- Université Bourgogne Europe, CHU Dijon Bourgogne, Department of Clinical Biochemistry, INSERM, CTM UMR 1231, PADYS team, Dijon, France
The development of once-weekly basal insulin analogues, such as insulin icodec and efsitora alfa, represents a promising strategy to reduce injection burden and improve adherence in diabetes management. This mini-review summarizes the recent findings from clinical trials evaluating once-weekly insulin therapies in both type 1 and type 2 diabetes. In type 1 diabetes, available data remain limited; however, the ONWARDS 6 and QWINT-5 trials demonstrated that once-weekly icodec and efsitora, respectively, achieved comparable reductions in HbA1c to once-daily insulin degludec, when used in combination with prandial insulin. In type 2 diabetes, accumulating evidences from randomized clinical trials supports the efficacy of once-weekly icodec and efsitora, showing non-inferiority—and in some cases, superiority—compared to once-daily basal insulin, both in insulin-naïve individuals and in those previously treated with insulin. Safety profiles of once-weekly insulins in type 2 diabetes are reassuring, with similar rates of clinically significant and severe hypoglycemia compared to once-daily regimens. In contrast, trials in type 1 diabetes reported higher hypoglycemia rates with once-weekly insulins. Recent findings from the COMBINE program demonstrated that the fixed-ratio combination of icodec and semaglutide (IcoSema) produced superior HbA1c reductions compared to either agent alone, though not superior to a basal-bolus regimen with glargine and aspart insulin. However, several important questions remain to be addressed regarding once-weekly insulins, including their long-term efficacy on cardiovascular outcomes and overall long-term safety.
1 Introduction
Type 1 diabetes necessitates lifelong basal and prandial insulin therapy to maintain glycemic control, typically requiring multiple daily injections—a factor that significantly impacts quality of life. Type 2 diabetes is characterized by insulin resistance and the gradual decline of pancreatic β-cell function. For many patients, this trajectory leads to the need for insulin therapy—approximately one in three individuals with type 2 diabetes will require insulin therapy within seven years of diagnosis (1). While the therapeutic landscape has expanded with several non-insulin glucose-lowering agents, real-world data show that insulin use remains the cornerstone of treatment for many individuals with type 2 diabetes. Unfortunately, psychological and practical barriers—most notably the fear of hypoglycemia and the burden of daily injections—often discourage timely insulin initiation (2).
To address these challenges, the development of once-weekly basal insulin analogues has emerged as a promising strategy to reduce treatment burden and improve adherence. These novel formulations use chemical modifications to reduce insulin receptor affinity, resulting in slower clearance and enabling a weekly dosing schedule.
Recent advances have brought two contenders to the forefront: insulin efsitora alfa (Eli Lilly™) and insulin icodec (Novo Nordisk™), the latter also studied in combination with semaglutide. This mini-review synthetizes the latest findings from six phase 3 trials published in 2025—QWINT-1, QWINT-3 and QWINT-4 for efsitora and COMBINE 1, COMBINE 2 and COMBINE 3 for icodec-semaglutide—to offer an updated perspective on the clinical promise of once-weekly insulins (3–7). Table 1 summarizes the key characteristics and outcomes of clinical trials involving once-weekly insulins in both type 1 and type 2 diabetes.
