Abstract
Objective:
Previous studies have shown an association between polycystic ovary syndrome (PCOS) and the use of various medications. However, there is still a lack of systematic research exploring this relationship in depth. This study aims to identify and evaluate drugs that may influence the risk of PCOS using the US FDA Adverse Event Reporting System (FAERS) database.
Methods:
Adverse events (AEs) related to drug-induced PCOS were retrieved from the FAERS database (Q1–2014 to Q4 2024). Four statistical methods (ROR, PRR, BCPNN, and MGPS) were used for imbalance analysis to identify drugs significantly associated with PCOS risk. Additionally, a latency (TTO) analysis was conducted to assess the timing of onset and the risk characteristics of PCOS-related adverse reactions.
Results:
This study identified 18 drugs significantly associated with PCOS-related AEs from a total of 1,516 cases through imbalance analysis. These drugs span various categories, including respiratory, antipsychotic, and anticonvulsant medications. Among them, Mecasermin (ROR = 67.54) and Ciclesonide (ROR = 62.10) presented the highest risk, followed by Valproic acid (ROR = 20.78) and Olanzapine (ROR = 10.27). Adverse events were most commonly observed either after 360 days of medication use or within 30 days. The median time to onset for the top three drugs with the highest signal frequency was as follows: Olanzapine (155.5 days), Quetiapine (335 days), and Valproic acid (905 days).
Conclusion:
This study is the first large-scale, systematic exploration of drug signals related to PCOS using the FAERS database. The drugs identified are primarily associated with the nervous system, followed by respiratory system medications and other types of drugs. These findings provide new warning evidence and references for clinical drug safety, suggesting that enhanced monitoring of female patients should be implemented when prescribing such drugs.
1 Introduction
PCOS is a common endocrine disorder of the female reproductive system, with an estimated prevalence ranging from 4% to 12% (1). Its typical clinical manifestations include irregular menstruation, hyperandrogenism, and polycystic ovarian changes (2). Additionally, PCOS increases the risk of various long-term complications in women, including infertility, type 2 diabetes, atherosclerosis, dyslipidemia, and cardiovascular diseases (3–6). The exact pathogenesis of PCOS is not yet fully understood, but it is widely believed that genetic, environmental, and lifestyle factors play a major role in its development (7). It is worth noting that drug side effects can be a potential risk factor for multiple diseases and may also extend to the realm of PCOS (8). Existing research indicates that certain hormonal drugs, antipsychotics, antiepileptic drugs, and others may interfere with ovarian function in women through complex physiological mechanisms, thereby triggering symptoms related to PCOS (9–11).
Current studies on drug-related PCOS mainly come from clinical trials and observational research, but these studies have limited sample sizes, disease coverage, and drug evaluation ranges. In real-world settings, large-scale, comprehensive research and sufficient data on drug-induced PCOS are still lacking. The FAERS database, one of the largest drug adverse event databases in the world, can identify potential associations between drugs and PCOS in a broad patient population. Research based on FAERS effectively overcomes the limitations of traditional clinical trials, such as small sample sizes and short observation periods (12). To date, there has been no extensive analysis from FAERS on drugs associated with an increased risk of PCOS. Therefore, this study aims to analyze FAERS data to identify potential risk signals of drugs that may increase the risk of PCOS, assess the associated risk levels, and systematically examine the relationship between these drugs and PCOS. This analysis will help clinicians assess the risk of PCOS when choosing treatment options and provide guidance for future basic research on potential mechanisms, ultimately advancing drug risk assessment and management strategies.
2 Materials and methods
2.1 Data source and preprocessing
The data for this study was sourced from the FAERS database, which can be downloaded from the FDA website (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). The database offers two download formats: ASCII and XML. For statistical analysis, this study downloaded the raw ASCII data from the first quarter of 2014 to the fourth quarter of 2024. The dataset includes patient demographics, drug information, adverse events, outcomes, report sources, treatment duration, and usage indications. A deduplication protocol was implemented according to FDA guidelines (13). Key fields such as PRIMARYID, CASEID, and FDADT were meticulously extracted and organized from the source database. For reports with the same CASEID, the entry with the latest FDADT was retained. For records with identical CASEID and FDADT, the record with the highest PRIMARYID was selected. In cases where both CASEID and FDADT were identical, the record with the highest PRIMARYID was selected (as shown in Table 1).
Table 1
| CASEID | FDA_DT | PRIMARYID | Delete |
|---|---|---|---|
| 4070800 | 20040113 | 4271953 | Yes |
| 4070800 | 20040113 | 4271960 | Yes |
| 4070800 | 20040130 | 4283861 | Yes |
| 4070800 | 20040308 | 4314767 | No |
Example of duplicate report removal criteria.
2.2 Adverse reaction identification
In this study, MedDRA dictionary (MedDRA 27.1) preferred terms (PTs) were used to encode adverse event names in the FAERS database. Standardized queries were used to identify PTs related to Drug-induced Polycystic Ovary Syndrome, and the term “Drug-induced Polycystic Ovary Syndrome” was selected for analysis. Cases related to this PT were extracted from the DEMO file using their PRIMARYID, and duplicate reports were removed using a deduplication algorithm based on patient ID and report event time, ensuring that each event was counted only once. For the drug involvement analysis, only cases labeled as “primary suspected drug” were included, while entries labeled as “secondary suspected drug,” “concomitant drug,” or “interaction drug” were excluded to reduce result uncertainty.
2.3 Statistical analysis
This study employed four methods to identify potential drug-event associations: Reporting Odds Ratio (ROR) (14), Proportional Reporting Ratio (PRR) (15), Bayesian Confidence Propagation Neural Network (BCPNN) (16), and Multi-item Gamma Poisson Shrinker (MGPS) (17). These methods evaluate the statistical relationship between a specific drug and a specific adverse event (AE) by calculating the relative frequency of target drug-related target AEs in the database over a period of time. a refers to the number of reports containing both the target drug and target AE. b refers to the number of reports containing the target drug and other drug AEs. c refers to the number of reports containing other drugs and the target AE. d refers to the number of reports containing other drugs and other drug AEs (as shown in Table 2). The formulas for these four methods are presented in Table 3. Drugs that met the criteria from all four methods were included in the study, indicating a significant association with Drug-induced Polycystic Ovary Syndrome.
