- 1Division of Endocrinology, Diabetes, and Bone & Mineral Disorders, Detroit, MI, United States
- 2Bone and Mineral Research Laboratory, Henry Ford Health, Detroit, MI, United States
- 3Department Pathology, Henry Ford Health, Detroit, MI, United States
- 4Michigan State University College of Human Medicine, Lansing, MI, United States
Background: Primary hyperparathyroidism (PHPT) is associated with an increased risk of kidney stones. However, the clinical utility of 24-hour urinary calcium (24h-UCa) as a predictor of nephrolithiasis, and its role in surgical decision-making, remains uncertain due to inconsistent findings.
Objective: To evaluate whether 24h-UCa is a reliable disease-specific predictor of kidney stone risk in patients with PHPT and to identify demographic and biochemical determinants of urinary calcium excretion.
Methods: This retrospective study included 306 PHPT patients who underwent curative parathyroidectomy for confirmed adenoma. Demographics, kidney stone history, and biochemical data were collected. T-tests, chi-square tests, multivariate logistic regression, and multiple regression were used to analyze associations of 24h-UCa with kidney stones, demographic, and biochemical indices.
Results: Kidney stones were present in 22% of patients. No significant difference in 24h-UCa was observed between stone-formers and non-stone-formers, even after adjustment for age, gender, and race. In contrast, 24h-UCa was significantly higher in men and white patients, with hypercalciuria more prevalent among white individuals. Serum calcium and eGFR were also significantly positively associated with 24h-UCa.
Conclusion: Our results suggest that 24h-UCa is not an independent predictor of kidney stone risk in PHPT and is largely influenced by demographic and biochemical factors. Accordingly, routine 24h-UCa measurement for evaluating patients with sporadic PHPT or for guiding parathyroidectomy decisions is not recommended. This study is limited by its retrospective design, reliance on a single urine collection, and lack of detailed dietary or genetic data, which may have introduced variability and reduced power to detect weak associations.
1 Introduction
Hypercalcemic primary hyperparathyroidism (PHPT) is the third most common endocrine disorder and is characterized by elevated serum calcium levels with high or inappropriately normal serum parathyroid hormone (PTH) levels (1–3). Hypercalcemia, regardless of its cause, necessarily increases the glomerular filtered load of calcium and consequent rise in urine calcium. Elevated PTH, as occurs in PHPT, increases renal tubular reabsorption of calcium, partially negating the rise in urine calcium due to higher filtered load. When the filtered load exceeds the tubular maximum for calcium reabsorption, urine calcium will rise, usually when serum calcium is >12 mg/dl. In addition, the amount of calcium excreted in the urine varies widely depending on calcium and sodium intakes, serum calcium level, gender and ethnicity. Thus, the 24-hour urinary calcium (24h-UCa) is not a reliable indicator of PHPT severity as is commonly assumed.
One of the known complications of PHPT is kidney stone formation (nephrolithiasis), and to a lesser extent nephrocalcinosis, which can significantly impact patient health and quality of life (4–6). Clinical guidelines—including those from the 5th International Workshop on PHPT, the Endocrine Society, and the American Association of Endocrine Surgeons—recommend thorough biochemical evaluations to assess the risk of kidney stones in PHPT (7, 8). Given the higher prevalence of nephrolithiasis in patients with PHPT, it is crucial to identify reliable diagnostic measures in evaluating and mitigating this risk. The most commonly used diagnostic tool is the measurement of 24h-UCa excretion to evaluate calcium metabolism (9, 10).
Nevertheless, the relationship between 24h-UCa levels and kidney stone risk in PHPT still remains unclear. Some studies have shown a correlation between elevated 24h-UCa levels and an increased risk of nephrolithiasis (11, 12), while others have not consistently found this association (13, 14). Additionally, the variability in methods used to measure 24 h-UCa and define hypercalciuria complicated the interpretation of these findings. Given the inconsistent evidence regarding the predictive capacity of 24-hour-UCa in PHPT (14–16), we attempted to determine whether 24-hour-UCa serves as an independent predictor of kidney stone formation in patients with PHPT undergoing parathyroidectomy. We hypothesized that 24h-UCa would not exhibit a significant association with kidney stone status after controlling for demographic and biochemical variables.
2 Materials and methods
2.1 Patient characteristics
PHPT in this study was defined by unambiguous hypercalcemia with either elevated or non-suppressed serum PTH levels. We included only those patients with surgically confirmed PHPT due to parathyroid adenoma(s) with no evidence of recurrence or persistence of PHPT after at least one year of follow-up evaluation. Three hundred and six adult patients with PHPT, as defined, underwent parathyroidectomy at Henry Ford Health between January 1, 2005, and December 31, 2015. We excluded patients 1) with normocalcemic hyperparathyroidism (15), 2) taking medications that are known to affect calcium homeostasis, 3) with kidney stones without PHPT; and 4) other than black and white people, due an insufficient number of other ethnic groups, 5) with familial PHPT syndromes, 6) with secondary or tertiary hyperparathyroidism (HPT), and 7) with chronic kidney disease, as assessed by serum creatinine level >1.5 mg/dl). Demographic (age, race, and gender), surgical (parathyroid adenoma), medical history (presence or absence of kidney stone history), and relevant biochemical data were extracted from the participant’s electronic health records.
