Abstract
Objective:
This systematic review and meta-analysis examined the prevalence of depression among individuals with diabetes and identified associated risk factors.
Methods:
Five databases (PubMed, Web of Science, Cochrane, ProQuest, Embase) were searched for observational studies reporting depression prevalence and multivariable-adjusted risk factors in diabetic populations. Two reviewers independently screened and extracted data. Analyses were conducted using R software.
Results:
Thirty-nine studies involving 17,486 diabetic patients were included. The pooled prevalence of depression was 35% (95% CI: 30%–41%). Risk factors included age ≤60 years, female sex, being single, unemployment, physical inactivity, anxiety, limited social support, poor medication adherence, complications (neuropathy, nephropathy, retinopathy, foot ulcers), physical disability, insulin therapy, combined insulin–oral treatment, and fasting glucose ≥126 mg/dL.
Conclusion:
Depression affects over one-third of diabetic patients and is associated with sociodemographic, psychological, and clinical factors. Our study provides updated global evidence and identifies specific high-risk profiles (e.g., females, those with complications, or on combination therapy), supporting the need for targeted screening beyond general recommendations. These findings support the integration of standardized depression screening tools such as the PHQ-9 into routine diabetes care, particularly in resource-limited settings. For patients with identified risk factors, regular follow-up screening is recommended to enable early detection and timely intervention. Routine screening and timely intervention are essential, especially for high-risk groups. Longitudinal studies are needed to clarify causal links and inform targeted prevention.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier CRD420250656589.
1 Introduction
Diabetes mellitus is a global health challenge, with prevalence projected to rise to 783 million adults by 2045 (1). Beyond its physical health and economic impacts (2, 3), diabetes imposes a significant psychological burden, as evidenced by its frequent co-occurrence with depression. This dual burden exacerbates disease management and worsens clinical outcomes.
Depression, defined as at least two weeks of low mood or reduced interest that impairs functioning (4), is frequently comorbid with diabetes. The two conditions share biological mechanisms, including CPE gene dysfunction (5), inflammatory pathways (6), and HPA axis dysregulation (7). Interventions such as anti-inflammatory diets and acupuncture have shown benefits for both HbA1c and depressive symptoms (8). Compared to diabetes alone, comorbidity is linked to greater glucose variability (9), poor adherence (10), and higher vascular risk (11). A 2024 UK cohort study reported that major depressive disorder accounted for 7.8% of new vascular events, and depressive symptoms for 3.8% (12).
Although numerous meta-analyses exist, most focus on single regions, reporting varied prevalence—e.g., 42% in Bangladesh (13), 34.6% in Ethiopia (14), and 25.9% in China (15)—reflecting differences in healthcare access and sociodemographic factors. Many lack multivariable analyses and fail to adjust for confounders (16, 17), limiting comparability. This study updates the global prevalence and integrates data from multiple countries to construct a multilevel model of sociodemographic, psychosocial, clinical, and biochemical correlates, offering evidence to support precise, targeted interventions.
2 Methods
This meta-analysis adhered to PRISMA guidelines (Appendix 1) and was prospectively registered in PROSPERO (CRD420250656589). A systematic search of PubMed, Web of Science, Cochrane Library, ProQuest, and Embase was performed to locate studies on depression prevalence and related risk factors among adults with diabetes. Both subject terms and free-text terms were employed. The initial search was performed between February 28 and March 7, 2025, and updated on June 17, 2025. Full search strategies are presented in Appendix 2.
Studies were eligible if they met the following criteria (1): observational design (cross-sectional or cohort) (2); published in English (3); participants aged 18 years or older (4); reported depression prevalence and risk estimates (ORs with 95% CIs, or data sufficient for calculation); and (5) utilized validated depression assessment tools. Exclusion criteria included: lack of full text, duplicate records, incomplete data, non-English language, or poor methodological quality.
All records were imported into EndNote 21 to remove duplicates. Titles and abstracts were independently screened by two reviewers, followed by evaluation of full texts. Data extracted comprised study title, author, publication year, design, setting, sample size, number of depression cases, prevalence and related influencing factors. Any disagreements were settled through discussion or consultation with a third reviewer.
Methodological quality was appraised using JBI checklists for prevalence and analytical cross-sectional studies. Items were scored as “yes,” “no,” or “unclear,” with “yes” responses assigned 1 point. Based on the proportion of positive responses, studies were categorized as high (≤49%), moderate (50–69%), or low (≥70%) risk. Studies deemed high risk on both tools were excluded.
Data were organized in Microsoft Excel and analyzed using R software (version 4.4.3) with the meta, metafor, dplyr, and metaprop packages. The Freeman–Tukey double arcsine transformation (sm = “PFT”) was used to stabilize variances in prevalence estimates. Between-study heterogeneity was assessed using the DerSimonian–Laird method (method.tau = “DL”). Subgroup analyses and pooled adjusted odds ratios (AORs) were calculated using random-effects models with restricted maximum likelihood estimation (REML), which improves precision in small samples or when heterogeneity is pronounced. The Hartung–Knapp adjustment was applied for random-effects confidence intervals, except in cases involving only two studies or low heterogeneity (I² < 50%), where standard methods were retained to prevent overly conservative intervals.All R code used for the meta-analyses is available for review at: https://dedi-meta.github.io/, ensuring full transparency and reproducibility.
Heterogeneity was assessed using Cochran’s Q test and the I² statistic, with P < 0.1 and I² > 50% considered indicative of substantial heterogeneity. To explore the sources of this heterogeneity, we pre-specified two strategies: subgroup analyses and multivariate meta-regression.
Subgroup analyses were stratified by age, sex, geographic region, publication year, study duration, setting, and depression measurement tools. Additionally, multivariate meta-regression was pre-planned to examine the potential moderating effects of study-level mean age, survey year, and geographic region on the prevalence estimates, as these variables represent key sources of clinical and methodological heterogeneity. Risk factors were synthesized using AORs derived from multivariable logistic regression. Heterogeneity was assessed using Cochran’s Q test and the I² statistic, with P < 0.1 and I² > 50% considered indicative of substantial heterogeneity. Inter-rater agreement for quality assessment was measured by Cohen’s kappa coefficient. Publication bias was assessed using Begg’s and Egger’s tests.To assess the robustness of the results, a leave-one-out sensitivity analysis was carried out. The results of these analyses were visually presented using forest plots.
3 Results
A total of 19,829 articles were retrieved from five databases: PubMed (2,562), Web of Science (3,425), Cochrane (408), ProQuest (267), and Embase (13,167). After removing duplicates in EndNote 21, 17,386 unique records remained. Following title and abstract screening, 466 articles were selected for full-text review. Full texts of 5 articles could not be retrieved, leaving 461 articles for full-text screening. Of these, 423 were excluded for reasons such as unclear inclusion and exclusion criteria, unclear diagnostic criteria, lack of multivariate analysis, or inclusion of ineligible populations. Ultimately, 38 articles met the inclusion criteria. One study separately analyzed two independent regional datasets, resulting in a total of 39 included studies (Figure 1).
Figure 1

PRISMA 2020 flowchart depicting the study selection process.
These studies were all cross-sectional in design and involved 17,486 diabetic patients. They were published between 2007 and 2025, with study durations ranging from 1 to 12 months. Geographically, the studies were conducted in Asia (22 items), Africa (11 items), Europe (2 items), and North America (4 items). Sample sizes ranged from 148 to 2,182 participants. All studies underwent dual quality assessment using the JBI tools: JBI 1 for prevalence studies and JBI 2 for analytical cross-sectional studies. All were rated low risk in the JBI 2 assessment; five were rated high risk in JBI 1(Table 1). Inter-rater agreement was assessed to ensure consistency in quality ratings. For JBI 1, the unweighted Cohen’s kappa was 0.891, reflecting high concordance. No discrepancies were found with JBI 2, so further statistical analysis was unnecessary.
