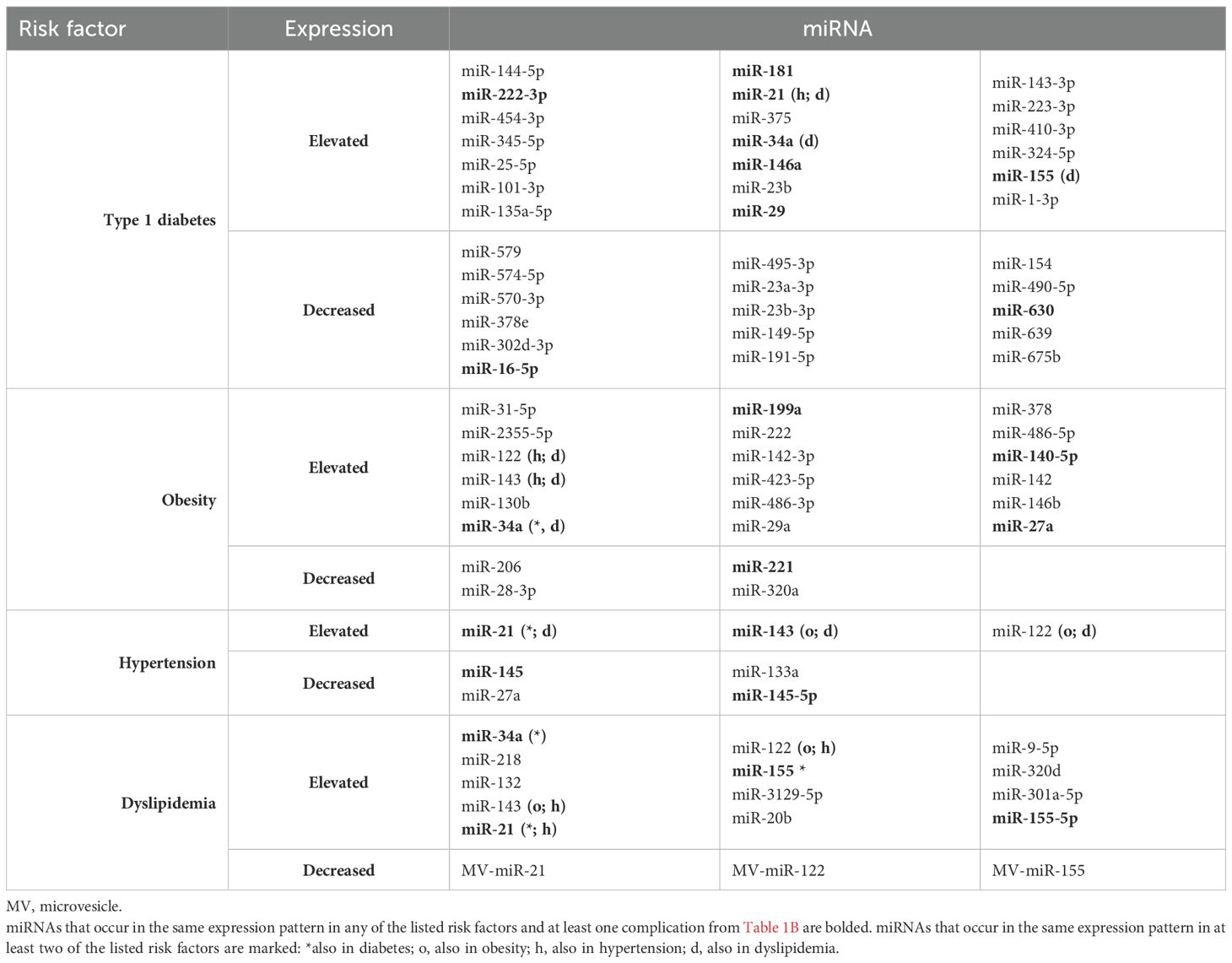
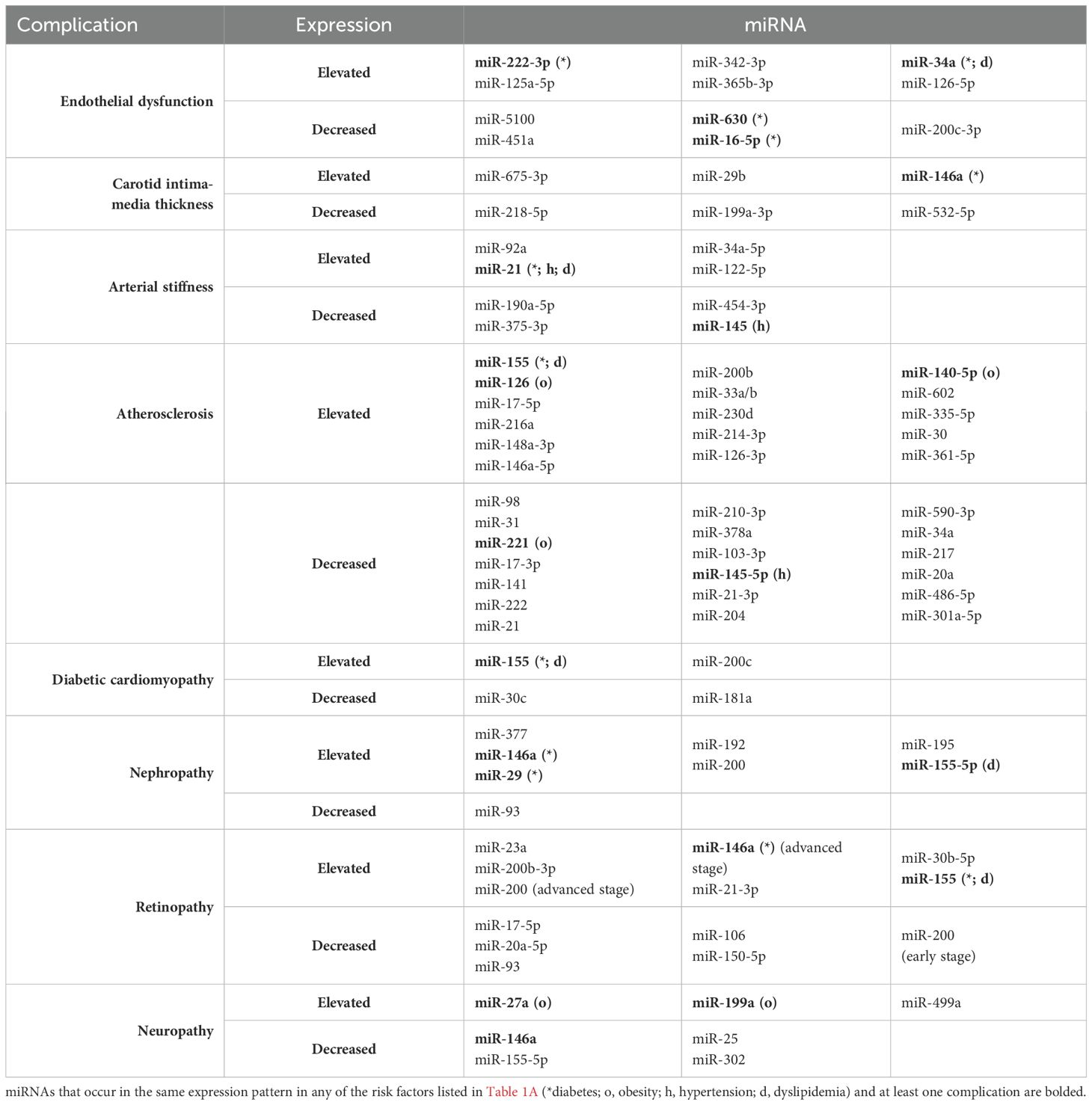
We would like to emphasize that the use of miRNA determination could become a routine approach in the prognosis, early diagnosis and monitoring the progression of cardiovascular complications and would also provide the development of better therapeutic strategies, implementing precision medicine conception. In the following review, we aimed to discuss the latest findings regarding miRNAs and their possible role as biomarkers in cardiovascular complications in children and adolescents with type 1 diabetes mellitus. We have decided to focus on highlighting the most relevant miRNAs that have been described in many studies and seem promising for clinical applications in this specific group of patients. The miRNAs described in this article and their expression in individual risk factors and complications are summarized in Table 1A (risk factors) and Table 1B (complications).
2 Type 1 diabetes as a cause of cardiovascular complications in pediatric population
Type 1 diabetes is undoubtedly a serious factor influencing the occurrence and development of cardiovascular complications in children and adolescents (22). The most unfavorable factors influencing diabetes complications are early age of onset and long duration of the disease. For patients diagnosed before the age of 10, life expectancy was reduced by almost 18 years for women and about 14 years for men (20). The most important cardiovascular complications of childhood diabetes include ED, increased cIMT, aortic stiffness, and progressive atherosclerosis, which may lead to an increased risk of myocardial infarction or stroke in early adulthood (6). Also worth mentioning are, diabetic cardiomyopathy, and microvascular complications such as retinopathy, neuropathy and nephropathy.
In recent years, many miRNAs have been studied for the prognosis of childhood T1D. Among the studied miRNAs that would play a key role in accurate prediction of diabetes, miR-146a was highlighted as the most important, followed by miR-29, along with miR-17, miR-20, miR-24, miR-126, miR-133, miR-210, miR-223, miR-320, miR-375 (23, 24). Moreover, significantly increased levels of miR-144-5p, miR-222-3p, miR-454-3p and miR-345-5p were found in sera of children with recently diagnosed T1D compared to the non-diabetic control group (25). In relation to long-term disease in children, miR-25-5p was shown to be increased, while miR-579, miR-574-5p, miR-570-3p, miR-378e, miR-302d-3p and miR-16-5p were decreased in these patients (26). Six more miRNAs were identified with differential levels between young people with long-term illness and healthy controls; miR-101-3p, miR-135a-5p, miR-143-3p, miR-223-3p, and miR-410-3p were upregulated, and miR-495-3p were downregulated (27). In direct comparison with healthy peers, miR-181 overexpression was also found in T1D patients, and due to its negative correlation with C-peptide levels, studies indicate that it may play a significant role in pancreatic β-cell dysfunction (28). Another study indicated that doubling of miR-197-3p at 3 months was the strongest predictor of residual beta-cell function 12 months after diagnosis in children with T1D and corresponded to a six-fold higher stimulated C-peptide level (29). Moreover, doubling of miR-24-3p and miR-146a-5p at 3 months corresponded to a 4.2% and 3.5% lower insulin-corrected HbA1c level at 12 months, respectively. It has been proven that the preservation of C-peptide may play a substantial role in delaying the occurrence of vascular complications in type 1 diabetes (30), therefore, the miRNAs discussed above may be of great importance for analyzing the possibility of maintaining residual β-cell secretion in long-term disease and thus for preventing its complications. However, it is still unknown which panel of miRNAs may have a protective effect in chronic childhood diabetes, or which miRNA expression may negatively influence the acceleration of the development of cardiovascular diseases in later life.
β-cells in the pathogenesis of type 1 diabetes are continuously exposed to inflammatory cytokines influencing their apoptosis. After exposure to interleukin-1b and interferon-γ, miRNA expression assessment showed increased levels of miR-21, miR-23b, miR-34a, and miR-146a, whereas miR-23a-3p, miR-23b-3p, and miR-149-5p were decreased (31). Other studies suggest that increased expression of miR-155 in immune cells may also contribute to β-cell death in type 1 diabetes (8). Studies indicate that miR-21 influences macrophage metabolism, resulting in a shift towards oxidative phosphorylation in naive and inhibition of glycolysis in pro-inflammatory macrophages, which plays a key role in the inflammatory response mediated by them (19). Circulating plasma miR-1-3p and miR-16-5p have also been found to directly influence genes associated with cardiovascular pathologies, such as GOSR2, ANK3, MTMR3 (32) and thus, in combination with glycemic control, may potentially serve as prognostic biomarkers to help prevent the development of these particular complications. Bellini et al. found reduced levels of miR-191-5p in diabetic patients compared with controls, as well as an inverse correlation between the levels of this molecule and chronic vascular complications of diabetes (33).
