Abstract
Background:
Insulin resistance (IR) and metabolic syndrome (MetS) are highly prevalent and pathophysiologically central features of polycystic ovary syndrome (PCOS). However, their assessment is challenged by the limitations of gold-standard diagnostic methods. The clinical utility of the novel triglyceride glucose index - body mass index (TyG-BMI) for predicting IR, MetS, and its association with fertility outcomes in Chinese women with PCOS remains unexplored and warrants investigation.
Objective:
To evaluate the association between TyG-BMI and IR and MetS, and fertility outcomes in women with PCOS.
Methods:
We used data of 855 participants of the Acupuncture and Clomiphene for Chinese Women with Polycystic Ovary Syndrome (PCOSAct) trial. Linear trend tests and logistic regression evaluated relationships between TyG-BMI and anthropometric, hormonal, metabolic, and fertility outcomes. Receiver operating characteristic (ROC) curves assessed TyG-BMI’s predictive value for IR and MetS. RCS analysis was used to examine threshold effects between TyG-BMI and IR, MetS, and ovulation. A likelihood ratio test was further incorporated to validate the model fit.
Results:
TyG-BMI was positively association with IR (OR: 2.747, 95% CI: 1.942–3.887) and MetS (OR: 4.176, 95% CI: 2.278–7.653). TyG-BMI had a strong predictive performance, with AUCIR of 0.841 and AUCMetS of 0.899. For fertility outcomes, after adjusting for confounders, only ovulation showed a significant negative association (OR: 0.984, 95% CI: 0.973–0.994). The study revealed significant nonlinear associations between TyG-BMI and both IR and MetS, but a linear link with ovulation status. The inflection point occurred at a TyG-BMI of 203. Below this, IR risk increased progressively with TyG-BMI, plateauing above it. Above 203, MetS prevalence continued to increase, while ovulation rates declined inversely.
Conclusion:
Elevated TyG-BMI is strongly associated with worsened IR and MetS in PCOS women, serving as a practical screening tool for these conditions, while also demonstrating a potential negative impact on ovulation.
Introduction
Polycystic ovary syndrome (PCOS), the most prevalent endocrine disorder in reproductive-aged women, affects up to 15% of women worldwide, depending on diagnostic criteria (1, 2). It is clinically characterized by hyperandrogenism, oligo-ovulation, and polycystic ovarian morphology, with heterogeneous manifestations ranging from obesity, menstrual irregularities, and infertility to metabolic complications such as insulin resistance (IR), dyslipidemia, metabolic syndrome (MetS), and an increased risk of cardiovascular disease (CVD) (3).
IR is a key pathophysiological feature of PCOS, contributing to both endocrine dysfunction and metabolic disturbances (4). Epidemiological data indicate a wide prevalence range of IR (12%-60%) in women with PCOS, influenced by diagnostic criteria, assessment methods, and population characteristics (5). While the hyperinsulinemic-euglycemic clamp (HIEC) remains the gold standard for IR assessment (6), its clinical utility is limited by invasiveness, high cost, and technical complexity. The more commonly used homeostasis model assessment of insulin resistance (HOMA-IR) provides a practical alternative but has significant limitations, particularly in insulin-treated patients or those with β-cell dysfunction (7). The diagnostic challenges extend to MetS, where incidence rates vary substantially across ethnic groups and diagnostic criteria. Notably, metabolic dysregulation is highly prevalent in PCOS, with 54.9% of patients classified as overweight and 25.7% meeting MetS criteria (8). Importantly, Chinese women with PCOS demonstrate a distinct metabolic profile, showing higher rates of metabolic disorders but lower MetS prevalence when assessed by IDF criteria (9). Given the proven impact of weight control and metabolic improvement on reproductive outcomes in PCOS (10, 11), early identification of IR and MetS is critical. However, current diagnostic limitations underscore the urgent need for simple, reliable tools to stratify metabolic risk in this population.
The triglyceride-glucose index (TyG), calculated from fasting triglyceride and glucose levels, has been validated as a reliable surrogate marker for IR (12). It exhibits broader clinical significance, demonstrating an association with higher mortality risk in Cardiovascular-Kidney-Metabolic (CKM) syndrome stages 0-3 (13). Notably, TyG shows U-shaped correlations with all-cause and cardiovascular mortality, suggesting intervention thresholds at 9.104 and 8.758, respectively (14). Furthermore, elevated TyG status correlates with early clinical manifestations of pancreatic ductal adenocarcinoma (PDAC) (15), while emerging evidence links it to metabolic dysregulation in post-COVID-19 syndrome (16).
In PCOS populations, where obesity frequently coexists with metabolic disturbances such as glucose intolerance and IR (17, 18), integrating BMI may enhance diagnostic utility. BMI is a fundamental measure of obesity and indicates IR through its association with adipose tissue lipolysis and free fatty acid-mediated insulin sensitivity modulation.
This rationale underlies the proposed TyG-BMI composite index as a potentially superior IR marker (19). Recent studies indicate that TyG-BMI outperforms TyG alone in metabolic risk assessment. For instance, research in Korean adults demonstrated TyG-BMI’s superior predictive power for IR compared to other parameters (TyG, TyG-WC, and TyG-WHtR) (20). Further supporting this, another study incorporating additional adiposity indicators found TyG-BMI achieved the highest AUC (0.801) among all evaluated metrics (21). The clinical utility of TyG-BMI in PCOS, particularly regarding its diagnostic accuracy for IR and MetS and its association with fertility outcomes following ovulation induction, remains unexplored. Addressing these knowledge gaps could provide valuable insights for PCOS management, warranting systematic investigation.
This study had two primary objectives: firstly, to systematically examine the relationships between TyG-BMI and baseline characteristics, including anthropometric measures, metabolic profiles, and reproductive characteristics in infertile women with PCOS; secondly, to determine the diagnostic accuracy of TyG-BMI for predicting IR and MetS in this population.
Patients and methods
Participants
This secondary analysis utilized data from the PCOS Acupuncture and Clomiphene Trial (PCOSAct), a multicenter, randomized, double-blind controlled clinical trial (ClinicalTrials.gov NCT01573858; Chinese Clinical Trial Registry ChiCTR-TRC-12002081). The study enrolled 1,000 infertile women aged 20–40 years meeting the modified Rotterdam criteria (22) for PCOS across 27 hospitals in mainland China (2012–2015). The trial protocol (23) and primary outcomes (24) have been previously published. Exclusion criteria included: endocrine comorbidities; recent (past 2 months) use of hormonal/Chinese herbal therapies; peripartum status (delivery/miscarriage ≤6 weeks); active breastfeeding; missing metabolic data; and pregnancy loss to follow-up.
Anthropometric measurements
At baseline, all participants received physical examinations that included anthropometric measurements (weight, height, waist circumference [WC], and hip circumference [HC]) and blood pressure assessment (systolic [SBP] and diastolic [DBP]). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m²), while waist-to-hip ratio (WHR) was derived from WC and HC measurements.
