- 1Department of Clinical Laboratory, Guangdong Women and Children Hospital, Guangzhou, China
- 2Women and Children’s Hospital, Southern University of Science and Technology, Guangzhou, China
Background: The impact of serum vitamin D levels on the success of assisted reproductive technology (ART) among women with polycystic ovary syndrome (PCOS) has not been fully explored.
Methods: This cohort study enrolled 111 patients with PCOS who underwent in vitro fertilization (IVF) or intracyto–plasmic sperm injection (ICSI) treatment at Guangdong Women and Children Hospital from January 2022 to October 2024. Serum 25–hydroxyvitamin D [25(OH)D] concentrations were measured before embryo transfer (ET). The outcomes included the pregnancy rate, live birth rate, and early miscarriage rate. Multivariable logistic regression and the receiver operating characteristic (ROC) curve analyses were performed to evaluate predictive thresholds.
Results: Patients achieving clinical pregnancy (n=76, 68.47%) exhibited significantly higher pre–ET 25(OH)D levels (65.28 ± 18.90 vs. 52.22 ± 15.14, nmol/L, P < 0.001) and luteinizing hormone (LH) levels (9.52 ± 6.37 vs. 6.94 ± 4.52, IU/L, P = 0.021) compared to non–pregnant counterparts. Logistic regression, after adjusting for LH, demographics and hormonal profiles, revealed that 25(OH)D was significantly associated with pregnancy (B = 0.13, P = 0.030). ROC analysis identified 67.95 nmol/L as the optimal 25(OH)D cutoff for predicting pregnancy (with an area under the curve of 0.703, a specificity of 94.3%, and a sensitivity of 36.8%). Furthermore, pregnancies with pre–ET 25(OH)D ≥67.95 nmol/L had significantly higher live birth rates (75.00% vs. 52.08%, P = 0.049). Live birth cases maintained higher 25(OH)D levels than early miscarriages (67.63 ± 19.32 vs. 58.22 ± 16.01, nmol/L, P = 0.042).
Conclusions: Pre–ET serum vitamin D levels may serve as a modifiable biomarker for optimizing ART success in women with PCOS, associated with enhanced pregnancy likelihood and live birth outcomes. Systematic vitamin D supplementation prior to ET warrants further investigation as a potential adjuvant strategy.
Clinical Trial Registration: https://www.medicalresearch.org.cn, identifier MR-44-25-016940.
Introduction
Polycystic ovary syndrome (PCOS) constitutes one of the most commonly encountered endocrine and metabolic disorders in reproductive–aged women, with estimated prevalence rates ranging between 10% and 13% (1). The syndrome manifests through heterogeneous clinical features, typically including hyperandrogenemia, ovulatory dysfunction and polycystic ovarian morphology, and is frequently complicated by obesity, insulin resistance, and elevated cardiovascular risk. While the precise pathophysiology remains incompletely understood, current evidence supports a multifactorial etiology involving genetic predisposition, environmental influences, and lifestyle factors (2).
Reproductive impairment, affecting 70–80% of patients with POCS (3), is largely attributed to endocrine dysregulation characterized by reduced follicle–stimulating hormone (FSH) levels impairing follicular maturation, coupled with elevated luteinizing hormone (LH) and androgen concentrations. This hormonal imbalance disrupts the menstrual cycle, leading to oligo/anovulation and subsequent infertility. For patients who fail to conceive through lifestyle adjustments and ovulation induction therapy, assisted reproductive technology (ART)–particularly in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI)–has become essential for achieving conception. These techniques assist conception through a series of complex procedures, including ovarian stimulation with medication, in vitro fertilization, and embryo transfer (ET). However, treatment outcomes remain compromised due to PCOS–related pathophysiological complexities, with lower–than–anticipated pregnancy rates and heightened risks of both procedural complications and adverse gestational outcomes (4–6). These challenges underscore the need for systematic exploration of adjustable prognostic determinants to optimize therapeutic strategies.
Accumulating evidence indicates that vitamin D plays a crucial role in human reproductive health. The ubiquitous distribution of vitamin D receptors in female reproductive tissues underpins their regulatory roles in ovarian steroidogenesis, folliculogenesis, endometrial receptivity maturation, and embryo implantation, thereby influencing critical reproductive phases from menstrual cyclicity to fertility outcomes (7–9). Notably, PCOS populations exhibit higher prevalence of vitamin D deficiency compared to the general population (10, 11). Emerging evidence links this deficiency to suboptimal ART outcomes. Some studies indicate that maintaining an adequate level of vitamin D might be helpful in improving the pregnancy rate and live birth rate of PCOS populations after undergoing ART (7, 12, 13). Nevertheless, current evidence remains limited regarding vitamin D’s specific impact on ART outcomes in PCOS populations.
This study is designed to investigate the association between serum vitamin D status and pregnancy outcomes of ART in PCOS populations. Our findings may enhance understanding of vitamin D’s therapeutic potential in PCOS–related infertility management and inform strategies for optimizing ART protocols.
Methods
Study design
This prospective cohort study investigated the association between pre-embryo transfer serum 25-hydroxyvitamin D [25(OH)D] concentrations and IVF/ICSI outcomes in women with PCOS. Consecutively enrolled PCOS patients undergoing fresh or frozen-thawed embryo transfer cycles at Guangdong Women and Children Hospital (January 2022–October 2024) were included. This study was approved by the Ethics Committee of Guangdong Women and Children Hospital (Approval No. 20251009) and conducted in accordance with the ethical principles of the Declaration of Helsinki.
Patients
We collected data of patients with PCOS who received IVF/ICSI treatment at Guangdong Women and Children Hospital from January 2022 to October 2024.
Inclusion criteria
(1) Meeting the Rotterdam diagnostic criteria: PCOS is diagnosed if two of the following three conditions are satisfied: hyperandrogenism, oligoovulation, and polycystic ovarian changes as assessed by ultrasound (>12 cysts or ovarian volume >10 mL); (2) aged 18–45 years; and (3) receiving IVF/ICSI treatment at Guangdong Women and Children Hospital.
