Abstract
Diabetic kidney disease (DKD) is the primary microvascular complication of diabetes and a leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide, with its prevalence on the rise. Recent evidence has highlighted the crucial involvement of gut microbiota (GM) dysbiosis in the pathogenesis and progression of DKD, mediated through the gut-kidney axis. At the core of this process is a dynamic network involving metabolic, immune, and barrier dysfunction. Renal impairment—such as that seen in uremia—disrupts gut microbial composition and metabolic function. In turn, dysbiosis compromises intestinal barrier integrity, resulting in increased exposure to endotoxins and a reduction in the production of beneficial metabolites, notably short-chain fatty acids (SCFAs). This triad manifests as: (1) impaired metabolism, marked by decreased SCFAs (e.g., acetate), which weaken anti-inflammatory and immunomodulatory effects, alongside an accumulation of uremic toxins like trimethylamine N-oxide (TMAO) that trigger inflammatory pathways and renal fibrosis; (2) immune dysregulation, where increased endotoxin translocation (e.g., lipopolysaccharide, LPS) provokes systemic inflammation, oxidative stress, and immune cell infiltration (such as macrophages), contributing to renal inflammatory and fibrotic responses; and (3) barrier dysfunction, in which compromised intestinal barrier accelerates the translocation of detrimental microbial components, perpetuating a vicious cycle that exacerbates glomerulosclerosis, tubular injury, and renal function decline.Collectively, metabolic, immune, and barrier alterations reinforce one another and drive DKD progression via gut-derived metabolites and immune activation. Targeted interventions aiming to modulate the GM—using probiotics, prebiotics, or synbiotics—show promise in improving metabolic profiles, restoring gut barrier function, and mitigating DKD phenotypes. This review systematically elucidates the metabolism–immunity–barrier mechanisms by which GM dysbiosis contributes to DKD and discusses the translational potential of microbiome-targeted therapies. Further studies are needed to validate these findings and assess their long-term clinical efficacy.
1 Introduction
DKD, a major microvascular complication of diabetes, remains the leading cause of ESRD globally (1, 2). Its high prevalence not only necessitates long-term renal replacement therapy but also markedly increases the incidence of cardiovascular events in affected individuals (2, 3). In recent years, mounting evidence has highlighted the pivotal role of the gut–kidney axis in the pathogenesis and progression of DKD. Specifically, GM dysbiosis contributes to DKD evolution through metabolic disturbances, immune dysregulation, and barrier dysfunction:
1.1 Metabolic dimension
DKD is associated with a distinct gut microbial profile, notably a decreased abundance of butyrate-producing bacteria, which leads to reduced synthesis of beneficial SCFAs (4–6). Simultaneously, the accumulation of uremic toxins, such as indoxyl sulfate, exacerbates renal injury (2, 7, 8). These changes drive systemic activation of the renin–angiotensin system (RAS) and foster renal fibrosis (2, 7, 9).
1.2 Immune dimension
SCFA deficiency impairs their anti-inflammatory effects and the inhibition of histone deacetylases (HDACs) (4, 6, 10). Meanwhile, accumulation of microbial toxins acts synergistically with hyperactivation of the TLR4/NF-κB pathway (9, 11), leading to chronic systemic inflammation and oxidative stress that accelerate renal damage.
1.3 Barrier dimension
Dysbiosis impairs the integrity of intestinal epithelial tight junctions—such as zonula occludens-1 (ZO-1)—facilitating translocation of endotoxins into the bloodstream (8, 12). This establishes a deleterious “leaky gut–renal injury” cycle, which is further aggravated in DKD by hyperglycemia-induced depletion of the intestinal mucous layer, setting DKD apart from non-diabetic kidney diseases (NDKD) (5, 12, 13).
Compared with NDKD, the gut–kidney axis in DKD exhibits unique metabolic features: chronic hyperglycemia selectively suppresses butyrate metabolic pathways (6, 14), and the synergy between advanced glycation end products (AGEs)-RAGE signaling and gut-derived toxins accelerates the development of renal fibrosis (2, 7). In this review, we systematically explore the mechanisms whereby the interplay of metabolism, immunity, and barrier dysfunction propels DKD progression to ESRD. Additionally, we critically appraise the translational potential of microbiota-targeted interventions, such as dietary fiber supplementation to enhance SCFAs–GPR43 signaling, and probiotic modulation of Akkermansia to promote barrier restoration.
2 Clinical evidence linking gm dysbiosis to diabetic nephropathy
2.1 Characteristics of GM in patients with DN
2.1.1 Alterations in microbial composition
Marked reduction in beneficial bacteria: Patients with DN exhibit a distinct gut microbial profile characterized by a significant decrease in SCFA-producing genera, such as Akkermansia, Roseburia, and Alistipes. Notably, a reduction in Akkermansia abundance is associated with the progression of renal fibrosis (p < 0.05), while decreased Alistipes correlates with heightened systemic inflammatory responses (p < 0.05) (15). These changes may contribute to compromised intestinal barrier function and immune dysregulation, thereby accelerating the pathological progression of nephropathy.
Enrichment of pathogenic bacteria: Conversely, the abundance of pathogenic bacteria, particularly Escherichia-Shigella, is increased in DN patients. These bacteria secrete endotoxins, such as LPS, which activate proinflammatory pathways in renal tissue (notably the NF-κB signaling pathway), thereby promoting glomerulosclerosis and tubular injury. Overall, gut dysbiosis exacerbates systemic inflammation and renal fibrosis in DN (16, 17).
Several studies have demonstrated a robust association between changes in microbial composition and renal function parameters. For instance, reduced Roseburia abundance is linked to an increased risk of DN (P = 0.00118; OR = 0.513, 95% CI: 0.343–0.768), suggesting a negative relationship with eGFR decline (18). Similarly, lower Dialister abundance is inversely correlated with DN risk (OR = 0.513, P = 0.00118) (18), further substantiating the detrimental impact of microbial dysbiosis on renal function. Moreover, Mendelian randomization (MR) analyses have confirmed a direct, negative causal effect of decreased SCFA-producing bacteria on DN progression (P < 0.05) (19), associating gut microbial alterations with renal function deterioration.
2.1.2 Potential as biomarkers
Characteristic microbial profiles hold significant promise for distinguishing DN from NDKD. For instance, reduced abundance of Verrucomicrobia in DN patients serves as a phylum-level discriminator (18, 20). Notably, species-level biomarkers within the Prevotella genus exhibit strong clinical relevance. Prevotella copri is markedly enriched in diabetic patients with poor glycemic control (HbA1c >7.0%) and inversely correlates with healthy dietary patterns (e.g., fish-vegetable intake) 21. Its diagnostic power is robust, with an AUC of 0.93 (95% CI: 0.88–0.98) for distinguishing DN from non-DKD subjects via qPCR validation (21).
Additionally, Prevotella_9 demonstrates significant alterations in DKD. Metagenomic analyses reveal that Prevotella_9 species (e.g., Prevotella sp. MSX73) are enriched in DKD patients and contribute to a combinatorial biomarker model (AUC = 0.889) differentiating DN from T2DM without nephropathy 22. The decline in Prevotella-associated butyrate production further exacerbates renal dysfunction by impairing anti-inflammatory pathways (22, 23).
Comprehensive integration of microbial signatures (e.g., Prevotella copri, Prevotella_9, and g_Prevotella) with serum metabolites (e.g., imidazolepropionic acid) enhances early DN detection (AUC >0.94) (22, 23). This multi-omics approach underscores the potential for developing non-invasive diagnostic models leveraging GM dysbiosis (3, 24).
2.2 Unresolved causality: insights from MR studies
MR has become a crucial approach for establishing causality in the relationship between GM and DN. While accumulating evidence from MR analyses suggests the existence of causal links at the genetic level, several key areas remain contentious, particularly concerning the directionality of effects and the clarification of underlying molecular mechanisms.
2.2.1 Evidence supporting a causal relationship
Multiple two-sample MR studies have provided substantial evidence supporting a causal relationship between specific gut microbial taxa and the risk of DN. For instance, studies have identified potential causal links between certain gut microbial taxa and DN, which were confirmed through MR analysis (25–27). Quantitative evaluations have further shown that an increased abundance of taxa such as Catenibacterium is significantly associated with a decreased risk of DN (OR =0.513; 95% CI, 0.343–0.768) (26, 27). Moreover, MR analyses have revealed that genetic variants influencing the composition of the gut microbiome, ranging from phylum to genus level, have both positive and negative causal effects on the risk of DN (27, 28). These data reinforce the view that gut microbial dysbiosis may act as a potential driver in DN pathogenesis. Robust causal associations have also been validated by MR studies employing inverse variance weighted methods (28).
2.2.1.1 Controversy: the bidirectionality of causality
Despite the above findings, one of the major points of contention is the potential bidirectional nature of the relationship between GM dysbiosis and DN. On the one hand, dysbiosis may be an initiating factor in DN: reduced production of SCFAs has been shown to promote renal inflammation and fibrosis, thereby accelerating DN progression (9, 12). Experimental studies have demonstrated that SCFA supplementation can attenuate renal fibrosis in DN models, supporting the causal role of the GM in DN onset (5). On the other hand, renal dysfunction itself may influence the gut microbiome: studies have found that declining renal function, such as a decreased glomerular filtration rate, can alter microbial diversity (29, 30). As such, reverse causation becomes a possibility, making it challenging to fully distinguish causality using MR analysis alone (31). To address this issue, Li Q has emphasized the need for longitudinal studies, such as repeated measures of renal function, to clarify the temporal and causal relationships (3). Additionally, fecal microbiota transplantation (FMT) experiments provide direct evidence for this interaction: transplantation of microbiota from patients with DKD increased the urinary albumin-to-creatinine ratio (UACR) in recipient animals by approximately 2.1-fold compared with transplantation from healthy donors, further supporting the role of dysbiosis as a driving factor in DN (32).
2.2.1.2 Controversy: unclear molecular mechanisms
Another unresolved issue lies in the lack of detailed understanding of the molecular mechanisms underlying GM-host interactions in DN. Although numerous studies have postulated that GM-derived metabolites, such as SCFAs, may modulate host pathways—including the renin-angiotensin system (RAS)—direct experimental evidence remains limited. For example, Huang L speculated that SCFAs might influence DN progression via immunometabolic pathways, but this has yet to be verified. Similarly, while Das S investigated the protective effects of SCFAs in DN models, their mechanisms—such as possible regulation of the RAS—require further elucidation (5, 12). As noted by Jin Y, “the precise pathways by which dysbiotic microbiota may induce and exacerbate DN remain undefined,” underscoring the need for mechanistic studies in cellular and animal models to move beyond mere genetic associations (27). Although FMT studies revealing elevated UACR in recipient animals offer a useful starting point for exploring these mechanistic links, the downstream pathways—potentially involving inflammation or metabolic disturbances—have not yet been clearly defined.
2.3 Cross-disease comparison: microbial differences between DN and NDKD
Comparative analysis between DN and NDKD reveals disease-specific patterns of GM dysbiosis, which are critical for understanding pathogenesis and guiding personalized treatment strategies. Current evidence indicates that DN and NDKD display distinct alterations in both metabolic and pathogenic microbial taxa, resulting in different responses to microbial interventions.
2.3.1 Disease-specific microbial features in DN
2.3.1.1 Metabolic dysfunction
DN is characterized by a notably greater depletion of short-chain fatty acid (SCFA)-producing bacteria, such as butyrate-producing Firmicutes species, compared to NDKD. Experimental studies have shown that DN mouse models exhibit significantly lower fecal SCFA levels (especially acetic acid), which correlate positively with the severity of renal fibrosis (6). Furthermore,it has been summarized that this reduction is strongly associated with insulin resistance—a relationship more pronounced in DN than in NDKD—and may impact glucose homeostasis through the “gut-kidney axis” (17). The observational data further confirm that gut microbial metabolic disturbances are a fundamental and unique aspect of DN, with SCFA depletion representing a core feature rather than a common trait of NDKD (25).
2.3.1.2 Differences in pathogenic bacteria
The enrichment of pathogenic bacteria also differs between DN and NDKD. In NDKD, dysbiosis mainly affects immune-modulating bacteria; for instance, enrichment of Clostridium has been shown to activate immune pathways and contribute to nephritis (1). In contrast, DN is characterized by an increase in metabolically relevant pathogenic taxa, notably Enterobacteriaceae (6, 25). Specifically, studies have identified immune dysregulation-associated dysbiosis in NDKD (e.g., Clostridium perfringens Clostridium enrichment disturbing T cell balance), whereas in DN, the dysbiosis is more strongly related to metabolic pathogens such as Enterobacteriaceae, which can directly aggravate insulin resistance and renal injury (1). Systematic review corroborates these findings, indicating that DN features more pronounced shifts in metabolic taxa, while NDKD primarily exhibits changes in immune-related microbes (33).
Therapeutic Implications: Microbiota-targeted interventions appear to be more effective in DN, underscoring the value of disease-specific strategies. For example, butyrate supplementation significantly alleviated renal fibrosis in DN models (6), and SCFA treatment was found to slow DN progression by correcting metabolic imbalances (34). However, similar interventions in NDKD yielded limited results; studies (17, 31) point out that the pathogenesis of NDKD is primarily immune-mediated, making SCFA supplementation less effective in mitigating immune-driven kidney damage. These findings emphasize the importance of tailoring microbial interventions to the underlying pathophysiology of each kidney disease subtype.
2.4 Key controversies and knowledge gaps
Despite significant advances in understanding the gut microbiota - diabetic nephropathy relationship, several critical controversies and knowledge gaps persist. First, while MR studies provide genetic evidence of causality, experimental validation using animal models remains essential. Integrated studies have demonstrated associations between clinical GM profiles and DN, yet in vivo models are necessary to elucidate causal mechanisms (33). Although FMT represents a promising therapeutic approach, standardized protocols for DN are lacking, necessitating additional animal studies to validate causal relationships through approaches such as transplanting DN-associated microbiota into healthy recipients (17, 35).
The translation of microbiota-targeted therapies to clinical practice faces significant challenges. Clinical trials of probiotics and prebiotics have yielded inconsistent results, likely reflecting inter-individual microbiota variability and disease heterogeneity. While short-chain fatty acid (SCFA) supplementation demonstrates benefits in DN animal models (34), FMT outcomes in human studies remain variable due to substantial individual microbiome diversity (35). Recent investigations have proposed personalized probiotic formulations based on microbiome profiling; however, population variability and the need for standardized interventions continue to impede clinical translation (35, 36).