2.2 Biochemical measurements and kidney stone assessment
Serum calcium (Ca), creatinine (Cr), and 24 h-UCa, Cr, and Sodium (Na) were measured in the Hospital laboratory by standard methods. The estimated glomerular filtration rate (eGFR) was calculated using the MDRD formula. Serum 25-hydroxyvitamin D (25(OH)D) was measured by competitive immunoassay using sheep monoclonal anti-25(OH)D antibody on Beckman Coulter UniCel Dxl platform. Serum PTH was measured by chemiluminescent assay on Roche-Cobas e801 platform. The coefficient variation ranged from 1.9-3.4% for 25(OH)D and 3.0-3.4% for PTH depending upon the concentrations of the respective hormone levels. The reference range for 24h-UCa is 50–300 mg/day for men and 50–250 mg/day for women. All the measurements closest to the parathyroidectomy (PTX) date were selected for each patient.
The kidney stone information was collected from the patient’s history during the period from recruitment into the study through the end of surgery. All data were gathered within 2 years following the detection of PHPT. Stones identified before the detection of PHPT were not included in this study. Kidney stones were diagnosed using ultrasound or CT scan.
2.3 Statistical analysis
Numerical data are expressed as mean and standard deviation (SD) and compared across kidney stone presence, gender, and race using Student’s t-tests, with the Mann–Whitney test applied for non-normally distributed variables. Categorical variables were analyzed using chi-square tests. Multivariate logistic regression analysis was performed to assess the relationship between 24h-UCa levels and kidney stone presence, adjusting for gender, race, and age. Multiple regression analysis was utilized to predict the demographic and serum biochemical factors that influence 24 h-UCa levels. We applied the Benjamini–Hochberg procedure to control the false discovery rate at 5% (16, 17).
3 Results
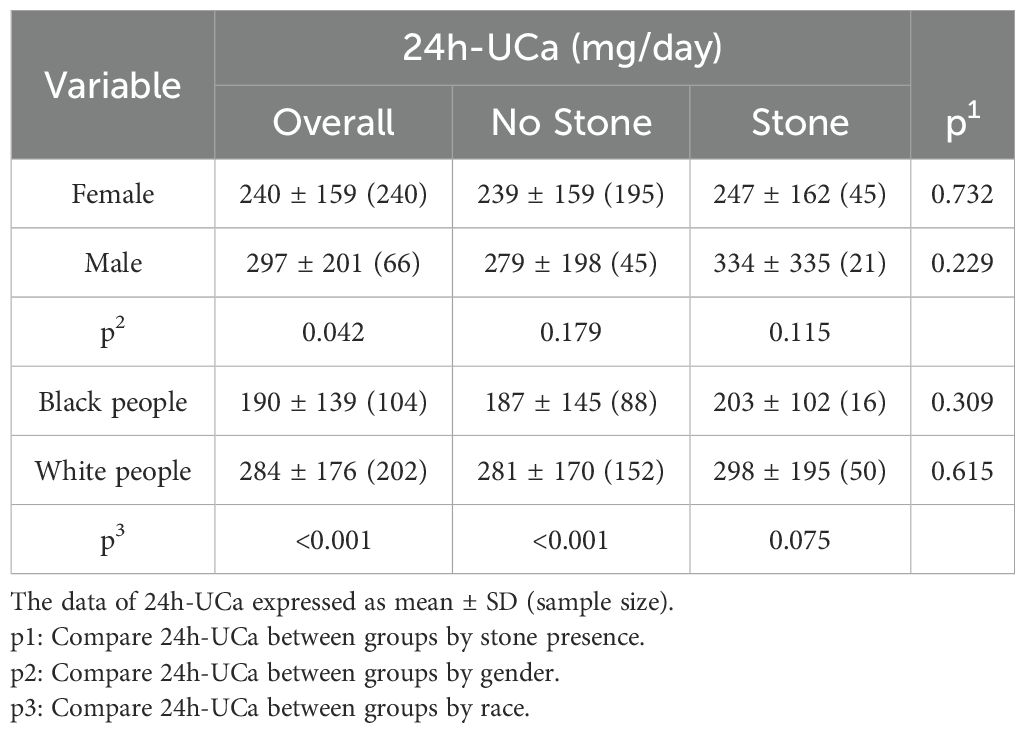
The cohort of 306 PHPT patients exclusively included black and white individuals with benign parathyroid adenoma. Among these, 66 (22%) were women, and 240 (78%) were white people. In addition, 66 (22%) of PHPT patients presented with or had a history of kidney stones. Table 1 shows that patients with kidney stones were significantly older (67.3 years) than those without stones (64.0 years). The proportion of men in the group with kidney stones (32%) was significantly higher than that in the group without kidney stones (19%)(p < 0.05), suggesting that male gender might be a risk factor for kidney stone formation. Although the proportion of white people in the group with kidney stones (76%) was higher than that in the group without kidney stones (63%), the difference did not quite reach significance (p = 0.06). None of the serum biochemical variables, including Calcium, Creatinine, eGFR, PTH, and 25(OH)D, showed statistically significant differences between stone and no-stone groups (p > 0.05)(Table 1).