Exclusion criteria
(1) Individuals with systemic diseases such as metabolic syndrome, diabetes, hyperlipidemia, and cardiovascular diseases; (2) patients with thyroid diseases or congenital adrenal hyperplasia; (3) patients with severe endometriosis, ovarian or cervical tumors, or HPV infection; (4) patients who smoke and/or consume alcohol; (5) patients who use any drugs that affect reproductive physiology within three months before the study, except for the treatment of PCOS; (6) patients who employ any medications that have an impact on reproductive physiology within three months preceding the study, with the exception of those for the treatment of PCOS; (7) patients with any autoimmune diseases; and (8) patients undergoing ART due to male factor infertility.
Clinical data
The clinical data of patients, including age, body mass index (BMI), sex hormone–binding globulin (SHGB), and hormone levels, including serum follicle–stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), testosterone, anti–Müllerian hormone (AMH), and androgens, were collected.
Measurement of the 25(OH)D concentration
The acquisition of blood samples during the IVF/ICSI treatment of patients for the purpose of evaluating vitamin D levels. All the vitamin D assays were carried out punctually in our clinical laboratory. Serum vitamin D was determined by chemiluminescence technology to measure total 25–hydroxyvitamin D [25(OH)D] (Architect, Abbott Diagnostics, USA). Its detection sensitivity is 8.8 nmol/L, and the inter–assay and intra–assay coefficients of variation are 9.0% and 5.5%, respectively.
IVF/ICSI–ET procedure and outcomes
The IVF/ICSI–ET procedure and its outcomes are referred to in the published literature by Xu et al. (14) Clinical pregnancy was defined as positive serum human chorionic gonadotropin (hCG) and the presence of a gestational sac and fetal heart activity on ultrasound examination. The primary outcome was pregnancy, and the secondary outcomes included live birth and early miscarriage. Early miscarriage refers to miscarriage that occurs within 12 weeks of gestation.
Statistical analysis
All the data were statistically analyzed via SPSS 24.0, and P < 0.05 was considered statistically significant. Continuous variables with a normal distribution are expressed as the mean ± SD, and Student’s t test was used to compare the differences in patient characteristics between groups. Data with a non-normal distribution are represented by the median and confidence interval, and the Mann–Whitney U test was used. Categorical variables are expressed as numbers and percentages, and the Pearson chi–square test or Fisher’s exact test was used for comparisons. Univariate