- 1The First People’s Hospital of Jiande, Hangzhou, China
- 2Office of Research and Innovation, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China
- 3Zhejiang Key Laboratory of Medical Epigenetics, Department of Cardiology, Affiliated Hospital of Hangzhou Normal University, Hangzhou Institute of Cardiovascular Diseases, Engineering Research Center of Mobile Health Management System & Ministry of Education, Hangzhou Normal University, Hangzhou, China
- 4Department of Cardiology, Hangzhou Lin’an Fourth People’s Hospital, Hangzhou, Zhejiang, China
Background: Metabolic dysfunction–associated fatty liver disease (MAFLD) is one of the most common chronic liver diseases. The relationship between MAFLD and thyroid function parameters remains controversial.
Aim: This study aimed to explore the influence of metabolic parameters and thyroid dysfunction on the development of MAFLD and examine the relationship between them in different age groups.
Methods: The PubMed, Embase, Web of Science, China National Knowledge Infrastructure(CNKI), and Cochrane Library databases were searched. Standardized mean difference (SMD) and odds ratio with a 95% confidence interval (CI) were calculated.
Results: A total of 36 studies involving 198,254 participants were eligible. Compared with controls, the patients with MAFLD had significantly higher thyroid-stimulating hormone (TSH) levels (MAFLD vs controls: SMD = 0.02, 95% CI = 0.01–0.03); significantly higher free triiodothyronine levels (MAFLD vs controls: SMD = 0.19, 95% CI = 0.18–0.20); significantly lower free thyroxine levels (MAFLD vs controls: SMD = −0.41, 95% CI = −0.42 to −0.40); significantly higher total triiodothyronine levels (MAFLD vs controls: SMD = 0.26, 95% CI = 0.23–0.29); and significantly lower total thyroxine levels (MAFLD vs controls: SMD = −0.10, 95% CI = −0.13 to −0.07).
Conclusions: The TSH level may be an important risk factor for the occurrence and development of MAFLD. The relationship between them is influenced by age.
1 Introduction
Metabolic dysfunction–associated fatty liver disease (MAFLD) encompasses a range of liver diseases closely related to abnormal metabolic disorders. It is considered a hepatic manifestation of the metabolic syndrome (1). MAFLD is characterized by hepatic steatosis (detected by imaging techniques, blood biomarkers/scores, or liver histology) associated with type 2 diabetes mellitus (T2DM) and overweight/obesity, regardless of alcohol intake or excluding other causes of chronic liver disease (2). The introduction of the new concept of MAFLD aims to include metabolic dysfunction as a diagnostic criterion and covers a larger and more diverse population than non-alcoholic fatty liver disease (NAFLD) (3). A meta-analysis involving 3.3 million people revealed that the global prevalence of MAFLD was 38.77% (4). Compared with the general population, patients with MAFLD experience higher liver-related morbidity or mortality and are strongly associated with extrahepatic diseases such as cancer, cardiovascular events, stroke, chronic kidney disease, and obstructive sleep apnea (5–8).
The rationale for MAFLD is to include metabolic dysfunction as a diagnostic criterion, including abdominal obesity and T2DM. The thyroid gland regulates body weight, lipid-like substance metabolism, and insulin resistance. Therefore, thyroid hormone may be closely related to the occurrence and development of MAFLD (9). However, the relationship between MAFLD and thyroid function still remains controversial. Three published meta-analyses have explored the relationship between NAFLD and thyroid function, but their findings were inconsistent (10–12). The patients with NAFLD/NASH had significantly higher thyroid-stimulating hormone (TSH) levels than controls in adults. Previous studies found no significant difference in thyroid hormone levels between patients with NAFLD and non-NAFLD. This might be mainly attributed to the heterogeneity of sample sizes and patient characteristics. MAFLD covers a much broader spectrum, and no studies demonstrating an association between MAFLD and thyroid function parameters have been reported. Therefore, this study conducted a new systematic review and meta-analysis, including a lot of research and exploration of several thyroid function parameters [free triiodothyronine (FT3), free thyroxine (FT4), total triiodothyronine (TT3), total thyroxine (TT4), and TSH levels].
2 Methods
2.1 Protocol
This systematic review and meta-analysis was conducted based on the meta-analysis of observational studies following epidemiology guidelines.
2.2 Eligibility criteria
The inclusion criteria were as follows: (1) original studies that explored the association between MAFLD, NAFLD, and NASH with thyroid function; (2) studies independent of sex and age; and (3)studies in which MAFLD was diagnosed by histology (liver biopsy), imaging modalities (such as ultrasonography, CT, or MRI), or validated biochemical indices. The exclusion criteria were as follows: (1) reviews and meta-analyses, (2) irrelevant pieces of literature, (3) duplicate studies, (4) experimental studies, (5) editorials/comments and case reports, and (6) original studies including patients with MAFLD/NAFLD/NASH but with competing etiologies for steatosis and coexisting causes for other chronic liver diseases, such as significant alcohol consumption, hepatitis C, and medications.
2.3 Search strategy
We searched the PubMed, Embase, Web of Science, CNKI, and Cochrane Library databases from the inception dates to March 1, 2025, with no restrictions on language. Conference abstracts were hand searched to identify potentially eligible studies. The search strategy was as follows: (“MAFLD” [All Fields] OR “Metabolic associated fatty liver disease” [All Fields]) OR (“NASH” [All Fields] OR “Nonalcoholic Steatohepatitis” [All Fields]) OR (“NAFLD” [All Fields] OR “Non-alcoholic Fatty Liver Disease” [All Fields]) AND (“thyroid” [All Fields] OR “thyroid-stimulating hormone” [All Fields] OR “free thyroxine” [All Fields] OR “free triiodothyronine” [All Fields] OR “total triiodothyronine” [All Fields] OR “total thyroxine” [All Fields]).
2.4 Study selection
Two reviewers (J.T. and C.C.) independently screened the studies based on the titles and abstracts. If the studies were potentially fit, full texts were further retrieved and screened. Disagreements were resolved by consensus.
