- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Background: Prader–Willi syndrome (PWS) is a rare genetic disorder characterized by severe multisystem comorbidities and increased mortality. Although growth hormone therapy (GHT) is widely used as standard care, population-based evidence on its long-term safety, particularly in relation to mortality and type 2 diabetes mellitus (T2DM), remains limited. We aimed to investigate the associations between GHT duration, mortality, and T2DM incidence in PWS.
Methods: This is a nationwide cohort study using the Korean National Health Insurance Service database. A total of 385 individuals with PWS were identified between January 2005 and February 2023. GHT duration was the primary exposure. All-cause mortality was analyzed using Cox proportional hazards models, and T2DM risk was evaluated using multivariable logistic regression adjusted for age, comorbidities, and GHT duration.
Results: GHT duration did not directly impact mortality (OR 1.00, 95% CI: 0.99–1.00); however, peripheral vascular disease (aOR 10.66, 95% CI: 1.07–106.56), renal disease (aOR 17.45, 95% CI: 1.17–259.93), adrenal insufficiency (aOR 23.90, 95% CI: 3.19–178.34), and behavioral disorders (aOR 29.51, 95% CI: 2.64–329.95) were significant predictors of all-cause mortality. Longer GHT duration was independently associated with higher T2DM risk (aOR 1.06, 95% CI: 1.02–1.11). Older age, age at PWS diagnosis, and comorbidities (peptic ulcer disease, mild liver disease, and diabetes insipidus) were additional risk factors.
Conclusions: GHT was not a direct predictor of mortality in PWS, which was instead influenced by comorbidities. However, its prolonged use was linked to increased T2DM. These findings support individualized risk assessment and metabolic monitoring in patients with PWS receiving GHT.
1 Introduction
Prader–Willi Syndrome (PWS) is a rare genetic disorder (OMIM#176270) with an estimated incidence of 1 in 21,000 live births. It results from the lack of expression of paternally inherited genes on chromosome 15q11–q13 and is characterized by hypothalamic dysfunction, hyperphagia, obesity, intellectual disability, and endocrine abnormalities (1).
PWS is an indication for growth hormone therapy (GHT), as its clinical features, such as impaired growth, abnormal body composition, hypotonia, and low energy expenditure, closely resemble those seen in growth hormone deficiency (GHD) unrelated to PWS. Studies have shown that nearly all individuals with PWS exhibit reduced spontaneous growth hormone (GH) secretion and low insulin-like growth factor-1 (IGF)-1 levels, primarily due to hypothalamic dysfunction and potential defects in neuroendocrine convertase 1 (2, 3). In many countries, including the United States, United Kingdom, and European nations, GHT is initiated in patients with PWS during infancy or early childhood to maximize benefits. In South Korea, GHT for PWS has been covered by the national insurance system starting at age 2, recognizing its critical role in managing this condition (4). These include improvements in growth, body composition, motor and cognitive development, and overall quality of life. The long-term benefits of GHT include reduced fat accumulation, increased lean body mass, and improved metabolic and cardiovascular health.
However, GH exerts counter-regulatory effects on insulin action by stimulating hepatic glucose production and reducing peripheral glucose uptake, thereby raising concerns regarding its long-term metabolic safety. In prepubertal children with PWS, GHT has been associated with increases in insulin resistance, with occasional cases of impaired glucose tolerance reported after approximately two years of treatment (5). Data from KIGS registry have documented rare instances of hyperglycemia and type 2 diabetes mellitus (T2DM) in GH-treated PWS (6). In adults, a longitudinal study of 12 GH-treated patients showed a statistically significant rise in fasting glucose and a modest increase in HbA1c (from 5.6% to 5.8%) (7). A meta-analysis focusing on adult GHT further identified a small increase in fasting glucose and trends toward higher insulin resistance (8). By contrast, an Italian cohort study found that disorders of glucose metabolism in PWS were more strongly associated with age and obesity than with GH exposure (9). A recent systematic review similarly reported that GHT improves body composition without significantly impairing glucose homeostasis (10).
However, GH exerts counter-regulatory effects on insulin action by stimulating hepatic glucose production and reducing peripheral glucose uptake, raising concerns about its long-term metabolic safety. In prepubertal children with PWS, GHT has been associated with increased insulin resistance, with occasional cases of impaired glucose tolerance reported after approximately two years of treatment (5). Data from the KIGS registry have documented rare instances of hyperglycemia and type 2 diabetes mellitus (T2DM) in GH-treated individuals with PWS (6). In adults, a longitudinal study of 12 GH-treated patients showed a statistically significant rise in fasting glucose and a modest increase in HbA1c (from 5.6% to 5.8%) (7). A meta-analysis of GH therapy in adults further reported small increases in fasting glucose and a trend toward greater insulin resistance (8). Conversely, an Italian cohort study suggested that glucose metabolism disorders in PWS are more strongly related to age and obesity than to GH exposure (9). A recent systematic review also concluded that GHT improves body composition without significantly impairing glucose homeostasis (10).
These findings suggest that while GH clearly benefits body composition, its long−term metabolic safety and impact on serious outcomes such as all-cause mortality and T2DM remain limited. In addition, how factors such as timing of diagnosis or duration of therapy influence these outcomes is not well understood.
We hypothesized that prolonged exposure to GHT may be associated with altered risks of mortality and T2DM in individuals with PWS. To address this, we conducted a nationwide population-based study to examine the associations between GHT duration and these long-term outcomes, aiming to inform risk stratification and long-term management in this population.
2 Methods
2.1 Ethics approval and patient consent
The study protocol was approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2023-07-036). The requirement for informed consent was waived due to the use of anonymized data.
2.2 Data source
This study utilized data from the Korean National Health Insurance Service (NHIS), which covers 97% of the population and includes diagnostic codes, prescription records, procedures, and demographic information. The NHIS database is linked to the National Death Registry, the National Health Screening Program, and the Rare Incurable Disease Registry (11). Under the Rare Disease Management Act, patients with conditions affecting fewer than 20,000 individuals, including PWS, are eligible for financial support and registered under a special reimbursement code (12).
2.3 Study population and design
We identified individuals with PWS using the Korean NHIS database from January 2004 to February 2023 using ICD-10 Q87.1 and reimbursement code V158. Because Q87.1 encompasses other congenital syndromes associated with short stature (e.g., Noonan syndrome, Aarskog syndrome), we enhanced diagnostic specificity by including only those treated with somatropin products explicitly approved for PWS: Genotropin (Pfizer), Eutropin (LG Chem), or SciTropin (SciGen Korea). In Korea, such prescriptions require both genetic and clinical confirmation, thus serving as a reliable proxy for diagnosis in NHIS data. Patients treated for other indications (eg, Noonan syndrome), with non-approved GH products, or with GH prescriptions during the designated 2024 washout period were excluded. GH therapy for PWS is typically initiated from age 2 without GH stimulation testing due to its syndromic indication. It is administered at a standard dose of 0.035 mg/kg/day or 1.0 mg/m²/day (maximum 2.7 mg/day), and discontinued when growth velocity falls below 1 cm or near epiphyseal closure. These practices are in line with international guidelines (4) and were applied consistently during the study period. The final study cohort consisted of 385 individuals (Figure 1).