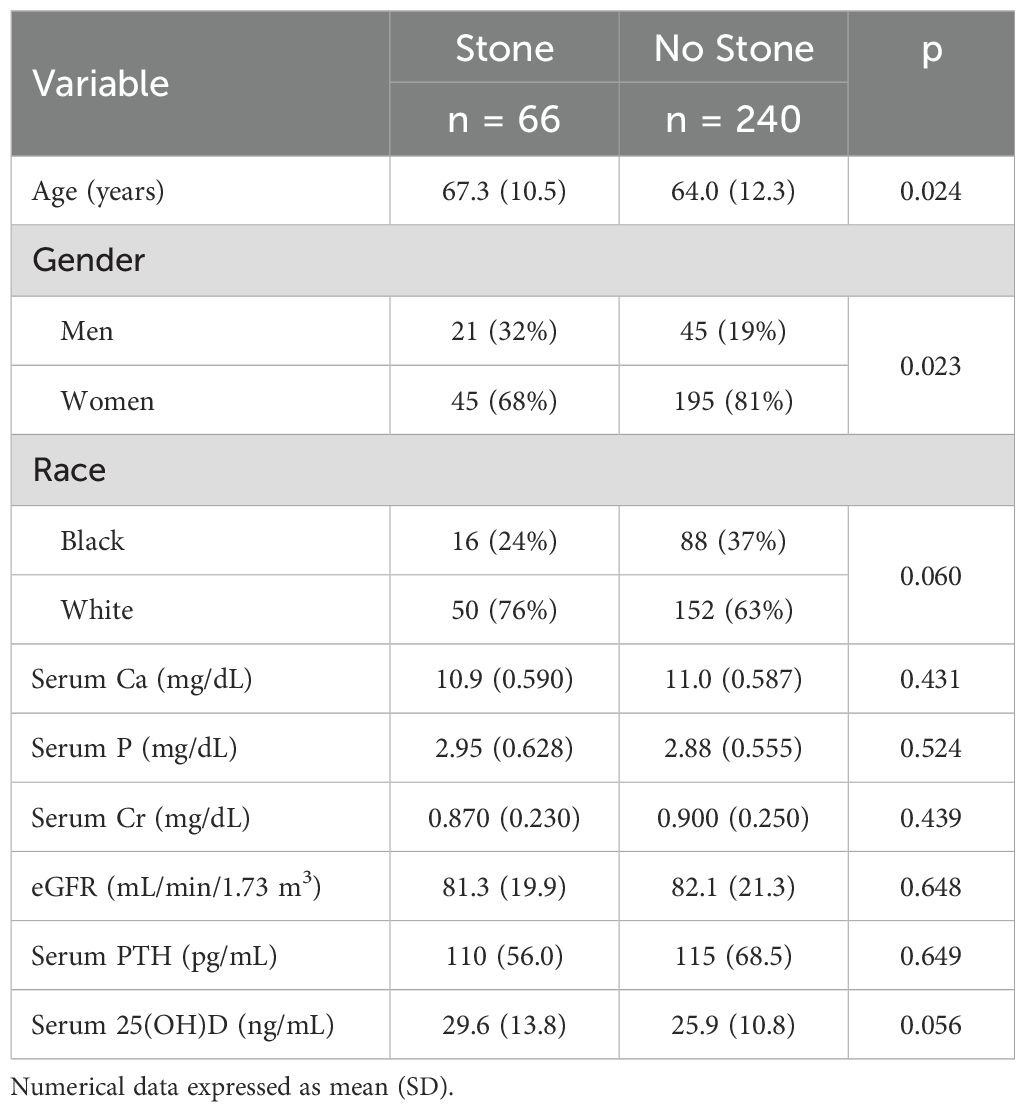
Table 1. Differences in demographic and serum biochemical variables between the PHPT patients with and without kidney stones.
Contrary to the pervasive general assumption and expectation, there was no significant difference in 24h-UCa levels between PHPT patients with and without kidney stones across all subgroups (Table 2). However, a significant difference in 24h-UCa levels was observed between genders, with men having higher 24 h-UCa levels than women (p <0.05), but the gender difference became insignificant when considering the presence of kidney stones. Additionally, 24h-UCa levels were significantly higher in white people compared to black people (Table 2), although this racial difference was only marginally significant in patients with kidney stones (p = 0.075).