Table 1
| Author + year | Country | N | Assessment | Prevalence (%) |
|---|---|---|---|---|
| Mohamed Abd-Elgawad2023 | Egypt | 679 | HADS≥8 | 34.17 |
| Shahad Abduljalil Abualhamael2024 | KSA | 251 | DASS-21≥10 | 49.40 |
| Hesham Abuhegazy2022 | KSA | 350 | PHQ-9>10 | 36.57 |
| Seid Yimam Ali | Ethiopia | 263 | PHQ-9≥5 | 47.15 |
| Abdullahi S. Aminu2017 | India | 200 | PHQ-9≥5 | 37.50 |
| Muhammad Atif2018 | Pakistan | 400 | GDS-15≥5 | 67.50 |
| Gedion Asnake Azeze2020 | Ethiopia | 410 | PHQ-9≥5 | 29.27 |
| anteneh Messele Birhanu2016 | Ethiopia | 415 | PHQ-9≥5 | 15.42 |
| Habtamu Birhanu2022 | Ethiopia | 310 | PHQ-9≥10 | 41.61 |
| Tania Dehesh2020 | Iran | 1500 | BDI-II≥18 | 59.00 |
| Mohamed Ebrahim2021 | Ethiopia | 401 | PHQ-9≥5 | 48.88 |
| Mohamed Hassan Elnaem2025 | Indonesia/Malaysia | 606 | PHQ-9≥10 | 56.60 |
| Nigus Alemnew Engidaw2020 | Ethiopia | 403 | PHQ-9≥5 | 21.34 |
| Annie C. H. Fung2018 | China | 325 | GDS-15≥7 | 12.92 |
| Malgorzata Gorska-Ciebiada2014 | Poland | 276 | GDS-30≥10 | 29.71 |
| Sheikh Mohammed Shariful Islam2015 | Bangladesh | 515 | PHQ-9≥5 | 61.94 |
| Firdous Jahan2011 | Pakistan | 320 | self-reported validated questionnaire≥9 | 17.50 |
| Mihyun Jeong2021 | Korean | 1529 | PHQ-9≥10 | 9.74 |
| Ashmita Karki2024 | Nepal | 481 | PHQ-9≥5 | 25.57 |
| Kankana Karpha2022 | India | 152 | PHQ-9≥5 | 39.47 |
| Nuket Bayram Kayar2017 | Turkey | 154 | SCID-I scale | 18.18 |
| Steven M. Kogan2007 | America | 200 | CES-D≥16 | 36.00 |
| Rehanguli Maimaitituerxun2023 | China | 496 | HADS-D≥8 | 27.22 |
| Makda Abate Belew2023 | Ethiopia | 426 | PHQ-9≥5 | 47.65 |
| Eva O. Melin2017 | Sweden | 148 | HADS-D≥8 | 11.49 |
| Nelda Mier2008① | Mexico | 200 | CES-D≥16 | 40.50 |
| Nelda Mier2008② | America | 172 | CES-D≥16 | 38.95 |
| Nur Adam Mohamed2024 | Somalia | 360 | DASS-21≥10 | 44.72 |
| Lili Husniati Yaacob2012 | Malaysia | 260 | HADS-D≥9 | 20.77 |
| Mussa R. Mussa2023 | Tanzania | 267 | PHQ-9≥5 | 72.66 |
| Kabtamu Nigussie2023 | Ethiopia | 416 | HADS≥8 | 42.31 |
| Hina Sharif2023 | Pakistan | 493 | PHQ-9≥5 | 30.83 |
| Avinash K. Sunny2019 | Nepal | 278 | BDI-II≥16 | 22.66 |
| Waleed M Sweileh2014 | Palestine | 294 | BDI-II≥16 | 40.82 |
| Thitiphan Thaneerat2009 | Thailand | 250 | HADS-D≥8 | 28.00 |
| Nhu Minh Hang Tran2021 | Vietnam | 216 | PHQ-9≥10 | 23.15 |
| Allan Oliver Dampil2019 | Philippines | 476 | PHQ-9≥5 | 81.09 |
| Yiting Wang2016 | America | 2182 | PHQ-9≥10 | 11.73 |
| Weijun Zhang2015 | China | 412 | BDI-II≥14 | 34.47 |
Summary of characteristics of included studies. (Full details of the 39 included studies are provided in Appendix 3).
N, Sample Size; Assessment, Depression Assessment and Cut-off Score.
3.1 Prevalence of depression in diabetic patients
The pooled prevalence of depression among individuals with diabetes was 35% (95% CI: 30%–41%), based on a random-effects model. Substantial heterogeneity was observed across studies (I² = 98.8%, τ² = 0.0351, P < 0.0001) (Figure 2). Publication bias was evaluated using Begg’s and Egger’s tests, with no significant bias detected (Begg’s p = 0.5778; Egger’s p = 0.1351). The non-significant results of Begg’s and Egger’s tests suggest no substantial publication bias was detected.
Figure 2
![Forest plot showing the prevalence of depression among patients with diabetes across multiple studies. Each study is represented by a blue square with horizontal lines indicating the 95% confidence interval. Studies vary in proportion, with weights mostly around 2.6%. At the bottom, a diamond represents the common and random effect models with prevalence summary estimates of 0.33 and 0.35, respectively, and confidence intervals of [0.32, 0.34] and [0.30, 0.41]. The plot includes a vertical line at 0.35, indicating overall effect size. Heterogeneity statistics are provided.](https://www.frontiersin.org/files/Articles/1660478/xml-images/fendo-16-1660478-g002.webp)
Forest plot presenting the pooled prevalence of depression in individuals with diabetes mellitus.
3.2 Subgroup analyses
To explore sources of heterogeneity, subgroup analyses were conducted based on age, gender, publication year, study duration, setting, geographic region, and depression assessment tools (Figure 3).
Figure 3

Forest plot of subgroup analysis on depression prevalence in individuals with diabetes.
Age: Depression prevalence increased slightly with age: 29% (under 40) (19–27), 30% (40–60) (19–28), and 36% (≥60) (19–29, 38, 46, 47). However, differences among age groups were not statistically significant (P = 0.4906). Heterogeneity remained high within subgroups (I² ≥ 85.9%).
Gender (18, 19, 21–28, 30, 32, 33, 36, 38, 39, 46–55): The prevalence was higher among female patients (34%, 95% CI: 28%–41%) than male patients (26%, 95% CI: 21%–32%), with a statistically significant difference (P = 0.0392). The pooled OR for females compared to males was 1.51 (95% CI: 1.31–1.74), with moderate between-study heterogeneity (I² = 59%).
Depression Screening Tools/Cut-off Values: Six subgroup categories were formed based on tools and cut-offs used in at least two studies. The highest prevalence was found with DASS-21 ≥10 (32, 56) (47%, 95% CI: 19%–75%), and the lowest with PHQ-9 ≥10 (23, 38, 40, 47, 51) (27%, 95% CI: 6%–54%). Differences across tools were statistically significant (P = 0.0041).
Study Settings: Among the 36 studies with available setting information, 31 (19–23, 25, 27–34, 36–39, 46, 48–50, 52–59) were hospital-based and 5 (18, 24, 26, 35, 60) community-based. Depression prevalence was higher in hospital settings (38%, 95% CI: 31%–44%) compared to community settings (30%, 95% CI: 23%–38%), though this difference was not statistically significant (P = 0.0856).
Study Duration: Of the 32 studies with duration data, 15 (20–23, 25, 27, 29, 32, 35, 48–50, 57–59) had a duration ≤3 months, 8 (18, 19, 33, 34, 36, 37, 51, 56) lasted 3–6 months, and 9 (26, 28, 30, 31, 38, 39, 46, 52) were >6 months. Prevalence was highest in studies with durations of 3–6 months (43%) and lowest in studies longer than 6 months (35%). However, no significant difference was observed among groups (P = 0.7340).
Region: Studies conducted in Africa (20–23, 25, 29, 32, 34, 48, 50, 58) showed the highest pooled prevalence (40%, 95% CI: 29%–51%), followed by Asia (20, 24, 46, 47, 51, 53, 54, 57, 59, 60) (36%), North America (31, 40, 60) (31%), and Europe (30, 53) (20%). Although point estimates varied, the differences were not statistically significant (P = 0.3092), and heterogeneity within regions remained high.
