- 1Department of Obstetrics and Gynecology, Dongguan Maternal and Child Health Care Hospital, Dongguan, Guangdong, China
- 2The First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, China
- 3State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, Macao SAR, China
- 4Department of Obstetrics and Gynecology, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, Guangdong Provincial Clinical Research Center for Obstetrics and Gynecology, Guangzhou Medical University, Guangzhou, China
- 5Guangdong-Hong Kong-Macao Greater Bay Area Higher Education Joint Laboratory of Maternal-Fetal Medicine, The Third Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
Background: The impact of hypothyroidism, which can be divided into overt hypothyroidism and subclinical hypothyroidism, on severe postpartum hemorrhage (SPPH) remains unknown.
Methods: A total of 34,303 pregnant women from the Third Affiliated Hospital of Guangzhou Medical University between 2016 and 2020 were included in this retrospective cohort study. We employed three logistic regression models incorporating different covariates to explore the relationship between hypothyroidism and SPPH, followed by an interaction analysis to identify potential modifiers. Then, we performed a stratified analysis to examine the influence of potential modifiers on the association between hypothyroidism and SPPH. Finally, sensitivity analyses were conducted to evaluate the robustness of our findings.
Results: In the logistic regression analysis, we found that hypothyroidism was correlated with an elevated risk of SPPH (OR = 1.609, 95% CI: 1.111–2.329, p = 0.012). In addition, overt hypothyroidism had a significant impact on the increased risk of SPPH (OR = 1.688, 95% CI: 1.137–2.507, p = 0.009), whereas no significant association with SPPH was observed for subclinical hypothyroidism (OR = 1.208, 95% CI: 0.443–3.291, p = 0.712). In the relationship between hypothyroidism and SPPH, we observed that age and history of radiation exposure acted as potential modifiers (p for interaction < 0.05). Additionally, the correlation between hypothyroidism and SPPH was stronger in pregnant women aged < 35 years (OR = 2.412, 95% CI: 1.583–3.673, p < 0.001) than in those aged ≥ 35 years (OR = 0.755, 95% CI: 0.364–1.567, p = 0.450).
Conclusions: Overt hypothyroidism had a significant impact on the elevated risk of SPPH, especially in pregnant women aged < 35 years. In contrast, subclinical hypothyroidism showed no significant association with SPPH, which may reflect limited statistical power rather than the absence of risk. These findings offer valuable insights into the relationship between hypothyroidism and SPPH, potentially optimizing maternal outcomes by preventing and intervening in the occurrence of SPPH.
1 Introduction
Postpartum hemorrhage (PPH), a major contributor to maternal mortality and severe morbidity, accounts for nearly a third of the deaths among pregnant and postpartum women (1, 2). Severe postpartum hemorrhage (SPPH) can lead to complications such as hemorrhagic shock, disseminated intravascular coagulation, acute renal failure, loss of fertility, pituitary necrosis (Sheehan syndrome), and even maternal or neonatal death (3). In recent years, the incidence of PPH incidence has been on the rise, with an increase of at least 26% over the past decade in the United States (4).
Maternal hypothyroidism is a common endocrine disorder of pregnancy, with a steadily increasing incidence (5). Overt hypothyroidism is estimated to affect 0.3–1.0% of pregnancies, while subclinical hypothyroidism ranges from 4.0% to 17.8% (6). Hypothyroidism, both overt and subclinical, have been associated with adverse maternal outcomes, including placental abruption (7, 8), polyhydramnios (8, 9), gestational diabetes mellitus (5, 7), and premature rupture of membranes (5, 10).
However, the relationship between hypothyroidism and PPH, particularly SPPH, is less frequently discussed. Some studies suggested that pregnant women with hypothyroidism had a higher risk of PPH. A Canadian study (5), involving more than 18,400 participants, reported that women with hypothyroidism were more likely to experience PPH during labor. Similarly, Kiran et al. (11) showed a significant association between PPH and hypothyroidism. On the contrary, Gur et al. (12) and Wang et al. (13) indicated that no statistical difference was observed in the incidence of PPH between hypothyroidism and euthyroid pregnant women. In these studies, PPH was defined as a blood loss of 500 mL or more within 24 hours after birth. The research data on hypothyroidism and SPPH remain very limited.
Thus, we aimed to investigate the relationship between hypothyroidism (including overt and subclinical hypothyroidism) and SPPH through a retrospective cohort study, utilizing data from the institutional medical record database of all women delivering at the Third Affiliated Hospital of Guangzhou Medical University from 2016 to 2020. This work could offer valuable insights into the influence of hypothyroidism on SPPH, potentially optimizing maternal outcomes by preventing and intervening in its occurrence.
