- 1Dept of Medical Biotechnology and Translational Medicine, University of Milan, Milan, Italy
- 2Istituto Auxologico Italiano IRCCS, Division of Endocrine and Metabolic Diseases, Milan, Italy
- 3Department of Life Science, Helath and health Professions, Università degli Studi Link, Rome, Italy
Introduction: We report the case of a 71-year-old woman with acromegalic facies, referred following bilateral idiopathic lens luxation (LL). Subsequent investigations revealed a 15-mm pituitary adenoma, along with biochemical evidence of massive growth hormone hypersecretion (Growth Hormone (GH): 93.22 µg/L; insulin-like growth factor 1 [IGF-1]: 748 µg/L), consistent with acromegaly. She exhibited multiple comorbidities, including arterial hypertension, chronic heart failure secondary to dilated cardiomyopathy—compatible with acromegalic heart disease (AHD)—osteoporosis, and type 2 diabetes mellitus (T2DM), reflecting a long-standing and high-burden disease.
Treatment and clinical course: Since the patient was not eligible for surgery and daily subcutaneous injections were unfeasible due to the patient’s lack of autonomy and limited caregiver support, therapy with lanreotide was initiated despite complete resistance to high doses of the classic analogue. We switched to pasireotide, achieving excellent disease control with 60 mg administered every 28 days. Progressive reduction in IGF-1 levels subsequently allowed a dose tapering to 40 mg every 28 days. Biochemical control of acromegaly was accompanied by improvement in disease-related complications (most notably T2DM), as well as the development of secondary hypocortisolism.
Ocular complications: Bilateral lens dislocation is not a known acromegaly complication; however, its bilateral occurrence suggests an underlying systemic cause. A plausible pathogenetic mechanism may involve chronic GH hypersecretion and IGF-1 overexpression, with subsequent interaction with ocular receptors. IGF-1 exerts an antiapoptotic and pro-proliferative action on lenticular cells through interaction with the IGF-1 receptor and the intracellular PI3K/Akt pathway. It is a regulatory factor in the synthesis and degradation of fibrillin-1, a glycoprotein abundantly expressed in the extracellular matrix of the ciliary zonule, whose altered synthesis may underlie weakness of the lens suspensory apparatus. This is the first reported case of its genre, although bilateral intraocular lens subluxation (LS) in a patient with acromegaly and elevated intraocular pressure has previously been reported.
Conclusions: Bilateral lens dislocation may represent an atypical presentation of acromegaly. It may indicate advanced disease and, if confirmed in other cohorts, could be considered among the suggestive signs of acromegaly. In our case, the use of pasireotide allowed adherence to therapy and optimal therapeutic response in a multicomplicated, non-self-sufficient patient.
Introduction
Acromegaly is a rare disease caused by hypersecretion of GH of pituitary or, in rare instances, of extra-pituitary origin. The disease is characterised by progressive somatic overgrowth involving bones, cartilage, and soft tissues, and is frequently associated with a broad spectrum of systemic comorbidities (1). Here, we report the case of a patient with acromegaly and a substantial disease burden, reflected by markedly elevated insulin-like growth factor 1 (IGF-1) levels and multiple severe comorbidities, who presented with spontaneous bilateral lens luxation (LL). To the best of our knowledge, this is the first documented case of such an occurrence. A prior report in 2020 reported bilateral intraocular lens subluxation (LS) in a patient with acromegaly and elevated intraocular pressure. We propose that bilateral lens dislocation may represent an atypical presentation of acromegaly, especially in cases of advanced disease.
Case description
In September 2021, a 71-year-old woman came to our clinic, as suggested by her ophthalmologist, following the recognition of acromegaloid features in the context of recent idiopathic bilateral lens dislocation. On examination, the patient exhibited macroglossia and frontal bossing. There was no family history of pituitary adenomas or other endocrine disorders. Her clinical profile was notable for multiple comorbidities commonly associated with acromegaly, including: essential hypertension, for which she was on antihypertensive medication; chronic heart failure secondary to dilated cardiomyopathy (consistent with AHD), managed with diuretics; obstructive sleep apnoea syndrome (OSAS) coupled with chronic pneumopathy due to severe kyphoscoliosis; osteoporosis with a recent low-energy femoral fracture; and type 2 diabetes mellitus (T2DM), managed with basal insulin and the glucagon-like peptide-1 receptor agonist liraglutide. Additional medical history included intellectual disability (oligophrenia), reduced mobility, and a prior colectomy with permanent colostomy following volvulus-induced bowel obstruction.
Diagnostic assessment
Biochemical evaluation revealed a massive hypersecretion of GH and IGF-1 (GH: 93.22 µg/L [reference range: < 4.00], IGF-1: 748 µg/L [reference range: 51–176]). Associated findings included hypogonadotropic hypogonadism and a cortisol level in the intermediate cortisol level, prompting dynamic testing. An adrenocorticotropic hormone (ACTH) stimulation test subsequently confirmed preserved adrenal function.
Neuroimaging with computed tomography—performed instead of magnetic resonance imaging (MRI) due to the patient’s severe kyphoscoliosis—revealed a 15-mm expansive lesion in the sellar area, which appeared slightly hyperdense on baseline imaging, with homogeneous contrast enhancement.
The diagnosis of acromegaly was confirmed, defined by IGF-1 > 1.3 times the upper limit of normal for age in a patient with typical signs and symptoms of acromegaly (2).
