- 1The Department of Endocrinology, Jiangsu University Affiliated People’s Hospital, Zhenjiang, Jiangsu, China
- 2Jiangsu Provincial Xuzhou Pharmaceutical Vocational College, Xuzhou, Jiangsu, China
Background: Antenatal depression, defined as clinically significant depressive symptoms occurring during pregnancy, has been suggested to increase the risk of gestational diabetes mellitus (GDM), a glucose intolerance disorder with onset or first recognition during pregnancy. However, evidence regarding its relationship with GDM remains inconsistent. This meta-analysis aimed to quantitatively assess the association between antenatal depression and the risk of GDM.
Methods: We systematically searched PubMed, Embase, Wanfang, and the Cochrane Library from inception to June 12, 2025, for observational studies reporting the association between depression during pregnancy and GDM. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using a random-effects model.
Results: A total of eight studies were included in the meta-analysis. The pooled analysis showed that maternal depression during pregnancy was significantly associated with an increased risk of GDM (OR = 1.37, 95% CI: 1.20-1.54). Subgroup analyses based on country, depression assessment tool, and study design showed consistent results. Sensitivity analyses confirmed the stability of the results. No significant publication bias was detected.
Conclusion: This meta-analysis suggests that maternal depression during pregnancy is associated with a significantly increased risk of developing GDM. Screening for depression in early pregnancy may represent a potential strategy to reduce the risk of GDM and improve maternal health outcomes.
Introduction
Gestational diabetes mellitus (GDM) is one of the most common metabolic complications of pregnancy, affecting approximately 6.1%-15.2% of pregnancies worldwide (1). GDM is associated with increased risks of adverse maternal and fetal outcomes, including preeclampsia, macrosomia, cesarean delivery, and a heightened lifetime risk of developing type 2 diabetes mellitus (2). Understanding modifiable risk factors for GDM is essential for early intervention and prevention.
Maternal mental health, particularly antenatal depression, has gained increasing attention in recent years (3, 4). Affecting up to 20% of pregnant women worldwide, antenatal depression is associated with poor prenatal care, substance use, and adverse neonatal outcomes including preterm birth and low birth weight (5, 6). Emerging evidence further suggests its potential link to impaired glucose metabolism, possibly mediated by hypothalamic–pituitary–adrenal (HPA) axis activation, elevated cortisol, inflammation, and unhealthy lifestyle behaviors (7–10). In addition, the genome-wide association study, which combined the metabolomics and genetics data of pregnant women, found that the rs1260326 mutation of the glucose kinase regulatory protein (GCKR) gene was significantly associated with insulin sensitivity in pregnant women of multiple ethnicities (11). This indicates that genetic and metabolic factors are the basis for glucose regulation during pregnancy.
Despite growing interest in the interplay between mental health and metabolic disorders during pregnancy, findings on the relationship between antenatal depression and GDM risk remain inconsistent (12–14). Some studies have reported a positive association, while others found no significant link after adjusting for confounders such as age or BMI (12–18). Previous meta-analyses was constrained by small samples, and the absence of subgroup or sensitivity analyses, limiting the robustness of their conclusions (19). Therefore, we conducted a meta-analysis to quantitatively synthesize available evidence on the association between maternal depression during pregnancy and the risk of GDM. This study aims to clarify whether prenatal depression is an independent risk factor for GDM. The findings may provide important implications for integrated prenatal care addressing both psychological and metabolic health.
Materials and methods
Search strategy
A comprehensive literature search was conducted to identify studies examining the association between maternal depression during pregnancy and the risk of GDM. The databases PubMed, Embase, Cochrane Library, and Wanfang databases were systematically searched from inception to June 12, 2025. The search terms included combinations of keywords and MeSH terms related to “antenatal depression,” “maternal depression,” “gestational diabetes mellitus,” and “GDM”. No language or geographic restrictions were applied. In addition, the reference lists of included articles and relevant reviews were manually screened to identify additional eligible studies.
Inclusion and exclusion criteria
Studies were included if they met the following criteria. Study design: cohort, case–control, or cross-sectional studies. Population: pregnant women. Exposure: depression diagnosed during pregnancy. Comparison: pregnant women without depression. Outcome: GDM incidence. Studies were excluded if they (1) included women with preexisting diabetes or depression; (2) lacked a comparison group; (3) are reviews, case reports, conference abstracts, or animal studies.
