- 1Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of General Medicine, Heze Hospital Affiliated to Shandong First Medical University, Heze, Shandong, China
- 3Department of Endocrinology and Metabolism, Tianjin University Central Hospital, Tianjin, China
- 4Department of Cardiology, Heze Municipal Hospital, Heze, Shandong, China
- 5Department of Urology, Heze Hospital Affiliated to Shandong First Medical University, Heze, Shandong, China
Objective: The free triiodothyronine to free thyroxine (FT3/FT4) ratio is an indicator of peripheral thyroid hormone sensitivity. However, its prognostic value in heart failure (HF) remains unclear.
Methods: This single center prospective cohort study included a total of 402 HF patients. The primary composite outcome was established as either mortality from any cause or HF-related hospitalization within one year. Multivariate Cox regression and Kaplan-Meier analysis assessed associations between the FT3/FT4 ratio and composite endpoint risks, with restricted cubic splines (RCS) exploring potential non-linear relationships.
Results: Among 402 heart failure patients, 188 (46.8%) experienced the primary composite endpoint. The highest FT3/FT4 tertile (T3) had 38% lower risk than the lowest tertile (T1) (adjusted HR 0.62, 95% CI 0.41-0.94). In the subgroup of patients with subclinical hypothyroidism (SCH), T3 individuals showed an 84% lower risk compared to T1 (adjusted HR 0.16, 95% CI 0.03–0.81). Both the overall cohort and SCH subgroup exhibited an inverse association between FT3/FT4 ratios and adverse outcomes, whereas euthyroid patients demonstrated a U-shaped relationship with composite endpoint hazards (P for nonlinear = 0.004).
Conclusions: Our findings suggest that maintaining or restoring higher FT3/FT4 levels may improve clinical outcomes in HF patients. Regular monitoring of this ratio, coupled with tailored interventions based on thyroid functional status, could enhance risk stratification and therapeutic decision-making.
Introduction
Thyroid hormones directly modulate cardiovascular function by enhancing myocardial contractility while also exerting significant indirect effects through sympathetic nervous system activation (1). Even subtle disturbances in thyroid homeostasis are linked to adverse cardiovascular outcomes (2). Heart failure (HF) patients frequently exhibit alterations in thyroid hormone metabolism, including low T3 syndrome and subclinical hypothyroidism (SCH), both established predictors of poor prognosis (3–5). Furthermore, thyrotropin (TSH) levels above 7.0 mU/L show dose-dependent associations with increased coronary heart disease mortality (6, 7), overt hyperthyroidism elevates cardiovascular mortality (8), and high-normal free thyroxine (FT4) correlates with increased mortality in the elderly (9).
Thyroid hormone homeostasis is regulated by the hypothalamic-pituitary-thyroid (HPT) axis (10). Triiodothyronine (T3), the biologically active hormone, is predominantly generated peripherally via deiodination of thyroxine (T4) (11). The FT3/FT4 ratio quantifies peripheral T4-to-T3 conversion efficiency and tissue-level thyroid hormone bioavailability (12, 13), serving as a surrogate marker of peripheral thyroid sensitivity (14, 15). This ratio may be more sensitive than isolated FT3 or FT4 measurements in detecting subtle thyroid metabolic perturbations (16). Emerging evidence supports the FT3/FT4 ratio’s prognostic value in cardiovascular disease for predicting adverse events across multiple populations, including euthyroid acute coronary syndrome patients (17, 18), general cardiovascular disease cohorts (19), and specific groups such as dilated cardiomyopathy (20).
Despite this, the prognostic significance of FT3/FT4 across the spectrum of thyroid function in HF remains underexplored. Notably, HF has an average 1-year mortality rate of up to 33% (21), underscoring the prognostic usefulness for precise, thyroid status-specific risk stratification tools. Defining thyroid function-dependent FT3/FT4 thresholds could enable personalized risk assessment and guide targeted therapies (22).
To address these gaps, this study aimed to: (1) Elucidate the relationship between the FT3/FT4 ratio and composite endpoint risk, and (2) Characterize the nature (linear vs. non-linear) and magnitude of associations of the FT3/FT4 ratio with adverse outcomes.
Methods
Study population
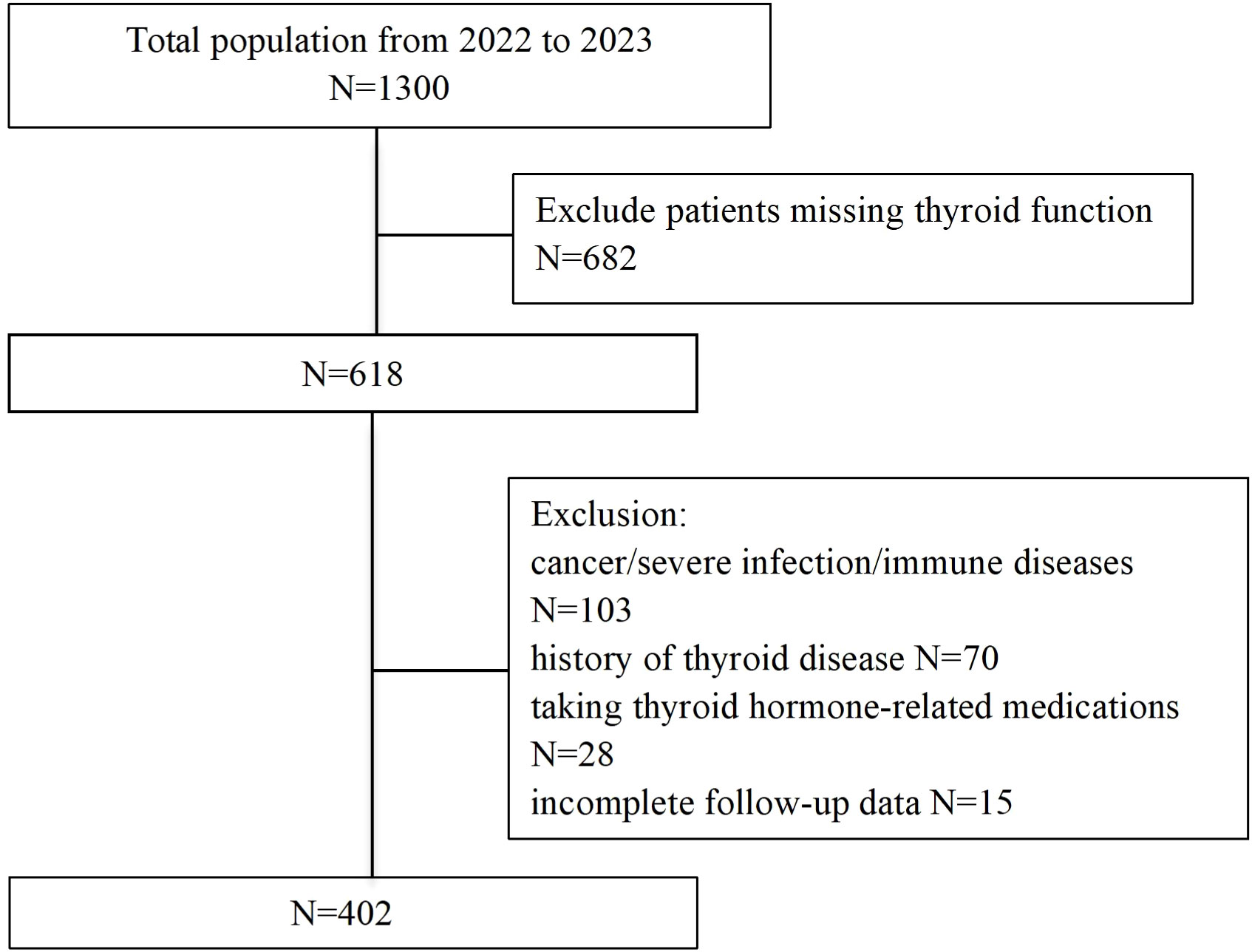
This prospective cohort study consecutively enrolled 1,300 patients admitted to the Cardiology Department of Heze Hospital, Affiliated to Shandong First Medical University, between January 2022 and December 2023. Inclusion criteria required participants to be ≥18 years old, fulfilling both the 2018 Chinese HF diagnostic guidelines (23) and the 2021 European Society of Cardiology (ESC) HF criteria (24), and New York Heart Association (NYHA) functional class I-IV. Exclusion criteria comprised: (1) unavailable baseline thyroid function data; (2) pre-existing thyroid disorders, malignancy, or severe infections; (3) pregnancy; (4) loss to follow-up; (5) use of thyroid-affecting medications. After exclusions, 402 patients constituted the final analytical cohort. Approved by the Ethics Committee of Heze Hospital Affiliated to Shandong First Medical University (No. 2024-KY001-079). All participants provided written informed consent.
