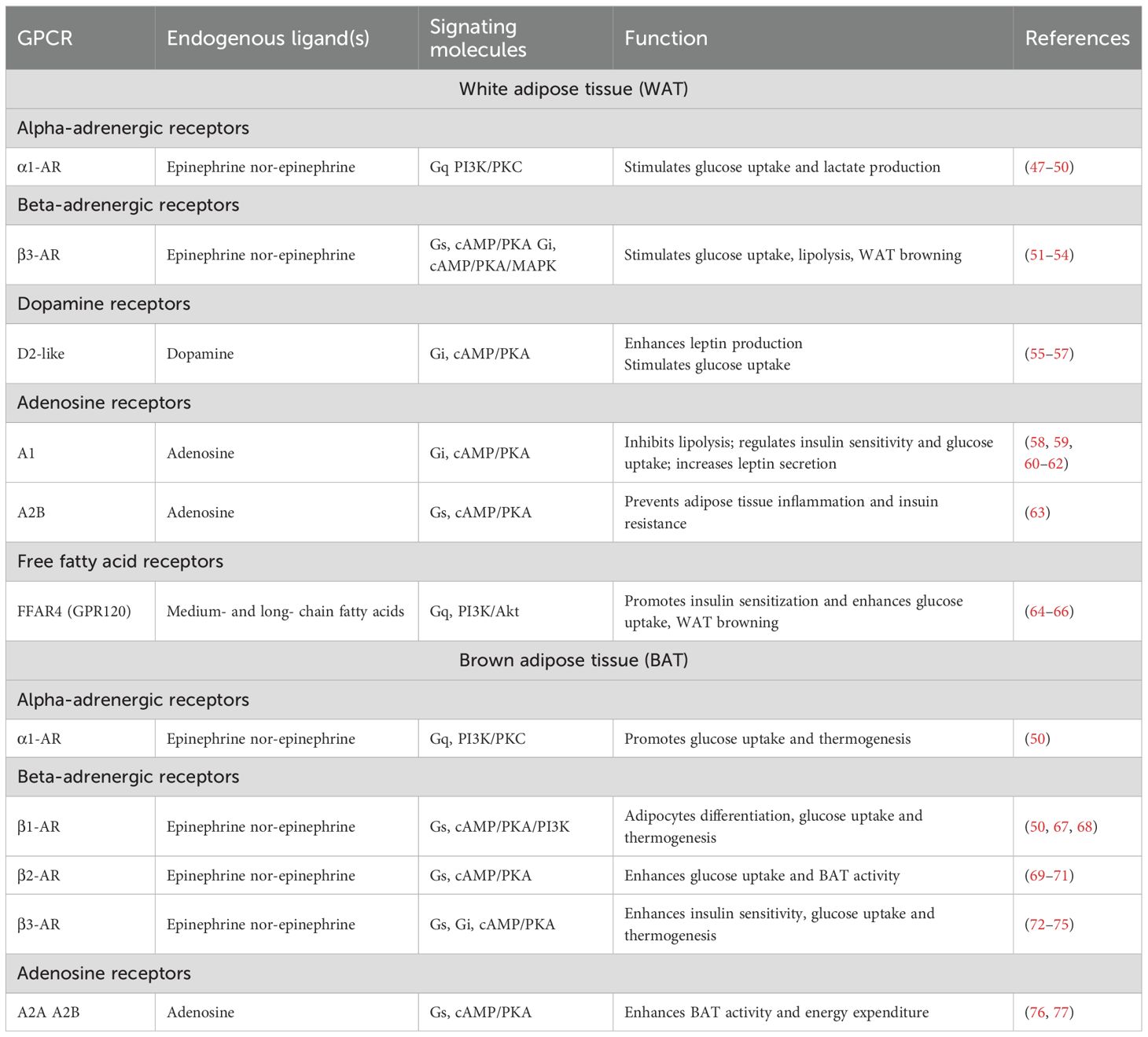
- 1Department of Pharmacology and Nutritional Sciences, University of Kentucky, Lexington, KY, United States
- 2Barnstable Brown Diabetes and Obesity Research Center, University of Kentucky, Lexington, KY, United States
The adipose tissue regulates energy homeostasis, which is one of the vital processes for organismal survival, and its dysregulation causes metabolic diseases including obesity and type 2 diabetes. Glucose is utilized by the adipose tissue for energy production and storage to regulate systemic glucose homeostasis. The G-protein-coupled receptors (GPCRs) expressed in the adipose tissues play a crucial role in adipocyte function by responding to hormonal, neural, and metabolic signals; thereby, influencing insulin sensitivity, glucose uptake and lipid metabolism. The specific contribution of adipocyte GPCRs to glucose sensing and its utilization is incompletely understood. Therefore, in this review we explore the diverse molecular and integrative mechanisms through which GPCR signaling in the adipose tissue senses glucose to regulate systemic glucose homeostasis. We first discuss the major GPCR families that modulate intracellular second messenger cascades in response to glucose and nutrient availability in the adipose tissue, and their metabolic implications in pathophysiological conditions like obesity and diabetes. These GPCRs regulate glucose sensing, lipid metabolism, adipokine secretion, and thereby coordinating metabolic responses with other central and peripheral tissues including the brain, pancreas, intestine and liver. Subsequently, we review the molecular mechanisms through which the adipocyte GPCR regulates systemic glucose homeostasis, from glucose sensing to its utilization. Determining how the GPCRs in the adipose tissue sense glucose will offer new and better therapeutic approaches for treating metabolic diseases including diabetes and obesity.
Introduction
G-protein-coupled receptors (GPCRs) are the largest known cell surface receptor family in humans, which transmit extracellular signals (such as presence or absence of hormones, metabolites, neurotransmitters, sensory stimuli such as taste and odors) to inside of the cell to regulate physiological processes. Structurally, they are composed of seven transmembrane α-helical domains in addition to an extracellular amino- and an intracellular carboxy-terminus, therefore they are also known as seven-transmembrane receptors (1). This diverse group of receptors responds to their specific ligands and thus exerts their physiological functions. Upon ligand binding, they undergo some conformational changes to activate intracellular G-proteins (i.e., Gs, Gi, Gq/11, or G12/13). These changes include the dissociation of Gα from the receptor and the Gβγ dimer, and the subsequent exchange of GTP for the bound GDP, which leads to Gα activation. The activated Gα then influences various downstream intracellular signaling and ultimately affects cellular function (2). At least 800 GPCRs have been identified in humans (1, 3), which are generally classified into five different groups according to the GRAFS (Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2 and Secretin) classification system (4). These receptors are involved in nearly every physiological process, including the metabolic pathways, which makes them attractive targets for drug development. Over one-third of the drugs approved by the U.S. Food and Drug Administration target GPCRs to treat a variety of disorders (5).
Glucose is a vital macronutrient for organismal survival, providing fuel for energy production and carbon skeletons for various cellular components. To effectively sense and respond to changes in circulating glucose levels, the body employs a combination of hormonal signaling, neuronal pathways, and cellular mechanisms, which are essential for regulating glucose and energy homeostasis. Cellular glucose utilization is facilitated by the glucose sensors and receptors, both centrally and peripherally. Centrally, the brain hypothalamus and brainstem regions are well-known sites for glucose sensing and uptake, primarily through the glucose-excited (GE) and glucose-inhibited neurons (6–9). Peripherally, critical sites for glucose homeostasis include the pancreas, liver, skeletal muscle, kidneys, and the adipose tissue (10). A number of GPCRs have been identified as regulators of glucose homeostasis. For example, glucagon-like peptide-1 receptor (GLP-1R) is a widely studied GPCR, activated by GLP-1 in response to nutrients (e.g., glucose), which increase pancreatic insulin secretion and lowers blood glucose levels (11–13). Taste 1 receptors, particularly TAS1R2 and TAS1R3, another GPCR family members, are also involved in glucose sensing in pancreas, intestine, and skeletal muscle, and control glucose metabolism (14–16). Adhesion G-protein-coupled receptor L1 (ADGRL1) was recently reported to function as a hypothalamic glucose receptor that controls energy homeostasis in mice (17). ADGRL1-deficiency increases food intake, impairs glucose sensing and homeostasis, and causes obesity in mice (17, 18). These findings were also confirmed in pathogenesis of human obesity (18). Glucose-ADGRL1 binding was also validated using different methods (19) and the differences between available transgenic mouse models targeting Adgrl1 were discussed recently (20).
Although the contribution of GPCRs to glucose homeostasis is widely recognized, the precise role of adipocyte GPCR signaling in direct glucose sensing is incompletely understood. In this review, we provide an overview of the current understanding of GPCRs in the adipose tissue biology with a focus on their role in glucose sensing and homeostasis. First, we summarize the key GPCRs expressed in various adipose tissue depots and their metabolic implications in the pathogenesis of obesity and diabetes. Subsequently, we discuss the signaling mechanisms through which the adipocyte GPCRs sense glucose, respond to different glucose levels, and interact with other tissues to regulate overall energy balance.