Hyperglycemia itself, which is both a symptom and a consequence of diabetes, induces a series of pathological changes in small vessels, arteries and peripheral nerves and causes tissue damage in endothelial cells by increasing reactive oxygen species. Moreover, hyperglycemia-induced oxidative stress promotes LDL oxidation, which is associated with increased cIMT in youths with T1D and poor glycemic control (34), and promotes atherosclerosis in several ways. In the study by Erener et al., HbA1c levels significantly negatively correlated with miR-324-5p, which was present in high levels in the serum of children with newly diagnosed type 1 diabetes, while, interestingly, they showed a positive correlation with miR-154, miR-490-5p, miR-630, miR-639, miR-675b, which expression in serum was low (25). It can therefore be suspected that these miRNAs may be released from endothelial cells in a hyperglycemia-induced process, reflecting early tissue damage.
As can be seen, miRNAs play a key role in pancreatic β-cell function, regulating their response to immunological, inflammatory, and metabolic factors. This highlights the importance of miRNAs as potential biomarkers in the diagnosis, prediction, and progression of diabetes complications, as well as therapeutic targets.
3 Risk factors for early vascular changes in childhood type 1 diabetes and future cardiovascular complications
Not only diabetes itself, but also comorbidities such as obesity, hypertension or dyslipidemia, the incidence of which is much higher in children with T1D than in their healthy peers, constitute additional risk factors provoking the development of later cardiovascular complications. In this paragraph, we would like to focus on the miRNA alterations that occur in the above-mentioned conditions and may influence early vascular changes that can become apparent already at a young age, ultimately leading to cardiovascular diseases in patients with diabetes.
3.1 miRNAs in obesity and obesity-mediated inflammation
Obesity has become a growing problem among children, especially those with type 1 diabetes, and is a documented risk factor for many diseases, including metabolic and cardiovascular disorders (35). Both diabetes and obesity alone are undisputed threats for cardiovascular health of the population, consequently the overlap of the two above conditions magnified the likelihood of complications (36). A multicenter, longitudinal study found that a large proportion of young people with type 1 diabetes who were overweight or obese during adolescence had increased body weight also in adulthood (37). Research suggests that, as in adulthood, elevated body mass index (BMI) in childhood and adolescence appears to be a key factor in higher cardiovascular risk in later life (38, 39). In adolescents and young adults with diabetes, central obesity may contribute to the development of hypertension (40). In the DCCT, excessive weight gain was associated not only with increased blood pressure but also with dyslipidemia and, in the follow-up study, with more extensive atherosclerosis (41). Furthermore, in adolescents with type 1 diabetes, the only modifiable cardiovascular risk factor that predicted cIMT was BMI z-score (42).
The regulation of adipocyte differentiation, lipid metabolism, and energy homeostasis has been shown to be mediated by miRNAs. Furthermore, excessive nutrition can alter the expression of miRNAs in adipocytes, leading to abnormal adipose tissue function (43). Studies show that circulating miRNAs are differentially expressed in obese/overweight children compared to their normal weight peers. One study found a two-fold augmentation in miR-31-5p, a three-fold augmentation in miR-2355-5p, and a 0.5-fold decline in miR-206 expressions in corpulent children (44). A significant increase in the concentration of miR-122, miR-143 and miR-199a was also observed in obese children compared to the control group (45). Additionally, miR-200c-3p, miR-190a, and miR-95 showed differential regulation in obese preschool children with insulin resistance both in the fasting state and 2 hours after an oral glucose tolerance test, potentially acting as biomarkers of insulin resistance in this group (46). The results presented in the review by Oses et al. unveil that miR-222, miR-142-3p, and miR-140-5p are significantly overexpressed in children and adolescents with obesity (47). Moreover, decreased levels of miR-28-3p and miR-221 as well as increased levels of miR-130b, miR-142-3p, miR-423-5p, miR-486-3p and miR-486-5p may prove to be novel biomarkers for estimating the risk of childhood obesity. Both miR-142-3p and miR-486-5p inhibit the expression of the transcription factor FoxO1 (48), which happens to be one of the main mediators of triglyceride metabolism and/or insulin action (49). Elevated plasma miR-486 and miR-142 levels in obese children may be involved in the development of obesity complications such as subclinical inflammation, insulin resistance, and hypertension in later life. Reduced levels of circulating miR-28-3p and miR-221 have been detected in obese children, which seem to be responsible for compensatory mechanisms that reduce the metabolic aberrations of obesity. It has been shown that miR-28-3p is negatively correlated with adiponectin levels, therefore its lower expression may to some extent induce the production of this antidiabetic adipokine, which may have a positive effect not only on insulin sensitivity in prepubertal age but also ultimately protect against diabetes in adulthood (48). In the case of miR-221, its decreased levels were also found in adults with morbid obesity, whereas its increased expression was shown after surgical intervention (50).
In addition, studies indicate that obesity may play a role in increasing the secretion of proinflammatory cytokines, causing chronic low-grade inflammation, as well as the potential role of miRNAs in this process. In a study conducted on a pediatric population, compared to the control group, obese children had increased levels of L - 6, hsCRP, and TNF-α, which positively correlated with levels of miR-29a and miR-122 (51). miR-378 as well as miR-146b may prove to be potential novel targets in controlling inflammation in adipose tissue, as increased expression of both was observed in response to IL - 6 and TNF-α in mature human adipocytes (52, 53). Similarly, in mice studies, TNF-α, the level of which increases already at a very early stage of obesity, upregulates miR-34a, and furthermore, miR-34a expression in visceral fat gradually rises with the development of obesity, while its selective deletion protects against the exacerbation of meta-inflammation induced by dietary stress (54). An increase in miR-27a levels was also observed along with aggravated expression of pro-inflammatory cytokines, macrophage influx and M1 macrophage polarization (55).
3.2 miRNAs in hypertension
Children with type 1 diabetes are more susceptible to hypertension than their healthy peers. Higher blood pressure can consequently lead to many microvascular complications and is also a major risk factor for serious macrovascular diseases, including cardiovascular diseases. There are many studies on hypertension and its associated miRNAs, although few studies specifically focus on the pediatric population. A study conducted on 22 children with hypertension showed that increased expression of miR-145 after exercise negatively correlated with both systolic and diastolic blood pressure (56). On the other hand, experimental work in rats suggests an unfavorable role of miR-145 in the development of systemic hypertension and may be involved in epigenetic changes influencing hypertension-induced organ damage (57). In adult patients with hypertension, miR-21 levels have been shown to be elevated and positively correlated with systolic and diastolic blood pressure, CRP levels, and parameters reflecting asymptomatic organ damage, such as microalbuminuria and cIMT (58), which may contribute to its potential as a biomarker heralding this damage without prior clinical manifestation. In another study, Nemecz et al. found a negative correlation between diastolic blood pressure and both plasma miR-143 and miR-122 concentrations (59). Moreover, serum concentrations of miR-27a and miR-133a were lower in individuals with newly diagnosed hypertension compared with normotensive individuals, making them potential biomarkers for predicting or preventing hypertension (60). In the study by Barruta et al. conducted on a large group of individuals with long-term type 1 diabetes, the level of miR-145-5p in serum was significantly lower in hypertensive patients than in normotensive patients and inversely correlated with hypertension, regardless of age, sex and duration of diabetes (61). Nevertheless, more research is needed to determine whether the above considerations are confirmed in the pediatric T1D population.
3.3 miRNAs in dyslipidemia
Dyslipidemia is an immense problem in children and adolescents with T1D. A recent study showed that the prevalence of dyslipidemia was 67.3%, with HbA1c being the most important modifiable factor determining its occurrence (62).
Both small and large blood vessels can be affected by lipid imbalances, increasing the risk of atherosclerotic plaque formation and subsequent cardiovascular disease. miR-34a expression was higher in patients with type 1 diabetes and dyslipidemia compared with those without dyslipidemia and showed a strong independent association with total cholesterol and low-density lipoprotein cholesterol (63). Overexpression of miR-34a is thought to be associated with the severity of several dyslipidemia-related disorders, e.g., nonalcoholic fatty liver disease or coronary artery disease (64, 65). In addition, the expression of miR-218, miR-132, and miR-143 was found to be significantly increased in both circulating microvesicles (MV) and plasma of diabetic patients with dyslipidemia (59). Furthermore, three more miRNAs in MV were downregulated: miR-21, miR-122, and miR-155, while the same plasma miRNAs were increased in diabetic dyslipidemia. Moreover, MV-miR-21 positively correlated with plasma cholesterol levels, MV-miR-122 negatively correlated with LDL-C levels, and MV-miR-155 positively correlated with cholesterol levels and negatively with HDL-C levels (59). Macrophage cholesterol efflux capacity has been identified as a predictor of cardiovascular diseases. Interestingly, changes in this process in adolescents are not associated with BMI (occurring in both obese and non-obese youths), inflammation or insulin resistance and occur before the main changes in lipid profiles, and may be partially driven by adipocyte-derived EV miRNAs, in particular miR-3129-5p miR-20b, miR-9-5p, miR-320d, miR-301a-5p, miR-155-5p (66).
4 Early preclinical vascular changes in childhood type 1 diabetes
4.1 miRNAs in endothelial dysfunction
Endothelial dysfunction (ED) refers to the loss of normal homeostatic function of blood vessels and is characterized by altered vasodilatory/constrictive functions and increased inflammatory activity. It is associated with the development of vascular complications such as hypertension, coronary artery disease, and chronic heart failure. The main factors responsible for the pathogenesis of ED in T1D are uncontrolled hyperglycemia, glycemic variability and low endogenous insulin concentration. All of the above lead to the generation of multiple biochemical factors, increased expression of classes of genes associated with inflammation and cellular stress, which disrupts the structural and functional integrity of the endothelium of vascular structures, thereby increasing progressive low-grade inflammation, inhibiting endothelium-dependent relaxation, and causing potential susceptibility to apoptosis (67). Data suggest that endothelial dysfunction and changes in peripheral vascular dynamics may occur as early as 5 years after the onset of T1D (68). Numerous miRNAs have been studied with the potential to play a significant role in regulating endothelial cell function, inflammation, and vascular integrity, making them potential indicators of endothelial health. Increased levels of circulating miR-222-3p have been shown to cause endothelial dysfunction and were found to be elevated in hyperglycemia (25), which may serve as a useful biomarker to monitor the effects of glycemic control on the vascular endothelium. Khalyfa et al. identified three miRNAs previously associated with cardiomyopathy, namely miR-125a-5p, miR-342-3p, and miR-365b-3p, as potential biomarkers encompassing three biological pathways that may have pathophysiological relevance to abnormal endothelial function in children (69). The researchers also found that in children with ED, the levels of miR-5100, miR-451a, miR-630, and the previously mentioned miR-16-5p were reduced, while after effective treatment there was a significant increase in the levels of the latter two miRNAs to the levels found in healthy individuals. Conversely, transfection of exosomes from individuals without evidence of endothelial dysfunction with a miR-630 inhibitor provoked a functional phenotype of endothelial dysfunction. Gene target discovery experiments conducted by this research group additionally demonstrated that miR-630 regulates as many as 416 gene targets in endothelial cells, including nuclear factor erythroid 2-related factor 2 (Nrf2), AMP-kinase, and tight junction pathways (70). Other miRNA, videlicet miR-34a, showed higher levels in children with T1D compared to their healthy peers in relation to serum endoglin and intercellular adhesion molecules (ICAM) (63), which are known to be involved in, among others, angiogenesis, vascular remodeling and endothelial cell activation (Figure 2).
