- 1Division of Infertility, Lee Women’s Hospital, Taichung, Taiwan
- 2Department of Post-Baccalaureate Medicine, National Chung Hsing University, Taichung, Taiwan
- 3Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 4Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 5Department of Obstetrics and Gynecology and Women’s Health, Taichung Veterans General Hospital, Taichung, Taiwan
- 6School of Medicine, National Yang-Ming Chiao Tung University, Taipei, Taiwan
Background: Achieving an optimal balance between oocyte yield and embryo quality is central to donor IVF. Although anti-Müllerian hormone (AMH) predicts oocyte quantity, donor characteristics and stimulation parameters may influence embryo developmental competence. We aimed to identify clinical and protocol-related factors associated with oocyte yield and embryo quality in a large donor cohort.
Methods: We retrospectively analyzed 584 donor IVF cycles at a single center (Jan 1, 2018–Dec 31, 2023). Donor variables included age, AMH, BMI (body mass index), and baseline hormones. Stimulation used gonadotropin-releasing hormone (GnRH) antagonist (27.1%) or progestin-primed ovarian stimulation (PPOS)(72.9%); recombinant human luteinizing hormone (LH) was used in 86% of cycles; final maturation was GnRH agonist (GnRHa) (59.4%) or dual trigger (40.6%). Outcomes were oocyte yield and embryo quality metrics (maturation, two-pronuclear (2PN) fertilization, Day 3 good-quality embryo rate, blastocyst formation rate, and top-quality blastocyst rate). Multivariable linear regression and propensity score matching (PSM) compared protocols and LH supplementation.
Results: Donors were 25.6 ± 3.7 years with AMH 6.1 ± 2.9 ng/mL and BMI 21.6 ± 2.8 kg/m². Per cycle: 27.1 ± 11.1 oocytes, 20.8 ± 8.3 MII (maturation 78.2 ± 13.4%), 2PN fertilization 73 ± 18%, Day 3 good-quality embryos 10.6 ± 6.0 (69.5 ± 23.5%), blastocysts 9.5 ± 5.5 (56.7 ± 22.5%), and top-quality blastocysts 6.1 ± 4.2 (36.5 ± 20.1%). First transfers (n=491) yielded 55.4% clinical pregnancy and 44.4% live birth; miscarriage 12.2%. AMH independently predicted oocyte number; higher BMI was associated with lower fertilization. PPOS produced lower Day 3 good-quality embryo (67.9% vs 72.8%) and top-quality blastocyst rates (59.7% vs 74.1%) versus antagonist in PSM, with similar blastocyst formation rate (64.0% vs 60.9%). LH supplementation modestly increased fertilization (74.4% vs 69.5%) without downstream differences. Dual trigger was associated with reduced blastocyst formation rate but a higher proportion of top-quality blastocysts.
Conclusions: In donor IVF, AMH is the principal predictor of oocyte yield, whereas BMI, stimulation protocol, and trigger method influence embryo quality. Despite protocol-dependent morphology differences, pregnancy and live birth rates remained high. Findings support individualized stimulation strategies that consider donor profile and protocol effects to optimize efficiency and outcomes.
1 Background
Oocyte donor cycles provide a well-controlled model to examine factors influencing ovarian response and oocyte quality, as donors are typically young, healthy, and undergo controlled ovarian stimulation under standardized conditions. While maximizing oocyte yield is essential to meet recipient demand, the quality of retrieved oocytes is equally critical to ensure optimal fertilization, embryo development, and pregnancy outcomes. Although ovarian reserve markers such as anti-Müllerian hormone (AMH) and antral follicle count are well-established predictors of oocyte quantity (1, 2), the extent to which donor characteristics and specific stimulation protocols affect oocyte developmental competence remains less well understood.
Young donors typically yield a high number of oocytes; however, an excessive response may be associated with diminishing per-oocyte viability. Prior studies suggest an optimal range of 12–18 oocytes for maximizing implantation, beyond which embryo quality may plateau or decline (3). Donors often exceed this threshold, raising concerns about whether high responders experience compromised oocyte maturation or embryo potential (4). Higher BMI, even within a non-obese range, has been linked to reduced fertilization and blastocyst development in autologous IVF (5, 6). Whether these findings apply to donor IVF cycles remains unclear.
Beyond donor characteristics, stimulation protocols and cycle management strategies may also impact oocyte and embryo quality (7). The progestin-primed ovarian stimulation (PPOS) protocol is widely used in donor cycles for its scheduling advantages, but its effect on embryo competence is debated. Some studies report pregnancy rates comparable to those achieved with GnRH antagonist protocols (8), while others suggest reduced blastocyst quality in certain populations (9). Similarly, the routine use of r-hLH supplementation in young oocyte donors—who typically have intact hypothalamic-pituitary function—has not consistently demonstrated clinical benefit. Nonetheless, its potential impact on fertilization and embryo development may still warrant investigation, as some studies have reported improved embryo quality and live birth rates with LH supplementation even in GnRH antagonist protocols (10–12). The optimal approach for final oocyte maturation triggering in donors, whether using a GnRH agonist alone or a dual trigger (GnRH agonist plus low-dose hCG), also remains unclear. While dual triggers have demonstrated benefits in specific subgroups, such as poor responders or those with previous suboptimal outcomes (13), evidence in young donor populations is limited.
In summary, while many predictors of oocyte yield are known, their relationship to oocyte and embryo quality in the donor population is not fully elucidated. Subtle differences in donor profile and cycle management may influence outcomes, warranting a comprehensive analysis. In this retrospective cohort study, we aimed to identify which donor characteristics and stimulation protocol factors are associated with oocyte yield and embryo developmental competence in donor IVF cycles.
2 Materials and methods
2.1 Study design and study population
This retrospective study reviewed the records of 939 oocyte donors who underwent ovarian stimulation for egg banking at an IVF clinic in Taiwan between January 1, 2018, and December 31, 2023. (Figure 1) Of 939 donor stimulation cycles screened, 584 donor–recipient pairs proceeded to embryo creation and were included in the final analysis; 355 were excluded at screening for procedural reasons (no embryo creation/no pairing; protocol not of interest; cancellation before retrieval) prior to outcome ascertainment.