Adipose tissue types and their association with metabolic diseases
White adipose tissue (WAT), brown adipose tissue (BAT), and beige or brite (brown-in-white) adipose tissue are the three major adipose tissue types in mammals, with distinct morphological and functional characteristics. WAT comprises the highest portion of body fat and be further subdivided into subcutaneous and visceral WAT, according to their anatomical location (21, 22). Subcutaneous WAT is found under the skin, while visceral WAT resides in the abdominal cavity and surroundings of different intrabdominal organs. WAT acts as an energy storage depot, as it stores excess energy as triglycerides (TAGs), which are released as free fatty acids and glycerol during energy deficits. Subcutaneous WAT preferably stores excess fats and expands in size by hypertrophy and/or hyperplasia. But when this expansion halts due to reaching the limit or impairment of the expandability, fats start to deposit in visceral depots and other organs like kidneys, liver, heart, skeletal muscle, and pancreas. This ectopic fat deposition in non-fat tissues exacerbates lipotoxicity, resulting in insulin resistance, localized and systemic inflammation, and apoptotic cell death (23). Development of metabolic syndromes, including insulin resistance due to this excess visceral fat deposition is well known (24, 25) and leads to cardiovascular diseases and type 2 diabetes (26–28). Moreover, dysregulation in WAT’s endocrine functions also contributes to these metabolic diseases (28, 29).
BAT, the second adipose tissue type, was historically considered to only exist in hibernating animals, rodents, and to a lesser extent in infants, but its presence in adult humans has now been confirmed (30–33). Its amount is comparatively smaller (<3% of total fat mass) than the WAT and located in cervical, supraclavicular, axillary, mediastinal, paraspinal, and abdominal areas (32, 33). BAT is a metabolically highly active tissue that dissipates excess energy, mostly by thermogenesis involving its unique uncoupling protein 1 (UCP1). In addition to the well-established thermogenic property of BAT, UCP1-independent thermogenesis has also been reported recently (34–36). Like BAT, beige/brite adipose tissue exerts similar roles in thermogenesis and energy metabolism, mostly found in the subcutaneous WAT depots of rodents, and in cervical and supraclavicular regions in adult humans (37, 38). Beyond chronic cold exposure, other factors like adrenergic stimulation, diet, and exercise can also activate their thermogenic programming (34, 39, 40). These adipose tissues utilize glucose and fatty acids as fuel sources and play crucial roles in metabolic homeostasis (34, 41). Their activation improves insulin sensitivity and glucose uptake, increases lipolysis and fatty acid beta-oxidation, and reduces ectopic fat deposition and systemic inflammation (31, 34, 42–44). In contrast, dysfunction or inadequate activation of these adipose tissues reduces energy expenditure, and therefore, exacerbating metabolic and obesity-related complications. For example, BAT whitening - a condition when BAT loses its morphological and molecular characteristics and acts like WAT- exacerbates obesity complications in mice (45).
Major GPCRs in the adipose tissue and their roles in glucose homeostasis
About 250 GPCRs have been identified in human (subcutaneous) WAT, while mice express over 270 and 290 GPCRs in WAT and BAT, respectively (46). Here, we are focusing on the major GPCRs, summarized in Table 1, involved in adipose tissue regulation of glucose and energy homeostasis.
Beta-adrenergic receptors (β-ARs: β1, β2, and β3 subtypes) are one of the highly expressed and well-investigated GPCRs in both human and mouse adipose tissues (68). Ligand (e.g., norepinephrine) mediated activation of β-ARs, particularly β3-AR, promotes lipolysis, mitochondrial respiration, and browning of WAT (51, 54, 78, 79). β3-AR activation also stimulates glucose uptake in WAT through insulin-dependent and -independent mechanisms (80). Pharmacological stimulation with the β3-AR agonist CL316,243 increases glucose uptake in rats mesenteric WAT (53), while Trecadrine (another β3-AR agonist) promotes insulin-dependent glucose uptake in cultured rat white adipocytes (81). Conversely, in brown and beige adipose tissue, β3-AR activation enhances thermogenesis by upregulating uncoupling protein 1 (UCP1) expression, and increasing lipolysis-derived free fatty acids and glucose utilization (72, 82, 83). These free fatty acids and glucose serve as fuels for the adaptive thermogenic process, which is crucial for maintaining the energy balance. However, β3-AR-mediated glucose uptake in BAT primarily takes place through dual mechanisms: cAMP-mediated upregulation of GLUT1 expression and mTORC2-dependent translocation of GLUT1 to the plasma membrane, independent of the classical insulin/PI3K/Akt pathway (84–86). Interestingly, β3-AR–stimulated glucose uptake occurs even in the absence of UCP1 (87), indicating that acute glucose uptake is not strictly coupled to thermogenesis but rather mediated by distinct signaling mechanisms. Clinical trials have also confirmed that treatment with mirabegron, a β3-AR agonist, helps to improve metabolic health by enhancing insulin sensitivity, WAT lipolysis, and BAT thermogenesis (72–74). While the glucoregulatory role of β2-AR in WAT remains unclear, it is well characterized in BAT. β2-AR stimulation by its selective agonist, salbutamol, increases both glucose uptake and BAT activity in mice (69), which is further confirmed in human BAT as well (70, 88). In contrast, β1-AR signaling is primarily linked to WAT lipolysis (89) and adipocyte differentiation in both WAT and BAT (67, 68). Although β1-AR may not be the most significant AR in terms of maintaining glucose homeostasis, it has been shown to facilitate glucose uptake in cultured brown adipocytes lacking β3-AR (50). Notably, the distribution and function of β-AR subtypes differs between species, for example, in human, β1- and β2-ARs, but not β3-AR (90), mediate lipolysis in WAT (71). On the other hands, all three subtypes of β-ARs are found in both WAT and BAT of rodents.
In addition to β-ARs, α-adrenergic receptors (α1- and α2-AR) are also expressed in both white and brown adipocytes. Stimulation of α1-AR increases glucose uptake and lactate production in rat white adipocytes that were resistant to insulin, indicating an insulin independent glucose uptake mechanism (47, 48). In support of this finding, α1-AR stimulated increase in glucose uptake and metabolism were also confirmed in human WAT (49, 91). This process is thought to be mediated via the phosphoinositide 3-kinase (PI3K)/protein kinase C (PKC) pathway, which is activated through α1-AR signaling induced by specific agonists and/or neurotransmitters (92). However, while β-adrenoceptors are often considered the primary regulators of thermogenesis in BAT, α-adrenoceptors and their downstream signaling pathways are also crucial for this process. A study conducted by Chernogubova et al. showed that stimulation of α1-adrenergic receptor, in association with β1-AR stimulation, is also involved in glucose uptake using the PI3K/PKC signaling in cultured β3-KO brown adipocytes, and can compensates the lack of β3-AR signaling (50). In addition, dopamine receptors (D1- and D2-subtypes) are also expressed in both humans and rodents white and brown adipocytes, which are involved in the regulation of glucose uptake and lipid metabolism, and adipocyte browning as well (56, 57, 93–95).
Both human and rodent adipose tissue express adenosine receptors (A1, A2A, A2B, and A3 subtypes), which bind to different G-proteins in adipocytes to stimulate or inhibit adenylyl cyclase activity and, consequently, influence glucose homeostasis. The A1-adenosine receptors are highly expressed in WAT and have inhibitory effects on lipolysis and may promote fat storage in adipocytes (96–98). Although a number of studies have investigated the role of A1-adenosine signaling in insulin action and glucose metabolism in white adipocytes in vitro, the findings remain controversial. Pharmacological activation of A1 adenosine receptor in white adipocytes isolated from rats showed decreased insulin sensitivity and glucose uptake (92, 99). Conversely, others found that adenosine increases insulin-stimulated glucose uptake and lipogenesis (62, 100, 101). In vivo studies in rodents have suggested that A1-adenosine receptor activation in WAT improves glucose tolerance and insulin sensitivity, and its deficiency leads to glucose intolerance and impaired insulin action (60, 62, 102). Although A2A and A2B adenosine receptors are expressed in both WAT and BAT; A2A is predominantly expressed in BAT and facilitates thermogenesis and promotes energy expenditure. Deletion or inhibition of A2A receptors reduces BAT thermogenesis, while their activation promotes WAT browning in mice (76). A2B receptors are also abundant in BAT and regulate adipogenesis and BAT functioning in mice and humans (77, 97). The activation/stimulation of A2B receptors protects mice from high-fat diet-induced obesity by increasing BAT-mediated energy expenditure (77). Moreover, it also prevents insulin resistance by inhibiting inflammation in the adipose tissue and regulates glucose homeostasis in diabetic and obese conditions (63).
Free fatty acid receptors (FFARs) are another group of GPCRs highly expressed in the adipose tissue and are crucial for regulating glucose homeostasis by influencing insulin sensitivity. These receptors include FFAR1 (GPR40), FFAR2 (GPR43), FFAR3 (GPR41), and FFAR4 (GPR120). Each receptor is activated by different types of fatty acids, with FFAR1 and FFAR4 responding to medium- and long-chain fatty acids, while FFAR2 and FFAR3 are primarily activated by short-chain fatty acids (SCFAs), and leading to several metabolic outcomes (103, 104). For instance, GPR120 (FFAR4) activation enhances insulin sensitivity and promotes anti-inflammatory responses in adipocytes, which is particularly important in the context of obesity and insulin resistance (65, 66). Dysfunction of GPR120 has been linked to obesity and metabolic disorders, as evidenced by studies demonstrating that its ablation leads to increased adiposity and insulin resistance in both mice and humans (64, 65, 105). Moreover, both increased BAT activity and WAT browning were also reported in mice due to GPR120 activation, which supports its role in thermogenesis (106). FFAR2 and FFAR3 activation have also been shown to influence lipolysis and energy expenditure in the adipose tissue. For example, acetate (a short-chain fatty acid) mediated activation of FFAR2 inhibits lipolysis in human white adipocytes by reducing phosphorylation of hormone-sensitive lipase (107). Furthermore, FFARs can regulate the secretion of adipokines, which are critical for maintaining metabolic balance and responding to changes in nutrient availability (65, 66).
