- 1Nacional’nij medicnij universitet imeni O Bohomol’ca, Kyiv, Ukraine
- 2Uniwersytet Jagiellonski w Krakowie Collegium Medicum, Kraków, Poland
- 3Corewell Health William Beaumont University Hospital, Royal Oak, MI, United States
- 4Willamette University, Portland, OR, United States
Background: Diabetic retinopathy (DR) is a leading cause of vision loss in patients with diabetes mellitus (DM). Hypoxia-driven overexpression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) is central to diabetic retinopathy (DR) pathogenesis. The use of cellular protein kinase inhibitors is a promising approach for correcting pathological changes in DR.
Objective: To determine the effect of pharmacological blockade of cellular protein kinases with sorafenib on the expression of VEGF and HIF-1α in the retina in experimental diabetic retinopathy.
Material and methods: Diabetes mellitus (DM) was induced in male rats by administration of streptozotocin (50 mg/kg). Animals were divided into three groups: in group 1 (n=15) rapid-acting insulin at a dose of 30 U was injected intraperitoneally, in group 2 (n=15) insulin was combined with sorafenib (per os 200 mg), and in the control group (n=15) no treatment of hyperglycemia was performed. 5 animals were used to obtain baseline data (intact). The drugs were administered every other day, starting from day 7 after streptozotocin injection, for 3 months. Immunohistochemical studies were performed using monoclonal mouse antibodies against VEGF. Sections were additionally stained with hematoxylin. The content of VEGF and HIF-1α in retinal tissue lysates was determined by Western blotting. Membranes with proteins were incubated with monoclonal antibodies against VEGF and HIF-1α. After the initial incubation, the membranes were washed and treated with anti-species secondary antibodies conjugated to horseradish peroxidase. Statistica 10 software was used for statistical analysis. Descriptive statistics were calculated, including means and their standard errors. Sample averages were compared using analysis of variance (ANOVA), with p-values less than 0.05 considered statistically significant.
Results: Under the conditions of experimental DR, the content of VEGF in retinal tissues increased significantly and after 3 months of observation increased 6,8-fold for the dimeric form and 27.1-fold for the monomeric form (p<0,05) compared to intact animals. Under the same conditions, the level of HIF-1α was also significantly increased (39.6-fold; p<0.05). When insulin was administered, the content of VEGF fractions in the retina decreased by an average of 1,4-1,5 times (p<0,05), and the heterogeneity of the response to its administration was observed. The use of sorafenib with insulin in all cases blocked the increase in VEGF content caused by DR. Insulin administration reduced HIF-1α levels by 1,4-fold (p<0,05) compared to the control, whereas combined sorafenib and insulin treatment reduced HIF-1α expression to undetectable levels. Immunohistochemical examination revealed a progressive increase in the intensity of VEGF-positive staining in the retina during experimental DR, as well as the development of its degenerative changes - edema, ischemia, pathological angiogenesis, neurodegeneration, and disruption of retinal layer organization. The use of insulin did not cause changes in the retinal pattern, whereas the combined use of sorafenib and insulin prevented the development of both morphological signs of DR and an increase in the intensity of VEGF-positive staining.
Conclusion: The significance of VEGF and HIF-1α upregulation in the pathogenesis of DR and the effectiveness of their correction by pharmacological blockade of cellular protein kinases with sorafenib have been established.
Introduction
Diabetic retinopathy (DR) is one of the leading causes of vision loss in patients with diabetes mellitus and represents a significant challenge for healthcare systems (1–3). The central pathobiological driver of early vascular and neuroretinal damage in DR is hypoxia-induced upregulation of the HIF1α–VEGF axis (hypoxia-inducible factor alpha – vascular endothelial growth factor), which promotes angiogenesis and increases blood-retinal barrier (BRB) permeability, leading to edema, microangiopathy, and progressive neurodegeneration (4–12). Under normoxia, HIF1α is rapidly degraded via PHD/pVHL (prolyl-4-hydroxylase domain/Von Hippel–Lindau protein)-dependent mechanisms; however, under hypoxic conditions it becomes stabilized and transcriptionally upregulates VEGF and other adaptive genes (5–7, 13, 14). Upregulation of VEGFR2 modulates endothelial survival, proliferation, and adhesive contacts (VE-cadherin), directly affecting BRB permeability and edema formation (9–11). Thus, the HIF1α–VEGF axis remains the most validated target for preventing early retinal damage in DR (4–7, 11).
Under physiological conditions, VEGF is predominantly localized in the ganglion cell layer and retinal pigment epithelium; in DR, its expression expands to Müller cells, astrocytes, and ischemic regions, correlating with BRB dysfunction and neurovascular unit remodeling (15–20). These changes underlie the structural and functional visual impairments; thus, targeting hypoxia–angiogenesis signaling nodes is a logical strategy for modifying DR progression (11). Current therapeutic approaches focus mainly on post-receptor VEGF blockade (intravitreal anti-VEGF agents), which effectively reduce edema and neovascularization but have limitations: variability of response, injection burden, and incomplete control of upstream hypoxic signaling (9–12, 21–23). An additional clinical challenge is the effect of insulin therapy: intensive glycemic normalization may transiently exacerbate BRB disruption via HIF1α/VEGF-dependent mechanisms, and insulin use is associated with a risk of macular edema — a context that underscores the need for adjuvant strategies beyond glycemic control (24–26). Conceptually, multikinase inhibition is attractive as it acts higher in the regulatory cascade (interrupting MAPK/ERK signaling and HIF1α stabilization) and at the level of VEGFR/PDGFR, potentially complementing anti-VEGF therapy (9–12, 21–23).
Sorafenib is a multikinase inhibitor that targets RAF (cRAF [cellular RAF serine/threonine-protein kinase], BRAF [B-RAF proto-oncogene serine/threonine-protein kinase]) and angiogenic receptors (VEGFR1/2/3, PDGFRβ), as well as other tyrosine kinases sustaining angiogenesis (27–30). Preclinical studies have shown that sorafenib reduces HIF1α/VEGF levels, microvascular density, and cellular invasiveness; mechanistically, these effects are attributed to blockade of the MAPK/ERK axis, disruption of nuclear translocation/binding of HIF1α to HRE (hypoxia response element), and inhibition of HIF1α translation via suppression of the mTOR/RPS6KB1/4EBP1 pathway (mammalian target of rapamycin/ribosomal protein S6 kinase B1/eukaryotic translation initiation factor 4E-binding protein 1) (28, 31). Concomitant inhibition of VEGFR2/3 and PDGFRβ may attenuate permeability, endothelial proliferation, and pathological neovascularization—events critical for DR progression (9–12, 21–23, 27, 28, 30, 31).
Despite this mechanistic rationale, a knowledge gap remains: whether sorafenib-mediated multikinase blockade can suppress HIF1α/VEGF specifically in the retina under diabetic conditions and preserve its architecture over time compared with standard glycemic correction, and to what extent such a strategy conceptually complements anti-VEGF therapy through both upstream and receptor-level actions (9–12, 21–23, 27, 28, 30, 31). To address this question, we employed a streptozotocin (STZ)-induced diabetes model in Wistar rats and compared standard insulin therapy with a combination of insulin plus sorafenib. Quantitative evaluation of HIF1α/VEGF was performed by Western blotting, while VEGF localization was assessed by immunohistochemistry at predefined time points. This design allowed integration of quantitative biomarkers with early morphological hallmarks of DR and enabled us to test the hypothesis of targeted modulation of the HIF1α–VEGF axis.
Thus, we hypothesized that sorafenib, as a multikinase inhibitor, would reduce HIF1α/VEGF expression in the retina and mitigate early DR-like damage beyond the effect of insulin therapy, thereby potentially complementing anti-VEGF agents by acting upstream at the RAF/ERK cascade and at the level of VEGFR/PDGFR (9–12, 21–23, 27, 28, 30, 31, 86, 87). At the same time, we acknowledge the translational limitations of systemic multikinase therapy in DR and aim for further evaluation of locally targeted or pathway-selective strategies for drug delivery/target inhibition (11). Notably, adverse effects of systemic sorafenib therapy in humans include diarrhea, arterial hypertension, skin reactions, thyroid dysfunction, and proteinuria (32, 33), as well as peripheral neuropathy, the pain of which is unrelieved by standard analgesics and frequently leads to treatment discontinuation (34). This positioning is consistent with contemporary clinical approaches and emphasizes a rational combination of glycemic control with targeted modification of hypoxia–angiogenesis pathways in DR.
The aim of this study was to determine the retinal content of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) in experimental diabetic retinopathy and to assess the effect of pharmacological blockade of cellular protein kinases with sorafenib on these parameters.
