- 1Department of Pathology, The Second Hospital of Longyan, Longyan, China
- 2Molecular Biology Laboratory, The Second Hospital of Longyan, Longyan, China
- 3Department of Pathology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
Purpose: Pituitary metastasis of malignant melanoma (MM) is rare. This study aimed to explore its diagnostic features using a multimodal approach and retrospectively analyzed previously reported cases to summarize its pathogenesis and diagnostic challenges.
Methods: We screened all published case reports and case series on pituitary metastatic MM using PubMed, focusing on cases with detailed clinical data, imaging features, pathological examination, and molecular test results. A total of 24 cases of MM with pituitary metastasis, including our case, were retrospectively analyzed. Additionally, the index patient underwent histopathological, immunohistochemical (S100, SOX10, Melan-A, HMB-45, BRAF V600E), and BRAF V600E PCR analyses.
Results: This case involved a 65-year-old female patient whose pathological examination revealed tumor cells with epithelioid and spindle cell features. Immunohistochemical analysis showed diffuse positivity for S-100, vimentin, and BRAF V600E, with focal positivity for Melan-A and HMB-45. The Ki-67 proliferation index was approximately 15%. Molecular testing confirmed BRAF V600E mutation. The patient died 12 months postoperatively. Our literature review indicated that MM with pituitary metastasis demonstrates male predominance, a median onset age of 62 years, a frequent association with BRAF V600E mutation, and a median survival time of 12 months.
Conclusion: Diagnosing MM with pituitary metastasis requires integrating detailed clinical history, imaging features, pathological examination, and molecular testing. Our findings highlight the importance of a comprehensive diagnostic approach with multidisciplinary collaboration when managing atypical pituitary masses, along with detailed investigation of a patient’s previous tumor history, to improve diagnostic accuracy and patient outcomes.
Introduction
The sellar region of the central nervous system is anatomically and functionally critical owing to its proximity to numerous vital structures. Pituitary adenomas are the most common tumors in this area, followed by craniopharyngiomas. Less common tumors include granulosa cell tumors, pituitary cell tumors, spindle cell eosinophilic tumors, and, rarely, pituitary metastases. Pituitary metastases account for only 1%–4% of all pituitary tumors (1, 2), with breast and lung cancers being the most common primary tumors (3, 4). Renal and prostate cancers are other frequent sources of metastasis (5), and virtually any type of tumor can metastasize to the pituitary region (6), including malignant melanoma (MM) (7, 8). Cutaneous melanoma most frequently metastasizes to lung, liver, brain, and bone. Approximately 50% of advanced melanomas harbour BRAF V600E mutations, making BRAF V600E analysis crucial for both diagnosis and targeted therapy. Pituitary metastasis of MM is particularly rare, and its imaging features often overlap with those of pituitary adenomas, making preoperative diagnosis extremely challenging.
The clinical presentation of pituitary metastasis is often nonspecific, with approximately 20% of patients presenting with symptoms that typically emerge in the advanced stages of the disease (3). Common manifestations include headache, visual impairment, cranial neuropathy, and pituitary dysfunction (3). These nonspecific symptoms pose significant diagnostic challenges, particularly when the primary tumor is unidentified. In this context, a comprehensive diagnostic approach is essential for accurately identifying and managing these rare cases.
In recent years, continuous advancements in imaging, pathological examination, and molecular diagnostic techniques have led to the gradual adoption of a multimodal diagnostic system for identifying nervous system tumors (9). Integrating detailed clinical history, imaging findings, pathological features, and molecular detection results can significantly improve the diagnostic accuracy of rare metastases. Additionally, targeted therapies against the BRAF V600E mutation have yielded remarkable progress in MM treatment. A landmark study demonstrated that patients with metastatic MM harboring the BRAF V600E mutation treated with dabrafenib–trametinib achieved a 5-year survival rate of 28% and an overall response rate of 76%, with 17% of the patients achieving complete remission (10). Long-term complete remission has also been reported, even after the treatment had been discontinued for 18 months (11). These findings indicate the significance of detecting the presence of a BRAF V600 mutation in MM with pituitary metastasis.
In this study, we report a case of MM with pituitary metastasis, discuss its clinical and imaging features, and highlight its diagnostic challenges. Combined with a literature review, we emphasize the value of a multimodal diagnostic system in accurately identifying rare pituitary metastases. Through this case study, we aim to provide clinicians and pathologists with a reference for diagnosing and treating such rare cases.