Biochemical measurements
Fasting blood samples were collected at baseline following a 12-hour overnight fast. For regularly cycling women, samples were obtained on day 3 of the menstrual cycle, while women with amenorrhea provided samples on their enrollment day. All samples were analyzed at the core laboratory of Heilongjiang University of Chinese Medicine. The comprehensive biochemical analysis included: sex hormones and gonadotropins [total testosterone (TT), free testosterone (FT), sex hormone-binding globulin (SHBG), estradiol (E2), progesterone (P), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and anti-Müllerian hormone (AMH)]; and metabolic parameters [fasting plasma glucose (FPG), fasting insulin (FIN), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), lipoprotein A, apolipoprotein A1 (ApoA1), and apolipoprotein B (ApoB)]. Derived indices included: (a) free androgen index (FAI = TT [nmol/L]/SHBG [nmol/L] × 100); (b) triglyceride-glucose index (TyG = Ln[TG (mg/dL) × FPG (mg/dL)/2]); and (c) TyG-BMI (TyG × BMI). Conversion factors: FPG (1 mg/dL = 0.0555 mmol/L) and TG (1 mg/dL = 0.0113 mmol/L).
Insulin resistance and metabolic syndrome
IR was defined as a HOMA-IR value ≥2.69 (25), calculated using the formula: HOMA-IR = FIN (mIU/L) × FPG (mol/L)/22.5 (26). MetS was diagnosed according to the Adult Treatment Panel III (ATP III) criteria (22), requiring the presence of at least three of the following five components: WC >88 cm; TG ≥150 mg/dL (1.7 mmol/L); HDL <50 mg/dL (1.3 mmol/L); blood pressure ≥130/85 mmHg; and FPG 100–126 mg/dL (5.6-6.9 mmol/L). For unit conversion, 1 mIU/L of FIN equals 6.965 pmol/L.
Interventions
In the PCOSAct trial, participants were randomized (1:1:1:1 ratio) into four treatment arms: (a) clomiphene plus active acupuncture, (b) placebo plus active acupuncture, (c) clomiphene plus sham acupuncture, and (d) placebo plus sham acupuncture (n=250 per group). All groups underwent four consecutive treatment cycles, with pregnancy outcomes evaluated after each cycle. Upon achieving conception, interventions were discontinued while pregnancy outcomes were monitored through either miscarriage or live delivery.
Fertility outcomes
Reproductive outcomes were defined as follows: ovulation - serum progesterone >5 ng/mL (15.9 nmol/L) during a treatment cycle; conception - serum hCG >10 IU/L (at which point all interventions ceased); clinical pregnancy - confirmed intrauterine gestation with fetal cardiac activity via transvaginal ultrasound; pregnancy loss - encompassing spontaneous abortion (<20 weeks), fetal death (≥20 weeks), and stillbirth (≥20 weeks); and live birth - delivery of a viable neonate at ≥20 weeks’ gestation.
Statistical analyses
Statistical analyses were performed using SPSS 25.0 (IBM Corp.) and R Studio. Continuous variables were expressed as mean ± standard deviation or median (interquartile range), while categorical variables were presented as numbers (percentages). Intergroup comparisons were conducted using one-way ANOVA or Kruskal-Wallis test for continuous variables, and chi-square or Fisher’s exact test for categorical variables, as appropriate. Linear trend analyses were employed to examine the relationships between TyG-BMI quartiles and: anthropometric/biochemical parameters, prevalence of IR and MetS, and reproductive outcomes (ovulation and pregnancy). Binary logistic regression assessed associations between TyG-BMI and IR/MetS, while multinomial and ordinal logistic regression evaluated risk trends across TyG-BMI quartiles. The predictive performance of TyG-BMI for IR and MetS was determined using receiver operating characteristic (ROC) curve analysis, with calculation of the area under the curve (AUC), optimal cutoff values, sensitivity, specificity, and Youden index. Adjusted odds ratios (ORs) for outcomes were derived from logistic regression models incorporating covariates identified as significant in univariate analyses. Given the demonstrated effect of clomiphene (but not acupuncture) on live birth rates in PCOSAct, additional adjustment for clomiphene treatment was performed, and potential interactions between clomiphene and TyG-BMI were assessed using generalized linear models. The relationships between TyG-BMI and IR, MetS, and ovulation were investigated using restricted cubic spline (RCS) analysis, and likelihood ratio tests were performed. All tests were two-tailed, with P < 0.05 considered statistically significant.
Results
Among the 1,000 women initially enrolled in PCOSAct, 145 were excluded due to missing glycolipid metabolic data or loss to follow-up, resulting in 855 PCOS patients included in the final analysis. The TyG-BMI was calculated for all participants and stratified into quartiles (Q1–Q4) with near-equal distribution (Q1: n = 214; Q2: n = 214; Q3: n = 213; Q4: n = 214).
The anthropometric, biochemical characteristics, prevalence of IR, MetS and fertility outcomes of PCOS women across the quartiles of TyG - BMI
As presented in Table 1, all anthropometric parameters exhibited significant progressive increases across TyG-BMI quartiles (P and P-trend < 0.05 for all). Sex hormones demonstrated similar trends: FT and FAI levels rose with higher TyG-BMI quartiles, whereas E2, P, LH, LH/FSH, AMH, and SHBG showed inverse relationships (P and P-trend < 0.05). Metabolic parameters followed comparable patterns, with FPG, FIN, HOMA-IR, TC, TG, LDL, and ApoB displaying ascending trends, while HDL and ApoA1 progressively declined (P and P-trend < 0.05). The prevalence of both IR and MetS escalated markedly with increasing TyG-BMI quartiles (IR: Q1 = 7.94%, Q2 = 25.70%, Q3 = 54.46%, Q4 = 80.37%; MetS: Q1 = 0%, Q2 = 1.4%, Q3 = 11.74%, Q4 = 50.93%; P and P-trend < 0.05). Reproductive outcomes varied significantly among quartiles (P < 0.05 for ovulation, conception, and clinical pregnancy). Notably, the Q4 group exhibited higher ovulation and clinical pregnancy rates than Q1–Q2, and greater conception rates than Q1. Linear trend analyses revealed TyG-BMI was inversely associated with ovulation, conception, clinical pregnancy, and live birth, but positively correlated with pregnancy loss (P-trend < 0.05).