Year of Publication: Depression prevalence was slightly higher in studies published after 2020 (19, 20, 23–25, 28, 29, 32, 34, 35, 38, 47, 48, 50, 51, 56, 59) (39%) compared to those before 2020 (18, 21–23, 26, 27, 30, 31, 33, 36, 37, 39, 40, 46, 49, 52–55, 57, 58, 60) (33%), but the difference was not statistically significant (P = 0.2354).
This study performed a multivariate meta-regression on mean age, survey year, and region. Results showed no significant effects of age (β = -0.0006, p = 0.921), survey year (β = 0.0118, p = 0.178), or region (QM = 3.83, p = 0.574) on depression prevalence in diabetic patients (Appendix 4).
3.3 Sensitivity analysis
A leave-one-out sensitivity analysis was conducted to evaluate the impact of each study on the overall prevalence estimate. Exclusion of individual studies did not significantly alter the pooled prevalence, which remained stable between 35% (95% CI: 30%–40%) and 37% (95% CI: 32%–42%). The overall effect estimate (theta = 0.35) remained within the 95% CIs of all iterations. The stability of the pooled prevalence upon successive exclusion of each study indicates that the overall result was not disproportionately influenced by any individual study (Appendix 5).
3.4 Influencing factors
In this meta-analysis, 30 variables were identified as potential factors influencing depression among diabetic patients. These were categorized into four domains: sociodemographic characteristics, psychosocial conditions, diabetes-related factors, and biochemical indicators. All included variables were derived from multivariable logistic regression models, ensuring adjustment for potential confounders. The following summarizes the pooled results (Figure 4).
Figure 4
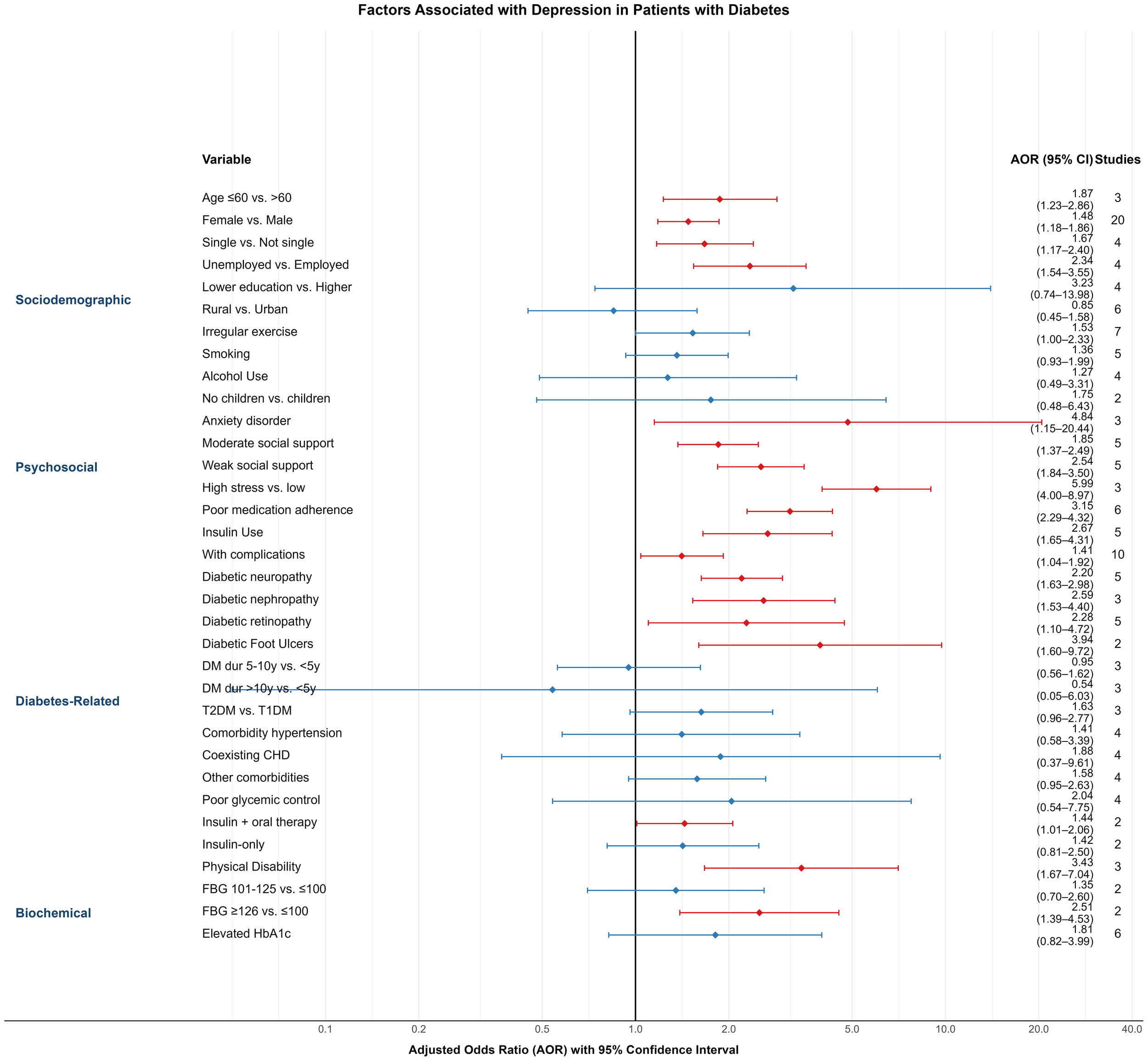
Forest plot of variables linked to depression prevalence among individuals with diabetes.
3.4.1 Sociodemographic factors
Age: Three studies (28, 38, 46) reported age as a predictor of depression. Patients aged ≤60 years had a significantly higher risk compared to those >60 years (AOR = 1.87; 95% CI: 1.23–2.86).
Gender: Based on 20 studies (18, 22, 23, 25–27, 31, 32, 34, 36, 39, 40, 46, 47, 49–51, 53, 56, 60), female diabetic patients had a higher likelihood of depression than males (AOR = 1.48; 95% CI: 1.18–1.86).
Marital Status: In four studies (22, 27, 53, 57), being single was associated with increased depression risk compared to being married or in a partnership (AOR = 1.67; 95% CI: 1.17–2.40).
Employment Status: Four studies (18, 27, 36, 47) found that unemployed individuals had a higher risk of depression than those employed (AOR = 2.34; 95% CI: 1.54–3.55).
Educational Attainment (26, 27, 31, 37): Lower education levels were associated with a non-significantly elevated risk (AOR = 3.23; 95% CI: 0.74–13.98).
Place of Residence: Six studies (18, 22, 24, 35, 51, 58) examined urban–rural differences. Patients living in rural areas had a slightly lower—but non-significant—risk of depression compared to urban counterparts (AOR = 0.85; 95% CI: 0.45–1.58).
Exercise Habits: Seven studies (22, 23, 32, 49, 51, 53, 56) reported that irregular physical activity was linked to increased depression risk (AOR = 1.53; 95% CI: 1.00–2.33).
Smoking and Alcohol Use: While both behaviors showed trends toward higher depression risk—smoking (24, 27, 49, 53, 56) (AOR = 1.36; 95% CI: 0.93–1.99) and alcohol use (23, 27, 29, 49) (AOR = 1.27; 95% CI: 0.49–3.31)—the results were not statistically significant.
Children: In two studies (21, 22), patients without children had a higher, but non-significant, risk of depression (AOR = 1.75; 95% CI: 0.48–6.43).
3.4.2 Psychosocial factors
Anxiety Disorder: Three studies (30, 37, 48) found a strong association between comorbid anxiety and depression in diabetic patients (AOR = 4.84; 95% CI: 1.15–20.44).
Social Support: Five studies (23, 25, 35, 50, 58) assessed social support levels. Compared to high support, moderate (AOR = 1.85; 95% CI: 1.37–2.49) and low support (AOR = 2.54; 95% CI: 1.84–3.50) were significantly associated with higher depression risk.
Stress (34, 38, 47): High perceived stress was significantly associated with depression (AOR = 5.99; 95% CI: 4.00–8.97; 3 studies).
Medication Adherence (19, 34, 37, 38, 51, 56): Non-adherence was significantly associated with depression (AOR = 3.15; 95% CI: 2.29–4.32; 6 studies).
