- 1Reproductive Medicine Center, Dazhou Central Hospital, Dazhou, China
- 2Department of Gynecology, Dazhou Central Hospital, Dazhou, China
Objective: Polycystic Ovary Syndrome (PCOS) is the most prevalent endocrine disorder affecting women of reproductive age. Lifestyle modifications, particularly exercise, are cornerstone management strategies, with High-Intensity Interval Training (HIIT) and Moderate-Intensity Continuous Training (MICT) being commonly recommended modalities. Despite their widespread use, high-quality evidence directly comparing HIIT and MICT in women with PCOS is limited. This meta-analysis aims to rigorously compare the effects of HIIT versus MICT in women with PCOS to provide precise and robust evidence for clinical recommendations.
Methods: This meta-analysis adhered to PRISMA guidelines, conducting a comprehensive search across PubMed, EMBASE, Web of Science, and Cochrane Library databases up to April 15, 2025. Randomized controlled trials (RCTs) directly comparing supervised HIIT and MICT interventions of at least 12 weeks in premenopausal women (18–50 years) diagnosed with PCOS were included. Outcome data covered anthropometric measures, cardiorespiratory fitness, glucose and insulin metabolism, lipid profile, and hormonal parameters. Methodological quality was assessed using the Cochrane Risk of Bias Tool (RoB 2), and overall evidence certainty was determined via GRADE methodology. Statistical analyses were performed using Review Manager (RevMan) 5.4.1, with continuous variables analyzed as Weighted Mean Differences (WMD) with 95% Confidence Intervals (CIs).
Results: A total of six RCTs were included in the meta-analysis. The main findings indicate no statistically significant superiority of either HIIT or MICT across anthropometric outcomes (weight, BMI, waist circumference, hip circumference, WHR), cardiorespiratory fitness (VO2max, SBP, DBP), glucose and insulin metabolism (fasting glucose, fasting insulin, HOMA-IR), lipid profile (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides), or hormonal parameters (testosterone, SHBG, FAI). The certainty of evidence for these outcomes ranged from very low to low.
Conclusion: Based on the current low to very low certainty evidence from RCTs, there is no statistically significant superiority of HIIT over MICT for improving anthropometric, cardiorespiratory, metabolic, or hormonal outcomes in women with PCOS. These findings suggest that either HIIT or MICT can be recommended based on patient preference, but larger RCTs are needed due to low evidence certainty. This study received no funding.
1 Introduction
Polycystic Ovary Syndrome (PCOS) is a common and complex endocrine disorder, characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovaries (1, 2). With an estimated prevalence of 11-13% among women worldwide (3), PCOS represents a significant public health and economic burden (4, 5). PCOS stands as the most prevalent endocrine disorder affecting women of reproductive age (6). Its diverse clinical presentation encompasses reproductive dysfunctions like infertility and menstrual irregularities, alongside significant metabolic complications including insulin resistance, type 2 diabetes, cardiovascular disease, and obesity (1, 2). Critically, PCOS is the leading cause of anovulatory infertility (7), and a substantial contributor to early-onset type 2 diabetes and various psychological disorders (8). The chronic nature of PCOS and its associated comorbidities necessitate effective and sustainable management strategies.
The underlying pathophysiology of PCOS is multifactorial, involving a complex interplay of genetic predispositions and environmental factors. Key mechanisms include insulin resistance, compensatory hyperinsulinemia, hyperandrogenism, chronic low-grade inflammation, and altered adipose tissue function (1, 2). Current non-pharmacological management recommendations for PCOS primarily focus on lifestyle modifications, including dietary interventions and regular exercise, to address these core pathological features (3). Exercise is recognized as a cornerstone of PCOS management due to its ability to improve insulin sensitivity, reduce androgen levels, mitigate inflammation, enhance body composition, and improve cardiovascular health (9–11).
Among the various exercise modalities, High-Intensity Interval Training (HIIT) and Moderate-Intensity Continuous Training (MICT) are two commonly recommended approaches (3). Typically, HIIT involves bursts at 80-95% of maximum heart rate (HRmax) for 30 seconds to 4 minutes, alternated with recovery at 50-60% HRmax, for 20–40 minutes per session, 3–5 times weekly. MICT entails continuous exercise at 50-70% HRmax for 30–60 minutes per session at similar frequencies (9, 11). HIIT offers notable time efficiency, potentially achieving comparable physiological benefits in less total exercise time, and its perceived enjoyability relative to MICT further contributes to its enhanced adherence potential. Both exercise patterns have been shown to improve insulin sensitivity, reduce hyperandrogenism, exert anti-inflammatory effects, improve body composition, and enhance cardiorespiratory fitness in women with PCOS (12, 13).
Despite the growing interest in exercise as a therapeutic intervention for PCOS, there remains limited high-quality evidence directly comparing the effects of HIIT and MICT head-to-head. Previous systematic reviews, often limited by small sample sizes and the inclusion of broad exercise interventions or non-direct comparisons, have yielded diverse and sometimes inconclusive findings regarding the relative superiority of one modality over another for specific PCOS outcomes (14–17). Therefore, a comprehensive and updated meta-analysis exclusively focusing on randomized controlled trials (RCTs) directly comparing HIIT and MICT in women with PCOS is critically needed to provide precise and robust evidence for clinical recommendations.
This meta-analysis aims to rigorously compare the effects of HIIT versus MICT in women with PCOS. We hypothesize that HIIT and MICT will lead to comparable improvements in PCOS-related outcomes.
2 Methods
2.1 Study design and registration
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18) and was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration ID: CRD42025649165. This study received no funding, and all authors declared no conflict of interest. Two independent reviewers (YZ and YL) conducted the literature search, extracted data, assessed the methodological quality of included studies, and performed statistical analyses, with any discrepancies resolved through discussion with a third reviewer (JL).
2.2 Literature search
A comprehensive search was conducted across PubMed, EMBASE, Web of Science, and Cochrane Library databases, including all records available up to April 15, 2025. The search strategy used the following main search terms: (“high-intensity interval training” OR “HIIT” OR “high-intensity intermittent exercise” OR “interval training”) AND (“moderate-intensity continuous training” OR “MICT” OR “moderate-intensity exercise” OR “continuous aerobic training”) AND (“polycystic ovary syndrome” OR “PCOS” OR “polycystic ovarian syndrome” OR “hyperandrogenic anovulation”). Terms like ‘intermittent’ and ‘interval’ were included to account for synonymous usage in literature, while ‘continuous aerobic’ captured MICT variants. Screening ensured conceptual consistency by verifying that HIIT protocols featured high-intensity bursts (typically >80% HRmax or equivalent) with recovery, and MICT involved moderate continuous exercise (typically 50-75% HRmax), though we accommodated minor variations as per study reporting to reflect practical implementations. Criteria were applied consistently, with all studies verified for direct HIIT/MICT comparisons. Search queries were tailored to meet the specific requirements of each database (Supplementary Table 1).
2.3 Inclusion and exclusion criteria
Inclusion criteria: (1) studies involving premenopausal women aged 18 to 50 years, diagnosed with PCOS per the Rotterdam Criteria (19); (2) RCTs directly comparing HIIT with MICT as supervised exercise interventions lasting at least 12 weeks, ensuring consistency in all other variables such as diet and medication except the exercise protocol; (3) studies reporting at least one outcome with baseline and endpoint data, covering anthropometric measures, cardiorespiratory fitness, glucose and insulin metabolism, lipid profile, or hormonal parameters.
Exclusion criteria: (1) studies including pregnant participants; (2) studies involving participants using antihypertensive medications, insulin sensitizers, dietary supplements, weight loss medications, or hormonal contraceptives within 3 months prior to enrolment; (3) non-English studies; (4) studies incorporating additional forms of exercise beyond HIIT or MICT.
