Abstract
Sodium glucose cotransporter-2 inhibitors (SGLT2i) have been found to have a range of benefits, including improving obesity and insulin resistance, hyperuricemia, hypertension, hyperlipidemia, and other metabolic disorders. Initially used for the hypoglycemic effects, they are now found to benefit atherosclerotic cardiovascular disease and chronic kidney disease. Additionally, SGLT2i has been found to have multiple functions, such as improving liver metabolism, affecting brain function, protecting islet β cell function, anti-tumor, and affecting immune system function. This review provides an overview of the protective effects of SGLT2i on different organs and tissues, as well as the potential mechanisms underlying the functional improvement induced by SGLT2i in recent years.
1 Introduction
SGLT2i are commonly used as hypoglycemic drugs in clinical settings. They work by promoting the excretion of glucose in the urine, thereby reducing glucose level in the blood. Recent studies have demonstrated that SGLT2i possesses multiple functions except hypoglycemic effects, such as lowering uric acid, blood pressure, blood lipids, and weight. Furthermore, SGLT2i can delay the progression of chronic kidney disease, reduce the risk of cardiovascular events, and lower all-cause mortality (1). These effects have also been observed in non-diabetic patients (2).
Although the targets of SGLT2i are limited compared to other drugs, its effects are systemic and can impact multiple organs. While SGLT2 is predominantly found in the kidney’s proximal tubule, studies have also found expression in the intestinal mucosa and brain, with rare expression in other tissues or organs (3). This analysis seeks to consolidate the function of SGLT2i across various organs and elucidate their underlying mechanisms, establishing a groundwork for future fundamental and applied studies on SGLT2i. To craft this review, we embarked on an extensive literature trawl through PubMed, Embase, and Web of Science, culling through articles up to May of 2025. Our search was spearheaded by a blend of key terms, such as “SGLT2 inhibitors,” “sodium-glucose cotransporter-2,” and terms like “cardiorenal protection,” “NAFLD,” “neuroprotection,” “pancreatic β-cells,” “cancer,” and “immune response.” We used a mix of controlled vocabularies (like MeSH/Emtree) and free-text keywords, cleverly weaving in Boolean operators to tie them together. This strategy was like casting a wide net, ensuring that we had a comprehensive and scientifically robust grasp of the relevant studies.
2 Pharmacological features and clinical overview of SGLT2i
SGLT2i form a category of oral, small-molecule hypoglycemic drugs that target SGLT2 in the kidney’s tubules, which in turn cuts down on glucose reabsorption and encourages its elimination through urine. Furthermore, these compounds spark a little natriuresis and osmotic diuresis, leading to a synergistic impact that’s akin to shifting metabolic fuel and fine-tuning blood flow—a combo that has a host of health benefits, such as lowering blood sugar, trimming the waistline, reducing blood pressure, and lowering uric acid levels (4, 5).
What they all have in common pharmacologically is that they’re super selective for SGLT2 rather than its lessor relative, SGLT2i offer convenient once-daily oral administration. They are absorbed reliably and rarely cause hypoglycemia when used alone. These drugs are primarily metabolized through glucuronidation and have minimal interaction with cytochrome P450 enzymes, reducing the risk of drug interactions (6). Furthermore, their glucose-lowering effectiveness is closely tied to kidney function; as kidney function decreases, their blood sugar-lowering effect diminishes, though their heart and kidney protection benefits are partly maintained (7).
These inhibitors aren’t just about dropping sugar levels; they’re like a Swiss Army knife for health, thanks to their multi-faceted action. They quell inflammation and oxidative stress, boost fat-burning and ketone production, and kickstart the body’s self-cleaning process, autophagy (2). This versatility is what gives them their broad-spectrum protection across different organs.
To further exemplify these pharmacological attributes, the key features of representative agents are summarized in Table 1.
Table 1
| Drug | SGLT2:SGLT1 selectivity | Half-life (t1/2) | Major metabolism/elimination | Approved indications | Key notes | References |
|---|---|---|---|---|---|---|
| Empagliflozin | ~2500:1 | ~12 h | UGT2B7/1A3/2B17; renal/fecal | T2DM, HF, CKD | Robust CV/renal benefit (EMPA-REG OUTCOME) | (111–113) |
| Dapagliflozin | ~1200:1 | ~12–13 h | UGT1A9 → 3-O-glucuronide | T2DM, HF, CKD | CV benefit (DAPA-HF); ongoing NAFLD studies | (111, 114, 115) |
| Canagliflozin | ~160–200:1 | ~10–13 h | UGT1A9/UGT2B4; minor CYP | T2DM | CV/renal benefit (CANVAS); partial SGLT1 inhibition | (111, 116, 117) |
| Phlorizin | Non-selective (SGLT1/2) | Short | Hydrolyzed to phloretin | None | Tool compound, not clinically used | (118) |
Key SGLT2 inhibitors and their pharmacokinetic profiles.
3 Multidimensional roles of SGLT2 inhibitorssglt2
3.1 Liver
Nonalcoholic fatty liver disease (NAFLD) is a common condition among people who are obese, especially those dealing with type 2 diabetes (T2DM). If left unchecked, NAFLD can progress to a more severe form known as nonalcoholic steatohepatitis (NASH), which may eventually lead to serious liver complications like fibrosis and cirrhosis. Numerous clinical studies have reported that SGLT2i can aid in improving NAFLD progression (8–10). According to an open-label randomized controlled trial, 24 weeks of dapagliflozin treatment decreased hepatic steatosis by 8%. a15% decrease in liver fibrosis score and a 25% decline in liver enzyme levels (9). The E-LIFT study discovered that empagliflozin could decrease liver fat content and enhance alanine aminotransferase (ALT) levels in patients with T2DM and NAFLD (10). These suggest that SGLT2i may be effective in improving liver health. Some experts believe that SGLT2i can delay the onset of NAFLD by promoting weight loss and decreasing glycemia (11). However, the EMPA-REG OUTCOME trial demonstrated empagliflozin’s efficacy in lowering ALT, regardless of alterations in HbA1c or body weight (12). Additionally, the analysis of the E-LIFT study found that the loss of liver fat was not related to HbA1c improvement or weight loss (10). Therefore, SGLT2i may have other mechanisms to influence the occurrence and progression of NAFLD.
3.1.1 Hepatic fat metabolism altered and deposition reduced
SGLT2i can cause a partial loss of glucose from the body, affecting energy metabolism patterns in various tissues, including the liver. SGLT2i can reduce the insulin/glucagon ratio (2) and increase insulin sensitivity, altering lipid metabolism (13, 14). Hüttl M et al. conducted a study using a In a non-overweight, pre-diabetic rat study, we examined how the drug empagliflozin affects liver metabolism. The findings indicated that empagliflozin therapy decreased both neutral triglycerides and lipotoxic diglycerides within the liver while increasing the transcription of fatty acid synthase (Fas) (15). Fas is the key enzyme of fatty acid synthesis in the liver and the determinant of the maximum ability of the liver to synthesize fatty acids. According to Teruo Jojima et al., canagliflozin significantly reduces the expression of the Fas gene, which is involved in lipogenesis, and the improvement of canagliflozin on NASH may be related to the reduction of fatty acid production (16). Additionally, research indicates that Daxigliflozin enhances the production of acyl-CoA oxidase-1 (ACOX1), the key regulatory enzyme involved in lipid catabolism.This up-regulation leads to an increase in fatty acid β-oxidation, which reduces fat deposition (17). In TallyHo mice on a high-milk-fat diet, EMPA normalized key liver metabolites, including orotate (pyrimidine synthesis) and dihydrofolate (folate/methionine pathways), and normalized dysregulated acylcarnitines in females. These metabolic benefits were absent under low-fat diet conditions, suggesting that SGLT2i-induced protection is particularly effective during states of metabolic stress. EMPA also reversed the elevation of circulating amino acids induced by lipotoxic diet and improved ketone body metabolism, underscoring its systemic regulatory potential (18).