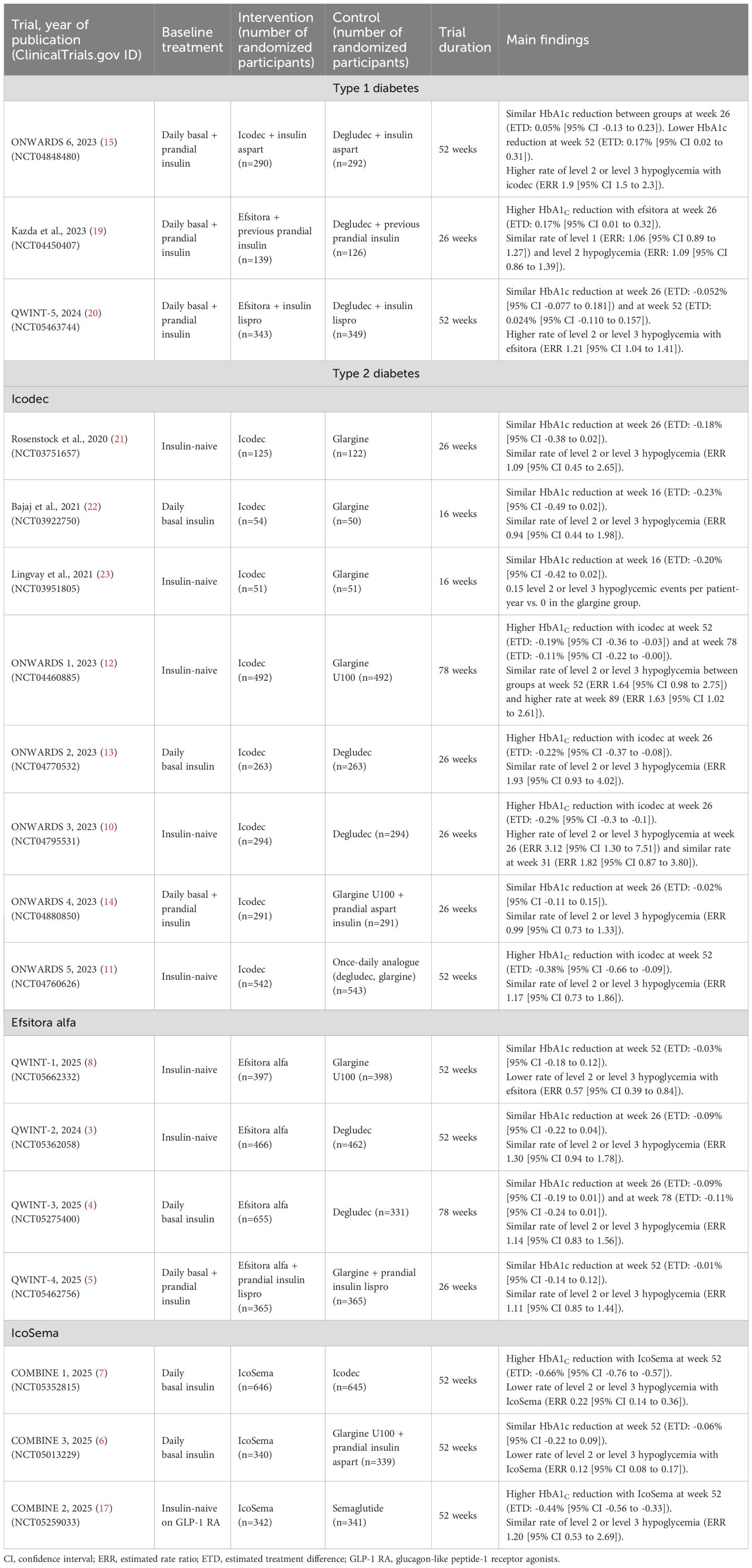
Table 1. Summary of randomized controlled trials investigating once-weekly insulin in type 1 and type 2 diabetes.
2 Efsitora alfa
Insulin efsitora alfa represents a new generation of basal insulin therapy, uniquely engineered as a fusion protein combining the Fc region of human IgG with a single-chain insulin variant. Its receptor binding is about 100 times weaker than that of natural insulin, enabling a pharmacokinetic profile suitable for once-weekly administration.
Over the past year, a robust body of evidence has emerged from the QWINT phase 3 clinical trial programme (QWINT-1 to QWINT-4 in type 2 diabetes and QWINT-5 in type 1 diabetes), collectively demonstrating that efsitora is an effective alternative to conventional daily insulins.