2.4 Data extraction
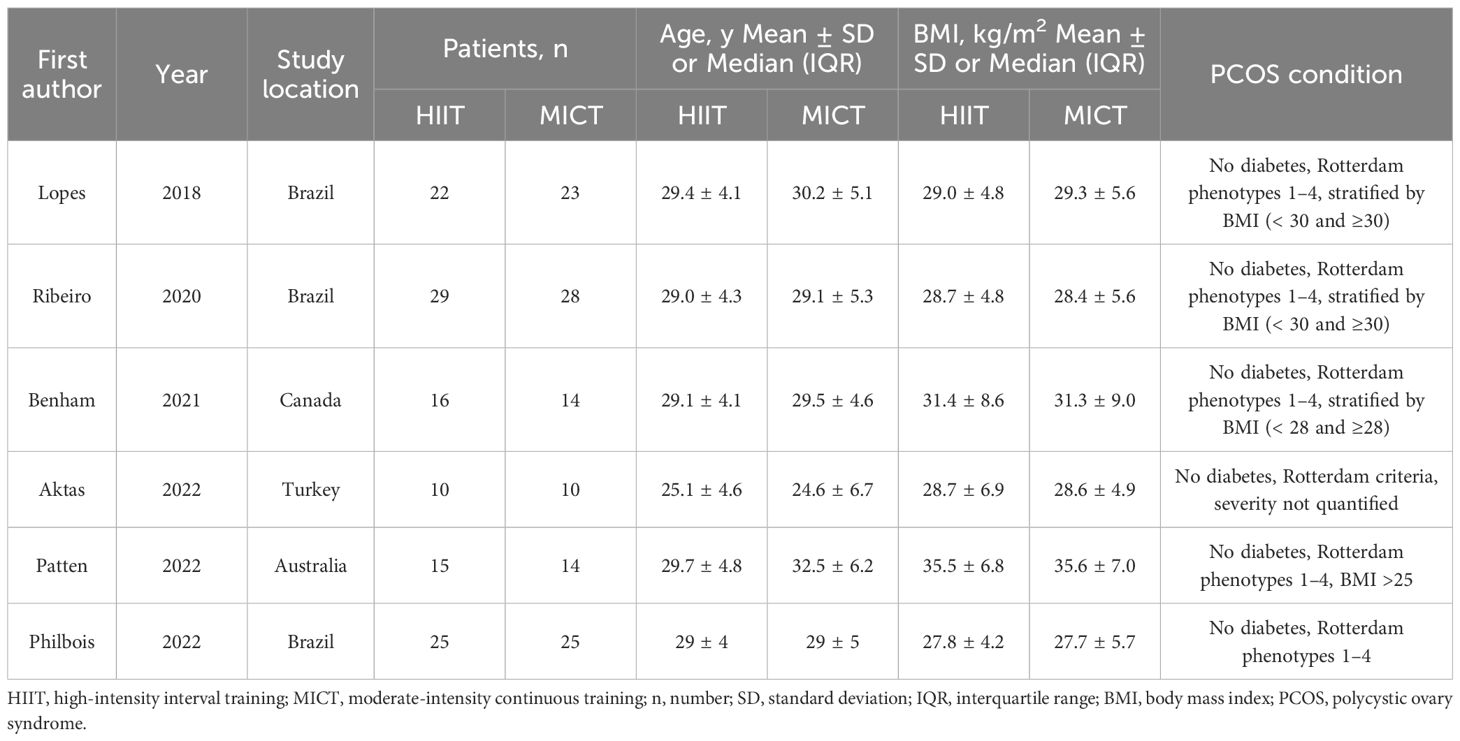
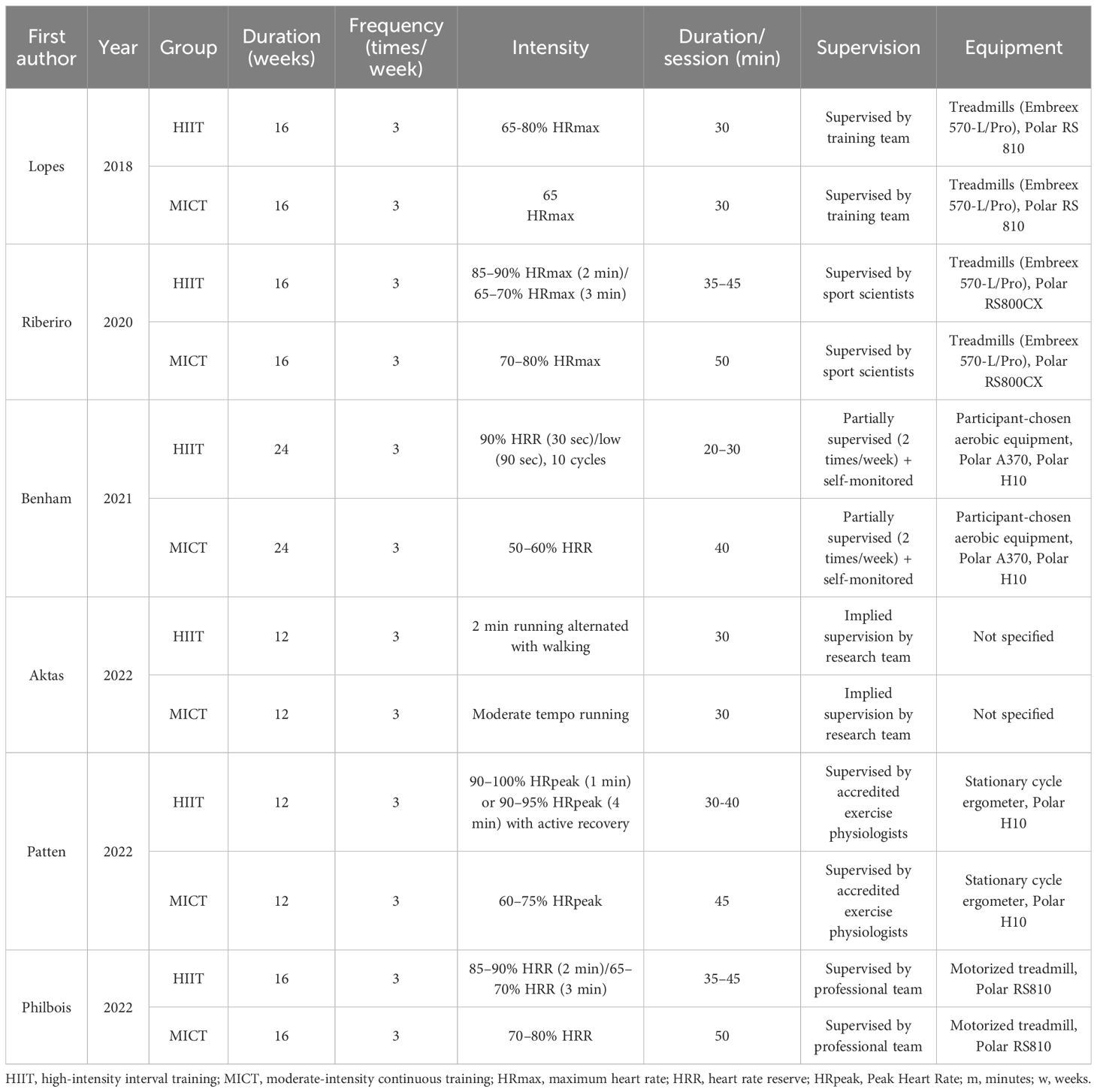
Extracted study details included the first author’s name, publication year, study location, sample size, patient demographics (age and body mass index [BMI]), PCOS condition, and specifics of both HIIT and MICT intervention. Outcome data were derived as the difference (Δ) between endpoint and baseline values (endpoint values – baseline values) for comparisons between groups, categorized into five major domains: anthropometric measures, cardiorespiratory fitness, glucose and insulin metabolism, lipid profile, and hormonal parameters.
Anthropometric measures included body mass index (BMI in kg/m²), waist and hip circumference (in cm), as well as waist-to-hip ratio (WHR). Cardiorespiratory fitness included maximal oxygen uptake (VO2max in ml/kg/min), systolic blood pressure (SBP) and diastolic blood pressure (DBP) in mm Hg. Glucose and insulin metabolism included fasting glucose (in mmol/L), fasting insulin (in µIU/mL), and homeostatic model assessment of insulin resistance (HOMA-IR). Lipid profile comprised total cholesterol (in mmol/L), high-density lipoprotein cholesterol (HDL cholesterol in mmol/L), low-density lipoprotein cholesterol (LDL cholesterol in mmol/L), and triglycerides (in mmol/L). Hormonal parameters included testosterone (in nmol/L), sex hormone-binding globulin (SHBG in nmol/L), and free androgen index (FAI).
2.5 Quality assessment
The methodological quality of included studies was appraised using the revised Cochrane Risk of Bias Tool (RoB 2) for RCTs (20). This tool evaluated bias across five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was classified as low risk, high risk, or some concerns based on RoB 2 guidelines. The overall evidence certainty was determined using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology, factoring in risk of bias, inconsistency, indirectness, imprecision, and publication bias, with quality rated as high, moderate, low, or very low. Publication bias was assessed via funnel plots and Egger’s test for outcomes with ten or more studies.
2.6 Statistical analysis
Meta-analyses were performed using Review Manager (RevMan) version 5.4.1 (The Cochrane Collaboration, Oxford, UK). Continuous variables were analyzed as Weighted Mean Differences (WMD) with 95% Confidence Intervals (CIs), while dichotomous variables were expressed as pooled Odds Ratios (ORs) with 95% CIs. Heterogeneity was evaluated using Cochrane’s Q test and the I² statistic. For analyses with I² <50%, a fixed-effects model was employed, whereas a random-effects model was adopted for I² ≥50%. Forest plots were used to present the pooled effect sizes, with statistical significance defined as P <0.05.