3.1.2 Inflammation and oxidative stress decreased
In the onset and worsening of NAFLD, chronic inflammation and oxidative damage are key drivers. When fat tissue becomes inflamed and the body resists insulin, it sets off a chain reaction that floods the liver with excessive fatty acids and sugar, leading to endoplasmic reticulum stress, which activates the inflammasome and leads to hepatocyte death. As hepatocellular injury signals continue to activate inflammatory cells, liver fibrosis can occur. Recent studies indicate that administering empagliflozin to obese diabetic rats can lead to decreased interleukin 6 (IL-6) expression while inducing a decrease in the expression of adipokine chemokines and chemokine receptors (19). Empagliflozin combined with dulaglutide can reduce the pro-inflammatory activation of the immune system in the liver, manifesting as a decrease in regulatory T cells (Treg), pro-inflammatory macrophages, and Kupffer cells (20). Inflammation can damage cell structure, resulting in electron leaks within the mitochondria that can generate excessive superoxide. In animal models, Ipragliflozin treatment enhances liver levels of superoxide dismutase and catalase expression.These enzymes can degrade most reactive oxygen species and reduce oxidative stress levels in the liver (21). Research has shown that empagliflozin stimulates CAMKK2, a calcium/calmodulin-dependent kinase, which in turn up-regulates the expression of anti-superoxide dismutase, thereby reducing oxidative stress and lipotoxicity (22).A recent study using db/db mice and diet-induced NAFLD models revealed that dapagliflozin and canagliflozin not only reduced hepatic steatosis and fibrosis but also modulated the hepatic immune microenvironment. SGLT2i therapy diminished liver tissue levels of M1 macrophage inflammation indicators and enhanced levels of M2 macrophage anti-inflammatory markers. In vitro, SGLT2i promoted M1-to-M2 macrophage polarization through metabolic reprogramming, primarily by inhibiting PFKFB3, a key glycolytic enzyme. Co-culture experiments further confirmed that macrophage-mediated crosstalk suppressed hepatocyte lipogenesis (23).
3.1.3 Autophagy activated and cellular senescence inhibited
Compared to patients with isolated steatosis or normal livers, patients with NASH exhibit a decrease in autophagy in liver cells. Recent studies have demonstrated that impaired mitophagy may contribute to liver injury during NAFLD and result in the formation of giant mitochondria (24). Inhibition of autophagy leads to elevated levels of TG and LDL cholesterol, causing abnormal lipid deposition in hepatocytes and further progression of NAFLD (25). Research indicates a rise in the mRNA and protein levels linked to autophagy in mice following 5 weeks of empagliflozin therapy, apoptosis resistance markers were markedly elevated in the empagliflozin group versus controls (26). Research revealed that empagliflozin boosted autophagy in liver macrophages via the AMPK-mTOR signaling pathway in diabetic mice suffering from NAFLD. This mechanism effectively curbed inflammation driven by the IL-17/IL-23 axis, ultimately alleviating hepatic injury (27).
Recent years have seen a marked increase in focus on the connection between hepatocyte senescence and NAFLD/NASH. Studies have found that liver cells in NAFLD patients exhibit signs of aging, such as shortened telomeres, increased expression of aging markers, and altered DNA methylation patterns. Additionally, aging liver cells secrete inflammatory factors and chemokines that accelerate the aging process in neighboring cells (28). One study showed that topagliflozin could reduce the expression of cyclin-dependent kinase inhibitor p21 in a mouse model, inhibiting liver cell senescence in diabetes and obesity and delaying the advancement of NASH (29) However, research on SGLT2i’s role in slowing NAFLD progression through cellular senescence inhibition remains scarce, requiring further validation.
3.2 Brain
The SGLT family, the most common glucose transport receptors other than glucose transporters (GLUTs), is widely distributed in the brain. All isoforms excluding SGLT5 are present in the brain (30). A study examining the protein composition of tiny blood vessels extracted from rat brain tissue showed that SGLT2 proteins were present in both nerve cell bodies and their branching extensions across various areas of the brain. What’s more, researchers using RT-PCR techniques also identified SGLT2 expression in the human cerebellum (31). D SGLT2 receptors in the CNS have been noted, but the impact of SGLT2i on the brain remains underrecognized.
3.2.1 Neuroprotective effects of SGLT2i
The study demonstrates that a history of hyperglycemia or diabetes worsens cerebral ischemia in patients (32). Yamazaki et al. administered phlorizin via intraperitoneal injection in a murine focal cerebral ischemia model. They observed that this treatment improved cerebral infarction and reduced neuron damage. Furthermore, their findings proved that phlorizin directly inhibits SGLT receptors in the brain, thereby offering protection against cerebral ischemia, independent of any improvements in peripheral blood glucose levels (33). Empagliflozin has been shown to significantly impact on reducing cerebral tissue damage after cerebral ischemia/reperfusion (I/R) injury, attributing to its ability to inhibit oxidative stress, inflammation, and down-regulate apoptosis markers (34). Recent studies indicate that empagliflozin’s neuroprotective effects likely stem from its dual action of suppressing neuronal caspase-3 protein levels while boosting both hypoxia-induced factor-1α (HIF-1α) and its downstream target, vascular endothelial growth factor (VEGF). This two-pronged mechanism appears to play a key role in shielding neural tissue from damage (35).
3.2.2 SGLT2i influences neurophysiology
Sodium ions are transported across the cell membrane alongside glucose through the SGLT receptor, resulting in depolarization of the cell membrane potential and increased excitability (36). SGLT2i can prevent this effect. Additionally, SGLT2i alters energy metabolism, shifting substrate utilization from carbohydrates to fatty acid oxidation and increasing ketone body production (37, 38). Ketone bodies play a role in inhibiting abnormal neuronal firing by interacting with cellular excitatory-inhibitory processes (39). The use of a ketogenic diet for epilepsy can be traced back to 500 BC (40). The clinical observation revealed that the ketogenic diet led to a 50% reduction in the number of relapses in patients with refractory epilepsy within 3 months (41). Dapagliflozin significantly reduces seizure activity in the epileptic rat model, which may be related to the reduction of neuronal glucose metabolism and neuronal cell membrane excitability (36). However, no study currently that compares the effectiveness of the ketogenic diet and SGLT2i in reducing epileptic seizures. The impact of SGLT2i on neuronal electrophysiology and its associated mechanisms remain unclear.
3.2.3 SGLT2i and cognitive function in diabetes
SGLT2i has been shown to have several benefits, including impaired cognitive dysfunction, reduced oxidative stress and inflammation, and enhanced neuronal plasticity (42, 43). Clinical studies have demonstrated that SGLT2i has the potential to improve the Montreal Cognitive Assessment Scale (MoCA) score and repetitive neuropsychological status test scores (44, 45). A mechanism study showed that empagliflozin significantly increased the concentration of brain-derived neurotrophic factor and prevented cognitive impairment in obese diabetic mice (43). Additionally, SGLT2i restores mTOR to an activated physiological state, which helps prevent the onset or progression of neurodegenerative diseases (46). Furthermore, apart from its direct impact on the central nervous system, SGLT2i also exhibits inhibitory effects on acetylcholinesterase, thereby protecting cognitive function (47). Nevertheless, a limited number of clinical trials explore the application of SGLT2i for managing diabetes-related cognitive deficits. Additional studies are needed to determine whether SGLT2i can meaningfully slow cognitive deterioration in diabetic patients. A landmark study by Kim et al., drawing from nationwide population data, offers compelling insights into this question. The research examined more than 1.34 million Korean adults aged 40+ with T2DM, ultimately analyzing a matched cohort of 359,000 patients to assess how SGLT2i use impacts the likelihood of developing Alzheimer’s, vascular dementia, and Parkinson’s. The findings revealed that SGLT2i outperformed other oral diabetes medications, showing substantially lower risks for these neurodegenerative conditions: a 19% decrease for Alzheimer’s (aHR 0.81, 95% CI 0.76–0.87), 31% for vascular dementia (aHR 0.69, 95% CI 0.60–0.78), and 20% for Parkinson’s (aHR 0.80, 95% CI 0.69–0.91). When looking at the combined outcome of all-cause dementia and Parkinson’s, the reduction reached 22% (aHR 0.78, 95% CI 0.73–0.83). Crucially, these protective effects held strong even after accounting for numerous variables—from gender and overall health status to diabetes-related complications, coexisting conditions, lab results, and other medications. Subgroup analyses further revealed that the neuroprotective effects of SGLT2i were consistent across different age groups, comorbidity burdens, and metabolic conditions, suggesting that SGLT2i may exert its beneficial impact on the central nervous system through multiple synergistic mechanisms, thereby contributing to the prevention of neurodegenerative diseases (48).
3.2.4 Improves brain insulin resistance
Insulin resistance serves as the key underlying mechanism in T2DM, with cerebral insulin sensitivity acting as a major player in maintaining metabolic balance throughout the body. When the brain’s ability to respond to insulin becomes compromised, it throws off the central control of metabolic processes, ultimately influencing emotional state, mental sharpness, and behavioral patterns (49). However, owing to the existence of the blood-brain barrier, progress in researching therapeutic methods to improve brain insulin resistance has been slow. Previous studies have focused on nasal insulin delivery primarily (50). Interestingly, SGLT2i is the first drug discovered to have the potential to reverse brain insulin resistance. A randomized controlled study, it was found that empagliflozin 25mg/d treatment for 8 weeks significantly improved hypothalamic insulin resistance, thereby reducing fasting blood sugar levels and liver fat content (51).
3.3 Protection of islet β cells function
The impairment and decline of pancreatic beta cell function are central to the development of diabetes. Consequently, safeguarding these insulin-producing cells is critical for effective diabetes management. Extensive research has demonstrated that many glucose-lowering medications exhibit protective effects capable of supporting beta cell health (52–54).
