- 1Department of Ophthalmology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Hepatobiliary and Pancreatic Surgery, The Affiliated Hospital of Qingdao University, Qingdao, China
Diabetic retinopathy (DR), a prevalent microvascular complication affecting diabetic patients, imposes a significant global burden. Current therapies like anti-vascular endothelial growth factor (anti-VEGF) agents, offering limited efficacy in early stages and posing challenges related to invasiveness and recurrence. This underscores the urgent need for novel strategies targeting early intervention. This review proposes a unifying hypothesis: microRNAs (miRNAs) function as master regulators that integrate and amplify hyperglycemia-induced damage across multiple pathological axes—oxidative stress, inflammation, neurodegeneration, and vascular dysfunction. Dysregulation of specific miRNAs not only contribute to DR pathogenesis through multi-target modulation of key pathways but also exhibit stage-specific expression patterns in biofluids, positioning them as promising non-invasive biomarkers. Furthermore, miRNA-based therapeutic interventions, leveraging tools like quantitative reverse transcriptase PCR (qRT-PCR), droplet-based digital PCR (ddPCR), and microarrays for profiling, hold revolutionary potential to modulate key pathological cascades, and ultimately enable precision management strategies for early intervention and prevention of DR progression.
1 Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder pathologically defined by persistent hyperglycemia resulting from peripheral insulin resistance (hallmark of type 2 diabetes mellitus, T2DM) or insulin deficiency (characteristic of type 1 diabetes mellitus, T1DM). According to the International Diabetes Federation Diabetes Atlas (10th Edition), DM affected 10.5% of the global population aged 20–79 years (536.6 million individuals) in the baseline year, with epidemiological modeling projecting an alarming 16.2% relative increase to 12.2% (783.2 million cases) by 2045 (1). Diabetic retinopathy (DR), the most prevalent microvascular complication of DM, affects approximately 90% of T1DM patients and 60% of T2DM patients within 20 years of diagnosis (2).
Hyperglycemia impairs the integrity of retinal microvasculature and induces pathological angiogenesis (3). As a consequence of these vascular pathologies, the progression of DR unfolds in a stepwise manner. Initially, non - proliferative DR (NPDR), characterized by the presence of microaneurysms and intraretinal hemorrhages, gradually evolves into proliferative DR (PDR), marked by the emergence of neovascularization (4). Despite current therapies such as anti-vascular endothelial growth factor (anti-VEGF) therapy offering clinical benefits, their invasive nature and adverse effects—including vitreous hemorrhage, endophthalmitis, and retinal detachment from repeated injections (5)—highlight the pressing need to vigorously explore therapeutic strategies that either intervene at the early stage of DR or effectively prevent its development.
MicroRNAs (miRNAs), a class of small non-coding RNAs, are evolutionarily conserved post-transcriptional regulators that fine-tune gene expression by binding to target mRNAs, leading to translational repression or degradation (6). These molecules are integral to diverse biological processes such as cellular growth, apoptosis, fibrosis, and senescence, and their dysregulation has been implicated in numerous diseases, including cancer, diabetes, and cardiovascular disorders (7, 8). Since the landmark discovery of miRNA involvement in chronic lymphocytic leukemia in 2002, research has expanded to uncover their roles in complex ocular diseases, particularly DR (9). Recent studies highlight miRNAs as pivotal mediators of DR pathogenesis, influencing critical mechanisms like angiogenesis, inflammation, oxidative stress, and neurodegeneration. This review synthesizes current research on miRNA-mediated regulatory networks in DR, evaluates their diagnostic and therapeutic applicability.
2 Global epidemiology of DR
Global epidemiological modeling projects DR to be a persistent global public health crisis. As of 2020, an estimated 130 million individuals worldwide were afflicted with DR. Moreover, it is forecasted that by 2045, the number of patients developing DR will be near 160 million (10). Vision-threatening complications, including vision-threatening diabetic retinopathy (VTDR) and clinically significant macular edema (CSME), collectively impair over 47 million individuals, with VTDR affecting 6.17% (28.54 million; 95% CI: 25.12–32.34 million) and CSME 4.07% (18.83 million; 95% CI: 3.42–4.82%) of the global diabetic population (10).
As the fifth leading cause of blindness among working-age adults (50 years of age and older) (11). DR exhibits striking geographical disparities: Asia shoulders the highest global burden, propelled by rapid urbanization, dietary transformations, and a burgeoning population of individuals with diabetes (12, 13). In China, with its large diabetic population and aging demographic, approximately 19.5 million people with diabetes are affected by DR. Among them, one - fifth have reached the VTDR stage (14). India demonstrates stark regional contrasts, with DR prevalence ranging from 12.27% in central regions to 34.06% in northern states, alongside an urban-rural divide (17.4% vs. 14.0%) (15). In contrast to the escalating DR burden in low- and middle-income regions, high-income nations paradoxically confront persistent DR challenges despite their comprehensive screening programs and widespread access to anti-VEGF therapies. In the United States, where 37.6 million adults were diagnosed with diabetes in 2021, epidemiological modeling revealed that 9.60 million individuals (95% UI: 7.90–11.55 million) — representing 26.43% (95% UI: 21.95–31.60) of the diabetic population — were affected by DR. Alarmingly, 1.84 million patients (95% UI: 1.41–2.40 million) progressed to VTDR, translating to a 5.06% prevalence rate (95% UI: 3.90–6.57) among diabetics (16). In Europe, DR is observed in 25.7% of individuals diagnosed with type 1 or 2 diabetes. Among this population, 18.5% exhibit mild to moderate NPDR, while 3.7% develop DME. Despite a steady decline in DR prevalence across Japan over the past decade, the prevalence rate remains alarmingly high — affecting 23.5% of Japanese diabetes patients (17).
3 Updated diagnosis and therapy of DR
ETDRS classification remains the gold standard for DR due to robust validation predicting progression, but its complexity impedes clinical adoption. This led to the ICDR Severity Scale— a streamlined adaptation of the ETDRS framework—which now serves as the most commonly adopted benchmark in clinical workflows due to its simplified five-tier staging (18, 19). Modern diagnostics integrate multimodal imaging: ultra-widefield (UWF) retinal imaging captures peripheral lesions, and optical coherence tomography angiography (OCTA) visualizes microvasculature (20, 21). AI enables automated lesion detection and predictive modeling for scalable screening (22, 23). These innovations enhance staging accuracy, facilitate progression monitoring, and optimize therapeutic decisions.
Anti-VEGF therapy (ranibizumab, bevacizumab, aflibercept) is the established first-line treatment for center-involved DME and a validated option for PDR, with extensive evidence demonstrating efficacy in edema reduction and vision improvement (24, 25). However, the DRCR.net Protocol W trial indicated that while proactive anti-VEGF treatment for NPDR prevents progression to PDR or DME, it does not yield superior long-term visual outcomes compared to initial observation with as-needed treatment upon complication development (26). Furthermore, the apparent improvement in DR severity with anti-VEGF often masks persistent underlying retinal ischemia, and lesions frequently recur rapidly after therapy cessation (24). For PDR, panretinal photocoagulation (PRP) remains the standard, effectively reducing vision loss risk (27). Emerging therapies focus on enhancing efficacy and durability while reducing injection frequency. Faricimab, a bispecific antibody inhibiting both VEGF and angiopoietin-2 (Ang-2)/Tie pathways, achieved visual gains comparable to aflibercept in DME but with superior anatomic outcomes and significantly extended dosing intervals (≥12–16 weeks for >50-70% of eyes at 1 year), addressing vascular instability more effectively (28–30). Nevertheless, treatment selection must weigh adherence challenges, injection burden, and patient preference against PRP’s durability.
Recent research focuses on identifying novel biomarkers – defined as biological molecules indicating physiological or pathological processes – to enable early detection of DR, halt progression, and prognostic outcomes (e.g., predicting NPDR→PDR transition), thereby guiding resource allocation and treatment strategies (31). Multiple clinical studies have identified 12-HETE and 2-piperidone in plasma/serum as potential DR biomarkers, which exhibited superior diagnostic performance to HbA1c in DR assessment using multiplatform metabolomics approaches (32). Circulating miRNAs represent a revolutionary frontier. Emerging evidence demonstrates that dynamic alterations in miRNA expression profiles in biofluids (e.g., serum, aqueous humor) strongly correlate with specific DR progression stages. These miRNAs exhibit highly sensitive and specific differential expression patterns, enabling non-invasive liquid biopsies to distinguish NPDR from PDR (33). This molecular stratification provides a crucial complement to structural imaging modalities, offering profound potential not only for earlier and more precise diagnosis but also for unveiling novel therapeutic targets to address the underlying pathophysiology of DR.
4 miRNAs biogenesis and silencing mechanisms
miRNAs are small non-coding RNA molecules (≈22 nucleotides) that play a crucial role in post-transcriptional gene regulation, primarily by inhibiting protein translation or promoting mRNA cleavage (34). Their multi-step process of biogenesis involving both nuclear and cytoplasm. Initially, miRNA genes are transcribed by the RNA polymerase II (RNA poly II) into primary miRNA transcripts (pri-miRNAs), which are large, hairpin-structured RNA molecules capped with a 7-methylguanosine moiety and polyadenylated at the 3′ end. These pri-miRNAs are then recognized and processed in the nucleus by the microprocessor complex, comprising the RNase III enzyme Drosha and its cofactor DGCR8. The microprocessor complex cleaves the pri-miRNA near the base of its stem-loop structure to generate a ≈70-nucleotide precursor miRNA (pre-miRNA). The pre-miRNA is subsequently transported to the cytoplasm via Exportin-5 protein. In the cytoplasm, another RNase III enzyme Dicer, along with its partner TRBP (TAR RNA-binding protein), cleaves the pre-miRNA to produce a shorter RNA duplex. This duplex consists of the mature miRNA (guide strand) and its complementary strand (passenger strand). The mature strand is selectively incorporated into the RNA-induced silencing complex (RISC), where Argonaute (AGO) proteins serve as core components, while the complementary strand is typically degraded. Mature miRNAs within the RISC silence or regulate gene expression by binding to complementary sequences in target mRNAs. This interaction leads to gene silencing through two primary pathways: (1) target mRNA degradation, which involves perfect or near-perfect complementarity between the miRNA “seed region” (nucleotides 2–8) and the 3’UTR of target mRNAs. Upon incorporation into the RISC with AGO proteins, miRNAs guide the complex to these complementary sites, triggering endonucleolytic cleavage of the mRNA by AGO; (2) translation repression, when miRNA-mRNA binding is imperfect, the miRNA-RISC complex can suppress translation even with partial complementarity (35). Notably, miRNAs exhibit the capacity to regulate multiple target mRNAs concurrently, while a single mRNA may be subject to regulation by multiple miRNAs — dynamics that underscore the complexity of the miRNA-mRNA regulatory network (36) (Figure 1).