Expression of adhesion GPCRs (aGPCRs) is also evident (about 37% of all aGPCRs) in human and mouse adipose tissues, where a substantial proportion of these receptors is differentially regulated under conditions of obesity and high-fat diets (108). In addition to their expression patterns, the functional relevance of aGPCRs in the adipose tissue is becoming increasingly clear. These receptors are implicated in various signaling pathways that regulate adipocyte function, including adipogenesis, lipolysis, and inflammation (109, 110). For instance, GPR116 has been identified as a key player in mediating insulin-sensitizing effects in white adipose tissue (111). While RNA sequence data revealed the presence of several aGPCRs in the adipose tissue, including ADGRL1-3/LPHN1-3, CD97, GPR125, GPR56, GPR64, and GPR97, their functional relevance in adipose biology is yet to be investigated. Although the role of ADGRL1 in regulating glucose and energy homeostasis was reported recently (17, 18) the contribution of adipocytic ADGRL1 to glucose sensing and responding to changes in blood glucose levels remains to be determined. It is likely that the local adipocyte ADGRL1 is involved in glucose signaling pathways and pathogenesis of type 2 diabetes and obesity.
In summary, the extensive diversity of GPCRs expressed in the adipose tissue and their ability to mediate complex signaling pathways to regulate glucose homeostasis underscore their potential as therapeutic targets for type 2 diabetes, obesity and related metabolic disorders. Future research is expected to elucidate the specific roles of individual GPCRs in the adipose tissue function and their interactions with other metabolic pathways in regulating energy and glucose homeostasis.
Glucose sensing by adipocyte GPCRs
Although glucose sensing and uptake may seem to be the same phenomenon, they occur at different spatial and temporal levels (Figure 1) to complement each other or to accomplish their individual functions. For example, adipocyte glucose sensing involves monitoring systemic or local glucose levels by plasma membrane receptors including GPCRs to influence downstream signaling pathways and thereby, maintain glucose homeostasis. In contrast, glucose uptake and utilization facilitate intracellular metabolism and energy production to support cell growth and proliferation.
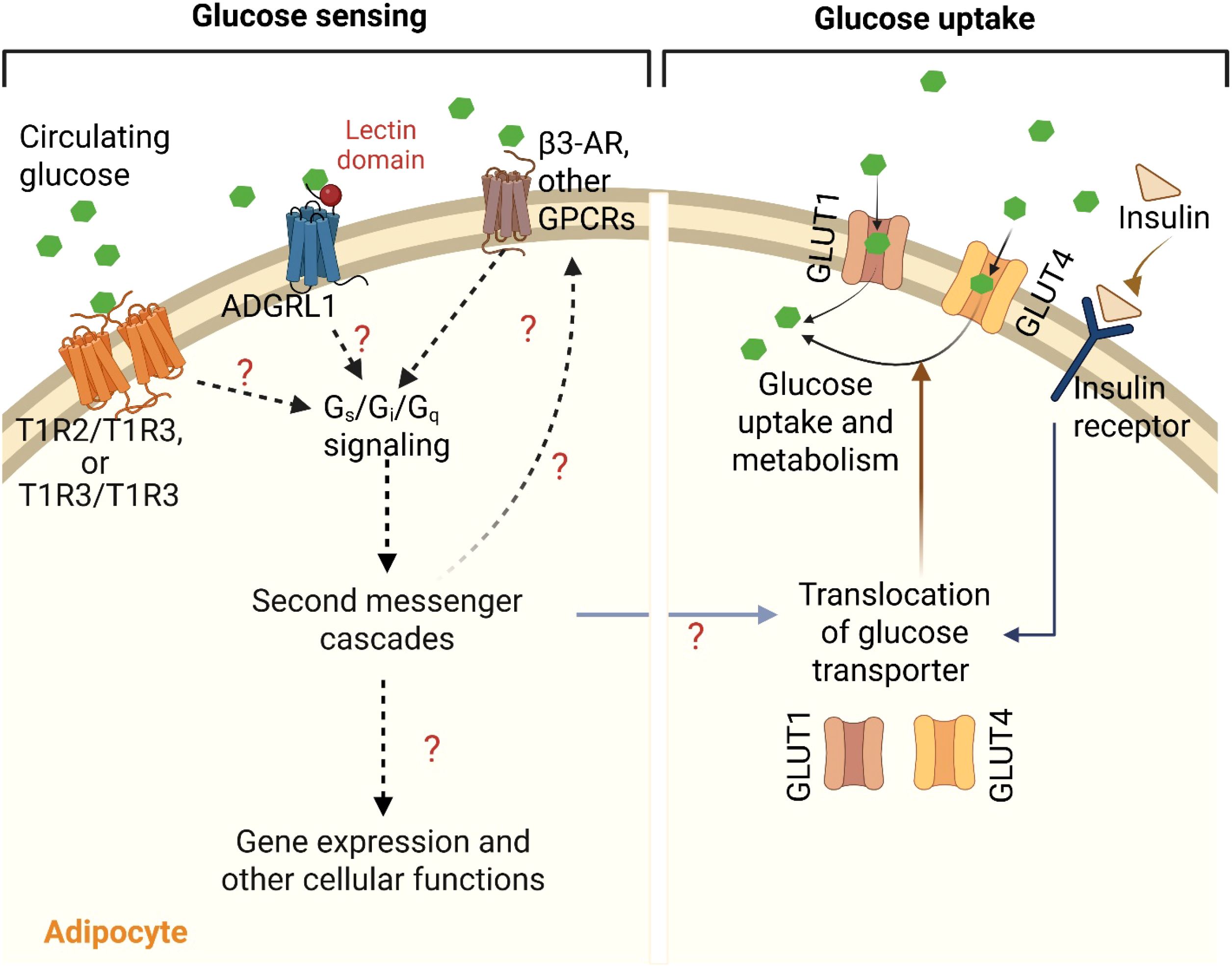
Figure 1. G-protein coupled receptors (GPCRs)-mediated sensing and uptake of glucose in adipocyte. Various GPCRs such as homo- or heterodimers of taste receptors (T1R2/T1R2 and/or T1R2/T1R3) and adhesion G-protein coupled receptor L1 (ADGRL1) sense and bind to the circulating blood glucose and activate Gs, Gi and/or Gq signaling pathways. The resulting second messenger cascades may then regulate insulin sensitivity, translocation of glucose transporters (GLUT1 and GLUT4), activities of other GPCRs including β3-adrenergic receptor, and other cellular and molecular functions in the adipocytes to control glucose uptake, either by insulin-dependent or -independent mechanisms. This figure was created with BioRender.com.
The adipose tissue utilizes a significant amount of glucose for either storage or energy production. The processes of glucose sensing and cellular glucose uptake are key steps involved in glucose homeostasis by the adipose tissue. In postprandial state, upon sensing blood glucose levels, pancreatic beta-cells secrete insulin that binds and activates insulin receptors on the adipocyte’s membrane. Activated insulin receptors then initiate a signaling cascade involving phosphoinositide 3-kinase (PI3K) and protein kinase B (Akt), which are critical for the translocation of GLUT4 on the cell surface and thus, allows glucose entry into the adipocytes from the blood (112, 113). While GLUT4 is the predominant glucose transporter in adipocytes, another transporter -GLUT1 - also contributes to an insulin-independent glucose uptake (114). However, beyond insulin signaling, GPCRs act as integral mediators of glucose sensing and metabolism within the adipose tissue, influencing glucose sensing, insulin sensitivity and glucose uptake through diverse signaling pathways. Several GPCR families in adipocytes participate in glucose sensing through Gq, Gi, and/or Gs signaling. Chemogenetic stimulation of Gs signaling in adipocytes resulted in a significant reduction in blood glucose levels, indicating its role in improving glucose tolerance (115). Likewise, Kimura et al. have also reported the involvement of Gq signaling on glucose uptake in the adipose tissue to improve glucose homeostasis in mice (116). In addition to Gs and Gq signaling, Gi signaling is also crucial for maintaining glucose homeostasis in adipocytes. Wang et al. have shown that Gi signaling is essential for preserving insulin sensitivity and regulating glucose metabolism in the adipose tissue (117). Therefore, the balance between these GPCR signaling pathways is crucial for the proper metabolic function of adipocytes.
How adipocytes detect changes in blood glucose levels, and how adipocytic GPCRs and their signaling pathways respond to these changes to regulate glucose homeostasis remain unclear. The sweet taste receptors (particularly T1R2 and T1R3) expressed in the adipose tissue are potential candidates for direct glucose sensing by adipocytes. Several studies have shown their roles in regulating glucose homeostasis, adipogenesis and lipolysis (118–121). Although the precise mechanism by which these taste receptors sense glucose in adipocytes is yet to be elucidated, their capacity to sense sugars including glucose by forming hetero (T1R2/T1R3)- and homo (T1R3/T1R3)-dimers in other tissues like intestine and skeletal muscle are well known (16, 122). Masubuchi et al. reported that activation of T1R3 homomeric receptors reduces insulin-induced GLUT4 translocation and glucose transport in a Gs-dependent, cAMP-independent manner (123).
T1R3 knockout mouse models exhibit impaired glucose clearance, reduced insulin sensitivity, and increased adiposity, highlighting the importance of T1R3 in maintaining glucose homeostasis (124, 125). Moreover, activation of T1R2/T1R3 can also indirectly impact glucose uptake by stimulating the release of incretin hormones like GLP-1, which enhances insulin secretion and glucose uptake in peripheral tissues, including the adipose tissue (126–128). Future studies targeting adipocyte-specific knockout of T1R2 and T1R3 may provide better mechanistic insights regarding how these GPCRs are involved in glucose homeostasis. In addition, ADGRL1 may also contribute to glucose sensing in adipocytes as the receptor was recently shown to bind and sense glucose in the hypothalamus (17, 20), which also warrants further investigation.