3.3.1 improve insulin sensitivity
Research indicates an enhancement in insulin responsiveness with the use of SGLT2i. Phase 3 clinical trial results of canagliflozin demonstrate its ability to significantly increase the insulin secretion index and improve insulin resistance (55). Similar improvements in insulin sensitivity have been observed with empagliflozin, dapagliflozin, and other SGLT2i (56, 57). A key point to remember is that SGLT2i don’t trigger insulin release directly. Instead, they work by promoting glucose elimination through urine, which helps alleviate the harmful impact of elevated blood sugar and lessens the strain on pancreaticβcells (58). Additionally, SGLT2i can enhance the glucose utilization rate of peripheral tissues by improving hypothalamic insulin resistance (51). Jahn et al. found that 12 weeks of empagliflozin improved insulin’s vascular effects, as evidenced by improved endothelial function, reduced arterial blood pressure, and increased microvascular perfusion in skeletal and cardiac muscle. These findings support the systemic extension of SGLT2i’s effects on insulin sensitivity from a hemodynamic perspective. This suggests that SGLT2i not only alleviate β-cell stress by lowering blood glucose levels, but also enhance overall insulin efficacy through improvements in vascular function, tissue perfusion, and hormonal signaling pathways (59).
3.3.2 Increase the number of islet β cells
Beyond preserving the functionality of pancreaticβcells, SGLT2i have demonstrated the ability to boostβcell mass in preclinical studies. Research by Wei R and colleagues revealed that diabetic mice treated with dapagliflozin for six weeks exhibited significant expansion of pancreatic islets andβcell volume. Subsequent investigations suggested this growth likely stems from multiple mechanisms: stimulatingβcell replication, convertingαcells intoβcells, and facilitating the differentiation of ductal cells into functionalβcells (60). Tanday N et al. conducted a study using lineage tracing technology and found that dapagliflozin intervention effectively reduces the proportion of dedifferentiated islet β cells in mice, leading to a decrease in cell loss (61). SGLT2i has also been observed to protect the remaining islet β cells by inhibiting the infiltration of inflammatory cells in the islet tissue and preventing apoptosis (62, 63).
3.3.3 Promotes glucagon-like peptide-1 secretion
GLP-1, a hormone predominantly synthesized in the gut, serves a dual role in pancreatic function. Beyond triggering insulin release from beta cells, it actively supports their growth while simultaneously mitigating cellular damage caused by oxidative stress (64). Animal studies indicate that dapagliflozin enhances GLP-1 secretion by upregulating essential enzymes for its production (60). Clinical studies have also demonstrated that SGLT2i can elevate plasma GLP-1 levels (65, 66). Since SGLT2 is hardly expressed in islet α cells, whether SGLT2i affects GLP-1 content through islet α cells is still controversial. Recent evidence suggests that SGLT2i with low SGLT2/SGLT1 selectivity increases circulating GLP-1 levels, possibly through inhibition of intestinal SGLT1 production (67). Another study discovered that islet α cells could express SGLT1 receptors, and the administration of dapagliflozin can enhance its expression level. This indicates that dapagliflozin may increase the secretion of GLP-1 by significantly up-regulating the expression of SGLT1 through an SGLT1-dependent mechanism (68). By evaluating the response of insulin and C-peptide to GLP-1 after 8 weeks of dapagliflozin intervention, it was found that dapagliflozin can increase the sensitivity of islet β cells to GLP-1 in patients with T2DM (69).
3.4 Anti-tumor effects
In addition to its clinically proven antidiabetic effects, SGLT2i has also demonstrated therapeutic potential against various solid malignancies (70, 71). Both in vivo and in vitro studies demonstrate canagliflozin’s suppression of prostate proliferation (72), pancreatic (73), lung (74), and hepatocellular carcinomas (71). Additionally, dapagliflozin suppresses kidney cancer cell growth (75).
3.4.1 Inhibition directly
Studies have found that SGLT2 is highly expressed in pancreatic cancer (43), prostate cancer (73), breast cancer (76), and highly differentiated lung adenocarcinoma (74). SGLT2i target the SGLT2 receptor in cancer cells, effectively halting glucose absorption, which in turn slashes tumor expansion and longevity. One research paper revealed that these inhibitors stifle the spread of breast cancer cells by bringing the cell cycle to a halt in the G1/G0 phase, thanks to AMPK activation, and by prompting cell death, or apoptosis (76). Similarly, Leona Yamamoto et al. obtained similar results in lung cancer cell lines, where Canagliflozin inhibited the proliferation of lung cancer cells dose-dependently (77). SGLT2i lower glucose absorption in cancer cells, alter the tumor’s cellular surroundings, and impact the energy processes within tumor cells, ultimately leading to a delay in tumor growth. While SGLT2i inhibits the transport of glucose by the SGLT2 receptor, the transport of sodium ions is also affected. Shiho Komatsu et al. found that Ipragliflozin shuts down sodium absorption through the SGLT2 receptor and induces hyperpolarization of cancer cell membranes, and at the same time, causes mitochondrial membrane potential instability sex, leading to host cell apoptosis and necrosis (78). A major retrospective study in Hong Kong involving more than 60,000 participants used propensity score matching to compare outcomes between patients on SGLT2i and those taking dipeptidyl peptidase-4 inhibitors (DPP4i). The results showed that SGLT2i use was linked to a substantially lower risk of developing hepatocellular carcinoma (HCC) in individuals with type 2 diabetes, with a hazard ratio (HR) of just 0.42. Notably, the protective effect was even stronger among high-risk groups—such as patients with cirrhosis, advanced fibrosis, or hepatitis B or C—where the HR dropped to a striking 0.12. These findings highlight the potential of SGLT2i as a promising therapeutic option for preventing liver cancer in diabetic patients, particularly those with pre-existing liver conditions (79).
3.4.2 Other mechanisms
In addition to directly inhibiting the SGLT2 receptor expressed on tumor cells, studies have found that SGLT2i can also indirectly inhibit tumors through other mechanisms. Cancer cells can increase the body’s local glucose transport to the tumor to meet their energy needs while increasing the body’s insulin resistance. The use of hypoglycemic drugs can reduce insulin resistance and lower blood sugar levels, which in turn inhibits tumor proliferation and growth (80). New research has revealed that dapagliflozin effectively curbs tumor progression in obese mice with cancer. The study, led by Ali R. Nasiri and colleagues, found that this anticancer mechanism didn’t stem from heightened ketosis or direct interference with cancer cell proliferation. Rather, the drug’s efficacy came from correcting excessive insulin levels, achieved by reducing glucose absorption and metabolism within tumors (81). A recent investigation led by David Papadopoli and his team revealed that canagliflozin’s ability to prevent cell growth is linked to its impact on glutamine metabolism, a process that remains unaffected by glucose availability or the amount of SGLT2 being expressed (82). Additionally, advanced metabolomic and proteomic analyses demonstrated that canagliflozin suppresses the growth of hepatocellular carcinoma by disrupting key metabolic pathways—specifically, electron transport chain activity, fatty acid breakdown, and DNA/RNA production. The findings suggest this drug targets cancer cell proliferation through multiple interconnected biological mechanisms (83). Jingyi Luo et al. discovered that canagliflozin can reduce the metastasis, angiogenesis, and metabolism of hepatocellular carcinoma under hypoxic conditions. This decrease is realized by preventing the formation of HIF-1α protein in reprogramming contexts, potentially by modulating the AKT/mTOR signaling pathway (84, 85).
It’s important to recognize that existing research indicates only select SGLT2i show promise against particular cancer types. More extensive studies are required to fully understand how different SGLT2i combat tumors and their precise mechanisms across various cancers. Beyond efficacy, we must also examine the safety profile and practical application of integrating these drugs into cancer therapies, while thoroughly assessing their clinical benefits.
3.5 Effects on the immune system
SGLT2 is known to be almost absent in immune cells (3). Thus far, the influence of SGLT2i on immune system components has been insufficiently recognized. However, recent studies have increasingly discovered the involvement of immune cells in the various mechanisms through which SGLT2i protects the heart, kidney, and liver (86), although the exact mechanism is still unclear. The following outlines the potential ways in which SGLT2i may influence immune cells.