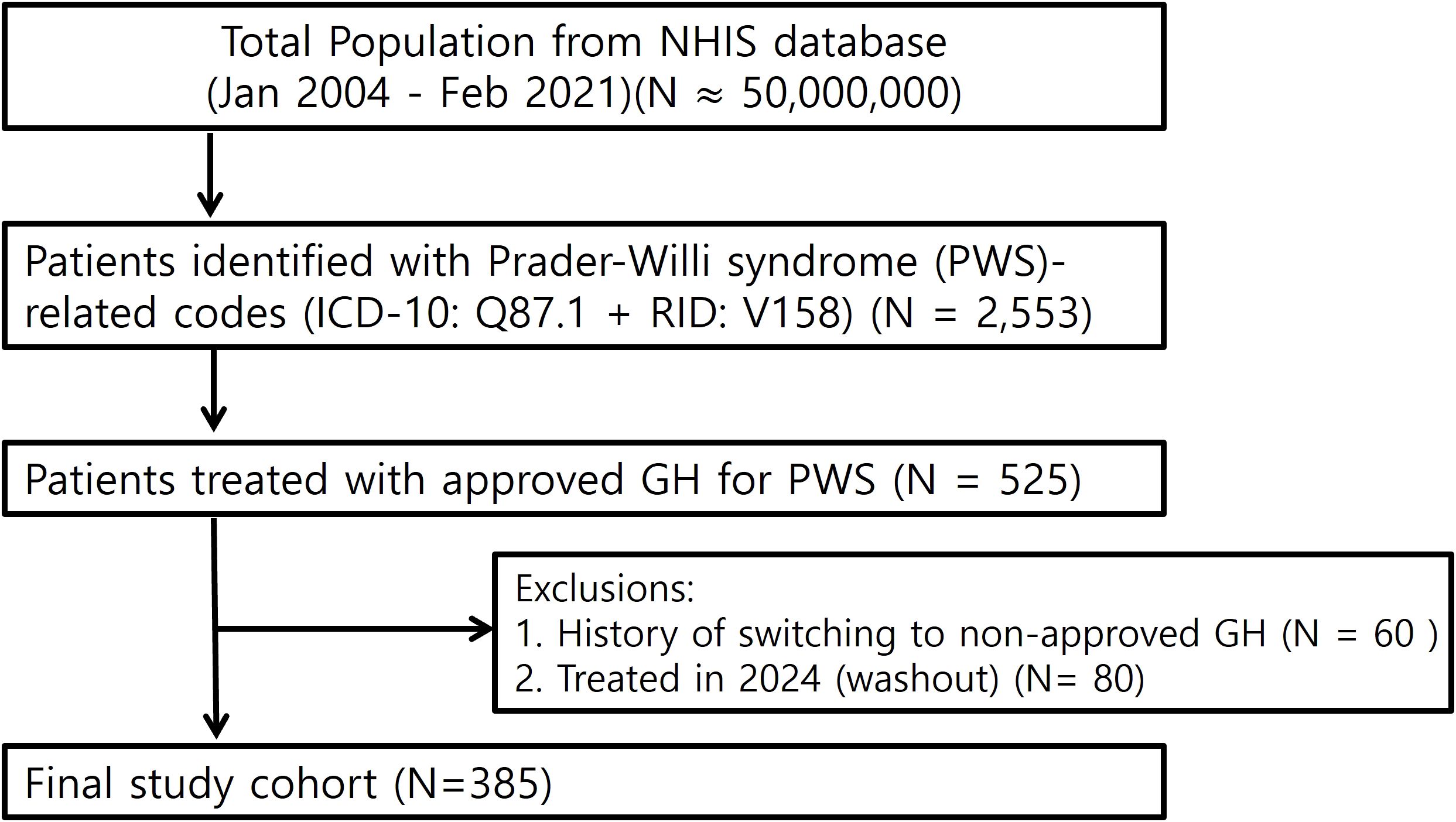
Figure 1. Study flow chart. Flow diagram of patient selection from the NHIS database (January 2004–February 2023). A total of 2,553 patients were identified with Prader–Willi syndrome (PWS)-related codes (ICD-10: Q87.1 and reimbursement code V158). To enhance diagnostic specificity, only patients who received GH products approved for PWS (N = 525) were included. After excluding 60 patients with a history of switching to non-approved GH and 80 patients treated during the 2024 washout period, the final study cohort comprised 385 individuals.
2.4 Data collection and definitions
Collected variables included sex, age at diagnosis, age at initiation of GHT, duration of GHT, age at last follow-up, and age at death. Comorbidities were identified using International Classification of Diseases, 10th Revision (ICD-10) diagnostic codes, ATC medication codes, and procedure codes, and summarized using the Charlson comorbidity index (CCI) according to Quan’s algorithm (13, 14). Key comorbidities were defined as follows: diabetes insipidus by desmopressin use; behavioral disorders by prescription of psychotropic medications; adrenal insufficiency by ICD-10 codes E27.3 or E27.4; and obstructive sleep apnea (OSA) by diagnosis with concurrent use of continuous positive airway pressure (CPAP). Full definitions and code sets are provided in Supplementary Table S1.
2.5 Outcome measures
The primary outcome was all-cause mortality, determined through linkage with the National Death Registry. The secondary outcome was the incidence of T2DM, defined by a diagnosis of ICD-10 code E11, followed by the prescription of at least one antidiabetic medication (ATC codes A10A or A10B) within 1 year of diagnosis. The observation period extended from January 2005 to February 2023, capturing a maximum follow-up duration of 16 years.