Data collection
Detailed clinical information was retrieved from the electronic medical records. Clinical information included sex, age, body mass index (BMI), lifestyle factors (smoking, alcohol use), parameters including heart rate (HR), systolic/diastolic blood pressure (SBP/DBP), NYHA class, HF etiology (hypertension, diabetes, coronary heart disease), along with medication records of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), β-blockers, and statins. Venous blood collected within 24 hours of admission underwent analysis for: lipid profiles (total cholesterol [TC], triglycerides [TG], high-density lipoprotein [HDL-C], low-density lipoprotein [LDL-C]), fasting blood glucose [FBG], uric acid [UA], creatinine [Cr], N-terminal pro-B-type natriuretic peptide [NT-proBNP], aspartate aminotransferase [AST], and thyroid function (TSH, FT3, FT4 via direct chemiluminescence; reference ranges: FT3 1.8-4.2 pg/ml, FT4 0.87-1.85 ng/dL, TSH 0.35-5.1 μIU/ml) (Roche Diagnostics Gmbh, Japan). The study population included euthyroid patients and those with thyroid dysfunction (hyperthyroidism, hypothyroidism, subclinical thyroid dysfunction, and other thyroid abnormalities). Euthyroid status is defined as having TSH, FT4, and FT3 all within their respective normal ranges. SCH is characterized by elevated TSH levels (>5.1 uIU/ml) in conjunction with normal FT4. Echocardiography assessed left ventricular ejection fraction (LVEF) and left ventricular end-diastolic dimension (LVDD) using the Biplane Simpson method. All missing values for these covariates are less than 1%.
Outcomes
The key composite outcome was defined as death from any cause or rehospitalization due to HF within 1 year, selected based on established prognostic relevance in prior studies (25, 26). Endpoint assessors were not blinded to thyroid data.
Statistical analysis
Statistical analyses were performed using SPSS 25.0 and R 0.5.6 (rms package). Patients were stratified into tertiles based on FT3/FT4 ratio. This equidistant grouping method produced consistent intervals and automatically assigned samples into groups of nearly equal size, due to the continuous distribution of values in the population. Similar approaches have been used in previous studies, such as those by Okoye et al. and Qin et al., which also reported balanced sample distributions across groups as a result of this method (27, 28). Continuous variables were summarized as median and interquartile range (IQR) and compared using the non-parametric Kruskal-Wallis test; categorical variables were presented as counts and percentages, with group comparisons performed using χ² or Fisher’s exact test as appropriate (29). Cox regression was used to assess the association between FT3/FT4 and composite outcomes. Model 1 remained unadjusted, whereas Model 2 incorporated adjustments for sex, age, and BMI. Model 3 added smoking, drinking, SBP, DBP, HR, NYHA class, LVEF, LVDD, TC, TG, LDL-C, HDL-C, FBG, UA, Cr, logNT-proBNP, AST, comorbidities (hypertension, diabetes, coronary heart disease), and HF medications (ACEIs/ARBs, β-blockers, statins). The results are presented as hazard ratios (HRs) along with 95% confidence intervals (CIs). Survival differences were tested with Kaplan-Meier/log-rank methods, while RCS (knots: 10th/50th/90th percentiles) examined nonlinear associations using Model 3 adjustments. Statistical significance threshold was P<0.05.
Results
FT3/FT4 ratio in the overall cohort
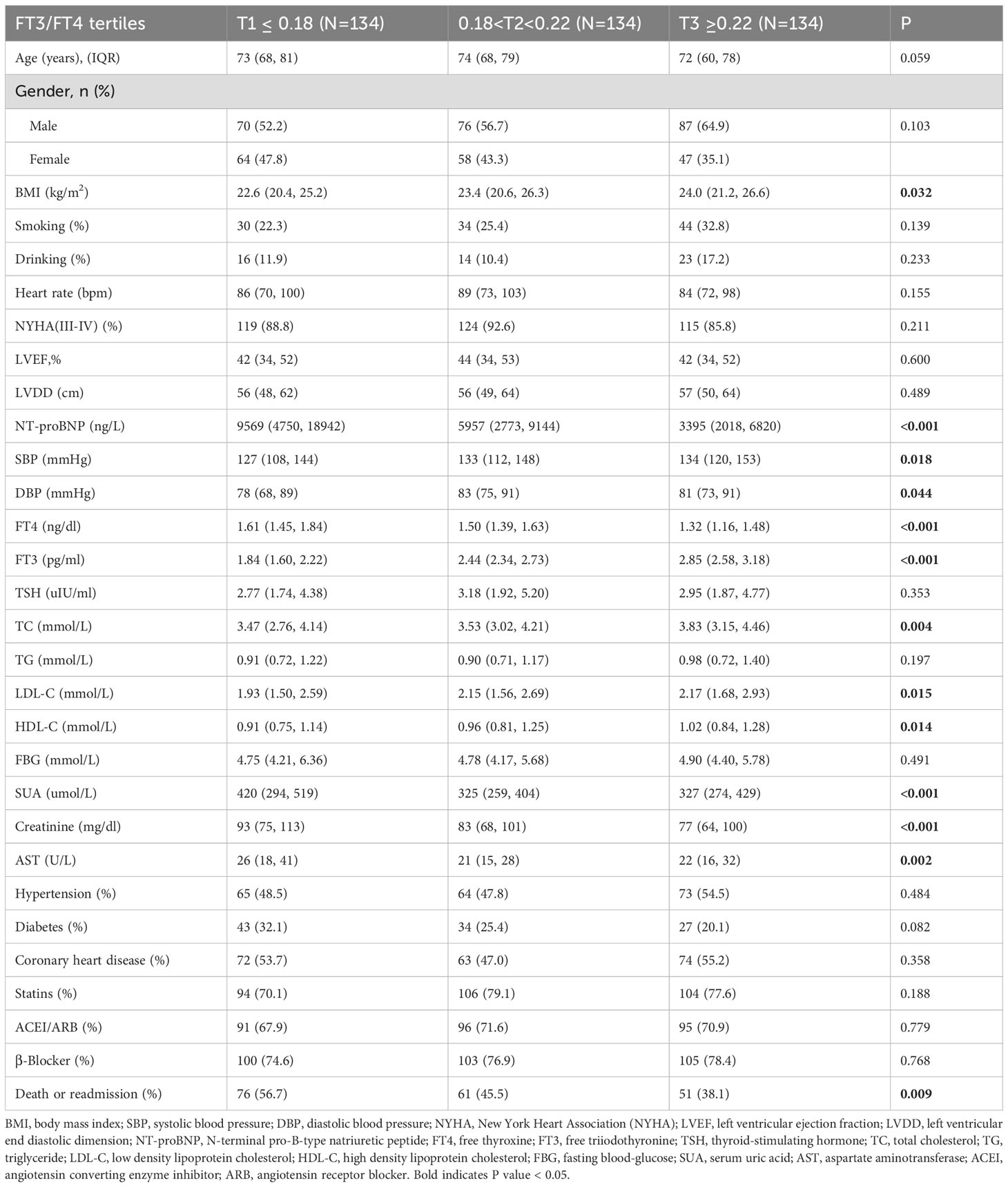
The patient selection flowchart is shown in Figure 1. The final analysis included 402 HF patients with a median age of 73 years (IQR: 67-79), of whom 58.0% were male. During follow-up, 188 (46.8%) experienced the composite events. Participants were stratified into tertiles: T1 (≤0.18), (0.18<T2<0.22), and T3 (≥0.22). Baseline characteristics across tertiles are detailed in Table 1. Higher FT3/FT4 ratios were associated with elevated BMI, SBP, DBP, LDL-C, and HDL-C levels, alongside lower uric acid, creatinine, NT-proBNP, and AST levels (all P <0.05) (Table 1). Notably, the T3 group demonstrated the lowest incidence of composite events (T1: 56.7% vs. T3: 38.1%; P=0.009).