Table 1
| Characteristics | Q 1 N = 214 | Q 2 N = 214 | Q 3 N = 213 | Q 4 N = 214 | P-value | P-trend |
|---|---|---|---|---|---|---|
| Anthropometric characteristics | ||||||
| Age(year) | 27.31 ± 3.09 | 27.99 ± 3.14 | 28.10 ± 3.44 | 28.45 ± 3.43* | 0.004 | <0.001 |
| BMI (kg/m2) | 19.60(2.05) | 22.31(1.94)* | 25.04(2.04)*△ | 28.89(4.26)*△# | <0.001 | <0.001 |
| WC (cm) | 74(9) | 80.65(8)* | 86(9.5)*△ | 97(12.3)*△# | <0.001 | <0.001 |
| HC (cm) | 90.76 ± 5.68 | 95.486 ± 5.42* | 99.96 ± 5.66*△ | 107.06 ± 5.66*△# | <0.001 | <0.001 |
| WHR | 0.82(0.08) | 0.85(0.07)* | 0.87(0.07)*△ | 0.91(0.07)*△# | <0.001 | <0.001 |
| SBP (mmHg) | 110(15) | 110(10)* | 110(10)* | 120(10)*△# | <0.001 | <0.001 |
| DBP (mmHg) | 70(10) | 73(10) | 75(10)* | 80(10)*△# | <0.001 | <0.001 |
| Sex hormone profiles | ||||||
| Estradiol (pmol/L) | 207.4(121.2) | 208.4(107.43) | 195.5(104.2) | 185.65(87.83)* | <0.001 | <0.001 |
| Progesterone (nmol/L) | 1.90(1.29) | 1.73(1.15) | 1.74(1.21) | 1.60(1.02)*△ | 0.004 | 0.003 |
| LH (mIU/mL) | 11.29(9.36) | 10.58(8.35) | 9.62(8.18) | 8.10(6.29)*△# | <0.001 | <0.001 |
| FSH (mIU/mL) | 6.25 ± 1.76 | 6.21 ± 1.60 | 5.87 ± 1.68* | 6.01 ± 1.55 | 0.058 | 0.035 |
| LH/FSH ratio | 1.85(1.45) | 1.71(1.32) | 1.63(1.34) | 1.30(0.95)*△# | <0.001 | <0.001 |
| AMH (ng/mL) | 12.79(9.77) | 12.31(8.94) | 11.07(7.67)* | 9.46(8.76)*△ | <0.001 | <0.001 |
| Total testosterone (nmol/L) | 1.53(0.91) | 1.67(0.85) | 1.58(0.85) | 1.62(0.77) | 0.475 | 0.829 |
| Free testosterone (pg/ml) | 2.12 ± 0.83 | 2.31 ± 0.87 | 2.28 ± 0.85 | 2.45 ± 0.77* | <0.001 | <0.001 |
| SHBG(nmol/L) | 52.6(38.7) | 41.5(32.78)* | 28.2(21.85)*△ | 21.95(12.95)*△# | <0.001 | <0.001 |
| Free androgen Index | 2.77(2.96) | 4.00(4.06)* | 5.50(5.52)* | 7.36(5.62)*△# | <0.001 | <0.001 |
| Glycolipid metabolic profiles | ||||||
| Fasting glucose (mmol/L) | 4.70(0.92) | 4.95(0.95)* | 5.17(0.88)*△ | 5.34(1.08)*△ | <0.001 | <0.001 |
| Fasting insulin (pmol/L) | 43.01(29.50) | 63.03(45.21)* | 86.39(58.16)*△ | 129.25(80.47)*△# | <0.001 | <0.001 |
| HOMA-IR | 1.28(0.95) | 1.96(1.55)* | 2.80(2.09)*△ | 4.53(3.11)*△# | <0.001 | <0.001 |
| Cholesterol (mmol/L) | 4.31(1.05) | 4.68(1.33)* | 4.77(1.28)* | 5.02(1.57)*△ | <0.001 | <0.001 |
| Triglyceride (mmol/L) | 0.88(0.43) | 1.20(0.71)* | 1.51(1.04)*△ | 2.08(1.30)*△# | <0.001 | <0.001 |
| TyG-BMI | 161.76(18.88) | 187.83(15.10) | 218.72(15.72) | 259.19(36.15) | <0.001 | <0.001 |
| High-density lipoprotein(mmol/L) | 1.44(0.48) | 1.29(0.46)* | 1.16(0.44)*△ | 1.10(0.39)*△ | <0.001 | <0.001 |
| Low-density lipoprotein(mmol/L) | 2.55(0.96) | 2.90(1.19)* | 3.00(0.98)* | 3.21(1.34)*△ | <0.001 | <0.001 |
| Lipoproteins A(mg/L) | 97.15(77.73) | 101.35(76.88) | 102.20(80.95) | 109.80(88.70) | 0.509 | 0.586 |
| Apolipoprotein A1 (g/L) | 1.56(0.42) | 1.51(0.41)* | 1.41(0.46) | 1.45(0.37)* | 0.001 | 0.001 |
| Apolipoprotein B (g/L) | 0.69(0.25) | 0.82(0.34)* | 0.92(0.35)*△ | 1.04(0.39)*△# | <0.001 | <0.001 |
| IR(Yes) | 17(7.94) | 55(25.70)* | 116(54.46)*△ | 172(80.37)*△# | <0.001 | <0.001 |
| MetS(Yes) | 0 | 3(1.40) | 25(11.74)*△ | 109(50.93)*△# | <0.001 | <0.001 |
| Fertility outcomes | ||||||
| Ovulation | 184(85.98) | 186(86.92) | 175(82.16) | 156(72.90)*△ | <0.001 | <0.001 |
| Conception | 81(37.85) | 79(36.92) | 78(36.62) | 55(25.70)* | 0.025 | 0.011 |
| Clinical pregnancy | 61(28.50) | 58(27.10) | 47(22.07) | 34(15.89)*△ | 0.008 | 0.001 |
| Pregnancy loss | 21/81(25.92) | 24/79(30.38) | 34/78(43.59) | 21/55(38.18) | 0.111 | 0.024 |
| Live birth | 55/81(67.90) | 52/79(65.82) | 40/78(51.28) | 30/55(54.55) | 0.078 | 0.023 |
Anthropometric, biochemical characteristics, prevalence of IR, MetS and fertility outcomes of the women with PCOS.
IR, MetS, Ovulation and Pregnancy Outcomes n (%) are presented. Significant differences are denoted as follows: *P<0.05 vs. Q1 group; △P<0.05 vs. Q2 group; #P<0.05 vs. Q3 group.
The correlation between TyG-BMI and IR, MetS in PCOS women
The TyG-BMI demonstrated significant associations with both IR and MetS (Supplementary Table S1). To further investigate these relationships, we performed multinomial and ordinal logistic regression analyses with adjustment for potential confounders (Figure 1). Ordinal logistic regression revealed that higher TyG-BMI quartiles were strongly associated with increased IR risk, with adjusted and unadjusted odds ratios (ORs) of 2.747 (95% CI: 1.942-3.887) and 10.487 (95% CI: 7.852-14.004), respectively. Notably, participants in the highest TyG-BMI quartile had a 10.485-fold increased risk of IR compared to those in the lowest quartile. Similarly, TyG-BMI showed a significant positive association with MetS. The adjusted OR for MetS was 4.176 (95% CI: 2.278-7.653), with the highest quartile exhibiting a 7.894-fold greater risk compared to the second quartile.
