- 1Department of Pharmacy Practice, College of Pharmacy, Taibah University, Madinah, Saudi Arabia
- 2Prince Mohammad Bin Abdulaziz Hospital, National Guard Health Affairs, Madinah, Saudi Arabia
- 3King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 4Department of Pharmacy Practice, College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 5King Abdulaziz Medical City, National Guard Health Affairs, Riyadh, Saudi Arabia
- 6Pharmaceutical Care Department, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 7Department of Pharmacy Practice, College of Pharmacy, Qassim University, Qassim, Saudi Arabia
Introduction: Hyperuricemia, characterized by elevated serum uric acid (UA) levels, is associated with cardiovascular–kidney–metabolic syndrome and remains challenging to manage due to medication side effects and adherence issues. SGLT2 inhibitors (SGLT2i), primarily prescribed for diabetes (DM), heart failure (HF), and chronic kidney disease (CKD), have demonstrated potential UA-lowering effects, though their precise impact is not well established.
Methods: This multicenter retrospective cohort study used a pre-and-post analysis to evaluate the effect of SGLT2i on UA levels. Data were collected from four major healthcare centers in Saudi Arabia. The study included adult patients who initiated SGLT2i therapy between January 2022 and January 2024, excluding those with active gout flares, a history of cancer, or recent changes in UA-lowering therapy. The primary outcome was the percentage change in serum UA levels post- i initiation, with secondary outcomes including subgroup analyses, metabolic effects, univariate and multivariate modeling, and longitudinal trend evaluations.
Results: Among 2,400 patients screened, 454 were included in the final analysis. SGLT2i significantly reduced UA levels by 4.5% (p=0.006), with the most pronounced reduction in patients with baseline elevated UA (10%, p=0.001) and those with HF (9%, p=0.001). Univariate analysis identified DM & HF, DM & CKD, and DM, HF & CKD as predictors of response, but multivariate analysis confirmed only DM & HF as an independent predictor (OR = 2.2, 95% CI: 1.2–4.04).
Conclusion: These findings suggest that SGLT2i may serve as an adjunct therapy for hyperuricemia, especially in patients with DM & HF, highlighting the need for further research on long-term benefits.
1 Introduction
Hyperuricemia is defined as an elevated serum uric acid (UA) level, typically exceeding 357 μmol/L in women and 416 μmol/L in men (1). Its prevalence varies widely worldwide, ranging from 2.6% to 36%, with a reported rate of 8.4% in Saudi Arabia (2–4). This condition arises from either increased UA synthesis or impaired excretion, ultimately leading to deposition of urate crystals in joints (gout) and kidneys (nephrolithiasis) (5, 6).
Hyperuricemia presents along a clinical spectrum, ranging from asymptomatic elevation of serum UA to manifestations such as gout and nephrolithiasis (7, 8). Importantly, elevated serum UA is independently associated with an increased risk of cardiovascular morbidity and mortality, even in the absence of gout (8–10). Non-pharmacologic interventions play a critical role in managing asymptomatic hyperuricemia, focusing on dietary and lifestyle modifications (11, 12). For symptomatic cases, xanthine oxidase inhibitors remain the first-line pharmacological treatment (13). However, the effectiveness of current urate-lowering therapies is often limited by dosing challenges, adverse effects, and patient nonadherence (14). This underscores the need for exploring alternative therapies that are both safer and more effective.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i), recommended for patients with type 2 diabetes (T2D), heart failure (HF), or chronic kidney disease (CKD), have been shown to reduce serum uric acid (UA) levels through enhanced urinary urate excretion and modulation of metabolic pathways, potentially lowering the risk of incident gout (15–17). However, the magnitude and clinical relevance of this effect, particularly in patients with cardiovascular–kidney–metabolic syndrome, remain uncertain. Although mechanistic and clinical studies suggest that SGLT2i may lower UA through pathways distinct from conventional urate-lowering therapies, findings have not been consistently replicated across diverse populations or real-world settings. Moreover, while large, randomized trials have primarily focused on cardiovascular and renal outcomes, data on the impact of SGLT2i on UA levels in routine clinical practice—especially among patients with multiple comorbidities—remain limited (10, 16). Thus, robust, population-based evidence is needed to clarify the role of SGLT2i in managing hyperuricemia.
Despite advances in hyperuricemia management and a deeper understanding of its relationship with cardiovascular and renal comorbidities, data remain limited regarding the practical effects of SGLT2i on UA levels in real-world patient populations. Observational studies and clinical trials have hinted at the potential of SGLT2i to reduce hyperuricemia through mechanisms distinct from traditional urate-lowering therapies, yet these findings have not been consistently replicated in diverse healthcare settings or in patients with multiple comorbidities such as diabetes, HF, and CKD. Furthermore, while randomized trials have typically focused on cardiovascular and renal endpoints, the impact of SGLT2i on UA levels in routine clinical practice is not fully established, underscoring the need for robust, population-based data in this area. To address this knowledge gap, this study conducted a comprehensive multicenter retrospective cohort utilizing electronic medical records from diverse healthcare institutions across the Kingdom of Saudi Arabia. The primary aim of this study was to evaluate the effect of SGLT2i on serum UA levels in patients with diabetes, heart failure and chronic kidney diseases in different healthcare settings.
2 Methods
2.1 Study design
This was a multicenter retrospective cohort study of patients who received an SGLT2i for any indication. Only patients newly initiated on an SGLT2i between January 2022 and January 2024 were eligible; patients already receiving therapy prior to this period were excluded. Data were collected from three tertiary care hospitals and one cardiac center in Saudi Arabia. The study was approved by the Institutional Review Board (NRC24R/081/02) on July 4th, 2024. Eligible patients were aged 18 years or older and received an SGLT2i for DM, HF, or CKD. Exclusion criteria included active gout flares, a history of cancer, or initiation or dose adjustment of urate-lowering therapy within 90 days before SGLT2i initiation or any changes in urate-lowering therapy while on SGLT2i. Baseline uric acid was defined as the most recent measurement within six months before SGLT2i initiation; if multiple values were available, the average was recorded. The included patients had a minimum follow-up duration of 10 months. Elevated serum UA levels were defined as >420 μmol/L (~7.0 mg/dL) in males or >360 μmol/L (~6.0 mg/dL) in females (18). Chronic NSAID use was defined as daily or most-days of the week for at least 3 months, consistent with widely accepted definitions (19).