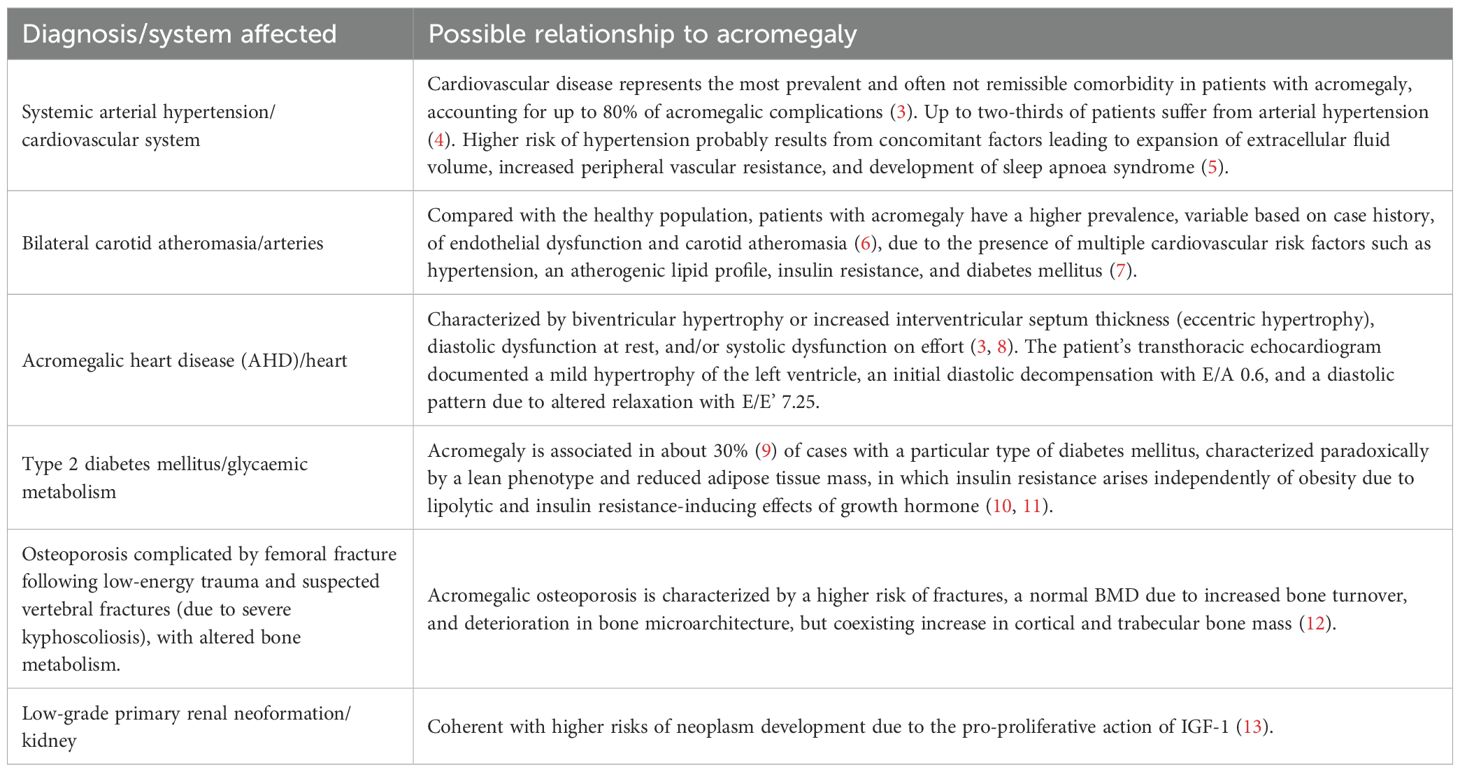
Follow-up of known acromegalic complications and screening for previously undiagnosed ones was initiated, with Table 1 reporting the most clinically relevant findings.
Regarding ocular involvement, in August 2021, the patient underwent a routine ophthalmologic examination as part of her annual diabetic retinopathy screening. During the visit, she reported a prior episode of sudden visual impairment. Due to her longstanding intellectual disability, she had not previously communicated any visual complaints; however, upon detailed questioning, she reported that the visual impairment had developed spontaneously within the preceding days to weeks. Slit-lamp examination revealed bilateral lens dislocation into the vitreous cavity. The patient was promptly referred to the nearest ophthalmologic emergency department for further evaluation and management. The LLs were addressed with the implantation of scleral-fixated intraocular lenses (IOLs); the right eye subsequently experienced a bulbar rupture, resulting in loss of function, while the left eye recovered without complications, preserving vision.
Given the idiopathic nature of the bilateral lens dislocation and the absence of trauma or other identifiable causes, the ophthalmologist referred the patient for endocrinological evaluation to investigate a possible underlying systemic etiopathogenesis.
Therapeutic intervention
According to the 2025 Consensus, surgery remains the first-line treatment for acromegaly (14); however, our patient was not a candidate for surgical intervention due to her severe comorbidities and poor general health status. Current clinical guidelines emphasise a personalised approach to acromegaly management, considering factors such as patient preferences (e.g., oral vs. injectable formulations), comorbid conditions (e.g., type 2 diabetes mellitus), disease-specific features (e.g., MRI findings, degree of IGF-1 elevation), and the potential adverse effects of therapy (e.g., hyperglycaemia). Somatostatin receptor ligands (SRLs) are considered the cornerstone of medical therapy. However, pegvisomant monotherapy may be a valuable alternative in patients with significantly impaired glucose metabolism, no concerns regarding adenoma mass, and markedly elevated IGF-1 levels (14). In the present case, due to the patient’s lack of autonomy and limited caregiver support, daily injections were not feasible. Consequently, long-acting lanreotide was initiated at a dose of 90 mg every 28 days.