Figure 1

The correlation between TyG-BMI and IR, MetS in women with PCOS. IR: Adjusted for age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, MetS: Adjusted for age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, FIN, HOMA-IR, IR.
Diagnostic performance of TyG-BMI for MetS, IR
Figure 2 presents the predictive performance of TyG-BMI and other metabolic parameters for IR and MetS. For IR prediction (panel A), TyG-BMI demonstrated superior diagnostic accuracy among the four evaluated indicators, showing the largest area under the curve (AUC = 0.841) with an optimal cutoff value of 206.708 (sensitivity: 78.6%; specificity: 75.8%). Regarding MetS prediction (panel B), TyG-BMI, TyG, and TG levels showed overlapping ROC curves, though TyG-BMI maintained a marginal advantage (AUC = 0.899; sensitivity: 90.5%; specificity: 78.4%). Notably, TG exhibited the highest sensitivity (94.2%), while TyG showed the greatest specificity (80.4%) for MetS detection. Complete comparative data are provided in Table 2.
Figure 2
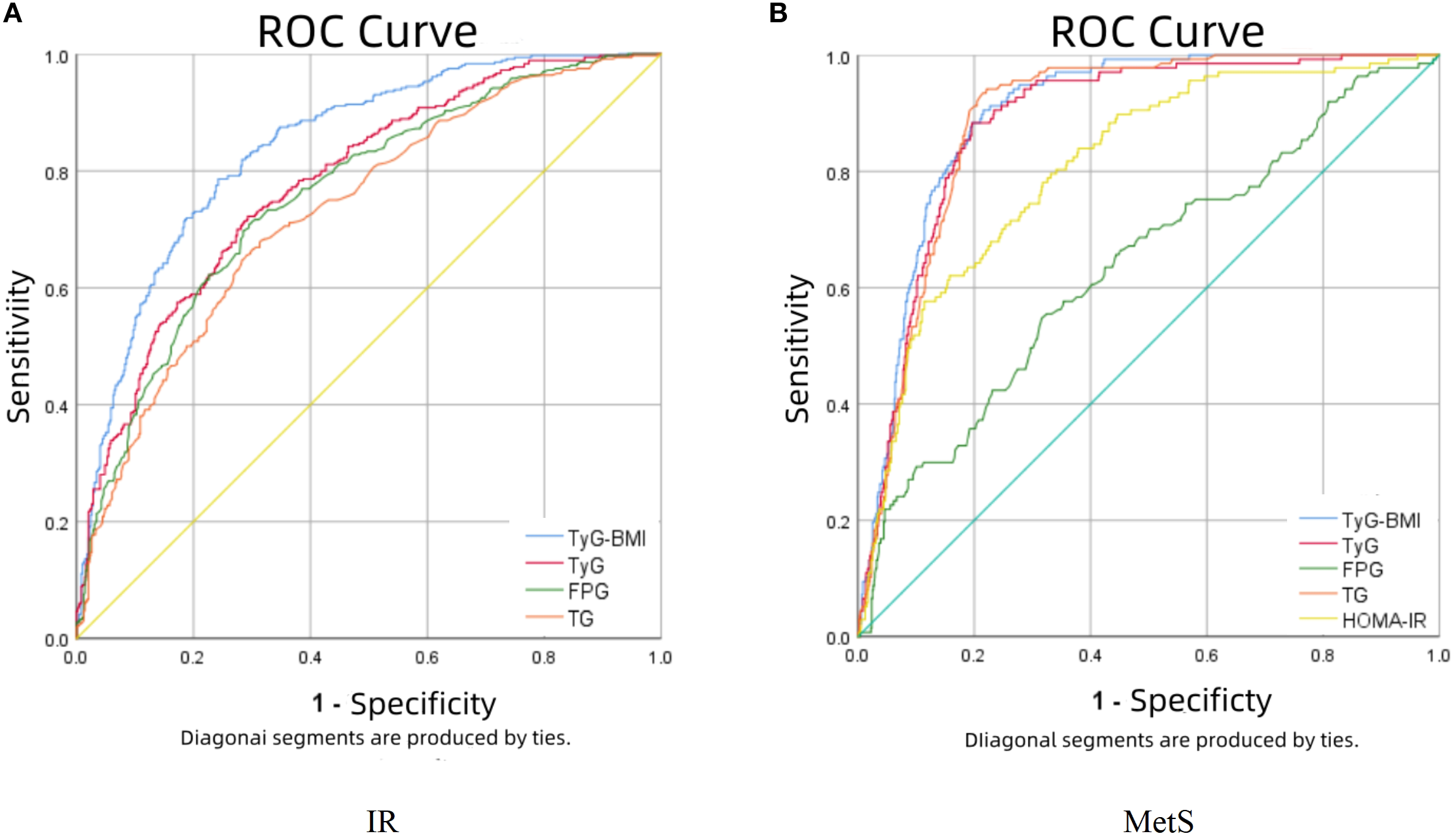
The results of ROC curve analysis regarding the predictability of TyG-BMI in IR (A) and MetS (B).
Table 2
| Outcomes | Variables | AUC(95%CI) | Cutoff value | Sensitivity | Specificty | You den Index |
|---|---|---|---|---|---|---|
| IR | TyG-BMI | 0.841(0.815-0.867) | 206.708 | 0.786 | 0.758 | 0.544 |
| TyG | 0.781(0.750-0.811) | 8.585 | 0.722 | 0.707 | 0.429 | |
| Fasting glucose | 0.759(0.727-0.792) | 5.105 | 0.714 | 0.699 | 0.413 | |
| triglyceride | 0.732(0.698-0.765) | 1.355 | 0.681 | 0.685 | 0.366 | |
| MetS | TyG-BMI | 0.899(0.877-0.921) | 225.104 | 0.905 | 0.784 | 0.689 |
| TyG | 0.884(0.858-0.910) | 8.903 | 0.883 | 0.804 | 0.687 | |
| Fasting glucose | 0.637(0.585-0.689) | 5.315 | 0.547 | 0.684 | 0.231 | |
| triglyceride | 0.890(0.867-0.913) | 1.695 | 0.942 | 0.779 | 0.721 | |
| HOMA-IR | 0.809(0.771-0.847) | 2.773 | 0.796 | 0.669 | 0.465 |
TyG-BMI and other metabolic indicators predict AUC, cutoff value, sensitivity, and specificity prediction values for IR and MetS.
The correlation between TyG-BMI and fertility outcomes in PCOS women
Amongst 855 PCOS women, reproductive outcomes included ovulation (n=701, 82.0%), conception (n=293, 34.3%), clinical pregnancy (n=200, 23.4%), pregnancy loss (n=100, 34.1% of conceptions), and live birth (n=177, 20.7%). Analysis of TyG-BMI quartiles revealed significant treatment effects for clomiphene versus placebo across outcomes: for ovulation, odds ratios (ORs) were Q1 = 10.11 (95%CI 3.39-30.18), Q2 = 8.13 (2.71-24.36), Q3 = 8.00 (3.18-20.13), and Q4 = 3.34 (1.72-6.51) (Supplementary Table S2). Significant treatment differences were also observed for conception (Q2 OR = 2.48 [1.40-4.41]; Q3 OR = 3.70 [2.03-6.75]; Q4 OR = 3.59 [1.87-6.92]) and clinical pregnancy (Q2 OR = 2.16 [1.16-4.04]; Q3 OR = 2.66 [1.33-5.33]; Q4 OR = 3.36 [1.52-7.44]).
