- 1Sport Science School, Beijing Sport University, Beijing, China
- 2School of Physical Education, Shanxi Normal University, Taiyuan, China
Background: Polycystic ovary syndrome (PCOS) is a prevalent endocrine-metabolic disorder characterized by Insulin Resistance (IR), hyperandrogenism, and ovulatory dysfunction, with gut dysbiosis emerging as a key pathophysiological driver. Exercise, a non-pharmacological intervention, ameliorates PCOS symptoms, yet the molecular mechanisms linking exercise-induced gut microbiota remodeling to metabolic improvements remain elusive.
Objective: This review synthesizes evidence on how exercise reshapes gut microbiota to reverse core PCOS pathologies through integrated molecular pathways.
Results: Exercise enriches beneficial taxa (e.g., Faecalibacterium, Roseburia, Akkermansia muciniphila) and reduces pro-inflammatory pathogens (e.g., Proteobacteria), elevating short-chain fatty acids (SCFAs) and secondary bile acids (BAs) while suppressing lipopolysaccharide (LPS) translocation. We propose three core mechanisms:(1) SCFAs network reconstruction: Butyrate/propionate enhance gut barrier integrity (via ZO-1/Occludin), inhibit histone deacetylases (suppressing CYP17A1), activate GLP-1 secretion (FFAR3-dependent), and mitigate inflammation. (2) BA-FXR axis activation: Exercise increases secondary BAs (e.g., deoxycholic acid), activating hepatic FXR to inhibit gluconeogenesis (*PEPCK/G6Pase*) and upregulate androgen-clearance enzymes (*SULT2A1/CYP3A4*). (3) LPS-inflammation inhibition: Reduced LPS blunts TLR4/NF-κB signaling and NLRP3 inflammasome activation, resolving chronic inflammation. These axes converge to improve tissue-specific PCOS features: ovarian androgen synthesis (HDAC/NF-κB inhibition), hepatic IR (FXR/PI3K-Akt), and ovulatory function (AhR-mediated Treg/Th17 balance). Exercise modality differentially impacts PCOS subtypes—endurance training benefits IR-dominant phenotypes via SCFAs producers, while resistance training reduces inflammation in obese PCOS.
Conclusion: Exercise remodels the gut microbiota-metabolism-immune network to reverse PCOS pathophysiology. Targeting microbial metabolites (e.g., butyrate, BAs) or their receptors (FXR, GPR43) offers novel therapeutic strategies. Future research must address PCOS heterogeneity and optimize exercise protocols for microbiota-directed precision medicine.
Highlights
● Exercise enriches SCFA-producers (Faecalibacterium, Roseburia) and Akkermansia while reducing Proteobacteria.
● SCFAs activate FFAR3/GPR43 to enhance GLP-1 secretion, gut barrier integrity, and HDAC inhibition.
● Secondary bile acids activate FXR to suppress hepatic gluconeogenesis and upregulate androgen clearance.
● Exercise inhibits LPS-TLR4/NF-κB and NLRP3 inflammasome, resolving chronic inflammation in PCOS.
● Modality-specific effects: Endurance training targets IR; resistance training reduces inflammation in obese PCOS.
1 Introduction
Polycystic Ovary Syndrome (PCOS) is a prevalent endocrine-metabolic disorder affecting millions of women of reproductive age (1, 2). Its core features include metabolic dysfunction, notably insulin resistance (IR), hormonal imbalances such as hyperandrogenism, and chronic low-grade inflammation. These disruptions contribute to significant health risks, including infertility, type 2 diabetes, and cardiovascular disease (1, 3–6). Global prevalence estimates range from 8% to 18%, varying significantly with diagnostic criteria and study populations (7). The etiology of PCOS is multifactorial, involving genetic, environmental, and lifestyle factors. Notably, gut microbiota dysbiosis has emerged as a key pathophysiological driver, exacerbating inflammation, oxidative stress, and metabolic abnormalities central to the syndrome (8, 9).
The gut microbiota, a complex ecosystem integral to host metabolism and immunity (10, 11), can directly modulate pathways involved in PCOS pathogenesis, including steroid hormone metabolism and insulin sensitivity (12). Accumulating evidence confirms that gut dysbiosis is a hallmark of PCOS and is mechanistically linked to its development and progression (8, 9).
Current PCOS treatments, while effective, often carry risks of adverse effects, highlighting the need for safer alternatives. Exercise, a non-pharmacological intervention, has demonstrated efficacy in alleviating PCOS symptoms (13, 14). However, the precise molecular mechanisms through which exercise-induced gut microbiota remodeling confers these metabolic benefits remain incompletely defined.
A recent comprehensive review has extensively summarized the role of gut microbiota in the pathogenesis and metabolic disorders of PCOS, including insulin resistance, hormonal imbalances, bile acid metabolism dysregulation, IL-22-mediated immune dysregulation, and brain-gut axis disturbances (15). Furthermore, as a non-pharmacological intervention, exercise has been demonstrated to significantly alter the composition and function of the gut microbiota, suggesting a novel mechanistic pathway through which it may ameliorate PCOS symptoms. While that review provides a broad overview of microbial contributions to PCOS, the present review distinctively focuses on the mechanistic links between exercise-induced gut microbiota remodeling and the amelioration of core PCOS symptoms.Therefore, this review aims to systematically integrate the current knowledge on the complex interrelationships among exercise intervention, gut microbiota composition/function (including microbial metabolites), and key PCOS pathological features, with a specific focus on the underlying molecular mechanisms driving metaboic remodeling. Our primary objective is to elucidate the specific molecular pathways through which exercise modulates the gut microbiome to improve metabolic homeostasis (notably insulin sensitivity), reduce hyperandrogenism, and mitigate inflammation in PCOS. By establishing a unified mechanistic framework linking exercise-induced microbial shifts to metabolic and endocrine improvements, this review seeks to fill a critical knowledge gap and provide robust scientific evidence essential for developing novel, effective, and microbiota-targeted therapeutic strategies, including optimized exercise prescriptions, for the management of PCOS.
2 The pathological association between gut dysbiosis and PCOS
2.1 Gut microbiota characteristics in PCOS patients
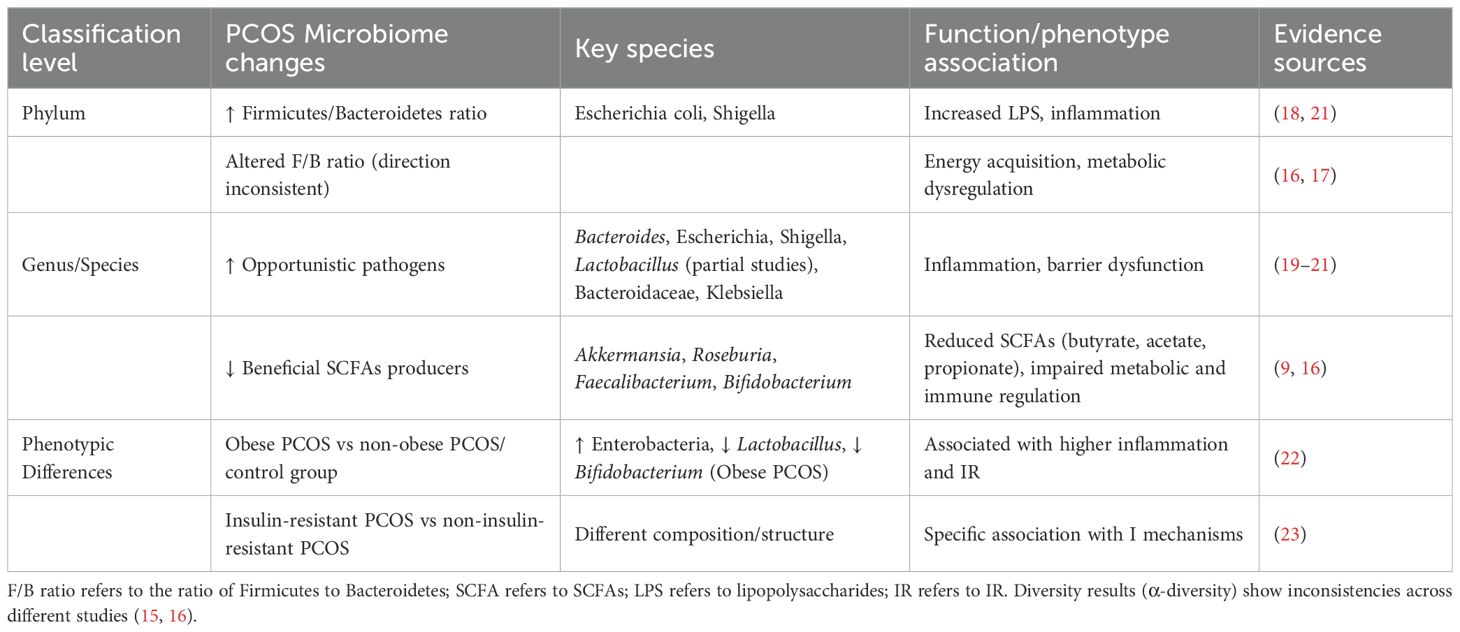
Current research on gut microbiota diversity in PCOS patients shows conflicting results. While most cross-sectional studies report a significant decrease in α-diversity in PCOS patients compared to healthy individuals (15), a meta-analysis found no statistically significant differences in overall diversity (16). This discrepancy likely arises from the high heterogeneity within the PCOS patient population and inadequate control of confounding factors such as dietary habits and antibiotic use in study designs (17).
Characteristic alterations are observed across multiple taxonomic ranks. At the phylum level, several studies confirm a significant increase in Proteobacteria abundance, particularly Gram-negative bacteria like Escherichia spp. and Shigella spp., whose lipopolysaccharides (LPS) may contribute to PCOS pathogenesis by triggering systemic chronic inflammation (18). Additionally, an imbalanced Bacteroidetes to Firmicutes ratio (F/B ratio) is widely reported, potentially exacerbating metabolic dysregulation (17). At the genus/species level, there is enrichment of opportunistic pathogens (e.g., Bacteroides spp., Escherichia spp., Shigella spp., Lactobacillus, Bacteroidaceae, and Klebsiella spp.) (19–21) alongside depletion of beneficial bacteria such as Akkermansia and Roseburia (11). A pronounced reduction is observed in short-chain fatty acid (SCFAs)-producing bacteria, including Roseburia, Faecalibacterium, and Bifidobacterium, whose functional loss may exacerbate PCOS-related metabolic and endocrine abnormalities (16).
Gut microbiota composition also differs according to PCOS phenotype. Obese PCOS patients exhibit increased Enterobacteriaceae and reduced Lactobacillus and Bifidobacterium compared to non-obese PCOS patients and healthy controls, changes associated with elevated inflammation and IR (22). Differences in gut microbiota composition and structure are also noted between PCOS patients with and without IR (23). These phenotype-specific microbial patterns highlight the need for targeted interventions that can modulate the gut ecosystem.
These characteristic shifts in gut microbiota composition (Table 1), including the reduction in SCFAs-producing bacteria and the increase in pro-inflammatory taxa, have been suggested to play a significant role in driving the metabolic dysfunction central to PCOS pathology.
2.2 Hyperandrogenism
Hyperandrogenism, a core pathological feature of PCOS, is linked to gut dysbiosis through a complex interaction network involving inflammation, epigenetic regulation, bile acid metabolism, and neuroendocrine interactions.
Chronic inflammation serves as a central mechanism linking gut dysbiosis to excessive androgen synthesis. Gut barrier disruption facilitates translocation of bacterial metabolites, particularly LPS from Gram-negative bacteria (e.g., Escherichia, Shigella), into systemic circulation (18, 21, 24). Circulating LPS targets the ovaries and activates the TLR4/NF-κB pathway, triggering local inflammatory responses (25). Specifically, (1) LPS activates ovarian NF-κB signaling via the TLR4-MyD88-dependent pathway, directly upregulating key androgen synthesis enzymes (CYP17A1 and CYP11A1) in granulosa cells (26).(2) (2) LPS-induced inflammatory cytokines, particularly IL-6 and TNF-α, synergistically amplify androgen effects - IL-6 activates JAK/STAT or MAPK signaling to induce androgen synthesis enzyme expression (27, 28), while TNF-α inhibits aromatase (CYP19A1) via the NF-κB pathway, blocking androgen-to-estrogen conversion (29). These mechanisms increase androgen production while inhibiting its clearance. Furthermore, chronic inflammation reduces sex hormone-binding globulin (SHBG) synthesis, enhancing free testosterone bioactivity (30).