Furthermore, emerging evidence suggests that DN and NDKD involve distinct dysbiosis-driven immunological mechanisms. DN-associated gut dysbiosis appears to influence host immunity through metabolic-immune crosstalk, whereas NDKD may be more directly linked to immune dysregulation, particularly Th17/Treg cell axis imbalances (1). Although experimental studies demonstrate that microbiota modulation can restore Treg/Th17 balance in inflammatory conditions, these models lack kidney disease specificity. Consequently, comparative mechanistic studies examining these immunological pathways and their roles in renal fibrosis across DN and NDKD represent critical research priorities requiring further investigation.
3 Key Mechanisms by which GM dysbiosis drives the progression of DN
3.1 Disruption of the intestinal barrier and translocation of endotoxins
3.1.1 Gut microbiota dysbiosis and intestinal barrier impairment
In the context of diabetes, GM dysbiosis is typified by a notable reduction in beneficial bacteria—particularly those producing SCFAs—accompanied by an overgrowth of Gram-negative pathogenic taxa (9, 12). This altered microbial composition leads to the downregulation of critical tight junction proteins within intestinal epithelial cells, such as ZO-1, occludin, and claudin-1, thereby compromising the integrity of the intestinal mucosal barrier (37–39) and resulting in enhanced intestinal permeability (40). Compelling evidence has demonstrated a depleted abundance of protective genera, such as Akkermansia, and an increased proportion of LPS-producing Gram-negative bacteria (Such as Bacteroides stercoris) in the gut of patients with DN (23). Animal studies further corroborate that high-fat diet or diabetic status markedly reduces the expression of tight junction proteins and exacerbates gut barrier dysfunction (41, 42).
3.3.2 Endotoxemia and activation of systemic inflammatory responses
Intestinal barrier disruption allows bacterial endotoxins, particularly LPS, to enter the systemic circulation, leading to metabolic endotoxemia (40, 43). Circulating LPS engages Toll-like receptor 4 (TLR4) on monocytes and macrophages, triggering the MyD88/NF-κB signaling pathway (25, 44, 45). This activation induces the release of pro-inflammatory cytokines, including TNF-α and IL-6 (25, 46). Clinical investigations have demonstrated a positive correlation between serum LPS levels and the degree of renal injury in DN, including declines in glomerular filtration rate and aggravation of proteinuria (3).
3.3.3 Renal inflammation and fibrosis
Systemic inflammatory cytokines, including TNF-α and IL-6, drive the infiltration of immune cells into renal tissue and activate local inflammatory pathways (17). Inflammatory responses cause direct damage to glomerular endothelial cells and podocytes, accelerate the progression of glomerulosclerosis, and promote the transdifferentiation of tubular epithelial cells into interstitial fibroblasts, thereby exacerbating tubulointerstitial fibrosis (1, 18). Experimental evidence suggests that interventions using probiotics or plant-derived extracts can effectively mitigate renal inflammation and fibrosis in diabetic models. Specifically, probiotic supplementation, such as Lactobacillus ATCC 4356, has been shown to reduce kidney inflammation and fibrosis in diabetic rats by modulating GM, restoring microbial diversity, and decreasing markers of DNA damage, as evidenced by histological analyses that revealed improved kidney structure and reduced fibrosis (47). Additionally, probiotic combinations including strains like Lactobacillus TYCA06 and Bifidobacterium BLI-02 attenuate renal function deterioration and blood-glucose fluctuations in diabetic CKD models, thereby suppressing inflammatory pathways (48). For plant-derived extracts, bioactive compounds from medicinal plants, such as flavonoid-rich preparations, demonstrate nephroprotective effects by suppressing oxidative stress and regulating proinflammatory pathways (e.g., through modulation of sirtuins and claudin-1 expression), thus reducing renal injury in streptozotocin-induced DN (48, 49). These interventions work through mechanisms like inhibition of inflammatory signaling cascades and modulation of the gut-kidney axis, as they reduce pro-inflammatory cytokines and oxidative stress markers, contributing to the amelioration of fibrosis (36, 50). The specific mechanistic process is summarized in Figure 1.
Figure 1

Mechanism of gut microbiota dysbiosis-induced systemic inflammation and renal damage in diabetes. Left: Diabetic conditions drive gut microbiota dysbiosis, characterized by diminished beneficial bacteria (blue) and expansion of pathogenic bacteria (red). This imbalance disrupts intestinal tight junctions, permitting translocation of bacterial fragments (e.g., LPS) into systemic circulation. Center: Key molecular pathway: Circulating bacterial fragments activate the TLR4/MyD88/NF-kB signaling cascade (detailed in dashed- line inset), triggering release of pro- inflammatory cytokines (TNF-α, IL-6). Right: Systemic inflammation propagates renal injury, culminating in inflammatory tubulointerstitial fibrosis. TLR4, Toll-like receptor 4; MyD88, Myeloid differentiation primary response 88; NF-κB, Nuclear factor kappa-light- chain-enhancer of activated B cells; TNF-a, Tumor necrosis factor-alpha; IL-6, Interleukin-6; LPS, Lipopolysaccharide.
3.2 Nephrotoxic effects of microbial metabolites
Gut-derived microbial metabolites, particularly the reduction of SCFAs and the accumulation of uremic toxins, play critical roles in the development and progression of DN. Their pathogenic mechanisms involve oxidative stress, inflammatory responses, and fibrosis, in part through modulation of key renal signaling pathways (44, 51).
3.2.1 Pathogenic mechanisms of reduced SCFAs
A decrease in SCFAs, such as butyrate and propionate, exacerbates renal injury primarily by impairing renal antioxidant defense systems and disrupting immune homeostasis. SCFA deficiency attenuates the activation of major regulatory pathways, such as nuclear factor erythroid 2-related factor 2 (Nrf2), leading to the accumulation of reactive oxygen species (ROS) and enhancing oxidative stress within the renal microenvironment (52). Reduced SCFA levels impair immunomodulatory functions by inhibiting regulatory T cell (Treg) differentiation and decreasing anti-inflammatory cytokines such as IL-10. This shift promotes the production of pro-inflammatory mediators, including IL-1β, IL-6, and TNF-α, leading to sustained renal inflammation and tissue damage (6, 44, 53). This immune dysregulation is increasingly recognized as a pivotal feature in the interplay between GM and renal pathology (51, 54).
3.2.2 Nephrotoxic mechanisms of uremic toxin accumulation
Uremic toxins, such as indoxyl sulfate (IS) and p-cresyl sulfate (PCS), exhibit a strong inverse correlation with estimated glomerular filtration rate (eGFR) (total IS: r = -0.819; free PCS: r = -0.753) (55), further aggravate renal damage in DN by promoting mitochondrial dysfunction and profibrotic remodeling. IS activates the aryl hydrocarbon receptor (AhR) pathway in renal tubular epithelial cells, enhancing oxidative stress, impairing mitochondrial function, and inducing apoptosis (53, 54, 56).Critically, serum IS ≥50 μmol/L predicts peritoneal dialysis technique failure with 70.4% sensitivity and 87.9% specificity (p<0.0001), highlighting its clinical relevance in renal functional decline 57. PCS, on the other hand, drives fibroblast activation and extracellular matrix deposition, thereby accelerating tubulointerstitial fibrosis (54, 57). Moreover, IS accumulation downregulates organic anion transporter 3 (OAT3) expression at the blood-brain barrier, impairing toxin efflux and exacerbating systemic accumulation (58). Importantly, IS and PCS can synergistically activate the NF-κB signaling pathway, upregulating pro-inflammatory cytokines such as IL-17 and IL-6, which directly correlate with peritoneal dialysate IL-6 levels (r = 0.92) (59), perpetuating a vicious cycle of inflammation and fibrosis (51, 57).
In DKD, GM dysbiosis promotes uremic toxin production (e.g., IS/PCS) (60, 61), while AST-120 adsorbent therapy significantly lowers serum IS (SMD = -1.75, p<0.001) and improves creatinine clearance (SMD = 0.41, p<0.001) in CKD models (62). This dysbiosis compromises intestinal barrier function, facilitating systemic toxin entry and exacerbating renal inflammation8,16. Dietary modulation (e.g., high-fiber diets, resistant starch) reduces toxin generation by altering microbiota composition (60, 63, 64), effectively lowering serum total cholesterol (SMD = -0.28, p=0.013) in diabetic CKD models (62), thereby mitigating DKD progression.
3.2.3 Interventional strategies and therapeutic potential
Targeted interventions aimed at restoring microbial metabolic balance have shown promising renoprotective effects, mainly through pharmacological, nutritional, and non-pharmacological approaches.
3.2.3.1 Pharmacological and nutritional modulation
Traditional Chinese herbal formulations (e.g., Xiaoyu Xiezhuo decoction, XXD) have been reported to lower plasma IS and PCS levels while increasing colonic SCFA (such as butyrate) concentrations, consequently ameliorating tubular injury and suppressing pro-inflammatory cytokines such as IL-17 and TNF-α (65, 66). Specific probiotic strains (e.g., ATCC 4356) facilitate the re-establishment of microbial diversity and enhance SCFA biosynthesis, thus mitigating oxidative stress and glomerulosclerosis (47). Similarly, natural compounds like resveratrol may reshape the GM structure, elevate fecal SCFA levels, and reduce tubulointerstitial fibrosis (6).
3.2.3.2 Non-pharmacological toxin removal
Use of intestinal adsorbents such as AST-120 effectively reduces circulating IS by binding its precursors in the gut, while extracorporeal techniques including hemodialysis and hemofiltration directly eliminate accumulated IS and PCS from the bloodstream (54). These interventions, by targeting the gut–kidney axis, present innovative strategies for metabolic regulation and renoprotection among patients with DN (44, 57).
3.3 Activation of immune-inflammatory pathways
GM dysbiosis triggers a cascade of immune-inflammatory responses through both innate and adaptive mechanisms, with the NLRP3–IL-1β axis serving as a central regulatory hub. Dysbiosis increases host exposure to endotoxins such as LPS and uremic toxins, which enter systemic circulation and function as pathogen-associated or damage-associated molecular patterns (42, 67–69). These molecules initiate innate immune activation through a two-signal process: LPS activates the TLR4/NF-κB pathway (Signal 1), inducing transcription of NLRP3 and pro-IL-1β, while uremic toxins trigger potassium efflux and mitochondrial ROS production (Signal 2), promoting NLRP3 oligomerization and caspase-1 activation (69–71).
This activation specifically promotes assembly of the NLRP3–ASC–pro-caspase-1 complex via the P2X7 receptor, leading to caspase-1-dependent maturation of IL-1β and IL-18 (68, 69). Activated caspase-1 cleaves gasdermin D, forming membrane pores that induce pyroptosis in glomerular endothelial cells—a form of inflammatory cell death characterized by cellular swelling, lysis, and massive cytokine release, resulting in direct renal tissue injury (42, 72, 73). The ensuing inflammatory milieu recruits neutrophils into the renal parenchyma, amplifying oxidative stress and contributing to podocyte injury (42, 67, 74).
Concurrently, altered GM disrupts adaptive immune homeostasis. Reduced beneficial metabolites, particularly SCFAs, skews T cell differentiation toward excessive Th17 activation while impairing regulatory T cell (Treg) function (74, 75). Hyperactivated Th17 cells release elevated IL-17A, which acts on kidney-resident cells to induce neutrophil chemoattractants (CXCL1, CXCL2) (73, 74). Infiltrating neutrophils release proteases and ROS that degrade podocyte cytoskeleton proteins and disrupt the slit diaphragm, culminating in proteinuria (73, 74). Critically, IL-17A upregulates NLRP3 expression in glomerular cells, establishing an IL-17A–NLRP3–IL-1β positive feedback loop that perpetuates renal inflammation (73–75).
The NLRP3–IL-1β axis thus bridges innate and adaptive immunity in DN pathogenesis. Therapeutically, targeting the NLRP3/caspase-1 pathway significantly reduces cytokine release and ameliorates glomerulosclerosis and proteinuria, emerging as a core target for mitigating GM-driven inflammation in DN (67, 76). The “gut–kidney axis”—encompassing primary microbiota alterations, secondary metabolite regulation, and tertiary pathological effects—is illustrated in Figure 2.
