- 1Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 3Rizhao Hospital of Traditional Chinese Medicine, Rizhao, China
- 4Shanghai University of Traditional, Chinese Medicine, Shanghai, China
- 5Shandong University of Traditional, Chinese Medicine Affiliated Hospital, Jinan, China
This review identifies the mechanosensitive ion channel Piezo1 as the central regulator of bone homeostasis. Piezo1 senses mechanical loads in osteocytes, osteoblasts, and bone marrow mesenchymal stem cells (BMSCs), converting them into Ca2+-dependent signals that activate key pathways, including CaMKII, YAP/TAZ, Wnt/β-catenin, and ERK. These cascades collectively promote osteoblast differentiation and suppress osteoclastogenesis via OPG/RANKL modulation. Age-related Piezo1 decline impairs bone mechanoresponsiveness, driving both senile and disuse osteoporosis. Piezo1 also integrates bone metabolism with vascular–immune interactions (e.g., promoting VEGFA release from bone marrow macrophages via the CaN/NFAT/HIF-1α pathway) and the gut–bone axis (e.g., intestinal Piezo1 deletion relieves osteoblast proliferation inhibition by reducing serotonin levels). Therapeutically, Piezo1 agonists restore bone mass in osteoporosis models by reactivating mechanotransduction, while physical interventions achieve similar effects. Outstanding challenges include optimizing mechanical parameters (e.g., vibration frequency, ultrasound intensity) for individualized therapy, disentangling pathway crosstalk under aging and inflammation, and developing bone-targeted delivery systems for Piezo1 modulators. Overall, Piezo1 emerges as a pivotal therapeutic target for osteoporosis.
1 Introduction
Osteoporosis (OP) is characterized by reduced bone mass and deteriorated microarchitecture, leading to increased fragility and fracture risk (1, 2). Its pathology arises from an imbalance in bone remodeling: excessive osteoclast-mediated resorption coupled with insufficient osteoblast-driven formation (3, 4). Mechanical stress is a fundamental determinant of skeletal remodeling—loading (e.g., exercise) enhances bone mass and strength, while unloading (e.g., bed rest, spaceflight) induces rapid bone loss (1, 5).
The mechanosensitive ion channel Piezo1 shows age-related decline, which correlates with impaired skeletal responsiveness to mechanical cues and contributes to bone loss in aging (6). In pathological models, the Piezo1 agonist Yoda1 restores mechanotransduction, improving bone mass and strength in glucocorticoid-induced and disuse osteoporosis (7).
Current studies mostly focus on Piezo1’s local role in bone cells, while its systemic regulatory mechanisms in the gut-bone axis and vascular-immune axis have not been systematically summarized; existing Piezo1 agonists (e.g., Yoda1) have off-target risks and long-term safety controversies, and the clinical translation path remains unclear—this review aims to address these gaps by integrating Piezo1’s local and systemic functions in osteoporosis.
Piezo1 is broadly expressed in osteocytes, chondrocytes, and BMSCs. By converting mechanical forces such as fluid shear stress (FSS) into cellular signals, Piezo1 regulates diverse processes including skeletal development, angiogenesis, and immune responses (8). In osteocytes and chondrocytes, Piezo1 modulates osteogenesis and cartilage homeostasis (9); in BMSCs, it promotes osteogenic differentiation while inhibiting adipogenesis (10). Importantly, Piezo1-mediated FSS suppresses osteoporosis progression by reducing RANKL secretion from osteocytes (11). In ovariectomized animal models, Piezo1 also exhibits anti-osteoporotic effects. Genetic studies show Piezo1 polymorphisms (e.g., rs4238686, rs11643303) are associated with human OP: the rs4238686 variant reduces Piezo1 channel opening efficiency, leading to decreased mechanosensitivity of osteocytes and significant correlation with reduced bone mineral density (BMD) in elderly women (12); additionally, Piezo1 expression is markedly reduced in patient bone tissue (9, 12, 13).
1.1 Piezo1-mediated molecular mechanisms of bone mechanical adaptation
Wolff’s law posits that bone adapts structurally to stress: growth occurs in regions of high load, while resorption predominates where stress is low (14). Frost’s “mechanostat” concept further emphasizes that bone senses mechanical cues and adjusts accordingly (15).
Piezo1, a mechanosensitive cation channel with a trimeric propeller-shaped structure, is expressed in tissues such as lung, kidney, bladder, vasculature, and bone (16–18). Its single transmembrane protein consists of 2,521 amino acids—the largest known transmembrane molecule—organized into 38 helices per subunit, forming peripheral blades that constitute the mechanosensing module. This structure is highly conserved across evolution (19).
Multiple studies identify Piezo1 as a core component of the skeletal mechanostat. Osteoblast-specific Piezo1 deficiency leads to bone loss, spontaneous fractures, and increased resorption, while conferring resistance to unloading-induced bone loss in mice (9). Mechanistically, Piezo1 influences type II and IX collagen expression through the YAP pathway: Activated YAP promotes the synthesis of type II and IX collagens, enhancing bone matrix integrity; meanwhile, collagens activate FAK signaling in osteoclasts via integrin αvβ3, inhibiting their excessive differentiation and ultimately maintaining the balance between bone resorption and formation. Deletion of Piezo1 in osteoblasts disrupts osteogenesis, causes skeletal fragility, and its expression declines with age in human OP patients (20). These findings firmly establish Piezo1 as a molecular bridge linking mechanical force to bone homeostasis.
At the functional level, Piezo1 maintains skeletal integrity via dual mechanisms: it upregulates osteoprotegerin (OPG) to inhibit osteoclastogenesis and simultaneously promotes osteoblast activity, particularly protecting against age-related cortical bone loss (21). Developmental studies show that Piezo1 deletion during embryogenesis (global knockout: Piezo1fl/fl; Sox2-Cre) induces bone deformities and fractures, whereas loss of function in adulthood (osteoblast-specific knockout: Piezo1fl/fl; OCN-Cre) directly causes osteoporosis (6). Together, evidence from both developmental biology and adult bone metabolism underscores Piezo1 as a central regulator of skeletal health. Its age-dependent expression provides a strong rationale for anti-osteoporosis therapies targeting Piezo1 activation.
2 Mechanical regulatory functions of Piezo1 and other mechanosensors in bone metabolism
2.1 Dominant role of Piezo1 in bone metabolism
Hindlimb suspension (HS) experiments show that unloading reduces bone strength in wild-type mice but not in Piezo1-knockout (KO) mice, suggesting that Piezo1 primarily regulates skeletal remodeling via osteoblasts (9). In osteocalcin (OCN)-specific KO mice, Piezo1 deletion caused shortened and weakened long bones, reduced bone mass, impaired osteoblast differentiation, and abolished mechanical loading–induced osteoblast–osteoclast coupling. By contrast, Piezo2 deletion had no significant impact on bone mass or bone length (22, 23). These results establish Piezo1 as the dominant mechanosensor in bone, with Piezo2 playing only a minor role.
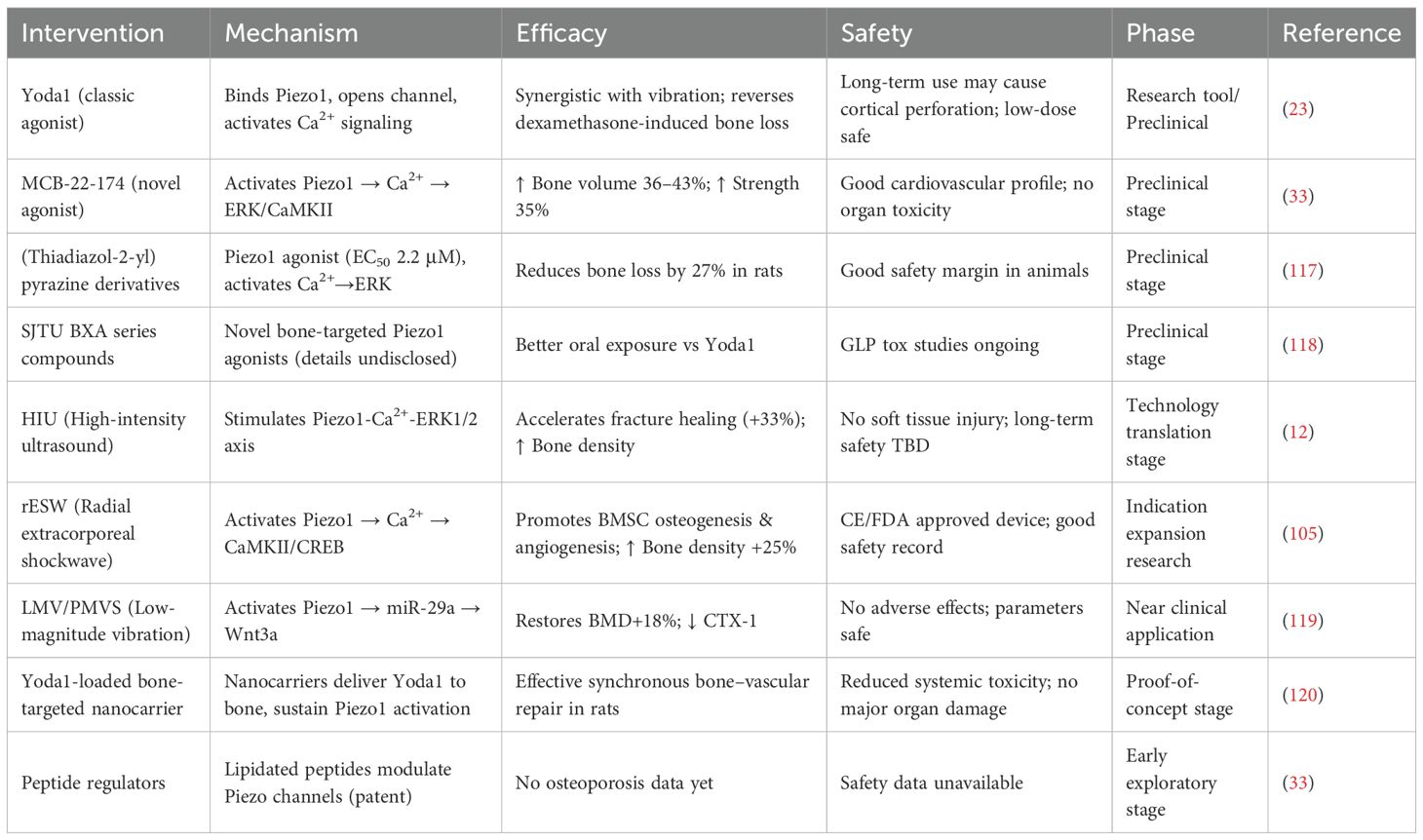
Piezo1 and Piezo2 form mechanosensitive cation channels (24), but Piezo1 is the primary transducer of membrane tension. Upon mechanical stimulation, Piezo1 opens to mediate Ca2+ influx, converting external forces into intracellular signals that drive mechanotransduction and cellular adaptation (25–27). This process is indispensable for bone-forming cell survival, differentiation, and matrix mineralization, as well as skeletal remodeling and regeneration (28) (Figure 1).To provide a comprehensive overview, Figure 1 illustrates both the structural characteristics of Piezo1—highlighting its trimeric architecture, central ion pore, and curved blades—and its expression patterns in bone tissue, where it shows cell type–specific localization and functions.