Data extraction and quality assessment
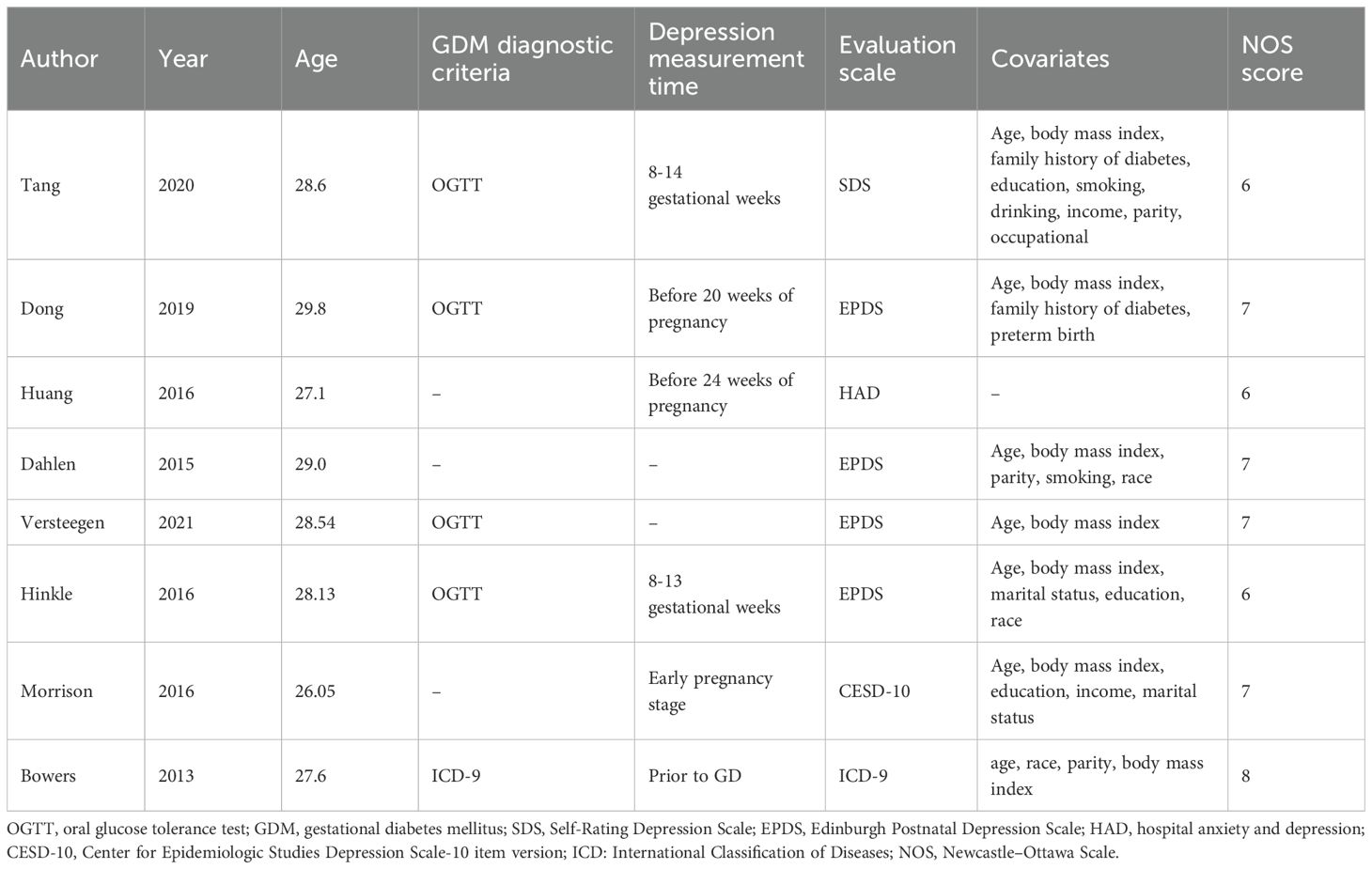
Two independent reviewers screened the titles and abstracts, assessed full-text articles for eligibility, and extracted relevant data using a standardized data extraction form. Extracted information included first author, year of publication, country, study design, age, sample size, diagnostic criteria for depression and GDM, adjusted confounders, and effect estimates (OR) with 95% CI. Discrepancies were resolved by discussion or by a third reviewer. The quality of included studies was assessed using the Newcastle–Ottawa Scale (NOS). Studies scoring ≥6 on NOS was classified as high-quality study (20).
