- 1Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, State Key Laboratory of Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Cardiology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 3Peking University Fifth School of Clinical Medicine, Beijing, China
- 4Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 5Department of Gerontology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 6Department of Clinical Trial Center, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 7Department of Pharmacy, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Key Laboratory of Assessment of Clinical Drugs Risk and Individual Application (Beijing Hospital), Beijing, China
Background: Recently, the Stress Hyperglycemia Ratio (SHR) — which integrates acute increases in blood glucose with long-term glycemic control levels — has shown independent predictive value for adverse events in patients with acute coronary syndrome (ACS). However, the long-term prognostic significance of SHR in a broader population of coronary artery disease (CAD) remains unclear. This study aimed to explore the role of SHR in prediction of long-term prognosis of CAD.
Methods: In this cohort study, we enrolled 23,591 participants diagnosed with CAD from January, 2016, to December, 2021 in Beijing Hospital. After excluding patients lacking data, with cancers, or missing follow-ups, 7,162 patients were finally enrolled into the analyses. The SHR was calculated using the following equation: SHR = admission glucose (mmol/L)/(1.59 × HbA1c [%]-2.59). The 7,162 participants were divided into three groups based on SHR tertiles: Tertile 1 (SHR ≤ 0.72, n=2391), Tertile 2 (0.73≤SHR ≤ 0.82, n=2388), and Tertile 3 group (SHR≥0.83, n=2383). The primary endpoint was all-cause mortality and cardiovascular death (CVD), while the second endpoint was major adverse cardiovascular events (MACE). The median follow-up was 28 months.
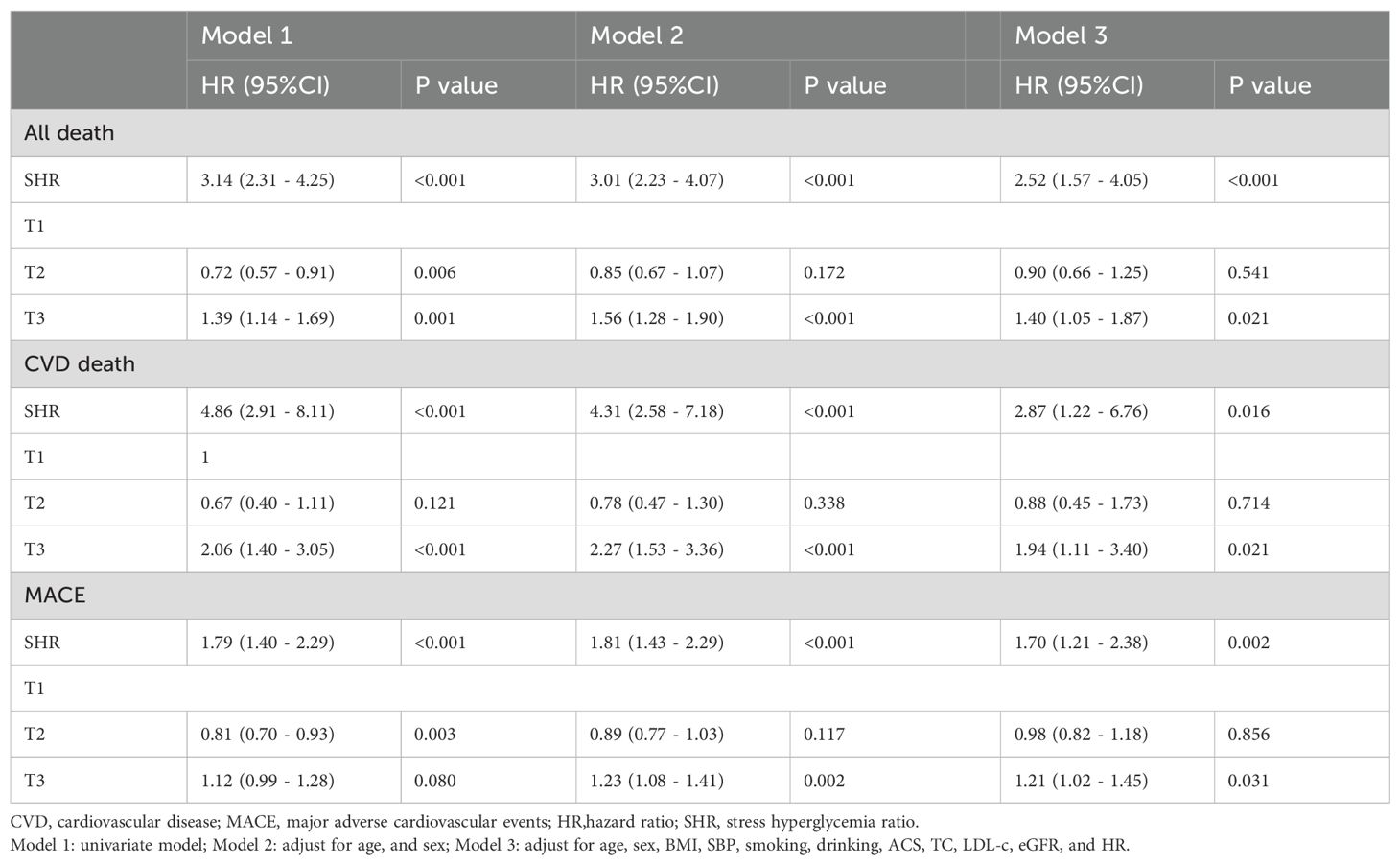
Results: Our results suggest that SHR was significantly associated with increased risks of long-term all-cause death, CVD death, and MACE. The Kaplan-Meier curves revealed that the highest tertile (T3) group had the highest risk of all-cause death, CVD death, and MACE, while the lowest tertile (T1) group had the lowest risk (all log-rank P < 0.05). After adjusting risk factors, the results of cox regression analyses showed that SHR was significantly associated with all three outcomes (all P < 0.05). For all-cause death, SHR was associated with an increased risk of all-cause death in the fully adjusted model (Model 3: HR = 2.52, 95% CI: 1.57 – 4.05, P < 0.001). Compared to the lowest tertile (T1), participants in the highest tertile (T3) had a likely higher risk of all-cause death (HR = 1.40, 95% CI: 1.05 – 1.87, P = 0.021). SHR also demonstrated a positive association with CVD death (Model 3: HR = 2.87, 95% CI: 1.22 – 6.76, P = 0.016), and participants in T3 had a significantly higher risk of CVD death compared to T1 (HR = 1.94, 95% CI: 1.11 – 3.40, P = 0.021). Additionally, SHR was also independently associated with MACE (Model 3: HR = 1.70, 95% CI: 1.21 – 2.38, P = 0.002). The risk of MACE was significantly higher in T3 compared to T1 (HR = 1.21, 95% CI: 1.02 – 1.45, P = 0.031). The restricted cubic spline (RCS) analysis further confirmed a positive nonlinear association between SHR and these adverse outcomes (all-cause death, CVD death, and MACE) and exhibited a J-shaped curve.