Table 2
| Types of drugs | Target adverse event reports | Other adverse event reports | Total |
|---|---|---|---|
| Target drug | a | b | a+b |
| Other drugs | c | d | c+d |
| Total | a+c | b+d | a+b+c+d |
Contingency table for proportional imbalance measurement method.
Table 3
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | 95% CI (lower limit) > 1, a ≥ 3 | |
| PRR | a ≥ 3, 95% CI (lower limit) > 1, and PRR ≥ 2 | |
| BCPNN | IC-2SD >0 | |
| MGPS | EBGM05>2 |
Four main algorithms for evaluating the potential association between drugs and drug-induced polycystic ovary syndrome.
ROR, Reporting Odds Ratio; PRR, Proportional Reporting Ratio; BCPNN, Bayesian Confidence Propagation Neural Network; MGPS, Multi-item Gamma Poisson Shrinker; EBGM, Empirical Bayesian Geometric Mean; CI, Confidence Interval; χ2, Chi-square; IC, Information Component; IC025, the lower limit of the 95% one-sided confidence interval for IC; EBGM05, the lower limit of the 95% CI for EBGM.
3 Results
3.1 Data retrieval results
A total of 1,516 reports of drug-induced polycystic ovary syndrome (PCOS) were retrieved from the FDA Adverse Event Reporting System (FAERS) database spanning Q1–2014 to Q4 2024. These reports include patients’ demographic information such as age, weight, adverse reaction onset time, and adverse reaction outcomes. The data retrieval workflow is illustrated in Figure 1.
Figure 1

Flowchart of data acquisition and processing for drug-related PCOS.
3.2 General information of drug-related polycystic ovary syndrome reports
The reports of drug-related Polycystic Ovary Syndrome (PCOS) came from 48 countries and regions, with the highest number of reports from the United States, followed by Canada. The number of reports has shown an increasing trend year by year (as shown in Figure 2). The majority of the reporters were consumers (50.53%), followed by doctors (20.71%) and healthcare professionals (10.22%). In these reports, the most common age group was 20–29 years (18.73%), followed by 30–39 years (13.92%), and so on. The majority of reported weights were >100kg (7.59%), followed by 60-69kg (5.34%), and others. The outcomes of these reports were primarily categorized as “other” (46.90%), followed by “hospitalization” (27.84%), and so on (as shown in Figure 3).
Figure 2

Basic information of drug-related polycystic ovary syndrome reports. (A) World Distribution Map of Reporting Countries; (B) Number of Reports by Year.
Figure 3

Basic information of drug-related PCOS reports. (A) Age Distribution of Drug-related PCOS Reports; (B) Distribution of Reporters’ Identities for Drug-related PCOS; (C) Weight Distribution in Drug-related PCOS Reports; (D) Outcome Distribution of Drug-related PCOS Reports.
3.3 Drug risks and classification for drug-related PCOS
Based on the four methods (ROR, PRR, BCPNN, and MGPS), 18 potential drugs that may induce PCOS were identified as positive. According to the ROR values, the drugs with the highest risk are Mecasermin (ROR = 67.54), followed by Ciclesonide (ROR = 62.1). In terms of frequency ranking, the drugs are: Valproic acid (ROR = 20.78), Olanzapine (ROR = 10.27), Lamotrigine (ROR = 5.25), Quetiapine (ROR = 3.52), Clonazepam (ROR = 12.90), Salbutamol (ROR = 3.18), Montelukast (ROR = 10.29), Levothyroxine (ROR = 3.36), Aciclovir (ROR = 15.96), Budesonide; Formoterol (ROR = 4.26), Tramadol (ROR = 3.88), Aclidinium (ROR = 25.76), Modafinil (ROR = 32.94), Budesonide (ROR = 4.68), Ciclesonide (ROR = 62.1), Ofatumumab (ROR = 3.72), Mecasermin (ROR = 67.54), and Ziprasidone (ROR = 5.10) (as shown in Table 4).
Table 4
| Drug | Number | ROR (95%CI) | PRR (χ2) | EBGM (EBGM05) | IC (IC025) |
|---|---|---|---|---|---|
| Valproic acid | 56 | 20.78 (15.91 - 27.14) | 20.75 (1013.71) | 20.02 (16.01) | 4.32 (3.93) |
| Olanzapine | 39 | 10.27 (7.47 - 14.11) | 10.26 (317.54) | 10.02 (7.68) | 3.32 (2.86) |
| Lamotrigine | 22 | 5.25 (3.45 - 8) | 5.25 (74.56) | 5.19 (3.65) | 2.37 (1.77) |
| Quetiapine | 22 | 3.52 (2.31 - 5.36) | 3.52 (39.1) | 3.48 (2.45) | 1.8 (1.19) |
| Clonazepam | 20 | 12.9 (8.3 - 20.06) | 12.89 (216.5) | 12.73 (8.8) | 3.67 (3.04) |
| Salbutamol | 19 | 3.18 (2.02 - 5) | 3.18 (28.06) | 3.15 (2.16) | 1.66 (1.01) |
| Montelukast | 18 | 10.29 (6.47 - 16.38) | 10.28 (149.1) | 10.17 (6.9) | 3.35 (2.68) |
| Levothyroxine | 14 | 3.36 (1.99 - 5.69) | 3.36 (23) | 3.34 (2.15) | 1.74 (0.99) |
| Aciclovir | 13 | 15.96 (9.24 - 27.56) | 15.94 (180.49) | 15.81 (10.01) | 3.98 (3.21) |
| Budesonide; Formoterol | 11 | 4.26 (2.35 - 7.7) | 4.26 (27.2) | 4.23 (2.58) | 2.08 (1.25) |
| Tramadol | 11 | 3.88 (2.14 - 7.02) | 3.88 (23.34) | 3.86 (2.35) | 1.95 (1.11) |
| Aclidinium | 9 | 25.76 (13.37 - 49.64) | 25.71 (212.48) | 25.56 (14.76) | 4.68 (3.76) |
| Modafinil | 9 | 32.94 (17.09 - 63.49) | 32.85 (276.33) | 32.66 (18.86) | 5.03 (4.11) |
| Budesonide | 8 | 4.68 (2.34 - 9.37) | 4.68 (23.01) | 4.66 (2.6) | 2.22 (1.25) |
| Ciclesonide | 8 | 62.1 (30.94 - 124.61) | 61.79 (475.96) | 61.47 (34.32) | 5.94 (4.97) |
| Ofatumumab | 8 | 3.72 (1.85 - 7.44) | 3.71 (15.79) | 3.7 (2.07) | 1.89 (0.92) |
| Sodium Chloride | 5 | 8.13 (3.38 - 19.57) | 8.13 (31.15) | 8.1 (3.89) | 3.02 (1.84) |
| Mecasermin | 4 | 67.54 (25.25 - 180.68) | 67.18 (260.1) | 67 (29.41) | 6.07 (4.77) |
| Ziprasidone | 4 | 5.1 (1.91 - 13.6) | 5.09 (13.13) | 5.08 (2.24) | 2.35 (1.05) |
Statistical values and distribution of drugs inducing PCOS.