The involvement of β-ARs in regulating glucose uptake in adipocytes, either by insulin dependent or independent mechanisms, is well known as we discussed in the earlier section. For example, β3-AR, coupled to Gs protein, stimulates GLUT1 translocation to the membrane in brown adipocytes, increasing glucose uptake independently of insulin, through cAMP-dependent mechanisms and mTORC2 activation (84–86, 129). Similarly, ligand (salbutamol)-mediated activation of β2-AR, has also been shown to increase glucose uptake in human BAT, but not in WAT (70). In tissues such as the brain, liver, and intestine, the β-ARs are involved in glucose sensing (130–132), which may be tied to the role of the receptors in affecting glucose uptake by their interactions with glucose transporters through downstream signaling pathways. Based on these reports, we speculate that the effects of β-ARs on glucose uptake in the adipose tissue may be associated with glucose sensing via communications between the receptors and glucose transporters (Figure 1). This may explain the adaptability of the adipose tissue under different glucose levels.
Altogether, adipocyte GPCRs may contribute to direct glucose sensing in addition to their role in glucose uptake and metabolism in the adipose tissue (Figure 1). Determining the molecular mechanisms involved in interactions between adipocyte GPCRs, glucose sensing, glucose transporters, and glucose metabolites will help develop more effective strategies to manage metabolic disorders associated with impaired energy and glucose balance.
GPCR-mediated crosstalk of the adipose tissue with other central and peripheral organs to regulate glucose homeostasis
The brain-adipose tissue axis
Depending on the glycemic status, the hypothalamus regulates the secretion of the pancreatic hormones (e.g., insulin and glucagon) through the autonomic [parasympathetic (PNS) and sympathetic (SNS)] nervous systems to maintain euglycemia. The mechanisms governing PNS- and SNS-mediated insulin secretion have been comprehensively reviewed by Valentine S. Moullé (133). Neurotransmitters released due to the activation of these autonomic pathways, such as acetylcholine and adrenaline, activate specific GPCRs (e.g., muscarinic and adrenergic receptors) in pancreatic β-cells, triggering downstream signaling cascades through G-proteins (Gq, Gi, and Gs) to regulate insulin release. For instance, activation of α1-adrenergic receptor (α1-AR) and muscarinic receptor 3 (M3R) leads to Gq and Gi signaling, which enhances insulin secretion by increasing intracellular Ca²+ levels. Conversely, α2-adrenergic receptor (α2-AR)-mediated Gs signaling inhibits insulin secretion by elevating intracellular K+ levels (133). These pancreas-secreted hormones are then transported throughout the body via circulation and regulate systemic glucose homeostasis through different tissues including the adipose tissue.
Given that glucose uptake in the adipose tissue is largely insulin-dependent (134) and the insulin sensitivity decreases with an increased adiposity (135, 136), an enhanced insulin secretion from the β-cells and/or an increase in insulin sensitivity are necessary for maintaining glucose homeostasis (137). This is accomplished through the combined contribution of the hypothalamus, β-cells, and the adipose tissue. For example, decreased insulin-dependent glucose uptake in WAT of hypothalamic ADGRL1-deficient mice was reported recently (17). The mice also had impaired insulin secretion probably associated with enhanced vagus nerve activity, since pancreatic vagotomy reversed insulin hypersecretion in the ADGRL1-deficient mice. Further studies are required to investigate whether hypothalamic ADGRL1 regulates SNS activity to influence insulin-induced glucose transport in adipocytes (Figure 2).
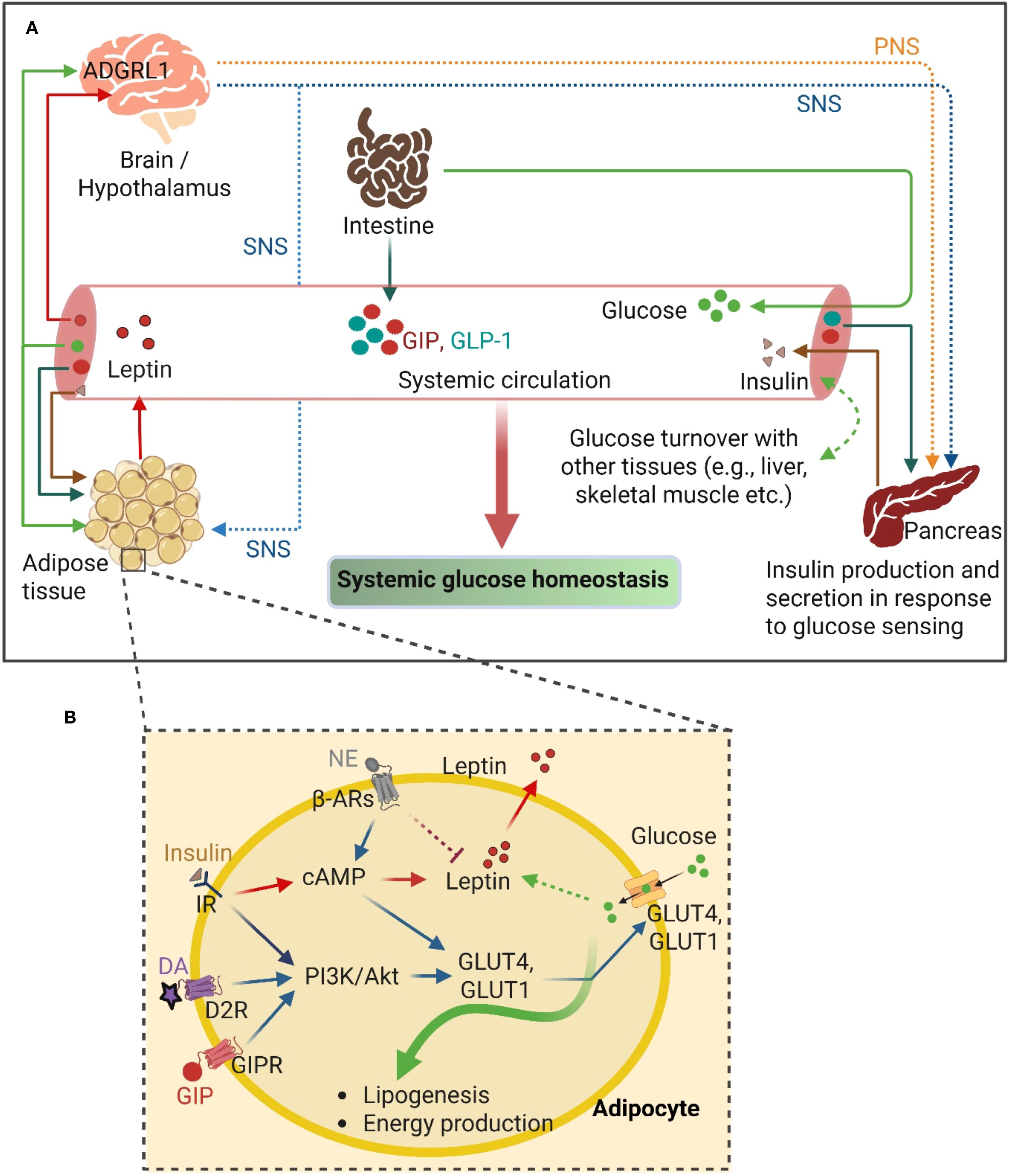
Figure 2. GPCR-mediated crosstalk between the adipose tissue and other organs to regulate glucose homeostasis. (A) The overview of the interconnecting pathways between adipose tissue and other central and peripheral organs, including the intestine and pancreas, regulating the systemic glucose homeostasis. (B) GPCR-mediated signaling mechanisms in adipocyte involving glucose uptake and utilization. PNS, Parasympathetic nervous system; SNS, Sympathetic nervous system; GIP, Glucose-dependent insulinotropic polypeptide; GIPR, Glucose-dependent insulinotropic polypeptide receptor; GLP-1, Glucagon-like peptide-1; NE, Norepinephrine; DA, Dopamine; D2R, Dopamine receptor D2; β-ARs, β-adrenergic receptors; PI3K, Phosphoinositide 3-Kinase; Akt, Protein kinase B; cAMP, Cyclic adenosine monophosphate; IR, Insulin receptor; GLUT4 and GLUT1, Glucose Transporter 4 and 1; respectively. ADGRL1, Adhesion G-protein coupled receptor L1. This figure was created with BioRender.com.
Norepinephrine secreted from the activated SNS functions through the adipocytic β3-AR to enhance glucose uptake and thermogenesis (87). In addition, dopamine, a neurotransmitter, secreted from SNS activation binds to the D2-like dopamine receptors (mainly D2R and D3R, members of the GPCR family) in the pancreatic beta-cells and negatively regulates glucose-stimulated insulin secretion (138–140). Dopamine also directly regulates glucose uptake in insulin-sensitive tissues, including WAT, liver, and skeletal muscle, acting through the dopamine receptors. Dopamine administration has been reported to directly enhance glucose uptake in WAT and the liver via D2R (57). D2R activation also modulates the secretion of adipokines, including leptin and adiponectin, the critical regulators of systemic energy balance (56) (Figure 2).