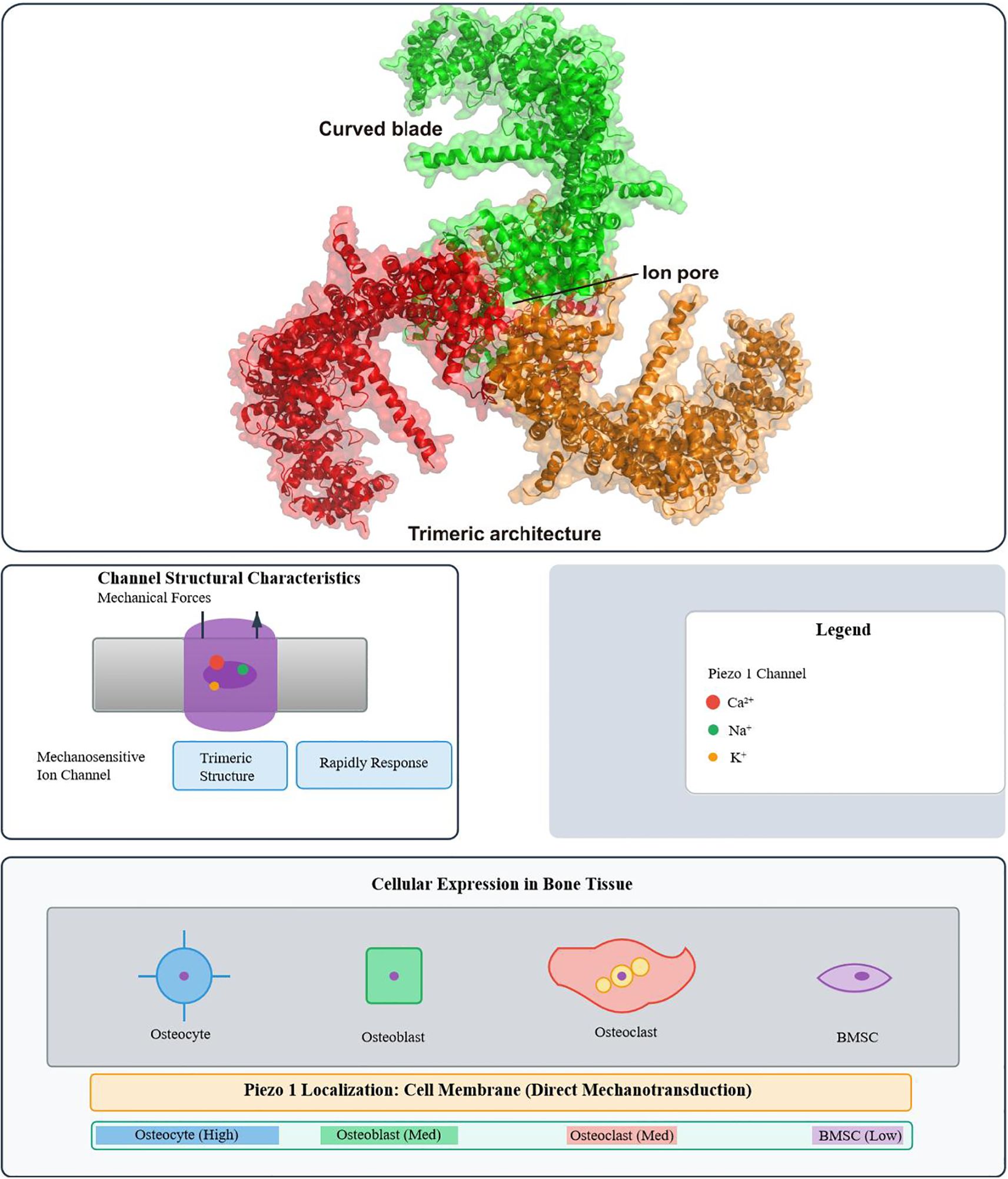
Figure 1. Structural characteristics of the Piezo1 ion channel and its distribution in bone tissue. (Top) Cryo-EM structure of Piezo1, highlighting its trimeric architecture, central ion pore, and curved blade domains. (Bottom) Schematic representation of Piezo1 expression in bone tissue. Piezo1 mediates rapid ion transport (Ca2+, Na+, K+) in response to mechanical force. It is highly expressed in osteocytes (the core mechanosensors), moderately in osteoblasts and osteoclasts, and weakly in bone marrow MSCs (BMSCs). Localized primarily at the cell membrane (direct mechanotransduction) and at low levels in the endoplasmic reticulum (involved in calcium homeostasis), Piezo1 converts mechanical cues into biochemical signals—with cell-specific functions (osteocytes, sensing fluid shear stress; BMSCs, regulating differentiation fate)—to regulate bone metabolism.
Osteocyte apoptosis critically affects bone homeostasis. Excessive apoptosis, such as that induced by glucocorticoids (GCs), disrupts the lacunar–canalicular network, reducing fluid flow and connectivity, ultimately impairing bone quality (29, 30). This process involves caspase-3 activation and phosphorylation of proline-rich tyrosine kinase 2 (PYK2) and c-Jun N-terminal kinase (JNK). Conversely, mechanical stress promotes production of anti-apoptotic mediators (e.g., nitric oxide and prostaglandin E2), helping preserve osteocyte viability (31, 32).
In BMSCs, Piezo1 also mediates proliferation and osteogenic differentiation. In vitro cyclic mechanical stretch (CMS) increases proliferation and upregulates osteogenic markers (COL1A1, OSX, RUNX2) in rat BMSCs. Piezo1 knockdown significantly reduces these effects, underscoring its critical role in mechanotransduction (33). Osteocytes sense fluid shear forces via Piezo1, triggering Ca2+ signaling cascades that regulate bone remodeling (11). In osteoblasts and chondrocytes, Piezo1-mediated Ca2+ influx activates downstream ERK1/2 and PI3K/Akt pathways, promoting osteogenesis and regulating cartilage metabolism (34). In periodontal ligament cells, Piezo1 responds to orthodontic pressure, modulating alveolar bone remodeling (35, 36).
Bone mechanotransduction is a multi-level system. Osteocytes form a mechanosensing complex with dendritic networks, integrins, ion channels (e.g., ANO1), and primary cilia. Mechanical loading also induces osteocytes to release exosomes carrying regulatory miRNAs, potentially contributing to systemic homeostasis. In osteoblasts, Piezo1-mediated Ca2+ signaling interacts with ANO1 chloride channels to influence osteoclast regulation (37, 38). Moreover, bone microvascular endothelial cells participate in signal transmission, and unloading disrupts this function (39).
Wnt/β-catenin and RANKL signaling pathways are central to Piezo1-mediated mechanical regulation. Mechanical loading activates Wnt/β-catenin signaling, promoting osteoblast differentiation, while suppressing RANKL-mediated osteoclastogenesis (40). YAP/TAZ also function as mechanosensitive transcriptional regulators, guiding BMSC fate via Runx2. For example, loading enhances expression of Fgf23 and Mepe, genes critical for phosphate metabolism and bone mineralization (41).
2.2 Other mechanosensors in bone metabolism
2.2.1 TRPV4 as a complementary mechanosensor
TRPV4 senses low-intensity, physiological mechanical deformation (0.1–1 dyne/cm², e.g., bone tissue hydrostatic pressure) and regulates chondrocyte differentiation, extracellular matrix metabolism, and osteogenic gene expression through Ca2+ influx. In contrast, Piezo1 responds to supraphysiological or injurious forces (≥5 dyne/cm², e.g., exercise-induced fluid impact). When Piezo1 is impaired (e.g., aging, knockout), TRPV4 partially compensates to sustain tissue homeostasis: mice with double knockout of Piezo1 and TRPV4 exhibit significantly more severe bone loss than Piezo1 single-knockout mice, with a 2.3-fold increase in osteoclast number (42, 43).
2.2.2 GPR68 as a supplementary mechanosensor
GPR68 provides additional compensation by responding to mechanically associated environmental changes such as pH shifts and fluid shear stress, especially under inflammatory conditions. GPR68 activation reduces osteoclast-related factors via PLC–IP3 signaling. In osteoarthritis, upregulation of GPR68 suppresses aberrant cartilage degradation through Rap1A-dependent pathways, offering a non-Ca2+-dependent compensatory mechanism—under inflammatory conditions, this pathway can partially reverse the enhanced bone resorption caused by Piezo1 deficiency (44–46).
2.2.3 Synergy among Piezo1, TRPV4, and GPR68
Piezo1 remains the central mechanotransducer, regulating bone remodeling, survival, and inflammatory responses (6, 47). TRPV4 and GPR68 act as compensatory systems: TRPV4 maintains Ca2+ signaling during physiological stimuli (0.1–1 dyne/cm²), while GPR68 compensates through pH-sensitive G-protein pathways. This redundancy across stimulus intensity and signaling modes ensures skeletal balance even when Piezo1 function declines.
3 Direct and systemic regulatory mechanisms of Piezo1 in osteoporosis
3.1 Direct regulation of bone cells by Piezo1
3.1.1 Osteoblast differentiation
Under hydrostatic pressure, Piezo1 functions as a signaling hub that rapidly initiates osteogenic programs. Piezo1-mediated Ca2+ influx activates ERK1/2 phosphorylation cascades and promotes F-actin assembly—F-actin assembly further promotes the G1/S phase transition of osteoblasts by activating YAP nuclear translocation (upregulating Cyclin D1 expression) and enhances cell adhesion, providing cytoskeletal support for osteoblast proliferation. Agonists such as Yoda1 significantly enhance BMP2 expression, directing BMSCs toward osteogenesis while suppressing adipogenesis. Conversely, Piezo1 silencing reduces BMP2 expression and cell migration (48).