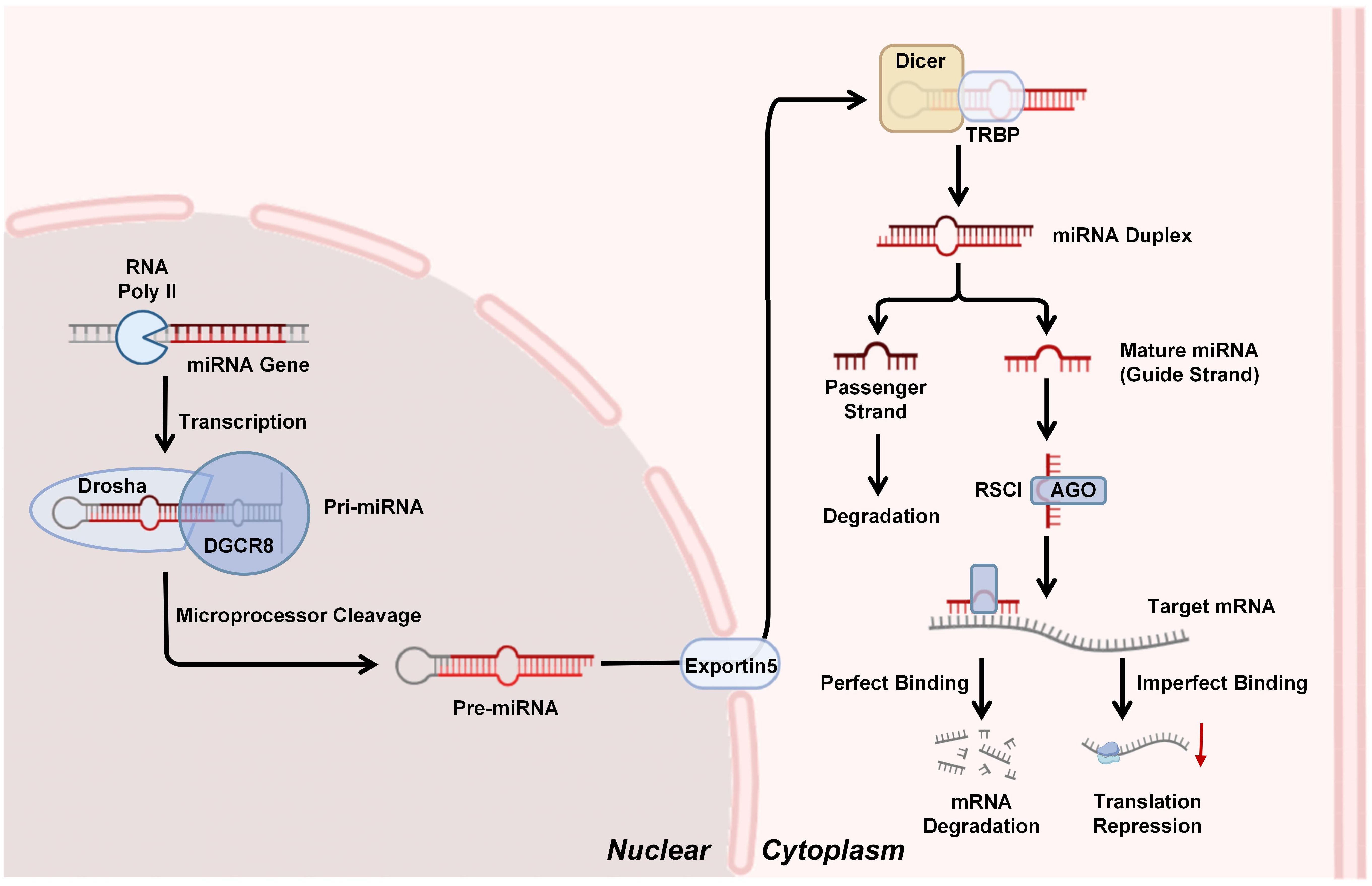
Figure 1. miRNAs biogenesis and silencing mechanism: miRNA genes are transcribed by RNA polymerase II (Pol II) into primary miRNAs (pri-miRNAs), which are processed by the Drosha-DGCR8 complex into precursor miRNAs (pre-miRNAs). Pre-miRNAs are exported to the cytoplasm by Exportin-5 and cleaved by Dicer-TRBP into miRNA duplexes. The mature miRNA strand is loaded into the RNA-induced silencing complex (RISC), where Argonaute (AGO) proteins facilitate target mRNA degradation or translational repression through complementary base pairing.
5 miRNA profiling tools
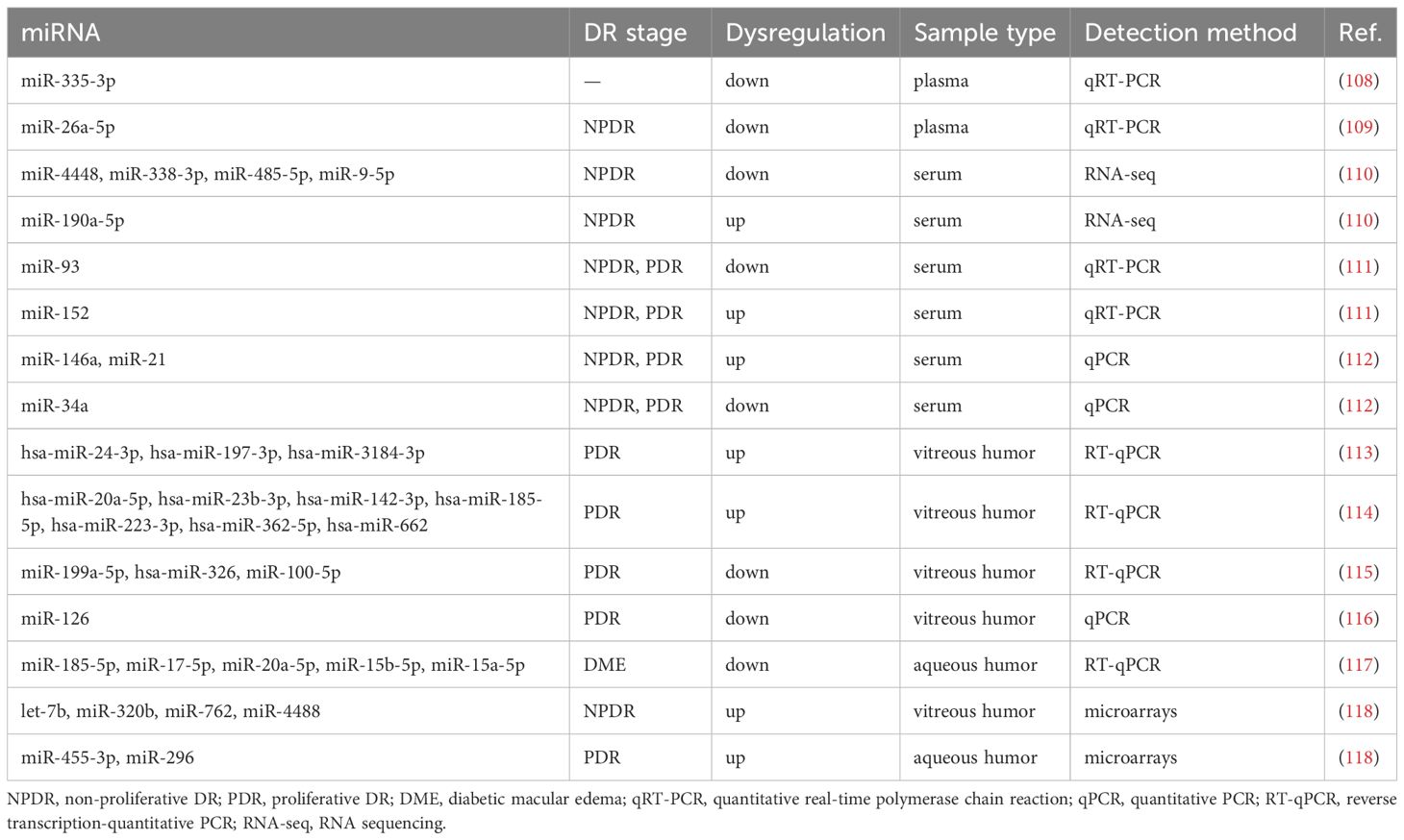
miRNA profiling has evolved into a sophisticated field with diverse methodologies tailored to detect and quantify miRNAs at different stages (e.g., primary, precursor, and mature miRNAs). The exploration of miRNA profiling tools in DR has provided critical insights into disease mechanisms and therapeutic targets. Numerous technologies have been utilized for miRNA detection and quantification including Northern blot, quantitative reverse transcriptase PCR (qRT-PCR), droplet-based digital PCR (ddPCR), miRNA microarrays, and other technologies. Below is an overview of each method, highlighting its strengths and limitations (Table 1).
5.1 Northern blot
Northern blot, the first method for miRNA quantification following the initial discovery of lin-4 in 1993, remains a gold standard for validating novel miRNAs (37). It confirms RNA size via denaturing gel electrophoresis and miRNA-specific probes. Its advantages include widespread laboratory availability without specialized equipment. However, limitations include low sensitivity due to high RNA requirements (≥5-10 μg) and time-consuming procedures (2–3 days). This technique’s utility persists in miRNA verification despite being superseded by newer high-throughput methods in profiling studies (38).
5.2 Quantitative reverse transcriptase PCR
Compared to Northern blot, qRT-PCR offers rapid and sensitive detection of miRNAs, leveraging stem-loop primers or oligonucleotide-based approaches for reverse transcription and target-specific amplification, followed by quantification via sequence-specific probes. While stem-loop designs enhance primer binding specificity, locked nucleic acid (LNA)-modified primers further improve target recognition but may compromise amplification efficiency, posing challenges for quantifying low-abundance miRNAs. Although cost-efficient and technically accessible, qRT-PCR suffers from limited throughput, requires reference gene normalization for accurate relative quantification, and relies on predefined miRNA sequences—restricting discovery of novel miRNAs (39, 40).
5.3 Droplet-based digital PCR
ddPCR enables absolute miRNA quantification by partitioning reactions into thousands of nanoliter-scale droplets. This technique eliminates the need for standard curves or reference genes and achieves exceptional sensitivity and precision. However, its high cost, reliance on specialized instrumentation, and limited scalability for large-scale studies pose challenges for routine use in DR biomarker discovery (41).
5.4 miRNA microarrays
Fluorescently labeled miRNAs hybridize to array-immobilized probes, allowing simultaneous profiling of thousands of miRNAs. Though it is cost-efficient for high-throughput screening, microarrays require substantial RNA input, lack sensitivity for low-abundance miRNAs, and are confined to known sequences. Relative quantification and a narrow dynamic range further limit their utility in comprehensive DR studies (42).
6 Mechanistic roles of miRNAs in DR
miRNA biogenesis and silencing mechanisms are critical to post-transcriptional gene regulation. Dysregulation of these processes can exert profound impacts on various diseases, including systemic metabolic eye disorders such as DR. Within DR pathogenesis, miRNAs mediate key roles in modulating the major effects of DR progression (inflammation, oxidative stress, and vascular dysfunction), highlighting their involvement in orchestrating complex molecular pathways that underpin disease progression (43–45). Changes of miRNA levels in various tissues, organs, and blood have been reported across multiple studies in diabetic patients and animal models. In patients with T1DM, circulating miR-346, miR-148a, miR-181a, and miR-208 are upregulated, while miR-16, miR-93, miR-191, and miR-146a are downregulated (46, 47). Approximately 350 miRNAs are expressed, with at least 86 miRNAs dysregulated in the retinas of streptozotocin (STZ)-induced diabetic rats (48, 49). A growing focus lies in deciphering how miRNAs interact with target mRNAs to regulate cellular dysfunction, vascular permeability, neovascularization, and retinal neurodegeneration, thereby unraveling the underlying pathobiological mechanisms of DR (50). Figure 2 illustrates the mechanistic roles of miRNAs in DR.
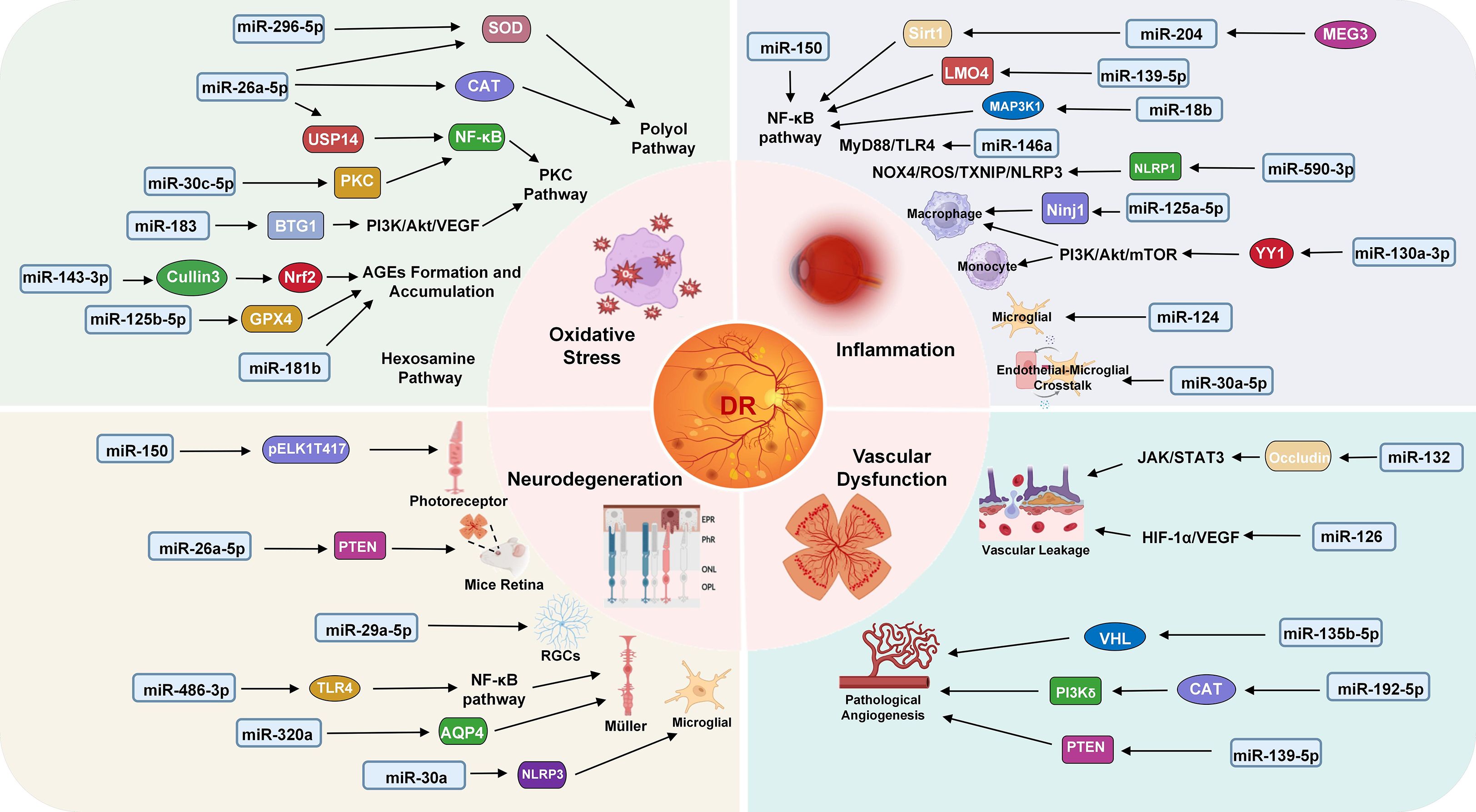
Figure 2. Mechanism of miRNAs in DR. Key miRNAs are involved in four major pathological processes: oxidative stress (miR-296-5p, miR-26a-5p, miR-30c-5p, miR183, miR-143-3p, miR-125b-5p, miR-181b), inflammation (miR-150, miR-204, miR-139-5p, miR-18b, miR-146a, miR-590-3p, miR-125a-5p, miR-130a-3p, miR-124, miR-30a-5p), neurodegeneration (miR-150, miR-26a-5p, miR-29a-5p, miR-486-3p, miR-320a, miR-30a) and vascular dysfunction (miR-132, miR-126, miR-135b-5p, miR-192-5p, miR-139-5p). Each miRNA modulates specific target genes and signaling pathways, contributing to DR progression.