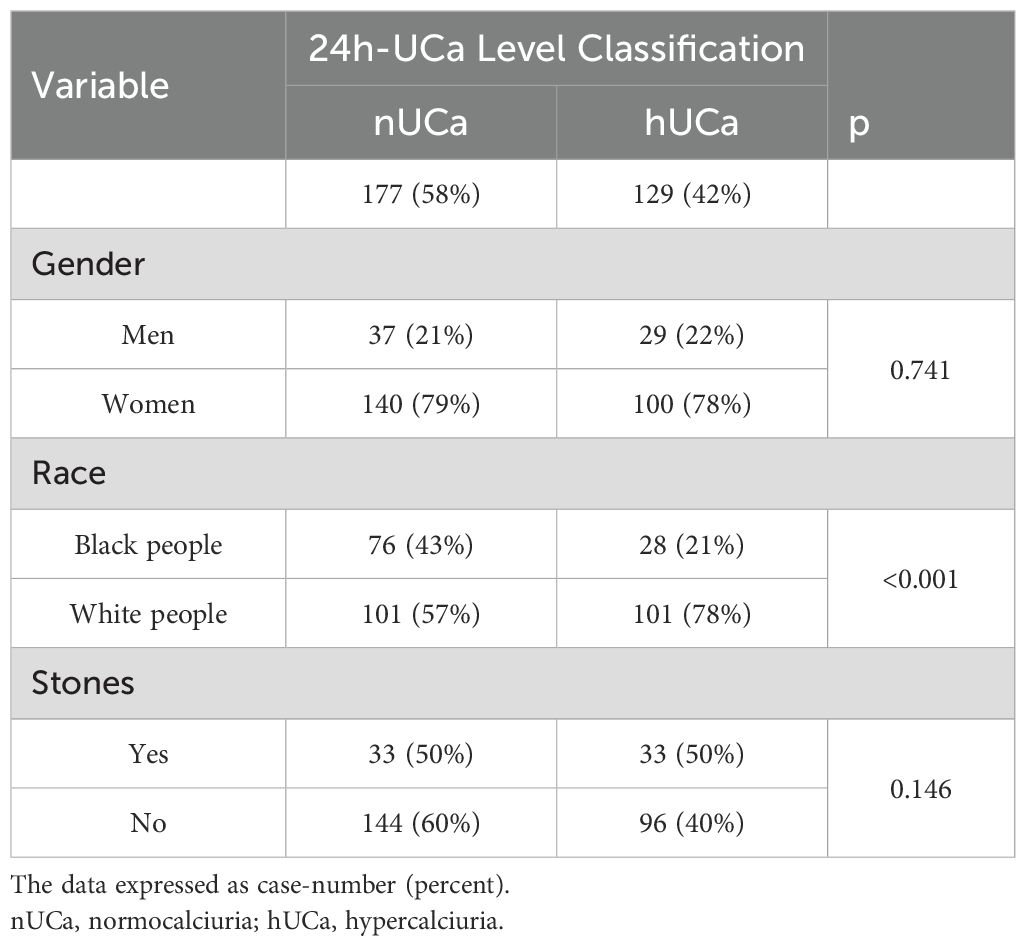
Table 3 shows that 42% of the overall PHPT patients had hypercalciuria. The level classification of 24 h-UCa for all patients presents the prevalence of hypercalciuria (hUCa) across genders, races, and kidney stones. There was no significant difference in hypercalciuria prevalence between men and women, or among individuals with and without kidney stones. However, a significant racial difference was found (p < 0.001) with hypercalciuria being more prevalent in white people compared to black people (Table 3).
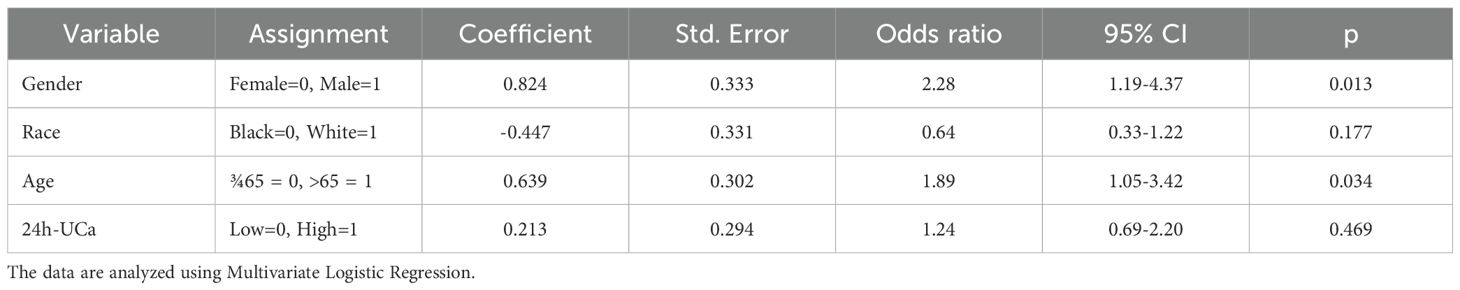
Table 4 presents the results of multivariate logistic regression analysis. This model examined whether gender, race, age, and 24h-UCa level independently predicted kidney stone formation in PHPT patients, adjusting for all variables simultaneously. The results demonstrated that male gender (OR = 2.28, 95% CI 1.19–4.37, p = 0.013) and age > 65 years (OR = 1.89, 95% CI 1.05–3.42, p = 0.034) were identified as independent predictors of kidney stone formation in PHPT patients. Race and 24h-UCa level were not significantly associated with stone risk after adjustment (p > 0.05).