logistic regression was used to calculate the OR for the preliminary test of the association between VD levels and IVF/ICSI treatment outcomes. Multivariate logistic regression was used to adjust the OR estimates of relevant covariates. For the primary outcome of treatment, pregnancy, we created two models: Model 1 included factors with significant differences in univariate logistic regression, namely, LH; Model 2 included adjusted Model 1, age, BMI, vitamin D supplement medications, SHBG, testosterone, AMH, PRL, LH, FSH, androstenedione, and the homeostasis model assessment insulin resistance index (HOMA–IR).
Results
General characteristics of PCOS patients undergoing ART
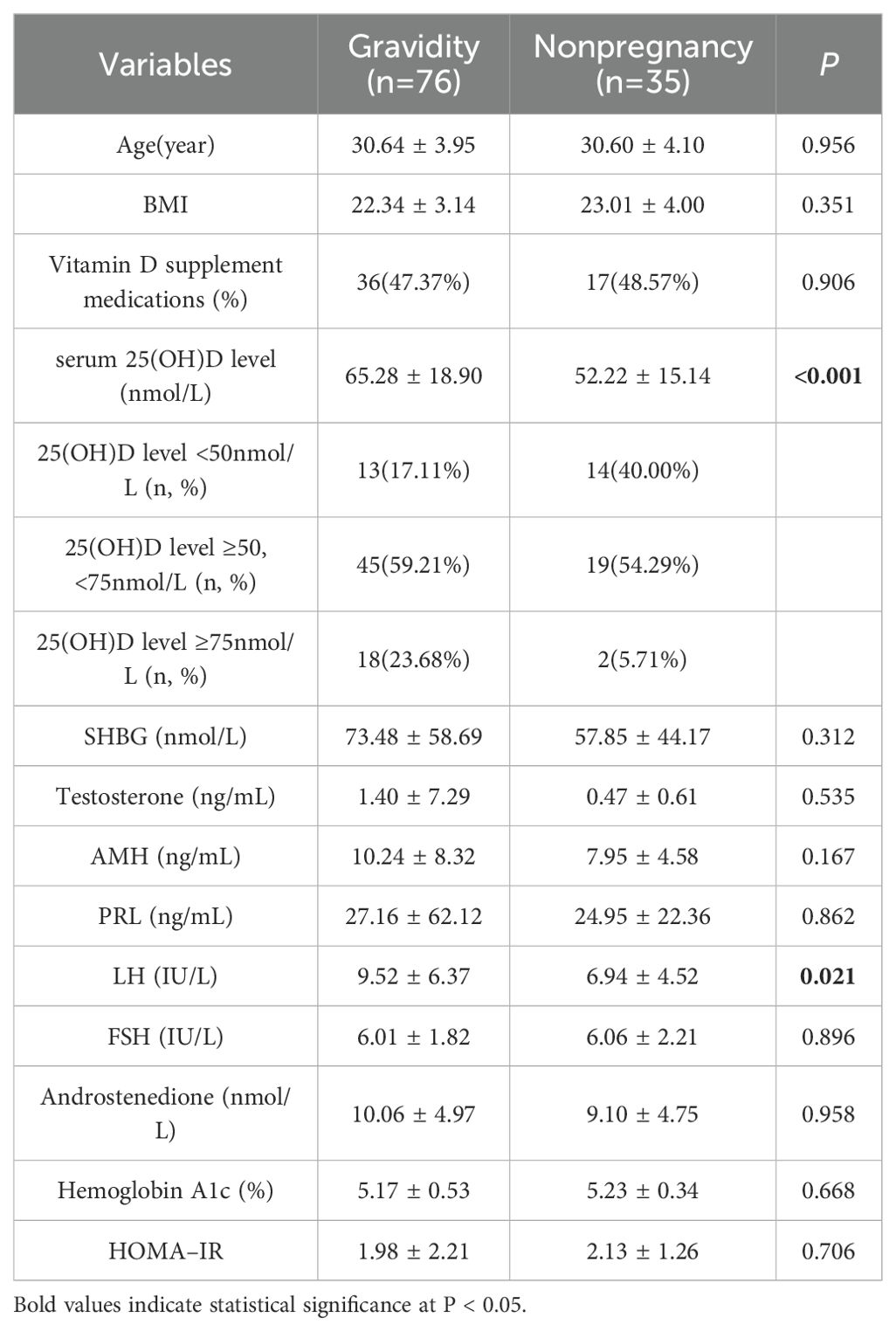
A total of 111 patients with PCOS who were undergoing IVF/ICSI treatment were included in this study. Prior to ET, tests for serum 25(OH) D and other hormone levels were carried out. The details of the general characteristics, including demographics and laboratory data, are summarized in Table 1. In accordance with the clinical pregnancy diagnostic criteria, these patients were divided into two groups: the gravidity group (with a mean age of 30.64 ± 3.95 years, a mean BMI of 22.34 ± 3.14, and a total of 76 patients, accounting for 68.47%) and the nonpregnancy group (with a mean age of 30.60 ± 4.10 years, a mean BMI of 23.01 ± 4.00, and a total of 35 patients, accounting for 31.53%). There was no significant difference between the two groups with respect to the use of vitamin D supplement medications before ET (P = 0.906). Compared with those in non-pregnant patients, 25(OH)D (65.28 ± 18.90 vs. 52.22 ± 15.14, nmol/L, P < 0.001) and LH (9.52 ± 6.37 vs. 6.94 ± 4.52, IU/L, P = 0.021) levels in pregnant patients were significantly elevated. Other clinical indicators, such as SHBG (73.48 ± 58.69 vs. 57.85 ± 44.17, nmol/L, P = 0.312), testosterone (1.40 ± 7.29 vs. 0.47 ± 0.61, ng/mL, P = 0.535), AMH (10.24 ± 8.32 vs. 7.95 ± 4.58, ng/mL, P = 0.167), PRL (27.16 ± 62.12 vs. 24.95 ± 22.36, ng/mL, P = 0.862), FSH (6.01 ± 1.82 vs. 6.06 ± 2.21, IU/L, P = 0.896), androstenedione (10.06 ± 4.97 vs. 9.10 ± 4.75, nmol/L, P = 0.958), hemoglobin A1c (5.17 ± 0.53 vs. 5.23 ± 0.34, %, P = 0.668) and HOMA–IR (1.98 ± 2.21 vs. 2.13 ± 1.26, P = 0.706) levels were did not exhibit significant differences.
Associations between vitamin D and gravidity in PCOS patients who had undergone ART
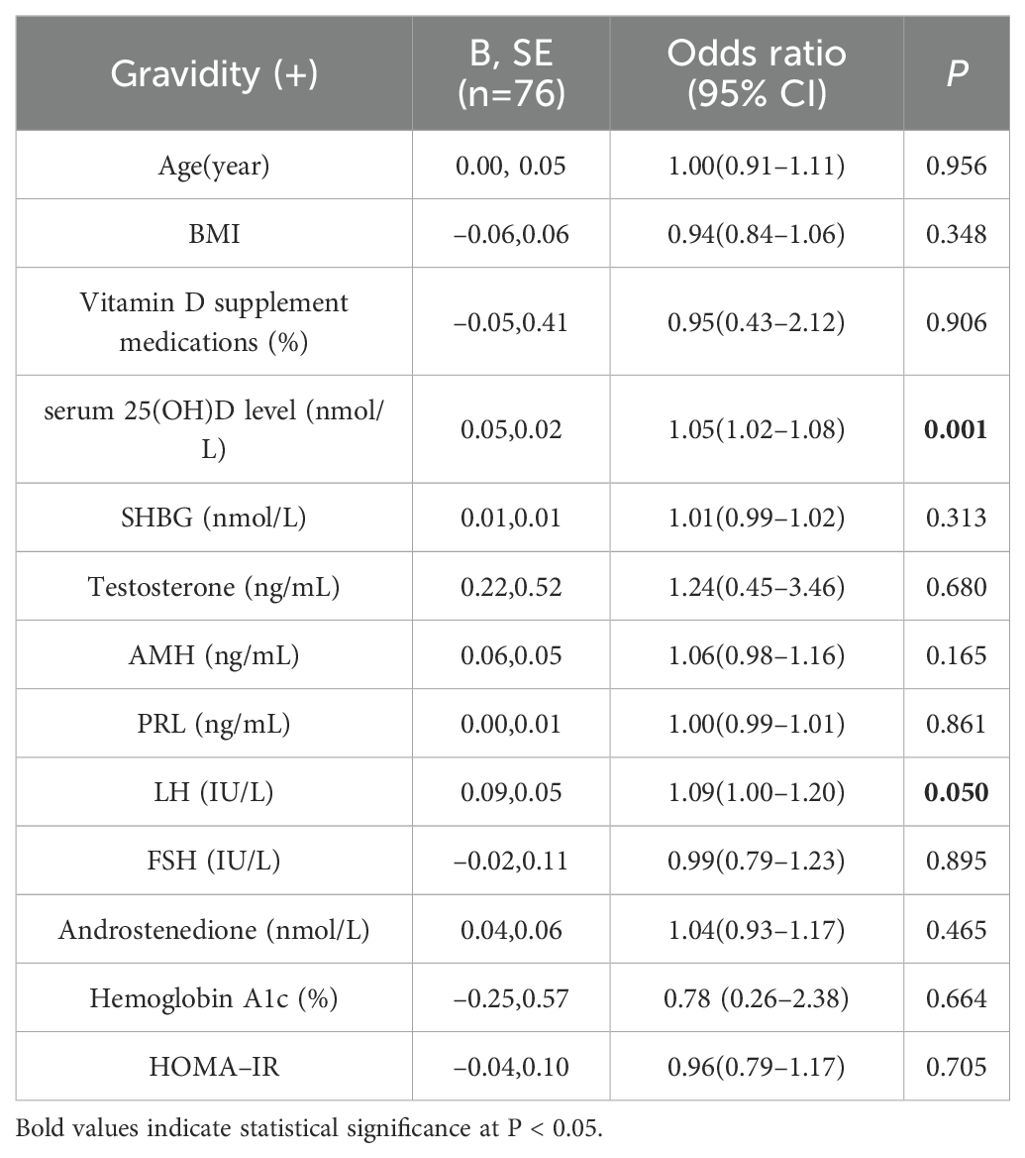
Univariate logistic regression analysis revealed that the serum 25(OH)D (B = 0.05, P = 0.001) and LH (B = 0.09, P = 0.050) levels were strongly correlated with pregnancy in PCOS patients who had undergone IVF/ICSI (Table 2).