Materials and methods
To address the aim of the study, we used a streptozotocin (STZ)-induced diabetes model in Wistar rats and compared standard insulin therapy with a combination of insulin + sorafenib. Quantitative assessment of HIF1α/VEGF was performed using Western blotting, and VEGF localization was evaluated by immunohistochemistry at predefined time points. This design allowed us to integrate quantitative biomarkers with early morphological features of DR and to test the hypothesis of targeted modulation of the HIF1α–VEGF axis.
All experimental procedures were carried out in accordance with the norms and principles of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986), the Council Directive 86/609/EEC (1986), the Law of Ukraine No. 3447-IV “On Protection of Animals from Cruelty,” the general ethical principles of animal experimentation adopted by the First National Congress of Bioethics of Ukraine (2001), and the expert approval of the Commission on Bioethical Expertise and Ethics of Scientific Research at the Bogomolets National Medical University.
The study involved 50 three-month-old male Wistar rats weighing 140–160 g. Experimental diabetes mellitus (DM) was induced in 45 animals by a single intraperitoneal injection of streptozotocin (50 mg/kg; Sigma-Aldrich, Co, China) dissolved in cold 0.1 M citrate buffer (pH 4.5). Five animals were used to obtain baseline (intact) values. Animals were fasted for 16 h before injection and received 5% glucose solution for 24 h afterwards. Blood glucose levels were subsequently monitored every three days using a glucometer and disposable test strips (ACCU-Check Instant, Roche, Mannheim, Germany) from tail vein blood samples collected in the fasting state. Three days after streptozotocin administration, blood glucose levels in treated rats were not lower than 15 mmol/L. Animals were observed for a total of three months.
In this study, we deliberately included only male rats to minimize hormonally driven variability in the results (estrogen-dependent regulation of VEGF/HIF1α, differences in DR pathogenesis between males and females) (35–38), and we plan a separate validation in females taking into account their cycle status. Under these conditions, comparing insulin versus insulin + sorafenib makes it possible to distinguish the contribution of glycemic control from upstream/receptor-level modulation of angiogenic signaling (24–26).
Seven days after induction, animals with persistent hyperglycemia (n = 45) were randomly assigned to three groups of 15 animals each using simple blinded randomization. The researchers performing the allocation (technical staff of the vivarium) were unaware of the experimental interventions and did not know which group would serve as the control or experimental group. Group 1 (control) remained untreated for hyperglycemia. Group 2 received short-acting insulin (Actrapid HM Penfill, Novo Nordisk A/S, Bagsværd, Denmark) intraperitoneally every other day at a dose of 30 U. Group 3 received the same insulin regimen as Group 2 and, in addition, were administered sorafenib (Cipla, India) orally once daily at a dose of 50 mg/kg, prepared ex tempore as a sachet solution. One tablet of the drug (200 mg) was homogenized in a porcelain mortar, dissolved in 20 ml of sterile physiological saline (pH 6.7), and immediately administered to the animal via a gavage needle at a dose of 0.5 ml per 100 g of body weight.
Animals were euthanized at 7 and 28 days, and at 3 months, with five animals from each group sacrificed via lethal thiopental injection (75 mg/kg). For morphological analysis, the eyes were fixed in 10% neutral formalin and embedded in paraffin. Serial histological sections 2–3 μm thick were prepared from the paraffin blocks using a rotary microtome HM 325 (Thermo Shandon, UK). Morphological examinations were performed by two independent teams of expert morphologists, who provided assessments for each specimen. The slides were numbered, but the morphologists were blinded to group allocation. The study was conducted at the Department of Morphology, National University of Health of Ukraine (Kyiv), under the supervision of Prof. Olena Dyadyk.
Immunohistochemical analysis was performed using monoclonal mouse antibodies against VEGF (Invitrogen, VEGF Monoclonal Antibody (VG1), VG1, Catalog #MA1-16629) at 1:200 dilution in 0.1% BSA, Thermo Fisher Scientific, Waltham, Massachusetts, USA) on adhesive Super Frost Plus slides (Menzel, Germany). High-temperature antigen retrieval was carried out using citrate buffer (pH 6.0) or EDTA buffer (pH 8.0), and detection was performed with the Master Polymer Plus Detection system (Master Diagnostica, Spain). Sections were additionally counterstained with hematoxylin. Microscopic examination and image archiving were conducted using light microscopes ZEISS (Germany) equipped with the Axio Imager.A2 imaging system at objective magnifications of 5×, 10×, 20×, and 40×, a binocular 1.5× attachment, and 10× ocular lenses, with ERc 5s cameras, as well as Carl Zeiss Primo Star and Axiocam 105 color cameras, and an Olympus BX 40 light microscope additionally equipped with a digital Olympus C3030-ADU camera and Olympus DP-Soft software.
The evaluation of staining intensity in cells was performed according to the recommendations of D. Dabbs (2021) using a visual analog scale (39). A score of 0 indicated no staining, 1 – weak, 2 – moderate, and 3 – strong staining intensity. The number of VEGF-positive ganglion cells with different staining intensities was counted using an object micrometer, and the results were calculated as the number of cells per mm² and expressed as a percentage.
The determination of VEGF and HIF-1α content in retinal tissue lysates was carried out by Western blotting at the Department of Enzyme Chemistry & Biochemistry, Palladin Institute of Biochemistry, Kyiv, Ukraine, under the supervision of Prof. Artem Tykhomyrov. Tissue samples were preserved in liquid nitrogen, minced, and homogenized in 50 mmol Tris-HCl buffer (pH 7.4) supplemented with phosphatase and protease inhibitors (Pierce Protease and Phosphatase Inhibitor, Thermo Scientific, USA, #A32961), followed by additional disruption using ultrasound. After centrifugation, the total protein concentration in the supernatant was determined spectrophotometrically (40). Electrophoresis was performed in an 8% polyacrylamide gel with sodium dodecyl sulfate (SDS-PAGE) (41) using a vertical gel electrophoresis chamber (Bio-Rad, USA). Sample stacking was performed at 30–35 V (15–18 mA), and separation at 45–50 V (30–35 mA). Proteins were transferred from the gel onto a nitrocellulose membrane by electroblotting using a buffer solution containing 0.025 mol Tris-HCl, 0.192 mol glycine, and 25% methanol (42). The membranes were blocked with 5% non-fat dry milk solution in phosphate-buffered saline (PBS), followed by incubation with monoclonal antibodies against VEGF (Invitrogen, USA, no. MA5-12184, mouse, 1:3,000 dilution) and HIF-1α (Sigma Aldrich, USA, no. HPA001275, rabbit, 1:2,500 dilution). Antibodies against β-actin (loading control, Invitrogen, USA, no. MA5-15739, mouse, 1:3,000 dilution) were used as a control for protein loading. After primary incubation, membranes were washed and treated with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit or anti-mouse IgG, Invitrogen, USA, cat. nos. G-21234 and 31430, respectively, 1:8,000 dilution). Semiquantitative analysis was performed densitometrically using TotalLab software (TL120, Nonlinear Inc., USA). Background subtraction was performed using by TotalLab (TL120, Nonlinear Inc.), and signal intensities were validated to fall within the linear dynamic range of detection. To ensure reproducibility, each blot was quantified in duplicate, and technical replicates were included for key experimental conditions. Inter-blot variability was minimized by using consistent exposure times, identical antibody batches, and internal loading controls across all experiments. Protein molecular weights were determined by comparing their migration on the nitrocellulose membrane with stained markers of the PageRuler™ Plus Prestained Protein Ladder (Thermo Scientific, Lithuania, cat. no. 26619) in the 10–230 kDa range. The results of Western blot analysis of VEGF and HIF-1α content were expressed as percentages relative to the control optical density value of the corresponding polypeptide band (in arbitrary units) on the blots, normalized to actin content in each sample (VEGF/actin and HIF-1α/actin). During the laboratory examination, retinal samples were blinded by assigning random numbering, so that the researchers had no information about the group allocation of the samples.
For statistical analysis, EZR v. 1.68 software (a graphical user interface for R statistical software version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria) was used. In this study, statistical comparisons were performed for the group assessment results of VEGF26/actin, VEGF45/actin, and HIF-1α/actin ratios in rat retinal tissue, as well as for the number of cells with different intensities of VEGF-positive staining in the inner nuclear layer. Data were recorded as median values (Me) and interquartile range (QI–QIII). The Shapiro–Wilk test was applied to assess normality. The Kruskal–Wallis test was used to determine differences among groups; when the test result was significant, post hoc pairwise comparisons were performed using Dunn’s test. Statistical experts conducted group comparisons in a blinded manner and had no information regarding group allocation.