In addition to its roles in regulating insulin action and glucose uptake in adipose tissue, SNS also triggers others metabolic pathways including- lipolysis, browning of WAT and thermogenesis in adipose tissues, which are crucial for the maintenance of systemic glucose homeostasis. In WAT, SNS-mediated stimulation of adrenergic signaling promotes lipolysis and provides free fatty acids and glycerol for systemic energy supply and gluconeogenesis, respectively (78, 141, 142). Furthermore, adrenergic signaling also stimulates thermogenic UCP1 expression in brown and beige adipocytes, which facilitates thermogenesis (83). A significant amount of glucose and free fatty acids are utilized in this thermogenic process, and thereby improving systemic glucose clearance. Collectively, these SNS-driven processes integrate brain-adipose tissue communication to regulate lipid and glucose metabolism, ultimately contributing to whole-body energy homeostasis.
The intestine-adipose tissue axis
Incretin hormones (GIP and GLP-1) are secreted from the intestinal cells upon glucose sensing by the gastrointestinal tract postprandially (143). The glucose homeostatic regulatory function of these incretins is largely mediated by their insulinotropic and glucagonotropic functions on the pancreas, such as by augmenting the insulin secretion from the pancreatic beta-cells (144, 145). At the pancreatic endocrine cells, GIP and GLP-1 bind to their respective G-protein coupled receptors (GIPR and GLP-1R), and transduce signal to increase cAMP production and protein kinase B (AKT) activation, which finally enhances insulin secretion from the beta-cells (146–148). Interestingly, the incretins - specifically GIP – also directly regulate glucose uptake in the adipose tissue by binding to GIPR in adipocytes. For instance, GIP-stimulated glucose uptake in 3T3-L1 adipocytes (in the presence of insulin) was reported over 20 years ago by Miyawaki et al (149). Later, Song et al. demonstrated that GIP has insulin-mimetic effects on glucose uptake in 3T3-L1 adipocytes, which is mediated through the activation of Akt via wortmannin (a potent inhibitor of PI3K)-sensitive pathway, at least partly, which promotes GLUT4 translocation to the adipocyte membrane to enhance glucose uptake (150). Although an earlier study reported that GIPR is predominantly expressed in non-adipocytes in the adipose tissue (151), recently Regmi et al. have reconfirmed the expression of functional GIPR in both human and mouse adipocytes (152). The authors demonstrated that activation of GIPR-signaling upon binding through GIP and/or tirzepatide (a dual agonist of GIPR/GLP-1R) enhances both insulin-dependent and -independent glucose uptake differentiated in human adipocytes (152). Moreover, abolishment of the gluco- and lipo-regulatory effects of GIP in lean human adipose tissue was demonstrated with the infusion of GIP(3-30)NH2, an antagonist of human GIPR, during hyperglycemic-hyperinsulinemic clamps (153). In contrast, BAT-specific deletion of GIPR in mice showed no significant alteration in glucose homeostasis (154). These findings indicate that GIPR signaling in BAT may be dispensable for glucose regulation, while GIPR signaling in WAT contributes to adipose tissue glucose uptake predominantly. Collectively, the adipose tissue receives glucose-sensing signals from the intestine through incretins to modulate the local adipocyte regulation of glucose homeostasis via GPCRs (Figure 2).
Future perspective and concluding remarks
Most studies have focused on glucose uptake, utilization, and its metabolism to establish the role of the adipose tissue in regulating glucose homeostasis. Although great progress has been made in this area of research, glucose sensing aspects of the adipose tissue remain unclear. For example, the following questions are largely unaddressed: how does the adipose tissue sense blood or local glucose levels? What are the molecular mechanisms through which the adipose tissue responds to the changes in glucose levels to restore homeostasis? Investigating adipocytic GPCRs and associated transduction pathways including the transcription factors may provide novel insights into the molecular machinery involved in glucose sensing and responding to changes in systemic or local glucose levels. This topic will open new research avenues for investigating glucose signaling pathways in the adipose tissue independently of glucose metabolism or its transport.
Author contributions
NH: Funding acquisition, Conceptualization, Writing – original draft, Writing – review & editing, Investigation. KC: Funding acquisition, Project administration, Supervision, Resources, Writing – review & editing, Writing – original draft, Investigation, Visualization, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Gatton Foundation Post-doctoral Fellowship to NH, start-up funds from the University of Kentucky, the National Institutes of Health grants DK124619 and DK140148, and the Del Monte Institute for Neuroscience Pilot Research Award, University of Rochester to KC. The funders had no role in study design, data collection, data analysis, or manuscript preparation.
Acknowledgments
Figures were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu S, Anderson PJ, Rajagopal S, Lefkowitz RJ, and Rockman HA. G protein-coupled receptors: A century of research and discovery. Circ Res. (2024) 135:174–97. doi: 10.1161/CIRCRESAHA.124.323067
2. Weis WI and Kobilka BK. The molecular basis of G protein–coupled receptor activation. Annu Rev Biochem. (2018) 87:897–919. doi: 10.1146/annurev-biochem-060614-033910
3. Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin J-P, Davenport AP, et al. International union of pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. (2005) 57:279–88. doi: 10.1124/pr.57.2.5
4. Schiöth HB and Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. (2005) 142:94–101. doi: 10.1016/j.ygcen.2004.12.018
5. Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, and Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. (2017) 16:829–42. doi: 10.1038/nrd.2017.178
6. Mayer J. Glucostatic mechanism of regulation of food intake. New Engl J Med. (1953) 249:13–6. doi: 10.1056/NEJM195307022490104
7. Anand BK, Chhina GS, Sharma KN, Dua S, and Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiology-Legacy Content. (1964) 207:1146–54. doi: 10.1152/ajplegacy.1964.207.5.1146
8. Melnick IV, Price CJ, and Colmers WF. Glucosensing in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. (2011) 34:272–82. doi: 10.1111/j.1460-9568.2011.07742.x
9. Choi JH and Kim M-S. Homeostatic regulation of glucose metabolism by the central nervous system. Endocrinol Metab. (2022) 37:9–25. doi: 10.3803/EnM.2021.1364
10. Sohn J-W and Ho W-K. Cellular and systemic mechanisms for glucose sensing and homeostasis. Pflügers Archiv - Eur J Physiol. (2020) 472:1547–61. doi: 10.1007/s00424-020-02466-2
11. Scrocchi LA, Marshall BA, Cook SM, Brubaker PL, and Drucker DJ. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes. (1998) 47:632–9. doi: 10.2337/diabetes.47.4.632
12. Doyle ME and Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. (2007) 113:546–93. doi: 10.1016/j.pharmthera.2006.11.007
13. Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduction Targeted Ther. (2024) 9. doi: 10.1038/s41392-024-01931-z
14. Daly K, Al-Rammahi M, Arora DK, Moran AW, Proudman CJ, Ninomiya Y, et al. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am J Physiology-Regulatory Integr Comp Physiol. (2012) 303:R199–208. doi: 10.1152/ajpregu.00031.2012
15. Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, and Bravo D. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr. (2014) 111:S8–S15. doi: 10.1017/S0007114513002286
16. Serrano J, Boyd J, Brown IS, Mason C, Smith KR, Karolyi K, et al. The TAS1R2 G-protein-coupled receptor is an ambient glucose sensor in skeletal muscle that regulates NAD homeostasis and mitochondrial capacity. Nat Commun. (2024) 15. doi: 10.1038/s41467-024-49100-8
17. Chhabra KH, Bathina S, Faniyan TS, Samuel DJ, Raza MU, De Souza Cordeiro LM, et al. ADGRL1 is a glucose receptor involved in mediating energy and glucose homeostasis. Diabetologia. (2024) 67:170–89. doi: 10.1007/s00125-023-06010-6
18. Dietzsch AN, Al-Hasani H, Altschmied J, Bottermann K, Brendler J, Haendeler J, et al. Dysfunction of the adhesion G protein-coupled receptor latrophilin 1 (ADGRL1/LPHN1) increases the risk of obesity. Signal Transduction Targeted Ther. (2024) 9. doi: 10.1038/s41392-024-01810-7
19. Rashid N and Chhabra KH. A protocol to isolate, identify, and verify glucose- or carbohydrate-binding receptors. Biol Methods Protoc. (2024) 9. doi: 10.1093/biomethods/bpae045
20. Chhabra KH. Adhesion G protein-coupled receptor latrophilin 1 (ADGRL1): a novel regulator of glucose and energy homeostasis. Signal Transduction Targeted Ther. (2024) 9. doi: 10.1038/s41392-024-01925-x
21. Zwick RK, Guerrero-Juarez CF, Horsley V, and Plikus MV. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. (2018) 27:68–83. doi: 10.1016/j.cmet.2017.12.002
22. Hagberg CE and Spalding KL. White adipocyte dysfunction and obesity-associated pathologies in humans. Nat Rev Mol Cell Biol. (2024) 25:270–89. doi: 10.1038/s41580-023-00680-1
23. Valenzuela PL, Carrera-Bastos P, Castillo-García A, Lieberman DE, Santos-Lozano A, and Lucia A. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol. (2023) 20:475–94. doi: 10.1038/s41569-023-00847-5
24. Chait A and Den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7. doi: 10.3389/fcvm.2020.00022
25. Raheem J, Sliz E, Shin J, Holmes MV, Pike GB, Richer L, et al. Visceral adiposity is associated with metabolic profiles predictive of type 2 diabetes and myocardial infarction. Commun Med. (2022) 2. doi: 10.1038/s43856-022-00140-5
26. Després J-P and Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. doi: 10.1038/nature05488
27. Gadgil MD, Cheng J, Herrington DM, Kandula NR, and Kanaya AM. Adipose tissue-derived metabolite risk scores and risk for type 2 diabetes in South Asians. Int J Obes. (2024) 48:668–73. doi: 10.1038/s41366-023-01457-4
28. Park S and Shimokawa I. Influence of adipokines on metabolic dysfunction and aging. Biomedicines. (2024) 12:873. doi: 10.3390/biomedicines12040873
29. Castela I, Morais J, Barreiros-Mota I, Silvestre MP, Marques C, Rodrigues C, et al. Decreased adiponectin/leptin ratio relates to insulin resistance in adults with obesity. Am J Physiology-Endocrinology Metab. (2023) 324:E115–9. doi: 10.1152/ajpendo.00273.2022
30. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. New Engl J Med. (2009) 360:1509–17. doi: 10.1056/NEJMoa0810780
31. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. New Engl J Med. (2009) 360:1518–25. doi: 10.1056/NEJMoa0808949
32. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci. (2017) 114:8649–54. doi: 10.1073/pnas.1705287114
33. Cypess AM. Reassessing human adipose tissue. New Engl J Med. (2022) 386:768–79. doi: 10.1056/NEJMra2032804
34. Cohen P and Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol. (2021) 22:393–409. doi: 10.1038/s41580-021-00350-0
35. Rahbani JF, Roesler A, Hussain MF, Samborska B, Dykstra CB, Tsai L, et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature. (2021) 590:480–5. doi: 10.1038/s41586-021-03221-y
36. Rahbani JF, Bunk J, Lagarde D, Samborska B, Roesler A, Xiao H, et al. Parallel control of cold-triggered adipocyte thermogenesis by UCP1 and CKB. Cell Metab. (2024) 36:526–540.e527. doi: 10.1016/j.cmet.2024.01.001
37. Vargas-Castillo A, Torres N, and Tovar AR. Endocrine Regulation of Brown and Beige Adipose Tissue. Elsevier (2021) p. 247–59.