Figure 2
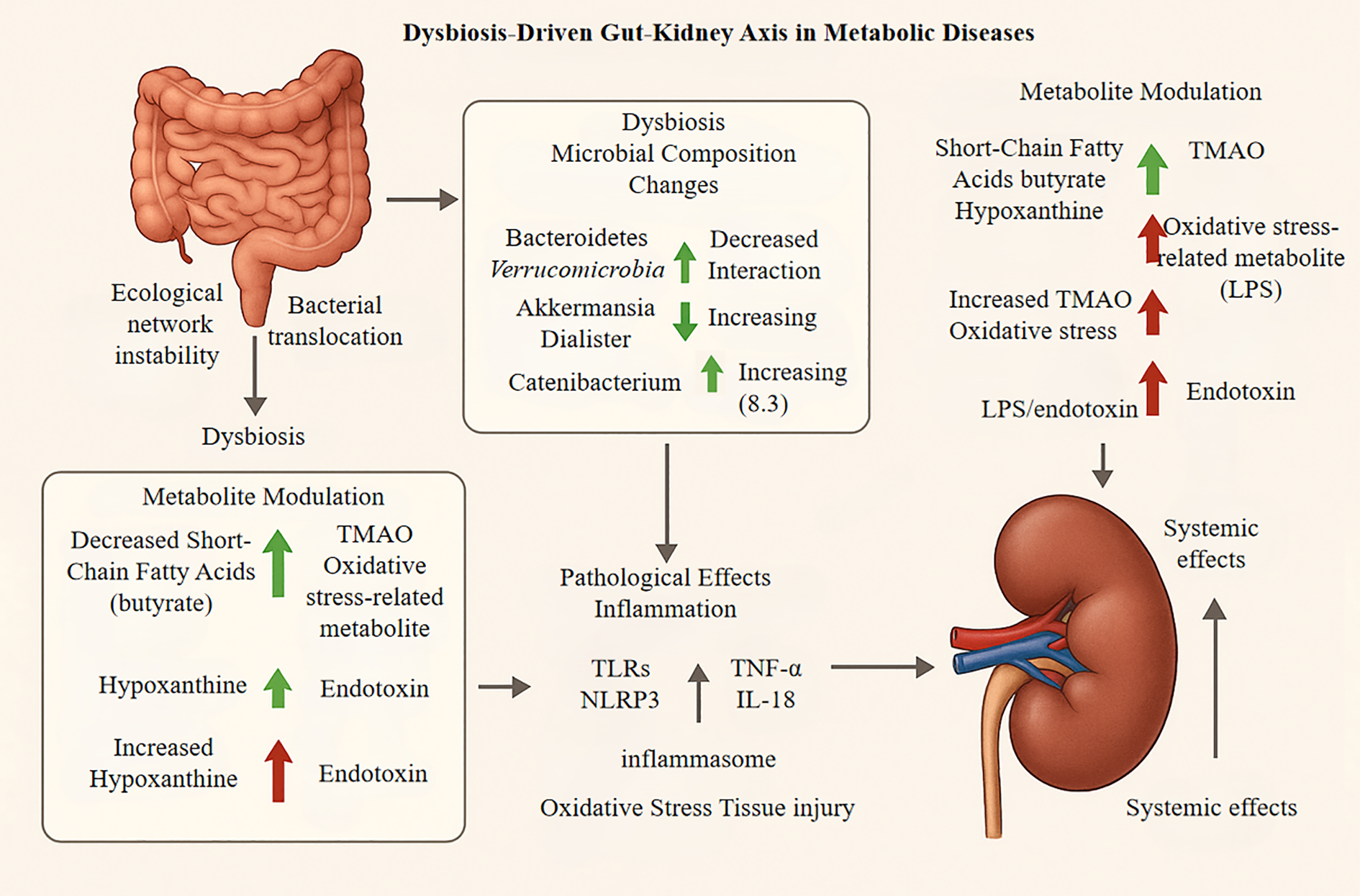
Downstream pathological effects, encompassing both renal injury and systemic consequences, involve several key processes. First, an amplification of inflammation occurs as microbial metabolites, such as lipopolysaccharide (LPS) and trimethylamine N-oxide (TMAO), activate renal-resident immune cells like macrophages. This activation triggers the release of pro-inflammatory cytokines, including TNF-α and IL-1β. Concurrently, the NLRP3 inflammasome is activated, promoting renal cell apoptosis and fibrosis. Metabolic imbalances also induce oxidative stress through the accumulation of reactive oxygen species (ROS), which damages the glomerular filtration barrier and leads to tubular epithelial cell injury and proteinuria under chronic conditions. These effects create a systemic feedback loop; for instance, a decline in the glomerular filtration rate (GFR) exacerbates toxin accumulation, which in turn worsens gut dysbiosis. This highlights complex inter- organ crosstalk, such as the kidney-brain axis, driving disease progression.
3.4 Role of metabolic dysregulation as a mediator
GM dysbiosis drives DN progression through interconnected metabolic pathways involving insulin resistance and lipid dysregulation. The disruption of gut microbiota significantly reduces SCFA synthesis, particularly acetate and butyrate (35, 51). SCFAs normally engage G protein-coupled receptors (GPR41/43) on intestinal L cells to promote glucagon-like peptide-1 (GLP-1) secretion, which is crucial for glycemic control (34). GLP-1 deficiency diminishes its inhibitory effect on hepatic gluconeogenesis, increasing activity of rate-limiting enzymes like phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), thereby exacerbating hyperglycemia (34, 77). Notably, when serum butyrate levels fall below 10 μM, renal IL-6 expression significantly increases (34).
Concurrently, gut dysbiosis facilitates LPS translocation into systemic circulation, activating TLR4 signaling pathways (78). This triggers the JNK signaling cascade in macrophages, leading to increased tumor necrosis factor-alpha (TNF-α) release. TNF-α promotes serine phosphorylation of insulin receptor substrate-1 (IRS-1), disrupting the downstream PI3K-AKT pathway and impairing glucose uptake in peripheral tissues (7, 44, 78). The resulting chronic insulin resistance induces sustained hyperinsulinemia, which causes afferent arteriolar dilation and efferent arteriolar constriction, increasing glomerular capillary pressure. These hemodynamic alterations promote proteinuria and glomerulosclerosis, accelerating renal injury progression (11).
Parallel to glucose metabolism disruption, gut dysbiosis profoundly alters lipid homeostasis through impaired bile acid metabolism. The reduced formation of secondary bile acids, particularly deoxycholic acid, impairs farnesoid X receptor (FXR) activation in the intestine (77, 79, 80). Inadequate FXR signaling upregulates hepatic sterol regulatory element-binding protein 1c (SREBP-1c), driving transcription of lipogenic enzymes including fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC). This promotes triglyceride synthesis and hepatic lipid accumulation (77, 79, 81). Under physiological conditions, intestinal FXR activation stimulates fibroblast growth factor 19 (FGF19) secretion, which inhibits hepatic CYP7A1-mediated bile acid synthesis and preserves lipid homeostasis (35, 77). In DN, suppressed FXR signaling decreases FGF19 levels, leading to insufficient SREBP-1c inhibition and systemic lipid overload (77, 79, 81).The elevated circulating free fatty acids (FFA) resulting from this metabolic disruption enhance renal lipid deposition, triggering mesangial cell lipotoxicity and mitochondrial oxidative stress. This stimulates release of inflammatory mediators including IL-6 and monocyte chemoattractant protein-1 (MCP-1), culminating in mesangial matrix expansion (19, 81). The synergistic effects of insulin resistance and lipid dysregulation accelerate renal deterioration through multiple mechanisms: insulin resistance-induced intraglomerular hypertension combines with lipotoxicity-mediated mesangial cell injury to promote glomerular basement membrane thickening and excessive extracellular matrix accumulation (11, 19). Furthermore, concomitant activation of the JNK pathway (linked to insulin resistance) and FFA-driven NLRP3 inflammasome assembly (associated with lipid dysregulation) synergistically promotes macrophage infiltration in renal tissue, hastening tubulointerstitial fibrosis (78). This integrated metabolic dysfunction underscores the critical role of the gut-kidney axis in DN pathogenesis.
4 Strategies targeting the GM in the management of DN
4.1 Probiotics, prebiotics, and synbiotics and postbiotics
Probiotics, prebiotics, and synbiotics have gained increasing attention as microbiota-targeted interventions for the management of DN. An accumulating body of clinical evidence suggests that these approaches, through modulation of the gut–kidney axis, can reshape intestinal microbial composition, reduce oxidative stress, and ultimately improve renal function parameters such as serum creatinine and blood urea nitrogen (47, 82). Probiotics, by restoring the balance of the GM, have been shown to lower the risk of renal injury. Synbiotics, which combine the complementary actions of probiotics and prebiotics, produce synergistic effects by regulating the production of key microbial metabolites, such as SCFAs and secondary bile acids, thereby mitigating systemic inflammatory responses (35, 83, 84).
Several randomized controlled trials have demonstrated that these interventions may also promote glycemic control (evidenced by reduced HbA1c levels) and improve lipid metabolism (e.g., lowering low-density lipoprotein cholesterol), underscoring the close interplay between the GM and host metabolic pathways (84, 85). Nevertheless, substantial heterogeneity and limitations persist across studies. The therapeutic efficacy of specific bacterial strains varies, potentially due to inter-individual differences in baseline gut microbial communities (47, 82). Furthermore, clinical protocols regarding optimal dosing, strain selection, and duration of interventions are not yet standardized, thereby affecting the reproducibility and robustness of clinical outcomes.
Given these challenges, future research should prioritize large-scale, multicenter randomized controlled trials to comprehensively assess the impact of targeted microbiome interventions on hard endpoints in DN and to facilitate the development of next-generation precision microbiome therapeutics (86). In summary, while microbiota-targeted interventions represent a promising avenue for DN management, further rigorous clinical validation is warranted to substantiate their efficacy.
4.2 Mechanistic insights into the role of high-fiber diets in DN
Emerging evidence highlights the therapeutic role of high-fiber diets in delaying the progression of DN, primarily through modulation of GM-derived metabolites such as SCFAs and restoration of intestinal barrier integrity. The underlying mechanisms are summarized as follows:
4.2.1 Enhancement of SCFA production
High-fiber diets, particularly those rich in fermentable fibers like pectin and inulin, are metabolized by colonic microbiota, leading to increased production of SCFAs such as acetate, propionate, and butyrate (53, 87, 88). These dietary interventions selectively enrich SCFA-producing bacteria, including Akkermansia muciniphila and members of the Bacteroides genus, thereby optimizing the gut microbial landscape (89, 90). Notably, butyrate has been demonstrated to improve insulin sensitivity and alleviate renal fibrosis and inflammatory responses by inhibiting HDACs (14, 34).
4.2.2 Restoration of intestinal barrier function
SCFAs are crucial for intestinal barrier integrity by enhancing tight junction protein expression, which decreases endotoxin translocation, such as LPS, into systemic circulation (10, 34, 91). They activate GPR41/43, aid regulatory T cell (Treg) differentiation, and increase anti-inflammatory cytokines like interleukin-10 (IL-10), thereby reducing systemic inflammation (92–94). In preclinical models, these effects are associated with marked decreases in proteinuria and tubular injury (14, 34, 95).
4.2.3 Modulation of the gut–kidney axis
High-fiber dietary interventions have been shown to rebalance GM composition, characterized by a reduction in pathogenic bacteria such as Enterobacter and an increase in beneficial taxa like Bacteroidetes (1, 3, 5). Through the action of SCFAs, these interventions further inhibit renal RAS activation and attenuate oxidative stress in renal tissues (14, 96). Clinical studies corroborate these findings, demonstrating that high-fiber interventions can significantly decrease glycemic parameters (e.g., reduction in HbA1c by 0.99%) and inflammatory markers in patients with DN (97). Nevertheless, individual variability in gut microbiome composition warrants personalized optimization of fiber type, with current evidence suggesting that soluble fibers confer greater efficacy (98).
In summary, high-fiber diets exert multifaceted renoprotective effects in DN via the microbiota–SCFA–intestinal barrier axis, ultimately ameliorating pathological progression. Future directions should focus on the development of individualized fiber-based dietary strategies and targeted SCFA delivery approaches to maximize therapeutic benefit.
4.3 Regulatory effects of microbiota-derived metabolites: the renoprotective role of butyrate
Butyrate, a key short-chain fatty acid derived from gut microbial fermentation, has shown notable renoprotective effects in multiple animal models of DKD and related renal injuries (34, 99). Studies have demonstrated that butyrate supplementation can markedly alleviate glomerular hypertrophy, podocyte injury, and interstitial fibrosis, as well as improve mitochondrial function and reduce serum creatinine levels in both diabetic and acute kidney injury models (100, 101). These results suggest that targeting microbial metabolites like butyrate may offer novel strategies for preserving kidney function.
Mechanistic investigations indicate that butyrate exerts its renoprotective roles via several pathways. It effectively suppresses renal inflammation and oxidative stress by downregulating pro-inflammatory mediators, such as TNF-α, and inhibits fibrosis by modulating TGF-β signaling in renal tubular cells and podocytes through GPR43 and GPR109A receptors (34, 102). In addition, butyrate acts as a histone deacetylase inhibitor, promoting beneficial epigenetic modifications and upregulating protective genes including Klotho and PGC-1α (100, 103), thereby restoring mitochondrial homeostasis primarily via the AMPK/PGC-1α pathway (80, 104).
Furthermore, butyrate reinforces the gut–kidney axis by enhancing intestinal barrier integrity and attenuating systemic inflammation, with evidence pointing to a receptor-dependent mechanism and the need for sustained supplementation to maintain its benefits (105, 106). In an adenine-induced model of CKD, butyrate has been shown to attenuate renal fibrosis by suppressing activation of the NLRP3/STING/NF-κB signaling pathway (107, 108). Collectively, these findings highlight the therapeutic potential of butyrate in DKD, supporting further clinical studies to optimize its application in patient care.
4.4 Exploration of novel targeted therapies
In recent years, increasing attention has been paid to the interaction between DKD and the GM, leading to the exploration of novel targeted therapeutic strategies. These strategies, primarily based on natural compounds and traditional Chinese medicine interventions, aim to regulate gut microbial homeostasis through multi-target mechanisms. Such interventions not only help restore microbial balance but also alleviate systemic inflammation and metabolic disturbances, ultimately contributing to the delay of DKD progression (109, 110).
4.4.1 Intervention with natural compounds
4.4.1.1 Intervention with natural compounds
Natural compounds demonstrate significant potential in modulating the gut microbiome and ameliorating DKD progression, primarily by regulating microbial-derived metabolites. Clinical and preclinical studies consistently report decreased abundance of probiotic genera Bifidobacterium and Lactobacillus (e.g., L. johnsonii, L. reuteri, B. animalis) in patients with CKD including DKD, which correlates with impaired renal function and increased uremic toxins (111, 112). For instance, plant polysaccharides and related natural products reshape GM composition, enriching SCFA-producing bacteria such as Bifidobacterium and Lactobacillus (35, 113, 114). TCM interventions like Moshen granule and specific compounds (e.g., barleriside A, 5,6,7,8,3’,4’-hexamethoxyflavone, Thonningianin A) counteract this dysbiosis by selectively increasing Lactobacillus/Bifidobacterium abundance, restoring microbial balance and gut barrier integrity (115, 116). This modulation reduces accumulation of diverse uremic toxins implicated in DKD pathogenesis, including:
Phenylacetylglutamine (PAGln): Derived from phenylalanine metabolism by gut microbes (e.g., Clostridium spp.), PAGln promotes cardiovascular complications and renal fibrosis via activation of G-protein-coupled receptors, exacerbating DKD progression (117).
p-Cresyl glucuronide and PCS: Protein-bound toxins produced from tyrosine metabolism by Bacteroides and Clostridium. They induce oxidative stress, endothelial dysfunction, and insulin resistance, accelerating renal injury (118, 119).
Hippuric acid: Generated from polyphenol metabolism (e.g., quercetin, chlorogenic acid), it contributes to tubular damage and inflammation in diabetic kidneys (119).
These interventions further suppress proteobacteria (e.g., Escherichia-Shigella) and reduce LPS release, thereby mitigating systemic inflammation via NLRP3/ASC/Caspase-1 pathway inhibition (116). TMAO, a gut-derived metabolite, remains a key contributor to renal injury in DKD. Elevated TMAO levels correlate strongly with glomerular filtration rate decline (117, 119). Natural polyphenols (e.g., resveratrol, curcumin) suppress TMAO generation by inhibiting microbial trimethylamine (TMA) production and hepatic flavin monooxygenase activity, thereby mitigating renal oxidative stress and inflammation (17, 114).