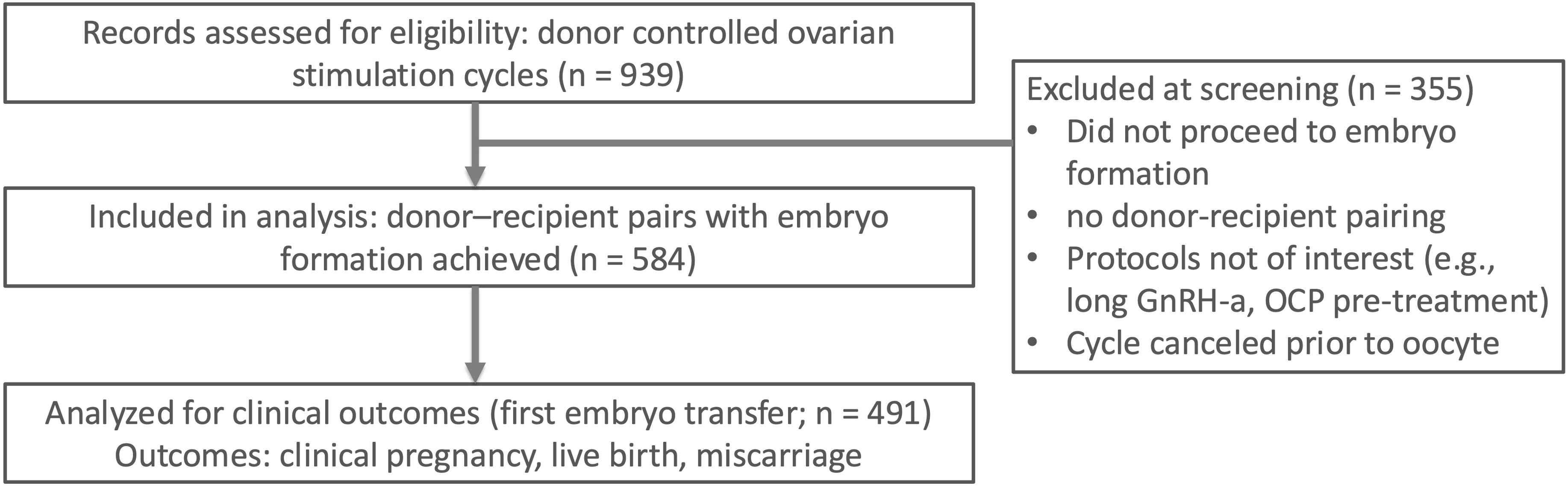
Figure 1. Of 939 donor controlled ovarian stimulation cycles screened, 584 donor–recipient pairs with embryo formation were included in the analysis. A subset of 491 first embryo transfers contributed to clinical outcomes (clinical pregnancy, live birth, miscarriage). Screening exclusions (n = 355) were due to no embryo formation, no donor–recipient pairing, non-study protocols (e.g., long GnRH agonist protocol or OCP pre-treatment), or cycle cancellation before oocyte retrieval. OCP, oral contraceptive pill.
Only cycles utilizing either a GnRH antagonist or a progestin-primed ovarian stimulation (PPOS) protocol were included. Exclusion criteria comprised the use of long GnRH agonist protocols, pre-treatment regimens such as oral contraceptive scheduling, or cancellation of the cycle prior to oocyte retrieval. In addition to donor records, the clinical outcomes of oocyte recipients whose embryos were derived from these donated oocytes were assessed.
Eligible donors were between 20 and 35 years of age, had regular menstrual cycles (25–35 days), and a BMI between 18 and 25 kg/m². All donors had a normal karyotype, adequate ovarian reserve (AMH > 4 ng/mL), no hereditary disorders, and no history of hormonal treatment in the three months preceding donation. According to Taiwan’s Assisted Reproduction Act, oocytes from a single donor may be assigned to only one recipient, thereby preventing multiple allocations from the same donation. This regulation aims to uphold ethical standards, minimize associated risks, and ensure traceability and accountability in the donation process. This study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (IRB: CS2-20201). Due to its retrospective nature, the requirement for written informed consent was waived.
2.2 Controlled ovarian hyperstimulation procedures
Controlled ovarian stimulation was conducted using either a GnRH antagonist or a PPOS protocol. In both protocols, stimulation began on day 3 of the menstrual cycle with 225–300 IU/day of recombinant FSH, either alone or in combination with recombinant LH (r-hFSH + r-hLH; Pergoveris, Merck-Serono, Germany), for five days. Thereafter, 225 IU/day of r-hFSH alone (Gonal-F, Merck-Serono, Germany) was continued until the day of final oocyte maturation triggering.
In the GnRH antagonist group, 0.25 mg of cetrorelix acetate (Cetrotide, Merck-Serono, Germany) was administered daily starting on stimulation day 6 for four consecutive days to suppress premature LH surges. In the PPOS group, oral progestin (medroxyprogesterone acetate, 5 mg twice daily; Provera) was initiated on cycle day 5 and continued until the trigger day.
In both protocols, ovarian response was monitored through serial transvaginal ultrasonography and serum hormone measurements. Final oocyte maturation was triggered when at least two leading follicles reached a mean diameter of ≥17 mm. Triggering was achieved via subcutaneous injection of 0.2 mg triptorelin (GnRH agonist; Decapeptyl, Ipsen, France), either alone or combined with 1500–3000 IU of recombinant human chorionic gonadotropin (hCG) (Ovidrel, Merck, Germany), depending on ovarian response and the risk of ovarian hyperstimulation syndrome (OHSS). Ovarian hyperstimulation syndrome (OHSS) was defined and graded according to the 2024 American Society for Reproductive Medicine (ASRM) Practice Committee guideline on prevention and management of moderate–severe OHSS. For incidence reporting, we present moderate–severe OHSS consistent with the ASRM definitions (14).
2.3 In vitro fertilization, embryo culture, TE biopsy, and frozen embryo transfer
Oocyte retrieval, fertilization, embryo culture, trophectoderm (TE) biopsy, and embryo vitrification/warming were performed according to established protocols (15). Retrieved oocytes were denuded and classified as metaphase II (MII), metaphase I (MI), or germinal vesicle (GV) stage. Mature oocytes (MII) were vitrified using the Cryotec system (Cryotec, Japan) according to the manufacturer’s protocol. Vitrification and warming followed a standard equilibration and ultra-rapid cooling process as described by Gandhi et al (16). All warmed oocytes underwent intracytoplasmic sperm injection (ICSI). Normally fertilized zygotes were defined by the presence of two pronuclei (2PN). Day 3 good-quality embryos were defined as those with ≥8 cells, <20% fragmentation, and symmetrical blastomeres. Blastocyst grading was performed on day 5 or 6 using the Gardner blastocyst morphological scoring system (17). A blastocyst was considered top-quality if it reached expansion grade ≥3 and had an inner cell mass and trophectoderm graded A or B. Top-quality blastocysts were transferred or cryopreserved for frozen embryo transfer. The first embryo transfer per recipient was analyzed to evaluate clinical outcomes. Endometrial preparation was conducted using either a natural cycle or hormone replacement therapy with estradiol and progesterone. An endometrial thickness of ≥7 mm was required prior to embryo transfer. Recipient outcomes are summarized for the first embryo transfer only. Because the first transfer uses the highest-quality embryo available, protocol-level differences upstream are expected to be minimized at this endpoint.
2.4 Data collection and outcome measurements
Two categories of outcomes were evaluated: (a) Oocyte yield: the total number of oocytes retrieved per donor cycle, including all maturation stages. (b) Oocyte and embryo quality outcomes, including: maturation rate = MII oocytes/total oocytes retrieved (%), normal fertilization rate = 2PN zygotes/MII oocytes (%), Day 3 good-quality embryo rate = good-quality cleavage-stage embryos/2PN embryos (%), blastocyst formation rate = blastocysts/2PN embryos (%), top-quality blastocyst rate = top-quality blastocysts/total blastocysts (%). In addition, the clinical pregnancy rate per cycle was recorded and defined as the presence of a gestational sac with a fetal heartbeat detected by ultrasound at 6–8 weeks of gestation following the first embryo transfer, whether from fresh or frozen donor-derived embryos.
Covariates included donor age, AMH, BMI, total administered doses of FSH and LH, serum estradiol (E2) and progesterone (P4) levels on the trigger day, stimulation protocol (GnRH antagonist vs PPOS), LH supplementation (yes/no), and trigger method (GnRH agonist alone, hCG alone, or dual trigger).
2.5 Statistical analysis
We summarized donor characteristics and cycle outcomes with descriptive statistics. Between-group comparisons used independent-sample t tests or χ² tests, as appropriate. Multivariable linear regression evaluated predictors of (1) oocyte yield and (2) embryo-quality outcomes (oocyte maturation, 2-pronuclear [2PN] fertilization, Day 3 good-quality embryo, blastocyst formation, and top-quality blastocyst rates). Coefficients are unstandardized and reported with 95% confidence intervals (CI); outcomes are percentages, so each β is interpreted as an absolute percentage-point change per unit of the predictor (e.g., per 1 ng/mL AMH, per 1 kg/m² BMI, per 100 IU total gonadotropin; PPOS vs antagonist; dual trigger vs GnRHa). Skewed variables were log-transformed before analysis. For the five prespecified embryo-quality endpoints, multiplicity was controlled using Bonferroni (family-wise α=0.05; P<0.005 significant). All other analyses are exploratory with P<0.05 reported for context.