Statistical analysis
Pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated to estimate the association between maternal depression during pregnancy and the risk of GDM based on the random-effects model. Heterogeneity across studies was assessed using Cochran’s Q test (p < 0.05 considered significant) and the I² statistic (with I² > 50% indicating substantial heterogeneity). Sensitivity analyses were performed by sequentially removing each study to evaluate the stability of the pooled estimates. We have also conducted subgroup analyses based on the information available, such as countries, depression scale, or study design. Publication bias was assessed through visual inspection of funnel plots and statistically tested using Egger’s regression test and Begg’s rank correlation test. All statistical analyses were conducted using STATA software, version 12.0 (StataCorp, College Station, TX, USA), with a two-tailed p-value < 0.05 considered statistically significant.
Result
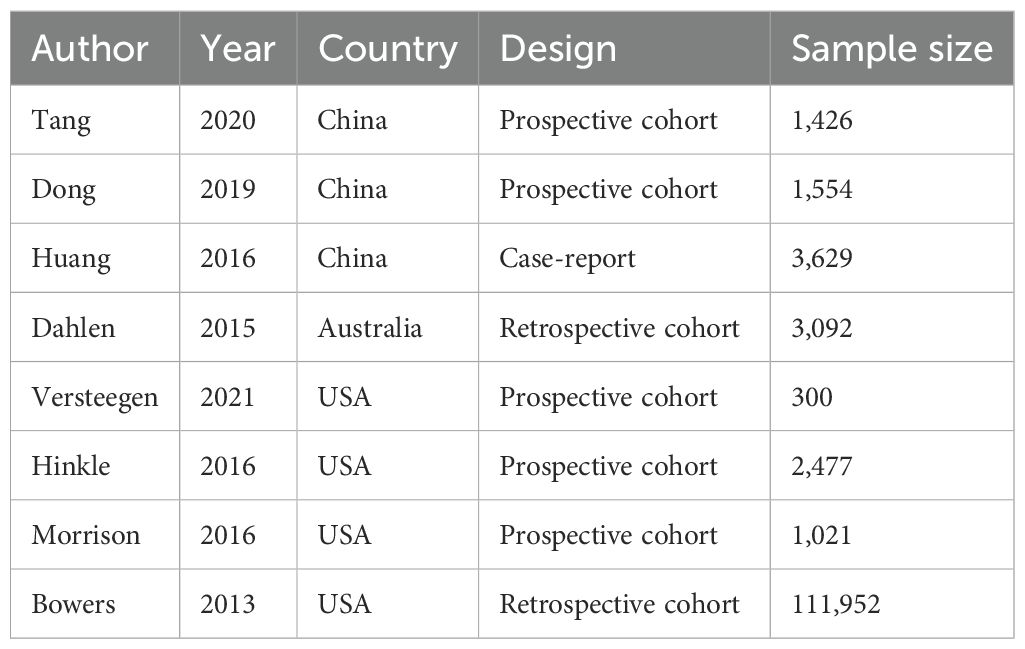
Study selection and study characteristics
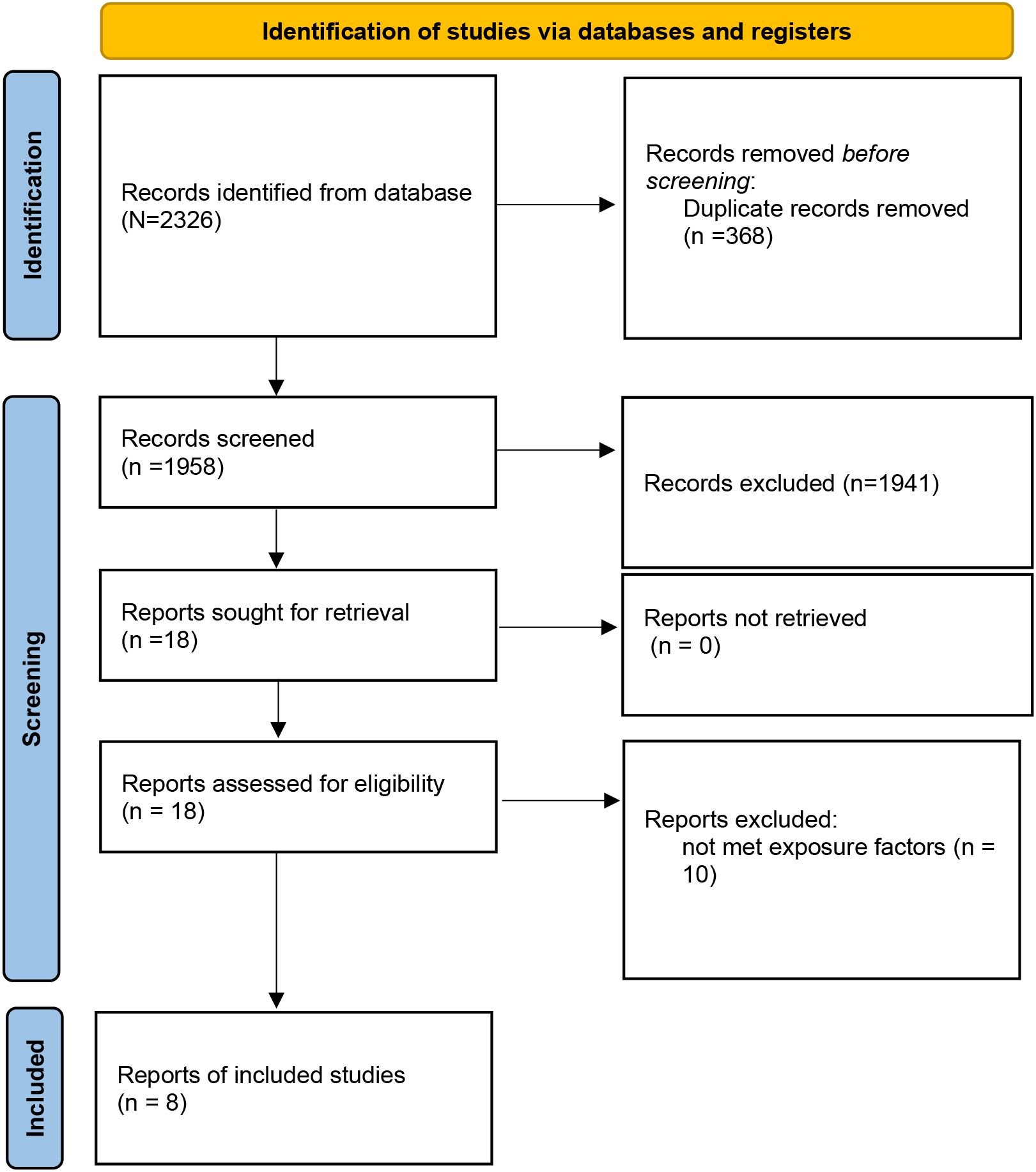
A total of 2,326 records were identified through database searching. After removing duplicates (n = 368), 1,958 articles remained for title and abstract screening. Based on inclusion and exclusion criteria, 18 full-text articles were assessed for eligibility, and finally, 8 studies (12–18, 21) were included in the meta-analysis (Figure 1). The included studies were published between 2013 and 2021, comprising a total of 125,451pregnant women. Study designs included five prospective cohort studies, one retrospective cohort study, and one case–control study. Depression was assessed using standardized tools such as the Edinburgh Postnatal Depression Scale (EPDS), Self-Rating Depression Scale (SDS), hospital anxiety and depression (HAD), International Classification of Diseases (ICD), or Center for Epidemiologic Studies Depression Scale-10 item version (CESD-10). In terms of participant characteristics, none of the included studies reported whether women received antidepressant treatment. Regarding established GDM risk factors among participants, only limited information was available across studies; age, body mass index, family history of diabetes, preterm birth, marital status, and miscarriage were included in two trials (16, 17). The methodological quality assessment of included studies according to NOS is shown in Tables 1, 2, and eight studies were high-quality.