3.4.3 Diabetes-related factors
(1) Insulin Use (26, 27, 53, 59, 60): Insulin-treated patients had significantly higher depression risk compared to those not using insulin (AOR = 2.67; 95% CI: 1.65–4.31).
(2) Complications (18, 20, 22, 27, 29, 33, 48–50, 57): The presence of diabetic complications was associated with higher depression risk (AOR = 1.41; 95% CI: 1.04–1.92; 10 studies), as were specific complications:
Neuropathy (19, 39, 48, 54, 59): AOR = 2.20 (95% CI: 1.63–2.98)
Retinopathy (21, 34, 39, 48, 59): AOR = 2.28 (95% CI: 1.10–4.72)
(3) Duration of Diabetes (22, 29, 51): No statistically significant association was observed for disease duration (5–10 years: AOR = 0.95; >10 years: AOR = 0.54).
(4) Type of Diabetes (22, 30, 58): Type 2 diabetes was associated with a higher—but non-significant—risk compared to Type 1 (AOR = 1.63; 95% CI: 0.96–2.77).
(5) Comorbid Conditions:
Hypertension (21, 39, 47–49, 52, 54, 59) (AOR = 1.41; 95% CI: 0.58–3.39),
Coronary heart disease (30, 39, 47, 59) (AOR = 1.88; 95% CI: 0.37–9.61),
Other comorbidities (18, 22, 50, 52) (AOR = 1.58; 95% CI: 0.95–2.63),
were not significantly associated with depression.
(6) Glycemic Control (20–22, 39): Poor glycemic control was linked to higher, but non-significant, depression risk (AOR = 2.04; 95% CI: 0.54–7.75).
(7) Treatment Regimen (19, 49): Patients using both insulin and oral agents had a significantly higher depression risk (AOR = 1.44; 95% CI: 1.01–2.06), while insulin-only users did not (AOR = 1.42; 95% CI: 0.81–2.5).
(8) Physical Disability: Three studies (34, 35, 58) reported a strong association between disability and depression (AOR = 3.43; 95% CI: 1.67–7.04).
3.4.4 Biochemical indicators
(1) Fasting Blood Glucose (35, 58): Patients with fasting glucose ≥126 mg/dL had significantly higher depression risk (AOR = 2.51; 95% CI: 1.39–4.53) compared to those with levels ≤100 mg/dL.
(2) HbA1c (27, 30, 49, 52, 56, 57): While elevated HbA1c levels were associated with a higher risk (AOR = 1.81; 95% CI: 0.82–3.99), the association was not statistically significant.
4 Discussion
Previous meta-analyses have firmly established the high comorbidity between depression and diabetes (41, 42). Our study builds upon this foundation by providing several critical advancements that refine our understanding and inform clinical practice. First, by including 39 cross-sectional studies from Asia, Africa, Europe, and North America (N = 17,486), we offer a more updated and geographically diverse synthesis. Our pooled prevalence estimate of 35% (95% CI: 30%–41%) is substantially higher than previous reports (41, 42), reflecting the contemporary and growing burden of this comorbidity. Second, and most importantly, unlike prior reviews that often relied on univariate analyses susceptible to confounding, our meta-analysis exclusively synthesizes evidence from multivariable-adjusted models. This methodological rigor allows us to identify a hierarchy of independent risk factors—spanning sociodemographic, psychosocial, and clinical domains—that persist after accounting for confounders. Consequently, our primary novel contribution lies in moving beyond the established recommendation for screening by providing the evidence necessary to implement stratified, risk-based screening protocols in clinical practice.
The high pooled prevalence underscores the substantial clinical burden. With the continuing rise in diabetes prevalence worldwide, the absolute number of individuals affected by both conditions will also grow, highlighting the urgent need for integrated care models that address their well-established bidirectional relationship (9, 11). The generalizability of our pooled estimate, however, should be considered in the context of the geographical distribution of the included studies, a point we expand upon in the Limitations section.
The pooled prevalence of depression in our diabetic cohort (35%) is substantially higher than estimates reported for the general global population, which typically range from 4% to 5% (43). This disparity underscores the immense psychological burden associated with diabetes. The etiological pathways are likely multifactorial, encompassing the relentless psychological stress of managing a chronic illness, the financial toxicity of treatment, disease-related stigma, and shared biological pathways such as chronic inflammation and HPA axis dysregulation (7, 8).
Furthermore, our analysis allows for a distinction between risk factors that are generalizable from the general population and those that may be amplified or more specific to the diabetic context. For instance, female gender is a well-established risk factor for depression in both general and diabetic populations, a finding corroborated in our study. In contrast, factors such as elevated fasting blood glucose, the presence of diabetes-specific complications (e.g., neuropathy), and insulin therapy appear to represent disease-specific amplifiers of depression risk. These factors likely contribute to the elevated prevalence observed in diabetes by interacting with underlying general vulnerabilities, creating a unique risk profile that necessitates tailored screening and intervention strategies.
A notable finding from our subgroup analysis was the higher pooled prevalence of depression in studies published after 2020 compared with earlier studies (39% vs 33%), although this difference did not reach statistical significance. While the cross-sectional design of the included studies limits causal inference, this temporal trend warrants attention. The onset of the COVID-19 pandemic in 2020 likely contributed to this increase. Patients with diabetes were particularly vulnerable to the pandemic’s multiple stressors, including elevated psychosocial burden (e.g., lockdowns, social isolation, financial insecurity), disruption of routine healthcare services, and a feedback loop whereby pandemic-related stress could worsen glycemic control, potentially amplifying depressive symptoms (44, 45). Additionally, heightened clinical and research focus on mental health during this period may have increased detection rates. If supported by future longitudinal studies, this pattern highlights the disproportionate mental health impact of global crises on vulnerable populations and emphasizes the need for healthcare systems to strengthen resilience and incorporate psychological support into chronic disease management.
Gender subgroup analysis showed a higher prevalence in females (34%) than males (26%), with an OR of 1.51 (95% CI: 1.31–1.74). This is consistent with broader evidence indicating that women have approximately double the risk of developing depression (61–64), potentially due to hormonal fluctuations (61), caregiving roles (62), emotional processing differences, and structural determinants such as the disproportionate burden of unpaid work (65). Routine screening in female patients is recommended.
Tool-based subgroup analysis showed highest prevalence with DASS-21 ≥ 10 (47%) and lowest with PHQ-9 ≥10 (27%). Differences may relate to timeframes (DASS-21: past week; PHQ-9: past two weeks), focus (subjective distress vs. functional impairment), and cultural responses—e.g., avoidance of suicide-related items in East Asian populations may lower PHQ-9 scores (66). These differences highlight the importance of culturally sensitive tool selection in clinical screening, and suggest that PHQ-9 may require adaptation or complementary methods in East Asian populations. Future research should adjust for such heterogeneity to improve comparability.
Multivariate meta-regression found no significant association between depression prevalence and mean age, survey year, or geographic region. Notably, this null finding is itself informative. It suggests that the drivers of heterogeneity are likely more complex and operate at a level not fully captured by these aggregate variables. Potential explanations include the preeminence of individual-level psychosocial and clinical factors (as identified in our risk factor analysis), nuanced cultural and socioeconomic differences that are obscured by broad regional categorizations, and fundamental methodological variations such as the use of different depression assessment tools. This aligns with other meta-analytic findings in psychiatric epidemiology (14, 67). Furthermore, this underscores the limitation of meta-regression (an ecological analysis) and highlights the necessity for future research utilizing individual patient data (IPD meta-analysis) to better elucidate these complex relationships.
Unlike most prior studies, our analysis included only factors adjusted by multivariable logistic regression. The following were associated with increased depression risk: age ≤60 years, female gender, single status, unemployment, physical inactivity, anxiety disorder, weak/moderate social support, poor medication adherence, complications (neuropathy, nephropathy, retinopathy, foot ulcers), physical disability, and fasting glucose ≥126 mg/dL. as well as treatment-related factors such as insulin use and combined oral and insulin therapy. Collectively, this set of independently associated factors provides a practical evidence base for the risk-stratified screening approach proposed in the introduction of this discussion.These findings suggest that clinicians should adopt individualized screening protocols, considering psychosocial and clinical risk profiles in routine practice.