Case report
A 65-year-old female patient presented with a 5-month history of dizziness. Hormonal evaluation revealed abnormal cortisol levels of 66.93 nmol/L, 130.50 nmol/L, and 124.84 nmol/L at 0, 8, and 16 h, respectively, as measured using the electrochemiluminescence immunoassay method (reference range: 171.0–536.0 nmol/L). All other hormone levels were within the normal limits. Cranial magnetic resonance imaging (MRI) (Figure 1) showed a round, abnormal signal shadow in the left sellar region. T1-weighted imaging (T1WI) revealed isointense to slightly hyperintense signals, while T2-weighted imaging (T2WI) showed slightly hyperintense signals. The lesion had clear boundaries and measured approximately 1.7 × 1.5 cm (coronal measurement). Contrast-enhanced scans revealed progressive, uneven, and marked enhancement without evidence of sellar floor bone absorption or destruction. A preliminary diagnosis of pituitary macroadenoma was made.
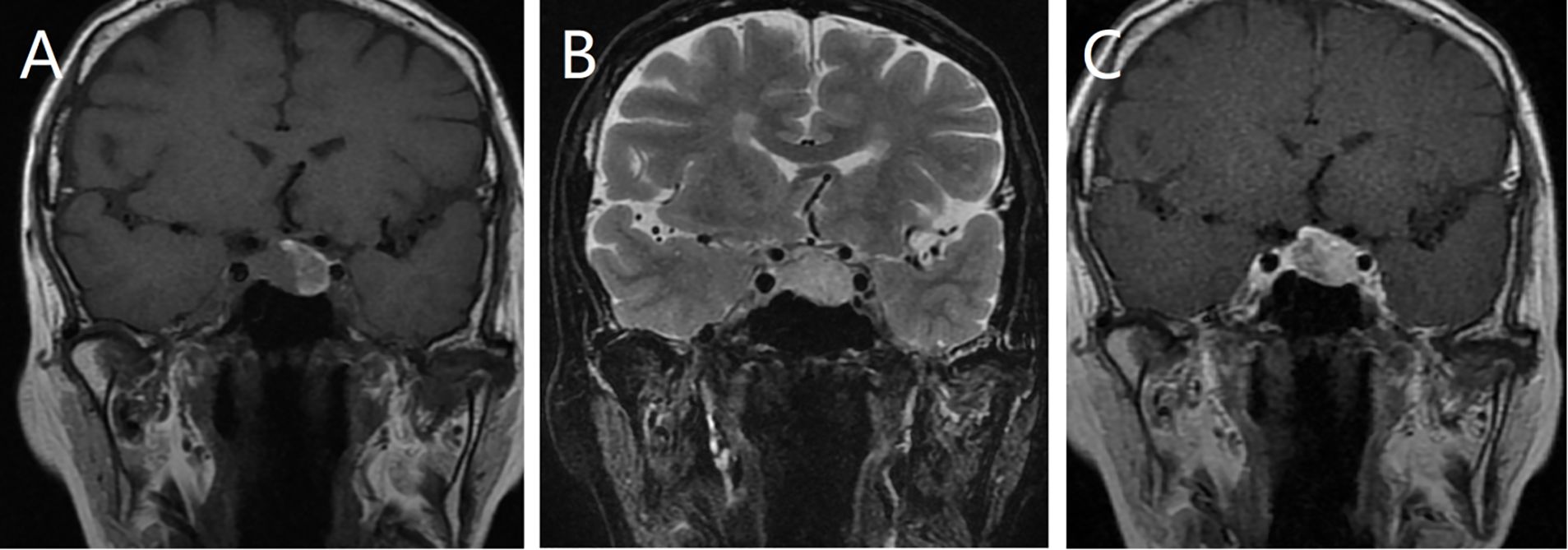
Figure 1. Magnetic resonance imaging (MRI) scans showing a circular abnormal signal shadow on the left side of the sellar area. The lesion exhibits an equal to slightly higher signal on T1-weighted imaging (T1WI) (A) and a slightly higher signal on T2-weighted imaging (T2WI) (B), with clear boundaries. Contrast-enhanced MRI showing progressive inhomogeneous enhancement (C).