2 Materials and methods
2.1 Study participants
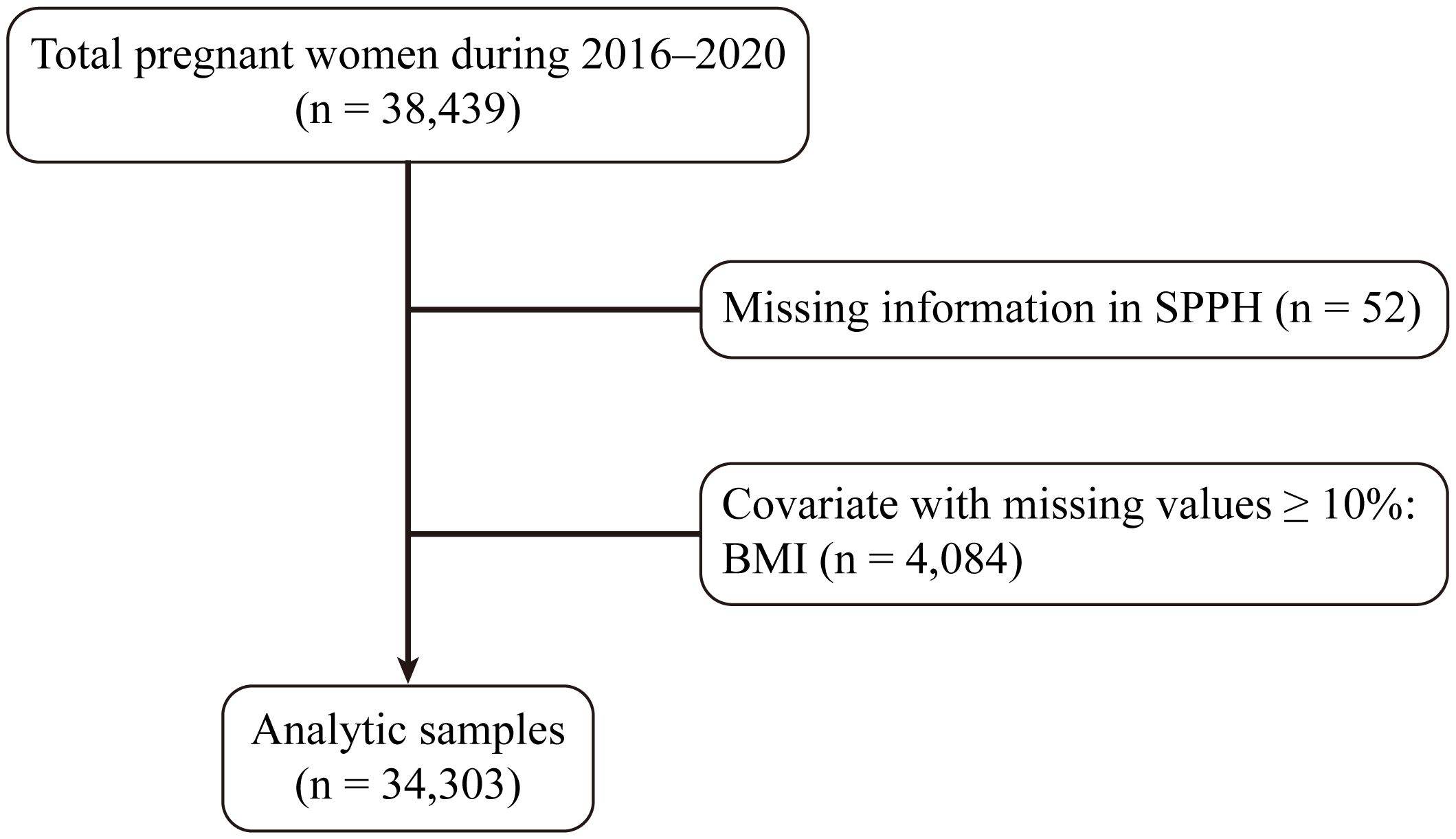
Ethical approval (approval number: [2024] 142) was obtained for this analysis and each participant signed informed consent. This study examined the institutional database encompassing all women who gave birth at the Third Affiliated Hospital of Guangzhou Medical University (Guangzhou Medical Centre for Critical Pregnant Women) from January 2016 to December 2020. In total, 38,439 women were included in the analysis. Women lacking SPPH information (n = 52) were excluded from the analysis. Additionally, pregnancies with missing values comprising ≥ 10% of covariates, such as pregestational body mass index (BMI) (n = 4,084), were removed. Subsequently, missing values for covariates comprising < 10% were imputed using the random forest method. Thus, our final analysis included a total of 34,303 pregnant women (Figure 1).
2.2 Definitions
The outcome of the research focused on SPPH, defined as an estimated hemorrhage of ≥ 1,000 mL within 24 hours after childbirth. Blood loss assessment was based on a combination of visual estimation, gravimetric method, estimated blood loss volume, shock index, and hemoglobin levels, as documented by the attending healthcare providers, including physicians, midwives, and nurses. The final estimated value was recorded by the resident physician who attended the delivery.
The diagnosis of hypothyroidism during pregnancy was based on the Guidelines on Diagnosis and Management of Thyroid Diseases during Pregnancy and Postpartum (14, 15). Overt hypothyroidism was defined as serum thyroid-stimulating hormone (TSH) levels above the trimester-specific reference ranges with free thyroxine (FT4) levels below the corresponding lower limit, while subclinical hypothyroidism was defined as elevated TSH levels with normal FT4. Measurements of TSH and FT4 were performed employing a chemiluminescent immunoassay with reagents from Abbott Laboratories. Trimester-specific reference ranges are shown in Supplementary Tables 1, 2. Laboratory test reports from hospitals of equivalent level were also acceptable. Cases of hypothyroidism were ascertained using the corresponding International Classification of Diseases (ICD) codes from the electronic health records. Regardless of thyroid peroxidase antibody (TPOAb) status (positive or negative), all pregnant women diagnosed with hypothyroidism routinely received levothyroxine (LT4) therapy, with dose adjustment to maintain TSH within the guideline-recommended levels.
2.3 Covariates
To reduce potential biases in our analysis, we chose covariates based on previous research findings and clinical plausibility. The analysis incorporated the following covariates: age, pregestational body mass index (BMI), education level, history of radiation exposure, previous cesarean delivery, number of fetuses, gestational age, birth weight, head circumference of newborns, stillbirth, hypertensive disorders of pregnancy, HELLP syndrome, diabetes, intrahepatic cholestasis of pregnancy (ICP), anemia, thrombocytopenia, placenta previa, placental abruption, placenta accreta spectrum (PAS), polyhydramnios, fetal anomalies, malposition, cesarean delivery, prolonged second stage of labor, and fetal distress. Full list of covariates investigated in analysis are provided in Supplementary Table 3.