To reduce confounding in protocol contrasts, we performed propensity score matching (PSM) for PPOS vs antagonist (1:2; 154 vs 308 cycles) and LH+ vs LH− (1:2; 162 vs 81 cycles). Propensity scores were estimated from donor age, AMH and BMI; matching used nearest-neighbor with caliper = 0.2 × standard deviation (SD) of the logit(PS). To preserve common support and avoid overfitting in a finite matched sample, the propensity-score model was intentionally limited to baseline determinants (age, AMH, BMI). Covariate balance was assessed by standardized mean differences (SMD < 0.10). We conducted sensitivity analyses using stabilized inverse probability weighting with the same covariates, which yielded directionally consistent estimates. (Follicle counts were not uniformly available; peak estradiol and total gonadotropin dose were used as proxies for follicular response.)
For tables, we additionally report absolute between-group differences (expressed in percentage points) with 95% CI for the two-sample difference in means, alongside group summaries and P values. Based on the observed variability of percentage outcomes (SD approximately 22%), the matched protocol comparison (154 vs 308) provides approximately 80–85% power (two-sided α=0.05) to detect an absolute difference of approximately 6 percentage points; effects near this magnitude are interpreted cautiously. All analyses were performed with SPSS v25 (IBM) and R software.
3 Results
3.1 Baseline characteristics
Among the 939 oocyte donor cycles performed for egg banking, the incidence of suboptimal ovarian response (≤9 oocytes retrieved) was low at 6.4% (n = 61). Moderate to severe ovarian hyperstimulation syndrome (OHSS) occurred in 1.5% of cases, while other complications—including intra-abdominal bleeding, ovarian torsion, infection, procedural injury, or severe pain—were reported in 1.0% of cycles. Of these, 584 donor–recipient pairs who proceeded to oocyte warming and embryo creation were included in the final analysis.
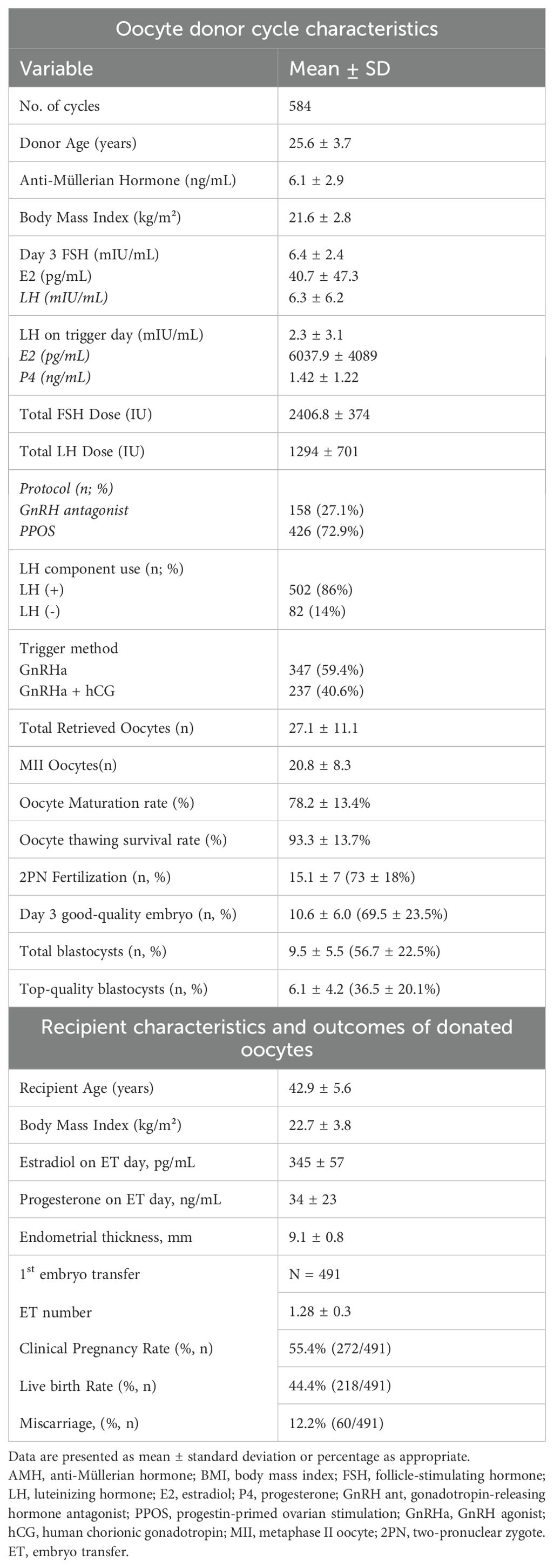
Table 1 summarizes the clinical characteristics and outcomes of the 584 oocyte donor stimulation cycles. Donors had a mean age of 25.6 years, with an average AMH of 6.1 ± 3.0 ng/mL and BMI of 21.6 kg/m². The majority of cycles (73%) utilized a progestin-primed ovarian stimulation (PPOS) protocol, while 27% employed a GnRH antagonist protocol. LH supplementation was used in 86% of cycles. A GnRH agonist trigger was applied in 60% of cases, and a dual trigger (GnRH agonist plus low-dose hCG) in the remaining 40%. On the trigger day, mean peak estradiol and progesterone levels were 6040 pg/mL and 1.4 ng/mL, respectively.
Each cycle yielded an average of 27.1 oocytes, with 20.8 reaching the MII stage, corresponding to a mean maturation rate of 78%. An average of 15.1 oocytes fertilized normally (2PN), yielding a fertilization rate of 73%. On Day 3, 10.5 good-quality embryos were obtained per cycle. Blastocyst formation averaged 9.5 per cycle, with a blastocyst formation rate of 63% per 2PN zygote. Of these, 6.1 blastocysts were graded as top-quality, accounting for 64% of all blastocysts. These findings indicate a robust ovarian response and embryo yield in this donor cohort. Recipients had a mean age of 42.9 ± 5.6 years and a BMI of 22.7 ± 3.8 kg/m². At the time of embryo transfer, the mean endometrial thickness was 9.1 mm, and serum estradiol and progesterone levels averaged 345 ± 57 pg/mL and 34 ± 23 ng/mL, respectively.
Among 491 first embryo transfer cycles, an average of 1.28 ± 0.3 embryos were transferred per cycle. Clinical pregnancy and live birth rates were 55.4% (272/491) and 44.4% (218/491), respectively. The miscarriage rate was 12.2% (60/491).
3.2 Predictors of oocyte yield
Table 2 presents the results of univariate and multivariable linear regression analyses identifying predictors of total oocyte yield. In both models, anti-Müllerian hormone (AMH) emerged as the strongest independent predictor (95% CI: 0.81–1.38; P < 0.001). Peak estradiol (E2) levels were also positively associated with oocyte count (β = 0.0010 per pg/mL; P < 0.001). A higher total LH dose showed a modest but statistically significant negative association with oocyte yield in the adjusted model (β = –0.0019 per IU; P = 0.032), suggesting that higher LH requirements may reflect reduced ovarian responsiveness.
