- 1Department of Traditional Chinese Medicine, Qinghai Unversity Medical College, Xining, Qinghai, China
- 2Andrology Department of Integrative Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, China
With the global prevalence of diabetes mellitus (DM) steadily increasing, its impact on male reproductive health has become a growing area of concern. Diabetes-induced testicular damage involves alterations in testicular cell function, hormone levels, and the integrity of the blood-testis barrier (BTB), ultimately disrupting spermatogenesis. The key pathogenic factors include hyperglycemia, oxidative stress, chronic inflammation, mitochondrial dysfunction, and the accumulation of advanced glycation end products (AGEs).This review synthesizes the latest research on diabetes-induced testicular dysfunction and spermatogenic impairment, while also exploring potential therapeutic strategies. Current interventions are primarily focused on glycemic control, with supplementary treatments involving Chinese medicine, nanoparticles, and probiotics. Although most of the current evidence is derived from preclinical studies, these findings provide important insights that may inform future clinical research on diabetes-related male reproductive dysfunction.
1 Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder that includes both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) (1). T1DM is characterized by absolute insulin deficiency resulting from autoimmune destruction of pancreatic β-cells, whereas T2DM primarily results from insulin resistance, commonly associated with overweight, obesity, and unhealthy lifestyle factors (2). In recent years, the global prevalence of diabetes has continued to rise, with T2DM accounting for the majority of cases (3, 4). By 2045, it is projected that the global number of individuals with diabetes will reach 693 million. Notably, the prevalence of diabetes is consistently higher in men than in women across all age groups (5). The pathogenesis of diabetes involves a complex interplay of multiple factors, including hyperglycemia, oxidative stress, chronic inflammation, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress, all of which contribute to multi-organ complications (6, 7). In addition to the well-established microvascular and macrovascular complications, mounting evidence suggests that diabetes also negatively impacts male reproductive health.
Epidemiological data suggest that approximately 15% of couples worldwide experience infertility, with male factors contributing to 40–50% of cases. Diabetes has been linked to an increased incidence of male infertility. Recent clinical and preclinical studies have demonstrated that diabetes induces widespread damage to the male reproductive system (7, 8). Diabetic testicular dysfunction is primarily characterized by impaired spermatogenesis and disruption of the sperm microenvironment, including altered testosterone secretion and structural damage to testicular tissue. These alterations are mainly associated with metabolic dysregulation of Sertoli cells, increased apoptosis of Leydig cells, and compromised integrity of the blood-testis barrier (BTB) (9, 10).
While glycemic control remains the cornerstone of diabetes management, increasing attention is being given to exploring therapeutic interventions aimed at mitigating diabetes-induced damage to the reproductive system. This review aims to summarize the current research progress, elucidate underlying mechanisms, assess the clinical applicability of emerging treatments, and explore potential strategies that may inform future clinical interventions.
2 Sperm damage
DM exerts a profoundly detrimental effect on male reproductive function (2). Both T1DM and T2DM have been shown to impair spermatogenesis, characterized by reduced semen volume, sperm count, concentration, and motility, along with increased DNA fragmentation and abnormal morphology (11–15). Notably, T1DM appears to more significantly impair spermatozoa progressive motility and is associated with a higher rate of semen anti‐sperm antibody positivity, whereas T2DM is more strongly linked to decreased sperm concentration and an increased proportion of late apoptotic spermatozoa (16). The underlying mechanisms include chronic inflammation, oxidative stress, mitochondrial dysfunction, and ER stress. Additionally, T1DM may be associated with a lack of physiological contraction of the cranial and caudal portion of the epididymis after ejaculation (17, 18). However, the mechanisms underlying hormonal disturbances, gonadal dysfunction, and male accessory gland infection (MAGI) remain incompletely understood (15). Spermatogenesis is highly dependent on metabolic support from SC, which utilize glucose as a primary energy substrate. Glucose transporters (GLUTs) facilitate the uptake of extracellular glucose into sertoli cell (SC), where it is converted into pyruvate and subsequently reduced to lactate by lactate dehydrogenase (LDH). Lactate is then exported via monocarboxylate transporters (MCTs) to developing germ cells, serving as their primary energy source. DM has been shown to significantly disrupt the expression of key components in this metabolism pathway, leading to decreased lactate availability and energy supply to germ cells (19–22). This metabolic disturbance contributes to ejaculatory dysfunction (including anejaculation, retrograde ejaculation, and asthenozoospermia) and erectile dysfunction (16). Furthermore, T2DM is frequently accompanied by metabolic syndrome and cardiovascular disease, compounding its negative effects on male reproductive health (18) (Table 1).
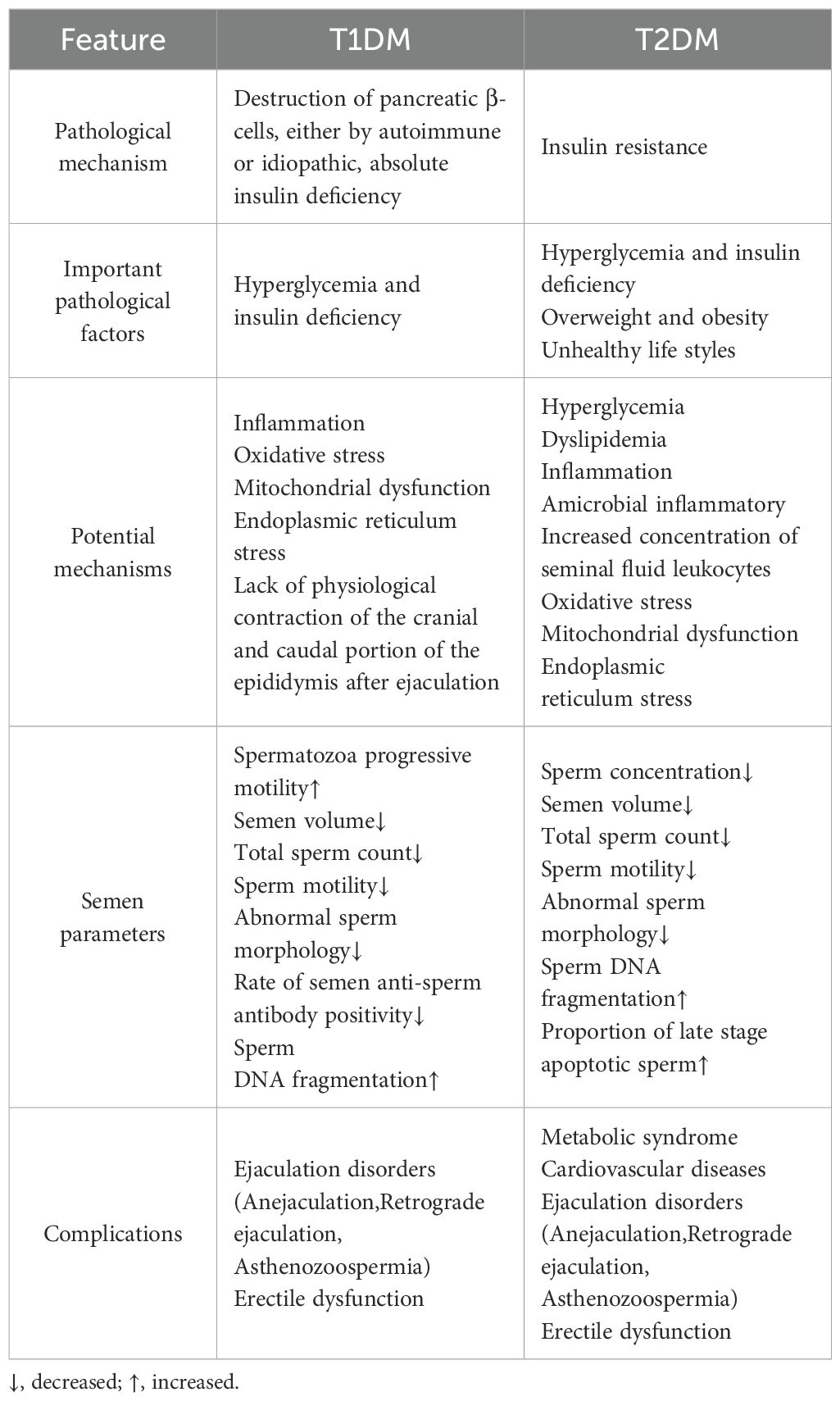
Table 1. Differential pathological mechanisms and sperm damage profiles in type 1 and type 2 diabetes.
2.1 Sperm DNA damage and abnormal sperm morphology
Chronic hyperglycemia and insulin resistance lead to systemic immune dysregulation and sustained inflammation within the male reproductive tract (23). This state of inflammation, characterized by abnormal activation of leukocytes, leads to an overproduction of reactive oxygen species (ROS). These ROS oxidize protamines, crucial for the correct condensation of sperm nuclei, leading to the disruption of disulfide bond formation. Consequently, incomplete chromatin packaging occurs, leading to heightened DNA fragility and genomic instability (24). These nuclear abnormalities are closely associated with morphological defects, particularly affecting the sperm head (25). Additionally, elevated levels of advanced glycation end products (AGEs) and DNA fragmentation markers have been consistently detected in the sperm of diabetic men, indicating substantial nuclear instability and chromatin disruption (26). Prolonged hyperglycemia disrupts normal spermatogenesis by interfering with the development of key structural components, including the sperm head, midpiece, and tail, leading to a significant increase in morphologically abnormal sperm (27).