Synthesis of results
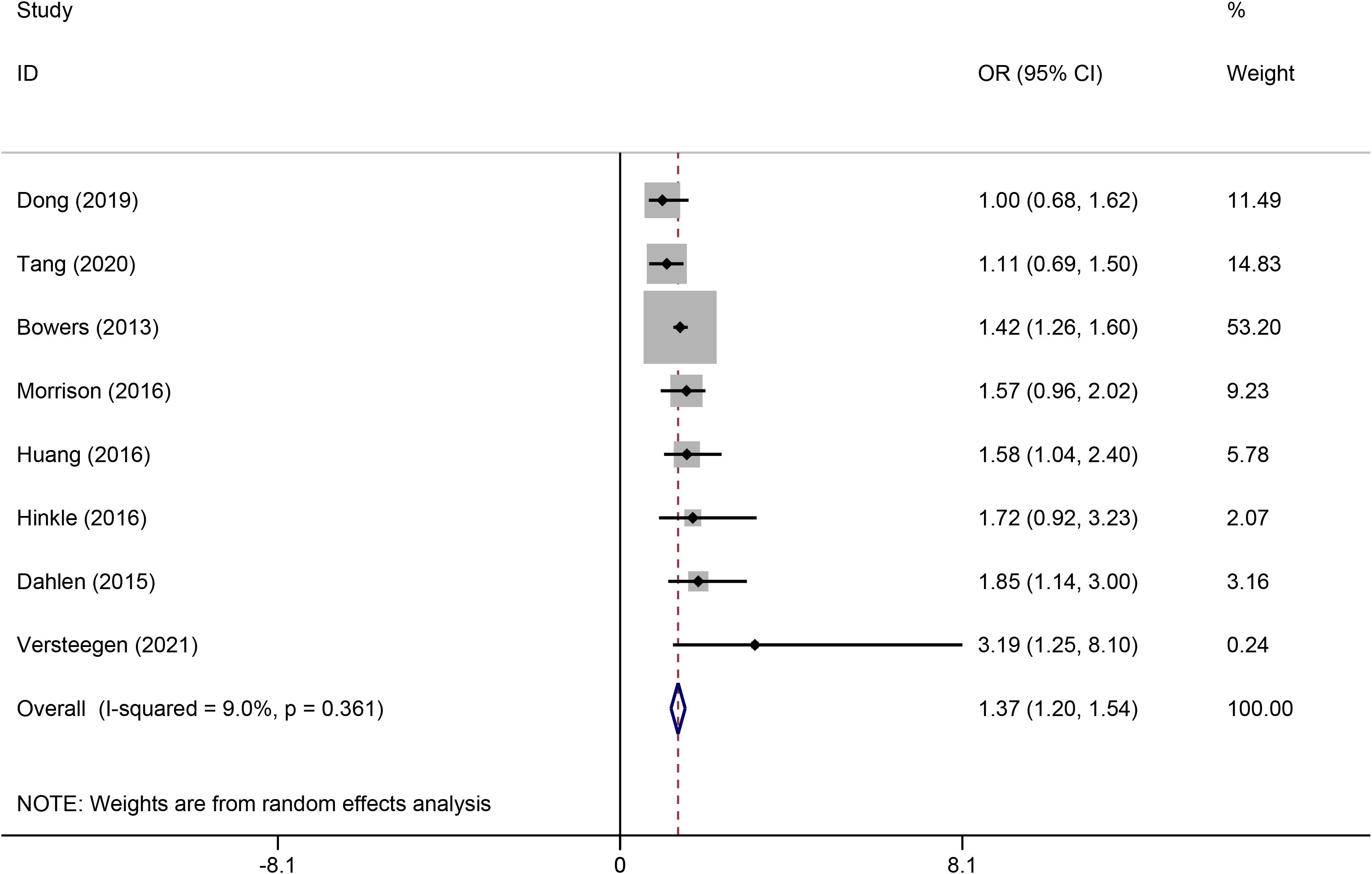
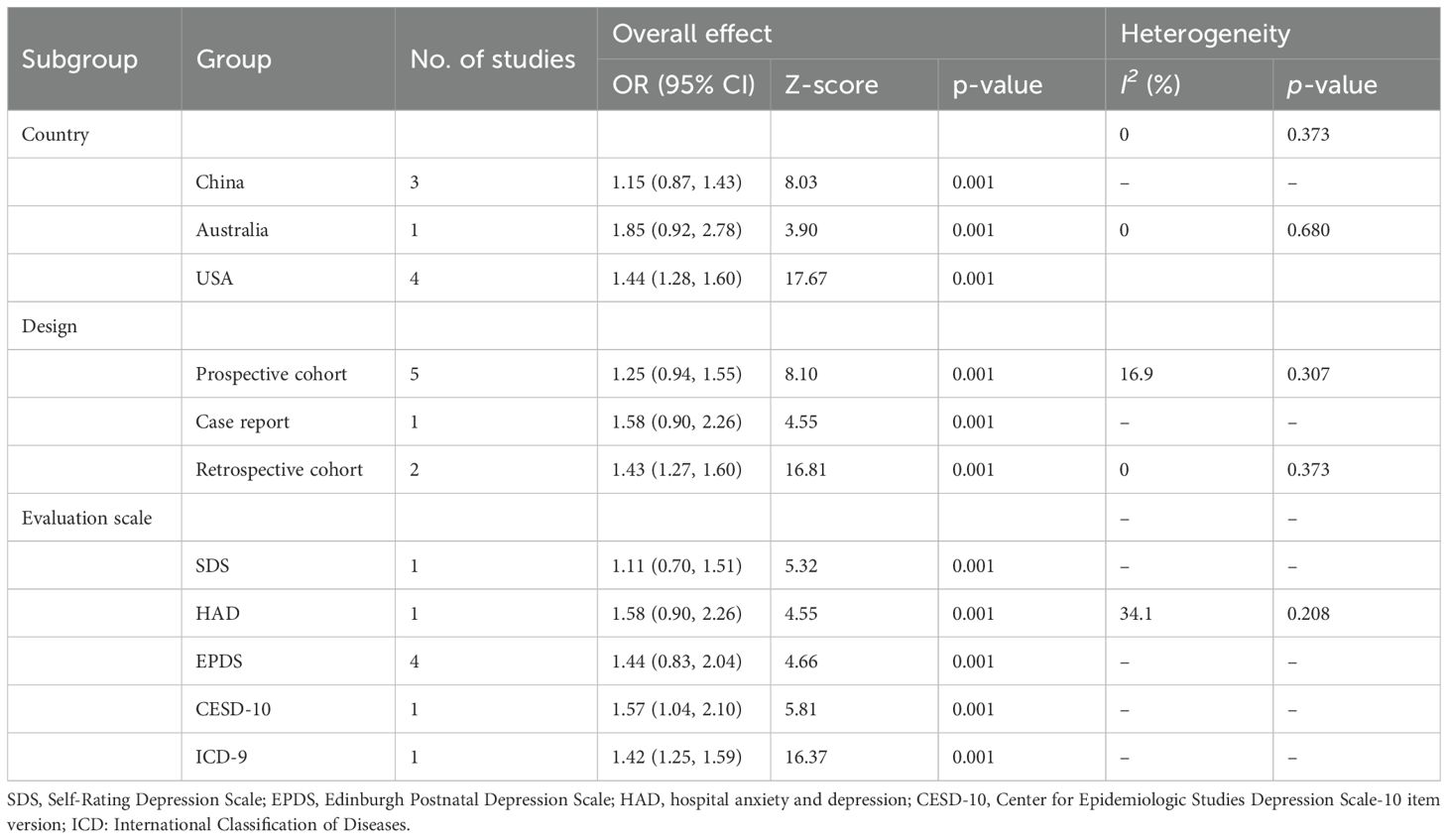
The pooled analysis showed that maternal depression during pregnancy was significantly associated with an increased risk of GDM. The combined effect estimate was OR = 1.37, 95% CI: 1.20-1.54, p < 0.001 (Figure 2), with a low heterogeneity (I2 = 9.0%; P = 0.361). In addition, subgroup analyses were stratified by country, depression assessment tool, and study design; the pooled results remained consistent, with no significant differences observed across subgroups (Table 3).
Sensitivity analysis
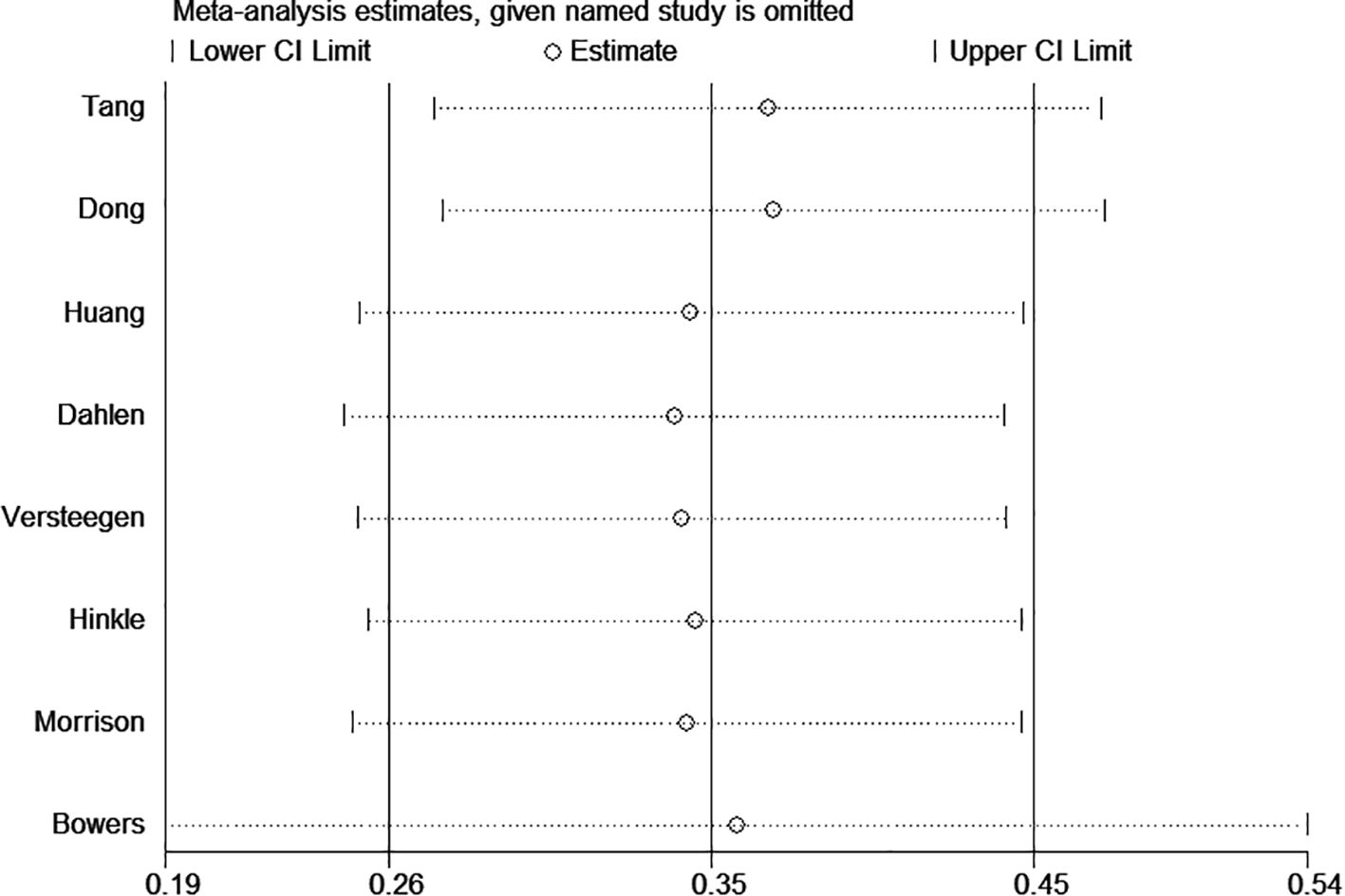
To assess the robustness of the pooled results, we performed a sensitivity analysis by sequentially excluding individual studies. Specifically, we excluded the study with the largest sample size (21) and the study with an excessively wide 95% confidence interval (18) (Figure 3). The exclusion of these studies did not materially alter the overall effect estimates, suggesting that the findings of this meta-analysis are stable and not driven by any single study.