Collectively, TCM-based modulation of the gut-kidney axis operates through a “microbiota-metabolite-inflammation” cascade (1): Correction of dysbiosis enriches beneficial taxa (2); Restoration of gut barrier reduces toxin translocation (3); Downregulation of inflammatory pathways (e.g., NLRP3, AhR) alleviates renal fibrosis (120, 121). The multitargeted actions of natural compounds constitute their primary advantage: they concurrently enhance microbial diversity (e.g., reducing Enterobacteriaceae while increasing SCFA producers), directly scavenge uremic toxins, and modulate host immune pathways (e.g., NLRP3 inflammasome suppression). These findings underscore microbiota–kidney axis-targeted interventions using natural compounds as a promising strategy for DKD management (3, 114, 118).
4.4.2 Potential of traditional Chinese medicine interventions
TCM has demonstrated distinct advantages in the regulation of GM for the management of DN. Mounting evidence highlights that TCM interventions target intestinal-derived metabolites (e.g., SCFAs, tryptophan derivatives, and LPS) through microbiota remodeling, thereby ameliorating gut-kidney axis dysregulation (116, 122). Owing to its multi-component and multi-target characteristics, TCM can simultaneously enhance intestinal barrier integrity, suppress the release of inflammatory mediators, and attenuate renal injury. Accumulating evidence from clinical and preclinical studies suggests that the therapeutic effects of TCM compound prescriptions are primarily mediated by modulation of gut microbial composition and SCFA metabolism.
Recent studies elucidate that TCM formulas significantly modulate tryptophan metabolism pathways. For instance, Tang Shen Formula (TSF) reduces accumulation of uremic toxins (IS and p-CS) by enriching Lactobacillus and Bifidobacterium, while increasing serum indole-3-aldehyde (IAld)—an AHR ligand that inhibits renal inflammation and fibrosis (1, 3). Similarly, Shenyan Kangfu Tablet downregulates gut-derived TMAO and suppresses NLRP3 inflammasome activation via the LPS-TLR4 pathway (55). For example, Shenqi Dihuang Decoction has been shown to increase the abundance of Roseburia in the GM, thereby promoting the production of SCFAs such as butyrate. This modulation improves intestinal barrier function, reduces pro-inflammatory cytokines such as TNF-α and IL-6, and effectively alleviates renal fibrosis (123, 124). Notably, natural compounds like Thonningianin A (from Penthorum chinense) ameliorate intestinal barrier impairment by restoring tight junction proteins (claudin-1, occludin, ZO-1), subsequently reducing fecal and serum LPS levels and inhibiting renal NLRP3/ASC/caspase-1 signaling (125). In clinical practice, Huangkui Capsule has demonstrated efficacy in lowering serum creatinine and blood urea nitrogen levels. The underlying mechanism is closely related to the inhibition of gut-derived uremic toxin generation, as well as the suppression of inflammation, consequently mitigating renal damage (110, 126). A multicenter trial further confirms that Yi-Shen-Hua-Shi Granule reduces proteinuria by elevating anti-inflammatory indole-3-propionic acid (IPA) and decreasing pro-fibrotic kynurenine, highlighting the role of tryptophan metabolism in DN progression (116). Qiditangshen Granules, by targeting the GM–SCFA axis and activating receptors such as GPR43, can inhibit overactivation of the renin-angiotensin system (RAS), thereby reducing glomerulosclerosis and tubular injury (123, 126).
The holistic approach of TCM thus addresses the multifaceted pathophysiology of DN through coordinated regulation of gut microecology, microbial metabolite profiles (SCFAs, tryptophan derivatives, LPS), and immune homeostasis (123, 124, 126). Such interventions not only restore microbial diversity but also attenuate inflammation and oxidative stress by modulating metabolite-mediated pathways (e.g., AHR/NF-κB, NLRP3, RAS) (55, 116, 122, 125). Future research should aim to further elucidate the specific molecular mechanisms through which TCM regulates GM, with the goal of optimizing clinical interventions. (For details of relevant clinical TCM studies, see Table 1).
Table 1
| Corresponding author | Year | Study type | Sample size | Intervention type | Core mechanism | Key evidence source |
|---|---|---|---|---|---|---|
| Pan LM | 2024 | Review | Not Stated | Traditional Chinese Medicine (TCM) | Modulation of gut microbiota | Literature review and summary |
| Wang Y | 2024 | Review | Not Stated | No intervention | Gut microbiota dysbiosis, altered metabolites, immune-inflammation | Literature review and mechanistic discussion |
| Li X | 2024 | Review | Not Stated | TCM | Modulation of gut microbiota, metabolites, and related signaling pathways | Literature review and summary |
| Tian Z | 2023 | Bibliometric Analysis | 1009 publications | No intervention | No intervention | VOSviewer analysis of literature data |
| Dong Y | 2025 | Bibliometric Analysis | 1289 publications | No intervention | No intervention | CiteSpace & VOSviewer analysis of literature data |
| Zhang G | 2024 | Review with Evidence Mapping | 139 studies | TCM | No intervention | Summary and analysis of clinical evidence |
| Gong YX | 2025 | Review | Not Stated | Active components of TCM | Inhibition of renal tubular epithelial cell apoptosis | Literature review and summary |
| Feng Z | 2025 | Bibliometric Analysis | 1585 publications | TCM-mediated gut microbiota regulation | No intervention | CiteSpace & VOSviewer analysis of literature data |
| Du J | 2024 | Bibliometric Analysis | 711 publications | TCM | No intervention | VOSviewer & CiteSpace analysis of literature data |
| Wang Y | 2024 | Review | Not Stated | TCM, Gut microbiota | Gut-lung axis regulation | Literature review and mechanistic discussion |
| Wu J | 2023 | Preclinical Study | Non-obese diabetic (NOD) mice | Abelmoschus manihot | Modulation of gut microbiota and circulating metabolites | 16S rRNA sequencing, metabolomics |
| Chang H | 2024 | Review | Not Stated | Chinese herbal medicine | No intervention | Review of clinical evidence and potential mechanisms |
| Xue M | 2025 | Preclinical Study | db/db mice | Danggui Buxue decoction | Regulation of autophagy via the miR-27a/PI3K/AKT pathway | Animal model experiments |
| Liu Y | 2022 | Network Pharmacology | N/A | Yishen capsules | Multi-target, multi-pathway regulation | Analysis of network pharmacology databases and software |
| Xu J | 2024 | Review | Not Stated | Edible TCM | Modulation of gut microbiota metabolites | Literature review and summary |
| Xu D | 2024 | Review | Not Stated | TCM | Modulation of gut microbiota and the microbiota-gut-x axis | Literature review and summary |
| Han J | 2022 | Review | Not Stated | Herbal medicine | Modulation of gut microbiota | Literature review and summary |
| Qin Y | 2024 | Systematic Review & Meta-analysis | 30 RCTs (2306 patients) | TCM decoction | No intervention | Meta-analysis of randomized controlled trials (RCTs) |
| Gao S | 2024 | Preclinical Study | db/db mice | Jiang Tang San Hao Formula | Affecting the gut-microbiota-brain axis | 16S rRNA sequencing, metabolomics, behavioral tests |
| Zhang L | 2024 | Review | Not Stated | TCM | Adjusting gut microbiota to improve immune imbalance | Literature review and summary |
| Chen Y | 2023 | Review | Not Stated | TCM | Gut microbiota-based therapy against bacteria | Literature review and summary |
| Tao P | 2025 | Network Pharmacology | N/A | Gut microbiota metabolites | Synergistic effects of multi-component, multi-target interactions | Analysis of databases such as TCMSP and DrugBank |
| Yang J | 2023 | Review | Not Stated | Gut microbiota metabolites | No intervention | Literature review and mechanistic discussion |
| Hui S | 2024 | Preclinical Study | db/db mice | Resveratrol | Modulation of the gut microbiota-short-chain fatty acids (SCFAs) axis | 16S rRNA sequencing, targeted metabolomics |
Summary of literature on the modulation of gut microbiota by natural compounds and Traditional Chinese Medicine (TCM).
4.5 Potential value of FMT in DN
FMT has demonstrated significant therapeutic potential in the management of DN by facilitating the reconstruction of a healthy gut microbial ecosystem and modulating relevant metabolic and immune pathways (36, 127). The current evidence is summarized below from aspects of efficacy in animal models and the progress and challenges of clinical translation.
4.5.1 Efficacy in animal models
Experimental studies have provided robust evidence that FMT can improve renal outcomes and restore intestinal microbial homeostasis in DN models. For instance, in DN animal models such as 5/6 nephrectomized rats, FMT intervention has been shown to remodel gut microbial composition, ameliorate dysbiosis, and attenuate glomerulosclerosis and interstitial fibrosis by regulating metabolic pathways such as serum amino acid metabolism (128, 129). Similarly, in type 1 diabetes models, FMT improved the overall metabolic profile through modulation of the gut-metabolic axis, which indirectly reduced the risk of renal injury (130, 131). These findings underscore the role of FMT in restoring microbial diversity and suppressing DN progression, while also providing mechanistic insights for future investigations (17, 129).
4.5.2 Clinical translation: advances and challenges
Preliminary clinical data suggest that FMT may delay the progression of CKD, including diabetes-related CKD, highlighting its potential as an alternative approach to restore GM balance (132, 133). However, several key obstacles remain to be overcome in clinical practice:
(1) Safety concerns: The risk of infection associated with FMT procedures and uncertainty regarding long-term safety profiles represent major challenges that require careful evaluation and ongoing surveillance (128, 134). (2) Standardization: The absence of unified protocols for the preparation and delivery of FMT, such as microbial capsules, hampers the reproducibility and consistency of therapeutic outcomes across studies (135, 136). (3) Mechanistic ambiguity: Immunoregulatory and metabolic benefits observed in preclinical models need further substantiation, as the causal pathways and long-term efficacy in humans remain unexplored. High-quality randomized controlled trials are warranted to address these gaps (18).
In summary, while FMT holds considerable promise for the treatment of DN—as substantiated by preclinical evidence—its broader application requires further optimization of safety, standardization of protocols, and mechanistic clarification. Future research combining multi-omics approaches with large-scale clinical studies will be essential to advance the development of precise FMT-based interventions for DN management.
5 Current limitations and challenges in research
5.1 Ambiguity of causal relationships
The ambiguity of causal relationships stands as a central bottleneck in current research on DN and GM, significantly impeding clinical translation. This ambiguity arises from several layers of complexity.
Firstly, most available evidence is derived from observational studies, which inherently lack the ability to establish definitive causal links. For example, cross-sectional designs have reported alterations in GM abundance among DN patients (such as fluctuations in specific microbial taxa) (26, 28). However, such methodologies are inherently limited in clarifying the directionality of causation or adequately accounting for confounding factors such as dietary patterns or pharmacotherapy (18). As highlighted in the literature, “despite emerging evidence supporting an association, the causal relationship between them hasn’t been clarified yet”, underscoring that correlational findings alone are insufficient to define the underlying mechanism of causality (18).
Secondly, the confounding effect of extraneous variables and the possibility of reverse causality further exacerbate this uncertainty. Factors such as metabolic status may generate spurious associations (137), while reverse causality—whereby DN may induce gut microbial dysbiosis—complicates the interpretation of the microbiota as either a pathogenic driver or a biomarker (18, 137). This is exemplified by reverse MR analyses: as reported in the literature (18), such analyses assessing the impact of DN on GM “did not identify any significant associations”, suggesting that observed correlations could be driven by reverse causality rather than direct causative effects.
Finally, while MR represents a promising tool for disentangling causality, practical limitations persist. MR leverages genetic variants (e.g., GM-related SNPs) to minimize confounding (18, 138); however, its utility is constrained by the strength and heterogeneity of the instrumental variables. For instance, forward MR analyses have identified putative causal effects of certain gut microbes (18, 124), yet reverse MR analyses often yield null results, highlighting ongoing difficulties in resolving the precise directionality of the relationship. In summary, such causal ambiguity remains a major limitation hindering the development of microbiota-targeted therapies in DN, emphasizing the need to integrate more robust MR approaches for advanced causal inference.
5.2 Fragmentation of mechanistic studies: a systemic lack of integration among metabolic, immune, and barrier pathways
Despite considerable advances in DN research, the mechanistic understanding remains fragmented, particularly regarding the integration of metabolic, immune, and barrier pathways. Current studies predominantly address these axes independently, resulting in a lack of comprehensive and unified framework for DN pathogenesis.
Research on metabolic pathways exemplifies this segregation. While GM-derived metabolites such as pyrroline and glycine-conjugated bile acids have been causally linked to DN progression (139), these investigations focus primarily on the microbiota–metabolite axis without addressing interactions with immune responses or barrier integrity. Potential synergistic mechanisms remain largely unexplored (51, 139). Similarly, immunoinflammatory activation, including NLRP3 inflammasome activation and macrophage infiltration, represents a well-established driver of DN (140, 141). However, most studies assess immune factors in isolation, seldom considering how immune cell dynamics interact with intestinal or renal barrier disturbances or fluctuations in microbial metabolites (139, 142). Although gut-kidney axis activation has been shown to modulate immunity (143), comprehensive elucidation of how metabolites modulate immune signaling through TLR pathways leading to barrier dysfunction remains lacking (139, 141). The investigation of barrier integrity follows a similarly narrow approach. Impairment of tight junction proteins and resulting dysfunctions of intestinal and glomerular barriers are hallmarks of DN (144, 145). Yet current research often fails to dissect how barrier injury is co-regulated by both microbial metabolites (such as acylcarnitines) and immune-inflammatory signals (such as NF-κB activation) (146, 147). For instance, while glomerular barrier damage is associated with dysregulated endothelial S1pR1 signaling (145) and certain gut-derived metabolites can restore intestinal barrier function via the AHR receptor (148), integrated models addressing cross-talk between barriers in DN are still missing.