ROR, Reporting Odds Ratio; PRR, Proportional Reporting Ratio; BCPNN, Bayesian Confidence Propagation Neural Network; MGPS, Multi-item Gamma Poisson Shrinker; EBGM, Empirical Bayesian Geometric Mean; CI, Confidence Interval; χ2, Chi-square; IC, Information Component; IC025, the lower limit of the 95% one-sided confidence interval for IC; EBGM05, the lower limit of the 95% CI for EBGM.
The selected high-risk drugs were classified as follows: Bronchodilators and respiratory medications include 6 types, Antipsychotic drugs include 3 types, Antiepileptic drugs include 2 types, Hormonal drugs include 1 type, Analgesic drugs include 1 type, Antiviral drugs include 1 type, Anxiolytic drugs include 1 type, Cancer treatment drugs include 1 type, Cognitive enhancers and narcolepsy medications include 1 type, and Growth factors include 1 type (as shown in Figure 4).
Figure 4

Forest plot of PCOS induced by different drugs under ROR classification. Abbreviation: ROR, Reporting Odds Ratio; CI, Confidence Interval.
3.4 Drug-related PCOS induction time
Among the reports on drug-related PCOS, 303 reports documented the onset time of drug-induced PCOS. The highest number of cases occurred after >360 days (129 cases), followed by 0–30 days (68 cases) (as shown in Figure 5). In this study, we further analyzed the induction time of the top 3 drugs based on signal frequency ranking. For Olanzapine, the median induction time for PCOS was 155.5 days, for Quetiapine, it was 335.00 days, and for Valproic acid, it was 905.00 days (as shown in Table 5).
Figure 5
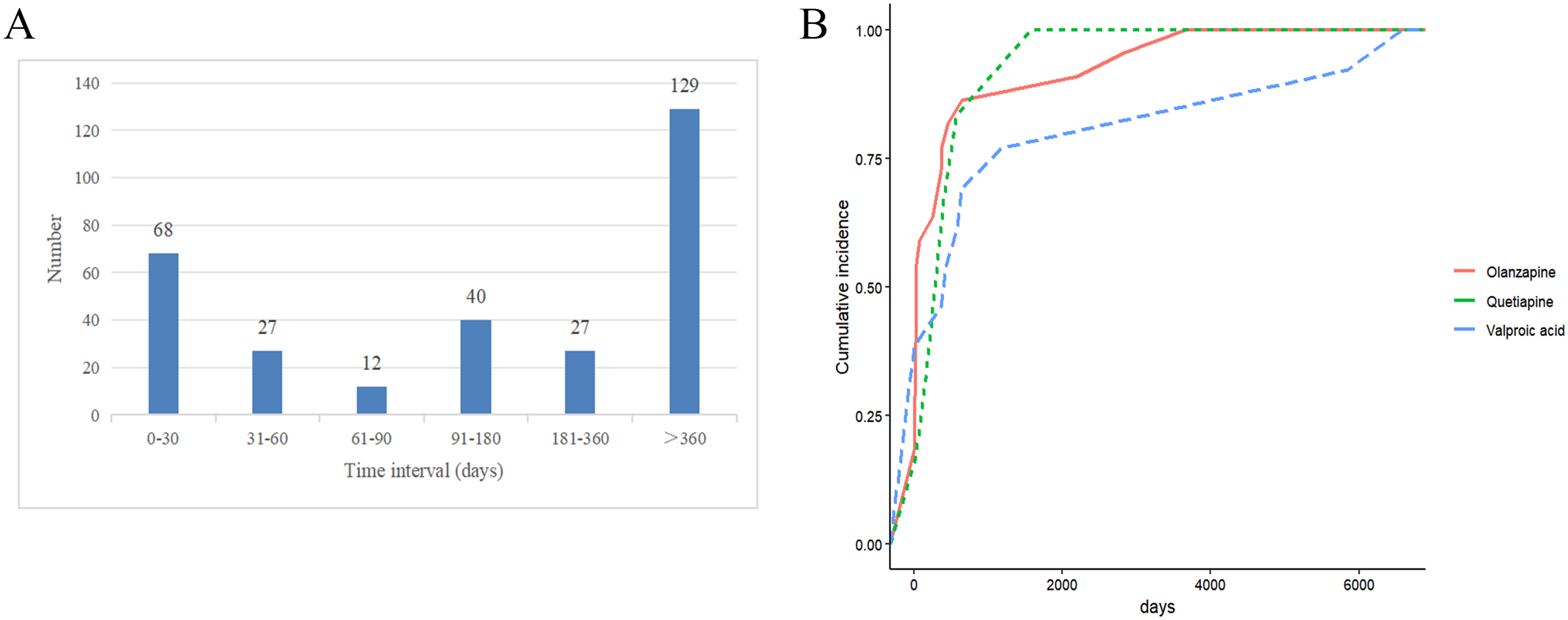
Drug-related PCOS induction time for risk drugs. (A) Drug-Induced Onset Time; (B) Cumulative Risk Timeline of PCOS Induced by Different Drugs.