Conclusions: SHR is significantly associated with long-term all-cause death, CVD death, and MACE in CAD patients. Our findings highlight SHR can be used as a valuable tool for long-term prognosis risk stratification in CAD, potentially influencing clinical decision-making and patient management strategies.
1 Introduction
Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide (1). The progression of CAD is dynamic and may lead to major adverse cardiovascular events (MACE), death or cardiovascular death (CVD). The early identification of high-risk populations for adverse outcomes in CAD remains a key strategy to optimize CAD management. Stress hyperglycemia, defined as transient hyperglycemia occurring in response to acute physiological stress, has been considered a predictor for adverse events, especially in patients with emergency and critical illness (2, 3). To distinguish from individuals with poor chronic glycemic control, Robert et al. proposed the stress hyperglycemia ratio (SHR), which is calculated as the admission blood glucose (ABG) divided by the estimated average glucose derived from HbA1c, to serve as a more accurate indicator of acute glycemic dysregulation (4). In recent years, SHR has been reported to be associated with increased risks of MACE and mortality in patients with ACS and chronic coronary syndromes (CCS) (5, 6). However, conflicting findings exist regarding the prognostic value of SHR across different CAD subtypes and glycemic statuses. Thus, further investigation is warranted to elucidate the role of SHR in predicting long-term cardiovascular outcomes among patients with CAD. This study serves as a natural extension of our previous research (6). In our prior investigation, we examined the correlation between the SHR and in-hospital mortality within the identical cohort. Our findings revealed that SHR exhibited a significant association with in-hospital mortality among patients with ACS or CCS, particularly among those with prediabetes and diabetes mellitus. Upon completion of the follow-up period, the current study builds upon and expands the insights garnered from our initial exploration, delving deeper into the associations between SHR and long-term prognosis.
The aim of this study was to evaluate the association between SHR and long-term all-cause death, CVD, and MACE in a large cohort of CAD patients.
2 Methods
2.1 Study design and population
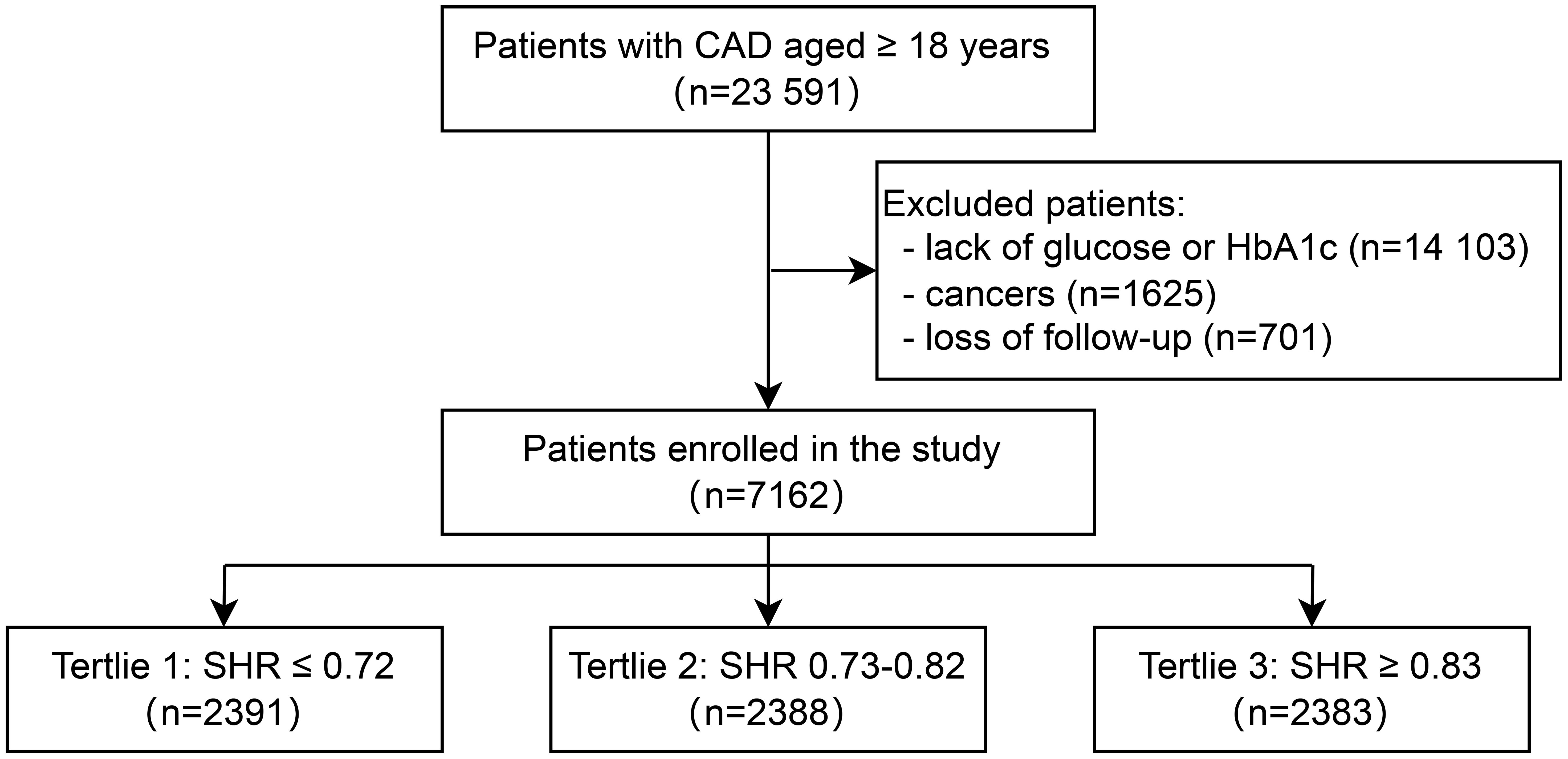
This study was conducted at Beijing Hospital from January 2016 to December 2021. A total of 23,591 patients diagnosed with CAD were enrolled. All CAD patients were from the inpatient department of Beijing Hospital and had at least one major coronary artery stenosis ≥50% diagnosed by coronary CT or angiography. After excluding patients with missing blood glucose or HbA1c data (n=14,103), those diagnosed with cancer (n=1,625), and those who were lost to follow-up (n=701), a total of 7,162 patients were included in the final analysis (Figure 1). Based on the tertiles of the SHR, the 7,162 patients were divided into three groups: Tertile 1 (SHR ≤ 0.72, n=2,391), Tertile 2 (0.73≤SHR ≤ 0.82, n=2,388), and Tertile 3 (SHR≥0.83, n=2,383). The median follow-up duration was 28 months. The primary outcomes of this study were all-cause death and CVD. The secondary outcome was MACE. The study was approved by the Ethics Committee of Beijing Hospital and conducted in accordance with the Declaration of Helsinki.