SCFAs deficiency exacerbates these effects. Depletion of SCFAs-producing bacteria (e.g., Roseburia, Faecalibacterium) significantly reduces butyrate levels, removing its inhibitory effects on androgen synthesis through multiple pathways: (1) Butyrate deficiency weakens gut barrier function by impairing tight junction protein expression, increasing permeability and LPS translocation (31). (2) (2) As a histone deacetylase (HDAC) inhibitor, butyrate deficiency increases histone H3K9 acetylation in the CYP17A1 promoter region, abnormally activating androgen synthesis (32). (3) SCFAs activate free fatty acid receptors (FFAR2/3), stimulating glucagon-like peptide-1 (GLP-1) secretion - loss of this function exacerbates IR, which further stimulates ovarian androgen secretion (33).
Bile acid metabolism reprogramming provides another mechanism for microbiota-mediated androgen regulation (34)(Figure 1c). Under dysbiosis, conversion of primary bile acids to secondary bile acids (e.g., deoxycholic acid) by bacteria like Bacteroides and Clostridia is reduced (35). This leads to insufficient activation of the Farnesoid X receptor (FXR), triggering dual pathological effects: (1) In the liver, loss of negative feedback results in excessive bile acid synthesis (upregulated CYP7A1/CYP27A1) (36). (2) In the ovary, insufficient local FXR activation weakens its inhibition of CYP17A1 transcription, directly promoting testosterone synthesis (36). Additionally, bile acid dysregulation impairs hepatic androgen inactivation (37).
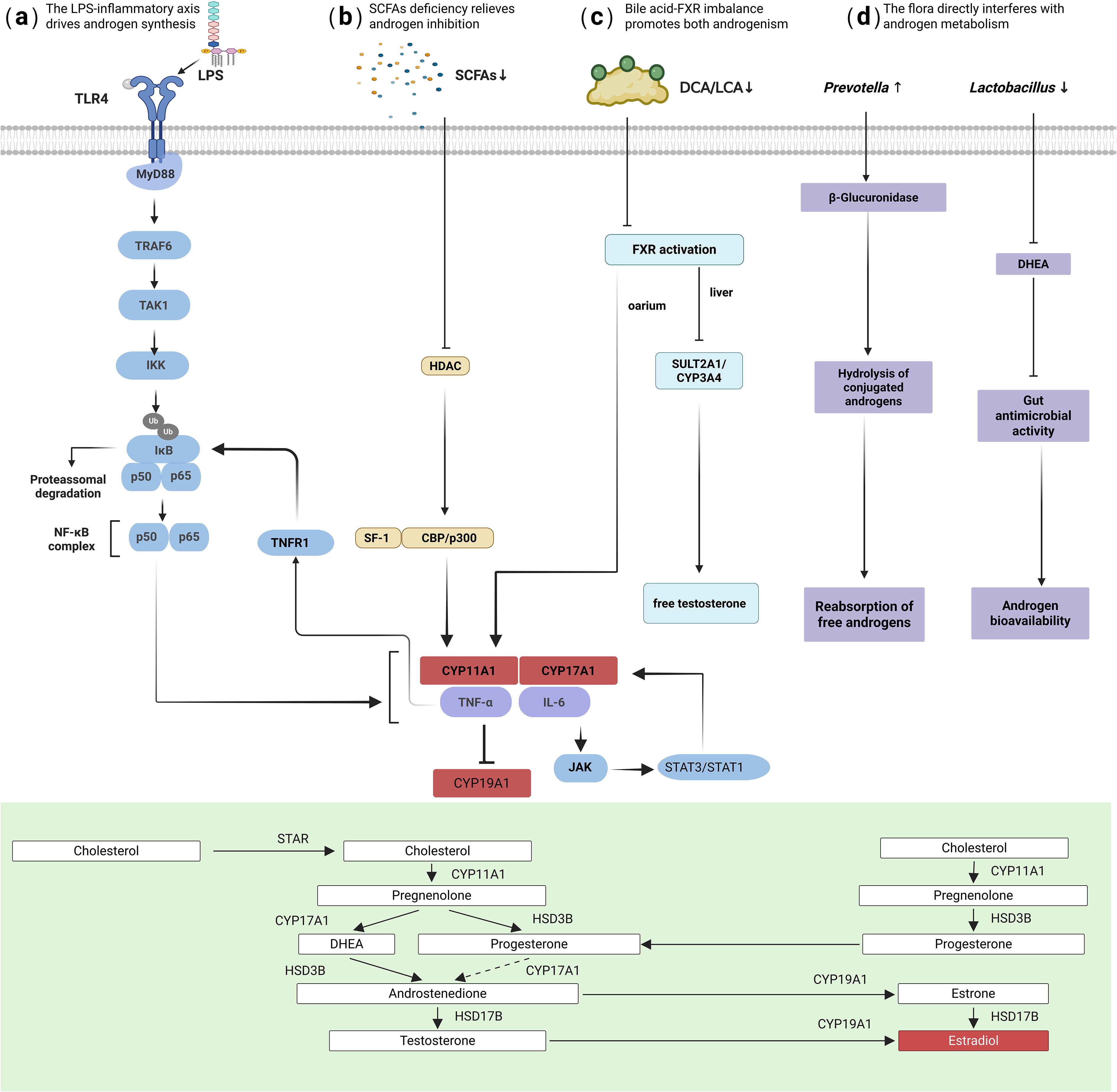
Figure 1. Mechanisms of hyperandrogenism driven by gut dysbiosis. The key pathways illustrated include: (a) LPS-TLR4/NF-κB activation: LPS from gut pathogens activates ovarian TLR4/NF-κB signaling, upregulating key androgen-synthesis enzymes (CYP17A1, CYP11A1). (b) Inflammatory cytokine signaling: TNF-α and IL-6 inhibit aromatase (CYP19A1) and synergistically amplify androgen synthesis. (c) Bile acid-FXR axis imbalance: Reduced secondary bile acids lead to insufficient FXR activation, weakening its inhibition of ovarian CYP17A1 and impairing hepatic androgen clearance. (d) Direct microbial enzymatic regulation: Bacterial β-glucuronidase hydrolyzes conjugated androgens into free, active forms.
Direct microbial enzymatic modulation also contributes: enrichment of Prevotella may promote hydrolysis of conjugated androgens to free forms via β-glucuronidase activity (Figure 1d) (38, 39), while reduction of Lactobacillus diminishes its protective effects, including competitive consumption of androgen precursors and intestinal acidification to inhibit pathogens (40).
These multifaceted mechanisms driven by gut dysbiosis not only elevate circulating androgens but also exacerbate underlying metabolic disturbances, creating a vicious cycle that underpins the PCOS phenotype and suggesting that interventions targeting gut microbiota could break this cycle.
2.3 Insulin resistance
Gut microbiota dysbiosis drives insulin resistance onset and progression through interconnected pathways. SCFAs deficiency is a core initiating factor. Butyrate deficiency impairs gut barrier integrity (downregulating ZO-1 and Occludin), exacerbates hepatic gluconeogenesis via AMPK signaling deactivation (evidenced by upregulated PEPCK and G6Pase expression), and impairs peripheral glucose uptake. Propionate deficiency reduces activation of free fatty acid receptors FFAR2/3, diminishing GLP-1 secretion, which leads to insufficient insulin secretion and accelerated gastric emptying, disrupting glucose homeostasis (41). Furthermore, SCFAs deficiency-induced barrier damage promotes LPS translocation into the bloodstream, triggering chronic inflammation. In this inflammatory state: TNF-α induces serine phosphorylation of IRS-1, blocking PI3K/Akt signaling, while IL-6 upregulates SOCS3 to accelerate insulin receptor degradation, establishing a “metabolic-inflammation” vicious cycle (Figure 2).
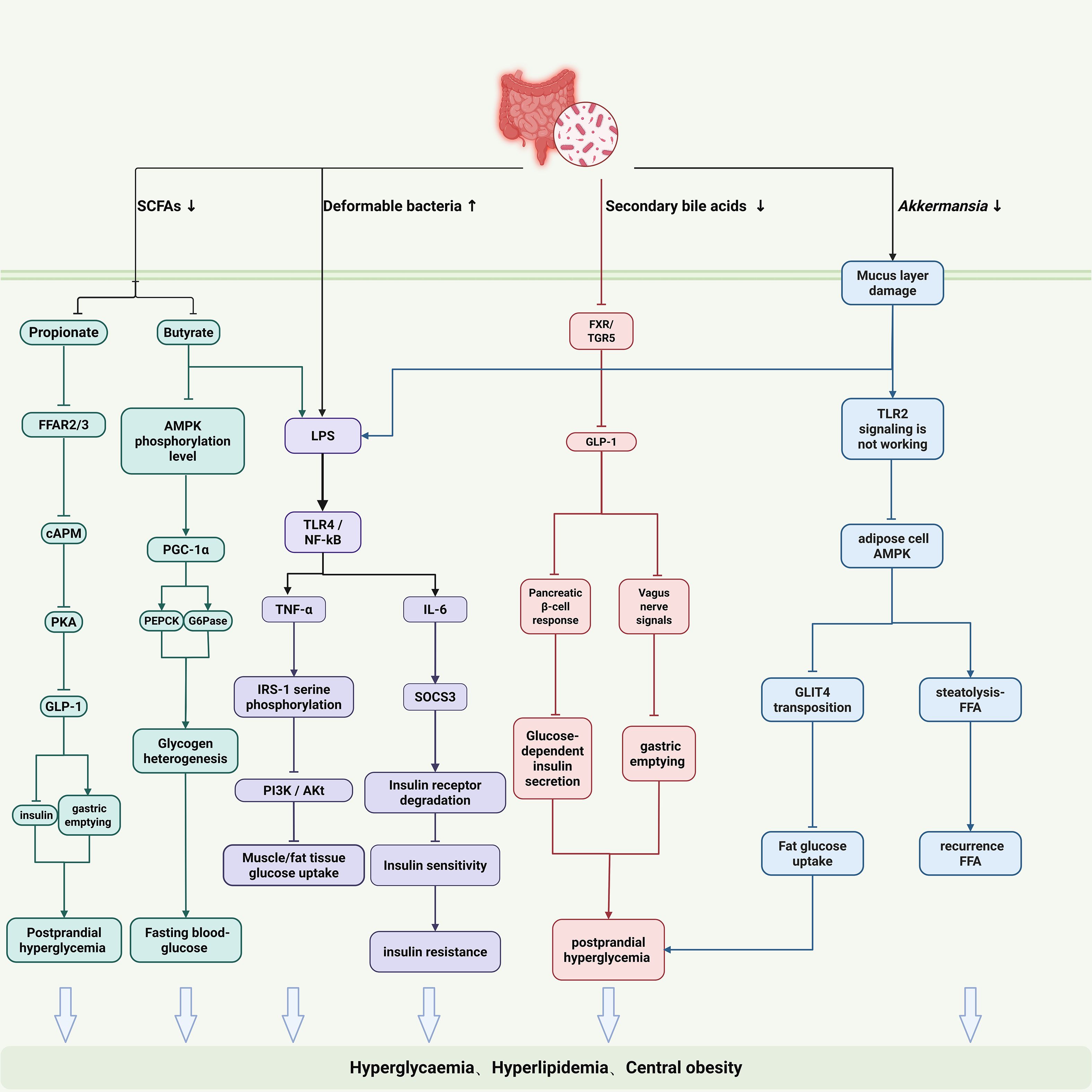
Figure 2. Gut microbiota-driven mechanisms of insulin resistance in PCOS. The illustrated pathways include: (1) SCFA deficiency: Impairs gut barrier, leading to LPS translocation, and reduces key metabolic signals (e.g., GLP-1). (2) LPS-induced inflammation: Circulating LPS and cytokines (TNF-α, IL-6) cause insulin signaling defects via serine phosphorylation of IRS-1 and SOCS3-mediated receptor degradation. (3) BA-FXR/TGR5 dysregulation: Affects glucose and lipid metabolism and gut hormone secretion. (4) Gut-brain axis disruption: Involving Akkermansia, SCFAs, and microbial metabolites, impacts central appetite regulation, fat storage, and energy expenditure.
