Abstract
Purpose:
Yerba Maté, a traditional South American herbal infusion abundant in bioactive compounds, has been suggested to offer health benefits including lipid regulation and weight management. However, existing evidence remains inconclusive. This systematic review and meta-analysis seeks to conduct a comprehensive evaluation of the effects of Yerba Maté consumption on metabolic health outcomes using data from randomized controlled trials (RCTs).
Methods:
In accordance with the PRISMA guidelines, a comprehensive systematic review and meta-analysis of RCTs was conducted, encompassing studies published up to January 2025. Studies were systematically retrieved from MEDLINE, EMBASE and The Cochrane Central Register of Controlled Trials without any language restrictions. The review included RCTs that evaluated the impact of Yerba Maté on metabolic health indicators. Meta-analyses were performed using Review Manager (RevMan 5.4) when two or more studies from the same comparator provided sufficient data. Quality assessment were assessed using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) tool. The overall quality of evidence was evaluated using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) method.
Results:
A total of 1294 studies were initially identified, of which 13 RCTs met the inclusion criteria. The study population includes dyslipidemic volunteers, overweight and obese and non-dyslipidemic, normal-weight volunteers. The results with pre-diabetes patients suggest significant decreases in postprandial glucose (MD -12.76, 95% CI -16.78, -8.74; N = 2), HbA1c (MD -0.37, 95% CI -0.56, -0.18; N = 2), and the homeostatic model assessment index (HOMA index) (MD -0.24, 95% CI -0.37, -0.11; N = 2), though further research is needed to confirm these findings. No significant effects were found on triglycerides, total cholesterol, HDL-C, LDL-C, fasting glucose, fasting insulin, waist circumference, or BMI. Adverse events included mucosal irritation, insomnia, tachycardia, angina, headache, and gastrointestinal discomfort.
Conclusion:
Yerba Maté consumption may demonstrate favorable effects on glycemic control, though its impact on lipid profiles and weight management appears to be limited.
Systematic Review Registration:
https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023369270.
Introduction
Ilex paraguariensis, or Yerba Maté, is a South American plant mainly grown in northern Argentina, Paraguay, Uruguay, and the south of Brazil (1). The history of this plant is long, and its cultural roots are deep in South America, where it is extensively used in social activities and day-to-day consumption. Because of its unique bitter taste and pleasant aroma, it’s a beverage popular not only in South America but also increasingly globally. This beverage constitutes a sophisticated matrix comprising bioactive constituents, including chlorogenic acid, polyphenolic compounds, saponins, and methylxanthine derivatives such as caffeine, theobromine, and theophylline. The predominant bioactive fraction consists of chlorogenic acids, which account for 71–76% of total polyphenols, together with methylxanthines, saponins and flavonoids (2, 3). Of them, chlorogenic acid is noteworthy as a lipid regulator and lipid oxidizing reduction that may be beneficial in preventing cardiovascular disease (4–6). Polyphenols combat oxidative stress and inflammation by neutralizing reactive oxygens species (7, 8); saponins may influence lipid metabolism by modulating intestinal lipid absorption (9, 10).
Epidemiological and clinical studies indicate Yerba Maté may influence lipid homeostasis, decreasing total cholesterol, LDL-C (low-density lipoprotein cholesterol) and triglycerides as well as increasing HDL-C (high-density lipoprotein cholesterol) (11–14). The behavior of signaling pathways may mediate these effects, e.g., the AMPKα-LXRα/SREBP-1c axis controlling lipolysis in adipose tissue (15, 16). According to the IDF (International Diabetes Federation) Diabetes Atlas 2025, in 2024, there were 589 million adults aged 20–79 living with diabetes globally, with a prevalence rate of approximately 11.1%. Globally diabetes and its complications caused over 3.4 million deaths, accounting for 9.3% of total global deaths. Although there is preliminary evidence that suggests Yerba Maté may play a role in modulating postprandial glycemic responses and decreasing HbA1c (glycated hemoglobin) levels (17, 18), we argue that these effects are likely due to the psychostimulant effect of the included xanthines. Furthermore, the mechanisms underlying these effects may include enhanced insulin sensitivity and the mechanism of action of glucose metabolism, which can be regulated by the PI3K-AKT signaling pathway that controls insulin resistance-associated genetic expression (19). Preliminary clinical investigations suggest that Yerba Maté may exert beneficial effects on weight management through multiple mechanisms, including pancreatic lipase inhibition, modulation of gastric motility, and enhancement of energy expenditure (20, 21). Several pilot studies have demonstrated modest yet statistically significant body weight and waist-to-hip ratio decreases in the supplemented group vs. placebo cohorts (20).
Existing studies also indicate that consumption of Yerba Maté may offer potential health benefits, but the results of these studies are inconsistent. As such, the aim of this systematic review is to provide a comprehensive review of the effects of Yerba Maté about health outcomes, such as lipid profiles, blood glucose levels, weight management and potentially harmful effects of consumption, by searching for evidence published in randomized controlled trials (RCTs).
Methods
This systematic review was conducted in strict adherence to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number CRD42023369270.
Search strategy
We searched all publications from database inception to 15 January 2025 using three electronic databases: EMBASE, MEDLINE, and The Cochrane Central Register of Controlled Trials. No language restrictions were applied. The search strategy included the following terms: (exp ilex paraguariensis/or ((“mate” or “chimarrao” or “ilex paraguariensis” or “terere” or “yerba-mate”).ab.)) AND (randomized controlled trial.pt. or controlled clinical trial.pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randmo*.ab. or trial.ab. or groups.ab. not exp animals/not humans.sh.). To optimize the search outcomes, suitable term combinations and truncations were carefully chosen and customized for each database. The detailed search strategies are comprehensively outlined in Supplementary Table 1. To ensure the identification of all potentially relevant studies, reference lists of included publications were manually scrutinized. Other study sources are mainly supplemented through reference list searching.
Eligibility criteria
The present review included RCTs that assessed the effects of Yerba Maté intake on metabolic health indicators. The inclusion criteria for the studies are systematically presented in Table 1 according to the PICOS principle. No restrictions were placed on language or time. Studies were excluded based on the following criteria: (1) Non-original research publications including reviews, letters, posters, conference abstracts, case reports and opinion pieces; (2) Studies where Yerba Maté was combined with other plants or supplements; (3) Studies without available information of interest.
Table 1
| Parameter | Criteria |
|---|---|
| Condition or domain being studied | dyslipidemia, T2DM/pre-diabetes, lFG or lGT (pre-diabetes status), at most one criteria of metabolic syndrome, obese. consumption of Yerba Maté as a possible lipid-lowering/glucose-reducing/anti-obesity compound |
| Patient/population | Individuals of all ages and genders |
| Intervention | Any form of Yerba Maté consumption (tea, extract, tablets, or capsules) |
| Comparison | Placebo, water, or other dietary interventions |
| Outcomes | Serum levels of triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting glucose, fasting insulin, postprandial glucose, glycated hemoglobin (HbA1c), homeostatic model assessment index (HOMA index), waist circumference, body mass index (BMI), and adverse events (AEs) |
| Study design | Randomized controlled trials (parallel and crossover designs) |
PICOS criteria for inclusion of studies.
Data extraction
Two investigators (Li Huang and Yadong Liu) independently screened titles and abstracts, followed by full-text reviews of eligible studies. Data were extracted according to predefined criteria. Disagreements were resolved through consensus discussions involving a third independent reviewer (Jirong Yue). The extracted data included: first author’s last name, publication year, country, study design, sample size (intervention and control groups), mean age, gender, dosage of Yerba Maté, study duration, treatment duration, and baseline and outcome data for triglycerides, total cholesterol, HDL-C, LDL-C, fasting glucose, fasting insulin, postprandial glucose, HbA1c, waist circumference, body mass index (BMI), homeostasis model assessment (HOMA) index, and adverse events (AEs).
Quality assessment
Two investigators (Daiping Li and Liantian Yue) independently assessed the risk of bias in the included RCTs using the RoB 2 tool. The risk of bias in parallel RCTs was assessed using the RoB 2.0 tool for RCTs (Version dated 22 August 2019). For crossover trials, the RoB 2.0 tool specific to crossover studies was utilized (Version dated 18 March 2021). Both tools evaluate the risk of bias across five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of reported results. Disagreements were resolved through discussion with a third author (Jirong Yue). In cases where pertinent information was lacking, we proactively contacted the respective authors for clarification.
The overall quality of the evidence was evaluated using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (21–23). The GRADE framework assesses the quality of evidence across five domains: risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. The quality of evidence was independently evaluated by two reviewers (Daiping Li and Liantian Yue). In cases of discrepancies, an additional experienced rater was consulted (Jirong Yue).
Statistical analysis
The primary outcomes assessed in this study included triglycerides, total cholesterol, HDL-C, LDL-C, fasting glucose, fasting insulin, HbA1c, postprandial glucose, waist circumference, BMI, and AEs. All outcomes were reported as the change from baseline values. The incidence of adverse events was also analyzed.
Meta-analyses were performed using Review Manager (RevMan 5.4) when two or more studies from the same comparator provided sufficient data (i.e., mean difference [MD], standard deviation [SD], and number of participants in each intervention group). The I² statistic was calculated to quantify between-trial heterogeneity and inconsistency. When summary measures were not reported as MD and SD, previously published conversion tools were utilized (24). The randomized crossover trial used data from both intervention periods for analysis in the meta-analysis.
We quantified heterogeneity with I², interpreting 0-40% as low, 40-60% as moderate, and 60-90% as high (25). All meta-analyses were conducted using a random-effects model irrespective of I² value, in accordance with Cochrane Handbook recommendations. The chi-square P value was calculated to assess the statistical significance of heterogeneity, with P ≤ 0.05 indicating significant heterogeneity among the studies included in the meta-analysis. Sensitivity analyses were performed to evaluate the robustness of the results by: (1) excluding low-quality studies and (2) excluding studies with fewer than 10 participants.
Results
Study selection
A total of 1451 citations were identified through the initial database search. After eliminating duplicates and screening titles, 1294 citations were considered potentially relevant. During the title/abstract screening phase, 1265 studies were excluded due to failure to meet the study objectives. Full-text screening further excluded 16 studies (12, 26–40). The reasons for excluding these studies are detailed in Supplementary Table 2. Ultimately, 13 RCTs (17, 21, 41–51) fulfilled the inclusion criteria for this systematic review and meta-analysis. Figure 1 is a flow diagram of the study selection process.