In Cox regression analyses, both T2 and T3 groups exhibited progressively lower risks of composite outcomes compared to T1. In unadjusted Cox analysis, the T3 group had significantly lower composite risk compared to T1 (HR=0.57, 95% CI 0.40-0.81; P for trend=0.002). Adjustment for sex, age, and BMI (Model 2) yielded similar results. In the fully adjusted model (Model 3), the T2 (HR=0.68, 95%CI 0.46-0.99, P=0.047) and T3 (HR=0.62, 95%CI 0.41-0.94, P=0.023) groups both showed significantly lower risks than the T1 group, with a significant decreasing trend across tertiles (P for trend=0.02) (Figure 2). When analyzed as a continuous variable, the FT3/FT4 ratio demonstrated an inverse association with composite outcomes after multivariable adjustment (adjusted HR:0.11, 95%CI: 0.07-0.99, P=0.049). Kaplan-Meier analysis confirmed significantly better event-free survival with higher FT3/FT4 ratios (log-rank P=0.009; Figure 3A). RCS analysis demonstrated an inverse association between the continuous FT3/FT4 ratio and composite risk (P for nonlinear=0.568; Figure 4A).
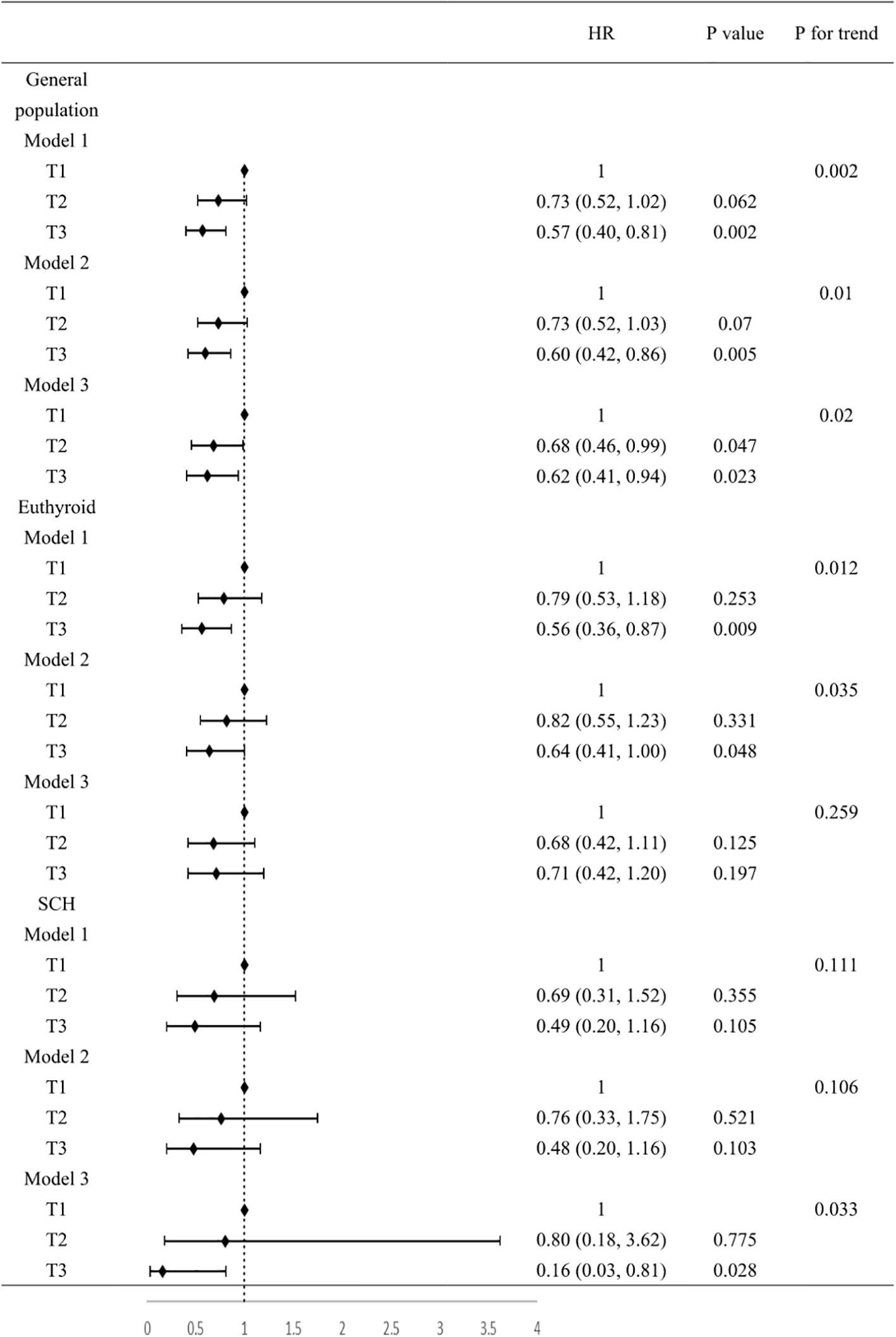
Figure 2. Association between FT3/FT4 value and composite outcomes. Model 1, no covariates were adjusted. Model 2, age, gender, BMI were adjusted. Model 3, age, gender, BMI, smoking, drinking, SBP, DBP, HR, NYHA class, LVEF, LVDD, TC, TG, LDL-C, HDL-C, FBG, UA, Cr, NT-proBNP, AST, ACEI/ARB, β-blockers, statins, hypertension, diabetes, coronary heart disease were adjusted. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart ratio; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LVDD, left ventricular end-diastolic dimension; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; FBG, fasting blood glucose; UA, uric acid; Cr, creatinine; NT-proBNP, N-terminal pro-B-type natriuretic peptide; AST, aspartate aminotransferase; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
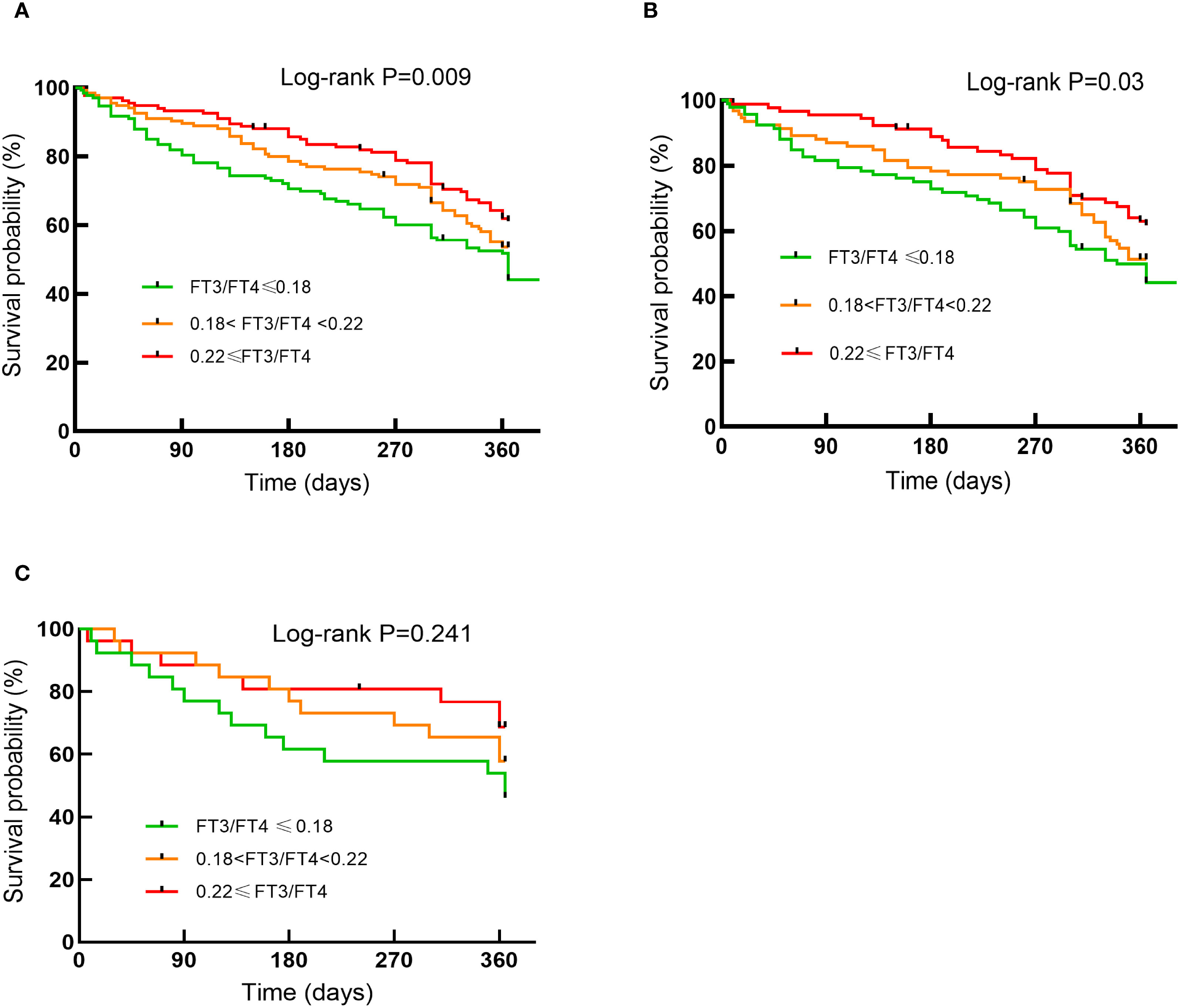
Figure 3. Kaplan-Meier curves for endpoint events by tertiles of FT3/FT4 ratio in the general population (A) in the euthyroid population (B) in the SCH population (C).
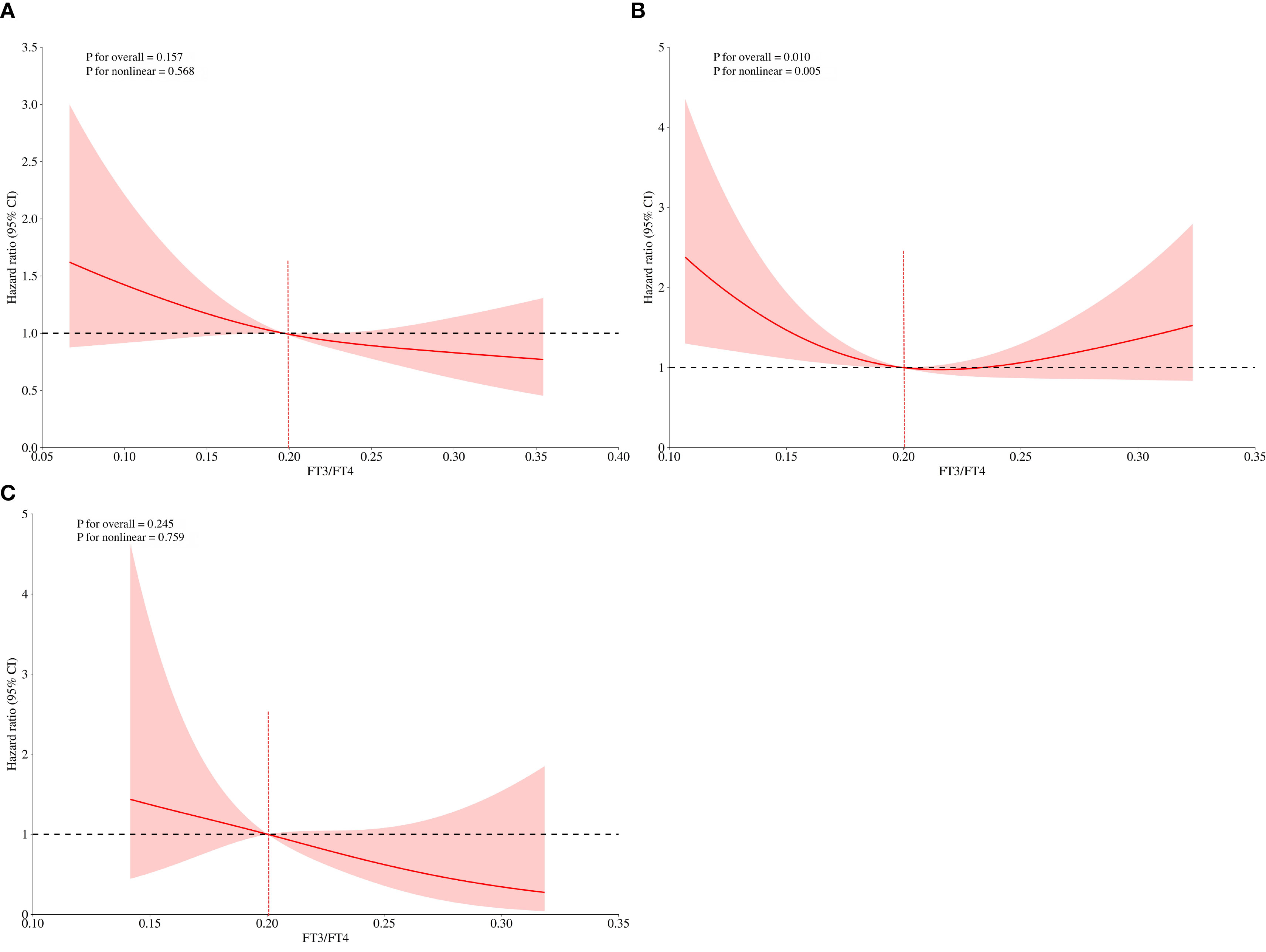
Figure 4. FT3/FT4 and the risk of composite endpoints derived from RCS with 3 knots in the general population (A) in the euthyroid population (B) in the SCH (C). The dotted lines represent 95% confidence intervals. Spline analyses were adjusted for age, gender, BMI, smoking, drinking, SBP, DBP, HR, NYHA class, LVEF, LVDD, TC, TG, LDL-C, HDL-C, FBG, UA, Cr, NT-proBNP, AST, ACEI/ARB, β-blockers, statins, hypertension, diabetes, coronary heart disease. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart ratio; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LVDD, left ventricular end-diastolic dimension; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; FBG, fasting blood glucose; UA, uric acid; Cr, creatinine; NT-proBNP, N-terminal pro-B-type natriuretic peptide; AST, aspartate aminotransferase; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
FT3/FT4 ratio in euthyroid population
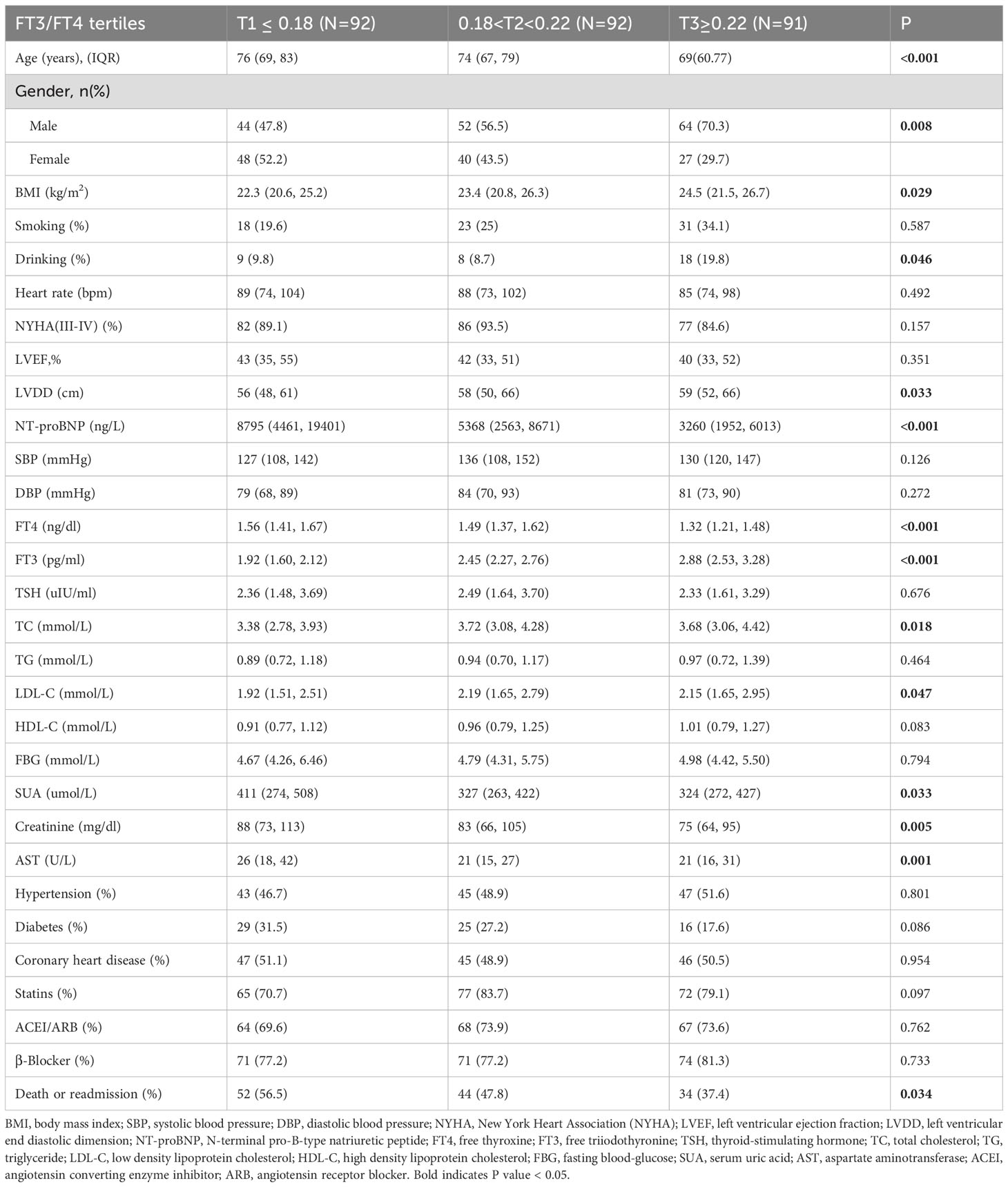