Bile acid-FXR/TGR5 signaling dysregulation exacerbates glucose/lipid imbalance. Dysbiosis hinders microbial conversion of primary to secondary bile acids, leading to insufficient FXR activation (42, 43). Consequences include: (1) Impaired hepatic FXR signaling fails to inhibit CYP7A1 via negative feedback, resulting in excessive bile acid synthesis; (2) Dysfunctional intestinal FXR/TGR5 pathway reduces GLP-1 secretion, aggravating insulin secretion defects (44, 45).
Disrupted gut-brain axis regulation provides a central mechanism for IR. Reduced Akkermansia muciniphila abundance damages intestinal mucus layer integrity and diminishes its vesicle-mediated, TLR2-dependent improvement of adipose tissue insulin sensitivity (46). SCFAs deficiency suppresses pro-opiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus, promoting appetite enhancement and energy excess via neuropeptide Y (NPY) and GLP-1 pathways (47, 48). Microbial metabolites (e.g., secondary bile acids, indole derivatives) modulate sympathetic nervous activity via vagus nerve afferents, impairing brown adipose tissue thermogenesis and energy expenditure (49).This intricate network of peripheral and central mechanisms highlights how gut microbiota dysbiosis orchestrates IR through multiple parallel pathways, suggesting that effective therapeutic strategies must address this complexity.
2.4 Ovulatory disorders
Gut microbiota dysbiosis interferes with follicular development and ovulation primarily through inflammatory microenvironment remodeling, endocrine axis disruption, and direct cytotoxic effects. (Figure 3). An inflammatory microenvironment particularly impairs folliculogenesis. Gut-derived LPS activates ovarian TLR4/MyD88/NF-κB signaling, causing granulosa cells to secrete pro-inflammatory cytokines such as TNF-α and IL-1β. Specifically, TNF-α inhibits aromatase (CYP19A1), consequently reducing estrogen production, impairing follicle development, and hindering dominant follicle formation. IL-1β further induces mitochondrial reactive oxygen species (ROS) generation, resulting in oocyte developmental abnormalities (50). Additionally, inflammatory processes are synergistically exacerbated by hyperinsulinemia and hyperandrogenism. Elevated insulin levels increase ovarian theca cell sensitivity to IGF-1, enhancing CYP17A1 activity and promoting androgen synthesis (51). Hyperandrogenism subsequently downregulates follicle-stimulating hormone (FSH) receptor expression, inhibiting granulosa cell proliferation and further compromising follicular maturation (51).
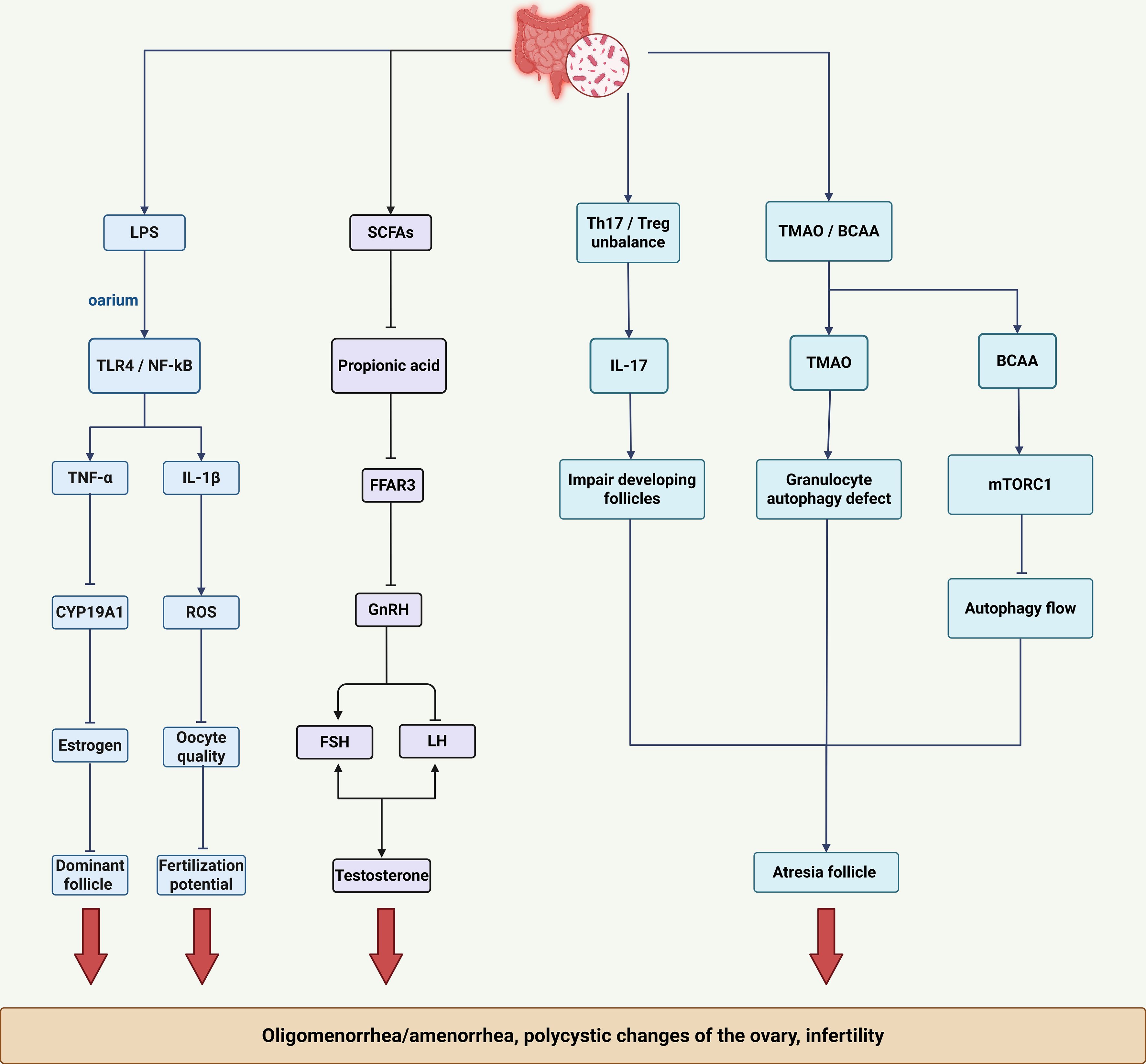
Figure 3. Mechanisms of ovulatory dysfunction mediated by gut dysbiosis. The key factors highlighted are: Inflammatory microenvironment: LPS, TNF-α, and IL-1β impair follicle development and oocyte quality. SCFAs deficiency: Disrupts endocrine rhythms (GnRH pulsatile secretion, LH/FSH ratio) and estrogen response. Immune-metabolic imbalance: Th17/Treg imbalance and cytotoxic metabolites (TMAO, BCAA) directly contribute to follicle atresia through mechanisms such as mitochondrial autophagy defects (TMAO) and inhibition of autophagic flux (BCAA/mTORC1).
Gut microbiota dysbiosis leads to loss of SCFAs, impairing their essential metabolic regulatory functions and disrupting reproductive endocrine homeostasis. Butyrate deficiency impairs histone deacetylase (HDAC) inhibition, leading to dysregulated estrogen receptor (ER) expression and reduced endometrial responsiveness to estrogen (52). Concurrently, propionate deficiency inhibits FFAR3 signaling, disrupting hypothalamic gonadotropin-releasing hormone (GnRH) pulsatility, increasing the LH/FSH ratio, and stimulating premature androgen synthesis in ovarian theca cells.
Gut dysbiosis-related immunometabolic disturbances directly drive follicular atresia. Dysbiosis-induced imbalance between T helper 17 (Th17) and regulatory T (Treg) cells exacerbates follicular damage: elevated Th17 cells produce IL-17, directly damaging developing follicles, while reduced Treg cells diminish follicular protection (53). Furthermore, cytotoxic gut-derived metabolites autophagy defects in granulosa cells, accelerating follicular degeneration. Similarly, accumulation of branched-chain amino acids (BCAAs) activates mTORC1 signaling, suppressing autophagic flux and thereby enhancing follicular damage. The multifactorial disruption of ovulatory function by gut dysbiosis is intrinsically linked to accompanying endocrine and metabolic imbalances, further compromising fertility and overall metabolic health in PCOS and identifying multiple potential targets for therapeutic intervention.
3 Exercise and gut microbiota
Given the established role of gut microbiota dysbiosis in the pathogenesis of PCOS, investigating the therapeutic potential of exercise is crucial. As a non-pharmacological intervention, exercise demonstrably influences the composition and function of the gut microbiota, suggesting a promising strategy for ameliorating PCOS symptoms. This section examines the mechanisms by which exercise modulates the gut microbiota and its functional consequences.
3.1 Animal model evidence
Extensive rodent research indicates that exercise training modifies gut microbiota composition and function (54–68). Key findings in animal models include:
F/B Ratio Variability: Changes in the Firmicutes/Bacteroidetes ratio are common but directionally inconsistent (increase (55, 56, 67), decrease (57–59), or no change (61, 62)), influenced by exercise modality, diet, age, and host genetics. For instance, voluntary wheel running (VWR) increased microbial diversity specifically in high-fat diet-fed mice (57), and juvenile rats showed more pronounced genus-level changes than adults after VWR (60).
Exercise Modality Matters: The type of exercise (voluntary vs. forced) differentially shapes microbial communities (63).
Beneficial Shifts: A consistent finding is the enrichment of beneficial bacteria, particularly SCFAs-producers (e.g., increased butyrate levels (62) and butyrate-producing taxa (57, 67)).
Pathogen Reduction: Exercise can decrease opportunistic pathogens (e.g., Pseudomonas, Enterobacteriaceae, Aeromonas after resistance training (68)).
3.2 Human evidence
Human studies corroborate exercise’s impact on gut microbiota.
Cross-sectional Evidence: Higher fitness levels correlate with greater diversity and abundance of butyrate-producing bacteria (69). Active individuals (e.g., women exercising ≥3h/week, athletes) show enrichment of beneficial taxa (e.g., Faecalibacterium prausnitzii, Roseburia hominis, Akkermansia muciniphila) and elevated fecal SCFAs concentrations compared to sedentary controls (70, 71). Athletes also exhibit distinct microbial metabolic pathways (70).
Longitudinal Interventions: Structured exercise programs induce specific changes. Endurance training (6 weeks, 3x/week) increased Faecalibacterium in lean individuals and butyrate producers/SCFAs (lean only), while decreasing them in obese participants where Bacteroides increased (72). Cycling (6 weeks) increased A. muciniphila and decreased Proteobacteria (73). An 8-week program increased Firmicutes abundance and specific butyrate producers (Ruminococcus gauvreauii, Firmicutes FCS020, Anaerobiospirillum) (74).
Collectively, evidence from both animal models and human studies demonstrates that exercise modulates the gut microbiota with core shared features: enhanced diversity (under specific conditions), enrichment of beneficial taxa (particularly SCFAs-producers like Faecalibacterium, Roseburia, and butyrate-producing Lachnospiraceae), increased abundance of Akkermansia muciniphila, and reduction in pro-inflammatory or opportunistic pathogens (e.g., Proteobacteria). This exercise-induced microbial profile is consistently associated with improved metabolic health parameters, positioning gut microbiota remodeling as a key mediator of exercise’s benefits.
4 Molecular mechanisms of exercise-induced gut microbiota remodeling in ameliorating PCOS
Having established the impact of exercise on gut microbiota composition, elucidating the underlying molecular mechanisms is essential to understand its therapeutic potential for PCOS. This section focuses on how exercise-induced microbial remodeling modulates metabolic networks, immune responses, and key signaling pathways to ameliorate PCOS pathology.