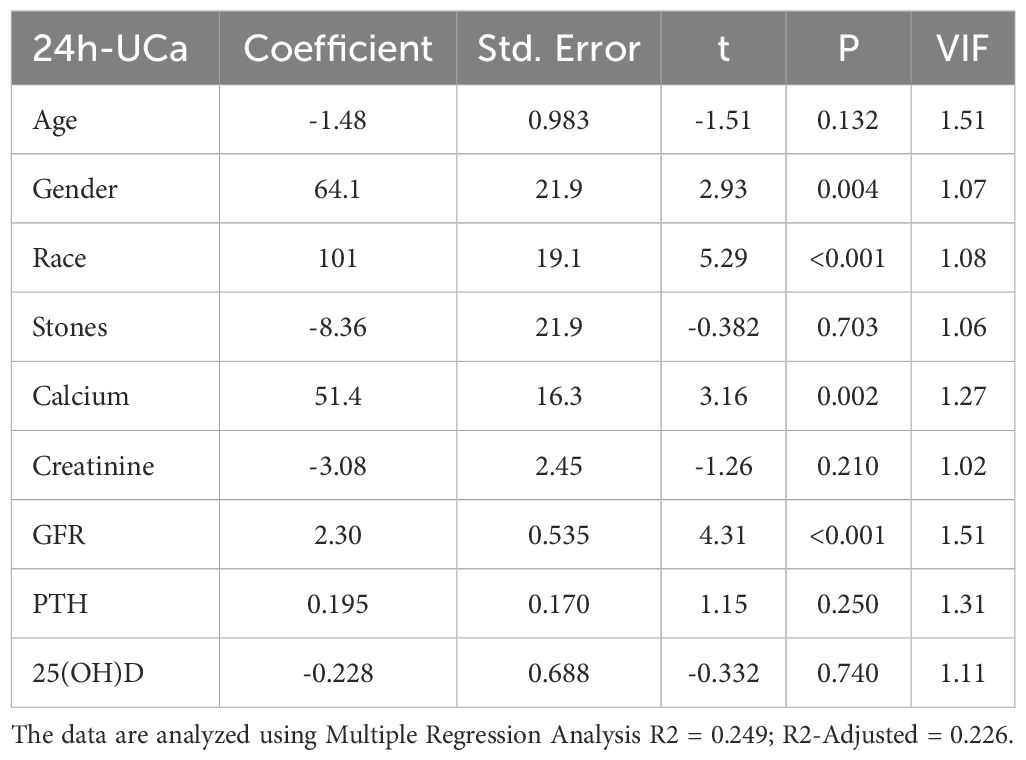
Table 5 presents the results of a multiple regression analysis examining the relationship between various demographic and serum biochemical factors and 24-hour urinary calcium (24h-UCa) levels. The analysis identified several significant predictors of high 24h-UCa, including race (p < 0.001), gender (p = 0.004), eGFR (p < 0.001), and serum calcium levels (p = 0.002). Age, serum creatinine, PTH, 25(OH)D, and stone history were not significant after adjustment. When applying the Benjamini–Hochberg false discovery rate (q = 0.05), these four associations remained significant (their p-values were ≤ the BH critical values 0.0056, 0.011, 0.017, and 0.022, respectively); all other predictors were not significant after FDR correction. The R2 = 0.249 and R2-Adjisted = 0.226. The variance inflation factor (VIF) was all <2.5, indicating that multicollinearity was not an issue.
4 Discussion
The prevalence of kidney stones in our large, well-characterized, and surgically confirmed patients with PHPT was 22%, which was similar to or significantly lower than the rates reported by other studies (5, 6, 18, 19). However, the prevalence of kidney stones in our PHPT individuals is still notably higher than the 10% in the general American population (20), emphasizing the potential role of PHPT in predisposing individuals to kidney stone formation. Male patients with PHPT were more likely to develop kidney stones (32%) than female patients (19%), a pattern consistent with the general population but more pronounced (20, 21). Racial differences were also observed, with the expected higher prevalence among white people (37%) than black people (24%). Our study indicated that patients with PHPT who had kidney stones were significantly older than those without stones. These findings suggest that demographic factors, such as age, gender, and race, are more important for kidney stone risk in PHPT, although the underlying mechanisms remain unclear. In contrast, none of the serum biochemical variables showed significant differences between patients with and without kidney stones.
Published data on the utility of 24 h-UCa as a predictor of kidney stone formation in PHPT have been inconsistent (11–14, 22). Variations in patient inclusion criteria, methods for diagnosing kidney stones and techniques for measuring 24 h-UCa, as well as differing dietary and lifestyle factors, can all lead to conflicting results that complicate the overall understanding of this issue (5, 12, 14, 23–30). In our study, we only included patients with hypercalcemic PHPT with serum Ca levels higher than the reference range (10.2 mg/dL) (26) and patients of different genders and races. Across all subgroups, no significant difference in 24h-UCa levels was observed between the patients with and without kidney stones. This lack of association persisted even after adjusting for factors such as age, gender, and race in the multivariate logistic regression analysis, suggesting that 24h-UCa is not a reliable standalone predictor of kidney stone formation as previously reported (11, 31). However, the multivariate logistic regression model demonstrated that gender and age were independently significant predictors of changes in 24h-UCa levels.