38. Ghesmati Z, Rashid M, Fayezi S, Gieseler F, Alizadeh E, and Darabi M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev Endocrine Metab Disord. (2024) 25:279–308. doi: 10.1007/s11154-023-09850-0
39. Harms M and Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. (2013) 19:1252–63. doi: 10.1038/nm.3361
40. Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C, et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes. (2016) 40:1655–61. doi: 10.1038/ijo.2016.124
41. Blondin DP, Tingelstad HC, Noll C, Frisch F, Phoenix S, Guérin B, et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun. (2017) 8:14146. doi: 10.1038/ncomms14146
42. Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. (2014) 63:4089–99. doi: 10.2337/db14-0746
43. Sidossis LS, Porter C, Saraf M, Børsheim E, Radhakrishnan RS, Chao T, et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. (2015) 22:219–27. doi: 10.1016/j.cmet.2015.06.022
44. Chondronikola M, Volpi E, Børsheim E, Porter C, Saraf MK, Annamalai P, et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab. (2016) 23:1200–6. doi: 10.1016/j.cmet.2016.04.029
45. Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res. (2018) 59:784–94. doi: 10.1194/jlr.M079665
46. Al Mahri S, Okla M, Rashid M, Malik SS, Iqbal J, Al Ibrahim M, et al. Profiling of G-protein coupled receptors in adipose tissue and differentiating adipocytes offers a translational resource for obesity/metabolic research. Cells. (2023) 12. doi: 10.3390/cells12030377
47. Faintrenie G and Géloën A. Alpha-1 adrenergic regulation of lactate production by white adipocytes. J Pharmacol Exp Ther. (1996) 277:235–8. doi: 10.1016/S0022-3565(25)12814-0
48. Faintrenie G and Géloën A. Alpha-1 adrenergic stimulation of glucose uptake in rat white adipocytes. J Pharmacol Exp Ther. (1998) 286:607–10. doi: 10.1016/S0022-3565(24)37629-3
49. Boschmann M, Krupp G, Luft FC, Klaus S, and Jordan J. In vivo response to α1-adrenoreceptor stimulation in human white adipose tissue. Obes Res. (2002) 10:555–8. doi: 10.1038/oby.2002.75
50. Chernogubova E, Hutchinson DS, Nedergaard J, and Bengtsson T. α1- and β1-adrenoceptor signaling fully compensates for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology. (2005) 146:2271–84. doi: 10.1210/en.2004-1104
51. Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper M-E, et al. Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem. (1995) 270:29483–92. doi: 10.1074/jbc.270.49.29483
52. Soeder KJ, Snedden SK, Cao W, Della Rocca GJ, Daniel KW, Luttrell LM, and Collins S. The β3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. Journal of Biological Chemistry. (1999) 274(17):12017–22. doi: 10.1074/jbc.274.17.12017
53. Ishino S, Sugita T, Kondo Y, Okai M, Tsuchimori K, Watanabe M, et al. Glucose uptake of the muscle and adipose tissues in diabetes and obesity disease models: evaluation of insulin and β3-adrenergic receptor agonist effects by 18F-FDG. Ann Nucl Med. (2017) 31:413–23. doi: 10.1007/s12149-017-1169-0
54. Ceddia R and Collins S. A compendium of G-protein–coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin Sci. (2020) 134:473–512. doi: 10.1042/CS20190579
55. Neve KA, Seamans JK, and Trantham-Davidson H. Dopamine receptor signaling. Journal of Receptors and Signal Transduction. (2004) 24(3):165–205. doi: 10.1081/rrs-200029981
56. Wang X, Villar VA, Tiu A, Upadhyay KK, and Cuevas S. Dopamine D2 receptor upregulates leptin and IL-6 in adipocytes. J Lipid Res. (2018) 59:607–14. doi: 10.1194/jlr.M081000
57. Tavares G, Martins FO, Melo BF, Matafome P, and Conde SV. Peripheral dopamine directly acts on insulin-sensitive tissues to regulate insulin signaling and metabolic function. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.713418
58. Cheng JT, Liu IM, Chi TC, Shinozuka K, Lu FH, Wu TJ, et al. Role of adenosine in insulin-stimulated release of leptin from isolated white adipocytes of Wistar rats. Diabetes. (2000) 49:20–4. doi: 10.2337/diabetes.49.1.20
59. Rice AM, Fain JN, and Rivkees SA. A1 adenosine receptor activation increases adipocyte leptin secretion. Endocrinology. (2000) 141(4):1442–5. doi: 10.1210/endo.141.4.7423
60. Dong Q, Ginsberg HN, and Erlanger BF. Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes Obes Metab. (2001) 3:360–6. doi: 10.1046/j.1463-1326.2001.00158.x
61. Eisenstein A and Ravid K. G protein-coupled receptors and adipogenesis: a focus on adenosine receptors. J Cell Physiol. (2014) 229:414–21. doi: 10.1002/jcp.24473
62. Granade ME, Hargett SR, Lank DS, Lemke MC, Luse MA, Isakson BE, et al. Feeding desensitizes A1 adenosine receptors in adipose through FOXO1-mediated transcriptional regulation. Mol Metab. (2022) 63:101543. doi: 10.1016/j.molmet.2022.101543
63. Csóka B, Koscsó B, Törő G, Kókai E, Virág L, Németh ZH, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. (2014) 63:850–66. doi: 10.2337/db13-0573
64. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. (2010) 142:687–98. doi: 10.1016/j.cell.2010.07.041
65. Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. (2012) 483:350–4. doi: 10.1038/nature10798
66. Miyamoto J, Kasubuchi M, Nakajima A, and Kimura I. Anti-Inflammatory and Insulin-Sensitizing Effects of Free Fatty Acid Receptors. Cham: Springer International Publishing (2016) p. 221–31.