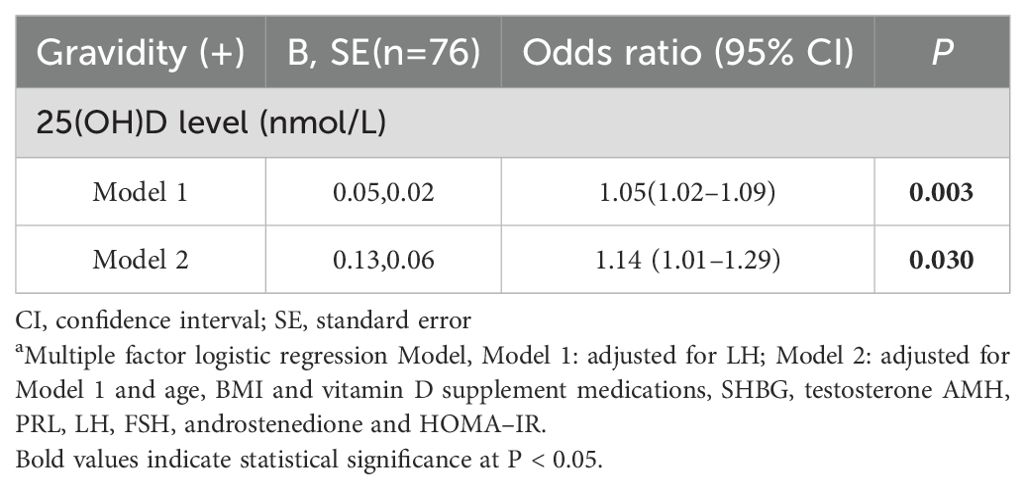
The relationship between vitamin D and gravidity in PCOS patients who had undergone ART was evaluated via a multivariate logistic regression model. After adjusting for LH, it was revealed that 25(OH)D levels were significantly linked with pregnancy (Model 1, P = 0.003). Similar results were obtained after further adjustment for age, BMI, history of taking vitamin D supplement medications, SHBG, testosterone, AMH, PRL, FSH, androstenedione and HOMA–IR (Model 2, P = 0.030) (Table 3).
In accordance with the analysis of the receiver operating characteristic (ROC) curve model, the cutoff point of serum 25(OH)D levels for predicting whether PCOS patients could achieve pregnancy subsequent to ART (Youden’s index) was 67.95 nmol/L, which demonstrated the optimal predictive effect (area under the curve: 0.703; specificity: 94.3%; sensitivity: 36.8%) (Figure 1).
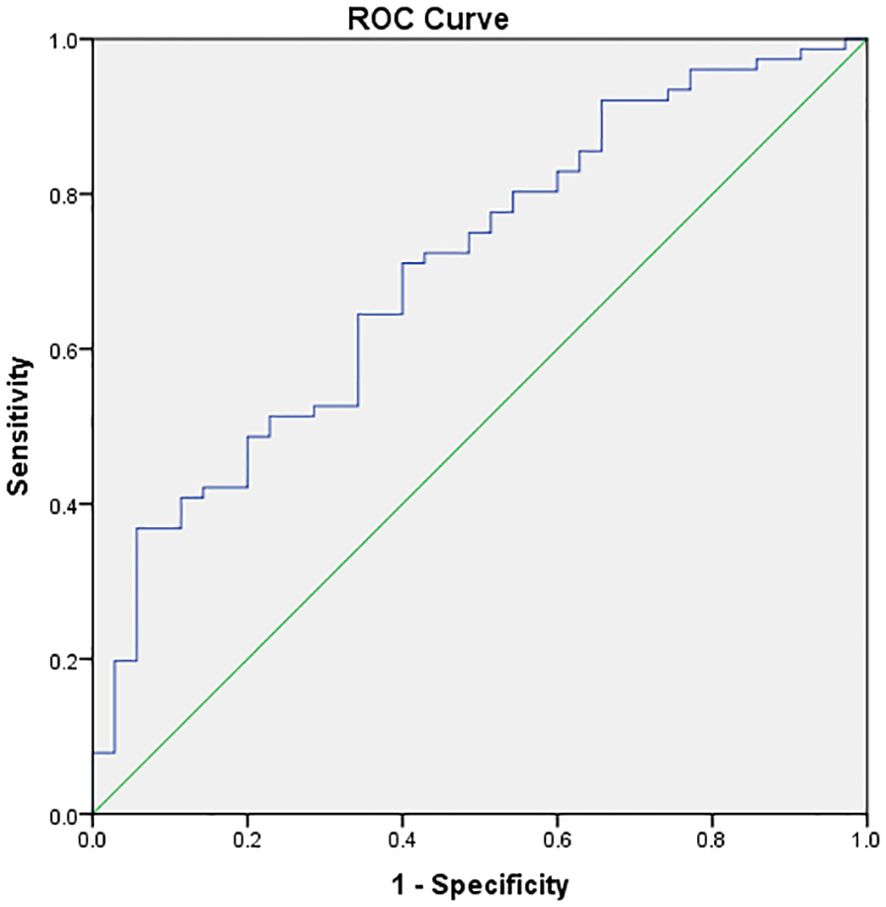
Figure 1. ROC curve model analysis of vitamin D and pregnancy. The X–axis represents 1–specificity, and the Y–axis represents sensitivity. The cutoff point of vitamin D for predicting pregnancy (Youden’s index) was 67.95 nmol/L, which was the best predictive value (area under the curve: 0.703, specificity: 94.3% and sensitivity: 36.8%).