2.2 Study objective and data collection
The primary outcome was the percentage change in serum UA levels following SGLT2i initiation compared to baseline prior of medications initiation. Secondary outcomes included changes in metabolic parameters and adverse effects. Baseline clinical data collected included age, sex, body mass index (BMI), comorbidities, type and dose of SGLT2i. Laboratory parameters included UA (μmol/L), eGFR (mL/min/1.73 m²), left ventricular ejection fraction (LVEF%) and glycated hemoglobin (A1C%). Data on gout medications and anti-inflammatory therapies were also collected.
2.3 Statistical analysis
Nominal data were analyzed using Chi-square or Fisher’s exact tests, while continuous data were analyzed using the Mann–Whitney U test, Student t-test, or Wilcoxon signed-rank test, as appropriate, with a significance level of α=0.05. The Shapiro-Wilk test was used to assess normality of continuous data. As UA levels were not normally distributed, the Wilcoxon signed-rank test was used for the primary outcome. For univariate analysis, responders were defined as patients achieving a ≥8.5% reduction in UA levels after SGLT2i initiation. This threshold was selected to ensure clinically significant reductions were captured even among patients with normal or near-normal baseline UA levels. Similar percentage-based thresholds have been utilized in previous studies and guidelines evaluating urate-lowering therapies that highlighted the clinical relevance of percentage reductions when assessing treatment response in gout and hyperuricemia management (20, 21). Multivariate analysis was performed to adjust for potential confounders, including all variables with P ≤ 0.2 in univariate analysis. Longitudinal regression modeling was also conducted to assess trends in UA reductions over time. Longitudinal analyses were based on routine uric acid measurements obtained after SGLT2i initiation, which were organized into 2-month intervals; values within each interval were plotted to assess temporal trends. All statistical analyses were performed using Stata Statistical Software or SPSS Statistics (licensed by the affiliated universities).