Table 5
| Drug | Median (day) | Q1 (day) | Q3 (day) |
|---|---|---|---|
| Olanzapine | 155.50 | 30.00 | 501.75 |
| Quetiapine | 335.00 | 127.25 | 826.50 |
| Valproic acid | 905.00 | 465.50 | 5259.00 |
Time distribution of PCOS induced by different drugs.
Q1,25th percentile; Q3,75th percentile.
4 Discussion
PCOS is a common reproductive endocrine disorder that significantly affects women’s reproductive health (18). Its etiology is complex; while genetic and environmental factors are widely regarded as the primary causes, drug-induced PCOS should not be overlooked (19). In this study, we utilized large-scale real-world data from the FAERS database to systematically analyze the potential risk drugs associated with the onset of PCOS. Through screening, we identified 18 drugs that may trigger PCOS, spanning categories such as respiratory drugs, psychiatric medications, antiepileptic drugs, and hormone regulators. Our findings provide important reference information for clinical drug safety evaluations.
Previous studies have shown that long-term use of various psychiatric and antiepileptic drugs may affect female reproductive endocrinology and lead to PCOS (20). In our study, we observed that antiepileptic drugs, such as valproic acid (ROR = 20.78) and lamotrigine (ROR = 5.25), were associated with PCOS. Previous reports have indicated that the risk of PCOS associated with lamotrigine is lower than that associated with valproic acid, which is consistent with our findings (11, 21). Although the exact mechanisms through which these two drugs induce PCOS remain unclear, several small-scale clinical studies have reported PCOS-like symptoms—such as ovulatory dysfunction, hyperandrogenemia, increased body mass index, and insulin resistance—associated with valproic acid and lamotrigine (22–25). Valproic acid may also disrupt the balance of the hypothalamic-pituitary-ovarian (HPO) axis by affecting the secretion of gonadotropin-releasing hormone (GnRH), potentially leading to PCOS or other reproductive endocrine disorders in women (26). Moreover, some studies have suggested that Valproic acid may cause follicular development disorders by disrupting the balance between luteinizing hormone (LH) and follicle-stimulating hormone (FSH), resulting in polycystic ovaries (27). Therefore, female patients using antiepileptic drugs long-term should regularly monitor their endocrine levels and closely observe for any PCOS-related symptoms. In this study, the median onset time for PCOS after using Valproic acid was 905.00 days, which provides an important time reference for clinical management. This study also observed that antipsychotic drugs such as Olanzapine (ROR = 10.27), Ziprasidone (ROR = 5.1), and Quetiapine (ROR = 3.52) were associated with PCOS onset. Previous research has shown that these drugs may trigger metabolic disorders, such as weight gain and insulin resistance, with Olanzapine showing the highest incidence rate (28). Several rat experiments have linked Olanzapine to severe metabolic abnormalities, such as weight gain, insulin resistance, and hyperlipidemia (29–31). In one experiment on female rats, Olanzapine might cause insulin-resistant PCOS by regulating the IGF1/p-AKT/FOXO1 and NF-KB/IL-1β/TNF-α signaling pathways (32). In our study, we also observed the association of the analgesic Tramadol (ROR = 3.88) and the anti-anxiety drug Clonazepam (ROR = 12.9) with PCOS. Tramadol is a centrally acting analgesic that relieves pain by acting on neurotransmitters in the brain and spinal cord and also has some antidepressant effects (33). Clonazepam is a benzodiazepine that enhances the inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the brain to alleviate anxiety and induce sedation (34). However, there is currently very limited research on the relationship between these drugs and PCOS in populations. Modafinil, a drug commonly used to treat narcolepsy, insomnia, and attention deficit disorders, improves alertness and attention by affecting neurotransmitters in the brain (35). Common side effects include headaches, nausea, diarrhea, nervousness, anxiety, indigestion, and insomnia (36). In addition, some studies suggest that modafinil may help maintain wakefulness and enhance alertness by acting on the hypothalamus and regulating the orexin system (37–39). For patients with PCOS, research shows that the hypothalamus may play a significant role in the onset and progression of the condition, with abnormal hormone regulation, such as elevated androgen levels, being closely linked to hypothalamic dysfunction (40). Modafinil’s effects might influence this regulatory process, potentially affecting hormone levels in PCOS patients. However, no studies have yet clearly demonstrated the relationship between modafinil and PCOS, and further research is needed to explore this potential link.
We also observed that bronchodilators and respiratory medications such as Salbutamol (ROR = 3.18), Montelukast (ROR = 10.29), Budesonide; formoterol (ROR = 4.26), Budesonide (ROR = 4.68), Ciclesonide (ROR = 62.1), and Aclidinium (ROR = 25.76) were associated with PCOS. Budesonide and Ciclesonide are steroid medications (41)that may interfere with hormonal balance in the body. Long-term use may lead to weight gain, insulin resistance, and other issues that are associated with the onset and exacerbation of PCOS (42, 43). Salbutamol, a β2-adrenergic receptor agonist, is primarily used to relieve bronchoconstriction (44). Previous studies have shown that, during hypoglycemic reactions, salbutamol may affect hormone secretion and the counterregulatory response by acting on the hypothalamus, specifically by regulating lactate levels and the hormonal response to hypoglycemia (45). Since the occurrence of PCOS is related to hypothalamic regulation of hormones, this suggests a possible link between salbutamol and PCOS. However, no studies have yet confirmed this hypothesis. Montelukast is a leukotriene receptor antagonist widely used in the treatment of allergic diseases and asthma. Leukotrienes play a crucial role in immune responses, particularly in allergic inflammation (46). Studies suggest that abnormal activation of leukotrienes may contribute to the chronic low-grade inflammation observed in PCOS patients (47). This mechanism may indirectly relate to the development of PCOS, but further research is needed to confirm this. Aclidinium is an anticholinergic drug that works by inhibiting the parasympathetic nervous system, reducing airway constriction and secretion production. It is commonly used in the treatment of chronic obstructive pulmonary disease (COPD) (48). Additionally, Aclidinium has been shown to possess anti-inflammatory properties (49). PCOS is closely associated with chronic low-grade inflammation; however, the direct impact of Aclidinium on PCOS still requires further research for validation. Currently, there is no research directly linking the use of salbutamol, montelukast, and aclidinium with the occurrence of PCOS. Therefore, future clinical trials will help further explore the relationship between them.