This fragmented approach generates significant limitations in both mechanistic understanding and therapeutic development. Single-pathway analyses, while facilitating understanding of specific molecular events—such as brown adipose tissue-derived NRG4 suppressing podocyte apoptosis through the Akt-GSK3β pathway (149) —overlook the broader impact of cross-talk among metabolic, immune, and barrier pathways (150, 151). Consequently, therapies targeting isolated pathways, such as PI3K-Akt or HIF-1 signaling, often fail to achieve optimal efficacy due to neglect of feedback and interaction between pathways (152, 153). For example, while PFKFB3 contributes to glomerular barrier protection (145), its interactions with microbial metabolic profiles, including circulating acylcarnitine levels (139), remain absent from therapeutic considerations.
In summary, the current fragmented approach constrains both mechanistic insights and identification of effective therapeutic targets. Comprehensive, multi-dimensional studies integrating metabolism, immunity, and barrier biology are urgently needed to clarify DN pathogenesis and guide the development of more effective interventions.
5.3 Barriers to clinical translation
The clinical translation of GM-based interventions for DN faces significant hurdles, primarily arising from the disconnect between findings in animal models and human clinical realities, as well as the lack of longitudinal, stage-specific data in disease progression. These barriers substantially impede the transition from bench to bedside.
One core challenge lies in the differences between animal and human GM, which restrict the reproducibility and translatability of preclinical findings. The GM composition in common animal models—such as rodents—differs markedly from that of humans, resulting in inconsistencies in intervention efficacy. Mechanisms such as short-chain fatty acid metabolism or intestinal barrier repair, which often demonstrate efficacy in animal studies, may not be directly reproduced in clinical studies due to these interspecies differences (154, 155). This limitation raises concerns regarding the extrapolation of preclinical results to human disease contexts.
Additionally, there is a conspicuous lack of systematic, longitudinal data capturing the dynamic shifts of GM throughout the various stages of DN. Most current studies are cross-sectional in design, typically comparing diabetic patients to healthy controls, and fail to longitudinally track changes in GM across clinical stages—from microalbuminuria to established renal failure (156, 157). This gap obscures the understanding of how microbial communities evolve with disease progression and limits the identification of stage-specific therapeutic targets.
The absence of robust, disease stage-oriented microbial evolution data hinders the development of tailored intervention strategies—for example, metabolic modulation in early-stage DN versus barrier restoration in advanced stages. Consequently, the true potential of personalized, microbiota-based therapies remains largely unrealized. The clinical translation workflow is summarized in Figure 3.
Figure 3
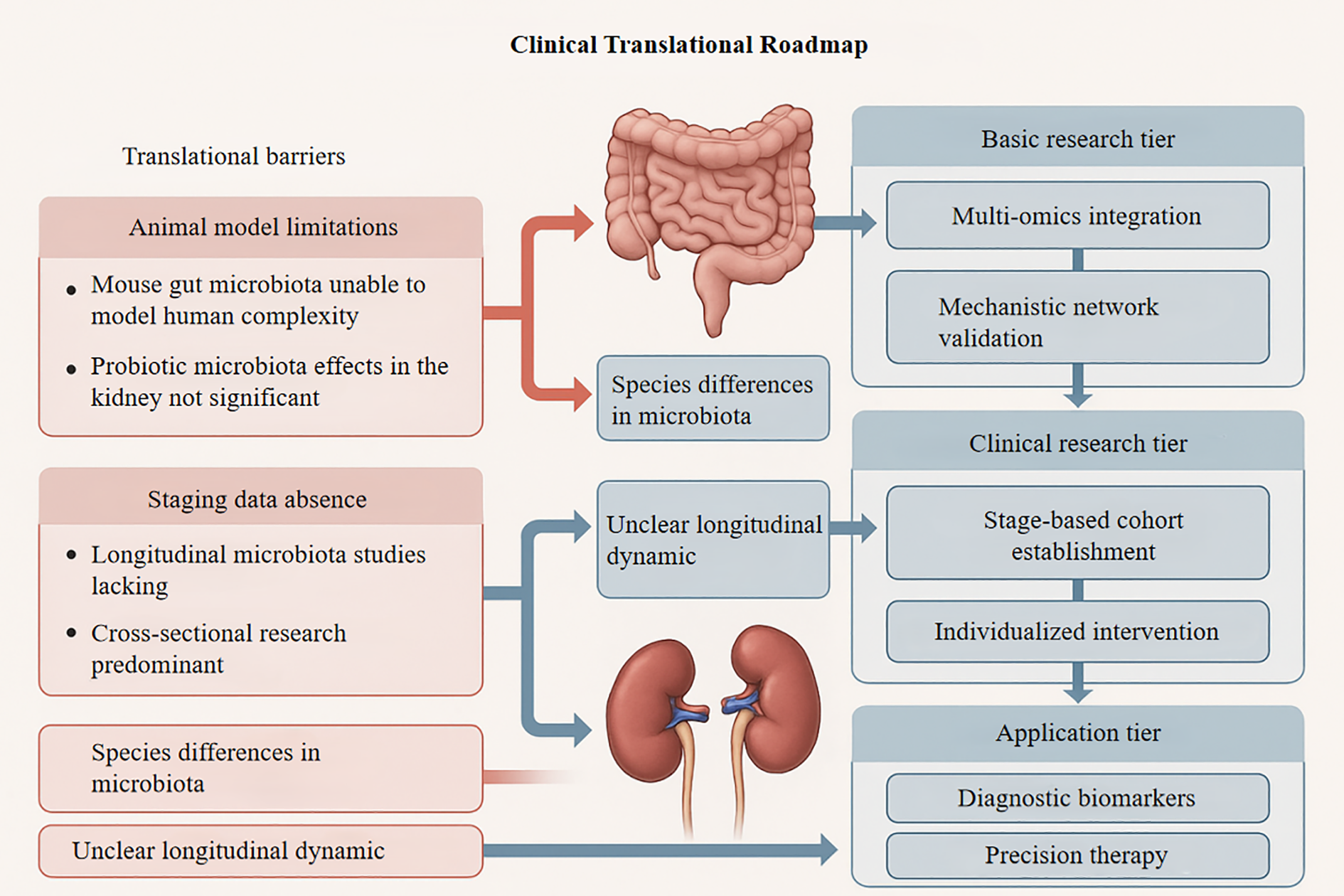
Roadmap for translating microbiota- kidney research into clinical applications, with key barriers identified. This schematic outlines a three-tiered framework for advancing gut-kidney axis research. Left panel highlights translational barriers (red boxes): (i) Animal model limitations (non-human microbiota complexity; limited probiotic efficacy in renal contexts), (ii) Staging data gaps (predominance of cross- sectional studies; longitudinal data scarcity), and (iii) Unresolved microbial dynamics (species-specific variations; undefined longitudinal trajectories). Center depicts gut- kidney anatomical targets. Right vertical axis details progressive research phases: Basic research (multi-omics integration; mechanistic network validation) → Clinical research (stage- stratified cohorts; individualized interventions) → Application (diagnostic biomarkers; precision therapies). Blue arrows denote barrier impact on translational stages. Kidney protection seen in animal models using probiotics, antibiotics, or fecal transplants often proves less effective in human trials. This is partly because animal models inadequately capture the complexity of the human gut- kidney axis and host-microbe co-evolution, making therapeutic targets identified in animals less reliable for clinical application.
6 Innovation and future directions
With the growing recognition of the GM’s pivotal role in DN, innovative research is increasingly oriented toward integrating mechanistic insights, multi-omics approaches, and personalized interventions. The following subsections systematically summarize key innovations and emerging trends in this field.
6.1 Integration of gut–kidney axis mechanisms and targeted interventions
At the mechanistic level, recent studies have, for the first time, systematically linked gut dysbiosis in DN with targeted intervention strategies. Specifically, current literature has demonstrated that gut microbial imbalance exacerbates glomerulosclerosis and tubular injury through both metabolic products (e.g., uremic toxins) and immune-inflammatory pathways (e.g., activation of Toll-like receptors) (17, 44). On this basis, targeted interventions aimed at restoring the gut–kidney axis—such as probiotics and prebiotics—may modulate microbial metabolism (e.g., enhancing short-chain fatty acid synthesis) and improve renal injury biomarkers, thereby attenuating kidney damage (158, 159).
6.2 Multi-omics research paradigms across scales
Multi-omics approaches have ushered in a new framework for dissecting the mechanisms of GM in DN. Metagenomics can reveal shifts in microbial community structure among DN patients, such as alterations in the abundance of specific genera (23, 51). However, coupling with metabolomics is required to delineate the specific nephrotoxic effects of microbial metabolites like indoxyl sulfate (79). Moreover, metatranscriptomics allows for the identification of key microbe–host interaction pathways, including the regulation of inflammatory responses via the AhR pathway (18, 160), while single-cell techniques enable detailed analysis of intestinal immune cells (e.g., Th17/Treg) and their association with renal immune infiltration (160, 161). Collectively, integrated multi-omics approaches hold promise for elucidating cross-scale regulatory networks linking microbial structure, function, and host physiology (79, 162).
6.3 Stage-specific and precision intervention strategies
Strategic interventions must be tailored to distinct DN stages. In early DN, supplementation with short-chain fatty acid-producing bacteria (such as Butyricicoccus) or their metabolic products (such as butyrate) has the potential to improve insulin resistance and alleviate glomerular hyperfiltration (158, 159). In contrast, late-stage interventions require the elimination of toxin-producing bacteria (such as Escherichia species) and blockade of toxin absorption (44). Nonetheless, major obstacles remain, including inter-individual variability in response to microbiota-based therapies due to genetic and dietary factors, as well as incomplete evidence regarding long-term safety (35, 163).
6.4 Potential of advanced technologies
Frontier technologies offer new avenues for GM-targeted therapy. Phage therapy can specifically lyse pathogenic bacteria (such as endotoxin-producing Klebsiella), and animal models have confirmed its ability to reduce serum LPS levels and proteinuria (25). Meanwhile, engineered probiotic therapies—where symbiotic bacteria are modified to express therapeutic molecules such as antioxidant enzymes—demonstrate potential in locally mitigating renal oxidative stress (45, 159). Going forward, the development of synergistic systems (e.g., coordinated phage–engineered bacteria interventions) may enable dynamic modulation of gut microbial ecology (25).
In terms of clinical translation, future directions include stratified and personalized probiotic regimens based on enterotype (161, 162), incorporation of microbiota-derived biomarkers (such as fecal butyrate) as efficacy indicators (164), and the application of artificial intelligence models to integrate multi-omics data for predictive risk assessment of DN progression (162).
7 Conclusion
The gut-kidney axis plays a pivotal role in DN pathogenesis through distinct yet interconnected mechanisms. Gut dysbiosis drives DN progression via three pathways: metabolic dysfunction through loss of protective SCFAs and accumulation of nephrotoxic compounds like indoxyl sulfate; compromised intestinal barrier integrity leading to endotoxin translocation and systemic inflammation; and immune dysregulation affecting Th17/Treg balance and macrophage polarization. MR studies have validated specific microbial signatures associated with DN risk, including protective effects of Bacteroidota and detrimental impacts of reduced Akkermansia abundance.
Current therapeutic approaches demonstrate promise but require optimization. Probiotic supplementation and TCM interventions have shown benefits in improving renal function markers and restoring gut barrier integrity, yet standardization of protocols remains a critical challenge. Future research priorities include elucidating molecular mechanisms linking specific bacterial strains and their metabolites to renal pathology, integrating multi-omics approaches for patient stratification, and developing DN-specific microbial biomarkers for early diagnosis.
Advancing the field necessitates interdisciplinary collaboration between microbiome researchers, nephrologists, and nutrition scientists to develop personalized therapeutic strategies. Key challenges include addressing regional microbiota heterogeneity and the complexity of host-microbe interactions through large-scale prospective cohorts and mechanistically driven randomized controlled trials. As our understanding of the gut-kidney axis deepens, microbiota-targeted interventions represent a promising frontier for DN management, offering potential for both prevention and treatment through precision medicine approaches.