3 Results
3.1 Study selection and characteristics
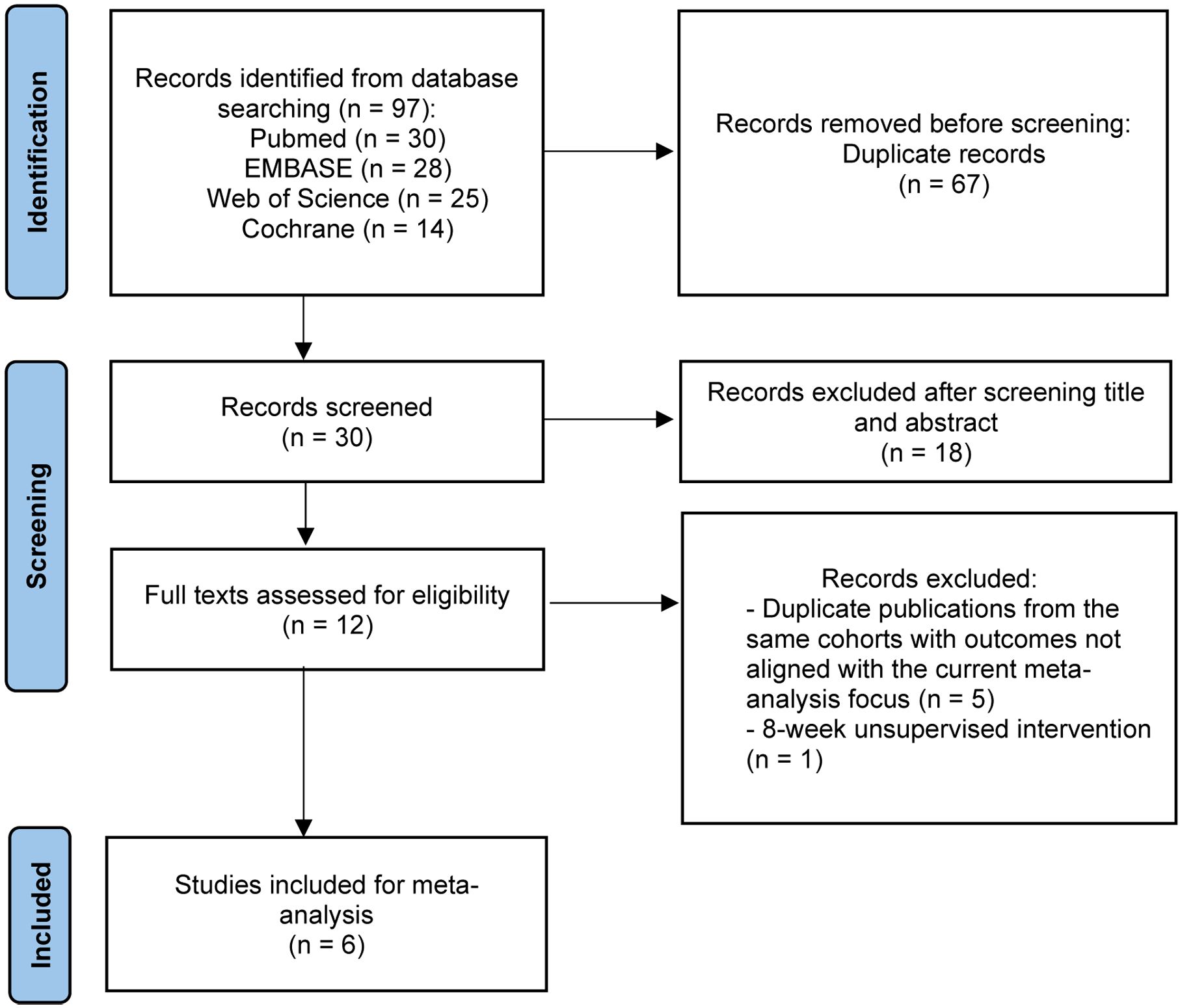
A search of PubMed, EMBASE, The Cochrane Library, and Web of Science found 97 studies. After removing 67 duplicates, 30 studies were screened by title and abstract. Of these, 18 were excluded, leaving 12 studies for full-text review and reference checking. After reviewing the full texts, 6 studies were excluded, with 5 (21–25) excluded due to being duplicate publications or secondary analyses of the same cohort (retaining only the initial study with the most comprehensive outcome data) and 1 (26) excluded due to an 8-week unsupervised home-based HIIT and MICT intervention. Finally, a total of 6 RCTs (27–32) were included (Figure 1). The main details of these studies are presented in Table 1 and Table 2.
3.2 Quality assessment
The quality of the included studies was assessed using the RoB 2 criteria, as illustrated in Figure 2, with no study exhibiting high risk in any domain. The primary “some concerns” were concentrated in the areas of missing outcome data and measurement of the outcomes. Overall, the quality of the included studies was considered moderate.
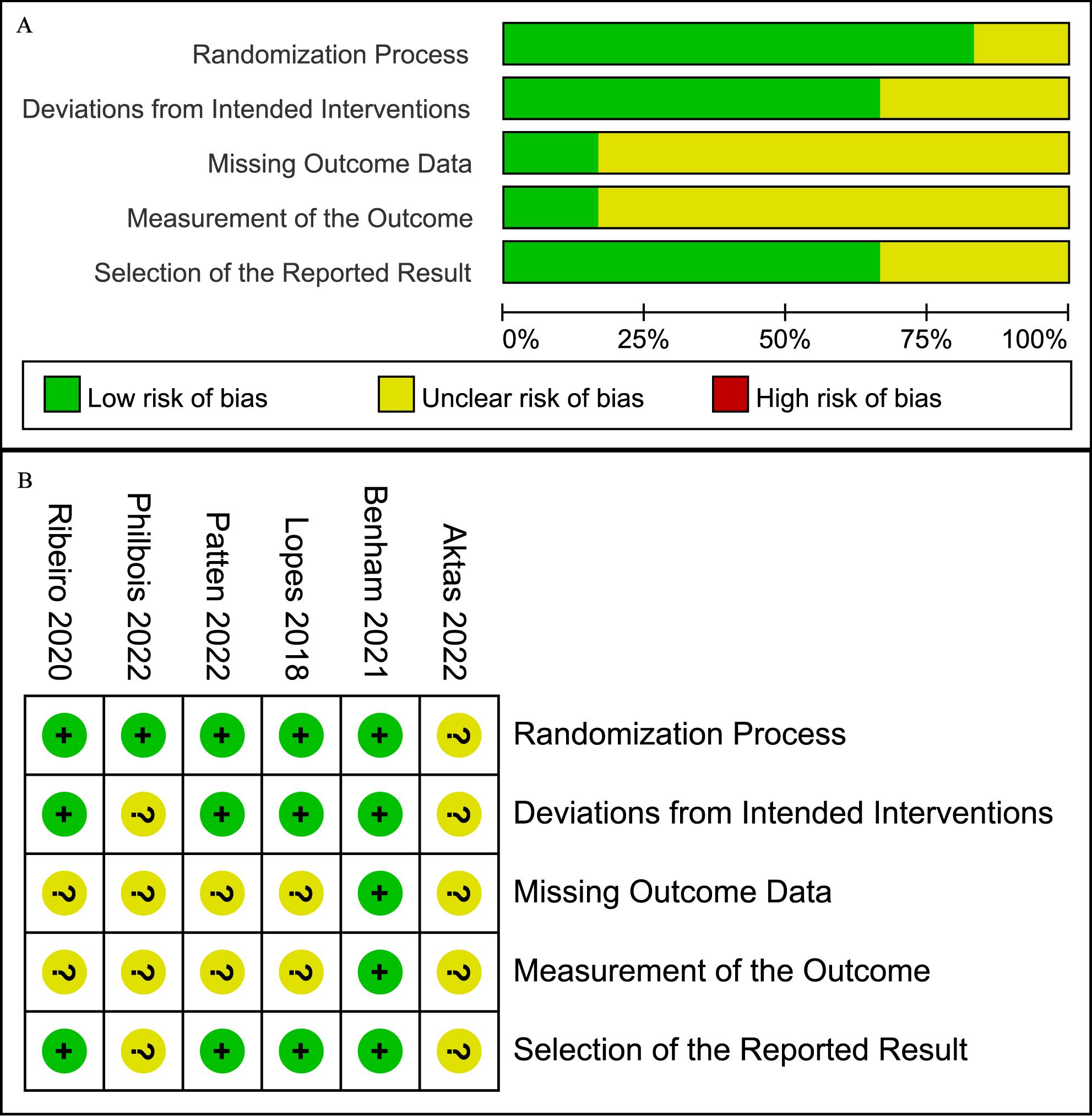
Figure 2. Risk of bias graph (A) Graph of the risk of bias summary for the included studies, (B) Graph of the risk of bias for each included study.