Table 3 presents the association between TyG-BMI and fertility outcomes across progressively adjusted models. In the unadjusted Model 1, TyG-BMI demonstrated negative correlations with ovulation (OR: 0.992, 95% CI: 0.988-0.995), conception (OR: 0.995, 95% CI: 0.992-0.998), clinical pregnancy (OR: 0.993, 95% CI: 0.989-0.997), and live birth (OR: 0.993, 95% CI: 0.989-0.997), while showing a weak positive association with pregnancy loss (OR: 1.006, 95% CI: 1.000-1.012). After adjustment for clomiphene treatment and basic anthropometrics (Model 2), TyG-BMI remained significantly associated with ovulation (OR: 0.989, 95% CI: 0.981-0.997), conception (OR: 0.993, 95% CI: 0.987-0.999), and clinical pregnancy (OR: 0.992, 95% CI: 0.985-0.998). Further adjustment for sex hormones (Model 3) maintained the significant association with ovulation (OR: 0.990, 95% CI: 0.981-0.998). The fully adjusted Model 4 (including glycolipid metabolism parameters) continued to show TyG-BMI’s negative association with ovulation (OR: 0.984, 95% CI: 0.973-0.994). No significant interaction was observed between clomiphene and TyG-BMI for any fertility outcome (Supplementary Table S3).
Table 3
| Fertility outcomes | Model 1 | P-value | Model 2 | P-value | Model 3 | P-value | Model 4 | P-value |
|---|---|---|---|---|---|---|---|---|
| OR; 95% CI | OR; 95% CI | OR; 95% CI | OR; 95% CI | |||||
| Ovulation | 0.992(0.988-0.995) | <0.001 | 0.989(0.981-0.997) | 0.005 | 0.990(0.981-0.998) | 0.015 | 0.984(0.973-0.994) | 0.004 |
| Conception | 0.995(0.992-0.998) | 0.002 | 0.993(0.987-0.999) | 0.017 | 0.994(0.987-1.000) | 0.051 | 0.995(0.988-1.003) | 0.200 |
| Clinical pregnancy | 0.993(0.989-0.997) | <0.001 | 0.992(0.985-0.998) | 0.016 | 0.994(0.987-1.001) | 0.072 | 0.998(0.989-1.006) | 0.606 |
| Pregnancy loss | 1.006(1.0-1.012) | 0.043 | 1.002(0.992-1.013) | 0.654 | 0.999(0.987-1.010) | 0.807 | 0.987(0.973-1.002) | 0.082 |
| Live birth | 0.993(0.989-0.997) | <0.001 | 0.995(0.988-1.002) | 0.165 | 0.997(0.990-1.004) | 0.421 | 1.002(0.993-1.011) | 0.703 |
The correlation between TyG-BMI and ovulation, fertility outcomes in women with PCOS.
Model 1: Non-adjusted.
Model 2: Adjusted for clomiphene, age, WC, HC, WHR, SBP, DBP.
Model 3: Adjusted for clomiphene, age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI.
Model 4: Adjusted for clomiphene, age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, FIN, HOMA-IR, IR.
RCS analysis investigating the relationship between TyG-BMI and IR, MetS, and ovulation
Given the significant associations between TyG-BMI and IR, MetS, and ovulation, a piecewise regression analysis and RCS were performed (Table 4, Figure 3). The analysis identified a consistent inflection point at TyG-BMI = 203 for all three outcomes. Below this threshold, IR prevalence demonstrated a progressive increase with rising TyG-BMI values, reaching an odds ratio plateau beyond the inflection point. Notably, distinct patterns emerged post-inflection: while MetS prevalence continued to rise with increasing TyG-BMI, ovulation rates showed an inverse relationship. RCS analyses, after full covariate adjustment, revealed significant nonlinear associations between TyG-BMI and both IR (P-overall <0.001; P-nonlinear <0.001) and MetS (P-overall <0.001; P-nonlinear <0.001). In contrast, TyG-BMI exhibited a strictly linear correlation with ovulation status (P-overall <0.05; P-nonlinear >0.05). The likelihood ratio tests yielded statistically significant resulted for IR, MetS, and ovulation (P-overall <0.001).
Table 4
| Outcomes | TyG-BMI ≤ 203 N = 424 | P-value | TyG-BMI > 203 N = 431 | P-value | Likelihood ratio test | ||
|---|---|---|---|---|---|---|---|
| Event no(%) | OR; 95% CI | Event no(%) | OR; 95% CI | ||||
| IR | 56(13.2) | 1.062(1.034-1.090) | <0.001 | 239(67.5) | 1.014(1.001-1.027) | 0.040 | <0.001 |
| MetS | 3(0.7) | – | 0.997 | 134(31.1) | 1.020(1.006-1.033) | 0.003 | <0.001 |
| Ovulation | 367(86.6) | 0.995(0.967-1.023) | 0.720 | 334(77.4) | 0.977(0.963-0.992) | 0.002 | <0.001 |
Threshold effect analysis between TyG-BMI and IR, MetS and ovulation.
IR: Adjusted for age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB.
MetS: Adjusted for age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, FIN, HOMA-IR, IR.
Ovulation: Adjusted for clomiphene, age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, FIN, HOMA-IR, IR.