Our study indicated that demographic factors were dominant predictors of 24h-UCa levels in PHPT patients as in the general population. Men exhibited higher urinary calcium excretion than women (10, 32), although this difference was insignificant when considering the presence or absence of kidney stones. Similarly, white individuals showed significantly higher 24h-UCa levels and a greater prevalence of hypercalciuria than black individuals (33). These findings highlight the influence of genetic, hormonal, and environmental factors on calcium metabolism and kidney stone risk (34, 35). Notably, racial differences in hypercalciuria and kidney stone prevalence (22, 36) underscore the need for demographic-specific approaches to PHPT management.
The occurrence of hypercalciuria in patients with PHPT is somewhat paradoxical, since the primary renal effect of PTH is to increase renal tubular reabsorption Ca (TRCa). Thus, for hypercalciuria to occur in PHPT, the PTH-dependent increased TRCa must be overcome by the increased filtered load, which usually does not occur until serum Ca level exceeds 12 mg/dl (12, 37, 38). Alternatively, some patients with PHPT may have unrecognized co-existent renal tubular leak hypercalciuria, which explains the variable response of nephrolithiasis and hypercalciuria to curative PTX (38, 39). In addition, there is good evidence to suggest that patients with PHPT have an increased intestinal absorption of calcium due to PTH-mediated increased production of 1,25-dihydroxyvitamin D (40, 41). However, the relative contributions of the three mechanisms (i.e., increased filtered load, renal tubular leak, and increased intestinal absorption of Ca) to hypercalciuria in patients with PHPT are not well studied and require further research (38, 39, 42–44).
This study has several limitations, most of which are related to its retrospective design. First, our cohort was limited to patients with surgically confirmed PHPT, which may limit the generalizability of the findings to patients with mild or normocalcemic disease who are not surgical candidates. Second, kidney stone status was determined through medical history, record review, and available imaging, which may have missed silent or remote stones and could not adequately distinguish between past and recent stone events. Such non-differential misclassification would likely bias results toward a null hypothesis and may explain weak associations. Third, we relied on a single 24-hour urine collection closest to surgery, an approach that is vulnerable to collection errors and day-to-day variability, introducing random noise that may reduce statistical power. Fourth, we lacked detailed dietary and urinary data, including calcium intake, and urine oxalate and citrate measurements. Although 24-hour urinary sodium was measured, extensive missing data precluded its inclusion in the models, limiting our ability to adjust for the known effect of urine sodium on calcinuria. Finally, race was abstracted from the medical record and categorized as “Black” or “White,” a social rather than biological classification that cannot separate genetic, hormonal, cultural, or socio-environmental influences.
Collectively, these limitations likely increased random variability, which would tend to diminish rather than exaggerate observed associations. Therefore, our null findings should be considered conservative. Future prospective studies with standardized dietary assessments, repeated urine collections, genetic ancestry data, and social determinant measures are needed to more precisely define predictors of urinary calcium excretion and stone risk across the full spectrum of PHPT.
5 Conclusion
Our study shows that 24h-UCa is not a reliable predictor of kidney stone formation in patients with PHPT. No significant differences in 24h-UCa levels were found between stone-formers and non-stone-formers. In contrast, demographic factors—especially gender and race—were strong indicators of urinary calcium excretion, with men and white individuals exhibiting higher levels and a greater prevalence of hypercalciuria. The positive relationships of 24h-UCa with serum calcium and eGFR highlight the role of systemic calcium metabolism but also present the limitations of using 24h-UCa alone as a diagnostic tool. Future research should investigate additional urinary risk factors that may contribute to stone formation in PHPT. Overall, the routine use of 24h-UCa to guide parathyroidectomy decisions should be reconsidered.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The Institutional Review Board of Henry Ford Hospital approved the study and deemed it exempt from written informed consent due to the de-identified data (NO. 14544-01).