2.5 Data extraction
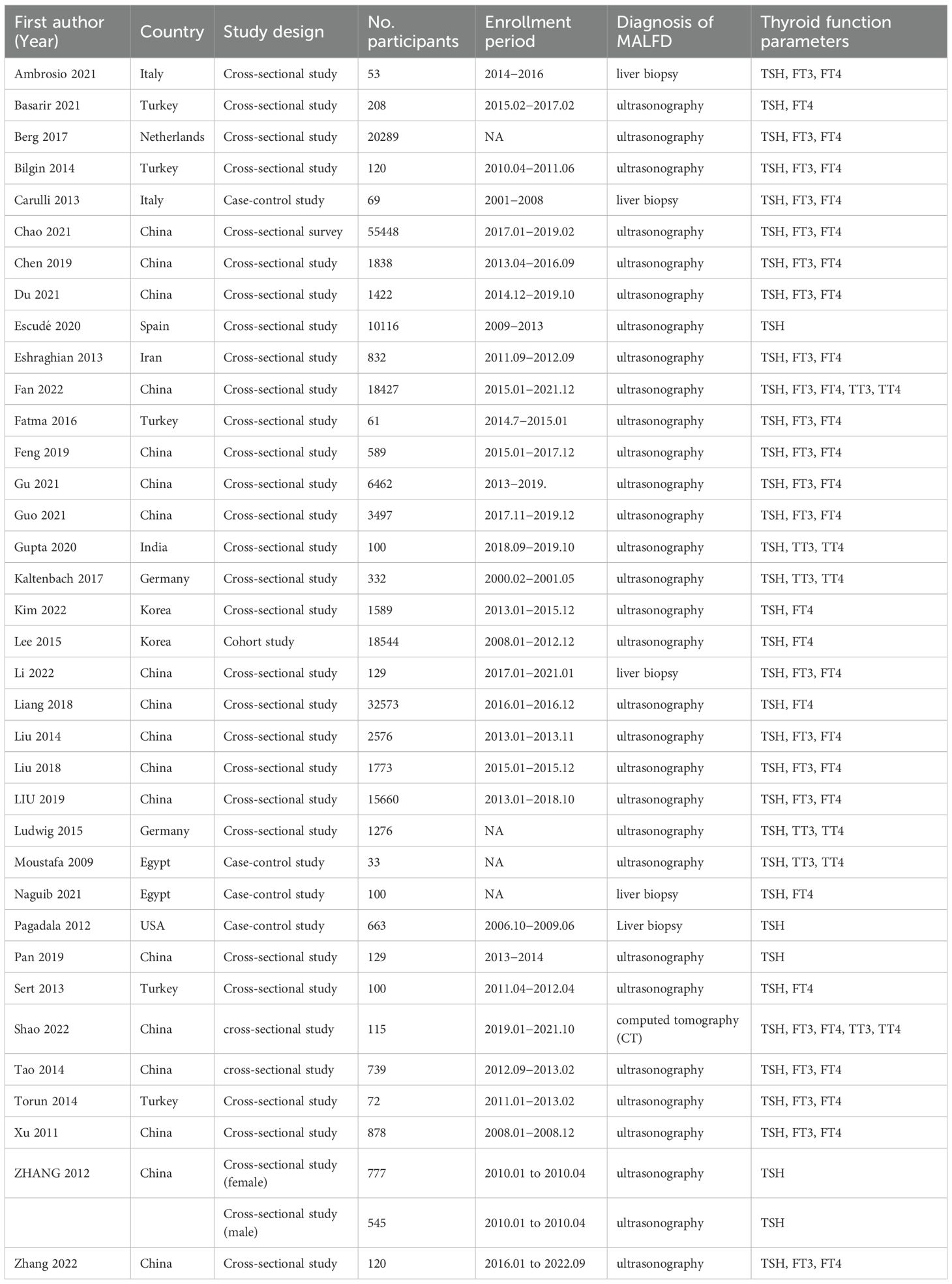
Two reviewers (J.T. and C.C.) extracted all the data independently. We extracted the following data from each study: study characteristics, first author, year of publication, country, study design, study population, number of participants, age, MAFLD diagnosis, and thyroid function parameters (TSH, FT3, FT4, TT3, and TT4). If necessary, the reviewers contacted for relevant data.
If some data were expressed as mean with a 95% confidence interval (CI) alone, the methods provided by the Cochrane Handbook were used to calculate the standard deviation (SD). The formula used is as follows:
If some data were expressed as median with range or interquartile range alone, the methods provided by Wan et al. (13) and Luo et al. (14) were used to estimate the mean and SD. All formulas are available on the website http://www.comp.hkbu.edu.hk/∼xwan/MedianRange.html.
2.6 Statistical analysis
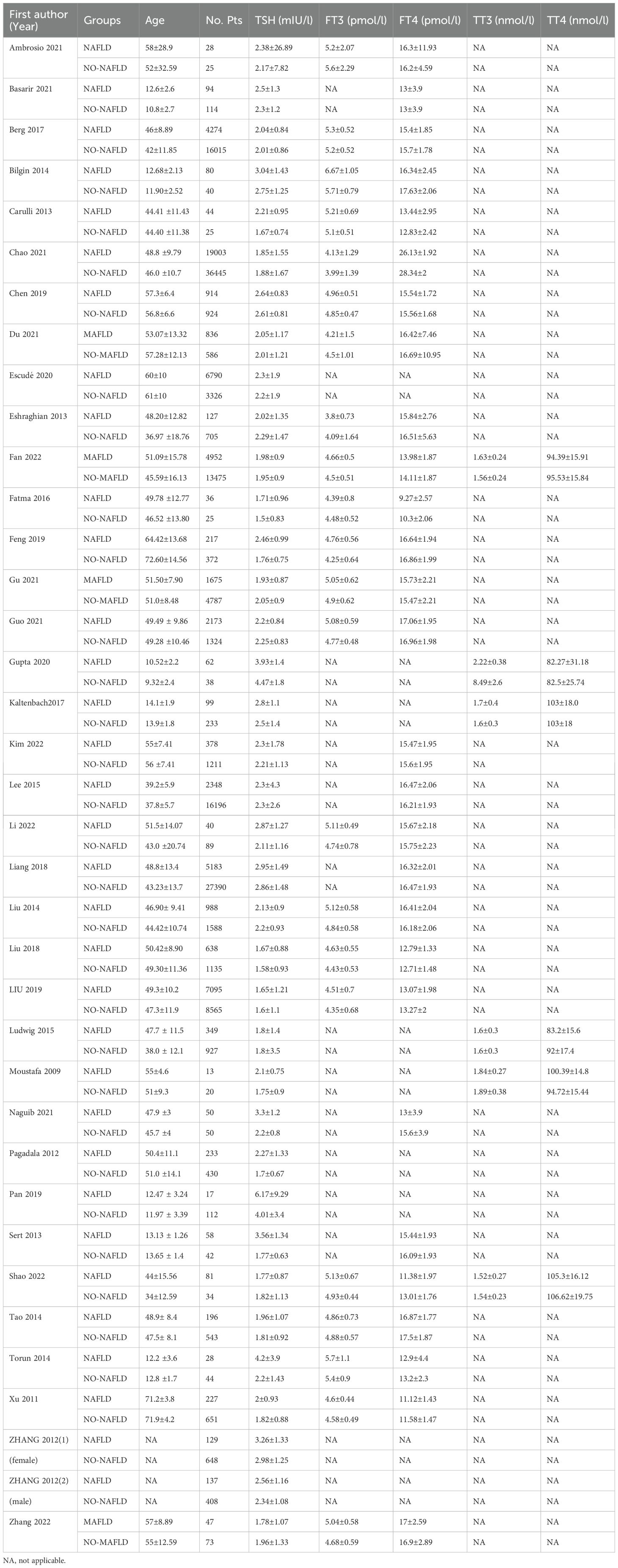
The association of MAFLD/NASH/NAFLD with thyroid function parameters (including FT3, FT4, TT3, TT4, and TSH) was assessed. For continuous data, standardized mean difference (SMD) with 95% CI was calculated using the inverse variance statistical method. For dichotomous variable data, the odds ratio (OR) with 95% CI was calculated using the Mantel–Haenszel statistical method. The data were pooled using a random-effects model to obtain a more conservative effect estimate. The I2 statistic and Q test were used to measure the heterogeneity across the included studies, where I2 >50% and P value <0.1 were considered to be of significant heterogeneity. If more than five eligible studies were included in a meta-analysis, the publication bias was assessed using funnel plots. All statistical analyses were performed using Review Manager version 5.2 software.