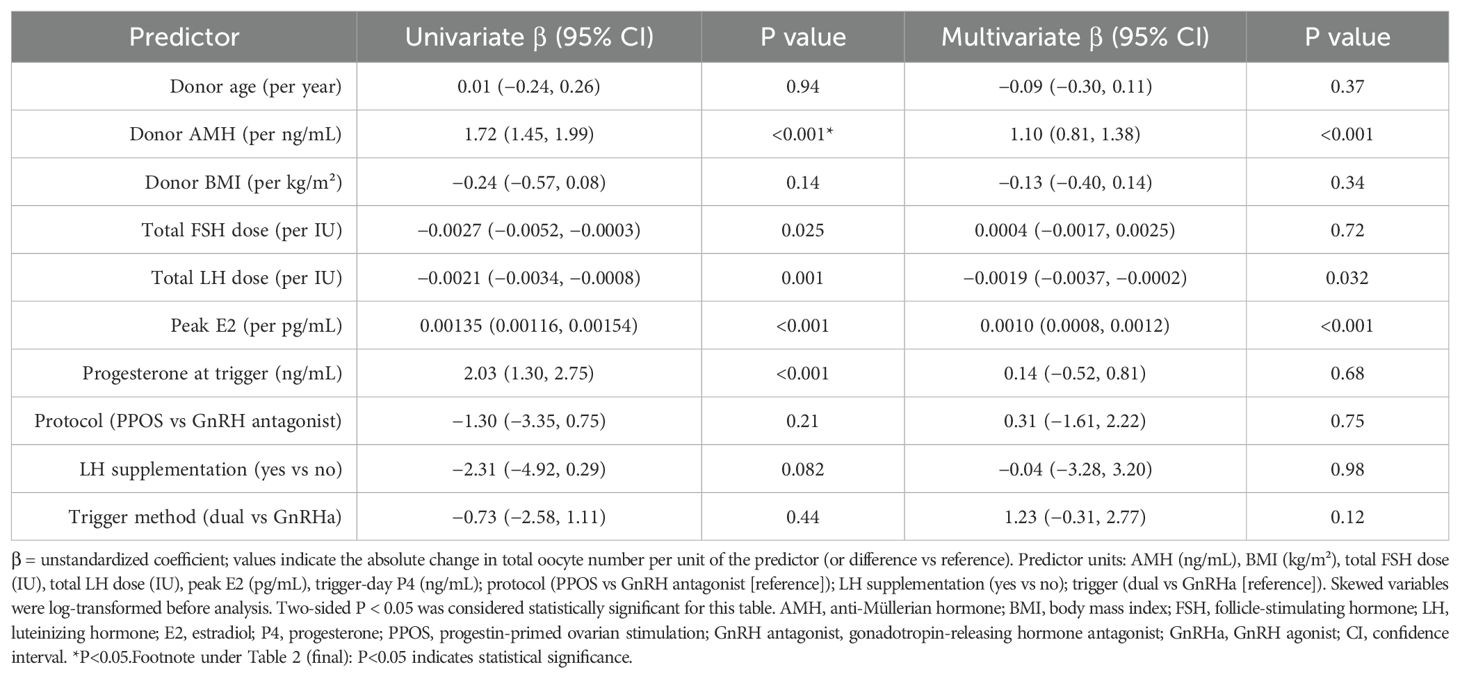
Table 2. Multivariable linear regression analysis identifying independent predictors of oocyte number.
In contrast, donor age, BMI, total FSH dose, trigger-day progesterone, stimulation protocol, LH supplementation, and trigger method were not significantly associated with oocyte yield in the multivariable model (P > 0.05 for all). Several variables—such as total gonadotropin doses and progesterone—reached significance in univariate analysis but lost significance after adjustment, indicating potential confounding. These findings underscore AMH and estradiol as key predictors of oocyte yield, while elevated LH dose may indicate decreased ovarian efficiency.
3.3 Predictors of oocyte quality outcomes
Table 3 presents multivariable regression results for five quality outcomes: oocyte maturation rate, 2PN fertilization rate, Day 3 good-quality embryo rate, blastocyst formation rate, and top-quality blastocyst rate.
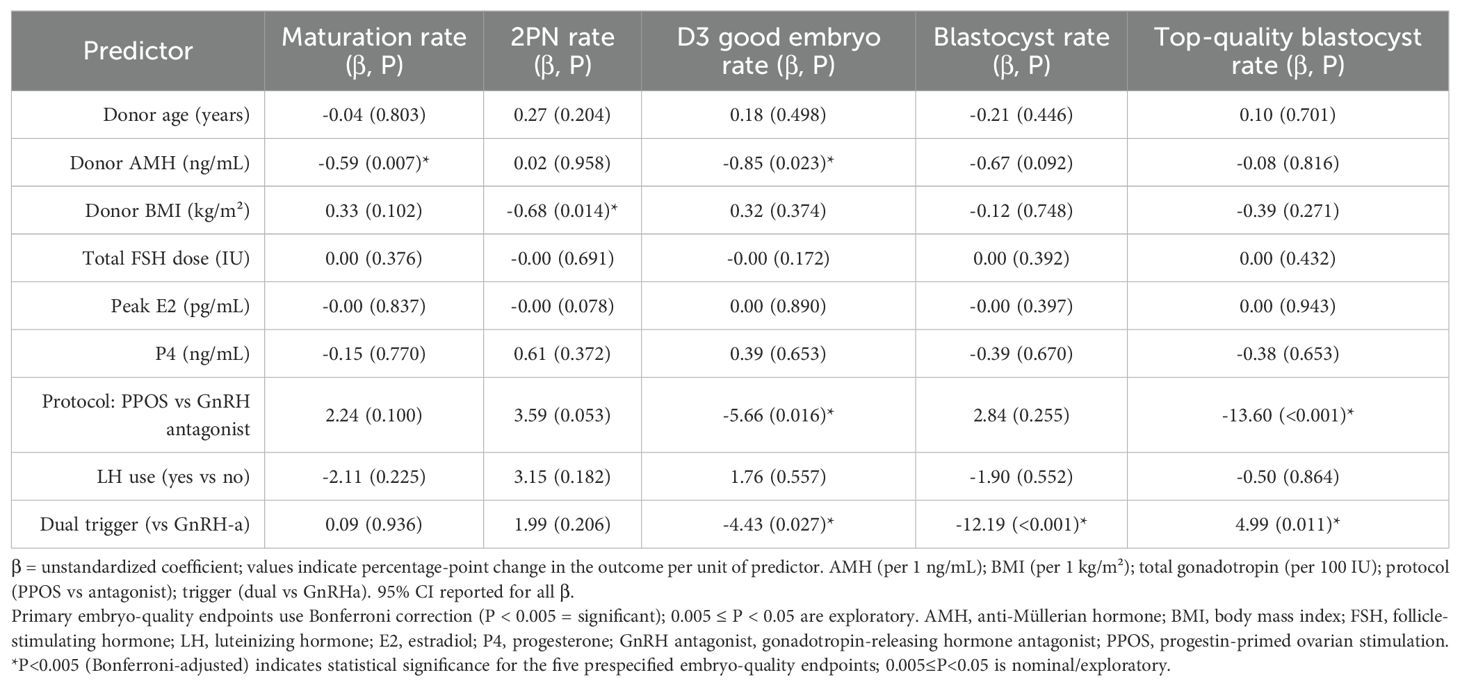
Table 3. Multivariable linear regression analysis identifying predictors of oocyte and embryo quality outcomes.
Donor AMH was negatively associated with oocyte maturation (β = –0.59; P = 0.007), suggesting that higher ovarian reserve may be linked to reduced maturation efficiency. Donor BMI was the only significant predictor of 2PN fertilization rate, showing a negative association (β = –0.68; P = 0.014), indicating that even in healthy donors, elevated BMI may impair fertilization potential.