We also observed a potential association between bronchodilators and respiratory system medications, such as salbutamol (ROR = 3.18), montelukast (ROR = 10.29), budesonide (ROR = 4.68), formoterol (ROR = 4.26), ciclesonide (ROR = 62.1), and aclidinium (ROR = 25.76), and PCOS (polycystic ovary syndrome). Budesonide and ciclesonide are corticosteroids (40) that may interfere with the body’s hormonal balance. Long-term use may lead to weight gain, insulin resistance, and other issues related to the onset and exacerbation of PCOS (41, 42) (43). Previous studies suggest that salbutamol may influence hormone secretion and counter-regulatory responses during hypoglycemic reactions by acting on the hypothalamus, particularly by regulating lactate levels and hormone reactions (44). Montelukast, a leukotriene receptor antagonist, is widely used to treat allergic diseases and asthma. Leukotrienes play an important role in immune responses, particularly in allergic inflammation (45). Studies indicate that abnormal activation of leukotrienes may contribute to the chronic low-grade inflammation seen in PCOS patients (46). This mechanism may indirectly relate to the development of PCOS, but further research is needed to confirm this. Aclidinium, an anticholinergic drug, is commonly used to treat chronic obstructive pulmonary disease (COPD) (47) by inhibiting the parasympathetic nervous system to reduce airway constriction and secretion. Additionally, aclidinium has anti-inflammatory properties (48). Given that PCOS is closely related to chronic low-grade inflammation, further studies are needed to determine whether aclidinium has a direct impact on PCOS. Currently, no studies have directly linked the use of salbutamol, montelukast, and aclidinium to the occurrence of PCOS. Therefore, future clinical trials will help further explore the relationship between these medications and PCOS.
Aciclovir is an antiviral drug commonly used to treat infections caused by the herpes simplex virus, such as shingles and mouth ulcers (50). Aciclovir works by converting into its active form, aciclovir triphosphate, which competitively inhibits viral DNA polymerase, preventing viral replication, and has minimal impact on human cells with low toxicity (51). While aciclovir is highly effective against viruses, its effects on the immune system and potential relationship with endocrine disorders have not been thoroughly studied. Furthermore, there is no clear research establishing a direct relationship between aciclovir and PCOS. Ofatumumab is a monoclonal antibody used to treat certain types of blood cancers, such as chronic lymphocytic leukemia, by targeting B cells, inducing cell death, and modulating immune responses (52). Ofatumumab is also used to treat certain autoimmune diseases (53). There is currently no research confirming a direct link between Ofatumumab and PCOS. However, since PCOS has been associated with chronic low-grade ovarian inflammation (54), this mechanism may relate to the action of Ofatumumab, although further experimental studies are required to validate this hypothesis. Mecasermin is a recombinant human insulin-like growth factor 1 (IGF-1) that promotes skeletal and soft tissue growth and enhances cell proliferation, metabolism, and development by stimulating growth factor receptors (55). Previous studies have shown that elevated IGF-1 activity may lead to chronic anovulation, insulin resistance, and increased adrenal androgen secretion, causing PCOS-like symptoms (56). Another study indicated that chronic inflammation of tissue cells caused by IGF-1 could be one of the mechanisms of chronic inflammation in PCOS patients (57). Furthermore, another study found that miR-323-3p targeting IGF-1 regulates steroidogenesis and CCs activity, potentially improving CCs dysfunction in PCOS (58). Therefore, Mecasermin, as an IGF-1 class drug, may be associated with the onset of PCOS, and further studies could explore its specific mechanisms of action.
Although this study provides us with an analysis of the correlation between drug use and the occurrence of PCOS, it also faces potential influences from indication bias and confounding factors caused by comorbidities or concomitant medication use. Firstly, we must point out that there may be indication bias, which could influence our results. For example, valproate sodium, a commonly used medication for treating bipolar disorder, is often prescribed to patient populations with mental illnesses in clinical practice. However, studies have shown that bipolar disorder itself may be associated with endocrine disorders, which are considered major risk factors for PCOS (59, 60). Therefore, the use of valproate sodium is not just a result of treating bipolar disorder itself, but more a reflection of the endocrine disease state in these patients, which may directly or indirectly increase the risk of PCOS. Additionally, another potential source of bias could arise from confounding factors associated with comorbidities or the use of multiple medications. For instance, many patients with depression are on long-term antipsychotic medications, and a significant proportion of them also suffer from obesity, which is a major risk factor for PCOS. At the same time, some patients may be receiving treatments with antidiabetic drugs, antihypertensive medications, or hormonal therapies. These medications may interact with each other, influencing their pharmacological effects and potentially altering the incidence. Moreover, the FAERS database relies on voluntary reports, which may lead to underreporting, especially of events deemed insignificant or mild by healthcare professionals or patients. As a result, FAERS cannot provide precise incidence rates nor establish causal relationships between drugs and diseases. Furthermore, while proportional imbalance analysis reveals statistical associations, it does not account for confounding factors or the temporal sequence of events. The lack of detailed patient-level information, such as comorbidities, concomitant treatments, or pre-existing metabolic conditions, further complicates causal inferences. Based on the above analysis, future research should focus on overcoming the challenges posed by reporting bias and confounding factors, particularly by integrating prospective studies, clinical data, and data from a broader patient population to further validate the relationship between drugs and PCOS. By combining multiple data sources, researchers will be better equipped to clarify the causal relationship between drug treatments and the occurrence of PCOS, providing more comprehensive and precise guidance for clinical practice.