3 Results
3.1 Study selection
A total of 1246 studies were retrieved, 36 studies were eligible (Figure 1). MAFLD was diagnosed by hepatic steatosis (including imaging techniques, blood biomarkers/scores, or liver histological tests). Patients without MAFLD rather than healthy individuals were randomly selected as controls. Ultimately, 198,254 participants were included in the quantitative analysis.
3.2 Study characteristics
The characteristics of the 36 included studies are depicted(Table 1). Among these, 7 were cohort studies (15–21), 25 were cross-sectional studies (22–46), and 4 were case–control studies (47–50). These clinical trials were conducted globally: 6 in Europe [Germany (23, 26), Italy (18, 47), Netherlands (30), and Spain (39)], 28 in Asia (5 in Turkey (15–17, 27, 29), 2 in South Korea (24, 36), 1 in India (20), 1 in Iran (22), 19 in China [19, 21, 25, 28, 31–35, 37, 38, 40–46)], 2 in Africa [Egypt (48, 50)], and 1 in the United States (49). MAFLD was diagnosed by different methods: through liver biopsies in 5 studies (18, 37, 47, 49, 50), through computed tomography (CT) in 1 study (41), and through ultrasound technology in 31 studies. All the 36 included studies reported detailed data regarding thyroid function parameters (Table 2). All studies reported detailed data on the TSH parameter, 22 studies reported data on the FT3 parameter, 28 on the FT4 parameter, and 6 on the TT3 and TT4 parameters.
3.2.1 Thyroid-stimulating hormone
A total of 36 studies with 198,254 patients were included in the meta-analysis. The patients with MAFLD had significantly higher levels of TSH than controls (SMD = 0.02; 95% CI = 0.01, 0.03; P < 0.001) (Figure 2). Heterogeneity was statistically significant (I2 = 89%, P < 0.001). The publication bias was not statistically significant (Figure 3). Meanwhile, it was found that compared with the controls, the patients with MAFLD had significantly higher levels of TSH in the children/youth group according to the age subgroup analysis (SMD = 0.32, 95% CI = 0.19, 0.045, P < 0.001); no significant difference was observed in the level of TSH in the middle-aged group (SMD = 0.01, 95% CI = 0.00, 0.02, P = 0.02); and significantly higher levels of TSH was observed in the elderly group (SMD = 0.10, 95% CI = 0.06, 0.14, P < 0.001).
3.2.2 Free triiodothyronine
A total of 22 studies with 121,169 patients were included in the meta-analysis. The patients with MAFLD had significantly higher levels of FT3 than controls (SMD = 0.19, 95% CI = 0.18, 0.20, P < 0.001) (Figure 4). Heterogeneity was statistically significant (I2 = 89%, P < 0.001). Compared with the controls, the patients with MAFLD had significantly higher levels of FT3 in the children/youth group (SMD = 0.70, 95% CI = 0.39, 1.01, P < 0.001); the middle-aged group (SMD = 0.19, 95% CI = 0.18, 0.2, P < 0.001); and the elderly group (SMD = 0.38, 95% CI = 0.27, 0.50, P < 0.001).
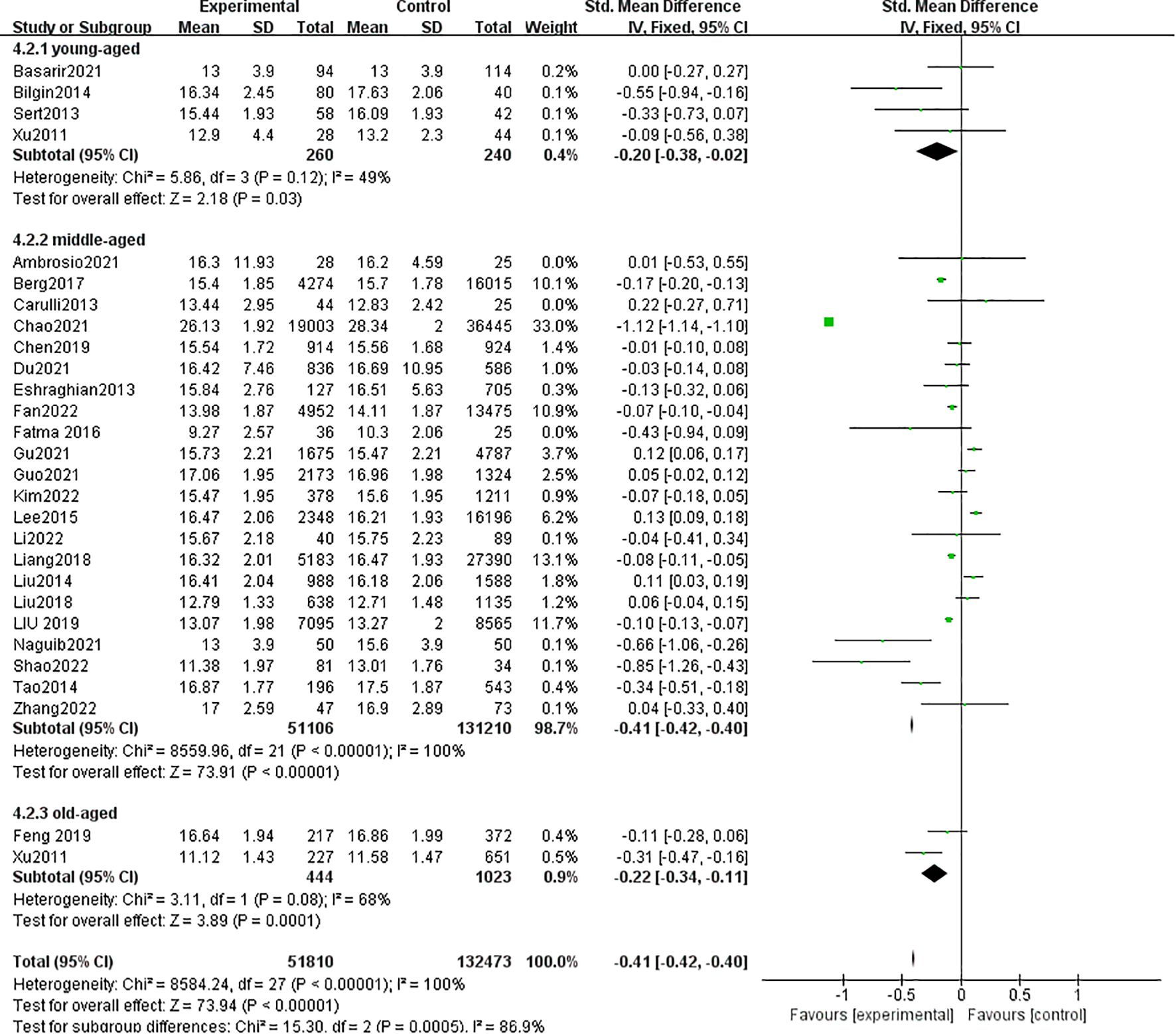
3.2.3 Free thyroxine
A total of 28 studies with 184,283 patients were included in the meta-analysis. The patients with MAFLD had significantly lower levels of FT4 than controls (SMD = −0.41, 95% CI = −0.42, −0.40, P < 0.001) (Figure 5). Heterogeneity was statistically significant (I2 = 100%, P < 0.001). Compared with controls, the patients with MAFLD had significantly lower levels of FT4 in the children/youth group (SMD = −0.20, 95% CI = −0.38, −0.02, P < 0.03); the middle-aged group (SMD = −0.41, 95% CI = −0.42, −0.40, P < 0.001); and the elderly group (SMD = −0.22, 95% CI = −0.34, −0.11, P < 0.001).