Author contributions
AB: Writing – original draft, Investigation, Data curation, Supervision, Writing – review & editing, Conceptualization. RS: Writing – original draft, Methodology, Data curation, Conceptualization, Writing – review & editing. AY: Data curation, Writing – review & editing, Writing – original draft. BC: Validation, Data curation, Writing – review & editing, Writing – original draft. SQ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. SR: Formal Analysis, Conceptualization, Project administration, Supervision, Writing – original draft, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was partly supported by the National Institute of Health (NIH) grant (DK43858) to Sudhaker D, Rao, Henry Ford Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JEM, Rejnmark L, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporosis Int. (2016) 28:1–19. doi: 10.1007/s00198-016-3716-2
2. Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab. (2018) 103:3993–4004. doi: 10.1210/jc.2018-01225
3. Rao DS, Phillips ER, Divine GW, and Talpos GB. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. (2004) 89:5415–22. doi: 10.1210/jc.2004-0028
4. Verdelli C and Corbetta S. Mechanisms in endocrinology: kidney involvement in patients with primary hyperparathyroidism: an update on clinical and molecular aspects. Eur J endocrinology. (2017) 176:R39–52. doi: 10.1530/EJE-16-0430
5. Castellano E, Attanasio R, Latina A, Visconti GL, Cassibba S, and Borretta G. Nephrolithiasis in primary hyperparathyroidism: a comparison between silent and symptomatic patients. Endocrine Practice. (2017) 23:157–62. doi: 10.4158/EP161476.OR
6. Zabolotniuk T, Guo M, Kwon M, Watanabe A, Teichman JM, and Wiseman SM. Screening for asymptomatic nephrolithiasis in primary hyperparathyroidism patients is warranted. Am J Surgery. (2024) 231:91–5. doi: 10.1016/j.amjsurg.2024.03.007
7. Bilezikian JP, Khan AA, Silverberg SJ, Fuleihan GE, Marcocci C, Minisola S, et al. Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the fifth international workshop. J Bone Miner Res. (2022) 37:2293–314. doi: 10.1002/jbmr.4677
8. Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, et al. The american association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. (2016) 151:959–68. doi: 10.1001/jamasurg.2016.2310
9. Walker MD and Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinology. (2018) 14:115–25. doi: 10.1038/nrendo
10. Shen L, Zhang H, Lu Q, Li S, Mei Y, Gao C, et al. Reference intervals for 24-hour urinary calcium excretion and its association with bone metabolism: A multicenter study. J Clin Endocrinol Metab. (2025) 110:e3783–e3793. doi: 10.1210/clinem/dgae805
11. Perez AA, Schneider DF, Long KL, Pitt SC, and Sippel RS. Timely evaluation and management of primary hyperparathyroidism in patients with kidney stones. J Surg Res. (2018) 232:564–9. doi: 10.1016/j.jss.2018.07.028
12. Söreide JA, Söreide JA, Heerden J, Heerden J, Grant CS, Grant CS, et al. Characteristics of patients surgically treated for primary hyperparathyroidism with and without renal stones. Surgery. (1996) 120 6:1033–7. doi: 10.1016/s0039-6060(96)80051-1
13. Berger AD, Wu W, Eisner BH, Cooperberg MR, Duh QY, and Stoller ML. Patients with primary hyperparathyroidism–why do some form stones? J Urol. (2009) 181:2141–5. doi: 10.1016/j.juro.2009.01.028
14. Black CE, Berg RL, and Urquhart AC. 24-hour urinary calcium in primary hyperparathyroidism. Clin Med Res. (2013) 11:219–25. doi: 10.3121/cmr.2013.1164
15. Zavatta G and Clarke BL. Normocalcemic hyperparathyroidism: A heterogeneous disorder often misdiagnosed? JBMR Plus. (2020) 4:e10391. doi: 10.1002/jbm4.10391
16. Thissen D, Steinberg L, and Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. J Educ Behav statistics. (2002) 27:77–83.
17. Benjamini Y and Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. (2001) 33:1165–88. doi: 10.1214/aos/1013699998
18. Tran H, Grange JS, Adams-Huet B, Nwariaku FE, Rabaglia JL, Woodruff SL, et al. The impact of obesity on the presentation of primary hyperparathyroidism. J Clin Endocrinol Metab. (2014) 99:2359–64. doi: 10.1210/jc.2013-3903
19. Ejlsmark-Svensson H, Bislev LS, Lajlev S, Harslof T, Rolighed L, Sikjaer T, et al. Prevalence and risk of vertebral fractures in primary hyperparathyroidism: A nested case-control study. J Bone Miner Res. (2018) 33:1657–64. doi: 10.1002/jbmr.3461
20. Chewcharat A and Curhan GC. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis. (2020) 49:27–39. doi: 10.1007/s00240-020-01210-w
21. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D’Andrea D, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007–2018 data. Eur Urol Focus. (2021) 7:1468–75. doi: 10.1016/j.euf.2020.08.011
22. Yeh MW, Ituarte PHG, Zhou H, Nishimoto S, Liu I-LA, Harari A, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. (2013) 98 3:1122–9. doi: 10.1210/jc.2012-4022
23. Cong X, Shen L, and Gu X. Current opinions on nephrolithiasis associated with primary hyperparathyroidism. Urolithiasis. (2018) 46:453–7. doi: 10.1007/s00240-018-1038-x
