- Department of Thyroid Surgery, General Surgery Center, The First Hospital of Jilin University, Changchun, Jilin, China
Background: Studies examining the relationship between overt hypothyroidism (oHT) and the risk of cognitive impairment (CI) have yielded mixed results. This study aimed to evaluate the association between oHT and the risk of CI.
Methods: We systematically searched relevant studies published up to March 2025. Data were extracted independently by two investigators. Overall odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated using a random-effects model. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of cohort and case-control studies, while the Agency for Healthcare Research and Quality (AHRQ) scale was used for cross-sectional studies. Results were reported following PRISMA guidelines.
Results: A total of 11 studies involving 1,190,059 participants were included in the systematic review. Meta-analysis revealed that oHT was associated with an increased risk of CI (OR = 1.18, 95%CI=1.04–1.34). When CI was categorized into mild cognitive impairment (MCI) and severe cognitive impairment (Alzheimer’s disease (AD) or all-cause dementia), oHT was associated with an increased risk of MCI (OR = 1.24, 95%CI=1.13–1.36) but not with AD (OR = 1.03, 95%CI=0.77–1.38) or all-cause dementia (OR = 1.20, 95%CI=0.94–1.53). Subgroup analysis based on diagnostic methods for oHT showed that oHT diagnosed solely by TSH levels was associated with a reduced risk of CI (OR = 0.87, 95%CI=0.79–0.95).
Conclusion: Available evidence suggests an association between oHT and an increased risk of cognitive impairment, particularly MCI. However, given the observational nature and significant heterogeneity of this study, the strength of this association still requires high-quality prospective studies for final confirmation and precise quantification.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251012792.
1 Introduction
Cognitive impairment (CI) refers to a significant decline or dysfunction in memory, learning, attention, language, executive function, visuospatial ability, and other cognitive domains (1, 2). It can result from various factors, including neurodegenerative diseases [e.g., AD (3, 4), Parkinson’s disease (5, 6)], cerebrovascular diseases (7, 8) [e.g., stroke (9)], traumatic brain injury, infections, metabolic abnormalities (10, 11), drug side effects, and psychiatric disorders. The severity of CI ranges from mild cognitive impairment (MCI), which may only affect daily living efficiency, to severe dementia, which can lead to a complete loss of independent living ability. According to the World Health Organization (WHO), approximately 50 million people worldwide live with dementia, and this number is expected to rise to 152 million by 2050 (12). CI not only significantly impacts patients’ quality of life but also imposes substantial economic and caregiving burdens on families and society. Therefore, identifying modifiable risk factors and potentially reversible causes is crucial for delaying disease progression and improving patients’ quality of life.
Thyroid dysfunction is considered a potentially reversible cause of CI (13, 14). Guidelines from the American Psychiatric Association (APA), European Association of Neurology (EAN), World Health Organization (WHO), and National Institute for Health and Clinical Excellence (NICE) recommend thyroid function testing during CI evaluation. While the association between hypothyroidism and CI has been extensively studied (15, 16), whether hypothyroidism is an independent risk factor for CI remains controversial. Some studies suggest that hypothyroidism increases the risk of CI (14, 17), while others report no significant association (18, 19).
Previous meta-analyses have primarily focused on the association between subclinical hypothyroidism (sHT) and CI, with limited analysis of overt hypothyroidism (oHT) (20–22). There are significant differences in the pathophysiological mechanisms and clinical severity between oHT and sHT. Patients with oHT exhibit markedly insufficient thyroid hormone levels, which have a more pronounced impact on systemic metabolism and nervous system function (23). Therefore, this meta-analysis aimed to, as a preliminary effort, separately analyze the association between oHT and varying degrees of CI, which may have important clinical implications for preventing or delaying the progression of cognitive impairment.
2 Materials and methods underscore the importance of early diagnosis and treatment
2.1 Standard protocol approvals, registrations, and guidelines
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered prospectively with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD420251012792).
2.2 Search strategy
We systematically searched PubMed, Web of Science, Scopus, Cochrane Library, and EMBASE for relevant studies published up to March 14, 2025. The complete search strategy is provided in the Supplementary Table S1. Additionally, references from included studies and existing systematic reviews were manually searched to identify additional relevant articles. No filters were applied to limit language, study type, country, or publication year.
2.3 Study selection and data extraction
Two independent investigators screened the literature, and any discrepancies in study inclusion were resolved through discussion or consultation with a third reviewer. After removing duplicates, studies were excluded if their titles or abstracts were irrelevant. Full texts were reviewed for eligible studies or when relevance could not be determined from the abstract alone.
Inclusion Criteria:
1. Cohort, case-control, or cross-sectional studies examining the association between hypothyroidism and CI.
2. Studies providing relative risk (RR), hazard ratio (HR), odds ratio (OR), or raw data to calculate these metrics, along with 95% confidence intervals (CIs).
3. Studies involving adults (age ≥ 18 years).
4. Studies reporting diagnostic criteria for oHT.
5. Studies published within the last 20 years (2005–2025).
Exclusion criteria:
1. Reviews, case reports, animal studies, letters, conference abstracts, or guidelines.
2. Studies lacking complete data or full-text availability.
3. Studies focusing solely on subclinical hypothyroidism (sHT).
4. Studies involving baseline conditions that may cause secondary hypothyroidism (e.g., pituitary or hypothalamic diseases).
Outcome definitions:
Cognitive Impairment: A broad term encompassing any clinically diagnosed decline in cognitive domains (memory, attention, executive function, etc.), assessed by standardized tools (e.g., MMSE, CDR). Mild Cognitive Impairment: Defined according to Petersen criteria or equivalent, characterized by subjective memory complaints with objective cognitive deficits not interfering with daily activities. Alzheimer’s Disease: Diagnosed based on NIA-AA criteria or ICD-10 codes. All-cause Dementia: Diagnosed using DSM-IV/ICD-10 criteria, regardless of etiology.
Extracted information included study year, author, country, study type, total sample size, follow-up duration, population age (median or mean), proportion of females, diagnostic criteria for oHT and CI, type of CI, and adjustment factors. In addition, we will employ specific formulas to convert HR values from cohort studies into OR values (24, 25), ensuring compatibility for subsequent pooled effect size analysis. When extracting OR values from the literature, both adjusted OR values (with the maximum number of covariates) and unadjusted OR values were comprehensively recorded.
2.4 Quality assessment
Two investigators independently assessed the quality of cohort and case-control studies using the NOS (26), which evaluates study population selection, group comparability, and exposure/outcome ascertainment. Studies were categorized as low (0–3 points), medium (4–6 points), or high quality (7–9 points). Cross-sectional studies were evaluated using the AHRQ checklist (27), which consists of 11 items scored as “yes,” “no,” or “unclear.” Studies were classified as low (0–5 points), medium (6–7 points), or high quality (8–11 points).
2.5 Statistical analysis
STATA 18.0 was used for data analysis and forest plots were plotted to visually display the pooled results. We preferentially extracted data from each study with adjusted OR values and 95%CI to assess the risk of cognitive impairment in patients with overt hypothyroidism. Heterogeneity was assessed with the use of the chi-square test and I2 values, with p values of less than 0.1 or I2 values of more than 50% considered to indicate heterogeneity, with the use of a random-effects model (28). Otherwise, a fixed effects model was used (29). Subgroup analyses were performed on the basis of population characteristics, study types, criteria for overt hypothyroidism, and whether adjustment for vascular disease to account for potential heterogeneity. In addition, sensitivity analyses were performed to verify the robustness of the overall results. Finally, funnel plots and Egger’s test were used to detect publication bias (30).