6.1 Oxidative stress
Oxidative stress, marked by pro-oxidants’ dominance over antioxidant defenses, drives cellular dysfunction via redox imbalance. While reactive oxygen species (ROS) normally regulate mitochondrial-lysosomal crosstalk during ATP production, excessive ROS accumulation damages biomolecules, exacerbates mitochondrial dysfunction, and causes uncontrolled oxidative tissue injury (51, 52). The core pathological mechanisms of DR are intimately linked to hyperglycemia-induced oxidative stress, with the polyol, hexosamine, protein kinase C (PKC) pathways, and formation of advanced glycation end products (AGEs) representing four central molecular networks (53–55). This section systematically discusses the action mechanisms of each pathway and research progress of miRNAs in DR oxidative stress.
6.1.1 Activation of polyol pathway
Importantly, hyperglycemia activates the polyol pathway via aldose reductase (AR), leading to NADPH depletion and increased ROS (53, 56). The overexpression of miR-26a-5p attenuates DR by enhancing superoxide dismutase (SOD) and catalase (CAT) activities. In HG-exposed retinal Müller cells, miR-26a-5p overexpression elevated SOD/CAT levels, reduced oxidative markers (MDA, ROS), and inhibited mitochondrial cytochrome c release, alleviating oxidative stress. In STZ-induced diabetic male mice, miR-26a-5p agomir improved retinal histopathology and reduced oxidative markers (57). Another study found that miR-296-5p was downregulated in diabetic male mice retinal tissues, exacerbating DR progression. Restoring miR-296-5p alleviated DR pathology by targeting retinal ganglion cells: it reduced their apoptosis (altered Bcl-2/Bax/Caspase-3 expression) and decreased Evans blue leakage, indicating improved vascular integrity. Additionally, miR-296-5p upregulation suppressed oxidative stress (lowered VEGF and MDA, enhanced SOD activity) and inhibited GNAI2 expression by binding its 3’UTR (58).
6.1.2 Activation of PKC pathway
PKC drives diabetic complications through hyperglycemia-induced diacylglycerol/ROS in retinal vasculature, triggering microvascular dysfunction via Nox, NF-κB, and VEGF (59, 60). Recent studies highlight miR-26a-5p as a critical regulator in DR pathogenesis. Specifically, in vitro experiments using HG-treated Müller cells exhibited downregulated miR-26a-5p, elevated oxidative stress, mitochondrial cytochrome c release, and NF-κB-driven inflammation (TNF-α, IL-1β, IL-6↑). Overexpression of miR-26a-5p mitigated these effects by directly targeting USP14, thereby suppressing USP14-mediated NF-κB activation (p-IκBα, p-p65↓) and nuclear translocation. Extending these findings to in vivo models, STZ-induced diabetic male mice displayed similar miR-26a-5p downregulation, retinal oxidative injury, and inflammatory responses, which were ameliorated by miR-26a-5p agomir treatment (57). The overexpression of miR-183 in DR rats activates the PI3K/Akt/VEGF signaling pathway by directly targeting and suppressing BTG1, thereby promoting vascular endothelial cell proliferation, pathological angiogenesis (via upregulated CD34 and eNOS), and ROS accumulation. Conversely, miR-183 silencing upregulated BTG1, inhibiting oxidative damage and pro-angiogenic pathways (61). Furthermore, miR-30c-5p acts as a critical regulator of DR-associated vascular dysfunction by suppressing PLCG1, thereby inhibiting the PKC/NF-κB pathway in HG-treated human retinal endothelial cells (HRECs) (62).
6.1.3 Formation and accumulation of AGEs
AGEs disrupt extracellular matrix (ECM) integrity and bind to RAGE to activate the NADPH oxidase/NF-κB/MAPK pathways, thereby exacerbating DR (53). Studies have demonstrated that AGEs-stimulated Müller cells exhibited elevated ROS and inflammation, which were mitigated by miR-143-3p-mediated inhibition of Cullin3 neddylation, thereby stabilizing Nrf2 to enhance antioxidant responses (63). Under AGEs-induced HRECs, miR-125b-5p suppresses P53-mediated ferroptosis by restoring glutathione peroxidase 4 (GPX4) activity and blocking lipid peroxidation, thereby preserving blood-retina barrier (BRB) integrity (64). Beyond retinal cells, systemic investigations in STZ-induced diabetic male mice revealed miR-181b downregulation exacerbated endothelial dysfunction by amplifying oxidative stress and vascular inflammation. AGEs suppressed miR-181b expression in human renal arteries and human umbilical vein endothelial cells, correlating with elevated superoxide production and impaired vasodilation. miR-181b mimics reversed these effects, reducing oxidative stress and restoring endothelial function. These findings suggest that miR-181b may mitigate oxidative stress in vascular endothelial cells, highlighting its therapeutic potential for DR (65).
6.1.4 Activation of hexosamine pathway
Hyperglycemia activates the hexosamine pathway via glutamine: fructose-6-phosphate amidotransferase (GFAT), producing UDP-N-acetylglucosamine (UDP-GlcNAc) that drives pathological O-glycosylation (66). O-GlcNAc modifications drive pathogenesis by activating AMPK and inducing photoreceptor degeneration in retinal neurons (67). To date, no studies have reported that miRNAs are involved in DR via activation of the hexosamine pathway; this still warrants further investigation.
6.2 Inflammation and immune cell activation
Hyperglycemia directly promotes inflammatory gene expression and activates immune cells via multiple pathways, amplifying inflammatory mediator production and impairing vascular endothelial cell structural and functional integrity (68). miRNAs further exert multidimensional regulatory roles in DR pathology by targeting inflammatory mediators, signaling pathways, and immune cell functions (69, 70). This section systematically discusses its mechanisms from three dimensions: inflammatory signaling pathway modulation, immune cell recruitment and activation, and miRNA-endothelial-immune cell crosstalk.
6.2.1 Inflammatory signaling pathway modulation
The dysregulation of inflammatory pathways in DR is centrally orchestrated by miRNAs through their targeted modulation of the NF-κB pathway, a master regulator of pro-inflammatory cytokine production (e.g., TNF-α, IL-1β, IL-6). miR-150, an intrinsic anti-inflammatory regulator, suppresses NF-κB activation under physiological conditions; however, its diabetes-associated depletion exacerbates ocular inflammation by disinhibiting NF-κB-driven responses, as evidenced by LPS-induced endothelial cell models (71–73). Furthermore, the MEG3/miR-204/Sirt1 axis mitigates inflammation via NF-κB pathway that disrupts pro-inflammatory cytokine production (e.g., TNF-α, IL-6) (74). Similarly, miR-18b suppresses MAP3K1-dependent phosphorylation of NF-κB p65, reducing vascular leakage and retinal thickening in STZ-induced diabetic SD rats, while miR-139-5p targets LMO4 to block NF-κB activation and downstream inflammatory cascades (TNF-α, IL-6, Cox-2) in HG-incubated human retinal pigment epithelial (ARPE-19) cells (75, 76). Apart from NF-κB, miR-146a attenuates inflammation in HG-treated primary human retinal microvascular endothelial cells (hRMECs) by inhibition of MyD88/TLR4 signaling and suppression of TNF-α production, positioning it as a pivotal regulator of retinal endothelial dysfunction (77). Additionally, miR-590-3p plays an active role in inflammation by directly targeting NLRP1, leading to the inhibition of pyroptosis via the NOX4/ROS/TXNIP/NLRP3 signaling cascade (78, 79). Collectively, the dysregulation of inflammatory signaling pathways in DR is centrally orchestrated by miRNAs through their targeted modulation of key transcriptional regulators and downstream effectors.
6.2.2 Immune cell recruitment and activation
The regulation of immune cells dynamics in DR involves miRNA-mediated modulation of chemokine signaling, cellular adhesion, and inflammatory cascades. miR-125a-5p directly targets Ninj1, a key mediator of macrophage adhesion and pro-inflammatory factor release. By suppressing Ninj1, miR-125a-5p reduces macrophage infiltration into inflamed retinas and attenuates vascular leakage in both endotoxin-induced and STZ-induced diabetic mice models, underscoring its role in preserving vascular integrity (80). Concurrently, overexpression of miR-130a-3p attenuated DR progression by targeting YY1 to inhibit the PI3K/Akt/mTOR pathway, thereby promoting macrophage autophagy, reducing M1 polarization, and suppressing inflammation in HG-treated human monocyte (THP-1) and STZ-induced diabetic male mice (81). miR-124 further modulates immune homeostasis by normalizing HG-induced microglial hyperactivity, suppressing inflammatory mediators (e.g., Tnf-α, Ccl2, Ccl3), and downregulating transcription factors (PU.1) and lipid raft proteins (Flot1), thereby preventing vasoregression and neuroretinal dysfunction (48).
6.2.3 miRNA-endothelial-immune cell crosstalk
In DR, miRNA-mediated regulation of intercellular adhesion molecule-1 (ICAM-1) and endothelial-immune crosstalk critically drives inflammatory pathology. Studies reveal elevated ICAM-1 expression in retinal vessels, promoting leukocyte adhesion, vascular leakage, and endothelial injury (82). miR-146a directly modulates this pathway by targeting IRAK1 and ICAM-1, with its rhythmic expression in diabetic STZ-induced diabetic rat retinas inversely correlating with ICAM-1 oscillation, suggesting circadian regulation of vascular inflammation (83). Apart from ICAM-1, miR-30a-5p emerges as a dual regulator of endothelial-microglial crosstalk. In a mice model of ischemic retinopathy, miR-30a-5p inhibition reduced pathological neovascularization by enhancing FasL+ microglial interactions with Fas+ endothelial cells, promoting endothelial apoptosis and microglial phagocytosis (84).
6.3 Retinal neurodegeneration
Emerging evidence reveals that neurodegeneration in DR, characterized by neuronal dysfunction, apoptosis, and reactive gliosis, precedes microvascular changes (85). Hyperglycemia-induced metabolic stress triggers tau hyperphosphorylation and mitochondrial dysfunction in retinal ganglion cells (RGCs), while oxidative stress and neuroinflammation reduce neurotrophic factors (86, 87). Activated microglia and Müller cells exacerbate neuronal damage through cytokine release and glutamate disruption (88–90).
Previous studies have shown that miRNAs critically regulate the neurodegenerative mechanisms (91). Specifically, in T2D mice, deletion of miR-150 (miR-150-/-) exacerbates photoreceptor apoptosis detected by increased TUNEL staining in the retina. This effect occurs because decreased miR-150 promotes nuclear pELK1T417 translocation, identified as the key step triggering photoreceptor apoptosis in response to the diabetic/high-fat conditions (92). Similarly, miR-26a-5p is downregulated in STZ-induced diabetic mice retina, exacerbating neuronal apoptosis. Mechanistically, miR-26a-5p directly targets PTEN, suppressing its upregulation in inner/outer nuclear layers and dampening glial activation (via GFAP reduction) and inflammatory markers (IL-1β, NF-κB, VEGF) (93). Transitioning to human studies, miR-29a-5p levels were markedly elevated in DR patients’ blood, correlating with hyperglycemia and dyslipidemia. HG upregulates miR-29a-5p expression in RGCs. This upregulation induced RGCs apoptosis, oxidative stress (increased ROS/MDA, decreased SOD), and inflammation (elevated TNF-α/IL-6) via SIRT3 suppression (94). Moreover, in HG-treated Müller cells, miR-486-3p overexpression reduced oxidative stress, inflammation, and apoptosis by targeting TLR4 to repress NF-κB signaling (95). Similarly, miR-320a overexpression reduced hypoxia-induced damage of Müller cells by suppressing aquaporin-4 (AQP4) expression, inhibiting superoxide anion production, enhancing cell viability, and promoting AQP4 internalization, thereby alleviating retinal edema. Conversely, miR-30a activates microglia in an NLRP3-dependent manner, thus promoting the progress of DR (96).