3.5.1 Affect immune inflammation
Inflammatory factors are released by immune cells under inflammatory conditions. Many diseases, such as rheumatoid arthritis, atherosclerosis, and diabetes, are associated with chronic inflammation (87, 88). Chen Xu et al. found that canagliflozin has a robust anti-inflammatory effect on human immune cells, leading to reduced production and release of IL-1, IL-6, and tumor necrosis factor-α, which may be related to canagliflozin’s inhibition of intracellular glycolysis and autophagy (89). An intervention study conducted in Iran observed that Empagliflozin not only reduced the production of pro-inflammatory factors by helper T cells but also hindered their proliferative ability (90). A recent study found that canagliflozin can directly inhibit the transcription of NLR family pyrin domain-containing protein 3 (NLRP3) inflammasome-related proteins by inhibiting the NFκB signaling pathway and can also up-regulate autophagy to affect inflammation directly (91). Clinical evidence further supports the anti-inflammatory effects of SGLT2i. A study in patients with severe heart failure showed that those treated with SGLT2i exhibited significantly reduced expression of pro-inflammatory genes and decreased immune cell infiltration in epicardial adipose tissue (EAT). Metabolomic analysis revealed an enrichment of ether lipids containing oleic acid in the EAT of the treated group, suggesting a reduced tendency toward ferroptosis, which may contribute to alleviating oxidative stress. Overall, SGLT2i exert their immunomodulatory and organ-protective effects in chronic inflammatory diseases through multiple mechanisms, including inhibition of the NF-κB signaling pathway, regulation of immune cell function, suppression of pro-inflammatory cytokine release, improvement of adipose tissue metabolism, and attenuation of ferroptosis (92).
3.5.2 Affect immune cell energy metabolism
The function of immune cells is closely related to their energy metabolism. For instance, naive T cells primarily rely on aerobic phosphorylation, while glycolysis becomes the main metabolic pathway for activated T cells (93). Canagliflozin inhibits intracellular glycolysis levels, promotes autophagy, and plays an anti-inflammatory role in immune cells in mice and humans (89). The switch in the energy metabolism of immune cells occurs as a result of antigenic stimulation, which leads to metabolic reprogramming. In patients with diabetes, high glucose toxicity, inflammation, and oxidative stress can activate immune cells and alter immune metabolism (94). Results from a cohort study found abnormally enhanced peripheral blood T cell glucose uptake in patients with type 1 diabetes, which was correlated with C-peptide and HbA1c levels (95). SGLT2i can potentially reduce the abnormal activation of immune cells by improving glucotoxicity and lipotoxicity, thereby altering the energy metabolism pattern of immune cells (96). In immune thrombocytopenia, empagliflozin can modify the energy metabolism of CD4+ T cells in peripheral blood, shifting it from oxidative phosphorylation to glycolysis. The mechanism behind this effect may be associated with the inhibition of the mTOR signaling pathway by empagliflozin (97).
SGLT2i causes partial glucose loss, enhanced fatty acid oxidation, and increased ketone body production (37). In addition to being metabolites, ketone bodies can serve as energy substrates for specific cells. This utilization of ketone bodies for energy supply is a mechanism that protects cells (98). Karagiannis F et al. discovered that severe patients infected with SARS-CoV-2 experienced a decrease in the synthesis of β-hydroxybutyrate (BHB), an increase in the level of glycolysis in T cells, and impairment in the immune function of CD4+ T cells. Mechanism research revealed that BHB, acting as a surrogate dye, enhances the production of energy-donating amino acids (glutamate and aspartate) and glutathione while promoting oxidative phosphorylation to restore cellular function (99). Furthermore, it has been discovered that the ketogenic diet impacts immune function. Ketone bodies can enhance T cell reactivity and cell lysis ability by activating mitochondrial oxidative metabolism-based metabolic reprogramming, additionally regulating cell differentiation and increasing the generation of memory T cells (100). While there is currently no evidence to suggest that SGLT2i can serve as a substitute for the ketogenic diet, it is worth noting that it exhibits exceptional safety and feasibility as a ketogenic diet mimic (101). A prospective pilot study systematically evaluated the changes in mitochondrial function of immune cells before and after 6 months of SGLT2i treatment in kidney transplant recipients with T2DM, aiming to explore its potential immunometabolic regulatory mechanisms. The study revealed that SGLT2i significantly preserved mitochondrial membrane potential in lymphocytes, reduced reactive oxygen species (ROS) levels, and enhanced mitochondrial biogenesis. These improvements were closely associated with reductions in body weight and LDL-C levels. Under PHA-induced immune activation, SGLT2i notably enhanced the metabolic adaptability of lymphocytes. This study, for the first time in human transplant recipients, demonstrated that SGLT2i may participate in immunometabolic reprogramming by improving mitochondrial homeostasis and oxidative stress in immune cells, thereby offering a potential cellular and metabolic explanation for its cardio-renal protective effects. These findings expand our understanding of the “beyond glycemic control” benefits of SGLT2i and provide new insights into its application as an immunometabolic intervention in chronic diseases (102).
4 Key molecular pathways modulated by SGLT2i
4.1 Anti-inflammatory pathways
SGLT2i reliably exhibit strong anti-inflammatory properties in various disease contexts. One of their primary actions involves blocking the NF-κB signaling cascade. By interrupting this pathway, SGLT2i effectively curb the production of inflammatory markers like TNF-α, IL-6, and IL-1β (103). Additionally, these drugs directly interfere with the NLRP3 inflammasome—a key molecular assembly responsible for processing IL-1β—through two distinct mechanisms: decreasing mitochondrial oxidative stress and enhancing the autophagy-driven removal of inflammasome elements (86, 104). The resulting decline in inflammatory cytokines helps alleviate persistent systemic inflammation, which plays a central role in the development of cardiometabolic and renal disorders.
4.2 Metabolic adaptation and bioenergetic regulation
SGLT2i fundamentally alter cellular energy dynamics by mimicking a fasting state. These drugs trigger AMPK, the body’s master metabolic regulator, which in turn suppresses the mTOR pathway—a key promoter of cell growth and synthetic processes (105). This metabolic switch through the AMPK/mTOR pathway not only stimulates cellular cleanup but also dials down inflammation while boosting insulin responsiveness (106).
What’s more, SGLT2i rev up peroxisome proliferator-activated receptor alpha (PPARα), shifting the body’s energy preference toward burning fats and producing ketones (107). This metabolic pivot offers the heart and brain a cleaner, more efficient fuel source—especially when they’re under duress—effectively upgrading the body’s energy economy under stress (2).
4.3 Mitigating oxidative stress
Oxidative stress plays a pivotal role in cellular damage associated with diabetes and aging. SGLT2i significantly bolster the body’s antioxidant defenses by stimulating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (108). Once activated, Nrf2 moves into the nucleus and binds to antioxidant response elements (ARE), triggering the production of key antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT) (109). By ramping up these protective mechanisms, SGLT2i help counteract harmful ROS, minimizing oxidative damage to vital cellular components—lipids, proteins, and DNA—and ultimately safeguarding cellular integrity (110).
4.4 Insulin signaling and survival pathways
Enhancements in insulin sensitivity throughout the body are largely thanks to the improved signaling process involving IRS-1, PI3K, and Akt (59). This trio plays a pivotal role in glucose absorption, protein formation, and the sustenance of cellular life. By easing glucotoxicity and inflammation, medications like SGLT2i can prevent IRS-1 from getting phosphorylated at the serine site—a move that’s a hallmark of insulin resistance (105). This in turn ensures the restoration of effective insulin signaling. Furthermore, the activated Akt route helps quash apoptosis and maintains the delicate balance of metabolism.
4.5 Anti-tumor mechanisms
SGLT2i don’t just impact metabolism; they also pack a punch against cancer through various mechanisms. By cutting off glucose supplies, they stop the stabilization of HIF-1α, a key player in how tumors cope with low oxygen levels. Lower HIF-1α levels then bring down the production of its targets, such as VEGF, effectively shutting down the growth of new blood vessels in tumors and preventing the spread of cancer (71). Moreover, these inhibitors hit the brakes on the Akt/mTOR pathway, which is crucial for cancer cells to multiply, survive, and make proteins (76, 84). Certain SGLT2 inhibitors even go the extra mile by directly blocking important enzymes like PFKFB3, throwing a wrench into the aerobic glycolysis, or Warburg effect, that many cancer cells rely on (23).
Table 2 outlines the principal molecular pathways influenced by SGLT2i.
Table 2
| Pathway | Key components | Biological effect | References |
|---|---|---|---|
| Inflammation | NF-κB, NLRP3, IL-6, TNF-α | Reduces cytokine production, attenuates chronic inflammation | (86, 103, 104) |
| Energy Sensing | AMPK, mTOR | Promotes catabolism, inhibits anabolism, induces autophagy | (105, 106) |
| Oxidative Stress | Nrf2, SOD, CAT | Enhances antioxidant defense, reduces ROS damage | (108, 109) |
| Insulin Signaling | IRS-1, PI3K, Akt | Improves insulin sensitivity, promotes cell survival | (59, 105) |
| Tumorigenesis | HIF-1α, VEGF, Akt/mTOR, PFKFB3 | Inhibits angiogenesis, metastasis, and tumor metabolism | (23, 71, 76, 84) |
Key molecular pathways modulated by SGLT2i.