A newly identified agonist, MCB-22-174, activates the Piezo1/CaMKII/ERK axis, offering a therapeutic approach for disuse osteoporosis (49). Collectively, Piezo1 acts as a central conductor of osteogenic differentiation, orchestrating signaling pathways that coordinate bone formation.
3.1.2 Cartilage differentiation and ossification balance
Piezo1 is highly expressed in chondrocytes, where it regulates responses to mechanical strain. Inhibition with GsMTx4 markedly diminishes chondrocyte mechanosensitivity (50). In inflammatory conditions, IL-1α enhances Piezo1 expression, causing Ca2+ overload and chondrocyte dedifferentiation, which predisposes to osteoarthritis (51).
During endochondral ossification, Piezo1 deletion disrupts key gene expression (e.g., Sox9, Col10a1), damaging growth plate structure and increasing fracture susceptibility (52). In osteoarthritis models, mechanical overload induces Piezo1-mediated Ca2+ influx that destabilizes the cytoskeleton and upregulates MMP13, accelerating cartilage degeneration (53). These findings underscore Piezo1 as a guardian of cartilage mechanohomeostasis, with dysfunction closely linked to degenerative joint disease.
3.2 Phenotypic differences of Piezo1 in skeletal development
3.2.1 Developmental vs. adult bone homeostasis
During embryogenesis, Piezo1 is indispensable for skeletal development. Its deletion (Piezo1fl/fl; Sox2-Cre) causes cranial defects, cortical porosity, reduced strength, and aberrant STAT3 activation (54–56). In adults, Piezo1 inactivation (Piezo1fl/fl; OCN-Cre) results in cortical thinning, increased porosity, decreased trabecular bone volume, and reduced bone formation—hallmarks of high-turnover osteoporosis (57–59). These findings demonstrate stage-specific functions: Piezo1 orchestrates development early, and maintains homeostasis later in life.
3.2.2 Aging and sex differences
Piezo1 expression declines with age, impairing osteoblast function and aggravating cortical bone loss. Activation of Piezo1 can reverse glucocorticoid-induced osteoporosis by restoring Wnt/β-catenin signaling (6). Genetic variants of Piezo1 are also linked to bone mineral density and fracture risk (60).
Estrogen deficiency further reduces Piezo1 expression, particularly in aging females: estrogen binds to the estrogen response element (ERE) in the Piezo1 promoter via ERα to promote its transcription; after estrogen deficiency, ERα-mediated transcriptional regulation of Piezo1 is lost, and simultaneous activation of the ROCK pathway leads to F-actin depolymerization, weakening cytoskeletal remodeling and reducing suppression of osteoclastogenesis (6, 61, 62). Estrogen deficiency also increases oxidative stress and reduces osteogenic activity, which synergize with Piezo1 loss. Mechanistically, Piezo1 deletion disrupts metabolism through the SIRT3–SDHA–OXPHOS axis, exacerbating impaired bone formation (63, 64). Furthermore, the Wnt/Ca2+ pathway, normally activated by Piezo1, is suppressed under estrogen deficiency, reducing osteogenesis (12, 36).
4 Indirect regulation of Piezo1 through non-bone cell networks
4.1 Vascular–immune axis: coordinated regulation of the bone microenvironment
In endothelial cells, Piezo1 functions as a mechanosensor that regulates vascular tone and blood flow distribution (65). Following radiation-induced bone injury, Piezo1 activation in bone marrow macrophages stimulates VEGFA release through the CaN/NFAT/HIF-1α pathway, thereby promoting vascular regeneration (66). Conversely, Piezo1 deletion downregulates PI3K–Akt and Notch signaling during fracture healing, impairing osteoblast maturation (67).
Under mechanical loading, periosteal myeloid cells differentiate into CD68+F4/80+ macrophages, which release thrombospondin-1 (TSP1) to activate TGF-β1 signaling, synergistically promoting bone formation (68). These findings indicate that Piezo1 regulates skeletal remodeling not only through direct mechanotransduction in bone cells but also by modulating the vascular–immune axis.
4.2 Gut–bone axis
Piezo1 also influences skeletal metabolism through the intestinal system. Intestine-specific Piezo1 deletion reduces serum serotonin (5-HT) levels—serotonin normally inhibits osteoblast proliferation—thus enhancing osteoblast activity and producing a high bone mass phenotype (69, 70). This finding identifies intestinal Piezo1 as a negative regulator of osteogenesis and highlights the gut–bone axis as an inter-organ regulatory network influencing skeletal health.
5 Core mechanosignaling pathways mediated by Piezo1
Piezo1 integrates pathways into a unified mechanosignaling network that regulates osteogenesis and osteoclast activity (Figure 2). The following subsections detail the key components of this network.
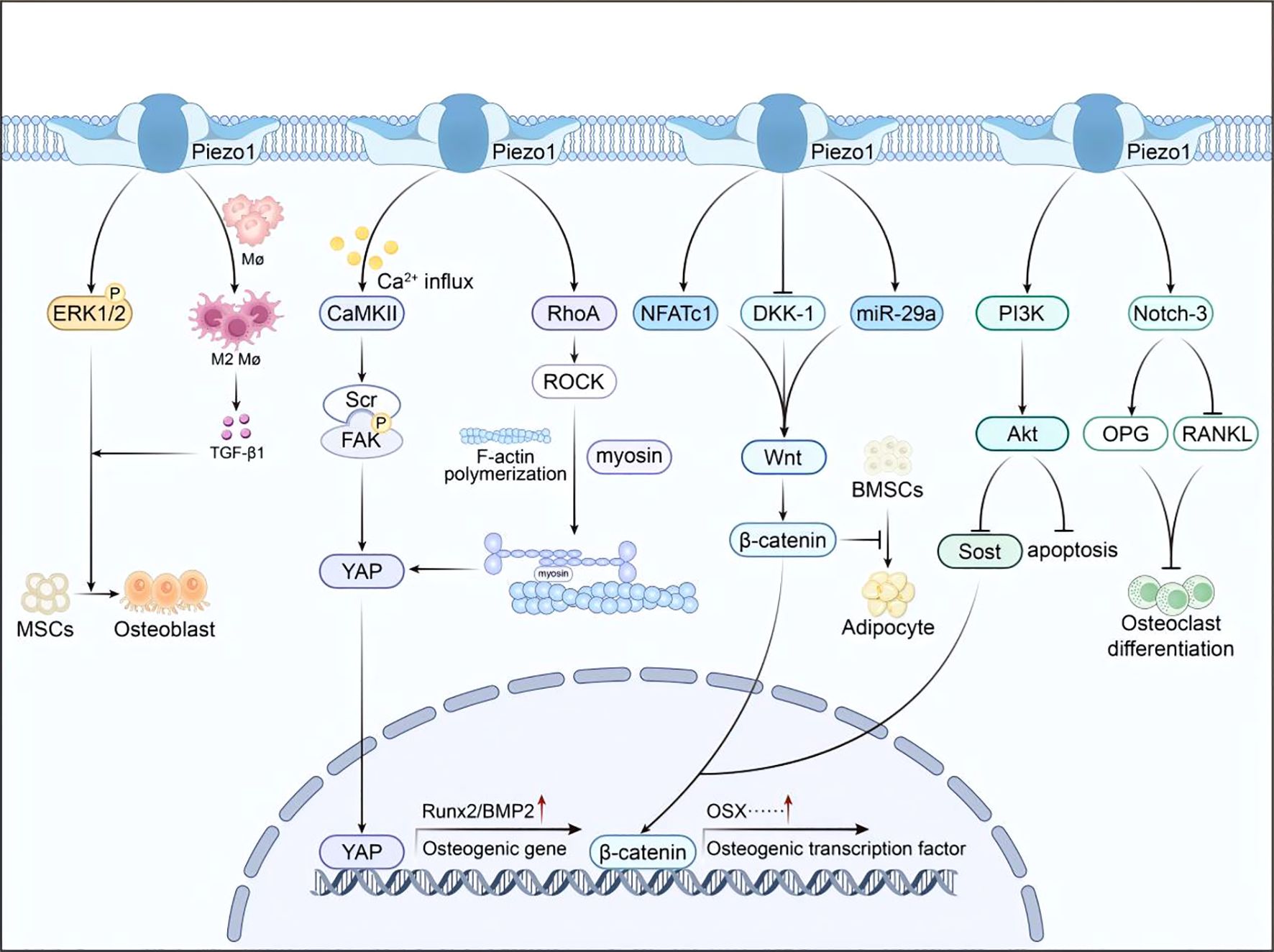
Figure 2. Integrated Piezo1 signaling network. Mechanical activation of Piezo1 triggers multiple downstream pathways (CaMKII–YAP, RhoA/ROCK, Wnt/β-catenin, ERK1/2, PI3K/Akt), collectively promoting osteogenesis and suppressing osteoclastogenesis.
5.1 CaMKII pathway: calcium signaling hub
Mechanical stimulation activates Piezo1, leading to Ca2+ influx and subsequent activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (71). Activated CaMKII phosphorylates focal adhesion kinase (FAK) and Src—phosphorylated FAK/Src inhibits the activity of Hippo pathway kinase MST1/2, reducing YAP phosphorylation at Ser127 and thereby driving YAP nuclear translocation to regulate osteogenic gene expression (72). This pathway plays a critical role in pathological ossification, such as ankylosing spondylitis, where aberrant mechanical signaling promotes osteophyte formation. In osteoporosis, insufficient Piezo1 activation reduces CaMKII signaling, YAP nuclear localization, and osteogenic gene expression, resulting in impaired bone formation.
5.2 YAP/TAZ pathway: cytoskeletal remodeling switch
Piezo1-mediated mechanical stimulation activates the RhoA/ROCK pathway, inducing cytoskeletal remodeling through F-actin polymerization and myosin reorganization (47). This structural reorganization facilitates YAP nuclear translocation, which upregulates osteogenic transcription factors such as Runx2 and BMP2 (73). For example, triangular micropatterns enhance BMSC osteogenesis through this mechanism (74). In osteoporosis, weakened mechanical stimulation reduces Piezo1 activity, restricting cytoskeletal remodeling and YAP signaling, thereby impairing osteogenic differentiation and promoting adipogenesis.