3 Results
3.1 Patient population
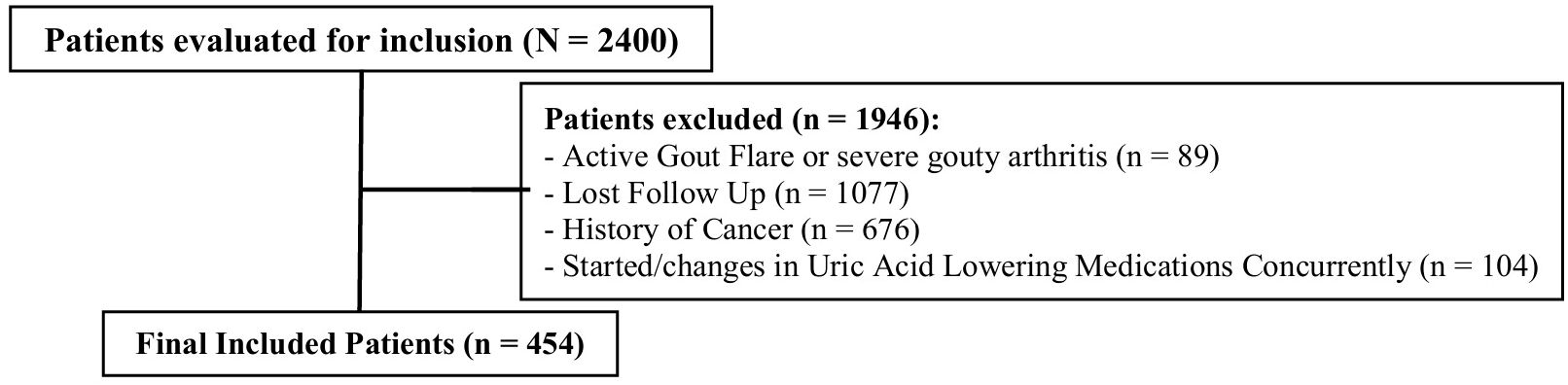
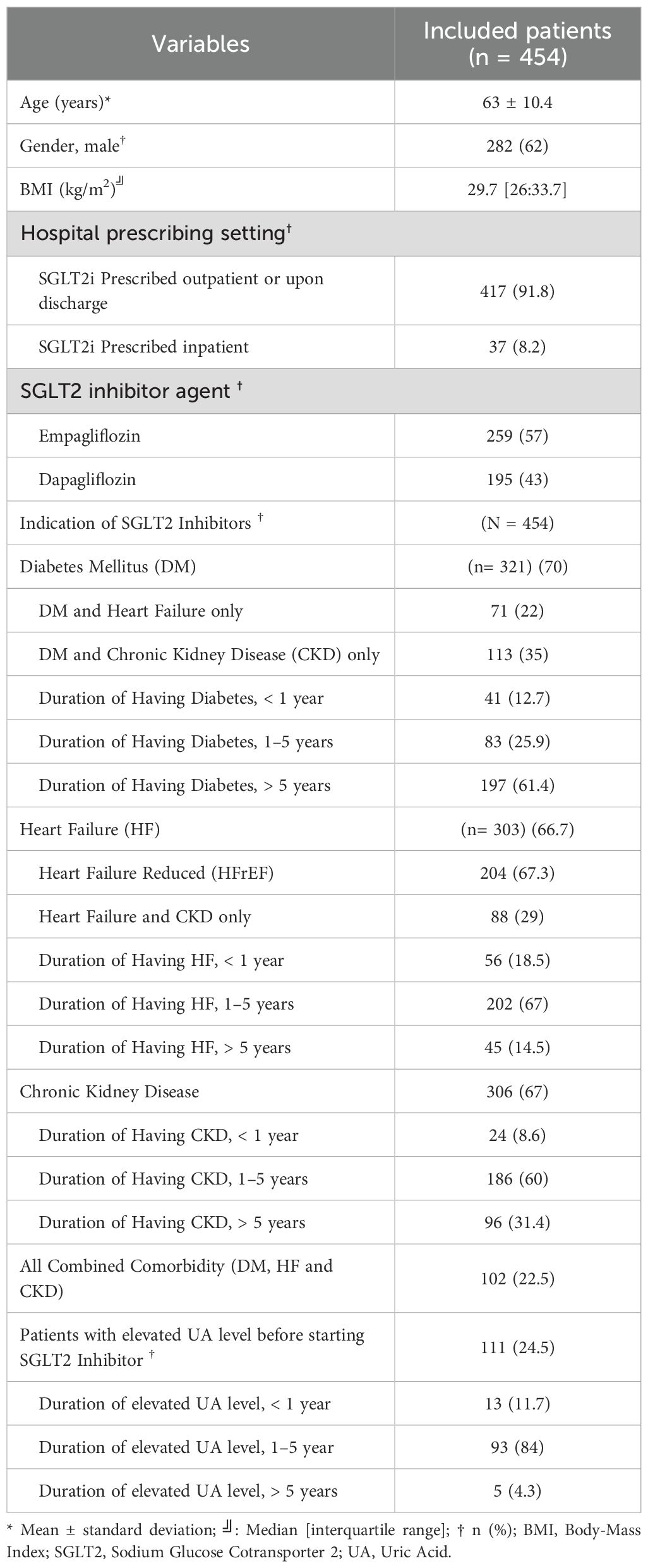
Data were collected from three tertiary care hospitals and one cardiac center in Saudi Arabia. Of the 2,400 patients screened, 1,946 were excluded, primarily due to loss to follow-up. The second most common exclusion criterion was a history of cancer. Additional details on exclusion criteria are shown in Figure 1. Ultimately, 454 patients were included in the final analysis. Most patients (91.8%) initiated SGLT2i in the outpatient setting. The baseline characteristics of the included patients are summarized in Table 1. The median age was 63 years, with 62% male and a median BMI of 29.7 kg/m². Empagliflozin was the more frequently used SGLT2i (57%), compared to dapagliflozin (43%), largely driven by formulary differences across sites. All patients received the standard dose of dapagliflozin (10 mg daily) or empagliflozin (10 mg daily), with no dose adjustments during follow-up. A diagnosis of DM was documented in 70% of patients, with most having been diagnosed more than 5 years prior to SGLT2i initiation. Heart failure (HF) and chronic kidney disease (CKD) were reported in 66.7% and 67% of patients, respectively, with disease durations typically between 1–5 years. Among HF patients, reduced ejection fraction was more common (67.3%) than preserved ejection fraction (32.7%). Only 3 patients initiated SGLT2i therapy exclusively for CKD without other comorbidities, while 23% had the combined comorbidity of DM, HF, and CKD. Elevated UA levels before SGLT2i initiation were observed in 25% of patients.
3.2 Outcomes
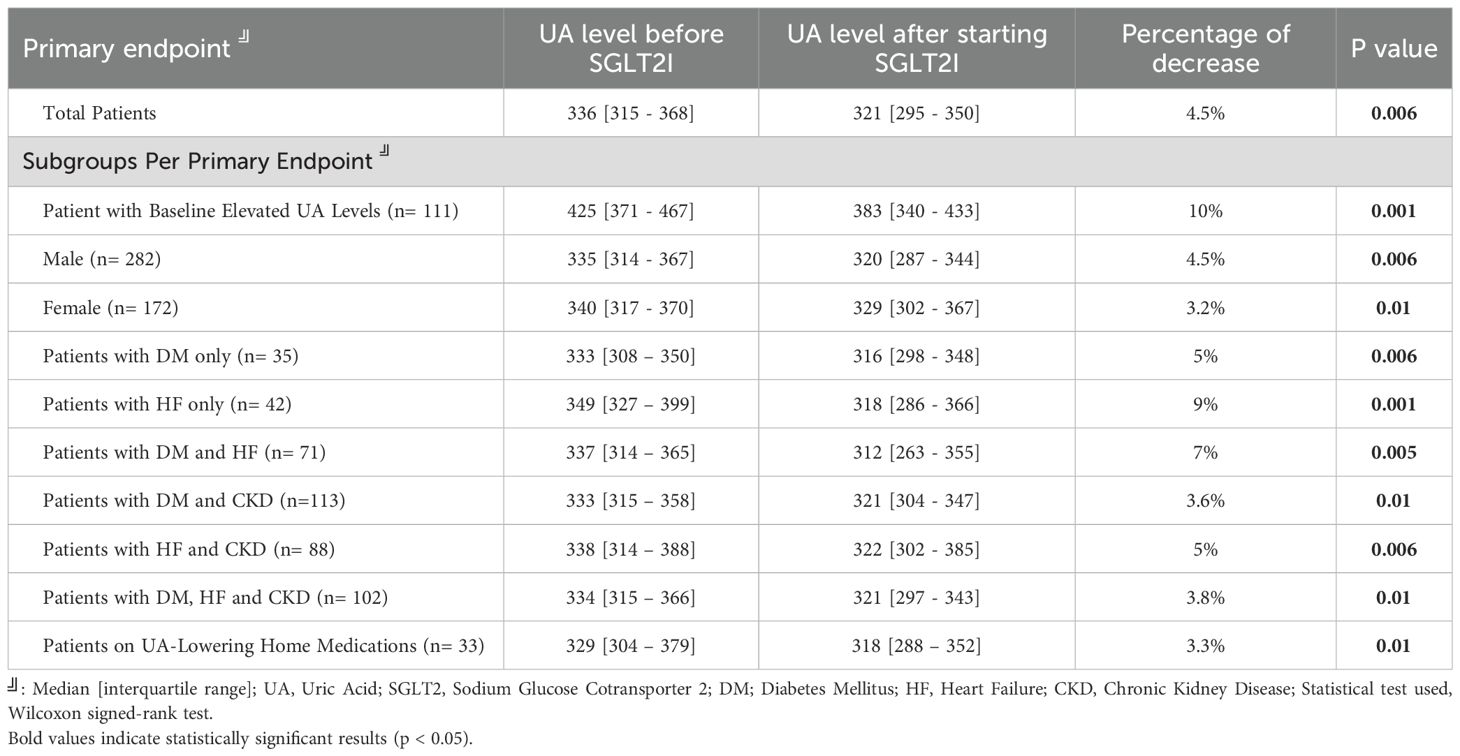