6.4 Vascular dysfunction
Pathological angiogenesis and vascular hyperpermeability are hallmark features of DR, driven by dysregulated miRNAs that modulate endothelial proliferation, junctional integrity, and angiogenic signaling cascades. In angiogenesis, miR-139-5p emerges as a critical promoter of retinal neovascularization, where its upregulation in HG-exposed RMECs enhances VEGF production, cell migration, and tube formation by repressing PTEN, a key suppressor of the PI3K/Akt pathway (97). Similarly, miR-135b-5p promotes endothelial cell proliferation and angiogenesis by inhibiting Von Hipp-el-Lindau (VHL) expression, as demonstrated through in vitro retinal endothelial cell isolation from DR mice transfected with miR-135b-5p inhibitor or VHL-overexpressing plasmids, and in vivo using a STZ-induced diabetic mice model (98). Conversely, miR-192-5p acts as a protective regulator by suppressing ELAVL1-mediated PI3Kδ stabilization, thereby inhibiting endothelial proliferation and migration in HG-treated hRMECs, with its overexpression mitigating pathological angiogenesis (99).
Interestingly, in the field of vascular permeability and stability, miR-132 disrupts retinal barrier integrity by directly targeting occludin, a tight junction protein, via JAK/STAT3 activation in HG-stressed ARPE-19 cells, exacerbating vascular leakage and epithelial mobility. Pharmacological inhibition of miR-132 restores occludin/E-cadherin expression and reduces permeability (100). Meanwhile, miR-126 downregulation in diabetic retinas plays a pivotal role in vascular destabilization. Extracellular vesicles (EVs) from mesenchymal stem cells under diabetic-like conditions suppress miR-126 in retinal pericytes, amplifying HIF-1α/VEGF-driven pericyte loss and BRB dysfunction. miR-126 deficiency correlates with enhanced vascular leakage, independent of Ang-2/PDGF pathways, positioning miR-126 restoration as a strategy to counteract EV-mediated BRB breakdown (101).
7 Circulating miRNAs: DR biomarkers
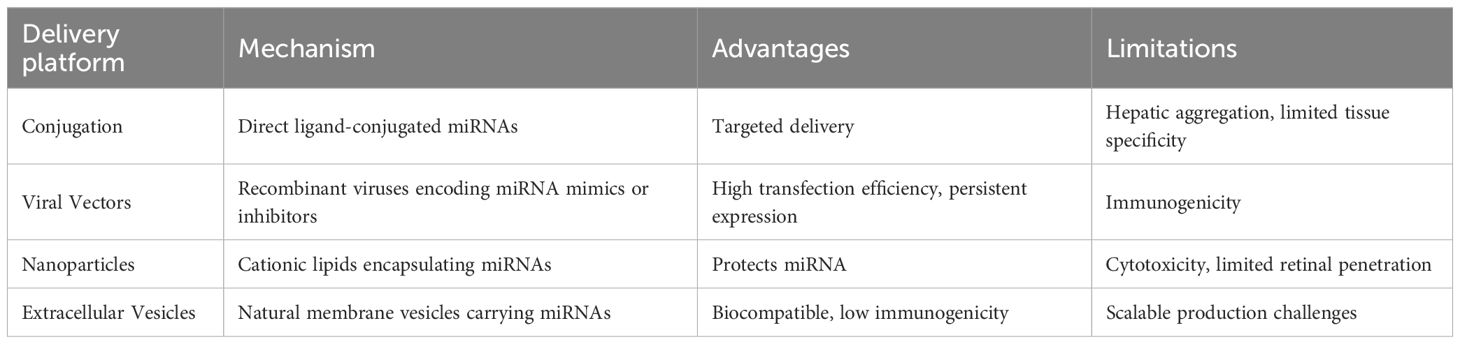
miRNAs, identified as highly stable molecules in various biological fluids, can be efficiently extracted from blood and other liquid biopsies (102). Emerging evidence demonstrates that dynamic alterations in miRNA expression profiles reflect pathological progression across diverse diseases, including cancers, cardiovascular diseases, and even DR (103, 104). For instance, miR-29a-3p exhibits stage-specific dysregulation during colorectal cancer progression, correlating with tumor invasiveness and metastatic potential (105). Similarly, miR-21 has emerged as a pivotal regulator in cardiovascular pathologies, driving cardiac remodeling (106). Notably, while the functional roles of these miRNAs in retinal tissues require further elucidation, their diagnostic utility is underscored by large-scale studies. Circulating miRNAs in biofluids (e.g., plasma, serum, aqueous humor, vitreous humor) not only reflect pathological states but also hold represent promising non-invasive biomarkers for early diagnosis and therapeutic monitoring (107). Table 2 summarizes the literature review regarding the expression profiles of miRNAs in DR.
Some studies have shown that blood-derived miRNAs possess remarkable predictive value for the diagnosis of DR. Through qRT-PCR analysis, plasma miR-335-3p levels were significantly decreased in DR patients and demonstrated specificity in distinguishing DR cases from healthy individuals and T2DM patients, suggesting its utility as a non-invasive biomarker for DR screening (108). Similarly, qRT-PCR quantification showed markedly reduced plasma miR-26a-5p levels in T2DM patients with NPDR compared to those without retinopathy, correlating with superior retinal nerve fiber layer (RNFL) thickness (109). RNA-seq profiling of serum identified five differentially expressed miRNAs (miR-4448, miR-338-3p, miR-485-5p, miR-9-5p, and miR-190a-5p) in NPDR patients, with the first four downregulated and miR-190a-5p upregulated (110). Furthermore, qRT-PCR analysis revealed dynamic expression patterns of miR-93 and miR-152 during diabetes progression in serum: miR-93 levels decreased (OR=0.25, p = 0.028), while miR-152 levels increased (OR=1.37, p < 0.001) across diabetes, NPDR, and PDR cohorts (111). Notably, qPCR assays revealed serum miR-146a and miR-21 levels correlating with DR severity (PDR > severe NPDR > moderate > mild > normal fundus), while miR-34a is in the opposite tendency (112).
The available data on the expression profiles of miRNAs in the vitreous humor and aqueous humor of eyes with DR remain scarce, primarily due to ethical considerations that preclude the use of healthy individuals as controls. Using RT-qPCR, studies with non-diabetic controls (macular hole cases) identified upregulated miRNAs in vitreous analyses of PDR patients: hsa-miR-24-3p, hsa-miR-197-3p, hsa-miR-3184-3p correlated with VEGF-A/TGF-β levels, while hsa-miR-20a-5p, hsa-miR-23b-3p, hsa-miR-142-3p, hsa-miR-185-5p, hsa-miR-223-3p, hsa-miR-362-5p, and hsa-miR-662 showed elevation compared to controls (113, 114). Conversely, miR-199a-5p and hsa-miR-326 were downregulated in PDR vitreous, and miR-100-5p demonstrated diagnostic potential through its reduction (114, 115). qPCR analysis stratifying PDR severity stages (IV-VI) against idiopathic macular hole controls revealed progressive miR-126 downregulation correlating with advanced fibrovascular proliferation in vitreous (116). In contrast, aqueous humor studies employed different control cohorts: RT-qPCR comparisons between diabetic macular edema (DMO) patients and cataract controls identified five markedly downregulated miRNAs (miR-185-5p, miR-17-5p, miR-20a-5p, miR-15b-5p, miR-15a-5p) (117). A comprehensive pilot study employing miRNA 3.0 microarrays systematically profiled DR subtypes (T1DM with PDR, T2DM with PDR, T2DM with NPDR) against non-diabetic vitreoretinal surgery controls, revealing fluid-specific patterns: vitreous showed predominant miRNA upregulation (e.g., let-7b, miR-320b, miR-762, miR-4488), while aqueous exhibited subtype-unique markers (miR-455-3p in T2DM with NPDR; miR-296 in T2DM with PDR) (118). Though limited by heterogeneous control groups (macular pathologies, cataracts), these findings collectively suggest specific miRNA signatures reflecting DR progression, with vitreous and aqueous humor profiles changes potentially serving as accessible proxies for intraocular pathophysiology.
8 miRNA-targeted retinal therapies
Emerging understanding of miRNAs in biology and their dysregulation in many diseases has prompted scientists to investigate their potential use in DR. Current strategies focus on three principal modalities: (1) miRNA restoration therapy, which introduces synthetic oligonucleotides to supplement downregulated or non-functional miRNAs; (2) miRNA inhibition therapy, which employs antagonists to suppress overexpressed miRNAs; (3) miRNA delivery therapy, where advances in systems like extracellular vesicles and exosomes enhance therapeutic precision by improving miRNA bioavailability and minimizing off-target effects. Figure 3 illustrates miRNA-targeted therapeutic strategies in DR.
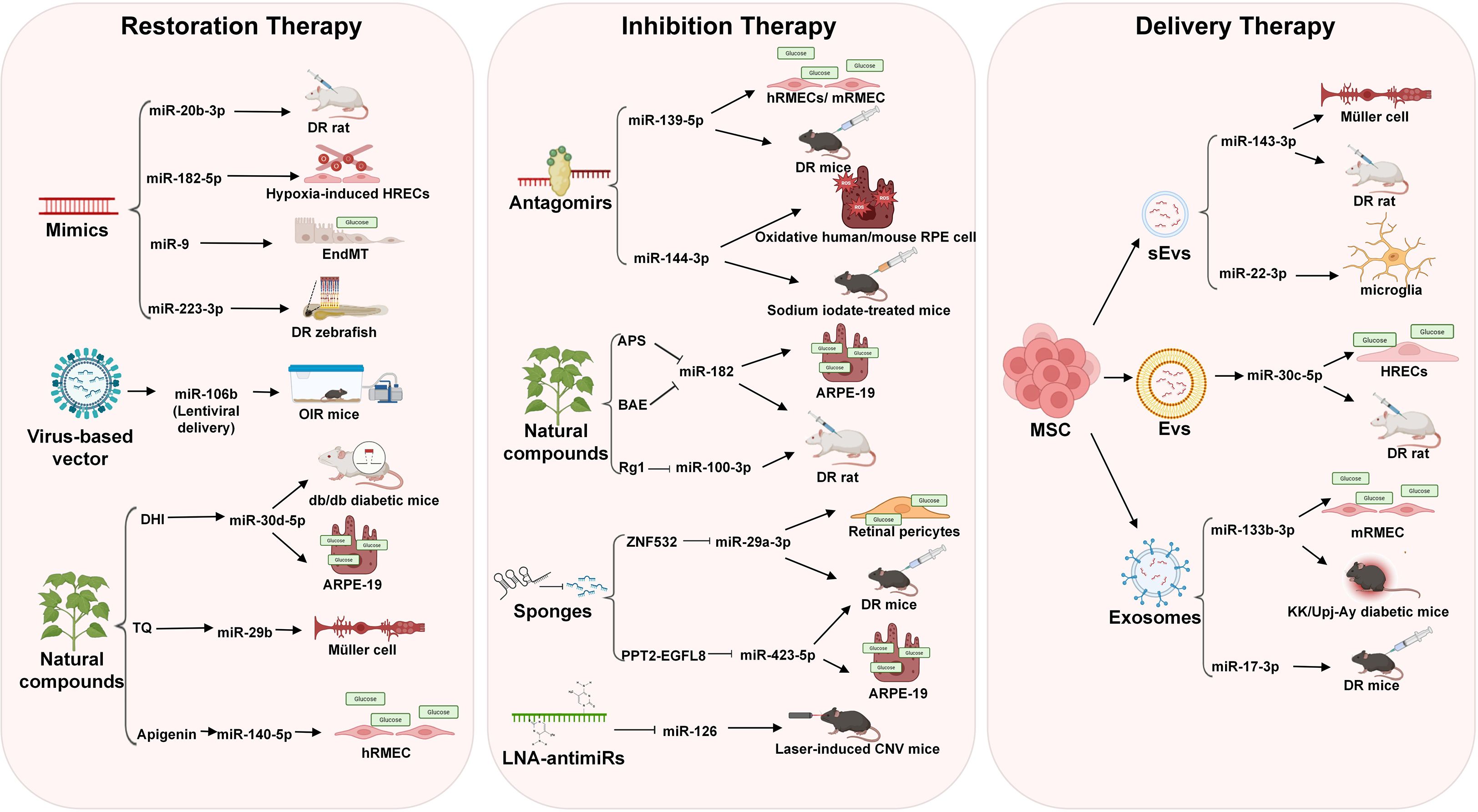
Figure 3. Major strategies for miRNA-targeted therapy in DR. Three main approaches include: miRNA restoration therapy (including miRNA mimics, natural compounds, and virus-based vectors), miRNA inhibition therapy (including miRNA antagomirs, natural compounds, miRNA sponges, and LNA-antimiRs), and miRNA delivery therapy (including mesenchymal stem cell (MSC)-derived extracellular vesicles (EVs), small extracellular vesicles (sEVs), and exosomes).