2.4 Statistical analysis
Continuous data with a normal distribution were presented as mean (± standard deviation [SD]) and were compared using the independent samples t-test. Variables with skewed distributions were expressed as medians [first quartile (P25) and third quartile (P75)] and were compared using the nonparametric Wilcoxon rank-sum test. Categorical variables were expressed as percentages (%) and were compared using either the chi-square test or Fisher’s exact test, as appropriate. We utilized three logistic regression models to explore the impact of overt or subclinical hypothyroidism on SPPH. Model 1 did not adjust for any covariates. Model 2 made adjustments for age, pregestational BMI, education level, history of radiation exposure, previous cesarean delivery, number of fetuses, gestational age, and birth weight. Model 3 further adjusted for head circumference of newborns, stillbirth, hypertensive disorders of pregnancy, HELLP syndrome, diabetes, ICP, anemia, thrombocytopenia, placenta previa, placental abruption, PAS, polyhydramnios, fetal anomalies, malposition, cesarean delivery, prolonged second stage of labor, and fetal distress based on Model 2. Subsequently, we conducted an interaction analysis, finding that age and history of radiation exposure acted as potential modifiers in the relationship between hypothyroidism and SPPH. Considering the relatively small number of patients with SPPH in the history of radiation exposure, stratified analysis was done only for age. To examine the influence of age on the association between hypothyroidism and SPPH, we stratified the study cohort based on age, employing a cut-off threshold of 35 years. Furthermore, sensitivity analyses were conducted to evaluate the robustness of our findings; In one analysis, participants who delivered twins or triplets were excluded, while in the other sensitivity analysis, participants with a history of radiation exposure and an unknown radiation exposure history were removed. All p values reported were two-sided, with a significance level established at 0.05. Statistical analyses were performed using IBM SPSS Statistics 26.
3 Results
3.1 Population characteristics
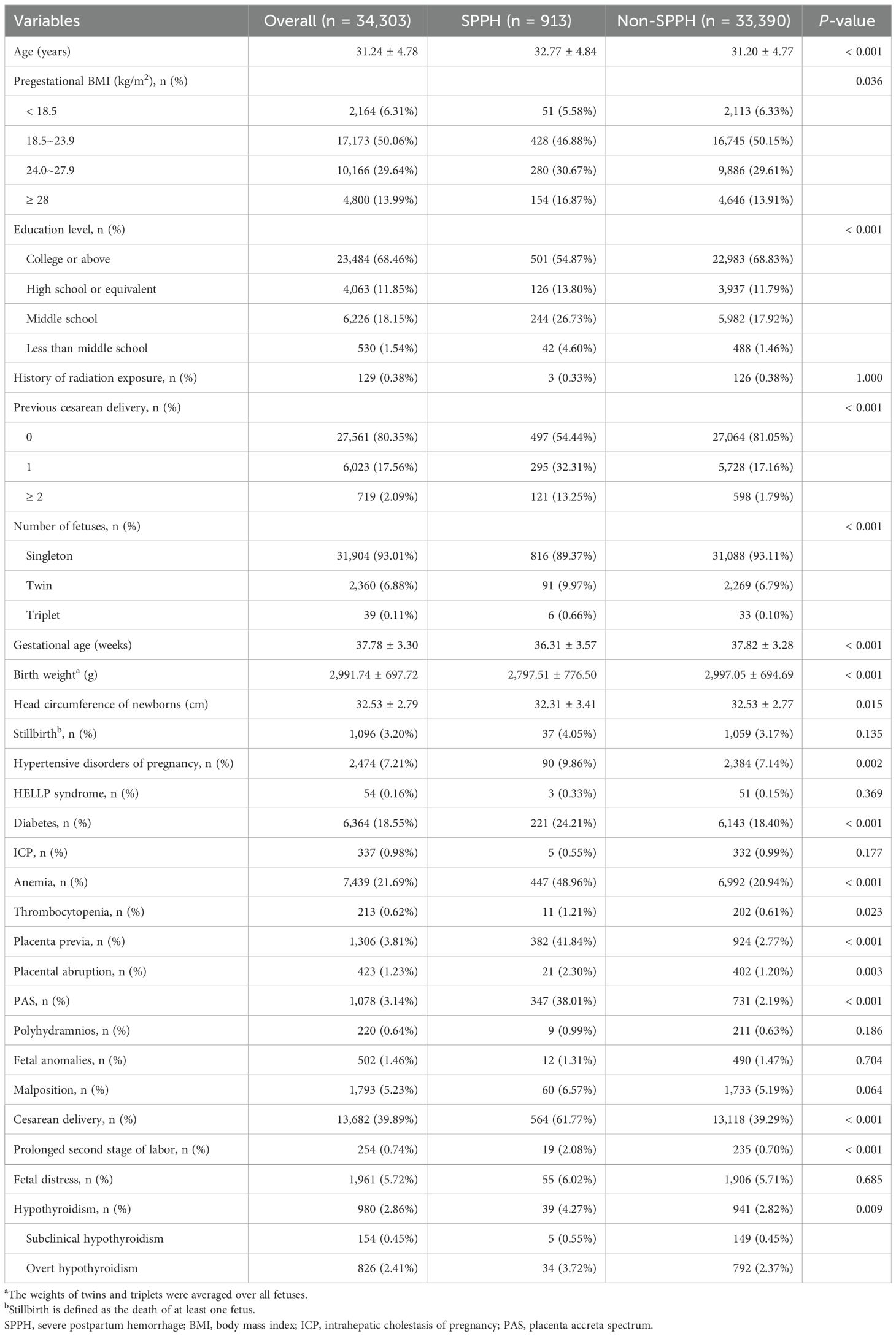
The baseline characteristics of the study population are shown in Table 1. In our analysis, we included 34,303 pregnant women, among whom 913 were diagnosed with SPPH, and the average age of the participants with SPPH was 32.77 ± 4.84 years. Overweight women (pregestational BMI exceeding 23.9) were more frequently observed in the SPPH group. Low educational level, history of radiation exposure, and previous cesarean delivery were more common among pregnant women with SPPH. Furthermore, the SPPH group exhibited higher rates of hypertensive disorders of pregnancy, diabetes, anemia, thrombocytopenia, placenta previa, placental abruption, PAS, cesarean delivery, prolonged second stage of labor, and hypothyroidism. Baseline characteristics stratified by thyroid status are provided in Supplementary Table 4.