5.3 Wnt/β-catenin pathway: bridge to bone metabolism
Piezo1 may activate the Wnt/β-catenin pathway via NFATc1 (75). Wnt activation drives β-catenin nuclear translocation, promoting transcription of osteogenic genes such as OSX while inhibiting adipogenesis (76). In osteoporosis, reduced Piezo1 activity attenuates Wnt/β-catenin signaling, leading to diminished osteogenesis and increased marrow adiposity.
5.4 ERK1/2 phosphorylation pathway: rapid response channel
Piezo1 activation by hydrostatic pressure or Yoda1 induces ERK1/2 phosphorylation, promoting BMSC osteogenic differentiation (77). Under physiological fluid shear stress, Piezo1 is upregulated in osteocytes, which activate Notch3 signaling to enhance OPG expression and suppress RANKL, thereby inhibiting osteoclastogenesis (11). Mechanical stretch also activates the PI3K/Akt pathway via Piezo1, downregulating Sost while enhancing Wnt/β-catenin signaling, thus driving osteogenesis (78). Moreover, Piezo1 activation reverses dexamethasone-induced osteocyte apoptosis through PI3K/Akt-mediated Ca2+ signaling (79, 80).
5.5 Multi-pathway synergy under mechanical stimulation
Piezo1 integrates multiple pathways during mechanical interventions. For instance, piezoelectric microvibration (PMVS) activates Wnt/β-catenin signaling by upregulating miR-29a and suppressing DKK-1 (81, 82). Additionally, Piezo1 polarizes macrophages toward the M2 phenotype: Piezo1-mediated Ca2+ influx activates the STAT6 pathway, upregulating M2 markers (Arg1, IL-10) and promoting TGF-β1 precursor maturation, thereby stimulating TGF-β1 secretion to enhance BMSC osteogenesis (83). This coordinated multi-pathway network ensures precise bone responses to mechanical stimuli. In osteoporosis, disruption of this network leads to impaired bone remodeling (Figure 3).
Figure 3. Mechanical loading–Piezo1 axis. Mechanical forces such as fluid shear stress, compression, and matrix stiffness deform the bone matrix, increasing membrane tension and triggering Piezo1 activation—with force-specific cellular targets (gravitational loading: osteoblasts; muscle contraction: osteocytes; FSS: osteocytes + vascular endothelial cells). Piezo1 then mediates Ca2+ influx, which activates downstream mechanosignaling cascades (YAP/TAZ, Calcineurin-NFAT, Wnt/β-catenin), converting physical loading into biochemical responses that regulate bone homeostasis. In the “Loss of Function” state, molecular changes include reduced YAP nuclear translocation, decreased Wnt3a expression, and increased RANKL/OPG ratio, leading to impaired bone structural integrity.
6 Interactions of Piezo1 with different pathways in specific bone cell types
6.1 Interaction with the CaMKII pathway
In adipose-derived stem cells (ADSCs), Piezo1-mediated Ca2+ influx activates CaMKII phosphorylation, enhancing β-catenin transcriptional activity and nuclear translocation, ultimately promoting osteogenesis (84). In osteoblasts and related cells, Piezo1 activation under stress also triggers CaMKII signaling, which synergizes with the Wnt/β-catenin pathway to regulate osteogenic differentiation (71).
6.2 Interaction with the YAP/TAZ pathway
In human dental follicle cells (hDFCs), cyclic tensile stress activates Piezo1, inducing Ca2+ influx that promotes YAP nuclear translocation and upregulates osteogenic genes (85). In BMSCs, Piezo1 integrates with YAP signaling, regulating target genes such as ATF4 via β-catenin and influencing proliferation and osteogenesis (7, 86). In valvular interstitial cells (VICs), Piezo1 activation drives Ca2+-dependent YAP signaling, enhancing osteogenesis through GLS1-mediated glutamine metabolism (87). In osteoblasts and osteosarcoma cells, Piezo1-mediated Ca2+ influx is essential for YAP/TAZ activation, which regulates cell motility and bone-associated processes (88).
6.3 Interaction with the Wnt/β-catenin pathway
In periodontal ligament cells (PDLCs), compressive force upregulates Piezo1 and β-catenin, while Piezo1 inhibition decreases β-catenin activity and osteogenic differentiation, modulating alveolar bone remodeling (35). In hDFCs, Piezo1 activation (e.g., Yoda1) enhances Wnt3a and β-catenin expression, activating canonical osteogenesis (89). In BMSCs and osteoblasts, Piezo1 promotes β-catenin nuclear translocation via Ca2+ influx, cooperating with CaMKII to support osteogenesis. Importantly, Piezo1 restores suppressed Wnt/β-catenin activity under microgravity, mitigating bone loss (75). In ADSCs, compressive stress–induced Piezo1 activation enhances β-catenin transcriptional activity and contributes to bone remodeling (36).
6.4 Interaction with the ERK1/2 pathway
In BMSCs, Piezo1 activation by hydrostatic pressure or Yoda1 triggers Ca2+ influx and ERK1/2 phosphorylation, promoting osteogenesis; this effect is abolished under Ca2+ deficiency (49, 90). In osteoblasts and osteosarcoma cells, Piezo1-mediated Ca2+ entry activates ERK via the MAPK cascade, which also cross-talks with YAP and Wnt/β-catenin signaling (91). In periodontal ligament cells and chondrocytes, Piezo1 activation engages ERK1/2 via PI3K–Akt/NF-κB, indirectly regulating proliferation and bone-related processes (92–94).
7 Therapeutic implications
Building upon the detailed molecular mechanisms of Piezo1 signaling, we propose a comprehensive pathophysiological framework that links mechanical input to skeletal outcomes (Figure 4). This model delineates how the spectrum of mechanical loading—from physiological stimulation to overload or absence of force—dictates Piezo1 activation states, thereby governing the fate of bone cells and ultimately determining bone mass and quality. Crucially, this framework incorporates critical modifiers of bone homeostasis, including hormonal status (e.g., estrogen deficiency), bone site-specific remodeling patterns, and common comorbidities.
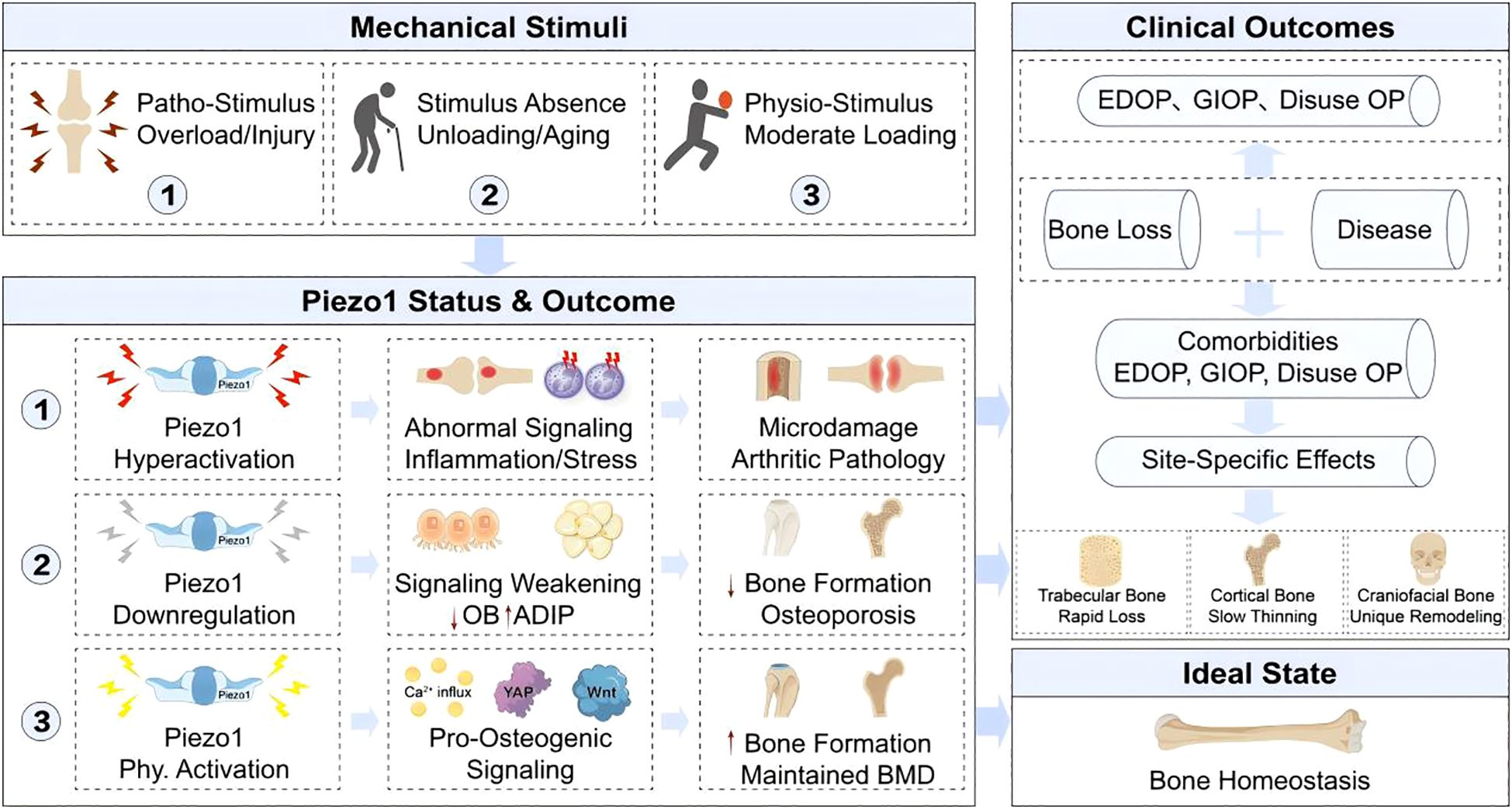
Figure 4. Pathophysiological outcomes of Piezo1 dysregulation and therapeutic implications. The spectrum of mechanical loading determines Piezo1 activity states, which govern skeletal fate. Physiological stimuli maintain Piezo1 activation and pro-osteogenic signaling, ensuring homeostasis. Loss of loading (e.g., aging, disuse) causes Piezo1 downregulation, shifting MSC fate toward adipogenesis and leading to bone loss (e.g., EDOP, GIOP). Conversely, pathological overloading hyperactivates Piezo1, inducing aberrant signaling and inflammation that contribute to arthritic pathology. This framework highlights key modifiers, including sex hormones (estrogen deficiency), bone site-specific responses (craniofacial vs. long bone remodeling), and comorbidities, underscoring the therapeutic goal of achieving precise Piezo1 modulation.