Table 2 representing the primary endpoint, shows a significant reduction in serum UA levels following the initiation of SGLT2i. For the total patient cohort, the median UA level decreased from 336 [315–368] μmol/L to 321 [295–350] μmol/L, reflecting a 4.5% reduction (p=0.006). The most pronounced decrease was observed in patients with baseline elevated UA levels, with a 10% reduction (from 425 [371–467] to 383 [340–433] μmol/L, p=0.001). Subgroup analysis demonstrated statistically significant reductions in UA levels across various patient groups. Males experienced a 4.5% decrease (p=0.006), while females had a 3.2% reduction (p=0.01). Patients with HF exhibited a 9% reduction (p=0.001), with median levels decreasing from 349 [327–399] to 318 [286–366] μmol/L. Reductions were also notable in patients with both HF and DM. Additional subgroup analyses are detailed in Table 2.
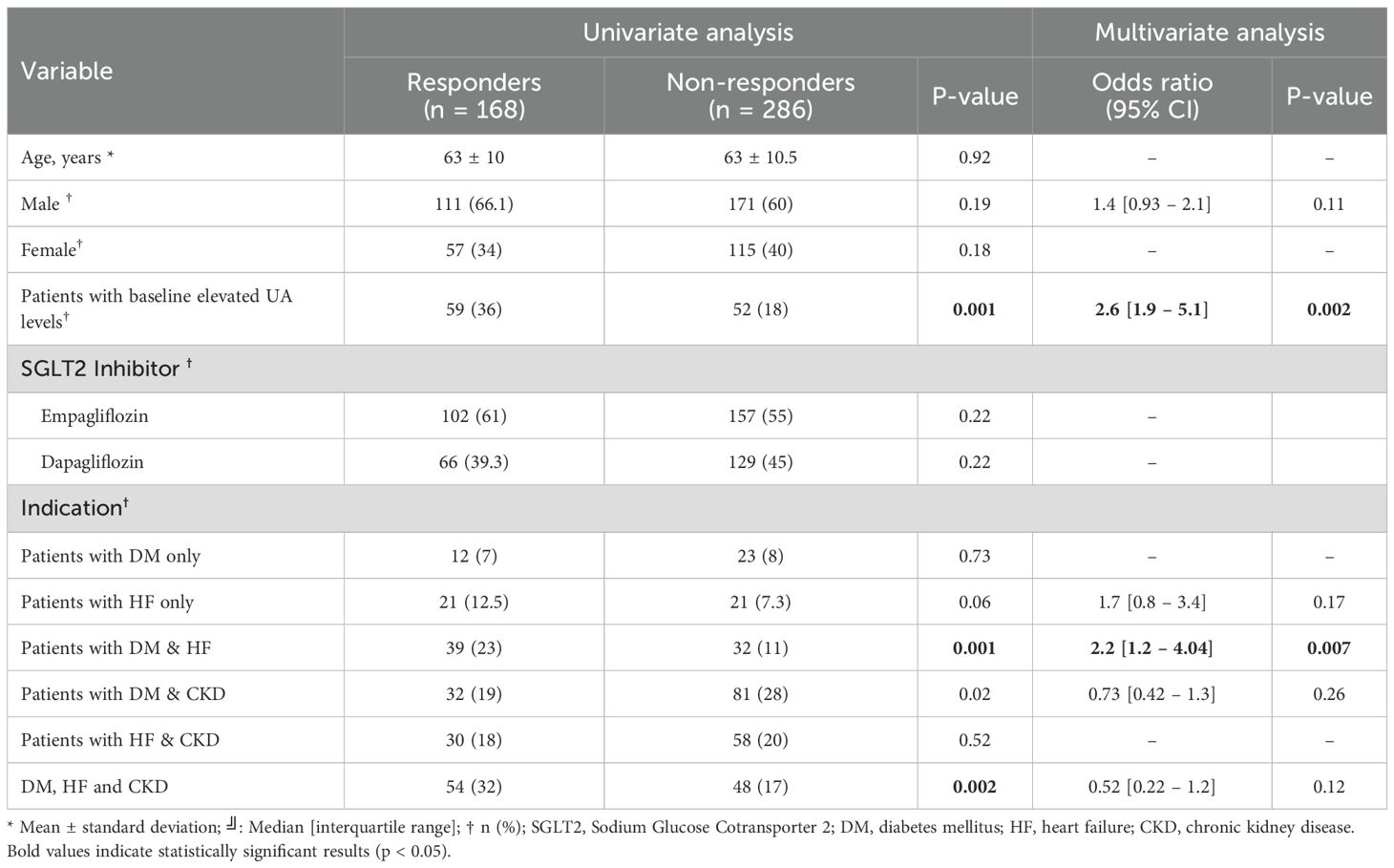
Univariate analysis revealed that patients with baseline elevated UA level (p=0.001), those with DM & HF (p=0.001), DM & CKD (p=0.02), and DM, HF, & CKD (p=0.002) were significantly more likely to be responders, while other variables, including age, sex, and SGLT2i type, did not show significant associations. In multivariate analysis, baseline elevated UA level (OR = 2.6, 95% CI: 1.9-5.1, p=0.002) and DM & HF (OR = 2.2, 95% CI: 1.2–4.04, p=0.007) remained an independent predictor of response. Associations for DM & CKD (OR = 0.73, p=0.26), DM, HF, & CKD (OR = 0.52, p=0.12), HF-only patients (OR = 1.7, p=0.17) and male sex (OR = 1.4, p=0.11) did not demonstrate significant independent effects (Table 3). Figure 2 illustrates UA level reductions over time for three patient groups. The line for “Total Patients” shows a consistent, gradual decline over 10 months. The line for “Elevated Baseline UA” shows a more rapid decrease, especially around months 5 to 6. The line for “DM and HF” shows a steep initial decline around month 3, then a stable pattern through month 10.