Regarding early embryo development, the PPOS protocol was associated with significantly lower Day 3 good-quality embryo rates compared to the GnRH antagonist protocol (β = –5.66; P = 0.016). The use of a dual trigger (GnRH agonist plus low-dose hCG) was also associated with reduced Day 3 embryo quality (β = –4.43; P = 0.027), possibly due to endocrine alterations during oocyte maturation. In terms of blastocyst formation, the trigger method had the most notable impact. Dual trigger was associated with a significantly lower blastocyst formation rate (β = –12.19; P < 0.001), while no other covariates reached significance. Interestingly, for top-quality blastocyst rate, PPOS was again associated with a markedly lower rate (β = –13.60; P < 0.001), whereas dual trigger use was positively associated (β = +4.99; P = 0.011), suggesting that although blastocyst formation rate may be reduced, embryos that develop may be of higher morphological quality.
In summary, AMH, BMI, stimulation protocol, and trigger method were independently associated with embryo quality parameters. Notably, PPOS was consistently linked to inferior embryo morphology, while dual trigger exerted divergent effects depending on the developmental stage assessed.
3.4 Propensity score–matched analyses of stimulation protocols and LH supplementation
Propensity score–matched comparisons are summarized in Table 4 between stimulation protocols (GnRH antagonist vs PPOS) and LH supplementation groups (LH+ vs LH–) are presented in Table 4A and Table 4B. Effect sizes are reported as absolute differences (percentage points) with 95% confidence intervals, alongside group summaries and P values. Statistical significance for the five prespecified embryo-quality endpoints is defined by Bonferroni (P < 0.005); results with 0.005 ≤ P < 0.05 are exploratory.
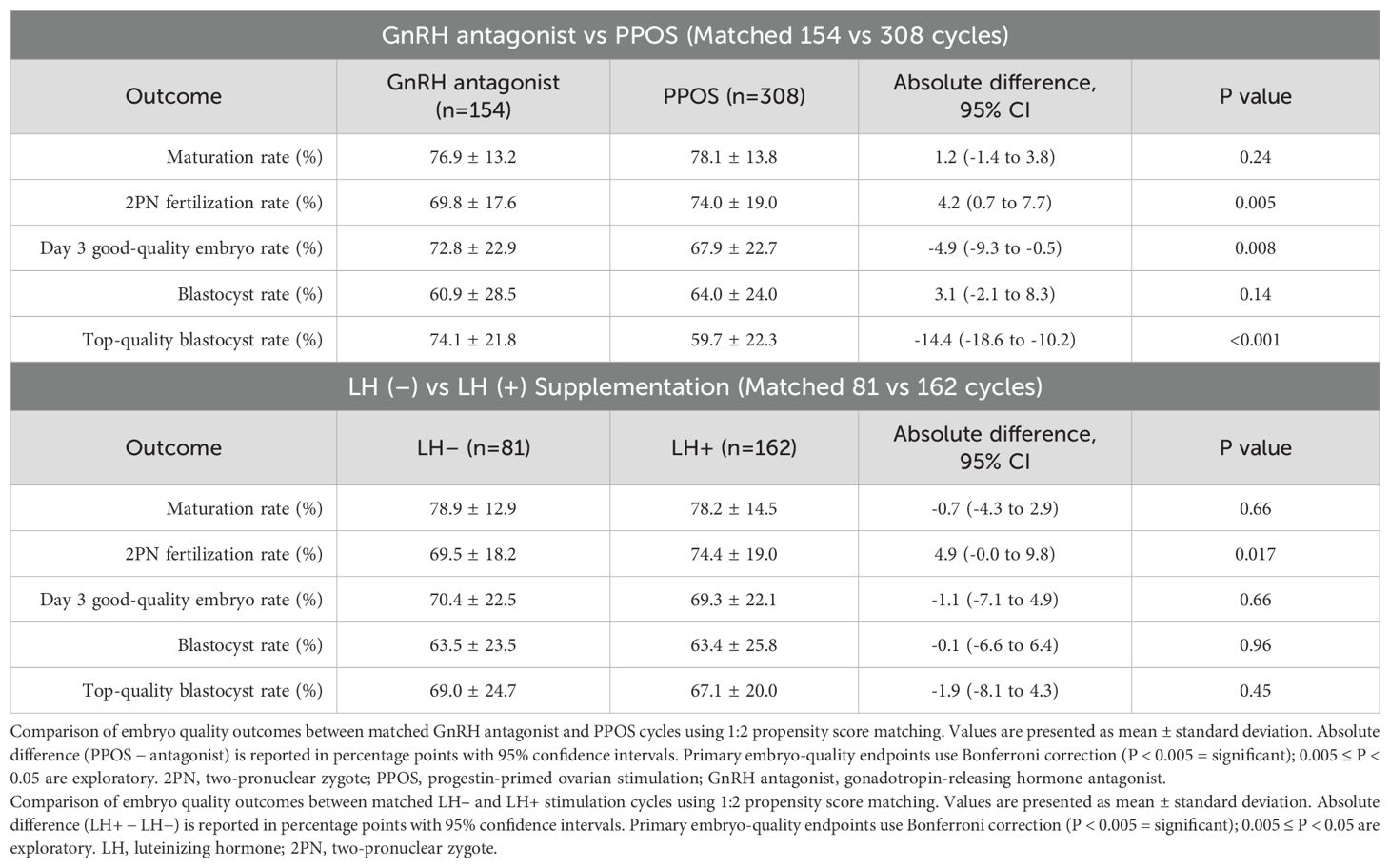
Table 4. Propensity score–matched comparison of embryo quality outcomes by protocol and LH supplementation.
In the protocol-matched cohort (Table 4A), 154 GnRH antagonist cycles were matched with 308 PPOS cycles based on donor age, AMH, and BMI. Fertilization rates were significantly higher in the PPOS group (74.0% vs 69.8%; P = 0.005), suggesting enhanced fertilization efficiency. However, this was offset by significantly reduced embryo quality: Day 3 good-quality embryo rate (67.9% vs 72.8%; P = 0.008) and top-quality blastocyst rate (59.7% vs 74.1%; P < 0.001) were both significantly lower in the PPOS group. No significant differences were found in maturation rate (78.1% vs 76.9%; P = 0.24) or overall blastocyst formation (64.0% vs 60.9%; P = 0.14). These findings suggest that although PPOS yields comparable numbers of embryos, their morphological quality may be compromised.
In the LH supplementation cohort (Table 4B), 81 FSH-only cycles were matched with 162 cycles receiving added LH, again balanced for donor age, AMH, and BMI. LH supplementation was associated with a significantly higher fertilization rate (74.4% vs 69.5%; P = 0.017), suggesting improved oocyte competence at fertilization. However, no statistically significant differences were found in oocyte maturation (78.2% vs 78.9%), Day 3 good-quality embryo rate (69.3% vs 70.4%), blastocyst formation (63.4% vs 63.5%), or top-quality blastocyst rate (67.1% vs 69.0%) between groups (all P > 0.4).
Together, these findings indicate that while PPOS and GnRH antagonist protocols yield similar oocyte yield and blastocyst formation rates, PPOS is associated with lower embryo quality. Conversely, LH supplementation may modestly enhance fertilization potential without significantly affecting downstream embryo development.
4 Discussion
This retrospective analysis of 584 oocyte donor IVF cycles provides meaningful insights into how donor characteristics and stimulation protocols influence oocyte yield and embryo quality. Our findings confirm established predictors—particularly AMH for oocyte quantity—while also uncovering more nuanced associations between stimulation strategies and embryologic outcomes. These results hold practical value for optimizing donor stimulation, especially in balancing treatment efficiency with embryo developmental competence.