8.1 miRNA restoration therapy
miRNA mimics are double-stranded RNA oligonucleotides that exactly copy the mature miRNA duplex to replenish downregulated miRNAs (119). Studies found that miR-20b-3p mimics attenuated retinal inflammation in DR rat models by targeting TXNIP, leading to reduced inflammatory cytokine levels and improved retinal pathology (120). In HRECs, miR-182-5p mimics suppressed hypoxia-induced angiogenesis by downregulating ANG and BDNF, thereby enhancing cell viability and stabilizing vascular integrity (121). Furthermore, miR-9 mimics counteracted glucose-induced endothelial-to-mesenchymal transition (EndMT) by inhibiting TGF-β and proinflammatory pathways, effectively reducing retinal vascular leakage (122). Interestingly, miR-223-3p mimics in zebrafish models exhibited dual effects: while elevating glucose levels, they simultaneously mitigated retinal vascular degeneration and restored structural abnormalities in ganglion and nuclear layers through VEGF-A/NRP-1 pathway modulation (123). Furthermore, a miRNA-encoding plasmid or virus-based vector could be exploited for purposes requiring miRNA delivery for a specific period. In the oxygen-induced retinopathy (OIR) mice model, lentiviral delivery of miR-106b reduced pathological neovascularization by approximately 45-50%, achieving a comparable therapeutic efficacy to anti-VEGF treatment, which underscores the therapy potential in counteracting pathological angiogenesis in PDR (124).
While miRNA mimics hold therapeutic potential for DR, their clinical application is hampered by side-effects linked to the passenger strand activity, raising concerns over safety and specificity (125). In contrast, natural compounds derived from traditional medicine offer a promising alternative by restoring endogenous miRNA expression. Danhong injection (DHI) alleviates DR by upregulating miR-30d-5p to suppress JAK1 expression, as demonstrated in both db/db diabetic mice and HG-induced ARPE-19 cells, through suppressing inflammation, improving renal/retinal injury, and inhibiting pathological angiogenesis (126). Similarly, thymoquinone (TQ), a bioactive phytochemical, alleviates Müller cell apoptosis in diabetic mice by elevating miR-29b levels, thereby repressing SP1-mediated activation of pro-apoptotic factors (e.g., Bax and Caspase-3) and enhancing anti-apoptotic Bcl-2 expression (127). Furthermore, Apigenin attenuates DR by elevating miR-140-5p expression to inhibit the HDAC3/PTEN/PI3K/AKT pathway, as demonstrated in HG-induced hRMECs through reduced angiogenesis, proliferation, and migration in vitro (128). These findings highlight the capacity of herbal constituents to fine-tune miRNA networks without exogenous oligonucleotide delivery, thereby preserving retinal homeostasis through multi-target mechanisms while minimizing side effects.
8.2 miRNA inhibition therapy
miRNA inhibition therapy represents a strategic approach to counteract disease progression by selectively suppressing the activity of aberrant miRNAs within target tissues, as demonstrated in various pathologies. For example, the synergistic downregulation of miR-92a and miR-18a in non-small cell lung cancer inhibits EMT and suppresses tumor advancement through SPRY4 targeting (129). In diabetic complications like nephropathy, the downregulation of miR-21 enhances PPARα expression to improve mitochondrial function and alleviate lipid metabolism disorders, thereby mitigating disease progression — a mechanism highly relevant to targeting similar pathways in DR (130). This therapy is achievable through diverse methodologies such as miRNA antagomirs, natural compounds, miRNA sponges, and LNA-antimiRs, which effectively disrupt miRNA-mRNA interactions (102).
Antagomirs achieve miRNA suppression through sequence-specific degradation of target miRNAs by utilizing chemically engineered, cholesterol-conjugated antisense oligonucleotides that bind complementarily to miRNAs (131). For example, the miR-139-5p antagomir ameliorates DR by suppressing miR-139-5p expression to upregulate PTEN and inhibit VEGF-driven retinal neovascularization, as demonstrated in HG-treated hRMECs/mice retinal microvascular endothelial cells (mRMECs) through reduced migration, tube formation, and VEGF levels, and further validated in diabetic mice models by attenuating acellular capillaries and pathological blood vessel formation (97). Similarly, The miR-144-3p antagomir mitigates oxidative stress-induced retinal degeneration in DR by suppressing miR-144-3p to activate Nrf2-dependent antioxidant signaling (e.g., NQO1, GCLC), demonstrated in oxidatively stressed human/mice RPE cells (reduced apoptosis) and sodium iodate-treated mouse (preserved retinal integrity via subretinal delivery) (132).
Emerging evidence highlights natural compounds as promising miRNA-modulating agents for DR management. Astragalus polysaccharide (APS) mitigates mitochondrial apoptosis in ARPE-19 cells by suppressing the miR-182/Bcl-2 axis under high glucose conditions. APS downregulates miR-182, restoring Bcl-2 expression, reducing cytochrome-c release, and attenuating apoptosis markers (Bax, cleaved caspases) (133). Similarly, blueberry anthocyanins (BAE) alleviate DR via the miR-182/OGG1 axis, suppressing HG-induced ROS, endoplasmic reticulum stress (ERS), and apoptosis in ARPE-19 cells. BAE inhibits miR-182 to upregulate OGG1, confirmed by luciferase assays, and ameliorates retinal oxidative damage in DR rats (134). Complementarily, ginsenoside Rg1 targets the miR-100-3p/FBXW7/c-MYC axis, inhibiting angiogenesis in hRMECs and DR rat models. Rg1 downregulates miR-100-3p, elevating FBXW7 to promote c-MYC degradation, thereby reducing vascular leakage and pathological neovascularization (135). These studies collectively demonstrate that natural compounds modulate distinct miRNA pathways to address multifactorial DR pathogenesis.
miRNA sponges represent a class of artificially engineered RNA molecules meticulously designed to specifically inhibit the function of one or more target miRNAs, thereby serving as potent tools in miRNA inhibition therapy; these constructs typically comprise long RNA chains embedded with multiple (ranging from 4 to 10 or more) tandem, fully or nearly fully complementary binding sites that exhibit high affinity for the designated miRNAs, functioning analogously to a “sponge” to efficiently sequester and bind the target miRNAs within cellular environments through competitive interactions (136, 137)—crucially, this sequestration prevents the miRNAs from binding to their natural downstream mRNA targets, effectively nullifying their gene silencing capabilities and restoring normal gene expression (138). For instance, circular RNA ZNF532 acts as a miR-29a-3p sponge to sequester and reduce miR-29a-3p activity, thereby upregulating NG2, LOXL2, and CDK2 expression to rescue pericyte function in HG-exposed retinal pericytes and STZ-induced diabetic mice, highlighting its relevance in mitigating early DR complications (139). Similarly, in the context of PDR, studies reveal that the long noncoding RNA PPT2-EGFL8 functions as a sponge for miR-423-5p. In PDR, long noncoding RNA PPT2-EGFL8 functions as a miR-423-5p sponge, suppressing hypoxia-induced hRMECs proliferation and ameliorating pathological neovascularization in STZ-induced diabetic mice models by modulating PPARD/ANGPTL4 signaling, thereby illustrating the targeted efficacy of miRNA sponges in addressing advanced DR pathologies (140).
LNA-antimiRs, characterized by their 20-O and 40-C methylene-bridged ribose ring modifications that confer structural rigidity, exhibit enhanced nuclease resistance and superior miRNA binding affinity compared to antagomirs (141). The study by Zhou et al. demonstrated that LNA-anti-miRs for miR-126 effectively suppressed pathological choroidal neovascularization (CNV) in a laser-induced mice model and reduced VEGF-A production through αB-Crystallin promoter regulation in RPE cells (142). Notably, while this study validates LNA-antimiRs as potent tools for modulating ocular angiogenesis, direct evidence for LNA-mediated miRNA inhibition in DR remains unexplored.
8.3 miRNA delivery therapy
Current miRNA delivery approaches include conjugation (direct ligand-conjugated miRNAs enabling targeted delivery but inducing hepatic aggregation), viral vectors (efficacy limited by safety concerns), and nanoparticles (cationic carriers protecting miRNAs but risking cytotoxicity) (143–145).
Emerging non-viral strategies, such as EVs and exosomes, offer promising alternatives due to their biocompatibility and targeted delivery potential for DR treatment (146). MSC-sEVs demonstrated therapeutic efficacy by delivering miR-143-3p to suppress NEDD8-mediated Cullin3 neddylation, thereby stabilizing Nrf2 to reduce oxidative stress and inflammation in Müller cells - both in vitro (restoring AGEs-induced endothelial barrier dysfunction) and in diabetic mice (alleviating retinal inflammation and gliosis) (63). Similarly, MSC-sEVs attenuated DR progression in diabetic rats through miR-22-3p transfer, which inhibited NLRP3 inflammasome activation in microglia, subsequently reducing retinal inflammation and blood-retinal barrier damage (147). Human umbilical cord mesenchymal stem cell-derived extracellular vesicles (MSC-Evs) were shown to enrich miR-30c-5p, targeting PLCG1 to suppress PKC/NF-κB signaling pathways, thereby ameliorating inflammatory responses in both STZ-induced diabetic rats and HG-treated HRECs (62). Exosomes, as a specialized subset of EVs, have demonstrated enhanced targeting capabilities in DR models. Bone marrow MSC-derived exosomes delivered miR-133b-3p to mRMECs, effectively suppressing FBN1-mediated angiogenesis and oxidative stress while promoting apoptosis in hyperglycemic conditions, as evidenced by both in vitro cellular assays and KK/Upj-Ay diabetic mice models (148). Similarly, umbilical cord MSC exosomes enriched with miR-17-3p demonstrated therapeutic efficacy by modulating the STAT1/miR-17-3p/VEGF axis to suppress pathological neovascularization in DR mice (149). These findings position EV/exosome-mediated miRNA delivery as a transformative approach for DR treatment, combining the physiological benefits of natural biomaterials with precise molecular targeting capabilities to address multifactorial retinal pathophysiology. A summary of the current miRNA delivery platforms is provided in Table 3.
9 Limitations and translational challenges
There are several critical limitations that impede clinical translation of miRNA-based therapies. Firstly, the heavy reliance on animal models—such as STZ-induced diabetic rodents—poses inherent constraints. These models do not fully recapitulate the complex, multifactorial, and chronic nature of human DR, particularly in terms of metabolic comorbidities, genetic diversity, and the slow progression of microvascular and neuroglial pathology (150). Species-specific differences in retinal anatomy, miRNA expression profiles, and disease progression limit the direct applicability of these findings to humans. Secondly, while circulating miRNAs show promise as non-invasive biomarkers, their clinical utility is hampered by preanalytical variability (e.g., sample collection, processing, and storage), lack of standardized profiling methods, and heterogeneity in expression profiles across different patient populations and stages of DR (151, 152). Moreover, current miRNA therapeutic strategies face substantial hurdles in clinical implementation. Key challenges include ensuring efficient, targeted, and sustained delivery to retinal tissues while minimizing off-target effects and immune activation (119). Current delivery systems—such as viral vectors, lipid nanoparticles, and extracellular vesicles—still face limitations in bioavailability, tissue specificity, and potential cytotoxicity (153).Furthermore, the risk of off-target effects due to the pleiotropic nature of miRNAs, which can regulate multiple mRNA targets, raises concerns about unintended consequences on physiological processes (154). Long-term safety profiles, including immunogenicity and cumulative toxicity, remain largely unexplored.
10 Discussion and future directions
While the compelling evidence underscores the immense potential of miRNAs as sensitive diagnostic biomarkers for non-invasive liquid biopsy-based staging and multi-target therapeutic agents capable of modulating key hyperglycemia-induced pathways (oxidative stress, inflammation, angiogenesis, neurodegeneration) in DR, significant translational challenges necessitate focused future research, including rigorously validating the diagnostic accuracy, specificity, and predictive power of candidate miRNAs across diverse, large-scale longitudinal cohorts and ethnic populations using standardized, sensitive profiling techniques like ddPCR to overcome limitations of variability and establish clinically actionable thresholds; developing efficient, targeted, and stable delivery systems capable of safely and persistently delivering miRNA mimics or inhibitors specifically to retinal cells while minimizing off-target effects and immune responses; conducting comprehensive preclinical studies evaluating long-term efficacy, safety, and potential synergies or antagonisms with existing therapies (e.g., anti-VEGF, faricimab); advancing robust miRNA-based combinatorial strategies that integrate multi-miRNA therapeutics or miRNA modulation with emerging modalities like sustained-release devices or gene editing technologies to enhance durability and efficacy; and fostering the integration of validated circulating miRNA signatures with cutting-edge diagnostic tools like AI-driven retinal imaging and metabolomics into unified, accessible platforms for precision risk stratification, early detection, personalized intervention, and real-time monitoring of DR progression to ultimately shift the paradigm towards prevention and halt the relentless global burden of this vision-threatening complication.