3.3 Anthropometric measures
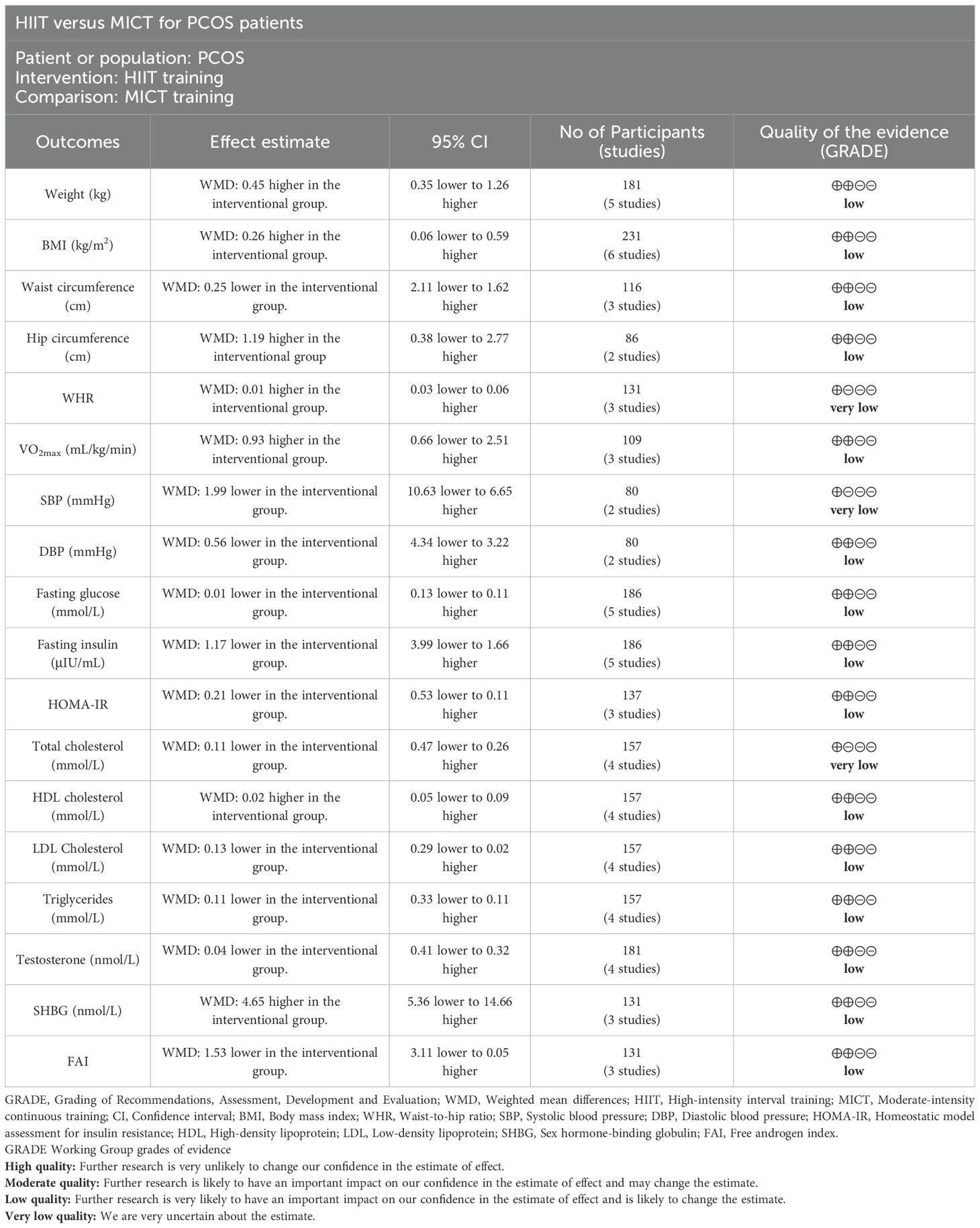
Five studies (27–30, 32) involving 181 patients reported body weight (kg). The results showed no significant difference in weight change between the HIIT and MICT groups (WMD: 0.45; 95% CI: -0.35 to 1.26; I² = 0%; P = 0.27) (Figure 3A). Similarly, six studies (27–32) with 231 patients reported BMI (kg/m2), indicating no significant difference between groups (WMD: 0.26; 95% CI: -0.06 to 0.59; I² = 0%; P = 0.11) (Figure 3B). Three studies (28, 30, 32) involving 116 patients assessed waist circumference (cm), showing no significant difference between the HIIT and MICT groups (WMD: -0.25; 95% CI: -2.11 to 1.62; I² = 0%; P = 0.80) (Figure 3C). Two studies (30, 32) with 86 patients reported hip circumference (cm), with no significant difference observed (WMD: 1.19; 95% CI: -0.38 to 2.77; I² = 0%; P = 0.14) (Figure 3D). Three studies (29, 30, 32) involving 131 patients reported WHR, showing no significant difference (WMD: 0.01 higher; 95% CI: -0.03 to 0.06; I² = 77%; P = 0.57) (Figure 3E).
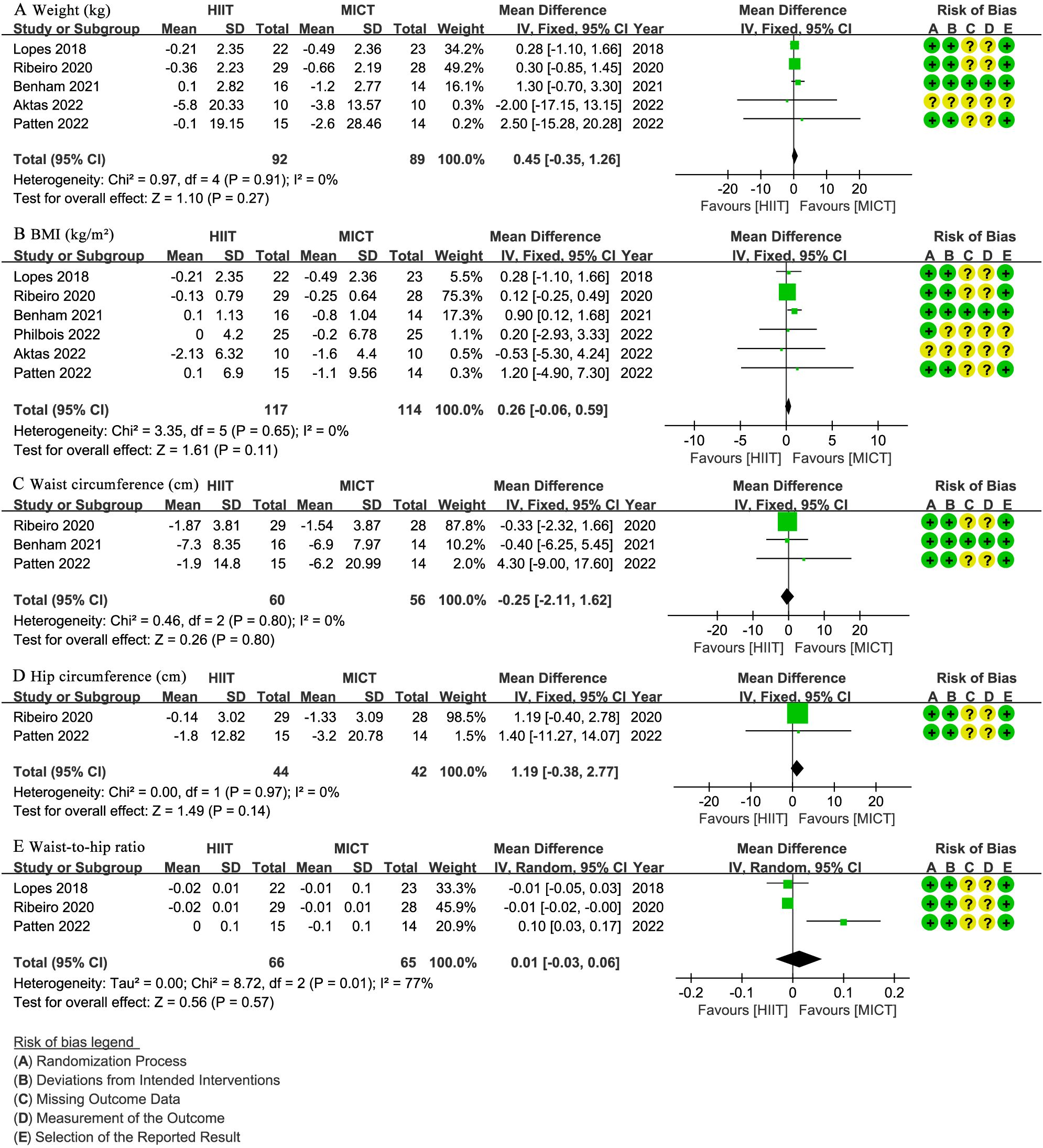
Figure 3. Meta-analysis of: (A) weight (kg), (B) body mass index (BMI) (kg/m2), (C) waist circumference (cm), (D) hip circumference (cm), (E) waist-to-hip ratio (WHR).