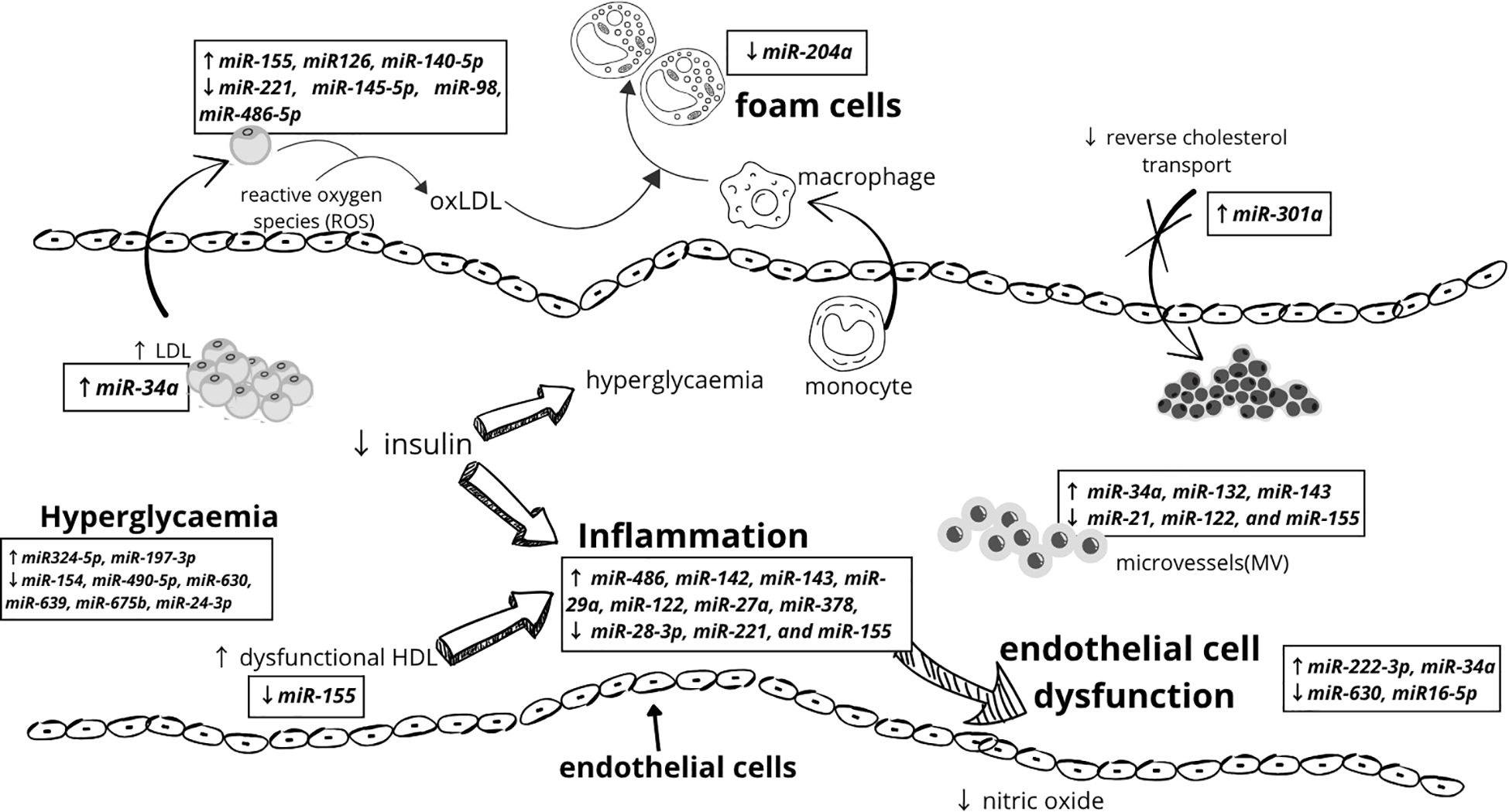
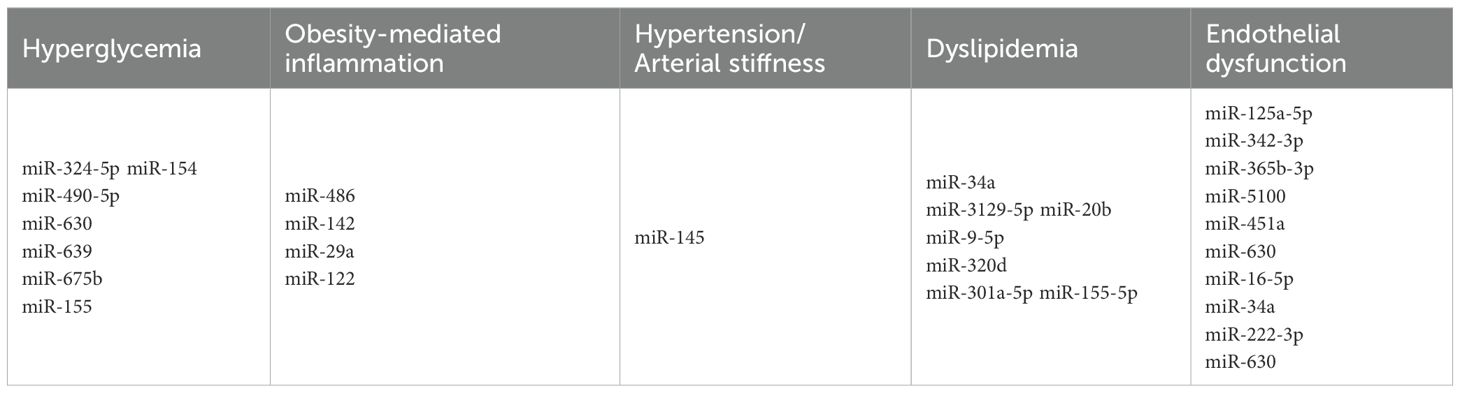
Figure 2. Mechanisms underlying cardiovascular diseases early pathogenesis in children with type 1 diabetes and miRNAs involved in the process.
Bakhashab et al. concluded that miR-200c-3p may be an early marker of subclinical ED in T1D patients, finding that it was significantly reduced in peripheral blood mononuclear cells of these patients and negatively correlated with HbA1c, interleukin-7, vascular endothelial growth factor-C, and soluble vascular cell adhesion molecule-1, whereas positively correlated with many markers of vascular health: circulating endothelial progenitor cells as well as proangiogenic cells (71). Furthermore, overexpression of miR-200c in nondiabetic endothelial cells leads to reduced endothelium-dependent relaxation, and inversion of this effect was achieved with anti-miR-200c in diabetic mice, thus postulating that it could be a potential target for intervention in vascular diseases (72). The last miRNA we want to discuss that has been shown to have proangiogenic and cardioprotective effects is miR-126-5p. Its lower expression has been shown to be associated with better vascular health, whereas its upregulation, likely enhanced by hyperglycemia and inflammation in T1D, may be a compensatory response (73).
4.2 miRNAs in carotid intima-media thickness
CIMT, i.e. the thickness of the common carotid arteries, is considered an important indicator of vascular complications in type 1 diabetes, which is measured primarily by ultrasound (74). Studies show that this marker is often significantly elevated in children with diabetes due to the pathogenesis of long-term hyperglycemia compared to healthy people of a similar age, and the highest results occur in the group with pre-existing vascular complications and in children with obesity (6). It has also been shown that the occurrence of insulin resistance and high fasting glycemia intensifies the increase in cIMT (74). Additionally, boys show higher cIMT values compared to girls, especially in late adolescence. Increased cIMT is associated with an increased risk of stroke and heart attack already in young adulthood (75). Studies have shown a strong correlation of cIMT with the expression of circulating miRNA, due to increased activation of the pathways regulating the activity of vascular endothelium and reduced regulation of its apoptosis. According to studies, the occurrence of miR-675-3p currently correlates most with atherosclerotic loss (76). MiR-29b and miR-146a may also prove to be potentially useful in diagnostics (75). On the other hand, miR-218-5p and miR-199a-3p (77) negatively correlate with cIMT (78) and miR-532-5p (79) demonstrated even in the asymptomatic phase of atherosclerosis. These miRNAs might serve as potential biomarkers for detecting early stages of atherosclerosis before clinical symptoms appear.
4.3 miRNAs in arterial stiffness
Young patients with type 1 diabetes have been found to have increased arterial stiffness compared to healthy individuals (80), which is considered a prognostic factor for developing atherosclerosis and an independent predictor of all-cause and cardiovascular mortality in later life. The primary effect of arterial stiffening is a loss of compliance and buffering potential, which results in increased pulse wave propagation and accelerates tissue damage in organs with numerous low-resistance capillaries, namely the kidneys, heart or brain, because they are more sensitive to pressure oscillations and more susceptible to damage from excessive, unbuffered pulsation of large arteries (81). Commonly used non-invasive methods of assessing arterial stiffness, including pulse wave velocity (PWV) and augmentation index (AIx), have been reported to predict future cardiovascular events. Importantly, a significant increase in PWV of 0.145 m/s/year over only a 5-year period was observed in adolescents with type 1 diabetes (82). Although the exact mechanism of early arterial remodeling toward stiffer walls remains unclear, additional information suggests an association between PWV in T1D and the additional cardiovascular risk factors mentioned earlier. Less commonly used magnetic resonance imaging (MRI) techniques can directly assess central aortic stiffness by measuring hemodynamic parameters. Of possible clinical importance is the growing evidence suggesting that MRI-based central aortic stiffness may be reversible in response to medical intervention or exercise (83, 84), hence it is worthwhile to explore new methods that can reverse these vascular changes. It has been speculated that circulating levels of miR-92a may be an important biomarker and potential therapeutic target for both hypertension and arterial stiffness. In an adult study, extracellular endothelium-derived miR-92a, which levels were positively correlated with PWV, may play an active role in promoting arterial stiffness by regulating the communication between endothelial cells and vascular smooth muscle cells. Furthermore, exogenously administered to mice locked nucleic acid-modified antisense miR-92a ameliorated angiotensin II-induced hypertension and arterial stiffness (85). In a study evaluating children with hypertension, an increase in miR-145 expression following physical exercise was described, which negatively correlated with 24-h peripheral PWV (56). Recently, in adults, reduced miR-21 expression was observed to lead to lower arterial stiffness in patients with hypertension at baseline as well as after antihypertensive treatment, regardless of blood pressure (86). Both studies offer hope for enabling the use of miRNA as a potential prognostic marker and future therapeutic target. In individuals over 50 years of age, higher expression of miR-190a-5p, miR-375-3p, and miR-454-3p was associated with lower PWV, whereas miR-34a-5p and miR-122-5p were associated with higher PWV (87).
5 Threatening future cardiovascular complications in type 1 diabetes
Various studies confirm that the incidence of cardiovascular risk factors is elevated among children and adolescents with diabetes, and in many cases these factors are already present at the time of diagnosis (88). Diabetes escalates the risk of cardiovascular disease, premature cardiovascular mortality, by at least twofold, and all-cause mortality by fourfold in young people. Furthermore, cardiovascular outcomes and mortality are inversely correlated with age at onset of diabetes; suggesting that the earlier the onset, the greater the risk (20). The additional comorbidity of obesity increases the risk of both disease-specific and all-cause mortality (89). In this chapter, we will discuss and attempt to summarize recent findings on cardiovascular complications of diabetes that seem to be regulated by miRNAs.
5.1 miRNAs in atherosclerosis
Atherosclerosis is a pathological process characterized by the accumulation of atherosclerotic plaques within the arterial walls, leading to their progressive hardening and luminal narrowing. This process may commence significantly earlier than commonly assumed - often during childhood or even in fetal development (90). While severe cardiovascular complications, such as CAD, manifest later in life, the underlying pathogenic mechanisms are initiated at an early age (91).
Numerous miRNAs regulate metabolic pathways associated with plaque formation. Thus, early identification of these biomarkers may help prevent adverse consequences, including myocardial infarction (90), which have been documented already in the second decade of life (92). In newborns and children in the first decade of life, the early stages of atherosclerosis are characterized by the presence of fatty streaks (93). These changes have even been demonstrated in the aorta and coronary arteries (94). As a consequence, this leads to vascular stiffening and accelerated vascular aging (92, 93). Significantly, the presence of fatty streaks is often observed despite normal blood pressure, BMI and cholesterol levels (95).
The initial phase of atherosclerosis involves the accumulation of oxidized low-density lipoprotein (oxLDL) within the subendothelial layer, regulated by miR-155 and miR-148a-3p, miR-146a-5p (96). The effect of miR-155 is expression-dependent, with low expression exerting an anti-atherosclerotic effect (97–99). Conversely, miR-98 inhibition enhances the secretion of chemokines and adhesion molecules in human umbilical vein endothelial cells (HUVECs) by suppressing HMGB1 expression (100). These processes are counteracted by miR-31, which regulates E-selectin expression, and miR-221, miR-17-3p, miR-141, and miR-222, which inhibit ICAM - 1 expression, collectively reducing leukocyte adhesion (96, 101).
A particularly intriguing biomarker is miR-126, which exerts dual effects: it offers protective benefits by inhibiting VCAM - 1 expression, yet excessive expression may promote angiogenesis and plaque instability (17, 96, 97).
Following this stage, unstable plaque formation occurs. Several miRNAs contribute to this process, including miR-17-5p, which modulates macrophage proliferation and apoptosis (17, 96); miR-155, which activates TNF-alfa and NLRP3 inflammasomes, exacerbating inflammation and plaque destabilization (101); and miR-216a, which promotes M1 macrophage polarization by activating telomerase in the Smad3/NF-κB pathway (102). Additionally, miR-200b is activated under hypoxic conditions, leading to eNOS destabilization, reduced nitric oxide (NO) levels, and vasoconstriction (97). Furthermore, miR-21 deficiency increases apoptosis and necrosis within the atherosclerotic plaque (102).