Intraoperative findings identified a solid mass in the sellar region with friable, fish-like tissue. Pathological examination revealed gray-brown, fragmented tumor tissue measuring 3 × 3 × 0.3 cm that was soft in texture. Microscopic evaluation revealed diffuse and patchy tumor cells, predominantly polygonal epithelioid cells with an eosinophilic cytoplasm (Figure 2A). Some cells exhibited a foamy cytoplasm with pigment deposition (Figure 2B). The nuclei were pleomorphic, including round, oval, and irregular shapes, with nucleoli visible in some cells (Figure 2B). A small number of spindle-shaped cells were interwoven with epithelioid cells, with deeply stained nuclei and inconspicuous nucleoli (Figure 2C). Mitotic figures were observed in both epithelioid and spindle cells, and focal lymphocytic aggregation and necrotic areas were present in the stroma. Immunohistochemical analysis showed diffuse positivity for S-100 and vimentin, with strong galectin-3 positivity. TTF-1, broad-spectrum CK, EMA, CgA, Syn, and GFAP were not expressed. The Ki-67 proliferation index was approximately 15%.
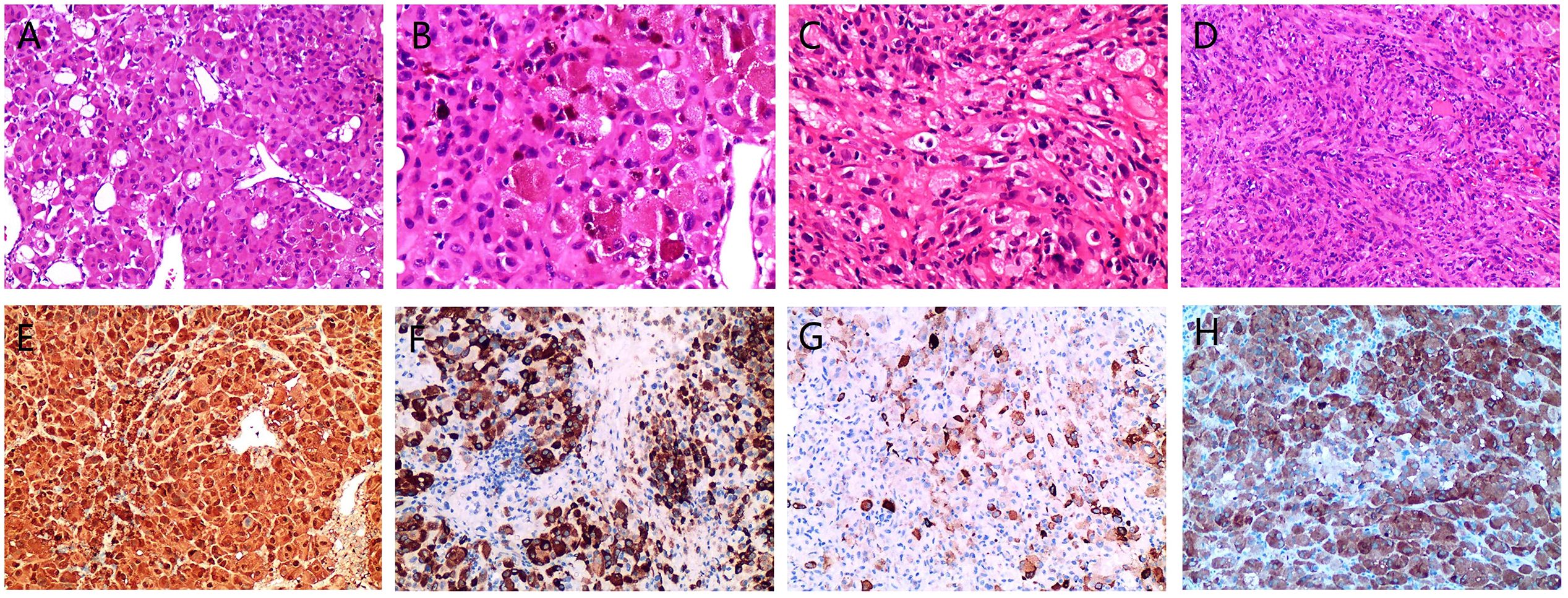
Figure 2. Microscopic morphology and immunohistochemical expression of pituitary and axillary metastatic malignant melanoma (MM). Tumor cells arranged in sheets with epithelioid morphology and thin-walled blood vessels are visible, HE, ×200 (A). Epithelioid, polygonal cells displaying varying degrees of eosinophilia, with some cells containing pigment in the cytoplasm, HE, ×400 (B). Spindle-shaped cells interwoven with epithelioid cells, HE, ×400 (C). The axillary mass is predominantly composed of spindle-shaped cells, HE, ×200 (D). Strongly positive for S-100, EnVision, ×200 (E). Melan-A is strongly positive in most areas, EnVision,×200 (F). HMB-45 exhibits varying degrees of expression in scattered cells, EnVision, ×200 (G). Strongly positive for BRAF V600E,EnVision, ×200 (H).