Figure 3
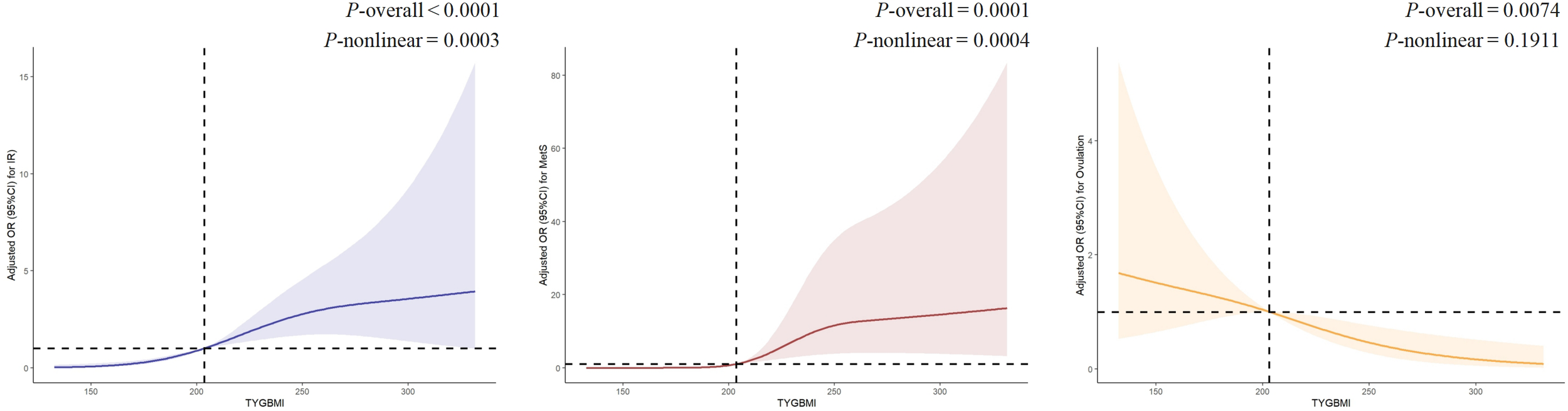
The RCS between TyG-BMI and IR, MetS and ovulation. IR: Adjusted for age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB. MetS: Adjusted for age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, FIN, HOMA-IR, IR Ovulation: Adjusted for clomiphene, age, WC, HC, WHR, SBP, DBP, E2, P, LH, LF/FSH, AMH, FT, SHBG, FAI, TC, HDL, LDL, ApoA1, ApoB, FIN, HOMA-IR, IR.
Discussion
In women with PCOS, we evaluated the association between TyG-BMI and multiple clinical parameters including circulating sex steroids, glucose/lipid metabolism, IR, MetS, and fertility outcomes. Our findings demonstrate that elevated TyG-BMI is significantly associated with more severe IR and MetS, establishing its value as a predictive marker for these metabolic disorders in PCOS patients. Additionally, we observed that higher TyG-BMI levels may impair ovulation, suggesting a potential role in reproductive dysfunction. Notably, these associations exhibited threshold effects: while IR prevalence plateaued beyond a certain TyG-BMI level, MetS prevalence continued to increase linearly, and ovulation rates showed a progressive decrease with rising TyG-BMI values.
Our findings establish TyG-BMI as a clinically valuable biomarker for identifying individuals at high risk of IR. The study demonstrates that both TyG and BMI independently correlate with IR, and their composite index (TyG-BMI) significantly enhances IR assessment by synergistically integrating adiposity-related metabolic effects (21). This improvement proves particularly relevant in obesity-prone populations, given obesity’s well-documented role in IR and metabolic dysfunction (27, 28). Clinically, TyG-BMI’s strong performance aligns with weight management strategies, which are fundamental to IR treatment, potentially guiding targeted interventions for obesity-related IR. ROC analyses confirmed TyG-BMI’s superior diagnostic accuracy over TyG alone in IR prediction. Notably, we observed that PCOS women with elevated BMI faced increased IR risk even when TyG levels were relatively low, highlighting BMI’s substantial contribution to IR development in this population. Furthermore, TyG-BMI demonstrated a dose-response relationship, with the effect potentially attenuating beyond a certain threshold point.
MetS represents a complex endocrine disorder characterized by multiple interrelated risk factors, including obesity (particularly visceral and abdominal adiposity), dyslipidemia, IR, hypertension, endothelial dysfunction, and systemic inflammation (29). While its precise pathogenesis remains incompletely understood, women with PCOS exhibit significantly elevated MetS risk (30), which is further exacerbated by concurrent overweight/obesity (31, 32). The TyG has demonstrated effectiveness in MetS identification (33), and visceral adiposity recognized is recognized as a key driver of MetS-related metabolic pathways (34). Our findings extend this understanding by revealing a positive correlation between increasing TyG-BMI levels and MetS risk progression, particularly above the inflection point. This observation supports the clinical rationale for combining BMI with TyG to enhance MetS prediction. This was evidenced by our results showing TyG-BMI’s superior diagnostic performance (AUC = 0.899 vs. TyG’s 0.884), with TyG-BMI offering better sensitivity while TyG maintained higher specificity.
This study demonstrates that elevated TyG-BMI is associated with poorer ovulation induction (more significant after the turning point), likely mediated through concurrent metabolic disturbances including obesity, IR, hyperandrogenism, and hyperlipidemia. Overweight/obesity frequently leads to ovulatory dysfunction - a primary infertility factor - through endocrine disruption and hypothalamic-pituitary- ovarian (HPO) axis dysregulation (35, 36). Specifically, obesity alters GnRH pulsatility and gonadotropin secretion. This occurs via increased peripheral androgen aromatization to estrogens, reduced SHBG, and elevated adipocyte leptin production. Concuruently, obesity-related IR exacerbates hyperandrogenemia (37, 38). These mechanisms are further supported by pregnant rat models showing androgen-induced uterine artery vasoconstriction and hypoxia-responsive gene upregulation (39). Our findings reveal that decreased SHBG levels with increased free testosterone and FAI correlate with elevated TyG-BMI. Weight gain exacerbates PCOS phenotype severity (40), and the obesity-PCOS synergy aggravates both metabolic and reproductive dysfunction (41), consistent with our observed TyG-BMI effects. IR plays a central role in PCOS-related lipid metabolism disorders by stimulating lipolysis and altering lipoprotein/hepatic lipase expression (40). The consequent dyslipidemia impairs oocyte quality and endometrial receptivity, partly through reduced adiponectin during implantation (42). Additionally, hyperinsulinemia directly compromises endometrial function and implantation efficacy (43), collectively contributing to the observed reproductive impairments. Although the association between TyG-BMI and ovulation is significant, its association with conception and live birth become non-significant in the adjusted models. This may be attributed to standardized ovulation protocols reducing the impact of metabolic factors on final delivery outcomes. Alternatively, male factors affecting embryo quality may have influenced conception and live birth rates.
This study possesses several notable strengths, including a large sample size representative of the Chinese PCOS population and comprehensively evaluating MetS, IR, and fertility outcomes through BMI-integrated assessment. Our findings suggest that TyG-BMI, a simple index derived from routine clinical measurements (triglycerides, fasting glucose, BMI), shows promise as a practical screening tool for identifying Chinese women with PCOS who are at heightened risk of significant insulin resistance and metabolic syndrome. A TyG-BMI value exceeding approximately 203 should prompt heightened clinical vigilance. This threshold may justify more intensive metabolic assessment and early intervention strategies, particularly those focused on lifestyle modification (weight control, dietary modification, and physical activity). Furthermore, the association between elevated TyG-BMI and reduced ovulation rates highlights the potential role of this integrated metabolic marker in assessing PCOS-related reproductive dysfunction. While further validation is needed, incorporating TyG-BMI assessment could help clinicians achieve a more comprehensive metabolic evaluation when managing infertility in this population.