2.6 Statistical analyses
Categorical variables are summarized as frequencies and percentages and were analyzed using chi-square or Fisher’s exact tests. Continuous variables are presented as means ± standard deviations or medians with interquartile ranges (IQRs), depending on their distribution, and were compared using Student’s t-test or the Wilcoxon rank-sum test. Univariate and multivariate logistic regression analyses were conducted to identify risk factors for mortality and T2DM. Variables with a univariable P <0.05 were included in the multivariable models. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as a two-sided P <0.05. All analyses were conducted using R (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline characteristics
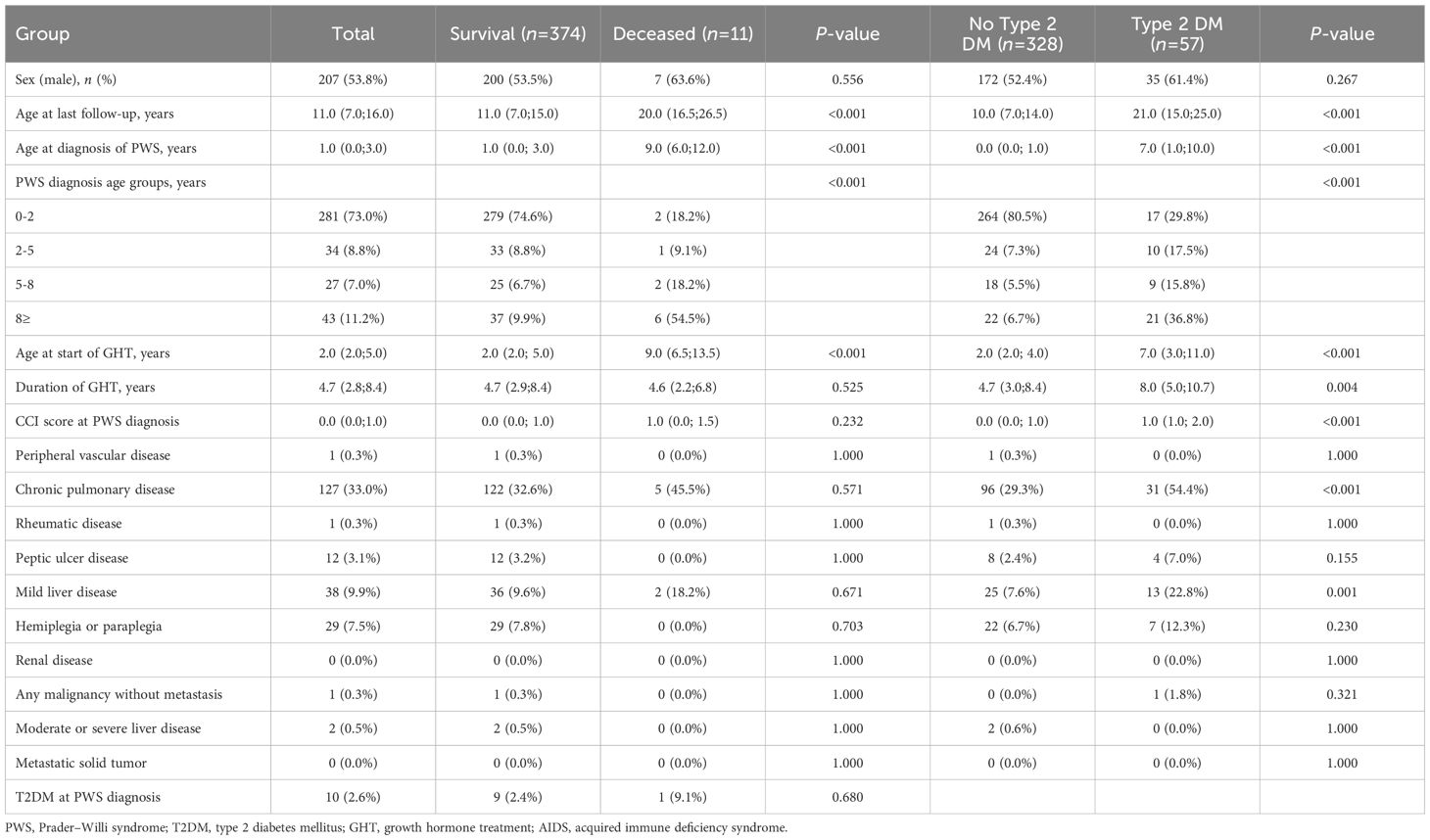
During the study period, 385 patients with PWS were included in the analysis. Among them, 207 (53.8%) were male. The median age at diagnosis was 1.0 years (interquartile range [IQR]: 0.0–3.0), and median age at last follow-up was 11.0 years (IQR: 7.0–16.0). Table 1 presents the baseline characteristics of the patients, categorized based on mortality and the occurrence of T2DM.
Comparison based on survival status revealed that the median age at PWS diagnosis was significantly higher in deceased patients than in survivors (9.0 years [IQR: 6.0–12.0] vs. 1.0 years [IQR: 0.0–3.0], P < 0.001), and the last follow-up age was also longer in deceased patients (20.0 year [IQR: 16.5–26.5] vs. 11.0 year [IQR: 7.0–15.0], P < 0.001). Furthermore, the median age at GH initiation was significantly higher in deceased patients than in survivors (9.0 years [IQR: 6.5–13.5] vs. 2.0 years [IQR: 2.0–5.0], P < 0.001), while the duration of GH treatment did not differ significantly between the groups (4.6 years [IQR: 2.2–6.8] vs. 4.7 years [IQR: 2.9–8.4], P = 0.525). T2DM prevalence at the time of PWS diagnosis did not differ significantly between survivors and deceased patients (P = 0.680).
Patients who developed T2DM were diagnosed with PWS at a significantly older age (7.0 years [IQR: 1.0–10.0] vs. 0.0 years [IQR: 0.0–1.0], P < 0.001), and had a higher age at last follow-up (21.0 years [IQR: 15.0–25.0] vs. 10.0 years [IQR: 7.0–14.0], P < 0.001) compared to those without T2DM. Additionally, the T2DM group had a higher age at the start of GHT and a longer duration of GHT compared to the non-T2DM group (7.0 years [IQR: 3.0–11.0] vs. 2.0 years [IQR: 2.0–4.0], P < 0.001, and 8.0 years [IQR: 5.0–10.7] vs. 4.7 years [IQR: 3.0–8.4], P = 0.004, respectively). In terms of underlying diseases at PWS diagnosis, the T2DM group had a higher CCI score (1.0 [IQR: 1.0–2.0] vs. 0.0 [IQR: 0.0–1.0], P < 0.001) and a higher prevalence of chronic pulmonary disease (54.4% [n=31] vs. 29.3% [n=96], P < 0.001) and mild liver disease (22.8% [n=13] vs. 7.6% [n=25], P = 0.001) compared to the non-T2DM group.
3.2 Comorbidities and clinical outcomes
Table 2 presents the clinical outcomes and the occurrence of comorbidities at the end of the observation period. Non-survivors had significantly higher CCI scores (median: 3.0 [IQR: 2.0–6.0]) than survivors (median: 2.0 [IQR: 1.0–3.0], P = 0.029). Additionally, non-survivors had significantly higher occurrences of T2DM (45.5% [n=5] vs. 13.9% [n=52], P = 0.013), peripheral vascular disease (18.2% [n=2] vs. 1.9% [n=7], P = 0.012), renal disease (18.2% [n=2] vs. 1.1% [n=4], P = 0.001), behavior disorders (90.9% [n=10] vs. 24.6% [n=92], P < 0.001), OSA (36.4% [n=4] vs. 10.7% [n=40], P = 0.031), and adrenal insufficiency (18.2% [n=2] vs. 0.8% [n=3], P < 0.001) compared to survivors.