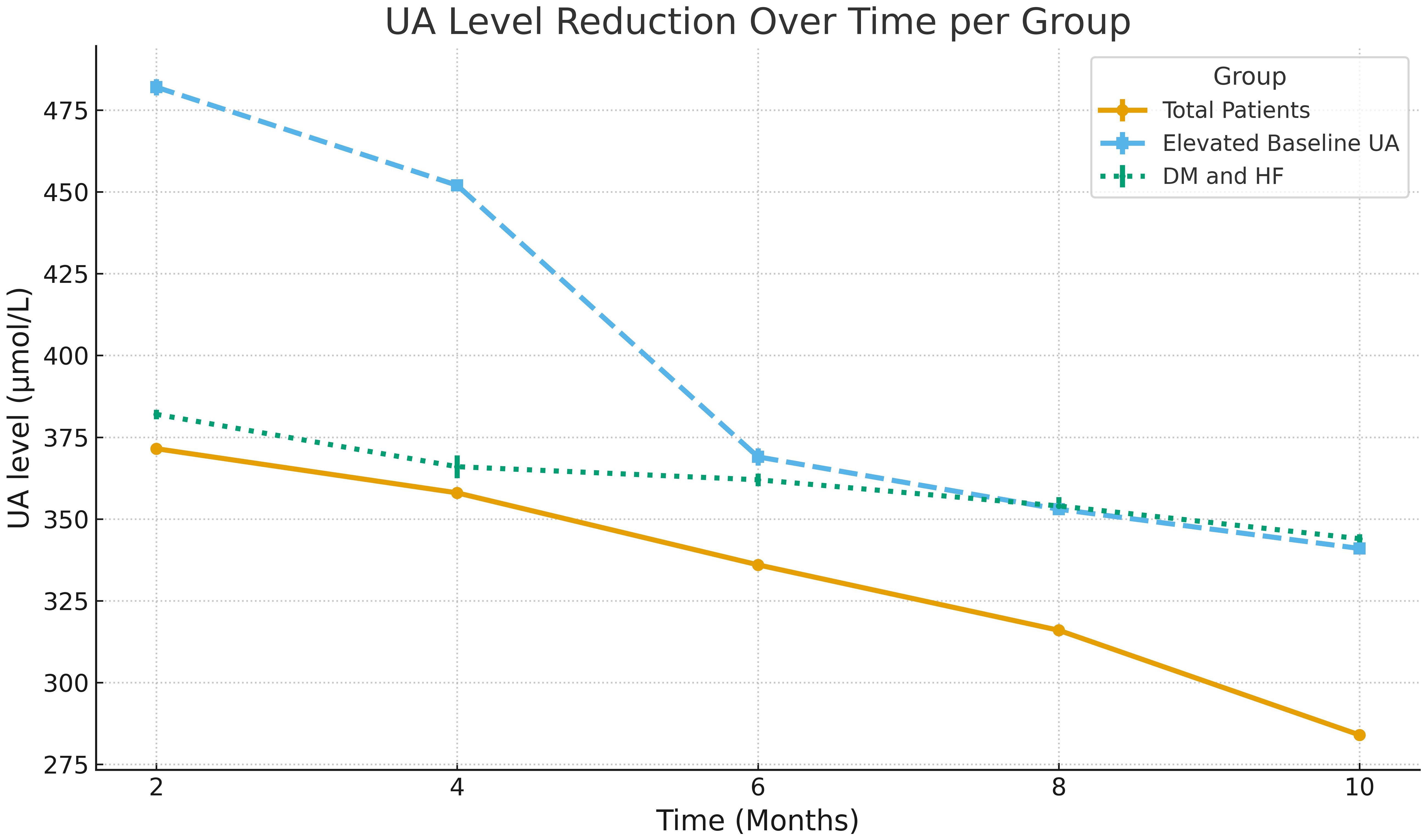
Figure 2. Longitudinal regression showing the reduction in serum uric acid (UA) levels over time across patient subgroups.