Of all the participants included in the analysis, 275 had normal thyroid function and a subgroup analysis was performed for this euthyroid cohort. Subjects had a median age of 73 years (IQR: 65-79), with 58.20% being male. A total of 130 composite outcomes were recorded. Baseline characteristics across tertiles (T1 ≤ 0.18; (0.18<T2<0.22); T3≥0.22) are shown in Table 2. Elevated FT3/FT4 ratio demonstrated positive associations with BMI, TC, and LDL levels, while correlating inversely with UA, Cr, NT-proBNP, AST, and incidence of composite events (Table 2). In addition, euthyroid individuals exhibiting elevated FT3/FT4 ratios were typically younger, predominantly male, and demonstrated higher alcohol consumption prevalence, and elevated LVDD (all P <0.05). Model 1 showed the T3 group exhibited a significant risk reduction in contrast to the T1 group (HR: 0.56, 95% CI 0.36-0.87, P=0.009, P for trend=0.012). This association persisted after sex/age/BMI adjustment in Model 2 (HR: 0.64, 95% CI 0.41-1.00, P=0.048). However, after fully adjusting for variables, no significant association was observed (HR: 0.71, 95% CI 0.42-1.20, P=0.197; Figure 2). When analyzed as a continuous variable, the FT3/FT4 ratio showed no significant association with composite outcomes in the multivariable-adjusted model (adjusted HR: 0.17, 95% CI: 0.71–9.72, P=0.394). Kaplan-Meier analysis revealed significant survival disparities across groups (log-rank test: P=0.03, Figure 3B). RCS analysis, however, uncovered a U-shaped relationship (P for nonlinear=0.005; Figure 4B). The minimum risk was observed when the FT3/FT4 ratio was 0.22 (HR: 0.95, 95% CI: 0.77-1.18).