The first published trial, QWINT-2 (NCT05362058), compared once-weekly efsitora to daily degludec in 928 insulin-naive adults with type 2 diabetes (3). After 52 weeks, efsitora achieved non-inferior HbA1c reduction (-1.26% vs -1.17%; difference -0.09%, 95% confidence interval [CI] -0.22 to 0.04) and showed a slight advantage in time-in-range (64.3% vs 61.2%), with comparable hypoglycemia and adverse event profiles. Interestingly, subgroup analyses confirmed noninferiority in those using or not using GLP-1 receptor agonists.
The QWINT-1 trial (NCT05662332), published in June 2025, confirmed these findings against daily insulin glargine (8). In 795 adults with type 2 diabetes naïve to insulin therapy, efsitora again achieved non-inferior HbA1c reduction (-1.19% vs. -1.16%; between-group difference -0.03%, 95% CI -0.18 to 0.12) at 52 weeks, but with fewer clinically significant or severe hypoglycemia episodes (0.50 vs. 0.88 events/person-year; rate ratio 0.57, 95% CI 0.39 to 0.84). However, reescalation of efsitora doses after a dose reduction due to hypoglycemia was prohibited, likely reducing the incidence of hypoglycemia in this group. Patient-reported outcomes favored efsitora, and treatment satisfaction scores improved in both groups. Adverse event rates and body weight gain were comparable.
The QWINT-3 trial (NCT05275400), which had the longest treatment duration trial among the studies conducted on once-weekly insulins, evaluated efsitora in 986 individuals with type 2 diabetes inadequately controlled on basal insulin (4). Over 78 weeks, efsitora was non-inferior to degludec in HbA1c reduction (-0.81% vs. -0.72% at week 26; estimated treatment difference -0.09%, 95% CI -0.19 to 0.01). Continuous glucose monitoring indicated similar improvements in time-in-range between groups, and patient-reported outcomes favored efsitora in terms of treatment satisfaction. Rates of clinically significant or severe hypoglycaemia and serious adverse events were similar. However, slight hypoglycemia was more frequent in the efsitora group. Despite a higher incidence of mild side effects such as headache and anaemia, no treatment-related deaths occurred.
Finally, the QWINT-4 trial (NCT05462756) extended the evidence base to people with type 2 diabetes already on basal-bolus therapy. In 730 participants, efsitora was non-inferior to glargine in HbA1c reduction over 26 weeks (-1.01% vs -1.00%; treatment difference -0.01%, 95% CI -0.14 to 0.12). It also showed numerically fewer nocturnal hypoglycemic events. Continuous glucose monitoring revealed similar time-in-range, with a slight advantage for efsitora during daytime hours. Once again, patient preference strongly leaned toward the simplicity of weekly dosing.
In type 1 diabetes, available data on efsitora alfa remain limited. The QWINT-5 trial (NCT05463744) demonstrated comparable reductions in HbA1c levels at 52 weeks between efsitora and degludec, both administered alongside with insulin lispro (-0.39% vs. -0.44%; treatment difference 0.024% [95% CI -0.110 to 0.157]). Notably, treatment satisfaction scores favored efsitora over degludec. However, a major safety concern emerged regarding hypoglycemia: efsitora was associated with higher rates of level 1 (<70 mg/dL), combined level 2 (<54 mg/dL) and level 3 (severe episodes requiring external assistance) hypoglycemia, including a greater non-nocturnal level 3 events. Interestingly, the incidence of nocturnal level 1 hypoglycemia with efsitora was lower in patients with type 2 diabetes than in those with type 1 diabetes (9).
Taken together, the QWINT trials position efsitora as a clinically effective once-weekly basal insulin that addresses common barriers to insulin initiation and persistence—namely, injection burden, and treatment satisfaction. Nevertheless, safety concerns remain regarding the risk of hypoglycemia in type 1 diabetes, which warrants further investigation. Additionally, all of these trials were open-label, which may introduce biases, including those related to the self-reporting of hypoglycemic events.