Results
Under the conditions of experimental DR, the content of VEGF in retinal tissues increased significantly and after 3 months of observation compared with the initial level (intact animals) was increased (Figure 1) – for the dimeric form (precursor protein) by 6,8 times, for the monomeric form (active form) by 27,1 times (p<0,05 for both cases).
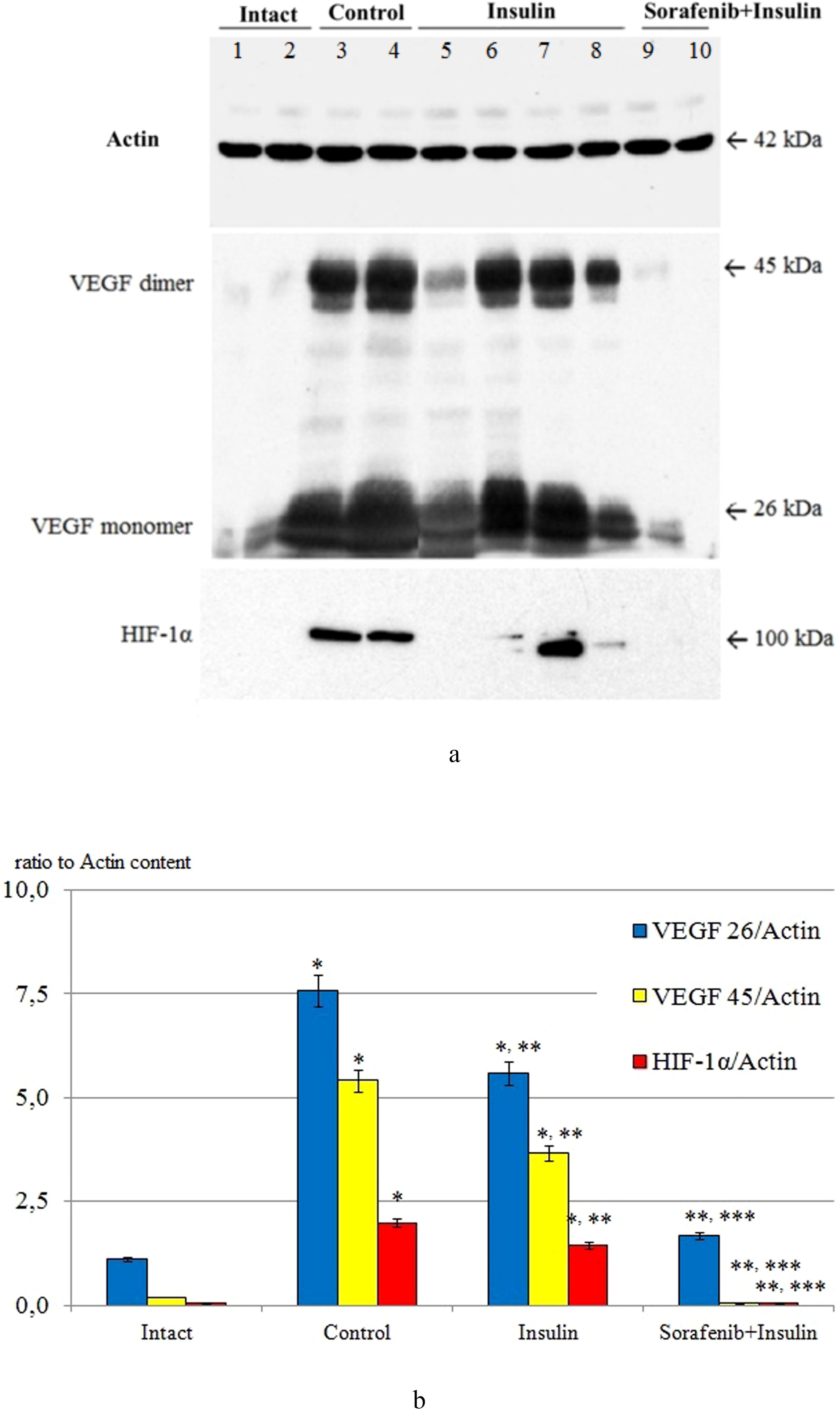
Figure 1. The ratio of VEGF 26/actin, VEGF 45/actin and HIF-1α/actin in the retinal tissue of intact animals (Intact; lanes 1, 2), after 3 months in the control group (Control; lanes 3, 4), the group with insulin administration (Insulin; lanes 5-8) and the group with combined administration of sorafenib with insulin (Sorafenib+Insulin; lanes 9, 10); (A) representative blotograms of actin, VEGF, and HIF-1α; (B) results of densitometric analysis of blotograms (ratio to actin content); *p<0,05 compared with intact; **p<0,05 compared with control; ***p<0,05 compared with insulin.
When insulin was administered, the content of VEGF fractions in the retina on average exceeded the initial data (see Figure 1) by 5.0 and 18.3 times, respectively (p < 0.05 for both cases). The difference compared with the control group was statistically significant (p < 0.05), indicating a certain effect of insulin on reducing diabetic retinal VEGF overexpression (1.4–1.5 times; p < 0.05). It should be noted that in 40% of animals, the VEGF content was significantly reduced (2.7-fold for the dimeric form and 1.7-fold for the monomeric form, respectively; p < 0.05 for both cases), while in the rest of the animals it did not differ from the control group. This fact pointed to the heterogeneity of the response of VEGF expression to insulin and requires separate discussion.
The use of sorafenib with insulin in all cases blocked the increase in VEGF content caused by diabetes (see Figure 1) – the content of this growth factor in the retina did not differ from the baseline data (p>0,05).
The increase in VEGF expression is closely related to the upregulation of the powerful transcription factor HIF-1α under hypoxic conditions (43).
Its content in retinal tissues was also studied by Western blotting (see Figure 1). The initial level of HIF-1α (intact animals) was below the detection level of the method, indicating a low level of expression in normal rat retinal tissues. After 3 months, in the control group, its level was significantly increased (39.6-fold compared with the densitometric optical density indices of samples from intact animals; p < 0.05), which, in our opinion, corresponded to the development of tissue hypoxia (44, 45).
Insulin administration decreased HIF-1α levels by 1.4-fold (p<0,05) compared to control group, whereas the combined administration of sorafenib and insulin was associated with the absence of HIF-1α expression (see Figure 1).
The results of the immunohistochemical study confirmed the upregulation of VEGF expression in retinal tissues during DR modeling (Figures 2-4). A qualitative trend toward increased intensity of VEGF-positive staining in the retina was observed at 7 and 28 days, as well as at 3 months (see Figures 2B, 3A, B).