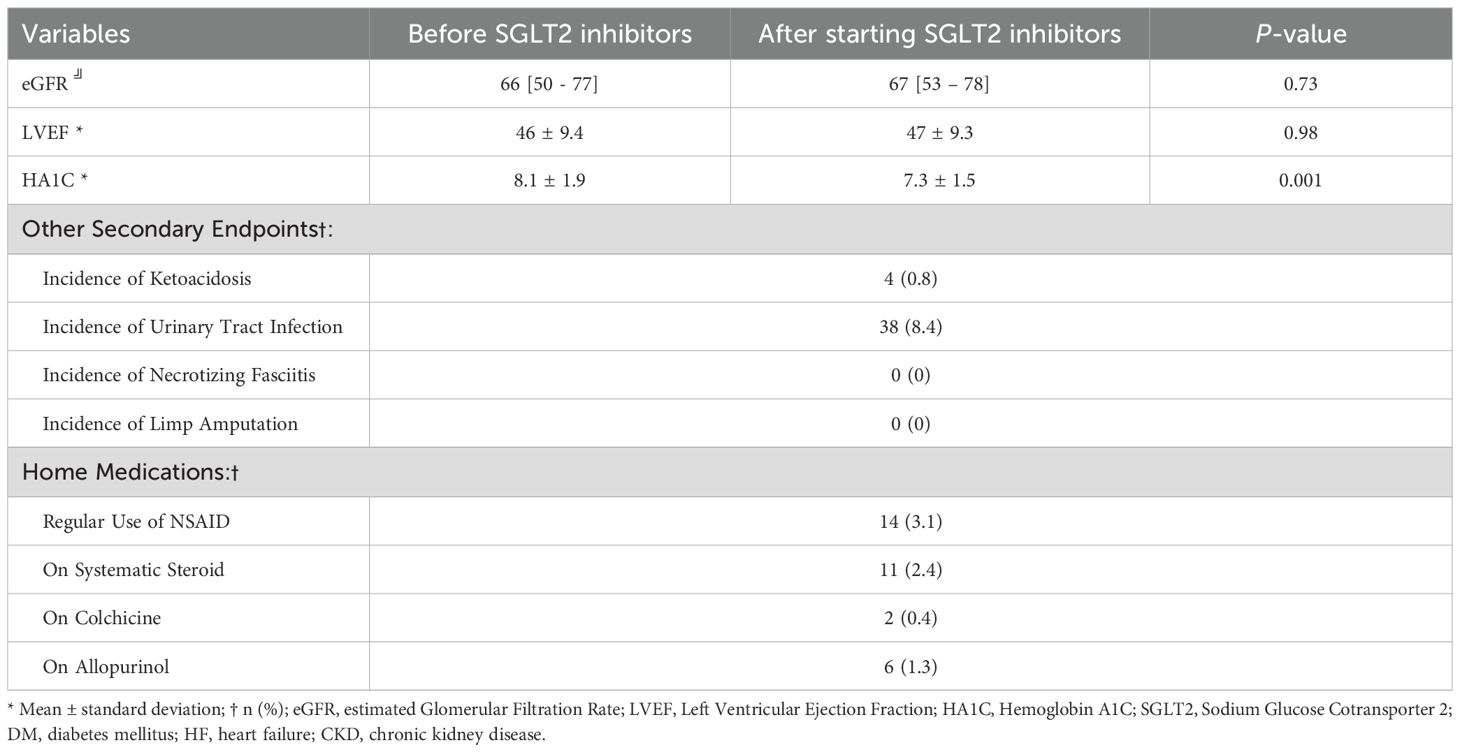
Table 4 presents the secondary endpoints evaluated in this study. There were no significant changes in eGFR 66 [50 - 77] vs 67 [53 - 78] (p=0.73) or LVEF (p=0.98) following SGLT2i initiation. However, there was a significant reduction in HbA1c levels from 8.1 ± 1.9% to 7.3 ± 1.5% (p=0.001). Adverse events were rare, with four cases (0.8%) of ketoacidosis and 38 cases (8.4%) of urinary tract infections. No cases of necrotizing fasciitis or limb amputation were observed. Regarding home medications, 33 patients were receiving agents that may influence uric acid, including NSAIDs (n=14, 3.1%), systemic steroids (n=11, 2.4%), colchicine (n=2, 0.4%), and allopurinol (n=6, 1.3%). No changes in dosing were observed during the study period based on chart review and refill history.
4 Discussion
This study examines the effect of SGLT2i on UA levels in a real-world, multicenter cohort in Saudi Arabia. Conducted across four major medical centers, this analysis demonstrated that SGLT2i therapy reduced UA levels regardless of the indication for use. Although patients with elevated baseline UA experienced the most pronounced reductions, UA-lowering effects were consistent across multiple subgroups. Additionally, the presence of both DM and HF emerged as an independent predictor of a favorable UA response. Given the high prevalence of hyperuricemia in patients with DM, HF, and CKD, - and its known association with worse outcomes - these findings support the use of SGLT2i in these patient populations (22–24).
In this cohort, about 35% of patients with DM also had CKD, aligning with previous findings in the Saudi population (25). Similarly, approximately 25% of patients with DM in this study also had HF, reflecting the well-documented bidirectional relationship between these conditions, where diabetes can contribute to structural and functional changes in the heart, and HF can exacerbate metabolic abnormalities, further increasing diabetes risk (26). For patients with combined diabetes, HF, and CKD, the prevalence was estimated at around 15–20% in this cohort, consistent with the concept of the “cardiorenal-metabolic syndrome,” where these interconnected conditions significantly worsen outcomes due to shared pathophysiological mechanisms (27). Additionally, among patients with HF, CKD prevalence in this study was 23%, which is within the reported range in previous epidemiological studies (28).
In this study, SGLT2i treatment led to a 4.5% reduction in UA levels over a 10-month follow-up. While this reduction is modest, it aligns with previous post hoc analyses of randomized clinical trials that reported approximately 13% reduction in UA levels with canagliflozin compared to placebo in type 2 DM patients (29). Similarly, empagliflozin lowered UA levels in the EMPA-REG OUTCOME study (30). A recent meta-analysis of patients with and without type 2 diabetes (n ≈ 464,009) showed that SGLT2i reduce the risk of clinically relevant hyperuricemic events by 33% (HR, 0.67; 95% CI, 0.59–0.77), reinforcing the UA-lowering effects beyond diabetic populations (31). Real-world studies have also confirmed these effects, and empagliflozin has demonstrated UA-lowering benefits in HF patients regardless of ejection fraction phenotype (24, 29).
Although this study did not assess gout incidence directly, prior analyses of trials such as EMPA-REG OUTCOME, EMPEROR-Reduced, CANVAS, and EMPEROR-Preserved—as well as real-world data—have shown that SGLT2i use reduces gout incidence (24, 32–34). Notably, SGLT2i have also been associated with lower risk of recurrent gout flares compared to GLP-1 receptor agonists and DPP-4 inhibitors in type 2 DM patients (35).