This mechano-dependent duality is exemplified in the context-specific, bidirectional regulation of osteoclasts by Piezo1. For example, in peri-prosthetic models, fluid shear stress mediated by Piezo1 exhibits frequency-dependent effects: low-frequency, high-amplitude stimulation enhances resorption, whereas high-frequency, low-amplitude stimulation promotes bone formation (95). In orthodontic models, Piezo1 activation increases the RANKL/OPG ratio, elevating TRAP+ osteoclast numbers (96). Conversely, prolonged high-amplitude fluid shear stress induces osteoclast formation by inhibiting the sarcoplasmic reticulum Ca2+ pump (97).
Piezo1 can also suppress osteoclastogenesis by upregulating OPG; its deficiency increases endocortical resorption (6). Thus, selective Piezo1 agonists (e.g., Yoda1 and its optimized derivatives) represent promising candidates for anti-osteoporosis therapies (6). In fracture repair, Piezo1 regulates endochondral ossification by modulating HIF-1α signaling in chondrocytes (98).
7.1 Broad regulatory effects
Engineered biomaterials exploit Piezo1 signaling to enhance bone repair. For example, oleic acid–modified iron oxide nanoparticles (IO-OA/PLGA) increase Piezo1 expression under magnetic fields: magnetic fields induce local mechanical vibration (10–50 Hz) of IO-OA/PLGA particles, activating Piezo1 channels via membrane tension, while particles slowly release oleic acid to promote Piezo1 transcription (99). Similarly, 3D-printed Ti2448 alloy scaffolds enhance angiogenesis and osteogenesis via Piezo1/YAP signaling, while titanium dioxide nanotubes stimulate Piezo1-mediated osteogenesis (100).
In fracture healing, reduced Piezo1 expression delays callus mineralization, whereas Yoda1 treatment increases BV/TV and bone mineral density, accelerating cartilage and callus maturation (101). Mechanical interventions such as low-intensity pulsed ultrasound or piezoelectric microvibration (PMVS) also activate Piezo1, promoting osteoblast precursor proliferation and migration, thereby improving bone strength (48).
7.2 Exercise therapy and rehabilitation
Exercise activates Piezo1 through cyclic loading, enhancing Ca2+ influx and Akt phosphorylation, which promote osteogenesis and skeletal muscle protein synthesis (102–104). Radial extracorporeal shock wave (R-ESW) therapy stimulates Piezo1/CaMKII/CREB signaling in senile osteoporosis (SOP) patient-derived BMSCs, enhancing their osteogenic and angiogenic capacity while reducing bone loss in animal models (105). These findings highlight Piezo1 as a therapeutic target linking musculoskeletal rehabilitation and osteoporosis treatment (Tables 1, 2).

Table 1. Evidence summary of exercise and physical interventions targeting Piezo1 for osteoporosis rehabilitation.
8 Difficulties and challenges
Despite significant progress, research on Piezo1 faces several challenges that hinder its translation into clinical therapies. A major obstacle is the precise modulation of mechanical stimulation parameters. Mechanical loading exerts bidirectional effects: low-frequency, high-amplitude stress tends to promote osteoclast-mediated resorption, whereas high-frequency, low-amplitude stress favors osteogenesis. Current studies on vibration frequency focus on 20–100 Hz, and ultrasound intensity on 0.5–2 W/cm², but optimal parameters vary significantly among populations of different ages (young vs. elderly) and genders (male vs. postmenopausal female), and an individualized parameter database is lacking—determining optimal parameters to maximize the osteogenic benefits of Piezo1 activation remains unresolved (60).
Another challenge lies in the complexity of signaling crosstalk. Piezo1 activates multiple downstream pathways, including PI3K/Akt, ERK, and YAP, through Ca2+ influx. The relative contribution and interaction of these cascades in osteocytes, BMSCs, and osteoclasts are still poorly defined. Moreover, aging and inflammation further complicate regulation: declining Piezo1 expression reduces Wnt/β-catenin signaling, while pro-inflammatory cytokines such as IL-1α can cause Piezo1 overactivation, leading to Ca2+ overload and chondrocyte apoptosis (51). Dissecting the molecular switches that govern this imbalance is critical for targeted intervention.
Piezo1 also interacts with other mechanosensing systems. Evidence suggests potential cross-talk with focal adhesion complexes and proteins such as Kindlin-2, as well as with other ion channels like connexin 43 hemichannels, but the specific molecular cascades remain largely unknown. In addition, Piezo1 plays a role in coordinating systemic regulatory axes, including the vascular–immune–bone and gut–bone axes. However, their dynamic integration is incompletely understood. For example, Piezo1 regulates macrophage polarization, VEGFA release, and vascular regeneration, yet how these processes interact during bone repair remains unclear. Similarly, intestine-specific deletion enhances osteogenesis by reducing circulating serotonin, which paradoxically contrasts with Piezo1’s direct pro-osteogenic role in bone tissue—single-cell sequencing can be used to analyze differences in downstream target genes of Piezo1 between intestinal epithelial cells and osteocytes, clarifying the regulatory hierarchy of serotonin-dependent and independent pathways (69, 70). Clarifying the hierarchy of such inter-organ signals is an urgent research priority.
Finally, pharmacological limitations represent a major bottleneck. Current Piezo1 agonists, such as Yoda1, have been tested primarily in animal models, but specific bone-targeted delivery systems are lacking. Given Piezo1’s broad expression across multiple tissues, concerns about off-target effects remain significant. Furthermore, the regulatory mechanisms controlling Piezo1’s dynamic expression during aging and disease progression are poorly characterized, complicating the selection of therapeutic timing (7).
9 Summary and perspectives
Piezo1 has emerged as the central mechanotransducer in bone tissue, converting external mechanical stimuli such as fluid shear stress and mechanical stretch into Ca2+ influx and activating downstream signaling pathways including PI3K/Akt, ERK, YAP/TAZ, and Wnt/β-catenin. Through these mechanisms, Piezo1 orchestrates the balance between osteoblast and osteoclast activity, regulates BMSC differentiation, and coordinates vascular–immune interactions, thereby playing a pivotal role in the pathogenesis and progression of osteoporosis. Declining Piezo1 expression with age or under pathological conditions such as estrogen deficiency directly impairs skeletal mechanoresponsiveness, contributing to reduced osteogenesis, increased bone resorption, and ultimately bone fragility.
Therapeutically, Piezo1 offers a promising target for intervention. Agonists such as Yoda1 and MCB-22–174 restore mechanotransduction and ameliorate bone loss in disuse, glucocorticoid-induced, and aging-related osteoporosis models. Mechanical therapies—including exercise, vibration, ultrasound, and shock wave treatment—also act through Piezo1 to promote osteogenesis, providing a theoretical basis for rehabilitation strategies. Furthermore, Piezo1’s systemic roles extend beyond bone tissue. In the gut–bone axis, intestine-specific Piezo1 deletion reduces circulating serotonin, indirectly enhancing osteoblast proliferation, while in the vascular–immune axis, Piezo1 regulates macrophage polarization and angiogenic factor release, contributing to bone repair. These findings suggest that Piezo1 functions not only as a local mechanosensor but also as a systemic regulator of skeletal homeostasis.
Future research should focus on several key directions. First, the development of selective Piezo1 agonists with optimized pharmacokinetic properties and bone-targeted delivery systems—e.g., modifying nanocarriers with bisphosphonates (high affinity for bone hydroxyapatite) or designing pH-sensitive carriers (bone microenvironment pH ≈ 5.5)—is crucial to reduce off-target risks (120). Second, building a database of individualized mechanical parameters based on genotype, age, and hormonal status could enable precision therapies using mechanical interventions or Piezo1 modulators. Third, advanced tools such as single-cell sequencing and in vivo Ca2+ imaging are needed to map Piezo1’s spatiotemporal activation patterns and to clarify its crosstalk with other key signaling pathways. Fourth, resolving the paradox between intestinal Piezo1 and bone Piezo1, as well as delineating the hierarchy of inter-organ regulatory networks, will be essential for fully understanding its systemic roles. Finally, the integration of Piezo1 agonists with established osteoporosis drugs, such as bisphosphonates—their combination may synergistically enhance BMD via Wnt/β-catenin (agonists: promote osteogenesis; bisphosphonates: inhibit resorption), with caution for Piezo1 overactivation-induced Ca2+ overload (51)—and the development of wearable mechanosensing devices for real-time feedback may provide novel strategies for long-term management and personalized rehabilitation (6, 121).
In summary, Piezo1 represents a pivotal molecular hub at the interface of biomechanics and bone biology. By bridging mechanical loading, cellular signaling, and systemic regulation, it not only provides new insights into the pathogenesis of osteoporosis but also opens avenues for innovative therapeutic strategies that combine pharmacological, mechanical, and bioengineering approaches.