Depression risk was higher in patients ≤60 years, consistent with prior finding (68). Younger patients may face greater life pressure and role burdens (69). Gender-related vulnerability was again confirmed, consistent with prior studies (15, 70). Single status also increased risk, likely due to reduced emotional and social support (15).
Unemployment was associated with elevated depression risk. A Taiwanese cohort study showed that employment reduced depressive symptoms by 32% over 3–4 years (71), consistent with other research (72). Depression and unemployment may interact bidirectionally through financial strain, loss of routine, and impaired work function.
Physical inactivity was another significant risk factor. Meta-analysis of 17 RCTs showed that physical activity significantly reduced depressive symptoms in T2DM patients (SMD = -0.57) (73). A 2025 cross-sectional study found that walking 4–7 days per week reduced poor mood likelihood by 57% (74). Mechanisms include increased BDNF, serotonin, and dopamine, and reduced inflammation (75). However, the observed association must be interpreted with caution due to the potential for reverse causality. While physical activity has consistently been shown to reduce the risk of depression (76), depressive symptoms such as anhedonia, fatigue, and diminished motivation may themselves lead to reduced engagement in physical activity. Longitudinal evidence supports this pathway; for example, Chen et al. (77) reported that depressive symptoms significantly predicted subsequent decreases in physical activity among older adults. These findings underscore the bidirectional nature of the relationship, suggesting a vicious cycle in which depression and physical inactivity reinforce one another. From a clinical perspective, this highlights the need for integrated management approaches that simultaneously address both mood disturbances and barriers to physical activity in patients with diabetes.
Psychosocial factors also played key roles. Anxiety, low social support, and poor medication adherence significantly increased depression risk. Anxiety may mediate the link between social support and depression, weakening the protective effect of support (78). Social support improves adherence and psychological resilience (79), while poor adherence is associated with depression (r = 0.21) (10). These factors may interact and reinforce each other in a vicious cycle.Given the multifaceted interaction among anxiety, social support, and adherence, integrated care models incorporating psychoeducation, peer support, and behavioral counseling may help break this cycle and improve mental health outcomes in diabetic patients.
Complications and disability significantly increased depression risk. A Danish cohort found T2DM complications raised depression/anxiety risk (HR = 1.77), with amputation having the strongest effect (HR = 2.16) (80). A meta-analysis confirmed increased risk in nephropathy patients (81). Mechanisms may involve chronic pain, loss of function, and treatment burden, reducing quality of life and increasing depression risk. Evidence regarding the impact of cardiovascular comorbidities like hypertension and coronary artery disease on depression among patients with diabetes remains inconclusive. While some studies suggest that comorbid conditions may exacerbate the psychological burden (82), others found no consistent association between depression and objective cardiovascular indicators (83, 84). A population-based study indicated that the history of cardiovascular events, rather than the mere presence of hypertension, was linked to depression (85). This suggests that the functional impact and severity of complications may be more critical than the simple presence of a comorbidity.
Treatment Regimen: Insulin use—especially combined oral and insulin therapy—was associated with higher depression risk. For example, A Korean study showed combined therapy patients had the highest depression rates (OR = 1.41), higher than insulin-only or oral-only users (86). Interestingly, this association was observed despite the lack of a significant relationship with HbA1c, suggesting the psychological impact may be related to the burdens of intensive treatment itself rather than glycemic control. Initiating insulin is often perceived by patients as a sign of disease progression or personal failure. Furthermore, the increased complexity, cost, and lifestyle rigidity associated with managing a combined regimen can be a source of distress (87, 88). This underlines the need for psychosocial support when initiating complex treatment regimens.
Fasting glucose ≥126 mg/dL was linked to higher depression risk; HbA1c was not. The significant association between elevated fasting blood glucose and depression is intriguing, though its interpretation is complex. This finding must be viewed in the context of an inconsistent literature regarding HbA1c; while some studies have reported positive correlations (89), others—including our pooled analysis of adjusted estimates—found no significant association after accounting for confounders (90). The discrepancy between FBG and HbA1c may reflect their distinct physiological correlates. FBG, particularly when measured in the morning after an overnight fast, may capture recent glycemic excursions and acute stress-related metabolic fluctuations involving cortisol and catecholamines (7, 89, 91). This mechanism is supported by evidence suggesting that acute glucose fluctuations (glycemic variability) are more strongly linked to negative psychological outcomes than mean glucose levels alone (91). In contrast, HbA1c represents average glycemic control over the preceding 2–3 months, and its long-term integrative nature may dilute the influence of acute psychological stress and is shaped by diverse clinical and behavioral factors, such as erythrocyte turnover, medication adherence, and diet. These findings underscore the importance of dynamic measures of glycemic variability in future research on the diabetes-depression nexus.
Although our pooled analysis indicated a non-significant trend toward lower depression risk in rural areas, this result should be interpreted with caution due to the small number of studies and wide confidence intervals. Prior meta-analyses suggest that urban–rural differences in depression are context-dependent. A global meta-analysis found that depression was significantly more prevalent in urban residents of developed countries, whereas no such association was observed in developing countries (92). Likewise, a systematic review and meta-analysis of older adults reported similar patterns (93). These findings imply that socioeconomic and community-level factors may underlie the heterogeneity of rural–urban differences.
This meta-analysis confirms the high comorbidity of depression in diabetes globally and identifies a suite of independent risk factors that contribute to this risk. The consistency of these findings across diverse settings underscores their potential utility in clinical practice. The implications of these results for developing targeted screening strategies are further elaborated in the conclusion.
Advantages and Limitations.
Advantages:
First, this study comprehensively included relevant literature on depression prevalence and associated risk factors among diabetic patients across multiple countries and regions.
Second, only multivariable logistic regression–adjusted ORs were included, which helped reduce the impact of confounding factors.
Third, the Hartung–Knapp adjustment was applied when heterogeneity exceeded 50%, yielding more conservative and reliable confidence intervals. This avoids the underestimation of uncertainty seen with traditional methods like DerSimonian–Laird in small or highly heterogeneous samples.
Fourth, the findings offer practical implications for clinical practice, including identifying high-risk individuals and informing stepped-care approaches.
Limitations:
As noted in the Discussion, the geographical distribution of included studies was uneven, with a predominance of research from Asia and Africa and fewer from Western countries and none from Latin America. This likely reflects global disparities in research funding and capacity, as well as differing regional priorities in public health research. It may limit the generalizability of our pooled prevalence estimate to high-income Western populations. However, this distribution also constitutes a unique strength of our study: it provides a much-needed synthesis of the evidence from low- and middle-income countries (LMICs), where the burden of diabetes is rising most rapidly and healthcare resources are often most strained. The risk factors identified (e.g., limited social support, unemployment) may be particularly relevant in these resource-limited settings. The absence of studies from Latin America highlights a significant gap in the literature that future research should aim to fill.
Second, only English-language publications were included, potentially omitting valuable data from non-English sources.
Third, substantial heterogeneity was present, partly due to differences in depression screening tools, which may have influenced the overall results.
Fourth, all studies were cross-sectional, limiting causal inferences.
5 Conclusion
This systematic review and meta-analysis found a high pooled prevalence of depression (35%) among patients with diabetes mellitus. More importantly, it identified a profile of specific, independent risk factors associated with significantly higher odds of depression, including sociodemographic (e.g., age ≤60 years, female gender, unemployment), psychosocial (e.g., anxiety, limited social support, poor medication adherence), and clinical factors (e.g., diabetic complications, insulin use, combination therapy, elevated fasting glucose).
Rather than reiterating the established need for routine screening, our findings provide an evidence-based framework for implementing risk-stratified screening protocols. Clinicians can use these identified risk factors to prioritize high-risk individuals (e.g., unemployed females with complications on insulin therapy) for more frequent and thorough assessment using standardized tools like the PHQ-9. This approach enables a move beyond blanket recommendations towards smarter, more efficient resource allocation, particularly in resource-constrained settings. For these high-risk groups, a stepped-care model—incorporating routine screening, brief interventions, and prompt referral—is essential.