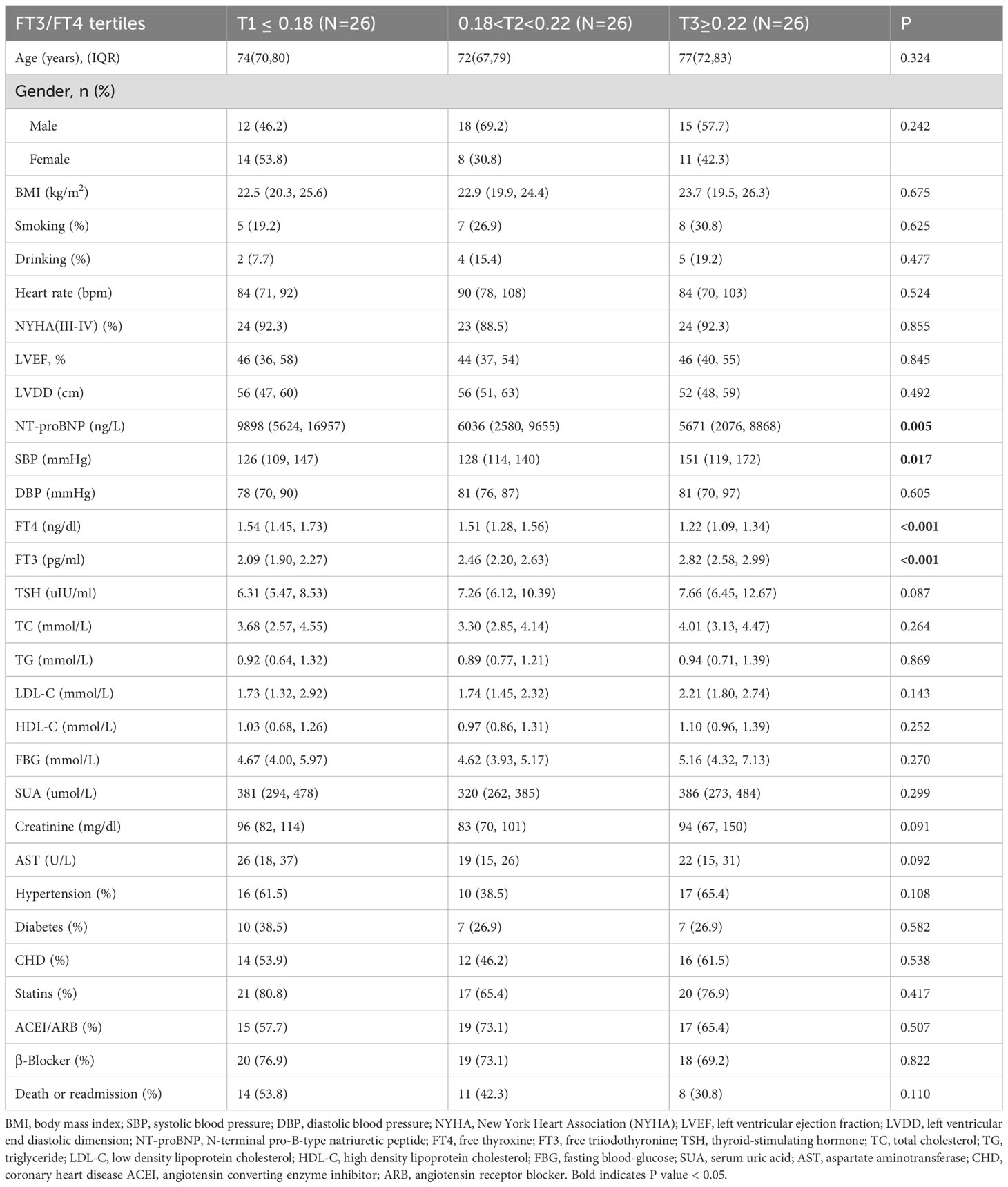
FT3/FT4 ratio in SCH population
The SCH subgroup included 78 patients with median age 75 years (IQR:68-80); 57.8% male), with 33 composite events (42.3%). Increasing FT3/FT4 ratios predicted increased SBP and NT-proBNP levels (P<0.05) (Table 3). In Model 3, the T3 group (≥0.22) had a substantially lower composite risk compared to T1 (≤0.18) (HR: 0.16, 95% CI 0.03–0.81; P=0.028) with progressive risk attenuation (P for trend=0.033; Figure 2). When analyzed as a continuous variable, the FT3/FT4 ratio demonstrated an inverse association with composite outcomes after multivariable adjustment (adjusted HR:0.45, 95%CI: 0.23-0.87, P=0.018). Kaplan-Meier survival curves showed no significant difference (log-rank P=0.241; Figure 3C), but RCS analysis demonstrated an inverse correlation (P for nonlinear=0.759; Figure 4C).
FT3/FT4 ratio in the remaining 49 patients
Among the remaining 49 patients, the median age was 74 (IQR:65-78) years, 57.1% were male, and 25 composite endpoint events were recorded. Baseline characteristics across tertiles are detailed in Supplementary Table 1. Since our initial exclusion criteria applied only to patients with pre-existing thyroid disease, rather than those with newly identified dysfunction in this study, the 49 patients were included in the overall population but were not analyzed separately due to limited sample size, so as not to compromise statistical power in subgroup analyses.
Discussion
This prospective study demonstrates that the FT3/FT4 ratio, a marker of peripheral thyroid hormone sensitivity, exhibits thyroid status-dependent associations with 1-year mortality rehospitalization risk in heart failure patients. Our key novel findings are: (1) An inverse relationship was observed between the FT3/FT4 ratio and composite risk across the overall HF cohort, particularly among patients with SCH; (2) A significant U-shaped relationship is observed in euthyroid HF patients.
HF is characterized by metabolic derangements including reduced nutrient intake (30), chronic inflammation, and oxidative stress (31), which can impair micronutrient absorption (iodine, selenium, zinc, iron) crucial for thyroid hormone synthesis and conversion (32). Thyroid hormones, in turn, profoundly impact cardiac electrophysiology, contractility, and structure (33). Hence, Subtle fluctuations in thyroid hormone bioavailability can therefore significantly influence cardiac function and HF progression (34). The inverse FT3/FT4 ratio-adverse outcome relationship likely involves multifactorial pathways. Reduced ratios indicate impaired peripheral T4-to-T3 conversion, directly contributing to tissue-level hypothyroidism during acute/chronic disease states (18). T3, acting via nuclear receptors (TRα/TRβ) in the heart, enhances the expression of key proteins involved in calcium handling (SERCA2a, RYR2) and mitochondrial function/biogenesis; processes often compromised in HF (35). Lower FT3 levels directly contributes to HF pathogenesis by impairing left ventricular relaxation and increasing myocardial stiffness (36). Furthermore, HF is associated with sympathetic overactivation and Renin-Angiotensin-Aldosterone System (RASS) hyperactivity (37); T3 restores autonomic balance in heart failure by: (1) Attenuating sympathetic overactivation through downregulation of myocardial β adrenergic receptor density and reduction of circulating norepinephrine levels (38); (2) Recovering baroreflex function via upregulation of neuronal nitric oxide synthase (nNOS) in the nucleus tractus solitarius (39), thereby mitigating these detrimental neurohormonal axes (40).
Our findings align with and extend previous research. Studies linked lower FT3/FT4 ratios to increased mortality in dilated cardiomyopathy (20). A study using propensity matching demonstrated that a reduced ratio of FT3/FT4 is a robust predictor of all-cause mortality in heart failure patients (41). Another study discovered that the FT3/FT4 ratio independently predicts all-cause mortality in the general population. In addition, a study noted that in euthyroid patients with type 2 diabetes mellitus, a low FT3/FT4 ratio independently contributed to major adverse cardiac events following acute myocardial infarction (42). However, our study is the first to comprehensively evaluate this ratio across distinct thyroid functional states (euthyroid vs. SCH) within an HF cohort and to identify state-specific risk patterns. The protective association observed in SCH patients is particularly noteworthy and suggests that maintaining adequate peripheral conversion is crucial in this subgroup, potentially outweighing the risks associated with mildly elevated TSH. This finding resonates with experimental data showing T3 reduces infarct size and activates cardio-protection during ischemia/reperfusion (43), and clinical evidence that low-dose T3 replacement improved LV function post-AMI (44). Notably, in patients with SCH, multivariate Cox regression demonstrated an inverse association between the FT3/FT4 ratio and the risk of composite outcomes, whereas KM analysis revealed no significant survival difference among the three groups. This discordance likely reflects the inherent limitation of KM analysis: as a non-parametric method estimating unadjusted survival probabilities from observed events, it does not account for confounding factors.