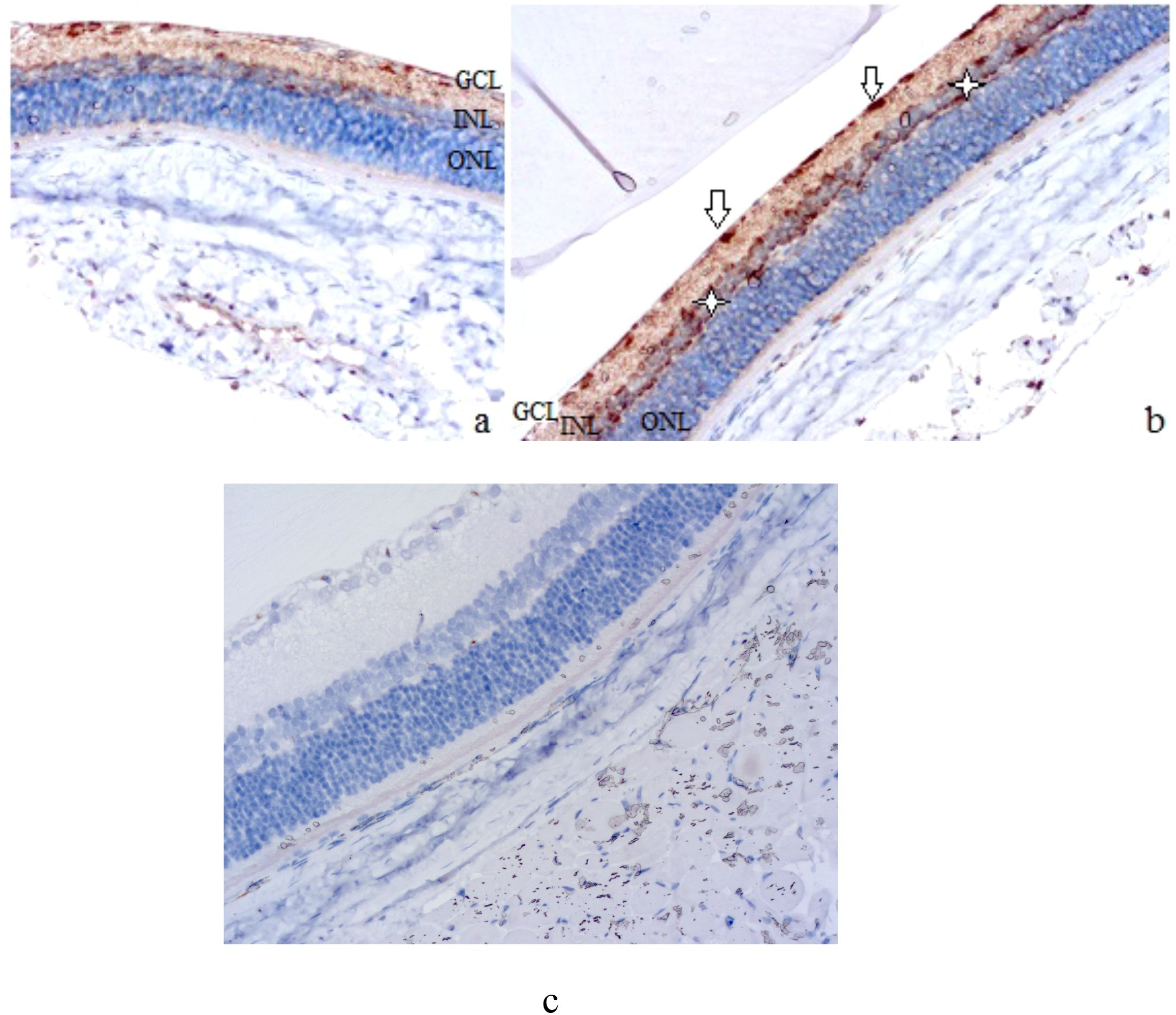
Figure 2. Rat retinal micropreparations (A – intact retina; B – day 7; C – day 7, negative control). Representative results of VEGF immunohistochemical staining, counterstained with hematoxylin; ×200. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. In (A) minimal VEGF-positive staining in the ganglion cell layer; in (B) white arrows indicate VEGF-positive ganglion cells, white asterisks indicate Müller cells at the periphery of the inner nuclear layer; (C) absence of specific staining.
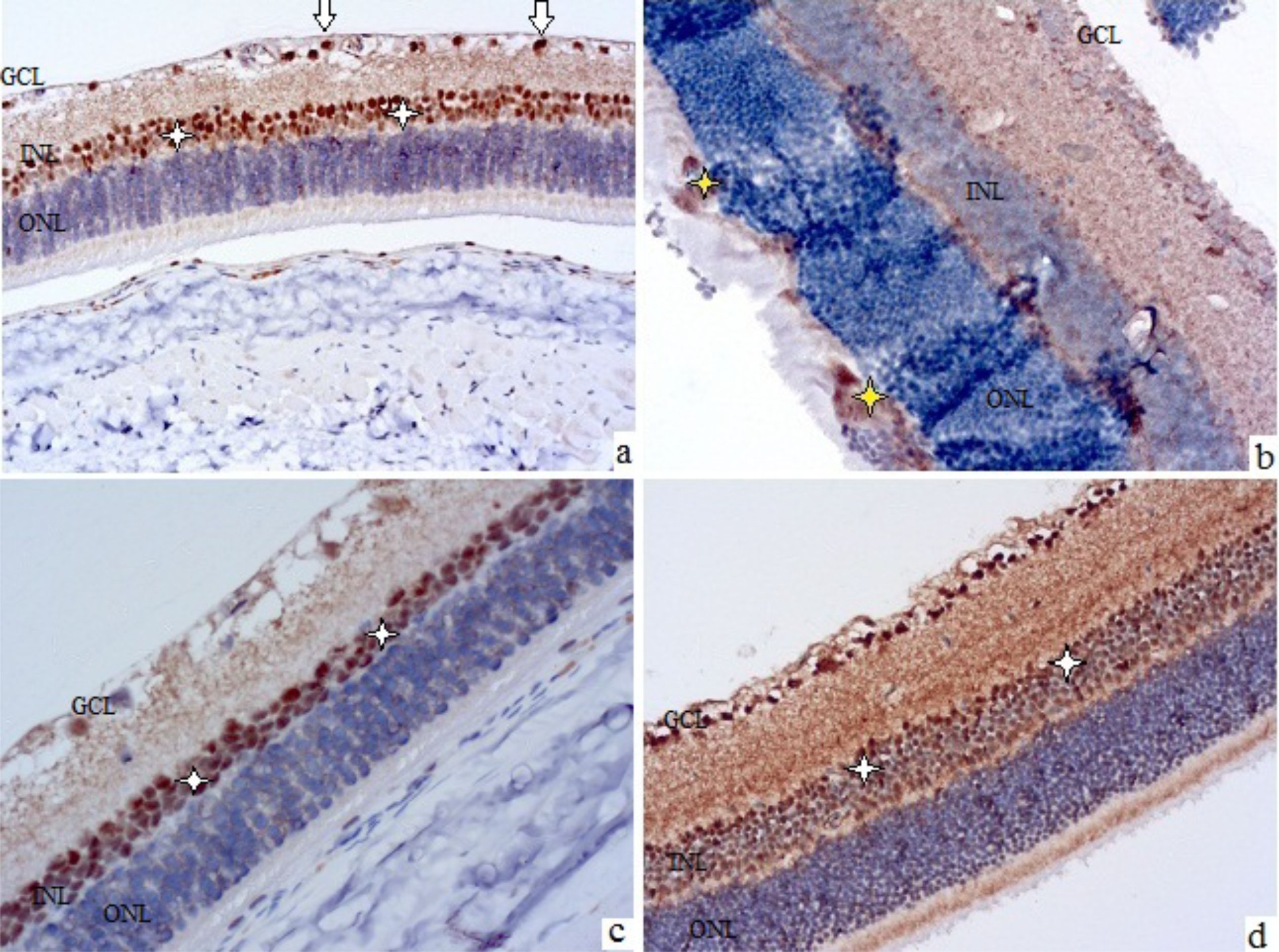
Figure 3. Rat retinal micropreparations at 28 days (A, C) and 3 months (B, D). Representative results of VEGF immunohistochemical staining, counterstained with hematoxylin; ×200. GCL – ganglion cell layer, INL – inner nuclear layer, ONL – outer nuclear layer. (A, B) control; (C, D) treatment with sorafenib and insulin. In (A, C, D) white arrows indicate VEGF-positive cells in the nerve fiber layer, white asterisks indicate Müller cells in the inner nuclear layer; in (B) yellow asterisks indicate cellular proliferates in the outer nuclear layer with VEGF-positive staining elements.
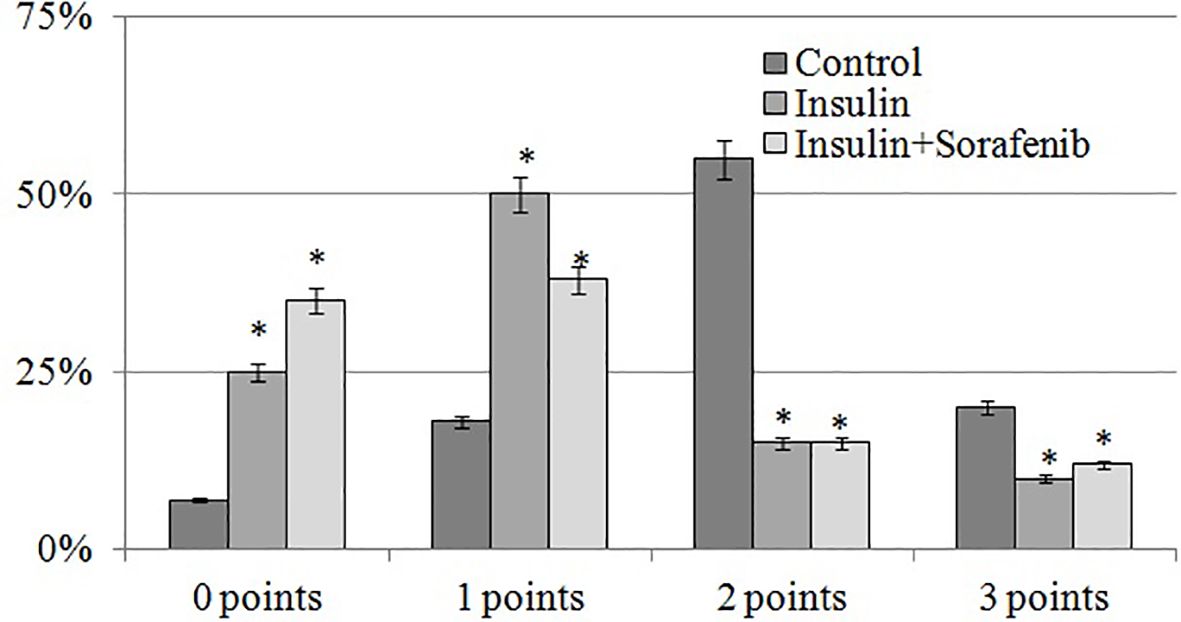
Figure 4. Histogram of the distribution of the number of VEGF-positive cells (%) depending on the intensity of immunospecific staining according to the scale of D.J. Dabbs at the inner nuclear layer in the 3 months; *p<0,05 compared to the control.