Author contributions
JC: Data curation, Formal Analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing. JZ: Data curation, Methodology, Writing – original draft. CL: Data curation, Project administration, Writing – original draft. LW: Formal Analysis, Resources, Writing – original draft. LT: Data curation, Writing – original draft. SX: Data curation, Project administration, Writing – original draft. FW: Formal Analysis, Methodology, Resources, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Supported by Qingdao Key Health Discipline Development Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Ko GY, Yu F, Bayless KJ, and Ko ML. MicroRNA-150 (miR-150) and diabetic retinopathy: is miR-150 only a biomarker or does it contribute to disease progression? Int J Mol Sci. (2022) 23:12099. doi: 10.3390/ijms232012099
3. Antonetti DA, Silva PS, and Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. (2021) 17:195–206. doi: 10.1038/s41574-020-00451-4
4. Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. (2020) 8:337–47. doi: 10.1016/S2213-8587(19)30411-5
5. Zehden JA, Mortensen XM, Reddy A, and Zhang AY. Systemic and ocular adverse events with intravitreal anti-VEGF therapy used in the treatment of diabetic retinopathy: a review. Curr Diab Rep. (2022) 22:525–36. doi: 10.1007/s11892-022-01491-y
6. Wonnacott A, Denby L, Coward RJM, Fraser DJ, and Bowen T. MicroRNAs and their delivery in diabetic fibrosis. Advanced Drug delivery Rev. (2022) 182:114045. doi: 10.1016/j.addr.2021.114045
7. Ilieva M, Panella R, and Uchida S. MicroRNAs in cancer and cardiovascular disease. Cells. (2022) 11:3551. doi: 10.3390/cells11223551
8. Pan G, Liu Y, Shang L, Zhou F, and Yang S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun (London England). (2021) 41:199–217. doi: 10.1002/cac2.12138
9. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. (2002) 99:15524–9. doi: 10.1073/pnas.242606799
10. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
11. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet. (2021) 9:e144–60. doi: 10.1016/S2214-109X(20)30489-7
12. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
13. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108072. doi: 10.1016/j.diabres.2020.108072
14. Hou X, Wang L, Zhu D, Guo L, Weng J, Zhang M, et al. Prevalence of diabetic retinopathy and vision-threatening diabetic retinopathy in adults with diabetes in China. Nat Commun. (2023) 14:4296. doi: 10.1038/s41467-023-39864-w
15. Setia S and Tidake P. Prevalence and awareness of diabetic retinopathy in diabetic patients visiting tertiary care hospitals in central India. Cureus. (2023) 15:e39414. doi: 10.7759/cureus.39414
16. Lundeen EA, Burke-Conte Z, Rein DB, Wittenborn JS, Saaddine J, Lee AY, et al. Prevalence of diabetic retinopathy in the US in 2021. JAMA Ophthalmol. (2023) 141:747–54. doi: 10.1001/jamaophthalmol.2023.2289
17. Yokoyama H, Araki SI, Kawai K, Yamazaki K, Tomonaga O, Shirabe SI, et al. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res Care. (2018) 6:e000521. doi: 10.1136/bmjdrc-2018-000521
18. Grading diabetic retinopathy from stereoscopic color fundus photographs - an extension of the modified airlie house classification: ETDRS report number 10. Ophthalmology. (2020) 127:S99–s119. doi: 10.1016/j.ophtha.2020.01.030
19. Yang Z, Tan TE, Shao Y, Wong TY, and Li X. Classification of diabetic retinopathy: Past, present and future. Front Endocrinol. (2022) 13:1079217. doi: 10.3389/fendo.2022.1079217
20. Cai S and Liu TYA. The role of ultra-widefield fundus imaging and fluorescein angiography in diagnosis and treatment of diabetic retinopathy. Curr Diab Rep. (2021) 21:30. doi: 10.1007/s11892-021-01398-0
21. Qi Z, Si Y, Feng F, Zhu J, Yang X, Wang W, et al. Analysis of retinal and choroidal characteristics in patients with early diabetic retinopathy using WSS-OCTA. Front Endocrinol. (2023) 14:1184717. doi: 10.3389/fendo.2023.1184717
22. Huang X, Wang H, She C, Feng J, Liu X, Hu X, et al. Artificial intelligence promotes the diagnosis and screening of diabetic retinopathy. Front Endocrinol. (2022) 13:946915. doi: 10.3389/fendo.2022.946915
23. Yang WH, Zheng B, Wu MN, Zhu SJ, Fei FQ, Weng M, et al. An evaluation system of fundus photograph-based intelligent diagnostic technology for diabetic retinopathy and applicability for research. Diabetes Ther. (2019) 10:1811–22. doi: 10.1007/s13300-019-0652-0
24. Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. (2018) 125:1608–22. doi: 10.1016/j.ophtha.2018.04.007
25. Lin KY, Hsih WH, Lin YB, Wen CY, and Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Invest. (2021) 12:1322–5. doi: 10.1111/jdi.13480
26. Maturi RK, Glassman AR, Josic K, Antoszyk AN, Blodi BA, Jampol LM, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the protocol W randomized clinical trial. JAMA Ophthalmol. (2021) 139:701–12. doi: 10.1001/jamaophthalmol.2021.0606
27. Everett LA and Paulus YM. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. (2021) 21:35. doi: 10.1007/s11892-021-01403-6
29. Uludag G, Hassan M, Matsumiya W, Pham BH, Chea S, Trong Tuong Than N, et al. Efficacy and safety of intravitreal anti-VEGF therapy in diabetic retinopathy: what we have learned and what should we learn further? Expert Opin Biol Ther. (2022) 22:1275–91. doi: 10.1080/14712598.2022.2100694
30. Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. (2022) 399:741–55. doi: 10.1016/S0140-6736(22)00018-6
31. Du X, Yang L, Kong L, Sun Y, Shen K, Cai Y, et al. Metabolomics of various samples advancing biomarker discovery and pathogenesis elucidation for diabetic retinopathy. Front Endocrinol. (2022) 13:1037164. doi: 10.3389/fendo.2022.1037164
32. Xuan Q, Ouyang Y, Wang Y, Wu L, Li H, Luo Y, et al. Multiplatform metabolomics reveals novel serum metabolite biomarkers in diabetic retinopathy subjects. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2020) 7:2001714. doi: 10.1002/advs.202001714
33. Smit-McBride Z and Morse LS. MicroRNA and diabetic retinopathy-biomarkers and novel therapeutics. Ann Trans Med. (2021) 9:1280. doi: 10.21037/atm-20-5189
34. Dexheimer PJ and Cochella L. MicroRNAs: from mechanism to organism. Front Cell Dev Biol. (2020) 8:409. doi: 10.3389/fcell.2020.00409
35. Dobrzycka M, Sulewska A, Biecek P, Charkiewicz R, Karabowicz P, Charkiewicz A, et al. miRNA studies in glaucoma: A comprehensive review of current knowledge and future perspectives. Int J Mol Sci. (2023) 24:14699. doi: 10.3390/ijms241914699
36. O'Brien J, Hayder H, Zayed Y, and Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. (2018) 9:402. doi: 10.3389/fendo.2018.00402
37. Lee RC, Feinbaum RL, and Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. (1993) 75:843–54. doi: 10.1016/0092-8674(93)90529-Y
38. Hunt EA, Broyles D, Head T, and Deo SK. MicroRNA detection: current technology and research strategies. Annu Rev Anal Chem (Palo Alto Calif.). (2015) 8:217–37. doi: 10.1146/annurev-anchem-071114-040343
39. Cao Y, Zhao F, Feng P, Hong Y, Zhang Y, Zhang Z, et al. Stem-loop RT-qPCR system for multiplex miRNA profiling and its application in wound healing-specific biomarker identification. Anal Biochem. (2023) 678:115267. doi: 10.1016/j.ab.2023.115267
40. Kramer MF. Stem-loop RT-qPCR for miRNAs. Curr Protoc Mol Biol Chapter. (2011) 15:Unit 15.10. doi: 10.1002/0471142727.mb1510s95
41. Campomenosi P, Gini E, Noonan DM, Poli A, D'Antona P, Rotolo N, et al. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. (2016) 16:60. doi: 10.1186/s12896-016-0292-7
42. Siddika T and Heinemann IU. Bringing microRNAs to light: methods for microRNA quantification and visualization in live cells. Front bioengineering Biotechnol. (2020) 8:619583. doi: 10.3389/fbioe.2020.619583
43. Zhao X, Ling F, Zhang GW, Yu N, Yang J, and Xin XY. The correlation between microRNAs and diabetic retinopathy. Front Immunol. (2022) 13:941982. doi: 10.3389/fimmu.2022.941982
44. Kang Q and Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. (2020) 37:101799. doi: 10.1016/j.redox.2020.101799
45. Peng H, Hu Q, Zhang X, Huang J, Luo S, Zhang Y, et al. Identifying therapeutic targets and potential drugs for diabetic retinopathy: focus on oxidative stress and immune infiltration. J Inflammation Res. (2025) 18:2205–27. doi: 10.2147/JIR.S500214
46. Santos AS, Ferreira LRP, da Silva AC, Alves LI, Damasceno JG, Kulikowski L, et al. Progression of type 1 diabetes: circulating microRNA expression profiles changes from preclinical to overt disease. J Immunol Res. (2022) 2022:2734490. doi: 10.1155/2022/2734490
47. Miao C, Chang J, Zhang G, and Fang Y. MicroRNAs in type 1 diabetes: new research progress and potential directions. Biochem Cell Biol. (2018) 96:498–506. doi: 10.1139/bcb-2018-0027
48. Chen Y, Schlotterer A, Kurowski L, Li L, Dannehl M, Hammes HP, et al. miRNA-124 prevents rat diabetic retinopathy by inhibiting the microglial inflammatory response. Int J Mol Sci. (2023) 24:2291. doi: 10.3390/ijms24032291
49. Kovacs B, Lumayag S, Cowan C, and Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Visual Sci. (2011) 52:4402–9. doi: 10.1167/iovs.10-6879
50. He X, Kuang G, Wu Y, and Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Trans Med. (2021) 11:e468. doi: 10.1002/ctm2.468
51. Karam-Palos S, Andrés-Blasco I, Campos-Borges C, Zanón-Moreno V, Gallego-Martínez A, Alegre-Ituarte V, et al. Oxidative stress mediates epigenetic modifications and the expression of miRNAs and genes related to apoptosis in diabetic retinopathy patients. J Clin Med. (2023) 13:74. doi: 10.3390/jcm13010074
52. Jia G, Bai H, Mather B, Hill MA, Jia G, and Sowers JR. Diabetic vasculopathy: molecular mechanisms and clinical insights. Int J Mol Sci. (2024) 25:804. doi: 10.3390/ijms25020804
53. Haydinger CD, Oliver GF, Ashander LM, and Smith JR. Oxidative stress and its regulation in diabetic retinopathy. Antioxidants (Basel Switzerland). (2023) 12:8643–55. doi: 10.3390/antiox12081649
54. Andrés-Blasco I, Gallego-Martínez A, MaChado X, Cruz-Espinosa J, Di Lauro S, Casaroli-Marano R, et al. Oxidative Stress, Inflammatory, angiogenic, and apoptotic molecules in proliferative diabetic retinopathy and diabetic macular edema patients. Int J Mol Sci. (2023) 24:8227. doi: 10.3390/ijms24098227
55. Ren J, Zhang S, Pan Y, Jin M, Li J, Luo Y, et al. Diabetic retinopathy: Involved cells, biomarkers, and treatments. Front Pharmacol. (2022) 13:953691. doi: 10.3389/fphar.2022.953691
56. Zhu H, Li B, Huang T, Wang B, Li S, Yu K, et al. Update in the molecular mechanism and biomarkers of diabetic retinopathy. Biochim Biophys Acta Mol basis Dis. (2025) 1871:167758. doi: 10.1016/j.bbadis.2025.167758