2.2 Reduction in sperm count
DM, particularly T2DM, significantly reduces sperm count through multiple interrelated mechanisms. It disrupts the hypothalamic-pituitary-gonadal (HPG) axis, resulting in decreased levels of testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) (28). The reduction in hormone levels directly impairs critical stages of spermatogenesis, particularly the primary meiotic division, and disrupts the function of both SC and Leydig cell(LC). A reduction in the number of SC, combined with impaired lactate production, leads to an inadequate energy supply for germ cells, thereby promoting germ cell death (29, 30). Furthermore, oxidative stress activates apoptotic signaling pathways—such as the MAPK/p38 pathway— upregulating the expression of the pro-apoptotic protein Bax while downregulating the anti-apoptotic protein Bcl-2. This dysregulation ultimately triggers extensive programmed cell death among spermatogenic cells, including SC and LC (24). Structural and functional defects in the sperm tail further impair sperm motility and viability. Ultimately, hormonal imbalances, SC dysfunction, oxidative stress, defective chromatin maturation, excessive activation of apoptotic pathways, and abnormalities in sperm structure and function collectively lead to severe disruption of spermatogenesis. This results in a substantial decline in daily sperm production, sperm reserve quantity, and impaired overall testicular spermatogenic function (27, 31).
2.3 Decreased sperm motility
DM impairs sperm motility through a complex interplay of hormonal imbalance, oxidative stress, metabolic dysfunction, and inflammation (23, 27, 32). It compromises testicular antioxidant capacity and promotes germ cell apoptosis, leading to reduced testosterone levels. This testosterone deficiency disrupts epididymal function, weakens reproductive antioxidant defenses, and alters the expression of proteins critical for sperm flagellar movement and acrosome integrity, ultimately diminishing sperm motility (27, 33).
Trace element imbalances also contribute to diabetic testicular dysfunction. Ghasemi et al. observed significantly reduced concentrations of zinc and magnesium in the seminal plasma of diabetic individuals. Zinc levels exhibited a strong positive correlation with sperm motility and morphology, whereas magnesium was significantly associated with parameters of sperm motility (34).
Inflammation is another important factor. The accumulation of AGEs activates macrophages through the receptor for AGEs (RAGE), triggering the release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α). In vitro studies have demonstrated that TNF-α can directly suppress sperm motility by 40%–60% (35, 36).
Furthermore, sperm motility is highly dependent on energy derived from glucose metabolism. Insulin deficiency or resistance interferes with glucose utilization, limiting energy availability for sperm motility (29).
3 Mechanisms of spermatogenesis disorders
3.1 Mitochondria and oxidative stress
Mitochondria play a pivotal role in regulating sperm function by modulating essential processes, including metabolism, signal transduction, energy production, and responses to oxidative stress. The midpiece of sperm is rich in mitochondria, which generate ATP through the electron transport chain (ETC), providing the energy required for sperm motility, capacitation, and overall functional capability (37). Additionally, mitochondria are essential for maintaining DNA integrity, regulating calcium homeostasis, mediating apoptotic signaling, and supporting the differentiation and maturation of spermatogenic cells (38). As the primary cellular energy generators, mitochondria are also the main source of ROS within the cell. Physiological levels of ROS are essential for sperm capacitation and fertilization. The antioxidant defense systems in semen generally maintain redox homeostasis under normal conditions. However, excessive ROS production can inhibit the activity of key antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), leading to oxidative stress and mitochondrial dysfunction. Therefore, maintaining mitochondrial integrity is crucial for preserving sperm quality, and its impairment is widely recognized as a major cause of male infertility (39–41).
3.2 Hypothalamic-pituitary-gonad axis function
Insulin regulates reproductive function in both males and females by modulating the HPG axis. This effect is probably mediated by the MAPK/Erk1/2 pathway, whereby insulin stimulates the hypothalamus to secrete gonadotropin-releasing hormone (GnRH) (42). GnRH subsequently acts on the pituitary gland to promote the synthesis and release of LH and FSH, which in turn facilitate the maturation of seminiferous tubules and LC (2). Insulin resistance disrupts the function of the HPG axis, directly leading to decreased secretion of LH and FSH, reduced testosterone levels, and an imbalance in the androgen-to-estrogen ratio (43, 44). Meanwhile, persistent hyperglycemia further exacerbates reproductive dysfunction by impairing the pulsatile release of GnRH from the hypothalamus and suppressing LH and FSH synthesis in the pituitary. These disturbances ultimately impair testosterone production by LC and compromise the supportive function of SC in spermatogenesis, resulting in impaired spermatogenic function (2).
3.3 Advanced glycation end products
AGEs can severely impair sperm function through multiple mechanisms. Upon binding to RAGE, AGEs not only activate inflammatory signaling pathways but also directly disrupt key cellular processes and structural components of sperm. Notably, AGEs exposure significantly reduces sperm motility, particularly progressive motility (45, 46). This decline may result from AGEs-induced mitochondrial dysfunction, crosslinking with membrane proteins or flagellar components, and the induction of DNA damage and activation of apoptotic pathways. In addition, AGEs–RAGE interactions may interfere with essential fertilization-related processes such as capacitation and the acrosome reaction, further compromising the fertilizing potential of sperm (35, 37).
4 Damage to the environment surrounding spermatogenesis
4.1 Sertoli cell
The number of SC in the adult testis determines daily sperm production, as each SC can maintain a limited number of germ cells (47). SC play a critical role in forming the BTB and providing nutrients essential for spermatogenesis. SC can metabolize a variety of substances, including glucose, lactic acid, fatty acids, and amino acids, and possess specific glucose sensing mechanisms that are highly sensitive to extracellular glucose levels (48). The glucose transporters GLUT1 and GLUT3 work synergistically in SC to maintain glucose uptake and ensure lactate production. Under diabetic conditions, the metabolic homeostasis of SC is disrupted, potentially leading to structural and functional impairment of the BTB in patients with T2DM (49). Both in vivo and in vitro studies have demonstrated that high glucose levels upregulate Apelin (APLN) in SC, which subsequently downregulates cell junction–related genes such as connexin 43 (Cx43) and zonula occludens-1 (ZO-1), ultimately compromising the integrity of the BTB (50).
4.2 Increased permeability of blood-testis barrier
The blood–testis barrier (BTB), also referred to as the SC barrier, is a critical structural and functional component of the seminiferous tubules. Located at the basal compartment of the seminiferous epithelium, the BTB is primarily formed by junctional complexes between SC, including tight junctions, gap junctions, and basal ectoplasmic specializations (51). The primary function of the BTB is to segregate the lumen of the seminiferous tubules from the systemic circulation, thereby establishing an immune-privileged microenvironment that protects developing germ cells from harmful substances and maintains the sterile conditions essential for spermatogenesis (52, 53).
During spermatogenesis, spermatogonia migrate across the BTB within the seminiferous tubules, the functional units of the mammalian testis. To facilitate this translocation, cell junctions are dynamically disassembled and reassembled, enabling the movement of immature germ cells from the basal compartment to the luminal compartment as they undergo meiosis and subsequent differentiation, ultimately culminating in the release of mature spermatozoa (54, 55). This process involves proteases, protease inhibitors, and cell-junction components (56).
The interaction between Cx43 and ZO-1 is crucial for the maintenance of BTB integrity. Hyperglycemia and AGEs have been shown to impair the expression and function of junctional proteins such as Cx43 and ZO-1 in SC by inducing oxidative stress, inflammation, and hormonal disturbances, ultimately compromising BTB function. Genetic deletion of Cx43 leads to BTB disruption and subsequent failure of spermatogenesis (11). Ke Song’s STRT-seq analysis of testicular tissue from diabetic patients at single-cell resolution revealed significant alterations in SC gene expression profiles and disruption of the BTB structure. In vivo biotin tracer assays in hyperglycemic (HGM) mice demonstrated a substantial increase in biotin-positive seminiferous tubules and deeper biotin penetration, indicating a loss of BTB integrity induced by hyperglycemia (50).
4.3 Alterations in Leydig cell secretory function
Basel A. Abdel-Wahab et al. reported histopathological changes in diabetic rat testes, including seminiferous tubule atrophy and significant degeneration and necrosis of LC (57).LC located in the testicular stroma, are regulated by LH to produce testosterone, essential for maintaining spermatogenesis. Androgens have a bidirectional relationship with glucose regulation (58). Androgen deficiency is a risk factor for T2DM, while hyperglycemia promotes the formation of AGEs through non-enzymatic glycation. These AGEs bind to receptors such as RAGE in the testes, triggering overactivation of downstream signaling pathways that downregulate key steroidogenic enzymes, ultimately impairing testosterone secretion (59, 60). Furthermore, chronic hyperglycemia itself induces ER stress within LC. This ER stress disrupts LC function, causing cell cycle arrest, apoptosis, and further suppression of testosterone synthesis. Collectively, these mechanisms disrupt the testicular reproductive environment and impair sperm production, contributing to male reproductive dysfunction in diabetes (59, 61, 62). Insulin resistance further exacerbates this by reducing insulin sensitivity and triggering compensatory hyperinsulinemia, which promotes facilitates the conversion of testosterone to dihydrotestosterone (DHT) and suppresses LH and FSH secretion, thereby disrupting the synthesis and regulation of reproductive hormones (63, 64). Consequently, men with T2DM frequently present with hypogonadism, which is closely associated with early LC dysfunction.