Compared with the slight staining in intact animals, which was mainly grouped in the ganglion cell layer (see Figure 2A), VEGF expression was much more intense after 7 days (see Figure 2B). VEGF-positive retinal elements could be clearly distinguished, including rounded cells located along the inner surface of the retina (see white arrows in Figure 2B) and small but numerous cells located along the periphery of the inner nuclear layer, probably Müller cells (see white asterisks in Figure 2B).
These characteristics became more evident after 28 days, when VEGF-positive astrocytes of the nerve fiber layer (see white arrows in Figure 3A) and Müller cells (see white asterisks in Figure 3A) were clearly visualized. Also, at this time, swelling and stratification of the inner layers of the retina, and the formation of foci of angiogenesis along the nerve fiber layer could be noted.
After 3 months of observation, the retinas of the control group animals (see Figure 3B) showed marked edema, areas of ischemia in the plexiform layers, signs of ganglion cell degeneration, and discomplexity of the layers, especially the outer nuclear layer, which contained disordered cell proliferations, some of which were VEGF-positive (see yellow asterisks in Figure 3B).
The use of insulin was accompanied by a retinal picture similar to that of the control group, while the combined use of sorafenib and insulin prevented the development of both morphological signs of diabetic retinopathy (DR) and an increase in the intensity of VEGF-positive staining (see Figures 3C, D). There were no signs of edema, ischemia, pathological angiogenesis, neurodegeneration, or layer discomplexity in the retinas of sorafenib+insulin-treated animals. Moderate VEGF-positive staining was preserved in the inner nuclear layer (white asterisks in Figures 3C, D) and the ganglion cell layer.
The intensity of VEGF-specific staining was most pronounced in the inner nuclear layer, and its semi-quantitative assessment using the D.J. Dabbs scale demonstrated a significant predominance of cells with strong staining (2–3 points, Figure 4) in the control group. Administration of insulin and insulin combined with sorafenib led to a marked increase in the proportion of unstained and weakly stained cells (0–1 point), accompanied by a decrease in the number of intensely stained cells (2–3 points). These results, in turn, confirmed the inhibitory effect of insulin and sorafenib on VEGF overexpression.
Thus, the levels of VEGF and HIF-1α in the retina were significantly elevated in experimental DR, which was attenuated by insulin administration and further prevented by the additional use of the protein kinase inhibitor sorafenib. Morphological analysis revealed the development of early DR signs associated with increased intensity of VEGF-positive staining in the retina, which was mitigated by sorafenib treatment.
Discussion
Key findings in the context of DR pathobiology
In the STZ-diabetes model, combined multikinase inhibition alongside insulin administration was associated with suppression of HIF1α and VEGF in the retina and preservation of retinal architecture compared with diabetic controls (Figures 1-3). These observations are consistent with the central role of the HIF1α–VEGF hypoxia–angiogenesis axis in DR and BRB dysfunction (4–6, 9–12) and align with known patterns of VEGF immunoreactivity redistribution from the ganglion cell layer/RPE under normal conditions to Müller cells, astrocytes, and ischemic regions in DR (15–20). We further focus on mechanisms, clinical relevance, and limitations.
Pathophysiological mechanisms
Under normoxic conditions, HIF1α is hydroxylated by PHD1–3 and FIH1 (factor inhibiting HIF-1) and degraded via pVHL-dependent proteolysis; under hypoxia, it is stabilized, translocates to the nucleus, dimerizes with HIF1β, and activates transcription of VEGF and other adaptive genes (5–7, 13, 14). VEGFR2 is a key downstream mediator: phosphorylation of tyrosine Y949 (Y951 in humans) forms a TSAd (T-cell-specific adaptor)–cSrc complex, which subsequently phosphorylates VE-cadherin, disrupting inter-endothelial adhesive contacts. Phosphorylation of tight junction proteins (claudins, occludin, ZO-1) further increases BRB permeability (9–11, 21–23). Pericytes stabilize microvessels and the BRB, and their dysfunction exacerbates microangiopathy (12, 46–49). At the neurogliovascular unit level, NO (nitric oxide)/glutamate-mediated pathways (NOGC/cGMP/PKG; NO-sensitive guanylate cyclases/cyclic GMP/protein kinase G) and impaired glutamate transport contribute to neurodistress and may act synergistically with VEGF-mediated barrier dysfunction (43, 50–54). This background directly supports the interpretation of HIF1α/VEGF suppression observed following kinase blockade in our model.
Hyperglycemia- and hypoxia-induced upregulation of HIF1α/VEGF and their modulation by insulin
Chronic hyperglycemia creates a pro-angiogenic microenvironment through inflammation (IL1β/TNFα/IL18/IL17A – interleukin-1β/tumor necrosis factor-α/interleukin-18/interleukin-17A, NFκB – nuclear factor kappa B-dependent mechanisms), oxidative stress (polyol pathway, hexosamine pathway, PKCβ – protein kinase Cβ activation), the AGEs-RAGE axis (advanced glycation end-products and their receptors), as well as GPR91 (G protein-coupled receptor 91)–MAPK/COX2 (cyclooxygenase-2)/PGE2 (prostaglandin E2) (44, 45, 55–63). Early microvascular events (leukostasis, vasoconstriction) exacerbate hypoperfusion and stabilize HIF1α, increasing VEGF and NOS levels; in ocular tissues, HIF1α rises earlier than VEGF and correlates with DR progression (44, 45, 64, 65). Additionally, PKC/MAPK, PI3K/Akt/mTOR (phosphoinositide 3-kinase/protein kinase B/mTOR), and NFκB enhance HIF1α transcription/translation, further amplifying VEGF signaling (66–70).
On this background, the effect of insulin is ambiguous: in clinical settings, early worsening of DR and increased risk of macular edema may occur with rapid glycemic improvement (24–26), while in retinal endothelium, insulin and high glucose exert non-additive and dose-dependent opposing effects on VEGF/ROS (reactive oxygen species) (71, 72). The involvement of NOX4 (an NADPH oxidase isoform) in the HIF1α/VEGF response, as well as variability in genetic determinants of VEGF/VEGFR, may explain individual differences (73, 74). In our experiment, this is reflected in the heterogeneity of VEGF response to insulin and explains why monotherapy targeting metabolic control only partially affects HIF1α/VEGF.
Mechanisms and sites of action of sorafenib (multi-kinase blockade)
Sorafenib acts on two levels. Upstream: inhibition of cRAF/BRAF disrupts the MAPK/ERK node, which supports HIF1α-dependent VEGF expression and expansion of the angiogenic transcriptome (27, 30, 75). Receptor level:blockade of VEGFR1/2/3 and PDGFRβ suppresses post-receptor events (permeability, endothelial proliferation, neo-(lymph)angiogenesis, and pericyte recruitment) (9–12, 21–23, 27–30, 75–77). Additionally, suppression of HIF1α translation via mTOR/RPS6KB1/4EBP1 without altering mRNA levels, as well as interference with nuclear translocation/binding of HIF1α to HRE sites, has been described (28, 29, 31). Collectively, these mechanisms logically explain the attenuation of the hypoxia-driven angiogenic response in our model. We do not ascribe a direct antioxidant effect to the drug; the potential reduction in ROS/HIF1α stabilization is considered secondary to inhibition of RAF/MAPK/VEGFR (78). At the microvascular level, PDGFRβ-mediated pericyte recruitment and stabilization of newly formed vessels may also be suppressed (46, 48), whereas VEGFR3 is involved in pathological neo-(lymph)angiogenesis (77). This “multi-node” effect is consistent with normalization of VEGF/HIF1α localization and preservation of retinal layer organization in the insulin+sorafenib group.