3.4 Cardiorespiratory fitness
Three studies (28, 30, 31) with 109 patients evaluated VO2max (mL/min/kg), indicating no significant difference between groups (WMD: 0.93; 95% CI: -0.66 to 2.51; I² = 0%; P = 0.25) (Figure 4A). Two studies (28, 31) involving 80 patients reported SBP and DBP (mmHg), with no significant difference observed for SBP (WMD: -1.99; 95% CI: -10.63 to 6.65; I² = 61%; P = 0.65) (Figure 4B) or DBP (WMD: -0.56; 95% CI: -4.34 to 3.22; I² = 0%; P = 0.77) (Figure 4C).
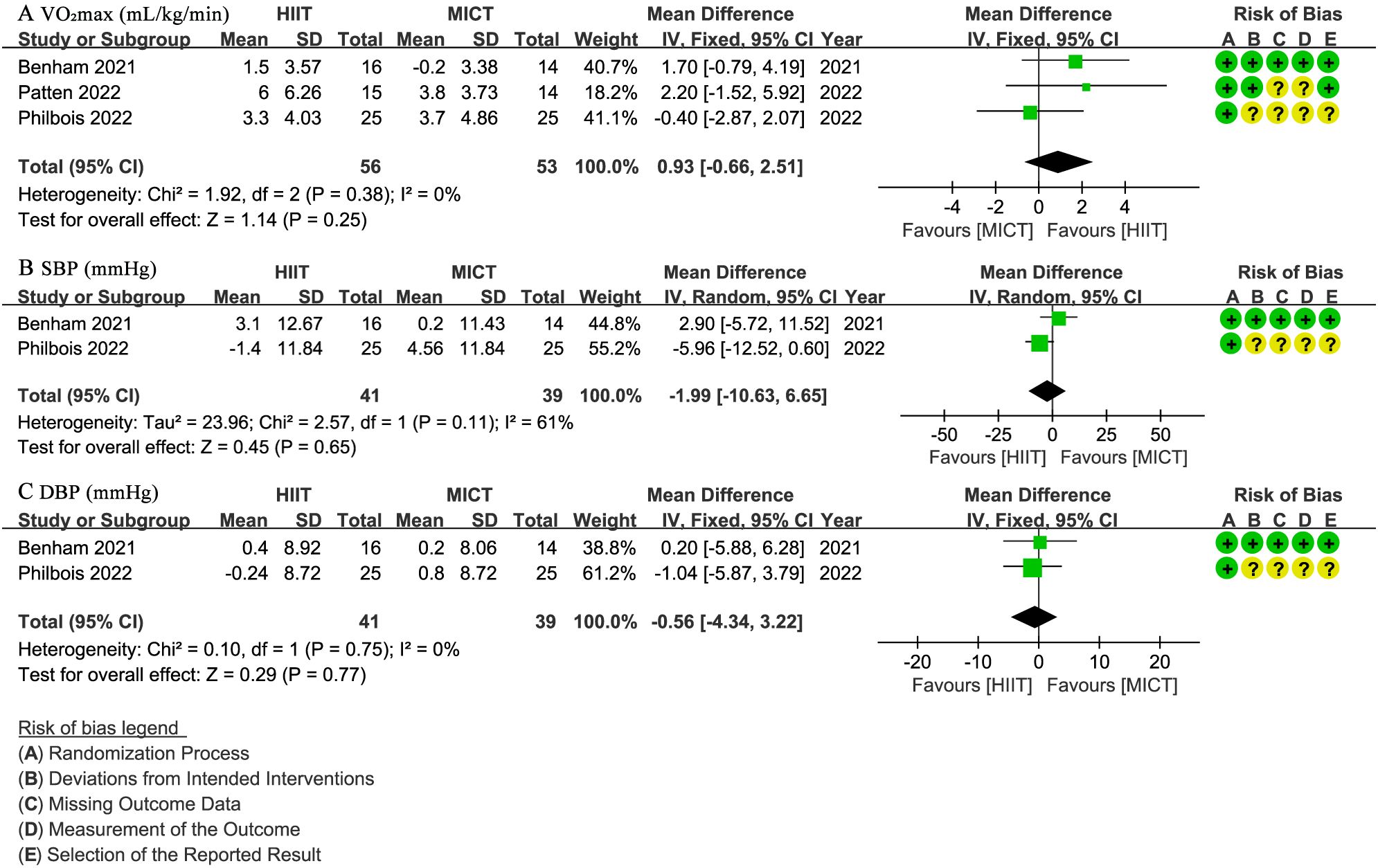
Figure 4. Meta-analysis of: (A) VO2max (mL/min/kg), (B) systolic blood pressure (SBP) (mmHg), (C) diastolic blood pressure (DBP) (mmHg).
3.5 Glucose and insulin metabolism
Five studies (27, 28, 30–32) involving 186 patients assessed fasting glucose (mmol/L), indicating no significant difference between the HIIT and MICT groups (WMD: -0.01; 95% CI: -0.13 to 0.11; I² = 0%; P = 0.89) (Figure 5A). The same five studies (27, 28, 30–32) reported fasting insulin (µIU/mL), with no significant difference observed (WMD: -1.17; 95% CI: -3.99 to 1.66; I² = 0%; P = 0.42) (Figure 5B). Three studies (28, 31, 32) with 137 patients evaluated HOMA-IR, showing no significant difference (WMD: -0.21; 95% CI: -0.53 to 0.11; I² = 0%; P = 0.20) (Figure 5C).
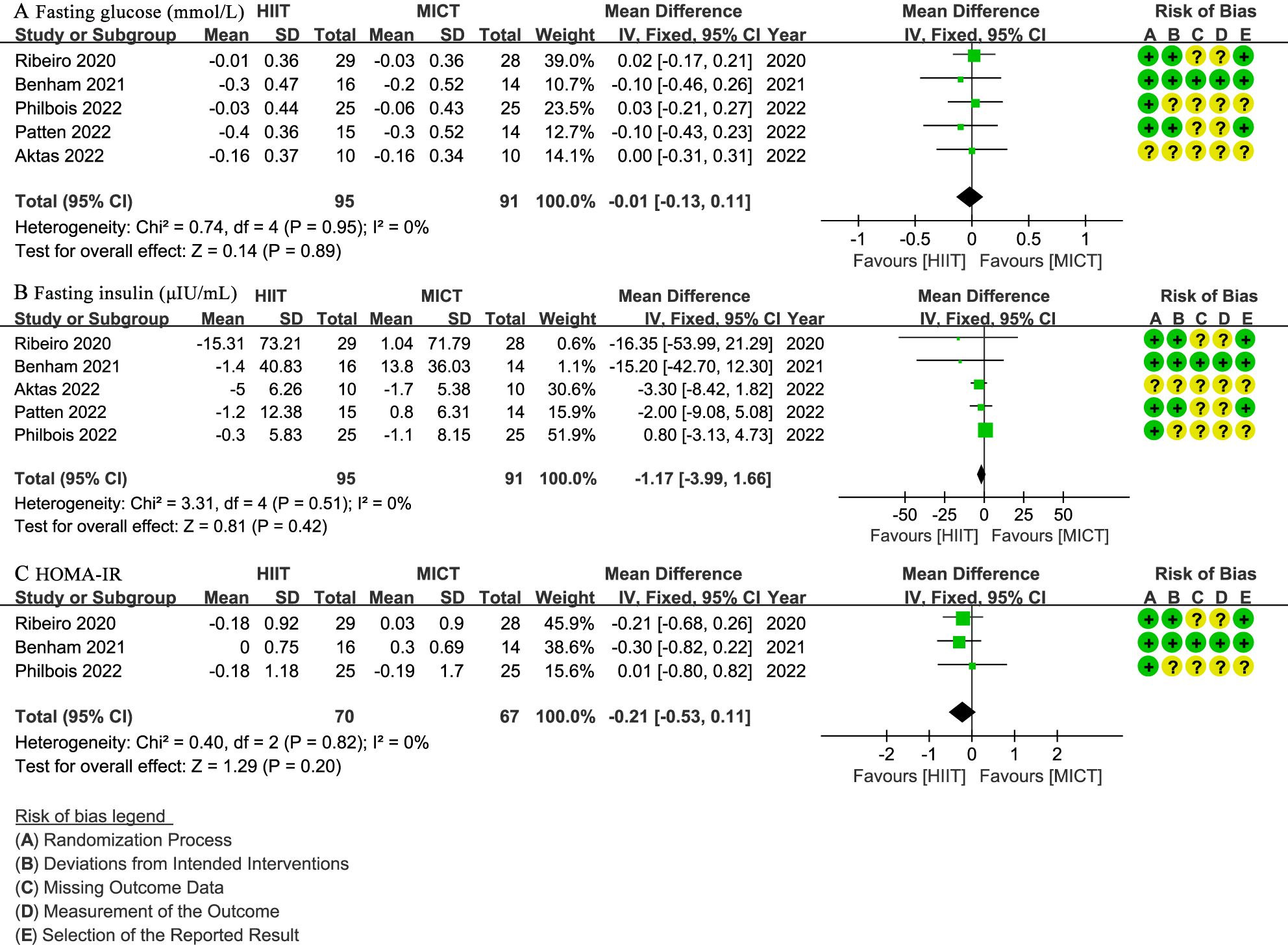
Figure 5. Meta-analysis of: (A) fasting glucose (mmol/L), (B) fasting insulin (µIU/mL), (C) homeostatic model assessment for insulin resistance (HOMA-IR).