Statements
Author contributions
HJ: Writing – original draft. XW: Writing – original draft, Funding acquisition. WeiZ: Visualization, Writing – review & editing. ZH: Visualization, Writing – review & editing. WenZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors declare that they received funding support for research, writing, and/or publication of this article. This work was sponsored by research grants from the Hangzhou Science and Technology Bureau (20241029Y164).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. For the 3D view of the kidneys in the article’s generated images, we used ChatGPT 4.0, developed by OpenAI.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Das S Gnanasambandan R . Intestinal microbiome diversity of diabetic and non-diabetic kidney disease: Current status and future perspective. Life Sci. (2023) 316:121414. doi: 10.1016/j.lfs.2023.121414
2
Zhang Y Zhao L Jia Y Zhang X Han Y Lu P et al . Genetic evidence for the causal relationship between gut microbiota and diabetic kidney disease: A bidirectional, two-sample mendelian randomisation study. J Diabetes Res. (2024) 2024:4545595. doi: 10.1155/2024/4545595
3
Li Q Xie S Liu Y Yue W Wang L Liang Y et al . Gut microbiota profiling reflects the renal dysfunction and psychological distress in patients with diabetic kidney disease. Front Endocrinol (Lausanne). (2024) 15:1410295. doi: 10.3389/fendo.2024.1410295
4
Coll E Cigarran S Portolés J Cases A . Gut dysbiosis and its role in the anemia of chronic kidney disease. Toxins (Basel). (2024) 16:495. doi: 10.3390/toxins16110495
5
Das S Devi Rajeswari V Venkatraman G Elumalai R Dhanasekaran S Ramanathan G . Current updates on metabolites and its interlinked pathways as biomarkers for diabetic kidney disease: A systematic review. Transl Res. (2024) 265:71–87. doi: 10.1016/j.trsl.2023.11.002
6
Yan H Zhang Y Lin X Huang J Zhang F Chen C et al . Resveratrol improves diabetic kidney disease by modulating the gut microbiota-short chain fatty acids axis in db/db mice. Int J Food Sci Nutr. (2024) 75:264–76. doi: 10.1080/09637486.2024.2303041
7
Wu X Zhao L Zhang Y Li K Yang J . The role and mechanism of the gut microbiota in the development and treatment of diabetic kidney disease. Front Physiol. (2023) 14:1166685. doi: 10.3389/fphys.2023.1166685
8
Lee TH Chen JJ Wu CY Lin TY Hung SC Yang HY . Immunosenescence, gut dysbiosis, and chronic kidney disease: Interplay and implications for clinical management. BioMed J. (2024) 47:100638. doi: 10.1016/j.bj.2023.100638
9
Zhang Y Qing J Saed YA Li Y . Gut microbiota implication in diabetic kidney disease: mechanisms and novel therapeutic strategies. Ren Fail. (2025) 47:2517402. doi: 10.1080/0886022X.2025.2517402
10
Chen J Chen B Lin B Huang Y Li J Li J et al . The role of gut microbiota in prostate inflammation and benign prostatic hyperplasia and its therapeutic implications. Heliyon. (2024) 10:e38302. doi: 10.1016/j.heliyon.2024.e38302
11
Mao ZH Gao ZX Liu DW Liu ZS Wu P . Gut microbiota and its metabolites - molecular mechanisms and management strategies in diabetic kidney disease. Front Immunol. (2023) 14:1124704. doi: 10.3389/fimmu.2023.1124704
12
Huang L Wu W Wang X . Analysis of the microecological mechanism of diabetic kidney disease based on the theory of ‘gut-kidney axis’: A systematic review. Open Life Sci. (2024) 19:20220909. doi: 10.1515/biol-2022-0909
13
Balint L Socaciu C Socaciu AI Vlad A Gadalean F Bob F et al . Metabolite profiling of the gut-renal-cerebral axis reveals a particular pattern in early diabetic kidney disease in T2DM patients. Int J Mol Sci. (2023) 24:6212. doi: 10.3390/ijms24076212
14
Zhou T Xu H Cheng X He Y Ren Q Li D et al . Sodium butyrate attenuates diabetic kidney disease partially via histone butyrylation modification. Mediators Inflamm. (2022) 2022:7643322. doi: 10.1155/2022/7643322
15
Cai C Cheng W Shi T Liao Y Zhou M Liao Z . Rutin alleviates colon lesions and regulates gut microbiota in diabetic mice. Sci Rep. (2023) 13:4897. doi: 10.1038/s41598-023-31647-z
16
Ni Y Zheng L Nan S Ke L Fu Z Jin J . Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim Biophys Sin (Shanghai). (2022) 54:1406–20. doi: 10.3724/abbs.2022140
17
Cheng G Liu Y Guo R Wang H Zhang W Wang Y . Molecular mechanisms of gut microbiota in diabetic nephropathy. Diabetes Res Clin Pract. (2024) 213:111726. doi: 10.1016/j.diabres.2024.111726
18
Chen K Wang X Shang Z Li Q Yao W Guo S et al . Exploring the causal effects of gut microbiota on diabetic nephropathy: a two-sample Mendelian randomization study. Comb Chem High Throughput Screen. (2025) 28:1026–38. doi: 10.2174/0113862073311197240425073859
19
Song S Ning L Yu J . Elucidating the causal relationship between gut microbiota, metabolites, and diabetic nephropathy in European patients: Revelations from genome-wide bidirectional Mendelian randomization analysis. Front Endocrinol (Lausanne). (2025) 15:1391891. doi: 10.3389/fendo.2024.1391891
20
Hong J Fu T Liu W Du Y Bu J Wei G et al . Specific alternation of gut microbiota and the role of Ruminococcus gnavus in the development of diabetic nephropathy. J Microbiol Biotechnol. (2024) 34:547–61. doi: 10.4014/jmb.2310.10028
21
Tsai CY Liu PY Huang MC Chang CI Chen HY Chou YH et al . Abundance of Prevotella copri in gut microbiota is inversely related to a healthy diet in patients with type 2 diabetes. J Food Drug Anal. (2023) 31:599–608. doi: 10.38212/2224-6614.3484
22
Hu Y Ni X Chen Q Qu Y Chen K Zhu G et al . Predicting diabetic kidney disease with serum metabolomics and gut microbiota. Sci Rep. (2025) 15:12179. doi: 10.1038/s41598-025-91281-9
23
Zhang L Wang Z Zhang X Zhao L Chu J Li H et al . Alterations of the gut microbiota in patients with diabetic nephropathy. Microbiol Spectr. (2022) 10:e0032422. doi: 10.1128/spectrum.00324-22
24
Lin R Chen R . Exploring the causal connection: insights into diabetic nephropathy and gut microbiota from whole-genome sequencing databases. Ren Fail. (2024) 46:2385065. doi: 10.1080/0886022X.2024.2385065
25
Yan W Ge Y Wang L Wang Y He D . Causal relationship of gut microbiota with diabetic nephropathy: a Mendelian randomization analysis. Front Microbiol. (2024) 14:1281361. doi: 10.3389/fmicb.2023.1281361
26
Yan S Wang H Feng B Ye L Chen A . Causal relationship between gut microbiota and diabetic nephropathy: a two-sample Mendelian randomization study. Front Immunol. (2024) 15:1332757. doi: 10.3389/fimmu.2024.1332757
27
Jin Y Han C Yang D Gao S . Association between gut microbiota and diabetic nephropathy: a mendelian randomization study. Front Microbiol. (2024) 15:1309871. doi: 10.3389/fmicb.2024.1309871
28
Fang Y Zhang Y Liu Q Zheng Z Ren C Zhang X . Assessing the causal relationship between gut microbiota and diabetic nephropathy: insights from two-sample Mendelian randomization. Front Endocrinol (Lausanne). (2024) 15:1329954. doi: 10.3389/fendo.2024.1329954
29
Wu IW Liao YC Tsai TH Lin CH Shen ZQ Chan YH et al . Machine-learning assisted discovery unveils novel interplay between gut microbiota and host metabolic disturbance in diabetic kidney disease. Gut Microbes. (2025) 17:2473506. doi: 10.1080/19490976.2025.2473506
30
Han S Chen M Cheng P Zhang Z Lu Y Xu Y et al . A systematic review and meta-analysis of gut microbiota in diabetic kidney disease: Comparisons with diabetes mellitus, non-diabetic kidney disease, and healthy individuals. Front Endocrinol (Lausanne). (2022) 13:1018093. doi: 10.3389/fendo.2022.1018093
31
Song X Cui J Li S Huang B . Causal relationships between gut microbiota, metabolites, and diabetic nephropathy: insights from a two-sample Mendelian randomization analysis. Int J Nephrol Renovasc Dis. (2024) 17:319–32. doi: 10.2147/IJNRD.S489074
32
Liu X Zhang M Wang X Liu P Wang L Li Y et al . Fecal microbiota transplantation restores normal fecal composition and delays Malignant development of mild chronic kidney disease in rats. Front Microbiol. (2022) 13:1037257. doi: 10.3389/fmicb.2022.1037257
33
Kim JE Nam H Park JI Cho H Lee J Kim HE et al . Gut microbial genes and metabolism for methionine and branched-chain amino acids in diabetic nephropathy. Microbiol Spectr. (2023) 11:e0234422. doi: 10.1128/spectrum.02344-22
34
Li YJ Ma J Loh YW Chadban SJ Wu H . Short-chain fatty acids directly exert anti-inflammatory responses in podocytes and tubular epithelial cells exposed to high glucose. Front Cell Dev Biol. (2023) 11:1182570. doi: 10.3389/fcell.2023.1182570
35
Tian E Wang F Zhao L Sun Y Yang J . The pathogenic role of intestinal flora metabolites in diabetic nephropathy. Front Physiol. (2023) 14:1231621. doi: 10.3389/fphys.2023.1231621
36
Qumsani AT . Gut microbiome engineering for diabetic kidney disease prevention: a Lactobacillus rhamnosus GG intervention study. Biol (Basel). (2025) 14:723. doi: 10.3390/biology14060723
37
Shukla PK Rao RG Meena AS Giorgianni F Lee SC Raju P et al . Paneth cell dysfunction in radiation injury and radiomitigation by human α-defensin 5. Front Immunol. (2023) 14:1174140. doi: 10.3389/fimmu.2023.1174140
38
Erkert L Gamez-Belmonte R Kabisch M Schödel L Patankar JV Gonzalez-Acera M et al . TRR241 IBDome Consortium; Neurath MF, Wirtz S, Becker C. Alzheimer’s disease-related presenilins are key to intestinal epithelial cell function and gut immune homoeostasis. Gut. (2024) 73:1618–31. doi: 10.1136/gutjnl-2023-331622
39
Ferreira YAM Jamar G Estadella D Pisani LP . Proanthocyanidins in grape seeds and their role in gut microbiota-white adipose tissue axis. Food Chem. (2023) 404. doi: 10.1016/j.foodchem.2022.134405
40
Rao PL Shen YH Song YJ Xu Y Xu HX . Prunella vulgaris L. attenuates gut dysbiosis and endotoxin leakage against alcoholic liver disease. J Ethnopharmacol. (2024) 319:117237. doi: 10.1016/j.jep.2023.117237
41
Koshida T Gohda T Kaga N Taka H Shimozawa K Murakoshi M et al . Inappropriate diet and hygiene status affect the progression of diabetic kidney disease by causing dysbiosis. Nutr. (2025) 131:112633. doi: 10.1016/j.nut.2024.112633
42
Li J Lv JL Cao XY Zhang HP Tan YJ Chu T et al . Gut microbiota dysbiosis as an inflammaging condition that regulates obesity-related retinopathy and nephropathy. Front Microbiol. (2022) 13:1040846. doi: 10.3389/fmicb.2022.1040846
43
Kong J Yang J He C Zhou B Fang S Salinas M et al . Regulation of endotoxemia through the gut microbiota: The role of the Mediterranean diet and its components. APMIS. (2024) 132:948–55. doi: 10.1111/apm.13473
44
Liu J Guo M Yuan X Fan X Wang J Jiao X . Gut microbiota and their metabolites: the hidden driver of diabetic nephropathy? Unveiling gut microbe’s role in DN. J Diabetes. (2025) 17:e70068. doi: 10.1111/1753-0407.70068
45
Chen PP Zhang JX Li XQ Li L Wu QY Liu L et al . Outer membrane vesicles derived from gut microbiota mediate tubulointerstitial inflammation: a potential new mechanism for diabetic kidney disease. Theranostics. (2023) 13:3988–4003. doi: 10.7150/thno.84650
46
Mázala-de-Oliveira T Jannini de Sá YAP Carvalho VF . Impact of gut-peripheral nervous system axis on the development of diabetic neuropathy. Mem Inst Oswaldo Cruz. (2023) 118:e220197. doi: 10.1590/0074-02760220197
47
Al-Ghamdi SB . Probiotics as renal guardians: modulating gut microbiota to combat diabetes-induced kidney damage. Biol (Basel). (2025) 14:122. doi: 10.3390/biology14020122
48
Gali S Kundu A Sharma S Ahn MY Puia Z Kumar V et al . Therapeutic potential of bark extracts from Macaranga denticulata on renal fibrosis in streptozotocin-induced diabetic rats. J Toxicol Environ Health A. (2024) 87:911–33. doi: 10.1080/15287394.2024.2394586
49
Ajiboye BO Famusiwa CD Nifemi DM Ayodele BM Akinlolu OS Fatoki TH et al . Nephroprotective effect of hibiscus sabdariffa leaf flavonoid extracts via KIM-1 and TGF-1β Signaling pathways in streptozotocin-induced rats. ACS Omega. (2024) 9:19334–44. doi: 10.1021/acsomega.4c00254
50
Putra IMWA Fakhrudin N Nurrochmad A Wahyuono S . A review of medicinal plants with renoprotective activity in diabetic nephropathy animal models. Life (Basel). (2023) 13:560. doi: 10.3390/life13020560
51
Chu C Behera TR Huang Y Qiu W Chen J Shen Q . Research progress of gut microbiome and diabetic nephropathy. Front Med (Lausanne). (2024) 11:1490314. doi: 10.3389/fmed.2024.1490314
52
Tokarek J Budny E Saar M Kućmierz J Młynarska E Rysz J et al . Does the composition of gut microbiota affect hypertension? Molecular mechanisms involved in increasing blood pressure. Int J Mol Sci. (2023) 24:1377. doi: 10.3390/ijms24021377
53
Chen CY Ho HC . Roles of gut microbes in metabolic-associated fatty liver disease. Tzu Chi Med J. (2023) 35:279–89. doi: 10.4103/tcmj.tcmj_86_23
54
Cha RH . Pharmacologic therapeutics in sarcopenia with chronic kidney disease. Kidney Res Clin Pract. (2024) 43:143–55. doi: 10.23876/j.krcp.23.094
55
Zhao H Zhao T Li P . Gut microbiota-derived metabolites: A new perspective of traditional chinese medicine against diabetic kidney disease. Integr Med Nephrol Andrology. (2024) 11:e23–00024. doi: 10.1097/IMNA-D-23-00024