Lanreotide only slightly reduced GH levels while failing to control IGF-1 concentrations. In accordance with current guidelines, gradual dose escalations were implemented, reaching up to 120 mg every 25 days; however, therapy remained ineffective, indicating complete resistance to the drug.
In cases of suboptimal response to SRLs and a high risk of new-onset or worsening diabetes mellitus, current guidelines recommend considering pegvisomant monotherapy or the addition of cabergoline if disease control is only marginally insufficient (14). However, persistent marked IGF-1 hypersecretion and the patient’s inability to adhere to daily injections precluded both options. Pasireotide is recognised as a valuable treatment for patients with acromegaly who remain uncontrolled despite maximally titrated doses of first-generation SRLs, as demonstrated in the PAOLA study (2014) (15) and supported by a recent systematic review (16). Nevertheless, pasireotide administration requires careful monitoring due to its association with increased glycaemia, glycated hemoglobin (HbA1c) levels, and a higher incidence of T2DM; its use should be carefully monitored in patients with uncontrolled T2DM (17). Consequently, second-line therapy with pasireotide long-acting release (LAR) was initiated, with close monitoring of T2DM throughout follow-up. Treatment with this new-generation SRL at a dose of 60 mg every 28 days achieved excellent disease control. This effect is likely due to pasireotide’s greater affinity for somatostatin receptor subtype 5 (SSTR-5), which allowed it to overcome the resistance to classic SRL (18).
IGF-1 levels progressively decreased, allowing concurrent reduction of pasireotide dosage to 40 mg (see Figure 1). Current consensus on criteria for acromegaly diagnosis and remission recommends assessing IGF-1 levels at 3 months after therapy initiation, followed by evaluations every 6–12 months after biochemical control is achieved. Biochemical control is defined as normalisation of IGF-1 concentrations within the age-adjusted reference range. Random GH evaluation is considered helpful only in cases of concern about adenoma behaviour (2). In this case, IGF-1 was monitored every 3–4 months, coinciding with the patient’s quarterly outpatient visits for in-hospital drug dispensing. This approach enabled closer clinical surveillance, considered necessary due to the patient’s overall frailty and complex clinical profile. Additionally, random GH levels were periodically measured to indirectly monitor pituitary adenoma activity, since imaging was not feasible.
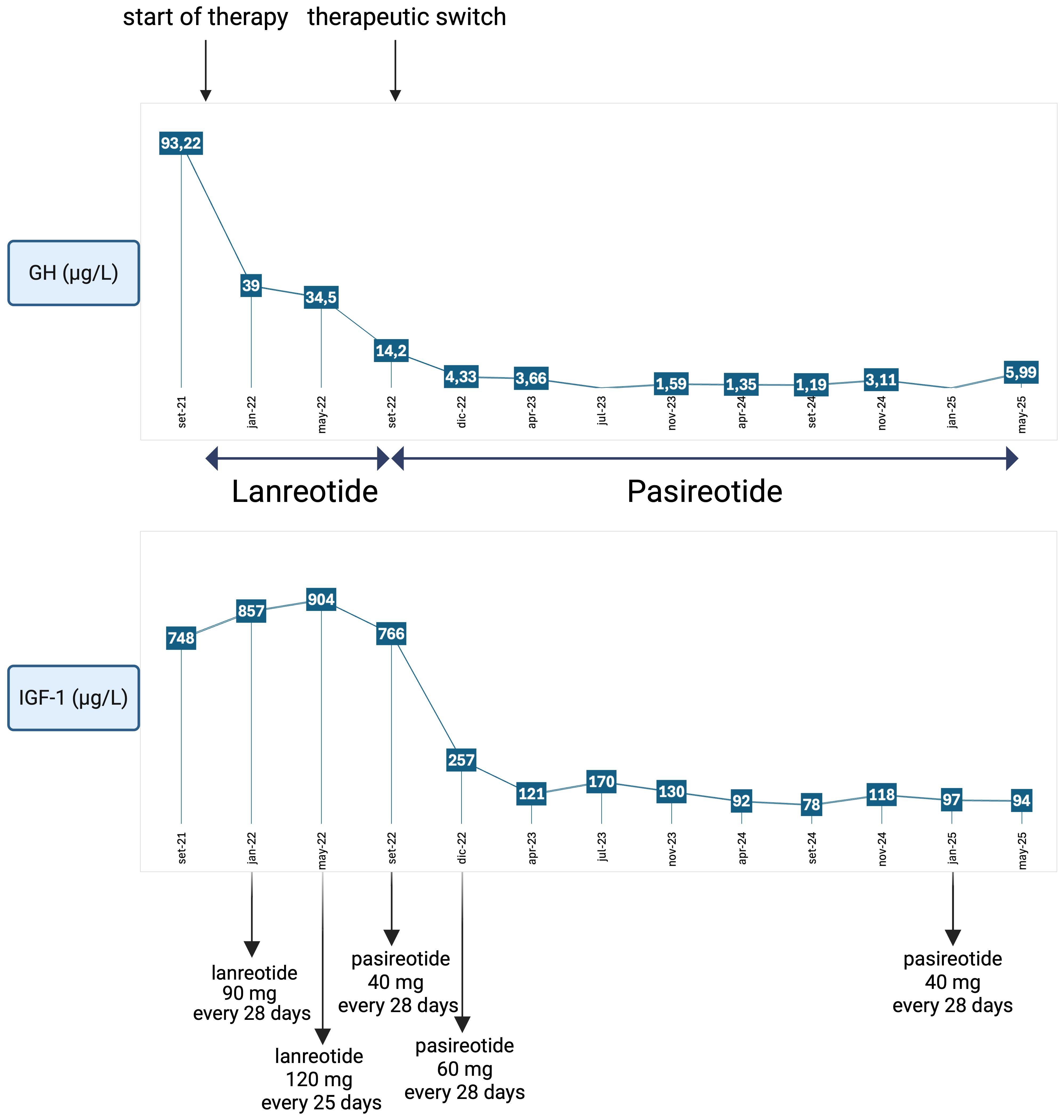
Figure 1. Trend of GH and IGF-1 values during therapy with a first-generation somatostatin analogue (lanreotide) at incremental dosages and with a new-generation somatostatin analogue (pasireotide), showing excellent disease control even after dosage reduction. Reference ranges: IGF-1: 51–176 µg/L; GH: < 4.00 µg/L. Created in BioRender. Vitale, L. (2025) https://BioRender.com/q1ke5xg.