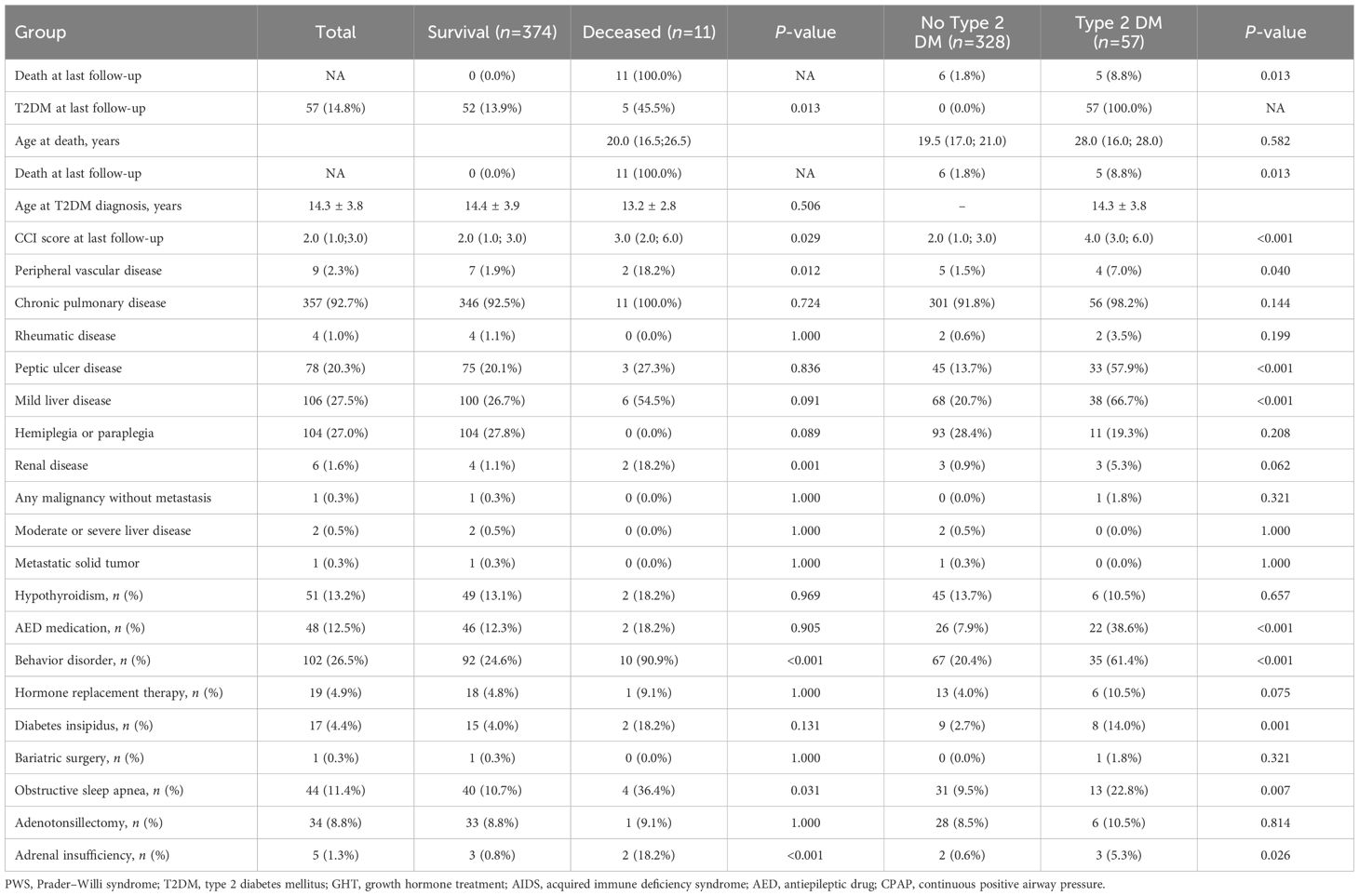
Table 2. Distribution of comorbidities in patients with Prader–Willi syndrome by survival and T2DM status.
In the comparison between T2DM and non-T2DM patients, there was no significant difference in age at death (19.5 vs. 28.0 years, P = 0.582). However, those with T2DM had significantly higher CCI scores (4.0 [IQR: 3.0–6.0] vs. 2.0 [IQR: 1.0–3.0], P < 0.001) and a higher prevalence of peripheral vascular disease (7.0% [n=4] vs. 1.5% [n=5], P = 0.040), peptic ulcer disease (57.9% [n=33] vs. 13.7% [n=45], P < 0.001), and mild liver disease (66.7% [n=38] vs. 20.7% [n=68], P < 0.001). Furthermore, patients with T2DM had higher AED medication use (38.6% [n=22] vs. 7.9% [n=26], P < 0.001), behavioral disorders (61.4% [n=35] vs. 20.4% [n=67], P < 0.001), diabetes insipidus (14.0% [n=8] vs. 2.7% [n=9], P = 0.001), OSA (22.8% [n=13] vs. 9.5% [n=31], P = 0.007), and adrenal insufficiency (5.3% [n=3] vs. 0.6% [n=2], P = 0.026).