Association between live birth and vitamin D in pregnant patients after receiving ART
To further investigate the relationship between vitamin D levels and pregnancy outcomes in patients with PCOS who underwent IVF/ICSI, we subdivided the pregnant patients into two subgroups according to the optimal predicted 25(OH) D levels (Table 4). Patients with 25(OH)D ≥ 67.95 nmol/L were more prone to live birth after a successful pregnancy (75.00% vs. 52.08%, P = 0.049).
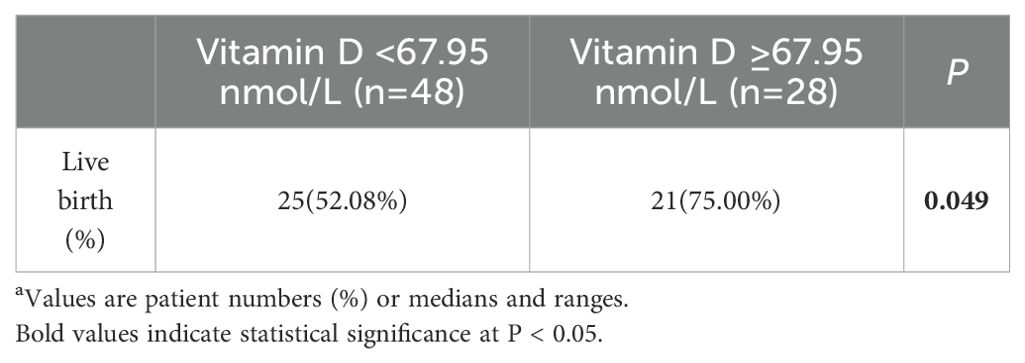
Table 4. Live birth rate after pregnancy in PCOS patients with high serum vitamin D levels a.
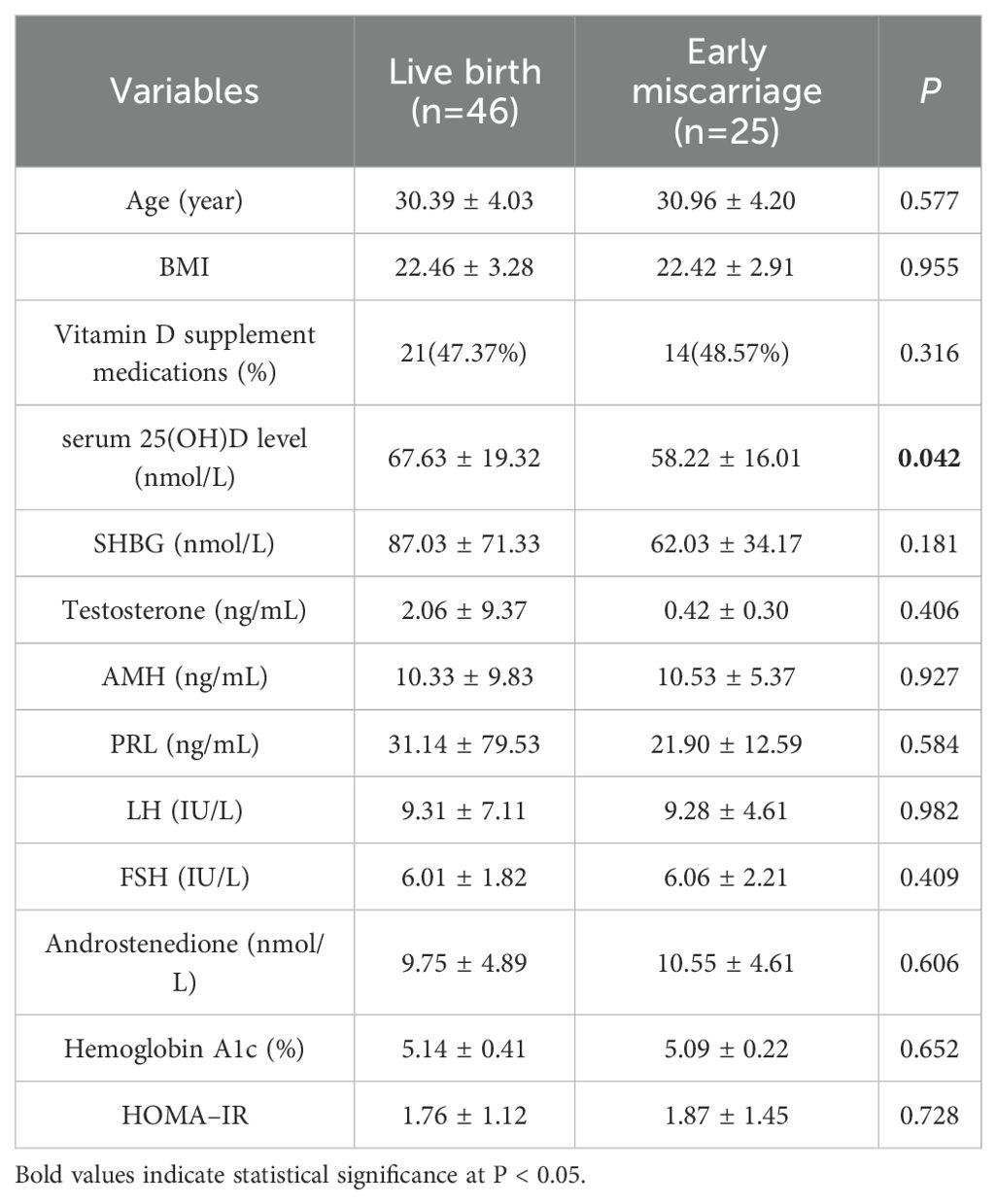
We subsequently compared the levels of vitamin D and other hormones between live births and early miscarriages within the pregnancy group (Table 5). The results demonstrated that the serum 25(OH)D levels in patients with live births were markedly greater than those in patients with early miscarriages (67.63 ± 19.32 vs. 58.22 ± 16.01, nmol/L, P = 0.042), whereas the other indicators were not significantly different.