67. Bronnikov G, Houstĕk J, and Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J Biol Chem. (1992) 267:2006–13. doi: 10.1016/S0021-9258(18)46046-2
68. Evans BA, Merlin J, Bengtsson T, and Hutchinson DS. Adrenoceptors in white, brown, and brite adipocytes. Br J Pharmacol. (2019) 176:2416–32. doi: 10.1111/bph.14631
69. Ernande L, Stanford KI, Thoonen R, Zhang H, Clerte M, Hirshman MF, et al. Relationship of brown adipose tissue perfusion and function: a study through β2-adrenoreceptor stimulation. J Appl Physiol. (2016) 120:825–32. doi: 10.1152/japplphysiol.00634.2015
70. Straat ME, Hoekx CA, Van Velden FHP, Pereira Arias-Bouda LM, Dumont L, Blondin DP, et al. Stimulation of the beta-2-adrenergic receptor with salbutamol activates human brown adipose tissue. Cell Rep Med. (2023) 4:100942. doi: 10.1016/j.xcrm.2023.100942
71. De Jong KA, Siddig S, Pfeifer A, and Nikolaev VO. The role of compartmentalized β-AR/cAMP signaling in the regulation of lipolysis in white and brown adipocytes. FEBS J. (2025) 292:261–71. doi: 10.1111/febs.17157
72. Cypess A, Weiner L, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn P, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. (2015) 21:33–8. doi: 10.1016/j.cmet.2014.12.009
73. O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest. (2020) 130:2209–19. doi: 10.1172/JCI131126
74. Cero C, Lea HJ, Zhu KY, Shamsi F, Tseng Y-H, and Cypess AM. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.139160
75. Peres Valgas Da Silva C, Calmasini F, Alexandre EC, Raposo HF, Delbin MA, Monica FZ, et al. The effects of mirabegron on obesity-induced inflammation and insulin resistance are associated with brown adipose tissue activation but not beiging in the subcutaneous white adipose tissue. Clinical and Experimental Pharmacology and Physiology. (2021) 48(11):1477–87. doi: 10.1111/1440-1681.13566
76. Gnad T, Scheibler S, Von Kügelgen I, Scheele C, Kilić A, Glöde A, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. (2014) 516:395–9. doi: 10.1038/nature13816
77. Gnad T, Navarro G, Lahesmaa M, Reverte-Salisa L, Copperi F, Cordomi A, et al. Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metab. (2020) 32:56–70.e57. doi: 10.1016/j.cmet.2020.06.006
78. Collins S. β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front Endocrinol. (2011) 2. doi: 10.3389/fendo.2011.00102
79. Barella LF, Jain S, Kimura T, and Pydi SP. Metabolic roles of G protein-coupled receptor signaling in obesity and type 2 diabetes. FEBS J. (2021) 288:2622–44. doi: 10.1111/febs.15800
80. Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. (2000) 43:533–49. doi: 10.1007/s001250051341
81. Moreno-Aliaga MJ, Lamas O, Marti A, and Martínez JA. Effects of a β3-adrenergic agonist on glucose uptake and leptin expression and secretion in cultured adipocytes from lean and overweight (Cafeteria) rats. Biochem Biophys Res Commun. (2002) 291:1201–7. doi: 10.1006/bbrc.2002.6577
82. Lowell MPBB and Flier MJS. Brown Adipose Tissue, β3-Adrenergic Receptors, And Obesity. Annu Rev Med. (1997) 48:307–16. doi: 10.1146/annurev.med.48.1.307
83. Sveidahl Johansen O, Ma T, and Gerhart-Hines Z. Leveraging GPCR signaling in thermogenic fat to counteract metabolic diseases. Mol Metab. (2022) 60:101474. doi: 10.1016/j.molmet.2022.101474
84. Dallner OS, Chernogubova E, Brolinson KA, and Bengtsson T. β3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology. (2006) 147:5730–9. doi: 10.1210/en.2006-0242
85. Olsen JM, Sato M, Dallner OS, Sandström AL, Pisani DF, Chambard J-C, et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2–promoted GLUT1 translocation. J Cell Biol. (2014) 207:365–74. doi: 10.1083/jcb.201403080
86. Olsen JM, Åslund A, Bokhari MH, Hutchinson DS, and Bengtsson T. Acute β-adrenoceptor mediated glucose clearance in brown adipose tissue; a distinct pathway independent of functional insulin signaling. Mol Metab. (2019) 30:240–9. doi: 10.1016/j.molmet.2019.10.004
87. Olsen JM, Csikasz RI, Dehvari N, Lu L, Sandström A, Öberg AI, et al. β 3 -Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: Mediation through the mTOR pathway. Mol Metab. (2017) 6:611–9. doi: 10.1016/j.molmet.2017.02.006
88. Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, et al. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab. (2020) 32:287–300.e287. doi: 10.1016/j.cmet.2020.07.005
89. Louis SN, Jackman GP, Nero TL, Iakovidis D, and Louis WJ. Role of beta-adrenergic receptor subtypes in lipolysis. Cardiovasc Drugs Ther. (2000) 14:565–77. doi: 10.1023/A:1007838125152
90. Rosenbaum M, Malbon CC, Hirsch J, and Leibel RL. Lack of beta 3-adrenergic effect on lipolysis in human subcutaneous adipose tissue. J Clin Endocrinol Metab. (1993) 77:352–5. doi: 10.1210/jcem.77.2.8393882
91. Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, and Ditschuneit HH. Sympathetic regulation of glucose uptake by the α1-adrenoceptor in human obesity. Obes Res. (2004) 12:612–20. doi: 10.1038/oby.2004.70
92. Cheng J-T, Liu IM, Yen S-T, and Chen P-C. Role of α1A-adrenoceptor in the regulation of glucose uptake into white adipocyte of rats in vitro. Autonomic Neurosci. (2000) 84:140–6. doi: 10.1016/S1566-0702(00)00197-1
93. Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J, et al. Dopamine receptors in human adipocytes: expression and functions. PloS One. (2011) 6:e25537. doi: 10.1371/journal.pone.0025537
94. Brizuela M, Antipov A, Blessing WW, and Ootsuka Y. Activating dopamine D2 receptors reduces brown adipose tissue thermogenesis induced by psychological stress and by activation of the lateral habenula. Sci Rep. (2019) 9. doi: 10.1038/s41598-019-56125-3
95. Yu J, Zhu J, Deng J, Shen J, Du F, Wu X, et al. Dopamine receptor D1 signaling stimulates lipolysis and browning of white adipocytes. Biochem Biophys Res Commun. (2022) 588:83–9. doi: 10.1016/j.bbrc.2021.12.040
96. Dhalla AK, Chisholm JW, Reaven GM, and Belardinelli L. A1 Adenosine Receptor: Role in Diabetes and Obesity. Berlin, Heidelberg: Springer (2009) p. 271–95.
97. Eisenstein A, Carroll SH, Johnston-Cox H, Farb M, Gokce N, and Ravid K. An adenosine receptor-Krüppel-like factor 4 protein axis inhibits Adipogenesis. J Biol Chem. (2014) 289:21071–81. doi: 10.1074/jbc.M114.566406
98. Antonioli L, Blandizzi C, Csóka B, Pacher P, and Haskó G. Adenosine signalling in diabetes mellitus—pathophysiology and therapeutic considerations. Nat Rev Endocrinol. (2015) 11:228–41. doi: 10.1038/nrendo.2015.10
99. Koupenova M and Ravid K. Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism. J Cell Physiol. (2013) 228:1703–12. doi: 10.1002/jcp.24352
100. Joost HG and Steinfelder HJ. Modulation of insulin sensitivity by adenosine. Effects on glucose transport, lipid synthesis, and insulin receptors of the adipocyte. Mol Pharmacol. (1982) 22:614–8. doi: 10.1016/S0026-895X(25)15176-6
101. Heseltine L, Webster JM, and Taylor R. Adenosine effects upon insulin action on lipolysis and glucose transport in human adipocytes. Mol Cell Biochem. (1995) 144:147–51. doi: 10.1007/BF00944394
102. Faulhaber-Walter R, Jou W, Mizel D, Li L, Zhang J, Kim SM, et al. Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes. (2011) 60:2578–87. doi: 10.2337/db11-0058
103. Kimura I, Ichimura A, Ohue-Kitano R, and Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. (2020) 100:171–210. doi: 10.1152/physrev.00041.2018
104. Al Mahri S, Malik SS, Al Ibrahim M, Haji E, Dairi G, and Mohammad S. Free fatty acid receptors (FFARs) in adipose: physiological role and therapeutic outlook. Cells. (2022) 11:750. doi: 10.3390/cells11040750
105. Codoñer-Alejos A, Carrasco-Luna J, Carrasco-García Á, and Codoñer-Franch P. Reduced free fatty acid receptor 4 gene expression is associated with extreme obesity and insulin resistance in children. J Pediatr Gastroenterol Nutr. (2022) 74:535–40. doi: 10.1097/MPG.0000000000003360
106. Quesada-López T, Cereijo R, Turatsinze J-V, Planavila A, Cairó M, Gavaldà-Navarro A, et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun. (2016) 7:13479. doi: 10.1038/ncomms13479
107. Jocken JWE, González Hernández MA, Hoebers NTH, van der Beek CM, Essers YPG, Blaak EE, et al. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front Endocrinol. (2018) 8. doi: 10.3389/fendo.2017.00372
108. Suchý T, Zieschang C, Popkova Y, Kaczmarek I, Weiner J, Liebing A-D, et al. The repertoire of Adhesion G protein-coupled receptors in adipocytes and their functional relevance. Int J Obes. (2020) 44:2124–36. doi: 10.1038/s41366-020-0570-2
109. Nie T, Hui X, Gao X, Li K, Lin W, Xiang X, et al. Adipose tissue deletion of Gpr116 impairs insulin sensitivity through modulation of adipose function. FEBS Lett. (2012) 586:3618–25. doi: 10.1016/j.febslet.2012.08.006
110. Al Hasan M, Roy P, Dolan S, Martin PE, Patterson S, and Bartholomew C. Adhesion G-protein coupled receptor 56 is required for 3T3-L1 adipogenesis. J Cell Physiol. (2020) 235:1601–14. doi: 10.1002/jcp.29079
111. Georgiadi A, Lopez-Salazar V, Merahbi RE, Karikari RA, Ma X, Mourão A, et al. Orphan GPR116 mediates the insulin sensitizing effects of the hepatokine FNDC4 in adipose tissue. Nat Commun. (2021) 12. doi: 10.1038/s41467-021-22579-1
112. Leto D and Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. (2012) 13:383–96. doi: 10.1038/nrm3351
113. Klip A, McGraw TE, and James DE. Thirty sweet years of GLUT4. J Biol Chem. (2019) 294:11369–81. doi: 10.1074/jbc.REV119.008351