Conversely, miR-210-3p exerts protective effects by inhibiting lipid accumulation and inflammation through IGF2 suppression. miR-378a also regulates macrophage phagocytosis via the CD47-SIRPα pathway (102). Lipid metabolism dysregulation significantly contributes to atherosclerosis progression. miR-33a/b enhances lipid accumulation, impairs apoptotic cell clearance, and reduces cholesterol uptake by downregulating CYP7A1 and CYP8B1 (96, 102). Similarly, miR-230d promotes lipid accumulation within the vascular walls. In contrast, miR-103-3p protects endothelial cells via the Notch1 pathway (103); miR-145-5p supports vascular smooth muscle cells (VSMCs) by reducing their proliferation and migration (96). miR-21-3p – also stimulates VSMC (104); and miR-204 inhibits foam cell formation by reducing SR-A and CD36 expression (104, 105).
Oxidative stress, driven by excessive reactive oxygen species (ROS) production, also plays a crucial role in atherogenesis. For instance, miR-214-3p inhibits GPX4 activity, miR-140-5p and miR-602 influence Nrf2 and SIRT2 expression, while miR-335-5p downregulates SIRT7, all contributing to disease progression (106). Additionally, excessive miR-30 expression has been associated with carotid atherosclerosis in hypertensive patients (17). MiR-20a and miR-486-5p shield endothelial cells from oxLDL-induced apoptosis, while miR-301a-5p modulates ABCA1/ABCG1 transporter expression, facilitating cholesterol efflux from macrophages (106). Notably, specific miRNAs have been linked to distinct pathological conditions. For example, miR-126-3p has been frequently associated with albuminuria (107), whereas elevated miR-361-5p levels have been observed in patients with acute myocardial infarction (17), including young individuals in their second decade of life, among whom substantial atherosclerotic lesions had already been identified during the first decade of life (94).
5.2 miRNAs in diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is a condition that refers to the structural and functional changes of the heart muscle associated with diabetes, including ventricular hypertrophy, myocardial fibrosis, and reduced heart function, and even heart failure. Type 1 diabetes causes initially impaired diastolic function, then subclinical systolic function with preserved ejection fraction (EF), progressing to heart failure with reduced EF. Children and adults with type 1 diabetes exhibit early signs of cardiomyopathy despite normal EF, because it is a crude marker of ventricular function that declines only late in the disease process. EF may be maintained by myocardial remodeling, despite the presence of significant reductions in longitudinal myocardial shortening located in the subendocardium, which is more susceptible to ischemic injury due to coronary microvascular dysfunction, commonly associated with hyperglycemia and insulin resistance (108). Early detection of diastolic dysfunction and subclinical systolic dysfunction is a priority, as both early pathologies strongly predict poor clinical outcomes (109). DCM occurrence is independent of other risk factors, such as hypertension or coronary heart disease, which is why the search for biomarkers for DCM prediction, prevention or treatment absorbs a lot of scientific efforts, including the search for appropriate miRNAs.
In vitro studies have shown that hyperglycemia increases miR-155 expression in cardiomyoblasts, leading to increased expression of profibrotic genes (e.g.: NRF2/HO-1 signaling pathway) and mitochondrial dysfunction. Furthermore, a miR-155 inhibitor can attenuate cardiac fibrosis, making miR-155 a promising target for the prevention or treatment of DCM (110). Moreover, diabetic heart cells showed significantly reduced expression of miR-181a and miR-30c and increased expression of their target genes p53 and p21, which are key regulators of cardiomyocyte hypertrophy, mediating the pro-hypertrophic effects of hyperglycemia (111). Furthermore, increased expression of miR-200c was observed in the diabetic cardiomyopathy model and in exposed patients cardiomyocytes to high glucose levels, while subsequent reversal of cardiomyocyte hypertrophy was achieved by inhibition of miR-200c (112).
DCM is an urgent problem because changes in the heart are a significant factor influencing mortality among the diabetic population, hence the need to continue research on its indicators in the earlier stages of type 1 diabetes in children, as such studies are unfortunately scarce.
6 Microvascular complications in type 1 diabetes
Statistics on vascular complications in type 1 diabetes in children and adolescents may vary depending on the study group. According to one study, in a group of 155 individuals with a mean age of 14.4 ± 2.1 years, the incidence of microangiopathy was 16.1% (113); 50% of patients developed complications within 5 years of diagnosis of type 1 diabetes, and 25% within 2 years (114). Effective diabetes treatment has significantly reduced the number of patients with microvascular complications in recent years. The percentage of patients with diabetic retinopathy has decreased from 45% to 5%, with neuropathy from 50% to 15%, and with nephropathy from 25% to 4 - 9%, depending on the study group (115).
6.1 miRNAs in nephropathy
Diabetic nephropathy (DN) is characterized by pathological changes including thickening of the glomerular and tubular basement membranes, mesangial matrix expansion, and eventual progression to glomerulosclerosis and tubulointerstitial fibrosis (61). DN is a leading cause of end-stage renal disease (116, 117). Evidence suggests that structural glomerular alterations often precede the clinical manifestation of microalbuminuria and abnormal albumin-to-creatinine ratio (ACR) values in adolescents often precede the clinical onset of microalbuminuria. A correlation has been shown between ACR and markers of glomerular hyperfiltration. Higher ACR activity in adolescents has been associated with the future development of advanced stages of diabetic nephropathy and cardiovascular disease. This is confirmed by the marker hs-CRP (high sensitivity C-reactive protein; a factor measuring the risk of cardiovascular diseases), which increases in parallel with the increase in ACR level (118).
Recent studies have identified significant alterations in the urinary levels of miRNAs such as elevated level of miR-377 and reduced level of miR-216a in pediatric patients with type 1 diabetes mellitus (T1DM). miR-377 has been implicated in mesangial cell dysfunction and oxygenase-1 (HO - 1), a critical antioxidant enzyme, exacerbating podocyte injury and albuminuria (117).
miRNAs such as miR-29, miR-192, and miR-200 regulate extracellular matrix (ECM) synthesis, with reduced expression of miR-29 in DN leading to increased collagen production and ECM accumulation (119, 120). It operates through the mechanism of renal fibrosis. Similar effects have been included by increased expression of miR-146a (9). miRNAs also regulate other pathogenic processes in DN, including oxidative stress (miR-377) and podocyte apoptosis (miR-195). Furthermore, reduced expression of miR-93 in podocytes and endothelial cells increases vascular endothelial growth factor (VEGF) expression, enhancing glomerular permeability and albuminuria, which are characteristic features of diabetic nephropathy (117). Addictionaly, miR-155-5p, exhibits increased expression in hyperglycemia. This leads to irreversible fibrotic changes in the renal tubular epithelium. The expression of miR-155-5p has also been observed in glomerular mesangial cells (121).
6.2 miRNAs in retinopathy
Microvascular dysfunction, characterized by altered interactions between endothelial cells and pericytes, is a key event in the pathogenesis of diabetic retinopathy. According to a study conducted between 2000 and 2020 on approximately 150,000 young people, its prevalence is 5.8% (122), while for severe retinopathy it is less than 1%. EVs derived from mesenchymal stem cells cultures can enter pericytes, causing their detachment, migration, and stimulating angiogenesis. Studies have shown that the miRNA profiles in EVs from diabetic retinopathy patients differ significantly from those of healthy controls and non-complicated patients with diabetes (123, 124).
It has been shown that lowering the level of miR-17-5p, miR-20a-5p (125), miR-93 and miR-106 causes retinal angiogenesis and thus the development of retinopathy already in premature infants (126). The results of another study showed that the expression of miR-23a and miR-200b-3p was significantly elevated in patients with retinopathy (126). Notably, miR-21-3p, miR-30b-5p, and miR-150-5p play a crucial role in vessel destabilization and angiogenesis. Hypoxia, a characteristic feature of the diabetic eye, activates HIF - 1α (a critical regulator of proangiogenic genes such as VEGF) and associated miRNAs, including miR-21-3p and miR-30b-5p, further contributing to vascular instability and abnormal vessel growth. In contrast, miR-150-5p, which normally inhibits angiogenesis by upregulating ITGA6, is downregulated in patients with DR (124).
It is in different way with miR-200. It has a dynamic expression pattern dependent on the stage of the disease. In the early stages of retinopathy, its reduced expression leads to increased VEGF levels and vascular permeability in infants (126). In advanced stages, miR-200b is overexpressed, contributing to increased oxidative stress by inhibiting Oxr1 (127).
MiR-146a plays a protective role by reducing the activation of inflammatory genes, such as MCP - 1 and ICAM - 1, through negative feedback in the NF-κB pathway. In addition, animal models have shown that overexpression of miR-146a limits pathological changes in the retina, such as overproduction of extracellular matrix proteins (128).
Diabetic retinopathy is currently a rare complication of type 1 diabetes in children. The incidence varies, because it depends on the age, gender and social environment of the population. According to studies conducted in the USA, it is estimated at <4% in children and adolescents with type 1 diabetes. Additionally, maintaining HbA1c <8% significantly reduces the risk of this complication, which confirms how important it is to maintain normal glycemia in the long term (129).
6.3 miRNAs in neuropathy
Dysregulation of miRNAs in the central nervous system causes progressive neuronal loss, resulting in neurodegenerative diseases. miR-124 and miR-298 (130) is responsible for the most common one, Alzheimer’s syndrome, while miR-133b affects Parkinson’s disease (131). miRNAs regulate neurogenesis and dendritic growth. Their insufficient concentration contributes to neuronal apoptosis. Moreover, studies indicate their role in maintaining synaptic plasticity (132).
Changes have not been demonstrated in children, however, elevated plasma levels of miR-21 and miR-210, along with decreased miR-126 concentrations, correlate with an increased risk of nervous system diseases in early adulthood (133). In diabetic polyneuropathy (DPN), microR-146a exerts neuroprotective effects by reducing inflammation and improving myelination and preserving nerve structure (128, 134). MiR-146 located in circulating mononuclear cells modulates inflammatory response in diabetic peripheral neuropathy. Additionally, miR-146a (97)and miR-155-5p exhibits a protective role against early cardiovascular autonomic neuropathy (CAN) (121). A protective role is also played by higher concentrations of miR-25 (134), miR-302, which through various metabolic pathways lead to reduced oxidative stress (119). This is unlike miR-27a, miR-499a and miR-199a, which are prothrombotic factors (132, 134). Dysregulated miRNAs may contribute to the development of diabetic foot, which is the most common complication of DPN (135).
7 Prevention and interventions
Cardiovascular disease accounts for up to 25 – 50% of deaths in people with type 1 diabetes who have had diabetes for even less than 20 years, and this percentage increases with longer duration of the disease (20). Prevention of cardiovascular events in people with type 1 diabetes is crucial and is based on the promotion of a healthy lifestyle, healthy diet, physical activity, maintaining a healthy body weight, strict glycemic control, maintaining normal blood pressure and LDL-C levels, and monitoring patients during follow-up visits: ACR and examination for neuropathy every year, fundus examination every 1 – 2 years, carotid artery ultrasound recommended every 5 years in the absence of significant preclinical atherosclerosis (136). As most early changes are subclinical, screening for cardiovascular complications is critical to identify adolescents at risk and initiate treatment before irreversible changes occur. The ADA considers the use of age-approved statins, along with nutritional therapy and lifestyle changes, in youth with type 1 diabetes >10 years of age whose LDL-C is ≥130 mg/dL (≥3.4 mmol/L) (4). Current treatment strategies and goals for T1D are based on the results of The Diabetes Control and Complications Trial (DCCT) and its follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) trial, which showed that intensive insulin therapy to achieve glycemic control as close to normoglycemia as possible improves cardiovascular risk factors and effectively delays the onset and progression of microvascular and macrovascular complications, with benefits that persist for decades and are associated with reduced all-cause mortality (137). Nevertheless, an association has been demonstrated between glycemic variability, cardiovascular risk factors, future complications, and mortality, regardless of adherence to recommendations for maintaining glycemic targets (138). In addition, hypoglycemia may contribute to cardiovascular complications, including myocardial ischemia and arrhythmias, by causing changes in hemodynamics, coagulation, arterial stiffness, cardiac electrophysiology, and autonomic function (139).