3.2 Impact of overt and subclinical hypothyroidism on SPPH
Table 2 illustrates the association between hypothyroidism and SPPH using three logistic regression models. In model 1, a positive association between hypothyroidism and SPPH was observed (odds ratio [OR] = 1.539, 95% confidence interval [CI]: 1.109–2.134, p = 0.009). Upon dividing hypothyroidism into overt hypothyroidism and subclinical hypothyroidism, it was found that compared to controls, the OR for SPPH with overt hypothyroidism and subclinical hypothyroidism were 1.594 (95% CI: 1.123–2.261, p = 0.009) and 1.246 (95% CI: 0.510–3.045, p = 0.630), respectively. After adjusting for age, pregestational BMI, education level, history of radiation exposure, previous cesarean delivery, number of fetuses, gestational age and birth weight in model 2, it was found that the association between hypothyroidism and increased SPPH risk persisted (OR = 1.477, 95% CI: 1.056–2.066, p = 0.023). Consistent with the findings of model 1, overt hypothyroidism was associated with an increased risk of SPPH (OR = 1.587, 95% CI: 1.109–2.271, p = 0.012) while subclinical hypothyroidism didn’t have a prominent impact on SPPH (OR = 0.993, 95% CI: 0.397–2.483, p = 0.988), compared to controls. Moreover, model 3 further adjusted for head circumference of newborns, stillbirth, hypertensive disorders of pregnancy, HELLP syndrome, diabetes, ICP, anemia, thrombocytopenia, placenta previa, placental abruption, PAS, polyhydramnios, fetal anomalies, malposition, cesarean delivery, prolonged second stage of labor, and fetal distress based on model 2. Subsequently, we observed a consistent finding that hypothyroidism (OR = 1.609, 95% CI: 1.111–2.329, p = 0.012), particularly overt hypothyroidism (OR = 1.688, 95% CI: 1.137–2.507, p = 0.009), was correlated with an elevated risk of SPPH. Nevertheless, subclinical hypothyroidism showed no significant association with SPPH.
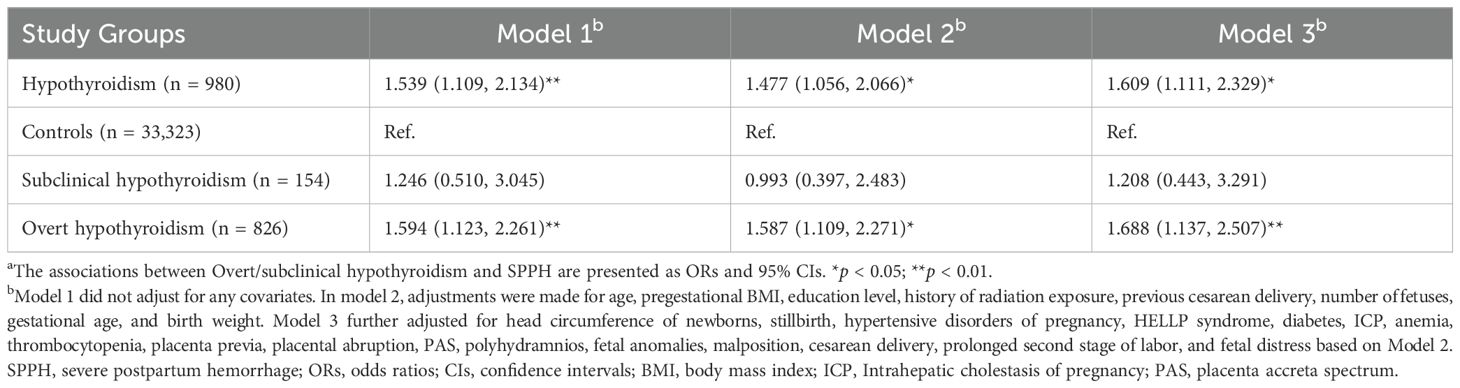
Table 2. Association between overt/subclinical hypothyroidism and SPPH by logistic modelsa.
3.3 Stratified analysis
We conducted an interaction analysis and found that age and history of radiation exposure acted as potential modifiers in the relationship between hypothyroidism and SPPH (p for interaction < 0.05). Due to the relatively small number of patients with SPPH in the history of radiation exposure, stratified analysis was performed only for age. Table 3 indicates the correlation between hypothyroidism and SPPH stratified by age, using 35 years as the cut-off (p for interaction < 0.05). The ORs were stronger in women aged < 35 years (OR = 2.412, 95% CI: 1.583–3.673, p < 0.001) compared to those aged ≥ 35 years (OR = 0.755, 95% CI: 0.364–1.567, p = 0.450). For participants aged < 35 years, the ORs for SPPH with overt hypothyroidism and subclinical hypothyroidism were 2.492 (95% CI: 1.585–3.916, p < 0.001) and 2.006 (95% CI: 0.671–5.996, p = 0.213), respectively. For women aged ≥ 35 years, the ORs for SPPH with overt and subclinical hypothyroidism were 0.807 (95% CI: 0.372–1.749, p = 0.586) and 0.487 (95% CI: 0.058–4.119, p = 0.509), respectively.