4.1 Donor AMH and oocyte quantity vs. quality
Anti-Müllerian hormone (AMH) remains the most reliable predictor of ovarian response. In our cohort, each 1 ng/mL increase in AMH was associated with an average increase of approximately one additional retrieved oocyte, consistent with large-scale studies linking AMH to follicular recruitment and response to stimulation (2, 18, 19). As expected, donors with high AMH levels—commonly selected in donor programs—enabled retrieval of numerous oocytes suitable for banking or multiple transfers. However, our results also revealed a modest inverse relationship between AMH and oocyte/embryo quality. Elevated AMH was significantly associated with reduced oocyte maturation and fewer good-quality embryos at both the cleavage and blastocyst stages (20, 21).
4.2 Donor BMI and fertilization rate
Interestingly, while donor BMI did not impact oocyte yield or blastocyst quality, it emerged as an independent predictor of impaired fertilization rate. This suggests that increased adiposity may impair oocyte competence, even in otherwise healthy young donors. Previous reports (6, 22) have linked higher donor BMI to decreased pregnancy and live birth rates. Our findings specifically implicate fertilization as the vulnerable stage, possibly due to metabolic dysregulation such as insulin resistance, altered lipid metabolism, or systemic inflammation. Notably, once fertilized, embryos from higher-BMI donors performed comparably in terms of blastocyst formation and morphological quality. This implies that the principal barrier may lie at or before fertilization. These findings underscore the importance of metabolic health prior to donation, even among donors with otherwise normal ovarian function. While many programs already impose BMI cutoffs for donor eligibility, our results suggest that optimizing BMI within the normal range could further improve fertilization outcomes and downstream embryo yield.
4.3 Stimulation protocol: PPOS vs. GnRH antagonist
Although PPOS offers logistical advantages and has been associated with comparable pregnancy outcomes in some studies (8), our data suggest potential embryologic disadvantages in young donor populations. The choice of ovarian suppression protocol had no significant effect on oocyte yield but had a notable impact on embryo quality. Compared to GnRH antagonist cycles, PPOS cycles resulted in 6% fewer Day 3 good-quality embryos and 13% fewer top-quality blastocysts, as shown by both multivariable and propensity-matched analyses. These findings align with previous reports (9, 23).
The likely mechanism involves supraphysiologic progesterone exposure impairing oocyte competence through altered granulosa cell signaling (24), disrupted oocyte gene expression and mitochondrial function (25), or impaired cumulus–oocyte communication (26). Additionally, more complete LH suppression in PPOS may disturb steroidogenesis compared to the partial suppression in antagonist cycles (27).
Although one smaller donor study reported similar oocyte and embryo counts between protocols (28), embryo quality was not evaluated. In our larger cohort, quality differences were evident despite comparable oocyte and blastocyst yields. Since all embryos were frozen before transfer, our findings reflect intrinsic differences in oocyte and embryo quality rather than endometrial receptivity.
Clinically, PPOS provides logistical and cost advantages, especially in donor settings where scheduling flexibility and avoiding daily injections are desirable (29). However, when maximizing embryo competence is the priority—for instance, in egg banking or efforts to maximize cumulative live birth per retrieval—GnRH antagonist protocols may be preferable. Our data suggest caution in assuming equivalence between protocols, and further studies should evaluate whether modifications to PPOS (e.g., progestin type, dose, or trigger timing) could mitigate its potential impact on embryo quality (30–32).
In our clinical workflow, the first transfer is intentionally performed with the top-quality embryo, which reduces the likelihood of detecting donor protocol/trigger effects at the first-transfer level. For translational relevance, cumulative live birth per retrieval may be a more sensitive endpoint if one protocol yields more transferable embryos. We plan to analyze cumulative outcomes once longitudinal follow-up is complete.
4.4 LH supplementation: limited utility in young donors
In our cohort, routine LH supplementation did not enhance oocyte maturation or embryo development. Although LH+ cycles showed slightly higher fertilization rates, the difference was not statistically significant after adjustment. This supports previous findings suggesting that LH supplementation may benefit selected populations—such as older women or those with hypogonadotropic hyporesponsiveness (12, 33, 34)—but not necessarily young, normo-ovulatory donors. In such donors, endogenous LH levels are typically sufficient even under GnRH antagonist protocols, rendering exogenous LH unnecessary (35).
Moreover, excessive LH exposure may negatively affect follicular synchrony or promote premature luteinization (36). Consistent with this, we observed a modest inverse association between total LH dose and oocyte yield. Overall, FSH-only stimulation appears adequate for most young donors. LH supplementation may be selectively considered in cases of prior suboptimal fertilization or borderline ovarian reserve, but its routine use may not be necessary in young normo-responders. This approach may reduce medication costs and simplify protocols without compromising clinical outcomes.
4.5 Trigger strategy: GnRH agonist alone vs. dual trigger
Dual trigger—combining a GnRH agonist with low-dose hCG—was initially designed to improve oocyte maturation, particularly in poor responders. However, in our high-responder donor cohort, dual trigger was not associated with better oocyte or embryo outcomes (37). In fact, it was linked to significantly lower blastocyst formation rates and slightly reduced Day 3 embryo quality. Although the proportion of top-quality blastocysts was marginally higher, the absolute number of blastocysts was lower, suggesting a trade-off between embryo quality and quantity.
One possible explanation is selection bias, as dual trigger was often used in donors with fewer mature follicles or prior poor maturation. However, our adjusted models and matched analyses support a true physiological effect. Biologically, hCG prolongs LH receptor activation in granulosa cells, which may promote early luteinization or disrupt follicular signaling in ways that impair oocyte competence.
Given that GnRH agonist trigger alone achieved high maturation and embryo development rates while effectively reducing OHSS risk, it remains the preferred strategy for most donor cycles. Dual trigger may still be appropriate for specific indications such as prior poor oocyte maturation or asynchronous follicular development, but it does not confer consistent benefit in high-responder donors (38, 39).
4.6 Strengths and limitations
Key strengths of this study include its large donor cohort, use of standardized laboratory protocols, and robust statistical methodology, including multivariable adjustment and propensity score matching. The exclusive focus on donor cycles ensured a homogenous population, minimizing uterine-related confounding and enabling clearer evaluation of oocyte and embryo-level outcomes.
This study was conducted at a single high-volume IVF center, which ensured standardized clinical management and laboratory workflows but may limit the external generalizability of our findings to other populations or practice environments. Future multi-center studies including diverse donor and recipient populations are warranted to validate these associations. Additionally, the retrospective design precludes causal inference and introduces potential selection bias, particularly regarding stimulation protocol selection. Clinical decisions may have been influenced by physician preference or logistical constraints. We also did not stratify outcomes by extreme AMH levels, which could differentially affect embryo quality. While statistically significant, the absolute decrease in fertilization rate with increasing BMI was modest, and the clinical relevance of this finding warrants cautious interpretation. As our embryo assessment relied on morphological criteria rather than genetic analysis in this analysis, the relationship between stimulation protocols and euploidy remains uncertain. Further studies integrating PGT-A data are needed to determine whether the observed morphological differences reflect true differences in embryo euploidy. Furthermore, recipient outcomes were reported in aggregate and not stratified by stimulation protocol, due to heterogeneity in recipient characteristics and embryo origin. Future prospective or randomized studies—particularly those evaluating blastocyst yield, embryo aneuploidy, and recipient outcomes—are warranted to validate and expand upon these findings. Another limitation is that calendar time was not modeled explicitly; although clinical and embryology workflows were highly standardized and endpoints were laboratory-based, any residual secular/batch effects cannot be excluded. Because baseline data for cycles excluded at screening were not captured in the research registry, we could not compare included versus excluded cohorts. Although exclusions were procedural and occurred prior to outcome ascertainment, some selection bias cannot be excluded. Future prospective, multi-center registries that capture all screened donors are needed to directly evaluate this issue. To preserve common support and avoid overfitting in a finite matched sample, the propensity-score model was intentionally limited to baseline determinants (age, AMH, BMI) measured prior to stimulation; trigger-day estradiol and total gonadotropin (on-path variables), non-uniform follicle counts, and calendar year were not included in the PS but were handled in multivariable outcome models and acknowledged in the Limitations. Our matched cohort sizes and observed variance provide approximately 80–85% power to detect approximately 6% absolute differences in embryo-quality rates; larger effects (e.g., top-quality blastocyst rate) are estimated with good precision, while approximately 5% effects are near the detection boundary. Interval estimates and Bonferroni-adjusted inferences are provided to contextualize statistical uncertainty.