3.2.4 Total triiodothyronine
A total of 6 studies with 20,283 patients were included in the meta-analysis. The patients with MAFLD had significantly higher levels of TT3 than controls (SMD = 0.26, 95% CI = 0.23, 0.29, P < 0.001) (Figure 6). Heterogeneity was statistically significant (I2 = 97%, P < 0.001).
3.2.5 Total thyroxine
A total of 6 studies involving 20,283 patients were included in the meta-analysis. The patients with MAFLD had significantly lower levels of TT3 than controls (SMD = −0.10; 95% CI = −0.13, −0.07; P < 0.001) (Figure 7). Heterogeneity was statistically significant (I2 = 90%, P < 0.001).
4 Discussion
A total of 36 studies with 198,254 participants were included in the systematic review and meta-analysis. The findings revealed the following. (1) Increased TSH levels were significantly associated with a higher risk of MAFLD. However, no significant difference in the TSH level was found between the patients with MAFLD and the controls in the middle-aged population. In the adolescent and elderly population, the TSH level of patients with MAFLD significantly differed from that of the controls. (2) The increased levels of FT3 and TT3 were significantly associated with the risk of MAFLD in all populations. (3) The decreased levels of TT4 and FT4 were significantly associated with the risk of MAFLD in all populations.
The “second strike” hypothesis has been widely considered to be the classical pathogenesis of MAFLD. The progressive accumulation of lipids in the cytoplasm of hepatocytes triggers a cytotoxic event that triggers an inflammatory response. The liver, being the central organ regulating endocrine function and maintaining triglyceride metabolism, plays a crucial role. Abnormal triglyceride accumulation in hepatocytes forms fatty liver. Some studies have shown a positive correlation between TSH and triglyceride concentrations (51, 52), even within the normal reference range (53). TSH might play a key role in regulating triglycerides and cholesterol, thus promoting the development of MAFLD (54).
In terms of pathophysiology, the presence of thyroid hormones can contribute to the development of MAFLD through multiple pathways. Thyrotropin hormone regulates the thyrotropin receptor (TSHR) through highly specific interactions with thyroxine. TSHR is expressed primarily on the cell membrane of thyroid follicular cells and is also expressed to varying degrees in numerous extrathyroidal tissues and cells, such as liver, kidney, bone, adipose tissue, and fibroblasts (55, 56). The binding of TSH to TSHR on hepatocytes can increase triglyceride levels, leading to hepatic steatosis. Thus, the potential mechanism of TSH-induced hepatic triglyceride accumulation involves the binding of TSH to its receptor TSHR, which then triggers hepatic Sterol Regulatory Element Binding Protein-1c(SREBP-1c)activity via the cAMP/PKA/PPARa pathway, resulting in reduced AMPK activity and increased expression of genes associated with insulin resistance and adipogenesis (57). Meanwhile, another study showed that TSH could be involved in intrahepatic lipolysis by the activation of autophagy and β-oxidation of fatty acids (58). Thus, elevated TSH levels indicated hypothyroidism and reduced activity of hepatic lipase, leading to fat cell accumulation in hepatocytes (59). A study demonstrated that foreign bodies from TSH-stimulated hepatocytes increased and showed specific protein profile changes, many of which were involved in metabolism, signal transduction, apoptosis, and inflammation (60). TSH is also associated with the regulation of microrne (61). Adipokine levels were altered in patients with hypothyroidism (62). Recently, a high TSH level was significantly correlated with NASH in individuals with normal thyroid function and NAFLD confirmed by biopsy (63). In this study, TSH increased the risk of MAFLD significantly. However, the results of this study were controversial. The subgroup analysis in this study showed no significant association between TSH and MAFLD in the middle-aged population, but found an association between TSH and MAFLD in adolescents and older adults.
A feedback loop exists between TSH and thyroid gland in the body. An increase in the level of TSH usually suggests a low thyroid function (i.e., hypothyroidism) (12). Ferrandino et al. demonstrated the development of NAFLD in mice with mild hypothyroidism without the downregulation of hepatic TH signaling or reduced hepatic lipid utilization. This condition led to an increased shuttling of fatty acids (FAs) to the liver, where they were esterified and accumulated as triglycerides. On the contrary, mice with severe hypothyroid exhibited a downregulation of hepatic TH signaling and a severe inhibition of adipose tissue lipolysis, which reduced the delivery of FAs to the liver. The resulting lack of triglyceride esterification substrates protected mice with severely hypothyroid against NAFLD (64). We speculated that MAFLD was induced by insufficient release of TSH in adolescents and the elderly, when the thyroid was immature or in the process of degeneration, respectively. In adults, the pituitary-thyroid axis may be mature enough to maintain adequate TSH secretion, which helps regulate lipid metabolism in the liver.The association between TSH and MAFLD might be influenced by age. Selin et al. demonstrated that children with metabolic disease had lower levels of FT4 and higher levels of TSH than normal children (65). Gu et al. found that FT3 was a higher risk factor for NAFLD than those without NAFLD, while FT4 and TSH were not significantly correlated with NAFLD in middle-aged and elderly people (age >40) (46). This also explained why such a wide divergence was observed in the existing studies, with some suggesting no association and others suggesting an association. This might depend on the age distribution of the population included in previous studies and the differences in TSH concentrations.