3.6 Lipid profile
Four studies (27, 28, 31, 32) involving 157 patients reported total cholesterol (mmol/L), HDL cholesterol (mmol/L), LDL cholesterol (mmol/L), and triglycerides (mmol/L) (Figure 6). There was no significant difference between groups for total cholesterol (WMD: -0.11; 95% CI: -0.47 to 0.26; I² = 68%; P = 0.57) (Figure 6A), HDL cholesterol (WMD: 0.02; 95% CI: -0.05 to 0.09; I² = 0%; P = 0.55) (Figure 6B), LDL cholesterol (WMD: -0.13; 95% CI: -0.29 to 0.02; I² = 45%; P = 0.10) (Figure 6C), or triglycerides (WMD: -0.11; 95% CI: -0.33 to 0.11; I² = 33%; P = 0.31) (Figure 6D).
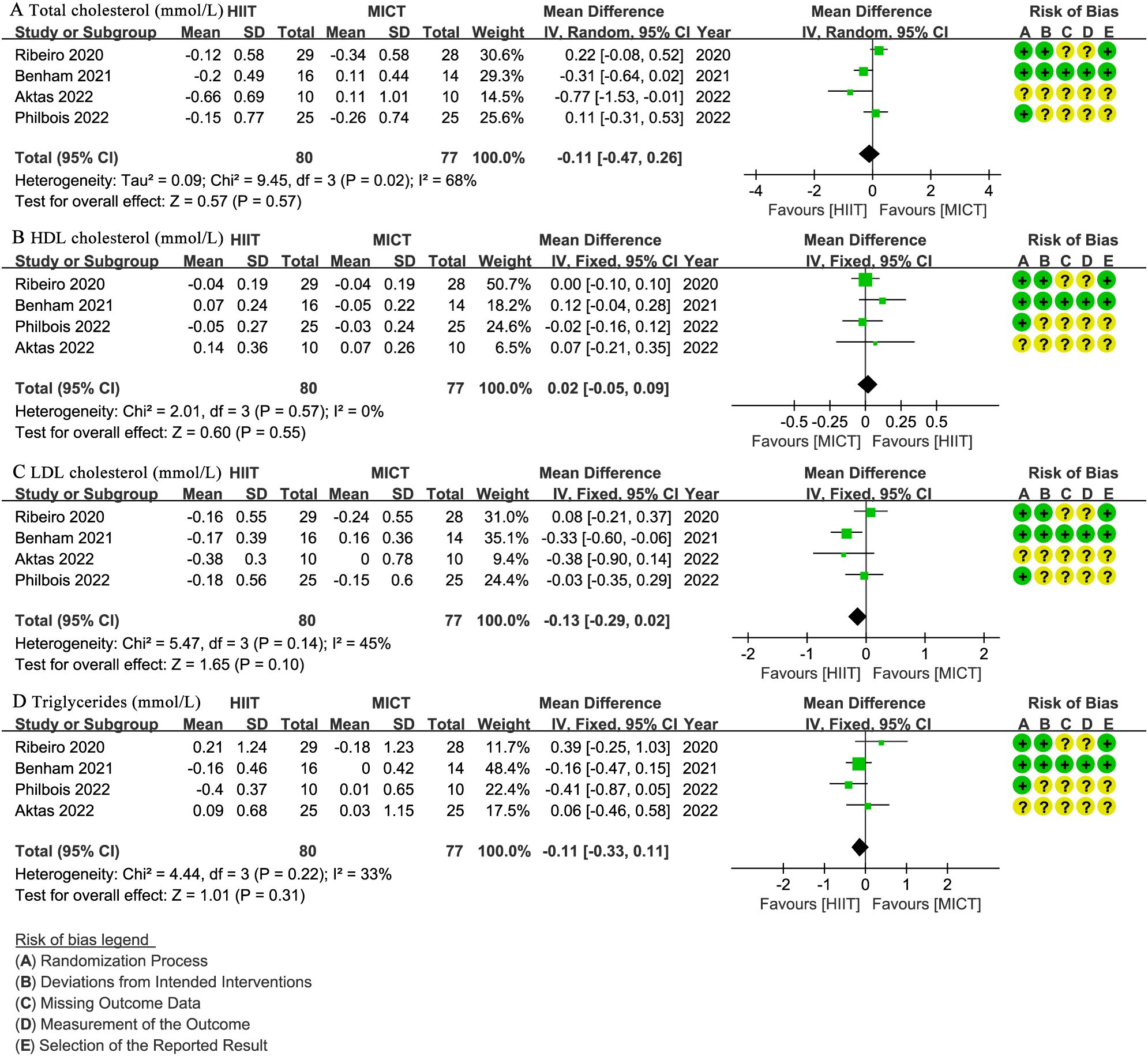
Figure 6. Meta-analysis of: (A) total cholesterol (mmol/L), (B) high-density lipoprotein (HDL) cholesterol (mmol/L), (C) low-density lipoprotein (LDL) cholesterol (mmol/L), (D) triglycerides (mmol/L).
3.7 Hormonal parameters
Four studies (29–32) involving 181 patients reported testosterone levels (nmol/L), with no significant difference observed (WMD: -0.04; 95% CI: -0.41 to 0.32; I² = 0%; P = 0.82) (Figure 7A). Three studies (29, 30, 32) with 131 patients assessed SHBG (nmol/L) and FAI, showing no significant difference for SHBG (WMD: 4.65; 95% CI: -5.36 to 14.66; I² = 0%; P = 0.36) (Figure 7B) or FAI (WMD: -1.53; 95% CI: -3.11 to 0.05; I² = 0%; P = 0.06) (Figure 7C).
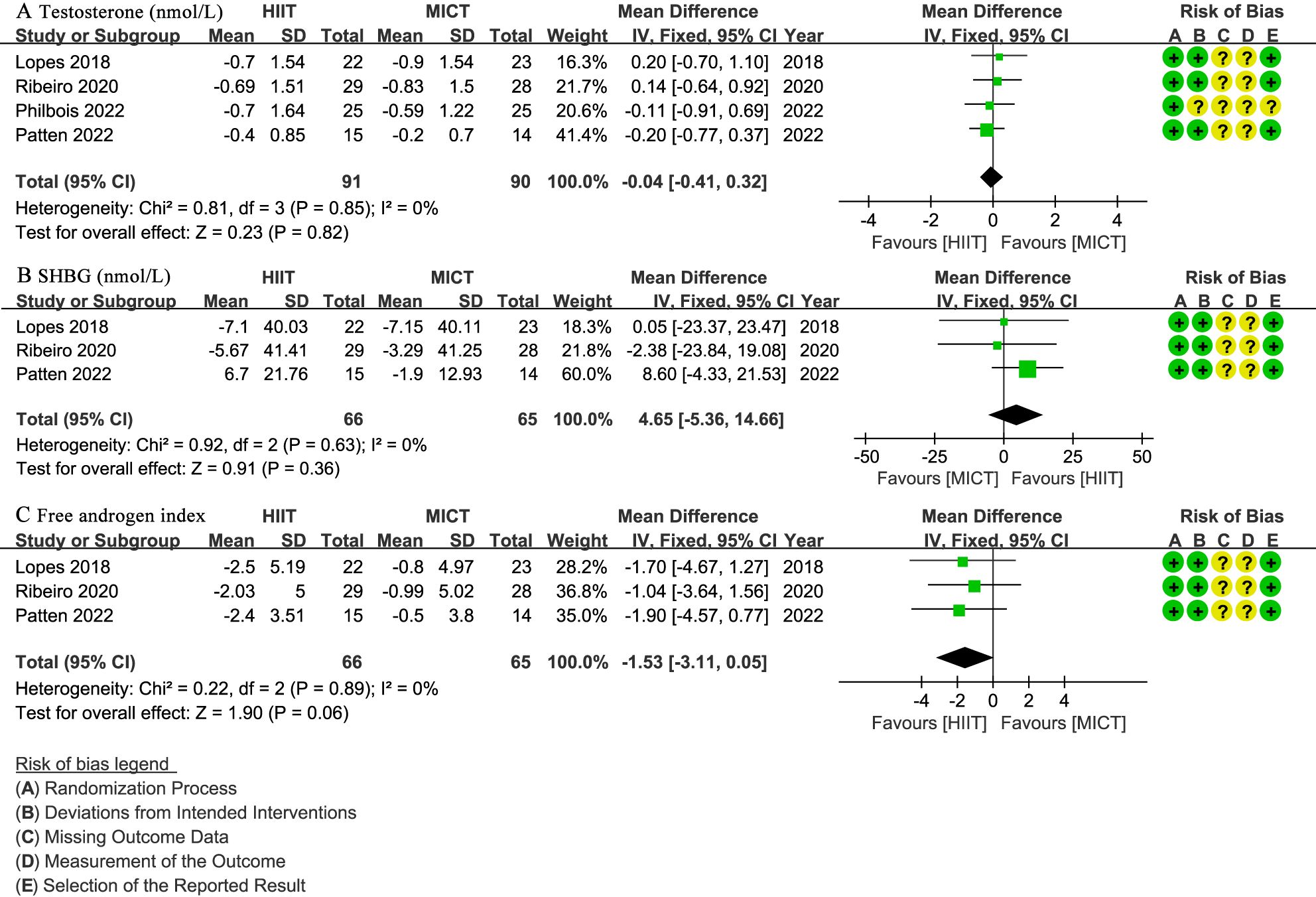
Figure 7. Meta-analysis of: (A) testosterone levels (nmol/L), (B) sex hormone binding globulin (SHBG) (nmol/L), (C) free testosterone index (FAI).