5 Conclusion
This study confirms that donor AMH is the principal predictor of oocyte yield, while embryo quality is influenced by both donor characteristics and stimulation protocols. Higher donor AMH levels and elevated BMI were modestly associated with reduced oocyte maturation and fertilization rates, underscoring the importance of tailoring stimulation strategies to individual donor profiles. Although PPOS yielded comparable oocyte yield to the GnRH antagonist protocol, it was consistently associated with lower embryo quality, warranting caution when embryo competence is a clinical priority. LH supplementation demonstrated limited benefit, and dual trigger did not improve outcomes compared to GnRH agonist alone in this young donor cohort. All protocols achieved high pregnancy and live birth rates, reflecting the overall efficacy of donor IVF. Nevertheless, individualizing stimulation strategies based on AMH, BMI, and prior cycle response may enhance oocyte yield and embryo developmental potential. Further prospective studies are warranted to validate these associations and inform optimal protocol selection in clinical donor IVF programs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (IRB No. CS2-20201) and was conducted in accordance with the Declaration of Helsinki and all applicable national regulations. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was waived due to the retrospective nature of the study.
Author contributions
P-YL: Investigation, Writing – review & editing, Methodology, Formal Analysis, Writing – original draft, Conceptualization. C-IL: Writing – review & editing, Visualization. H-HC: Writing – review & editing, Data curation, Project administration, Investigation. C-CH: Writing – review & editing, Project administration. M-JC: Visualization, Writing – review & editing, Supervision. T-NY: Visualization, Writing – review & editing, Project administration. T-HL: Writing – review & editing, Visualization, Supervision. M-SL: Supervision, Writing – review & editing, Investigation, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Medical writing support was funded by Merck Ltd. (Taiwan). The funder had no role in study design, data collection, analysis, interpretation, decision to publish, or manuscript preparation.
Acknowledgments
The authors thank the clinical and laboratory staff at the IVF center for their support in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sukur YE, Aslan B, Kaplan NB, Dogru M, Ozmen B, Sonmezer M, et al. Inter-cycle variability of anti-Mullerian hormone: implications for predicting controlled ovarian stimulation cycle outcomes. J Ovarian Res. (2024) 17:209. doi: 10.1186/s13048-024-01517-x
2. La Marca A and Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. (2014) 20:124–40. doi: 10.1093/humupd/dmt037
3. Law YJ, Zhang N, Kolibianakis EM, Costello MF, Keller E, Chambers GM, et al. Is there an optimal number of oocytes retrieved at which live birth rates or cumulative live birth rates per aspiration are maximized after ART? A systematic review. Reprod BioMed Online. (2021) 42:83–104. doi: 10.1016/j.rbmo.2020.10.008
4. Yuwen T, Yang Z, Cai G, Feng G, Liu Q, and Fu H. Association between serum AMH levels and IVF/ICSI outcomes in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2023) 21:95. doi: 10.1186/s12958-023-01153-y
5. Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology. (2008) 15:115–21. doi: 10.1016/j.pathophys.2008.04.004
6. Fabozzi G, Cimadomo D, Maggiulli R, Vaiarelli A, Badajoz V, Aura M, et al. Association between oocyte donors' or recipients' body mass index and clinical outcomes after first single blastocyst transfers-the uterus is the most affected. Fertil Steril. (2024) 121:281–90. doi: 10.1016/j.fertnstert.2023.07.029
7. Harvey AJ, Willson BE, Surrey ES, and Gardner DK. Ovarian stimulation protocols: impact on oocyte and endometrial quality and function. Fertil Steril. (2025) 123:10–21. doi: 10.1016/j.fertnstert.2024.08.340
8. Chen ZQ, Ai A, Zhang Y, Li H, Wang JY, Wang L, et al. A randomized controlled trial to compare the live birth rate of the first frozen embryo transfer following the progestin-primed ovarian stimulation protocol vs the antagonist protocol in women with an anticipated high ovarian response. Fertil Steril. (2024) 121:937–45. doi: 10.1016/j.fertnstert.2024.01.027
9. Zhou R, Dong M, Huang L, Wang S, Fan L, Liang X, et al. Comparison of cumulative live birth rates between progestin-primed ovarian stimulation protocol and gonadotropin-releasing hormone antagonist protocol in different populations. Front Endocrinol (Lausanne). (2023) 14:1117513. doi: 10.3389/fendo.2023.1117513
10. Canosa S, Carosso AR, Mercaldo N, Ruffa A, Evangelista F, Bongioanni F, et al. Effect of rLH Supplementation during Controlled Ovarian Stimulation for IVF: Evidence from a Retrospective Analysis of 1470 Poor/Suboptimal/Normal Responders Receiving Either rFSH plus rLH or rFSH Alone. J Clin Med. (2022) 11:1575. doi: 10.3390/jcm11061575
11. Wang M, Huang R, Liang X, Mao Y, Shi W, and Li Q. Recombinant LH supplementation improves cumulative live birth rates in the GnRH antagonist protocol: a multicenter retrospective study using a propensity score-matching analysis. Reprod Biol Endocrinol. (2022) 20:114. doi: 10.1186/s12958-022-00985-4
12. Alviggi C, Vigilante L, Cariati F, Conforti A, and Humaidan P. The role of recombinant LH in ovarian stimulation: what's new? Reprod Biol Endocrinol. (2025) 23:38. doi: 10.1186/s12958-025-01361-8
13. Lin MH, Wu FS, Hwu YM, Lee RK, Li RS, and Li SH. Dual trigger with gonadotropin releasing hormone agonist and human chorionic gonadotropin significantly improves live birth rate for women with diminished ovarian reserve. Reprod Biol Endocrinol. (2019) 17:7. doi: 10.1186/s12958-018-0451-x
14. Practice Committee of the American Society for Reproductive Medicine. Prevention of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. (2024) 121:230–45. doi: 10.1016/j.fertnstert.2023.11.013
15. Chen HH, Huang CC, Cheng EH, Lee TH, Chien LF, and Lee MS. Optimal timing of blastocyst vitrification after trophectoderm biopsy for preimplantation genetic screening. PloS One. (2017) 12:e0185747. doi: 10.1371/journal.pone.0185747
16. Gandhi GK, Kuwayama M, Kagalwala S, and Pangerkar P. Appendix A: Cryotech® vitrification thawing. In: Cryopreservation of mammalian gametes and embryos: methods and protocols. Springer New York, New York,NY (2017). pp. 281–295.