The thyroid hormone receptor (TR) is of two types, TRα and TRβ, and it is the main receptor in the liver (66). Studies have shown that M1 macrophages polarizably induce secreted phosphoprotein 1 (SPP1) secretion and downregulate hepatocyte TRβ in a paracrine manner, exacerbating lipid deposition in the liver and compensating for increased serum TSH. Increased levels of TSH can lead to SPP1 secretion by M1 macrophages. The positive feedback interaction between the thyroid and liver may play a significant role in maintaining and amplifying the pathological process of MAFLD (67). Therefore, TRβ agonists have been investigated as potential therapies for serum dyslipidemia and MAFLD (68). Chaves et al. demonstrated that mutations in the THR-beta gene of TR can induce that signal transduction damage in the liver, leading to hepatic steatosis. This indicates the influence of thyroid hormones on lipid metabolism in the liver (69). Our findings supported the effect of thyroxine on MAFLD. This study found that elevated levels of FT3 and TT3 were significantly associated with the risk of MAFLD, and decreased levels of TT4 and FT4 were significantly associated with the risk of MAFLD. Further clinical studies are needed to investigate whether TSH affects FT3, FT4, TT3, and TT4 and thus MAFLD.
In addition, recent evidence suggests that thyroid dysfunction may not only be associated with hepatic steatosis but also with ectopic fat deposition outside the liver. For example, Bayyigit et al. (Diabetes Metab Res Rev, 2024) reported that both hypothyroidism and subclinical hypothyroidism were significantly associated with an increased prevalence of non-alcoholic fatty pancreas disease (NAFPD) as well as MAFLD. Their findings indicate a potential bidirectional relationship between thyroid dysfunction and ectopic fat accumulation, further underscoring the need for future studies to comprehensively investigate the interplay among hypothyroidism, MAFLD, and NAFPD.
In conclusion, TSH plays a key role in the development and progression of MAFLD and may be influenced by age, which needs to be further elucidated by more clinical trials in different age groups. As thyroid replacement therapy improves elevated TSH levels, whether treating hypothyroidism in patients with MAFLD improves liver function or disease progression outcomes should be further elucidated by placebo-controlled clinical trials.
This study had some limitations. First, heterogeneity was significant in some meta-analyses. We tried to explore heterogeneity between studies through sensitivity and meta-regression analyses and pooled data through random-effects models to obtain more conservative effect estimates. Second, several noninvasive methods [e.g., elevated liver enzymes, fatty liver index, ultrasonography, CT magnetic resonance imaging, and spectroscopy] were commonly used to diagnose MAFLD because liver biopsy was not feasible in the general population (70). While liver biopsy remains the diagnostic gold standard, non-invasive approaches such as the Hepatic Steatosis Index or FibroScan, although practical, may introduce a degree of misclassification bias compared with histology, and this potential bias should be considered when interpreting our results.Third, we did not perform a subgroup analysis of TSH concentration because the upper normal limit of TSH concentration had long been controversial, and the diagnosis of subclinical hypothyroidism depended crucially on the definition of the upper normal limit of TSH concentration (71). We only compared TSH values between the MAFLD and the controls, irrespective of whether the population had hypothyroidism.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
WX: Writing – original draft, Investigation, Methodology. HF: Writing – review & editing, Resources, Software. JT: Formal Analysis, Methodology, Investigation, Writing – original draft. QY: Visualization, Resources, Validation, Software, Writing – review & editing. ZX: Writing – review & editing, Software, Resources. TT: Software, Writing – review & editing. XR: Methodology, Writing – review & editing, Investigation. LF: Methodology, Investigation, Writing – review & editing. YS: Validation, Writing – review & editing, Software. XT: Resources, Writing – review & editing. XF: Writing – review & editing, Visualization, Validation. XZ: Project administration, Supervision, Software, Writing – review & editing. MW: Project administration, Writing – review & editing, Methodology, Formal Analysis, Funding acquisition, Writing – original draft, Conceptualization. WG: Supervision, Conceptualization, Writing – review & editing, Funding acquisition, Formal Analysis, Project administration, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Hangzhou biomedicine and health industry development support science and technology project (No. 2022WJCY024; No.2021WJCY001; No. 2021WJCY238; No. 2021WJCY047;No. 2021WJCY115); Hangzhou Normal University Dengfeng Project “Clinical Medicine Revitalization Plan” Jiande Hospital Special Project (No. LCYXZXJH001); Medical and Technology Project of Zhejiang Province (No. 2024KY1348; No. 2024KY200); Zhejiang Traditional Chinese Medicine Scientific Research Fund Project (No. 2024ZL723); Hangzhou Natural Science Foundation of China under Grant (No.2024SZRZDH250001).
Acknowledgments
We thank EditorBar (https://www.editorbar.com/) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pipitone RM, Ciccioli C, Infantino G, La Mantia C, Parisi S, Tulone A, et al. MAFLD: a multisystem disease. Ther Adv Endocrinol Metab. (2023) 14:20420188221145549. doi: 10.1177/20420188221145549
2. Kawaguchi T, Tsutsumi T, Nakano D, and Torimura T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res. (2022) 52:422–32. doi: 10.1111/hepr.13706
3. Mendez-Sanchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. (2022) 7:388–90. doi: 10.1016/S2468-1253(22)00062-0
4. Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: A meta-analysis and systematic review of 10 739–607 individuals. J Clin Endocrinol Metab. (2022) 107:2691–700. doi: 10.1210/clinem/dgac321
5. Xiong KG, Ke KY, Chen LF, Kong JF, Lin TS, Lin QB, et al. The impact of metabolic dysfunction-associated fatty liver disease on the prognosis of patients with hepatocellular carcinoma after radical resection. Hepatobiliary Pancreat Dis Int. (2022) 22(4):366–72. doi: 10.1016/j.hbpd.2022.04.001
6. Pemmasani G, Tremaine WJ, and John S. Su1399: DOES THE ETIOLOGY OF CIRRHOSIS IMPACT THROMBOEMBOLIC RISK? Gastroenterology. (2022) 162:S–1208-S-1209. doi: 10.1016/s0016-5085(22)63577-7
7. Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, et al. MAFLD and risk of CKD. Metabolism. (2021) 115:154433. doi: 10.1016/j.metabol.2020.154433
8. Wang Y, Shen R, and Ge J. Association between self-reported snoring and metabolic-associated fatty liver disease: A cross-sectional analysis of the NHANES 2017-2018. Sleep Med. (2023) 101:414–20. doi: 10.1016/j.sleep.2022.11.029
9. Eshraghian A and Hamidian Jahromi A. Non-alcoholic fatty liver disease and thyroid dysfunction: a systematic review. World J Gastroenterol. (2014) 20:8102–9. doi: 10.3748/wjg.v20.i25.8102
10. He W, An X, Li L, Shao X, Li Q, Yao Q, et al. Relationship between hypothyroidism and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2017) 8:335. doi: 10.3389/fendo.2017.00335
11. Jaruvongvanich V, Sanguankeo A, and Upala S. Nonalcoholic fatty liver disease is not associated with thyroid hormone levels and hypothyroidism: A systematic review and meta-analysis. Eur Thyroid J. (2017) 6:208–15. doi: 10.1159/000454920
12. Guo Z, Li M, Han B, and Qi X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig Liver Dis. (2018) 50:1153–62. doi: 10.1016/j.dld.2018.08.012
13. Wan X, Wang W, Liu J, and Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
14. Luo D, Wan X, Liu J, and Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
15. Bilgin H and Pirgon Ö. Thyroid function in obese children with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. (2014) 6:152–7. doi: 10.4274/Jcrpe.1488
16. Gokmen FY, Ahbab S, Ataoglu HE, Türker BÇ, Çetin F, Türker F, et al. FT3/FT4 ratio predicts non-alcoholic fatty liver disease independent of metabolic parameters in patients with euthyroidism and hypothyroidism. Clinics (Sao Paulo). (2016) 71:221–5. doi: 10.6061/clinics/2016(04)08
17. Basarir G, Ozcabi B, Aksu Sayman O, Ozturkmen Akay H, and Yildiz FM. Evaluation of clinical, endocrine and metabolic findings in obese children with and without hepatosteatosis. J Pediatr Endocrinol Metab. (2021) 34:1081–7. doi: 10.1515/jpem-2021-0034
18. D’Ambrosio R, Campi I, Maggioni M, Perbellini R, Giammona E, Stucchi R, et al. The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liver disease (NAFLD). PloS One. (2021) 16:e0249614. doi: 10.1371/journal.pone.0249614
19. Feng YW and Liu DD. Correlation between thyroid hormone levels and nonalcoholic fatty liver disease in euthyroid middle-aged and older people. J Med Postgra. (2019) 32:73–7.