Author contributions
CL: Writing – original draft, Writing – review & editing. JY: Conceptualization, Investigation, Writing – review & editing. ZD: Data curation, Formal Analysis, Writing – review & editing. SZ: Methodology, Supervision, Writing – review & editing. ZT: Formal Analysis, Funding acquisition, Writing – review & editing. YL: Funding acquisition, Validation, Writing – review & editing. YH: Funding acquisition, Resources, Writing – review & editing. MW: Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the following funding sources: - The Doctoral Student Quality Improvement and Innovation Project of Shandong University of Traditional Chinese Medicine (Grant No. YJSTZCX2024031, awarded to ZT) - Natural Science Foundation of Shandong Province Project (Grant No. ZR2022MH096, awarded to YH) - Science and Technology Co-construction Project of the Science and Technology Department of the National Administration of Traditional Chinese Medicine (Grant No. GZY-KJS-SD-2024-025, awarded to MW) - Shandong Province Medical and Health Technology Project (Grant No. 202403100325, awarded to YL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vico L and Hargens A. Skeletal changes during and after spaceflight. Nat Rev Rheumatol. (2018) 14(4):229–45. doi: 10.1038/nrrheum.2018.37
2. Srivastava M and Deal C. Osteoporosis in elderly: Prevention and treatment. (2002) 18(3):529–55. doi: 10.1016/S0749-0690(02)00022-8
3. Raisz LG. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. (2005) 115(12):3318–25. doi: 10.1172/JCI27071
4. Brown C. Osteoporosis: staying strong. Nature. (2017) 550(7674):S15–7. doi: 10.1016/s0140-6736(03)15055-6
5. Nicogossian A. Medicine and space exploration. Lancet. (2003) 362 Suppl:S8–9. doi: 10.1016/s0140-6736(03)15055-6
6. Li X, Zhang C, Bowman HH, Stambough JB, Stronach BM, Mears SC, et al. Piezo1 opposes age-associated cortical bone loss. Aging Cell. (2023) 22(6):e13846. doi: 10.1111/acel.13846
7. Hu Y, Tian H, Chen W, Liu Y, Cao Y, Pei H, et al. The critical role of the Piezo1/β-catenin/ATF4 axis on the stemness of Gli1+ BMSCs during simulated microgravity-induced bone loss. Advanced Sci. (2023) 10(32):e2303375. doi: 10.1002/advs.202303375
8. Qu J, Zong HF, Shan Y, Zhang SC, Guan WP, Zhao HL, et al. Piezo1 suppression reduces demyelination after intracerebral hemorrhage. Neural Regener Res. (2022) 18(8):1750–6. doi: 10.4103/1673-5374.361531
9. Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife. (2019) 8:e47454. doi: 10.7554/eLife.47454
10. Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. (2017) 7(1):17696. doi: 10.1038/s41598-017-18089-0
11. Liu Z, Tang Y, He L, Geng B, Lu F, He J, et al. Piezo1-mediated fluid shear stress promotes OPG and inhibits RANKL via NOTCH3 in MLO-Y4 osteocytes. Channels. (2022) 16(1):127–36. doi: 10.1080/19336950.2022.2085379
12. Wu RW, Lian WS, Chen YS, Ko JY, Wang SY, Jahr H, et al. Piezoelectric microvibration mitigates estrogen loss-induced osteoporosis and promotes Piezo1, microRNA-29a and Wnt3a signaling in osteoblasts. Int J Mol Sci. (2021) 22(17):9476. doi: 10.3390/ijms22179476
13. Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. (2019) 51(2):258–66. doi: 10.1038/s41588-018-0302-x
14. Wolff J. The classic: on the theory of fracture healing. 1873. Clin Orthop Relat Res. (2010) 468(4):1052–5. doi: 10.1007/s11999-010-1240-9
15. Frost HM. Changing concepts in skeletal physiology: Wolff’s law, the mechanostat, and the ‘Utah paradigm. Am J Hum Biol. (1998) 10(5):599–605. doi: 10.1002/(SICI)1520-6300(1998)10:5<599::AID-AJHB6>3.0.CO;2-9
16. Fang XZ, Zhou T, Xu JQ, Wang YX, Sun MM, He YJ, et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. (2021) 11(1):13. doi: 10.1186/s13578-020-00522-z
17. Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, and Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. (2018) 554(7693):481–6. doi: 10.1038/nature25453
18. Dienes B, Bazsó T, Szabó L, and Csernoch L. The role of the piezo1 mechanosensitive channel in the musculoskeletal system. (2023) 24(7):6513. doi: 10.3390/ijms24076513
19. Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. (2015) 527(7576):64–9. doi: 10.1038/nature15247
20. Wang L, You X, Lotinun S, Zhang L, Wu N, and Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. (2020) 11(1):282. doi: 10.1038/s41467-019-14146-6
21. Nie X and Chung MK. Piezo channels for skeletal development and homeostasis: Insights from mouse genetic models. (2022) 126:10–5. doi: 10.1016/j.diff.2022.06.001
22. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife. (2020) 9:e52779. doi: 10.7554/eLife.52779
23. Li L, Han L, Noo Kaew I, Mannen E, Silva MJ, Almeida M, et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife. (2019) 8:e49631. doi: 10.7554/eLife.49631
24. Poole K, Herget R, Lapatsina L, Ngo HD, and Lewin GR. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat Commun. (2014) 5:3520. doi: 10.1038/ncomms4520
25. Du Y, Xu B, Li Q, Peng C, and Yang K. The role of mechanically sensitive ion channel Piezo1 in bone remodeling. (2024) 12:1342149. doi: 10.3389/fbioe.2024.1342149
26. Capponi G, Zambito M, Neri I, Cottone F, Mattarelli M, Vassalli M, et al. Cellular mechanosensitivity: validation of an adaptable 3D-printed device for microindentation. Nanomaterials. (2022) 12(15):2691. doi: 10.3390/nano12152691
27. Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, et al. Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron. (2016) 89(6):1248–63. doi: 10.1016/j.neuron.2016.01.046
28. Spyropoulou A, Karamesinis K, and Basdra EK. Mechanotransduction pathways in bone pathobiology. (2015) 1852(9):1700–8. doi: 10.1016/j.bbadis.2015.05.010
29. O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. (2004) 145(4):1835–41. doi: 10.1210/en.2003-0990
30. Schurman CA, Verbruggen SW, and Alliston T. Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF-β signaling. Proc Natl Acad Sci U S A. (2021) 118(25):e2023999118. doi: 10.1073/pnas.2023999118
31. Tan SD, Bakker AD, Semeins CM, Kuijpers-Jagtman AM, and Klein-Nulend J. Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem Biophys Res Commun. (2008) 369(4):1150–4. doi: 10.1016/j.bbrc.2008.03.007
32. Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways. J Bone Mineral Res. (2010) 25(12):2657–68. doi: 10.1002/jbmr.168
33. Hao R, Tang H, Ding C, Rajbanshi B, Liu Y, Ma D, et al. A novel Piezo1 agonist promoting mesenchymal stem cell proliferation and osteogenesis to attenuate disuse osteoporosis. Small Sci. (2024) 4:2400061. doi: 10.1002/smsc.202400061
34. Zeng Y, Riquelme MA, Hua R, Zhang J, Acosta FM, Gu S, et al. Mechanosensitive Piezo1 calcium channel activates connexin 43 hemichannels through PI3K signaling pathway in bone. Cell Biosci. (2022) 12(1):191. doi: 10.1186/s13578-022-00929-w
35. Jiang Y, Lin H, Chen Y, Lan Y, Wang H, Li T, et al. Piezo1 contributes to alveolar bone remodeling by activating β-catenin under compressive stress. Am J Orthodontics Dentofacial Orthopedics. (2024) 165(4):458–70. doi: 10.1016/j.ajodo.2023.10.020
36. Du Y and Yang K. Role of mechanosensitive ion channel Piezo1 in tension-side orthodontic alveolar bone remodeling in rats. Arch Oral Biol. (2023) 155:105798. doi: 10.1016/j.archoralbio.2023.105798
37. Nagai S, Kitamura K, Kimura M, Yamamoto H, Katakura A, and Shibukawa Y. Functional expression of mechanosensitive Piezo1/TRPV4 channels in mouse osteoblasts. Bull Tokyo Dental Coll. (2023) 64(1):1–11. doi: 10.2209/tdcpublication.2022-0015
38. Sun W, Li Y, Li J, Tan Y, Yuan X, Meng H, et al. Mechanical stimulation controls osteoclast function through the regulation of Ca2+-activated Cl– channel Anoctamin 1. Commun Biol. (2023) 6(1):407. doi: 10.1038/s42003-023-04806-1
39. Zhang X, Zhang L, Xu L, Li G, Wang K, Xue T, et al. Exosomes from Microvascular Endothelial Cells under Mechanical Unloading Inhibit Osteogenic Differentiation via miR-92b-3p/ELK4 Axis. J Pers Med. (2022) 12(12):2030. doi: 10.3390/jpm12122030
40. Seddiqi H, Klein-Nulend J, and Jin J. Osteocyte mechanotransduction in orthodontic tooth movement. (2023) 21(6):731–42. doi: 10.1007/s11914-023-00826-2
41. Nepal AK, van Essen HW, Reijnders CMA, Lips P, and Bravenboer N. Mechanical loading modulates phosphate related genes in rat bone. PLoS One. (2023) 18(3):e0282678. doi: 10.1371/journal.pone.0282678
42. Nims R, Palmer DR, Kassab J, Zhang B, and Guilak F. The chondrocyte ‘mechanome’: Activation of the mechanosensitive ion channels TRPV4 and PIEZO1 drives unique transcriptional signatures. FASEB J. (2024) 38(13):e23778. doi: 10.1096/fj.202400883R
43. Mukherjee S, Das SK, Srivastava AK, Chatterjee S, and Bandyopadhyay S. TRPV4 regulates osteoblast differentiation and mitochondrial function that are relevant for channelopathy. Front Cell Dev Biol. (2023) 11:1066788. doi: 10.3389/fcell.2023.1066788