Alongside monitoring biochemical disease control, follow-up also included assessment of acromegaly-related comorbidities and complications, in accordance with current clinical guidelines (2).
Follow-up and outcome
The broad spectrum of acromegaly-related comorbidities reflects both a substantial disease burden and a long-term condition, which often limits the reversibility of comorbidities. Nevertheless, alongside reductions in GH and IGF-1 levels, several comorbidities showed significant improvement (see Figure 2).
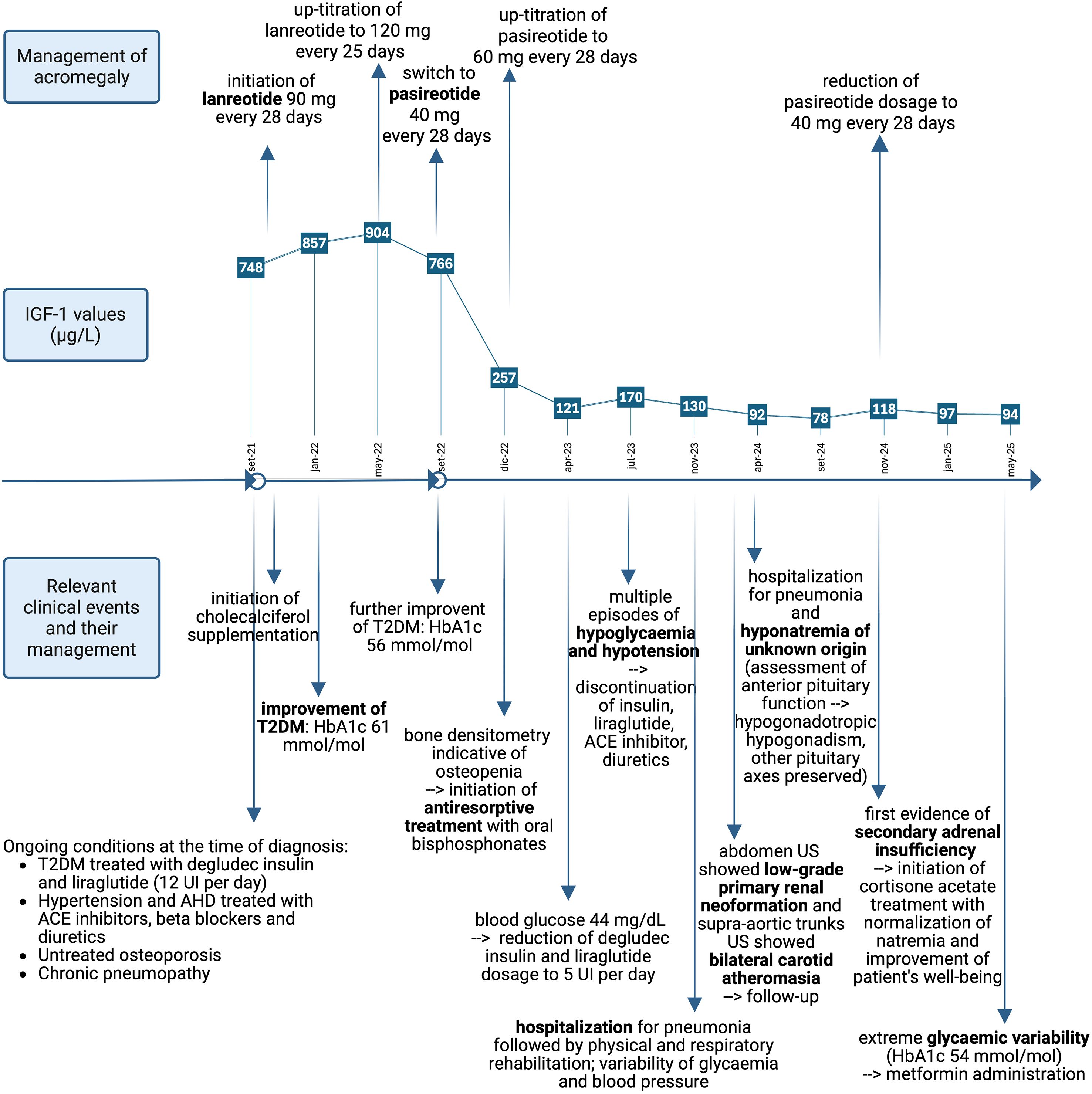
Figure 2. Timeline of key clinical events, their management, and concurrent IGF-1 values in the management of acromegaly. T2DM significantly improved during both lanreotide and pasireotide treatments. Cardiovascular conditions improved with the detection of hypotension, which led to suspension of diuretics and antihypertensive drugs. Variability of glycaemia and blood pressure persisted throughout the follow-up. Primary renal neoplasm and bilateral carotid atherosclerosis were detected, and follow-up was initiated. Hyponatremia and secondary adrenal insufficiency were detected, and replacement therapy was initiated. Osteoporosis was managed with antiresorptive treatment and cholecalciferol supplementation. Poor general health conditions led to hospitalisation due to pneumonia. T2DM, type 2 diabetes mellitus; AHD, acromegalic heart disease; HbA1c, glycated haemoglobin; US, ultrasound sonography. Reference ranges: IGF-1: 51–176 µg/L; HbA1c: < 48 mmol/mol. Created in BioRender. Vitale, L. (2025) https://BioRender.com/ten8nep.