Figure 1

Flow diagram of identified citations and included studies.
Study characteristics
A detailed overview of the study characteristics is presented in Table 2. The 13 studies enrolled participants with ages ranging from 28 to 57 years. Among these studies, 9 were parallel RCTs and 4 were crossover RCTs. The studies were conducted across various countries: five in Brazil (17, 43, 46, 50, 51), three in Korea (21, 47, 48), two in Italy (44, 45), one in Germany (49), one in Norway (41), and one in Argentina (42). The study sample comprised healthy adults (41, 49), overweight or obese individuals (21, 42, 47, 48), participants with metabolic abnormalities (17, 43–46), and people with HIV (50, 51). Interventions were delivered as Yerba Maté capsules (21, 41, 44–51) or as Yerba Maté tea (42, 43, 49). Daily capsule doses ranged from 0.5 g (44), 1.0 g (45), 2.25 g (46), 3.0 g (21, 47, 48, 50, 51) to 5 g (41); tea doses were 20 g (17, 43) or 100 g (42). The extract used by Opala 2006 (49) (containing asparagus, green tea, black tea, guarana, maté, and kidney beans) did not specify individual amounts. Treatment durations spanned 5 days (41), 15 days (50, 51), 4 weeks (46), 6 weeks (47, 48), 60 days (17), 3 months (44, 45), 12 weeks (21, 42, 49) and 90 days (43).
Table 2
| First author and year | Location | Study design | Number of patients | Sample size I/C | Type of patients | Mean age | Gender (M/F) | Intervention group | Control group | Study duration | Dosage/day | Outcomes of interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Areta,2018 (41) | Norway | Crossover RCT | 9 | 9/9 | Well-trained male cyclists | 30 | 9/0 | Yerba Maté capsules | Placebo | 5 days | 5 g | Glucose, Lactate, Free fatty acids, Glycerol, Caffeine, Paraxanthine, Adrenaline |
| Avena,2019 (42) | Argentina | Parallel RCT | 119 | not reported | Overweight (25.0 kg/m2 ≤BMI <32.5 kg/m2) | 36 | 0/119 | Yerba Maté tea | Water and dietary intervention | 12 weeks | 100g | Total cholesterol, Triglycerides, HDL-c, LDL-c, Waist circumference, Body weight, BMI, Body fat, BFM |
| Boaventura,2012 (43) | Brazil | Parallel RCT | 51 | 25/26 | Dyslipidemic volunteers | 47 | 9/42 | Yerba Maté tea | Dietary intervention | 90 days | 20g | Total cholesterol, LDL-c, HDL-c, non-HDL-c, Triglycerides, Ferric reducing antioxidant potential, Uric acid, Reduced glutathione, Lipid hydroperoxide, Protein carbonyl, Enzyme paraoxonase-1 |
| Derosa,2019 (44) | Italy | Parallel RCT | 130 | 66/64 | Patients with pre-diabetes | 53 | 63/67 | Glicoset 500 (Yerba Maté tablet) | Placebo | 3 months | 0.5g | Total cholesterol, Triglycerides, HDL-c, LDL-c, Body weight, BMI, Waist circumference, Hip circumference, Abdominal circumference, Fasting plasma glucose, Postprandial glucose, HbA1c, Fasting plasma insulin, HOMA index, Hs-CRP, AST, ALT, Glycemia, Impaired fasting glucose, Impaired glucose tolerance |
| Derosa,2021 (45) | Italy | Parallel RCT | 148 | 72/76 | Patients with pre-diabetes | 54 | 73/75 | Glicoset 1000 (Yerba Maté tablet) | Placebo | 3 months | 1g | Total cholesterol, Triglycerides, HDL-c, LDL-c, Body weight, BMI, Waist circumference, Hip circumference, Abdominal circumference, Fasting plasma glucose, Postprandial glucose, HbA1c, Fasting plasma insulin, HOMA index, Hs-CRP, AST, ALT, Glycemia, Impaired fasting glucose, Impaired glucose tolerance |
| Gebara,2021 (46) | Brazil | Crossover RCT | 34 | 34/34 | With at most one criteria of metabolic syndrome | 50 | 34/0 | Yerba Maté capsules | Placebo (starch) | 4 weeks | 2.25g (Maté extract) | Total cholesterol, Triglycerides, HDL-c, LDL-c, fasting glucose, SBP, DBP, Pulse, Waist circumference, Body weight, BMI, C-reactive protein, Intercellular adhesion molecule 1, Vascular cell adhesion molecule 1, Interleukin-6 |
| Jung,2016 (47) | Korea | Parallel RCT | 33 | 17/16 | Overweight (25.0 kg/m2 ≤BMI <30.0 kg/m2) | 44 | 0/33 | Yerba Maté tablet | Placebo (starch) | 6 weeks | 3g (Maté extract) | Total cholesterol, Fasting glucose, SBP, DBP, Waist circumference, Body weight, BMI, Hip circumference, Body fat, BFM, LBM, AST, ALT, SBP, DBP, Pulse rate, Total bilirubin, Albumin, Protein |
| Kim,2012 (48) | Korea | Parallel RCT | 60 | 30/30 | BMI≥ 25.0 kg/m2 | 28 | 0/60 | Yerba Maté capsules | Placebo(starch) | 6 weeks | 3g (Maté extract) | Waist circumference, Body weight, BMI, Body fat, BFM, LBM, Total cholesterol, Triglycerides, HDL-c, Fasting glucose, Safety |
| Kim, 2015 (21) | Korea | Parallel RCT | 30 | 15/15 | Obese | 43 | 4/26 | Yerba Maté capsules | Placebo | 12 weeks | 3g (Maté extract) | Visceral fat, Subcutaneous fat, Visceral subcutaneous ratio, Total cholesterol, LDL-c, HDL-c, non-HDL-c, Triglycerides, Free Fatty Acid |
| Klein,2011 (17) | Brazil | Parallel RCT | 36 | 9/9 10/8 |
T2DM/pre-diabetes | 57 | 10/26 | Yerba Maté tea | Dietary intervention | 60 days | 20g | Total cholesterol, LDL-c, HDL-c, non-HDL-c, Triglycerides |
| Opala,2006 (49) | Germany | Parallel RCT | 98 | 47/51 | Healthy volunteers | 42 | 21/77 | Yerba Maté tablets | Placebo | 12 weeks | two tablets | Body weight, Waist circumference, Hip, W/H, Body fat by SKF, Total cholesterol, LDL-c, HDL-c, Triglycerides, HDL/LDL, Lipoprotein (a), Fasting Insulin, Fasting glucose |
| Petrilli,2016 (50) | Brazil | Crossover RCT | 92 | 92/92 | HIV | 45 | 58/34 | Yerba Maté capsules | Mate-placebo | 15days | 3g | Hs-CRP, HDL-c, fibrinogen, Leukocytes, Lymphocytes, Neutrophils, Monocytes |
| Souza,2017 (51) | Brazil | Crossover RCT | 92 | 92/92 | HIV/AIDS | 45 | 58/34 | Yerba Maté tablets | Mate-placebo | 15days | 3g | Total cholesterol, Triglycerides, LDL-c, HDL-c, LDL(-), Apolipoprotein B-100, Apolipoprotein A1 |
Characteristics of studies included in the meta-analysis.
RCT, randomized controlled trial; I, intervention group; C, control group; HbA1c, glycated haemoglobin; HOMA index, homeostatic model assessment index; Hs-CRP, high sensitivity C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BFM, body fat mass; LBM, lean body mass; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; T2DM, type 2 diabetes mellitus.
Risk of bias
Among the nine parallel RCTs included, potential bias was identified across several domains in Supplementary Figure 1. Specifically, issues with the Randomization process (D1) were noted in three studies (17, 42, 43) and concerns regarding Deviations from the intended interventions (D2) were observed in two study (17, 43). One study (17) was rated as some concerns in the Missing outcome data (D3) domain. All studies, however, demonstrated a low risk of bias in the Measurement of the outcome (D4) domain. Furthermore, three studies (17, 42, 47) exhibited some concerns in the Selection of the reported result (D5) domain. Five studies (21, 44, 45, 48, 49) exhibited low risk of bias across all domains, increasing confidence in their findings.
Among the four crossover RCTs included, potential bias was identified across several domains in Supplementary Figure 2. Of the four crossover RCTs assessed, three studies raised some concerns about the Randomization process (D1) (41, 46, 50), and one study showed bias issues caused by menstruation and carryover effects (DS) (41). In terms of Deviations from the intended interventions (D2), one study presented a high risk of bias (50), while two studies indicated some concerns (41, 51). For Missing outcome data (D3), two studies were rated as high risk (50, 51). The Measurement of the outcome (D4) domain generally showed a low risk across studies, suggesting a reliable assessment of outcomes. As for the Selection of the reported result (D5) domain, three studies were identified with some concerns (41, 50, 51). Three studies (41, 50, 51) were rated as having some concerns in the domain of Selection of the reported result (D5). Two studies (50, 51) were classified as high risk and other two studies (41, 46) were identified with some concerns across all domains.
GRADE assessment
Applying the GRADE framework (Table 3), we rated the certainty of evidence as high, moderate, or low for all outcomes. We downgraded waist circumference evidence by one level because of marked inconsistency (I²>50%). The evidence for fasting insulin and HOMA index was downgraded by one level due to detected publication bias (more than half of the studies come from the same team). Total cholesterol, triglycerides, LDL-C, and fasting glucose were downgraded by two level for very serious limitations, combining serious inconsistency and serious imprecision (wide 95% CI), resulting in low certainty. Postprandial glucose and HbA1c were downgraded by two levels due to very serious limitations, combining serious inconsistency and detected publication bias, resulting in low certainty.