3 Results
3.1 Literature search
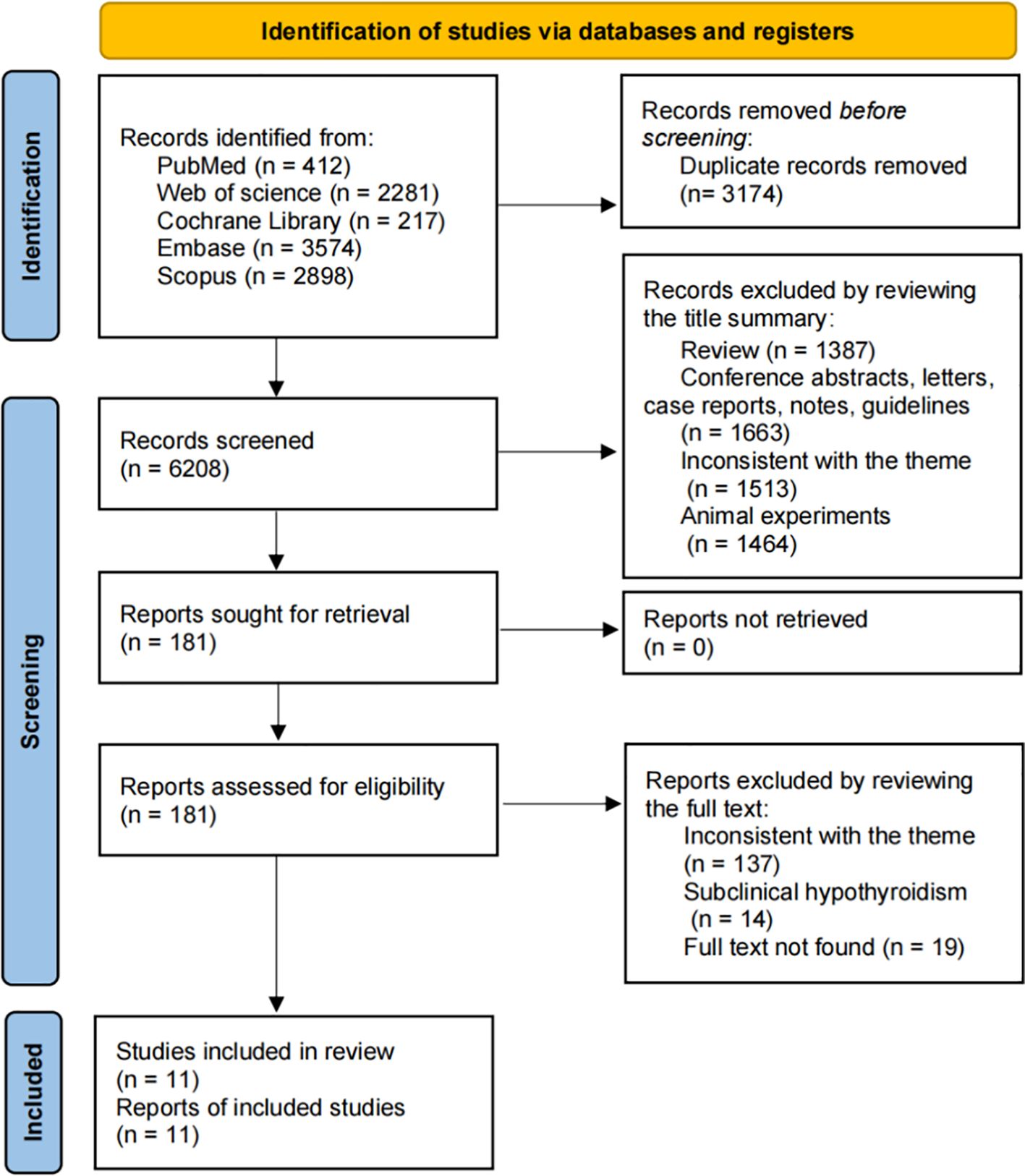
The search process is summarized in Figure 1. A total of 9,382 articles were identified, with 6,208 remaining after duplicate removal. After title and abstract screening, 181 articles were considered potentially relevant. Following full-text review, 11 studies from 9 articles were included in the meta-analysis (14, 17–19, 31–35).
3.2 Study characteristics
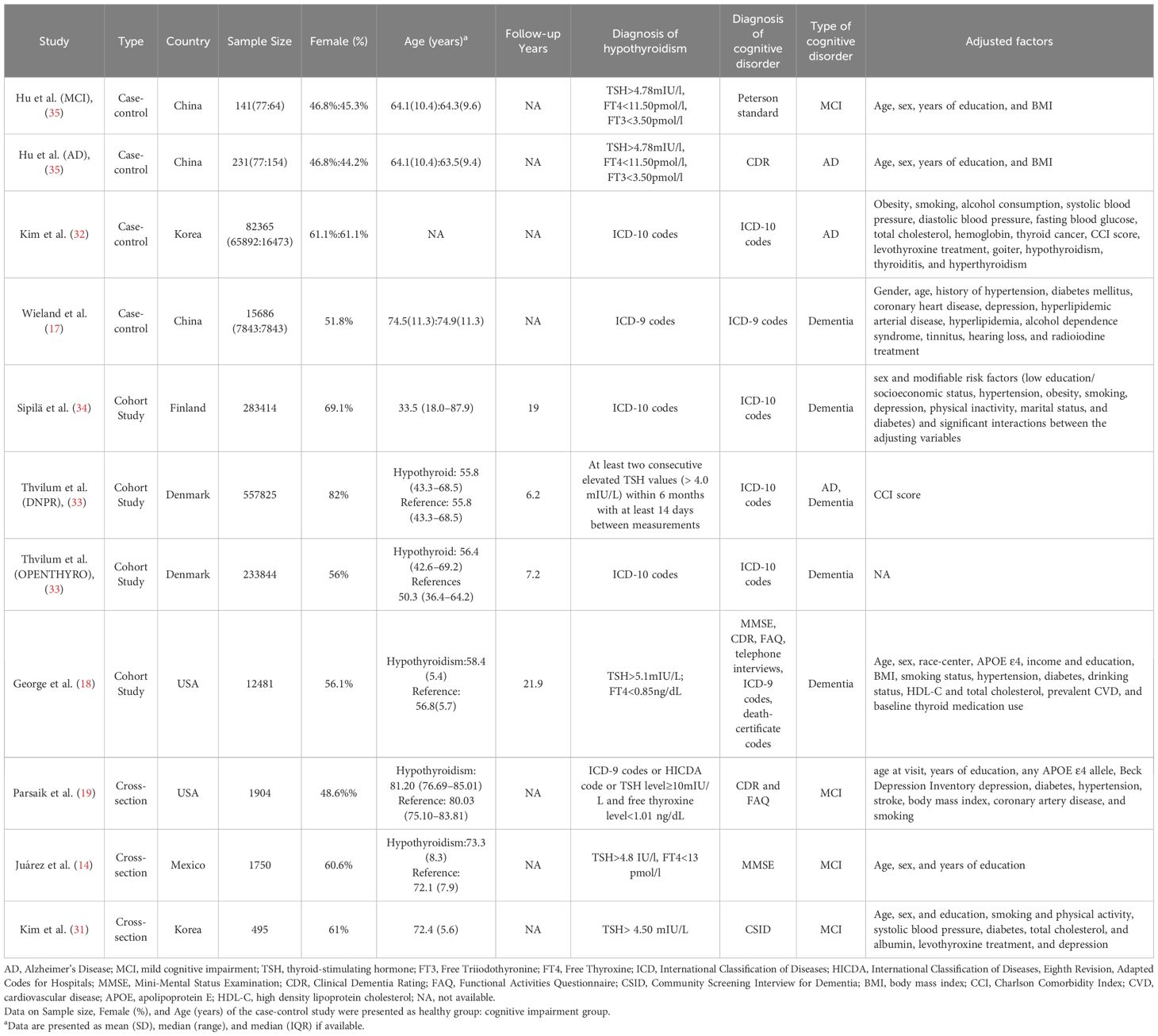
The characteristics of the included studies are presented in Table 1. The 11 studies [2 of which included 2 cohorts (33, 35)] comprised 4 case-control studies, 4 cohort studies, and 3 cross-sectional studies. These studies involved diverse populations from China, Korea, Finland, Denmark, the United States, and Mexico, published between 2010 and 2022, and included 1,190,059 participants. The age range of participants was 33.5 to 85 years, with females comprising 44.2% to 82% of the samples. Follow-up durations in cohort studies ranged from 6.2 to 21.9 years. The primary outcome was MCI in 4 studies and dementia in 7 studies (3 all-cause dementia, 3 AD, and 1 either all-cause dementia or AD). Only one study did not adjust for potential confounders; common adjustment variables included age, gender, education, BMI, smoking, hypertension, and diabetes. In addition, the quality assessments of the individual studies are provided in Supplementary Tables S2–S4.