Cardiovascular status improved to the extent that antihypertensive agents and diuretics were temporarily discontinued.
Antiresorptive therapy was administered without evidence of new fractures; however, the patient’s osteoporosis had already contributed to clinical frailty. Severe kyphoscoliosis—potentially exacerbated by suspected vertebral fractures (morphometric assessment was hardly performable)—led to hypomobility and chronic pulmonary disease, which required pulmonary and physical rehabilitation. Osteoporosis severity may be due to postmenopausal hypoestrogenism or hypopituitarism of uncertain onset (19), compounded by chronic GH/IGF-1 excess (12).
T2DM, diagnosed in 2019 and poorly controlled at acromegaly presentation (HbA1c: 85 mmol/mol; reference range: < 42 mmol/mol), improved significantly during lanreotide therapy (HbA1c: 56 mmol/mol). Pasireotide is known to induce or worsen T2DM in 57% (20)–63% (21) of patients due to its inhibitory effect on incretin secretion via SSTR-5 pancreatic cell receptors (22). Unexpectedly, with acromegaly control, blood glucose levels rapidly declined, allowing complete discontinuation of antidiabetic medications due to episodes of severe hypoglycaemia. This strongly suggests that the patient’s diabetes was secondary to acromegaly and GH hypersecretion. While T2DM improvement may be considered novel, pasireotide’s effectiveness in achieving disease control is well established (15, 16).
In this elderly, nonautonomous patient with multiple comorbidities, pasireotide offered several advantages, including ease of administration (one intramuscular injection every 28 days), rapid and sustained disease control, and reduction of disease burden with improvement in symptoms and complications, particularly T2DM.
Collaterally, central hypocortisolism was observed, and replacement therapy with cortisone acetate was initiated. This may be secondary to adenoma shrinkage or possible pituitary apoplexy; however, the absence of imaging data limits confirmation of this hypothesis.
Discussion
Given that pretreatment IGF-1 levels are important predictors of morbidity and mortality in acromegaly (23), our patient not only experienced prolonged exposure to elevated IGF-1 and a high disease burden but was also ineligible for first-line therapy. This sustained hormonal excess likely contributed to the development of progressively severe complications, including ocular manifestations.
Multiple studies have identified various GH and IGF-1 target sites within ocular tissues, supporting an association between acromegaly and a spectrum of ocular abnormalities. These include increased iris thickness, reduced iris curvature, a wider anterior chamber angle (24), as well as increased corneal and lens thickness. Additionally, retinal changes such as thinning of the retinal nerve fibre layer, ganglion cell layer, and inner plexiform layer have been reported (25).
Furthermore, evidence indicates that GH—and particularly its effector IGF-1—acts on lens cells and their suspensory apparatus via IGF-1 receptor interactions. In vitro studies using bovine and chicken lens epithelial cells demonstrated expression of IGF-1 binding sites and the ability to synthesise and release IGF-1, potentially establishing a concentration gradient maintained by locally produced IGF-1 and by IGF-1 in the vitreous humour, which may reflect systemic GH and IGF-1 levels (26, 27).
Specifically, IGF-1 exerts antiapoptotic and pro-proliferative effects on lens epithelial cells through activation of the phosphatidylinositol 3-kinase (PI3K) pathway. Downstream signalling diverges into two branches: one mediates antiapoptotic effects via the PI3K/Protein Kinase B (Akt) pathway, which inactivates the pro-apoptotic protein Bad and suppresses caspase activation; the other promotes cell proliferation through stimulation of the PI3K/p70 ribosomal S6 kinase (p70 S6) kinase cascade (28). Additionally, while Zang et al. reported a nonstatistically significant increase in lens thickness in patients with acromegaly compared to controls, Batur et al. later confirmed this finding with statistical significance, supporting the role of GH and IGF-1 in lens thickening. However, correlation analyses revealed only weak associations between lens thickness and disease duration, IGF-1, or GH levels. Moreover, IGF-1 has been shown to stimulate fibroblast growth factor, inducing lens fibre differentiation in rat models (29).