3.3 Risk factors for mortality
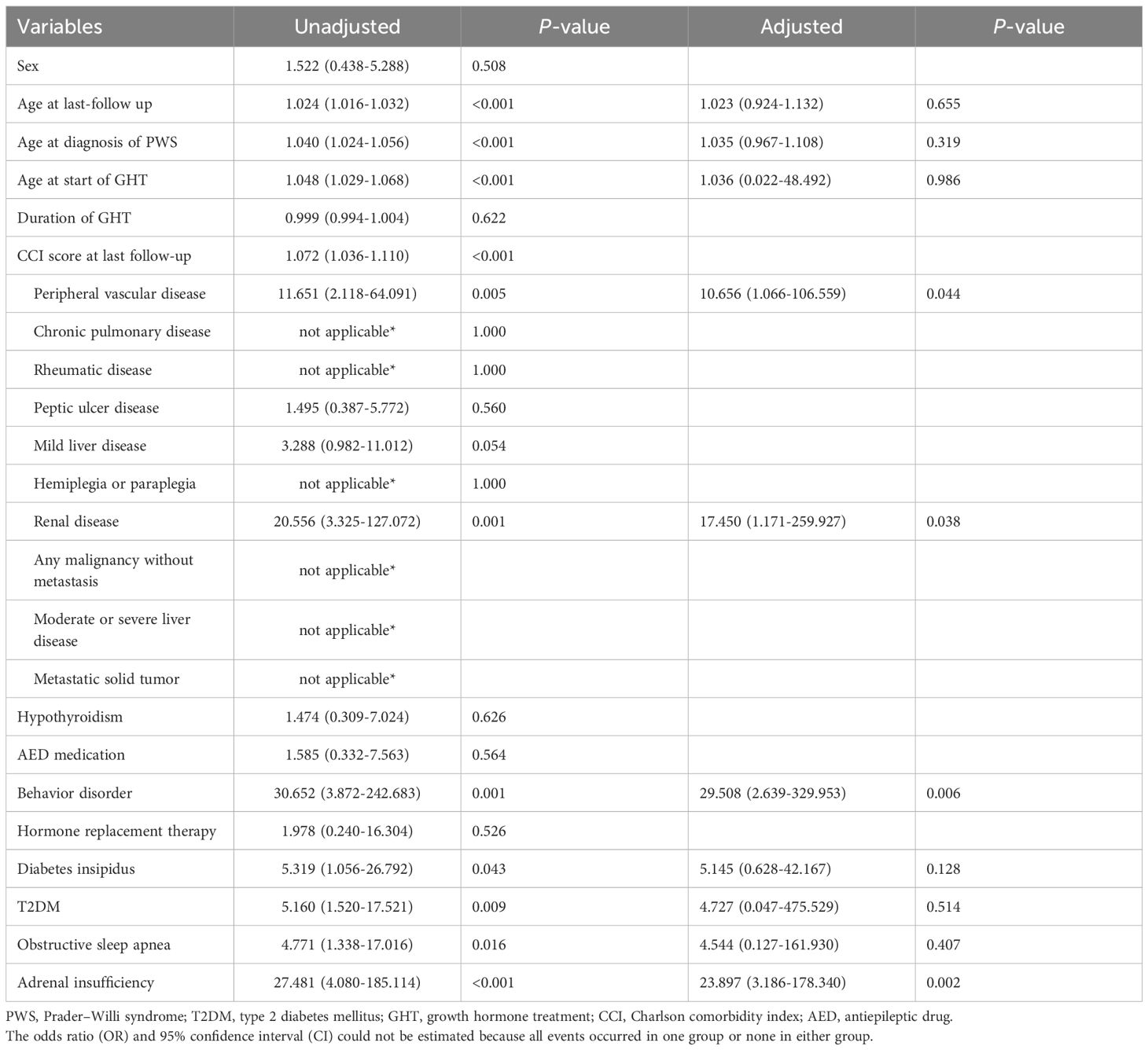
Table 3 presents the results of univariate and multivariate logistic regression analyses for all-cause mortality. In the univariate analysis, older age (OR: 1.024 [95% CI: 1.016–1.032]), later PWS diagnosis (OR: 1.040 [95% CI: 1.024–1.056]), delayed initiation of GHT (OR: 1.048 [95% CI: 1.029–1.068]), and higher CCI scores (OR: 1.072 [95% CI: 1.036–1.110]) were associated with all-cause mortality. In terms of underlying diseases, peripheral vascular disease (OR: 11.651 [95% CI: 2.118–64.091]), renal disease (OR: 20.556 [95% CI: 3.325–127.072]), behavior disorders (OR: 30.652 [95% CI: 3.872–242.683]), diabetes insipidus (OR: 5.319 [95% CI: 1.056–26.792]), T2DM (OR: 5.160 [95% CI: 1.520–17.521]), OSA (OR: 4.771 [95% CI: 1.338–17.016]), and adrenal insufficiency (OR: 27.481 [95% CI: 4.080–185.114]) were significantly associated with all-cause mortality.
In the adjusted analysis, peripheral vascular disease (OR: 10.656 [95% CI: 1.066–106.559]), renal disease (OR: 17.450 [95% CI: 1.171–259.927]), behavior disorders (OR: 29.508 [95% CI: 2.639–329.953]), and adrenal insufficiency (OR: 23.897 [95% CI: 3.186–178.340]) showed significant associations with all-cause mortality. However, age and GH use were not significantly associated with all-cause mortality (Table 3).
3.4 Risk factors for T2DM
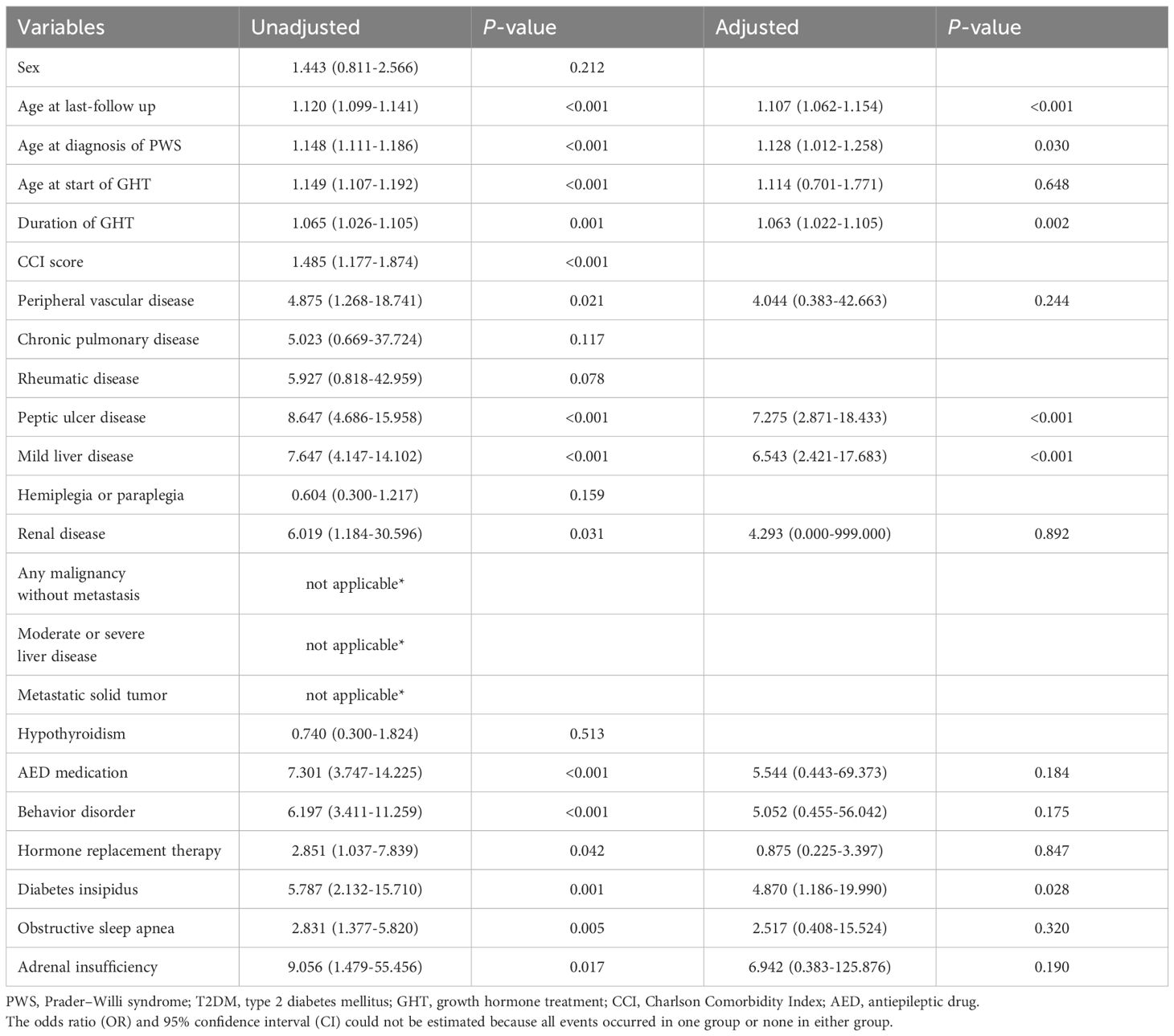
Table 4 presents the results of the logistic regression analysis for factors associated with the occurrence of T2DM. In the unadjusted analysis, older age (OR: 1.120 [95% CI: 1.099–1.141]), later PWS diagnosis (OR: 1.148 [95% CI: 1.111–1.186]), older age at GHT initiation (OR: 1.149 [95% CI: 1.107–1.192]), longer GHT duration (OR: 1.065 [95% CI: 1.026–1.105]), and higher CCI score (OR: 1.485 [95% CI: 1.177–1.874]) were significantly associated with T2DM occurrence. Among underlying diseases, several factors were significantly associated with T2DM occurrence, including peripheral vascular disease (OR: 4.875 [95% CI: 1.268–18.741]), peptic ulcer disease (OR: 8.647 [95% CI: 4.686–15.958]), mild liver disease (OR: 7.647 [95% CI: 4.147–14.102]), and renal disease (OR: 6.019 [95% CI: 1.184–30.596]). Additionally, the use of AEDs (OR: 7.301 [95% CI: 3.747–14.225]), behavior disorders (OR: 6.197 [95% CI: 3.411–11.259]), HRT use (OR: 2.851 [95% CI: 1.037–7.839]), diabetes insipidus (OR: 5.787 [95% CI: 2.132–15.710]), OSA (OR: 2.831 [95% CI: 1.377–5.820]), and adrenal insufficiency (OR: 9.056 [95% CI: 1.479–55.456]) were also significantly associated with T2DM occurrence.
However, in the multivariate analysis, older age (OR: 1.107 [95% CI: 1.062–1.154]), later PWS diagnosis (OR: 1.128 [95% CI: 1.012–1.258]), longer GHT duration (OR: 1.063 [95% CI: 1.022–1.105]), peptic ulcer disease (OR: 7.275 [95% CI: 2.871–18.433]), mild liver disease (OR: 6.543 [95% CI: 2.421–17.683]), and diabetes insipidus (OR: 4.870 [95% CI: 1.186–19.990]) showed significant associations with T2DM occurrence.
4 Discussion
This study investigated long-term outcomes of GHT on mortality and T2DM in patients with PWS using a nationwide cohort. Our analysis suggests that mortality in this population is more closely associated with comorbidities—especially peripheral vascular disease, renal disease, behavioral disorders, and adrenal insufficiency—than with GHT itself.