The UA-lowering effects of SGLT2i likely extend beyond their uricosuric action, involving reductions in UA synthesis via downregulation of pentose phosphate pathway enzymes (17, 36). Although some studies suggest that urate-lowering therapies like allopurinol can reduce cardiovascular events in hypertension, this effect has not consistently translated to HF populations (37, 38). Thus, the UA-lowering properties of SGLT2i may contribute to—but not solely account for—their cardiovascular benefits.
It is noteworthy that most patients with DM in this cohort initiated SGLT2i approximately 5 years after diagnosis. Earlier initiation may help prevent complications such as CKD, highlighting the importance of timely adherence to current guidelines (15, 39, 40). Given the high prevalence and adverse outcomes of hyperuricemia in patients with DM, HF, and CKD, the UA-lowering effect of SGLT2i may represent an additional pleiotropic benefit in these populations. While this study did not assess gout incidence or flare reduction, future prospective studies are warranted to determine whether these metabolic effects translate into improved gout-related outcomes (15, 39).
This study’s real-world, multicenter design across four major healthcare centers in Saudi Arabia enhances generalizability. The main limitation is the absence of a comparator group. As SGLT2i are now guideline-recommended for patients with diabetes, heart failure, and chronic kidney disease, an untreated control group was not feasible retrospectively. Instead, a pre–post design was used, allowing patients to serve as their own controls and reducing inter-individual variability. While this does not replace a formal comparator, the findings remain consistent with prior randomized trials. Another limitation is the lack of detailed data on diet and concomitant medications (e.g., diuretics), although we excluded patients with recent changes in urate-lowering therapy to minimize confounding. Moreover, the high exclusion rate, largely reflecting patients lost to follow-up. These exclusions were necessary to preserve data quality and ensure valid assessment of uric acid changes, but they may still introduce selection bias and affect generalizability.
5 Conclusion
SGLT2i were associated with a modest but consistent reduction in uric acid, most pronounced in patients with elevated baseline levels and those with both diabetes and heart failure. These findings suggest potential metabolic benefits and should be regarded as hypothesis-generating, warranting further prospective studies to confirm and clarify their implications for long-term patient management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by King Abdullah International Medical Research Center. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Retrospective chart review study.
Author contributions
EH: Conceptualization, Supervision, Writing – review & editing, Investigation, Writing – original draft, Project administration. AG: Writing – original draft, Data curation. AA: Methodology, Writing – review & editing, Writing – original draft. GA: Writing – original draft. AHAf: Data curation, Writing – review & editing. AHAl: Writing – review & editing. AAA: Writing – original draft, Conceptualization, Writing – review & editing. OA: Writing – original draft, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Song P, Wang H, Xia W, Chang X, Wang M, and An L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci Rep. (2018) 8:4314. doi: 10.1038/s41598-018-22662-5
2. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, and Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
3. Dehlin M, Jacobsson L, and Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16:380–90. doi: 10.1038/s41584-020-0441-1
4. Al-Arfaj AS. Hyperuricemia in Saudi Arabia. Rheumatol Int. (2001) 20:61–4. doi: 10.1007/s002960000076
5. Mandal AK and Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
6. Choi HK, Mount DB, and Reginato AM. Pathogenesis of gout. Ann Intern Med. (2005) 143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009
7. Stack AG, Hanley A, Casserly LF, Cronin CJ, Abdalla AA, Kiernan TJ, et al. Independent and conjoint associations of gout and hyperuricemia with total and cardiovascular mortality. QJM. (2013) 106:647–58. doi: 10.1093/qjmed/hct083
8. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, and Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). (2010) 62:170–80. doi: 10.1002/acr.20065
9. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, and Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheumatol. (2009) 61:885–92. doi: 10.1002/art.24612
10. Lu Z, Lu F, Zhang R, and Guo S. Interaction between anemia and hyperuricemia in the risk of all-cause mortality in patients with chronic kidney disease. Front Endocrinol (Lausanne). (2024) 15:1286206. doi: 10.3389/fendo.2024.1286206
11. Dincer HE, Dincer AP, and Levinson DJ. Asymptomatic hyperuricemia: to treat or not to treat. Cleve Clin J Med. (2002) 69:594–7. doi: 10.3949/ccjm.69.8.594
12. Paul BJ, Anoopkumar K, and Krishnan V. Asymptomatic hyperuricemia: is it time to intervene? Clin Rheumatol. (2017) 36:2637–44. doi: 10.1007/s10067-017-3825-0
13. Hainer BL, Matheson E, and Wilkes RT. Diagnosis, treatment, and prevention of gout. Am Fam Physician. (2014) 90:831–6.
14. Sharma G, Dubey A, Nolkha N, and Singh JA. Hyperuricemia, urate-lowering therapy, and kidney outcomes: a systematic review and meta-analysis. Ther Adv Musculoskelet Dis. (2021) 13:1759720X2110166. doi: 10.1177/1759720X211016619
15. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2024. Diabetes Care. (2024) 47:S158–78. doi: 10.2337/dc24-S009
16. Suijk DL, van Baar MJ, van Bommel EJ, Iqbal Z, Krebber MM, Vallon V, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol. (2022) 17:663–71. doi: 10.2215/CJN.11480821
17. Packer M. Hyperuricemia and gout reduction by SGLT2 inhibitors in diabetes and heart failure: JACC review topic of the week. J Am Coll Cardiol. (2024) 83:371–81. doi: 10.1016/j.jacc.2023.10.030
18. American Board of Internal Medicine. Uric acid levels and management guidelines. Philadelphia, PA: American Board of Internal Medicine (2022). Available online at: https://www.abim.org.
19. Papatheodorou I, Zampeli E, Voulgari PV, and Drosos AA. NSAID use in patients with rheumatic disease: a review of benefits and risks. Clin Exp Rheumatol. (2020) 38 Suppl 122:27–32.