2.2 Data collection and measurements
The SHR was calculated as the ABG (mmol/L) divided by the estimated average glucose (eAG), where eAG was determined using the formula: eAG = 1.59 × HbA1c [%] − 2.59 (4). The formula of eAG (mmol/l) was derived from the eAG (mg/dL) = 28.7 × HbA1c − 46.7, which was proposed by Nathan et al (7). We finally adopted the mmol/L version to match our laboratory units and ensure consistency between numerator and denominator in the SHR calculation. Baseline characteristics, including demographics (age, gender, height, weight, smoking status, and alcohol use) and vital signs (admission systolic blood pressure [SBP], diastolic blood pressure [DBP], and heart rate [HR]), were collected from medical records. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m²). HbA1c, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-c) were measured from a cubital vein blood sample after at least eight hours of fasting. Blood glucose, TC, TG, HDL-C, and LDL-C were analyzed using a LABOSPECT 008 system (Hitachi, Tokyo, Japan), while HbA1c levels were determined by high-performance liquid chromatography (G8, TOSOH, Tokyo, Japan) at the laboratory of Beijing Hospital. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.
2.3 Definitions
In this study, diabetes specifically refers to type 2 diabetes, which was defined as either a documented history of type 2 diabetes or an HbA1c level greater than 6.5% (14). Prediabetes mellitus (Pre-DM) was identified in patients who had never been diagnosed with diabetes but had an HbA1c level ranging from 5.7% to 6.4%. Normoglycemia (NGR) was assigned to those without a history of diabetes and with an HbA1c level of 5.7% or lower.
All-cause mortality included in-hospital deaths and deaths occurring during the follow-up period. CVD death in this study was defined as death resulting from cardiovascular diseases causes. MACE included rehospitalization due to cardiovascular causes or cerebrovascular causes, the occurrence of heart failure or cerebrovascular events (ischemic stroke or intracerebral hemorrhage) during follow-up, all-cause death, and CVD death.
2.4 Statistical analysis
Continuous variables were summarized as means ± standard deviations (SD) or medians with interquartile ranges (IQR), and categorical variables were counts and proportions. Differences for baseline characteristics across SHR tertiles were compared using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables, and the chi-square test for categorical variables. Survival curves were plot by Kaplan-Meier method, and differences among groups were compared by log-rank test. Cox proportional hazards regression models were used to evaluate the association of SHR with all-cause death, death caused by CVD, and MACE. SHR was analyzed both as a continuous variable and as tertiles (T1 ≤ 0.72, T2 0.73 - 0.82, and T3 ≥ 0.83). Univariate and multivariable adjusted HRs were assessed based on three models: Model 1 was unadjusted; Model 2 was adjusted for age and sex; and Model 3 was further adjusted for potential confounders, including BMI, SBP, smoking, drinking, ACS, TC, LDL-c, eGFR, and HR. Restricted cubic spline (RCS) regression was used to explore the relationship between SHR and the risk of all-cause death, CVD, and MACE. Subgroup analyses were further performed to explore the association of SHR with outcomes stratified by ACS status (ACS vs. CCS) and glucose status (NGR, Pre-DM, and DM). All analyses were conducted using SAS 9.4. A two-tailed P value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of participants
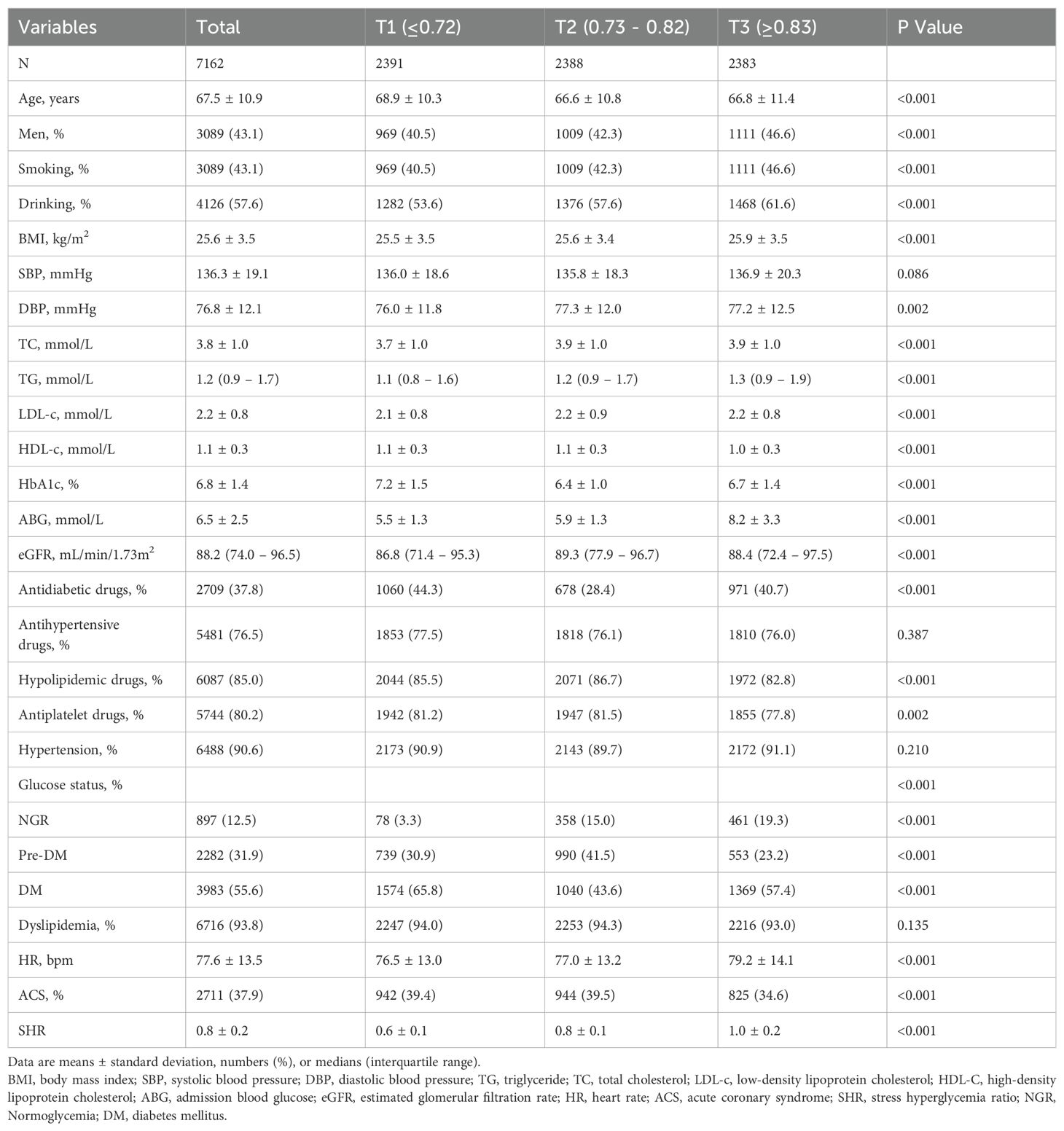
Patients with CAD aged ≥ 18 years were included (N = 23,591). After patients lack of glucose or HbA1c (N = 14,103), with cancers (N = 1625), and loss of follow-up (N = 701) were excluded, a total of 7162 participants were included (Figure 1). There were 2391, 2388, and 2383 participants in the T1 group, T2 group, and T3 group. The baseline characteristics of participants stratified by SHR tertiles (T1 ≤ 0.72, T2 0.73 – 0.82, T3 ≥ 0.83) are presented in Table 1. Participants in the highest tertile (T3) were younger, had a higher proportion of men, and were more likely to smoke and drink. Besides, participants in T3 had higher BMI, TC levels, and ABG. The use of antidiabetic drugs was more prevalent in T1, while hypolipidemic drug use was higher in T2. No significant differences were observed in SBP or hypertension prevalence across tertiles.