The impact of GHT on mortality in PWS remains unclear. Some studies have suggested that early GHT may improve survival (15), others report no significant effect (16–18). Research in patients with idiopathic short stature (ISS), isolated GHD, and small-for-gestational-age (SGA) highlights that GHT may increase long-term mortality related to cardiovascular and hematologic diseases, necessitating ongoing surveillance (19). Studies on PWS have reported adverse events and deaths during GHT, though no direct causal relationship has been established (6, 10). Our findings align with the prevailing consensus that GHT itself does not directly influence mortality rates (20). Instead, early diagnosis of PWS and effective management of comorbidities are crucial in improving survival outcomes.
Among the comorbidities observed, adrenal insufficiency, a common but often underrecognized complication in PWS, may have contributed to stress-related mortality from acute adrenal crisis in some cases (21–23). Although we could not distinguish central from primary adrenal insufficiency using claims data, the observed association with mortality emphasizes the importance of early detection and appropriate hydrocortisone coverage during acute illness or surgery (24). Behavioral disorders were also more common among non-survivors. While not traditionally considered direct mortality drivers, these conditions may increase the risk of choking, gastric perforation, and obesity-related morbidity (25, 26). Consistent with previous studies, respiratory failure remains the leading cause of mortality in PWS (31%–70%) (15, 26). Although no direct link has been confirmed, GHT may exacerbate respiratory dysfunction due to hypothalamic impairment (25), underscoring the need for respiratory assessment in those receiving GHT.
In contrast to mortality, GHT duration was independently associated with a higher risk of T2DM, even after adjusting for age, timing of diagnosis, and comorbid conditions. While GHT improves body composition and reduces visceral adiposity, it may also reduce insulin sensitivity through counter-regulatory mechanisms (27).
Clinical evidence regarding the metabolic effects of GHT has been mixed. Some observational studies have reported no significant deterioration in glucose metabolism during GHT, including in GH-treated children followed into adulthood (28), adults receiving long-term biosimilar GHT (29), and children born SGA (30).
However, other studies in SGA children (31) and adults with GHD (27, 32) have demonstrated impaired insulin action following GH administration. GH-induced lipolysis increases circulating free fatty acids, which interfere with skeletal muscle glucose uptake via the glucose–fatty acid cycle (27), and may promote intramyocellular lipid accumulation that disrupts insulin signaling (33).
In our PWS cohort, older age was independently associated with T2DM, independent of other metabolic factors. Moreover, certain comorbidities, including peptic ulcer disease, mild liver disease, and diabetes insipidus, were associated with an increased risk of T2DM, though their clinical significance in the context of PWS requires further investigation.
A later PWS diagnosis was independently linked to T2DM risk, likely due to prolonged exposure to metabolic risk factors such as obesity and insulin resistance. Early recognition and intervention, including lifestyle modifications and timely GHT initiation, may help reduce long-term metabolic complications.
In other conditions, such as Turner syndrome, GH-related insulin resistance may normalize after discontinuation (34), though large retrospective studies in pediatric populations have reported persistent T2DM (35, 36) and cardiovascular risks post-treatment (37).