5 Therapeutic strategies for diabetes testicular dysfunction
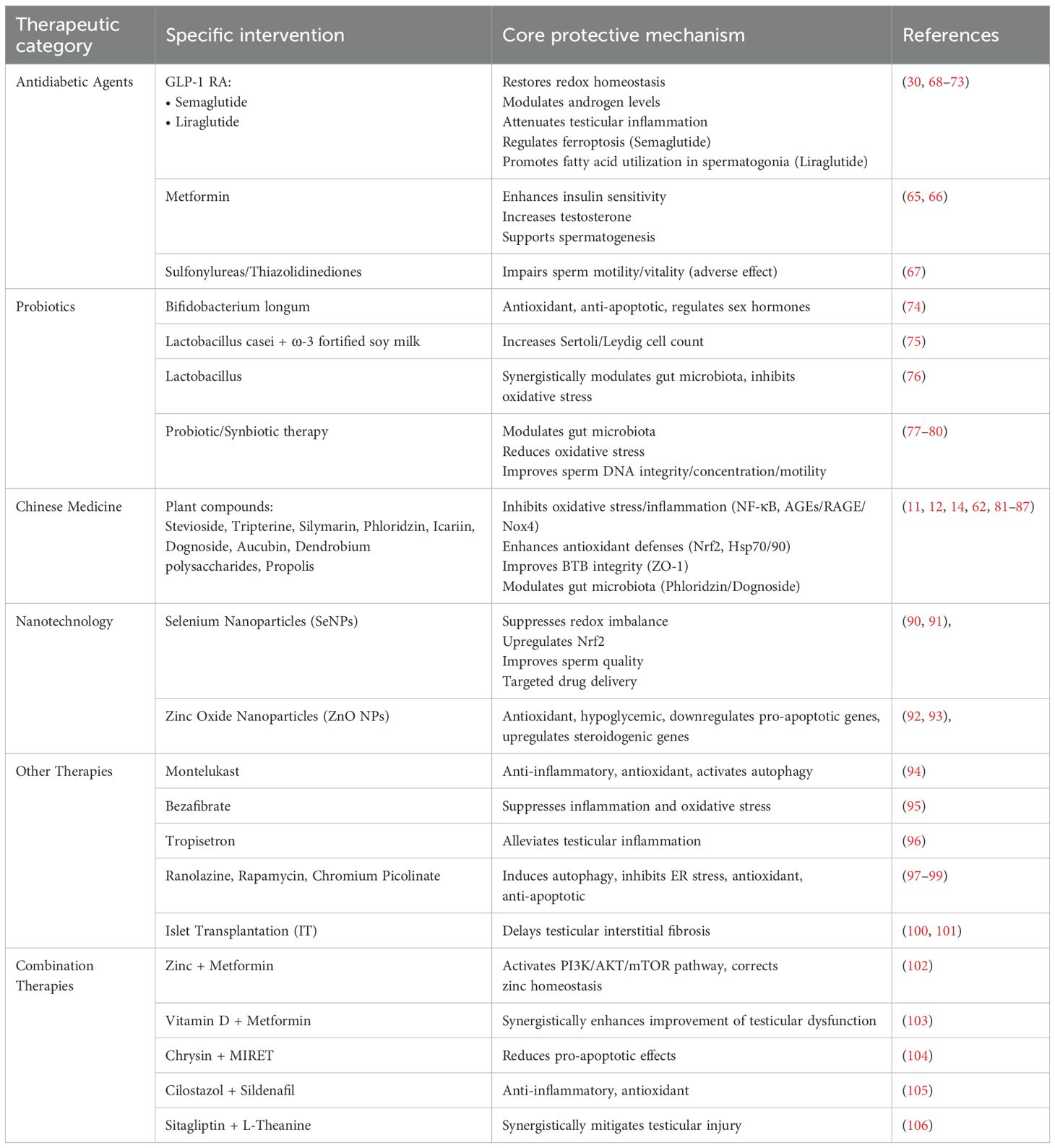
5.1 Antidiabetic agents treatments
Current therapeutic strategies for diabetes-associated testicular dysfunction remain limited, with a predominant emphasis on glycemic control (Table 2). Studies have demonstrated that metformin can improve sperm quality, an effect attributed to its ability to elevate testosterone levels and support spermatogenesis. This beneficial action is closely linked to the restoration of gonadotropic hormone and leptin system function within the testes. These effects are largely attributed to improved insulin sensitivity, which contributes to hormonal balance and promotes testicular function and integrity (65, 66). Conversely, evidence suggests that the use of sulfonylureas and thiazolidinediones may be associated with impaired sperm quality, exerting detrimental effects on motility and vitality (67). Emerging evidence suggests that semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), may offer a more comprehensive therapeutic approach for male infertility associated with diabetes and obesity. In diabetic rat models, semaglutide has been shown to restore redox homeostasis, modulate androgen levels, attenuate testicular inflammation, and alleviate diabetes-induced testicular damage by regulating the ferroptosis pathway (68). Furthermore, semaglutide improves the expression of key genes and proteins involved in spermatogenesis through multiple molecular mechanisms, thereby preventing testicular and sexual dysfunction induced by diabetes (69). In a 24-week clinical trial, semaglutide significantly improved sperm morphology in men with type 2 diabetes, obesity, and functional hypogonadism. Notably, its effects on sperm concentration and total sperm count were superior to those achieved with testosterone replacement therapy (TRT), offering a novel therapeutic option for this patient population (30). Liraglutide improves energy homeostasis by promoting fatty acid utilization in spermatogonia, thereby enhancing the energetic state of seminiferous tubules. Additionally, it exerts anti-inflammatory and antioxidant effects, contributing to the improvement of sperm quality and hormonal profiles (70, 71). A 16-week study demonstrated that liraglutide effectively facilitates weight loss, ameliorates sexual function, elevates testosterone levels, and restores HPT axis function. Furthermore, liraglutide positively impacts metabolic health by improving glycemic control, reducing HbA1c, alleviating insulin resistance, and mitigating metabolic syndrome (72). Simultaneously, another study investigating liraglutide treatment in obese men with metabolic hypogonadism reported significant improvements in sperm motility, semen parameters, and sexual function after four months of therapy, compared to both gonadotropin and transdermal testosterone groups. Moreover, increases in testosterone, sex hormone-binding globulin (SHBG), and gonadotropin levels were also observed (73). The GLP-1 peptide-based agents discussed above have demonstrated the potential to enhance spermatogenesis and testosterone synthesis in both preclinical and clinical studies, offering valuable insights for the development of therapeutic strategies in clinical practice. Consequently, addressing testicular dysfunction and abnormalities in spermatogenesis continues to represent a meaningful and promising direction for future research.
5.2 Probiotic treatments
Bifidobacterium longum exerts multifaceted anti-diabetic effects, with its antioxidant and anti-apoptotic properties contributing to improvements in reproductive function and the regulation of hormone levels (74). Soy milk fortified with Lactobacillus casei and omega-3 inhibits the infertility phenotype in a rat model of type 1 diabetes, increasing the number of SC and LC and enhancing sperm quality (75). The combination of Lactobacillus, montelukast, and metformin synergistically mitigates diabetes-induced testicular damage by modulating the intestinal microbiome and reducing oxidative stress (76).Multiple randomized controlled trials (RCTs) have demonstrated that probiotic supplementation significantly improves sperm parameters in infertile men, including sperm concentration, motility, and morphology. In addition, a marked reduction in oxidative stress biomarkers has been reported, suggesting that probiotics help restore redox homeostasis within the seminal microenvironment (77–79). Probiotics may also influence male hormonal profiles. Several studies have reported a mild increase in serum testosterone levels and improved hormonal balance following probiotic supplementation, which may support spermatogenesis. Furthermore, synbiotic therapy (a combination of probiotics and prebiotics) has shown beneficial effects in certain trials, including improvements in sperm DNA integrity and chromatin quality (80). Lactobacillus and Bifidobacterium species are among the most commonly used and well-studied probiotic strains. These bacteria can lower intestinal pH and significantly enhance sperm quality through antioxidant, anti-inflammatory, and gut microbiota-modulating mechanisms. Clinically, probiotic-based treatments are low-cost, safe, and offer promising potential as adjunctive therapies for improving sperm quality in men with diabetes. Nevertheless, large-scale clinical studies are still needed to determine the most effective strain combinations and to validate their long-term efficacy (77).