3 Icodec
Insulin icodec, another once-weekly basal insulin analogue, represents a significant pharmacological advancement owing to its albumin-binding fatty acid modifications and reduced affinity for the insulin receptor. These structural optimizations extend its half-life to approximately 196 hours, thereby enabling sustained glycemic control with a single weekly injection.
In type 2 diabetes, the efficacy and safety of icodec have been robustly evaluated through the ONWARDS phase 3 trials, published in 2023. In insulin-naïve individuals with type 2 diabetes (ONWARDS 1, 3, and 5), once-weekly icodec demonstrated superior HbA1c reductions compared to degludec (treatment difference -0.2% at week 26, 95% CI -0.3 to -0.1), glargine (treatment difference -0.19% at week 52, 95% CI -0.36 to -0.03), and to once-daily basal insulin analogues (treatment difference -0.38% at week 52, 95% CI -0.66 to -0.09) (10–12). In patients previously treated with insulin (ONWARDS 2 and 4), icodec was superior to degludec (treatment difference -0.22%, 95% CI -0.37 to -0.08) and non-inferior to glargine (treatment difference -0.02%, 95% CI -0.11 to 0.15) in HbA1c reduction after 26 weeks of treatment (13, 14).
In type 1 diabetes, available evidence is currently limited to the ONWARDS 6 trial (NCT04848480) (15). This study demonstrated comparable reductions in HbA1c levels at 26 weeks between icodec and degludec, both administered alongside with insulin aspart (-0.47% vs. -0.51%; treatment difference 0.05% [95% CI -0.13 to 0.23]) (15). However, at 52 weeks, icodec was less effective than degludec in lowering HbA1c (-0.37% vs. -0.54%; treatment difference 0.17% [95% CI 0.02 to 0.31]). Consistent with findings for efsitora in patients with type 1 diabetes, a notable safety concern with icodec was a higher incidence of hypoglycemia compared to degludec. Specifically, the percentage of time spent with blood glucose levels below 54 mg/dL was significantly greater with icodec (1.0% vs. 0.7%). A post-hoc analysis of the ONWARDS 6 trial reported no significant increase in the odds of hypoglycemia attributed to physical activity with once-weekly icodec compared to degludec (16).
The once-weekly fixed-ratio combination IcoSema (icodec + semaglutide) has recently emerged as a compelling intensification strategy. The first findings of phase 3 trials using IcoSema have just been published in 2025. In the COMBINE 1 trial (NCT05352815), IcoSema showed superior efficacy on HbA1c reduction compared to icodec alone (-1.55% vs. -0.89% at 52 weeks; treatment difference -0.66%, 95% CI -0.76 to -0.57) in 1291 adults with type 2 diabetes inadequately controlled on daily basal insulin (7). IcoSema also led to significant weight loss (-3.7 kg vs. +1.9 kg; treatment difference -5.59 kg) and a lower rate of clinically significant or severe hypoglycaemia (0.14 vs. 0.63 events/person-year; rate ratio 0.22). Time-in-range was notably greater (75.9% vs. 61.9%), and more participants achieved HbA1c <7.0% without weight gain or hypoglycaemia. While mild gastrointestinal adverse events were more frequent, overall safety was comparable, with no new safety concerns.
In the COMBINE 2 trial (NCT05259033), IcoSema also outperformed once-weekly semaglutide in 683 patients with type 2 diabetes inadequately controlled on GLP-1 receptor agonist, achieving a greater HbA1c reduction at 52 weeks (-1.35% vs. -0.90%; treatment difference -0.44%, 95% CI -0.56 to -0.33) (17). The rate of clinically significant or severe hypoglycaemia was similar between the two groups.