Future large-scale longitudinal studies are needed to confirm the causal relationships suggested by our cross-sectional data and to refine the precision of these targeted prevention strategies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KY: Writing – original draft, Formal Analysis, Conceptualization, Software, Methodology, Data curation. YF: Writing – review & editing, Data curation, Investigation. JH: Writing – review & editing, Visualization. JL: Project administration, Writing – review & editing, Methodology, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1660478/full#supplementary-material
References
1
SunHSaeediPKarurangaSPinkepankMOgurtsovaKDuncanBBet al. Erratum to IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045 [. Diabetes Res Clin Pract. (2023) 204:110945. doi: 10.1016/j.diabres.2023.110945
2
SaeediPSalpeaPKarurangaSPetersohnIMalandaBGreggEWet al. Mortality attribute able to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086
3
PatelMR. Financial toxicity in diabetes: the state of what we know. Curr Diabetes Rep. (2025) 25:32. doi: 10.1007/s11892-025-01588-0
4
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC (2018). Available online at: https://dsm.psychiatryonline.org/pb-assets/dsm/update/DSM5Update_October2018.pdf (Accessed June 20, 2025).
5
XiaoLLohYPNeurotrophic factor-α1/carboxypeptidase E functions in neuroprotection and alleviates depression. Front Mol Neurosci. (2022) 15:918852. doi: 10.3389/fnmol.2022.918852
6
StuartMJBauneBT. Depression and type 2 diabetes: Inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci Biobehav Rev. (2012) 36:658–76. doi: 10.1016/j.neubiorev.2011.10.001
7
MoultonCDPickupJCIsmailK. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. (2015) 3:461–71. doi: 10.1016/S2213-8587(15)00134-5
8
IrandoostPFirouzjaeiAHeshmatiJSadeghiEAyatiMHNamaziN. The effects of an anti-inflammatory diet alone or in combination with acupuncture on mental health, anthropometric indices, and metabolic status in diabetic patients with depression: a randomized, controlled clinical trial. Nutr Diabetes. (2025) 15:18. doi: 10.1038/s41387-025-00373-y
9
GilsanzPKarterAJBeeriMSQuesenberryCPWhitmerRA. The bidirectional association between depression and severe hypoglycemic and hyperglycemic events in type 1 diabetes. Diabetes Care. (2018) 41:446–52. doi: 10.2337/dc17-1566
10
GonzalezJSPeyrotMMcCarlLACollinsEMSerpaLMimiagaMJet al. Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care. (2008) 31:2398–403. doi: 10.2337/dc08-1341
11
NouwenAAdriaanseMCVan DamKIversenMMViechtbauerWPeyrotMet al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabetes Med. (2019) 36:1562–72. doi: 10.1111/dme.14054
12
LiGYuYLinCZhengSTuHXuW. Association between major depressive disorder or depressive symptoms and the risk of vascular complications among patients with type 2 diabetes, and the mediating role of metabolic biomarkers: an analysis of the UK Biobank cohort. eClinicalMedicine. (2025) 79:102982. doi: 10.1016/j.eclinm.2024.102982
13
Al-MamunFHasanMQuadrosSKaggwaMMMubarakMMdTSet al. Depression among Bangladeshi diabetic patients: a cross-sectional, systematic review, and meta-analysis study. BMC Psychiatry. (2023) 23:369. doi: 10.1186/s12888-023-04845-2
14
TegegneKDGebeyehuNAKassawMW. Depression and determinants among diabetes mellitus patients in Ethiopia, a systematic review and meta-analysis. BMC Psychiatry. (2023) 23:209. doi: 10.1186/s12888-023-04655-6
15
LiuXLiYGuanLHeXZhangHZhangJet al. A systematic review and meta-analysis of the prevalence and risk factors of depression in type 2 diabetes patients in China. Front Med. (2022) 9:759499. doi: 10.3389/fmed.2022.759499
16
RoyTLloydCEParvinMMohiuddinKGBRahmanM. Prevalence of co-morbid depression in out-patients with type 2 diabetes mellitus in Bangladesh. BMC Psychiatry. (2012) 12:123. doi: 10.1186/1471-244X-12-123
17
HabtewoldTDAlemuSMHaileYG. Sociodemographic, clinical, and psychosocial factors associated with depression among type 2 diabetic outpatients in Black Lion General Specialized Hospital, Addis Ababa, Ethiopia: a cross-sectional study. BMC Psychiatry. (2016) 16:103. doi: 10.1186/s12888-016-0809-6
18
AminuASChandrasekaranVNairS. Depression among patients with diabetes: A community-based study in South India. J Med Sci. (2017) 37:237–44. doi: 10.4103/jmedsci.jmedsci_118_16
19
AbuhegazyHMujairiABanahFAgdiYElkeshishiHKamelAet al. Depression and associated risk factors among type 2 diabetic patients: A cross sectional study on a convenience sample from the diabetic center, khamis mushait; Saudi Arabia. NDT. (2022) 18:1975–84. doi: 10.2147/NDT.S374752
20
AliSYSeidAMHassenKAbebeSTBanjawZIbrahimM. Depression and glycaemic control among adult patients with type 2 diabetes: a cross-sectional study in a comprehensive specialised hospital, Jigjiga, Ethiopia. BMJ Open. (2023) 13:e073123. doi: 10.1136/bmjopen-2023-073123
21
AzezeGAAdemaBGAdellaGADemissieBWObsaMS. Factors associated with untreated depression among type 2 diabetic patients at halaba kulito hospital, south Ethiopia: A cross-sectional study. DMSO. (2020) 13:2189–98. doi: 10.2147/DMSO.S255360
22
DachewBABirhanuAMAlemuFMBalchaSAAshenafiTD. Depression in diabetic patients attending University of Gondar Hospital Diabetic Clinic, Northwest Ethiopia. DMSO. (2016) 155:155–62. doi: 10.2147/DMSO.S97623
23
BirhanuHZenuSShelemeTTefera KefeniB. Magnitude of depression and its associated factors among patients with diabetes mellitus at public hospitals in Southwest Ethiopia, 2021. Sci Rep. (2022) 12:22134. doi: 10.1038/s41598-022-26330-8
24
KarkiAVandelanotteCRawalLB. Depressive symptoms, perceived stress, and associated socio-demographic and diabetes-related factors in people with type 2 diabetes in Nepal. Asia Pac J Public Health. (2024) 36:719–29. doi: 10.1177/10105395241277892
25
NigussieKSertsuAAyanaGMDessieYBeteTAbdisaLet al. Determinants of depression and anxiety among type 2 diabetes patients in governments’ hospitals at Harari regional state, Eastern Ethiopia: A multi-center cross-sectional study. BMC Psychiatry. (2023) 23:13. doi: 10.1186/s12888-022-04494-x
26
SunnyAKKhanalVKSahRBGhimireA. Depression among people living with type 2 diabetes in an urbanizing community of Nepal. PloS One. (2019) 14:e0218119. doi: 10.1371/journal.pone.0218119
27
ZhangWXuHZhaoSYinSWangXGuoJet al. Prevalence and influencing factors of co-morbid depression in patients with type 2 diabetes mellitus: a General Hospital based study. Diabetol Metab Syndr. (2015) 7:60. doi: 10.1186/s13098-015-0053-0
28
MaimaitituerxunRChenWXiangJKamingaACWuXYChenLet al. Prevalence of comorbid depression and associated factors among hospitalized patients with type 2 diabetes mellitus in Hunan, China. BMC Psychiatry. (2023) 23:158. doi: 10.1186/s12888-023-04657-4
29
BelewMAGetuRAGetahunSADessieAMAbejeEDEngdaASet al. Psychosocial and clinical factors associated with depression among diabetic patients in Amhara region comprehensive specialized hospitals, Ethiopia, 2022; a multicenter prevalence study. Health Sci Rep. (2024) 7:e2195. doi: 10.1002/hsr2.2195
30
MelinEOThunanderMLandin-OlssonMHillmanMThulesiusHO. Depression differed by midnight cortisol secretion, alexithymia and anxiety between diabetes types: a cross sectional comparison. BMC Psychiatry. (2017) 17:335. doi: 10.1186/s12888-017-1495-8
31
MierNBocanegra-AlonsoAZhanDWangSStoltzSMAcosta-GonzalezRIet al. Clinical depressive symptoms and diabetes in a binational border population. J Am Board Family Med. (2008) 21:223–33. doi: 10.3122/jabfm.2008.03.070255
32
MohamedNMohamudRYHilowleFMohamedYMohamedH. The prevalence and determinants of anxiety and depressive symptoms in patients with type II diabetes mellitus in mogadishu, Somalia: A cross-sectional study. DMSO. (2024) 17:3419–32. doi: 10.2147/DMSO.S479583
33
MohamedRAbdul KadirAYaacobLH. A study on depression among patients with type 2 diabetes mellitus in North-East Coast Malaysia. Int J Collab Res Internal Med Public Health. (2012) 4:1589–600.