Table 3
| Outcomes | Participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty | Absolute effects 95%CI |
|---|---|---|---|---|---|---|---|---|
| Total cholesterol | 926 (11RCTS) |
not serious | seriousa I2 = 89% |
not serious | seriousb | undetected | Low ⨁⨁◯◯ |
MD -3.97 (-12.06 to 4.12) |
| Triglycerides | 893 (10RCTS) |
not serious | seriousa I2 = 51% |
not serious | seriousb | undetected | Low ⨁⨁◯◯ |
MD -6.30 (-14.02 to 1.42) |
| HDL-C | 1077 (11RCTS) |
not serious | not serious I2 = 31% |
not serious | not serious | undetected | High ⨁⨁⨁⨁ |
MD 0.34 (-0.50 to 1.17) |
| LDL-C | 833 (9RCTS) |
not serious | seriousa I2 = 84% |
not serious | seriousb | undetected | Low ⨁⨁◯◯ |
MD -3.68 (-10.63 to 3.26) |
| Fasting glucose | 562 (7RCTS) |
not serious | seriousa I2 = 96% |
not serious | seriousb | undetected | Low ⨁⨁◯◯ |
MD -1.46 (-7.53 to 4.62) |
| Fasting insulin | 383 (3RCTS) |
not serious | not serious I2 = 0% |
not serious | not serious | detectedc | Moderate ⨁⨁⨁◯ |
MD -0.04 (-0.78 to 0.70) |
| Postprandial glucose | 285 (2RCTS) |
not serious | seriousa I2 = 91% |
not serious | not serious | detectedc | Low ⨁⨁◯◯ |
MD -12.76 (-16.78 to -8.74) |
| HbA1c | 285 (2RCTS) |
not serious | seriousa I2 = 64% |
not serious | not serious | detectedc | Low ⨁⨁◯◯ |
MD -0.37 (-0.56 to -0.18) |
| HOMA index | 285 (2RCTS) |
not serious | not serious I2 = 0% |
not serious | not serious | detectedc | Moderate ⨁⨁⨁◯ |
MD -0.24 (-0.37 to -0.11) |
| Waist circumference | 654 (8RCTS) |
not serious | seriousa I2 = 51% |
not serious | not serious | undetected | Moderate ⨁⨁⨁◯ |
MD -0.48 (-1.22 to -0.26) |
| BMI | 624 (7RCTS) |
not serious | not serious I2 = 0% |
not serious | not serious | undetected | High ⨁⨁⨁⨁ |
MD -0.17 (-0.36 to 0.02) |
GRADE’s summary of findings.
aDowngraded for high inconsistency (I2 >50%).
bDowngraded for high imprecision (if the 95% confidence interval was very wide).
cDowngraded for publication bias (if more than half of the studies come from the same team).
dHDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated haemoglobin; HOMA index, homeostatic model assessment index; BMI, body mass index.
Impact on lipid levels
A total of 11 RCTs were included to assess the impact of Yerba Maté on lipid levels, with follow-up durations ranging from 15 to 90 days (Figure 2). Comparative analysis of Yerba Maté versus control groups demonstrated non-significant differences in total cholesterol (Figure 2A), triglycerides (Figure 2B), HDL-C (Figure 2C) or LDL-C (Figure 2D).
Figure 2
![Four forest plots compare Yerba Maté vs. control groups on lipid profiles.A: Total cholesterol: mean difference -3.97 [95% Cl: -12.06, 4.12], showing non-significant differences Yerba Maté vs. control groups. B: Triglycerides: mean difference -6.30 [95% Cl: -14.02, 1.42], showing non-significant differences Yerba Maté vs. control groups. C: HDL-C: mean difference 0.34 [95% Cl: -0.50, 1.17], showing non-significant differences Yerba Maté vs. control groups. D: LDL-C: mean difference -3.68 [95%Cl:-10.63,3.26], showing non-significant differences Yerba Maté vs. control groups. Studies show varying heterogeneity.](https://www.frontiersin.org/files/Articles/1641592/xml-images/fendo-16-1641592-g002.webp)
Forest plot of Yerba Maté’s effect on lipid levels. (A) Total cholesterol; (B) Triglycerides; (C) HDL-C (high-density lipoprotein cholesterol); (D) LDL-C (low-density lipoprotein cholesterol).
Effects on blood glucose metabolism
Seven RCTs were included to evaluate the effects of Yerba Maté on glycemic status, with follow-up durations ranging from 5 days to 3 months. The quantitative analysis results are summarized in Figure 3. Meta-analysis revealed a significant reduction in postprandial glucose levels (MD -12.76, 95% CI -16.78 to -8.74, I² = 91%, p=0.001, N = 2) (Figure 3C), HbA1c levels (MD -0.37, 95% CI -0.56 to -0.18, I² = 64%, p=0.1; N = 2) (Figure 3D) and the HOMA index (MD -0.24, 95% CI -0.37 to -0.11, I² = 0%, p=0.82; N = 2) (Figure 3E). No significant differences were observed in fasting glucose levels (Figure 3A) or fasting insulin concentrations (Figure 3B) between the Yerba Maté group and the control group.
Figure 3

Forest plot of Yerba Maté’s effect on glycemic status. (A) Fasting glucose, (B) Fasting insulin, (C) Postprandial glucose, (D) HbA1c(glycated hemoglobin), and (E) HOMA index(homeostatic model assessment index).
Influence on weight management
Eight RCTs were included to assess the effects of Yerba Maté on waist circumference and BMI, with follow-up durations ranging from 4 weeks to 3 months (Figure 4). Comparative meta-analysis showed no significant differences in waist circumference (Figure 4A) or BMI (Figure 4B) between Yerba Maté and control groups.
Figure 4

Forest plot of Yerba Maté’s effect on waist circumference and BMI. (A) Waist circumference, (B) BMI (body mass index).
Subgroup analyses
We performed subgroup analyses of intervention population, intervention methods, and intervention duration. Subgroup analyses of intervention doses were not possible because most intervention doses were only available in 1–2 studies. We conducted a subgroup analysis of the intervention group for Yerba Maté (Figures 5, 6), including: dyslipidemic volunteers, overweight and obese and non-dyslipidemic, normal-weight volunteers. Subgroup analyses found a significant difference in triglycerides (MD -15.34, 95%CI -20.37, -10.31, I² =0%, p<0.001, n=5) (Figure 5B) and fasting glucose (MD -9.08, 95%CI -15.29, -2.88, I² = 95%, p=0.004, n=3) (Figure 6A) among dyslipidemic volunteers.
Figure 5
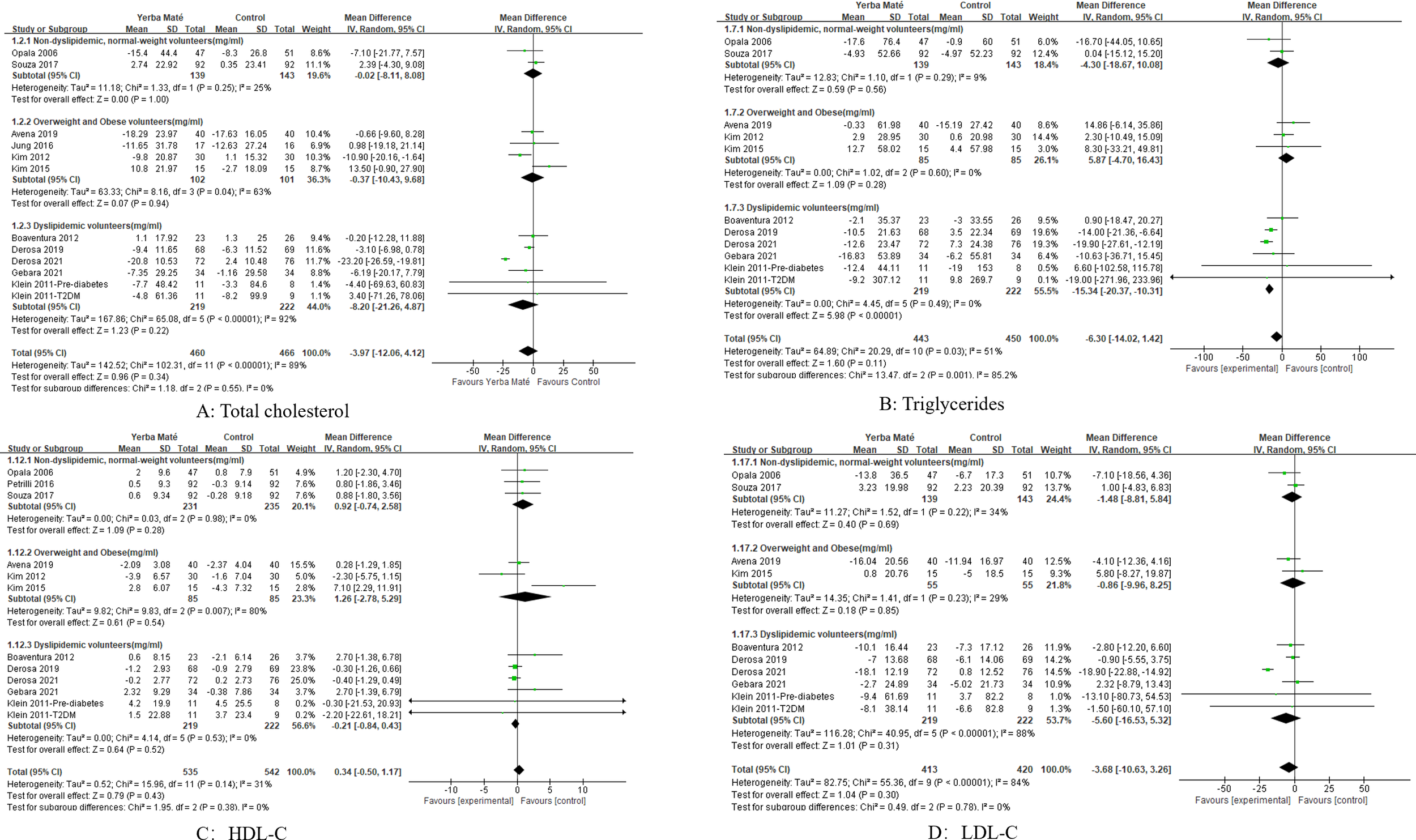
Forest plot for subgroup analysis of intervention populations on lipid levels. (A) Total cholesterol; (B) Triglycerides; (C) HDL-C (high-density lipoprotein cholesterol); (D) LDL-C (low-density lipoprotein cholesterol).