5 Future perspectives and clinical implications
Even with all the hype around SGLT2i and their potential across various health areas, we’re not out of the woods yet. For starters, a lot of the buzz is based on early lab work or smaller trials. We desperately need some big, robust randomized controlled trials to really prove these drugs work and are safe for all sorts of patients. Also, we’re still fuzzy on exactly how SGLT2i tinker with things like immune function, brain protection, and even how tumors behave. More digging is definitely needed there. And let’s not forget the big question mark hanging over what these drugs do in the long run to organs other than the kidneys and heart, especially in people who don’t have diabetes.
On the bright side, the fact that we’re using SGLT2i for more than just blood sugar control shows they might be true multi-organ protectors. Looking ahead, research should focus on combining them with other treatments, like GLP-1 receptor agonists or drugs that tweak the immune system. Figuring out which biomarkers can help us pick the right patients and seeing if these drugs are actually cost-effective in the real world are also key. Putting all this together will be vital to really unlock the full potential of SGLT2i and give patients the best possible results.
6 Summary
In addition to its hypoglycemic and cardiorenal protective effects, SGLT2i also exhibits multiple mechanisms for organ protection. A simplified graphic summary of the beneficial effects and possible mechanisms of SGLT2i is provided in Figure 1. It can delay the occurrence and development of NAFLD by altering liver fat metabolism, reducing inflammation and oxidative stress, activating autophagy, and inhibiting liver cell aging. SGLT2i has also shown the ability to protect neuron function, influence neurophysiological activity, and improve brain insulin resistance, which is associated with cognitive function. Furthermore, studies have found that SGLT2i can safeguard islet function, slow down tumor growth, and impact immune inflammation and energy metabolism of immune cells. In conclusion, despite the limited distribution of SGLT2 receptors, the roles of SGLT2i are extensive and varied, and its multi-organ protective mechanism holds significant potential for clinical application.
Figure 1
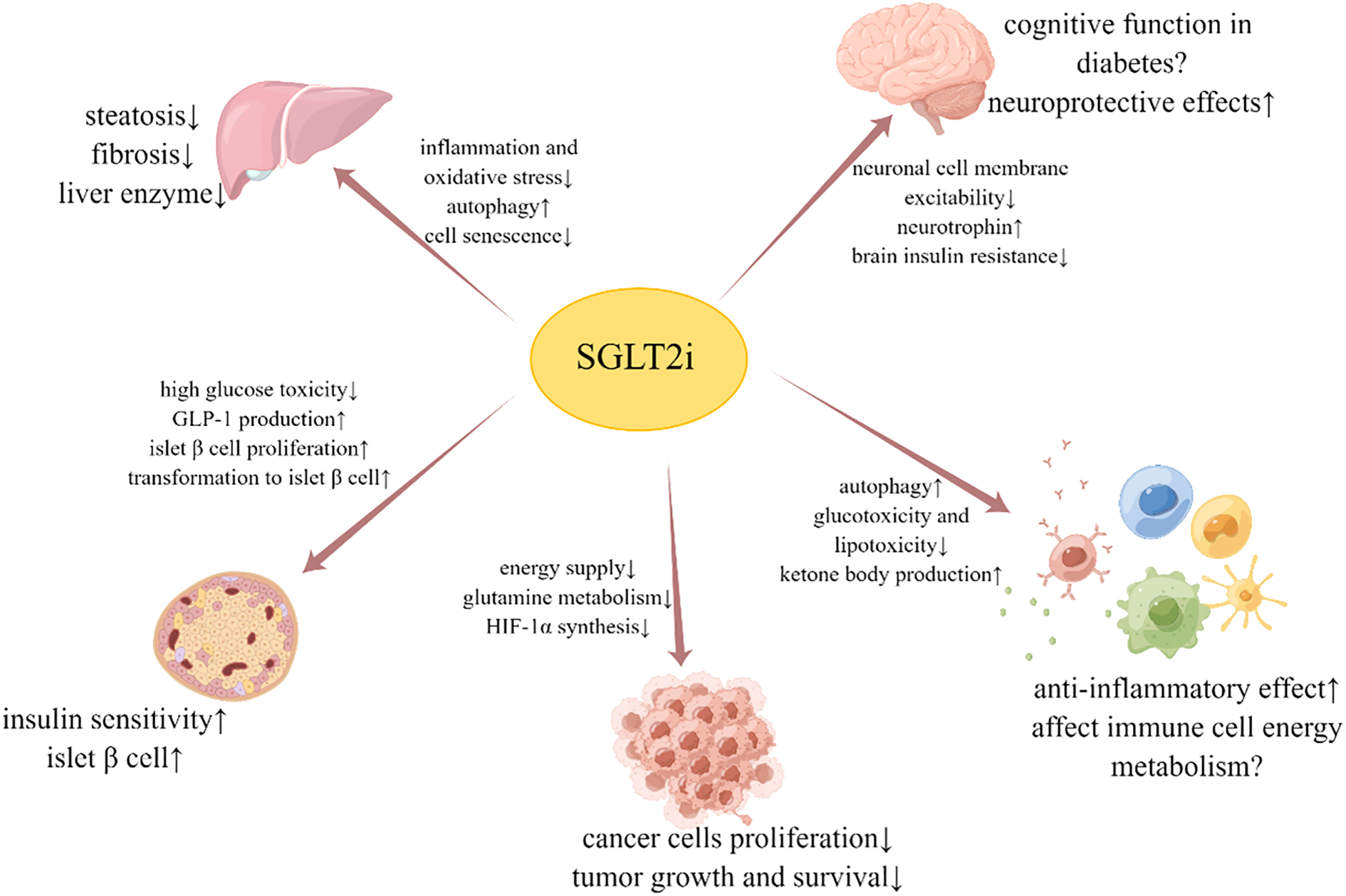
Beneficial effects and possible mechanisms of SGLT2i on liver, brain, islet β cell, tumor and immune cell.
Statements
Author contributions
SL: Writing – original draft. YW: Writing – original draft. QG: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Brown E Wilding JPH Alam U Barber TM Karalliedde J Cuthbertson DJ et al . The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. (2021) 53:2072–89. doi: 10.1080/07853890.2020.1841281
2
Santos-Gallego CG Requena-Ibanez JA San Antonio R Ishikawa K Watanabe S Picatoste B et al . Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. (2019) 73:1931–44. doi: 10.1016/j.jacc.2019.01.056
3
Čertíková Chábová V Zakiyanov O . Sodium glucose cotransporter-2 inhibitors: spotlight on favorable effects on clinical outcomes beyond diabetes. Int J Mol Sci. (2022) 23:2812–28. doi: 10.3390/ijms23052812
4
Lambers Heerspink HJ de Zeeuw D Wie L Leslie B List J . Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. (2013) 15:853–62. doi: 10.1111/dom.12127
5
Chino Y Samukawa Y Sakai S Nakai Y Yamaguchi J Nakanishi T et al . SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. (2014) 35:391–404. doi: 10.1002/bdd.1909
6
Kasichayanula S Liu X Zhang W Pfister M LaCreta FP Boulton DW . Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: an open-label, parallel-group, single-dose study. Clin Ther. (2011) 33:1798–808. doi: 10.1016/j.clinthera.2011.09.011
7
Toyama T Neuen BL Jun M Ohkuma T Neal B Jardine MJ et al . Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. (2019) 21:1237–50. doi: 10.1111/dom.13648
8
Taheri H Malek M Ismail-Beigi F Zamani F Sohrabi M Babaei MR et al . Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: A randomized, double-blind, placebo-controlled trial. Adv Ther. (2020) 37:4697–708. doi: 10.1007/s12325-020-01498-5
9
Shimizu M Suzuki K Kato K Jojima T Iijima T Murohisa T et al . Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. (2019) 21:285–92. doi: 10.1111/dom.13520
10
Kuchay MS Krishan S Mishra SK Farooqui KJ Singh MK Wasir JS et al . Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: A randomized controlled trial (E-LIFT trial). Diabetes Care. (2018) 41:1801–8. doi: 10.2337/dc18-0165
11
Leiter LA Forst T Polidori D Balis DA Xie J Sha S . Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. (2016) 42:25–32. doi: 10.1016/j.diabet.2015.10.003
12
Sattar N Fitchett D Hantel S George JT Zinman B . Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. (2018) 61:2155–63. doi: 10.1007/s00125-018-4702-3
13
Herring RA Shojaee-Moradie F Stevenage M Parsons I Jackson N Mendis J et al . The SGLT2 inhibitor dapagliflozin increases the oxidation of ingested fatty acids to ketones in type 2 diabetes. Diabetes Care. (2022) 45:1408–15. doi: 10.2337/figshare.19241664
14
Li D Wu T Wang T Wei H Wang A Tang H et al . Effects of sodium glucose cotransporter 2 inhibitors on risk of dyslipidemia among patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Pharmacoepidemiol Drug Saf. (2020) 29:582–90. doi: 10.1002/pds.4985
15
Hüttl M Markova I Miklankova D Zapletalova I Poruba M Haluzik M et al . In a prediabetic model, empagliflozin improves hepatic lipid metabolism independently of obesity and before onset of hyperglycemia. Int J Mol Sci. (2021) 22:11513–28. doi: 10.3390/ijms222111513
16
Jojima T Wakamatsu S Kase M Iijima T Maejima Y Shimomura K et al . The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci. (2019) 20:5237–52. doi: 10.3390/ijms20205237
17
Li L Li Q Huang W Han Y Tan H An M et al . Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front Pharmacol. (2021) 12:589273. doi: 10.3389/fphar.2021.589273
18
Mendez Espinoza I Choos END Ecelbarger CM Shepard BD . SGLT2 inhibition leads to a restoration of hepatic and circulating metabolites involved in the folate cycle and pyrimidine biosynthesis. Am J Physiol Gastrointest Liver Physiol. (2024) 327:G235–g253. doi: 10.1152/ajpgi.00029.2024
19
Aragón-Herrera A Otero-Santiago M Anido-Varela L Moraña-Fernández S Campos-Toimil M García-Caballero T et al . The treatment with the SGLT2 inhibitor empagliflozin modifies the hepatic metabolome of male zucker diabetic fatty rats towards a protective profile. Front Pharmacol. (2022) 13:827033. doi: 10.3389/fphar.2022.827033
20
Hupa-Breier KL Dywicki J Hartleben B Wellhöner F Heidrich B Taubert R et al . Dulaglutide alone and in combination with empagliflozin attenuate inflammatory pathways and microbiome dysbiosis in a non-diabetic mouse model of NASH. Biomedicines. (2021) 9:353–69. doi: 10.3390/biomedicines9040353
21
Morishita A Tadokoro T Fujihara S Iwama H Oura K Fujita K et al . Ipragliflozin attenuates non-alcoholic steatohepatitis development in an animal model. PloS One. (2022) 17:e0261310. doi: 10.1371/journal.pone.0261310
22