The distribution of SHR approximated a normal distribution (Figure 2A), with a mean ± SD of 0.8 ± 0.2. The means ± SDs for the T1, T2 and T3 group were 0.6 ± 0.1, 0.8 ± 0.1, and 1.0 ± 0.2, respectively.
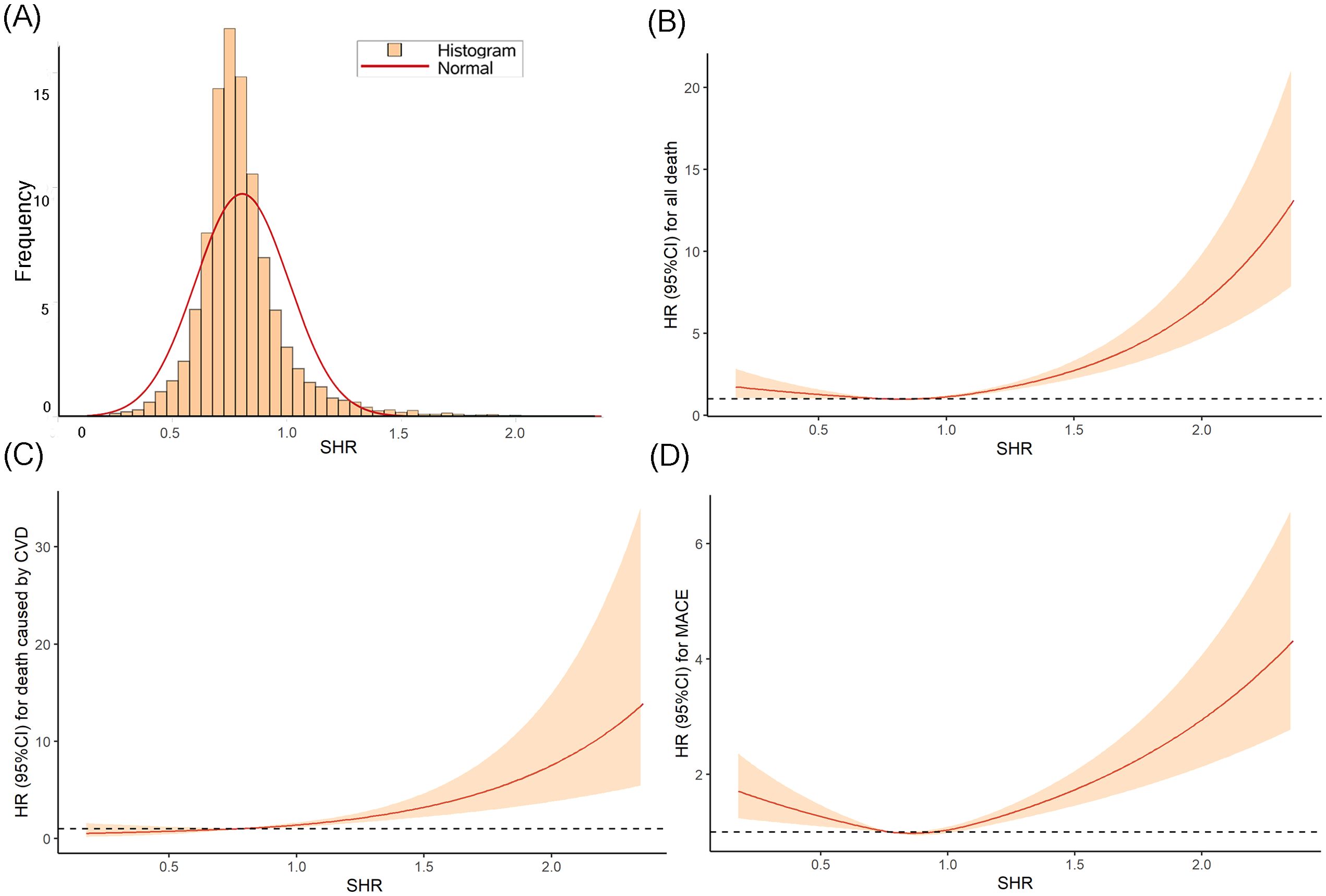
Figure 2. The distribution of SHR (A) and the RCS curves between SHR and all-cause death (B), CVD death (C), and MACE (D).