The U-shaped relationship observed in euthyroid patients is a novel and significant finding. The loss of significance in the fully adjusted multivariate Cox model for the euthyroid subgroup, particularly after accounting for age and NT-proBNP-both strongly associated with the ratio and outcomes, suggesting that the univariate association was partly confounded. This interpretation is primarily informed by the simultaneous measurement of NT-proBNP and thyroid indicators during the same blood draw, which inherently precludes establishing the temporal sequence required for mediation (where exposure must precede the mediator, which subsequently influences the outcome) (45). However, the highly significant U-curve revealed by RCS analysis indicates a complex, non-monotonic relationship. This suggests an optimal range for peripheral thyroid sensitivity in euthyroid HF. Ratios significantly below 0.22 likely indicate impaired conversion and tissue hypothyroidism, increasing risk. Conversely, ratios significantly above this point might reflect excessive peripheral conversion, potentially linked to hypermetabolic states or impaired hormone clearance, which could paradoxically increase cardiovascular strain or indicate other underlying metabolic disturbances detrimental in HF. Compensatory mechanisms preserving tissue-level thyroid hormone action within the normal functional range might also buffer the impact of ratio variations, except at the extremes (46). Potential mechanisms involve: (1) impaired enzymatic efficiency due to the Thr92Ala DIO2 polymorphism at low FT4/FT3 ratios, whereas DIO2 overexpression under high-T4 conditions depletes essential cofactors (e.g., glutathione), thereby exacerbating T4-to-T3 conversion failure (47); (2) pro-inflammatory cytokines (TNF-α, IL-6) suppressing deiodinase activity through NF-κB-mediated downregulation of DIO1 and DIO2 expression, impairing T3 generation and promoting thyroid hormone resistance during chronic inflammation (48). Further research is needed to elucidate the mechanisms underlying this U-shape.
To our knowledge, this is the first study to identify the optimal FT3/FT4 for stratifying mortality/readmission risk in HF patients with varying thyroid states. Limitations should be acknowledged. First, the single-center design necessitates cautious interpretation regarding generalizability. As iodine sufficiency varies significantly across global regions, and Asian populations often exhibit distinct iodine nutritional status compared to Western cohorts, our results should be interpreted within this contextual framework. Second, only baseline thyroid function was assessed; serial measurements might better capture dynamic changes relevant to prognosis. Third, the mechanisms linking the FT3/FT4 ratio to HF outcomes, particularly the U-shape in euthyroidism, require further elucidation. Fourth, In the SCH cohort, the observed hazard ratio (HR) of 0.16 should be interpreted with caution due to the limited sample size. Finally, observational design precludes causal inference.
In conclusion, the FT3/FT4 ratio is a thyroid status-dependent predictor of 1-year mortality and HF rehospitalization risk. Monitoring the FT3/FT4 ratio, offers a valuable tool for risk stratification. Future studies should validate these thresholds in diverse populations and explore whether interventions aimed at optimizing peripheral thyroid hormone sensitivity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Heze Hospital Affiliated to Shandong First Medical University (No. 2024-KY001-079). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LM: Writing – original draft, Formal analysis, Conceptualization. MG: Formal analysis, Writing – review & editing, Data curation. XL: Writing – review & editing, Supervision. LD: Conceptualization, Writing – review & editing, Supervision, Methodology. CM: Conceptualization, Writing – review & editing, Data curation, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We thank all the patients enrolled in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1652749/full#supplementary-material
Supplementary Table 1 | Baseline characteristics of the remaining 49 patients by FT3/FT4 tertiles.
References
1. Klein I and Danzi S. Thyroid disease and the heart. Circulation. (2007) 116:1725–35. doi: 10.1161/CIRCULATIONAHA.106.678326
2. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, and Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. (2017) 14:39–55. doi: 10.1038/nrcardio.2016.174
3. Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. (2003) 107:708–13. doi: 10.1161/01.cir.0000048124.64204.3f
4. Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R, et al. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des. (2008) 14:2686–92. doi: 10.2174/138161208786264142
5. Lubrano V, Pingitore A, Carpi A, and Iervasi G. Relationship between triiodothyronine and proinflammatory cytokines in chronic heart failure. BioMed Pharmacother. (2010) 64:165–69. doi: 10.1016/j.biopha.2009.09.001
6. Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. (2012) 126:1040–49. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. (2010) 304:1365–74. doi: 10.1001/jama.2010.1361
8. Dekkers OM, Horvath-Puho E, Cannegieter SC, Vandenbroucke JP, Sorensen HT, and Jorgensen JO. Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. Eur J Endocrinol. (2017) 176:1–09. doi: 10.1530/EJE-16-0576
9. Groothof D, Flores-Guerrero JL, Nolte IM, Bouma HR, Gruppen EG, Bano A, et al. Thyroid function and risk of all-cause and cardiovascular mortality: a prospective population-based cohort study. Endocrine. (2021) 71:385–96. doi: 10.1007/s12020-020-02397-z
10. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. (2018) 71:1781–96. doi: 10.1016/j.jacc.2018.02.045
11. Maia AL, Kim BW, Huang SA, Harney JW, and Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. (2005) 115:2524–33. doi: 10.1172/JCI25083
12. Le TN, Celi FS, and Wickham ER. Thyrotropin levels are associated with cardiometabolic risk factors in euthyroid adolescents. Thyroid. (2016) 26:1441–49. doi: 10.1089/thy.2016.0055
13. Bian N, Sun X, Zhou B, Zhang L, Wang Q, An Y, et al. Obese patients with higher TSH levels had an obvious metabolic improvement after bariatric surgery. Endocr Connect. (2021) 10:1326–36. doi: 10.1530/EC-21-0360
14. Lang X, Li Y, Zhang D, Zhang Y, Wu N, and Zhang Y. FT3/FT4 ratio is correlated with all-cause mortality, cardiovascular mortality, and cardiovascular disease risk: NHANES 2007-2012. Front Endocrinol (Lausanne). (2022) 13:964822. doi: 10.3389/fendo.2022.964822
15. Sun H, Zhu W, Liu J, An Y, Wang Y, and Wang G. Reduced sensitivity to thyroid hormones is associated with high remnant cholesterol levels in Chinese euthyroid adults. J Clin Endocrinol Metab. (2022) 108:166–74. doi: 10.1210/clinem/dgac523
16. Liu ZM, Li G, Wu Y, Zhang D, Zhang S, Hao YT, et al. Increased central and peripheral thyroid resistance indices during the first half of gestation were associated with lowered risk of gestational diabetes-analyses based on Huizhou birth cohort in south China. Front Endocrinol (Lausanne). (2022) 13:806256. doi: 10.3389/fendo.2022.806256
17. Han C, Wang L, Liu C, Qi W, Zhang R, Wei A, et al. FT3/FT4 enhances risk assessment in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention based on GRACE 2.0 score. Angiology. (2025) 76:125–40. doi: 10.1177/00033197231199228