Initiation of insulin pump therapy within 6 months of diagnosis of childhood type 1 diabetes was associated with reduced cardiovascular risk, particularly lower mean systolic blood pressure and higher high-density lipoprotein cholesterol (HDL-C), compared with those who initiated CSII within 2 – 3 years of disease onset (140). A multicenter study showed that patients using insulin pumps had increased HDL-C levels and reduced total cholesterol, LDL-C and triglyceride levels, and this effect was maintained after 8 years of follow-up when they also showed fewer cardiovascular events compared to diabetics using MDI (141). Similarly, in a large Swedish registry of 18,168 people with type 1 diabetes, a reduction in coronary heart disease mortality (45%), cardiovascular disease mortality (42%), and all-cause mortality (27%) was observed with pump use compared with MDI use over approximately 7 years of follow-up. It was hypothesized that this may have been due to a reduction in severe hypoglycemia with CSII (142). Interestingly, literature reports that compared to patients using MDI, CSII treatment was associated with lower arterial stiffness independent of other risk factors (143). The use of personal insulin pumps may provide cardiac benefits also by improving endothelial function and overall myocardial performance, as lower cIMT and anteroposterior diameter of the infrarenal abdominal aorta were observed compared to MDI use (144). Systematic use of modern solutions in the treatment of T1D by improving glycemic trends and beyond may positively influence cardiovascular risk factors such as hypertension and dyslipidemia or even protect against the development and progression of macrovascular complications such as circulatory system diseases. Recently, it has been suggested that increased glycemic variability, i.e. the number and amplitude of glycemic excursions measured by CGM as well as HbA1c above target values, may constitute undisputed cardiovascular risk factors (145). A previous study from our institution demonstrated that reducing glycemic variability in a population of young patients with type 1 diabetes using CSII and real-time CGM improves endothelial function by increasing flow-mediated dilation of the brachial artery (146). Current recommendations indicate that hybrid closed-loop systems are the most beneficial treatment option for T1D patients and should be the treatment of choice in the pediatric population since they provide optimal metabolic control while improving the quality of life of both diabetics and their families (147). Furthermore, for patients who have not achieved satisfactory metabolic control with traditional methods of intensive insulin therapy, the use of closed-loop systems may be the only way to improve metabolic control and reduce the risk of acute and chronic complications. A diagram summarizing the risk factors in type 1 diabetes with their subsequent complications leading to cardiovascular diseases, as well as methods of prevention and intervention is presented in Figure 3.

Figure 3. Risk factors and early vascular complications in children with type 1 diabetes predisposing to future CVD as well as possible prevention and interventions strategies in the management of CVD.
In the only study to assess differences in miRNA expression during insulin therapy in children with type 1 diabetes, 27 Australian adolescents without significant differences in baseline characteristics were randomized to CSII or MDI within 3 months of disease onset. There were no significant differences in baseline miRNA expression by treatment, but significantly altered expression of individual miRNAs (measured between baseline and end of follow-up) were noted between the CSII and MDI groups. Expression of miR-17, which has been proven to positively correlate with vascular complications, decreased in patients on CSII and increased in patients on MDI. In addition, miR-106a is seen in higher amounts in diabetics with vascular complications, and its levels increased in patients using both therapies, but to significantly higher levels in patients on MDI therapy. Although decreased expression of miR-24, miR-92a, miR-320, and miR-483-5p has been described in patients with cardiovascular complications, in this study the researchers observed that miR-24 decreased with both treatments, but more so with CSII therapy; miR-92a and miR-320 decreased with CSII and increased with MDI, whereas miR-483-5p increased regardless of treatment method. miR-21 levels decreased to a greater extent during the follow-up in CSII users than in MDI users, whereas increased expression of this miRNA was noted in individuals with nephropathy, retinopathy and high glucose variability. miR-28 and miR-99b, which were shown to predict an increased risk of microalbuminuria, were decreased from baseline in CSII users and increased in the MDI group. miR-328 associated with impaired wound healing in diabetic individuals showed reduced expression compared to baseline in both adolescent groups, but the degree of reduction was less in those using MDI. Compared to baseline, miR-375 levels, associated with beta-cell death, decreased in CSII users and increased in MDI users, but did not correlate with changes in C-peptide. In addition, CSII therapy was associated with lower glycemic variability than MDI therapy (148). Therefore, it can be concluded from this study that early CSII therapy in adolescents with T1D is associated with an altered miRNA expression pattern less adversely related to vascular complications and may be an early sign of better vascular health in adolescents treated with CSII. Nevertheless, replication and further studies as well as long-term follow-up are warranted to validate the data collected here and to explore preventive methods or possible therapeutic targets for preserving or prolonging healthy vasculature.
MiRNAs appear to be promising targets for therapeutic interventions. In obese children and adolescents, miR-320a expression significantly increased after 12 weeks of exercise training and correlated negatively with markers of endothelial dysfunction (149). Increased expression of miR-133a after exercise has been shown to positively correlate with left ventricular mass index, and thus may be a marker of left ventricular hypertrophy in children with primary hypertension (56). Furthermore, it has been suggested that reduced miR-133a expression is associated with the process of cardiac hypertrophy, because this molecule indirectly affects antihypertrophic genes (150), whereas increased miR-133a expression prevents early cardiac fibrosis in diabetes (151). Children with left ventricular hypertrophy may therefore have a greater potential to reverse adverse cardiac changes, and exercise seems to help in this matter. Similarly, obese male adolescents who underwent 6 weeks of exercise combined with a low-calorie diet showed an amelioration in their peripheral vasodilation capacity, indirectly indicating improved vascular endothelial function, which was associated with a reduction in miR-126 expression (152). Together, the data from these studies may indicate a protective effect of exercise, healthy diet and weight loss on endothelial health and reduced cardiovascular risk in children and adolescents as well as demonstrate the involvement of miRNAs in these processes.
8 Conclusions
Hitherto, no precise method has been developed to determine the risk of cardiovascular complications in children with type 1 diabetes, and they are usually diagnosed in the advanced stages. Notably, variable miRNAs are important in the pathogenesis, development and course of T1D, additional risk factors which are comorbidities such as obesity, hypertension or dyslipidemia, as well as early vascular changes occurring already in adolescence and later complications in old age. Based on the above considerations, miRNAs do not seem to be only a passive biomarker of developing complications, but may play an active role in the pathogenesis of cardiovascular complications and be of great importance in the development of precision medicine in the future. Knowledge of how the expression of specific miRNAs changes in individual risk factors and then after a specific intervention, identifying and developing anti-miRNAs and miRNA mimetics to inhibit, predict, and treat cardiovascular complications of diabetes in T1D is an active and alluring area of research. Disorder-causing aberrantly upregulated miRNAs can be targeted for silencing or sequestration, whereas those that are downregulated can be transfected to inhibit or cure the disturbances they induce (153, 154). MiRNA may therefore play a tremendous role in precise diagnosis, prevention, delaying cardiovascular complications or determining personalized therapy. Taking into account the above considerations, we hope that miRNA profiling in children with type 1 diabetes will be used in decision-making regarding cardiovascular diseases even in the coming years. However, before miRNA can be widely accepted to use in clinical practice, validation and standardization are necessary, as well as identification of potential factors that may influence false positive or false negative results due to the method of sample collection or storage or technical conditions. It’s important to note that while these findings are promising, they are exceptionally scarce in the discussed pediatric population. We summarize the potential miRNA biomarkers of cardiovascular risk in children with type 1 diabetes in Table 2. Further, more detailed analyses on the influence of selected miRNAs on specific risk factors for cardiovascular complications in people with type 1 diabetes, both in developmental and adult age, are necessary.
Author contributions
JP: Conceptualization, Investigation, Resources, Visualization, Writing – original draft, Methodology, Project administration, Data curation, Formal Analysis. EO: Investigation, Visualization, Writing – original draft, Methodology. KK: Investigation, Visualization, Writing – original draft. MJ: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing. AB: Funding acquisition, Supervision, Validation, Writing – review & editing. BG: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The APCs were funded by Medical University of Bialystok.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ogle GD, James S, Dabelea D, Pihoker C, Svennson J, Maniam J, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10. Diabetes Res Clin Pract. (2021) 183:109083. doi: 10.1016/j.diabres.2021.109083
2. Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. (2022) 10:741–60. doi: 10.1016/S2213-8587(22)00218-2
3. Ciężki S, Odyjewska E, Bossowski A, and Głowińska-Olszewska B. Not only metabolic complications of childhood obesity. Nutrients. (2024) 16:539. doi: 10.3390/nu16040539
4. Committee ADAPP. 14. Children and adolescents: standards of care in diabetes-2025. Diabetes Care. (2025) 48:S283–305. doi: 10.2337/dc25-S014
5. Grabia M, Markiewicz-Żukowska R, and Socha K. Prevalence of metabolic syndrome in children and adolescents with type 1 diabetes mellitus and possibilities of prevention and treatment: A systematic review. Nutrients. (2021) 13:1782. doi: 10.3390/nu13061782
6. Klonowska B, Charemska D, Jabłońska J, Banach A, Kącka A, Szynkarczuk E, et al. Carotid artery intima-media thickness (cIMT) in young type 1 diabetic patients in relation to comorbid additional autoimmune diseases and microvascular complications. Pediatr Endocrinol Diabetes Metab. (2016) 22. doi: 10.18544/PEDM-22.03.0057