5.3 Chinese medicine treatments
A variety of plant-derived compounds, including stevioside, tripterine, silymarin, phloridzin, icariin, dognoside, Aucubin, dendrobium polysaccharides, and propolis, have demonstrated protective effects against testicular damage induced by diabetes or inflammation (11, 12, 14, 62, 81–87). The core mechanisms underlying these protective effects involve inhibition of oxidative stress and inflammatory pathways (e.g., regulation of NF-κB, Nrf2, AGEs/RAGE/Nox4), reduction of apoptosis and necrosis, enhancement of endogenous antioxidant defenses (e.g., increased antioxidant enzyme activity and Hsp70/90 expression), and improvement of BTB integrity (61, 88). In some cases, the protective effects are associated with the regulation of intestinal microbiota, as observed with phloridzin and dognoside (89). Ultimately, these interventions help restore testosterone levels, promote spermatogenesis, and improve testicular tissue structure.
5.4 New nanotechnology treatments
Selenium plays a critical role in healthy spermatogenesis, testicular development, and sperm motility. Selenium nanoparticles (SeNPs), functionalized with active targeting ligands, exhibit excellent biocompatibility and enable efficient, targeted delivery of therapeutic agents (90). Recent studies have demonstrated that the combined administration of SeNPs and metformin confers protective effects against streptozotocin-induced testicular oxidative damage in diabetic rats. This protective mechanism involves the suppression of redox imbalance, improvement of sperm quality, and upregulation of nuclear factor erythroid 2–related factor 2 (Nrf2) expression (91). Similarly, zinc oxide nanoparticles (ZnO NPs) have been shown to exert protective effects against diabetes-induced testicular damage. These effects are attributed not only to the potent antioxidant and hypoglycemic properties of ZnO NPs, but also to their ability to downregulate pro-apoptotic gene expression and upregulate the expression of steroidogenesis-related regulatory genes within testicular tissue (92, 93). Collectively, selenium and zinc oxide nanoparticles demonstrate promising protective effects against diabetes-induced testicular oxidative damage and sperm dysfunction. Experimental evidence supports the role of these nanoparticles in improving sperm quality, highlighting their value as adjunctive agents in the management of male reproductive disorders associated with diabetes.
5.5 Other treatments
Montelukast, a cysteinyl leukotriene receptor 1 antagonist, has been shown to attenuate testicular injury through its anti-inflammatory, antioxidant, and autophagy-inducing properties (94). Similarly, bezafibrate, a peroxisome proliferator-activated receptor alpha (PPARα) agonist, has been shown to suppress inflammation and oxidative stress, thereby alleviating diabetic spermatogenesis disorder (95). Tropisetron, an antagonist of 5-Hydroxytryptamine (5-HT) type 3 receptor, has been demonstrated to reduce testicular inflammation induced by streptozotocin in diabetic rats. Furthermore, the late sodium current inhibitor ranolazine, as well as the mechanistic target of rapamycin (mTOR) inhibitor rapamycin and the chromium-based compound chromium picolinate, have demonstrated protective trends against diabetic testicular injury by inducing autophagy, inhibiting ER stress, oxidative damage, and apoptosis (96–99). Islet transplantation may also serve as an therapeutic strategy for diabetic complications in men by delaying diabetic testicular interstitial fibrosis (100, 101).
5.6 Combination treatments
Zinc combined with metformin restores zinc homeostasis by activating the PI3K/AKT/mTOR pathway, thereby enhancing steroidogenesis and improving semen quality in male mice with T2DM (102). Vitamin D enhances the efficacy of metformin in alleviating testicular dysfunction associated with T2DM (103). Co-administration of chrysin with MIRET significantly ameliorates diabetic testicular histopathological testicular histopathological and biochemical damage and diminishes pro-apoptotic effects (104). Cilostazol and sildenafil alleviate testicular damage in diabetes by exerting anti-inflammatory and antioxidant effects (105). The combination of sitagliptin and L-theanine demonstrates potential to significantly reduce diabetic testicular dysfunction, indicating a promising therapeutic approach (106).
5.7 Future perspectives
Although the aforementioned studies provide several potential therapeutic strategies for alleviating diabetic testicular dysfunction and impaired spermatogenesis, most of the current evidence is derived from animal models, with clinical data remaining relatively limited. Therefore, future research should focus on optimizing these interventions to enhance their clinical efficacy while minimizing potential side effects and safety concerns. Such efforts may contribute to the development of more personalized and targeted therapeutic approaches for diabetes-related complications, ultimately facilitating the clinical translation of these emerging treatments.
6 Discussion
DM is a metabolic disorder that induces multi-organ complications primarily through neuropathy and vascular damage, with significant adverse effects on the male reproductive system (107–109). Although the diabetes-induced male reproductive health remains incompletely understood, recent studies have highlighted diabetic testicular dysfunction, particularly in relation to impaired spermatogenesis and disruption of the testicular microenvironment. The pathogenesis of diabetes is primarily driven by hyperglycemia and insulin deficiency (82). Hyperglycemia disrupts the metabolic support provided by SC to developing germ cells, directly impairing sperm production and quality. Additionally, hyperglycemia results in reduced sperm count, abnormal sperm morphology, and a marked decline in sperm motility (14, 21).
Mitochondria play a vital role in providing energy for sperm motility. However, under diabetic conditions, mitochondrial dysfunction leads to excessive production of ROS, impaired ATP synthesis, and activation of mitochondria-dependent apoptotic pathways. This results in the apoptosis of germ cells and a decline in sperm parameters (110). Additionally, diabetes disrupts the tight junctions between Sertoli cells that constitute the BTB, compromising its integrity and permitting harmful systemic factors to infiltrate the seminiferous epithelium. This disruption impairs germ cell migration during spermatogenesis (52).
Endocrine dysregulation is another significant factor in diabetic testicular dysfunction. Diabetes affects the HPG axis, often leading to reduced levels of gonadotropins (LH, FSH) and testosterone (59). It also impairs the function of LC by suppressing the expression of steroidogenic enzymes such as steroidogenic acute regulatory protein (StAR), cholesterol side-chain cleavage enzyme (P450scc), and 3β-hydroxysteroid dehydrogenase (3β-HSD), ultimately reducing testosterone synthesis (111). The resulting low testosterone levels further exacerbate impairments in spermatogenesis and metabolic function, thereby perpetuating a vicious cycle (27).
Diabetes negatively impacts testicular function through multiple mechanisms, including hyperglycemia, oxidative stress, accumulation of AGEs, inflammation, endocrine disruption, ER stress, and mitochondrial dysfunction (112). These pathological processes directly impair sperm quality, including concentration, motility, morphology, and DNA integrity, while also damaging the BTB, LC, and SC, thus disrupting the testicular spermatogenic microenvironment. Ultimately, these cascades lead to reduced male fertility or infertility (Figure 1).
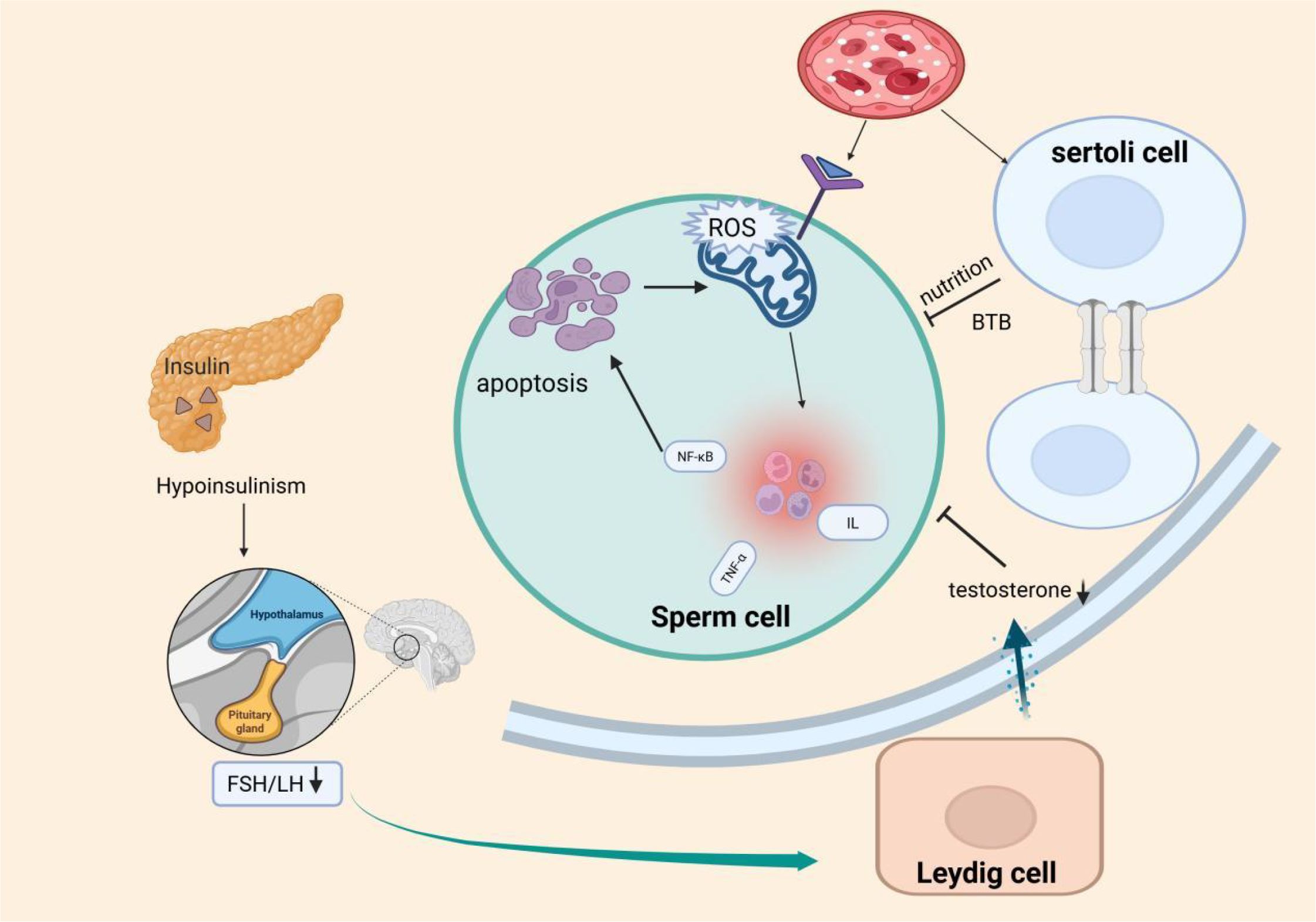
Figure 1. Proposed model of testicular injury and spermatogenic impairment induced by metabolic disturbances and insulin dysregulation in diabetes mellitus (DM).This schematic illustrates that hyperglycemia in DM primarily triggers oxidative stress, increased advanced glycation end products (AGEs), mitochondrial dysfunction, and inflammatory responses, ultimately disrupting the spermatogenic environment (e.g., blood-testis barrier impairment, BTB). Concurrently, insulin deficiency in diabetic patients impairs the hypothalamic-pituitary-gonadal (HPG) axis, reducing secretion of luteinizing hormone (LH) and testosterone (T). These factors may synergistically disrupt spermatogenesis, contributing to male infertility.