5 Conclusion
With its vast data volume, wide coverage, and open access, the FAERS database has become an important resource for studying adverse drug reactions. Through the FAERS database, we identified 18 drugs that may contribute to the occurrence of PCOS. These drugs are primarily related to the nervous system, followed by respiratory system medications and other types of drugs. This study provides a practical perspective for developing drug safety strategies and addressing drug-related harm. Future research should combine real-world data with experimental validation to develop a comprehensive safety profile for potential drugs related to PCOS, thereby supporting safer treatment options.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The data used in this study are from publicly available public databases, thus not requiring informed consent and ethical statements.
Author contributions
HZ: Validation, Data curation, Visualization, Supervision, Methodology, Project administration, Conceptualization, Investigation, Software, Resources, Formal Analysis, Writing – original draft, Writing – review & editing. MD: Resources, Formal Analysis, Validation, Data curation, Visualization, Writing – review & editing, Supervision, Conceptualization, Software. YW: Visualization, Validation, Resources, Project administration, Supervision, Software, Methodology, Investigation, Writing – original draft. YM: Data curation, Software, Methodology, Writing – original draft, Investigation, Conceptualization, Project administration, Resources, Formal Analysis. YG: Resources, Data curation, Validation, Visualization, Conceptualization, Supervision, Formal Analysis, Software, Writing – original draft. ZZ: Writing – review & editing, Validation, Formal Analysis, Project administration, Resources, Supervision, Software, Data curation, Methodology, Conceptualization, Writing – original draft, Investigation, Visualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Wang J Wang B Li C Meng T Liu C Chen J et al . Evolving global trends in PCOS burden: a three-decade analysis (1990-2021) with projections to 2036 among adolescents and young adults. Front Endocrinol (Lausanne). (2025) 16:1569694. doi: 10.3389/fendo.2025.1569694
2
Waldrop SW Buenaventura M Campoverde Reyes KJ Stanford FC . Disparities in the diagnosis and management of polycystic ovarian syndrome in adolescents. Endocrinol Metab Clin North Am. (2025) 54:233–50. doi: 10.1016/j.ecl.2025.02.016
3
Dokras A Luque-Ramírez M Escobar-Morreale HF . POLYCYSTIC OVARY SYNDROME: ORIGINS AND IMPLICATIONS: Long-term health outcomes in polycystic ovary syndrome. Reproduction. (2025) 170:e250118. doi: 10.1530/rep-25-0118
4
Yanachkova V Stankova T . Abnormally increased prolactin levels in women with polycystic ovarian syndrome are associated with risk of obesity, insulin resistance and prediabetes. Int J Mol Sci. (2025) 26:4239. doi: 10.3390/ijms26094239
5
Wang A Corley J Jaswa EG Lin J Smith DL McCulloch CE et al . Association of polycystic ovary syndrome with endothelial health, cardiovascular risk, and cellular aging. Fertil Steril. (2025) 123:1123–32. doi: 10.1016/j.fertnstert.2025.01.006
6
Guan C Zahid S Minhas AS Ouyang P Vaught A Baker VL et al . Polycystic ovary syndrome: a “risk-enhancing” factor for cardiovascular disease. Fertil Steril. (2022) 117:924–35. doi: 10.1016/j.fertnstert.2022.03.009
7
Sadeghi HM Adeli I Calina D Docea AO Mousavi T Daniali M et al . Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci. (2022) 23:583. doi: 10.3390/ijms23020583
8
Sipkoff M . Drug-induced diseases focus of pharmacy manual. Manag Care. (2010) 19:11–2.
9
Li Y Chen C Ma Y Xiao J Luo G Li Y et al . Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. (2019) 228:167–75. doi: 10.1016/j.lfs.2019.04.046
10
Ortiz Salas PA Rodríguez JH Florez SJB Suarez FE . Polycystic ovary syndrome and the new antiepileptic drugs: A systematic review. Epilep Res. (2022) 185:106968. doi: 10.1016/j.eplepsyres.2022.106968
11
Morrell MJ Hayes FJ Sluss PM Adams JM Bhatt M Ozkara C et al . Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. (2008) 64:200–11. doi: 10.1002/ana.21411
12
Kao D Bucher Bartelson B Khatri V Dart R Mehler PS Katz D et al . Trends in reporting methadone-associated cardiac arrhythmia, 1997-2011: an analysis of registry data. Ann Intern Med. (2013) 158:735–40. doi: 10.7326/0003-4819-158-10-201305210-00008
13
Khaleel MA Khan AH Ghadzi SMS Adnan AS Abdallah QM . A standardized dataset of a spontaneous adverse event reporting system. Healthcare (Basel). (2022) 10:420. doi: 10.3390/healthcare10030420
14
Zhang H Zhou Z Wang J Wang S Ren J Zhang M et al . Adverse drug reaction assessment of pembrolizumab in cervical cancer treatment: a real-world pharmacovigilance study using the FAERS database. Front Immunol. (2025) 16:1582050. doi: 10.3389/fimmu.2025.1582050
15
Li X Sun YQ Huang QL Zhang ZJ Shi LQ Tang JF et al . Drug-related macular edema: a real-world FDA Adverse Event Reporting System database study. BMC Pharmacol Toxicol. (2025) 26:23. doi: 10.1186/s40360-025-00856-9
16
Bate A . Bayesian confidence propagation neural network. Drug Saf. (2007) 30:623–5. doi: 10.2165/00002018-200730070-00011
17
Gao W Yu J Sun Y Song Z Liu X Han X et al . Adverse events in the nervous system associated with blinatumomab: a real-world study. BMC Med. (2025) 23:72. doi: 10.1186/s12916-025-03913-6
18
Helvaci N Yildiz BO . Polycystic ovary syndrome as a metabolic disease. Nat Rev Endocrinol. (2025) 21:230–44. doi: 10.1038/s41574-024-01057-w
19
Su P Chen C Sun Y . Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J Ovarian Res. (2025) 18:34. doi: 10.1186/s13048-025-01621-6
20
Ernst CL Goldberg JF . The reproductive safety profile of mood stabilizers, atypical antipsychotics, and broad-spectrum psychotropics. J Clin Psychiatry. (2002) 63 Suppl 4:42–55.