3.3 oHT and risk of CI
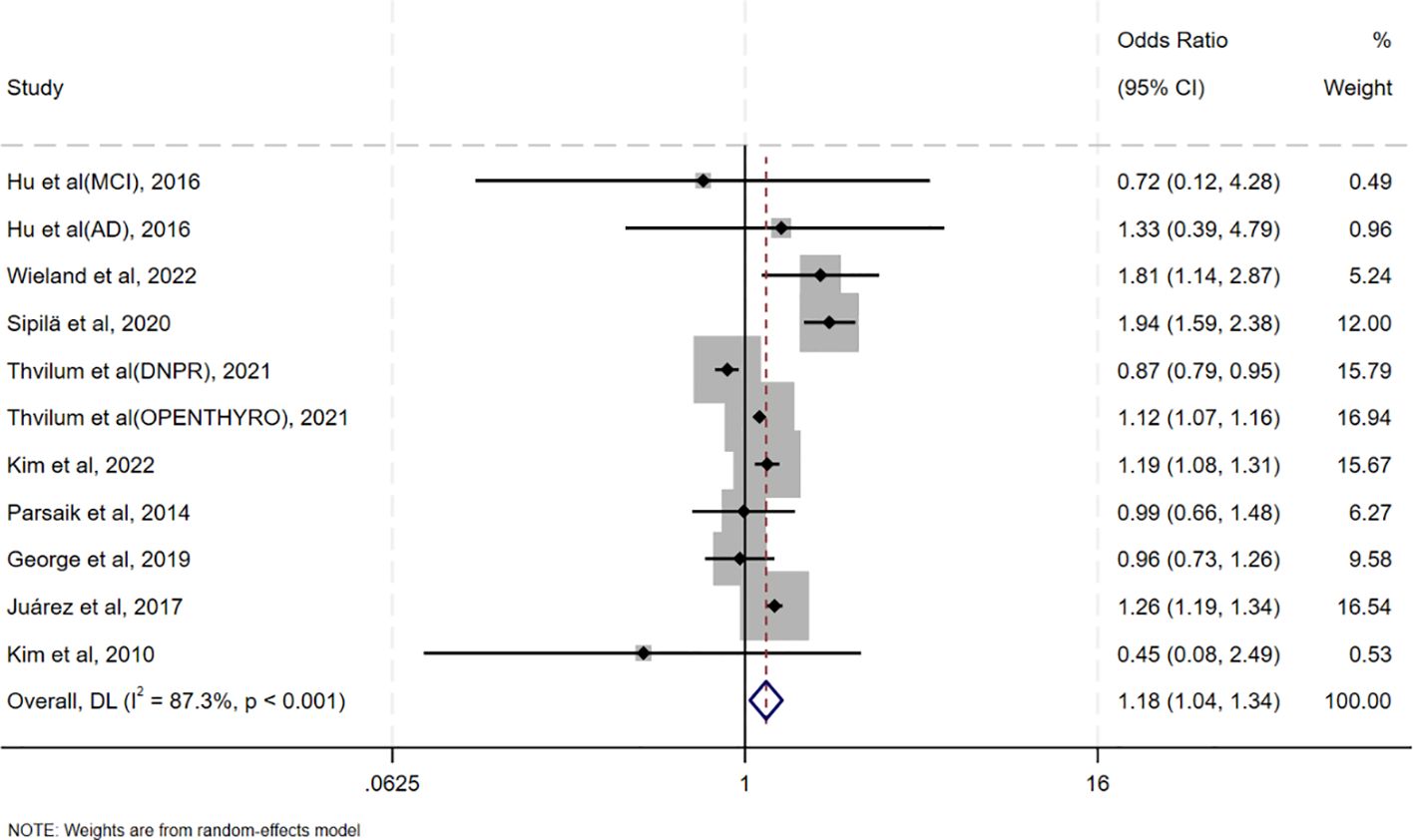
Pooled analysis of ORs adjusted for the maximum number of covariates showed that oHT was associated with an increased risk of CI (OR = 1.18, 95%CI=1.04–1.34, I²=87.3%, p<0.001; Figure 2). It is noteworthy that the meta-analysis reveals a high degree of statistical heterogeneity (I² =87.3%, p <0.001), indicating significant differences in the effect sizes among the included studies, rather than due to sampling error. Therefore, interpretation of the pooled effect size should be approached with caution, and there is an urgent need for an in-depth exploration of the sources of heterogeneity. Sensitivity analysis in Supplementary Figure S1 revealed that excluding individual studies resulted in point estimates ranging from 0.96 to 1.38, with 95% CIs consistently crossing 1, indicating non-significant results. However, point estimates slightly above 1 suggest a potential positive effect. A pooled analysis of unadjusted ORs yielded similar results (OR = 1.23, 95% CI = 1.12–1.35, I²=75.9%, p<0.001; Supplementary Figure S2).
3.4 oHT and risk of MCI, AD and dementia
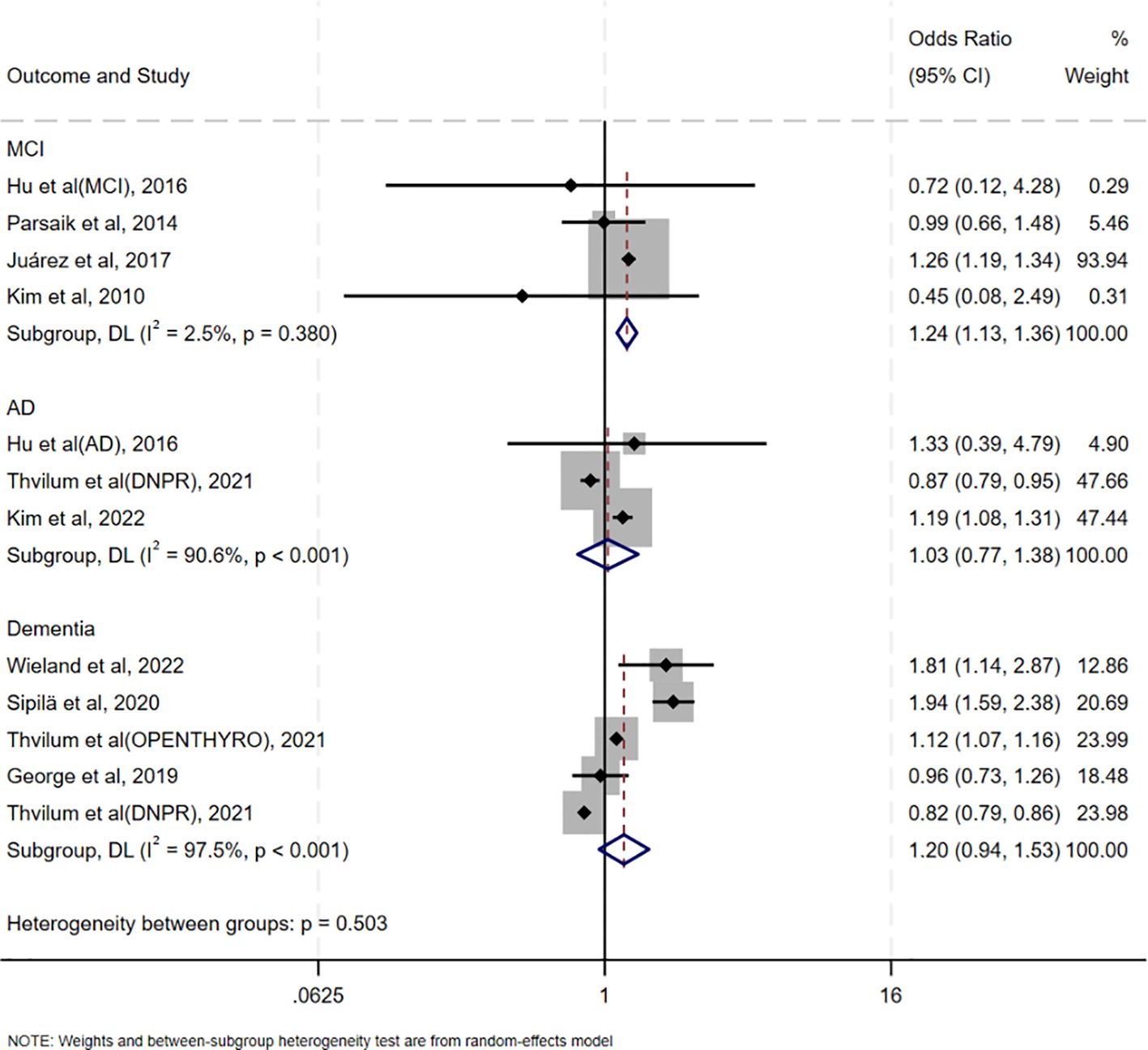
The association between oHT and MCI was evaluated in four studies conducted in China, the United States, Mexico, and Korea, respectively. Notably, these studies used diverse cognitive assessment tools (Peterson criteria, CDR, MMSE, and CSID), yet showed consistent direction of effect, supporting the robustness of the association. (OR = 1.24, 95% CI = 1.13–1.36, I² = 2.5%, p = 0.380; Figure 3). Three studies assessed the association between oHT and AD risk, revealing no significant increase (OR = 1.03, 95%CI=0.77–1.38, I²=90.6%, p<0.001; Figure 3). Five studies examined the association between oHT and all-cause dementia risk, also showing no significant increase (OR = 1.20, 95%CI=0.94–1.53, I²=97.5%, p<0.001; Figure 3).