Prior to surgery, the patient reported a palpable axillary mass. Ultrasonography revealed a hypoechoic nodule in the right axilla. Intraoperatively, a mass approximately 3 cm in size was identified under the skin of the right axilla, with a smooth, cystic-solid appearance and containing a dark red fluid. The solid area had a fish-like texture with clear boundaries from the surrounding adipose tissue. Microscopic cell morphology and immunohistochemical expression of the axillary mass were similar to those of the sellar mass: predominantly spindle cells with few mitotic figures and no apparent pigment deposition (Figure 2D).
Given the simultaneous presence of an axillary mass, metastasis was suspected. The presence of pigment in the cytoplasm of the pituitary tumor cells and immunohistochemical expression of S-100 and vimentin were consistent with the characteristics of MM. Further review of the patient’s medical history revealed surgical excision of a nevus from the calf over 10 years ago, with difficult postoperative wound healing. This information supported the hypothesis that both the pituitary and axillary masses were metastatic MM. To confirm this diagnosis, immunohistochemical tests for Melan-A (Figure 2F), HMB45 (Figure 2G), and BRAF V600E (Figure 2H) were performed, all of which showed strong positivity, thereby supporting the diagnosis of MM.
BRAF V600E mutational status was determined by allele-specific real-time PCR (TaqMan® SNP Genotyping Assay, ThermoFisher, sensitivity ≥1% mutant allele), validated in-house with known positive and negative controls, and detected the V600E mutation in both pituitary and axillary specimens. Ten days after surgery, the patient received dacarbazine plus ifosfamide; BRAF/MEK inhibitors were not used because the BRAF V600E status had not yet been determined. After two treatment cycles, the patient developed severe bone marrow suppression, discontinued therapy, and ultimately succumbed 12 months post-operatively.
Literature review
To better understand the clinical manifestations and diagnostic approaches for MM with pituitary metastasis, a comprehensive PubMed search was conducted without date restrictions (up to 30 April 2024) using the Boolean query (“melanoma” OR “melanoma metastasis”) AND (“pituitary” OR “sella” OR “brain”). After excluding duplicates, primary melanoma, and non-metastatic lesions, 24 histologically confirmed cases of pituitary metastatic melanoma were retained for retrospective analysis (5, 7, 11–28) (Table 1). Although we attempted to identify all relevant cases, the possibility of publication or database bias could not be excluded. The cohort comprised 16 male patients, 7 female patients, and 1 patient of unknown sex (22). The patients’ ages ranged from 25 to 78 years, with a median age of 62 years. The primary clinical manifestations included visual impairment and pituitary dysfunction (3, 29), followed by headache and dizziness.
Discussion
This study investigated the clinical, imaging, and pathological features of MM with pituitary metastasis and highlighted the diagnostic challenges associated with this rare condition. Through a comprehensive case analysis and literature review, we demonstrate the significant value of a multimodal diagnostic approach involving the integration of clinical, radiological, pathological, and molecular data in enhancing diagnostic accuracy and emphasize the need for a detailed patient history and a comprehensive diagnostic strategy.
MM is considered one of the most centrophilic tumors, with central nervous system metastases occurring in 10%–40% of patients with MM (30, 31). However, pituitary involvement remains rare. The literature reports several risk factors for the development of brain metastases in MM, including the primary tumor thickness (Breslow depth > 3 mm), the presence of ulceration, and the location of the primary tumor (32). In our case series, the primary site of MM was most commonly the skin (13 out of 14 cases), with the condition in all cases presenting as Clark stage IV cancer, with a Breslow depth ranging from 1.5 to 12 mm. The median time to MM brain metastasis is reported in the literature as 30 months (32). Of the 24 MM cases, the primary lesion was identified simultaneously in two cases (7, 25), and 15 cases had clearly documented metastasis to the pituitary. The median time from MM diagnosis to pituitary metastasis was 36 months.