Wang Y Ding Y Sun P Zhang W Xin Q Wang N et al . Empagliflozin-enhanced antioxidant defense attenuates lipotoxicity and protects hepatocytes by promoting foxO3a- and nrf2-mediated nuclear translocation via the CAMKK2/AMPK pathway. Antioxids (Basel). (2022) 11. doi: 10.3390/antiox11050799
23
Lin XF Cui XN Yang J Jiang YF Wei TJ Xia L et al . SGLT2 inhibitors ameliorate NAFLD in mice via downregulating PFKFB3, suppressing glycolysis and modulating macrophage polarization. Acta Pharmacol Sin. (2024) 45:2579–97. doi: 10.1038/s41401-024-01389-3
24
Jin K Shi Y Zhang H Zhangyuan G Wang F Li S et al . A TNFα/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates nonalcoholic steatohepatitis. J Hepatol. (2023) 79:403–16. doi: 10.1016/j.jhep.2023.03.039
25
Byrnes K Blessinger S Bailey NT Scaife R Liu G Khambu B et al . Therapeutic regulation of autophagy in hepatic metabolism. Acta Pharm Sin B. (2022) 12:33–49. doi: 10.1016/j.apsb.2021.07.021
26
Nasiri-Ansari N Nikolopoulou C Papoutsi K Kyrou I Mantzoros CS Kyriakopoulos G et al . Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed apoE((-/-)) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. (2021) 22:818–38. doi: 10.3390/ijms22020818
27
Meng Z Liu X Li T Fang T Cheng Y Han L et al . The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int Immunopharmacol. (2021) 94:107492. doi: 10.1016/j.intimp.2021.107492
28
Papatheodoridi AM Chrysavgis L Koutsilieris M Chatzigeorgiou A . The role of senescence in the development of nonalcoholic fatty liver disease and progression to nonalcoholic steatohepatitis. Hepatology. (2020) 71:363–74. doi: 10.1002/hep.30834
29
Yoshioka N Tanaka M Ochi K Watanabe A Ono K Sawada M et al . The sodium-glucose cotransporter-2 inhibitor Tofogliflozin prevents the progression of nonalcoholic steatohepatitis-associated liver tumors in a novel murine model. BioMed Pharmacother. (2021) 140:111738. doi: 10.1016/j.biopha.2021.111738
30
Koekkoek LL Mul JD la Fleur SE . Glucose-sensing in the reward system. Front Neurosci. (2017) 11:716. doi: 10.3389/fnins.2017.00716
31
Wright EM Loo DD Hirayama BA . Biology of human sodium glucose transporters. Physiol Rev. (2011) 91:733–94. doi: 10.1152/physrev.00055.2009
32
Doi Y Ninomiya T Hata J Fukuhara M Yonemoto K Iwase M et al . Impact of glucose tolerance status on development of ischemic stroke and coronary heart disease in a general Japanese population: the Hisayama study. Stroke. (2010) 41:203–9. doi: 10.1161/STROKEAHA.109.564708
33
Yamazaki Y Harada S Tokuyama S . Post-ischemic hyperglycemia exacerbates the development of cerebral ischemic neuronal damage through the cerebral sodium-glucose transporter. Brain Res. (2012) 1489:113–20. doi: 10.1016/j.brainres.2012.10.020
34
Amin EF Rifaai RA Abdel-Latif RG . Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam Clin Pharmacol. (2020) 34:548–58. doi: 10.1111/fcp.12548
35
Abdel-Latif RG Rifaai RA Amin EF . Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch Pharm Res. (2020) 43:514–25. doi: 10.1007/s12272-020-01237-y
36
Erdogan M.A Yusuf D Christy J Solmaz V Erdogan A Taskiran E et al . Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. (2018) 18:81. doi: 10.1186/s12883-018-1086-4
37
Op den Kamp YJM de Ligt M Dautzenberg B Kornips E Esterline R Hesselink MKC et al . Effects of the SGLT2 inhibitor dapagliflozin on energy metabolism in patients with type 2 diabetes: A randomized, double-blind crossover trial. Diabetes Care. (2021) 44:1334–43. doi: 10.2337/dc20-2887
38
Daniele G Xiong J Solis-Herrera C Merovci A Eldor R Tripathy D et al . Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care. (2016) 39:2036–41. doi: 10.2337/dc15-2688
39
Kumar A Kumari S Singh D . Insight into the cellular interactions and molecular mechanisms of ketogenic diet for comprehensive management of epilepsy. Curr Neuropharmacol. (2022) 20:2812–28. doi: 10.2174/1570159X20666220420130109
40
Zarnowska IM . Therapeutic use of the ketogenic diet in refractory epilepsy: what we know and what still needs to be learned. Nutrients. (2020) 12:2616–594. doi: 10.3390/nu12092616
41
Kossoff EH Rowley H Sinha SR Vining EP . A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. (2008) 49:316–9. doi: 10.1111/j.1528-1167.2007.01256.x
42
Khan T Khan S Akhtar M Ali J Najmi AK . Empagliflozin nanoparticles attenuates type2 diabetes induced cognitive impairment via oxidative stress and inflammatory pathway in high fructose diet induced hyperglycemic mice. Neurochem Int. (2021) 150:105158. doi: 10.1016/j.neuint.2021.105158
43
Lin B Koibuchi N Hasegawa Y Sueta D Toyama K Uekawa K et al . Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. (2014) 13:148. doi: 10.1186/s12933-014-0148-1
44
Mone P Lombardi A Gambardella J Pansini A Macina G Morgante M et al . Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. (2022) 45:1247–51. doi: 10.2337/dc21-2434
45
Low S Goh KS Ng TP Moh A Ang SF Wang J et al . Association between use of sodium-glucose co-transporter-2 (SGLT2) inhibitors and cognitive function in a longitudinal study of patients with type 2 diabetes. J Alzheimers Dis. (2022) 87:635–42. doi: 10.3233/JAD-215678
46
Esterline R Oscarsson J Burns J . A role for sodium glucose cotransporter 2 inhibitors (SGLT2is) in the treatment of Alzheimer’s disease? Int Rev Neurobiol. (2020) 155:113–40. doi: 10.1016/bs.irn.2020.03.018
47
Rizvi S.M Shakil S Biswas D Shakil S Shaikh S Bagga P et al . Invokana (Canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer’s disease- diabetes type 2 linkage via an enzoinformatics study. CNS Neurol Disord Drug Targets. (2014) 13:447–51. doi: 10.2174/18715273113126660160
48
Kim H.K Biessels GJ Yu MH Hong N Lee YH Lee BW et al . SGLT2 inhibitor use and risk of dementia and parkinson disease among patients with type 2 diabetes. Neurology. (2024) 103:e209805. doi: 10.1212/WNL.0000000000209805
49
Chen W Cai W Hoover B Kahn CR . Insulin action in the brain: cell types, circuits, and diseases. Trends Neurosci. (2022) 45:384–400. doi: 10.1016/j.tins.2022.03.001
50
Scherer T Sakamoto K Buettner C . Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. (2021) 17:468–83. doi: 10.1038/s41574-021-00498-x
51
Kullmann S Hummel J Wagner R Dannecker C Vosseler A Fritsche L et al . Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: A randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. (2022) 45:398–406. doi: 10.2337/dc21-1136
52
Kimura T Obata A Shimoda M Hirukawa H Kanda-Kimura Y Nogami Y et al . Durability of protective effect of dulaglutide on pancreatic β-cells in diabetic mice: GLP-1 receptor expression is not reduced despite long-term dulaglutide exposure. Diabetes Metab. (2018) 44:250–60. doi: 10.1016/j.diabet.2017.10.007
53
Hamamoto S Kanda Y Shimoda M Tatsumi F Kohara K Tawaramoto K et al . Vildagliptin preserves the mass and function of pancreatic β cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes Obes Metab. (2013) 15:153–63. doi: 10.1111/dom.12005
54
Shimoda M Kanda Y Hamamoto S Tawaramoto K Hashiramoto M Matsuki M et al . The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. (2011) 54:1098–108. doi: 10.1007/s00125-011-2069-9
55