The association between GHT and T2DM in PWS remains uncertain due to mixed findings. Several studies have reported low diabetes incidence during GHT (9, 10), including one involving 235 patients with only a single case attributed to preexisting obesity (38). In contrast, other studies have identified abnormal glucose metabolism and insulin resistance, particularly in prepubertal children or those with obesity (5, 6), and slightly impaired glucose homeostasis in adults receiving long-term GHT (8, 39). Thus, it has been established by expert consensus to include a diabetes risk assessment in patients with PWS receiving GHT, especially those who are obese, older than 12 years, or on antipsychotic medications (2, 5, 40).
This study has several strengths, including its large, nationally representative sample and linkage with mortality records. Use of validated diagnostic and treatment codes allowed for reliable cohort construction and longitudinal follow-up. However, several limitations merit consideration. Residual confounding due to unmeasured variables such as body mass index, laboratory data, or family history could not be fully addressed. In addition, the left-skewed distribution of age at PWS diagnosis, particularly among patients without T2DM, may have influenced group comparisons. Although age at diagnosis was included as a covariate in multivariate models, the potential for bias remains. Furthermore, the inability to differentiate PWS genetic subtypes may have limited our ability to explore phenotype–treatment interactions. Lastly, as with all observational studies, the associations observed cannot establish causality. Prospective studies are needed to validate our findings, explore alternative explanations, and inform risk stratification in the management of PWS.
5 Conclusion
This nationwide study found that while GHT was not directly associated with increased mortality in patients with PWS, it was linked to a higher likelihood of T2DM with longer treatment duration. These findings highlight the need for long-term, multidisciplinary monitoring of both endocrine and non-endocrine complications in this population. A multidisciplinary approach—including endocrinology, nutrition, behavioral management, and psychosocial support—remains essential to optimizing outcomes in patients receiving GHT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Kangbuk Samsung Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YC: Validation, Formal Analysis, Writing – review & editing, Writing – original draft, Methodology, Software, Visualization. AY: Data curation, Project administration, Methodology, Supervision, Conceptualization, Funding acquisition, Resources, Investigation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Data from the National Health Insurance Database were provided by the Korean National Health Insurance Service (KNHIS). The authors would like to thank the KNHIS for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1642129/full#supplementary-material
References
1. Bar C, Diene G, Molinas C, Bieth E, Casper C, and Tauber M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet J Rare Dis. (2017) 12:118. doi: 10.1186/s13023-017-0673-6
2. Deal CL, Tony M, Höybye C, Allen DB, Tauber M, and Christiansen JS. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. (2013) 98:E1072–87. doi: 10.1210/jc.2012-3888
3. Burnett LC, LeDuc CA, Sulsona CR, Paull D, Rausch R, Eddiry S, et al. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J Clin Invest. (2017) 127:293–305. doi: 10.1172/JCI88648
4. Yang A. Prader-Willi syndrome and growth hormone therapy: exploring the precise management of hypothalamic short stature: A review. Precis Future Med. (2023) 7:107–16. doi: 10.23838/pfm.2023.00079
5. Crinò A, Di Giorgio G, Manco M, Grugni G, and Maggioni A. Effects of growth hormone therapy on glucose metabolism and insulin sensitivity indices in prepubertal children with Prader-Willi syndrome. Horm Res. (2007) 68:83–90. doi: 10.1159/000100371
6. Craig ME, Cowell CT, Larsson P, Zipf WB, Reiter EO, Albertsson Wikland K, et al. Growth hormone treatment and adverse events in Prader-Willi syndrome: data from KIGS (the Pfizer International Growth Database). Clin Endocrinol (Oxf). (2006) 65:178–85. doi: 10.1111/j.1365-2265.2006.02570.x
7. Grugni G, Sartorio A, and Crinò A. Growth hormone therapy for Prader-willi syndrome: challenges and solutions. Ther Clin Risk Manage. (2016) 12:873–81. doi: 10.2147/TCRM.S70068
8. Sanchez-Ortiga R, Klibanski A, and Tritos NA. Effects of recombinant human growth hormone therapy in adults with Prader-Willi syndrome: a meta-analysis. Clin Endocrinol (Oxf). (2012) 77:86–93. doi: 10.1111/j.1365-2265.2011.04303
9. Fintini D, Grugni G, Bocchini S, Brufani C, Di Candia S, Corrias A, et al. Disorders of glucose metabolism in Prader–Willi syndrome: Results of a multicenter Italian cohort study. Nutrition Metab Cardiovasc Dis. (2016) 26:842–7. doi: 10.1016/j.numecd.2016.05.010
10. Passone CGB, Franco RR, Ito SS, Trindade E, Polak M, Damiani D, et al. Growth hormone treatment in Prader-Willi syndrome patients: systematic review and meta-analysis. BMJ Pediatr Open. (2020) 4:e000630. doi: 10.1136/bmjpo-2019-000630
11. Lee J, Lee JS, Park SH, Shin SA, and Kim K. Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. (2017) 46:e15. doi: 10.1093/ije/dyv319
12. Kwak JH, Choi YJ, Kim S, and Yang A. Long-term cardiovascular outcomes and mortality with enzyme replacement therapy in patients with mucopolysaccharidosis type II. J Inherit Metab Dis. (2025) 48:e12779. doi: 10.1002/jimd.12779
13. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. (2005) 43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
14. Choi YJ, Park HJ, Kim CY, Jung BM, Cho JH, and Byun MK. Features of lung cyst in birt-hogg-dubé Syndrome from patients with multiple lung cysts. Tuberc Respir Dis. (2024) 88(2):388–98. doi: 10.4046/trd.2024.0045
15. Proffitt J, Osann K, McManus B, Kimonis VE, Heinemann J, Butler MG, et al. Contributing factors of mortality in Prader-Willi syndrome. Am J Med Genet A. (2019) 179:196–205. doi: 10.1002/ajmg.a.60688
16. Quigley CA, Child CJ, Zimmermann AG, Rosenfeld RG, Robison LL, and Blum WF. Mortality in children receiving growth hormone treatment of growth disorders: data from the genetics and neuroendocrinology of short stature international study. J Clin Endocrinol Metab. (2017) 102:3195–205. doi: 10.1210/jc.2017-00214