3.2 Association of SHR with all-cause death, CVD death, and MACE
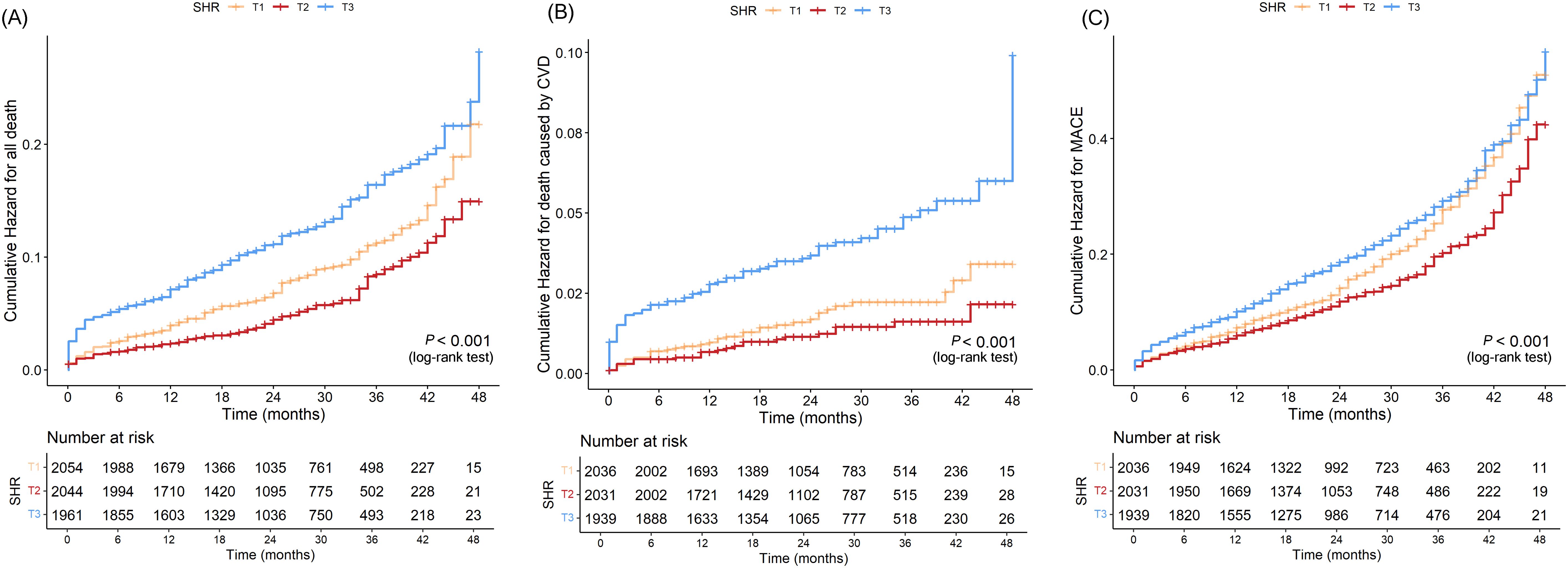
The Kaplan-Meier curves (Figure 3) revealed that the highest tertile (T3) group had the highest risk of all-cause death, CVD death, and MACE, while the lowest tertile (T1) group had the lowest risk (all log-rank P < 0.05). The association between SHR and all-cause death, death caused by CVD, and MACE, was shown in Table 2. After adjusting risk factors including age, sex, BMI, SBP, smoking, drinking, ACS, TC, LDL-c, eGFR, and HR in model 3, the results of cox regression analyses showed that SHR was significantly associated with all three outcomes (all P < 0.05). For all-cause death, SHR was associated with an increased risk of all-cause death in the fully adjusted model (Model 3: HR = 2.52, 95% CI: 1.57 – 4.05, P < 0.001). Compared to the lowest tertile (T1), participants in the highest tertile (T3) had a likely higher risk of all-cause death (HR = 1.40, 95% CI: 1.05 – 1.87, P = 0.021). SHR also demonstrated a positive association with CVD death (Model 3: HR = 2.87, 95% CI: 1.22 – 6.67, P = 0.016), and participants in T3 had a significantly higher risk of CVD death compared to T1 (HR = 1.94, 95% CI: 1.11 – 3.40, P = 0.021). Additionally, SHR was also independently associated with MACE (Model 3: HR = 1.70, 95% CI: 1.21 – 2.38, P = 0.002). The risk of MACE was significantly higher in T3 compared to T1 (HR = 1.21, 95% CI: 1.02 – 1.45, P = 0.031). Furthermore, the RCS curves showed a J-shaped positive association between SHR and all-cause death, CVD death, and MACE (Figures 2B–D).
3.3 Subgroup analysis
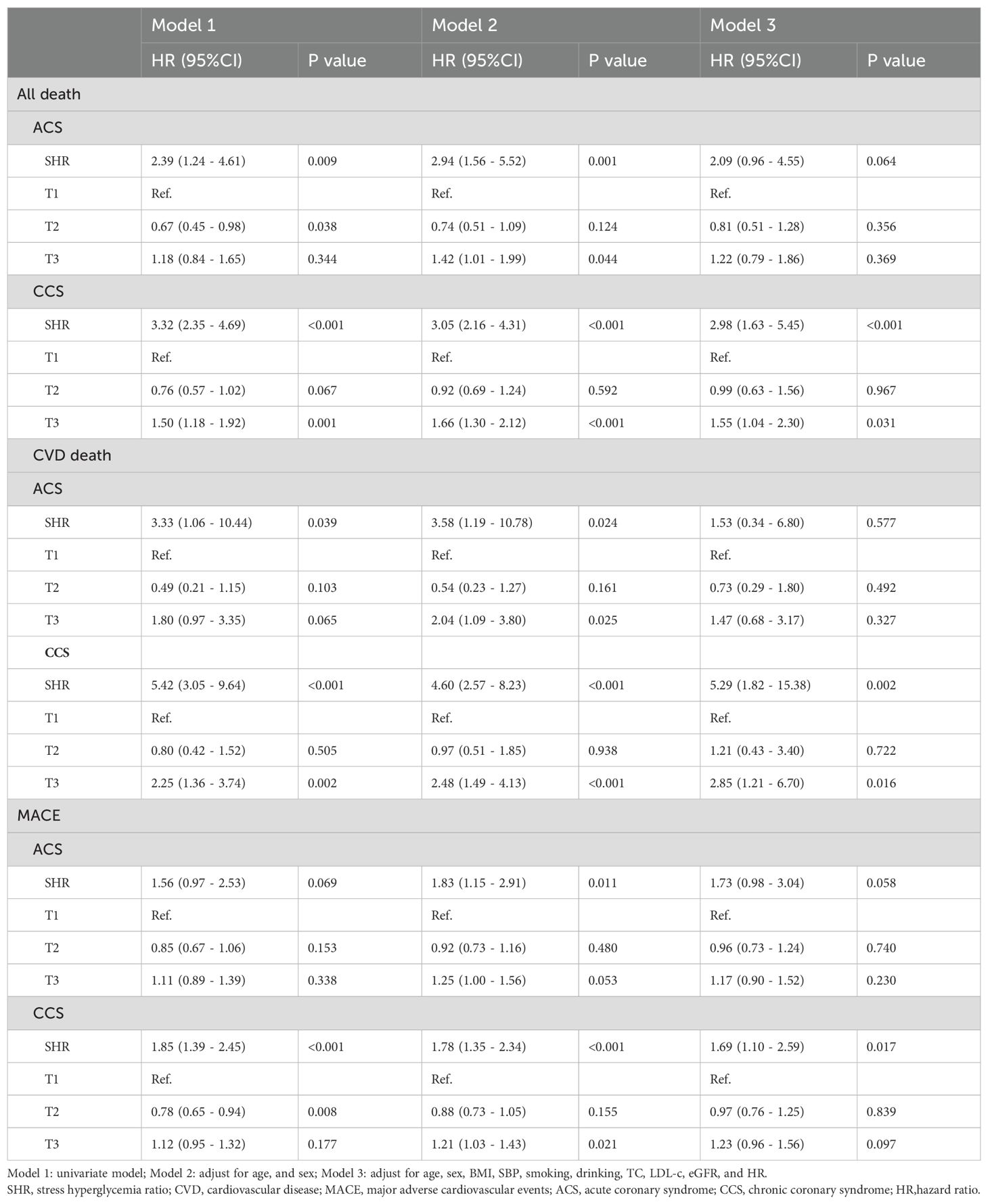
In subgroup analysis of ACS and chronic coronary syndrome (CCS), SHR was significantly associated with all-cause death, CVD death, and MACE in participants with CCS, but showed weaker associations in those with ACS (see Table 3).