114. Chadt A and Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Archiv - Eur J Physiol. (2020) 472:1273–98. doi: 10.1007/s00424-020-02417-x
115. Caron A, Reynolds RP, Castorena CM, Michael NJ, Lee CE, Lee S, et al. Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis. Mol Metab. (2019) 27:11–21. doi: 10.1016/j.molmet.2019.06.019
116. Kimura T, Pydi SP, Wang L, Haspula D, Cui Y, Lu H, et al. Adipocyte G(q) signaling is a regulator of glucose and lipid homeostasis in mice. Nat Commun. (2022) 13:1652. doi: 10.1038/s41467-022-29231-6
117. Wang L, Pydi SP, Zhu L, Barella LF, Cui Y, Gavrilova O, et al. Adipocyte G(i) signaling is essential for maintaining whole-body glucose homeostasis and insulin sensitivity. Nat Commun. (2020) 11:2995. doi: 10.1038/s41467-020-16756-x
118. Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, et al. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PloS One. (2013) 8:e54500. doi: 10.1371/journal.pone.0054500
119. Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. (2013) 288:32475–89. doi: 10.1074/jbc.M113.514034
120. Laffitte A, Neiers F, and Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. (2014) 17:379–85. doi: 10.1097/MCO.0000000000000058
121. Kimura S and Kato E. TAS2R expression profile in brown adipose, white adipose, skeletal muscle, small intestine, liver and common cell lines derived from mice. Gene Rep. (2020) 20:100763. doi: 10.1016/j.genrep.2020.100763
122. Smith K, Karimian Azari E, Lamoia TE, Hussain T, Vargova V, Karolyi K, et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol Metab. (2018) 17:98–111. doi: 10.1016/j.molmet.2018.08.009
123. Masubuchi Y, Ma J, Suzuki T, Kojima I, Inagaki T, and Shibata H. T1R3 homomeric sweet taste receptor negatively regulates insulin-induced glucose transport through Gαs-mediated microtubules disassembly in 3T3-L1 adipocytes. Endocrine J. (2022) 69:487–93. doi: 10.1507/endocrj.EJ21-0661
124. Simon BR, Learman BS, Parlee SD, Scheller EL, Mori H, Cawthorn WP, et al. Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLoS One. (2014) 9:e86454. doi: 10.1371/journal.pone.0086454
125. Murovets VO, Bachmanov AA, and Zolotarev VA. Impaired glucose metabolism in mice lacking the Tas1r3 taste receptor gene. PLoS One. (2015) 10:e0130997. doi: 10.1371/journal.pone.0130997
126. Gerspach AC, Steinert RE, Schönenberger L, Graber-Maier A, and Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiology-Endocrinology Metab. (2011) 301:E317–25. doi: 10.1152/ajpendo.00077.2011
127. Kohno D, Koike M, Ninomiya Y, Kojima I, Kitamura T, and Yada T. Sweet taste receptor serves to activate glucose- and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness. Front Neurosci. (2016) 10. doi: 10.3389/fnins.2016.00502
128. Raka F, Farr S, Kelly J, Stoianov A, and Adeli K. Metabolic control via nutrient-sensing mechanisms: role of taste receptors and the gut-brain neuroendocrine axis. Am J Physiology-Endocrinology Metab. (2019) 317:E559–72. doi: 10.1152/ajpendo.00036.2019
129. Mukaida S, Evans BA, Bengtsson T, Hutchinson DS, and Sato M. Adrenoceptors promote glucose uptake into adipocytes and muscle by an insulin-independent signaling pathway involving mechanistic target of rapamycin complex 2. Pharmacol Res. (2017) 116:87–92. doi: 10.1016/j.phrs.2016.12.022
130. Erraji-Benchekroun L, Couton D, Postic C, Borde I, Gaston J, Guillet J-G, et al. Overexpression of β2-adrenergic receptors in mouse liver alters the expression of gluconeogenic and glycolytic enzymes. Am J Physiology-Endocrinology Metab. (2005) 288:E715–22. doi: 10.1152/ajpendo.00113.2004
131. Szepietowska B, Zhu W, Chan O, Horblitt A, Dziura J, and Sherwin RS. Modulation of β-adrenergic receptors in the ventromedial hypothalamus influences counterregulatory responses to hypoglycemia. Diabetes. (2011) 60:3154–8. doi: 10.2337/db11-0432
132. Paulussen F, Kulkarni CP, Stolz F, Lescrinier E, De Graeve S, Lambin S, et al. The β2-adrenergic receptor in the apical membrane of intestinal enterocytes senses sugars to stimulate glucose uptake from the gut. Front Cell Dev Biol. (2023) 10. doi: 10.3389/fcell.2022.1041930
133. Moullé VS. Autonomic control of pancreatic beta cells: What is known on the regulation of insulin secretion and beta-cell proliferation in rodents and humans. Peptides. (2022) 148:170709. doi: 10.1016/j.peptides.2021.170709
134. Santoro A, McGraw TE, and Kahn BB. Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab. (2021) 33:748–57. doi: 10.1016/j.cmet.2021.03.019
135. Walton C, Godsland IF, Proudler AJ, Felton CV, and Wynn V. Effect of body mass index and fat distribution on insulin sensitivity, secretion, and clearance in nonobese healthy men. J Clin Endocrinol Metab. (1992) 75:170–5. doi: 10.1210/jcem.75.1.1619007
136. Fairchild TJ, Klakk H, Heidemann M, Grøntved A, and Wedderkopp N. Insulin sensitivity is reduced in children with high body-fat regardless of BMI. Int J Obes. (2018) 42:985–94. doi: 10.1038/s41366-018-0043-z
137. Morton GJ, Muta K, Kaiyala KJ, Rojas JM, Scarlett JM, Matsen ME, et al. Evidence that the sympathetic nervous system elicits rapid, coordinated, and reciprocal adjustments of insulin secretion and insulin sensitivity during cold exposure. Diabetes. (2017) 66:823–34. doi: 10.2337/db16-1351
138. Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. (2005) 280:36824–32. doi: 10.1074/jbc.M505560200
139. Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Javitch J, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. (2012) 26:1757–72. doi: 10.1210/me.2012-1101
140. Farino ZJ, Morgenstern TJ, Maffei A, Quick M, De Solis AJ, Wiriyasermkul P, et al. New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion. Mol Psychiatry. (2020) 25:2070–85. doi: 10.1038/s41380-018-0344-6
141. Lee EY, Sakurai K, Zhang X, Toda C, Tanaka T, Jiang M, et al. Unsuppressed lipolysis in adipocytes is linked with enhanced gluconeogenesis and altered bile acid physiology in InsrP1195L/+ mice fed high-fat-diet. Sci Rep. (2015) 5:17565. doi: 10.1038/srep17565
142. Wang Y, Kwon H, Su X, and Wondisford FE. Glycerol not lactate is the major net carbon source for gluconeogenesis in mice during both short and prolonged fasting. Mol Metab. (2020) 31:36–44. doi: 10.1016/j.molmet.2019.11.005
143. Fournel A, Marlin A, Abot A, Pasquio C, Cirillo C, Cani PD, et al. Glucosensing in the gastrointestinal tract: Impact on glucose metabolism. Am J Physiology-Gastrointestinal Liver Physiol. (2016) 310:G645–58. doi: 10.1152/ajpgi.00015.2016
144. Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, and Knop FK. Glucose-dependent insulinotropic polypeptide. Diabetes. (2011) 60:3103–9. doi: 10.2337/db11-0979
145. Rabbani N and Thornalley PJ. Unraveling the impaired incretin effect in obesity and type 2 diabetes: Key role of hyperglycemia-induced unscheduled glycolysis and glycolytic overload. Diabetes Res Clin Pract. (2024) 217:111905. doi: 10.1016/j.diabres.2024.111905
146. Ding W-G, Renström E, Rorsman P, Buschard K, and Gromada J. Glucagon-like peptide I and glucose-dependent insulinotropic polypeptide stimulate Ca2+-induced secretion in rat α-cells by a protein kinase A–mediated mechanism. Diabetes. (1997) 46:792–800. doi: 10.2337/diab.46.5.792
147. Nauck MA, Quast DR, Wefers J, and Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab. (2021) 23:5–29. doi: 10.1111/dom.14496
148. Mayendraraj A, Rosenkilde MM, and Gasbjerg LS. GLP-1 and GIP receptor signaling in beta cells – A review of receptor interactions and co-stimulation. Peptides. (2022) 151:170749. doi: 10.1016/j.peptides.2022.170749
149. Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. (2002) 8:738–42. doi: 10.1038/nm727
150. Song DH, Getty–Kaushik L, Tseng E, Simon J, Corkey BE, and Wolfe MM. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology. (2007) 133:1796–805. doi: 10.1053/j.gastro.2007.09.005
151. Campbell JE, Beaudry JL, Svendsen B, Baggio LL, Gordon AN, Ussher JR, et al. GIPR is predominantly localized to nonadipocyte cell types within white adipose tissue. Diabetes. (2022) 71:1115–27. doi: 10.2337/db21-1166
152. Regmi A, Aihara E, Christe ME, Varga G, Beyer TP, Ruan X, et al. Tirzepatide modulates the regulation of adipocyte nutrient metabolism through long-acting activation of the GIP receptor. Cell Metab. (2024) 36:1534–49.e1537. doi: 10.1016/j.cmet.2024.05.010
153. Asmar M, Asmar A, Simonsen L, Gasbjerg LS, Sparre-Ulrich AH, Rosenkilde MM, et al. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes. (2017) 66:2363–71. doi: 10.2337/db17-0480
Keywords: glucose sensing, glucose homeostasis, adipose tissue, GPCR (G protein coupled receptor), obesity, diabetes, metabolism, glucose transport
Citation: Hasan N and Chhabra KH (2025) Glucose sensing and homeostasis by adipocyte GPCR. Front. Endocrinol. 16:1657747. doi: 10.3389/fendo.2025.1657747
Received: 01 July 2025; Accepted: 02 September 2025;
Published: 18 September 2025.
Edited by:
Ken-ichiro Nakajima, Nagoya University, JapanReviewed by:
Sai Prasad Pydi, Indian Institute of Technology Kanpur, IndiaKunio Kondoh, Tottori University, Japan
Copyright © 2025 Hasan and Chhabra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nazmul Hasan, TmF6bXVsLkhhc2FuQHVreS5lZHU=; Kavaljit H. Chhabra, a2F2YWxqaXRfY2hoYWJyYUB1a3kuZWR1
 Nazmul Hasan
Nazmul Hasan Kavaljit H. Chhabra
Kavaljit H. Chhabra