However, certain limitations must be acknowledged. Firstly, as this is a secondary analysis of baseline data from the PCOSAct trial utilizing a cross-sectional design, our findings demonstrate associations but cannot establish causality between TyG-BMI and IR, MetS, or ovulation rates. The observed relationships may be bidirectional or influenced by unmeasured confounding factors. In the future, prospective longitudinal or interventional studies are needed to investigate whether changes in TyG-BMI cause alterations in metabolic or reproductive outcomes. Secondly, the absence of gold-standard IR diagnostic measures means our approach may not capture the full spectrum of insulin resistance dynamics. This is an important limitation affecting the precision of our primary outcome assessment related to insulin resistance. Thirdly, the lack of TyG-BMI measurements during second/third trimesters restrict pregnancy outcome analyses to baseline parameters, which limits the clinical applicability of findings regarding fertility outcomes. Consequently, the direct association between baseline TyG-BMI and long-term fertility success, including live birth rates, remains unknown and represents an important gap for future research. Furthermore, our findings are derived exclusively from a cohort of Chinese women with PCOS. Metabolic profiles, genetic factors, and PCOS phenotypic expression can vary significantly across different ethnicities and geographic regions. Therefore, the generalizability of our results—particularly the specific TyG-BMI threshold identified—to non-Chinese populations or other ethnicities with PCOS may be limited. This requires validation in diverse cohorts. Finally, due to incomplete data, potential confounding factors—such as dietary habits, physical activity levels, and medication use—were not adequately controlled for in the analysis. This limitation may affect the observed association between TyG-BMI and the study outcomes.
Conclusion
In conclusion, our findings demonstrate that elevated TyG-BMI levels are significantly associated with IR, hyperlipidemia, and hyperandrogenism in Chinese women with PCOS. These results position TyG-BMI as a comprehensive and clinically reliable screening tool for both IR and MetS in this population. Furthermore, our data reveal that increased TyG-BMI may adversely affect ovulation induction outcomes. Notably, these associations show a threshold effect. Metabolic and reproductive impacts become particularly pronounced beyond specific TyG-BMI values.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of the First Affiliated Hospital of Heilongjiang University of Chinese Medicine (Approval Number: 2010HZYLL-010). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MG: Methodology, Visualization, Writing – original draft, Writing – review & editing. JF: Writing – review & editing. MZ: Conceptualization, Data curation, Writing – review & editing. YW: Conceptualization, Project administration, Writing – review & editing. BS: Conceptualization, Project administration, Writing – review & editing. FL: Conceptualization, Project administration, Writing – review & editing. JY: Conceptualization, Project administration, Writing – review & editing. ZG: Conceptualization, Project administration, Writing – review & editing. XW: Project administration, Resources, Supervision, Writing – review & editing. HY: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is supported by (1) Project of Heilongjiang Province Innovation Team “TouYan”, (2) Heilongjiang Provincial Clinical Research Centre for Ovary Diseases, (3) Key discipline construction fund on Gynecology of Traditional Chinese Medicine from Department of Education of Heilongjiang Province, (4) TCM evidence-based Capacity Improvement Project (Note of Chinese Medicine Science and Technology (2023) 24), (5) National Key R&D Program - New Round of Provincial "Double World-Class" Initiative.
Acknowledgments
The Steering Committee members included Tai-Xiang Wu, Elisabet Stener-Victorin and Heping Zhang, and Richard S. Legro (Chair). The Data and Safety Monitoring Board (DSMB) members included Esther Eisenberg, Wei-Liang Weng, Su-Lun Sun, Wei Zou and Zi-Dan Chen, and Robert Rebar (Chair). Principal investigators participated in patient recruitment at local sites. Other personnel with administrative resource support included Song-Jiang Liu, Gui-Yuan Wang, Yan-Qiu Du, Yang Xia, Shu-Lai Li, Ke-Qiu Zhang and Jian-Hua Shen. Yan Li, Wen-Juan Shen, Wei Li and Jing Cong were involved in protocol preparation and blood sample management in Harbin office and core laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1653636/full#supplementary-material
References
1
Dapas M Dunaif A . Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev. (2022) 43:927–65. doi: 10.1210/endrev/bnac001
2
Guan C Zahid S Minhas AS Ouyang P Vaught A Baker VL et al . Polycystic ovary syndrome: a "risk-enhancing" factor for cardiovascular disease. Fertil Steril. (2022) 117(5):924–35. doi: 10.1016/j.fertnstert.2022.03.009
3
Chen W Pang Y . Metabolic syndrome and PCOS: pathogenesis and the role of metabolites. Metabolites. (2021) 11(12):869. doi: 10.3390/metabo11120869
4
Houston EJ Templeman NM . Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS. J Endocrinol. (2025) 265(2):e240269. doi: 10.1530/JOE-24-0269
5
Chen Y Zheng X Ma D Zheng S Han Y Su WJ et al . Neck circumference is a good predictor for insulin resistance in women with polycystic ovary syndrome. Fertil Steril. (2021) 115(3):753–60. doi: 10.1016/j.fertnstert.2020.07.027
6
DeFronzo RA Tobin JD Andres R . Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. (1979) 237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214
7
Minh HV Tien HA Sinh CT Thang DC Chen CH Tay JC et al . Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). (2021) 23(3):529–37. doi: 10.1111/jch.14155
8
Altintas KZ Dilbaz B Cirik DA Ozelci R Zengin T Erginay ON et al . The incidence of metabolic syndrome in adolescents with different phenotypes of PCOS. Ginekol Pol. (2017) 88(6):289–95. doi: 10.5603/GP.a2017.0055
9
Ni RM Mo Y Chen X Zhong J Liu W Yang D et al . Low prevalence of the metabolic syndrome but high occurrence of various metabolic disorders in Chinese women with polycystic ovary syndrome. Eur J Endocrinol. (2009) 161(13):411–8. doi: 10.1530/EJE-09-0298
10
Balen AH Morley LC Misso M Franks S Legro RS Wijeyaratne CN et al . The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. (2016) 22(6):687–708. doi: 10.1093/humupd/dmw025
11
Morgante G Massaro MG Di Sabatino A Cappelli V De Leo V . Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol Endocrinol. (2018) 34:4–9. doi: 10.1080/09513590.2017.1370644
12
Wang S Shi J Peng Y Fang Q Mu Q Gu W et al . Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. (2021) 20(1):82. doi: 10.1186/s12933-021-01274-x
13