21
Sidhu HS Srinivasa R Sadhotra A . Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either Valproate or Lamotrigine monotherapy: A prospective study. Epilep Res. (2018) 139:20–7. doi: 10.1016/j.eplepsyres.2017.10.016
22
Luef G Abraham I Haslinger M Trinka E Seppi K Unterberger I et al . Polycystic ovaries, obesity and insulin resistance in women with epilepsy. A comparative study of carbamazepine and valproic acid in 105 women. J Neurol. (2002) 249:835–41. doi: 10.1007/s00415-002-0731-3
23
Zhou JQ Zhou LM Chen LJ Han JD Wang Q Fang ZY et al . Polycystic ovary syndrome in patients with epilepsy: a study in 102 Chinese women. Seizure. (2012) 21:729–33. doi: 10.1016/j.seizure.2012.08.001
24
Joffe H Cohen LS Suppes T McLaughlin WL Lavori P Adams JM et al . Valproate is associated with new-onset oligoamenorrhea with hyperandrogenism in women with bipolar disorder. Biol Psychiatry. (2006) 59:1078–86. doi: 10.1016/j.biopsych.2005.10.017
25
Luef G Abraham I Trinka E Alge A Windisch J Daxenbichler G et al . Hyperandrogenism, postprandial hyperinsulinism and the risk of PCOS in a cross sectional study of women with epilepsy treated with valproate. Epilep Res. (2002) 48:91–102. doi: 10.1016/s0920-1211(01)00317-5
26
Li S Zhang L Wei N Tai Z Yu C Xu Z . Research progress on the effect of epilepsy and antiseizure medications on PCOS through HPO axis. Front Endocrinol (Lausanne). (2021) 12:787854. doi: 10.3389/fendo.2021.787854
27
Popovic V Spremovic S . The effect of sodium valproate on luteinizing hormone secretion in women with polycystic ovary disease. J Endocrinol Invest. (1995) 18:104–8. doi: 10.1007/bf03349709
28
Newcomer JW . Metabolic risk during antipsychotic treatment. Clin Ther. (2004) 26:1936–46. doi: 10.1016/j.clinthera.2004.12.003
29
Choi S DiSilvio B Unangst J Fernstrom JD . Effect of chronic infusion of olanzapine and clozapine on food intake and body weight gain in male and female rats. Life Sci. (2007) 81:1024–30. doi: 10.1016/j.lfs.2007.08.009
30
Houseknecht KL Robertson AS Zavadoski W Gibbs EM Johnson DE Rollema H . Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. (2007) 32:289–97. doi: 10.1038/sj.npp.1301209
31
Razavi BM Lookian F Hosseinzadeh H . Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. BioMed Pharmacother. (2017) 92:726–31. doi: 10.1016/j.biopha.2017.05.113
32
Mohyeldin RH Abdelzaher WY Sharata EE Mohamed HMA Ahmed MYM Attia JZ et al . Aprepitant boasted a protective effect against olanzapine-induced metabolic syndrome and its subsequent hepatic, renal, and ovarian dysfunction; Role of IGF(1)/p-AKT/FOXO(1) and NFκB/IL-1β/TNF-α signaling pathways in female Wistar albino rats. Biochem Pharmacol. (2024) 221:116020. doi: 10.1016/j.bcp.2024.116020
33
Fernández-Minotre L Montero-Aguilar M Vázquez-Vázquez FC Serrano-Bello J Vega-Baudrit J Pereira-Reyes R et al . Characterization of polylactic acid membranes for local release of tramadol. Int J Mol Sci. (2025) 26:6018. doi: 10.3390/ijms26136018
34
Sigfredsson AKV Palmqvist DF Dalhoff K . Poisonings with opioids and benzodiazepines in Denmark: A retrospective study from the danish poison information centre. Basic Clin Pharmacol Toxicol. (2025) 136:e70044. doi: 10.1111/bcpt.70044
35
Cao Y Li Q Liu L Wu H Huang F Wang C et al . Modafinil protects hippocampal neurons by suppressing excessive autophagy and apoptosis in mice with sleep deprivation. Br J Pharmacol. (2019) 176:1282–97. doi: 10.1111/bph.14626
36
Morais GCF Dantas da Silva Junior E Bruno Silva de Oliveira C Rodrigues-Neto JF Laino Fulco U Ivan Nobre Oliveira J . Modafinil: A closer look at its theoretical toxicological potential. J Psychopharmacol. (2023) 37:945–7. doi: 10.1177/02698811231187127
37
Patel V Sarkar P Siegel DM Teegala SB Hirschberg PR Wajid H et al . The antinarcolepsy drug modafinil reverses hypoglycemia unawareness and normalizes glucose sensing of orexin neurons in male mice. Diabetes. (2023) 72:1144–53. doi: 10.2337/db22-0639
38
Cohen S Ifergane G Vainer E Matar MA Kaplan Z Zohar J et al . The wake-promoting drug modafinil stimulates specific hypothalamic circuits to promote adaptive stress responses in an animal model of PTSD. Transl Psychiatry. (2016) 6:e917. doi: 10.1038/tp.2016.172
39
Ishizuka T Murotani T Yamatodani A . Action of modafinil through histaminergic and orexinergic neurons. Vitam Horm. (2012) 89:259–78. doi: 10.1016/b978-0-12-394623-2.00014-7
40
Vieyra E Silva CC Linares R Rosas G Espinoza JA Chaparro A et al . The hypothalamic nuclei implicated in the regulation of polycystic ovary syndrome: A review of its clinical, metabolic, and endocrine aspects. Molecules. (2025) 30:3407. doi: 10.3390/molecules30163407
41
Kimura H Kurusu H Sada M Kurai D Murakami K Kamitani W et al . Molecular pharmacology of ciclesonide against SARS-CoV-2. J Allergy Clin Immunol. (2020) 146:330–1. doi: 10.1016/j.jaci.2020.05.029
42