Figure 6
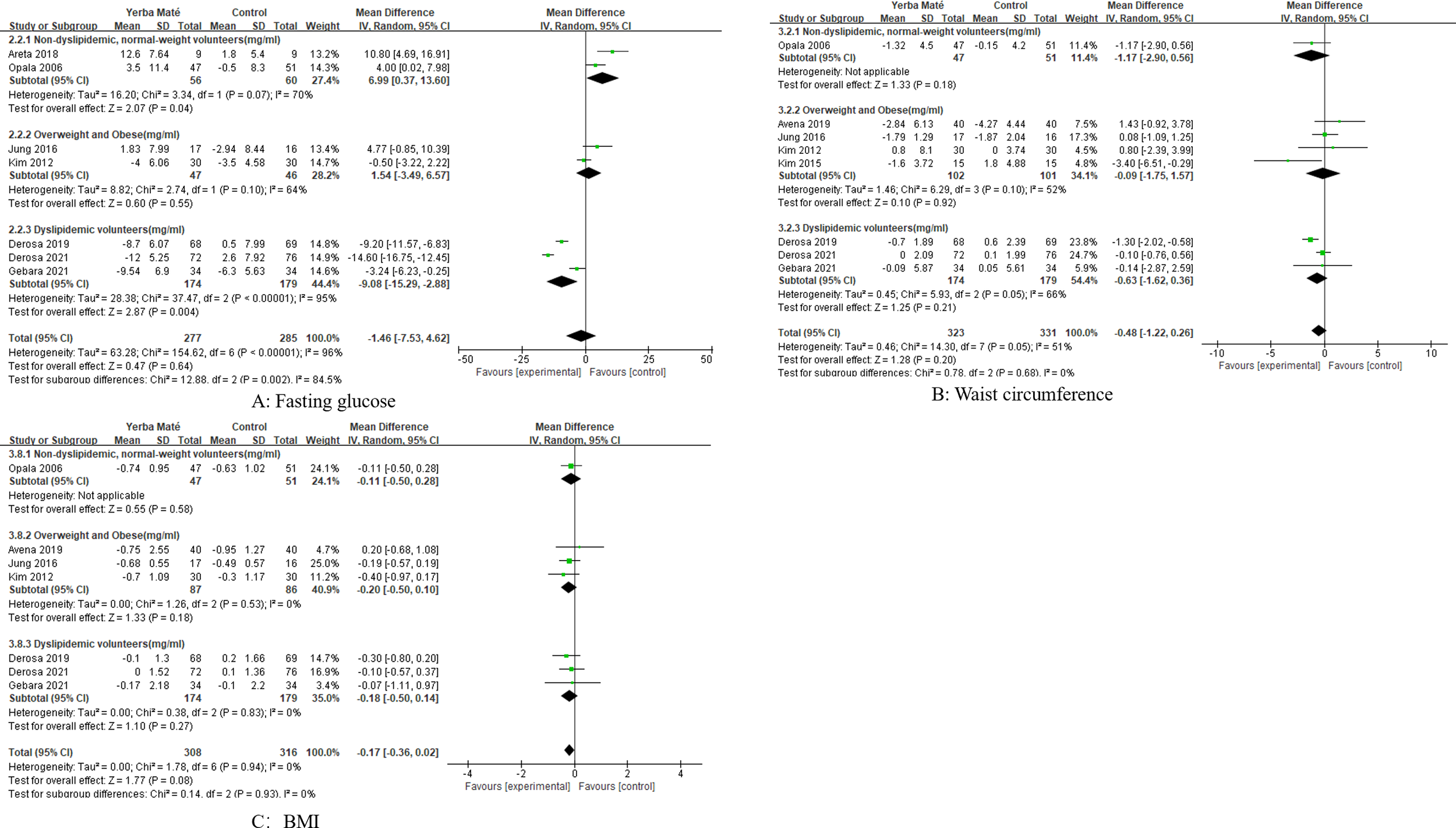
Forest plot for subgroup analysis of intervention populations on fasting glucose, waist circumference and BMI. (A) Fasting glucose, (B) Waist circumference, (C) BMI (body mass index).
We conducted a subgroup analysis of our intervention methods for Yerba Maté (Figure 7), including Yerba Maté extract and Yerba Maté. In this subgroup analysis, the intervention with Yerba Maté extract significantly reduced triglycerides(MD -9.75, 95%CI -17.60, -1.91, I² =52%, p=0.01, n=7) (Figure 7B). Most intervention methods included in the study were Yerba Maté extracts, with no more than one study involving Yerba Maté tea. Therefore, no subgroup analysis of intervention methods based on fasting glucose, waist circumference, and BMI was conducted.
Figure 7
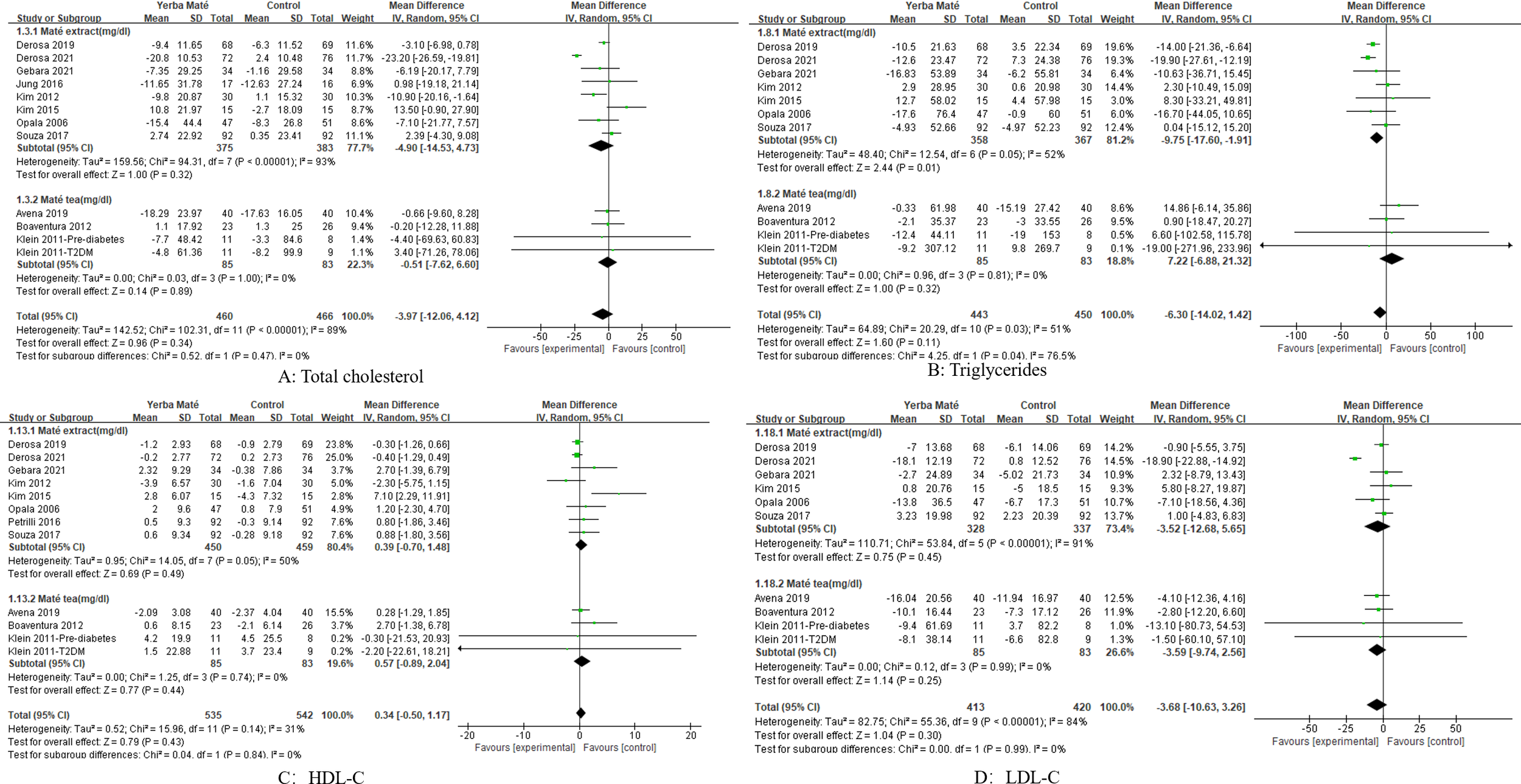
Forest plot for subgroup analysis of intervention methods on lipid levels. (A) Total cholesterol; (B) Triglycerides; (C) HDL-C (high-density lipoprotein cholesterol); (D) LDL-C (low-density lipoprotein cholesterol).
We conducted a subgroup analysis of the intervention duration for Yerba Maté, including periods of less than 12 weeks and 12 weeks or longer. However, no significant differences were found (Figures 8, 9).
Figure 8

Forest plot for subgroup analysis of intervention duration on lipid levels. (A) Total cholesterol; (B) Triglycerides; (C) HDL-C (high-density lipoprotein cholesterol); (D) LDL-C (low-density lipoprotein cholesterol).