24. Corbetta S, Baccarelli A, Aroldi A, Vicentini L, Fogazzi GB, Eller-Vainicher C, et al. Risk factors associated to kidney stones in primary hyperparathyroidism. J Endocrinological Invest. (2005) 28:122–8. doi: 10.1007/BF03345354
25. Islam AK. Advances in the diagnosis and the management of primary hyperparathyroidism. Ther Adv Chronic Dis. (2021) 12:20406223211015965. doi: 10.1177/20406223211015965
26. Ejlsmark-Svensson H, Bislev LS, Rolighed L, Sikjaer T, and Rejnmark L. Predictors of renal function and calcifications in primary hyperparathyroidism: a nested case-control study. J Clin Endocrinol Metab. (2018) 103:3574–83. doi: 10.1210/jc.2018-00923
27. Behrens M, Boyle S, Finger, et al. Evaluation for primary hyperparathyroidism in patients who present with nephrolithiasis. J Surg Res. (2021) 257:79–84. doi: 10.1016/j.jss.2020.07.049
28. Keller EX, De Coninck V, Pietropaolo A, Somani B, Haymann J-P, and Daudon M. Metabolic evaluation: place of the calcium load test: How, When, For Whom, and Why? Eur Urol Focus. (2021) 7:26–30. doi: 10.1016/j.euf.2020.12.019
29. Liu J, Tio MC, Verma A, Schmidt IM, Ilori TO, Knauf F, et al. Determinants and outcomes associated with urinary calcium excretion in chronic kidney disease. J Clin Endocrinol Metab. (2021) 107:e281–e92. doi: 10.1210/clinem/dgab574
30. Smith LM and Gallagher JC. Reference range for 24-h urine calcium, calcium/creatinine ratio, and correlations with calcium absorption and serum vitamin D metabolites in normal women. Osteoporosis Int. (2021) 32:539–47. doi: 10.1007/s00198-020-05615-6
31. Geraghty RM, Wilson I, Olinger E, Cook P, Troup S, Kennedy D, et al. Routine urinary biochemistry does not accurately predict stone type nor recurrence in kidney stone formers: A multicentre, multimodel, externally validated machine-learning study. J Endourol. (2023) 37:1295–304. doi: 10.1089/end.2023.0451
32. Rathod A, Bonny O, Guessous I, Suter PM, Conen D, Erne P, et al. Association of urinary calcium excretion with serum calcium and vitamin D levels. Clin J Am Soc Nephrology. (2015) 10:452–62. doi: 10.2215/CJN.12511213
33. Taylor EN and Curhan GC. Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol. (2007) 18:654–9. doi: 10.1681/ASN.2006080854
34. Taylor EN, Hoofnagle AN, and Curhan GC. Calcium and phosphorus regulatory hormones and risk of incident symptomatic kidney stones. Clin J Am Soc Nephrology: CJASN. (2015) 10 4:667–75. doi: 10.2215/CJN.07060714
36. Zisman AL, Coe FL, Cohen AJ, Riedinger CB, and Worcester EM. Racial differences in risk factors for kidney stone formation. Clin J Am Soc Nephrol. (2020) 15:1166–73. doi: 10.2215/CJN.12671019
37. Castellano E, Attanasio R, Latina A, Visconti GL, Cassibba S, and Borretta G. Nephrolithiasis in primary hyeperparathyroidism: A ccomparison beteen silent and symptomatic patients. Endocr Pract. (2017) 23:157–62. doi: 10.4158/EP161476.OR
38. Palmieri S, Eller-Vainicher C, Cairoli E, Morelli V, Zhukouskaya VV, Verga U, et al. Hypercalciuria may persist after successful parathyroid surgery and it is associated with parathyroid hyperplasia. J Clin Endocrinol Metab. (2015) 100:2734–42. doi: 10.1210/jc.2014-4548
39. Spivacow FR, Negri AL, del Valle EE, Fradinger E, Martinez C, and Polonsky A. Persistence of hypercalciuria after successful surgical treatment for primary hyperparathyroidism. Int Urol nephrology. (2012) 44:857–63. doi: 10.1007/s11255-011-9953-6
40. Kaplan RA, Haussler MR, Deftos LJ, Bone H, and Pak CY. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. (1977) 59:756–60. doi: 10.1172/JCI108696
41. Broadus AE, Horst RL, Lang R, Littledike ET, and Rasmussen H. The importance of circulating 1,25-dihydroxyvitamin D in the pathogenesis of hypercalciuria and renal-stone formation in primary hyperparathyroidism. New Engl J Med. (1980) 302:421–6. doi: 10.1056/NEJM198002213020801
42. Reid LJ, Muthukrishnan B, Patel D, Seckl JR, and Gibb FW. Predictors of nephrolithiasis, osteoporosis, and mortality in primary hyperparathyroidism. J Clin Endocrinol Metab. (2019) 104:3692–700. doi: 10.1210/jc.2018-02483
43. Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, and Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. Br Med J. (2002) 325:807. doi: 10.1136/bmj.325.7368.807
Keywords: hypercalcemic primary hyperparathyroidism, kidney stones, 24-hour urine calcium excretion, hypercalciuria, demographic factors
Citation: Bhan A, Simon R, Yaseen A, Cook B, Qiu S and Rao SD (2025) The predictive value of 24-hour urinary calcium for kidney stone risk in primary hyperparathyroidism: insight from a retrospective study of parathyroid adenoma cases. Front. Endocrinol. 16:1671905. doi: 10.3389/fendo.2025.1671905
Received: 23 July 2025; Accepted: 03 November 2025;
Published: 25 November 2025.
Edited by:
Giulia Lanzolla, University of Cagliari, ItalyReviewed by:
Wenjian Li, Xuzhou Medical University, ChinaManohar Bairy, Tan Tock Seng Hospital, Singapore
Copyright © 2025 Bhan, Simon, Yaseen, Cook, Qiu and Rao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arti Bhan, YWJoYW4yQGhmaHMub3Jn
 Arti Bhan
Arti Bhan Rebecca Simon1
Rebecca Simon1 Bernard Cook
Bernard Cook Shijing Qiu
Shijing Qiu Sudhaker D. Rao
Sudhaker D. Rao