Stenlöf K Cefalu WT Kim KA Alba M Usiskin K Tong C et al . Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. (2013) 15:372–82. doi: 10.1111/dom.12054
56
Al Jobori H Daniele G Adams J Cersosimo E Solis-Herrera C Triplitt C et al . Empagliflozin treatment is associated with improved β-cell function in type 2 diabetes mellitus. J Clin Endocrinol Metab. (2018) 103:1402–7. doi: 10.1210/jc.2017-01838
57
Ramírez-Rodríguez AM González-Ortiz M Martínez-Abundis E . Effect of dapagliflozin on insulin secretion and insulin sensitivity in patients with prediabetes. Exp Clin Endocrinol Diabetes. (2020) 128:506–11. doi: 10.1055/a-0664-7583
58
Obata A Kubota N Kubota T Iwamoto M Sato H Sakurai Y et al . Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. (2016) 157:1029–42. doi: 10.1210/en.2015-1588
59
Jahn LA Hartline LM Nguyen T Aylor K Horton WB Liu Z et al . Empagliflozin improves vascular insulin sensitivity and muscle perfusion in persons with type 2 diabetes. Am J Physiol Endocrinol Metab. (2024) 326:E258–e267. doi: 10.1152/ajpendo.00267.2023
60
Wei R Cui X Feng J Gu L Lang S Wei T et al . Dapagliflozin promotes beta cell regeneration by inducing pancreatic endocrine cell phenotype conversion in type 2 diabetic mice. Metabolism. (2020) 111:154324. doi: 10.1016/j.metabol.2020.154324
61
Tanday N Irwin N Flatt PR Moffett RC . Dapagliflozin exerts positive effects on beta cells, decreases glucagon and does not alter beta- to alpha-cell transdifferentiation in mouse models of diabetes and insulin resistance. Biochem Pharmacol. (2020) 177:114009. doi: 10.1016/j.bcp.2020.114009
62
Karlsson D Ahnmark A Sabirsh AC Andréasson AS Gennemark P Sandinge AS et al . Inhibition of SGLT2 preserves function and promotes proliferation of human islets cells in vivo in diabetic mice. Biomedicines. (2022) 10:203. doi: 10.3390/biomedicines10020203
63
Liu P Zhang Z Wang J Zhang X Yu X Li Y et al . Empagliflozin protects diabetic pancreatic tissue from damage by inhibiting the activation of the NLRP3/caspase-1/GSDMD pathway in pancreatic β cells: in vitro and in vivo studies. Bioengineered. (2021) 12:9356–66. doi: 10.1080/21655979.2021.2001240
64
Tan Q Akindehin SE Orsso CE Waldner RC DiMarchi RD Müller TD et al . Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front Endocrinol (Lausanne). (2022) 13:838410. doi: 10.3389/fendo.2022.838410
65
Osonoi T Tamasawa A Osonoi Y Ofuchi K Katoh M Saito M et al . Canagliflozin increases postprandial total glucagon-like peptide 1 levels in the absence of α-glucosidase inhibitor therapy in patients with type 2 diabetes: A single-arm, non-randomized, open-label study. Diabetes Ther. (2019) 10:2045–59. doi: 10.1007/s13300-019-00689-w
66
Ferrannini E Muscelli E Frascerra S Baldi S Mari A Heise T et al . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. (2014) 124:499–508. doi: 10.1172/JCI72227
67
Takebayashi K Inukai T . Effect of sodium glucose cotransporter 2 inhibitors with low SGLT2/SGLT1 selectivity on circulating glucagon-like peptide 1 levels in type 2 diabetes mellitus. J Clin Med Res. (2017) 9:745–53. doi: 10.14740/jocmr3112w
68
Solini A Sebastiani G Nigi L Santini E Rossi C Dotta F . Dapagliflozin modulates glucagon secretion in an SGLT2-independent manner in murine alpha cells. Diabetes Metab. (2017) 43:512–20. doi: 10.1016/j.diabet.2017.04.002
69
Ahn CH Oh TJ Kwak SH Cho YM . Sodium-glucose cotransporter-2 inhibition improves incretin sensitivity of pancreatic β-cells in people with type 2 diabetes. Diabetes Obes Metab. (2018) 20:370–7. doi: 10.1111/dom.13081
70
Nakachi S Okamoto S Tamaki K Nomura I Tomihama M Nishi Y et al . Impact of anti-diabetic sodium-glucose cotransporter 2 inhibitors on tumor growth of intractable hematological Malignancy in humans. BioMed Pharmacother. (2022) 149:112864. doi: 10.1016/j.biopha.2022.112864
71
Kaji K Nishimura N Seki K Sato S Saikawa S Nakanishi K et al . Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. (2018) 142:1712–22. doi: 10.1002/ijc.31193
72
Villani LA Smith BK Marcinko K Ford RJ Broadfield LA Green AE et al . The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol Metab. (2016) 5:1048–56. doi: 10.1016/j.molmet.2016.08.014
73
Scafoglio C Hirayama BA Kepe V Liu J Ghezzi C Satyamurthy N et al . Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U.S.A. (2015) 112:E4111–9. doi: 10.1073/pnas.1511698112
74
Scafoglio CR Villegas B Abdelhady G Bailey ST Liu J Shirali AS et al . Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med. (2018) 10:aat5933. doi: 10.1126/scitranslmed.aat5933
75
Kuang H Liao L Chen H Kang Q Shu X Wang Y . Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit. (2017) 23:3737–45. doi: 10.12659/MSM.902530
76
Zhou J Zhu J Yu SJ Ma HL Chen J Ding XF et al . Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. BioMed Pharmacother. (2020) 132:110821. doi: 10.1016/j.biopha.2020.110821
77
Yamamoto L Yamashita S Nomiyama T Kawanami T Hamaguchi Y Shigeoka T et al . Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol Int. (2021) 12:389–98. doi: 10.1007/s13340-021-00494-6
78
Komatsu S Nomiyama T Numata T Kawanami T Hamaguchi Y Iwaya C et al . SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J. (2020) 67:99–106. doi: 10.1507/endocrj.EJ19-0428
79
Chou OHI Ning J Chan RNC Chung CT Huang H Ng K et al . Lower risks of new-onset hepatocellular carcinoma in patients with type 2 diabetes mellitus treated with SGLT2 inhibitors versus DPP4 inhibitors. J Natl Compr Canc Netw. (2024) 22:e237118. doi: 10.6004/jnccn.2023.7118
80
Bora V Patel BM . Investigation into the role of anti-diabetic agents in cachexia associated with metastatic cancer. Life Sci. (2021) 274:119329. doi: 10.1016/j.lfs.2021.119329
81
Nasiri AR Rodrigues MR Li Z Leitner BP Perry RJ . SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. (2019) 7:10. doi: 10.1186/s40170-019-0203-1
82
Papadopoli D Uchenunu O Palia R Chekkal N Hulea L Topisirovic I et al . Perturbations of cancer cell metabolism by the antidiabetic drug canagliflozin. Neoplasia. (2021) 23:391–9. doi: 10.1016/j.neo.2021.02.003
83
Nakano D Kawaguchi T Iwamoto H Hayakawa M Koga H Torimura T . Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: Multi-omics analysis of metabolomics and absolute quantification proteomics (iMPAQT). PloS One. (2020) 15:e0232283. doi: 10.1371/journal.pone.0232283
84
Luo J Sun P Zhang X Lin G Xin Q Niu Y et al . Canagliflozin modulates hypoxia-induced metastasis, angiogenesis and glycolysis by decreasing HIF-1α Protein synthesis via AKT/mTOR pathway. Int J Mol Sci. (2021) 22:13336–50. doi: 10.3390/ijms222413336
85
Xu D Zhou Y Xie X He L Ding J Pang S et al . Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter−1 and lactate dehydrogenase A. Int J Oncol. (2020) 57:1223–33. doi: 10.3892/ijo.2020.5120
86
Xu L Nagata N Nagashimada M Zhuge F Ni Y Chen G et al . SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. (2017) 20:137–49. doi: 10.1016/j.ebiom.2017.05.028
87
Baldrighi M Mallat Z Li X . NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis. (2017) 267:127–38. doi: 10.1016/j.atherosclerosis.2017.10.027
88
Xie W Du L . Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. (2011) 13:289–301. doi: 10.1111/j.1463-1326.2010.01336.x