44. Yuan J, Krasnow MJ, Chen CC, Patapoutian A, and Plemper RK. Mechanical and chemical activation of GPR68 probed with a genetically encoded fluorescent reporter. Sci Adv. (2021) 7(10):eabi9775. doi: 10.1126/sciadv.abi9775
45. Covington RM, Dill KM, Hagan CR, and Imig JD. pH-sensing GPR68 inhibits vascular smooth muscle cell proliferation through Rap1A. Am J Physiol Heart Circ Physiol. (2021) 327(5):H1210–29. doi: 10.1152/ajpheart.00245.2021
46. Zhou G and Zha XM. GPR68 contributes to persistent acidosis-induced activation of AGC kinases and tyrosine phosphorylation in organotypic hippocampal slices. Front Neurosci. (2021) 15:692217. doi: 10.3389/fnins.2021.692217
47. Li X, Zhang C, Vail CE, Sherrill JT, and Xiong J. Piezo1 expression in mature osteocytes is dispensable for the skeletal response to mechanical loading. Bone. (2025) 190:117276. doi: 10.1016/j.bone.2024.117276
48. Zhang G, Li X, Wu L, and Qin YX. Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells. Bone Res. (2021) 9(1):16. doi: 10.1038/s41413-020-00124-y
49. Sugimoto A, Iwata K, Kurogoushi R, Tanaka M, Nakashima Y, Yamakawa Y, et al. C-terminus of PIEZO1 governs Ca2+ influx and intracellular ERK1/2 signaling pathway in mechanotransduction. Biochem Biophys Res Commun. (2023) 682:39–45. doi: 10.1016/j.bbrc.2023.09.080
50. Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. (2014) 111(47):E5114–22. doi: 10.1073/pnas.1414298111
51. Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F, et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci U S A. (2021) 118(13):e2001611118. doi: 10.1073/pnas.2001611118
52. Hendrickx G, Fischer V, Liedert A, von Kroge S, Haffner-Luntzer M, Brylka L, et al. Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J Bone Mineral Res. (2021) 36(2):369–84. doi: 10.1002/jbmr.4198
53. Ren X, Zhuang H, Li B, Jiang F, Zhang Y, and Zhou P. Gsmtx4 Alleviated Osteoarthritis through Piezo1/Calcineurin/NFAT1 Signaling Axis under Excessive Mechanical Strain. Int J Mol Sci. (2023) 24(4):4022. doi: 10.3390/ijms24044022
54. Jiang Q, Qin X, Nagano K, Komori H, Matsuo Y, Ito K, et al. Different requirements of CBFB and RUNX2 in skeletal development among calvaria, limbs, vertebrae and ribs. Int J Mol Sci. (2022) 23(21):13299. doi: 10.3390/ijms232113299
55. Wee NKY, de Lima TFC, McGregor NE, Walker EC, Poulton IJ, Blank M, et al. Leptin receptor in osteocytes promotes cortical bone consolidation in female mice. J Endocrinol. (2022) 255(1):25–37. doi: 10.1530/JOE-22-0084
56. Hariri H, Kose O, Bezdjian A, Daniel SJ, and St-Arnaud R. USP53 regulates bone homeostasis by controlling Rankl expression in osteoblasts and bone marrow adipocytes. J Bone Mineral Res. (2023) 38(4):578–96. doi: 10.1002/jbmr.4778
57. Cao Z, Niu X, Wang M, Yu S, Wang M, Mu S, et al. Anemoside B4 attenuates RANKL-induced osteoclastogenesis by upregulating Nrf2 and dampens ovariectomy-induced bone loss. Biomedicine Pharmacotherapy. (2023) 167:115454. doi: 10.1016/j.biopha.2023.115454
58. Hu TL, Chen J, Shao SQ, Li LL, Lai C, Gao WN, et al. Biomechanical and histomorphological analysis of the mandible in rats with chronic kidney disease. Sci Rep. (2023) 13(1):21886. doi: 10.1038/s41598-023-49152-8
59. Cunningham HC, Orr S, Murugesh DK, Hsia AW, Osipov B, Go L, et al. Differential bone adaptation to mechanical unloading and reloading in young, old, and osteocyte deficient mice. Bone. (2023) 167:116646. doi: 10.1016/j.bone.2022.116646
60. Bai WY, Wang LJ, Ying ZM, Hu B, Xu L, Zhang GQ, et al. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone. (2020) 133:115247. doi: 10.1016/j.bone.2020.115247
61. Mishra SK, Chatterjee S, Bandyopadhyay S, and Srivastava AK. ROCK-II inhibition suppresses impaired mechanobiological responses in early estrogen deficient osteoblasts. J Cell Physiol. (2020) 396(1):112264. doi: 10.1002/jcp.29645
62. Sun LQ, Zhang XD, and Yao WJ. Osteocytes and estrogen deficiency. Curr Osteoporos Rep. (2021) 19(6):592–603. doi: 10.1007/s11914-021-00645-x
63. Yang R, Li J, Zhang J, Xue Q, Qin R, Wang R, et al. 17β-estradiol plays the anti-osteoporosis role via a novel ESR1-Keap1-Nrf2 axis-mediated stress response activation and Tmem119 upregulation. J Bone Miner Res. (2022) 37:231–44. doi: 10.1002/jbmr.4543
64. Yang Q, Cao Y, Wang L, Dong Y, Zhao L, Geng Z, et al. Mechanical force receptor Piezo1 regulates T9 cell differentiation. J Immunol. (2023) 44(1):115136. doi: 10.4049/jimmunol.2200845
65. Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. (2017) 8(1):350. doi: 10.1038/s41467-017-00429-3
66. Zhang X, Hou L, Li F, Zhang W, Wu C, Xiang L, et al. Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury. Theranostics. (2022) 12(4):1621–38. doi: 10.7150/thno.64963
67. Chen P, Zhang G, Jiang S, Ning Y, Deng B, Pan X, et al. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium. (2021) 97:102431. doi: 10.1016/j.ceca.2021.102431
68. Deng R, Li C, Wang X, Chang L, Ni S, Zhang W, et al. Periosteal CD68+F4/80+ Macrophages are mechanosensitive for cortical bone formation by secretion and activation of TGF-β1. Advanced Sci. (2022) 9(3):e2103343. doi: 10.1002/advs.202103343
69. Sugisawa E, Takayama Y, Takemura N, Kondo T, Hatakeyama S, Kumagai Y, et al. RNA sensing by gut Piezo1 is essential for systemic serotonin synthesis. Cell. (2020) 182(3):609–24.e21. doi: 10.1016/j.cell.2020.06.022
70. Matute JD, Duan J, and Blumberg RS. Microbial RNAs pressure piezo1 to respond. (2020) 182(3):542–4. doi: 10.1016/j.cell.2020.07.015
71. Chen S, Li Z, Chen D, Cui H, Wang J, Li Z, et al. Piezo1-mediated mechanotransduction promotes entheseal pathological new bone formation in ankylosing spondylitis. Ann Rheum Dis. (2023) 82(4):533–45. doi: 10.1136/ard-2022-223428
72. Lan Y, Lu J, Zhang S, Jie C, Chen C, Xiao C, et al. Piezo1-mediated mechanotransduction contributes to disturbed flow-induced atherosclerotic endothelial inflammation. J Am Heart Assoc. (2024) 13:e035558. doi: 10.1161/JAHA.123.035558
73. Xu HQ, Guo ZX, Yan JF, Wang SY, Gao JL, Han XX, et al. Fibrotic matrix induces mesenchymal transformation of epithelial cells in oral submucous fibrosis. Am J Pathol. (2023) 193(9):1208–22. doi: 10.1016/j.ajpath.2023.05.014
74. Zeng Y, Shen J, Zhou X, Ouyang Z, Zhong J, Qin Y, et al. Osteogenic differentiation of bone mesenchymal stem cells on linearly aligned triangular micropatterns. J Mater Chem B. (2024) 12:8420–30. doi: 10.1039/d4tb01218f
75. Wu Z, Hou W, Lu J, Zhao H, Chen S, Guo T, et al. Piezo1 is an early mediator during topography-stimulated osteogenic differentiation of bone mesenchymal stem cells. J BioMed Mater Res A. (2025) 113:e37931. doi: 10.1002/jbm.a.37931
76. Ganguly K, Dutta SD, Randhawa A, Patel DK, Patil TV, and Lim KT. Transcriptomic Changes toward Osteogenic Differentiation of Mesenchymal Stem Cells on 3D-Printed GelMA/CNC Hydrogel under Pulsatile Pressure Environment. Adv Healthc Mater. (2023) 12:e2202163. doi: 10.1002/adhm.202202163
77. Alemán OR, Blanco-Camarillo C, Naranjo-Pinto N, Mora N, and Rosales C. Fc gamma receptors activate different protein kinase C isoforms in human neutrophils. J Leukoc Biol. (2025) 117:qiaf019. doi: 10.1093/jleuko/qiaf019
78. Song J, Liu L, Lv L, Hu S, Alkhatatbeh T, Dang X, et al. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol Int. (2020) 44(7):1491–502. doi: 10.1002/cbin.11344
79. Wang Z and Guo J. Mechanical induction of BMP-7 in osteocyte blocks glucocorticoid-induced apoptosis through PI3K/AKT/GSK3β Pathway. Cell Biochem Biophys. (2013) 67(2):567–74. doi: 10.1007/s12013-013-9543-6
80. Li C, Yang P, Liu B, Bu J, Liu H, Guo J, et al. Prednisolone induces osteocytes apoptosis by promoting Notum expression and inhibiting PI3K/AKT/GSK3β/β-catenin pathway. J Mol Histol. (2021) 52(5):1081–95. doi: 10.1007/s10735-021-10006-0
81. Fan J, Lee CS, Kim S, Chen C, Aghaloo T, and Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. (2020) 14(9):11973–84. doi: 10.1021/acsnano.0c05122
82. Kapinas K, Kessler C, Ricks T, Gronowicz G, and Delany AM. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem. (2010) 285:25221–31. doi: 10.1074/jbc.M110.116137
83. Cai GH, Lu YH, Zhong WJ, Wang T, Li YY, Ruan XL, et al. Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Prolif. (2023) 56(9):e13440. doi: 10.1111/cpr.13440
84. Tan B, Deng YY, Mao JW, Peng Y, Yang RY, Shen J, et al. The critical role of Piezo1/CaMKII/β-catenin axis in promoting osteogenic differentiation of ADSCs by pressure stimulation. J Cell Physiol. (2022) 10(29):31368–80. doi: 10.1002/jcp.31087
85. Xie BQ, He XY, Guo Y, Shen J, Yang BB, Cai R, et al. Cyclic tensile stress promotes osteogenic differentiation via upregulation of Piezo1 in human dental follicle stem cells. Cell Biol Int. (2022) 37(6):1649–62. doi: 10.1002/cbin.12145
86. Liu S, Xu X, Fang Z, Ning Y, Deng B, Pan X, et al. Piezo1 impairs hepatocellular tumor growth via deregulation of the MAPK-mediated YAP signaling pathway. Oncogene. (2021) 40:102367. doi: 10.1038/s41388-021-02102-x