Discussion
In the present study, we investigated the relationship between serum vitamin D levels and the therapeutic outcomes of ART in patients with PCOS. The results revealed that there was a positive correlation between vitamin D levels and the pregnancy rate of ART in PCOS patients. Moreover, among the PCOS patients who successfully conceived, those with high vitamin D levels presented a higher live birth rate, and the serum vitamin D level of the live birth patients was clearly higher than that of the patients with early miscarriage.
Vitamin D is a fat–soluble steroid hormone, and its receptors exist in various tissues. In addition to playing crucial roles in aspects such as bone health, cardiovascular health, and immune function, its function in female reproductive health has also drawn increasing attention. Research has revealed that vitamin D participates in the follicular development process and the proliferation process of granulosa cells through mechanisms such as regulating hormone levels and anti–inflammatory effects (8, 15). Furthermore, vitamin D is also involved in embryo implantation and the development of the endometrium; therefore vitamin D influences the diverse reproductive cycles of women (16).
Vitamin D deficiency is a risk factor for multiple gynecological and obstetrical diseases, including PCOS and infertility (15, 17). Clinical investigations have shown that in women with PCOS, vitamin D deficiency is related to the occurrence of hyperandrogenism, insulin resistance, metabolic and endocrine disorders, as well as oxidative stress and a proinflammatory environment (11, 18, 19). Nevertheless, some studies have revealed that supplementation with vitamin D has no beneficial effect on patients with PCOS (20, 21). In terms of PCOS–related pregnancies, in contrast to PCOS women with a normal vitamin D level who require ovulation induction, PCOS women with vitamin D deficiency exhibit a lower ovulation rate and live birth rate and an increased risk of early miscarriage (11). Moreover, supplementation with vitamin D can increase the ovulation rate in PCOS patients (22). However, at present, studies on the impact of vitamin D on IVF outcomes in PCOS patients are relatively rare. Hence, more studies are needed for further in–depth exploration. In this study, after excluding the influences of age, BMI, vitamin D supplementation and other hormones, we discovered that the serum vitamin D levels of PCOS patients undergoing IVF were significantly positively correlated with the pregnancy rate after treatment.
Currently, there remains controversy regarding the impact of vitamin D on the treatment outcomes of ART. The majority of studies have shown that maintaining an adequate level of vitamin D leads to a higher pregnancy rate and live birth rate after assisted reproductive treatment (7, 12). However, some studies suggest that the serum vitamin D level does not affect the treatment results of ART, including the pregnancy rate, live birth rate, and miscarriage rate (23). Some studies have indicated that there is a nonlinear correlation between vitamin D and ART results. Specifically, when the vitamin D level is low, it does not affect the ART results, and when an adequate vitamin D level is maintained during ART, the pregnancy rate and live birth rate tend to be higher. Researchers posit that this might be due to the threshold effect of the vitamin D concentration, which leads to inconsistent research results. Xu et al. proposed that 24 ng/ml (approximately 60 nM/L) might be a suitable threshold for predicting reproductive outcomes (7). In our study, we found that a vitamin D level exceeding 67.95 nmol/L before embryo transfer in PCOS patients was the best predictor of pregnancy and live birth. Compared with that of patients who ultimately experienced early miscarriage, the vitamin D level of live–birth patients was markedly greater.
Luteinizing hormone, a gonadotropin secreted by the anterior pituitary gland, stimulates follicular cells to produce androgens and promotes follicle maturation and granulosa cell ovulation (24). In ART, the administration of exogenous recombinant LH has been widely utilized to stimulate follicle development. Previous investigations have demonstrated that supplementing LH is helpful for improving oocyte quality, follicle growth, and pregnancy outcomes (25, 26). In this study, we observed that the LH level in the pregnant group was significantly greater than that in the non-pregnant group, which might be a consequence of the influence of ovulation induction drugs. These findings suggest that reasonable regulation of LH levels during the ART treatment of PCOS patients is beneficial for improving treatment outcomes.
Despite the fact that this study has provided valuable insights, there are nevertheless certain limitations.
First, this is a single–center study that only incorporates Chinese individuals and has a relatively restricted sample size, which might impact the universality of the results. Future multicenter studies with larger cohorts are needed to validate our findings.
Second, we were unable to completely rule out all potential confounding factors. Specifically, data on vitamin D supplementation, nutritional status, and sunlight exposure—key factors known to influence serum vitamin D concentrations —were not systematically collected (19, 27). Seasonal variations in vitamin D levels, a well-documented phenomenon (27), were also not accounted for in the analysis.
Third, although couples with known male infertility factors were excluded from this study, we did not evaluate subtle male parameters (e.g., sperm DNA fragmentation) or partners’ vitamin D status. Research indicates that vitamin D plays a critical role in maintaining male reproductive health by influencing spermatogenesis, sperm motility, and hormonal regulation (16, 28, 29).
Fourth, in ART, embryo transfer occurs at two key stages: cleavage-stage transfer and blastocyst-stage transfer. Frozen embryo transfer (FET) is increasingly preferred for achieving pregnancy post-IVF, particularly benefiting anovulatory women. In this study, the developmental stage of transferred embryos was not specified or analyzed; future research should incorporate this variable (30, 31).