56
Wiefels MD Furar E Eshraghi RS Mittal J Memis I Moosa M et al . Targeting gut dysbiosis and microbiome metabolites for the development of therapeutic modalities for neurological disorders. Curr Neuropharmacol. (2024) 22:123–39. doi: 10.2174/1570159X20666221003085508
57
Yan W Wen S Zhou L . Effect of intestinal flora on hyperuricemia-induced chronic kidney injury in type 2 diabetic patients and the therapeutic mechanism of new anti-diabetic prescription medications. Diabetes Metab Syndr Obes. (2023) 16:3029–44. doi: 10.2147/DMSO.S429068
58
Jiang L Sun XY Wang SQ Liu YL Lu LJ Wu WH et al . Indoxyl sulphate-TNFα axis mediates uremic encephalopathy in rodent acute kidney injury. Acta Pharmacol Sin. (2024) 45:1406–24. doi: 10.1038/s41401-024-01251-6
59
Stepanova N Driianska V Korol L Snisar L . Association between serum total indoxyl sulfate, intraperitoneal inflammation, and peritoneal dialysis technique failure: a 3-year prospective cohort study. BMC Nephrol. (2024) 25:475. doi: 10.1186/s12882-024-03935-x
60
Kędzierska-Kapuza K Grudniewska A Durma A Małecki R Franek E Szczuko M . Dietary and nutritional strategies to prevent uremic toxin formation and slow the progression of diabetic kidney disease. J Clin Med. (2025) 14:4701. doi: 10.3390/jcm14134701
61
Tsuji K Uchida N Nakanoh H Fukushima K Haraguchi S Kitamura S et al . The gut-kidney axis in chronic kidney diseases. Diagnostics (Basel). (2024) 15:21. doi: 10.3390/diagnostics15010021
62
Altunkaynak HO Karaismailoglu E Massy ZA . The ability of AST-120 to lower the serum indoxyl sulfate level improves renal outcomes and the lipid profile in diabetic and nondiabetic animal models of chronic kidney disease: A meta-analysis. Toxins (Basel). (2024) 16:544. doi: 10.3390/toxins16120544
63
Czaja-Stolc S Potrykus M Ruszkowski J Styburski D Dębska-Ślizień A Małgorzewicz S . The associations between nutrition and circulating gut microbiota-derived uremic toxins in patients undergoing kidney replacement therapy: An observational, cross-sectional study. Clin Nutr ESPEN. (2025) 65:105–14. doi: 10.1016/j.clnesp.2024.11.022
64
Hu D Liu W Yu W Huang L Ji C Liu X et al . Psyllium seed husk regulates the gut microbiota and improves mucosal barrier injury in the colon to attenuate renal injury in 5/6 nephrectomy rats. Ren Fail. (2023) 45:2197076. doi: 10.1080/0886022X.2023.2197076
65
Ji Y Xiao Y Li S Fan Y Cai Y Yang B et al . Protective effect and mechanism of Xiaoyu Xiezhuo decoction on ischemia-reperfusion induced acute kidney injury based on gut-kidney crosstalk. Ren Fail. (2024) 46:2365982. doi: 10.1080/0886022X.2024.2365982
66
Zhu N Duan H Feng Y Xu W Shen J Wang K et al . Magnesium lithospermate B ameliorates diabetic nephropathy by suppressing the uremic toxin formation mediated by gut microbiota. Eur J Pharmacol. (2023) 953:175812. doi: 10.1016/j.ejphar.2023.175812
67
Duan J He L Deng W Lu M Zhai Y Pei F et al . Natural swietenine attenuates diabetic nephropathy by regulating the NF-κB/NLRP3/Caspase-1 signaling pathways: In vivo and in vitro study. Environ Toxicol. (2022) 37:2977–89. doi: 10.1002/tox.23653
68
Afsari Y Atabi F Aghelan Z Khazaie H Vakili Z Abtahi SH et al . Serum levels of 1,3-β-D-glucan is correlated with NLRP3 inflammasome activation and insomnia severity in people with chronic insomnia disorder. Sleep Med. (2025) 129:187–91. doi: 10.1016/j.sleep.2025.02.046
69
Yu T Luo L Xue J Tang W Wu X Yang F . Gut microbiota-NLRP3 inflammasome crosstalk in metabolic dysfunction-associated steatotic liver disease. Clin Res Hepatol Gastroenterol. (2024) 48:102458. doi: 10.1016/j.clinre.2024.102458
70
Huang H Zhou T He F Wen B Yang Y Zhong W et al . The gut microbiota improves reproductive dysfunction in obese mice by suppressing the NLRP3/ASC/caspase-1 axis. Future Microbiol. (2024) 19:1389–405. doi: 10.1080/17460913.2024.2386867
71
Kounatidis D Vallianou N Evangelopoulos A Vlahodimitris I Grivakou E Kotsi E et al . SGLT-2 inhibitors and the inflammasome: what’s next in the 21st century? Nutrients. (2023) 15:2294. doi: 10.3390/nu15102294
72
Zhao Z Liu W Cheng G Dong S Zhao Y Wu H et al . Knockdown of DAPK1 inhibits IL-1β-induced inflammation and cartilage degradation in human chondrocytes by modulating the PEDF-mediated NF-κB and NLRP3 inflammasome pathway. Innate Immun. (2024) 30:21–30. doi: 10.1177/17534259221086837
73
Zhou KL He YR Liu YJ Liu YM Xuan LZ Gu ZY et al . IL-17A/p38 signaling pathway induces alveolar epithelial cell pyroptosis and hyperpermeability in sepsis-induced acute lung injury by activating NLRP3 inflammasome. Adv Biol (Weinh). (2023) 7:e2300220. doi: 10.1002/adbi.202300220
74
Jiang R Zhou H Kong X Zhou Z . Reactive Oxygen Species Modulate Th17/Treg Balance in Chlamydia psittaci Pneumonia via NLRP3/IL-1β/Caspase-1 Pathway Differentiation. Folia Biol (Praha). (2024) 70:74–83. doi: 10.14712/fb2024070010074
75
Liu X Chen J Yue S Zhang C Song J Liang H et al . NLRP3-mediated IL-1β in regulating the imbalance between Th17 and Treg in experimental autoimmune prostatitis. Sci Rep. (2024) 14:18829. doi: 10.1038/s41598-024-69512-2
76
Yang X Hong C Guan T Zhang C Yang Y Xiao P et al . Periplaneta americana (L.) extract PAS840 promotes ischemic stroke recovery by inhibiting inflammasome activation. Biol (Basel). (2025) 14:589. doi: 10.3390/biology14060589
77
Zhang Z He Y Zhao M He X Zhou Z Yue Y et al . Qinlian hongqu decoction modulates FXR/TGR5/GLP-1 pathway to improve insulin resistance in NAFLD mice: bioinformatics and experimental study. ACS Omega. (2024) 9:45447–66. doi: 10.1021/acsomega.4c07463
78
Zhu M Dagah OMA Silaa BB Lu J . Thioredoxin/glutaredoxin systems and gut microbiota in NAFLD: interplay, mechanism, and therapeutical potential. Antioxidants (Basel). (2023) 12:1680. doi: 10.3390/antiox12091680
79
Fu S Li F Yu J Ma S Zhang L Cheng Y . Investigating the role of gut microbiota in diabetic nephropathy through plasma proteome mediated analysis. Sci Rep. (2025) 15:5457. doi: 10.1038/s41598-025-90306-7
80
Ye K Zhao Y Huang W Zhu Y . Sodium butyrate improves renal injury in diabetic nephropathy through AMPK/SIRT1/PGC-1α signaling pathway. Sci Rep. (2024) 14:17867. doi: 10.1038/s41598-024-68227-8
81
Lv M Zheng Y Yuan M Zhang E Zheng M Liu G et al . Cassiae semen extract ameliorates hyperlipidemia in rats by modulating lipid metabolism and FcγR-mediated immune regulation. Front Pharmacol. (2025) 16:1546119. doi: 10.3389/fphar.2025.1546119
82
Paul P Kaul R Chaari A . Renal health improvement in diabetes through microbiome modulation of the gut-kidney axis with biotics: A systematic and narrative review of randomized controlled trials. Int J Mol Sci. (2022) 23:14838. doi: 10.3390/ijms232314838
83
Yadav M Sehrawat N Sharma AK Kumar S Singh R Kumar A et al . Synbiotics as potent functional food: recent updates on therapeutic potential and mechanistic insight. J Food Sci Technol. (2024) 61:1–15. doi: 10.1007/s13197-022-05621-y
84
Baghel K Khan A Kango N . Role of synbiotics (Prebiotics and probiotics) as dietary supplements in type 2 diabetes mellitus induced health complications. J Diet Suppl. (2024) 21:677–708. doi: 10.1080/19390211.2024.2340509
85
Ghosh A Muley A Ainapure AS Deshmane AR Mahajan A . Exploring the impact of optimized probiotic supplementation techniques on diabetic nephropathy: mechanisms and therapeutic potential. Cureus. (2024) 16:e55149. doi: 10.7759/cureus.55149
86
Stougaard EB Tougaard NH Sivalingam S Hansen CS Størling J Hansen TW et al . Effects of probiotics and fibers on markers of nephropathy, inflammation, intestinal barrier dysfunction and endothelial dysfunction in individuals with type 1 diabetes and albuminuria. ProFOS Study. J Diabetes Complications. (2024) 38:108892. doi: 10.1016/j.jdiacomp.2024.108892
87
Bo T Liu H Liu M Liu Q Li Q Cong Y et al . Mechanism of inulin in colic and gut microbiota of captive Asian elephant. Microbiome. (2023) 11:148. doi: 10.1186/s40168-023-01581-3
88
Zhu M Ouyang J Zhou F Zhao C Zhu W Liu C et al . Polysaccharides from Fu brick tea ameliorate obesity by modulating gut microbiota and gut microbiota-related short chain fatty acid and amino acid metabolism. J Nutr Biochem. (2023) 118:109356. doi: 10.1016/j.jnutbio.2023.109356
89
Yu Z Li D Sun H . Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. BioMed Pharmacother. (2023) 161:114409. doi: 10.1016/j.biopha.2023.114409
90
Chen L Liu B Ren L Du H Fei C Qian C et al . High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front Cell Infect Microbiol. (2023) 13:1069954. doi: 10.3389/fcimb.2023.1069954
91
Yang R Shan S An N Liu F Cui K Shi J et al . Polyphenols from foxtail millet bran ameliorate DSS-induced colitis by remodeling gut microbiome. Front Nutr. (2022) 9:1030744. doi: 10.3389/fnut.2022.1030744
92
Yadav SK Ito K Dhib-Jalbut S . Interaction of the gut microbiome and immunity in multiple sclerosis: impact of diet and immune therapy. Int J Mol Sci. (2023) 24:14756. doi: 10.3390/ijms241914756
93
Xu H Yang F Bao Z . Gut microbiota and myocardial fibrosis. Eur J Pharmacol. (2023) 940:175355. doi: 10.1016/j.ejphar.2022.175355
94
Budau R Okamura T Hasegawa Y Nakanishi N Hamaguchi M Fukui M . Supplementation of miso to a western-type diet stimulates ILC3s and decreases inflammation in the small intestine. Nutrients. (2024) 16:3743. doi: 10.3390/nu16213743
95
Li X Peng X Qiao B Peng M Deng N Yu R et al . Gut-Kidney Impairment Process of Adenine Combined with Folium sennae-Induced Diarrhea: Association with Interactions between Lactobacillus intestinalis, Bacteroides acidifaciens and Acetic Acid, Inflammation, and Kidney Function. Cells. (2022) 11:3261. doi: 10.3390/cells11203261
96
Li C Yao J Yang C Yu S Yang Z Wang L et al . Gut microbiota-derived short chain fatty acids act as mediators of the gut-liver-brain axis. Metab Brain Dis. (2025) 40:122. doi: 10.1007/s11011-025-01554-5
97
Xu X Zhang F Ren J Zhang H Jing C Wei M et al . Dietary intervention improves metabolic levels in patients with type 2 diabetes through the gut microbiota: a systematic review and meta-analysis. Front Nutr. (2024) 10:1243095. doi: 10.3389/fnut.2023.1243095
98
Fu J Zheng Y Gao Y Xu W . Dietary fiber intake and gut microbiota in human health. Microorganisms. (2022) 10:2507. doi: 10.3390/microorganisms10122507
99
Shi Y Xing L Zheng R Luo X Yue F Xiang X et al . Butyrate attenuates high-fat diet-induced glomerulopathy through GPR43-Sirt3 pathway. Br J Nutr. (2025) 133:1–10. doi: 10.1017/S0007114524002964
100
Favero C Pintor-Chocano A Sanz A Ortiz A Sanchez-Niño MD . Butyrate promotes kidney resilience through a coordinated kidney protective response in tubular cells. Biochem Pharmacol. (2024) 224:116203. doi: 10.1016/j.bcp.2024.116203
101
Chen WJ Chen YT Ko JL Chen JY Zheng JY Liao JW et al . Butyrate modulates gut microbiota and anti-inflammatory response in attenuating cisplatin-induced kidney injury. BioMed Pharmacother. (2024) 181:117689. doi: 10.1016/j.biopha.2024.117689
102
Tian X Zeng Y Tu Q Jiao Y Yao S Chen Y et al . Butyrate alleviates renal fibrosis in CKD by regulating NLRP3-mediated pyroptosis via the STING/NF-κB/p65 pathway. Int Immunopharmacol. (2023) 124. doi: 10.1016/j.intimp.2023.111010
103
Bashir AM Olaniyi KS . Butyrate alleviates renal inflammation and fibrosis in a rat model of polycystic ovarian syndrome by suppression of SDF-1. BMC Pharmacol Toxicol. (2023) 24:48. doi: 10.1186/s40360-023-00692-9
104
Yu Y Jia YY Li HJ . Sodium butyrate improves mitochondrial function and kidney tissue injury in diabetic kidney disease via the AMPK/PGC-1α pathway. Ren Fail. (2023) 45:2287129. doi: 10.1080/0886022X.2023.2287129
105
Liu J Gu QH Cui Z Zhao MH Jia XY . Short-chain fatty acids ameliorate experimental anti-glomerular basement membrane disease. Clin Immunol. (2024) 259:109903. doi: 10.1016/j.clim.2024.109903
106
Niu H Ren X Tan E Wan X Wang Y Shi H et al . CD36 deletion ameliorates diabetic kidney disease by restoring fatty acid oxidation and improving mitochondrial function. Ren Fail. (2023) 45:2292753. doi: 10.1080/0886022X.2023.2292753
107
Zhou K Zi X Song J Zhao Q Liu J Bao H et al . Molecular mechanistic pathways targeted by natural compounds in the prevention and treatment of diabetic kidney disease. Molecules. (2022) 27:6221. doi: 10.3390/molecules27196221
108
Ren N Wang WF Zou L Zhao YL Miao H Zhao YY . The nuclear factor kappa B signaling pathway is a master regulator of renal fibrosis. Front Pharmacol. (2024) 14:1335094. doi: 10.3389/fphar.2023.1335094
109
Gao LW Yang XY Yu YF Yin S Tong KK Hu G et al . Bibliometric analysis of intestinal microbiota in diabetic nephropathy. Eur Rev Med Pharmacol Sci. (2023) 27:8812–28. doi: 10.26355/eurrev_202309_33802
110
Wu XQ Zhao L Zhao YL He XY Zou L Zhao YY et al . Traditional Chinese medicine improved diabetic kidney disease through targeting gut microbiota. Pharm Biol. (2024) 62:423–35. doi: 10.1080/13880209.2024.2351946
111