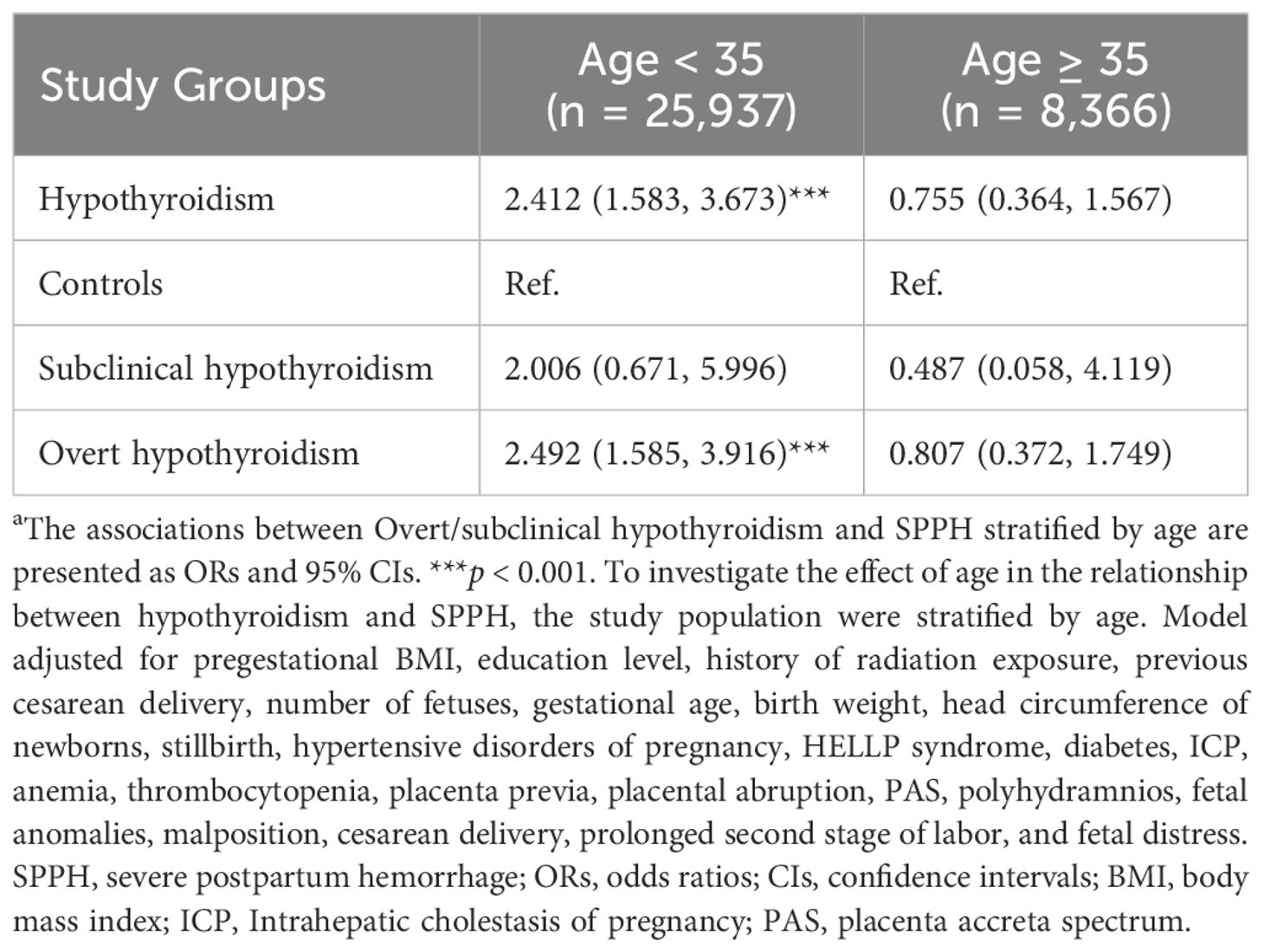
Table 3. Stratified analysis in the relationship between hypothyroidism and SPPH by logistic modelsa.
3.4 Sensitivity analyses
We conducted several sensitivity analyses to verify the stability of our results (Table 4). In one sensitivity analysis, we excluded participants who delivered twins or triplets. Additionally, another sensitivity analysis eliminated participants with a history of radiation exposure and those with an unknown radiation exposure history. The sensitivity analyses indicated that overt hypothyroidism remained a substantial influence on elevating the risk of SPPH, whereas subclinical hypothyroidism was not found to exert a notable impact on SPPH.
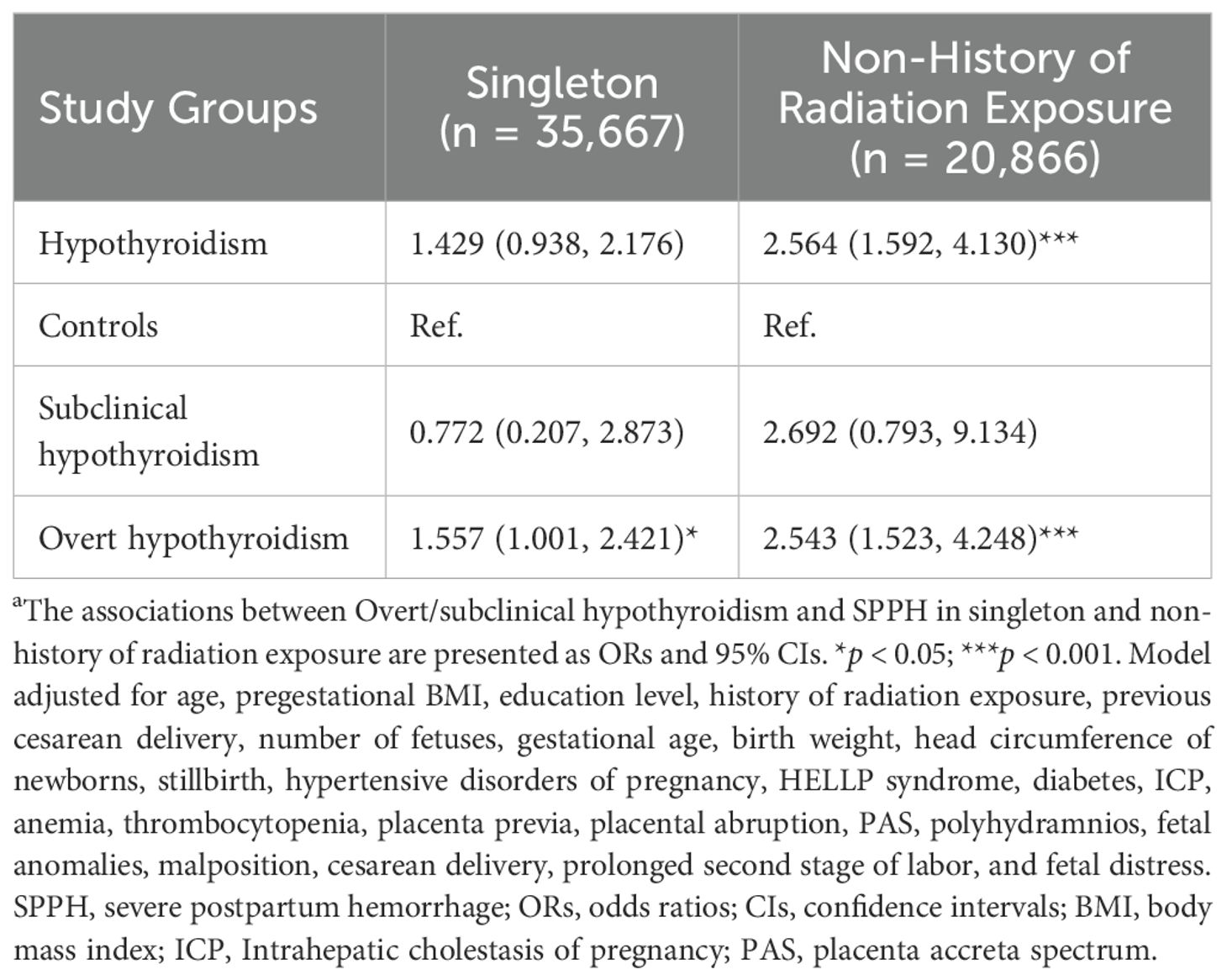
Table 4. Sensitivity analyses in the relationship between hypothyroidism and SPPH by logistic modelsa.