Exercise efficacy in PCOS management is well-supported by systematic reviews and meta-analyses (27, 75–77). Increasing evidence highlights its role in gut microbiota modulation. Clarke et al. demonstrated greater gut microbiota diversity in athletes compared to controls, a finding paralleled in animal models where six weeks of wheel running increased diversity and shifted abundances (increased Bacteroidetes, decreased Firmicutes) versus sedentary rats (78). Nevertheless, the precise molecular mechanisms linking exercise-induced microbial shifts to the reversal of core PCOS symptoms remain incompletely defined.
4.1 Core mechanism–systematic regulation of the gut microbiota-metabolism axis
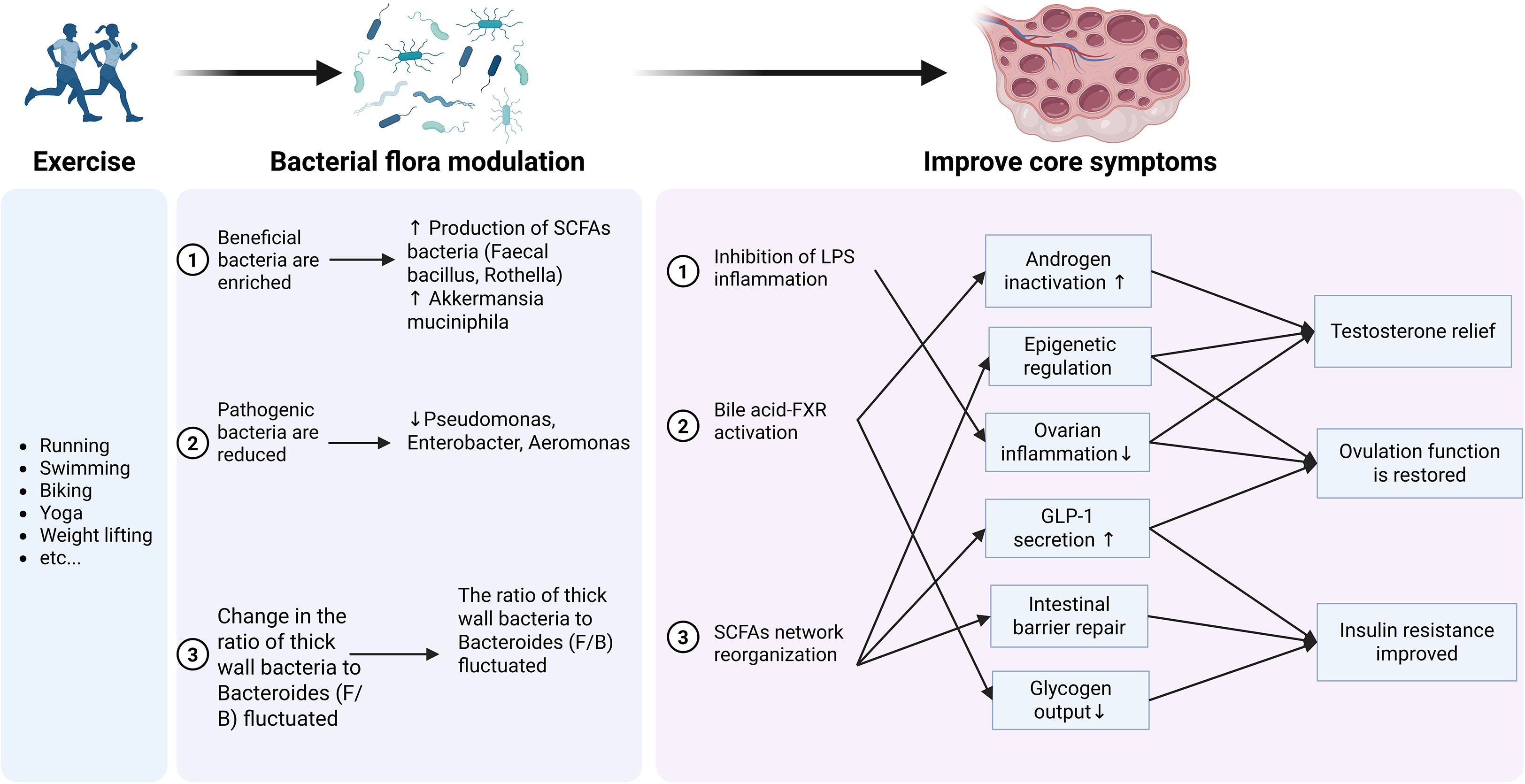
Exercise systematically regulates gut microbiota composition and function, converging on three core mechanisms: (1) reconstruction of the SCFAs metabolic network, (2) activation of the BA-FXR signaling axis, and (3) inhibition of the LPS-inflammation axis. These interconnected pathways form a unified regulatory framework underpinning metabolic and endocrine improvements in PCOS.
4.1.1 Reconstruction of the SCFAs metabolic network
Regular exercise significantly enriches Firmicutes phylum bacteria, particularly SCFAs-producing families like Ruminococcaceae, Lachnospiraceae, and Erysipelotrichaceae (74, 79, 80). Key functional examples include the enrichment of Faecalibacterium prausnitzii (Ruminococcaceae) in high-intensity female athletes and endurance runners, whose butyrate production promotes intestinal gluconeogenesis and glucose homeostasis (71). Similarly, Lachnospiraceae genera (Dorea, Coprococcus, Roseburia) demonstrate increased abundance in regular exercisers, producing acetate and butyrate to regulate blood glucose, mitigate allergic responses, and enhance gut immunity (81, 82). Marathon athletes further exhibit high enrichment of Veillonella, which utilizes lactate to produce SCFAs (83).
The resulting elevation in butyrate and propionate levels improves PCOS metabolic disturbances through three primary pathways. First, butyrate acts as a histone deacetylase (HDAC) inhibitor (84, 85), reducing the activity of HDAC1/2. This regulates chromatin accessibility and affects gene expression associated with processes such as androgen synthesis (e.g., CYP17A1 (32)) and gluconeogenesis. Second, propionate activates Free Fatty Acid Receptor 3 (FFAR3) on intestinal L-cells, stimulating GLP-1 secretion to enhance insulin sensitivity. GLP-1 enhances insulin secretion from pancreatic β-cells, inhibits glucagon release, delays gastric emptying, and promotes satiety, collectively contributing to improved glucose homeostasis and insulin sensitivity. Third, as the primary energy source for colonocytes, butyrate increases epithelial proliferation, upregulates tight junction proteins (ZO-1, Occludin), and reduces intestinal permeability (86, 87), preventing LPS translocation and subsequent systemic inflammation. Collectively, these SCFAs-mediated effects ameliorate metabolic dysfunction, inflammation, and barrier integrity, providing a multi-faceted foundation for PCOS symptom improvement.
The efficacy of exercise in modulating gut microbiota and metabolic health is indeed dose-dependent, with intensity serving as a critical modulator. While the term “high-intensity” applied to athletes denotes a level of training far exceeding general recommendations, the exercise intensity beneficial for PCOS management is both quantifiable and subject to an optimal range rather than a simple upper limit. Prescriptions are typically grounded in objective physiological metrics to ensure safety and efficacy. Common quantifiable measures include: (1)Percentage of Maximum Heart Rate (%HRmax): Moderate-intensity is defined as 64-76% HRmax; vigorous-intensity as 77-93% HRmax (88); (2)Percentage of Heart Rate Reserve (%HRR) or Oxygen Uptake Reserve (%VO2R): Moderate-intensity corresponds to 40-59% HRR/VO2R, while vigorous-intensity to 60-89% (89, 90); (3)Rating of Perceived Exertion (RPE): Using the Borg scale (6-20), moderate intensity targets 12-13 (“somewhat hard”), and vigorous intensity targets 14-16 (“hard” to “very hard”) (91).
For PCOS populations, evidence suggests that vigorous-intensity exercise (e.g., High-Intensity Interval Training(HIIT) protocols involving bouts at 80-90% HRmax) is highly effective for improving insulin sensitivity and cardiorespiratory fitness (76). However, the optimal intensity must be individualized. Factors such as baseline fitness, PCOS phenotype, joint health, and exercise tolerance necessitate personalization. The prevailing consensus recommends beginning with moderate-intensity exercise for sedentary individuals, progressively incorporating vigorous intervals as tolerance improves, while strictly avoiding excessive intensity that could provoke a negative stress response (92). This structured, quantifiable approach ensures that exercise intervention remains a potent, evidence-based strategy for gut microbiota and metabolic remodeling in PCOS.
4.1.2 Activation of the BA-FXR signaling axis
Exercise regulates the gut-liver axis BA pool composition and function, increasing BA excretion (93, 94). By elevating the Firmicutes/Bacteroidetes ratio (55, 56, 67), exercise promotes microbial conversion of primary to secondary BAs. Exercise promotes this shift towards secondary BAs primarily by enriching gut bacteria harboring bile salt hydrolase (BSH) and 7α-dehydroxylase activities (e.g., certain Firmicutes like Clostridium cluster XIVa and XVI), thereby enhancing microbial conversion of primary to secondary BAs. These secondary BAs act as endogenous ligands for the FXR, recruiting nuclear receptor coactivators to activate FXR signaling with dual organ effects. The activation of FXR signaling in both liver and intestine represents a master regulator of systemic glucose and lipid metabolism, integrating microbial BA metabolism with host metabolic homeostasis. In the liver, FXR activation suppresses cholesterol 7α-hydroxylase (CYP7A1) expression to curb excessive BA synthesis while concurrently upregulating fibroblast growth factor 19 (FGF19) secretion, further inhibiting synthesis via negative feedback. Intestinally, FXR activation maintains glucose homeostasis by inhibiting hepatic gluconeogenesis enzyme expression (95) and enhances hepatic detoxification capacity. This axis provides a molecular framework for tissue-specific, multi-organ regulation of metabolic balance.
4.1.3 Inhibition of the LPS-inflammation axis
Exercise reduces Proteobacteria abundance (96–98), with regular moderate-intensity resistance training specifically lowering circulating LPS levels (99). Crucially, exercise-induced increases in SCFAs, particularly butyrate, play a fundamental role in fortifying the intestinal barrier. Butyrate serves as the primary energy source for colonocytes, stimulating epithelial proliferation and upregulating tight junction proteins (ZO-1, Occludin), thereby significantly reducing intestinal permeability and preventing LPS translocation. This reduction disrupts inflammatory cascades through coordinated mechanisms. Diminished LPS impairs Toll-like receptor 4 (TLR4)/Myeloid differentiation primary response 88 (MyD88) complex formation, blocking nuclear factor kappa B (NF-κB) phosphorylation and subsequent pro-inflammatory cytokine transcription. Concurrently, SCFAs activate G-protein coupled receptor 43 (GPR43), inhibiting apoptosis-associated speck-like protein (ASC) oligomerization, caspase-1 activation, and NLRP3 inflammasome assembly. These changes collectively promote macrophage polarization from pro-inflammatory M1 to anti-inflammatory M2 phenotypes.Furthermore, SCFAs, particularly butyrate, contribute to the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) by inhibiting its cytoplasmic repressor Kelch-like ECH-associated protein 1 (KEAP1). This inhibition stabilizes Nrf2 and facilitates its translocation to the nucleus, where it binds to antioxidant response elements (ARE) in the promoter regions of target genes, boosting the transcription of antioxidant enzymes (e.g., heme oxygenase-1, NAD(P)H quinone dehydrogenase 1, glutathione peroxidase) and enhancing oxidative stress resistance (100, 101).
4.1.4 Integrative crosstalk and metabolic network remodeling
The three core mechanistic axes—SCFAs network reconstruction, BA-FXR signaling activation, and LPS-inflammation inhibition—do not operate in isolation but engage in extensive crosstalk, forming a dynamic and integrated metabolic network that underlies exercise’s ameliorative effects on PCOS.
SCFAs & BA/FXR: SCFAs, particularly butyrate, acting as HDAC inhibitors, may influence the epigenetic regulation of genes involved in BA metabolism (e.g., CYP7A1, FXR) and FXR target genes, potentially amplifying FXR signaling efficacy. Conversely, FXR activation in the intestine can modulate the expression of genes involved in mucosal defense and potentially influence the niche for SCFAs-producing bacteria.