87. Zhong GH, Su SW, Li JC, Zhao HL, Hu DT, Chen J, et al. Activation of Piezo1 promotes osteogenic differentiation of aortic valve interstitial cell through YAP-dependent glutaminolysis. Cardiovasc Res. (2023) 9(22):eadg0478. doi: 10.1093/cvr/cvac145
88. Kim OH, Choi YW, Park JH, Hong SA, Hong M, Chang IH, et al. Fluid shear stress facilitates prostate cancer metastasis through Piezo1-Src-YAP axis. Oncogene. (2022) 41:120936. doi: 10.1038/s41388-022-02502-8
89. Xing YY, Yang BB, He Y, Xie BQ, Zhao TQ, Chen JL, et al. Effects of mechanosensitive ion channel Piezo1 on proliferation and osteogenic differentiation of human dental follicle cells. J Cell Physiol. (2023) 238:151847. doi: 10.1002/jcp.30845
90. Ouyang MX, Zhang QY, Zhu YM, Luo MZ, Bu B, Deng LH, et al. α-catenin and Piezo1 mediate cell mechanical communication via cell adhesions. Cell. (2022) 13(5):357. doi: 10.1016/j.cell.2022.06.010
91. Kuang ZH, Bai JW, Ni LC, Hang K, Xu JX, Ying L, et al. Withanolide B promotes osteogenic differentiation of human bone marrow mesenchymal stem cells via ERK1/2 and Wnt/β-catenin signaling pathways. Phytomedicine. (2021) 88:106960. doi: 10.1016/j.phymed.2021.153664
92. Wu YJ, Li AL, Sun N, Jiang ZL, Li YF, Zhou ZW, et al. Unveiling the mechanisms of mechanical loading-induced knee osteoarthritis through transcriptomics. Osteoarthritis Cartilage. (2022) 30:114785. doi: 10.1016/j.joca.2022.08.015
93. Yuan XH, Zhang P, Yu TT, Huang HK, Zhang LL, Yang CM, et al. Lycorine inhibits tumor growth of human osteosarcoma cells by blocking Wnt/β-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway. J Exp Clin Cancer Res. (2020) 12(9):5381–98. doi: 10.1186/s13046-020-01792-8
94. Wang SX and Sun L. Silencing Aurora-kinase-A (AURKA) reinforced the sensitivity of diffuse large B-cell lymphoma cells to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) via suppressing β-Catenin and RAS-extracellular signal-regulated protein kinase (ERK1/2) pathway. Oncol Rep. (2021) 12(1):8296–308. doi: 10.3892/or.2021.7972
95. Xu H, Guan JN, Jin ZC, Yin C, Wu SN, Sun W, et al. Mechanical force modulates macrophage proliferation via Piezo1-AKT-Cyclin D1 axis. FASEB J. (2022) 36(8):e22423. doi: 10.1096/fj.202200314R
96. Jiang YK, Guan YZ, Lan YC, Chen S, Li TC, Zou SJ, et al. Mechanosensitive Piezo1 in periodontal ligament cells promotes alveolar bone remodeling during orthodontic tooth movement. Front Physiol. (2021) 12:767136. doi: 10.3389/fphys.2021.767136
97. Bratengeier C, Liszka A, Hoffman J, Bakker AD, and Fahlgren A. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J. (2020) 34. doi: 10.1096/fj.201901458R
98. Brylka LJ, Alimy A-R, Tschaffon-Müller ME, Jiang S, Ballhause TM, Baranowsky A, et al. Piezo1 expression in chondrocytes controls endochondral ossification and osteoarthritis development. Bone Res. (2024) 12(1):12. doi: 10.1038/s41413-024-00315-x
99. Hao LL, Li LL, Wang P, Wang ZL, Shi XC, Guo M, et al. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale. (2019) 11:23423–37. doi: 10.1039/C9NR07170A
100. Kong KY, Chang YY, Hu Y, Qiao H, Zhao C, Rong KW, et al. TiO(2) nanotubes promote osteogenic differentiation through regulation of Yap and Piezo1. Front Bioeng Biotechnol. (2022) 10:872088. doi: 10.3389/fbioe.2022.872088
101. Liu YL, Tian HT, Hu YX, Cao YL, Song H, Lan SH, et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int J Biol Sci. (2022) 18(10):3961–80. doi: 10.7150/ijbs.71390
102. Gorelick-Feldman J, Cohick W, and Raskin I. Ecdysteroids elicit a rapid Ca2+ flux leading to Akt activation and increased protein synthesis in skeletal muscle cells. Steroids. (2010) 75(10):632–7. doi: 10.1016/j.steroids.2010.03.008
103. Wu L, Zhang G, Guo C, and Pan Y. Intracellular Ca2+ signaling mediates IGF-1-induced osteogenic differentiation in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. (2020) 527(1):200–6. doi: 10.1016/j.bbrc.2020.04.048
104. Danciu TE, Adam RM, Naruse K, Freeman MR, and Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. (2003) 536(1-3):193–7. doi: 10.1016/S0014-5793(03)00055-3
105. Wang B, Shao W, Zhao Y, Li Z, Wang P, Lv X, et al. Radial extracorporeal shockwave promotes osteogenesis-angiogenesis coupling of bone marrow stromal cells from senile osteoporosis via activating the Piezo1/CaMKII/CREB axis. Bone. (2024) 187:117196. doi: 10.1016/j.bone.2024.117196
106. Cao S, Wang Z, Li C, and Wang Q. The effect of whole-body vibration exercise on postmenopausal women with osteoporosis: A protocol for systematic review and meta-analysis. (2021) 100(18):e25606. doi: 10.1097/MD.0000000000025606
107. Liu X, Gao X, Tong J, Yu L, Xu M, and Zhang J. Improvement of osteoporosis in rats with hind-limb unloading treated with pulsed electromagnetic field and whole-body vibration. Phys Ther. (2022) 102(10):pzac097. doi: 10.1093/ptj/pzac097
108. Maus U. Exercise therapy and basic treatment for osteoporosis. (2023) 52(10):793–8. doi: 10.1007/s00132-023-04432-z
109. Pinheiro MB, Oliveira J, Bauman A, Fairhall N, Kwok W, and Sherrington C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. (2020) 17(1):150. doi: 10.1186/s12966-020-01040-4
110. Yan Y, Tan B, Fu FY, Chen QL, Li WL, Chen WH, et al. Exercise vs conventional treatment for treatment of primary osteoporosis: A systematic review and meta-analysis of randomized controlled trials. (2021) 13(5):1474–87. doi: 10.1111/os.13036
111. Wang LQ, Jiang JJ, Li Y, Huang JM, Wang RJ, Liang YX, et al. Global trends and hotspots in research on osteoporosis rehabilitation: A bibliometric study and visualization analysis. Front Public Health. (2022) 10:1022035. doi: 10.3389/fpubh.2022.1022035
112. Manaye S, Cheran K, Murthy C, Bornemann EA, Kamma HK, Alabbas M, et al. The role of high-intensity and high-impact exercises in improving bone health in postmenopausal women: A systematic review. Cureus. (2023) 15(2):e34644. doi: 10.7759/cureus.34644
113. Bansod YD, Kebbach M, Kluess D, Bader R, and van Rienen U. Computational analysis of bone remodeling in the proximal tibia under electrical stimulation considering the piezoelectric properties. Front Bioeng Biotechnol. (2021) 9:705199. doi: 10.3389/fbioe.2021.705199
114. Sallehuddin H, Ong T, Md Said S, Ahmad Tarmizi NA, Loh SP, Lim WC, et al. Non-pharmacological interventions for bone health after stroke: A systematic review. (2022) 17(2):e0263935. doi: 10.1371/journal.pone.0263935
115. Li HL, Jiang HB, Wang JY, Zhou J, Liang H, Chen GX, et al. Effects of mind-body exercises for osteoporosis in older adults: A systematic review and meta-analysis of randomized controlled trials. (2023) 14:21514593231195237. doi: 10.1177/21514593231195237
116. Avin KG, Nithman RW, Osborne R, Betz SR, Lindsey C, and Hartley GW. Essential components of physical therapist management of patients with osteoporosis: A Delphi study. J Geriatric Phys Ther. (2022) 45(2):E120–6. doi: 10.1519/JPT.0000000000000347
117. Tang HR, Hao RH, Ma D, Yao YJ, Ding CY, Zhang XL, et al. Structural Modification and Pharmacological Evaluation of (Thiadiazol-2-yl) pyrazines as Novel Piezo1 Agonists for the Intervention of Disuse Osteoporosis. J Med Chem. (2024) 67(21):19837-19851. doi: 10.1021/acs.jmedchem.4c02224
118. Inoue S, Li CX, Hatakeyama J, Jiang HL, Kuroki H, and Moriyama H. Higher-intensity ultrasound accelerates fracture healing via mechanosensitive ion channel Piezo1. Bone. (2023) 38:177. doi: 10.1016/j.bone.2023.116916
119. Guan H-T, Wang W, Jiang Z-C, Zhang B-Y, Ye Z-P, Zheng J-D, et al. Magnetic Aggregation-Induced Bone-Targeting Nanocarrier with Effects of Piezo1 Activation and Osteogenic-Angiogenic Coupling for Osteoporotic Bone Repair. Adv Mater. (2024) 36(13):e2312081. doi: 10.1002/adma.202312081
120. Sun Y, Li X, and Xiao B. Piezo channel regulators: Mechanisms and therapeutic implications in bone diseases. Nat Rev Rheumatol. (2020) 16:673–86. doi: 10.1038/s41584-020-00493-0
Keywords: Piezo1, osteoporosis, mechanotransduction, bone homeostasis, therapeutic target
Citation: Liu C, Yang J, Dong Z, Zhao S, Tian Z-H, Li Y-Y, Hao Y-K and Wang M (2025) The central mechanotransducer in osteoporosis pathogenesis and therapy. Front. Endocrinol. 16:1658967. doi: 10.3389/fendo.2025.1658967
Received: 03 July 2025; Accepted: 05 September 2025;
Published: 23 September 2025.
Edited by:
Sandeep Kumar, Tulane University, United StatesReviewed by:
Rubi Gupta, Assam Agricultural University, IndiaNisha Thomas, University of Oklahoma, United States
Meghana Hosahalli Shivananda Murthy, Arizona State University, United States
Copyright © 2025 Liu, Yang, Dong, Zhao, Tian, Li, Hao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Ke Hao, aGFveWFua2VAMTI2LmNvbQ==; Mingliang Wang, bWluZ2xpYW5nMTk2NzQyMjk3MEBxcS5jb20=
 Chaoyue Liu
Chaoyue Liu Jihao Yang2
Jihao Yang2 Zeng-Hui Tian
Zeng-Hui Tian Ying-Ying Li
Ying-Ying Li Yan-Ke Hao
Yan-Ke Hao