34
MussaMRIseseloMKTarimoEAM. Depression and its associated factors among patients with diabetes: A cross-sectional survey at Mnazi Mmoja Referral Hospital in Zanzibar, Tanzania. PloS One. (2023) 18:e0284566. doi: 10.1371/journal.pone.0284566
35
SharifHJanSSSharifSSeemiTNaeemHJawedZ. Depression and suicidal ideation among individuals with type-2 diabetes mellitus, a cross-sectional study from an urban slum area of Karachi, Pakistan. Front Public Health. (2023) 11:1135964. doi: 10.3389/fpubh.2023.1135964
36
SweilehWMAbu-HadeedHMAl-JabiSWZyoudSH. Prevalence of depression among people with type 2 diabetes mellitus: a cross sectional study in Palestine. BMC Public Health. (2014) 14:163. doi: 10.1186/1471-2458-14-163
37
ThaneeratTTangwongchaiSWorakulP. Prevalence of depression, hemoglobin A1C level, and associated factors in outpatients with type-2 diabetes. Asian Biomedicine. (2009) 3:383–90.
38
TranNMHNguyenQNLVoTHLeTTANgoNH. Depression among patients with type 2 diabetes mellitus: prevalence and associated factors in hue city, Vietnam. DMSO. (2021) 14:505–13. doi: 10.2147/DMSO.S289988
39
St. Luke’s Medical Center, Quezon CityBernabe-Dela VictoriaGMDampilOA. Prevalence of Depression among Patients with Type 2 Diabetes Mellitus and its associated Clinical Factors. JAFES. (2019) 34:197–203. doi: 10.15605/jafes.034.02.11
40
WangYLopezJMSBolgeSCZhuVJStangPE. Depression among people with type 2 diabetes mellitus, US National Health and Nutrition Examination Survey (NHANES), 2005–2012. BMC Psychiatry. (2016) 16:88. doi: 10.1186/s12888-016-0800-2
41
AliSStoneMAPetersJLDaviesMJKhuntiK. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetic Med. (2006) 23:1165–73. doi: 10.1111/j.1464-5491.2006.01943.x
42
KhalediMHaghighatdoostFFeiziAAminorroayaA. The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. (2019) 56:631–50. doi: 10.1007/s00592-019-01295-9
43
World Health Organization. Depressive disorder (depression). In: Fact sheet. World Health Organization, Geneva (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed September 13, 2025).
44
BusiliAKumarKKudrnaLRabbaniU. Examining the impact of the COVID-19 pandemic on mental health among adults with type 2 diabetes: a systematic review of pre and during pandemic insights. Med (Baltimore). (2025) 104:e43112. doi: 10.1097/MD.0000000000043112
45
MoradianSTeufelMJahreLMuscheVFinkMDinseHet al. Mental health burden of patients with diabetes before and after the initial outbreak of COVID-19: predictors of mental health impairment. BMC Public Health. (2021) 21:2068. doi: 10.1186/s12889-021-12101-z
46
IslamSMSRawalLBNiessenLW. Prevalence of depression and its associated factors in patients with type 2 diabetes: A cross-sectional study in Dhaka, Bangladesh. Asian J Psychiatry. (2015) 17:36–41. doi: 10.1016/j.ajp.2015.07.008
47
JeongM. Factors associated with depressive symptoms in korean adults with diabetes mellitus: A cross-sectional study. Healthcare. (2021) 9:1049. doi: 10.3390/healthcare9081049
48
Abd-ElgawadMAbdelsattarNKGenedyGTMadeehAKKhamisMRyaadMet al. Prevalence of depression and anxiety among diabetic patients in Egypt: A cross-sectional study. Medicine. (2023) 102:e35988. doi: 10.1097/MD.0000000000035988
49
DeheshTDeheshPShojaeiS. Prevalence and associated factors of anxiety and depression among patients with type 2 diabetes in kerman, southern Iran. DMSO. (2020) 13:1509–17. doi: 10.2147/DMSO.S249385
50
EbrahimMTamiruDHawulteBMisganaT. Prevalence and associated factors of depression among diabetic outpatients attending diabetic clinic at public hospitals in Eastern Ethiopia: A cross-sectional study. SAGE Open Med. (2021) 9:20503121211066244. doi: 10.1177/20503121211066244
51
ElnaemMHBukhoriNASTengku Mohd. KamilTKRahayuSRamatillahDLElrggalME. Depression and anxiety in patients with type 2 diabetes in Indonesia and Malaysia: do age, diabetes duration, foot ulcers, and prescribed medication play a role? Psychology Health Med. (2025) 30:555–71. doi: 10.1080/13548506.2025.2450545
52
FungACHTseGChengHLLauESHLukAOzakiRet al. Depressive symptoms, co-morbidities, and glycemic control in hong kong chinese elderly patients with type 2 diabetes mellitus. Front Endocrinol. (2018) 9:261. doi: 10.3389/fendo.2018.00261
53
Gorska-CiebiadaMSaryusz-WolskaMCiebiadaMLobaJ. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res. (2014) 2014:1–7. doi: 10.1155/2014/179648
54
JahanFJabbarANaqviH. Depression in patients with diabetes mellitus and its impact on diabetes self-care, medication adherence and glycemic control. Int J Diabetes Developing Countries. (2011) 31:154–60. doi: 10.1007/s13410-011-0036-0
55
KayarYKayarNBErdenSCOnemREkinciIEmegilSet al. The relationship between depression and demographic risk factors, individual lifestyle factors, and health complications in patients with type 2 diabetes mellitus. BioMed Res. (2017) 28:1560–5.
56
AbualhamaelSABaigMAlghamdiWGazzazZJAl-HayaniMBaziA. Quality of life, stress, anxiety and depression and associated factors among people with type 2 diabetes mellitus in Western region Saudi Arabia. Front Psychiatry. (2024) 14:1282249. doi: 10.3389/fpsyt.2023.1282249
57
AtifMSaleemQScahillS. Depression and mild cognitive impairment (MCI) among elderly patients with type 2 diabetes mellitus in Pakistan: possible determinants. Int J Diabetes Developing Countries. (2018) 38:312–20. doi: 10.1007/s13410-017-0600-3
58
EngidawNAWubetuADBashaEA. Prevalence of depression and its associated factors among patients with diabetes mellitus at Tirunesh-Beijing general hospital, Addis Ababa, Ethiopia. BMC Public Health. (2020) 20:266. doi: 10.1186/s12889-020-8360-2
59
KarphaKBiswasJNathSDhaliASarkhelSDhaliGK. Factors affecting depression and anxiety in diabetic patients: A cross sectional study from a tertiary care hospital in Eastern India. Ann Med Surg. (2022) 84:104945. doi: 10.1016/j.amsu.2022.104945
60
KoganSMBrodyGHCrawleyCLoganPMurryVM. Correlates of elevated depressive symptoms among rural African American adults with type 2 diabetes. Ethn Dis. (2007) 17:106–12.