SCFAs & LPS/Inflammation: Butyrate’s enhancement of gut barrier integrity is fundamental to reducing LPS translocation and systemic inflammation. Simultaneously, reduced inflammation (via LPS inhibition and SCFAs-GPR43 signaling) creates a more favorable gut environment for beneficial SCFAs-producing bacteria to thrive. SCFAs also directly suppress NLRP3 inflammasome activation via GPR43, synergizing with reduced LPS/TLR4 signaling to quell inflammation.
BA/FXR & LPS/Inflammation: Secondary BAs activating FXR can exert anti-inflammatory effects in the liver and intestine. FXR activation suppresses hepatic inflammation and may contribute to maintaining gut barrier function. Reduced systemic inflammation, in turn, may improve BA metabolism and signaling.
Convergence on Gut-Brain Axis & Central Metabolism: Key metabolites from these axes (SCFAs, secondary BAs, reduced inflammatory signals) collectively signal to the central nervous system via the gut-brain axis (vagal afferents, circulating mediators). They modulate hypothalamic neurons in the arcuate nucleus (ARC) regulating appetite (POMC/CART, AgRP/NPY), energy expenditure, and HPG axis function (kisspeptin, GnRH neurons). This central integration coordinates peripheral metabolic improvements (glucose/lipid handling, insulin sensitivity) with endocrine normalization (reduced androgens, improved gonadotropin dynamics).
This intricate crosstalk ensures that exercise-induced gut microbiota remodeling orchestrates a coordinated multi-organ response, simultaneously targeting metabolic (liver, adipose, muscle), immune (systemic, local gut, ovarian), endocrine (ovary, adrenal, HPG axis), and neural (gut-brain) systems. The resultant metabolic network remodeling—characterized by enhanced insulin sensitivity, optimized hepatic glucose/lipid output, reduced adipose tissue inflammation, suppressed ovarian androgenesis, restored central energy balance, and normalized reproductive endocrine rhythms—collectively reverses the core pathological features of PCOS.
4.2 Tissue-specific effects targeting the improvement of core symptoms in PCOS
Building upon the regulatory effects of exercise on metabolic and immune pathways, this section elucidates the mechanisms by which exercise-induced changes directly ameliorate the core manifestations of polycystic ovary syndrome (PCOS)—Hyperandrogenism, IR, and ovulatory dysfunction—thereby establishing a multi-targeted therapeutic framework.
4.2.1 Improvement of hyperandrogenism
Hyperandrogenism pathologically characterizes an imbalance between increased androgen synthesis and reduced metabolic clearance. Exercise targets the metabolic pathways in the ovaries and liver to restore androgen homeostasis.
(1) Ovarian Targeting to Inhibit Androgen Synthesis
Exercise interventions have been shown to increase butyrate levels. Butyrate, as a histone deacetylase (HDAC) inhibitor (84, 85), has experimental evidence indicating that it can reduce histone H3K9 acetylation at the CYP17A1 promoter in relevant cell types (32), thereby blocking the transcriptional activation of this rate-limiting enzyme in androgen synthesis.
Exercise reduces plasma lipopolysaccharide (LPS) levels and diminishes LPS binding to ovarian Toll-like receptor 4 (TLR4), thereby inhibiting activation of the MyD88/NF-κB signaling axis. Consequently, this reduction alleviates the inhibitory effect of inflammatory cytokines (TNF-α, IL-6) on aromatase (CYP19A1), promoting the conversion of androgens to estrogens. Supporting this mechanism, clinical studies demonstrate that both sprint interval training and moderate-intensity continuous training significantly reduce plasma LPS and TNF-α levels after just two weeks (102).
(2) Liver Targeting to Enhance Androgen Clearance
Exercise induces the production of secondary BAs. These secondary BAs activate hepatic Farnesoid X receptor alpha (FXRα) (103), which upregulates the expression of androgen-metabolizing enzymes SULT2A1 and CYP3A4. This accelerates the sulfation and hydroxylation inactivation of testosterone.
Exercise can modulate the gut-liver axis by increasing beneficial gut bacteria and suppressing the growth of Prevotella, thereby reducing the production of bacterial β-glucuronidase. This enzyme, produced by bacteria such as Prevotella, hydrolyzes conjugated androgens back into their free, active forms. Reducing β-glucuronidase activity is crucial for limiting the enterohepatic circulation and reabsorption of androgens, thereby contributing to the regulation of systemic androgen levels.
4.2.2 Alleviation of IR
Improving IR involves a synergistic interaction between peripheral metabolic regulation and central appetite suppression. Exercise targets key metabolic organs through modulation of the gut-host axis to provide effective intervention.
(1) Peripheral Metabolic Regulation in the Liver and Adipose Tissue
Exercise promotes metabolic homeostasis through the actions of secondary BAs and SCFAs on dual targets: the liver and adipose tissue. In the liver, secondary BAs activate the FXR, which directly inhibits the transcription of genes encoding phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase). These enzymes are key rate-limiting factors in gluconeogenesis and glycogenolysis; their reduced activity significantly attenuates hepatic glucose production, thereby improving hyperglycemia (95). Meanwhile, in adipose tissue, SCFAs interact with the GPR43 receptor, establishing a multi-layered anti-inflammatory barrier: locally, GPR43 activation directly suppresses the assembly of the NLRP3 inflammasome, blocking the release of pro-inflammatory cytokines such as IL-1β and IL-18 (104); systemically, SCFAs regulate the migration threshold of monocytes/neutrophils and the dynamic balance between pro-inflammatory and anti-inflammatory cytokines, thereby curbing the spread of systemic inflammation (105). SCFAs and GPR43 engage in a bidirectional regulatory loop: physiological concentrations of SCFAs upregulate GPR43 expression via histone acetylation (106), and activated GPR43 further inhibits the NF-κB/MAPK signaling axis, reducing NLRP3 inflammasome activity (106). This hepatic-adipose metabolic synergy optimizes hepatic energy output via the FXR-PEPCK/G6Pase axis, while adipose tissue mitigates metabolic inflammation through the GPR43-NLRP3 pathway. Together, they form a dynamic equilibrium system maintaining “glucose-lipid metabolism-inflammation homeostasis.”
(2) Repairing the gut-brain axis to regulate central appetite
Exercise intervention enhances the production of SCFAs and BAs, regulating the gut-brain axis via a cooperative multi-receptor mechanism to maintain energy homeostasis. SCFAs activate free fatty acid receptors FFAR2/GPR43 and FFAR3/GPR41 on gut endocrine L-cells, stimulating the secretion of GLP-1 and peptide YY (PYY) (107–110). Acetate crosses the blood-brain barrier and accumulates in the hypothalamic arcuate nucleus (ARC), where it activates pro-opiomelanocortin (POMC) neurons through an FFAR2-dependent pathway (111), inhibiting agouti-related peptide (AgRP) neurons and enhancing central satiety signals. Butyrate exerts potent appetite suppression by upregulating PYY and inhibiting hypothalamic neuropeptide Y (NPY) neuron activity, largely dependent on vagus nerve-mediated FFAR3 signaling (112–114). Secondary BAs (e.g., DCA, LCA) activate TGR5 highly expressed on vagal afferents and in the ARC, suppressing AgRP/NPY neuron activity (115, 116). The BA-FXR axis further influences central metabolism via the FGF15/19 pathway (117). This integrated signaling dynamically reshapes central appetite regulation and energy homeostasis within the gut-brain axis (118, 119).
4.2.3 Restoration of ovulatory function
Restoring ovulatory function involves re-establishing follicular microenvironment homeostasis and regulating endocrine rhythms. Exercise exerts beneficial effects through dual pathways involving microbiota-derived metabolites and immune modulation.
(1) Repair of the follicular microenvironment
Propionate enhances autophagy by inhibiting the NF-κB and AKT/mTOR signaling pathways and increasing LC3B protein levels. This promotes the clearance of mitochondrial damage induced by trimethylamine N-oxide (TMAO) (120), thereby helping to improve the follicular microenvironment, maintain its homeostasis, and contribute to the amelioration of ovulatory dysfunction.
Immune-Endocrine Crosstalk Regulation. Studies show that gut microbial metabolites metabolize tryptophan into aryl hydrocarbon receptor (AhR) agonists, such as indole-3-lactic acid (ILA), that activate AhR to modulate the host immune response (121). Upon binding ILA, this ligand-dependent transcription factor translocates from the cytoplasm to the nucleus and dimerizes with the AhR nuclear translocator (ARNT) to regulate downstream gene expression. AhR activation promotes the differentiation of regulatory T cells (Treg) (122). Tregs help counterbalance the activity of T helper 17 (Th17) cells, a pro-inflammatory T cell subset whose overactivation can contribute to follicular atresia. By activating AhR, ILA can suppress the differentiation and function of Th17 cells (122), thereby reducing the occurrence of follicular atresia.
(2) Regulation of endocrine rhythms
Normalization of GnRH Pulsatile Secretion. The gut microbiota influences ovarian function partly by modulating the hypothalamic-pituitary-gonadal (HPG) axis. Gut microbiota-derived metabolites, particularly SCFAs and BAs, are potent regulators of hypothalamic GnRH neuron function. Research indicates that alterations in gut microbiota composition (e.g., reductions in Firmicutes and Bacteroidetes) can increase serum levels of GLP-1 (123). GLP-1, produced by intestinal L-cells, stimulates GnRH neurons, potentially involving kisspeptin neuron intermediation. Specifically, the SCFAs (e.g., propionate) and potentially microbial-modulated BAs signal via the gut-brain axis (likely involving vagal afferents and circulating factors) to influence kisspeptin neuron activity in the hypothalamus. Kisspeptin is a critical regulator of GnRH neuron pulsatility. Exercise-induced normalization of this signaling contributes to lowering the LH/FSH ratio and restoring physiological GnRH release patterns.
Enhanced Estrogen Response. Acetate inhibits histone deacetylases (HDACs), helping to maintain normal ovarian function and follicular development, leading to elevated circulating levels of 17β-estradiol (110). Supporting this, animal studies in PCOS models demonstrate that acetate treatment significantly inhibits HDAC activity in plasma and ovaries. This inhibition counteracts the pro-inflammatory effects of TNF-α and restores nuclear factor erythroid 2–related factor 2 (Nrf2) signaling along with related antioxidant defenses (glutathione - GSH, glutathione peroxidase - GPx) (124). Furthermore, acetate administration lowers plasma testosterone levels and the LH/FSH ratio while increasing circulating 17β-estradiol and sex hormone-binding globulin (SHBG) levels in these models (124). Collectively, acetate’s HDAC inhibition contributes to improved ovarian steroidogenesis and estrogen response.
This figure (Figure 4) summarizes the core mechanisms through which exercise-induced changes in the gut microbiota improve the core symptoms of PCOS. These include the reorganization of the SCFAs network, activation of the BA-FXR signaling axis, and inhibition of the LPS-inflammation axis, which collectively target hyperandrogenism, insulin resistance, and ovulatory dysfunction in a tissue-specific manner.
4.3 Ecological basis of exercise-driven microbial response and its implications for specificity
The interaction between exercise and the gut microbiome is far more than a simple change in composition. Exercise can induce physiological changes, reshape the gut environment, and drive microbial community restructuring.
4.3.1 Driving factors
Alterations in Gut Oxygen Supply: Exercise, particularly endurance training, improves cardiovascular function and may temporarily increase gut perfusion and oxygenation (125). This favors the expansion of facultative anaerobes (e.g., certain Lactobacillus spp.) and obligate aerobes, while potentially limiting strictly anaerobic bacteria, including many SCFAs producers. However, the net effect of exercise is generally an increase in SCFAs producers, suggesting the presence of compensatory mechanisms or ecological niche adaptation (78).
BA Flow and Composition: Exercise enhances the excretion of BAs (86, 87) and regulates the gut-liver axis. Changes in the BA profile (e.g., increased secondary BAs) can specifically inhibit or promote the growth of certain bacteria that are sensitive to these antimicrobial molecules (42, 93).
Immune System Regulation: Exercise alters both systemic and mucosal immunity. The release of exercise-induced myokines (e.g., IL-6) and changes in immunoglobulin A (IgA) secretion can influence microbial growth and adhesion (54).