20. Robinson PC and Stamp LK. The management of gout: much has changed. Aust Fam Physician. (2016) 45:299–302.
21. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). (2012) 64:1431–46. doi: 10.1002/acr.21772
22. Krishnan E. Reduced glomerular filtration rate increases serum uric acid concentrations: effect of dose-response relationship in the US general population. Kidney Int. (2012) 82:416–23. doi: 10.1038/ki.2012.63
23. Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. (2012) 14:179–88. doi: 10.1007/s11926-012-0241-z
24. Doehner W, Anker SD, Butler J, Zannad F, Filippatos G, Ferreira JP, et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR-reduced trial. Eur Heart J. (2022) 43:3435–46. doi: 10.1093/eurheartj/ehac320
25. Alqarni A, Alsharif A, Alalwan A, Alghamdi T, Alsulaimani O, Almalki M, et al. Prevalence and risk factors of chronic kidney disease in type 2 diabetes in Saudi Arabia. Saudi J Kidney Dis Transpl. (2022) 33:23–30.
26. Thrainsdottir IS, Aspelund T, Gudnason V, Sigurdsson G, Olafsson O, Eiriksdottir G, et al. The prevalence of heart failure in patients with diabetes: insights from the Reykjavik Study. Eur Heart J. (2005) 26:66–72. doi: 10.1093/eurheartj/ehi022
27. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of diabetes mellitus with heart failure and chronic kidney disease: results from the CANVAS Program. Circulation. (2020) 141:703–12. doi: 10.1161/CIRCULATIONAHA.119.042360
28. Elheet AA, Alosaimi MA, Alalawi WA, Almohamadi AA, Almutairi AA, Alharbi AA, et al. Association between cardiovascular disease and chronic kidney disease prevalence and characteristics in Saudi Arabia. Cureus. (2023) 15:e50205. doi: 10.7759/cureus.50205
29. Davies MJ, Trujillo A, Vijapurkar U, Damaraju CV, and Meininger G. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab. (2015) 17:426–9. doi: 10.1111/dom.12439
30. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
31. Ghukasyan H, Navalha DD, Pérez Romero I, Roca-Maza J, López Soler P, Flores T, et al. Reducing hyperuricemic events with SGLT2 inhibitors: an updated systematic review with meta-regression. Endocrinol Diabetes Nutr. (2025) 72:26–36. doi: 10.1016/j.endinu.2024.06.004
32. Ferreira JP, Zannad F, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. Empagliflozin and uric acid metabolism in diabetes: a post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes Metab. (2022) 24:135–41. doi: 10.1111/dom.14559
33. Li J, Badve SV, Zhou Z, Rodgers A, Jun M, Zoungas S, et al. The effects of canagliflozin on gout in type 2 diabetes: a post-hoc analysis of the CANVAS Program. Lancet Rheumatol. (2019) 1:e220–8. doi: 10.1016/S2665-9913(19)30078-5
34. Chung MC, Hung PH, Hsiao PJ, Lin YT, Lin KD, Pei D, et al. Association of sodium-glucose transport protein 2 inhibitor use for type 2 diabetes and incidence of gout in Taiwan. JAMA NetwOpen. (2021) 4:e2135353. doi: 10.1001/jamanetworkopen.2021.35353
35. Wei J, Choi HK, Dalbeth N, Stamp LK, Gaffo AL, Singh JA, et al. Gout flares and mortality after sodium-glucose cotransporter-2 inhibitor treatment for gout and type 2 diabetes. JAMA Netw Open. (2023) 6:e2333267. doi: 10.1001/jamanetworkopen.2023.33267
36. Novikov A, Fu Y, Huang W, Freeman B, Patel R, Thomas S, et al. SGLT2 inhibition and renal uric acid excretion: role of glucose and sodium transporters. Am J Physiol Renal Physiol. (2019) 316:F687–95. doi: 10.1152/ajprenal.00468.2018
37. MacIsaac RL, Salatzki J, Patel SK, Ord T, Hunter RW, Hayward C, et al. Allopurinol use and cardiovascular outcomes in patients with hypertension: a population-based cohort study. Hypertension. (2019) 73:566–74. doi: 10.1161/HYPERTENSIONAHA.118.12270
38. Hare JM, Mangal B, Brown J, Fisher C, Freudenberger R, Colucci WS, et al. Impact of oxypurinol in patients with symptomatic heart failure: results of the OPT-CHF study. J Am Coll Cardiol. (2008) 51:2301–9. doi: 10.1016/j.jacc.2008.01.068
39. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. (2022) 79:e263–421. doi: 10.1016/j.jacc.2021.12.012
Keywords: hyperuricemia, SGLT2 inhibitors, uric acid, diabetes, heart failure, chronic kidney disease
Citation: Habeeb EA, Ghaith AM, Alshehri AM, Aquil G, Afana AH, Alqarafi AH, Alahmed AA and Alkhezi OS (2025) Revealing the hidden impact of SGLT2 inhibitors on uric acid levels: a retrospective multicenter cohort study. Front. Endocrinol. 16:1667438. doi: 10.3389/fendo.2025.1667438
Received: 16 July 2025; Accepted: 02 October 2025;
Published: 20 October 2025.
Edited by:
Wanlu Ma, Endocrinology Department of China Japan Friendship Hospital, ChinaReviewed by:
Manuel Kraft, Heidelberg University Hospital, GermanyHamlet Ghukasyan, Hospital Francesc de Borja, Spain
Copyright © 2025 Habeeb, Ghaith, Alshehri, Aquil, Afana, Alqarafi, Alahmed and Alkhezi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulmajeed M. Alshehri, c2hlaHJpYWJkdWxAa3NhdS1ocy5lZHUuc2E=
 Ehsan A. Habeeb1,2,3
Ehsan A. Habeeb1,2,3 Abdulmajeed M. Alshehri
Abdulmajeed M. Alshehri