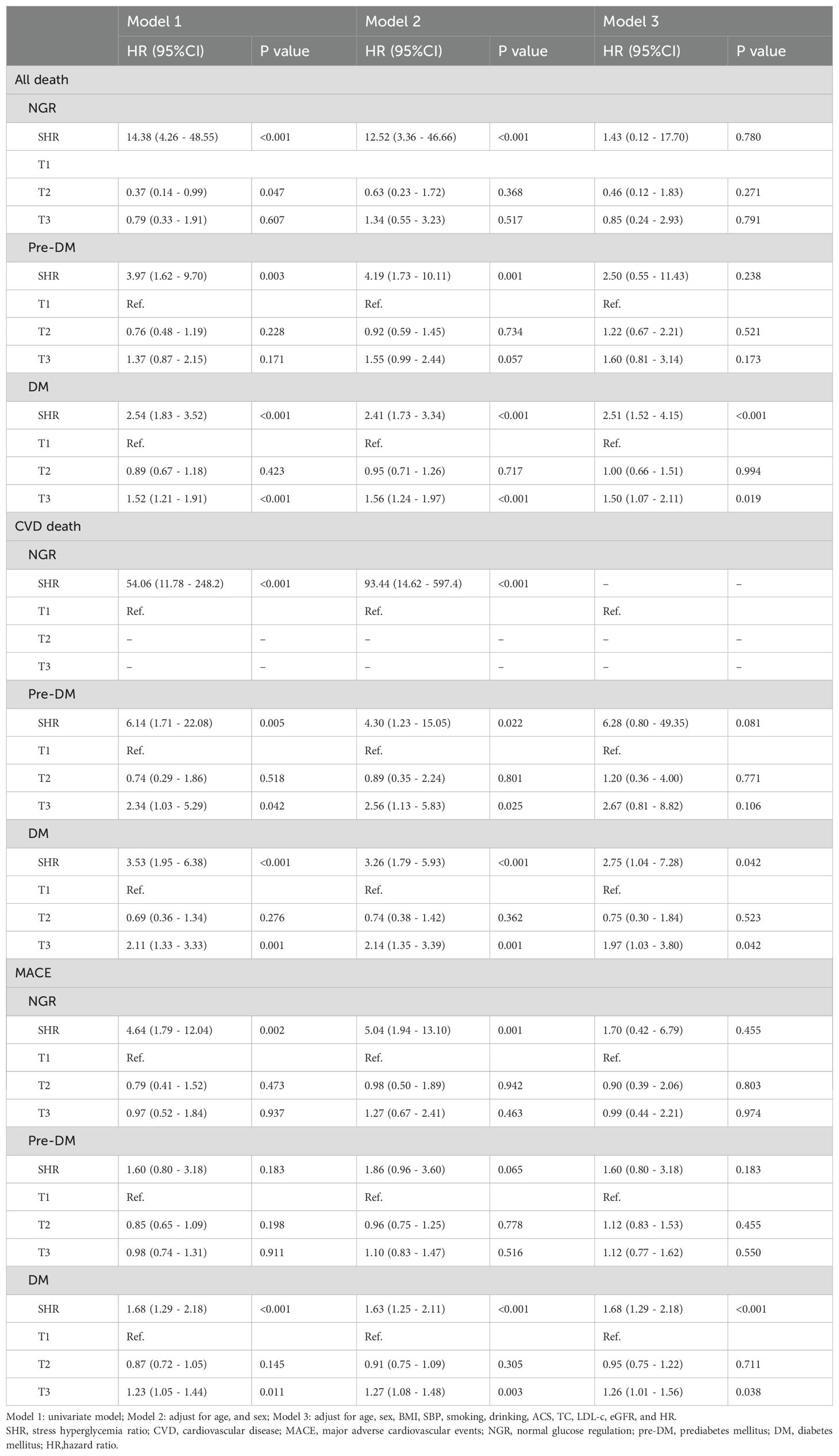
Besides, in the subgroup analysis of glucose status, SHR showed the stronger association with all-cause death, CVD death, and MACE in participants with DM. The association with CVD death in NGR could not be fully evaluated due to limited events (see Table 4).
4 Discussion
In this study, we explored the association between the SHR and long-term clinical outcomes of CAD, including all-cause death, CVD death, and MACE, in a large cohort of CAD during a median follow-up of 28 months. Our findings suggest that SHR was significantly associated with increased risks of long-term all-cause death, CVD death, and MACE. The RCS analysis further confirmed a positive nonlinear association between SHR and these adverse outcomes (all-cause death, CVD death, and MACE) and exhibited a J-shaped curve. The Kaplan-Meier curves revealed that the highest tertile (T3) group had the highest risk of all-cause death, CVD death, and MACE, while the lowest tertile (T1) group had the lowest risk (all log-rank P < 0.05). Further analysis using Cox proportional hazards regression showed that the T3 group had a significantly higher risk compared to the reference group (P < 0.05), while the differences between T2 and the reference group were not statistically significant. This indicates a clear risk gradient, with T3 having the highest risk and T1 having the lowest risk, suggesting that it has good stratification ability for identifying high-risk groups. Integrated with our previous study, the combined findings demonstrate that SHR is not only significantly associated with the risk of in-hospital mortality but also serves as an effective marker for risk stratification of long-term prognosis in patients with CAD (6).