While maintaining strict glycemic control remains critical, emerging therapeutic strategies targeting specific mechanisms of testicular damage offer promising avenues for enhancing reproductive health in diabetic men. These include the use of GLP-1 RA, Chinese Medicine, nanoparticles, probiotics, and targeted antioxidants and anti-inflammatory drugs. However, further research is required to evaluate the clinical translational potential and long-term safety of these novel interventions.
Author contributions
WZ: Conceptualization, Data curation, Writing – original draft. LT: Conceptualization, Formal Analysis, Writing – review & editing. BJ: Supervision, Writing – review & editing. DS: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China, grant numbers 82374459 and Collaborative Medical Research Project of Traditional Chinese and Western Medicine at Zhongda Hospital, School of Medicine, Southeast University, grant numbers 2023zxyxt14.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Yang Z, Kong D, Zhang Y, Yu W, and Zha W. Metformin ameliorates testicular damage in male mice with streptozotocin-induced type 1 diabetes through the PK2/PKR pathway. Oxid Med Cell Longev. (2019) 2019:5681701. doi: 10.1155/2019/5681701
2. Graziani A, Scafa R, Grande G, and Ferlin A. Diabetes and male fertility disorders. Mol Aspects Med. (2024) 99:101303. doi: 10.1016/j.mam.2024.101303
3. Lotti F and Maggi M. Effects of diabetes mellitus on sperm quality and fertility outcomes: Clinical evidence. Andrology. (2023) 11:399–416. doi: 10.1111/andr.13342
4. Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, and Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. (2020) 18:104–9. doi: 10.2174/1570161117666190405165911
5. Zhu Y, Du Q, Jiao N, Shu A, Gao Y, Chen J, et al. Catalpol ameliorates diabetes-induced testicular injury and modulates gut microbiota. Life Sci. (2021) 267:118881. doi: 10.1016/j.lfs.2020.118881
6. Li JP, Fu MM, Li XX, and Xu M. Cell-based therapy for diabetic cardiovascular complications: Prospects and challenges. Br J Pharmacol. (2024). doi: 10.1111/bph.16475
7. Huang R, Chen J, Guo B, Jiang C, and Sun W. Diabetes-induced male infertility: potential mechanisms and treatment options. Mol Med. (2024) 30:11. doi: 10.1186/s10020-023-00771-x
8. Chen ZF, Shen YF, Gao DW, Lin DF, Ma WZ, and Chang DG. Metabolic pathways and male fertility: exploring the role of Sertoli cells in energy homeostasis and spermatogenesis. Am J Physiol Endocrinol Metab. (2025) 329:E160–e78. doi: 10.1152/ajpendo.00074.2025
9. Wang D, Li Y, Zhai QQ, Zhu YF, Liu BY, and Xu Y. Quercetin ameliorates testosterone secretion disorder by inhibiting endoplasmic reticulum stress through the miR-1306-5p/HSD17B7 axis in diabetic rats. Bosn J Basic Med Sci. (2022) 22:191–204. doi: 10.17305/bjbms.2021.6299
10. Xu R, Wang F, Zhang Z, Zhang Y, Tang Y, Bi J, et al. Diabetes-induced autophagy dysregulation engenders testicular impairment via oxidative stress. Oxid Med Cell Longev. (2023) 2023:4365895. doi: 10.1155/2023/4365895
11. Wei J, Lu X, Bao X, Zhang C, Li J, Ren C, et al. Aucubin supplementation alleviate diabetes induced-disruption of blood-testis barrier and testicular damage via stabilizing cell junction integrity. Eur J Pharmacol. (2023) 938:175430. doi: 10.1016/j.ejphar.2022.175430
12. Ashour AM. Propolis attenuates diabetes-induced testicular injury by protecting against DNA damage and suppressing cellular stress. Front Pharmacol. (2024) 15:1416238. doi: 10.3389/fphar.2024.1416238
13. Lei X, Huo P, Wang Y, Xie Y, Shi Q, Tu H, et al. Lycium barbarum polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats. Front Endocrinol (Lausanne). (2020) 11:164. doi: 10.3389/fendo.2020.00164
14. Luo M, Liao B, Ma D, Wang J, Wang J, Liu J, et al. Dendrobium nobile-derived polysaccharides ameliorate spermatogenic disorders in mice with streptozotocin-induced diabetes through regulation of the glycolytic pathway. Int J Biol Macromol. (2022) 216:203–12. doi: 10.1016/j.ijbiomac.2022.06.193
15. Facondo P, Di Lodovico E, Delbarba A, Anelli V, Pezzaioli LC, Filippini E, et al. The impact of diabetes mellitus type 1 on male fertility: Systematic review and meta-analysis. Andrology. (2022) 10:426–40. doi: 10.1111/andr.13140
16. Delbarba A, Anelli V, Bambini F, Buoso C, Facondo P, Gatta E, et al. Type 1 diabetes mellitus and sperm quality: A case-control study. Andrology. (2025) 13:208–16. doi: 10.1111/andr.13681
17. Condorelli RA, La Vignera S, Mongioì LM, Alamo A, and Calogero AE. Diabetes mellitus and infertility: different pathophysiological effects in type 1 and type 2 on sperm function. Front Endocrinol (Lausanne). (2018) 9:268. doi: 10.3389/fendo.2018.00268
18. La Vignera S, Condorelli RA, Di Mauro M, Lo Presti D, Mongioì LM, Russo G, et al. Reproductive function in male patients with type 1 diabetes mellitus. Andrology. (2015) 3:1082–7. doi: 10.1111/andr.12097
19. Rato L, Alves MG, Dias TR, Cavaco JE, and Oliveira PF. Testicular metabolic reprogramming in neonatal streptozotocin-induced type 2 diabetic rats impairs glycolytic flux and promotes glycogen synthesis. J Diabetes Res. (2015) 2015:973142. doi: 10.1155/2015/973142
20. Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, and Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. (2012) 9:330–8. doi: 10.1038/nrurol.2012.77
21. Wang JY, Ma D, Luo M, Tan YP, Ou Z, Tian G, et al. Effect of spermidine on ameliorating spermatogenic disorders in diabetic mice via regulating glycolysis pathway. Reprod Biol Endocrinol. (2022) 20:45. doi: 10.1186/s12958-022-00890-w
22. Ma D, Hu L, Wang J, Luo M, Liang A, Lei X, et al. Nicotinamide mononucleotide improves spermatogenic function in streptozotocin-induced diabetic mice via modulating the glycolysis pathway. Acta Biochim Biophys Sin (Shanghai). (2022) 54:1314–24. doi: 10.3724/abbs.2022099
23. Gori PV, Raval M, Patel S, Patel A, Solanki N, Patel P, et al. Argyreia nervosa (Brum.f.) Bojer. alleviates diabetes mellitus-induced male infertility. Mol Biol Rep. (2025) 52:546. doi: 10.1007/s11033-025-10654-5
24. Bezerra LGP, Silva AM, Jurema AP, Dantas MRT, Pereira AG, Oliveira MF, et al. Changes in sperm morphology, morphometry, and motility from the epididymis to the vas deferens in rheas (Rhea americana, linnaeus, 1758). Anim (Basel). (2023) 13:1483. doi: 10.3390/ani13091483
25. Carvalho RPR, Guimarães-Ervilha LO, Viana AGA, Ribeiro IM, Ramirez-Lopez C, and MaChado-Neves M. A systematic review and meta-analysis of the effects of diabetes on human and murine epididymis. Andrology. (2025). doi: 10.1111/andr.70026
26. Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, and Amiri I. Increased receptor for advanced glycation end products in spermatozoa of diabetic men and its association with sperm nuclear DNA fragmentation. Andrologia. (2012) 44 Suppl 1:280–6. doi: 10.1111/j.1439-0272.2011.01178.x
27. Kiani M, Mehranjani MS, and Shariatzadeh MA. Myoinositol improves sperm parameters in diabetic rats by reducing oxidative stress and regulating apoptosis-related genes. J Mol Histol. (2025) 56:165. doi: 10.1007/s10735-025-10451-1
28. Ali BR, Alameri AN, Al Rumaidh S, and Ethaib S. Correlation between reproductive hormones levels and semen quality in patients with diabetes. J Med Life. (2022) 15:1507–10. doi: 10.25122/jml-2022-0079