61
KuehnerC. Why is depression more common among women than among men? Lancet Psychiatry. (2017) 4:146–58. doi: 10.1016/S2215-0366(16)30263-2
62
KuehnerC. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. (2003) 108:163–74. doi: 10.1034/j.1600-0447.2003.00204.x
63
SeedatSScottKMAngermeyerMCBerglundPBrometEJBrughaTSet al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. J Affect Disord. (2009) 118:61–9. doi: 10.1016/j.jad.2009.01.029
64
SalkRHHydeJSAbramsonLY. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. (2017) 143:783–822. doi: 10.1037/bul0000102
65
SeedatSRondonM. Women’s wellbeing and the burden of unpaid work. BMJ. (2021) 374:n1972. doi: 10.1136/bmj.n1972
66
ChengS-YSuhS-YMoritaTOyamaYChiuT-YKohSJet al. A cross-cultural study on behaviors when death is approaching in east asian countries: what are the physician-perceived common beliefs and practices? Medicine. (2015) 94:e1573. doi: 10.1097/MD.0000000000001573
67
OgunsakinREOlugbaraOOMoyoSIsraelC. Meta-analysis of studies on depression prevalence among diabetes mellitus patients in Africa. Heliyon. (2021) 7:e07085. doi: 10.1016/j.heliyon.2021.e07085
68
BaiSWangJLiuJMiaoYZhangAZhangZ. Analysis of depression incidence and influence factors among middle-aged and elderly diabetic patients in China: based on CHARLS data. BMC Psychiatry. (2024) 24:146. doi: 10.1186/s12888-023-05473-6
69
LloydCERoyTNouwenAChauhanAM. Epidemiology of depression in diabetes: International and cross-cultural issues. J Affect Disord. (2012) 142:S22–9. doi: 10.1016/S0165-0327(12)70005-8
70
AndersonRJFreedlandKEClouseRELustmanPJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. (2001) 24:1069–78. doi: 10.2337/diacare.24.6.1069
71
ShihPLinM-YGuoYL. Employment status and depressive symptoms in Taiwanese older adults: an 11-year prospective cohort study. BMC Geriatr. (2024) 24:671. doi: 10.1186/s12877-024-05258-w
72
IslamMDisuTRFarjanaSRahmanMM. Malnutrition and other risk factors of geriatric depression: a community-based comparative cross-sectional study in older adults in rural Bangladesh. BMC Geriatr. (2021) 21:572. doi: 10.1186/s12877-021-02535-w
73
ArshAAfaqSCarswellCBhattiMMUllahISiddiqiN. Effectiveness of physical activity in managing co-morbid depression in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. J Affect Disord. (2023) 329:448–59. doi: 10.1016/j.jad.2023.02.122
74
UlambayarBGhanemASTóthÁNagyAC. Impact of physical activity and dietary habits on mental well-being in patients with diabetes mellitus. Nutrients. (2025) 17:1042. doi: 10.3390/nu17061042
75
KandolaAAshdown-FranksGHendrikseJSabistonCMStubbsB. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. (2019) 107:525–39. doi: 10.1016/j.neubiorev.2019.09.040
76
PearceMGarciaLAbbasAStrainTSchuchFBGolubicRet al. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiatry. (2022) 79:550–9. doi: 10.1001/jamapsychiatry.2022.0609
77
ChenHLuTSuiHTaoBChenHYanJ. Bidirectional relationships between physical exercise and depressive symptoms among Chinese older adults: evidence from the China family panel studies. Sci Rep. (2025) 15:15689. doi: 10.1038/s41598-025-00947-x
78
ZhaoLXuFZhengXXuZOstenBJiKet al. Mediation role of anxiety on social support and depression among diabetic patients in elderly caring social organizations in China during COVID-19 pandemic: a cross-sectional study. BMC Geriatr. (2023) 23:790. doi: 10.1186/s12877-023-04502-z
79
GuLWuSZhaoSZhouHZhangSGaoMet al. Association of social support and medication adherence in chinese patients with type 2 diabetes mellitus. IJERPH. (2017) 14:1522. doi: 10.3390/ijerph14121522
80
HorsbølTAHoffmannSHThorstedABRosenkildeSLehnSFKofoed-EnevoldsenAet al. Diabetic complications and risk of depression and anxiety among adults with type 2 diabetes. Diabetes Med. (2024) 41:e15272. doi: 10.1111/dme.15272
81
ZhangXMaLMuSYinY. The hidden burden—Exploring depression risk in patients with diabetic nephropathy: A systematic review and meta-analysis. Diabetes Ther. (2023) 14:1481–502. doi: 10.1007/s13300-023-01436-y
82
PouwerFGeelhoed-DuijvestijnPHTackCJBazelmansEBeekmanAJHeineRJet al. Prevalence of comorbid depression is high in out-patients with type 1 or type 2 diabetes mellitus. Diabetes Med. (2010) 27:217–24. doi: 10.1111/j.1464-5491.2009.02903.x
83
KhanAPalkaJJoshiPHKheraABrownES. Association of depressive symptom severity with coronary artery calcium: the Dallas Heart Study. J Affect Disord. (2020) 276:267–71. doi: 10.1016/j.jad.2020.07.042
84
YangEHLermanSLennonRJSimariRDLermanLOLermanA. Relation of depression to coronary endothelial function. Am J Cardiol. (2007) 99:1134–6. doi: 10.1016/j.amjcard.2006.11.054
85
BrinkmannBPayneCFKohlerIHarlingGDaviesJWithamMet al. Depressive symptoms and cardiovascular disease: a population-based study of older adults in rural Burkina Faso. BMJ Open. (2020) 10:e038199. doi: 10.1136/bmjopen-2020-038199
86
LeeHJJangJLeeSAOhSSParkE-C. Association between the type of diabetes treatment and depressive symptoms among patients with diabetes: A cross-sectional study of korea community health surveys data, 2011–2016. IJERPH. (2019) 16:4441. doi: 10.3390/ijerph16224441
87
GumuskayaPOAltunOYildirimEYuztasNKOzsoyNKalyonSet al. The association between depression and antidiabetic treatments in type 2 diabetes patients with both good and poor glycemic control. J Clin Med. (2025) 14:3460. doi: 10.3390/jcm14103460
88
BaiXLiuZLiZYanD. The association between insulin therapy and depression in patients with type 2 diabetes mellitus: a meta-analysis. BMJ Open. (2018) 8:e020062. doi: 10.1136/bmjopen-2017-020062
89
ZhengCYinJWuLHuZZhangYCaoLet al. Association between depression and diabetes among American adults using NHANES data from 2005 to 2020. Sci Rep. (2024) 14:27735. doi: 10.1038/s41598-024-78345-y
90
LangbergJMuellerARodriguez de la VegaPCastroGVarellaM. The association of hemoglobin A1c levels and depression among adults with diabetes in the United States. Cureus. (2022) 14(2):e22688. doi: 10.7759/cureus.22688
91
KwonMLeeMKimEHChoiDWJungEKimKYet al. Risk of depression and anxiety disorders according to long-term glycemic variability. J Affect Disord. (2023) 343:50–8. doi: 10.1016/j.jad.2023.09.017
92
PurtleJNelsonKLYangYLangellierBStankovIDiez RouxAV. Urban-rural differences in older adult depression: a systematic review and meta-analysis of comparative studies. Am J Prev Med. (2019) 56:603–13. doi: 10.1016/j.amepre.2018.11.008
93
XuCMiaoLTurnerDDeRubeisR. Urbanicity and depression: a global meta-analysis. J Affect Disord. (2023) 340:299–311. doi: 10.1016/j.jad.2023.08.030
Summary
Keywords
diabetes mellitus, depression, prevalence, risk factors, mental health
Citation
Yang K, Fang Y, He J and Li J (2025) Prevalence and risk factors of depression in patients with diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 16:1660478. doi: 10.3389/fendo.2025.1660478
Received
06 July 2025
Accepted
02 October 2025
Published
20 October 2025
Volume
16 - 2025
Edited by
Maria Aparecida Bicalho, Federal University of Minas Gerais, Brazil
Reviewed by
Sridhar R. Gumpeny, Endocrine and Diabetes Centre, India
Natália Silva Dias, Universidade Federal de Minas Gerais, Brazil
Updates
Copyright
© 2025 Yang, Fang, He and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, jlsdxhxc@163.com
†These authors have contributed equally to this work and share first authorship
‡ORCID: Ke Yang, orcid.org/0009-0006-2708-615X; Yuyang Fang, orcid.org/0009-0000-5857-7046; Junbo He, orcid.org/0009-0008-6927-4284; Jing Li, orcid.org/0009-0008-1722-0196
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.