18. Yu T, Tian C, Song J, He D, Wu J, Wen Z, et al. Value of the fT3/fT4 ratio and its combination with the GRACE risk score in predicting the prognosis in euthyroid patients with acute myocardial infarction undergoing percutaneous coronary intervention: a prospective cohort study. BMC Cardiovasc Disord. (2018) 18:181. doi: 10.1186/s12872-018-0916-z
19. Yuan D, Zhang C, Jia S, Liu Y, Jiang L, Xu L, et al. Predictive value of free triiodothyronine (FT3) to free thyroxine (FT4) ratio in long-term outcomes of euthyroid patients with three-vessel coronary artery disease. Nutr Metab Cardiovasc Dis. (2021) 31:579–86. doi: 10.1016/j.numecd.2020.10.011
20. Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, et al. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. Eur J Heart Fail. (2005) 7:113–18. doi: 10.1016/j.ejheart.2004.04.016
21. Emmons-Bell S, Johnson C, and Roth G. Prevalence, incidence and survival of heart failure: a systematic review. Heart. (2022) 108:1351–60. doi: 10.1136/heartjnl-2021-320131
22. Abbey EJ, McGready J, Sokoll LJ, Simonsick EM, and Mammen J. Free thyroxine distinguishes subclinical hypothyroidism from other aging-related changes in those with isolated elevated thyrotropin. Front Endocrinol (Lausanne). (2022) 13:858332. doi: 10.3389/fendo.2022.858332
23. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi. (2018) 46:760–89. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004
24. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
25. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. (2016) 18:613–25. doi: 10.1002/ejhf.566
26. McDowell K, Kondo T, Talebi A, Teh K, Bachus E, de Boer RA, et al. Prognostic models for mortality and morbidity in heart failure with preserved ejection fraction. JAMA Cardiol. (2024) 9:457–65. doi: 10.1001/jamacardio.2024.0284
27. Okoye C, Arosio B, Carino S, Putrino L, Franchi R, Rogani S, et al. The free triiodothyronine/free thyroxine ratio is associated with frailty in older adults: A longitudinal multisetting study. Thyroid. (2023) 33:169–76. doi: 10.1089/thy.2022.0422
28. Qin Z, Muhanhali D, and Ling Y. Impaired thyroid hormone sensitivity increases risk of cardiovascular events in patients undergoing coronary angiography. J Clin Endocrinol Metab. (2024) 109:1550–64. doi: 10.1210/clinem/dgad735
29. Chandrasekar V, Mohammad S, Aboumarzouk O, Singh AV, and Dakua SP. Quantitative prediction of toxicological points of departure using two-stage machine learning models: A new approach methodology (NAM) for chemical risk assessment. J Hazard Mater. (2025) 487:137071. doi: 10.1016/j.jhazmat.2024.137071
30. Costa JO, Barbosa JS, Alves L, de Almeida RR, Oliveira VB, Pereira L, et al. Food patterns of hospitalized patients with heart failure and their relationship with demographic, economic and clinical factors in Sergipe, Brazil. Nutrients. (2022) 14. doi: 10.3390/nu14050987
31. Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, et al. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur J Prev Cardiol. (2020) 27:494–510. doi: 10.1177/2047487319870344
32. Lima LF, Barbosa FJ, Simoes MV, and Navarro AM. Heart failure, micronutrient profile, and its connection with thyroid dysfunction and nutritional status. Clin Nutr. (2019) 38:800–05. doi: 10.1016/j.clnu.2018.02.030
33. Vargas-Uricoechea H and Bonelo-Perdomo A. Thyroid dysfunction and heart failure: mechanisms and associations. Curr Heart Fail Rep. (2017) 14:48–58. doi: 10.1007/s11897-017-0312-5
34. Zhang M, Sara JD, Matsuzawa Y, Gharib H, Bell MR, Gulati R, et al. Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J. (2016) 37:2055–65. doi: 10.1093/eurheartj/ehv737
35. D’Angelo V, Martinez C, Arreche N, Balaszczuk AM, Del CFM, Burgos JI, et al. Thyroid hormone disorder and the heart: The role of cardiolipin in calcium handling. Exp Physiol. (2023) 108:412–19. doi: 10.1113/EP090817
36. Mantzouratou P, Malaxianaki E, Cerullo D, Lavecchia AM, Pantos C, Xinaris C, et al. Thyroid hormone and heart failure: charting known pathways for cardiac repair/regeneration. Biomedicines. (2023) 11. doi: 10.3390/biomedicines11030975
37. Resnick LM and Laragh JH. PLasma renin activity in syndromes of thyroid hormone excess and deficiency. Life Sci. (1982) 30:585–86. doi: 10.1016/0024-3205(82)90273-9
38. Ojamaa K, Klemperer JD, MacGilvray SS, Klein I, and Samarel A. Thyroid hormone and hemodynamic regulation of beta-myosin heavy chain promoter in the heart. Endocrinology. (1996) 137:802–08. doi: 10.1210/endo.137.3.8603588
39. de Castro AL, Tavares AV, Fernandes RO, Campos C, Conzatti A, Siqueira R, et al. T3 and T4 decrease ROS levels and increase endothelial nitric oxide synthase expression in the myocardium of infarcted rats. Mol Cell Biochem. (2015) 408:235–43. doi: 10.1007/s11010-015-2501-4
40. Fekete C and Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. (2014) 35:159–94. doi: 10.1210/er.2013-1087
41. Wang C, Han S, Li Y, Tong F, Li Z, and Sun Z. Value of FT3/FT4 ratio in prognosis of patients with heart failure: A propensity-matched study. Front Cardiovasc Med. (2022) 9:859608. doi: 10.3389/fcvm.2022.859608
42. He X, Gao R, Wu Y, Wu K, Sun J, Zhang X, et al. Low FT3/FT4 ratio is linked to poor prognosis of acute myocardial infarction in euthyroid patients with type 2 diabetes mellitus. J Clin Med. (2022) 11. doi: 10.3390/jcm11216530
43. Lieder HR, Braczko F, Gedik N, Stroetges M, Heusch G, and Kleinbongard P. Cardioprotection by post-conditioning with exogenous triiodothyronine in isolated perfused rat hearts and isolated adult rat cardiomyocytes. Basic Res Cardiol. (2021) 116:27. doi: 10.1007/s00395-021-00868-6
44. Pingitore A, Mastorci F, Piaggi P, Aquaro GD, Molinaro S, Ravani M, et al. Usefulness of triiodothyronine replacement therapy in patients with ST elevation myocardial infarction and borderline/reduced triiodothyronine levels (from the THIRST study). Am J Cardiol. (2019) 123:905–12. doi: 10.1016/j.amjcard.2018.12.020
45. Bohnke JR. Explanation in causal inference: Methods for mediation and interaction. Q J Exp Psychol (Hove). (2016) 69:1243–44. doi: 10.1080/17470218.2015.1115884
46. Fazio S, Palmieri EA, Lombardi G, and Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. (2004) 59:31–50. doi: 10.1210/rp.59.1.31
47. McAninch EA, Rajan KB, Miller CH, and Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: A systematic review and meta-analysis. J Clin Endocrinol Metab. (2018) 103:4533–42. doi: 10.1210/jc.2018-01361
Keywords: FT3/FT4 ratio, heart failure, mortality, readmission, peripheral thyroid sensitivity
Citation: Ma L, Gou M, Liu X, Ding L and Ma C (2025) Association between peripheral thyroid sensitivity defined by the FT3/FT4 ratio and composite adverse outcome among inpatients with heart failure. Front. Endocrinol. 16:1652749. doi: 10.3389/fendo.2025.1652749
Received: 24 June 2025; Accepted: 28 August 2025;
Published: 16 September 2025.
Edited by:
Jaideep Menon, Amrita Vishwa Vidyapeetham University, IndiaReviewed by:
Sunil Jit Ramamoorthy Jeewanlal Logantha, University of Central Lancashire, United KingdomAjay Vikram Singh, Federal Institute for Risk Assessment (BfR), Germany
A. Subandi, University of Jambi, Indonesia
Copyright © 2025 Ma, Gou, Liu, Ding and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Ma, bWFjaGFvXzk5MEAxNjMuY29t; Li Ding, ZGluZ2xpdG1sQHRtdS5lZHUuY24=
†ORCID: Li Ding, orcid.org/0000-0001-7982-4157
Chao Ma, orcid.org/0000-0001-6988-0497
 Li Ma
Li Ma Manting Gou3
Manting Gou3