7. Nocuń-Wasilewska K, Zwolińska D, Zubkiewicz-Kucharska A, and Polak-Jonkisz D. Evaluation of vascular endothelial function in children with type 1 diabetes mellitus. J Clin Med. (2021) 10:5065. doi: 10.3390/jcm10215065
8. Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A, et al. Lymphocyte-derived exosomal microRNAs promote pancreatic β Cell death and may contribute to type 1 diabetes development. Cell Metab. (2019) 29:348–61.e6. doi: 10.1016/j.cmet.2018.09.011
9. Čugalj Kern B, Trebušak Podkrajšek K, Kovač J, Šket R, Jenko Bizjan B, Tesovnik T, et al. The role of epigenetic modifications in late complications in type 1 diabetes. Genes (Basel). (2022) 13:705. doi: 10.3390/genes13040705
10. Pang H, Fan W, Pi L, Shi X, Wang Z, Luo S, et al. Plasma-derived exosomal miRNA profiles associated with type 1 diabetes. Diabetes Metab Res Rev. (2024) 40:e3774. doi: 10.1002/dmrr.3774
11. Isaac R, Reis FCG, Ying W, and Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. (2021) 33:1744–62. doi: 10.1016/j.cmet.2021.08.006
12. Cui Y, Huang T, and Zhang X. RNA editing of microRNA prevents RNA-induced silencing complex recognition of target mRNA. Open Biol. (2015) 5:150126. doi: 10.1098/rsob.150126
13. Gebert LFR and MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. (2019) 20:21–37. doi: 10.1038/s41580-018-0045-7
14. Kozomara A, Birgaoanu M, and Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. (2019) 47:D155–D62. doi: 10.1093/nar/gky1141
15. Morales-Sánchez P, Lambert C, Ares-Blanco J, Suárez-Gutiérrez L, Villa-Fernández E, Garcia AV, et al. Circulating miRNA expression in long-standing type 1 diabetes mellitus. Sci Rep. (2023) 13:8611. doi: 10.1038/s41598-023-35836-8
16. Lobera ES, Varela MA, Jimenez RL, and Moreno RB. miRNA as biomarker in lung cancer. Mol Biol Rep. (2023) 50:9521–7. doi: 10.1007/s11033-023-08695-9
17. Sharma AR, Sharma G, Bhattacharya M, Lee SS, and Chakraborty C. Circulating miRNA in atherosclerosis: A clinical biomarker and early diagnostic tool. Curr Mol Med. (2022) 22:250–62. doi: 10.2174/1566524021666210315124438
18. Hochreuter MY, Dall M, Treebak JT, and Barrès R. MicroRNAs in non-alcoholic fatty liver disease: Progress and perspectives. Mol Metab. (2022) 65:101581. doi: 10.1016/j.molmet.2022.101581
19. Chorley BN, Atabakhsh E, Doran G, Gautier JC, Ellinger-Ziegelbauer H, Jackson D, et al. Methodological considerations for measuring biofluid-based microRNA biomarkers. Crit Rev Toxicol. (2021) 51:264–82. doi: 10.1080/10408444.2021.1907530
20. Rawshani A, Sattar N, Franzén S, Hattersley AT, Svensson AM, Eliasson B, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. (2018) 392:477–86. doi: 10.1016/S0140-6736(18)31506-X
21. Prasher D, Greenway SC, and Singh RB. The impact of epigenetics on cardiovascular disease. Biochem Cell Biol. (2020) 98:12–22. doi: 10.1139/bcb-2019-0045
22. Pastore I, Bolla AM, Montefusco L, Lunati ME, Rossi A, Assi E, et al. The impact of diabetes mellitus on cardiovascular risk onset in children and adolescents. Int J Mol Sci. (2020) 21:4928. doi: 10.3390/ijms21144928
23. Li X, Dai A, Tran R, and Wang J. Text mining-based identification of promising miRNA biomarkers for diabetes mellitus. Front Endocrinol (Lausanne). (2023) 14:1195145. doi: 10.3389/fendo.2023.1195145
24. Margaritis K, Margioula-Siarkou G, Margioula-Siarkou C, Petousis S, Kotanidou EP, Christoforidis A, et al. Circulating serum and plasma levels of micro-RNA in type-1 diabetes in children and adolescents: A systematic review and meta-analysis. Eur J Clin Invest. (2021) 51:e13510. doi: 10.1111/eci.13510
25. Erener S, Marwaha A, Tan R, Panagiotopoulos C, and Kieffer TJ. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight. (2017) 2:e89656. doi: 10.1172/jci.insight.89656
26. Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep. (2017) 7:5998. doi: 10.1038/s41598-017-05787-y
27. Swolin-Eide D, Forsander G, Pundziute Lyckå A, Novak D, Grillari J, Diendorfer AB, et al. Circulating microRNAs in young individuals with long-duration type 1 diabetes in comparison with healthy controls. Sci Rep. (2023) 13:11634. doi: 10.1038/s41598-023-38615-7
28. Nabih ES and Andrawes NG. The Association Between Circulating Levels of miRNA-181a and Pancreatic Beta Cells Dysfunction via SMAD7 in Type 1 Diabetic Children and Adolescents. J Clin Lab Anal. (2016) 30:727–31. doi: 10.1002/jcla.21928
29. Samandari N, Mirza AH, Nielsen LB, Kaur S, Hougaard P, Fredheim S, et al. Circulating microRNA levels predict residual beta cell function and glycaemic control in children with type 1 diabetes mellitus. Diabetologia. (2017) 60:354–63. doi: 10.1007/s00125-016-4156-4
30. Lam A, Dayan C, and Herold KC. A little help from residual β cells has long-lasting clinical benefits. J Clin Invest. (2021) 131:e143683. doi: 10.1172/JCI143683
31. Scherm MG and Daniel C. miRNA-mediated immune regulation in islet autoimmunity and type 1 diabetes. Front Endocrinol (Lausanne). (2020) 11:606322. doi: 10.3389/fendo.2020.606322
32. Badacz R, Kleczyński P, Legutko J, Żmudka K, Gacoń J, Przewłocki T, et al. Expression of miR-1-3p, miR-16-5p and miR-122-5p as Possible Risk Factors of Secondary Cardiovascular Events. Biomedicines. (2021) 9:1055. doi: 10.3390/biomedicines9081055
33. Bellini S, Guarrera S, Matullo G, Schalkwijk C, Stehouwer CD, Chaturvedi N, et al. Serum microRNA-191-5p levels in vascular complications of type 1 diabetes: the EURODIAB prospective complications study. J Clin Endocrinol Metab. (2023) 109:e163–e74. doi: 10.1210/clinem/dgad468
34. Tanaka J, Qiang L, Banks AS, Welch CL, Matsumoto M, Kitamura T, et al. Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes. (2009) 58:2344–54. doi: 10.2337/db09-0167
35. Ciężki S, Kurpiewska E, Bossowski A, and Głowińska-Olszewska B. Multi-faceted influence of obesity on type 1 diabetes in children - from disease pathogenesis to complications. Front Endocrinol (Lausanne). (2022) 13:890833. doi: :10.3389/fendo.2022.890833
36. Marlow AL, Rowe CW, Anderson D, Wynne K, King BR, Howley P, et al. Young children, adolescent girls and women with type 1 diabetes are more overweight and obese than reference populations, and this is associated with increased cardiovascular risk factors. Diabetes Med. (2019) 36:1487–93. doi: 10.1111/dme.14133
37. Phelan H, Foster NC, Schwandt A, Couper JJ, Willi S, Kroschwald P, et al. Longitudinal trajectories of BMI z-score: an international comparison of 11,513 Australian, American and German/Austrian/Luxembourgian youth with type 1 diabetes. Pediatr Obes. (2020) 15:e12582. doi: 10.1111/ijpo.12582
38. Nuotio J, Laitinen TT, Magnussen CG, Sinaiko AR, Bazzano LA, Daniels SR, et al. Predictors in youth of adult cardiovascular events. Pediatrics. (2024) 154:e2024066736. doi: 10.1542/peds.2024-066736
39. Kartiosuo N, Raitakari OT, Juonala M, Viikari JSA, Sinaiko AR, Venn AJ, et al. Cardiovascular risk factors in childhood and adulthood and cardiovascular disease in middle age. JAMA Netw Open. (2024) 7:e2418148. doi: 10.1001/jamanetworkopen.2024.18148
40. Koebnick C, Imperatore G, Jensen ET, Stafford JM, Shah AS, Mottl AK, et al. Progression to hypertension in youth and young adults with type 1 or type 2 diabetes: The SEARCH for Diabetes in Youth Study. J Clin Hypertens (Greenwich). (2020) 22:888–96. doi: 10.1111/jch.13849
41. Purnell JQ, Zinman B, Brunzell JD, and Group DER. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. (2013) 127:180–7. doi: 10.1161/CIRCULATIONAHA.111.077487
42. Shah AS, Dabelea D, Fino NF, Dolan LM, Wadwa RP, D’Agostino R, et al. Predictors of increased carotid intima-media thickness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. (2016) 39:418–25. doi: 10.2337/dc15-1963
43. Kurylowicz A. microRNAs in human adipose tissue physiology and dysfunction. Cells. (2021) 10:3342. doi: 10.3390/cells10123342
44. Iacomino G, Russo P, Stillitano I, Lauria F, Marena P, Ahrens W, et al. Circulating microRNAs are deregulated in overweight/obese children: preliminary results of the I. Family study. Genes Nutr. (2016) 11:7. doi: 10.1186/s12263-016-0525-3
45. Thompson MD, Cismowski MJ, Serpico M, Pusateri A, and Brigstock DR. Elevation of circulating microRNA levels in obese children compared to healthy controls. Clin Obes. (2017) 7:216–21. doi: 10.1111/cob.12192
46. Masotti A, Baldassarre A, Fabrizi M, Olivero G, Loreti MC, Giammaria P, et al. Oral glucose tolerance test unravels circulating miRNAs associated with insulin resistance in obese preschoolers. Pediatr Obes. (2017) 12:229–38. doi: 10.1111/ijpo.12133
47. Oses M, Margareto Sanchez J, Portillo MP, Aguilera CM, and Labayen I. Circulating miRNAs as biomarkers of obesity and obesity-associated comorbidities in children and adolescents: A systematic review. Nutrients. (2019) 11:2890. doi: 10.3390/nu11122890
48. Prats-Puig A, Ortega FJ, Mercader JM, Moreno-Navarrete JM, Moreno M, Bonet N, et al. Changes in circulating microRNAs are associated with childhood obesity. J Clin Endocrinol Metab. (2013) 98:E1655–60. doi: 10.1210/jc.2013-1496
49. Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. (2004) 114:1493–503. doi: 10.1172/JCI200419992
50. Ortega FJ, Mercader JM, Catalán V, Moreno-Navarrete JM, Pueyo N, Sabater M, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. (2013) 59:781–92. doi: 10.1373/clinchem.2012.195776
51. Mohany KM, Al Rugaie O, Al-Wutayd O, and Al-Nafeesah A. Investigation of the levels of circulating miR-29a, miR-122, sestrin 2 and inflammatory markers in obese children with/without type 2 diabetes: a case control study. BMC Endocr Disord. (2021) 21:152. doi: 10.1186/s12902-021-00829-z
52. Jiang X, Xue M, Fu Z, Ji C, Guo X, Zhu L, et al. Insight into the effects of adipose tissue inflammation factors on miR-378 expression and the underlying mechanism. Cell Physiol Biochem. (2014) 33:1778–88. doi: 10.1159/000362957
53. Shi C, Zhu L, Chen X, Gu N, Chen L, Yang L, et al. IL - 6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J Interferon Cytokine Res. (2014) 34:342–8. doi: 10.1089/jir.2013.0078
54. Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. (2019) 129:834–49. doi: 10.1172/JCI123069
55. Yao F, Yu Y, Feng L, Li J, Zhang M, Lan X, et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARγ of insulin resistance induced by high-fat diet-associated obesity. Exp Cell Res. (2017) 355:105–12. doi: 10.1016/j.yexcr.2017.03.060
56. Szyszka M and Skrzypczyk P. MicroRNA-133a and microRNA-145 may be involved in the development of hypertension-mediated organ damage in children with primary hypertension-A preliminary study. J Clin Med. (2024) 13:6929. doi: 10.3390/jcm13226929
57. Wang Y and Jin L. miRNA-145 is associated with spontaneous hypertension by targeting SLC7A1. Exp Ther Med. (2018) 15:548–52. doi: 10.3892/etm.2017.5371
58. Yildirim E, Ermis E, Allahverdiyev S, Ucar H, Yavuzer S, and Cengiz M. Circulating miR-21 levels in hypertensive patients with asymptomatic organ damage. Med (Baltimore). (2019) 98:e17297. doi: 10.1097/MD.0000000000017297
59. Nemecz M, Stefan DS, Comariţa IK, Constantin A, Tanko G, Guja C, et al. Microvesicle-associated and circulating microRNAs in diabetic dyslipidemia: miR-218, miR-132, miR-143, and miR-21, miR-122, miR-155 have biomarker potential. Cardiovasc Diabetol. (2023) 22:260. doi: 10.1186/s12933-023-01988-0