3.5 Subgroup analysis
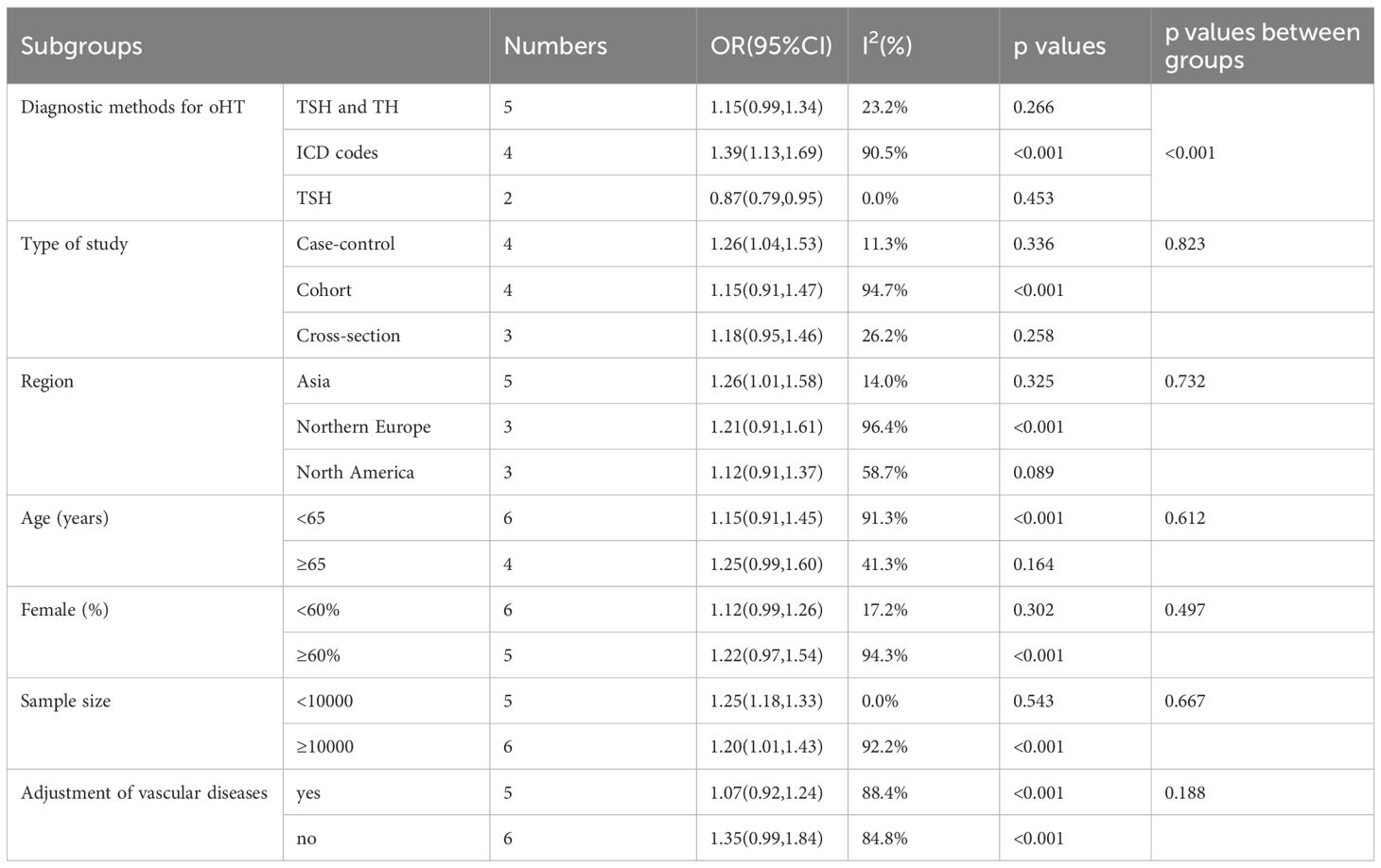
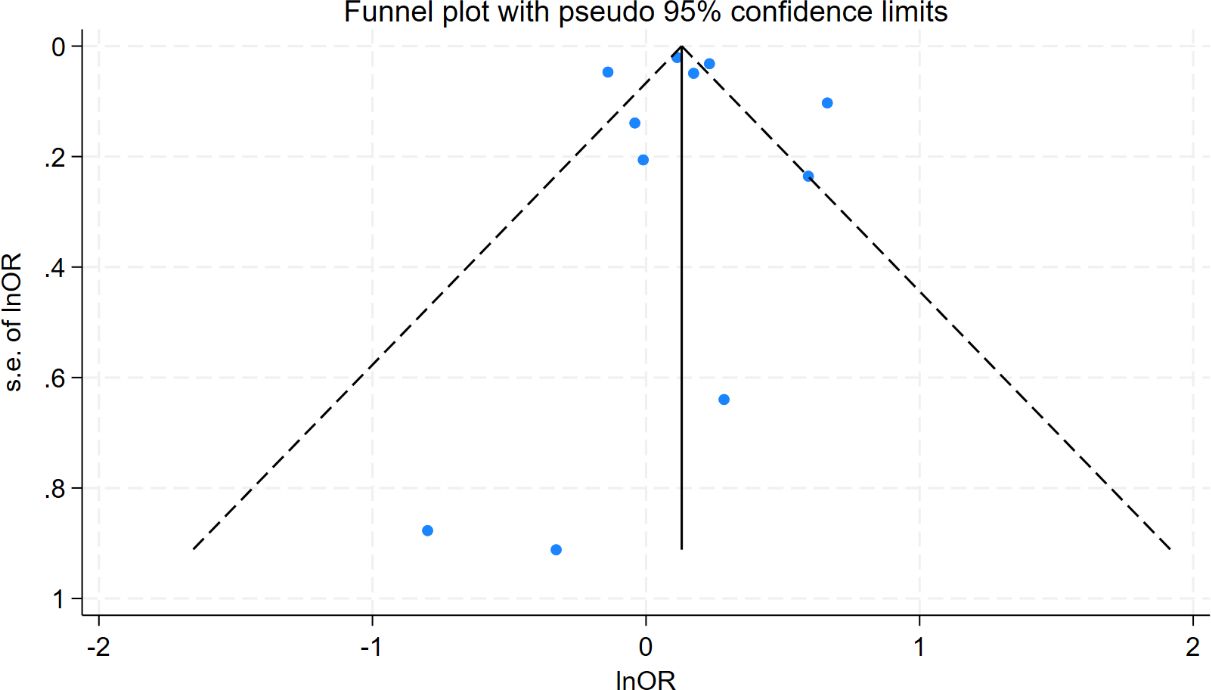
Table 2 summarizes the results of all subgroup analyses to explore potential sources of heterogeneity, including population characteristics (region, age distribution, proportion of females, sample size), study type, diagnostic methods for oHT, and adjustment factors. Significant between-group differences were observed only in diagnostic methods for oHT (p<0.001; Figure 4). Specifically, studies using thyroid stimulating hormone (TSH) and thyroid hormone (TH) levels for diagnosis showed a pooled effect size of 1.15 (95% CI = 0.99–1.34, I² = 23.2%), while those using ICD codes had a pooled effect size of 1.39 (95% CI = 1.13–1.69, I² = 90.5%). Studies using only TSH levels for diagnosis showed a reduced risk of CI (OR = 0.87, 95% CI = 0.79–0.95, I² = 0.0%). Excluding these studies yielded a pooled effect size of 1.25 (95%CI=1.12–1.40, I² = 80.3%, p < 0.001; Figure 5). The gradient change in effect size caused by different diagnostic methods (ICD code>TSH+TH>TSH only) strongly indicates that differences in diagnostic methodology are the main reason for the appearance of “inconsistent” overall results.