Finally, this was a cross-sectional study with vitamin D measured only pre-transfer; dynamic monitoring of vitamin D fluctuations during treatment was not performed, preventing assessment of its longitudinal impact on outcomes.
These limitations may contribute to the relatively low sensitivity of our predictive model. Future studies should incorporate these variables and employ longitudinal designs to better elucidate the causal relationship between vitamin D dynamics and ART outcomes.
In summary, this study provides a new perspective on the application of vitamin D in ART, suggesting that the serum vitamin D level in PCOS patients may have predictive value for their pregnancy outcomes. These findings might offer new evidence for optimizing reproductive treatment regimens for patients with PCOS.
Conclusion
In this prospective study of PCOS women undergoing IVF/ICSI treatment, pre-embryo transfer serum 25(OH)D concentrations demonstrated significant predictive utility for ART outcomes. Key findings revealed that higher 25(OH)D levels (≥68 nmol/L) were independently associated with increased clinical pregnancy rates, enhanced live birth probabilities, and reduced early miscarriage risks. The identification of a 25(OH)D threshold at 68 nmol/L—characterized by high specificity (94.3%) for pregnancy prediction—suggests its potential as a clinically actionable biomarker. While the modest sensitivity (36.8%) underscores the multifactorial nature of ART success, our data imply that optimizing vitamin D status prior to embryo transfer may represent a modifiable strategy to improve reproductive outcomes in this population. These findings advocate for systematic screening and targeted supplementation in vitamin D-deficient PCOS patients undergoing ART. Further randomized controlled trials are warranted to establish causality and refine evidence-based thresholds for clinical implementation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving human data was approved by the Ethics Committee of Guangdong Women and Children Hospital (Approval No. 20251009) and conducted in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived by the ethics committee as this retrospective study analyzed de-identified clinical records and serum vitamin D data obtained during routine clinical procedures. All data handling complied with national data protection regulations and institutional policies.
Author contributions
LG: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. XHZ: Data curation, Formal Analysis, Writing – original draft. CG: Data curation, Visualization, Writing – review & editing. ZZ: Data curation, Formal Analysis, Validation, Writing – review & editing. XDZ: Formal Analysis, Methodology, Software, Writing – review & editing. XR: Data curation, Investigation, Validation, Writing – review & editing. HL: Formal Analysis, Investigation, Methodology, Writing – review & editing. QM: Formal Analysis, Investigation, Software, Writing – review & editing. WW: Data curation, Formal Analysis, Writing – review & editing. ML: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article.
Acknowledgments
Thanks for the support of all the staff in the Clinical Laboratory of Guangdong Women and Children Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PCOS, polycystic ovary syndrome; ART, assisted reproductive technology; IVF, in vitro fertilization (IVF); ICSI, intracyto–plasmic sperm injection; ET, embryo transfer; ROC, receiver operating characteristic; LH, luteinizing hormone; FSH, follicle–stimulating hormone; BMI, body mass index; SHGB, sex hormone–binding globulin; PRL, prolactin; AMH, anti–Müllerian hormone; 25(OH)D, 25–hydroxyvitamin D; hCG, human chorionic gonadotropin; HOMA–IR, homeostasis model assessment insulin resistance index.
References
1. Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. (2023) 108:2447–69. doi: 10.1210/clinem/dgad463
2. Stanczak NA, Grywalska E, and Dudzinska E. The latest reports and treatment methods on polycystic ovary syndrome. Ann Med. (2024) 56:2357737. doi: 10.1080/07853890.2024.2357737
3. Melo AS, Ferriani RA, and Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo). (2015) 70:765–9. doi: 10.6061/clinics/2015(11)09
4. Liu S, Zhou X, Jie H, Zheng Z, Cai B, Mai Q, et al. Higher cumulative live birth rate but also higher late miscarriage risk in non-obese women with polycystic ovary syndrome undergoing the first IVF/ICSI cycle. Int J Womens Health. (2024) 16:289–98. doi: 10.2147/IJWH.S445021
5. Tang K, Wu L, Luo Y, and Gong B. In vitro fertilization outcomes in women with polycystic ovary syndrome: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2021) 259:146–52. doi: 10.1016/j.ejogrb.2021.02.023
6. Jiang L, Sun Y, Pan P, Li L, Yang D, Huang J, et al. Live birth rate per fresh embryo transfer and cumulative live birth rate in patients with PCOS under the POSEIDON classification: a retrospective study. Front Endocrinol (Lausanne). (2024) 15:1348771. doi: 10.3389/fendo.2024.1348771
7. Xu C, An X, Tang X, Yang Y, Deng Q, Kong Q, et al. Association between vitamin D level and clinical outcomes of assisted reproductive treatment: A systematic review and dose-response meta-analysis. Reprod Sci. (2024) 32(5):1446–58. doi: 10.1007/s43032-024-01578-9
8. Irani M and Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. (2014) 102:460–468.e3. doi: 10.1016/j.fertnstert.2014.04.046
9. Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, et al. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. (2010) 42:754–7. doi: 10.1055/s-0030-1262837
10. Li HW, Brereton RE, Anderson RA, Wallace AM, and Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism. (2011) 60:1475–81. doi: 10.1016/j.metabol.2011.03.002