The molecular mechanisms underlying brain metastasis in MM are multifaceted, encompassing oncogenic mutations, aberrant activation of signaling pathways, alterations in the intracranial microenvironment, and expression of nerve growth factor receptors (33, 34). We hypothesized that BRAF mutations may be associated with an increased propensity for pituitary metastasis in patients with MM. Studies have demonstrated that BRAF mutations activate downstream signaling via the MAPK pathway (35), which may indirectly regulate the expression of chemokine receptors such as CXCR4 and promote the migration of tumor cells to the CXCL12-rich pituitary microenvironment (36). BRAF is the most frequently mutated gene in melanocytic tumors, with approximately 50% of patients with metastatic MM harboring BRAF mutations, 95% of which are located in exon 15 at BRAF V600 (5). In our retrospective analysis, 80% (4 out of 5) of cases were BRAF-positive, indicating a potential association between MM with BRAF mutations and pituitary metastasis. However, this observation was based on only four mutation-positive cases and remains hypothesis-generating; larger studies are needed to establish any causal relationships.
The MRI features of MM with pituitary metastasis are dynamically influenced by melanin content owing to its paramagnetic properties. Melanin-rich tumors typically exhibit T1WI hyperintensity and T2WI hypointensity. In our case series, 38.9% (7 out of 18) of cases exhibited this typical “T1 hyperintensity/T2 hypointensity” pattern, while the present case displayed atypical slight T2 hyperintensity, likely related to a lower melanin content. These MRI features are closely associated with the pathological characteristics of MM, reflecting its growth and metabolic features within the pituitary gland. However, similar MRI features can also be observed in hemorrhagic pituitary macroadenomas (12, 37), making preoperative diagnosis challenging and necessitating close integration with clinical history.
In clinical practice, diagnosis is relatively straightforward when patients have a known history of MM. However, if the patient’s history of MM is unknown, the diagnosis becomes challenging due to the diverse histological structures and cellular morphologies of MM, which can readily be confused with those of other primary tumors. This diagnostic difficulty is further compounded in rare cases where the MM has metastasized to primary pituitary tumors, making differentiation particularly challenging. In the present case, the unknown MM history, along with significant microscopic morphological and immunophenotypic overlap with pituitary adenomas, contributed to the diagnostic difficulty.
Microscopically, the tumors exhibited typical morphologies, with pigment deposition observed in most cases. Among four cases involving collision tumors, three involved MM metastasis to a pituitary adenoma (15, 24, 28), and one involved metastasis to a pituitary eosinophilic tumor (18). Immunohistochemistry revealed diffuse positivity for vimentin, S-100, Melan-A, HMB45, and SOX10. BRAF V600E mutation detection also provided critical diagnostic support, If BRAF V600E immunohistochemistry is negative, PCR or NGS can be performed to confirm the mutation status. Of the 24 cases, 5 underwent BRAF testing (three via immunohistochemistry), and 4 exhibited positive results. There is significant overlap in the histological features of granulosa cell tumors, pituitary cell tumors, and spindle cell eosinophilic tumors. According to the 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (38), these tumors are grouped together due to their diverse cellular arrangements and morphologies, including epithelioid, round, and spindle-shaped cells with eosinophilic or pale pink cytoplasm, inconspicuous nucleoli, abundant interstitial blood vessels, and lymphocytic infiltration. Immunomarkers such as TTF-1, vimentin, S-100, and GFAP are highly specific to these tumors. While typically benign (WHO grade 1), atypia and mitosis can occur in recurrent cases (39), further increasing the difficulty of differential diagnosis. Therefore, integrating clinical history with immunohistochemistry and confirmatory molecular analysis (BRAF V600E) can be used to reliably distinguish metastatic melanoma from primary pituitary tumors.