Figure 9
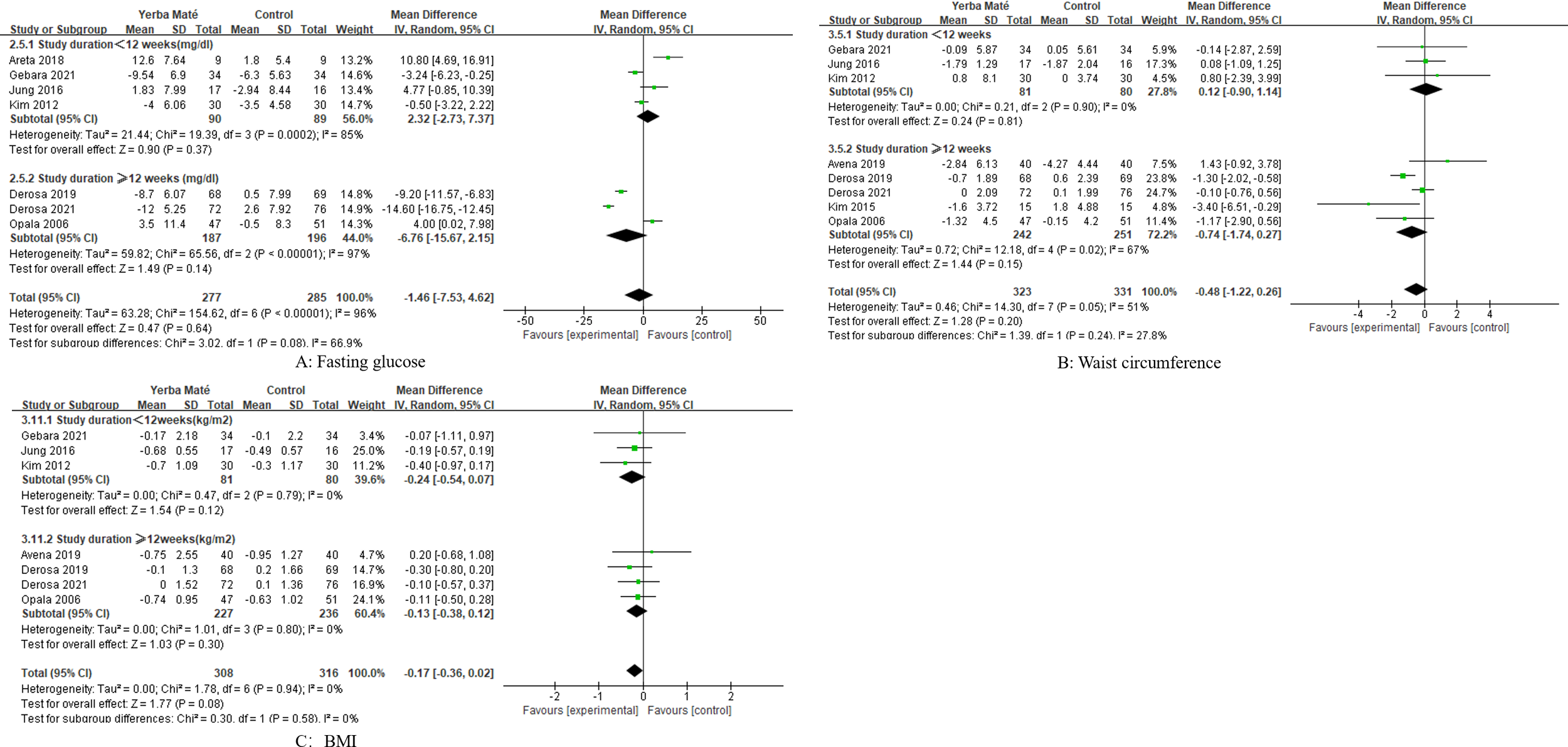
Forest plot for subgroup analysis of intervention duration on fasting glucose, waist circumference and BMI. (A) Fasting glucose, (B) Waist circumference, (C) BMI (body mass index).
Adverse events
The safety profile of Yerba Maté was reported in four studies (12, 18, 21, 49), with adverse effects summarized in Table 4. Yerba Maté tea consumption has been associated with adverse effects in certain individuals, including oral or gastric mucosal irritation, insomnia, nausea, acid reflux, tachycardia, angina pectoris, headache, and abdominal discomfort.
Table 4
| First author, year [reference] | Number of patients | Number of adverse events | Adverse effects |
|---|---|---|---|
| MORAIS, 2009 (12) | 118 | 4 | irritation of the oral or stomach mucosa, insomnia, or nausea |
| Klein, 2011 (17) | 85 | 8 | insomnia, heartburn, and tachycardia |
| Kim, 2015 (21) | 30 | 1 | adverse side effects |
| Opala, 2006 (49) | 98 | 8 | gastrointestinal discomfort |
Adverse effects of Yerba Maté.
Sensitivity analyses
To evaluate the reliability of the results regarding Yerba Maté’s effects on lipid profiles and fasting blood glucose levels, sensitivity analyses were performed (Figure 10). Excluding low-quality studies and those with fewer than 10 participants did not alter the overall findings, confirming the robustness of the meta-analysis results. No sensitivity analysis was performed on fasting insulin, postprandial glucose, HbA1c, HOMA index, waist circumference and BMI, since the studies did not include low-quality research or studies with fewer than 10 participants.
Figure 10
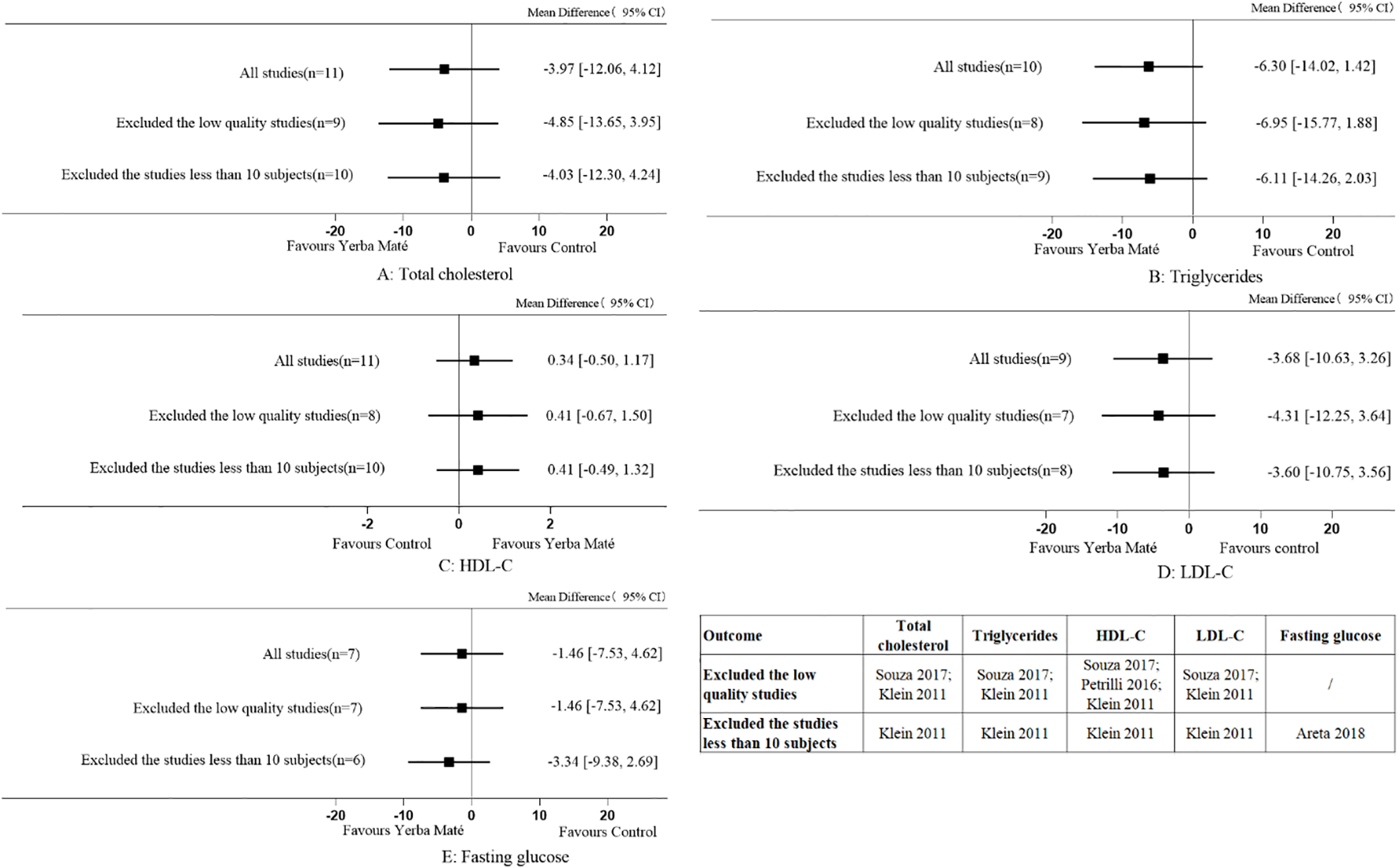
Sensitivity analyses for Yerba Maté consumption on lipid levels and fasting glucose. (A) Total cholesterol; (B) Triglycerides; (C) HDL-C (high-density lipoprotein cholesterol); (D) LDL-C (low-density lipoprotein cholesterol), (E) Fasting glucose.
Discussion
Principal findings
Our systematic review and meta-analysis demonstrate that Yerba Maté consumption may improve glycemic outcomes, including decreased postprandial glucose levels, HbA1c levels, and the HOMA index. However, no significant effects were observed on lipid profiles (triglycerides, total cholesterol, HDL-C, LDL-C), waist circumference, or BMI. These findings suggest that Yerba Maté may exert glycemic control benefits independent of lipid metabolism modulation.
It is important to highlight that the two studies indicating Yerba Maté’s beneficial effects on blood glucose were conducted by the same research team (44, 45), which may introduce reporting bias and warrants cautious interpretation. In these two studies (44, 45), the subjects were individuals with impaired fasting glucose and impaired glucose tolerance. While other studies (41, 46–49) included diverse populations such as healthy volunteers, athletes, and overweight individuals with normal or well-regulated blood glucose, who may respond less noticeably to interventions, leading to non-significant results. In addition, these two studies employed a longer intervention period of three months, providing a more extended window to observe potential changes in blood glucose indicators, whereas some shorter-term studies may not have been as effective in capturing significant improvements (41, 46–48). However, it should be noted that the two studies were conducted by the same research team and used a standardized formulation produced by the same company. This might lead to reporting bias. Therefore, their positive results should be interpreted with caution.
To contextualize our findings, we compared the effect sizes for fasting glucose and triglycerides with those reported for cinnamon and fenugreek (52, 53). Yerba Maté and cinnamon achieve identical fasting-glucose reductions (MD -9.08), surpassing fenugreek (MD -0.84); for triglycerides cinnamon (54) retains the largest effect (WMD -29.59), while yerba maté provides a moderate but clinically relevant benefit (MD -20.37).
Some systematic reviews have yielded inconsistent results concerning the metabolic impacts of Yerba Maté supplementation. For example, Masson et al. (10) showed a significant reduction in LDL-C in non-diabetic populations, Clemente et al. (15) found HDL-C improvements in obese populations using 3g/d standardized extracts, but José et al. (55) observed no effects on glycemic parameters in type 2 diabetes patients given <1g/d extracts. Four factors might account for the disparity between our findings and prior meta-analyses. First, there are population-specific differences in metabolic pathways. José et al. (55) focused on chronic disease populations but excluded the acute metabolic responses in our athlete subgroup. While Clemente et al. (15) reported HDL-C improvement in homogeneous obese cohorts, our study included HIV patients and athletes whose lipid metabolism may be affected by antiretroviral therapy (50) or exercise-induced adaptations (41). Second, dose-response heterogeneity is crucial. Unlike Luís et al. (21), who focused on 3g/d standardized extracts, our analysis included extreme dosing like 50g/d tea in Avena 2019 (42). Third, there were marked differences in outcome measurement timelines. The HbA1c reduction in our study mainly came from ≥84-day interventions (44, 45), whereas the negative waist circumference findings included short-term trials like the 5-day cycling protocol in Areta 2018 (41), which contrasts with Luís et al.’s (21) 90-day focused analysis. Fourth, heterogeneity in study design caused bias. Our review strictly included only parallel RCTs and crossover RCTs to maximize internal validity. In contrast, Masson et al. (14) combined RCTs and observational studies (e.g., cohort and case-control studies), which might have allowed lifestyle confounders such as diet and exercise to interfere with the independent link between Yerba Maté intervention and metabolic outcomes.