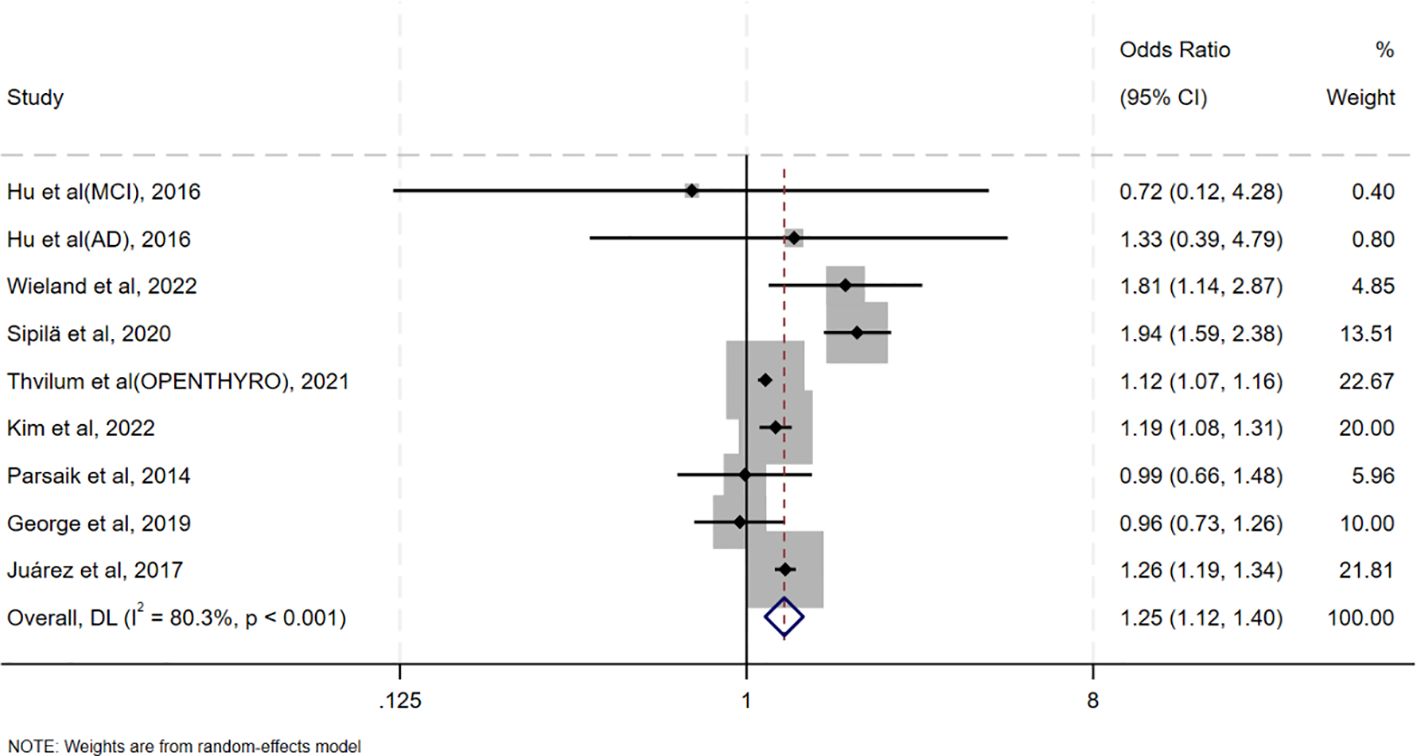
Figure 5. Forest plots to reexamine the association between oHT and CI after excluding studies reporting only TSH.
In other subgroup analyses, case-control studies showed that oHT was associated with an increased risk of CI (OR = 1.26, 95%CI=1.04–1.53, I²=11.3%, p=0.336; Supplementary Figure S3), whereas cohort and cross-sectional studies did not. oHT was associated with an increased risk of CI in Asian populations (OR = 1.26, 95%CI=1.01–1.58, I²=14.0%, p=0.325; Supplementary Figure S4) but not in Northern European or North American populations. Subgroup analyses based on age or proportion of females did not show a significant association between oHT and CI risk (Supplementary Figures S5, S6). Both small (OR = 1.25, 95%CI=1.18–1.33, I²=0.0%, p=0.543; Supplementary Figure S7) and large sample sizes (OR = 1.20, 95%CI=1.01–1.43, I²=92.2%, p<0.001; Supplementary Figure S7) suggested an increased risk of CI. Additionally, cerebrovascular disease, a significant risk factor for CI, was adjusted for in some studies, but oHT was not associated with an increased risk of CI in these analyses (Supplementary Figure S8).