Finally, the COMBINE 3 trial (NCT05013229) compared once-weekly IcoSema with conventional basal-bolus therapy (glargine + insulin aspart) in 679 patients with type 2 diabetes insufficiently controlled on basal insulin (6). IcoSema achieved non-inferior HbA1c reduction (-1.47% vs. -1.40% at week 52; treatment difference -0.06%, 95% CI -0.22 to 0.09) and demonstrated superiority in multiple secondary outcomes, including substantial weight reduction (-3.56 vs. +3.16 kg; treatment difference -6.72 kg; 95% CI -7.58 to -5.86), and a significant reduction in clinically significant or severe hypoglycaemia episodes (0.21 vs. 2.23 episodes per person-year of exposure; rate ratio 0.12, 95% CI 0.08 to 0.17). Continuous glucose monitoring showed similar time-in-range, with significantly less time spent below 54 mg/dL in the IcoSema group. Again, gastrointestinal side effects were more frequent but mostly mild, and consistent with known profiles of GLP-1 receptor agonists. Patient-reported outcomes consistently favored IcoSema for convenience and satisfaction.
Together, these findings position IcoSema as a powerful, once-weekly intensification option offering superior glycaemic control, meaningful weight benefits, and a lower risk of hypoglycaemia—an appealing alternative to traditional basal-bolus therapy in the management of type 2 diabetes. Similar to the QWINT trials, the COMBINE trials were open-label, which may introduce participant bias when reporting.
4 Discussion
The emergence of once-weekly basal insulin analogues represents a transformative shift in the treatment landscape of type 1 and type 2 diabetes, addressing long-standing barriers to insulin treatment. Both insulin efsitora alfa and insulin icodec offer simplified regimens with promising efficacy and safety profiles, as demonstrated in multiple recent phase 3 trials conducted in patients with type 2 diabetes. However, a notable safety concern with both efsitora and icodec was a higher incidence of hypoglycemia.
Efsitora alfa has shown consistent glycaemic control across a broad range of patient populations with type 2 diabetes—from insulin-naïve individuals (QWINT-1, QWINT-2) to those previously treated with basal or basal-bolus insulin (QWINT-3, QWINT-4). Across these trials, efsitora achieved non-inferior HbA1c reductions compared to daily basal insulin analogues, with additional benefits in hypoglycaemia risk, time-in-range, and treatment satisfaction. Importantly, patient-reported outcomes favored once-weekly dosing, highlighting the potential to improve long-term adherence.
Similarly, insulin icodec has demonstrated robust efficacy, with superior glycaemic outcomes in both insulin-naïve and insulin-experienced populations with type 2 diabetes, as shown in the ONWARDS programme. The addition of semaglutide in the fixed-ratio formulation IcoSema further enhances clinical outcomes. COMBINE trials have revealed superior HbA1c reduction, greater weight loss, and fewer hypoglycaemic events compared to either icodec alone, semaglutide monotherapy, or even conventional basal-bolus regimens.
Together, these findings suggest that once-weekly insulins could redefine insulin-based management strategies in diabetes, combining clinical efficacy with a reduced treatment burden. Nevertheless, several limitations merit further consideration. The fixed weekly administration schedule limits dosing flexibility, and the initiation of such therapies requires re-education of both patients and healthcare providers.
Once-weekly insulins beyond icodec and efsitora alfa are currently in development. Notably, GZR4 has recently demonstrated a favorable safety profile in a phase 1a trial (18). Several ongoing clinical trials are expected to more precisely define the efficacy and safety of these agents. In particular, the ONWARDS 11 trial (NCT07076199) will evaluate icodec versus glargine in individuals with type 1 diabetes, while the single-arm trial NCT06807190 will assess icodec in Japanese participants irrespective of diabetes type. Additionally, the COMBINE 4 trial (NCT06269107) will compare icosema with glargine in insulin-naïve individuals with type 2 diabetes. Beyond these ongoing investigations, further research is needed to assess long-term cardiovascular outcomes and safety profiles, cost-effectiveness, real-world adherence and head-to-head comparisons between efsitora and icodec. As these therapies advance toward broader clinical use, they hold the potential to significantly improve the quality of diabetes care.