Subgroup analysis
Subgroup analysis of the intervention population revealed significant differences in triglycerides and fasting glucose levels among dyslipidemic volunteers. Subgroup analysis of the intervention methods revealed Yerba Maté extracts were significantly differences in triglycerides. Dyslipidemic volunteers and Yerba Maté extracts as possible sources of heterogeneity. Future studies may explore larger-scale interventions with Yerba Maté extracts among dyslipidemic volunteers. However, subgroup analysis conducted in subgroups according to different intervention duration showed no significant differences in total cholesterol, triglycerides, HDL-C, LDL-C, fasting glucose, waist circumference and BMI between Yerba Maté and control groups.
Mechanism basis
Our systematic review and meta-analysis suggest that Yerba Maté consumption may be associated with improvements in glycemic control, as indicated by reductions in postprandial glucose, HbA1c levels, and HOMA index. However, no significant effects were observed on fasting glucose or insulin levels. These findings hint at potential mechanisms that might underlie the observed effects, such as enhanced insulin sensitivity and postprandial glucose regulation, though the exact mechanisms remain to be fully elucidated. Existing literature proposes that Yerba Maté polyphenols could potentially modulate hepatic insulin signaling by suppressing TNF-α (56), and may interact with the PI3K-AKT pathway, involving elements like Akt2 and Irs1, possibly contributing to improved peripheral insulin resistance (57). Regarding postprandial glucose control, it has been hypothesized that bioactive compounds in Yerba Maté might inhibit intestinal SGLT1-mediated glucose absorption (58). Additionally, its anti-glycation properties, which seem to surpass those of green tea (59), could potentially protect β-cells by inhibiting AGE formation during the secondary glycation phase. While these mechanistic insights offer plausible explanations for the observed effects, they are largely derived from preclinical studies and in vitro evidence. As such, they should be interpreted with caution and require further validation through well-designed clinical trials before Yerba Maté can be positioned as an adjuvant therapy for diabetes management.
The present study found no significant effects of Yerba Maté consumption on waist circumference or body mass index (BMI), suggesting limited efficacy in directly reducing abdominal adiposity or overall body weight. However, preclinical evidence indicates its potential to modulate energy metabolism through three interconnected mechanisms: (1) Caffeine-mediated activation of the sympathetic nervous system enhances lipolytic enzyme activity, though clinical relevance requires exceeding dose-dependent thresholds (>3 g/day of standardized extracts or >20 g/day of traditional infusion) to achieve thermogenic effects (60–62); (2) While acute interventions (e.g., 5-day trials) demonstrate efficacy in delaying gastric emptying and enhancing satiety via gastrointestinal modulation, these effects fail to translate into sustained body composition improvements in longitudinal studies, likely due to compensatory metabolic adaptations (28, 63); (3) Although Yerba Maté supplementation reduces chronic inflammation markers such as C-reactive protein (CRP) – a known correlate of visceral adiposity – its anti-inflammatory properties exhibit paradoxical dissociation from abdominal fat redistribution, necessitating extended clinical trials to validate causal relationships (55, 62).
Potential adverse effects
Although Yerba Maté (Ilex paraguariensis) is recognized for its numerous health benefits, its potential adverse effects and carcinogenic risks warrant attention. Studies have indicated that long-term, high-volume consumption of Yerba Maté may increase the risk of certain cancers, including oral, esophageal, laryngeal, and lung cancers (64–68). Notably, the risk of carcinogenesis is significantly elevated when Yerba Maté is consumed at temperatures exceeding 65 °C, which may be attributed to thermal injury to esophageal cells caused by hot beverages (65–68). Additionally, Yerba Maté contains various chemical constituents, such as tannins, nitrosamines, and polycyclic aromatic hydrocarbons, which may possess carcinogenic potential (68–72). However, other research has suggested that the polyphenolic compounds in Yerba Maté may exert protective effects against certain cancers (70–72). Despite these findings, the current evidence is inconsistent, with some studies indicating that the association between hot Yerba Maté consumption and increased cancer risk may be influenced by other factors, such as smoking and alcohol consumption (64, 73). The randomized controlled trials included in this systematic review all had intervention periods of no more than three months. Future research necessitates long-term follow-up studies to further investigate the safety profile of Yerba Maté, particularly its health impacts under different consumption temperatures and dosages.
Methodological quality of included trials
The methodological quality assessment revealed notable variations across study designs. Among the nine parallel RCTs, five studies (21, 44, 45, 48, 49) demonstrated low risk of bias across all domains, while the remaining four exhibited some concerns or high risk in specific domains (particularly randomization process and deviations from intended interventions). The crossover RCTs showed more substantial methodological limitations, with two studies (50, 51) classified as high risk and two (41, 46) having some concerns across multiple domains.
Sources of heterogeneity
Significant heterogeneity persisted in this study’s meta-analysis, despite conducting subgroup and sensitivity analyses. Several factors likely contributed to this heterogeneity. First, the studies encompassed a wide range of participant characteristics, including age, gender, and health status, from healthy individuals to those with conditions like obesity, prediabetes, metabolic syndrome, and HIV infection. Such diversity in metabolic status and disease burden can lead to varied responses to Yerba Maté interventions. Second, differences in the form of intake of Yerba Maté and study design, such as differences in the mode of use (tea infusions, extract or capsules), whole and part amounts used, dosage, and duration of intervention, could act as confounders responsible for differences between effects in the short term and the long term. Third, studies were marked by strong differences in baseline characteristics. Some studies may have included subjects with high baseline triglyceride levels. In contrast, others have lower baseline levels that could have resulted in different effects of Yerba Maté being observed. Finally, heterogeneity may be attributed to study design and methodology differences, e.g., randomization, blinding and data collection. These issues can be addressed by future research that standardizes intervention and measurement protocols, broadens the study population, controls all confounding variables, and strengthens the study’s design. Since this approach reduces heterogeneity, it will aid in increasing the reliability and the generalizability of findings.
Strengths and limitations
The present study has several strengths. Its exhaustive search strategy allows all relevant literature to be included. By minimizing the risk of publication bias, this approach guarantees that as many studies as possible are represented and thus makes the findings more robust. Moreover, the studies are assessed with rigorous evaluation methods, including the RoB 2 tool, for the risk of bias so that each study is thoroughly scrutinized. The GRADE approach adds to the analysis by practicing a systematic and transparent approach to evaluating the overall quality of evidence and, thus, the level of confidence in the results.
This review is subject to certain limitations. Given that research findings from South America may be published in languages such as Spanish, we ultimately decided to include studies without language restrictions. This is not consistent with our PROSPERO record. The major issue is a big heterogeneity in clinical and procedural aspects of the Yerba Maté consumption in the studies examined, including differences in the details of their participants, their Yerba Maté consumption regimens, and the different follow-up durations. Further, heterogeneity extends to assessing such outcomes as total cholesterol, LDL-C, fasting blood glucose, postprandial blood glucose, HOMA index and BMI, which may influence the comparison and the generalizability of the results. In addition, the study of outcomes, including postprandial blood glucose, HOMA index, and HbA1c, is limited to only two of the included studies, which may result in too few subjects to allow sound conclusions. Furthermore, three studies had a follow-up period under 20 days, which may raise a question of the length of follow-up was insufficient for the Yerba Maté consumption. Future research should strive to develop protocols and outcome measures that are standardized in order to facilitate comparison and reliability of results.
Implications for future research
Although consumption of Yerba Maté has shown health benefits, future research should go further in studying the long-term effects and risks of this consumption. Future research investigations on Yerba Maté and glycemic control should focus on extending the intervention duration, employing Yerba Maté extracts, and enrolling subjects with metabolic disorders. As far as it is carcinogenic, this matter is still inconclusive because some studies on Yerba Maté show that carcinogenic substances (Polycyclic aromatic hydrocarbons, or PAHs, and tannins) come not from Yerba Maté itself but from the contamination during processing. Yet more studies have come to the conclusion that perhaps it is not the tea that poses a problem but the high temperature with which it is traditionally consumed. Moreover, smoking and alcohol drinking may contribute to the observed cancer risk. Given the present study’s limitations, large-scale prospective cohort studies are required to clarify whether Yerba Maté carries a carcinogenic risk and to differentiate temperature, PAH, and other variables.
Furthermore, the role of Yerba Maté in blood pressure and other metabolic syndrome indices (uric acid) has been widely ignored. The current research focus mainly involves short-term interventions with limited systematic evaluation of long-term effects. RCTs that are well designed are necessary to validate the effects of Yerba Maté on the metabolic indicators and elucidate the physiological mechanisms. Evaluation of the role of Yerba Maté in preventing and treating chronic diseases has not been possible because of the lack of long-term intervention studies. Studies of the incidence of severe cardiovascular events in prospective cohort studies with extended follow-up could supply important information about its long-term health effects.
Then, the possible effects of Yerba Maté on several different populations, e.g., the older adults, the ones who are athletes, and the diabetic, are variant due to the differences in each person. Its mechanisms of action and effects in specific populations merit future research, focusing on the feasibility of a personalized intervention. In doing this, we will augment our entity’s understanding of the health implications of Yerba Maté.