57. Bian J, Ge W, and Jiang Z. miR-26a-5p attenuates oxidative stress and inflammation in diabetic retinopathy through the USP14/NF-κB signaling pathway. J Ophthalmol. (2024) 2024:1470898. doi: 10.1155/2024/1470898
58. Che S, Wu S, and Yu P. Downregulated HDAC3 or up-regulated microRNA-296-5p alleviates diabetic retinopathy in a mouse model. Regenerative Ther. (2022) 21:1–8. doi: 10.1016/j.reth.2022.04.002
59. Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. (2019) 20:247–60. doi: 10.1016/j.redox.2018.09.025
60. Hussain A, Ashique S, Afzal O, Altamimi MA, Malik A, Kumar S, et al. A correlation between oxidative stress and diabetic retinopathy: An updated review. Exp eye Res. (2023) 236:109650. doi: 10.1016/j.exer.2023.109650
61. Zhang ZZ, Qin XH, and Zhang J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. Am J Physiol Endocrinol Metab. (2019) 316:E1050–e1060. doi: 10.1152/ajpendo.00444.2018
62. He Y, Zhang Z, Yao T, Huang L, Gan J, Lv H, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells relieves diabetic retinopathy through a microRNA-30c-5p-dependent mechanism. Diabetes Res Clin Pract. (2022) 190:109861. doi: 10.1016/j.diabres.2022.109861
63. Chen Y, Tong J, Liu C, He C, Xiang J, Yao G, et al. MSC-derived small extracellular vesicles mitigate diabetic retinopathy by stabilizing Nrf2 through miR-143-3p-mediated inhibition of neddylation. Free Radic Biol Med. (2024) 219:76–87. doi: 10.1016/j.freeradbiomed.2024.04.216
64. Tong J, Chen Y, Ling X, Huang Z, Yao G, and Xie Z. MSC-derived exosomal miR-125b-5p suppressed retinal microvascular endothelial cell ferroptosis in diabetic retinopathy. Stem Cells. (2025) 43(6):sxaf023. doi: 10.1093/stmcls/sxaf023
65. Cheng CK, Shang W, Liu J, Cheang WS, Wang Y, Xiang L, et al. Activation of AMPK/miR-181b axis alleviates endothelial dysfunction and vascular inflammation in diabetic mice. Antioxidants (Basel Switzerland). (2022) 11:1137. doi: 10.3390/antiox11061137
66. Mrowicka M, Mrowicki J, and Majsterek I. Relationship between biochemical pathways and non-coding RNAs involved in the progression of diabetic retinopathy. J Clin Med. (2024) 13:292. doi: 10.3390/jcm13010292
67. Dong W, Imdad L, Xu S, Wang Y, Liu C, Song S, et al. O-glcNAc modification is a promising therapeutic target for diabetic retinopathy. Int J Mol Sci. (2024) 25:6286. doi: 10.3390/ijms25116286
68. Tang L, Xu GT, and Zhang JF. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural regeneration Res. (2023) 18:976–82. doi: 10.4103/1673-5374.355743
69. Ismail A, El-Mahdy HA, Eldeib MG, and Doghish AS. miRNAs as cornerstones in diabetic microvascular complications. Mol Genet Metab. (2023) 138:106978. doi: 10.1016/j.ymgme.2022.106978
70. Luo Y and Li C. Advances in research related to microRNA for diabetic retinopathy. J Diabetes Res. (2024) 2024:8520489. doi: 10.1155/2024/8520489
71. Yu F, Chapman S, Pham DL, Ko ML, Zhou B, and Ko GY. Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open Diabetes Res Care. (2020) 8:e001446. doi: 10.1136/bmjdrc-2020-001446
72. Liu L, Yan LN, and Sui Z. MicroRNA-150 affects endoplasmic reticulum stress via MALAT1-miR-150 axis-mediated NF-κB pathway in LPS-challenged HUVECs and septic mice. Life Sci. (2021) 265:118744. doi: 10.1016/j.lfs.2020.118744
73. Kaur P, Kotru S, Singh S, and Munshi A. Role of miRNAs in diabetic neuropathy: mechanisms and possible interventions. Mol Neurobiol. (2022) 59:1836–49. doi: 10.1007/s12035-021-02662-w
74. Tu Y, Song E, Wang Z, Ji N, Zhu L, Wang K, et al. Melatonin attenuates oxidative stress and inflammation of Müller cells in diabetic retinopathy via activating the Sirt1 pathway. BioMed Pharmacother. (2021) 137:111274. doi: 10.1016/j.biopha.2021.111274
75. Xu Z, Tian N, Li S, Li K, Guo H, Zhang H, et al. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. Int Immunopharmacol. (2021) 101:108234. doi: 10.1016/j.intimp.2021.108234
76. Shao K, Chen G, Xia L, Chen C, and Huang S. MicroRNA-139-5p alleviates high glucose-triggered human retinal pigment epithelial cell injury by targeting LIM-only factor 4. Mediators Inflammation. (2021) 2021:1629783. doi: 10.1155/2021/1629783
77. Ye EA and Steinle JJ. miR-146a attenuates inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediators Inflammation. (2016) 2016:3958453. doi: 10.1155/2016/3958453
78. Gu C, Draga D, Zhou C, Su T, Zou C, Gu Q, et al. miR-590-3p inhibits pyroptosis in diabetic retinopathy by targeting NLRP1 and inactivating the NOX4 signaling pathway. Invest Ophthalmol Visual Sci. (2019) 60:4215–23. doi: 10.1167/iovs.19-27825
79. Wang N, Ding L, Liu D, Zhang Q, Zheng G, Xia X, et al. Molecular investigation of candidate genes for pyroptosis-induced inflammation in diabetic retinopathy. Front Endocrinol. (2022) 13:918605. doi: 10.3389/fendo.2022.918605
80. Hwang SJ, Ahn BJ, Shin MW, Song YS, Choi Y, Oh GT, et al. miR-125a-5p attenuates macrophage-mediated vascular dysfunction by targeting Ninjurin1. Cell Death Differ. (2022) 29:1199–210. doi: 10.1038/s41418-021-00911-y
81. Xi X, Wang X, Ma J, Chen Q, Zhang Y, Song Y, et al. miR-130a-3p enhances autophagy through the YY1/PI3K/AKT/mTOR signaling pathway to regulate macrophage polarization and alleviate diabetic retinopathy. J Diabetes Invest. (2025) 16:392–404. doi: 10.1111/jdi.14381
82. Zhang M, Zhou M, Cai X, Zhou Y, Jiang X, Luo Y, et al. VEGF promotes diabetic retinopathy by upregulating the PKC/ET/NF-κB/ICAM-1 signaling pathway. Eur J Histochem. (2022) 66:3522. doi: 10.4081/ejh.2022.3522
83. Wang Q, Bozack SN, Yan Y, Boulton ME, Grant MB, and Busik JV. Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Invest Ophthalmol Visual Sci. (2014) 55:3986–94. doi: 10.1167/iovs.13-13076
84. Murinello S, Usui Y, Sakimoto S, Kitano M, Aguilar E, Friedlander HM, et al. miR-30a-5p inhibition promotes interaction of Fas(+) endothelial cells and FasL(+) microglia to decrease pathological neovascularization and promote physiological angiogenesis. Glia. (2019) 67:332–44. doi: 10.1002/glia.23543
85. Fragiotta S, Pinazo-Durán MD, and Scuderi G. Understanding neurodegeneration from a clinical and therapeutic perspective in early diabetic retinopathy. Nutrients. (2022) 14:729. doi: 10.3390/nu14040792
86. Simó R, Simó-Servat O, Bogdanov P, and Hernández C. Diabetic retinopathy: role of neurodegeneration and therapeutic perspectives. Asia-Pacific J Ophthalmol (Philadelphia Pa.). (2022) 11:160–7. doi: 10.1097/APO.0000000000000510
87. Sachdeva MM. Retinal neurodegeneration in diabetes: an emerging concept in diabetic retinopathy. Curr Diab Rep. (2021) 21:65. doi: 10.1007/s11892-021-01428-x
88. Bianco L, Arrigo A, Aragona E, Antropoli A, Berni A, Saladino A, et al. Neuroinflammation and neurodegeneration in diabetic retinopathy. Front Aging Neurosci. (2022) 14:937999. doi: 10.3389/fnagi.2022.937999
89. Huang Z, Liang J, Chen S, Ng TK, Brelén ME, Liu Q, et al. RIP3-mediated microglial necroptosis promotes neuroinflammation and neurodegeneration in the early stages of diabetic retinopathy. Cell Death Dis. (2023) 14:227. doi: 10.1038/s41419-023-05660-z
90. Zhu M, Li N, Wang Y, Gao S, Wang J, and Shen X. Regulation of inflammation by VEGF/BDNF signaling in mouse retinal Müller glial cells exposed to high glucose. Cell Tissue Res. (2022) 388:521–33. doi: 10.1007/s00441-022-03622-z
91. Alkhazaali-Ali Z, Sahab-Negah S, Boroumand AR, and Tavakol-Afshari J. MicroRNA (miRNA) as a biomarker for diagnosis, prognosis, and therapeutics molecules in neurodegenerative disease. BioMed Pharmacother. (2024) 177:116899. doi: 10.1016/j.biopha.2024.116899
92. Yu F, Ko ML, and Ko GY. Decreased microRNA-150 exacerbates neuronal apoptosis in the diabetic retina. Biomedicines. (2021) 9:1135. doi: 10.3390/biomedicines9091135
93. Shi R, Liu DD, Cao Y, and Xue YS. microRNA-26a-5p prevents retinal neuronal cell death in diabetic mice by targeting PTEN. Curr eye Res. (2022) 47:409–17. doi: 10.1080/02713683.2021.1975760
94. He J, Wei S, Ye L, Liao R, and Zeng Z. MiR-29a-5p engages in the mechanism of diabetic retinopathy by specifically targeting SIRT3. Gene. (2025) 963:149599. doi: 10.1016/j.gene.2025.149599
95. Li W, Jin L, Cui Y, Nie A, Xie N, and Liang G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J Endocrinol Invest. (2021) 44:1193–207. doi: 10.1007/s40618-020-01405-3
96. Dong N and Wang Y. MiR-30a regulates S100A12-induced retinal microglial activation and inflammation by targeting NLRP3. Curr eye Res. (2019) 44:1236–43. doi: 10.1080/02713683.2019.1632350
97. Zhang Z, Song C, Wang T, Sun L, Qin L, and Ju J. miR-139-5p promotes neovascularization in diabetic retinopathy by regulating the phosphatase and tensin homolog. Arch Pharm Res. (2021) 44:205–18. doi: 10.1007/s12272-021-01308-8
98. Liu L, Xu H, Zhao H, and Sui D. MicroRNA-135b-5p promotes endothelial cell proliferation and angiogenesis in diabetic retinopathy mice by inhibiting Von Hipp-el-Lindau and elevating hypoxia inducible factor α expression. J Drug Target. (2021) 29:300–9. doi: 10.1080/1061186X.2020.1833017
99. Fu XL, He FT, Li MH, Fu CY, and Chen JZ. Up-regulation of miR-192-5p inhibits the ELAVL1/PI3Kδ axis and attenuates microvascular endothelial cell proliferation, migration and angiogenesis in diabetic retinopathy. Diabet. Med. (2023) 40:e15077. doi: 10.1111/dme.15077
100. Wang SS, Liao X, Liu F, Zhang Q, Qiu JJ, and Fu SH. miR-132 mediates cell permeability and migration by targeting occludin in high-glucose -induced ARPE-19 cells. Endocr. J. (2021) 68:531–41. doi: 10.1507/endocrj.EJ20-0277
101. Mazzeo A, Beltramo E, Iavello A, Carpanetto A, and Porta M. Molecular mechanisms of extracellular vesicle-induced vessel destabilization in diabetic retinopathy. Acta Diabetol. (2015) 52:1113–9. doi: 10.1007/s00592-015-0798-9
102. Ho PTB, Clark IM, and Le LTT. MicroRNA-based diagnosis and therapy. Int J Mol Sci. (2022) 23:716. doi: 10.3390/ijms23137167
103. Martins B, Amorim M, Reis F, Ambrósio AF, and Fernandes R. Extracellular vesicles and microRNA: putative role in diagnosis and treatment of diabetic retinopathy. Antioxidants (Basel Switzerland). (2020) 9:705. doi: 10.3390/antiox9080705
104. Li CL, Yao ZY, Sun A, Cao JY, and Wang ZS. Targeting super-enhancers in liver cancer: from pathogenic mechanisms to clinical applications. Front Pharmacol. (2025) 16:1589455. doi: 10.3389/fphar.2025.1589455
105. Mo WY and Cao SQ. MiR-29a-3p: a potential biomarker and therapeutic target in colorectal cancer. Clin Transl Oncol. (2023) 25:563–77. doi: 10.1007/s12094-022-02978-6
106. He F and Guan W. The role of miR-21 as a biomarker and therapeutic target in cardiovascular disease. Clin Chim Acta. (2025) 574:120304. doi: 10.1016/j.cca.2025.120304