Author contributions
DD: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gentile S, Strollo F, Viazzi F, Russo G, Piscitelli P, Ceriello A, et al. Five-year predictors of insulin initiation in people with type 2 diabetes under real-life conditions. J Diabetes Res. (2018) 2018:7153087. doi: 10.1155/2018/7153087
2. Galdón Sanz-Pastor A, Justel Enríquez A, Sánchez Bao A, and Ampudia-Blasco FJ. Current barriers to initiating insulin therapy in individuals with type 2 diabetes. Front Endocrinol. (2024) 15:1366368. doi: 10.3389/fendo.2024.1366368
3. Wysham C, Bajaj HS, Del Prato S, Franco DR, Kiyosue A, Dahl D, et al. Insulin Efsitora versus Degludec in Type 2 Diabetes without Previous Insulin Treatment. N Engl J Med. (2024) 391:2201–11. doi: 10.1056/NEJMoa2403953
4. Philis-Tsimikas A, Bergenstal RM, Bailey TS, Jinnouchi H, Thrasher JR, Ilag L, et al. Once-weekly insulin efsitora alfa versus once-daily insulin degludec in adults with type 2 diabetes currently treated with basal insulin (QWINT-3): a phase 3, randomised, non-inferiority trial. Lancet. (2025) 405:2279–89. doi: 10.1016/S0140-6736(25)01044-X
5. Blevins T, Dahl D, Pérez Manghi FC, Murthy S, Ortiz Carrasquillo R, Li X, et al. Once-weekly insulin efsitora alfa versus once-daily insulin glargine U100 in adults with type 2 diabetes treated with basal and prandial insulin (QWINT-4): a phase 3, randomised, non-inferiority trial. Lancet. (2025) 405:2290–301. doi: 10.1016/S0140-6736(25)01069-4
6. Billings LK, Andreozzi F, Frederiksen M, Gourdy P, Gowda A, Ji L, et al. Once-weekly IcoSema versus multiple daily insulin injections in type 2 diabetes management (COMBINE 3): an open-label, multicentre, treat-to-target, non-inferiority, randomised, phase 3a trial. Lancet Diabetes Endocrinol. (2025) 13:556–67. doi: 10.1016/S2213-8587(25)00052-X
7. Mathieu C, Giorgino F, Kim SG, Larsen JH, Philis-Tsimikas A, Ramachandran A, et al. Once−weekly IcoSema versus once−weekly insulin icodec in type 2 diabetes management (COMBINE 1): an open−label, multicentre, treat−to−target, randomised, phase 3a trial. Lancet Diabetes Endocrinol. (2025) 13:568–79. doi: 10.1016/S2213-8587(25)00096-8
8. Rosenstock J, Bailey T, Connery L, Miller E, Desouza C, Wang Q, et al. Weekly fixed-dose insulin efsitora in type 2 diabetes without previous insulin therapy. N Engl J Med. (2025) 393:325–35. doi: 10.1056/NEJMoa2502796
9. de Oliveira HM, Gallo Ruelas M, Flávio-Reis VHP, Queiroz I, Cavalcante DVS, Barbosa LM, et al. Degludec insulin versus efsitora insulin in diabetes mellitus management: A systematic review and meta-analysis. J Diabetes Complications. (2025) 39:109115. doi: 10.1016/j.jdiacomp.2025.109115
10. Lingvay I, Asong M, Desouza C, Gourdy P, Kar S, Vianna A, et al. Once-weekly insulin icodec vs once-daily insulin degludec in adults with insulin-naive type 2 diabetes: the ONWARDS 3 randomized clinical trial. JAMA. (2023) 330:228–37. doi: 10.1001/jama.2023.11313
11. Bajaj HS, Aberle J, Davies M, Donatsky AM, Frederiksen M, Yavuz DG, et al. Once-weekly insulin icodec with dosing guide app versus once-daily basal insulin analogues in insulin-naive type 2 diabetes (ONWARDS 5): A randomized trial. Ann Intern Med. (2023) 176:1476–85. doi: 10.7326/M23-1288
12. Rosenstock J, Bain SC, Gowda A, Jódar E, Liang B, Lingvay I, et al. Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin. N Engl J Med. (2023) 389:297–308. doi: 10.1056/NEJMoa2303208