Conclusion
In summary, our systematic review and meta-analysis suggest that Yerba Maté consumption may significantly reduce postprandial glucose, HbA1c, and HOMA index. However, it did not show significant impacts on total cholesterol, triglycerides, HDL-C, LDL-C, fasting glucose, fasting insulin, waist circumference, or BMI, and further research is needed to confirm these preliminary findings. Although some adverse events were reported, the overall findings suggest that Yerba Maté may exert glycemic control benefits independent of lipid metabolism modulation. Future research should address the identified limitations and knowledge gaps through large-scale, rigorously designed RCTs and longitudinal cohort studies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DL: Writing – original draft, Writing – review & editing. LY: Writing – original draft, Writing – review & editing. XP: Data curation, Formal analysis, Writing – review & editing. LC: Methodology, Writing – review & editing. TL: Formal analysis, Writing – review & editing. LH: Data curation, Writing – review & editing. YL: Methodology, Writing – review & editing. JY: Funding acquisition, Supervision, Writing – review & editing. XH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financially supported by Sichuan Province science and technology innovation base project (2023ZYD0173), Sichuan-Chongqing Science and Technology Innovation Cooperation Plan (2024YFHZ0072), Xiamen Medical and Health Key Project (3502Z20234009), and Health and scientific research for cadres in Sichuan province, grant (no. 2024-105). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1641592/full#supplementary-material
Abbreviations
AEs, adverse events; BMI, body mass index; CI, confidence intervals; HbA1c, glycated hemoglobin; HOMA index, homeostatic model assessment index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MD, mean difference; SD, standard deviations; RCTs, randomized controlled trials; ROB, risk of bias; T2DM, type 2 diabetes mellitus; PRISMA, preferred reporting items for systematic reviews and meta-analyses.
References
1
Alves FE Scheer AD . Yerba mate (Ilex paraguariensis), science, technology and health: A systematic review on research, recent advances and possible paths for future studies. S Afr J Bot. (2024) 168:573–87. doi: 10.1016/j.sajb.2024.04.008
2
Meremäe K Raudsepp P Rusalepp L Laun T Roasto M . Polyphenolic profiles, antioxidant capacity, and antibacterial activity of green tea, matcha tea, black tea, and Yerba Maté extracts. Appl Food Res. (2025) 5:101080. doi: 10.1016/j.afres.2025.101080
3
Iommi C Chemical Profiles of Yerba Mate Infusions Across South American Countries . Chemistry and Safety of South American Yerba Mate Teas. SpringerBriefs in Molecular Science. Cham: Springer (2021) p. 39–48. doi: 10.1007/978-3-030-69614-6_3
4
Meng S Cao J Feng Q Peng J Hu Y . Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Alternat Med. (2013) 2013:801457. doi: 10.1155/2013/801457
5
Lu H Tian Z Cui Y Liu Z Ma X . Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr Rev Food Sci Food Saf. (2020) 19:3130–58. doi: 10.1111/1541-4337.12620
6
Quiñones M Miguel M Aleixandre A . Beneficial effects of polyphenols on cardiovascular disease. Pharmacol Res. (2013) 68:125–31. doi: 10.1016/j.phrs.2012.10.018
7
Serino A Salazar G . Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients. (2018) 11:53. doi: 10.3390/nu11010053
8
Rudrapal M Khairnar SJ Khan J Dukhyil AB Ansari MA Alomary MN et al . Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. (2022) 13:806470. doi: 10.3389/fphar.2022.806470
9
Zhang Y Hao R Chen J Li S Huang K Cao H et al . Health benefits of saponins and its mechanisms: perspectives from absorption, metabolism, and interaction with gut. Crit Rev Food Sci Nutr. (2024) 64:9311–32. doi: 10.1080/10408398.2023.2212063
10
Cao S Liu M Han Y Li S Zhu X Li D et al . Effects of saponins on lipid metabolism: the gut-liver axis plays a key role. Nutrients. (2024) 16:1514. doi: 10.3390/nu16101514
11
Messina D Soto C Mendez A Corte C Kemnitz M Avena V et al . Lipid-lowering effect of mate tea intake in dyslipidemic subjects. Nutr Hosp. (2015) 31:2131–9. doi: 10.3305/nh.2015.31.5.8386
12
de Morais EC Stefanuto A Klein GA Boaventura BC de Andrade F Wazlawik E et al . Consumption of Yerba Maté (Ilex paraguariensis) improves serum lipid parameters in healthy dyslipidemic subjects and provides an additional LDL-cholesterol reduction in individuals on statin therapy. J Agric Food Chem. (2009) 57:8316–24. doi: 10.1021/jf901660g
13
Masson W Barbagelata L Lobo M Nogueira JP Corral P Lavalle-Cobo A . Effect of Yerba Maté (Ilex paraguariensis) on lipid levels: A systematic review and meta-analysis. Plant Foods Hum Nutr. (2022) 77:353–66. doi: 10.1007/s11130-022-00991-2
14
Clemente M Wiens A Miguel D Bettega K Clemente E Gribner C et al . Efficacy of ilex paraguariensis versus placebo on lipid profile in randomized clinical trial: A systematic review and meta-analysis. Pharmacogn Rev. (2020) 14:91–6. doi: 10.5530/phrev.2020.14.13
15
Kim S Tang T Abbott MJ Viscarra JA Wang Y Sul HS . AMPK phosphorylates desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol Cell Biol. (2016) 36:1961–76. doi: 10.1128/MCB.00244-16
16
Gaidhu MP Anthony N Patel P Hawke TJ Ceddia RB . Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. (2010) 298:C961–71. doi: 10.1152/ajpcell.00547.2009
17
Klein GA Stefanuto A Boaventura BC de Morais EC Cavalcante LD de Andrade F et al . Mate tea (Ilex paraguariensis) improves glycemic and lipid profiles of type 2 diabetes and pre-diabetes individuals: A pilot study. J Am Coll Nutr. (2011) 30:320–32. doi: 10.1080/07315724.2011.10719975
18
Sarriá B Martínez-López S García-Cordero J González-Rámila S Mateos R Bravo L . Yerba mate may prevent diabetes according to a crossover, randomized, controlled study in humans. Proc Nutr Soc. (2020) 79:E245. doi: 10.1017/S0029665120001937
19
Taheri R Mokhtari Y Yousefi A Bashash D . The PI3K/Akt signaling axis and type 2 diabetes mellitus (T2DM): From mechanistic insights into possible therapeutic targets. Cell Biol Int. (2024) 48:1049–68. doi: 10.1002/cbin.12189
20
Luís  Domingues FC Amaral LM . The anti-obesity potential of Ilex paraguariensis: results from a meta-analysis. Braz J Pharm Sci. (2019) 55:e17615. doi: 10.1590/s2175-97902019000217615
21
Kim SY Oh MR Kim MG Chae HJ Chae SW . Anti-obesity effects of Yerba Mate (Ilex Paraguariensis): a randomized, double-blind, placebo-controlled clinical trial. BMC Complement Altern Med. (2015) 15:338. doi: 10.1186/s12906-015-0859-1
22
Guyatt GH Oxman AD Vist GE Kunz R Falck-Ytter Y Alonso-Coello P et al . GRADE: an emerging consensus on rating quality of evidence and strength of rcommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
23
Schünemann HJ Vist GE Higgins JP Santesso N Deeks JJ Glasziou P et al . Interpreting results and drawing conclusions. In: Cochrane Handbook for Systematic Reviews of InterventionsNew Jersey: Wiley-Blackwell (2019). p. 403–31. doi: 10.1002/9781119536604.ch15
24
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
25
Higgins JPT Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26
Alkhatib A . Yerba mate (Ilex paraguariensis) ingestion augments fat oxidation and energy expenditure during exercise at various submaximal intensities. Nutr Metab (Lond). (2014) 11:42. doi: 10.1186/1743-7075-11-42
27
Alkhatib A Seijo M Larumbe E Naclerio F . Acute effectiveness of a “fat-loss” product on substrate utilization, perception of hunger, mood state and rate of perceived exertion at rest and during exercise. J Int Soc Sports Nutr. (2015) 12:44. doi: 10.1186/s12970-015-0105-8
28
Alkhatib A Atcheson R . Yerba mate (Ilex paraguariensis) metabolic, satiety, and mood state effects at rest and during prolonged exercise. Nutrients. (2017) 9:882. doi: 10.3390/nu9080882
29
Andersen T Fogh J . Weight loss and delayed gastric emptying following a South American herbal preparation in overweight patients. J Hum Nutr Diet. (2001) 14:243–50. doi: 10.1046/j.1365-277x.2001.00290.x
30
Balsan G Pellanda LC Sausen G Galarraga T Zaffari D Pontin B et al . Effect of Yerba Maté and green tea on paraoxonase and leptin levels in patients affected by overweight or obesity and dyslipidemia: a randomized clinical trial. Nutr J. (2019) 18:5. doi: 10.1186/s12937-018-0426-y
31
Filipini RE José MF Speretta GFF . The acute ingestion of Yerba Maté (Ilex paraguariensis) infusion does not modify endothelial function, hemodynamics, or heart rate variability: A randomized crossover clinical trial. Plant Foods Hum Nutr. (2025) 80:17. doi: 10.1007/s11130-024-01254-y
32
Harrold JA Hughes GM O’Shiel K Quinn E Boyland EJ Williams NJ et al . Acute effects of a herb extract formulation and inulin fibre on appetite, energy intake and food choice. Appetite. (2013) 62:84–90. doi: 10.1016/j.appet.2012.11.018
33
Martinet A Hostettmann K Schutz Y . Thermogenic effects of commercially available plant preparations aimed at treating human obesity. Phytomedicine. (1999) 6:231–38. doi: 10.1016/S0944-7113(99)80014-2
34
Maufrais C Sarafian D Dulloo A Montani JP . Cardiovascular and metabolic responses to the ingestion of caffeinated herbal tea: drink it hot or cold? Front Physiol. (2018) 9:315. doi: 10.3389/fphys.2018.00315
35
Min B McBride BF Kardas MJ Ismali A Sinha V Kluger J et al . Electrocardiographic effects of an Ephedra-free, multicomponent weight-loss supplement in healthy volunteers. Pharmacotherapy. (2005) 25:654–36. doi: 10.1592/phco.25.5.654.63581
36
Pagliosa CM Vieira FGK Dias BV Brognoli Franco VK Ramos HP da Silva EL . Ilex paraguariensis (A. St.-Hil.) leaf infusion decreases iron absorption in patients with hereditary hemochromatosis: a randomized controlled crossover study. Food Funct. (2021) 12:7321–28. doi: 10.1039/d1fo00482d
37
Panza VP Diefenthaeler F Tamborindeguy AC Camargo CdQ de Moura BM Brunetta HS et al . Effects of mate tea consumption on muscle strength and oxidative stress markers after eccentric exercise. Br J Nutr. (2016) 115:1370–78. doi: 10.1017/S000711451600043X
38
Panza VP Brunetta HS de Oliveira MV Nunes EA da Silva EL . Effect of mate tea (Ilex paraguariensis) on the expression of the leukocyte NADPH oxidase subunit p47phox and on circulating inflammatory cytokines in healthy men: a pilot study. Int J Food Sci Nutr. (2018) 70:212–21. doi: 10.1080/09637486.2018.1486393
39
Schubert MM Astorino TA Azevedo JL Jr . The effects of caffeinated “energy shots” on time trial performance. Nutrients. (2013) 5:2062–75. doi: 10.3390/nu5062062
40
Yu S Yue SW Liu Z Zhang T Xiang N Fu H . Yerba mate (Ilex paraguariensis) improves microcirculation of volunteers with high blood viscosity: a randomized, double-blind, placebo-controlled trial. Exp Gerontol. (2015) 62:14–22. doi: 10.1016/j.exger.2014.12.016
41
Areta JL Austarheim I Wangensteen H Capelli C . Metabolic and performance effects of Yerba Maté on well-trained cyclists. Med Sci Sports Exerc. (2018) 50:817–26. doi: 10.1249/MSS.0000000000001482
42
Avena Alvarez MV Messina DN Corte C Mussi Stoizik JA Saez A Boarelli P et al . Association between consumption of Yerba Maté and lipid profile in overweight women. Nutr Hosp. (2019) 36:1300–06. doi: 10.20960/nh.02599
43
Boaventura BC Di Pietro PF Stefanuto A Klein GA de Morais EC de Andrade F et al . Association of mate tea (Ilex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutr. (2012) 28:657–64. doi: 10.1016/j.nut.2011.10.017
44
Derosa G D’Angelo A Maffioli P . Ilex paraguariensis, white mulberry and chromium picolinate in patients with pre-diabetes. Phytother Res. (2019) 34:1377–84. doi: 10.1002/ptr.6611
45
Derosa G D’Angelo A Maffioli P . Metabolic actions of a supplement of Ilex Paraguariensis (an extract of the leaf standardized to 2% I-deoxinojirimcina), white mulberry and chromium picolinate in nondiabetic subjects with dysglycemia: a randomized trial. Life. (2021) 11:18. doi: 10.3390/life11070709
46
Gebara KS Junior AG Palozi RAC Morand C Bonetti CI Gozzi PT et al . A randomized crossover intervention study on the effect of a standardized mate extract (Ilex paraguariensis A. St.-Hil.) in men predisposed to cardiovascular risk. Nutrients. (2021) 13:1–14. doi: 10.3390/nu13010014
47
Jung J Hur Y . The effect of maté extract on body weight and fat reduction in obese women: a randomized placebo-controlled clinical trial. Korean J Obes. (2016) 25:197–206. doi: 10.7570/kjo.2016.25.4.197
48
Hwa JK Jeongah K Charlotte S Hong JS Young GC . Effect of green mate in overweight volunteers: a randomized placebo-controlled human study. J Funct Foods. (2012) 4:287–93. doi: 10.1016/j.jff.2011.12.005
49
Opala T Rzymski P Pischel I Wilczak M Wozniak J . Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects: a randomized double-blind placebo-controlled clinical trial. Eur J Med Res. (2006) 11:343–50.