4.3.2 Differential impact of exercise modalities on PCOS subtypes
The heterogeneity of PCOS is well-recognized and is formally categorized into phenotypic subtypes based on the Rotterdam diagnostic criteria, which require the presence of at least two of the following three features: (1) hyperandrogenism (clinical and/or biochemical), (2) ovulatory dysfunction, and (3) polycystic ovarian morphology on ultrasound (126). This yields four distinct phenotypes: (1)Phenotype A (classic PCOS): Hyperandrogenism + Ovulatory dysfunction + Polycystic ovaries; (2)Phenotype B: Hyperandrogenism + Ovulatory dysfunction; (3)Phenotype C: Hyperandrogenism + Polycystic ovaries; (4)Phenotype D: Ovulatory dysfunction + Polycystic ovaries (non-hyperandrogenic). Beyond this phenotypic classification, stratification by the presence of obesity (e.g., BMI ≥25 kg/m² vs. lean) and the severity of insulin resistance (e.g., HOMA-IR >2.1 or based on glucose clamp studies) is crucial for understanding metabolic risk and tailoring therapy (127, 128). It is within this framework of phenotypic and metabolic heterogeneity that the differential effects of exercise modalities must be considered.
Given the heterogeneity of polycystic ovary syndrome (PCOS) (e.g., obesity vs. lean, IR-dominant vs. hyperandrogenism-dominant), and the unique physiological effects of different types of exercise, specific interventions for certain subtypes may be more effective. Critically, these exercise modalities engage distinct metabolic pathways, which underpins their differential therapeutic effects.
Endurance/Aerobic Training: This modality primarily enhances cardiovascular health and insulin sensitivity through AMPK activation and improved mitochondrial biogenesis in skeletal muscle. Its significant effects on increasing SCFAs producers (e.g., Faecalibacterium, Roseburia) and reducing systemic inflammation (72, 74, 102) may be especially beneficial for PCOS women with IR and metabolic dysfunction, regardless of obesity status. The potential increase in Akkermansia muciniphila (73) also supports metabolic health.
Resistance/Strength Training: This is effective in increasing muscle mass, improving basal metabolic rate, and glucose handling capacity primarily via mTORC1-mediated muscle protein synthesis. Its documented ability to reduce opportunistic pathogens (e.g., Pseudomonas, Aeromonas) and circulating LPS levels (68, 74) may offer an advantage for PCOS women with significant chronic low-grade inflammation and androgen excess, particularly in the context of obesity, where inflammation is often exacerbated.
HIIT: HIIT provides time-efficient improvements in metabolic health and cardiovascular function by stimulating both aerobic and anaerobic systems, leading to exaggerated post-exercise oxygen consumption (EPOC) and enhanced GLUT4 translocation. Although studies specifically targeting the relationship between PCOS and gut microbiome changes with HIIT are limited, its significant effects on insulin sensitivity and reduction of androgens (76) suggest it may be beneficial for various subtypes, though its intensity must be carefully personalized based on individual tolerance.
Understanding these ecological driving factors and the distinct microbial and metabolic signatures induced by specific exercise patterns (e.g., HIIT’s rapid metabolic improvement linked to specific SCFAs producers; resistance training’s anti-inflammatory effect via pathogen reduction) provides a molecular rationale for designing personalized exercise prescriptions. These prescriptions can be tailored to target the primary pathological drivers in different PCOS phenotypes: Endurance/aerobic training may be prioritized for IR-dominant phenotypes (regardless of BMI) due to its potent effects on SCFAs producers and systemic inflammation. Resistance training might offer advantages for obese PCOS women with significant chronic inflammation and androgen excess by reducing LPS and opportunistic pathogens. HIIT’s efficiency in improving insulin sensitivity and reducing androgens suggests broad applicability, but intensity should be personalized.
Furthermore, the efficacy of exercise is modulated by dietary patterns, creating a critical exercise-diet interaction that shapes the gut microbiota. For instance, the enrichment of SCFA-producing bacteria (e.g., Faecalibacterium) through exercise can be synergistically amplified by a high-fiber, prebiotic-rich diet, which provides essential substrates for microbial fermentation (129). Conversely, the benefits of exercise may be diminished by a Western-style diet high in saturated fats and refined sugars, which promotes dysbiosis and inflammation. Therefore, combining tailored exercise protocols with evidence-based dietary interventions (e.g., Mediterranean or low-glycemic index diets) represents a most promising strategy for optimizing gut microbiome remodeling and achieving sustainable metabolic and endocrine improvements in PCOS (130, 131).
It is noteworthy that the beneficial role of exercise presents a dose-dependent characteristic. This review focuses on the mechanisms by which moderate exercise ameliorates PCOS; however, it is observed in clinical practice that prolonged high-intensity exercise, often coupled with psychological stress (e.g., in some athletes), can conversely lead to endocrine dysfunction and menstrual disturbances. This apparent paradox highlights the critical importance of exercise intensity and individual tolerance. The underlying mechanism may involve excessive activation of the hypothalamic-pituitary-adrenal (HPA) axis, leading to elevated cortisol levels that suppress GnRH pulsatility and ovarian function (132), while also potentially exacerbating gut dysbiosis and inflammation, thereby counteracting the benefits brought by moderate exercise. Consequently, the exercise prescriptions recommended for PCOS management should be tailored to the individual, aiming to achieve the beneficial “eustress” that remodels the gut microbiota and metabolism, while avoiding excessive intensity that leads to detrimental “distress”.
5 Conclusion
In conclusion, this review synthesizes evidence establishing exercise-induced gut microbiota remodeling as a key mechanism ameliorating PCOS pathology. Up-to-date evidence demonstrates that exercise enriches beneficial taxa (e.g., SCFA-producing Faecalibacterium, Roseburia, Akkermansia) while suppressing pro-inflammatory pathogens (e.g., Proteobacteria), thereby elevating beneficial metabolites (SCFAs, secondary BAs) and reducing detrimental factors (LPS). These microbial shifts activate a coordinated molecular response through key receptors (FXR, GPR43, TGR5, AhR) and inhibit critical pathogenic pathways (TLR4/NF-κB, NLRP3, HDAC), culminating in a multi-organ reversal of PCOS features: improved insulin sensitivity and metabolic homeostasis, suppressed hyperandrogenism, restored ovulatory function, and resolved chronic inflammation.
Despite this compelling framework, significant limitations remain. Most mechanistic insights are derived from animal models, lacking robust translational human data. The profound heterogeneity of PCOS is often overlooked, and the optimal exercise protocol (intensity, frequency, duration) remains undefined. Furthermore, the long-term sustainability of exercise-induced microbial and symptomatic benefits is unclear.
Future research must prioritize human studies incorporating multi-omics approaches and interventional designs like FMT to establish causality. Leveraging gut microbial signatures and metabolite profiles as biomarkers will be crucial for developing personalized exercise prescriptions tailored to PCOS phenotypes. Ultimately, translating these mechanistic insights into microbiome-targeted therapies—whether through prebiotics, microbial metabolites, or their analogs—holds exceptional promise for revolutionizing the management of PCOS.
Author contributions
QL: Visualization, Writing – original draft, Writing – review & editing. LC: Supervision, Writing – review & editing. RW: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
AhR: Aryl Hydrocarbon Receptor
AMPK: AMP-activated Protein Kinase
BA: Bile Acid
BCAA: Branched-Chain Amino Acid
BSH: Bile Salt Hydrolase
FXR: Farnesoid X Receptor
GLP-1: Glucagon-Like Peptide-1
GnRH: Gonadotropin-Releasing Hormone
GPR43/FFAR3: G Protein-Coupled Receptor 43/Free Fatty Acid Receptor 3;HDAC, Histone Deacetylase
HPG: Hypothalamic-Pituitary-Gonadal Axis
IR: Insulin Resistance
LPS: Lipopolysaccharide;mTORC1, Mechanistic Target of Rapamycin Complex 1
NF-κB: Nuclear Factor Kappa B
NLRP3: NLR Family Pyrin Domain Containing 3
PCOS: Polycystic Ovary Syndrome
SCFAs: Short-Chain Fatty Acid
TLR4: Toll-Like Receptor 4
TMAO: Trimethylamine N-Oxide
A. muciniphila: Akkermansia muciniphila
F/B ratio: Firmicutes/Bacteroidetes Ratio
F. prausnitzii: Faecalibacterium prausnitzii
CYP17A1: 17α-Hydroxylase
CYP19A1: Aromatase
FGF19: Fibroblast Growth Factor 19
G6Pase: Glucose-6-Phosphatase
PEPCK: Phosphoenolpyruvate Carboxykinase
SULT2A1: Sulfotransferase 2A1
IL-6/IL-1β/TNF-α: Interleukin-6/1β/Tumor Necrosis Factor-α
Th17/Treg: T Helper 17/Regulatory T Cell
TLR4/MyD88: Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88
HIIT: High-Intensity Interval Training
VWR: Voluntary Wheel Running
FMT: Fecal Microbiota Transplantation
ROS: Reactive Oxygen Species
SHBG: Sex Hormone-Binding Globulin.
References
1. Stener-Victorin E, Teede H, Norman RJ, Legro R, Goodarzi MO, Dokras A, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. (2024) 10:27. doi: 10.1038/s41572-024-00511-3
2. Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14:270–84. doi: 10.1038/nrendo.2018.24
3. Dumesic DA and Lobo RA. Cancer risk and PCOS. Steroids. (2013) 78:782–5. doi: 10.1016/j.steroids.2013.04.004
4. Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-a systematic review, meta-analysis, and meta-regression. Obes Rev. (2019) 20:659–74. doi: 10.1111/obr.12829
5. Persson S, Elenis E, Turkmen S, Kramer MS, Yong E-L, and Sundström-Poromaa I. Fecundity among women with polycystic ovary syndrome (PCOS)-a population-based study. Hum Reprod. (2019) 34:2052–60. doi: 10.1093/humrep/dez159
6. Lee S, Tejesvi MV, Hurskainen E, Aasmets O, Plaza-Díaz J, Franks S, et al. Gut bacteriome and mood disorders in women with PCOS. Hum Reprod. (2024) 39:1291–302. doi: 10.1093/humrep/deae073
7. Lüll K, Arffman RK, Sola-Leyva A, Molina NM, Aasmets O, Herzig K-H, et al. The gut microbiome in polycystic ovary syndrome and its association with metabolic traits. J Clin Endocrinol Metab. (2021) 106:858–71. doi: 10.1210/clinem/dgaa848
8. Fang J and Liu H. Research progress on the occurrence and development of intestinal flora and polycystic ovary syndrome. J Reprod Med. (2024) 33:396–400.
9. Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. (2019) 25:1225–33. doi: 10.1038/s41591-019-0509-0
10. Lynch SV and Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
11. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalog established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
12. Barber TM, Dimitriadis GK, Andreou A, and Franks S. Polycystic ovary syndrome: Insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond). (2015) 15 Suppl 6:s72–76. doi: 10.7861/clinmedicine.15-6-s72
13. Hansen SL, Bojsen-Møller KN, Lundsgaard A-M, Hendrich FL, Nilas L, Sjøberg KA, et al. Mechanisms underlying absent training-induced improvement in insulin action in lean, hyperandrogenic women with polycystic ovary syndrome. Diabetes. (2020) 69:2267–80. doi: 10.2337/db20-0062
14. Wu X, Wu H, Sun W, and Wang C. Improvement of anti-müllerian hormone and oxidative stress through regular exercise in Chinese women with polycystic ovary syndrome. Hormones (Athens). (2021) 20:339–45. doi: 10.1007/s42000-020-00233-7
15. Chen K, Geng H, Liu J, and Ye C. Alteration in gut mycobiota of patients with polycystic ovary syndrome. Microbiol Spectr. (n.) 11:e02360–23. doi: 10.1128/spectrum.02360-23
16. Zhu Q and Zhang N. Gut microbiome composition in polycystic ovary syndrome adult women: A systematic review and meta-analysis of observational studies. Reprod Sci. (2024) 31:1800–18. doi: 10.1007/s43032-023-01440-4
17. Wang W, Shang X, Zeng Q, Liu D, and Li B. Intestinal flora and biochemical immune molecular characteristics of polycystic ovary syndrome patients. Acta Microbiologica Sin. (2021) 61:452–68. doi: 10.13343/j.cnki.wsxb.20200255
18. Tremellen K and Pearce K. Dysbiosis of gut microbiota (DOGMA) – a novel theory for the development of polycystic ovarian syndrome. Med Hypotheses. (2012) 79:104–12. doi: 10.1016/j.mehy.2012.04.016
19. Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril. (2020) 113:1286–1298.e4. doi: 10.1016/j.fertnstert.2020.01.027
20. Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. (2017) 8:324. doi: 10.3389/fmicb.2017.00324
21. Guo J, Shao J, Yang Y, Niu X, Liao J, Zhao Q, et al. Gut microbiota in patients with polycystic ovary syndrome: A systematic review. Reprod Sci. (2022) 29:69–83. doi: 10.1007/s43032-020-00430-0
22. Yurtdaş G and Akdevelioğlu Y. A new approach to polycystic ovary syndrome: The gut microbiota. J Am Coll Nutr. (2020) 39:371–82. doi: 10.1080/07315724.2019.1657515
23. Zhou L, Ni Z, Cheng W, Yu J, Sun S, Zhai D, et al. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr Connect. (2019) 9:63–73. doi: 10.1530/EC-19-0522
24. Duan L, An X, Zhang Y, Jin D, Zhao S, Zhou R, et al. Gut microbiota as the critical correlation of polycystic ovary syndrome and type 2 diabetes mellitus. Biomedicine Pharmacotherapy. (2021) 142:112094. doi: 10.1016/j.biopha.2021.112094
25. Zhou P, Feng P, Liao B, Fu L, Shan H, Cao C, et al. Role of polyphenols in remodeling the host gut microbiota in polycystic ovary syndrome. J Ovarian Res. (2024) 17:69. doi: 10.1186/s13048-024-01354-y
26. Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): A pilot study. Res Microbiol. (2019) 170:43–52. doi: 10.1016/j.resmic.2018.09.002
27. Vincent J, Harris J, Hill JS, and Lewis M. A case study of marcus rashford: The people’s champion, a “national treasure,” and an inspirational personal brand. Int J Sport Communication. (2024) 17:325–37. doi: 10.1123/ijsc.2024-0026
28. Zhao X, Jiang Y, Xi H, Chen L, and Feng X. Exploration of the relationship between gut microbiota and polycystic ovary syndrome (PCOS): A review. Geburtshilfe Frauenheilkd. (2020) 80:161–71. doi: 10.1055/a-1081-2036
29. Lang Q, Yidong X, Xueguang Z, Sixian W, Wenming X, and Tao Z. ETA-mediated anti-TNF-α therapy ameliorates the phenotype of PCOS model induced by letrozole. PloS One. (2019) 14:e0217495. doi: 10.1371/journal.pone.0217495
30. Lu M, Xu Z, Sun R, and Mo Z. advances in hyperandrogen-associated chronic inflammation and polycystic ovary syndrome. Prog Biochem Biophys. (2022) 49:767–74. doi: 10.16476/j.pibb.2021.0339
31. Du Y, He C, An Y, Huang Y, Zhang H, Fu W, et al. The role of short chain fatty acids in inflammation and body health. IJMS. (2024) 25:7379. doi: 10.3390/ijms25137379
32. Zhang D, Jian Y-P, Zhang Y-N, Li Y, Gu L-T, Sun H-H, et al. Short-chain fatty acids in diseases. Cell Commun Signal. (2023) 21:212. doi: 10.1186/s12964-023-01219-9
33. Yao Y, Cai X, Fei W, Ye Y, Zhao M, and Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. (2022) 62:1–12. doi: 10.1080/10408398.2020.1854675
34. Babu A, Devi Rajeswari V, Ganesh V, Das S, Dhanasekaran S, Usha Rani G, et al. Gut microbiome and polycystic ovary syndrome: Interplay of associated microbial-metabolite pathways and therapeutic strategies. Reprod Sci. (2024) 31:1508–20. doi: 10.1007/s43032-023-01450-2
35. Chiang JYL and Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. (2018) 18:71–87. doi: 10.3727/105221618X15156018385515
36. Fuchs CD, Simbrunner B, Baumgartner M, Campbell C, Reiberger T, and Trauner M. Bile acid metabolism and signalling in liver disease. J Hepatol. (2025) 82:134–53. doi: 10.1016/j.jhep.2024.09.032
37. Chiang JYL. Bile acid metabolism and signaling. Compr Physiol. (2013) 3:1191–212. doi: 10.1002/CPHY.C120023
38. Muccee F, Razzaq F, Iqbal R, Rafique F, Amjad A, and Nasir Q. DIVERSITY OF β-GLUCURONIDASE AMONG THE MICROBIOME OF HEALTHY INDIVIDUALS AND PCOS PATIENTS. Biol Clin Sci Res J. (2023) 2023:568–8. doi: 10.54112/bcsrj.v2023i1.568
39. Hu S, Ding Q, Zhang W, Kang M, Ma J, and Zhao L. Gut microbial beta-glucuronidase: a vital regulator in female estrogen metabolism. Gut Microbes. (2023) 15:2236749. doi: 10.1080/19490976.2023.2236749
40. Hu A, Liu J, Xu Y, and Wu Y. Research progress on the mechanism of lactic acid bacteria inhibiting bacteria. J Anim Nutr. (2021) 33:6690–8.
41. Canfora EE, Jocken JW, and Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. doi: 10.1038/nrendo.2015.128
42. Wahlström A, Sayin SI, Marschall H-U, and Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. doi: 10.1016/j.cmet.2016.05.005
43. Agus A, Clément K, and Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. (2021) 70:1174–82. doi: 10.1136/gutjnl-2020-323071
44. Chen M, Liu C, Wan Y, Yang L, Jiang S, Qian D, et al. Enterohepatic circulation of bile acids and their emerging roles on glucolipid metabolism. Steroids. (2021) 165:108757. doi: 10.1016/j.steroids.2020.108757
45. Jia W, Li Y, Cheung KCP, and Zheng X. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis. Sci China Life Sci. (2024) 67:865–78. doi: 10.1007/s11427-023-2353-0
46. Yan J, Sheng L, and Li H. Akkermansia muciniphila: Is it the holy grail for ameliorating metabolic diseases? Gut Microbes. (n.) 13:1984104. doi: 10.1080/19490976.2021.1984104
47. Han H, Yi B, Zhong R, Wang M, Zhang S, Ma J, et al. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome. (2021) 9:162. doi: 10.1186/s40168-021-01093-y
48. Sun Y, Gao S, Ye C, and Zhao W. Gut microbiota dysbiosis in polycystic ovary syndrome: Mechanisms of progression and clinical applications. Front Cell Infect Microbiol. (2023) 13:1142041. doi: 10.3389/fcimb.2023.1142041
49. Yu Z, Wang Y, Yu Z, Lu M, and Xu B. Crosstalk between adipose tissue and the microbiota-gut-brain axis in metabolic diseases. Int J Biol Sci. (2022) 18:1706–23. doi: 10.7150/ijbs.68786
50. Zhu Q, Li Y, Ma J, Ma H, and Liang X. Potential factors result in diminished ovarian reserve: A comprehensive review. J Ovarian Res. (2023) 16:208. doi: 10.1186/s13048-023-01296-x
51. Zhang D, Gao J, Liu X, Qin H, and Wu X. Effect of three androgen indexes (FAI, FT, and TT) on clinical, biochemical, and fertility outcomes in women with polycystic ovary syndrome. Reprod Sci. (2021) 28:775–84. doi: 10.1007/s43032-020-00316-1
52. Chen J, Ni T, and Yan J. Progress in the study of endometrial receptivity defects in Pcos patients. Chin J Reprod Contraception. (2024) 44:1021–6. doi: 10.3760/cma.j.cn101441-20240310-00089
53. Cui Y and Feng X. Research progress on the correlation between Th17/treg cell imbalance under intestinal flora regulation and unexplained recurrent miscarriage. Chin J Microecology. (2023) 35:1471–4. doi: 10.13381/j.cnki.cjm.202312020
54. Mailing LJ, Allen JM, Buford TW, Fields CJ, and Woods JA. Exercise and the gut microbiome: A review of the evidence, potential mechanisms, and implications for human health. Exercise Sport Sci Rev. (2019) 47:75. doi: 10.1249/JES.0000000000000183
55. Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. (2014) 15:511. doi: 10.1186/1471-2164-15-511
56. Lambert JE, Myslicki JP, Bomhof MR, Belke DD, Shearer J, and Reimer RA. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab. (2015) 40:749–52. doi: 10.1139/apnm-2014-0452
57. Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PloS One. (2014) 9:e92193. doi: 10.1371/journal.pone.0092193
58. Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PloS One. (2013) 8:e65465. doi: 10.1371/journal.pone.0065465
59. Denou E, Marcinko K, Surette MG, Steinberg GR, and Schertzer JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. (2016) 310:E982–993. doi: 10.1152/ajpendo.00537.2015
60. Mika A, Van Treuren W, González A, Herrera JJ, Knight R, and Fleshner M. Exercise is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PloS One. (2015) 10:e0125889. doi: 10.1371/journal.pone.0125889
61. Campbell SC, Wisniewski PJ, Noji M, McGuinness LR, Häggblom MM, Lightfoot SA, et al. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PloS One. (2016) 11:e0150502. doi: 10.1371/journal.pone.0150502
62. Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, et al. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. (2008) 72:572–6. doi: 10.1271/bbb.70474
63. Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, et al. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol (1985). (2015) 118:1059–66. doi: 10.1152/japplphysiol.01077.2014
64. Batacan RB, Fenning AS, Dalbo VJ, Scanlan AT, Duncan MJ, Moore RJ, et al. A gut reaction: The combined influence of exercise and diet on gastrointestinal microbiota in rats. J Appl Microbiol. (2017) 122:1627–38. doi: 10.1111/jam.13442
65. Welly RJ, Liu T-W, Zidon TM, Rowles JL, Park Y-M, Smith TN, et al. Comparison of diet vs. Exercise on metabolic function & gut microbiota in obese rats. Med Sci Sports Exerc. (2016) 48:1688–98. doi: 10.1249/MSS.0000000000000964
66. Liu T-W, Park Y-M, Holscher HD, Padilla J, Scroggins RJ, Welly R, et al. Physical activity differentially affects the cecal microbiota of ovariectomized female rats selectively bred for high and low aerobic capacity. PloS One. (2015) 10:e0136150. doi: 10.1371/journal.pone.0136150
67. Kang SS, Jeraldo PR, Kurti A, Miller MEB, Cook MD, Whitlock K, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegeneration. (2014) 9:36. doi: 10.1186/1750-1326-9-36
68. Castro AP, Silva KKS, Medeiros CSA, Alves F, Araujo RC, and Almeida JA. Effects of 12 weeks of resistance training on rat gut microbiota composition. J Exp Biol. (2021) 224:jeb242543. doi: 10.1242/jeb.242543
69. Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. (2016) 4:42. doi: 10.1186/s40168-016-0189-7
70. Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. (2018) 67:625–33. doi: 10.1136/gutjnl-2016-313627
71. Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PloS One. (2017) 12:e0171352. doi: 10.1371/journal.pone.0171352
72. Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. (2018) 50:747–57. doi: 10.1249/MSS.0000000000001495
73. Moitinho-Silva L, Wegener M, May S, Schrinner F, Akhtar A, Boysen TJ, et al. Short-term physical exercise impacts on the human holobiont obtained by a randomised intervention study. BMC Microbiol. (2021) 21:162. doi: 10.1186/s12866-021-02214-1
74. Verheggen RJHM, Konstanti P, Smidt H, Hermus ARMM, Thijssen DHJ, and Hopman MTE. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obes (Silver Spring). (2021) 29:1615–24. doi: 10.1002/oby.23252
75. Haqq L, McFarlane J, Dieberg G, and Smart N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Endocr Connect. (2014) 3:36–46. doi: 10.1530/EC-14-0010
76. Dos Santos IK, Ashe MC, Cobucci RN, Soares GM, de Oliveira Maranhão TM, and Dantas PMS. The effect of exercise as an intervention for women with polycystic ovary syndrome: A systematic review and meta-analysis. Med (Baltimore). (2020) 99:e19644. doi: 10.1097/MD.0000000000019644
77. Shetty D, Chandrasekaran B, Singh AW, and Oliverraj J. Exercise in polycystic ovarian syndrome: An evidence-based review. Saudi J Sports Med. (2017) 17:123. doi: 10.4103/sjsm.sjsm_10_17
78. Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. (2014) 63:1913–20. doi: 10.1136/gutjnl-2013-306541
79. Cheng R, Wang L, Le S, Yang Y, Zhao C, Zhang X, et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat Commun. (2022) 13:2555. doi: 10.1038/s41467-022-29968-0
80. Samuel BS and Gordon JI. A humanized gnotobiotic mouse model of host–archaeal–bacterial mutualism. Proc Natl Acad Sci. (2006) 103:10011–6. doi: 10.1073/pnas.0602187103
81. Dm K, Keohane DM, Woods T, O'Connor P, Underwood S, Cronin O, et al. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J Sci Med Sport. (2019) 22:1059–64. doi: 10.1016/j.jsams.2019.04.004
82. Zhao X, Zhang Z, Hu B, Huang W, Yuan C, and Zou L. Response of gut microbiota to metabolite changes induced by endurance exercise, Front. Microbiol. (2018) 9:765. doi: 10.3389/fmicb.2018.00765
83. Gross K, Santiago M, Krieger JM, Hagele AM, Zielinska K, Scheiman J, et al. Impact of probiotic veillonella atypica FB0054 supplementation on anaerobic capacity and lactate. iScience. (2024) 27:108643. doi: 10.1016/j.isci.2023.108643
84. Yuille S, Reichardt N, Panda S, Dunbar H, and Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PloS One. (2018) 13:e0201073. doi: 10.1371/journal.pone.0201073
85. Waldecker M, Kautenburger T, Daumann H, Busch C, and Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. (2008) 19:587–93. doi: 10.1016/j.jnutbio.2007.08.002
86. Peng L, Li Z-R, Green RS, Holzman IR, and Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
87. Säemann MD, Böhmig GA, Osterreicher CH, Burtscher H, Parolini O, Diakos C, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. (2000) 14:2380–2. doi: 10.1096/fj.00-0359fje
88. Magal M. ACSM’s Guidelines for Exercise Testing and Prescription (2018). Wolters kiuwer. Available online at: https://www.wolterskluwer.com/en/know/acsm/guidelines-for-exercise-testing-and-prescription (Accessed September 9, 2025).