Under the impact of emergency and critical illnesses such as severe trauma, infection, myocardial infarction, and stroke, the stress response leads to increased secretion of glucagon, adrenaline, and inflammatory factors, resulting in insulin resistance and ultimately causing an elevation in blood glucose levels (3, 8–10). As an important indicator of stress hyperglycemia, previous studies have sufficiently validated the relationship between the SHR and adverse prognosis in critical illnesses (11–14). Research on SHR in CAD has predominantly focused on patients with ACS or with limited follow-up duration, few studies focused on the entire CAD population both including ACS and CCS (5, 15–18). Yang et al. conducted a retrospective study for ACS patients who underwent drug-eluting stent and found that SHR presented U-shaped or J-shaped associations with early and late cardiovascular outcomes (5). Luo et al. enrolled 2,089 patients with acute myocardial infarction (AMI) and demonstrated that SHR can serve as a predictor of worse prognosis and may enhance the Global Registry of Acute Coronary Events (GRACE) score (16). During the median follow-up of 28 months, our study indicated a strong positive relationship between SHR and all-cause death, CVD death, and MACE among the CAD patients, with an approximately two-fold increased risk of all-cause death observed in the highest tertile (T3) compared to the lowest tertile (T1) in the fully adjusted model. Patients in T3 exhibited a significantly higher risk of all-cause death, CVD death, and MACE, compared to those in T1. These findings are consistent with prior research, highlighting the predictive value of SHR in worsening prognosis among patients with CAD.
4.1 Subgroup differences in SHR prognostic value
Subgroup analyses in this current study revealed a stronger association between SHR and long-term adverse outcomes (all-cause death, CVD death, and MACE) in patients with CCS compared to those with ACS. However, in our last study, we observed that SHR was significantly associated with in-hospital mortality in both ACS and CCS (6). The subgroup analyses from our two studies indicated that SHR held significant value in forecasting short-term prognosis in both ACS and CCS. However, its correlation with long-term prognosis appears to be more pronounced in CCS. This discrepancy likely stemmed from the different pathophysiological mechanisms underlying ACS and CCS. In CCS, chronic metabolic dysregulation and endothelial dysfunction may amplify the impact of stress hyperglycemia on long-term outcomes. Conversely, in ACS, the acute stress response gradually subsides after discharge, and chronic disease states may have a more significant impact on prognosis (19). The influence of stress hyperglycemia at admission on long-term prognosis may gradually diminish. In previously published literature, Schmitz et al. observed that stress hyperglycemia primarily had a significant impact on the short-term prognosis of patients with AMI, but barely had influence on long-term prognosis, validating the hypothesis that stress hyperglycemia affects poor prognosis through transient dynamic disorder when the acute event occurs (20). Additionally, the smaller scale of the ACS population in this study compared to the CCS group may have reduced statistical efficiency.
Furthermore, SHR exhibited a stronger predictive value for long-term adverse outcomes among individuals with DM in the subgroup analyses in this study. However, in our last study, SHR presented superior predictive power for in-hospital mortality in both the DM and pre-DM subgroups. In patients with DM, long-term blood glucose control is often difficult, and blood sugar levels may not return to normal even after an acute stress event. In contrast, patients with pre-DM have relatively lower glucose sugar levels and may more easily achieve normal levels through lifestyle and medication, which may lessen SHR’s impact on long-term prognosis. This underscores that in CAD patients with DM, those with higher SHR have both elevated short-term and long-term risks of adverse outcomes and require more attention, such as stricter blood glucose management. Besides, the limited number of CVD-related deaths in the NGR subgroup constrained our ability to fully assess this relationship. This finding aligns with prior studies suggesting that acute glucose fluctuations in DM may have detrimental cardiovascular effects beyond those observed in patients with chronic hyperglycemia.
4.2 Clinical implications and future directions
Our findings highlight the importance of SHR as a potential prognostic biomarker in CAD patients for predicting long-term outcomes, particularly those with CCS or DM. Given the increasing recognition of stress hyperglycemia as a cardiovascular risk factor, routine assessment of SHR in clinical practice may help identify high-risk individuals. Future studies should aim to validate our findings in larger, multi-center cohorts and explore the mechanistic pathways linking SHR to cardiovascular outcomes.
4.3 Limitations
The strengths of our study include a large sample size of CAD population and this study is an extension of our previous research on SHR and in-hospital mortality in CAD. We completed a median follow-up of 28 months, further exploring the impact of SHR on the long-term prognosis of CAD patients and conducted comprehensive analyses. However, several limitations should be acknowledged. First, the observational nature of this observational study may preclude causal inference. Second, glucose measurements were obtained at a single time point upon hospital admission, which may not fully capture dynamic glycemic changes over time. Third, the small number of CVD deaths in certain subgroups limited statistical power for subgroup analyses.
5 Conclusion
In conclusion, our study demonstrates that elevated SHR was significantly associated with increased risks of all-cause death, CVD death, and MACE in CAD patients during a median follow-up of 28 months, with stronger associations observed in those with CCS or DM. Our findings suggest that SHR may serve as a valuable marker of risk stratification for long-term prognosis in patients with CAD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WX: Writing – original draft, Conceptualization. XW: Writing – review & editing, Conceptualization. CX: Data curation, Writing – review & editing. XM: Writing – review & editing, Data curation. YL: Methodology, Writing – review & editing. YZ: Writing – review & editing, Methodology. CY: Methodology, Writing – review & editing. BF: Writing – review & editing, Writing – original draft. ZZ: Writing – review & editing. FW: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. National High-Level Hospital Clinical Research Funding. Grant Numbers: BJ - 2024-180, BJ - 2022-117, BJ - 2024-245. General Program of National Natural Science Foundation of China. Grant Number: 82470363. National Key Research and Development Program of China. Grant Numbers: 2020YFC2008100, 2020YFC2008106. Wei Xu gratefully acknowledges the support of the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (Grant No. GZC20240151) and Fundamental Research Funds for the Central Universities (2024-XHQN15).