3.6 Publication bias
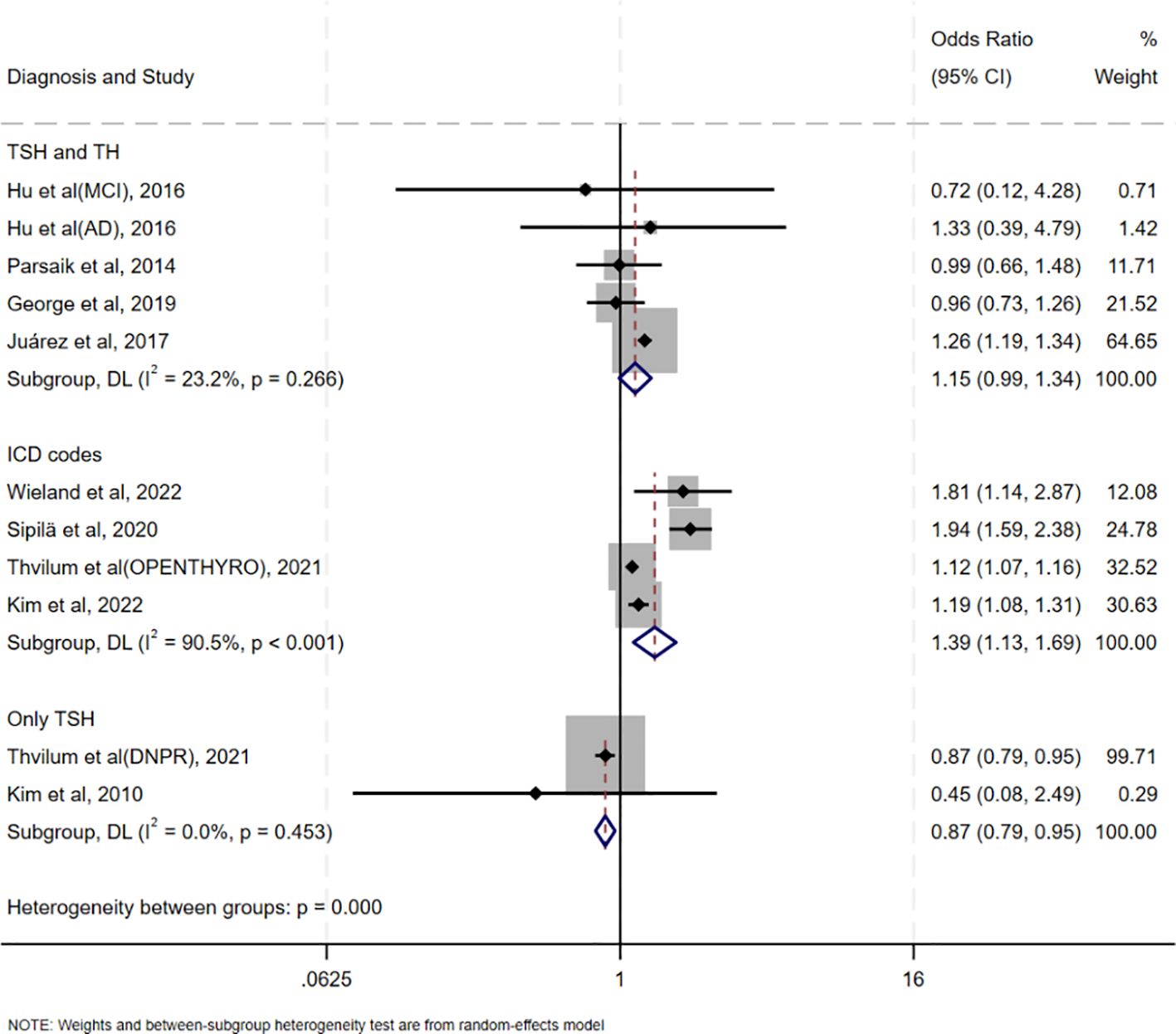
Funnel plot analysis showed symmetrical distribution of study points, with no significant outliers (Figure 6), suggesting low publication bias. Egger’s test further supported this finding (intercept coefficient=0.24, 95%CI=-2.53–3.01, p=0.849; Table 3), indicating no significant publication bias. Thus, the results of this meta-analysis are considered reliable.
4 Discussion
This meta-analysis aimed to explore the relationship between oHT and CI risk. To our knowledge, this is the first meta-analysis specifically focusing on the association between oHT and CI. As a preliminary study, it provides an initial estimate of the association and, more importantly, highlights critical methodological sources of heterogeneity that must be addressed in future work. The pooled analysis of 11 studies demonstrated that oHT was associated with an increased risk of CI, whether using adjusted (OR = 1.18, 95%CI=1.04–1.34) or unadjusted OR values (OR = 1.23, 95% CI = 1.12–1.35). This suggests that oHT may be an independent risk factor for CI. Further analysis by CI severity revealed that oHT was associated with an increased risk of MCI (OR = 1.24, 95% CI = 1.13–1.36) but not with AD (OR = 1.03, 95%CI=0.77–1.38) or all-cause dementia (OR = 1.20, 95%CI=0.94–1.53). Sensitivity analysis revealed that excluding individual studies resulted in point estimates ranging from 0.96 to 1.38, with 95% CIs consistently crossing 1, indicating that the statistical significance of the pooled effect was not robust. However, most point estimates remained above 1.0, suggesting a potential positive trend.
From a biological perspective, thyroid hormones play a critical role in maintaining brain function (36–39). They regulate neuronal metabolism, synaptic plasticity, neurotransmitter synthesis and release, and neuronal survival and differentiation (40–42). In oHT patients, significantly reduced thyroid hormone levels may slow neuronal metabolism, impairing energy supply and functional maintenance. Additionally, thyroid hormone deficiency can decrease synaptic plasticity (43–45), disrupting neuronal signaling and further impairing cognitive functions such as memory, learning, and executive function. Beyond direct neuronal effects, oHT may contribute to CI through indirect pathways, including impaired cerebrovascular function, increased oxidative stress (46–48), and heightened inflammatory responses (49–51). These mechanisms support the association between oHT and CI and highlight the potential importance of identifying and managing oHT. However, given the observational nature and limitations of this study, our findings do not directly demonstrate that intervening in oHT can effectively prevent dementia. This critical issue urgently needs to be addressed through rigorously designed randomized controlled trials (RCTs).
Our findings align with some previous studies showing an increased risk of CI with oHT (52, 53), while others report no significant association (19). This inconsistency may stem from differences in study design, sample characteristics, and diagnostic criteria for oHT. For instance, some studies relied solely on TSH levels for oHT diagnosis, whereas our subgroup analysis found that studies using combined TSH and TH levels showed a more significant effect size (OR = 1.15, 95%CI=0.99–1.34) compared to those using only TSH (OR = 0.87, 95%CI=0.79–0.95). This highlights the importance of diagnostic criteria in influencing study outcomes. Previous meta-analyses often combined various forms of hypothyroidism, such as subclinical hypothyroidism, overt hypothyroidism, and thyroiditis, without rigorously distinguishing between overt and subclinical hypothyroidism. This approach likely introduced confounding factors that obscured the true relationship between overt hypothyroidism and cognitive impairment. Additionally, the limited number of studies focusing specifically on overt hypothyroidism in prior analyses may have further reduced the statistical power to detect a significant association, potentially contributing to the negative conclusions drawn. In contrast, the present study specifically focused on overt hypothyroidism and analyzed it independently, thereby providing a clearer and more precise understanding of its potential association with cognitive impairment.
The high heterogeneity found in this study (I²=87.3%) is a core issue that needs to be considered when interpreting the results. Our subgroup analysis successfully identified the diagnostic method of oHT as its main source. This difference may stem from the fundamentally different patient populations captured by different diagnostic methods. The study using ICD codes is likely to include oHT patients who seek medical attention due to clinical symptoms, have a longer course of illness, are more severe, or have more complications, therefore their assessed risk is the highest (OR = 1.39). On the contrary, studies that rely solely on a single TSH elevation diagnosis may inadvertently include some subclinical, temporary, or mild thyroid dysfunction patients, thereby diluting the true risk and even obtaining a reverse association (OR = 0.87). The study using TSH combined with THs levels for diagnosis has the most accurate patient definition, and its risk estimate (OR = 1.15) may be the closest to the true association. Although diagnostic methods explain most of the heterogeneity, residual heterogeneity may still be related to population characteristics (such as age, race), assessment tools for cognitive impairment, and differences in the degree of adjustment for confounding factors (such as vascular disease). Therefore, the summary results of this study should be considered as an average of the reported effect sizes under different clinical backgrounds and research methods, which reveals the existence of associations but also emphasizes that their strength may vary significantly in different contexts. Funnel plot and Egger’s test revealed no significant publication bias (Egger’s test p=0.849), indicating that the results of this meta-analysis are highly reliable. Nevertheless, attention should be paid to other potential sources of bias, such as differences in study design and inadequate adjustment for confounding factors. In particular, some studies did not adjust for important confounders such as vascular comorbidities, which may have affected the accuracy of the results.