17. van Bunderen CC, van Nieuwpoort IC, Arwert LI, Heymans MW, Franken AA, Koppeschaar HP, et al. Does growth hormone replacement therapy reduce mortality in adults with growth hormone deficiency? Data from the Dutch National Registry of Growth Hormone Treatment in adults. J Clin Endocrinol Metab. (2011) 96:3151–9. doi: 10.1210/jc.2011-1215
18. van Bunderen CC and Olsson DS. Meta-analysis of mortality in adults with growth hormone deficiency: Does growth hormone replacement therapy really improve mortality rates? Best Pract Res Clin Endocrinol Metab. (2023) 37:101835. doi: 10.1016/j.beem.2023.101835
19. Sävendahl L, Cooke R, Tidblad A, Beckers D, Butler G, Cianfarani S, et al. Long-term mortality after childhood growth hormone treatment: the SAGhE cohort study. Lancet Diabetes Endocrinol. (2020) 8:683–92. doi: 10.1016/S2213-8587(20)30163-7
20. Lee P, Boldon T, Transduction S, and Syndrome B. Growth hormone and mortality in prader-willi syndrome. Growth, Genetics & Hormones (2006) 22(2):17–23
21. de Lind van Wijngaarden RF, Otten BJ, Festen DA, Joosten KF, de Jong FH, Sweep FC, et al. High prevalence of central adrenal insufficiency in patients with Prader-Willi syndrome. J Clin Endocrinol Metab. (2008) 93:1649–54. doi: 10.1210/jc.2007-2294
22. de Lind van Wijngaarden RF, Joosten KF, van den Berg S, Otten BJ, de Jong FH, Sweep CG, et al. The relationship between central adrenal insufficiency and sleep-related breathing disorders in children with Prader-Willi syndrome. J Clin Endocrinol Metab. (2009) 94:2387–93. doi: 10.1210/jc.2008-2808
23. Connell NA, Paterson WF, Wallace AM, and Donaldson MD. Adrenal function and mortality in children and adolescents with Prader–Willi syndrome attending a single center from 1991–2009. Clin Endocrinol. (2010) 73:686–8. doi: 10.1111/j.1365-2265.2010.03853.x
24. Kusz MJ and Gawlik AM. Adrenal insufficiency in patients with Prader-Willi syndrome. Front Endocrinol (Lausanne). (2022) 13:1021704. doi: 10.3389/fendo.2022.1021704
25. Nagai T, Obata K, Tonoki H, Temma S, Murakami N, Katada Y, et al. Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A. (2005) 136:45–8. doi: 10.1002/ajmg.a.30777
26. Butler MG, Manzardo AM, Heinemann J, Loker C, and Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. (2017) 19:635–42. doi: 10.1038/gim.2016.178
27. Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, and Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. (2003) 88:1455–63. doi: 10.1210/jc.2002-020542
28. Poidvin A, Weill A, Ecosse E, Coste J, and Carel JC. Risk of diabetes treated in early adulthood after growth hormone treatment of short stature in childhood. J Clin Endocrinol Metab. (2017) 102:1291–8. doi: 10.1210/jc.2016-3145
29. Beck-Peccoz P, Höybye C, Murray RD, Simsek S, Zabransky M, Zouater H, et al. No increased risk of glucose metabolism disorders in adults with growth hormone deficiency undergoing long-term treatment with biosimilar somatropin (Omnitrope®): data from an observational, longitudinal study. BMC Endocr Disord. (2019) 19:138. doi: 10.1186/s12902-019-0464-2
30. López-Siguero JP, Martínez-Aedo MJ, Bermúdez de la Vega JA, Bosch-Muñoz J, Lechuga-Sancho AM, and Villalobos T. Growth hormone treatment does not to lead to insulin resistance nor excessive rise in IGF-1 levels, while improving height in patients small for gestational age A long-term observational study. Clin Endocrinol (Oxf). (2022) 96:558–68. doi: 10.1111/cen.14626
31. Cutfield WS, Jackson WE, Jefferies C, Robinson EM, Breier BH, Richards GE, et al. Reduced insulin sensitivity during growth hormone therapy for short children born small for gestational age. J Pediatr. (2003) 142:113–6. doi: 10.1067/mpd.2003.8
32. Svensson J and Bengtsson B-AK. Growth hormone replacement therapy and insulin sensitivity. J Clin Endocrinol Metab. (2003) 88:1453–4. doi: 10.1210/jc.2003-030218
33. Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, et al. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab. (2007) 292:E920–7. doi: 10.1152/ajpendo.00374.2006
34. Van Pareren YK, De Muinck Keizer-Schrama SM, Stijnen T, Sas TC, and Drop SL. Effect of discontinuation of long-term growth hormone treatment on carbohydrate metabolism and risk factors for cardiovascular disease in girls with Turner syndrome. J Clin Endocrinol Metab. (2002) 87:5442–8. doi: 10.1210/jc.2002-020789
35. Cutfield WS, Wilton P, Bennmarker H, Albertsson-Wikland K, Chatelain P, Ranke MB, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. (2000) 355:610–3. doi: 10.1016/S0140-6736(99)04055-6
36. Luger A, Mattsson AF, Koltowska-Häggström M, Thunander M, Góth M, Verhelst J, et al. Incidence of diabetes mellitus and evolution of glucose parameters in growth hormone-deficient subjects during growth hormone replacement therapy: a long-term observational study. Diabetes Care. (2012) 35:57–62. doi: 10.2337/dc11-0449
37. Tidblad A, Bottai M, Kieler H, Albertsson-Wikland K, and Sävendahl L. Association of childhood growth hormone treatment with long-term cardiovascular morbidity. JAMA Pediatr. (2021) 175:e205199. doi: 10.1001/jamapediatrics.2020.5199
38. Lämmer C, Backeljauw P, Tauber M, Kanumakala S, Loche S, Otfried Schwab K, et al. Growth hormone treatment in children with Prader-Willi syndrome: safety and effectiveness data from the PATRO Children study. Ther Adv Endocrinol Metab. (2024) 15:20420188241264343.
39. Grugni G, Sartorio A, Soranna D, Zambon A, Grugni L, Zampino G, et al. Long-term effects of GH therapy in adult patients with Prader-Willi syndrome: a longitudinal study. Front Endocrinol (Lausanne). (2023) 14:1198616. doi: 10.3389/fendo.2023.1198616
Keywords: Prader-Willi syndrome, growth hormone, mortality, diabetes mellitus, type 2, cohort studies
Citation: Choi YJ and Yang A (2025) Long-term impact of growth hormone therapy on mortality and type 2 diabetes in Prader–Willi syndrome: a nationwide cohort study. Front. Endocrinol. 16:1642129. doi: 10.3389/fendo.2025.1642129
Received: 06 June 2025; Accepted: 31 July 2025;
Published: 22 August 2025.
Edited by:
Mohamed Taha Moutaoufik, Mohammed VI Polytechnic University, MoroccoReviewed by:
Stephanie Brown, University of Cambridge, United KingdomAbdallah Mound, University of Hassan II Casablanca, Morocco
Copyright © 2025 Choi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aram Yang, YXJhbS55YW5nQHNhbXN1bmcuY29t
 Yong Jun Choi
Yong Jun Choi Aram Yang
Aram Yang