Acknowledgments
The authors thank all the participants of the Beijing hospital for their contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABG, Admission blood glucose; ACS, Acute Coronary Syndrome; AMI, Acute Myocardial Infarction; ANOVA, One-way Analysis of Variance; BMI, Body Mass Index; CAD, Coronary Artery Disease; CCS, Chronic Coronary Syndromes; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, Cardiovascular Death; DBP, Diastolic Blood Pressure; DM, Diabetes Mellitus; eGFR, Estimated Glomerular Filtration Rate; GRACE, Global Registry of Acute Coronary Events; HbA1c, Glycated Hemoglobin; HDL-C, High-Density Lipoprotein Cholesterol; HR, Heart Rate; IQR, Interquartile Ranges; LDL-c, Low-Density Lipoprotein Cholesterol; MACE, Major Adverse Cardiovascular Events; NGR, Normoglycemia; Pre-DM, Prediabetes Mellitus; RCS, Restricted Cubic Spline; SBP, Systolic Blood Pressure; SD, Standard Deviations; SHR, Stress Hyperglycemia Ratio; TC, Total Cholesterol; TG, Triglycerides.
References
1. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990 - 2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/S0140-6736(24)00367-2
2. Guan Y, Liu G, Tang F, Wu X, Shi J, Huang Q, et al. Stress hyperglycemia in acute pancreatitis: From mechanisms to prognostic implications. Life Sci. (2025) 365:123469. doi: 10.1016/j.lfs.2025.123469
3. Dungan KM, Braithwaite SS, and Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373:1798 – 807. doi: 10.1016/S0140-6736(09)60553-5
4. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100:4490 – 7. doi: 10.1210/jc.2015-2660
5. Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. (2022) 45:947–956. doi: 10.2337/dc21-1526
6. Xu W, Song Q, Wang X, Zhao Z, Meng X, Xia C, et al. Association of stress hyperglycemia ratio and in-hospital mortality in patients with coronary artery disease: insights from a large cohort study. Cardiovasc Diabetol. (2022) 21:217. doi: 10.1186/s12933-022-01645-y
7. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. (2008) 31:1473 – 8. doi: 10.2337/dc08-0545
8. Marik PE and Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care Med. (2013) 41:e93–4. doi: 10.1097/CCM.0b013e318283d124
9. Yan F, Chen X, Quan X, Wang L, Wei X, Zhu J, et al. Association between the stress hyperglycemia ratio and 28-day all-cause mortality in critically ill patients with sepsis: a retrospective cohort study and predictive model establishment based on machine learning. Cardiovasc Diabetol. (2024) 23:163. doi: 10.1186/s12933-024-02265-4
10. Zhang Y, Yin X, Liu T, Ji W, and Wang G. Association between the stress hyperglycemia ratio and mortality in patients with acute ischemic stroke. Sci Rep. (2024) 14:20962. doi: 10.1038/s41598-024-71778-5
11. Merlino G, Smeralda C, Gigli GL, Lorenzut S, Pez S, Surcinelli A, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis. (2021) 51:789–797. doi: 10.1007/s11239-020-02252-y
12. Jiang Z, Wang K, Duan H, Du H, Gao S, Chen J, et al. Association between stress hyperglycemia ratio and prognosis in acute ischemic stroke: a systematic review and meta-analysis. BMC Neurol. (2024) 24:13. doi: 10.1186/s12883-023-03519-6
13. Aon M, Alsaeedi A, Alzafiri A, Al-Shammari A, Taha S, Al-Shammari O, et al. Stress hyperglycemia ratio as a prognostic marker in diabetic patients hospitalized with COVID - 19. Infect Dis Rep. (2022) 14:675–685. doi: 10.3390/idr14050073
14. Li L, Zhao M, Zhang Z, Zhou L, Zhang Z, Xiong Y, et al. Prognostic significance of the stress hyperglycemia ratio in critically ill patients. Cardiovasc Diabetol. (2023) 22:275. doi: 10.1186/s12933-023-02005-0
15. Şimşek B, Çınar T, Tanık VO, İnan D, Zeren G, Avcı İ, et al. The association of acute–to–chronic glycemic ratio with no-reflow in patients with ST–segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Kardiol Pol. (2021) 79:170–178. doi: 10.33963/KP.15736
16. Luo J, Xu S, Li H, Li Z, Gong M, Qin X, et al. Prognostic impact of stress hyperglycemia ratio in acute myocardial infarction patients with and without diabetes mellitus. Nutr Metab Cardiovasc Dis. (2022) 32:2356–2366. doi: 10.1016/j.numecd.2022.07.004
17. Zhang Y, Guo L, Zhu H, Jiang L, Xu L, Wang D, et al. Effects of the stress hyperglycemia ratio on long-term mortality in patients with triple-vessel disease and acute coronary syndrome. Cardiovasc Diabetol. (2024) 23:143. doi: 10.1186/s12933-024-02220-3
18. Xie E, Ye Z, Wu Y, Zhao X, Li Y, Shen N, et al. Predictive value of the stress hyperglycemia ratio in dialysis patients with acute coronary syndrome: insights from a multi-center observational study. Cardiovasc Diabetol. (2023) 22:288. doi: 10.1186/s12933-023-02036-7
19. Li Z, Chen R, Zeng Z, Wang P, Yu C, Yuan S, et al. Association of stress hyperglycemia ratio with short-term and long-term prognosis in patients undergoing coronary artery bypass grafting across different glucose metabolism states: a large-scale cohort study. Cardiovasc Diabetol. (2025) 24:179. doi: 10.1186/s12933-025-02682-z
Keywords: coronary artery disease, stress hyperglycemia ratio, all-cause death, cardiovascular disease death, major adverse cardiovascular events
Citation: Xu W, Wang X, Xia C, Meng X, Li Y, Zhao Y, Yang C, Feng B, Zhao Z and Wang F (2025) The role of SHR in risk stratification for long-term prognosis in patients with coronary artery disease: findings from a large cohort study. Front. Endocrinol. 16:1640725. doi: 10.3389/fendo.2025.1640725
Received: 04 June 2025; Accepted: 20 August 2025;
Published: 16 September 2025.
Edited by:
Galileo Escobedo, General Hospital of Mexico, MexicoReviewed by:
Ming Yi, Hunan Hospital of Traditional Chinese Medicine, ChinaJose Israel Leon Pedroza, Universidad Anáhuac México Campus Norte, Mexico
Copyright © 2025 Xu, Wang, Xia, Meng, Li, Zhao, Yang, Feng, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wang, YmpoX3dhbmdmYW5nQDE2My5jb20=; Zinan Zhao, YmVsbGF6aGFvMjAxNUAxNjMuY29t; Baoyu Feng, c3lzdWJhb3l1QDEyNi5jb20=
†These authors have contributed equally to this work
 Wei Xu
Wei Xu Xiang Wang
Xiang Wang Chenxi Xia
Chenxi Xia Xuyang Meng
Xuyang Meng Yi Li
Yi Li Yejing Zhao
Yejing Zhao Chenguang Yang
Chenguang Yang Baoyu Feng
Baoyu Feng Zinan Zhao
Zinan Zhao Fang Wang
Fang Wang