Lu L Chen Y Liu B Li X Wang J Nie Z et al . Association between cumulative changes of the triglyceride glucose index and incidence of stroke in a population with cardiovascular-kidney-metabolic syndrome stage 0-3: a nationwide prospective cohort study. Cardiovasc Diabetol. (2025) 24(1):202. doi: 10.1186/s12933-025-02754-0
14
Liu Q Zhang Y Chen S Xiang H Ouyang J Liu H et al . Association of the triglyceride-glucose index with all-cause and cardiovascular mortality in patients with cardiometabolic syndrome: a national cohort study. Cardiovasc Diabetol. (2024) 23(1):80. doi: 10.1186/s12933-024-02152-y
15
Song Y Jiang L Han Y Zhang S Li S . Triglyceride-glucose index and glycemic dynamics in pancreatic ductal adenocarcinoma: implications for disease progression and prognosis. J Transl Med. (2024) 22:708. doi: 10.1186/s12967-024-05524-w
16
Tudoran C Bende R Bende F Giurgi-Oncu C Enache A Dumache R et al . Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases. Biol (Basel). (2023) 12(3):370. doi: 10.3390/biology12030370
17
Lavie C Laddu D Arena R Ortega FB Alpert MA Kushner RF . Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol. (2018) 72(13):1506–31. doi: 10.1016/j.jacc.2018.08.1037
18
Huang T Qi Q Zheng Y Ley SH Manson JE Hu FB et al . Genetic predisposition to central obesity and risk of type 2 diabetes: two independent cohort studies. Diabetes Care. (2015) 38(7):1306–11. doi: 10.2337/dc14-3084
19
Morigny P Houssier M Mouisel E Langin D . Adipocyte lipolysis and insulin resistance. Biochimie. (2016) 125:259–66. doi: 10.1016/j.biochi.2015.10.024
20
Lim J Kim J Koo SH Kwon GC . Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PloS One. (2019) 14(3):e0212963. doi: 10.1371/journal.pone.0212963
21
Er L Wu S Chou H Hsu LA Teng MS Sun YC et al . Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PloS One. (2016) 11(3):e0149731. doi: 10.1371/journal.pone.0149731
22
Group REASPCW . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
23
Kuang H Li Y Wu X Hou L Wu T Liu J et al . Acupunctureand clomiphene citrate for live birth in polycystic ovary syndrome: study design of a randomized controlled trial. Evid Based Complement Alternat Med. (2013) 2013:527303. doi: 10.1155/2013/527303
24
Wu X-K Stener-Victorin E Kuang H-Y Ma HL Gao JS Xie LZ et al . Effect of acupuncture and clomiphene in chinese women with polycystic ovary syndrome: A randomized clinical trial. JAMA. (2017) 317(24):2502–14. doi: 10.1001/jama.2017.7217
25
Jayanthi R Srinivasan AR Hanifah M Maran AL . Associations among Insulin Resistance, Triacylglycerol/High Density Lipoprotein (TAG/HDL ratio) and Thyroid hormone levels-A study on Type 2 diabetes mellitus in obese and overweight subjects. Diabetes Metab Syndr. (2017) 11 Suppl 1:S121–6. doi: 10.1016/j.dsx.2016.12.020
26
Wallace TM Levy JC Matthews DR . Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. doi: 10.2337/diacare.27.6.1487
27
Song B Zhao X Yao T Lu W Zhang H Liu T et al . Triglyceride glucose-body mass index and risk of incident type 2 diabetes mellitus in Japanese people with normal glycemic level: a population-based longitudinal cohort study. Front Endocrinol. (2022) 13:907973. doi: 10.3389/fendo.2022.907973
28
Ahmed B Sultana R Greene MW . Adipose tissue and insulin resistance in obese. BioMed Pharmacother. (2021) 137:111315. doi: 10.1016/j.biopha.2021.111315
29
Islam MS Wei P Suzauddula M Nime I Feroz F Acharjee M et al . The interplay of factors in metabolic syndrome: understanding its roots and complexity. Mol Med. (2024) 30(1):279. doi: 10.1186/s10020-024-01019-y
30
Lim SS Kakoly NS Tan JWJ Fitzgerald G Bahri Khomami M Joham AE et al . Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev. (2019) 20(2):339–52. doi: 10.1111/obr.12762
31
Gilbert EW Tay CT Hiam DS Teede HJ Moran LJ . Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). (2018) 89(6):683–99. doi: 10.1111/cen.13828
32
Dietz de Loos A Jiskoot G Beerthuizen A Busschbach J Laven J . Metabolic health during a randomized controlled lifestyle intervention in women with PCOS. Eur J Endocrinol. (2021) 186:53–64. doi: 10.1530/EJE-21-0669
33
Yin Q Zheng J Cao Y Yan X Zhang H . Evaluation of novel obesity and lipid-related indices as indicators for the diagnosis of metabolic syndrome and premetabolic syndrome in chinese women with polycystic ovary syndrome. Int J Endocrinol. (2021) 2021:7172388. doi: 10.1155/2021/7172388
34
Chait A den Hartigh LJ . Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7:22. doi: 10.3389/fcvm.2020.00022
35
NICE (National Institute for Health and Care Excellence) . Fertility: assessment and treatment for people with fertility problems. London: NICE and RCOG, RCOG Press (2013). Available online at: https://www.nice.org.uk/guidance/cg156.
36
Mikhael S Punjala-Patel A Gavrilova-Jordan L . Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines. (2019) 7:1–9. doi: 10.3390/biomedicines7010005
37
Shukla KK Chambial S Dwivedi S Misra S Sharma P . Recent scenario of obesity and male fertility. Andrology. (2014) 2(6):809–18. doi: 10.1111/andr.270
38
Broughton DE Moley KH . Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017
39
Chinnathambi V Blesson CS Vincent KL Saade GR Hankins GD Yallampalli C Sathishkumar K . Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. (2014) 64(2):405–14. doi: 10.1161/HYPERTENSIONAHA.114.03283
40
Barber TM Hanson P Weickert MO et al . Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Heal. (2019) 13:1179558119874042. doi: 10.1177/1179558119874042
41
Rojas J Chavez M Olivar L Rojas M Morillo J Mejías et al . Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med. (2014) 2014:719050. doi: 10.1155/2014/719050
42
Catov JM Bodnar LM Ness RB Barron SJ Roberts JM . Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. (2007) 166(11):1312–9. doi: 10.1093/aje/kwm273
43
Chang EM Han JE Seok HH Lee DR Yoon TK Lee WS . Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin Endocrinol (Oxf). (2013) 79(1):93–9. doi: 10.1111/cen.12099
Summary
Keywords
polycystic ovary syndrome, triglyceride glucose index-body mass index, insulin resistance, metabolic syndrome, fertility outcomes
Citation
Guan M, Feng J, Zhu M, Wang Y, Shi B, Lu F, Yu J, Gao Z, Yu H and Wu X (2025) Triglyceride glucose index - body mass index predicts insulin resistance, metabolic syndrome and associates with impaired ovulation in Chinese women with polycystic ovary syndrome. Front. Endocrinol. 16:1653636. doi: 10.3389/fendo.2025.1653636
Received
25 June 2025
Accepted
03 September 2025
Published
18 September 2025
Volume
16 - 2025
Edited by
Omotayo Erejuwa, Alex Ekwueme Federal University Ndufu-Alike, Nigeria
Reviewed by
Giovanni Vitale, Italian Auxological Institute (IRCCS), Italy
Liwen Xiao, Chinese Academy of Sciences (CAS), China
Updates
Copyright
© 2025 Guan, Feng, Zhu, Wang, Shi, Lu, Yu, Gao, Yu and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoke Wu, xiaokewu2002@vip.sina.com; Hong Yu, hongyuaria@outlook.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.