29. Andlib N, Sajad M, Kumar R, and Thakur SC. Abnormalities in sex hormones and sexual dysfunction in males with diabetes mellitus: A mechanistic insight. Acta Histochem. (2023) 125:151974. doi: 10.1016/j.acthis.2022.151974
30. Gregorič N, Šikonja J, Janež A, and Jensterle M. Semaglutide improved sperm morphology in obese men with type 2 diabetes mellitus and functional hypogonadism. Diabetes Obes Metab. (2025) 27:519–28. doi: 10.1111/dom.16042
31. Odetayo AF, Ajibare AJ, Okesina KB, Akhigbe TM, Olugbogi EA, and Olayaki LA. Orange peel ethanolic extract and physical exercise prevent testicular toxicity in streptozocin and high fat diet-induced type 2 diabetes rats via Nrf2/NF-kB signaling: In silico and in vivo studies. Heliyon. (2024) 10:e39780. doi: 10.1016/j.heliyon.2024.e39780
32. Soetan OA, Ajao FO, and Ajayi AF. Erythritol attenuates testicular dysfunction in diabetic rat via suppression of oxidative stress, inflammation and apoptosis. Biochem Biophys Res Commun. (2024) 690:149254. doi: 10.1016/j.bbrc.2023.149254
33. Belhan S, Yıldırım S, Huyut Z, Özdek U, Oto G, and Algül S. Effects of curcumin on sperm quality, lipid profile, antioxidant activity and histopathological changes in streptozotocin-induced diabetes in rats. Andrologia. (2020) 52:e13584. doi: 10.1111/and.13584
34. Ghasemi H, Karimi J, Goodarzi MT, Khodadadi I, Tavilani H, Moridi H, et al. Seminal plasma zinc and magnesium levels and their relation to spermatozoa parameters in semen of diabetic men. Int J OF Diabetes IN DEVELOPING COUNTRIES. (2016) 36:34–9. doi: 10.1007/s13410-015-0408-y
35. Mori Y, Terasaki M, Osaka N, Fujikawa T, Yashima H, Saito T, et al. DNA aptamer raised against advanced glycation end products improves sperm concentration, motility, and viability by suppressing receptors for advanced glycation end product-induced oxidative stress and inflammation in the testes of diabetic mice. Int J Mol Sci. (2024) 25:5947. doi: 10.3390/ijms25115947
36. Wang F, Liu W, Jiang Q, Gong M, Chen R, Wu H, et al. Lipopolysaccharide-induced testicular dysfunction and epididymitis in mice: a critical role of tumor necrosis factor alpha†. Biol Reprod. (2019) 100:849–61. doi: 10.1093/biolre/ioy235
37. Mai Z, Yang D, Wang D, Zhang J, Zhou Q, Han B, et al. A narrative review of mitochondrial dysfunction and male infertility. Transl Androl Urol. (2024) 13:2134–45. doi: 10.21037/tau-24-262
38. Fujisawa Y, Kikuchi S, Kuba F, Oishi K, Murayama S, Sugiyama T, et al. Ectopic expression of the mitochondrial protein COXFA4L3 in human sperm acrosome and its potential application in the selection of male infertility treatments. Reprod Med Biol. (2024) 23:e12602. doi: 10.1002/rmb2.12602
39. Ayad B, Omolaoye TS, Louw N, Ramsunder Y, Skosana BT, Oyeipo PI, et al. Oxidative stress and male infertility: evidence from a research perspective. Front Reprod Health. (2022) 4:822257. doi: 10.3389/frph.2022.822257
40. Lopes-Ferreira JV, Matos JEM, Dias FCR, Siervo G, and Gomes MLM. Protective effects of phenolic phytochemicals on male fertility: a narrative review. Braz J Biol. (2025) 85:e288879. doi: 10.1590/1519-6984.288879
41. Pashapour S, Saberivand A, Khaki AA, and Saberivand M. Effect of saponin on spermatogenesis and testicular structure in streptozotocin-induced diabetic mice. Vet Res Forum. (2023) 14:601–6. doi: 10.30466/vrf.2023.1986019.3727
42. Oghbaei H, Fattahi A, Hamidian G, Sadigh-Eteghad S, Ziaee M, and Mahmoudi J. A closer look at the role of insulin for the regulation of male reproductive function. Gen Comp Endocrinol. (2021) 300:113643. doi: 10.1016/j.ygcen.2020.113643
43. Hammoud AO, Meikle AW, Reis LO, Gibson M, Peterson CM, and Carrell DT. Obesity and male infertility: a practical approach. Semin Reprod Med. (2012) 30:486–95. doi: 10.1055/s-0032-1328877
44. Khorrami A, Ghanbarzadeh S, Ziaee M, Arami S, Vajdi R, and Garjani A. Dietary cholesterol and oxidised cholesterol: effects on sperm characteristics, antioxidant status and hormonal profile in rats. Andrologia. (2015) 47:310–7. doi: 10.1111/and.12262
45. Darmishonnejad Z, Hassan-Zadeh V, Tavalaee M, Kobarfard F, Gharagozloo P, Drevet JR, et al. Effects of acute exposure to methylglyoxal or/and A diet rich in advanced glycation end products on sperm parameters in mice. Int J Fertil Steril. (2024) 18:263–70. doi: 10.22074/ijfs.2023.2005832.1485
46. Darmishonnejad Z, Zadeh VH, Tavalaee M, Kobarfard F, Hassani M, Gharagozloo P, et al. Effect of advanced glycation end products (AGEs) on sperm parameters and function in C57Bl/6 mice. Reprod Sci. (2024) 31:2114–22. doi: 10.1007/s43032-024-01507-w
47. Rindone GM, Gorga A, Pellizzari EH, Camberos MDC, Galardo MN, Da Ros VG, et al. Postnatal metformin treatment alters rat Sertoli cell proliferation and daily sperm production. Andrology. (2021) 9:965–76. doi: 10.1111/andr.12957
48. Alves MG, Martins AD, Cavaco JE, Socorro S, and Oliveira PF. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers. (2013) 1:e23992. doi: 10.4161/tisb.23992
49. Ribeiro JC, Martins AD, Jarak I, Carvalho RA, Alves MG, and Oliveira PF. Exenatide and dapagliflozin combination enhances sertoli cell secretion of key metabolites for spermatogenesis. Biomedicines. (2022) 10:1115. doi: 10.3390/biomedicines10051115
50. Song K, Yang X, An G, Xia X, Zhao J, Xu X, et al. Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models. Nat Commun. (2022) 13:7335. doi: 10.1038/s41467-022-34990-3
51. Cheng CY and Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. (2012) 64:16–64. doi: 10.1124/pr.110.002790
52. Wanjari UR and Gopalakrishnan AV. Blood-testis barrier: a review on regulators in maintaining cell junction integrity between Sertoli cells. Cell Tissue Res. (2024) 396:157–75. doi: 10.1007/s00441-024-03894-7
53. Miller SR and Cherrington NJ. Transepithelial transport across the blood-testis barrier. Reproduction. (2018) 156:R187–r94. doi: 10.1530/rep-18-0338
54. Stanton PG. Regulation of the blood-testis barrier. Semin Cell Dev Biol. (2016) 59:166–73. doi: 10.1016/j.semcdb.2016.06.018
55. Mruk DD and Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. (2015) 36:564–91. doi: 10.1210/er.2014-1101
56. Liu M, He Q, Yuan Z, Chen N, Ren S, Du Q, et al. HDAC3 promotes Sertoli cell maturation and maintains the blood-testis barrier dynamics. FASEB J. (2024) 38:e23526. doi: 10.1096/fj.202301349RR
57. Abdel-Wahab BA, El-Shoura EAM, Habeeb MS, Aldabaan NA, Ahmed YH, and Zaafar D. Piperazine ferulate impact on diabetes-induced testicular dysfunction: unveiling genetic insights, MAPK/ERK/JNK pathways, and TGF-β signaling. Naunyn Schmiedebergs Arch Pharmacol. (2025) 398:6719–37. doi: 10.1007/s00210-024-03654-y
58. Ermetici F, Donadio F, Iorio L, Malavazos AE, Dolci A, Peverelli E, et al. Peripheral insulin-like factor 3 concentrations are reduced in men with type 2 diabetes mellitus: effect of glycemic control and visceral adiposity on Leydig cell function. Eur J Endocrinol. (2009) 161:853–9. doi: 10.1530/eje-09-0203
59. Zhao YT, Qi YW, Hu CY, Chen SH, and Liu Y. Advanced glycation end products inhibit testosterone secretion by rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress. Int J Mol Med. (2016) 38:659–65. doi: 10.3892/ijmm.2016.2645