3.8 Quality of evidence
The GRADE methodology was applied to assess the certainty of evidence for each outcome (Table 3). The quality of evidence was very low for WHR, SBP, total cholesterol, and low for other outcomes.
4 Discussion
This meta-analysis synthesized current RCTs on the effects of HIIT versus MICT on women with PCOS. Our findings indicate that neither HIIT nor MICT demonstrated a statistically significant superiority over the other across anthropometric, cardiorespiratory, metabolic, or hormonal outcomes. Notably, the certainty of evidence for these outcomes ranged from very low to low, largely due to small sample sizes and inherent heterogeneity among studies.
Several recent systematic reviews have investigated exercise interventions in women with PCOS, each with distinct scopes and limitations. A 2021 review by Richards et al. (16) concluded Moderate-Intensity Steady State (MISS) exercise was superior for cardiorespiratory fitness and BMI in PCOS, but its scope was not limited to direct HIIT vs. MICT comparisons. Another 2021 review by Santos et al. (17) found HIIT alone significantly decreased HOMA-IR and BMI, but its primary objective was not head-to-head comparison of HIIT and MICT. A 2022 review by Breyley-Smith et al. (14) primarily compared exercise against non-exercising controls, concluding exercise improved cardiorespiratory fitness and waist circumference, with MICT showing greater or more significant improvements. Most recently, the 2023 review by Colombo et al. (15) found no statistically significant differences between HIIT and MICT for various parameters, with low or very low certainty of evidence. Notably, Colombo et al.’s meta-analysis included five RCTs. Three (28, 30, 32) directly compared HIIT and MICT (also in our review), while the other two compared HIIT to Resistance Training (RT) and MICT to MICT+RT.
To our best knowledge, our meta-analysis is the first to exclusively include RCTs directly comparing HIIT and MICT in women with PCOS. This rigorous focus on head-to-head comparisons, distinct from prior reviews that included broader exercise interventions or non-direct comparisons, ensures a more precise evaluation of their comparative effectiveness. Although the certainty of evidence for many outcomes remains very low to low due to limited studies and small sample sizes, our findings represent the highest quality evidence currently available in this specific comparative domain. Despite conclusions similar to Colombo et al. (15) regarding the lack of clear superiority, our expanded and updated dataset offers a more comprehensive and current perspective.
The observed absence of statistically significant superiority between HIIT and MICT, despite their distinct exercise protocols, can be largely attributed to a convergence in their underlying physiological mechanisms that target the core pathophysiological features of PCOS. Both modalities consistently improve insulin sensitivity, a cornerstone in PCOS management, by reducing markers such as fasting insulin and HOMA-IR (12, 13). This is crucial as it impacts the bidirectional link between hyperinsulinemia and hyperandrogenism, central to PCOS pathology (9–11). Beyond metabolic and hormonal improvements, both training types exert significant anti-inflammatory effects, mitigating chronic low-grade inflammation by modulating cytokine levels, which is vital for protecting against long-term cardiometabolic risks (14). In terms of body composition, both lead to favorable changes, including reductions in BMI, waist circumference, and overall body fat, while promoting increases in lean muscle mass (15–17). Last, both enhance cardiorespiratory fitness, indicated by improvements in maximal VO2max (15, 16). This broad spectrum of overlapping physiological benefits suggests that the body’s adaptive response to chronic exercise, irrespective of intensity, ultimately might converge on similar improvements in key PCOS indicators.
The “very low to low certainty of evidence” in our meta-analysis critically explains the lack of statistical superiority, primarily stemming from significant methodological limitations in existing RCTs. A key challenge is the inherent impossibility of participant blinding in exercise interventions, which invariably lowers the quality assessment of included studies. Another significant contributing factor is the consistently small sample sizes (typically 24–110 participants), reducing statistical power and hindering the detection of subtle yet clinically meaningful differences between HIIT and MICT. Heterogeneity across studies also manifests in variable designs, exercise protocols (intensity, duration, frequency, modality), participant characteristics (BMI, insulin resistance, PCOS phenotypes), and inconsistent outcome measurements. Finally, the relatively short duration of most studies (12–24 weeks) may be insufficient to reveal long-term or more significant differential effects in a chronic condition like PCOS.
Beyond statistical and methodological considerations, the practical implications of these findings warrant discussion. While our meta-analysis indicates no statistical superiority in efficacy, HIIT often achieves comparable physiological benefits in significantly less total exercise time compared to MICT. One study reported HIIT requiring 27.5% less total exercise time and approximately 25% less energy expenditure than MICT to achieve similar adaptations (33). This “time efficiency” is a crucial practical advantage that can significantly enhance patient adherence and long-term sustainability in real-world settings (34). However, a recent systematic review found that HIIT showed no advantage over MICT in unsupervised settings, as participants often exercised at lower-than-prescribed intensities (35). Despite our included studies being supervised and excluding those shorter than 12 weeks, these studies did not comprehensively report adherence, highlighting an important area for future research.
This meta-analysis has several limitations that warrant consideration. First, despite conducting a comprehensive search and including all eligible RCTs, the total number of studies and participants available for direct comparison between HIIT and MICT remains relatively small. Second, the heterogeneity in exercise protocols (such as exact intensity, duration of intervals, rest periods, overall weekly volume, and supervision levels) among the included studies, even within the broad categories of HIIT and MICT, makes it challenging to draw highly specific conclusions about optimal exercise prescription. This stems in part from methodological limitations of the review process, including our broad search strategy, which, while ensuring comprehensive capture of relevant studies, led to inclusion of trials with variations in practical parameters, with some minor adaptations as reported. Although inclusion criteria were consistently applied to verify direct HIIT versus MICT comparisons, these parameter variations may contribute to heterogeneity and compromise synthesis validity. Future meta-analyses could mitigate this by incorporating stricter subgroup analyses or protocol standardization. Third, while we aimed to isolate the effects of exercise, some studies included concurrent interventions like dietary advice, which, even if consistent across groups, could influence outcomes. Fourth, the majority of included studies involved women who were overweight or obese, and often had baseline cardiometabolic parameters within normal ranges. This limits the generalizability of our findings to lean women with PCOS or those with more severe metabolic abnormalities, where exercise might show more pronounced effects. Last, our review primarily focused on cardiometabolic and hormonal outcomes and did not extensively cover psychological aspects or long-term adherence beyond the intervention period, which are also crucial for comprehensive PCOS management.
5 Conclusion
Based on the current low to very low certainty evidence from RCTs, there is no statistically significant superiority of HIIT over MICT for improving anthropometric, cardiorespiratory, metabolic, or hormonal outcomes in women with PCOS. Given these findings, individuals with PCOS may select either HIIT or MICT based on personal preference and feasibility. Future large-scale, high-quality RCTs with standardized protocols and detailed reporting of participant phenotypes are needed.