60. Suzuki K, Yamada H, Fujii R, Munetsuna E, Yamazaki M, Ando Y, et al. Circulating microRNA-27a and -133a are negatively associated with incident hypertension: a five-year longitudinal population-based study. Biomarkers. (2022) 27:496–502. doi: 10.1080/1354750X.2022.2070281
61. Barutta F, Bellini S, Guarrera S, Matullo G, Schalkwijk C, Stehouwer CD, et al. Association of serum MicroRNA-145-5p levels with microvascular complications of type 1 Diabetes: The EURODIAB prospective complications study. Diabetes Res Clin Pract. (2022) 190:109987. doi: 10.1016/j.diabres.2022.109987
62. Selvaraj M, Prasad HK, White S, Prasanna B, and Sangaralingam T. Prevalence and determinants of occurrence of dyslipidemia in subjects with type 1 diabetes mellitus. Indian J Pediatr. (2023) 90:118–23. doi: 10.1007/s12098-022-04130-2
63. Ibrahim AA, Wahby AA, Ashmawy I, Saleh RM, and Soliman H. Association of exosomal miR-34a with markers of dyslipidemia and endothelial dysfunction in children and adolescents with T1DM. J Clin Res Pediatr Endocrinol. (2020) 12:401–9. doi: 10.4274/jcrpe.galenos.2020.2020.0134
64. Hendy OM, Rabie H, El Fouly A, Abdel-Samiee M, Abdelmotelb N, Elshormilisy AA, et al. The circulating micro-RNAs (-122, -34a and -99a) as predictive biomarkers for non-alcoholic fatty liver diseases. Diabetes Metab Syndr Obes. (2019) 12:2715–23. doi: 10.2147/DMSO.S231321
65. Tabuchi T, Satoh M, Itoh T, and Nakamura M. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond). (2012) 123:161–71. doi: 10.1042/CS20110563
66. Barberio MD, Kasselman LJ, Playford MP, Epstein SB, Renna HA, Goldberg M, et al. Cholesterol efflux alterations in adolescent obesity: role of adipose-derived extracellular vesical microRNAs. J Transl Med. (2019) 17:232. doi: 10.1186/s12967-019-1980-6
67. Bucciarelli LG, Pollreisz A, Kebschull M, Ganda A, Kalea AZ, Hudson BI, et al. Inflammatory stress in primary venous and aortic endothelial cells of type 1 diabetic mice. Diabetes Vasc Dis Res. (2009) 6:249–61. doi: 10.1177/1479164109338775
68. Cé GV, Rohde LE, da Silva AM, Puñales MK, de Castro AC, and Bertoluci MC. Endothelial dysfunction is related to poor glycemic control in adolescents with type 1 diabetes under 5 years of disease: evidence of metabolic memory. J Clin Endocrinol Metab. (2011) 96:1493–9. doi: 10.1210/jc.2010-2363
69. Khalyfa A, Kheirandish-Gozal L, Bhattacharjee R, Khalyfa AA, and Gozal D. Circulating microRNAs as potential biomarkers of endothelial dysfunction in obese children. Chest. (2016) 149:786–800. doi: 10.1378/chest.15-0799
70. Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, Philby MF, Alonso-Álvarez ML, Mohammadi M, et al. Circulating plasma extracellular microvesicle microRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. (2016) 194:1116–26. doi: 10.1164/rccm.201602-0323OC
71. Bakhashab S, Yuen Yeoh ML, Coulson DJ, Steel SC, Ray SL, and Weaver JU. Deciphering the role of miR-200c-3p in type 1 diabetes (Subclinical cardiovascular disease) and its correlation with inflammation and vascular health. Int J Mol Sci. (2022) 23:15659. doi: 10.3390/ijms232415659
72. Zhang H, Liu J, Qu D, Wang L, Luo JY, Lau CW, et al. Inhibition of miR-200c restores endothelial function in diabetic mice through suppression of COX - 2. Diabetes. (2016) 65:1196–207. doi: 10.2337/db15-1067
73. Coulson DJ, Bakhashab S, Latief JS, and Weaver JU. MiR-126, IL - 7, CXCR1/2 receptors, inflammation and circulating endothelial progenitor cells: The study on targets for treatment pathways in a model of subclinical cardiovascular disease (type 1 diabetes mellitus). J Transl Med. (2021) 19:140. doi: 10.1186/s12967-021-02785-7
74. Faienza MF, Acquafredda A, Tesse R, Luce V, Ventura A, Maggialetti N, et al. Risk factors for subclinical atherosclerosis in diabetic and obese children. Int J Med Sci. (2013) 10:338–43. doi: 10.7150/ijms.5181
75. Guo W, Li XN, Li J, Lu J, Wu J, Zhu WF, et al. Increased plasma miR-146a levels are associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. J Diabetes Complicat. (2020) 34:107725. doi: 10.1016/j.jdiacomp.2020.107725
76. Witarto BS, Visuddho V, Aldian FM, Atmaja MSS, Ariyanto MV, Witarto AP, et al. Blood-based circulating microRNAs as diagnostic biomarkers for subclinical carotid atherosclerosis: A systematic review and meta-analysis with bioinformatics analysis. Diabetes Metab Syndr. (2023) 17:102860. doi: 10.1016/j.dsx.2023.102860
77. Sun X, Zhang Y, Liu Z, Li S, and Wang L. MicroRNA-199a-3p exhibits beneficial effects in asymptomatic atherosclerosis by inhibiting vascular smooth muscle cell proliferation and migration. Mol Biotechnol. (2021) 63:595–604. doi: 10.1007/s12033-021-00323-w
78. Chen J, Tang Z, Chen Z, Wei Y, Liang H, Zhang X, et al. MicroRNA-218-5p regulates inflammation response via targeting TLR4 in atherosclerosis. BMC Cardiovasc Disord. (2023) 23:122. doi: 10.1186/s12872-023-03124-y
79. Sun H, Wu S, and Sun B. MicroRNA-532-5p protects against atherosclerosis through inhibiting vascular smooth muscle cell proliferation and migration. Cardiovasc Diagn Ther. (2020) 10:481–9. doi: 10.21037/cdt-20-91
80. Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. (2010) 156:731–7, 7.e1. doi: 10.1016/j.jpeds.2009.11.011
81. Chirinos JA, Segers P, Hughes T, and Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74:1237–63. doi: 10.1016/j.jacc.2019.07.012
82. Dabelea D, Talton JW, D’Agostino R, Wadwa RP, Urbina EM, Dolan LM, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. (2013) 36:3938–43. doi: 10.2337/dc13-0851
83. Bjornstad P, Schäfer M, Truong U, Cree-Green M, Pyle L, Baumgartner A, et al. Metformin improves insulin sensitivity and vascular health in youth with type 1 diabetes mellitus. Circulation. (2018) 138:2895–907. doi: 10.1161/CIRCULATIONAHA.118.035525
84. Bhuva AN, D’Silva A, Torlasco C, Jones S, Nadarajan N, Van Zalen J, et al. Training for a first-time marathon reverses age-related aortic stiffening. J Am Coll Cardiol. (2020) 75:60–71. doi: 10.1016/j.jacc.2019.10.045
85. Wang C, Wu H, Xing Y, Ye Y, He F, Yin Q, et al. Endothelial-derived extracellular microRNA-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells. Sci Rep. (2022) 12:344. doi: 10.1038/s41598-021-04341-1
86. Parthenakis F, Marketou M, Kontaraki J, Patrianakos A, Nakou H, Touloupaki M, et al. Low levels of microRNA-21 are a marker of reduced arterial stiffness in well-controlled hypertension. J Clin Hypertens (Greenwich). (2017) 19:235–40. doi: 10.1111/jch.12900
87. Streese L, Demougin P, Iborra P, Kanitz A, Deiseroth A, Kröpfl JM, et al. Untargeted sequencing of circulating microRNAs in a healthy and diseased older population. Sci Rep. (2022) 12:2991. doi: 10.1038/s41598-022-06956-4
88. Jones S, Khanolkar AR, Gevers E, Stephenson T, and Amin R. Cardiovascular risk factors from diagnosis in children with type 1 diabetes mellitus: a longitudinal cohort study. BMJ Open Diabetes Res Care. (2019) 7:e000625. doi: 10.1136/bmjdrc-2018-000625
89. Lindberg L, Danielsson P, Persson M, Marcus C, and Hagman E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PloS Med. (2020) 17:e1003078. doi: 10.1371/journal.pmed.1003078
90. Ferraro S, Benedetti S, Mannarino S, Marcovina S, Mario Biganzoli E, and Zuccotti G. Prediction of atherosclerotic cardiovascular risk in early childhood. Clin Chim Acta. (2024) 552:117684. doi: 10.1016/j.cca.2023.117684
91. Koskinen JS, Kytö V, Juonala M, Viikari JSA, Nevalainen J, Kähönen M, et al. Childhood risk factors and carotid atherosclerotic plaque in adulthood: The Cardiovascular Risk in Young Finns Study. Atherosclerosis. (2020) 293:18–25. doi: 10.1016/j.atherosclerosis.2019.11.029
92. Simeunovic A, Brunborg C, Heier M, Seljeflot I, Dahl-Jørgensen K, and Margeirsdottir HD. Sustained low-grade inflammation in young participants with childhood onset type 1 diabetes: The Norwegian atherosclerosis and childhood diabetes (ACD) study. Atherosclerosis. (2023) 379:117151. doi: 10.1016/j.atherosclerosis.2023.05.020
93. Luca AC, David SG, David AG, Ţarcă V, Pădureţ IA, Mîndru DE, et al. Atherosclerosis from newborn to adult-epidemiology, pathological aspects, and risk factors. Life (Basel). (2023) 13:2056. doi: 10.3390/life13102056
94. Santos MG, Pegoraro M, Sandrini F, and Macuco EC. Risk factors for the development of atherosclerosis in childhood and adolescence. Arq Bras Cardiol. (2008) 90:276–83. doi: 10.1590/s0066-782x2008000400012
95. Heier M, Margeirsdottir HD, Torjesen PA, Seljeflot I, Stensæth KH, Gaarder M, et al. The advanced glycation end product methylglyoxal-derived hydroimidazolone-1 and early signs of atherosclerosis in childhood diabetes. Diabetes Vasc Dis Res. (2015) 12:139–45. doi: 10.1177/1479164114560910
96. Wallace SR, Pagano PJ, and Kračun D. MicroRNAs in the regulation of NADPH oxidases in vascular diabetic and ischemic pathologies: A case for alternate inhibitory strategies? Antioxid (Basel). (2022) 12:70. doi: 10.3390/antiox12010070
97. Xourgia E, Papazafiropoulou A, and Melidonis A. Circulating microRNAs as biomarkers for diabetic neuropathy: A novel approach. World J Exp Med. (2018) 8:18–23. doi: 10.5493/wjem.v8.i3.18
98. Peters LJF, Biessen EAL, Hohl M, Weber C, van der Vorst EPC, and Santovito D. Small things matter: relevance of microRNAs in cardiovascular disease. Front Physiol. (2020) 11:793. doi: 10.3389/fphys.2020.00793
99. Hutny M, Hofman J, Zachurzok A, and Matusik P. MicroRNAs as the promising markers of comorbidities in childhood obesity-A systematic review. Pediatr Obes. (2022) 17:e12880. doi: 10.1111/ijpo.12880
100. Yu H and Cao H. MicroRNA-98 inhibition accelerates the development of atherosclerosis via regulation of dysfunction of endothelial cell. Clin Exp Hypertens. (2023) 45:2206068. doi: 10.1080/10641963.2023.2206068
101. Calabriso N, Massaro M, Scoditti E, Carluccio C, Verri T, and Carluccio MA. Epigenetic mechanisms in vascular inflammation: modulation of endothelial adhesion molecules and endothelium-leukocyte adhesion. Front Biosci (Landmark Ed). (2023) 28:194. doi: 10.31083/j.fbl2809194
102. Li Z, Zhao Y, Suguro S, and Suguro R. MicroRNAs regulate function in atherosclerosis and clinical implications. Oxid Med Cell Longev. (2023) 2023:2561509. doi: 10.1155/2023/2561509
103. Farina FM, Weber C, and Santovito D. The emerging landscape of non-conventional RNA functions in atherosclerosis. Atherosclerosis. (2023) 374:74–86. doi: 10.1016/j.atherosclerosis.2023.01.009
104. Qi L, Xing J, Yuan Y, and Lei M. Noncoding RNAs in atherosclerosis: regulation and therapeutic potential. Mol Cell Biochem. (2024) 479:1279–95. doi: 10.1007/s11010-023-04794-0
105. Wojtasińska A, Frąk W, Lisińska W, Sapeda N, Młynarska E, Rysz J, et al. Novel insights into the molecular mechanisms of atherosclerosis. Int J Mol Sci. (2023) 24:13434. doi: 10.3390/ijms241713434
106. Minjares M, Wu W, and Wang JM. Oxidative stress and microRNAs in endothelial cells under metabolic disorders. Cells. (2023) 12:1341. doi: 10.3390/cells12091341
107. Martinez-Arroyo O, Ortega A, Flores-Chova A, Sanchez-Garcia B, Garcia-Garcia AB, Chaves FJ, et al. High miR-126-3p levels associated with cardiovascular events in a general population. Eur J Intern Med. (2023) 113:49–56. doi: 10.1016/j.ejim.2023.04.013
108. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, et al. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol. (2017) 70:942–54. doi: 10.1016/j.jacc.2017.06.046
109. Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. (2014) 16:1301–9. doi: 10.1002/ejhf.154