Most brain metastases in MM are advanced at presentation, characterized by refractory disease and a poor prognosis (3). Follow-up data were available for 20 patients, with the follow-up durations ranging from 1 to 45 months. Among these patients, 11 died during the follow-up period, including 1 due to an unrelated accident. Of the 10 patients who died from disease-related causes, the survival times ranged from 1 to 34.8 months, with a median survival of 12 months. Among the remaining survivors, one patient survived for 22 months but with poor health. For brain metastases in MM, multimodal treatment approaches are recommended, including surgery, radiotherapy, hormone replacement therapy, chemotherapy (40), immunotherapy, and targeted drug therapy (12, 15). BRAF mutation testing not only aids in diagnosing MM but also provides a theoretical basis for targeted therapies. Small-molecule inhibitors targeting the BRAF V600 mutation have been shown to have remarkable efficacy (41), and the combination of a BRAF inhibitor (BRAFi) with a MEK inhibitor (MEKi) can mitigate drug resistance and improve prognosis. Among the cases retrospectively analyzed in this study, patients who received BRAFi–MEKi combination therapy achieved remission (5). Currently, three BRAFi–MEKi combination regimens—dabrafenib with trametinib, encorafenib with binimetinib, and vemurafenib with cobimetinib—are considered the standard treatment for advanced BRAF-mutated MM.
This study has some limitations. First, some of the case reports lacked detailed clinical follow-up information, and the literature review may be subject to publication bias, which limits the generalizability of our findings. Second, molecular data were incomplete for some patients, which may restrict the depth and universality of our analysis. Future studies should address these limitations by incorporating more comprehensive clinical and molecular data.
Conclusion
Our findings underscore the rarity of pituitary metastasis in MM and the complexity of its diagnosis. Despite numerous challenges, meticulous clinical observation, imaging, and pathological examination led to accurate diagnosis. A thorough patient history and comprehensive approach are essential when facing atypical presentations, and further studies are needed to identify early diagnostic markers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The retrospective study was conducted in accordance with the Declaration of Helsinki and local legislation. The Institutional Review Board of The Second Hospital of Longyan approved the protocol (Approval No. LYEY-KY-2025-017) and waived written informed consent because the patient had died before the study began. Only de-identified data collected during routine clinical care were used.
Author contributions
XL: Writing – original draft, Writing – review & editing. WJ: Writing – review & editing. XT: Writing – review & editing. MC: Writing – review & editing. WD: Writing – review & editing. YW: Writing – review & editing. XW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.cn) for the English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heshmati HM, Scheithauer BW, and Young WF. Metastases to the pituitary gland. Endocrinologists. (2002) 12:45–9. doi: 10.1097/00019616-200201000-00010
2. Valassi E, Biller BMK, Klibanski A, and Swearingen B. Clinical features of nonpituitary sellar lesions in a large surgical series. Clin Endocrinol. (2010) 73:798–807. doi: 10.1111/j.1365-2265.2010.03881.x
3. Komninos J, Varvara V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. (2004) 2004:574–80. doi: 10.1210/jc.2003-030395
4. Al-Aridi R, Sibai KE, Fu P, Khan M, Selman WR, and Arafah BM. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: an analytical review. Pituitary. (2014) 17:575–87. doi: 10.1007/s11102-013-0542-9
5. Mormando M, Puliani G, Barnabei A, Lauretta R, and Appetecchia M. A rare case of pituitary melanoma metastasis: a dramatic and prolonged response to dabrafenib-trametinib therapy. Front Endocrinol. (2020) 11:471. doi: 10.3389/fendo.2020.00471
6. Shimon I. Metastatic spread to the pituitary. Neuroendocrinology. (2020) 110:805–8. doi: 10.1159/000506810
7. Masui K, Yonezawa T, Shinji Y, Nakano R, and Miyamae S. Pituitary apoplexy caused hemorrhage from pituitary metastatic melanoma: case report. Neurol Med Chir (Tokyo). (2013) 53:695–8. doi: 10.2176/nmc.cr2012-0068
8. Cox HH and Sloan LH. Melanoma: report of a case apparently primary in the jejunum, the presenting symptoms resulting from metastasis in the hypophysis cerebri. JAMA. (1924) 82:2021–5. doi: 10.1001/jama.1924.02650510021007
9. Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
10. Long GV, Eroglu Z, Infante J, Patel S, Daud A, Johnson DB, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. (2018) 36:667–73. doi: 10.1200/JCO.2017.74.1025
11. Brugnara S, Sicher M, Bonandini EM, Donner D, and Caffo O. Treatment with combined dabrafenib and trametinib in BRAFV600E-mutated metastatic Malignant melanoma: a case of long-term complete response after treatment cessation. Drugs Context. (2017) 7:212515. doi: 10.7573/dic.212515
12. Giuffrida G, Ferraù F, Alessi Y, and Cannavò S. Shrinkage of a pituitary metastasis of melanoma induced by pembrolizumab: a case report. J Med Case Rep. (2021) 15:555. doi: 10.1186/s13256-021-03150-4
13. Mattogno PP, Giordano M, D'Alessandris QG, Ktari O, and Lauretti L. Solitary metastatic melanoma of the pituitary gland: report of two cases and literature review. World Neurosurg. (2020) 139:378–81. doi: 10.1016/j.wneu.2020.04.138
14. Ng S, Boetto J, Rigau V, Raingeard I, Crampette L, Favier V, et al. Pituitary metastasis of Malignant melanoma misdiagnosed as pituitary adenoma: a case report and systematic review of the literature. Neurochirurgie. (2020) 66:383–90. doi: 10.1016/j.neuchi.2020.06.129
15. Lamorie-Foote K, Rangwala SD, Kammen A, Gnass E, Kramer DR, Rutkowski M, et al. Melanoma metastasis to a nonfunctioning pituitary macroadenoma: illustrative case. J Neurosurg Case Lessons. (2021) 1:CASE2167. doi: 10.3171/CASE2167
16. Mayr NA, Yuh WTC, Muhonen MG, Koci TM, Tali ET, Nguyen HD, et al. Pituitary metastases: MR findings. J Comput Assist Tomogr. (1993) 17:432–7. doi: 10.1097/00004728-199305000-00018
17. Leung GK, Chow WS, Tan KC, Fan YW, and Lam KS. Metastatic melanoma of the pituitary gland. Case Rep J Neurosurg. (2003) 99:913–5. doi: 10.3171/jns.2003.99.5.0913
18. Jung SM, Hsu YY, Chuang CC, Chang CN, Hsueh C, and Kuo TT. A man in his mid-70s with a sellar mass. Brain Pathol. (2007) 17:115–6. doi: 10.1111/j.1750-3639.2007.00044_1.x
19. McCutcheon IE, Waguespack SG, Fuller GN, and Couldwell WT. Metastatic melanoma to the pituitary gland. Can J Neurol Sci. (2007) 34:322–7. doi: 10.1017/S0317167100006752
20. Guzel A, Maciaczyk J, Dohmen-Scheufler H, Senturk S, Volk B, Ostertag CB, et al. Multiple intracranial melanoma metastases: case report and review of the literature. J Neurooncol. (2009) 93:413–20. doi: 10.1007/s11060-008-9785-0
21. Kano H, Niranjan A, Kondziolka D, Flickinger JC, and Lunsford LD. Stereotactic radiosurgery for pituitary metastases. Surg Neurol. (2008) 72:248–255; discussion 255–256. doi: 10.1016/j.surneu.2008.06.003
22. Zoli M, Mazzatenta D, Faustini-Fustini M, Pasquini E, and Frank G. Pituitary metastases: role of surgery. World Neurosurg. (2013) 79:327–30. doi: 10.1016/j.wneu.2012.03.018
23. Burkhardt T, Henze M, Kluth LA, Westphal M, Schmidt NO, and Flitsch J. Surgical management of pituitary metastases. Pituitary. (2016) 19:11–8. doi: 10.1007/s11102-016-0001-8
24. Yang C, Liu L, Lan X, Zhang S, Li X, and Zhang B. Progressive visual disturbance and enlarging prolactinoma caused by melanoma metastasis: a case report and literature review. Medicine. (2017) 96:e6483. doi: 10.1097/MD.0000000000006483
25. Castle-Kirszbaum M, Goldschlager T, Ho B, Wang YY, and King J. Twelve cases of pituitary metastasis: a case series and review of the literature. Pituitary. (2018) 21:463–73. doi: 10.1007/s11102-018-0899-x
26. Lithgow K, Siqueira I, Senthil L, Chew HS, and Karavitaki N. Pituitary metastases: presentation and outcomes from a pituitary center over the last decade. Pituitary. (2020) 23:258–65. doi: 10.1007/s11102-020-01034-2
27. Yang K, Begley SL, Lynch D, Ye V, Saini J, Gutiérrez-Valencia E, et al. Pituitary metastases: a case series and scoping review. Pituitary. (2023) 26:538–50. doi: 10.1007/s11102-023-01349-w
28. Ramos R, MaChado MJ, Antunes C, Fernandes V, Marques O, and Almeida R. Metastasis of a dorsal melanoma to a pituitary adenoma mimicking pituitary apoplexy. Arq Bras Neurocirurgia. (2017) 36:238–42. doi: 10.1055/s-0037-1608907
29. He W, Chen F, Dalm B, Kirby PA, and Greenlee JD. Metastatic involvement of the pituitary gland: a systematic review with pooled individual patient data analysis. Pituitary. (2015) 18:159–68. doi: 10.1007/s11102-014-0552-2
30. Goulart CR, Mattei TA, and Ramina R. Cerebral melanoma metastases: a critical review on diagnostic methods and therapeutic options. ISRN Surg. (2011) 2011:276908. doi: 10.5402/2011/276908
31. Chiarion-Sileni V, Murr R, Pigozzo J, Sarti S, Tomassi O, and Romanini A. Brain metastases from Malignant melanoma. Forum (Genova). (2003) 13:170–182; quiz 190.