Author contributions
YZ: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft. YL: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. HZ: Writing – review & editing. RH: Writing – review & editing. YC: Writing – review & editing. JL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1672257/full#supplementary-material
References
1. Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. (2022) 10:668–80. doi: 10.1016/s2213-8587(22)00163-2
2. Stener-Victorin E, Teede H, Norman RJ, Legro R, Goodarzi MO, Dokras A, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. (2024) 10:27. doi: 10.1038/s41572-024-00511-3
3. Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertility sterility. (2023) 120:767–93. doi: 10.1016/j.fertnstert.2023.07.025
4. Riestenberg C, Jagasia A, Markovic D, Buyalos RP, and Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab. (2022) 107:575–85. doi: 10.1210/clinem/dgab613
5. Yadav S, Delau O, Bonner AJ, Markovic D, Patterson W, Ottey S, et al. Direct economic burden of mental health disorders associated with polycystic ovary syndrome: Systematic review and meta-analysis. eLife. (2023) 12:e85338 . doi: 10.7554/eLife.85338
6. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, and Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertility sterility. (2016) 106:6–15. doi: 10.1016/j.fertnstert.2016.05.003
7. Hamilton-Fairley D and Taylor A. Anovulation. BMJ (Clinical Res ed). (2003) 327:546–9. doi: 10.1136/bmj.327.7414.546
8. Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, et al. Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertility sterility. (2018) 109:888–99. doi: 10.1016/j.fertnstert.2018.01.038
9. Gu Y, Zhou G, Zhou F, Wu Q, Ma C, Zhang Y, et al. Life modifications and PCOS: old story but new tales. Front Endocrinol. (2022) 13:808898. doi: 10.3389/fendo.2022.808898
10. Patten RK, Boyle RA, Moholdt T, Kiel I, Hopkins WG, Harrison CL, et al. Exercise interventions in polycystic ovary syndrome: A systematic review and meta-analysis. Front Physiol. (2020) 11:606. doi: 10.3389/fphys.2020.00606
11. Woodward A, Klonizakis M, and Broom D. Exercise and polycystic ovary syndrome. Adv Exp Med Biol. (2020) 1228:123–36. doi: 10.1007/978-981-15-1792-1_8
12. Luo Y, Zhang J, Jia H, Mu X, and Huang J. Effects of high intensity interval training and moderate intensity continuous training on enjoyment and affective responses in overweight or obese people: a meta-analysis. Front Public Health. (2024) 12:1487789. doi: 10.3389/fpubh.2024.1487789
13. Yin M, Chen Z, Nassis GP, Liu H, Li H, Deng J, et al. Chronic high-intensity interval training and moderate-intensity continuous training are both effective in increasing maximum fat oxidation during exercise in overweight and obese adults: A meta-analysis. J Exercise Sci fitness. (2023) 21:354–65. doi: 10.1016/j.jesf.2023.08.001
14. Breyley-Smith A, Mousa A, Teede HJ, Johnson NA, and Sabag A. The effect of exercise on cardiometabolic risk factors in women with polycystic ovary syndrome: A systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19(3):1386. doi: 10.3390/ijerph19031386
15. Colombo GE, Bouzo XD, Patten RK, Mousa A, Tay CT, Pattuwage L, et al. Comparison of selected exercise training modalities in the management of PCOS: a systematic review and meta-analysis to inform evidence-based guidelines. JSAMS Plus. (2023) 2:100024. doi: 10.1016/j.jsampl.2023.100024
16. Richards CT, Meah VL, James PE, Rees DA, and Lord RN. HIIT’ing or MISS’ing the optimal management of polycystic ovary syndrome: A systematic review and meta-analysis of high- versus moderate-intensity exercise prescription. Front Physiol. (2021) 12:715881. doi: 10.3389/fphys.2021.715881
17. Santos IKD, Nunes F, Queiros VS, Cobucci RN, Dantas PB, Soares GM, et al. Effect of high-intensity interval training on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. PloS One. (2021) 16:e0245023. doi: 10.1371/journal.pone.0245023
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Res ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod (Oxford England). (2004) 19:41–7. doi: 10.1093/humrep/deh098
20. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. Benham JL, Booth JE, Goldfield G, Friedenreich CM, Rabi DM, and Sigal RJ. Self-reported sleep quality and exercise in polycystic ovary syndrome: A secondary analysis of a pilot randomized controlled trial. Clin Endocrinol. (2023) 98:700–8. doi: 10.1111/cen.14900
22. Patten RK, Bourke M, McIlvenna LC, Moreno-Asso A, Woessner MN, Stepto NK, et al. Longitudinal affective response to high-intensity interval training and moderate-intensity continuous training in overweight women with polycystic ovary syndrome: A randomised trial. Psychol sport Exercise. (2023) 64:102325. doi: 10.1016/j.psychsport.2022.102325
23. Patten RK, McIlvenna LC, Moreno-Asso A, Hiam D, Stepto NK, Rosenbaum S, et al. Efficacy of high-intensity interval training for improving mental health and health-related quality of life in women with polycystic ovary syndrome. Sci Rep. (2023) 13:3025. doi: 10.1038/s41598-023-29503-1
24. Ribeiro VB, Lopes IP, Dos Reis RM, Silva RC, Mendes MC, Melo AS, et al. Continuous versus intermittent aerobic exercise in the improvement of quality of life for women with polycystic ovary syndrome: A randomized controlled trial. J Health Psychol. (2021) 26:1307–17. doi: 10.1177/1359105319869806
25. Ribeiro VB, Pedroso DCC, Kogure GS, Lopes IP, Santana BA, Dutra de Souza HC, et al. Short-term aerobic exercise did not change telomere length while it reduced testosterone levels and obesity indexes in PCOS: A randomized controlled clinical trial study. Int J Environ Res Public Health. (2021) 18(21):11274. doi: 10.3390/ijerph182111274
26. Wang A, Noel M, Christ JP, Corley J, Lenhart N, Cedars MI, et al. Vigorous vs. moderate exercise to improve glucose metabolism in inactive women with polycystic ovary syndrome and insulin resistance: a pilot randomized controlled trial of two home-based exercise routines. F&S Rep. (2024) 5:80–6. doi: 10.1016/j.xfre.2023.12.004
27. Aktaş H, Uzun YE, Kutlu O, Pençe HH, Özçelik F, Çil E, et al. The effects of high intensity-interval training on vaspin, adiponectin and leptin levels in women with polycystic ovary syndrome. Arch Physiol Biochem. (2022) 128:37–42. doi: 10.1080/13813455.2019.1662450
28. Benham JL, Booth JE, Corenblum B, Doucette S, Friedenreich CM, Rabi DM, et al. Exercise training and reproductive outcomes in women with polycystic ovary syndrome: A pilot randomized controlled trial. Clin Endocrinol. (2021) 95:332–43. doi: 10.1111/cen.14452
29. Lopes IP, Ribeiro VB, Reis RM, Silva RC, Dutra de Souza HC, Kogure GS, et al. Comparison of the effect of intermittent and continuous aerobic physical training on sexual function of women with polycystic ovary syndrome: randomized controlled trial. J sexual Med. (2018) 15:1609–19. doi: 10.1016/j.jsxm.2018.09.002
30. Patten RK, McIlvenna LC, Levinger I, Garnham AP, Shorakae S, Parker AG, et al. High-intensity training elicits greater improvements in cardio-metabolic and reproductive outcomes than moderate-intensity training in women with polycystic ovary syndrome: a randomized clinical trial. Hum Reprod (Oxford England). (2022) 37:1018–29. doi: 10.1093/humrep/deac047
31. Philbois SV, Ribeiro VB, Tank J, Dos Reis RM, Gerlach DA, and Souza HCD. Cardiovascular autonomic modulation differences between moderate-intensity continuous and high-intensity interval aerobic training in women with PCOS: A randomized trial. Front Endocrinol. (2022) 13:1024844. doi: 10.3389/fendo.2022.1024844
32. Ribeiro VB, Kogure GS, Lopes IP, Silva RC, Pedroso DCC, de Melo AS, et al. Effects of continuous and intermittent aerobic physical training on hormonal and metabolic profile, and body composition in women with polycystic ovary syndrome: A randomized controlled trial. Clin Endocrinol. (2020) 93:173–86. doi: 10.1111/cen.14194
33. Sawyer BJ, Tucker WJ, Bhammar DM, Ryder JR, Sweazea KL, and Gaesser GA. Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. J Appl Physiol (Bethesda Md: 1985). (2016) 121:279–88. doi: 10.1152/japplphysiol.00024.2016
34. Bartlett JD, Close GL, MacLaren DP, Gregson W, Drust B, and Morton JP. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J sports Sci. (2011) 29:547–53. doi: 10.1080/02640414.2010.545427
Keywords: polycystic ovary syndrome, high-intensity interval training, moderate-intensity continuous training, meta-analysis, randomized controlled trials
Citation: Zhao Y, Long Y, Zhu H, He R, Chen Y and Li J (2025) High-intensity interval training versus moderate-intensity continuous training for polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Front. Endocrinol. 16:1672257. doi: 10.3389/fendo.2025.1672257
Received: 24 July 2025; Accepted: 06 October 2025;
Published: 16 October 2025.
Edited by:
Eduardo Piedrafita, Universidad San Jorge, SpainReviewed by:
Adriana Duaso-Iriarte, Saint George University, SpainAjith K, Yenepoya (Deemed to be University), India
Copyright © 2025 Zhao, Long, Zhu, He, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingrong Li, MjE0NzI5Mjg0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Yi Zhao1†
Yi Zhao1† Yu Long
Yu Long