Miao H Liu F Wang YN Yu XY Zhuang S Guo Y et al . Targeting Lactobacillus johnsonii to reverse chronic kidney disease. Signal Transduct Target Ther. (2024) 9:195. doi: 10.1038/s41392-024-01913-1
112
Miao H Wang YN Yu XY Zou L Guo Y Su W et al . Lactobacillus species ameliorate membranous nephropathy through inhibiting the aryl hydrocarbon receptor pathway via tryptophan-produced indole metabolites. Br J Pharmacol. (2024) 181:162–79. doi: 10.1111/bph.16219
113
Zhang X Wang J Zhang T Li S Liu J Li M et al . Updated progress on polysaccharides with anti-diabetic effects through the regulation of gut microbiota: sources, mechanisms, and structure-activity relationships. Pharm (Basel). (2024) 17:456. doi: 10.3390/ph17040456
114
Li L Peng P Ding N Jia W Huang C Tang Y . Oxidative stress, inflammation, gut dysbiosis: what can polyphenols do in inflammatory bowel disease? Antioxidants (Basel). (2023) 12:967. doi: 10.3390/antiox12040967
115
Stepanova N . Probiotic interventions in peritoneal dialysis: A review of underlying mechanisms and therapeutic potentials. World J Nephrol. (2024) 13:98719. doi: 10.5527/wjn.v13.i4.98719
116
Zhang S Zhang S Bai X Wang Y Liu Y Liu W . Thonningianin A ameliorated renal interstitial fibrosis in diabetic nephropathy mice by modulating gut microbiota dysbiosis and repressing inflammation. Front Pharmacol. (2024) 15:1389654. doi: 10.3389/fphar.2024.1389654
117
Mitrea L Medeleanu M Pop CR Rotar AM Vodnar DC . Biotics (Pre-, pro-, post-) and uremic toxicity: implications, mechanisms, and possible therapies. Toxins (Basel). (2023) 15:548. doi: 10.3390/toxins15090548
118
Rumanli Z Vural IM Avci GA . Chronic kidney disease, uremic toxins and microbiota. Microbiota Host. (2025) 3:e240012. doi: 10.1530/MAH-24-0012
119
Frąk W Dąbek B Balcerczyk-Lis M Motor J Radzioch E Młynarska E et al . Role of uremic toxins, oxidative stress, and renal fibrosis in chronic kidney disease. Antioxidants (Basel). (2024) 13:687. doi: 10.3390/antiox13060687
120
Tao P Huo J Chen L . Bibliometric analysis of the relationship between gut microbiota and chronic kidney disease from 2001–2022. Integr Med Nephrol Andrology. (2024) 11:e00017. doi: 10.1097/IMNA-D-23-00017
121
Ye Z So T Zhang T Gao X . Association between gut microbiota and diabetic nephropathy: a two-sample Mendelian randomization study. Front Endocrinol (Lausanne). (2024) 15:1361440. doi: 10.3389/fendo.2024.1361440
122
Li XJ Shan QY Wu X Miao H Zhao YY . Gut microbiota regulates oxidative stress and inflammation: a double-edged sword in renal fibrosis. Cell Mol Life Sci. (2024) 81:480. doi: 10.1007/s00018-024-05532-5
123
Li YY Guan RQ Hong ZB Wang YL Pan LM . Advances in the treatment of diabetic peripheral neuropathy by modulating gut microbiota with traditional Chinese medicine. World J Diabetes. (2024) 15:1712–6. doi: 10.4239/wjd.v15.i8.1712
124
Tahiri M Gilbert JA . Examining the potential prebiotic effect of almonds. J Appl Microbiol. (2025) 136:lxaf078. doi: 10.1093/jambio/lxaf078
125
Miao H Zhang SJ Wu X Li P Zhao YY . Tryptophan metabolism as a target in gut microbiota, ageing and kidney disease. Int J Biol Sci. (2025) 21:4374–87. doi: 10.7150/ijbs.115359
126
Sun X Sun W Huang Y Chen J . Traditional chinese medicine: an exogenous regulator of crosstalk between the gut microbial ecosystem and CKD. Evid Based Complement Alternat Med. (2022) 2022:7940684. doi: 10.1155/2022/7940684
127
Vassallo GA Dionisi T De Vita V Augello G Gasbarrini A Pitocco D et al . The role of fecal microbiota transplantation in diabetes. Acta Diabetol. (2025) 62:977–81. doi: 10.1007/s00592-025-02508-0
128
Li YG Yu ZJ Li A Ren ZG . Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications: Molecular mechanisms and therapeutic inventions. World J Gastroenterol. (2022) 28:3555–72. doi: 10.3748/wjg.v28.i28.3555
129
Shang J Cui W Guo R Zhang Y Wang P Yu W et al . The harmful intestinal microbial community accumulates during DKD exacerbation and microbiome-metabolome combined validation in a mouse model. Front Endocrinol (Lausanne). (2022) 13:964389. doi: 10.3389/fendo.2022.964389
130
Xie YC Jing XB Chen X Chen LZ Zhang SH Cai XB . Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: a case report. Ther Adv Chronic Dis. (2022) 13:20406223221117449. doi: 10.1177/20406223221117449
131
Ye XX Jiang QY Wu MJ Ye QH Zheng H . Transplant of fecal microbiota from healthy young mice relieves cognitive defects in late-stage diabetic mice by reducing metabolic disorders and neuroinflammation. Acta Pharmacol Sin. (2024) 45:2513–26. doi: 10.1038/s41401-024-01340-6
132
Arteaga-Muller GY Flores-Treviño S Bocanegra-Ibarias P Robles-Espino D Garza-González E Fabela-Valdez GC et al . Changes in the progression of chronic kidney disease in patients undergoing fecal microbiota transplantation. Nutrients. (2024) 16:1109. doi: 10.3390/nu16081109
133
Caggiano G Stasi A Franzin R Fiorentino M Cimmarusti MT Deleonardis A et al . Fecal microbiota transplantation in reducing uremic toxins accumulation in kidney disease: current understanding and future perspectives. Toxins (Basel). (2023) 15:115. doi: 10.3390/toxins15020115
134
Rakotonirina A Galperine T Allémann E . Fecal microbiota transplantation: a review on current formulations in Clostridioides difficile infection and future outlooks. Expert Opin Biol Ther. (2022) 22:929–44. doi: 10.1080/14712598.2022.2095901
135
Zhi W Li A Wang Q Yuan X Qing J Zhang C et al . Safety and efficacy assessment of fecal microbiota transplantation as an adjunctive treatment for IgA nephropathy: an exploratory clinical trial. Sci Rep. (2024) 14:22935. doi: 10.1038/s41598-024-74171-4
136
Wu L Li MQ Xie YT Zhang Q Lu XJ Liu T et al . Washed microbiota transplantation improves patients with high blood glucose in South China. Front Endocrinol (Lausanne). (2022) 13:985636. doi: 10.3389/fendo.2022.985636
137
Xu M Hao J Qi Y Wu B Li R Yang X et al . Causal effects of gut microbiota on diabetic neuropathy: a two-sample Mendelian randomization study. Front Endocrinol (Lausanne). (2024) 15:1388927. doi: 10.3389/fendo.2024.1388927
138
Yang CL Chen YJ Huang X Cheng Q Chen W Guo Z . Causal relationship between gut microbiota and diabetic nephropathy: A bidirectional mendelian randomization study. Cell Mol Biol (Noisy-le-grand). (2024) 70:127–33. doi: 10.14715/cmb/2024.70.4.20
139
Cao BN Zhang CY Wang Z Wang YX . Causal relationship between 412 gut microbiota, 1,400 blood metabolites, and diabetic nephropathy: a randomized Mendelian study. Front Endocrinol (Lausanne). (2025) 15:1450428. doi: 10.3389/fendo.2024.1450428
140
Hu X Chen S Ye S Chen W Zhou Y . New insights into the role of immunity and inflammation in diabetic kidney disease in the omics era. Front Immunol. (2024) 15:1342837. doi: 10.3389/fimmu.2024.1342837
141
Ma L Liu D Yu Y Li Z Wang Q . Immune-mediated renal injury in diabetic kidney disease: from mechanisms to therapy. Front Immunol. (2025) 16:1587806. doi: 10.3389/fimmu.2025.1587806
142
Zhuang M Zhang X Cai J . Microbiota-gut-brain axis: interplay between microbiota, barrier function and lymphatic system. Gut Microbes. (2024) 16:2387800. doi: 10.1080/19490976.2024.2387800
143
Pour-Reza-Gholi F Assadiasl S . Immunological approaches in the treatment of diabetic nephropathy. Curr Diabetes Rev. (2024) 21:e061123223172. doi: 10.2174/0115733998267893231016062205
144
Robles-Osorio ML Sabath E . Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: A narrative review. World J Diabetes. (2023) 14:1013–26. doi: 10.4239/wjd.v14.i7.1013
145
Yu B Shen K Li T Li J Meng M Liu W et al . Glycolytic enzyme PFKFB3 regulates sphingosine 1-phosphate receptor 1 in proangiogenic glomerular endothelial cells under diabetic condition. Am J Physiol Cell Physiol. (2023) 325:C1354–68. doi: 10.1152/ajpcell.00261.2023
146
Zhang J Chen S Xiang H Xiao J Zhao S Shu Z et al . S1PR2/Wnt3a/RhoA/ROCK1/β-catenin signaling pathway promotes diabetic nephropathy by inducting endothelial mesenchymal transition and impairing endothelial barrier function. Life Sci. (2023) 328:121853. doi: 10.1016/j.lfs.2023.121853
147
Li X Miao Y Fang Z Zhang Q . The association and prediction value of acylcarnitine on diabetic nephropathy in Chinese patients with type 2 diabetes mellitus. Diabetol Metab Syndr. (2023) 15:130. doi: 10.1186/s13098-023-01058-1
148
Zhang J Lin B Zhang Y Hu X Liu T Liu EH et al . Baitouweng decoction alleviates ulcerative colitis by regulating tryptophan metabolism through DOPA decarboxylase promotion. Front Pharmacol. (2024) 15:1423307. doi: 10.3389/fphar.2024.1423307
149
Ding S Xu JL Tong JY Cheng YY Shi LF Wei W et al . Brown adipose tissue alleviates podocyte apoptosis through NRG4 in a male mouse model of diabetic kidney disease. Diabetologia. (2025) 68:1057–75. doi: 10.1007/s00125-025-06385-8
150
Papaetis G . SGLT2 inhibitors and diabetic kidney disease: targeting multiple and interrelated signaling pathways for renal protection. Curr Mol Pharmacol. (2023). doi: 10.2174/0118761429261105231011101200
151
Zhang MY Zheng SQ . Network pharmacology and molecular dynamics study of the effect of the Astragalus-Coptis drug pair on diabetic kidney disease. World J Diabetes. (2024) 15:1562–88. doi: 10.4239/wjd.v15.i7.1562
152
Mao J Lu Q Gao F Liu H Tan J . Antagonistic effect of jiawei shengjiang san on a rat model of diabetic nephropathy: related to EGFR/MAPK3/1 signaling pathway. J Vis Exp. (2024) 207:110–6. doi: 10.3791/66179
153
Shi J Liu X Jiao Y Tian J An J Zou G et al . mTOR pathway: A key player in diabetic nephropathy progression and therapeutic targets. Genes Dis. (2024) 12:101260. doi: 10.1016/j.gendis.2024.101260
154
Jung YS Song NE Oh SY Park YK Kim YJ Seong H et al . Advances in in vitro cultivation techniques for comprehensive analysis of human gut microbiome. Biotechnol Adv. (2025) 82:108595. doi: 10.1016/j.bioteChadv.2025.108595
155
Rocha Martin VN Del’Homme C Chassard C Schwab C Braegger C Bernalier-Donadille A et al . A proof of concept infant-microbiota associated rat model for studying the role of gut microbiota and alleviation potential of Cutibacterium avidum in infant colic. Front Nutr. (2022) 9:902159. doi: 10.3389/fnut.2022.902159
156
Wang J Liu X Li Q . Interventional strategies for ischemic stroke based on the modulation of the gut microbiota. Front Neurosci. (2023) 17:1158057. doi: 10.3389/fnins.2023.1158057
157
Jiang QR Zeng DW . Gut microbiota shifts in hepatitis B-related portal hypertension after transjugular intrahepatic portosystemic shunt: Mechanistic and clinical implications. World J Gastroenterol. (2025) 31:100752. doi: 10.3748/wjg.v31.i3.100752
158
Yadav S Sapra L Srivastava RK . Polysaccharides to postbiotics: Nurturing bone health via modulating ‘gut-immune axis’. Int J Biol Macromol. (2024) 278. doi: 10.1016/j.ijbiomac.2024.134655
159
Zhao J Liao Y Wei C Ma Y Wang F Chen Y et al . Potential ability of probiotics in the prevention and treatment of colorectal cancer. Clin Med Insights Oncol. (2023) 17:11795549231188225. doi: 10.1177/11795549231188225
160
Zhao H Yang CE Liu T Zhang MX Niu Y Wang M et al . The roles of gut microbiota and its metabolites in diabetic nephropathy. Front Microbiol. (2023) 14:1207132. doi: 10.3389/fmicb.2023.1207132
161
Lu X Ma J Li R . Alterations of gut microbiota in biopsy-proven diabetic nephropathy and a long history of diabetes without kidney damage. Sci Rep. (2023) 13:12150. doi: 10.1038/s41598-023-39444-4
162
Dai M Wu J Ji Z Chen P Yang C Luo J et al . Construction of a metabolic-immune model for predicting the risk of diabetic nephropathy and study of gut microbiota. J Diabetes Investig. (2025) 16:863–73. doi: 10.1111/jdi.14401
163
Wang J Wei HJ Mao RF Chang X . Gut microbiota modulating therapy for diabetes mellitus should be individualized. World J Diabetes. (2024) 15:2152–6. doi: 10.4239/wjd.v15.i10.2152
164
Ma Y Xie H Xu N Li M Wang L Ge H et al . Large yellow tea polysaccharide alleviates HFD-induced intestinal homeostasis dysbiosis via modulating gut barrier integrity, immune responses, and the gut microbiome. J Agric Food Chem. (2024) 72:7230–43. doi: 10.1021/acs.jafc.4c00616
Summary
Keywords
diabetic kidney disease, gut microbiota, gut-kidney axis, metabolism–immunity–barrier interaction, microbiome-targeted therapy
Citation
Jiang H, Wang X, Zhou W, Huang Z and Zhang W (2025) Gut microbiota dysbiosis in diabetic nephropathy: mechanisms and therapeutic targeting via the gut-kidney axis. Front. Endocrinol. 16:1661037. doi: 10.3389/fendo.2025.1661037
Received
07 July 2025
Accepted
28 August 2025
Published
18 September 2025
Volume
16 - 2025
Edited by
Åke Sjöholm, Gävle Hospital, Sweden
Reviewed by
Laura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Lin Chen, Northwest University, China
Dinakaran Vasudevan, SKAN Research Trust, India
Updates
Copyright
© 2025 Jiang, Wang, Zhou, Huang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Zhang, 306762827@qq.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.