32. Qian M, Ma MW, Fleming NH, Lackaye DJ, Hernando E, Osman I, et al. Clinicopathological characteristics at primary melanoma diagnosis as risk factors for brain metastasis. Melanoma Res. (2013) 23:461–7. doi: 10.1097/CMR.0000000000000015
33. Mao LL and Si L. Basic and clinical research on brain metastasis of melanoma. Chin J Cancer Metastasis Invasion. (2019) 2019:5. doi: 10.3760/cma.j.issn.2096-5400.2019.02.011
34. Niu NN, Wu D, and Guo Z. Research progress on the molecular mechanisms of melanoma invasion and metastasis. Chin J Cancer Metastasis Invasion. (2020) 2020:4. doi: 10.3760/cma.j.cn101548-2020606-00071
35. Castellani G, Buccarelli M, Arasi MB, Rossi S, Pisanu ME, Bellenghi M, et al. BRAF mutations in melanoma: biological aspects, therapeutic implications, and circulating biomarkers. Cancers (Basel). (2023) 15:4026. doi: 10.3390/cancers15164026
36. Barbieri F, Thellung S, Würth R, Gatto F, Corsaro A, Villa V, et al. Emerging targets in pituitary adenomas: role of the CXCL12/CXCR4-R7 system. Int J Endocrinol. (2014) 2014:753524. doi: 10.1155/2014/753524
37. Claus M, van der Linden M, Van Dorpe J, Lapauw B, and T'Sjoen G. Primary sellar melanocytoma. Pituitary. (2021) 24:970–7. doi: 10.1007/s11102-021-01186-9
38. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
39. Viaene AN, Lee EB, Rosenbaum JN, Nasrallah IM, and Nasrallah MP. Histologic, immunohistochemical, and molecular features of pituicytomas and atypical pituicytomas. Acta Neuropathol Commun. (2019) 7:69. doi: 10.1186/s40478-019-0722-6
40. Patel KR, Zheng J, Tabar V, Cohen MA, and Girotra M. Extended survival after surgical resection for pituitary metastases: clinical features, management, and outcomes of metastatic disease to the sella. Oncologist. (2020) 25:e789–97. doi: 10.1634/theoncologist.2019-0520
Keywords: pituitary metastasis, malignant melanoma, multimodal diagnosis, BRAF V600E, case report
Citation: Li X, Jiang W, Tang X, Chen M, Deng W, Wang Y and Wang X (2025) Malignant melanoma with pituitary metastasis: A case report and literature review. Front. Endocrinol. 16:1661983. doi: 10.3389/fendo.2025.1661983
Received: 08 July 2025; Accepted: 18 September 2025;
Published: 06 October 2025.
Edited by:
Alfio Spina, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Ashutosh Rai, Queen Mary University of London, United KingdomZilu Chen, Rutgers, The State University of New Jersey, United States
Copyright © 2025 Li, Jiang, Tang, Chen, Deng, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingfu Wang, d2FuZ194ZnVAMTI2LmNvbQ==
†ORCID: Xingfu Wang, orcid.org/0000-0002-0734-4936
 Xiaoling Li
Xiaoling Li Wenhui Jiang1
Wenhui Jiang1 Xingfu Wang
Xingfu Wang