89
Xu C Wang W Zhong J Lei F Xu N Zhang Y et al . Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol. (2018) 152:45–59. doi: 10.1016/j.bcp.2018.03.013
90
Borzouei S Moghimi H Zamani A Behzad M . Changes in T helper cell-related factors in patients with type 2 diabetes mellitus after empagliflozin therapy. Hum Immunol. (2021) 82:422–8. doi: 10.1016/j.humimm.2021.03.004
91
Niu Y Zhang Y Zhang W Lu J Chen Y Hao W et al . Canagliflozin ameliorates NLRP3 inflammasome-mediated inflammation through inhibiting NF-κB signaling and upregulating bif-1. Front Pharmacol. (2022) 13:820541. doi: 10.3389/fphar.2022.820541
92
Kasperova BJ Mraz M Svoboda P Hlavacek D Kratochvilova H Modos I et al . Sodium-glucose cotransporter 2 inhibitors induce anti-inflammatory and anti-ferroptotic shift in epicardial adipose tissue of subjects with severe heart failure. Cardiovasc Diabetol. (2024) 23:223. doi: 10.1186/s12933-024-02298-9
93
Salio M Puleston DJ Mathan TS Shepherd D Stranks AJ Adamopoulou E et al . Essential role for autophagy during invariant NKT cell development. Proc Natl Acad Sci U.S.A. (2014) 111:E5678–87. doi: 10.1073/pnas.1413935112
94
Jing FY Weng YJ Zhang YQ . The protective effect of sericin on AML12 cells exposed to oxidative stress damage in a high-glucose environment. Antioxids (Basel). (2022) 11:712–23. doi: 10.3390/antiox11040712
95
Tang R Zhong T Fan L Xie Y Li J Li X . Enhanced T cell glucose uptake is associated with progression of beta-cell function in type 1 diabetes. Front Immunol. (2022) 13:897047. doi: 10.3389/fimmu.2022.897047
96
Xie L Xiao Y Tai S Yang H Zhou S Zhou Z . Emerging roles of sodium glucose cotransporter 2 (SGLT-2) inhibitors in diabetic cardiovascular diseases: focusing on immunity, inflammation and metabolism. Front Pharmacol. (2022) 13:836849. doi: 10.3389/fphar.2022.836849
97
Qin J Liu Q Liu A Leng S Wang S Li C et al . Empagliflozin modulates CD4(+) T-cell differentiation via metabolic reprogramming in immune thrombocytopenia. Br J Haematol. (2022) 198:765–75. doi: 10.1111/bjh.18293
98
Hiruma S Shigiyama F Hisatake S Mizumura S Shiraga N Hori M et al . A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the ASSET study. Cardiovasc Diabetol. (2021) 20:32. doi: 10.1186/s12933-021-01228-3
99
Karagiannis F Peukert K Surace L Michla M Nikolka F Fox M et al . Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19. Nature. (2022) 609:801–7. doi: 10.1038/s41586-022-05128-8
100
Hirschberger S Strauß G Effinger D Marstaller X Ferstl A Müller MB et al . Very-low-carbohydrate diet enhances human T-cell immunity through immunometabolic reprogramming. EMBO Mol Med. (2021) 13:e14323. doi: 10.15252/emmm.202114323
101
La Grotta R Frigé C Matacchione G Olivieri F de Candia P Ceriello A et al . Repurposing SGLT-2 inhibitors to target aging: available evidence and molecular mechanisms. Int J Mol Sci. (2022) 23:12325–38. doi: 10.3390/ijms232012325
102
Pérez-Flores I López-Pastor AR Gómez-Pinedo U Gómez-Infantes A Espino-Paisán L Calvo Romero N et al . Mitochondrial changes induced by SGLT2i in lymphocytes from diabetic kidney transplant recipients: A pilot study. Int J Mol Sci. (2025) 26:3351–69. doi: 10.3390/ijms26073351
103
Mroueh A Algara-Suarez P Fakih W Gong DS Matsushita K Park SH et al . SGLT2 expression in human vasculature and heart correlates with low-grade inflammation and causes eNOS-NO/ROS imbalance. Cardiovasc Res. (2025) 121:643–57. doi: 10.1093/cvr/cvae257
104
Ye Y Bajaj M Yang HC Perez-Polo JR Birnbaum Y . SGLT-2 inhibition with dapagliflozin reduces the activation of the nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. (2017) 31:119–32. doi: 10.1007/s10557-017-6725-2
105
Hawley SA Ford RJ Smith BK Gowans GJ Mancini SJ Pitt RD et al . The na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. (2016) 65:2784–94. doi: 10.2337/db16-0058
106
Zhou H Wang S Zhu P Hu S Chen Y Ren J . Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. (2018) 15:335–46. doi: 10.1016/j.redox.2017.12.019
107
Kawakami R Matsui H Matsui M Iso T Yokoyama T Ishii H et al . Empagliflozin induces the transcriptional program for nutrient homeostasis in skeletal muscle in normal mice. Sci Rep. (2023) 13:18025. doi: 10.1038/s41598-023-45390-y
108
Zhao X Liu Y Han X Wang X Qu C Liu X et al . Dapagliflozin attenuates the vulnerability to atrial fibrillation in rats with lipopolysaccharide-induced myocardial injury. Int Immunopharmacol. (2023) 125:111038. doi: 10.1016/j.intimp.2023.111038
109
Yaribeygi H Hemmati MA Nasimi F Pakdel R Jamialahmadi T Sahebkar A . Empagliflozin alleviates diabetes-induced cognitive impairments by lowering nicotinamide adenine dinucleotide phosphate oxidase-4 expression and potentiating the antioxidant defense system in brain tissue of diabetic rats. Behav Brain Res. (2024) 460:114830. doi: 10.1016/j.bbr.2023.114830
110
Liu H Jiang B Hua R Liu X Qiao B Zhang X et al . ALDH2 mediates the effects of sodium-glucose cotransporter 2 inhibitors (SGLT2i) on improving cardiac remodeling. Cardiovasc Diabetol. (2024) 23:380. doi: 10.1186/s12933-024-02477-8
111
Grempler R Thomas L Eckhardt M Himmelsbach F Sauer A Sharp DE et al . Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. (2012) 14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x
112
Heise T Seman L Macha S Jones P Marquart A Pinnetti S et al . Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. (2013) 4:331–45. doi: 10.1007/s13300-013-0030-2
113
Zinman B Wanner C Lachin JM Fitchett D Bluhmki E Hantel S et al . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
114
Kasichayanula S Liu X Pe Benito M Yao M Pfister M LaCreta FP et al . The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus. Br J Clin Pharmacol. (2013) 76:432–44. doi: 10.1111/bcp.12056
115
McMurray JJV Solomon SD Inzucchi SE Køber L Kosiborod MN Martinez FA et al . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
116
Devineni D Curtin CR Polidori D Gutierrez MJ Murphy J Rusch S et al . Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. (2013) 53:601–10. doi: 10.1002/jcph.88
117
Neal B Perkovic V Mahaffey KW de Zeeuw D Fulcher G Erondu N et al . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
118
Ehrenkranz JR Lewis NG Kahn CR Roth J . Phlorizin: a review. Diabetes Metab Res Rev. (2005) 21:31–8. doi: 10.1002/dmrr.532
Summary
Keywords
nonalcoholic fatty liver disease, neuroprotection, insulin β cell, tumor, immunity
Citation
Li S, Wang Y and Gong Q (2025) Multi-dimensional roles of sodium-glucose cotransporter 2 inhibitors: beyond hypoglycemic and cardiorenal protection. Front. Endocrinol. 16:1641699. doi: 10.3389/fendo.2025.1641699
Received
06 June 2025
Accepted
08 September 2025
Published
29 September 2025
Volume
16 - 2025
Edited by
Duuamene Nyimanu, University of Kansas Medical Center, United States
Reviewed by
Viji Remadevi, University of Kansas Medical Center, United States
Sulthan Al Rashid, Saveetha Medical College & Hospital, India
Updates
Copyright
© 2025 Li, Wang and Gong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Gong, gongquan1998@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.