17. Gardner DK and Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. (1999) 11:307–11. doi: 10.1097/00001703-199906000-00013
18. Broer SL, Dolleman M, Opmeer BC, Fauser BC, Mol BW, and Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. (2011) 17:46–54. doi: 10.1093/humupd/dmq034
19. Li R, Gong F, Zhu Y, Fang W, Yang J, Liu J, et al. Anti-Mullerian hormone for prediction of ovarian response in Chinese infertile women undergoing IVF/ICSI cycles: a prospective, multi-centre, observational study. Reprod BioMed Online. (2016) 33:506–12. doi: 10.1016/j.rbmo.2016.07.003
20. Tal R, Seifer CM, Khanimov M, Seifer DB, and Tal O. High serum Antimullerian hormone levels are associated with lower live birth rates in women with polycystic ovarian syndrome undergoing assisted reproductive technology. Reprod Biol Endocrinol. (2020) 18:20. doi: 10.1186/s12958-020-00581-4
21. Mashiach R, Amit A, Hasson J, Amzalzg S, Almog B, Ben-Yosef D, et al. Follicular fluid levels of anti-Mullerian hormone as a predictor of oocyte maturation, fertilization rate, and embryonic development in patients with polycystic ovary syndrome. Fertil Steril. (2010) 93:2299–302. doi: 10.1016/j.fertnstert.2009.01.125
22. Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, and Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. (2013) 28:2720–7. doi: 10.1093/humrep/det292
23. Yang AM, Feng TF, Han Y, Zhao ZM, Wang W, Wang YZ, et al. Progestin-primed ovarian stimulation protocol for patients with endometrioma. Front Endocrinol (Lausanne). (2022) 13:798434. doi: 10.3389/fendo.2022.798434
24. Foot NJ, Dinh DT, Emery-Corbin SJ, Yousef JM, Dagley LF, and Russell DL. Comparative analysis of the progesterone receptor interactome in the human ovarian granulosa cell line KGN and other female reproductive cells. Proteomics. (2025):e202400374. doi: 10.1002/pmic.202400374
25. Smith KM, Dinh DT, Akison LK, Nicholls M, Dunning KR, Morimoto A, et al. Intraovarian, isoform-specific transcriptional roles of progesterone receptor in ovulation. Cells. (2022) 11(9):1563. doi: 10.3390/cells11091563
26. Chen YC, Li JY, Li CJ, Tsui KH, Wang PH, Wen ZH, et al. Luteal Phase Ovarian Stimulation versus Follicular Phase Ovarian Stimulation results in different Human Cumulus cell genes expression: A pilot study. Int J Med Sci. (2021) 18:1600–8. doi: 10.7150/ijms.55955
27. Chen Q, Chai W, Wang Y, Cai R, Zhang S, Lu X, et al. Progestin vs Gonadotropin-Releasing Hormone Antagonist for the Prevention of Premature Luteinizing Hormone Surges in Poor Responders Undergoing in vitro Fertilization Treatment: A Randomized Controlled Trial. Front Endocrinol (Lausanne). (2019) 10:796. doi: 10.3389/fendo.2019.00796
28. Khurana RK, Rao V, Nayak C, Pranesh GT, and Rao KA. Comparing progesterone primed ovarian stimulation (PPOS) to gnRH antagonist protocol in oocyte donation cycles. J Hum Reprod Sci. (2022) 15:278–83. doi: 10.4103/jhrs.jhrs_85_22
29. Hendrickx S, De Vos M, De Munck N, Mackens S, Ruttens S, Tournaye H, et al. Progestin primed ovarian stimulation using dydrogesterone from day 7 of the cycle onwards in oocyte donation cycles: a longitudinal study. Reprod BioMed Online. (2024) 48:103732. doi: 10.1016/j.rbmo.2023.103732
30. Pai AH, Sung YJ, Li CJ, Lin CY, and Chang CL. Progestin Primed Ovarian Stimulation (PPOS) protocol yields lower euploidy rate in older patients undergoing IVF. Reprod Biol Endocrinol. (2023) 21:72. doi: 10.1186/s12958-023-01124-3
31. Yang L, Liang F, Yuan Y, Luo X, Wang Q, Yao L, et al. Efficacy of progestin-primed ovarian stimulation in women with polycystic ovary syndrome undergoing in vitro fertilization: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1224858. doi: 10.3389/fendo.2023.1224858
32. Ata B, Cakar A, Turkgeldi E, Yildiz S, Keles I, and Kalafat E. Should the trigger to oocyte retrieval interval be different in progestin-primed ovarian stimulation cycles? Reprod BioMed Online. (2024) 48:103626. doi: 10.1016/j.rbmo.2023.103626
33. Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril. (2012) 97:1108–14.e1. doi: 10.1016/j.fertnstert.2012.01.130
34. Mannaerts B, Yding Andersen C, and Howles CM. Does HCG and LH supplementation during ovarian stimulation improve clinical outcome? A evaluation of 30 years of clinical research. Reprod BioMed Online. (2024) 50:104782. doi: 10.1016/j.rbmo.2024.104782
35. Paterson ND, Foong SC, and Greene CA. Improved pregnancy rates with luteinizing hormone supplementation in patients undergoing ovarian stimulation for IVF. J Assist Reprod Genet. (2012) 29:579–83. doi: 10.1007/s10815-012-9740-z
36. Loumaye E, Engrand P, Shoham Z, Hillier SG, and Baird DT. Clinical evidence for an LH 'ceiling' effect induced by administration of recombinant human LH during the late follicular phase of stimulated cycles in World Health Organization type I and type II anovulation. Hum Reprod. (2003) 18:314–22. doi: 10.1093/humrep/deg066
37. Wang Y, Yi YC, Guu HF, Chen YF, Kung HF, Chang JC, et al. GnRH agonist-only trigger, compared to dual trigger, reduces oocyte retrieval rate in high responders without affecting cumulative live birth rate. Front Endocrinol (Lausanne). (2024) 15:1461317. doi: 10.3389/fendo.2024.1461317
38. Tu B, Zhang H, Chen L, Yang R, Liu P, Li R, et al. Co-administration of GnRH-agonist and hCG (double trigger) for final oocyte maturation increases the number of top-quality embryos in patients undergoing IVF/ICSI cycles. J Ovarian Res. (2024) 17:137. doi: 10.1186/s13048-024-01465-6
Keywords: oocyte donation, anti-Müllerian hormone, progestin-primed ovarian stimulation, body mass index, dual trigger, donor in vitro fertilization (IVF), propensity score matching
Citation: Lin P-Y, Lee C-I, Chen H-H, Huang C-C, Chen M-J, Yu T-N, Lee T-H and Lee M-S (2025) Factors influencing oocyte yield and embryo quality in donor IVF cycles: a retrospective cohort study. Front. Endocrinol. 16:1649523. doi: 10.3389/fendo.2025.1649523
Received: 18 June 2025; Accepted: 14 October 2025;
Published: 31 October 2025.
Edited by:
Eda Vrtacnik Bokal, Department Of Gynaecology University Medical Centre, SloveniaReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, ChinaHongBo Wu, Qinzhou Maternity and Child Health Care Hospital, China
Copyright © 2025 Lin, Lee, Chen, Huang, Chen, Yu, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maw-Sheng Lee, bXNsZWVwaGRAZ21haWwuY29t
 Pin-Yao Lin
Pin-Yao Lin Chun-I Lee
Chun-I Lee Hsiu-Hui Chen
Hsiu-Hui Chen Chun-Chia Huang
Chun-Chia Huang Ming-Jer Chen1,5,6
Ming-Jer Chen1,5,6 Tzu-Ning Yu
Tzu-Ning Yu Tsung-Hsien Lee
Tsung-Hsien Lee Maw-Sheng Lee
Maw-Sheng Lee