107. Ma L, Wen Y, Li Z, Wu N, and Wang Q. Circulating microRNAs as potential diagnostic biomarkers for diabetic retinopathy: A meta-analysis. Front Endocrinol. (2022) 13:929924. doi: 10.3389/fendo.2022.929924
108. Xia Z, Yang X, Zheng Y, Yi G, and Wu S. Plasma levels and diagnostic significance of miR-335-3p and EGFR in diabetic retinopathy. Clin Lab. (2022) 68. doi: 10.7754/Clin.Lab.2021.210447
109. Shi R, Chen L, Wang W, Deng Y, Liu Y, Zhou H, et al. Plasma miR-26a-5p is a biomarker for retinal neurodegeneration of early diabetic retinopathy. Eye (London England). (2021) 35:1587–99. doi: 10.1038/s41433-021-01393-5
110. Li Z, Dong Y, He C, Pan X, Liu D, Yang J, et al. RNA-seq revealed novel non-proliferative retinopathy specific circulating miRNAs in T2DM patients. Front Genet. (2019) 10:531. doi: 10.3389/fgene.2019.00531
111. Saleh AA, El-Hefnawy SM, Kasemy ZA, Alhagaa AA, Nooh MZ, and Arafat ES. Mi-RNA-93 and mi-RNA-152 in the diagnosis of type 2 diabetes and diabetic retinopathy. Br J Biomed Sci. (2022) 79:10192. doi: 10.3389/bjbs.2021.10192
112. Helal HG, Rashed MH, Abdullah OA, Salem TI, and Daifalla A. MicroRNAs (-146a, -21 and -34a) are diagnostic and prognostic biomarkers for diabetic retinopathy. Biomed J. (2021) 44:S242–s251. doi: 10.1016/j.bj.2020.11.003
113. Guo J, Zhou P, Pan M, Liu Z, An G, Han J, et al. Relationship between elevated microRNAs and growth factors levels in the vitreous of patients with proliferative diabetic retinopathy. J Diabetes Complications. (2021) 35:108021. doi: 10.1016/j.jdiacomp.2021.108021
114. Friedrich J, Steel DHW, Schlingemann RO, Koss MJ, Hammes HP, Krenning G, et al. microRNA expression profile in the vitreous of proliferative diabetic retinopathy patients and differences from patients treated with anti-VEGF therapy. Trans Vision Sci Technol. (2020) 9:16. doi: 10.1167/tvst.9.6.16
115. Dähmcke M, Busch M, Pfeil JM, Brauckmann T, Schulz D, Omran W, et al. Circulating microRNAs as biomarker for vessel-associated retinal diseases. Ophthalmologica. (2023) 246:227–37. doi: 10.1159/000533481
116. Liu R, Liu CM, Cui LL, Zhou L, Li N, and Wei XD. Expression and significance of MiR-126 and VEGF in proliferative diabetic retinopathy. Eur Rev Med Pharmacol Sci. (2019) 23:6387–93. doi: 10.26355/eurrev_201908_18518
117. Cho H, Hwang M, Hong EH, Yu H, Park HH, Koh SH, et al. Micro-RNAs in the aqueous humour of patients with diabetic macular oedema. Clin Exp Ophthalmol. (2020) 48:624–35. doi: 10.1111/ceo.13750
118. Smit-McBride Z, Nguyen AT, Yu AK, Modjtahedi SP, Hunter AA, Rashid S, et al. Unique molecular signatures of microRNAs in ocular fluids and plasma in diabetic retinopathy. PLoS One. (2020) 15:e0235541. doi: 10.1371/journal.pone.0235541
119. Seyhan AA. Trials and tribulations of microRNA therapeutics. Int J Mol Sci. (2024) 25:1469. doi: 10.3390/ijms25031469
120. Wang S, Du S, Lv Y, Wang W, and Zhang F. Elevated microRNA-20b-3p and reduced thioredoxin-interacting protein ameliorate diabetic retinopathy progression by suppressing the NLRP3 inflammasomes. IUBMB Life. (2020) 72:1433–48. doi: 10.1002/iub.2267
121. Li C, Lie H, and Sun W. Inhibitory effect of miR−182−5p on retinal neovascularization by targeting angiogenin and BDNF. Mol Med Rep. (2022) 25:12577. doi: 10.3892/mmr.2021.12577
122. Wang E, Feng B, and Chakrabarti S. MicroRNA 9 is a regulator of endothelial to mesenchymal transition in diabetic retinopathy. Invest Ophthalmol Visual Sci. (2023) 64:13. doi: 10.1167/iovs.64.7.13
123. Da'as SI, Ahmed I, Hasan WH, Abdelrahman DA, Aliyev E, Nisar S, et al. The link between glycemic control measures and eye microvascular complications in a clinical cohort of type 2 diabetes with microRNA-223-3p signature. J Transl Med. (2023) 21:171. doi: 10.1186/s12967-023-03893-2
124. Ménard C, Wilson AM, Dejda A, Miloudi K, Binet F, Crespo-Garcia S, et al. miR-106b suppresses pathological retinal angiogenesis. Aging (Albany N Y.). (2020) 12:24836–52. doi: 10.18632/aging.202404
125. Søkilde R, Newie I, Persson H, Borg Å, and Rovira C. Passenger strand loading in overexpression experiments using microRNA mimics. RNA Biol. (2015) 12:787–91. doi: 10.1080/15476286.2015.1020270
126. Deng W, Huang D, Xie H, Wang L, Shen Q, Zeng R, et al. Danhong injection represses diabetic retinopathy and nephropathy advancement in diabetic mice by upregulating microRNA-30d-5p and targeting JAK1. Bioengineered. (2022) 13:8187–200. doi: 10.1080/21655979.2021.2006964
127. Alkharfy KM, Ahmad A, Raish M, and Alenazy MF. Thymoquinone Mediates Müller Cell Apoptosis via miR-29b/SP1 Pathway: A Potential Therapeutic Approach in Diabetic Retinopathy. Drug Res (Stuttg). (2025) 75:76–83. doi: 10.1055/a-2507-5528
128. Fu C, Peng J, Ling Y, Zhao H, Zhao Y, Zhang X, et al. Apigenin inhibits angiogenesis in retinal microvascular endothelial cells through regulating of the miR-140-5p/HDAC3-mediated PTEN/PI3K/AKT pathway. BMC Ophthalmol. (2023) 23:302. doi: 10.1186/s12886-023-03046-5
129. Zhang X, Wang X, Chai B, Wu Z, Liu X, Zou H, et al. Downregulated miR-18a and miR-92a synergistically suppress non-small cell lung cancer via targeting Sprouty 4. Bioengineered. (2022) 13:11281–95. doi: 10.1080/21655979.2022.2066755
130. Xiang J, Zhang H, Zhou X, Wang D, Chen R, Tan W, et al. Atorvastatin restores PPARα Inhibition of lipid metabolism disorders by downregulating miR-21 expression to improve mitochondrial function and alleviate diabetic nephropathy progression. Front Pharmacol. (2022) 13:819787. doi: 10.3389/fphar.2022.819787
131. Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature. (2005) 438:685–9. doi: 10.1038/nature04303
132. Jadeja RN, Jones MA, Abdelrahman AA, Powell FL, Thounaojam MC, Gutsaeva D, et al. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biol. (2020) 28:101336. doi: 10.1016/j.redox.2019.101336
133. Gao LM, Fu S, Liu F, Wu HB, and Li WJ. Astragalus Polysaccharide Regulates miR-182/Bcl-2 Axis to Relieve Metabolic Memory through Suppressing Mitochondrial Damage-Mediated Apoptosis in Retinal Pigment Epithelial Cells. Pharmacology. (2021) 106:520–33. doi: 10.1159/000515901
134. Wang C, Wang K, and Li P. Blueberry anthocyanins extract attenuated diabetic retinopathy by inhibiting endoplasmic reticulum stress via the miR-182/OGG1 axis. J Pharmacol Sci. (2022) 150:31–40. doi: 10.1016/j.jphs.2022.06.004
135. Xue L, Hu M, Li Y, Zhu Q, Zhou G, Zhang X, et al. Ginsenoside Rg1 inhibits angiogenesis in diabetic retinopathy through the miR-100-3p/FBXW7/c-MYC molecular axis. J Diabetes Invest. (2025) 16:791–806. doi: 10.1111/jdi.70016
136. Venkatesh J, Wasson MD, Brown JM, Fernando W, and Marcato P. LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. (2021) 509:81–8. doi: 10.1016/j.canlet.2021.04.002
137. Wang X, Li H, Lu Y, and Cheng L. Circular RNAs in human cancer. Front Oncol. (2020) 10:577118. doi: 10.3389/fonc.2020.577118
138. Tay FC, Lim JK, Zhu H, Hin LC, and Wang S. Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Advanced Drug delivery Rev. (2015) 81:117–27. doi: 10.1016/j.addr.2014.05.010
139. Jiang Q, Liu C, Li CP, Xu SS, Yao MD, Ge HM, et al. Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction. J Clin Invest. (2020) 130:3833–47. doi: 10.1172/JCI123353
140. Xu Z, Yang J, Zheng H, Xie T, Yang Q, Cai J, et al. Long noncoding RNA PPT2-EGFL8 regulates pathological retinal neovascularization in PDR by functioning as a competing endogenous RNA. Diabetes. (2023) 72:1012–27. doi: 10.2337/db22-0342
141. Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. (2011) 43:371–8. doi: 10.1038/ng.786
142. Zhou Q, Anderson C, Hanus J, Zhao F, Ma J, Yoshimura A, et al. Strand and cell type-specific function of microRNA-126 in angiogenesis. Mol Ther. (2016) 24:1823–35. doi: 10.1038/mt.2016.108
143. Chen G, Roy I, Yang C, and Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. (2016) 116:2826–85. doi: 10.1021/acs.chemrev.5b00148
144. Taghdiri M and Mussolino C. Viral and non-viral systems to deliver gene therapeutics to clinical targets. Int J Mol Sci. (2024) 25:7333. doi: 10.3390/ijms25137333
145. Jeong JH, Mok H, Oh YK, and Park TG. siRNA conjugate delivery systems. Bioconjug Chem. (2009) 20:5–14. doi: 10.1021/bc800278e
146. Yan Y, Liu XY, Lu A, Wang XY, Jiang LX, and Wang JC. Non-viral vectors for RNA delivery. J Control Release. (2022) 342:241–79. doi: 10.1016/j.jconrel.2022.01.008
147. Chen Y, Yao G, Tong J, Xie H, Zheng X, Zhang H, et al. MSC-derived small extracellular vesicles alleviate diabetic retinopathy by delivering miR-22-3p to inhibit NLRP3 inflammasome activation. Stem Cells. (2024) 42:64–75. doi: 10.1093/stmcls/sxad078
148. Liang G, Qin Z, Luo Y, Yin J, Shi Z, Wei R, et al. Exosomal microRNA-133b-3p from bone marrow mesenchymal stem cells inhibits angiogenesis and oxidative stress via FBN1 repression in diabetic retinopathy. Gene Ther. (2022) 29:710–9. doi: 10.1038/s41434-021-00310-5
149. Li W, Jin LY, Cui YB, and Xie N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int Immunopharmacol. (2021) 90:107010. doi: 10.1016/j.intimp.2020.107010
150. Olivares AM, Althoff K, Chen GF, Wu S, Morrisson MA, DeAngelis MM, et al. Animal models of diabetic retinopathy. Curr Diab Rep. (2017) 17:93. doi: 10.1007/s11892-017-0913-0
151. Benesova S, Kubista M, and Valihrach L. Small RNA-sequencing: approaches and considerations for miRNA analysis. Diagnostics (Basel Switzerland). (2021) 11:964. doi: 10.3390/diagnostics11060964
152. Marzi MJ, Montani F, Carletti RM, Dezi F, Dama E, Bonizzi G, et al. Optimization and standardization of circulating microRNA detection for clinical application: the miR-test case. Clin Chem. (2016) 62:743–54. doi: 10.1373/clinchem.2015.251942
153. Chakraborty C, Sharma AR, Sharma G, and Lee SS. Therapeutic advances of miRNAs: A preclinical and clinical update. J advanced Res. (2021) 28:127–38. doi: 10.1016/j.jare.2020.08.012
Keywords: diabetic retinopathy, miRNA, oxidative stress, biomarkers, therapeutic targets
Citation: Chen J, Zhang J, Li C, Wang L, Tao L, Xue S and Wang F (2025) Targeting microRNAs in diabetic retinopathy: from pathogenic mechanisms to therapeutic potentials. Front. Endocrinol. 16:1664604. doi: 10.3389/fendo.2025.1664604
Received: 12 July 2025; Accepted: 05 September 2025;
Published: 23 September 2025.
Edited by:
Martina Tomić, Merkur University Hospital, CroatiaReviewed by:
Michael Telias, University of Rochester Medical Center, United StatesMohammad Sarif Mohiuddin, New York University, United States
Copyright © 2025 Chen, Zhang, Li, Wang, Tao, Xue and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenglei Wang, d2ZsYWl5eUAxMjYuY29t
 Jiajun Chen
Jiajun Chen Jingjing Zhang1
Jingjing Zhang1 Changlei Li
Changlei Li Ling Wang
Ling Wang Shasha Xue
Shasha Xue