Publication bias
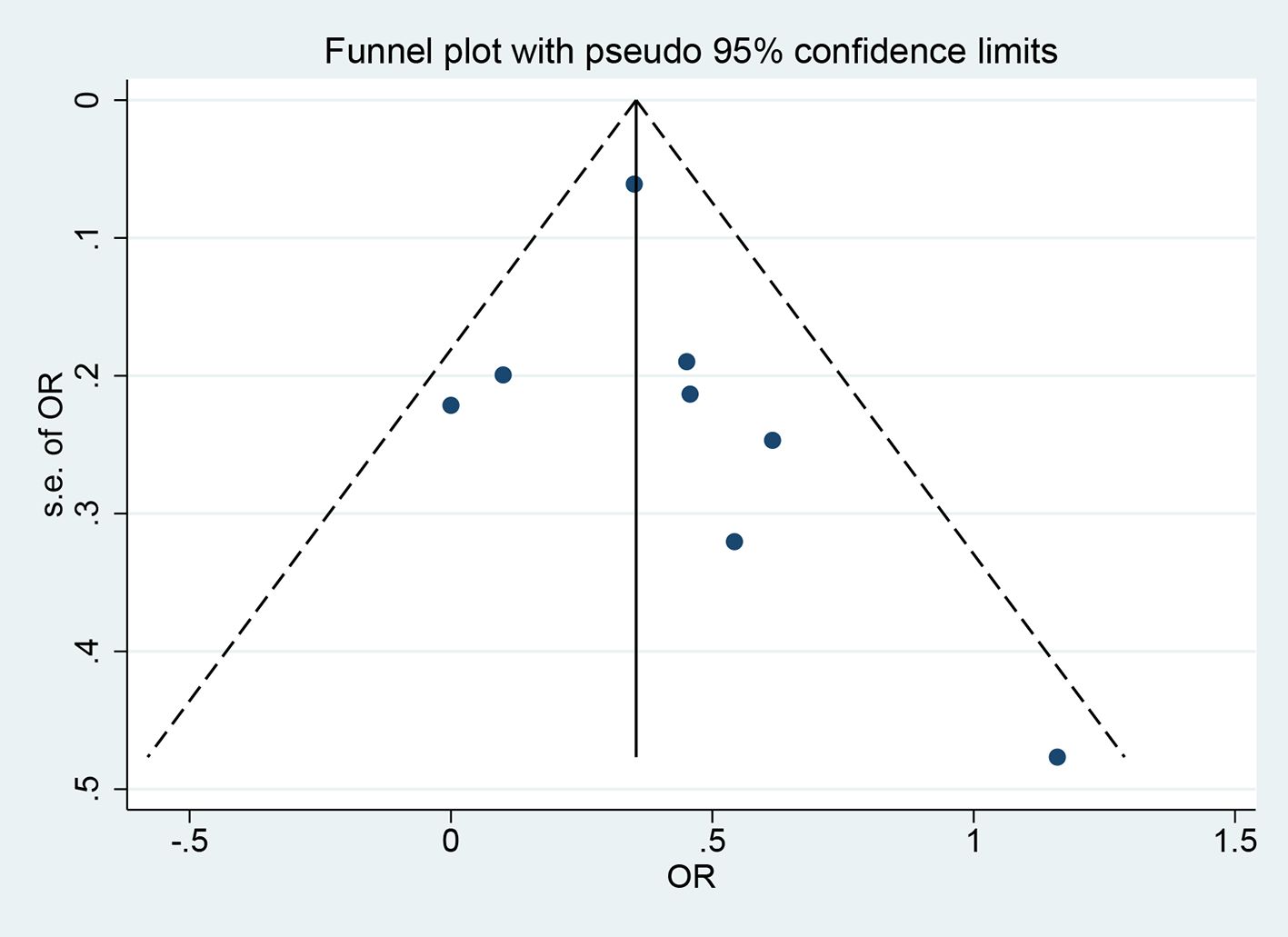
The funnel plot and Egger’s test were utilized to evaluate publication bias. Visual inspection of the funnel plot showed a roughly symmetrical distribution of the included studies (Figure 4). The Egger’s test (P = 0.509) and Begg’s test (P = 0.138) indicated no significant publication bias.
Discussion
In this meta-analysis, we found a significant association between maternal depression during pregnancy and an increased risk of GDM. Subgroup analyses based on country, depression assessment scale, and study design yielded consistent results, with no significant differences observed across subgroups. Sensitivity analyses, including the exclusion of the study with the largest sample size and the study with an excessively wide 95% confidence interval, did not affect the overall effect estimates. The pooled results across multiple studies indicated that women who experienced antenatal depression had a higher likelihood of developing GDM compared to those without depression (12, 14, 16). This finding