11. Butts SF, Seifer DB, Koelper N, Senapati S, Sammel MD, Hoofnagle AN, et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J Clin Endocrinol Metab. (2019) 104:369–78. doi: 10.1210/jc.2018-00750
12. Yu Z, Sun Y, Wang P, Hu Y, Zhou Y, Xie J, et al. Does vitamin D level associate with pregnancy outcomes in Chinese women undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer? A retrospective cohort study. J Obstet Gynaecol Res. (2023) 49:835–45. doi: 10.1111/jog.15521
13. Chu J, Gallos I, Tobias A, Tan B, Eapen A, Coomarasamy A, et al. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod. (2018) 33:65–80. doi: 10.1093/humrep/dex326
14. Xu H, Zhang XQ, Zhu XL, Weng HN, Xu LQ, Huang L, et al. Comparison of vaginal progesterone gel combined with oral dydrogesterone versus intramuscular progesterone for luteal support in hormone replacement therapy-frozen embryo transfer cycle. J Gynecol Obstet Hum Reprod. (2021) 50:102110. doi: 10.1016/j.jogoh.2021.102110
15. Li M, Hu S, Sun J, and Zhang Y. The role of vitamin D3 in follicle development. J Ovarian Res. (2024) 17:148. doi: 10.1186/s13048-024-01454-9
16. Farhangnia P, Noormohammadi M, and Delbandi AA. Vitamin D and reproductive disorders: a comprehensive review with a focus on endometriosis. Reprod Health. (2024) 21:61. doi: 10.1186/s12978-024-01797-y
17. Dragomir RE, Toader OD, Gheoca Mutu DE, and Stănculescu RV. The key role of vitamin D in female reproductive health: A narrative review. Cureus. (2024) 16:e65560. doi: 10.7759/cureus.65560
18. Piao C, Li J, Liang C, Zhang J, Li X, Zhao Z, et al. Effect of vitamin D on pregnancy in women with polycystic ovary syndrome: retrospective and prospective studies. Reprod BioMed Online. (2024) 49:103909. doi: 10.1016/j.rbmo.2024.103909
19. Sparic R, Andjic M, Vergara D, Morciano A, D'Oria O, Baldini GM, et al. PCOS and vitamin D: a clinical appraisal. Arch Gynecol Obstet. (2024) 309:907–15. doi: 10.1007/s00404-023-07227-x
20. Jia XZ, Wang YM, Zhang N, Guo LN, Zhen XL, Li H, et al. Effect of vitamin D on clinical and biochemical parameters in polycystic ovary syndrome women: A meta-analysis. J Obstet Gynaecol Res. (2015) 41:1791–802. doi: 10.1111/jog.12793
21. Pergialiotis V, Karampetsou N, Panagopoulos P, Trakakis E, and Papantoniou N. The effect of Vitamin D supplementation on hormonal and glycaemic profile of patients with PCOS: A meta-analysis of randomised trials. Int J Clin Pract. (2017) 71(6). doi: 10.1111/ijcp.12957
22. Yang M, Shen X, Lu D, Peng J, Zhou S, Xu L, et al. Effects of vitamin D supplementation on ovulation and pregnancy in women with polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1148556. doi: 10.3389/fendo.2023.1148556
23. Cozzolino M, Busnelli A, Pellegrini L, Riviello E, and Vitagliano A. How vitamin D level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril. (2020) 114:1014–25. doi: 10.1016/j.fertnstert.2020.05.040
24. Caglar GS, Asimakopoulos B, Nikolettos N, Diedrich K, and Al-Hasani S. Recombinant LH in ovarian stimulation. Reprod BioMed Online. (2005) 10:774–85. doi: 10.1016/S1472-6483(10)61123-6
25. Conforti A, Esteves SC, Humaidan P, Longobardi S, D'Hooghe T, Orvieto R, et al. Recombinant human luteinizing hormone co-treatment in ovarian stimulation for assisted reproductive technology in women of advanced reproductive age: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. (2021) 19:91. doi: 10.1186/s12958-021-00759-4
26. Wang M, Huang R, Liang X, Mao Y, Shi W, Li Q, et al. Recombinant LH supplementation improves cumulative live birth rates in the GnRH antagonist protocol: a multicenter retrospective study using a propensity score-matching analysis. Reprod Biol Endocrinol. (2022) 20:114. doi: 10.1186/s12958-022-00985-4
27. Alcalá-Santiago Á, García-Villanova B, Ruíz-López MD, Gil Á, Rodriguez-Barranco M, Sánchez MJ, et al. Dietary and lifestyle determinants of vitamin D status in the UK Biobank Cohort study for predictive modeling. J Nutr Biochem. (2025) 142:109919. doi: 10.1016/j.jnutbio.2025.109919
28. Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Jolles M, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update. (2023) 29:157–76. doi: 10.1093/humupd/dmac035
29. Lin M, Zhang Y, Wang H, Wang Y, Wang Y, Feng N, et al. Multivariate analyses on male factors and construction of a nomogram for predicting low in vitro fertilization rate. Heliyon. (2024) 10:e29271. doi: 10.1016/j.heliyon.2024.e29271
30. Yanai A, Horie A, Sakurai A, Imakita S, Nakamura M, Ikeda A, et al. Innovative AI models for clinical decision-making: predicting blastocyst formation and quality from time-lapse embryo images up to embryonic day 3. Comput Biol Med. (2025) 195:110637. doi: 10.1016/j.compbiomed.2025.110637
Keywords: polycystic ovary syndrome, assisted reproductive technology, vitamin D, pregnancy, live birth
Citation: Guo L, Zheng X, Gu C, Zhang Z, Zhang X, Ruan X, Li H, Mai Q, Wu W and Luo M (2025) Associations between serum 25– hydroxyvitamin D concentrations and assisted reproductive technology outcomes in women with polycystic ovary syndrome: a cohort study. Front. Endocrinol. 16:1639137. doi: 10.3389/fendo.2025.1639137
Received: 01 June 2025; Accepted: 26 September 2025;
Published: 15 October 2025.
Edited by:
Gedis Grudzinskas, Independent Researcher, London, United KingdomReviewed by:
Zeyad Habeeb, University of Kerbala, IraqLateef Akinola, Medison Specialist Women’s Hospital, Nigeria
Copyright © 2025 Guo, Zheng, Gu, Zhang, Zhang, Ruan, Li, Mai, Wu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyong Luo, bHVvJiN4MjAxMztteUAxNjMuY29t
 Li Guo
Li Guo Xianhua Zheng1,2
Xianhua Zheng1,2 Chunming Gu
Chunming Gu Hongyu Li
Hongyu Li Weixiang Wu
Weixiang Wu Mingyong Luo
Mingyong Luo