20. Gupta N, Jindal G, Nadda A, Bansal S, Gahukar S, and Kumar A. Prevalence and risk factors for nonalcoholic fatty liver disease in obese children in rural Punjab, India. J Family Community Med. (2020) 27:103–8. doi: 10.4103/jfcm.JFCM_287_19
21. Zhang X, Chen Y, Ye H, Luo Z, Li J, Chen Z, et al. Correlation between thyroid function, sensitivity to thyroid hormones and metabolic dysfunction-associated fatty liver disease in euthyroid subjects with newly diagnosed type 2 diabetes. Endocrine. (2022) 80(2):366–79. doi: 10.1007/s12020-022-03279-2
22. Eshraghian A, Dabbaghmanesh MH, Eshraghian H, Fattahi MR, and Omrani GR. Nonalcoholic fatty liver disease in a cluster of Iranian population: thyroid status and metabolic risk factors. Arch Iran Med. (2013) 16:584–9.
23. Kaltenbach TE, Graeter T, Oeztuerk S, Holzner D, Kratzer W, Wabitsch M, et al. Thyroid dysfunction and hepatic steatosis in overweight children and adolescents. Pediatr Obes. (2017) 12:67–74. doi: 10.1111/ijpo.12110
24. Lee KW, Bang KB, Rhee EJ, Kwon HJ, Lee MY, and Cho YK. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study. Clin Mol Hepatol. (2015) 21:372–8. doi: 10.3350/cmh.2015.21.4.372
25. Liu G, Zheng X, Guan L, Jiang Z, Lin H, Jiang Q, et al. Free triiodothyronine levels are positively associated with non-alcoholic fatty liver disease in euthyroid middle-aged subjects. Endocr Res. (2015) 40(4):188–93. doi: 10.3109/07435800.2014.987399
26. Ludwig U, Holzner D, Denzer C, Greinert A, Haenle MM, Oeztuerk S, et al. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord. (2015) 15:41. doi: 10.1186/s12902-015-0030-5
27. Sert A, Pirgon O, Aypar E, Yilmaz H, and Odabas D. Subclinical hypothyroidism as a risk factor for the development of cardiovascular disease in obese adolescents with nonalcoholic fatty liver disease. Pediatr Cardiol. (2013) 34:1166–74. doi: 10.1007/s00246-013-0638-z
28. Tao Y, Gu H, Wu J, and Sui J. Thyroid function is associated with non-alcoholic fatty liver disease in euthyroid subjects. Endocr Res. (2015) 40:74–8. doi: 10.3109/07435800.2014.952014
29. Torun E, Özgen IT, Gökçe S, Aydın S, and Cesur Y. Thyroid hormone levels in obese children and adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. (2014) 6:34–9. doi: 10.4274/Jcrpe.1155
30. van den Berg EH, van Tienhoven-Wind LJ, Amini M, Schreuder TC, Faber KN, Blokzijl H, et al. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: the Lifelines Cohort Study. Metabolism. (2017) 67:62–71. doi: 10.1016/j.metabol.2016.11.002
31. Xu C, Xu L, Yu C, Miao M, and Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clin Endocrinol (Oxf). (2011) 75:240–6. doi: 10.1111/j.1365-2265.2011.04016.x
32. Zhang J, Sun H, Chen L, Zheng J, Hu X, Wang S, et al. Relationship between serum TSH level with obesity and NAFLD in euthyroid subjects. J Huazhong Univ Sci Technolog Med Sci. (2012) 32:47–52. doi: 10.1007/s11596-012-0008-8
33. Chao G and Chen L. Study on the independent effect of thyroid hormone based on uric acid level on NAFLD. J Health Popul Nutr. (2021) 40:21. doi: 10.1186/s41043-021-00247-w
34. Chen P, Hou X, Wei L, Feng L, Zhong L, Jiao L, et al. Free triiodothyronine is associated with the occurrence and remission of nonalcoholic fatty liver disease in euthyroid women. Eur J Clin Invest. (2019) 49:e13070. doi: 10.1111/eci.13070
35. Guo W, Qin P, Li XN, Wu J, Lu J, Zhu WF, et al. Free triiodothyronine is associated with hepatic steatosis and liver stiffness in euthyroid Chinese adults with non-alcoholic fatty liver disease. Front Endocrinol (Lausanne). (2021) 12:711956. doi: 10.3389/fendo.2021.711956
36. Kim HJ, Park SJ, Park HK, Byun DW, Suh K, and Yoo MH. Association of thyroid autoimmunity with nonalcoholic fatty liver disease in euthyroid middle-aged subjects: A population-based study. J Gastroenterol Hepatol. (2022) 37:1617–23. doi: 10.1111/jgh.15865
37. Li R, Zhou L, Chen C, Han X, Gao M, Cheng X, et al. Sensitivity to thyroid hormones is associated with advanced fibrosis in euthyroid patients with non-alcoholic fatty liver disease: A cross-sectional study. Dig Liver Dis. (2023) 55:254–61. doi: 10.1016/j.dld.2022.06.021
38. Liu Y, Wang W, Yu X, and Qi X. Thyroid function and risk of non-alcoholic fatty liver disease in euthyroid subjects. Ann Hepatol. (2018) 17:779–88. doi: 10.5604/01.3001.0012.3136
39. Martinez Escude A, Pera G, Arteaga I, Expósito C, Rodríguez L, Torán P, et al. Relationship between hypothyroidism and non-alcoholic fatty liver disease in the Spanish population. Med Clin (Barc). (2020) 154:1–6. doi: 10.1016/j.medcli.2019.03.018
40. Pan YW, Tsai MC, Yang YJ, Chen MY, Chen SY, and Chou YY. The relationship between nonalcoholic fatty liver disease and pediatric congenital hypothyroidism patients. Kaohsiung J Med Sci. (2019) 35:778–86. doi: 10.1002/kjm2.12118
41. Shao C, Cheng Q, Zhang S, Xiang X, and Xu Y. Serum level of free thyroxine is an independent risk factor for non-alcoholic fatty liver disease in euthyroid people. Ann Palliat Med. (2022) 11:655–62. doi: 10.21037/apm-21-3890
42. Liang SS and Wang X. Correlation between subclinical hypothyroidism and nonalcoholic fatty liver disease. JSichuanUniv (Med Sci edi). (2018) 49:504–6. doi: 10.13464/j.scuxbyxb.2018.03.043
43. Liu YT and Wang W. Relationship between thyroid hormone levels and non-alcoholic fatty liver disease in euthyroid population. Chin Gen Practice. (2019) 22:3088–93. doi: 10.12114/j.issn.1007-9572.2019.00.494
44. Du J, Chai S, Zhao X, Sun J, Zhang X, and Huo L. Association between thyroid hormone levels and advanced liver fibrosis in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Diabetes Metab Syndr Obes. (2021) 14:2399–406. doi: 10.2147/DMSO.S313503