110. Li Y, Duan JZ, He Q, and Wang CQ. miR−155 modulates high glucose−induced cardiac fibrosis via the Nrf2/HO−1 signaling pathway. Mol Med Rep. (2020) 22:4003–16. doi: 10.3892/mmr.2020.11495
111. Raut SK, Singh GB, Rastogi B, Saikia UN, Mittal A, Dogra N, et al. miR-30c and miR-181a synergistically modulate p53-p21 pathway in diabetes induced cardiac hypertrophy. Mol Cell Biochem. (2016) 417:191–203. doi: 10.1007/s11010-016-2729-7
112. Singh GB, Raut SK, Khanna S, Kumar A, Sharma S, Prasad R, et al. MicroRNA-200c modulates DUSP - 1 expression in diabetes-induced cardiac hypertrophy. Mol Cell Biochem. (2017) 424:1–11. doi: 10.1007/s11010-016-2838-3
113. Demirel F, Tepe D, Kara O, and Esen I. Microvascular complications in adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. (2013) 5:145–9. doi: 10.4274/Jcrpe.994
114. Cho YH, Craig ME, Hing S, Gallego PH, Poon M, Chan A, et al. Microvascular complications assessment in adolescents with 2- to 5-yr duration of type 1 diabetes from 1990 to 2006. Pediatr Diabetes. (2011) 12:682–9. doi: 10.1111/j.1399-5448.2011.00762.x
115. Bjornstad P, Dart A, Donaghue KC, Dost A, Feldman EL, Tan GS, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Microvascular and macrovascular complications in children and adolescents with diabetes. Pediatr Diabetes. (2022) 23:1432–50. doi: 10.1111/pedi.13444
116. El-Samahy MH, Adly AA, Elhenawy YI, Ismail EA, Pessar SA, Mowafy ME, et al. Urinary miRNA-377 and miRNA-216a as biomarkers of nephropathy and subclinical atherosclerotic risk in pediatric patients with type 1 diabetes. J Diabetes Complicat. (2018) 32:185–92. doi: 10.1016/j.jdiacomp.2017.10.014
117. Ruiz MA and Chakrabarti S. MicroRNAs: the underlying mediators of pathogenetic processes in vascular complications of diabetes. Can J Diabetes. (2013) 37:339–44. doi: 10.1016/j.jcjd.2013.07.003
118. Marcovecchio ML, Chiesa ST, Armitage J, Daneman D, Donaghue KC, Jones TW, et al. Renal and cardiovascular risk according to tertiles of urinary albumin-to-creatinine ratio: the adolescent type 1 diabetes cardio-renal intervention trial (AdDIT). Diabetes Care. (2018) 41:1963–9. doi: 10.2337/dc18-1125
119. Qadir MMF, Klein D, Álvarez-Cubela S, Domínguez-Bendala J, and Pastori RL. The role of microRNAs in diabetes-related oxidative stress. Int J Mol Sci. (2019) 20:5423. doi: 10.3390/ijms20215423
120. Zeni L, Norden AGW, Prandi E, Canepa C, Burling K, Simpson K, et al. Exploration of a panel of urine biomarkers of kidney disease in two paediatric cohorts with Type 1 diabetes mellitus of differing duration. Diabetol Metab Syndr. (2022) 14:71. doi: 10.1186/s13098-022-00839-4
121. Jankauskas SS, Gambardella J, Sardu C, Lombardi A, and Santulli G. Functional role of miR-155 in the pathogenesis of diabetes mellitus and its complications. Noncoding RNA. (2021) 7:39. doi: 10.3390/ncrna7030039
122. Bratina N, Auzanneau M, Birkebaek N, de Beaufort C, Cherubini V, Craig ME, et al. Differences in retinopathy prevalence and associated risk factors across 11 countries in three continents: A cross-sectional study of 156,090 children and adolescents with type 1 diabetes. Pediatr Diabetes. (2022) 23:1656–64. doi: 10.1111/pedi.13416
123. Garavelli S, Prattichizzo F, Ceriello A, Galgani M, and de Candia P. Type 1 diabetes and associated cardiovascular damage: contribution of extracellular vesicles in tissue crosstalk. Antioxid Redox Signal. (2022) 36:631–51. doi: 10.1089/ars.2021.0053
124. Mazzeo A, Lopatina T, Gai C, Trento M, Porta M, and Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp Eye Res. (2019) 184:56–63. doi: 10.1016/j.exer.2019.04.015
125. Guo Y, Du F, Tan YL, Luo J, Xiong D, and Song WT. -mediated angiogenesis in retinopathy of prematurity is co-regulated by miR-17-5p and miR-20a-5p. Biochem Cell Biol. (2021) 99:414–23. doi: 10.1139/bcb-2020-0357
126. Metin T, Dinç E, Görür A, Erdoğan S, Ertekin S, Sarı AA, et al. Evaluation of the plasma microRNA levels in stage 3 premature retinopathy with plus disease: preliminary study. Eye (Lond). (2018) 32:415–20. doi: 10.1038/eye.2017.193
127. Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, and Ma JX. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest Ophthalmol Vis Sci. (2013) 54:1689–97. doi: 10.1167/iovs.12-10921
128. Ghaffari M, Razi S, Zalpoor H, Nabi-Afjadi M, Mohebichamkhorami F, and Zali H. Association of microRNA-146a with type 1 and 2 diabetes and their related complications. J Diabetes Res. (2023) 2023:2587104. doi: 10.1155/2023/2587104
129. Porter M, Channa R, Wagner J, Prichett L, Liu TYA, and Wolf RM. Prevalence of diabetic retinopathy in children and adolescents at an urban tertiary eye care center. Pediatr Diabetes. (2020) 21:856–62. doi: 10.1111/pedi.13037
130. Sidorkiewicz I, Niemira M, Maliszewska K, Erol A, Bielska A, Szalkowska A, et al. Circulating miRNAs as a predictive biomarker of the progression from prediabetes to diabetes: outcomes of a 5-year prospective observational study. J Clin Med. (2020) 9:2184. doi: 10.3390/jcm9072184
131. Venneri M and Passantino A. MiRNA: what clinicians need to know. Eur J Intern Med. (2023) 113:6–9. doi: 10.1016/j.ejim.2023.05.024
132. Simeoli R and Fierabracci A. Insights into the role of microRNAs in the onset and development of diabetic neuropathy. Int J Mol Sci. (2019) 20:4627. doi: 10.3390/ijms20184627
133. Osipova J, Fischer DC, Dangwal S, Volkmann I, Widera C, Schwarz K, et al. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J Clin Endocrinol Metab. (2014) 99:E1661–5. doi: 10.1210/jc.2013-3868
134. Bielska A, Niemira M, and Kretowski A. Recent highlights of research on miRNAs as early potential biomarkers for cardiovascular complications of type 2 diabetes mellitus. Int J Mol Sci. (2021) 22:3153. doi: 10.3390/ijms22063153
135. Kaur P, Kotru S, Singh S, and Munshi A. Role of miRNAs in diabetic neuropathy: mechanisms and possible interventions. Mol Neurobiol. (2022) 59:1836–49. doi: 10.1007/s12035-021-02662-w
136. Viñals C, Conget I, Granados M, Giménez M, and Amor AJ. Evaluation of cardiovascular risk in people with type 1 diabetes: A comprehensive and specific proposed practical approach. Diabetes Ther. (2024) 15:1831–44. doi: 10.1007/s13300-024-01616-4
137. Group DCaCTDEoDIaCESR. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. (2016) 39:686–93. doi: 10.2337/dc15-1990
138. Alatawi Z and Mirghani H. The association between glycemic variability and myocardial infarction: A review and meta-analysis of prospective studies and randomized trials. Cureus. (2020) 12:e11556. doi: 10.7759/cureus.11556
139. Yang SW, Park KH, and Zhou YJ. The impact of hypoglycemia on the cardiovascular system: physiology and pathophysiology. Angiology. (2016) 67:802–9. doi: 10.1177/0003319715623400
140. Kamrath C, Tittel SR, Kapellen TM, von dem Berge T, Heidtmann B, Nagl K, et al. Early versus delayed insulin pump therapy in children with newly diagnosed type 1 diabetes: results from the multicentre, prospective diabetes follow-up DPV registry. Lancet Child Adolesc Health. (2021) 5:17–25. doi: 10.1016/S2352-4642(20)30339-4
141. Derosa G, Catena G, Scelsi L, D’Angelo A, Raddino R, Cosentino E, et al. Glyco-metabolic control, inflammation markers, and cardiovascular outcomes in type 1 and type 2 diabetic patients on insulin pump or multiple daily injection (italico study). Diabetes Metab Res Rev. (2020) 36:e3219. doi: 10.1002/dmrr.3219
142. Steineck I, Cederholm J, Eliasson B, Rawshani A, Eeg-Olofsson K, Svensson AM, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. (2015) 350:h3234. doi: 10.1136/bmj.h3234
143. Rosenlund S, Theilade S, Hansen TW, Andersen S, and Rossing P. Treatment with continuous subcutaneous insulin infusion is associated with lower arterial stiffness. Acta Diabetol. (2014) 51:955–62. doi: 10.1007/s00592-014-0619-6
144. Faienza MF, Scicchitano P, Lamparelli R, Zaza P, Cecere A, Brunetti G, et al. Vascular and myocardial function in young people with type 1 diabetes mellitus: insulin pump therapy versus multiple daily injections insulin regimen. Exp Clin Endocrinol Diabetes. (2022) 130:415–22. doi: 10.1055/a-1523-7574
145. Huang L, Pan Y, Zhou K, Liu H, and Zhong S. Correlation between glycemic variability and diabetic complications: A narrative review. Int J Gen Med. (2023) 16:3083–94. doi: 10.2147/IJGM.S418520
146. Jamiołkowska M, Jamiołkowska I, Łuczyński W, Tołwińska J, Bossowski A, and Głowińska Olszewska B. Impact of real-time continuous glucose monitoring use on glucose variability and endothelial function in adolescents with type 1 diabetes: new technology–new possibility to decrease cardiovascular risk? J Diabetes Res. (2016) 2016:4385312. doi: 10.1155/2016/4385312
147. Szadkowska A, Chobot A, Głowińska-Olszewska B, Jarosz-Chobot P, Mianowska B, Myśliwiec M, et al. Guidelines of the Polish Society of Pediatric Endocrinology and Diabetology and Pediatric Section of Diabetes Poland on insulin therapy using hybrid closed-loop systems in children and adolescents with diabetes in Poland. Pediatr Endocrinol Diabetes Metab. (2024) 30:132–47. doi: 10.5114/pedm.2024.144041
148. Scott ES, Januszewski AS, Carroll LM, Fulcher GR, Joglekar MV, Hardikar AA, et al. Continuous subcutaneous insulin infusion alters microRNA expression and glycaemic variability in children with type 1 diabetes. Sci Rep. (2021) 11:16656. doi: 10.1038/s41598-021-95824-8
149. Zhao W, Yin Y, Cao H, and Wang Y. Exercise Improves Endothelial Function via the lncRNA MALAT1/miR-320a Axis in Obese Children and Adolescents. Cardiol Res Pract. (2021) 2021:8840698. doi: 10.1155/2021/8840698
150. Wang J, Liew OW, Richards AM, and Chen YT. Overview of microRNAs in cardiac hypertrophy, fibrosis, and apoptosis. Int J Mol Sci. (2016) 17:749. doi: 10.3390/ijms17050749
151. Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW, and Chakrabarti S. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med. (2014) 18:415–21. doi: 10.1111/jcmm.12218
152. Donghui T, Shuang B, Xulong L, Meng Y, Yujing G, Yujie H, et al. Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Microvasc Res. (2019) 123:86–91. doi: 10.1016/j.mvr.2018.10.009
153. Yu M, Liu Y, Zhang B, Shi Y, Cui L, and Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc Pathol. (2015) 24:375–81. doi: 10.1016/j.carpath.2015.06.003
Keywords: type 1 diabetes, children, cardiovascular risk factors, cardiovascular complications, miRNA, precise medicine
Citation: Peczyńska J, Odyjewska E, Koszykowska K, Jamiołkowska-Sztabkowska M, Bossowski A and Głowińska-Olszewska B (2025) Cardiovascular risk factors and modern therapeutic strategies in children and adolescents with type 1 diabetes to prevent future diabetic angiopathy in the era of innovative miRNAs biomarkers. Front. Endocrinol. 16:1638770. doi: 10.3389/fendo.2025.1638770
Received: 31 May 2025; Accepted: 18 August 2025;
Published: 08 September 2025.
Edited by:
Antonio Rossi, University of Milan, ItalyReviewed by:
Moufida Ben Nasr, University of Milan, ItalyLoredana Bucciarelli, University of Milan, Italy
Copyright © 2025 Peczyńska, Odyjewska, Koszykowska, Jamiołkowska-Sztabkowska, Bossowski and Głowińska-Olszewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Głowińska-Olszewska, YmFyYmFyYS5nbG93aW5za2Etb2xzemV3c2thQHVtYi5lZHUucGw=
 Joanna Peczyńska
Joanna Peczyńska Emilia Odyjewska
Emilia Odyjewska Kamila Koszykowska
Kamila Koszykowska Milena Jamiołkowska-Sztabkowska
Milena Jamiołkowska-Sztabkowska Artur Bossowski
Artur Bossowski Barbara Głowińska-Olszewska
Barbara Głowińska-Olszewska