Analytical and methodological aspects relevant for interpretation
The study design included time points at 7, 28 days, and 3 months, allowing the tracking of transitions from early molecular events to morphological features of diabetic retinopathy (DR); this approach is consistent with data showing a preceding increase in HIF1α levels compared to VEGF during retinal ischemia (64). Quantitative assessment of VEGF was performed by Western blot under reducing and non-reducing conditions to distinguish the monomer (~26 kDa) from the disulfide-linked dimer (~45 kDa), while immunohistochemistry confirmed the topographic expansion of VEGF-positive cells (ganglion cell layer, Müller cells, astrocytes, ischemic zones) in control DR, with a partial reversal following sorafenib treatment (15–20). The combination of these approaches minimizes the risk of misinterpretation from a single method and strengthens causal linkage to the HIF1α–VEGF axis.
Clinical relevance and safety
Intravitreal anti-VEGF therapy is the standard for macular edema and neovascularization; however, response variability, injection burden, and the persistence of upstream drivers remain challenges (9–12, 21–23). Our data support the concept of complementarity: multikinase blockade can reduce HIF1α-dependent pathways and VEGFR/PDGFR signaling, theoretically complementing anti-VEGF therapy in scenarios of residual hypoxia or high injection frequency (9–12, 21–23, 27–31, 70, 75, 76, 78, 79). At the same time, we do not propose systemic sorafenib as a ready treatment for DR: safety limitations for the multikinase inhibitor class upon systemic administration are well documented (dermatologic, cardiovascular, neurological, and other adverse effects; class-specific effects in oncology) (32–34). Therefore, translationally more feasible approaches appear to be local delivery modes (intraocular microdoses/depot formulations) or more selective agents targeting RAF/MAPK or VEGFR/PDGFR with minimal systemic impact (11, 27, 30, 76). Additionally, in the eye, prolonged PDGFRβ blockade should be avoided to prevent disruption of pericyte-mediated stabilization of newly formed vessels (46, 48).
Autophagy
The role of autophagy in microvascular remodeling in diabetes is actively discussed, particularly regarding interactions with HIF1α, mTOR, and the Bnip3/FoxO3a axis (80–84). We did not experimentally assess these pathways, and therefore refrain from causal claims, presenting them only as a potential modulatory context warranting further investigation.
Limitations and future directions
Animal sex. Studies were conducted on males to minimize hormonal variability and estrogen-dependent modulation of VEGF/HIF1α and BRB permeability; extrapolation to females requires validation with estrous stratification and hormonal profiling (11, 35–38).
Observation period. The study covered early-to-intermediate time points, so late-stage DR and long-term intervention outcomes were not assessed.
Functional endpoints. ERG and behavioral vision assessments were not included; bridging molecular/morphological effects to visual function requires further validation.
Potential off-target effects. Multikinase inhibition may have non-retinal consequences; given the class profile, systemic sorafenib for DR is limited, whereas local or more selective approaches appear more promising (11, 27–30, 75).
Future directions naturally arise from these limitations: inclusion of sex as a variable—replication in females with estrous stratification and hormonal profiling; extended observation periods to assess effect durability and interactions with late-stage DR; functional validation (ERG, optokinetic reflex, behavioral assays) to link HIF1α/VEGF dynamics and morphology to visual function; and delivery/selectivity platforms—testing local regimens (intravitreal, microdosing) and/or more selective inhibitors of upstream/receptor nodes as potentially safer alternatives to systemic multikinase blockade (9–12, 21–23, 27–30, 75–77, 85). This mechanistically and clinically oriented approach aligns with contemporary DR management strategies and may reduce injection burden and instances of anti-VEGF monotherapy resistance.
Concluding remarks and translational outlook
Our data support the notion that the HIF1α–VEGF axis is central to early hypoxia-driven angiogenic changes in DR. Insulin partially attenuates this activity but does not fully normalize it, whereas combining insulin with multikinase blockade prevents VEGF upregulation and topographical shifts, preserving retinal architecture over the studied period. Mechanistically, these effects are consistent with upstream (RAF/MAPK) and receptor-level (VEGFR/PDGFR) actions of sorafenib, reinforcing the rationale for complementarity with anti-VEGF strategies. Translationally, local or more selective targeting approaches appear promising, provided careful safety evaluation. Our study was male-only, short-term, and lacked functional visual metrics (ERG), highlighting the need for studies in females, extended observation periods, and functional validation before clinical translation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Bogomolets National Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KU: Writing – original draft, Software, Formal Analysis, Resources, Validation. OS: Software, Investigation, Formal Analysis, Writing – review & editing. SR: Methodology, Conceptualization, Formal Analysis, Writing – original draft. MB: Data curation, Validation, Investigation, Writing – original draft. KS: Validation, Writing – review & editing, Methodology, Data curation. OK: Writing – review & editing, Resources, Software, Visualization. SZ: Writing – original draft, Resources, Funding acquisition, Supervision. MK: Formal Analysis, Validation, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
2. GBD 2019 Blindness and Vision Impairment Collaborators and Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. (2021) 9:e144–60. doi: 10.1016/S2214-109X(20)30489-7
3. Vision Loss Expert Group of the Global Burden of Disease Study. GBD 2019 Blindness and Vision Impairment Collaborators. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 2000 to 2020. Eye (Lond). (2024) 38:2047–57. doi: 10.1038/s41433-024-03101-5
4. Gnanasambandam B, Prince J, Limaye S, Moran E, Lee B, Huynh J, et al. Addressing retinal hypoxia: pathophysiology, therapeutic innovations, and future prospects. Ther Adv Ophthalmol. (2024) 16:25158414241280187. doi: 10.1177/25158414241280187
5. Koyasu S, Kobayashi M, Goto Y, Hiraoka M, and Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. (2018) 109:560–71. doi: 10.1111/cas.13483
6. Lee D, Tomita Y, Miwa Y, Kunimi H, Nakai A, Shoda C, et al. Recent insights into roles of hypoxia-inducible factors in retinal diseases. Int J Mol Sci. (2024) 25:10140. doi: 10.3390/ijms251810140
7. Jucht AE and Scholz CC. PHD1–3 oxygen sensors in vivo-lessons learned from gene deletions. Pflugers Arch. (2024) 476:1307–37. doi: 10.1007/s00424-024-02944-x
8. Zhao Z, Nelson AR, Betsholtz C, and Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. (2015) 163:1064–78. doi: 10.1016/j.cell.2015.10.067
9. Simons M, Gordon E, and Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. (2016) 17:611–25. doi: 10.1038/nrm.2016.87
10. Smith RO, Ninchoji T, Gordon E, André H, Dejana E, Vestweber D, et al. Vascular permeability in retinopathy is regulated by VEGFR2 Y949 signaling to VE-cadherin. Elife. (2020) 9:e54056. doi: 10.7554/eLife.54056
11. Wang W and Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. (2018) 19:1816. doi: 10.3390/ijms19061816
12. Mrugacz M, Bryl A, and Zorena K. Retinal vascular endothelial cell dysfunction and neuroretinal degeneration in diabetic patients. J Clin Med. (2021) 10:458. doi: 10.3390/jcm10030458
13. Kiriakidis S, Henze AT, Kruszynska-Ziaja I, Skobridis K, Theodorou V, Paleolog EM, et al. Factor-inhibiting HIF-1 (FIH-1) is required for human vascular endothelial cell survival. FASEB J. (2015) 29:2814–27. doi: 10.1096/fj.14-252379
14. Mimura I, Nangaku M, Kanki Y, Tsutsumi S, Inoue T, Kohro T, et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. (2012) 32:3018–32. doi: 10.1128/MCB.06643-11
15. Kim I, Ryan AM, Rohan R, Amano S, Agular S, Miller JW, et al. Constitutive expression of VEGF, VEGFR-1, and VEGFR-2 in normal eyes. Invest Ophthalmol Vis Sci. (1999) 40:2115–21.
16. Lutty GA, McLeod DS, Merges C, Diggs A, and Plouét J. Localization of vascular endothelial growth factor in human retina and choroid. Arch Ophthalmol. (1996) 114:971–7. doi: 10.1001/archopht.1996.01100140179011
17. Rossino MG, Lulli M, Amato R, Cammalleri M, Monte MD, and Casini G. Oxidative stress induces a VEGF autocrine loop in the retina: relevance for diabetic retinopathy. Cells. (2020) 9:1452. doi: 10.3390/cells9061452
18. Kim YS, Sohn EJ, Kim CS, Lee YM, Jung DH, Kim NH, et al. Vascular endothelial growth factor (VEGF) and advanced glycation end products (AGEs) Overexpression in the retina and serum and lens opacities of streptozotocin-induced diabetic rats. Korean Diabetes J Korean Diabetes Association;. (2008) 32:44. doi: 10.4093/kdj.2008.32.1.44
19. Gerhardinger C, Brown LF, Roy S, Mizutani M, Zucker CL, and Lorenzi M. Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am J Pathol. (1998) 152:1453–62.
20. Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, and Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. (1996) 74:819–25.
21. Li X, Padhan N, Sjöström EO, Roche FP, Testini C, Honkura N, et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat Commun. (2016) 7:11017. doi: 10.1038/ncomms11017
22. Lampugnani MG, Dejana E, and Giampietro C. Vascular endothelial (VE)-cadherin, endothelial adherens junctions, and vascular disease. Cold Spring Harb Perspect Biol. (2018) 10:a029322. doi: 10.1101/cshperspect.a029322
23. Overgaard CE, Daugherty BL, Mitchell LA, and Koval M. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid Redox Signal. (2011) 15:1179–93. doi: 10.1089/ars.2011.3893
24. Zhang J, Ma J, Zhou N, Zhang B, and An J. Insulin use and risk of diabetic macular edema in diabetes mellitus: a systemic review and meta-analysis of observational studies. Med Sci Monit. (2015) 21:929–36. doi: 10.12659/MSM.892056
25. Akil H, Burgess J, Nevitt S, Harding SP, Alam U, and Burgess P. Early worsening of retinopathy in type 1 and type 2 diabetes after rapid improvement in glycaemic control: A systematic review. Diabetes Ther. (2022) 13:1–23. doi: 10.1007/s13300-021-01190-z
26. Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. (2002) 109:805–15. doi: 10.1172/JCI13776
27. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, and Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. (2008) 7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013
28. Xu M, Zheng YL, Xie XY, Liang JY, Pan FS, Zheng SG, et al. Sorafenib blocks the HIF-1α/VEGFA pathway, inhibits tumor invasion, and induces apoptosis in hepatoma cells. DNA Cell Biol. (2014) 33:275–81. doi: 10.1089/dna.2013.2184
29. Li N, Chen B, Lin R, Liu N, Dai HT, Tang KY, et al. The earlier, the better: the effects of different administration timepoints of sorafenib in suppressing the carcinogenesis of VEGF in rats. Cancer Chemother Pharmacol. (2018) 81:207–16. doi: 10.1007/s00280-017-3493-4
30. Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. (2006) 66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377
31. Liu LP, Ho RL, Chen GG, and Lai PB. Sorafenib inhibits hypoxia-inducible factor-1α synthesis: implications for antiangiogenic activity in hepatocellular carcinoma. Clin Cancer Res. (2012) 18:5662–71. doi: 10.1158/1078-0432.CCR-12-0552
32. Walko CM and Grande C. Management of common adverse events in patients treated with sorafenib: nurse and pharmacist perspective. Semin Oncol. (2014) 41 Suppl 2:S17–28. doi: 10.1053/j.seminoncol.2014.01.002
33. Abdel-Rahman O and Lamarca A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic-review and meta-analysis of the impact on survival. Expert Rev Gastroenterol Hepatol. (2017) 11:75–83. doi: 10.1080/17474124.2017.1264874
34. Di Cesare Mannelli L, Maresca M, Farina C, Scherz MW, and Ghelardini C. A model of neuropathic pain induced by sorafenib in the rat: Effect of dimiracetam. Neurotoxicology. (2015) 50:101–7. doi: 10.1016/j.neuro.2015.08.002
35. Aberdeen GW, Babischkin JS, Pepe GJ, and Albrecht ED. Estrogen stimulates fetal vascular endothelial growth factor expression and microvascularization. J Endocrinol. (2024) 262:e230364. doi: 10.1530/JOE-23-0364
36. Zhang J, Song H, Lu Y, Chen H, Jiang S, and Li L. Effects of estradiol on VEGF and bFGF by Akt in endometrial cancer cells are mediated through the NF-κB pathway. Oncol Rep. (2016) 36:705–14. doi: 10.3892/or.2016.4888
37. Chen Y, Schlotterer A, Lin J, Dietrich N, Fleming T, Lanzinger S, et al. Sex differences in the development of experimental diabetic retinopathy. Sci Rep. (2024) 14:22812. doi: 10.1038/s41598-024-73279-x
38. Ozawa GY, Bearse MA Jr, and Adams AJ. Male-female differences in diabetic retinopathy? Curr Eye Res. (2015) 40:234–46. doi: 10.3109/02713683.2014.958500
39. Dabbs DJ ed. Diagnostic immunohistochemistry : theranostic and genomic applications. Sixth edition. Philadelphia, PA: Elsevier (2021).
40. Stoscheck CM. Quantitation of protein. Methods Enzymol. (1990) 182:50–68. doi: 10.1016/0076-6879(90)82008-p
41. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. (1970) 227:680–5. doi: 10.1038/227680a0
42. Towbin H, Staehelin T, and Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. (1979) 76:4350–4. doi: 10.1073/pnas.76.9.4350
43. Passlick S, Rose CR, Petzold GC, and Henneberger C. Disruption of glutamate transport and homeostasis by acute metabolic stress. Front Cell Neurosci. (2021) 15:637784. doi: 10.3389/fncel.2021.637784
44. Arden GB and Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. (2011) 7:291–304. doi: 10.2174/157339911797415620
45. Fahmideh F, Marchesi N, Campagnoli LIM, Landini L, Caramella C, Barbieri A, et al. Effect of troxerutin in counteracting hyperglycemia-induced VEGF upregulation in endothelial cells: a new option to target early stages of diabetic retinopathy? Front Pharmacol. (2022) 13:951833. doi: 10.3389/fphar.2022.951833
46. Rey S and Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. (2010) 86:236–42. doi: 10.1093/cvr/cvq045
47. Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, and Heymach JV. Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res. (2023) 29:30–9. doi: 10.1158/1078-0432.CCR-22-1366
48. Santos GSP, Prazeres PHDM, Mintz A, and Birbrair A. Role of pericytes in the retina. Eye (Lond). (2018) 32:483–6. doi: 10.1038/eye.2017.220
49. Vernazza S, Oddone F, Tirendi S, and Bassi AM. Risk factors for retinal ganglion cell distress in glaucoma and neuroprotective potential intervention. Int J Mol Sci. (2021) 22:7994. doi: 10.3390/ijms22157994
50. Neitz A, Mergia E, Eysel UT, Koesling D, and Mittmann T. Presynaptic nitric oxide/cGMP facilitates glutamate release via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Eur J Neurosci. (2011) 33:1611–21. doi: 10.1111/j.1460-9568.2011.07654.x
51. Vielma AH, Agurto A, Valdés J, Palacios AG, and Schmachtenberg O. Nitric oxide modulates the temporal properties of the glutamate response in type 4 OFF bipolar cells. PloS One. (2014) 9:e114330. doi: 10.1371/journal.pone.0114330
52. Tewari D, Sah AN, Bawari S, Nabavi SF, Dehpour AR, Shirooie S, et al. Role of nitric oxide in neurodegeneration: function, regulation, and inhibition. Curr Neuropharmacol. (2021) 19:114–26. doi: 10.2174/1570159X18666200429001549
53. Boccuni I and Fairless R. Retinal glutamate neurotransmission: from physiology to pathophysiological mechanisms of retinal ganglion cell degeneration. Life (Basel). (2022) 12:638. doi: 10.3390/life12050638
54. Kou ZW, Mo JL, Wu KW, Qiu MH, Huang YL, Tao F, et al. Vascular endothelial growth factor increases the function of calcium-impermeable AMPA receptor GluA2 subunit in astrocytes via activation of protein kinase C signaling pathway. Glia. (2019) 67:1344–58. doi: 10.1002/glia.23609
55. Seo H, Park SJ, and Song M. Diabetic retinopathy (DR): mechanisms, current therapies, and emerging strategies. Cells. (2025) 14:376. doi: 10.3390/cells14050376
56. Yue T, Shi Y, Luo S, Weng J, Wu Y, and Zheng X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front Immunol. (2022) 13:1055087. doi: 10.3389/fimmu.2022.1055087
57. Yun JH. Interleukin-1β induces pericyte apoptosis via the NF-κB pathway in diabetic retinopathy. Biochem Biophys Res Commun. (2021) 546:46–53. doi: 10.1016/j.bbrc.2021.01.108
58. Thorne CA, Grey AC, Lim JC, and Donaldson PJ. The synergistic effects of polyol pathway-induced oxidative and osmotic stress in the aetiology of diabetic cataracts. Int J Mol Sci. (2024) 25:9042. doi: 10.3390/ijms25169042