45. Fan H, Li L, Liu Z, Cao L, Chen X, Suo C, et al. The association between thyroid hormones and MAFLD is mediated by obesity and metabolic disorders and varies among MAFLD subtypes. Dig Liver Dis. (2022). doi: 10.1016/j.dld.2022.11.020
46. Gu Y, Wu X, Zhang Q, Liu L, Meng G, Wu H, et al. High-normal thyroid function predicts incident nonalcoholic fatty liver disease among middle-aged and older euthyroid subjects. J Gerontol A Biol Sci Med Sci. (2022) 77:197–203. doi: 10.1093/gerona/glab037
47. Carulli L, Ballestri S, Lonardo A, Lami F, Violi E, Losi L, et al. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern Emerg Med. (2013) 8:297–305. doi: 10.1007/s11739-011-0609-4
48. Moustafa AH, Ali EM, Mohamed TM, and Abdou HI. Oxidative stress and thyroid hormones in patients with liver diseases. Eur J Intern Med. (2009) 20:703–8. doi: 10.1016/j.ejim.2009.08.003
49. Pagadala MR, Zein CO, Dasarathy S, Yerian LM, Lopez R, and McCullough AJ. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci. (2012) 57:528–34. doi: 10.1007/s10620-011-2006-2
50. Naguib R, Fayed A, Elkemary EZ, and Naguib H. Evaluation of thyroid function and thyroid autoimmune disease in patients with non-alcoholic fatty liver disease. Clin Exp Hepatol. (2021) 7:422–8. doi: 10.5114/ceh.2021.111169
51. Langen VL, Niiranen TJ, Puukka P, Sundvall J, and Jula AM. Association of thyroid-stimulating hormone with lipid concentrations: an 11-year longitudinal study. Clin Endocrinol (Oxf). (2017) 86:120–7. doi: 10.1111/cen.13151
52. Beukhof CM, Massolt ET, Visser TJ, Korevaar TIM, Medici M, de Herder WW, et al. Effects of thyrotropin on peripheral thyroid hormone metabolism and serum lipids. Thyroid. (2018) 28:168–74. doi: 10.1089/thy.2017.0330
53. Wang F, Tan Y, Wang C, Zhang X, Zhao Y, Song X, et al. Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J Clin Endocrinol Metab. (2012) 97:2724–31. doi: 10.1210/jc.2012-1133
54. Martinez-Escude A, Pera G, Costa-Garrido A, Rodríguez L, Arteaga I, Expósito-Martínez C, et al. TSH levels as an independent risk factor for NAFLD and liver fibrosis in the general population. J Clin Med. (2021) 10(13):2907. doi: 10.3390/jcm10132907
55. Williams GR. Extrathyroidal expression of TSH receptor. Ann Endocrinol (Paris). (2011) 72:68–73. doi: 10.1016/j.ando.2011.03.006
56. Zhang W, Tian LM, Han Y, Ma HY, Wang LC, Guo J, et al. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med. (2009) 13:4636–42. doi: 10.1111/j.1582-4934.2008.00670.x
57. Yan F, Wang Q, Lu M, Chen W, Song Y, Jing F, et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol Dec. (2014) 61:1358–64. doi: 10.1016/j.jhep.2014.06.037
58. Sinha RA, You SH, Zhou J, Siddique MM, Bay BH, Zhu X, et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. (2012) 122:2428–38. doi: 10.1172/JCI60580
59. Fuchs CD, Claudel T, and Trauner M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol Metab. (2014) 25:576–85. doi: 10.1016/j.tem.2014.08.001
60. Ma S, Shao S, Yang C, Yao Z, Gao L, and Chen W. A preliminary study: proteomic analysis of exosomes derived from thyroid-stimulating hormone-stimulated HepG2 cells. J Endocrinol Invest. (2020) 43:1229–38. doi: 10.1007/s40618-020-01210-y
61. Singh BK, Sinha RA, and Yen PM. Novel transcriptional mechanisms for regulating metabolism by thyroid hormone. Int J Mol Sci. (2018) 19(10):3284. doi: 10.3390/ijms19103284
62. Lonardo A, Mantovani A, Lugari S, and Targher G. NAFLD in some common endocrine diseases: prevalence, pathophysiology, and principles of diagnosis and management. Int J Mol Sci. (2019) 20(11):2841. doi: 10.3390/ijms20112841
63. Hu DS, Zhu SH, Liu WY, Pan XY, Zhu PW, Li YY, et al. PNPLA3 polymorphism influences the association between high-normal TSH level and NASH in euthyroid adults with biopsy-proven NAFLD. Diabetes Metab. (2020) 46:496–503. doi: 10.1016/j.diabet.2020.02.001
64. Ferrandino G, Kaspari RR, Spadaro O, Reyna-Neyra A, Perry RJ, Cardone R, et al. Pathogenesis of hypothyroidism-induced NAFLD is driven by intra- and extrahepatic mechanisms. Proc Natl Acad Sci U S A. (2017) 114:E9172–80. doi: 10.1073/pnas.1707797114
65. Elmaogullari S, Demirel F, and Hatipoglu N. Risk factors that affect metabolic health status in obese children. J Pediatr Endocrinol Metab. (2017) 30:49–55. doi: 10.1515/jpem-2016-0128
66. Tang Q, Zeng M, Chen L, and Fu N. Targeting thyroid hormone/thyroid hormone receptor axis: an attractive therapy strategy in liver diseases. Front Pharmacol. (2022) 13:871100. doi: 10.3389/fphar.2022.871100
67. Huang B, Wen W, and Ye S. TSH-SPP1/TRbeta-TSH positive feedback loop mediates fat deposition of hepatocyte: Crosstalk between thyroid and liver. Front Immunol. (2022) 13:1009912. doi: 10.3389/fimmu.2022.1009912
68. Zucchi R. Thyroid hormone analogues: an update. Thyroid. (2020) 30:1099–105. doi: 10.1089/thy.2020.0071
69. Chaves C, Bruinstroop E, Refetoff S, Yen PM, and Anselmo J. Increased hepatic fat content in patients with resistance to thyroid hormone beta. Thyroid. (2021) 31:1127–34. doi: 10.1089/thy.2020.0651
70. MaChado MV and Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A Crit appraisal. J Hepatology. (2013) 58:1007–19. doi: 10.1016/j.jhep.2012.11.021
Keywords: metabolic associated fatty liver disease, thyroid function, meta-analysis, systemic review, MAFLD
Citation: Xiong W, Fan H, Tang J, Yu Q, Xin Z, Tang T, Rao X, Feng L, Shi Y, Tong X, Fu X, Zhang X, Wang M and Gan W (2025) Association between thyroid function and metabolic associated fatty liver disease: a systemic review and meta-analysis. Front. Endocrinol. 16:1660437. doi: 10.3389/fendo.2025.1660437
Received: 06 July 2025; Accepted: 13 October 2025;
Published: 29 October 2025.