4 Discussion
In this retrospective cohort study, we had several important findings. First, we observed an elevated risk of SPPH in pregnant women with hypothyroidism. Second, we found that overt hypothyroidism was significantly associated with an elevated risk of SPPH, whereas no significant association with SPPH was detected for subclinical hypothyroidism. Finally, we observed that age and history of radiation exposure acted as potential modifiers in the relationship between hypothyroidism and SPPH. Moreover, the correlation between hypothyroidism and SPPH was stronger in pregnant women aged < 35 years than in those aged ≥ 35 years.
Multiple studies demonstrated that hypothyroidism was associated with adverse pregnancy outcomes (16–19), including preterm delivery, stillbirth, cesarean delivery, and PPH (5, 17), and perinatal complications such as abortion, gestational diabetes mellitus, and gestational hypertension (20–22). Moreover, overt hypothyroidism is associated with an increased risk of preterm complications and adverse pregnancy outcomes such as abortion, gestational diabetes mellitus, gestational hypertension, preeclampsia, preterm delivery, and cesarean delivery (9, 23–29). However, although some studies suggest an association between subclinical hypothyroidism and adverse pregnancy outcomes (30–32), this association has not been fully established (33).
Similar to a recent study of a population database including over 184000 women with hypothyroidism (5), we found that hypothyroidism is associated with an increased risk of SPPH. In a study of 702 pregnant women in Pakistan, it was found that PPH was the most common maternal outcome in pregnant women with hypothyroidism (11). However, none of these studies differentiated between overt or subclinical hypothyroidism. Nazarpour et al. found that overt hypothyroidism was associated with an increased incidence of adverse pregnancy outcomes, including PPH, while there is no consensus on subclinical hypothyroidism (33). In this retrospective cohort study of 38439 women from the Third Affiliated Hospital of Guangzhou Medical University, we observed that overt hypothyroidism was an important factor contributing to the elevated risk of SPPH, but subclinical hypothyroidism did not show a significant effect on the development of SPPH. However, Medenica et al. suggest that subclinical hypothyroidism may cause PPH (34). Additionally, treatment of pregnant women with subclinical hypothyroidism using LT4 can significantly reduce the incidence of adverse pregnancy outcomes (35), especially PPH (36). One possible explanation for this discrepancy is the small number of SPPH events among women with subclinical hypothyroidism in our cohort, which may have limited the statistical power to detect a true association. Therefore, for subclinical hypothyroidism, the absence of a significant association in our study should be interpreted with caution rather than as evidence of no risk. Some studies have shown that advanced maternal age is associated with an increased risk of PPH (37, 38). Additionally, Cai et al. found that maternal age ≥ 30 years was related to adverse pregnancy outcomes in pregnant women with hypothyroidism (39). In our study, we observed an age-stratified pattern in the association between hypothyroidism and SPPH. Among participants younger than 35 years, hypothyroidism was identified as a significant risk factor for SPPH. However, for those aged 35 years and older, no significant association was detected. Further examination of the raw data revealed that, within the ≥ 35 years group (n = 8,366), the number of SPPH events among women with overt hypothyroidism (n = 9) and subclinical hypothyroidism (n = 1) was small, which likely limited the statistical power to detect a true association. Thus, the lack of significance in this subgroup should be interpreted with caution.
Thyroid hormone is an important factor in the migration, proliferation, and invasion of trophoblast cells (11). In pregnant women with hypothyroidism, especially overt hypothyroidism, deficiency of thyroid hormones may lead to placental abnormalities (40), particularly placenta previa. Meanwhile, a study (41) showed a higher rate of placenta previa in hypothyroid women (p = 0.09). This may the relationship between hypothyroidism and PPH. Another major function regulated by thyroid hormones is mitochondrial energy production and biogenesis (42), and maternal thyroid hormone deficiency may cause PPH by affecting uterine muscle energy production, resulting in weak uterine contractions.
Our study has several strengths, as well as some limitations. Firstly, we selected a large cohort of women from the Guangzhou Medical Centre for Critical Pregnant Women, ensuring a representative sample of critical obstetric patients in the southern region and providing comprehensive data. Secondly, we employed three models incorporating different covariates to explore the relationship between hypothyroidism and SPPH, followed by stratified analysis to identify potential moderating effects of age. Finally, our results remained robust even after accounting for a wide range of potential confounders and performing sensitivity analyses.