Given its stimulatory effects on the proliferation and differentiation of lenticular cells, the same authors demonstrated that IGF-1 also acts on the ciliary zonule, the fibrillar structure connecting the lens to the ciliary body and maintaining lens positioning. IGF-1 serves as a key regulatory factor in the synthesis and degradation of fibrillin-1, a glycoprotein abundantly expressed in the extracellular matrix of the ciliary zonule. This regulatory role is mediated through the interaction between the IGF-1 receptor and the PI3K/Akt pathway, as well as mTOR and p70 S6 kinase signalling (30), with studies showing that inhibition of this pathway impairs fibrillin-1 synthesis. However, several factors regulate the synthesis and degradation of fibrillin-1, whose altered production can weaken the lens suspensory apparatus; nonetheless, IGF-1 is likely a major contributor to this process.
In particular, chronic exposure to elevated levels of GH and IGF-1—as seen in patients with a significant disease burden, characterised by persistently high hormone levels and multiple comorbidities indicative of longstanding disease—may contribute to ocular alterations such as lens thickening and dysfunction of the lens suspensory apparatus, potentially increasing the risk of LL (see Figure 3).
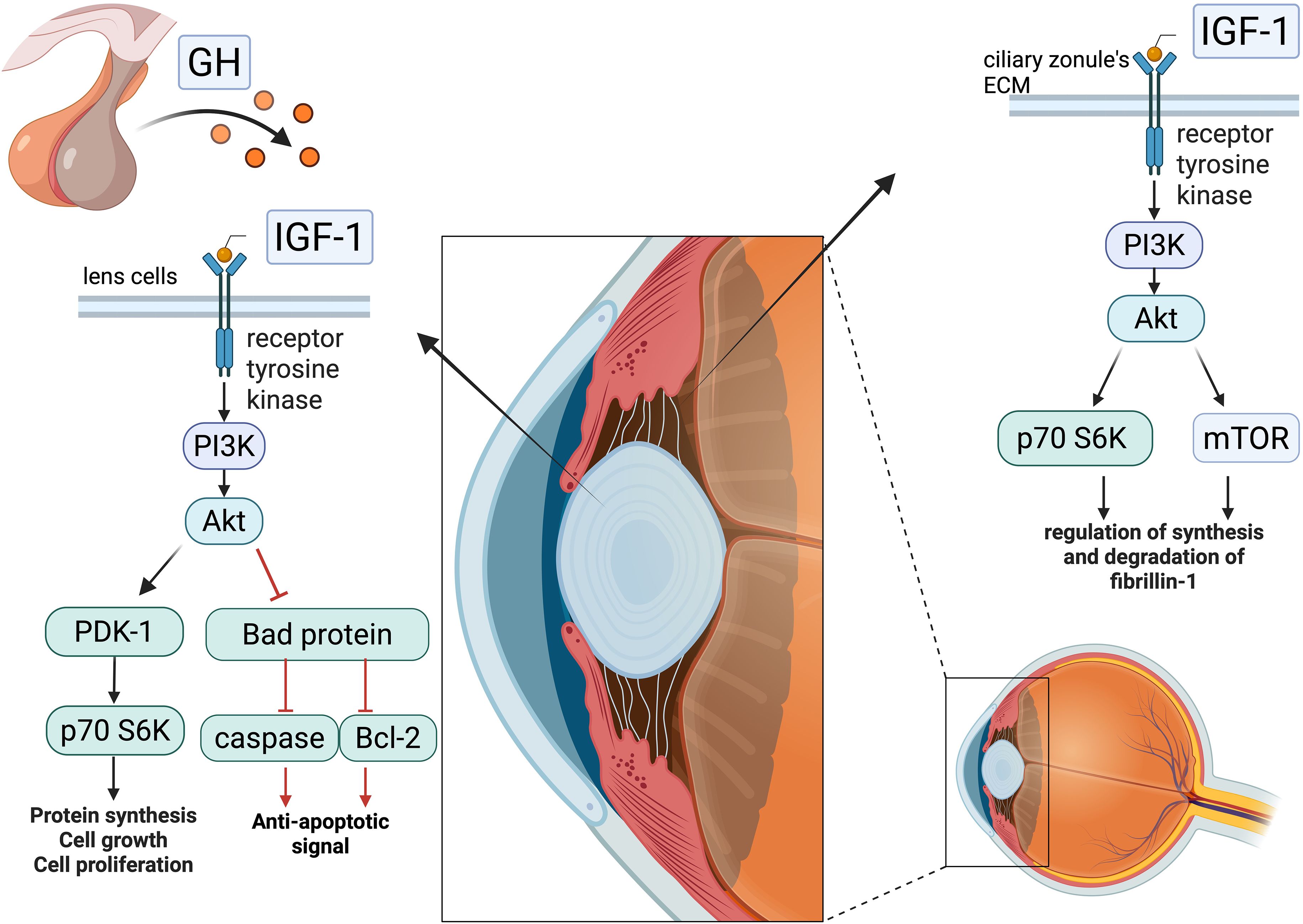
Figure 3. Effect of IGF-1’s interaction with cells of the lens and ciliary body: intracellular IGF-1 pathway—following interaction with the tyrosine kinase receptor, IGF-1 initiates a phosphorylation cascade that bifurcates, in lenticular cells, into a pro-proliferative signal and an antiapoptotic one, while in the ciliary zonule, it regulates the synthesis and degradation of fibrillin-1. In the presence of high IGF-1 concentrations, these events may contribute to the weakening of the lens suspensory apparatus and lead to increased lens thickness, promoting its dislocation. Created in BioRender. Vitale, l. (2025) https://BioRender.com/ez3w9xi.