Edited by:
Birsen Yilmaz, Çukurova University, TürkiyeReviewed by:
Mohamed Adel Elbasiony, Mansoura University, EgyptAkif Bayyigit, Prof. Dr. Cemil Tascioglu City Hospital, Türkiye
Copyright © 2025 Xiong, Fan, Tang, Yu, Xin, Tang, Rao, Feng, Shi, Tong, Fu, Zhang, Wang and Gan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingwei Wang, d213OTkwNTU2QGh6bnUuZWR1LmNu; Wentao Gan, d2ludGVyZ2FuQDEyNi5jb20=
†These authors have contributed equally to this work
 Wei Xiong1†
Wei Xiong1† Hua Fan
Hua Fan Xingwei Zhang
Xingwei Zhang Mingwei Wang
Mingwei Wang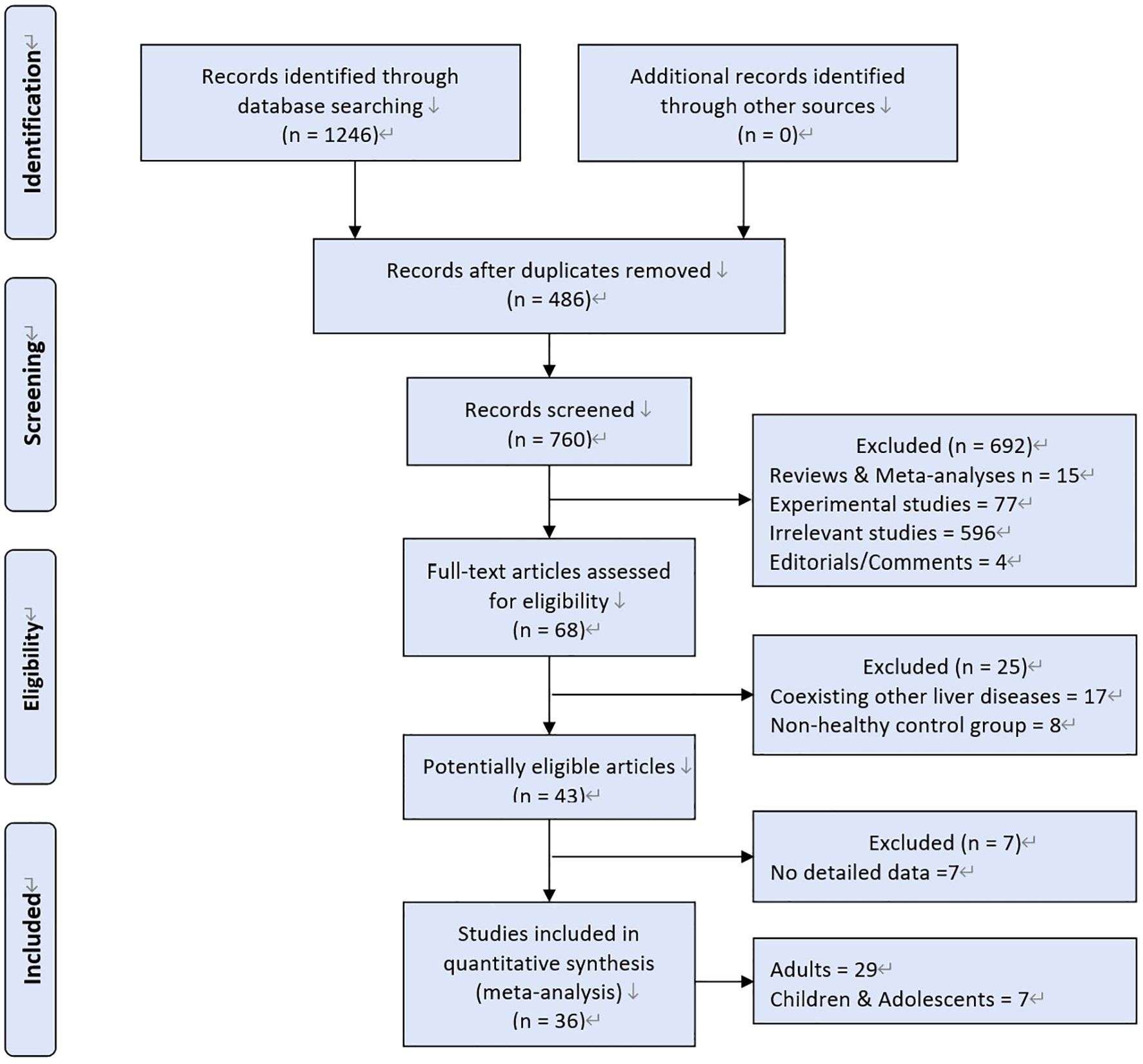

![Forest plot showing a meta-analysis of studies comparing experimental and control groups across three age subgroups: young-aged, middle-aged, and old-aged. Each study is represented by a square with 95% confidence intervals, and pooled estimates are represented by diamonds. The middle-aged subgroup shows the highest weight. Overall, the analysis favors the control group with a total effect size of 0.19 [0.18, 0.20]. Heterogeneity statistics indicate high variability among studies.](https://www.frontiersin.org/files/Articles/1660437/fendo-16-1660437-HTML-r1/image_m/fendo-16-1660437-g004.jpg)
![Forest plot showing the standardized mean difference between experimental and control groups for six studies. Each study's effect size and confidence interval are depicted. The overall effect favors the experimental group, with a summarized mean difference of 0.26 [0.23, 0.29]. Heterogeneity is high, with Chi-square of 165.59, degrees of freedom of 5, and I-squared of 97%.](https://www.frontiersin.org/files/Articles/1660437/fendo-16-1660437-HTML-r1/image_m/fendo-16-1660437-g006.jpg)
![Forest plot showing a meta-analysis of six studies comparing experimental and control groups. Standardized mean differences and confidence intervals are displayed, with a total effect favoring control at -0.10 [-0.13, -0.07]. High heterogeneity is indicated, with Chi-squared 48.87 and I-squared 90%.](https://www.frontiersin.org/files/Articles/1660437/fendo-16-1660437-HTML-r1/image_m/fendo-16-1660437-g007.jpg)