Although this meta-analysis provides valuable evidence supporting the association between overt hypothyroidism (oHT) and cognitive impairment (CI), several limitations should be acknowledged. First, the high heterogeneity among the included studies may compromise the stability of the results. Second, the vast majority of the original studies we included did not provide more detailed clinical characteristic data of the oHT patient population. Especially, there is a lack of information regarding the specific etiology of oHT, the use of levothyroxine, and the adequacy of treatment. Untreated oHT, under treatment but poorly controlled oHT, and adequately treated oHT may have different potential impact mechanisms and risk intensities on cognitive function. Combining all these patients with different states for analysis may lead to underestimation or misestimation of the true risk, which is another potential important source of heterogeneity observed in this study. Third, the geographic distribution of the included studies was predominantly limited to Asia, Northern Europe, and North America, potentially restricting the generalizability of the results to other populations. Fourth, the small sample sizes in some studies may have reduced the precision of the effect size estimates. To address these limitations, future research should have the following characteristics to provide higher-level evidence: (a) systematically collect data on the etiology, treatment status, treatment dose, treatment duration, and thyroid function (TSH) levels during oHT follow-up; (b) Adopting a standardized oHT diagnostic process based on international guidelines (which must be based on both TSH and free thyroxine FT4 levels) to ensure the accuracy of patient grouping and avoid mistakenly including subclinical hypothyroidism patients; (c) Large scale, multi-center samples to provide sufficient statistical power to detect effect size and allow for sufficient subgroup analysis.
5 Conclusion
Available evidence suggests an association between oHT and an increased risk of cognitive impairment, particularly MCI. However, given the observational nature and significant heterogeneity of this study, the strength of this association still requires high-quality prospective studies for final confirmation and precise quantification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JZ: Software, Investigation, Writing – original draft, Writing – review & editing, Methodology, Data curation. JX: Data curation, Writing – review & editing, Methodology. ZL: Writing – review & editing, Methodology, Software. JL: Resources, Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1643589/full#supplementary-material
References
1. Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J cardiac failure. (2017) 23:464–75. doi: 10.1016/j.cardfail.2017.04.007, PMID: 28433667
2. Jongsiriyanyong S and Limpawattana P. Mild cognitive impairment in clinical practice: A review article. Am J Alzheimers Dis Other Demen. (2018) 33:500–7. doi: 10.1177/1533317518791401, PMID: 30068225
3. Liu Y, Xiao X, Yang Y, Yao R, Yang Q, Zhu Y, et al. The risk of Alzheimer's disease and cognitive impairment characteristics in eight mental disorders: A UK Biobank observational study and Mendelian randomization analysis. Alzheimer's dementia: J Alzheimer's Assoc. (2024) 20:4841–53. doi: 10.1002/alz.14049, PMID: 38860751
4. Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, et al. Sleep, Cognitive impairment, and Alzheimer's disease: A Systematic Review and Meta-Analysis. Sleep. (2017) 40. doi: 10.1093/sleep/zsw032, PMID: 28364458
5. Goldman JG and Sieg E. Cognitive impairment and dementia in parkinson disease. Clin Geriatr Med. (2020) 36:365–77. doi: 10.1016/j.cger.2020.01.001, PMID: 32222308
6. Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. (2021) 7:47. doi: 10.1038/s41572-021-00280-3, PMID: 34210995
7. Zanon Zotin MC, Sveikata L, Viswanathan A, and Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management. Curr Opin neurology. (2021) 34:246–57. doi: 10.1097/WCO.0000000000000913, PMID: 33630769
8. Chang Wong E and Chang Chui H. Vascular cognitive impairment and dementia. Continuum (Minneap Minn). (2022) 28:750–80. doi: 10.1212/CON.0000000000001124, PMID: 35678401
9. McInnes K, Friesen CL, MacKenzie DE, Westwood DA, and Boe SG. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PloS One. (2017) 12:e0174847. doi: 10.1371/journal.pone.0174847, PMID: 28399158
10. Zheng W, Zhou X, Yin J, Liu H, Yin W, Zhang W, et al. Metabolic syndrome-related cognitive impairment with white matter hyperintensities and functional network analysis. Obes (Silver Spring Md). (2023) 31:2557–67. doi: 10.1002/oby.23873, PMID: 37724054
11. Khaleghzadeh-Ahangar H, Talebi A, and Mohseni-Moghaddam P. Thyroid disorders and development of cognitive impairment: A review study. Neuroendocrinology. (2022) 112:835–44. doi: 10.1159/000521650, PMID: 34963121
12. Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. (2020) 16:1078–94. doi: 10.1002/alz.12123, PMID: 32627328
13. van Vliet NA, van Heemst D, Almeida OP, Åsvold BO, Aubert CE, Bae JB, et al. Association of thyroid dysfunction with cognitive function: an individual participant data analysis. JAMA Internal Med. (2021) 181:1440–50. doi: 10.1001/jamainternmed.2021.5078, PMID: 34491268
14. Juárez-Cedillo T, Basurto-Acevedo L, Vega-García S, Sánchez-Rodríguez Martha A, Retana-Ugalde R, Juárez-Cedillo E, et al. Prevalence of thyroid dysfunction and its impact on cognition in older mexican adults: (SADEM study). J endocrinological Invest. (2017) 40:945–52. doi: 10.1007/s40618-017-0654-6, PMID: 28343318
15. Biondi B and Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine Rev. (2008) 29:76–131. doi: 10.1210/er.2006-0043, PMID: 17991805
16. Smith CD, Grondin R, LeMaster W, Martin B, Gold BT, and Ain KB. Reversible cognitive, motor, and driving impairments in severe hypothyroidism. Thyroid: Off J Am Thyroid Assoc. (2015) 25:28–36. doi: 10.1089/thy.2014.0371, PMID: 25381990
17. Wieland DR, Wieland JR, Wang H, Chen YH, Lin CH, Wang JJ, et al. Thyroid disorders and dementia risk: A nationwide population-based case-control study. Neurology. (2022) 99:e679–e87. doi: 10.1212/WNL.0000000000200740, PMID: 35794019
18. George KM, Lutsey PL, Selvin E, Palta P, Windham BG, and Folsom AR. Association between thyroid dysfunction and incident dementia in the atherosclerosis risk in communities neurocognitive study. J Endocrinol Metab. (2019) 9:82–9. doi: 10.14740/jem588, PMID: 32411312
19. Parsaik AK, Singh B, Roberts RO, Pankratz S, Edwards KK, Geda YE, et al. Hypothyroidism and risk of mild cognitive impairment in elderly persons: a population-based study. JAMA neurology. (2014) 71:201–7. doi: 10.1001/jamaneurol.2013.5402, PMID: 24378475
20. Pasqualetti G, Pagano G, Rengo G, Ferrara N, and Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab. (2015) 100:4240–8. doi: 10.1210/jc.2015-2046, PMID: 26305618
21. Ma LY, Zhao B, Ou YN, Zhang DD, Li QY, and Tan L. Association of thyroid disease with risks of dementia and cognitive impairment: A meta-analysis and systematic review. Front Aging Neurosci. (2023) 15:1137584. doi: 10.3389/fnagi.2023.1137584, PMID: 36993905
22. Ye Y, Wang Y, Li S, Guo J, Ding L, and Liu M. Association of hypothyroidism and the risk of cognitive dysfunction: A meta-analysis. J Clin Med. (2022) 11. doi: 10.3390/jcm11226726, PMID: 36431204
23. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Internal Med. (1998) 158:1413–8. doi: 10.1001/archinte.158.13.1413, PMID: 9665349
24. Zhang J and Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690, PMID: 9832001
25. Shor E, Roelfs D, and Vang ZM. The "Hispanic mortality paradox" revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med. (2017) 186:20–33. doi: 10.1016/j.socscimed.2017.05.049, PMID: 28577458
26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z, PMID: 20652370
27. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evidence-Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141, PMID: 25594108
28. Wang Z, Brumback BA, Alrwisan AA, and Winterstein AG. Model-based standardization using an outcome model with random effects. Stat Med. (2019) 38:3378–94. doi: 10.1002/sim.8182, PMID: 31150151
29. Kanters S. Fixed- and random-effects models. Methods Mol Biol. (2022) 2345:41–65. doi: 10.1007/978-1-0716-1566-9_3, PMID: 34550583
30. Lin L and Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817, PMID: 29141096
31. Kim JM, Stewart R, Kim SY, Bae KY, Yang SJ, Kim SW, et al. Thyroid stimulating hormone, cognitive impairment and depression in an older korean population. Psychiatry Invest. (2010) 7:264–9. doi: 10.4306/pi.2010.7.4.264, PMID: 21253410
32. Kim JH, Lee HS, Kim YH, Kwon MJ, Kim JH, Min CY, et al. The association between thyroid diseases and alzheimer's disease in a national health screening cohort in korea. Front endocrinology. (2022) 13:815063. doi: 10.3389/fendo.2022.815063, PMID: 35321339
33. Thvilum M, Brandt F, Lillevang-Johansen M, Folkestad L, Brix TH, and Hegedüs L. Increased risk of dementia in hypothyroidism: A Danish nationwide register-based study. Clin endocrinology. (2021) 94:1017–24. doi: 10.1111/cen.14424, PMID: 33484007
34. Sipilä PN, Lindbohm JV, Singh-Manoux A, Shipley MJ, Kiiskinen T, Havulinna AS, et al. Long-term risk of dementia following hospitalization due to physical diseases: A multicohort study. Alzheimer's dementia: J Alzheimer's Assoc. (2020) 16:1686–95. doi: 10.1002/alz.12167, PMID: 32886434
35. Hu Y, Wang ZC, Guo QH, Cheng W, and Chen YW. Is thyroid status associated with cognitive impairment in elderly patients in China? BMC endocrine Disord. (2016) 16:11. doi: 10.1186/s12902-016-0092-z, PMID: 26897535
36. de Souza JS. Thyroid hormone biosynthesis and its role in brain development and maintenance. Adv Protein Chem Struct Biol. (2024) 142:329–65. doi: 10.1016/bs.apcsb.2023.12.024, PMID: 39059990
37. Churilov LP, Sobolevskaia PA, and Stroev YI. Thyroid gland and brain: Enigma of Hashimoto's encephalopathy. Best Pract Res Clin Endocrinol Metab. (2019) 33:101364. doi: 10.1016/j.beem.2019.101364, PMID: 31801687
38. Bauer M, Goetz T, Glenn T, and Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. (2008) 20:1101–14. doi: 10.1111/j.1365-2826.2008.01774.x, PMID: 18673409
39. Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, and Ahmed RG. Thyroid hormones states and brain development interactions. Int J Dev Neurosci. (2008) 26:147–209. doi: 10.1016/j.ijdevneu.2007.09.011, PMID: 18031969
40. Liu YY and Brent GA. Thyroid hormone and the brain: Mechanisms of action in development and role in protection and promotion of recovery after brain injury. Pharmacol Ther. (2018) 186:176–85. doi: 10.3390/ijms241512352, PMID: 29378220
41. Alcaide Martin A and Mayerl S. Local thyroid hormone action in brain development. Int J Mol Sci. (2023) 24. doi: 10.1007/978-1-0716-1566-9_3, PMID: 37569727
42. Sawicka-Gutaj N, Zawalna N, Gut P, and Ruchała M. Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions. Pharmacol Rep. (2022) 74:847–58. doi: 10.1007/s43440-022-00377-w, PMID: 35771431
43. Vallortigara J, Chassande O, Higueret P, and Enderlin V. Thyroid hormone receptor alpha plays an essential role in the normalisation of adult-onset hypothyroidism-related hypoexpression of synaptic plasticity target genes in striatum. J neuroendocrinology. (2009) 21:49–56. doi: 10.1111/j.1365-2826.2008.01802.x, PMID: 19094093
44. Raymaekers SR and Darras VM. Thyroid hormones and learning-associated neuroplasticity. Gen Comp Endocrinol. (2017) 247:26–33. doi: 10.1016/j.ygcen.2017.04.001, PMID: 28390960
45. Alkadhi KA. Synaptic plasticity and cognitive ability in experimental adult-onset hypothyroidism. J Pharmacol Exp Ther. (2024) 389:150–62. doi: 10.1124/jpet.123.001887, PMID: 38508752
46. Cattani D, Goulart PB, Cavalli VL, Winkelmann-Duarte E, Dos Santos AQ, Pierozan P, et al. Congenital hypothyroidism alters the oxidative status, enzyme activities and morphological parameters in the hippocampus of developing rats. Mol Cell Endocrinol. (2013) 375:14–26. doi: 10.1016/j.mce.2013.05.001, PMID: 23693027
47. Sinha P, Chakrabarti N, Ghosh N, Mitra S, Dalui S, and Bhattacharyya A. Alterations of thyroidal status in brain regions and hypothalamo-pituitary-blood-thyroid-axis associated with dopaminergic depletion in substantia nigra and ROS formation in different brain regions after MPTP treatment in adult male mice. Brain Res Bull. (2020) 156:131–40. doi: 10.1016/j.brainresbull.2019.12.013, PMID: 31891753
48. Sun X, Zhao M, Wang X, Sun Y, Li J, Zhang Y, et al. Tyrosol ameliorates depressive-like behavior and hippocampal damage in perimenopausal depression rats by inhibiting oxidative stress and thyroid dysfunction. Neurosci Lett. (2025) 859-861:138266. doi: 10.1016/j.neulet.2025.138266, PMID: 40383458
49. Lai R, Yin B, Feng Z, Deng X, Lv X, Zhong Y, et al. The causal relationship between 41 inflammatory cytokines and hypothyroidism: bidirectional two-sample Mendelian randomization study. Front endocrinology. (2023) 14:1332383. doi: 10.3389/fendo.2023.1332383, PMID: 38317717
50. Hu XY, Liang YC, Zhang HH, Li HL, and Liu DL. Association between the systemic immune-inflammation index and thyroid function in U.S. Adults. Mediators Inflammation. (2023) 2023:5831858. doi: 10.1155/2023/5831858, PMID: 38022688
51. Türemen EE, Çetinarslan B, Şahin T, Cantürk Z, and Tarkun İ. Endothelial dysfunction and low grade chronic inflammation in subclinical hypothyroidism due to autoimmune thyroiditis. Endocr J. (2011) 58:349–54. doi: 10.1507/endocrj.K10E-333, PMID: 21490407
52. Dolatshahi M, Salehipour A, Saghazadeh A, Sanjeari Moghaddam H, Aghamollaii V, Fotouhi A, et al. Thyroid hormone levels in Alzheimer disease: a systematic review and meta-analysis. Endocrine. (2023) 79:252–72. doi: 10.1007/s12020-022-03190-w, PMID: 36166162
Keywords: hypothyroidism, cognitive impairment, Alzheimer’s disease, dementia, thyroid
Citation: Zhu J, Xu J, Li Z and Liu J (2025) Association of overt hypothyroidism with risks of cognitive impairment: a meta-analysis and systematic review. Front. Endocrinol. 16:1643589. doi: 10.3389/fendo.2025.1643589
Received: 09 June 2025; Accepted: 16 September 2025;
Published: 01 October 2025.
Edited by:
Tinghe Yu, Chongqing Medical University, ChinaReviewed by:
Ricardo V. Garcia-Mayor, Instituto de Investigación Sanitaria Galicia Sur (IISGS), SpainOkan İmre, Karamanoğlu Mehmetbey University, Türkiye
Copyright © 2025 Zhu, Xu, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Liu, bGl1X2ppYUBqbHUuZWR1LmNu
 Jinxin Zhu
Jinxin Zhu Jialu Xu
Jialu Xu Zhaoqing Li
Zhaoqing Li Jia Liu
Jia Liu