59. Paneque A, Fortus H, Zheng J, Werlen G, and Jacinto E. The hexosamine biosynthesis pathway: regulation and function. Genes (Basel). (2023) 14:933. doi: 10.3390/genes14040933
60. Donovan K, Alekseev O, Qi X, Cho W, and Azizkhan-Clifford J. O-GlcNAc modification of transcription factor Sp1 mediates hyperglycemia-induced VEGF-A upregulation in retinal cells. Invest Ophthalmol Vis Sci. (2014) 55:7862–73. doi: 10.1167/iovs.14-14048
61. Lim PS, Sutton CR, and Rao S. Protein kinase C in the immune system: from signalling to chromatin regulation. Immunology. (2015) 146:508–22. doi: 10.1111/imm.12510
62. Hu J, Li T, Du S, Chen Y, Wang S, Xiong F, et al. The MAPK signaling pathway mediates the GPR91-dependent release of VEGF from RGC-5 cells. Int J Mol Med. (2015) 36:130–8. doi: 10.3892/ijmm.2015.2195
63. Zong H, Ward M, and Stitt AW. AGEs, RAGE, and diabetic retinopathy. Curr Diabetes Rep. (2011) 11:244–52. doi: 10.1007/s11892-011-0198-7
64. Håkansson G, Gesslein B, Gustafsson L, Englund-Johansson U, and Malmsjö M. Hypoxia-inducible factor and vascular endothelial growth factor in the neuroretina and retinal blood vessels after retinal ischemia. J Ocul Biol Dis Infor. (2010) 3:20–9. doi: 10.1007/s12177-010-9050-6
65. Loukovaara S, Koivunen P, Inglés M, Escobar J, Vento M, and Andersson S. Elevated protein carbonyl and HIF-1α levels in eyes with proliferative diabetic retinopathy. Acta Ophthalmol. (2014) 92:323–7. doi: 10.1111/aos.12186
66. Xiao Q, Zeng S, Ling S, and Lv M. Up-regulation of HIF-1alpha and VEGF expression by elevated glucose concentration and hypoxia in cultured human retinal pigment epithelial cells. J Huazhong Univ Sci Technolog Med Sci. (2006) 26:463–5. doi: 10.1007/s11596-006-0422-x
67. Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, et al. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. (2007) 18:4691–7. doi: 10.1091/mbc.e07-04-0391
68. Xia J, Ozaki I, Matsuhashi S, Kuwashiro T, Takahashi H, Anzai K, et al. Mechanisms of PKC-mediated enhancement of HIF-1α Activity and its inhibition by vitamin K2 in hepatocellular carcinoma cells. Int J Mol Sci. (2019) 20:1022. doi: 10.3390/ijms20051022
69. Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, et al. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid Med Cell Longev. (2017) 2017:9702820. doi: 10.1155/2017/9702820
70. Huang W, Ding X, Ye H, Wang J, Shao J, and Huang T. Hypoxia enhances the migration and invasion of human glioblastoma U87 cells through PI3K/Akt/mTOR/HIF-1α pathway. Neuroreport. (2018) 29:1578–85. doi: 10.1097/WNR.0000000000001156
71. Wu H, Xia X, Jiang C, Wu J, Zhang S, Zheng Z, et al. High glucose attenuates insulin-induced VEGF expression in bovine retinal microvascular endothelial cells. Eye (Lond). (2010) 24:145–51. doi: 10.1038/eye.2009.157
72. Wu H, Jiang C, Gan D, Liao Y, Ren H, Sun Z, et al. Different effects of low- and high-dose insulin on ROS production and VEGF expression in bovine retinal microvascular endothelial cells in the presence of high glucose. Graefes Arch Clin Exp Ophthalmol. (2011) 249:1303–10. doi: 10.1007/s00417-011-1677-x
73. Meng D, Mei A, Liu J, Kang X, Shi X, Qian R, et al. NADPH oxidase 4 mediates insulin-stimulated HIF-1α and VEGF expression, and angiogenesis in vitro. PloS One. (2012) 7:e48393. doi: 10.1371/journal.pone.0048393
74. Sajovic J, Cilenšek I, Mankoč S, Tajnšek Š, Kunej T, Petrovič D, et al. Vascular endothelial growth factor (VEGF)-related polymorphisms rs10738760 and rs6921438 are not risk factors for proliferative diabetic retinopathy (PDR) in patients with type 2 diabetes mellitus (T2DM). Bosn J Basic Med Sci. (2019) 19:94–100. doi: 10.17305/bjbms.2018.3519
75. Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, and Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. (2020) 19:1997–2007. doi: 10.3892/etm.2020.8454
76. Elkaeed EB, Yousef RG, Khalifa MM, Ibrahim A, Mehany ABM, Gobaara IMM, et al. Discovery of new VEGFR-2 inhibitors: design, synthesis, anti-proliferative evaluation, docking, and MD simulation studies. Molecules. (2022) 27:6203. doi: 10.3390/molecules27196203
77. Donnan MD, Deb DK, Onay T, Scott RP, Ni E, Zhou Y, et al. Formation of the glomerular microvasculature is regulated by VEGFR-3. Am J Physiol Renal Physiol. (2023) 324:F91–F105. doi: 10.1152/ajprenal.00066.2022
78. Wang D, Wang L, Gu J, Yang H, Liu N, Lin Y, et al. Scutellarin inhibits high glucose-induced and hypoxia-mimetic agent-induced angiogenic effects in human retinal endothelial cells through reactive oxygen species/hypoxia-inducible factor-1α/vascular endothelial growth factor pathway. J Cardiovasc Pharmacol. (2014) 64:218–27. doi: 10.1097/FJC.0000000000000109
79. Hu J, Hameed MR, Agaram NP, Whiting KA, Qin LX, Villano AM, et al. PDGFRβ Signaling cooperates with β-catenin to modulate c-Abl and biologic behavior of desmoid-type fibromatosis. Clin Cancer Res. (2024) 30:450–61. doi: 10.1158/1078-0432.CCR-23-2313
80. Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. (2018) 75:669–88. doi: 10.1007/s00018-017-2639-1
81. Tanaka Y, Kume S, Kitada M, Kanasaki K, Uzu T, Maegawa H, et al. Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res. (2012) 2012:628978. doi: 10.1155/2012/628978
82. Saxena S, Anand SK, Sharma A, and Kakkar P. Involvement of Sirt1-FoxO3a-Bnip3 axis and autophagy mediated mitochondrial turnover in according protection to hyperglycemic NRK-52E cells by Berberine. Toxicol. In Vitro. (2024) 100:105916. doi: 10.1016/j.tiv.2024.105916
83. Zoncu R, Efeyan A, and Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. (2011) 12:21–35. doi: 10.1038/nrm3025
84. Rosa MD, Distefano G, Gagliano C, Rusciano D, and Malaguarnera L. Autophagy in diabetic retinopathy. Curr Neuropharmacol. (2016) 14:810–25. doi: 10.2174/1570159x14666160321122900
85. Yokota S, Yonezawa T, Momoi Y, and Maeda S. Sorafenib inhibits tumor cell growth and angiogenesis in canine transitional cell carcinoma. J Vet Med Sci. (2022) 84:666–74. doi: 10.1292/jvms.21-0478
86. Ziablitsev SV, Zhupan DB, Tykhomyrov AO, and Dyadyk OO. Benzodiazepine receptor agonist Carbacetam modulates the level of vascular endothelial growth factor in the retina of rats with streptozotocin-induced diabetes. Ukr Biochem J. (2023) 6:21–9. doi: 10.15407/ubj95.06.021
Keywords: diabetes mellitus, diabetic retinopathy, VEGF, HIF-1α, western blot, immunohistochemistry
Citation: Usenko KO, Strubchevska O, Rykov SO, Babenko MS, Strubchevska K, Kozyk O, Ziablitsev SV and Kozyk M (2025) Growth factor and hypoxia-inducible factor alpha content in the retina of male Wistar rats in experimental diabetic retinopathy and the effect of cellular protein kinase blockade. Front. Endocrinol. 16:1643445. doi: 10.3389/fendo.2025.1643445
Received: 08 June 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Ricardo Espinosa-Tanguma, Autonomous University of San Luis Potosí, MexicoReviewed by:
Carolina Dalmasso, University of Kentucky, United StatesAlShaimaa Mohamed Taha, Faculty of Science, Egypt
Copyright © 2025 Usenko, Strubchevska, Rykov, Babenko, Strubchevska, Kozyk, Ziablitsev and Kozyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: K. O. Usenko, dXNlbmtva285NTdAZ21haWwuY29t
 K. O. Usenko
K. O. Usenko Olena Strubchevska
Olena Strubchevska S. O. Rykov1
S. O. Rykov1 M. S. Babenko
M. S. Babenko S. V. Ziablitsev
S. V. Ziablitsev