13. Philis-Tsimikas A, Asong M, Franek E, Jia T, Rosenstock J, Stachlewska K, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. (2023) 11:414–25. doi: 10.1016/S2213-8587(23)00093-1
14. Mathieu C, Ásbjörnsdóttir B, Bajaj HS, Lane W, Matos ALSA, Murthy S, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet Lond Engl. (2023) 401:1929–40. doi: 10.1016/S0140-6736(23)00520-2
15. Russell-Jones D, Babazono T, Cailleteau R, Engberg S, Irace C, Kjaersgaard MIS, et al. Once-weekly insulin icodec versus once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): a phase 3a, randomised, open-label, treat-to-target trial. Lancet Lond Engl. (2023) 402:1636–47. doi: 10.1016/S0140-6736(23)02179-7
16. Sourij H, Bracken RM, Carstensen L, Pagliaro Rocha TM, Kehlet Watt S, and Philis-Tsimikas A. No evidence of increased hypoglycaemia attributed to physical activity with once-weekly insulin icodec versus once-daily basal insulin degludec in type 1 diabetes: A post hoc analysis of ONWARDS 6. Diabetes Obes Metab. (2025) 27:2882–6. doi: 10.1111/dom.16265
17. Lingvay I, Benamar M, Chen L, Fu A, Jódar E, Nishida T, et al. Once-weekly IcoSema versus once-weekly semaglutide in adults with type 2 diabetes: the COMBINE 2 randomised clinical trial. Diabetologia. (2025) 68:739–51. doi: 10.1007/s00125-024-06348-5
18. Tang C, Su R, Wan L, Zhu M, Pu J, Hao C, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of GZR4, a novel once-weekly basal insulin, in healthy participants: A randomized trial. Diabetes Obes Metab. (2025) 27:2515–22. doi: 10.1111/dom.16250
19. Kazda CM, Bue-Valleskey JM, Chien J, Zhang Q, Chigutsa E, Landschulz W, et al. Novel once-weekly basal insulin fc achieved similar glycemic control with a safety profile comparable to insulin degludec in patients with type 1 diabetes. Diabetes Care. (2023) 46:1052–9. doi: 10.2337/dc22-2395
20. Bergenstal RM, Weinstock RS, Mathieu C, Onishi Y, Vijayanagaram V, Katz ML, et al. Once-weekly insulin efsitora alfa versus once-daily insulin degludec in adults with type 1 diabetes (QWINT-5): a phase 3 randomised non-inferiority trial. Lancet Lond Engl. (2024) 404:1132–42. doi: 10.1016/S0140-6736(24)01804-X
21. Rosenstock J, Bajaj HS, Janež A, Silver R, Begtrup K, Hansen MV, et al. NN1436–4383 investigators. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. (2020) 383:2107–16. doi: 10.1056/NEJMoa2022474
22. Bajaj HS, Bergenstal RM, Christoffersen A, Davies MJ, Gowda A, Isendahl J, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: a phase 2 randomized controlled trial. Diabetes Care. (2021) 44:1586–94. doi: 10.2337/dc20-2877
Keywords: diabetes mellitus, insulin icodec, insulin efsitora, long-acting insulin, glycemic control
Citation: Denimal D (2025) Emerging perspectives on once-weekly insulins in type 1 and type 2 diabetes: a mini-review. Front. Endocrinol. 16:1656884. doi: 10.3389/fendo.2025.1656884
Received: 30 June 2025; Accepted: 11 August 2025;
Published: 27 August 2025.
Edited by:
Yun Shen, Pennington Biomedical Research Center, United StatesReviewed by:
Yoshifumi Saisho, Saisho Diabetes Clinic, JapanCopyright © 2025 Denimal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Damien Denimal, ZGFtaWVuLmRlbmltYWxAdS1ib3VyZ29nbmUuZnI=
 Damien Denimal
Damien Denimal