60. Du Z, Xu S, Hu S, Yang H, Zhou Z, Sidhu K, et al. Melatonin attenuates detrimental effects of diabetes on the niche of mouse spermatogonial stem cells by maintaining Leydig cells. Cell Death Dis. (2018) 9:968. doi: 10.1038/s41419-018-0956-4
61. Jiao N, Chen Y, Zhu Y, Wang W, Liu M, Ding W, et al. Protective effects of catalpol on diabetes mellitus-induced male reproductive damage via suppression of the AGEs/RAGE/Nox4 signaling pathway. Life Sci. (2020) 256:116736. doi: 10.1016/j.lfs.2019.116736
62. Chen Y, Wu Y, Gan X, Liu K, Lv X, Shen H, et al. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J Ethnopharmacol. (2016) 194:850–60. doi: 10.1016/j.jep.2016.10.079
63. Tahara N, Imaizumi T, Takeuchi M, and Yamagishi S. Insulin resistance is an independent correlate of high serum levels of advanced glycation end products (AGEs) and low testosterone in non-diabetic men. Oxid Med Cell Longev. (2010) 3:262–5. doi: 10.4161/oxim.3.4.12734
64. Kaltsas A, Koumenis A, Stavropoulos M, Kratiras Z, Deligiannis D, Adamos K, et al. Male infertility and reduced life expectancy: epidemiology, mechanisms, and clinical implications. J Clin Med. (2025) 14:3930. doi: 10.3390/jcm14113930
65. Derkach KV, Bakhtyukov AA, Romanova IV, Zorina II, Bayunova LV, Bondareva VM, et al. The effect of metformin treatment on the basal and gonadotropin-stimulated steroidogenesis in male rats with type 2 diabetes mellitus. Andrologia. (2020) 52:e13816. doi: 10.1111/and.13816
66. Nna VU, Bakar ABA, Ahmad A, and Mohamed M. Down-regulation of steroidogenesis-related genes and its accompanying fertility decline in streptozotocin-induced diabetic male rats: ameliorative effect of metformin. Andrology. (2019) 7:110–23. doi: 10.1111/andr.12567
67. Padwal LP, More A, Choudhary N, and Kalasakar GL. Study protocol: evaluating the impact of diabetic medications on semen quality: A comparative analysis of diabetic patients and nondiabetic controls with correlation to fertility potential. J Pharm Bioallied Sci. (2025) 17:S932–s5. doi: 10.4103/jpbs.jpbs_316_25
68. Zhou L, Dong M, Feng G, Zhang Y, Wang J, Kang H, et al. Semaglutide mitigates testicular damage in diabetes by inhibiting ferroptosis. Biochem Biophys Res Commun. (2024) 715:149996. doi: 10.1016/j.bbrc.2024.149996
69. Abdel-Wahab BA, El-Shoura EAM, Habeeb MS, Aldabaan NA, Ahmed YH, and Zaafar D. Unraveling the impact of semaglutide in a diabetic rat model of testicular dysfunction: Insights into spermatogenesis pathways and miRNA-148a-5p. Steroids. (2025) 213:109537. doi: 10.1016/j.steroids.2024.109537
70. Dasso ME, Centola CL, Galardo MN, Meroni SB, and Riera MF. Glucagon-like peptide-1 analog, liraglutide, regulates Sertoli cell energy metabolism. J Endocrinol. (2024) 263:e240274. doi: 10.1530/joe-24-0274
71. Harby SA, Fathelbab MH, Nawwar BM, Sheta E, Halwag DI, Elneily DAE, et al. Liraglutide and denatonium benzoate attenuate T2DM-induced metabolic, neurological, and testicular changes in rats: Targeting oxidative stress, inflammation, and BCRP transporter. J Mol Histol. (2025) 56:78. doi: 10.1007/s10735-025-10355-0
72. Jensterle M, Podbregar A, Goricar K, Gregoric N, and Janez A. Effects of liraglutide on obesity-associated functional hypogonadism in men. Endocr Connect. (2019) 8:195–202. doi: 10.1530/ec-18-0514
73. La Vignera S, Condorelli RA, Calogero AE, Cannarella R, and Aversa A. Sexual and reproductive outcomes in obese fertile men with functional hypogonadism after treatment with liraglutide: preliminary results. J Clin Med. (2023) 12:672. doi: 10.3390/jcm12020672
74. Ansari Z, Maleki MH, Roohy F, Ebrahimi Z, Shams M, Mokaram P, et al. Protective effects of artichoke extract and Bifidobacterium longum on male infertility in diabetic rats. Biochem Biophys Rep. (2024) 40:101834. doi: 10.1016/j.bbrep.2024.101834
75. Bayat M, Koohpeyma F, Montazeri-Najafabady N, Dabbaghmanesh MH, Asmarian N, and Hosseini SI. The effects of modest intake of soy milk enriched with Lactobacillus casei and omega-3 on the testis parameters in diabetic rats: a stereological study. Int Urol Nephrol. (2025) 57:1123–33. doi: 10.1007/s11255-024-04243-x
76. El-Baz AM, Shata A, Hassan HM, El-Sokkary MMA, and Khodir AE. The therapeutic role of lactobacillus and montelukast in combination with metformin in diabetes mellitus complications through modulation of gut microbiota and suppression of oxidative stress. Int Immunopharmacol. (2021) 96:107757. doi: 10.1016/j.intimp.2021.107757
77. Oliveira L, Costa EC, Martins FDG, Rocha ASD, and Brasil GA. Probiotics supplementation in the treatment of male infertility: A Systematic Review. JBRA Assist Reprod. (2024) 28:341–8. doi: 10.5935/1518-0557.20240013
78. Maretti C and Cavallini G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: a pilot study. Andrology. (2017) 5:439–44. doi: 10.1111/andr.12336
79. Helli B, Kavianpour M, Ghaedi E, Dadfar M, and Haghighian HK. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum Fertil (Camb). (2022) 25:499–507. doi: 10.1080/14647273.2020.1824080
80. Abbasi B, Abbasi H, and Niroumand H. Synbiotic (FamiLact) administration in idiopathic male infertility enhances sperm quality, DNA integrity, and chromatin status: A triple-blinded randomized clinical trial. Int J Reprod Biomed. (2021) 19:235–44. doi: 10.18502/ijrm.v19i3.8571
81. Elshafey M, Erfan OS, Risha E, Badawy AM, Ebrahim HA, El-Sherbiny M, et al. Protective effect of Stevia on diabetic induced testicular damage: an immunohistochemical and ultrastructural study. Eur Rev Med Pharmacol Sci. (2023) 27:11039–56. doi: 10.26355/eurrev_202311_34473
82. Faheem H, Alawadhi R, Basha EH, Ismail R, Ibrahim HA, Elshamy AM, et al. Ameliorating immune-dependent inflammation and apoptosis by targeting TLR4/MYD88/NF-κB pathway by celastrol mitigates the diabetic reproductive dysfunction. Physiol Genomics. (2025) 57:103–14. doi: 10.1152/physiolgenomics.00072.2024
83. Hejazi S, Rasekh M, Taghdisi A, Sabet A, Maroufi MM, Taghinasab S, et al. The effect of silymarin on diabetes mellitus-induced male rats reproductive impairment: Evidences for role of heat shock proteins 70 and 90. Pol J Vet Sci. (2024) 27:631–40. doi: 10.24425/pjvs.2024.152953
84. Guo Q, Li TF, Huang J, Li JC, Zhang ZC, and Qu YL. The protective role of phlorizin against lipopolysaccharide-induced acute orchitis in mice associated with changes in gut microbiota composition. Front Vet Sci. (2024) 11:1340591. doi: 10.3389/fvets.2024.1340591
85. Liu F, Liao B, Ling YL, Meng XZ, Wang JL, Hu LL, et al. Icariin protects testicular damage in streptozotocin-induced diabetic rats through regulation of glycolysis pathway. Int J Immunopathol Pharmacol. (2024) 38:3946320241279525. doi: 10.1177/03946320241279525
86. Lei X, Huo P, Xie YJ, Wang Y, Liu G, Tu H, et al. Dendrobium nobile Lindl polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats. Mol Reprod Dev. (2022) 89:202–13. doi: 10.1002/mrd.23556
87. Emil AB, Hassan NH, Ibrahim S, Hassanen EI, Eldin ZE, and Ali SE. Propolis extract nanoparticles alleviate diabetes-induced reproductive dysfunction in male rats: antidiabetic, antioxidant, and steroidogenesis modulatory role. Sci Rep. (2024) 14:30607. doi: 10.1038/s41598-024-81949-z
88. Zhou J, Xi Y, Zhang J, Tang J, Zhou X, Chen J, et al. Protective effect of Dioscorea zingiberensis ethanol extract on the disruption of blood-testes barrier in high-fat diet/streptozotocin-induced diabetic mice by upregulating ZO-1 and Nrf2. Andrologia. (2020) 52:e13508. doi: 10.1111/and.13508