89. American College of Sports Medicine Position Statement. Quantity and quality of exercise for developing and maintaining fitness in healthy adults. Physician Sportsmed. (1978) 6:39–41. doi: 10.1080/00913847.1978.11710773
90. American college of sports medicine position stand. American college of sports medicine position stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exercise. (1998) 30:975–91. doi: 10.1097/00005768-199806000-00032
91. Ritchie C. Rating of perceived exertion (RPE). J Physiother. (2012) 58:62. doi: 10.1016/S1836-9553(12)70078-4
92. Patten RK, Boyle RA, Moholdt T, Kiel I, Hopkins WG, Harrison CL, et al. Exercise interventions in polycystic ovary syndrome: A systematic review and meta-analysis. Front Physiol. (2020) 11:606. doi: 10.3389/fphys.2020.00606
93. Zhang M, Xiao B, Chen X, Ou B, and Wang S. Physical exercise plays a role in rebalancing the bile acids of enterohepatic axis in non-alcoholic fatty liver disease. Acta Physiologica. (2024) 240:e14065. doi: 10.1111/apha.14065
94. Meissner M, Lombardo E, Havinga R, Tietge UJF, Kuipers F, and Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis. (2011) 218:323–9. doi: 10.1016/j.atherosclerosis.2011.06.040
95. Li Y, Wang L, Yi Q, Luo L, and Xiong Y. Regulation of bile acids and their receptor FXR in metabolic diseases, Front. Nutr 11. (2024) 11:1447878. doi: 10.3389/fnut.2024.1447878
96. Munukka E, Ahtiainen JP, Puigbó P, Jalkanen S, Pahkala K, Keskitalo A, et al. Six-week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over-weight women. Front Microbiol. (2018) 9:2323. doi: 10.3389/fmicb.2018.02323
97. Donati Zeppa S, Amatori S, Sisti D, Gervasi M, Agostini D, Piccoli G, et al. Nine weeks of high-intensity indoor cycling training induced changes in the microbiota composition in non-athlete healthy male college students. J Int Soc Sports Nutr. (2021) 18:74. doi: 10.1186/s12970-021-00471-z
98. Zhong F, Wen X, Yang M, Lai H-Y, Momma H, Cheng L, et al. Effect of an 8-week exercise training on gut microbiota in physically inactive older women. Int J Sports Med. (2021) 42:610–23. doi: 10.1055/a-1301-7011
99. Khan MS, Ikram M, Park JS, Park TJ, and Kim MO. Gut microbiota, its role in induction of alzheimer’s disease pathology, and possible therapeutic interventions: Special focus on anthocyanins. Cells. (2020) 9:853. doi: 10.3390/cells9040853
100. Canning P, Sorrell FJ, and Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radical Biol Med. (2015) 88:101–7. doi: 10.1016/j.freeradbiomed.2015.05.034
101. Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. (2016) 7:11624. doi: 10.1038/ncomms11624
102. Motiani KK, Collado MC, Eskelinen J-J, Virtanen KA, Löyttyniemi E, Salminen S, et al. Exercise training modulates gut microbiota profile and improves endotoxemia. Med Sci Sports Exerc. (2020) 52:94–104. doi: 10.1249/MSS.0000000000002112
103. Baptissart M, Vega A, Martinot E, Baron S, Lobaccaro J-MA, and Volle DH. Farnesoid X receptor alpha: A molecular link between bile acids and steroid signaling? Cell Mol Life Sci. (2013) 70:4511–26. doi: 10.1007/s00018-013-1387-0
104. Shao N, Cai K, Hong Y, Wang K, Luo Q, and Wu L. Short-chain fatty acids inhibit NLRP3 inflammasome to alleviate inflammation and epithelial-mesenchymal transition in diabetic nephropathy rats via GPR41/43. J Biol Regulators Homeostatic Agents. (2024) 38:4757–70. doi: 10.23812/j.biol.regul.homeost.agents.20243806.380
105. He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. (2020) 21:6356. doi: 10.3390/ijms21176356
106. Lu Y, Fan C, Li P, Lu Y, Chang X, and Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. (2016) 6:37589. doi: 10.1038/srep37589
107. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. (2003) 278:11312–9. doi: 10.1074/jbc.M211609200
108. Bolognini D, Dedeo D, and Milligan G. Metabolic and inflammatory functions of short-chain fatty acid receptors. Curr Opin Endocr Metab Res. (2021) 16:1–9. doi: 10.1016/j.coemr.2020.06.005
109. Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. (2015) 39:424–9. doi: 10.1038/ijo.2014.153
110. Acharya A, Shetty SS, and Kumari N S. Role of gut microbiota derived short chain fatty acid metabolites in modulating female reproductive health. Hum Nutr Metab. (2024) 36:200256. doi: 10.1016/j.hnm.2024.200256
111. Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. (2014) 5:3611. doi: 10.1038/ncomms4611
112. Cook TM, Gavini CK, Jesse J, Aubert G, Gornick E, Bonomo R, et al. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol Metab. (2021) 54:101350. doi: 10.1016/j.molmet.2021.101350
113. Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. (2018) 8:74. doi: 10.1038/s41598-017-18259-0
114. Li Z, Yi C-X, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. (2018) 67:1269–79. doi: 10.1136/gutjnl-2017-314050
115. Christiansen CB, Trammell SAJ, Wewer Albrechtsen NJ, Schoonjans K, Albrechtsen R, Gillum MP, et al. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am J Physiol Gastrointest Liver Physiol. (2019) 316:G574–84. doi: 10.1152/ajpgi.00010.2019
116. Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Møller E, Andersen DB, et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. (2018) 11:84–95. doi: 10.1016/j.molmet.2018.03.007
117. Liu S, Marcelin G, Blouet C, Jeong JH, Jo Y-H, Schwartz GJ, et al. A gut–brain axis regulating glucose metabolism mediated by bile acids and competitive fibroblast growth factor actions at the hypothalamus. Mol Metab. (2018) 8:37–50. doi: 10.1016/j.molmet.2017.12.003
118. Perino A, Velázquez-Villegas LA, Bresciani N, Sun Y, Huang Q, Fénelon VS, et al. Central anorexigenic actions of bile acids are mediated by TGR5. Nat Metab. (2021) 3:595–603. doi: 10.1038/s42255-021-00398-4
119. Li S, Liu M, Cao S, Liu B, Li D, Wang Z, et al. The mechanism of the gut-brain axis in regulating food intake. Nutrients. (2023) 15:3728. doi: 10.3390/nu15173728
120. Pham CH, Lee J-E, Yu J, Lee SH, Yu K-R, Hong J, et al. Anticancer effects of propionic acid inducing cell death in cervical cancer cells. Molecules. (2021) 26:4951. doi: 10.3390/molecules26164951
121. Hang S, Paik D, Devlin AS, Jamma T, Lu J, Ha S, et al. Bile acid metabolites control Th17 and Treg cell differentiation. bioRxiv. (2018) 8:465344. doi: 10.1101/465344
122. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. (2008) 453:65–71. doi: 10.1038/nature06880
123. Tetel MJ, De Vries GJ, Melcangi RC, Panzica G, and O’Mahony SM. Steroids, stress and the gut microbiome-brain axis. J Neuroendocrinol. (2018) 30:e12548. doi: 10.1111/jne.12548
124. Olaniyi KS, Bashir AM, Areloegbe SE, Sabinari IW, Akintayo CO, Oniyide AA, et al. Short chain fatty acid, acetate restores ovarian function in experimentally induced PCOS rat model. PloS One. (2022) 17:e0272124. doi: 10.1371/journal.pone.0272124
125. Ribichini E, Scalese G, Cesarini A, Mocci C, Pallotta N, Severi C, et al. Exercise-induced gastrointestinal symptoms in endurance sports: A review of pathophysiology, symptoms, and nutritional management. Dietetics. (2023) 2:289–307. doi: 10.3390/dietetics2030021
126. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod (Oxf. Engl.). (2004) 19:41–7. doi: 10.1093/humrep/deh098
127. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, and Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. (2016) 106:6–15. doi: 10.1016/j.fertnstert.2016.05.003
128. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod (Oxf. Engl.). (2013) 28:777–84. doi: 10.1093/humrep/des463
129. Ross FC, Patangia D, Grimaud G, Lavelle A, Dempsey EM, Ross RP, et al. The interplay between diet and the gut microbiome: Implications for health and disease. Nat Rev Microbiol. (2024) 22:671–86. doi: 10.1038/s41579-024-01068-4
130. Wang L, Liu K, Wang G, and Yang L. Effects of exercise interventions on women with polycystic ovary syndrome: A systematic review and meta-analysis. Nurs. Health Sci. (2025) 27:e70209. doi: 10.1111/nhs.70209
131. Calcaterra V, Verduci E, Cena H, Magenes VC, Todisco CF, Tenuta E, et al. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: the role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients. (2021) 13:1848. doi: 10.3390/nu13061848
Keywords: pcos, gut microbiota, exercise, molecular mechanisms, IR
Citation: Li Q, Chen L and Wang R (2025) Exercise reshapes gut microbiota to ameliorate core symptoms in PCOS: molecular mechanisms and therapeutic implications. Front. Endocrinol. 16:1652731. doi: 10.3389/fendo.2025.1652731
Received: 24 June 2025; Accepted: 17 October 2025;
Published: 29 October 2025.
Edited by:
Giselle Adriana Abruzzese, CIC bioGUNE, SpainReviewed by:
Valentina Caputi, University College Cork, IrelandLei Han, Binzhou Medical University Hospital, China
Copyright © 2025 Li, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronghui Wang, d3JoLmJzdUAxNjMuY29t
 Qianqian Li
Qianqian Li Leqin Chen2
Leqin Chen2