50
Petrilli AA Souza SJ Teixeira AM Pontilho PM Souza JM Luzia LA et al . Effect of chocolate and Yerba Maté phenolic compounds on inflammatory and oxidative biomarkers in HIV/AIDS individuals. Nutrients. (2016) 8. doi: 10.3390/nu8050132
51
Souza SJ Petrilli AA Teixeira AM Pontilho PM Carioca AA Luzia LA et al . Effect of chocolate and mate tea on the lipid profile of individuals with HIV/AIDS on antiretroviral therapy: a clinical trial. Nutr. (2017) 43-44:61–8. doi: 10.1016/j.nut.2017.06.017
52
Moridpour AH Kavyani Z Khosravi S Farmani E Daneshvar M Musazadeh V et al . The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes mellitus: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Phytother research: PTR. (2024) 38:117–30. doi: 10.1002/ptr.8026
53
Gong J Fang K Dong H Wang D Hu M Lu F . Effect of fenugreek on hyperglycaemia and hyperlipidemia in diabetes and prediabetes: A meta-analysis. J Ethnopharmacol. (2016) 194:260–8. doi: 10.1016/j.jep.2016.08.003
54
Allen RW Schwartzman E Baker WL Coleman CI Phung OJ . Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. (2013) 11:452–9. doi: 10.1370/afm.1517
55
José MFB MaChado RP Araujo PAB Speretta GFF . Physiological effects of yerba maté (Ilex paraguariensis): a systematic review. Nutr Rev. (2023) 81:1163–79. doi: 10.1093/nutrit/nuac109
56
Arçari DP Bartchewsky W dos Santos TW Oliveira KA Funck A Pedrazzoli J et al . Anti-inflammatory effects of yerba maté extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Mol Cell Endocrinol. (2011) 335:110–5. doi: 10.1016/j.mce.2011.01.003
57
Arçari DP Santos JC Gambero A Ferraz LFC Ribeiro ML . Modulatory effects of yerba maté (Ilex paraguariensis) on the PI3K-AKT signaling pathway. Mol Nutr Food Res. (2013) 57:1882–5. doi: 10.1002/mnfr.201200834
58
Oliveira DM Freitas HS Souza MFF Arçari DP Ribeiro ML Carvalho PO et al . Yerba Maté (Ilex paraguariensis) Aqueous Extract Decreases Intestinal SGLT1 Gene Expression but Does Not Affect Other Biochemical Parameters in Alloxan-Diabetic Wistar Rats. J Agric Food Chem. (2008) 56:10527–32. doi: 10.1021/jf8021404
59
Lunceford N Gugliucci A . Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia. (2005) 76:419–27. doi: 10.1016/j.fitote.2005.03.021
60
Choi MS Park HJ Kim SR Kim DY Jung UJ . Long-term dietary supplementation with Yerba Maté ameliorates diet-induced obesity and metabolic disorders in mice by regulating energy expenditure and lipid metabolism. J Med Food. (2017) 20:1168–75. doi: 10.1089/jmf.2017.3995
61
Arçari DP Bartchewsky W dos Santos TW Oliveira KA Funck A Pedrazzoli J et al . Antiobesity effects of yerba maté extract (Ilex paraguariensis) in high-fat diet-induced obese mice. Obesity. (2009) 17:2127–33. doi: 10.1038/oby.2009.158
62
Gambero A Ribeiro ML . The positive effects of yerba maté (Ilex paraguariensis) in obesity. Nutrients. (2015) 7:730–50. doi: 10.3390/nu7020730
63
Hussein GM Matsuda H Nakamura S Akiyama T Tamura K Yoshikawa M . Protective and ameliorative effects of maté (Ilex paraguariensis) on metabolic syndrome in TSOD mice. Phytomedicine. (2011) 19:88–97. doi: 10.1016/j.phymed.2011.06.036
64
Loria D Barrios E Zanetti R . Cancer and Yerba Maté consumption: a review of possible associations. Rev Panam Salud Publ. (2009) 25:530–9. doi: 10.1590/s1020-49892009000600010
65
Mello FW Scotti FM Melo G Warnakulasuriya S Guerra ENS Rivero ERC . Maté consumption association with upper aerodigestive tract cancers: A systematic review and meta-analysis. Oral Oncol. (2018) 82:37–47. doi: 10.1016/j.oraloncology.2018.04.023
66
Dasanayake AP Silverman AJ Warnakulasuriya S . Maté drinking and oral and oro-pharyngeal cancer: a systematic review and meta-analysis. Oral Oncol. (2010) 46:82–6. doi: 10.1016/j.oraloncology.2009.07.006
67
Islami F Boffetta P Ren JS Pedoeim L Khatib D Kamangar F . High-temperature beverages and foods and esophageal cancer risk–a systematic review. Int J Cancer. (2009) 125:491–524. doi: 10.1002/ijc.24445
68
Castellsagué X Muñoz N De Stefani E Victora CG Castelletto R Rolón PA . Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. (2000) 88:658–64. doi: 10.1002/1097-0215(20001115)88:4<658:aid-ijc22>3.0.co;2-t
69
Oranuba E Deng H Peng J Dawsey SM Kamangar F . Polycyclic aromatic hydrocarbons as a potential source of carcinogenicity of mate. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. (2019) 37:26–41. doi: 10.1080/10590501.2019.1555323
70
Lopes AB Metzdorf M Metzdorf L Sousa MPR Kavalco C Etemadi A et al . Urinary concentrations of polycyclic aromatic hydrocarbon metabolites in maté Drinkers in rio grande do sul, Brazil. Cancer Epidemiol Biomarkers Prev. (2018) 27:331–7. doi: 10.1158/1055-9965.EPI-17-0773
71
Wróblewska K Wróblewski H Zajączkowska M Wójtowicz B Zimna AI Zygmunt-Siembida E et al . The correlation between Yerba Mate and cancer - a review. Qual Sport. (2023) 12:62–9. doi: 10.12775/QS.2023.12.01.007
72
Heck CI de Mejia EG . Yerba Mate Tea (Ilex paraguariensis): a comprehensive review on chemistry, health implications, and technological considerations. J Food Sci. (2007) 72:R138–51. doi: 10.1111/j.1750-3841.2007.00535.x
73
Rolón PA Castellsagué X Benz M Muñoz N . Hot and cold mate drinking and esophageal cancer in Paraguay. Cancer Epidemiol Biomarkers Prev. (1995) 4:595–605.
Summary
Keywords
Yerba Maté (Ilex paraguariensis), glycemic control, systematic review, meta-analysis, randomized controlled trials (RCT)
Citation
Li D, Yue L, Peng X, Chen L, Lin T, Huang L, Liu Y, Yue J and Huang X (2025) Yerba Maté and its impact on glycemic control and metabolic health: a systematic review and meta-analysis. Front. Endocrinol. 16:1641592. doi: 10.3389/fendo.2025.1641592
Received
05 June 2025
Accepted
16 October 2025
Published
30 October 2025
Volume
16 - 2025
Edited by
Sylvère Störmann, LMU Munich University Hospital, Germany
Reviewed by
Rachael Frost, University College London, United Kingdom
Ahmed A.M. Abdel-Hamid, Mansoura University, Egypt
Updates
Copyright
© 2025 Li, Yue, Peng, Chen, Lin, Huang, Liu, Yue and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Huang, huangxiaoli@scu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.