The limitations of this study are as follows. First, the observational nature of this retrospective cohort study precludes causal inference. Despite our efforts to enhance robustness through multiple models (adjusted for different covariates), stratified analyses, and sensitivity analyses, the observed association between hypothyroidism and SPPH should not be interpreted as causal and warrants cautious interpretation. Second, hypothyroidism was identified using ICD codes from electronic health records rather than original laboratory values (e.g., TSH and FT4), which may have resulted in diagnostic misclassification. Third, some important clinical information—such as thyroid disease history, prior treatments (e.g., thyroid surgery), and specific medication use—was often scattered across unstructured clinical notes, making systematic extraction unfeasible. As a result, these variables could not be adjusted for in the analysis and may have introduced unmeasured confounding. Fourth, although multiple potential confounders were adjusted for, several key obstetric variables—including induction of labor, chorioamnionitis, and use of oxytocin/uterotonics—were not accounted for, leaving residual confounding a possibility. Finally, this was a single-center retrospective study conducted in China. Variations in iodine intake, thyroid screening practices, and obstetric management worldwide may also limit the generalizability of our findings. Therefore, future prospective, multicenter studies are warranted to validate these results.
5 Conclusion
Overt hypothyroidism significantly increased the risk of SPPH, particularly among women aged < 35 years. For subclinical hypothyroidism, the absence of a significant association with SPPH should be interpreted with caution, as the small number of cases of SPPH in women with subclinical hypothyroidism may result in limited statistical power. These findings may offer valuable insights into the relationship between hypothyroidism and SPPH, potentially optimizing maternal outcomes by preventing and intervening in the occurrence of SPPH.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of the Third Affiliated Hospital of Guangzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Formal analysis, Writing – original draft, Writing – review & editing. QG: Formal analysis, Writing – original draft, Writing – review & editing. CL: Writing – review & editing, Formal analysis, Writing – original draft. YF: Writing – original draft, Data curation. YL: Writing – original draft, Data curation. LT: Conceptualization, Supervision, Writing – review & editing. FH: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Social Development Project of Science and Technology in Dongguan City (No.: 20221800905742), National Key Research and Development Program of China (No.: 2021YFC2701500), and Guangzhou fundamental research project jointly funded by School (Institution) (high-level university) (No.: 202102010131).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1654856/full#supplementary-material.
Abbreviations
PPH, Postpartum hemorrhage; SPPH, Severe postpartum hemorrhage; TSH, Thyroid-stimulating hormone; FT4, Free thyroxine; ICD, International Classification of Diseases; TPOAb, Thyroid peroxidase antibody; LT4, Levothyroxine; BMI, Body mass index; ICP, Intrahepatic cholestasis of pregnancy; PAS, Placenta accreta spectrum; SD, Standard deviation; OR, Odds ratio; CI, Confidence interval.
References
1. Högberg U. The world health report 2005: ``make every mother and child count’’ — including africans. Scand J Public Health. (2005) 33:409–11. doi: 10.1080/14034940500217037
2. Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. (2014) 2:e323–33. doi: 10.1016/S2214-109X(14)70227-X
3. Liu CN, Yu FB, Xu YZ, Li JS, Guan ZH, Sun MN, et al. Prevalence and risk factors of severe postpartum hemorrhage: a retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21:332. doi: 10.1186/s12884-021-03818-1
4. Bateman BT, Berman MF, Riley LE, and Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. (2010) 110:1368–73. doi: 10.1213/ANE.0b013e3181d74898
5. Hizkiyahu R, Badeghiesh A, Baghlaf H, and Dahan MH. Associations between hypothyroidism and adverse obstetric and neonatal outcomes: a study of a population database including over 184,000 women with hypothyroidism. J Matern Fetal Neonatal Med. (2023) 36:2278027. doi: 10.1080/14767058.2023.2278027
6. Dong AC and Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid. (2019) 29:278–89. doi: 10.1089/thy.2018.0475
7. Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, and Rovet J. Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies. Pediatr Res. (2005) 57:902–7. doi: 10.1203/01.PDR.0000157681.38042.B4
8. Poulasouchidou MK, Goulis DG, Poulakos P, Mintziori G, Athanasiadis A, Grimbizis G, et al. Prediction of maternal and neonatal adverse outcomes in pregnant women treated for hypothyroidism. Hormones (Athens). (2012) 11:468–76. doi: 10.14310/horm.2002.1379
9. Hirsch D, Levy S, Nadler V, Kopel V, Shainberg B, and Toledano Y. Pregnancy outcomes in women with severe hypothyroidism. Eur J Endocrinol. (2013) 169:313–20. doi: 10.1530/EJE-13-0228
10. Abalovich M, Vazquez A, Alcaraz G, Kitaigrodsky A, Szuman G, Calabrese C, et al. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancy. Thyroid. (2013) 23:1479–83. doi: 10.1089/thy.2013.0024
11. Kiran Z, Sheikh A, Malik S, Meraj A, Masood M, Ismail S, et al. Maternal characteristics and outcomes affected by hypothyroidism during pregnancy (maternal hypothyroidism on pregnancy outcomes, MHPO-1). BMC Pregnancy Childbirth. (2019) 19:476. doi: 10.1186/s12884-019-2596-9
12. Gur EB, Karadeniz M, Inceefe H, Tatar S, Turan GA, Genc M, et al. Thyroid antibodies in euthyroid and subclinical hypothyroidic pregnant women with autoimmune hypothyroidism: effects on hematological parameters and postpartum hemorrhage. Ginekol Pol. (2015) 86(9):666–71. doi: 10.17772/gp/57810
13. Wang S, Teng WP, Li JX, Wang WW, and Shan ZY. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest. (2012) 35:322–5. doi: 10.3275/7772