To the best of our knowledge, this is the first case of its kind reported to date. In 2020, Chang and Chen reported a case of late bilateral IOL subluxation in a patient with acromegaly. The patient had previously undergone IOL implantation for cataract correction and later developed elevated intraocular pressure—a known ocular complication of acromegaly—which was treated and controlled. However, subsequent slit-lamp examination revealed bilateral inferior subluxation of the IOLs. The authors attributed this finding to weakening of the IOL suspensory apparatus, likely resulting from IGF-1-mediated effects on myofibrils and the extracellular matrix (ECM) of the zonular fibres. Additionally, they proposed a potential role for matrix metalloproteinases, which are highly expressed in pituitary adenomas and may release growth factors anchored to the ECM, potentially degrading fibrillin-1 molecules and further contributing to ciliary apparatus weakness (31).
Bilateral LL may represent an atypical presentation of acromegaly. If confirmed in larger cohorts, it could indicate advanced disease and potentially be included among the suggestive signs of acromegaly.
Patient perspective
In this case, treatment with pasireotide facilitated adherence to therapy and achieved optimal disease control in a complex, nonautonomous patient with limited caregiver support. The patient also experienced significant improvement in disease symptoms and related complications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LV: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. LF: Investigation, Supervision, Writing – review & editing. MB: Conceptualization, Supervision, Writing – review & editing. SF: Supervision, Writing – review & editing. GV: Investigation, Supervision, Writing – review & editing. LP: Supervision, Writing – review & editing, Project administration. BC: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Italian Ministry of Health - Ricerca Corrente.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pivonello R, et al. Acromegaly. Nat Rev Dis Primers. (2019) 5:20. doi: 10.1038/s41572-019-0071-6
2. Giustina A, Biermasz N, Casanueva FF, Fleseriu M, Mortini P, Strasburger C, et al. Acromegaly consensus group. Consensus on criteria for acromegaly diagnosis and remission. Pituitary. (2024) 27:7–22. doi: 10.1007/s11102-023-01360-1
3. Colao A, Ferone D, Marzullo P, and Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. (2004) 25:102–52. doi: 10.1210/er.2002-0022
4. Vila G, Luger A, van der Lely AJ, Neggers SJCMM, Webb SM, Hey-Hadavi J, et al. Hypertension in acromegaly in relationship to biochemical control and mortality: global ACROSTUDY outcomes. Front Endocrinol (Lausanne). (2020) 11:577173. doi: 10.3389/fendo.2020.577173
5. Puglisi S and Terzolo M. Hypertension and acromegaly. Endocrinol Metab Clin North Am. (2019) 48:779–93. doi: 10.1016/j.ecl.2019.08.008
6. Otsuki M, Kasayama S, Yamamoto H, Saito H, Sumitani S, Kouhara H, et al. Characterization of premature atherosclerosis of carotid arteries in acromegalic patients. Clin Endocrinol (Oxf). (2001) 54:791–6. doi: 10.1046/j.1365-2265.2001.01281.x
7. Parolin M, Dassie F, Martini C, Mioni R, Russo L, Fallo F, et al. Preclinical markers of atherosclerosis in acromegaly: a systematic review and meta-analysis. Pituitary. (2018) 21:653–62. doi: 10.1007/s11102-018-0911-5
8. Abreu-Lomba A, Aristizábal-Colorado D, Sierra-Castillo S, Weir-Restrepo D, Gómez-Mercado CA, Trujillo DAV, et al. Prevalence and characteristics of heart disease in patients with acromegaly in Colombia (RAPACO HEART study). Cardiology. (2025) 10:1–10. doi: 10.1159/000545875
9. Gadelha MR, Kasuki L, Lim DST, and Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. (2019) 40:268–332. doi: 10.1210/er.2018-00115
10. Møller N and Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. (2009) 30:152–77. doi: 10.1210/er.2008-0027
11. Esposito D, Boguszewski CL, Colao A, Fleseriu M, Gatto F, and Johannsson G. Diabetes mellitus in patients with acromegaly: pathophysiology, clinical challenges and management. Nat Rev Endocrinol. (2024) 20:541–52. doi: 10.1038/s41574-024-00993-x
12. Mazziotti G, Maffezzoni F, Frara S, and Giustina A. Acromegalic osteopathy. Pituitary. (2017) 20:63–9. doi: 10.1007/s11102-016-0758-6
13. Ruchała M, Szczepanek-Parulska E, Fularz M, and Woliński K. Risk of neoplasms in acromegaly. Contemp Oncol (Pozn). (2012) 16:111–7. doi: 10.5114/wo.2012.28790
14. Melmed S, di Filippo L, Fleseriu M, Mercado M, Karavitaki N, Gurnell M, et al. Consensus on acromegaly therapeutic outcomes: an update. Nat Rev Endocrinol. (2025) 21:718–37. doi: 10.1038/s41574-025-01148-2
15. Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide C2402 Study Group. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. (2014) 2:875–84. doi: 10.1016/S2213-8587(14)70169-X
16. Aliyeva T, Muniz J, Soares GM, Firdausa S, and Mirza L. Efficacy and safety of pasireotide treatment in acromegaly: A systematic review and single arm meta-analysis. Pituitary. (2024) 27:468–79. doi: 10.1007/s11102-024-01461-5
17. Costanza F, Basile C, Chiloiro S, Hessman E, Chantzichristos D, Esposito D, et al. Impact of pasireotide on lipid and glucose metabolism in patients with acromegaly: a systematic review and meta-analysis. J Endocrinol Invest. (2025) 7. doi: 10.1007/s40618-025-02642-0
18. Puig-Domingo M, Bernabéu I, Picó A, Biagetti B, Gil J, Alvarez-Escolá C, et al. Pasireotide in the personalized treatment of acromegaly. Front Endocrinol (Lausanne). (2021) 12:648411. doi: 10.3389/fendo.2021.648411
19. Akirov A, Rudman Y, and Fleseriu M. Hypopituitarism and bone disease: pathophysiology, diagnosis and treatment outcomes. Pituitary. (2024) 27:778–88. doi: 10.1007/s11102-024-01391-2
20. Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, et al. Pasireotide C2305 Study Group: pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. (2014) 99:791–9. doi: 10.1210/jc.2013-2480
21. Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide C2402 Study Group: pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. (2014) 2:875–84. doi: 10.1016/S2213-8587(14)70169-X
22. Chisholm C and Greenberg GR. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am J Physiol Endocrinol Metab. (2002) 283:E311–7. doi: 10.1152/ajpendo.00434.2001
23. Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, De Marinis L, et al. Italian Study Group of Acromegaly. Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol. (2012) 167:189–98. doi: 10.1530/EJE-12-0084
24. Zhang X, Ma J, Li L, Gan L, He H, Shao E, et al. Elevated IGF-1 and GH levels are correlated with a thicker iris and wider anterior chamber angle in treatment-naïve acromegaly patients. Invest Ophthalmol Vis Sci. (2022) 63:27. doi: 10.1167/iovs.63.11.27
25. Batur M, Özer MD, Üçler R, Seven E, Tekin S, and Ünal F. Corneal parameters, ocular biometers, and retinal and choroidal thickness in acromegaly patients. Photodiagnosis Photodyn Ther. (2023) 44:103773. doi: 10.1016/j.pdpdt.2023.103773
26. Palmade F, Sechoy-Chambon O, Coquelet C, and Bonne C. Insulin-like growth factor-1 (IGF-1) specifically binds to bovine lens epithelial cells and increases the number of fibronectin receptor sites. Curr Eye Res. (1994) 13:531–7. doi: 10.3109/02713689408999885
27. Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation. (1996) 61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x
28. Chandrasekher G and Sailaja D. Phosphatidylinositol 3-kinase (PI-3K)/Akt but not PI-3K/p70 S6 kinase signaling mediates IGF-1-promoted lens epithelial cell survival. Invest Ophthalmol Vis Sci. (2004) 45:3577–88. doi: 10.1167/iovs.04-0279
29. Richardson NA, Chamberlain CG, and McAvoy JW. IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest Ophthalmol Visual Sci. (1993) 34:3303–12.
30. Schaefer L, Tsalastra W, Babelova A, Baliova M, Minnerup J, Sorokin L, et al. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am J Pathol. (2007) 170:301–15. doi: 10.2353/ajpath.2007.060497
Keywords: acromegaly, pasireotide, diabetes, lens dislocation, complication, Eye manifestations, Insulin-like growth factor I
Citation: Vitale L, Fatti LM, Bonomi M, Frara S, Vitale G, Persani L and Cangiano B (2025) Case Report: Bilateral lens dislocation as an atypical presentation of acromegaly and review of the ocular effects of GH/IGF-1 excess. Front. Endocrinol. 16:1666425. doi: 10.3389/fendo.2025.1666425
Received: 15 July 2025; Accepted: 08 October 2025;
Published: 27 October 2025.
Edited by:
Daniela Esposito, University of Gothenburg, SwedenReviewed by:
Magdalena Stasiak, Polish Mother’s Memorial Hospital Research Institute, PolandDavid Alexander Vernaza Trujillo, Pontificia Universidad Javeriana, Colombia
Copyright © 2025 Vitale, Fatti, Bonomi, Frara, Vitale, Persani and Cangiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biagio Cangiano, Yi5jYW5naWFub0BhdXhvbG9naWNvLml0
†ORCID: Laura Vitale, orcid.org/0009-0009-9419-3993
Marco Bonomi, orcid.org/0000-0001-5454-6074
Stefano Frara, orcid.org/0000-0002-1308-5598
Giovanni Vitale, orcid.org/0000-0003-2478-683X
Luca Persani, orcid.org/0000-0003-2068-9581
Biagio Cangiano, orcid.org/0000-0002-2658-744X
 Laura Vitale
Laura Vitale Letizia Maria Fatti
Letizia Maria Fatti Marco Bonomi
Marco Bonomi Stefano Frara
Stefano Frara Giovanni Vitale
Giovanni Vitale Luca Persani
Luca Persani Biagio Cangiano
Biagio Cangiano