Aigner E Bachofner N Klein K De Geyter C Hohla F Patsch W et al . Retinol-binding protein 4 in polycystic ovary syndrome–association with steroid hormones and response to pioglitazone treatment. J Clin Endocrinol Metab. (2009) 94:1229–35. doi: 10.1210/jc.2008-2156
43
Ottesen TG Rovsing AH Ulrik CS . Local and systemic adverse effects of inhaled corticosteroids - Does ciclesonide differ from other inhaled corticosteroids? Respir Med. (2025) 238:107962. doi: 10.1016/j.rmed.2025.107962
44
Ojanperä L Lehtimäki L Kelemen B Csonka P . Inhaled salbutamol delivery in small children with disposable and reusable spacers: an. Vitro. (2024) 20:328830. doi: 10.1136/archdischild-2025-328830
45
Sejling AS Wang P Zhu W Farhat R Knight N Appadurai D et al . Repeated activation of noradrenergic receptors in the ventromedial hypothalamus suppresses the response to hypoglycemia. Endocrinology. (2021) 162:bqaa241. doi: 10.1210/endocr/bqaa241
46
Biedritzky A Zhang Y Fuhr A Kleinmaier C Gamper-Tsigaras J Heck-Swain KL et al . Modulation of neutrophil recruitment and inflammatory signaling in acute respiratory distress syndrome by leukotriene inhibitors montelukast and zileuton. FASEB J. (2025) 39:e70934. doi: 10.1096/fj.202501684R
47
Xu M Liu D Wang L . Role of oxylipins in ovarian function and disease: A comprehensive review. BioMed Pharmacother. (2024) 178:117242. doi: 10.1016/j.biopha.2024.117242
48
Xiong Y Hu JQ Tang HL Zhao ZX Liu LH . Network meta-analysis of the efficacy and safety of monoclonal antibodies and traditional conventional dichotomous agents for chronic obstructive pulmonary disease. Front Med (Lausanne). (2024) 11:1334442. doi: 10.3389/fmed.2024.1334442
49
Li Y Ding S Wang Y . Targeting the cholinergic anti-inflammatory pathway: an innovative strategy for treating diseases. Mol Biol Rep. (2025) 52:199. doi: 10.1007/s11033-025-10288-7
50
Serris A Pouvaret A Loiseau C Abid H Burrel S Fourgeaud J et al . Pritelivir for recurrent aciclovir-resistant herpes simplex virus 2 infections in immunocompromised patients. J Antimicrob Chemother. (2022) 77:2303–5. doi: 10.1093/jac/dkac165
51
Kably B Briard M Francoz C Roux O Houhou N Mackiewicz V et al . Population pharmacokinetics of aciclovir and its major metabolite 9-carboxymethoxymethylguanine and safety profile of valaciclovir in early liver transplant recipients. J Antimicrob Chemother. (2025) 80:1302–8. doi: 10.1093/jac/dkaf070
52
van Imhoff GW McMillan A Matasar MJ Radford J Ardeshna KM Kuliczkowski K et al . Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. (2017) 35:544–51. doi: 10.1200/jco.2016.69.0198
53
Kang C Blair HA . Ofatumumab: A review in relapsing forms of multiple sclerosis. Drugs. (2022) 82:55–62. doi: 10.1007/s40265-021-01650-7
54
Rudnicka E Suchta K Grymowicz M Calik-Ksepka A Smolarczyk K Duszewska AM et al . Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. (2021) 22:3789. doi: 10.3390/ijms22073789
55
Miller BS Rogol AD Rosenfeld RG . The History of the Insulin-Like Growth Factor System. Horm Res Paediatr. (2022) 95:619–630. doi: 10.1159/000527123
56
Hatasaka HH Kazer RR Chatterton RT Unterman TG Glick RP . The response of the growth hormone and insulin-like growth factor I axis to medical castration in women with polycystic ovarian syndrome. Fertil Steril. (1994) 62:273–8. doi: 10.1016/s0015-0282(16)56878-2
57
Li X Lin S Yang X Chen C Cao S Zhang Q et al . When IGF-1 meets metabolic inflammation and polycystic ovary syndrome. Int Immunopharmacol. (2024) 138:112529. doi: 10.1016/j.intimp.2024.112529
58
Wang T Liu Y Lv M Xing Q Zhang Z He X et al . miR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene. (2019) 683:87–100. doi: 10.1016/j.gene.2018.10.006
59
Wang C Wu W Yang H Ye Z Zhao Y Liu J et al . Mendelian randomization analyses for PCOS: evidence, opportunities, and challenges. Trends Genet. (2022) 38:468–82. doi: 10.1016/j.tig.2022.01.005
60
Liu J Teng Z Xie H Yuan H Liu M Chen J et al . Prevalence and characteristics of polycystic ovarian syndrome in patients with bipolar disorder. J Affect Disord. (2023) 340:387–95. doi: 10.1016/j.jad.2023.08.007
Summary
Keywords
polycystic, ovary, syndrome, FAERS, adverse, events, disproportionality analysis, pharmacovigilance
Citation
Zhang H, Di M, Wang Y, Ma Y, Gou Y and Zhou Z (2025) Drug-induced polycystic ovary syndrome: a real-world pharmacovigilance study based on the FAERS database. Front. Endocrinol. 16:1671511. doi: 10.3389/fendo.2025.1671511
Received
23 July 2025
Revised
08 September 2025
Accepted
11 November 2025
Published
24 November 2025
Volume
16 - 2025
Edited by
Christos Kontogiorgis, Democritus University of Thrace, Greece
Reviewed by
Liu Chengzhi, Capital Medical University, China
Hicran Mutlu, University of Istanbul Medeniyet, Türkiye
Updates
Copyright
© 2025 Zhang, Di, Wang, Ma, Gou and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuo Zhou, 550677140@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.