14. Endocrinology CSO and Medicine CSOP. A guideline for diagnosis and management of thyroid diseases during pregnancy and postpartum. Chin J Perinat Med. (2012) 15:385–403. doi: 10.3760/cma.j.issn.1007-9408.2012.07.001
15. Endocrinology CSO, Perinatology CMAA, and Association CM. Guideline on diagnosis and management of thyroid diseases during pregnancy and postpartum (2nd edition). Chin J Endocrinol Metab. (2019) 35:636–65. doi: 10.3760/cma.j.issn.1000-6699.2019.08.003
16. Negro R and Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ. (2014) 349:g4929. doi: 10.1136/bmj.g4929
17. Chen S, Zhou X, Zhu H, Yang H, Gong F, Wang L, et al. Preconception TSH and pregnancy outcomes: a population-based cohort study in 184–611 women. Clin Endocrinol (Oxf). (2017) 86:816–24. doi: 10.1111/cen.13329
18. Turunen S, Vaarasmaki M, Mannisto T, Hartikainen AL, Lahesmaa-Korpinen AM, Gissler M, et al. Pregnancy and perinatal outcome among hypothyroid mothers: a population-based cohort study. Thyroid. (2019) 29:135–41. doi: 10.1089/thy.2018.0311
19. van den Boogaard E, Vissenberg R, Land JA, van Wely M, Ven DPJ, Goddijn M, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. (2016) 22:532–3. doi: 10.1093/humupd/dmw003
20. Maraka S, Ospina NM, O’Keeffe DT, Espinosa DYA, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. (2016) 26:580–90. doi: 10.1089/thy.2015.0418
21. Sharmeen M, Shamsunnahar PA, Laita TR, and Chowdhury SB. Overt and subclinical hypothyroidism among Bangladeshi pregnant women and its effect on fetomaternal outcome. Bangladesh Med Res Counc Bull. (2014) 40:52–7. doi: 10.3329/bmrcb.v40i2.25183
22. Zhang Y, Wang H, Pan X, Teng W, and Shan Z. Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: a systematic review and meta-analysis. PloS One. (2017) 12:e0175708. doi: 10.1371/journal.pone.0175708
23. Mannisto T, Mendola P, Grewal J, Xie Y, Chen Z, and Laughon SK. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J Clin Endocrinol Metab. (2013) 98:2725–33. doi: 10.1210/jc.2012-4233
24. Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. (2008) 112:85–92. doi: 10.1097/AOG.0b013e3181788dd7
25. Idris I, Srinivasan R, Simm A, and Page RC. Maternal hypothyroidism in early and late gestation: effects on neonatal and obstetric outcome. Clin Endocrinol (Oxf). (2005) 63:560–5. doi: 10.1111/j.1365-2265.2005.02382.x
26. Wolfberg AJ, Lee-Parritz A, Peller AJ, and Lieberman ES. Obstetric and neonatal outcomes associated with maternal hypothyroid disease. J Matern Fetal Neonatal Med. (2005) 17:35–8. doi: 10.1080/14767050400028642
27. Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, and Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. (2002) 12:63–8. doi: 10.1089/105072502753451986
28. Leung AS, Millar LK, Koonings PP, Montoro M, and Mestman JH. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. (1993) 81:349–53. doi: 10.1016/0020-7292(93)90343-U
29. Sahu MT, Das V, Mittal S, Agarwal A, and Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. (2010) 281:215–20. doi: 10.1007/s00404-009-1105-1
30. Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, and Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid. (2011) 21:1143–7. doi: 10.1089/thy.2011.0053
31. Casey BM and Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. (2006) 108:1283–92. doi: 10.1097/01.AOG.0000244103.91597.c5
32. Chang DL and Pearce EN. Screening for maternal thyroid dysfunction in pregnancy: a review of the clinical evidence and current guidelines. J Thyroid Res. (2013) 2013:851326. doi: 10.1155/2013/851326
33. Nazarpour S, Ramezani TF, Simbar M, and Azizi F. Thyroid dysfunction and pregnancy outcomes. Iran J Reprod Med. (2015) 13:387–96.
34. Medenica S, Nedeljkovic O, Radojevic N, Stojkovic M, Trbojevic B, and Pajovic B. Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility. Eur Rev Med Pharmacol Sci. (2015) 19:977–87.
35. Ju R, Lin L, Long Y, Zhang J, and Huang J. Clinical efficacy of therapeutic intervention for subclinical hypothyroidism during pregnancy. Genet Mol Res. (2016) 15. doi: 10.4238/gmr15049019
36. Geng X, Chen Y, Wang W, Ma J, Wu W, Li N, et al. Systematic review and meta-analysis of the efficacy and pregnancy outcomes of levothyroxine sodium tablet administration in pregnant women complicated with hypothyroidism. Ann Palliat Med. (2022) 11:1441–52. doi: 10.21037/apm-22-269
37. Li J, Yan J, and Jiang W. The role of maternal age on adverse pregnancy outcomes among primiparous women with singleton birth: a retrospective cohort study in urban areas of China. J Matern Fetal Neonatal Med. (2023) 36:2250894. doi: 10.1080/14767058.2023.2250894
38. Lu L, Li JH, Dai XF, Wei JB, Chen LH, and Hu JF. Impact of advanced maternal age on maternal and neonatal outcomes in preterm birth. Ginekol Pol. (2022) 93:134–41. doi: 10.5603/GP.a2021.0224
39. Cai L, Wang P, Xue C, Chen J, and Zhang Y. Clinical characteristics and risk factors associated with adverse pregnancy outcomes in patients with gestational hypothyroidism: a case-control study. Endocr Pract. (2024) 30:101–6. doi: 10.1016/j.eprac.2023.10.135
40. Matsuo H, Maruo T, Hayashi M, and Mochizuki M. Modification of endocrine function of trophoblasts by thyroid hormone. Nihon Sanka Fujinka Gakkai Zasshi. (1991) 43:1533–8.
41. Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. (2003) 189:1063–9. doi: 10.1067/S0002-9378(03)00838-X
Keywords: hypothyroidism, severe postpartum hemorrhage, subclinical hypothyroidism, overt hypothyroidism, risk factors
Citation: Zhuang J, Guo Q, Liu C, Fu Y, Liu Y, Tang L and He F (2025) Differential impact of overt and subclinical hypothyroidism on severe postpartum hemorrhage: a retrospective cohort study. Front. Endocrinol. 16:1654856. doi: 10.3389/fendo.2025.1654856
Received: 27 June 2025; Accepted: 24 October 2025;
Published: 05 November 2025.
Edited by:
Karin Windsperger, Medical University of Vienna, AustriaCopyright © 2025 Zhuang, Guo, Liu, Fu, Liu, Tang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang He, aGVmYW5nam51QDEyNi5jb20=; Li Tang, dGFuZ2RhaTAyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jungou Zhuang1,2†
Jungou Zhuang1,2† Chenning Liu
Chenning Liu Fang He
Fang He