89. Liu L, Shu A, Zhu Y, and Chen Y. Cornuside alleviates diabetes mellitus-induced testicular damage by modulating the gut microbiota. Evid Based Complement Alternat Med. (2021) 2021:5301942. doi: 10.1155/2021/5301942
90. Yuan S, Zhang Y, Dong PY, Chen Yan YM, Liu J, Zhang BQ, et al. A comprehensive review on potential role of selenium, selenoproteins and selenium nanoparticles in male fertility. Heliyon. (2024) 10:e34975. doi: 10.1016/j.heliyon.2024.e34975
91. Ebokaiwe AP, Obeten KE, Okori SO, David EE, Olusanya O, Chukwu CJ, et al. Co-administration of selenium nanoparticles and metformin abrogate testicular oxidative injury by suppressing redox imbalance, augmenting sperm quality and nrf2 protein expression in streptozotocin-induced diabetic rats. Biol Trace Elem Res. (2020) 198:544–56. doi: 10.1007/s12011-020-02082-2
92. El-Behery EI, El-Naseery NI, El-Ghazali HM, Elewa YHA, Mahdy EAA, El-Hady E, et al. The efficacy of chronic zinc oxide nanoparticles using on testicular damage in the streptozotocin-induced diabetic rat model. Acta Histochem. (2019) 121:84–93. doi: 10.1016/j.acthis.2018.10.010
93. Shaman AA, Zidan NS, Atteia HH, Alalawy AI, Alzahrani S, AlBishi LA, et al. Arthrospira platensis nanoparticles defeat against diabetes-induced testicular injury in rat targeting, oxidative, apoptotic, and steroidogenesis pathways. Andrologia. (2022) 54:e14456. doi: 10.1111/and.14456
94. Awad AM, Elshaer SL, Gangaraju R, Abdelaziz RR, and Nader MA. CysLTR1 antagonism by montelukast can ameliorate diabetes-induced aortic and testicular inflammation. Int Immunopharmacol. (2023) 125:111127. doi: 10.1016/j.intimp.2023.111127
95. Mu Y, Luo LB, Wu SJ, Gao Y, Qin XL, Zhao J, et al. Bezafibrate alleviates diabetes-induced spermatogenesis dysfunction by inhibiting inflammation and oxidative stress. Heliyon. (2024) 10:e28284. doi: 10.1016/j.heliyon.2024.e28284
96. Naderi R, Pourheydar B, and Moslehi A. Tropisetron improved testicular inflammation in the streptozotocin-induced diabetic rats: The role of toll-like receptor 4 (TLR4) and mir146a. J Biochem Mol Toxicol. (2023) 37:e23272. doi: 10.1002/jbt.23272
97. Samaha MM and Nour OA. Ranolazine ameliorates T1DM-induced testicular dysfunction in rats; role of NF-κB/TXNIP/GSDMD-N/IL-18/Beclin-1 signaling pathway. Eur J Pharmacol. (2024) 977:176744. doi: 10.1016/j.ejphar.2024.176744
98. Shi W, Guo Z, and Yuan R. Testicular injury attenuated by rapamycin through induction of autophagy and inhibition of endoplasmic reticulum stress in streptozotocin- induced diabetic rats. Endocr Metab Immune Disord Drug Targets. (2019) 19:665–75. doi: 10.2174/1871530319666190102112844
99. Zheng H, Hu Y, Shao M, Chen S, and Qi S. Chromium Picolinate Protects against Testicular Damage in STZ-Induced Diabetic Rats via Anti-Inflammation, Anti-Oxidation, Inhibiting Apoptosis, and Regulating the TGF-β1/Smad Pathway. Molecules. (2023) 28:7669. doi: 10.3390/molecules28227669
100. Zheng YC, Feng YL, Wang YH, Kong LJ, Zhou MS, Wu MM, et al. Islet transplantation ameliorates diabetes-induced testicular interstitial fibrosis and is associated with inhibition of TGF-β1/Smad2 pathway in a rat model of type 1 diabetes. Mol Med Rep. (2021) 23:376. doi: 10.3892/mmr.2021.12015
101. Zhu X, Guo F, Tang H, Huang C, Xie G, Huang T, et al. Islet transplantation attenuating testicular injury in type 1 diabetic rats is associated with suppression of oxidative stress and inflammation via nrf-2/HO-1 and NF-κB pathways. J Diabetes Res. (2019) 2019:8712492. doi: 10.1155/2019/8712492
102. Li H, Zhang M, Ma J, Li W, Liu X, Li Y, et al. Zinc combined with metformin corrects zinc homeostasis and improves steroid synthesis and semen quality in male type 2 diabetic mice by activating PI3K/AKT/mTOR pathway. Biol Trace Elem Res. (2025) 203:4659–70. doi: 10.1007/s12011-025-04518-z
103. Odetayo AF, Abdulrahim HA, Yusuf AM, Aromokhame WO, Olaitan AM, Ugoji MC, et al. Combination therapy with vitamin D and metformin: A potential approach to mitigate testicular dysfunction in type 2 diabetes mellitus. Reprod Sci. (2024) 31:3795–807. doi: 10.1007/s43032-024-01708-3
104. Cheraghi Abajlou S, Tofighi A, Tolouei Azar J, Khaki AA, and Razi M. Combined effects of chrysin supplementation and exercise training on diabetes-induced oxidative stress and apoptosis in rat testicular tissue. Int J Fertil Steril. (2025) 19:88–95. doi: 10.22074/ijfs.2024.2019906.1606
105. Mohamed MZ, Hafez HM, Zenhom NM, and Mohammed HH. Cilostazol alleviates streptozotocin-induced testicular injury in rats via PI3K/Akt pathway. Life Sci. (2018) 198:136–42. doi: 10.1016/j.lfs.2018.02.038
106. Oltulu C, Ersoy O, Akinci M, Cevikelli-Yakut ZA, Dasman M, and Bakar E. Effects of sitagliptin and L-theanine combination therapy on testicular tissue in rats with experimental diabetes. Toxicol Appl Pharmacol. (2024) 492:117119. doi: 10.1016/j.taap.2024.117119
107. Jin T, Li F, Wei W, Li Q, Gao Y, Yuwen C, et al. SDF2L1 downregulation mediates high glucose-caused Schwann cell dysfunction by inhibiting nuclear import of TFEB and CREB via KPNA3. Exp Neurol. (2025) 390:115273. doi: 10.1016/j.expneurol.2025.115273
108. Liu FY, Cho YL, Fridayana FR, Niloofar L, Vo MN, Huang Y, et al. MT-100, a human Tie2-agonistic antibody, improves penile neurovasculature in diabetic mice via the novel target Srpx2. Exp Mol Med. (2025) 57:104–17. doi: 10.1038/s12276-024-01373-1
109. Jena L, Kaur P, Singh T, Sharma K, Kotru S, and Munshi A. Gene expression analysis in T2DM and its associated microvascular diabetic complications: focus on risk factor and RAAS pathway. Mol Neurobiol. (2024) 61:8656–67. doi: 10.1007/s12035-024-04127-2
110. Nakadate K, Ito N, Kawakami K, and Yamazaki N. Anti-inflammatory actions of plant-derived compounds and prevention of chronic diseases: from molecular mechanisms to applications. Int J Mol Sci. (2025) 26:5206. doi: 10.3390/ijms26115206
111. Jalili C, Zamir Nasta T, Makalani F, Davoodi E, and Tabandeh MR. Mitigation of hyperglycemia-induced leydig cell dysfunction by gut derived indol 3- propionic acid: Targeting apoptosis, endoplasmic reticulum stress response and steroidogenesis. Reprod Toxicol. (2025) 137:108987. doi: 10.1016/j.reprotox.2025.108987
Keywords: diabetes mellitus, oxidative stress, AGEs, testicular damage, spermatogenesis impairment
Citation: Zhang W, Tong L, Jin B and Sun D (2025) Diabetic testicular dysfunction and spermatogenesis impairment: mechanisms and therapeutic prospects. Front. Endocrinol. 16:1653975. doi: 10.3389/fendo.2025.1653975
Received: 25 June 2025; Accepted: 04 August 2025;
Published: 25 August 2025.
Edited by:
Mohammad Ishraq Zafar, Zhejiang University, ChinaReviewed by:
Andrea Delbarba, Asst degli Spedali Civili di Brescia, ItalyIbrahim Mehmet Tuğlu, Manisa Celal Bayar University, Türkiye
Copyright © 2025 Zhang, Tong, Jin and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baofang Jin, aGV4aWtpbmdAMTI2LmNvbQ==; Dalin Sun, ZGFsaW5nMTFAMTI2LmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Li Tong, orcid.org/0000-0002-9557-8205
 Wenxiu Zhang
Wenxiu Zhang Li Tong1†‡
Li Tong1†‡ Baofang Jin
Baofang Jin Dalin Sun
Dalin Sun