was consistent across different geographic regions, and diagnostic methods for depression, suggesting a potentially independent relationship between maternal psychological status and glucose metabolism during pregnancy.
Our findings are in line with several recent cohort studies that reported a positive association between antenatal depression and increased GDM risk (12, 14). However, some studies failed to detect a significant link, possibly due to limited sample sizes or lack of adjustment for key confounding factors (15, 18). Notably, previous studies investigating GDM risk factors have primarily focused on biomedical or lifestyle determinants, often overlooking psychosocial variables such as antenatal depression. Our findings highlight the importance of including psychological factors in future research, which may provide a more comprehensive understanding of GDM etiology and support the development of integrative prevention strategies (22). Compared with the previous meta-analysis that included five studies (19), our study incorporated a total of eight studies. Among them, four studies were overlapping with those included in the previous analysis, while we also identified and included four additional studies that met our predefined inclusion criteria and were eligible. In addition, by integrating cohort studies from multiple databases, our research can more comprehensively assess the relationship between perinatal depression and the risk of GDM.
Several biological and behavioral mechanisms may underlie this association. Depression is known to activate the hypothalamic–pituitary–adrenal (HPA) axis, leading to elevated cortisol levels, which can induce insulin resistance and impair glucose tolerance (23, 24). In addition, depression is often accompanied by systemic inflammation and dysregulated immune responses, which have been implicated in the pathogenesis of GDM (25, 26). Lifestyle-related factors such as poor dietary habits, physical inactivity, reduced sleep quality, and non-adherence to prenatal care among women with depression may further contribute to metabolic disturbances during pregnancy (27, 28). Recent evidence suggests a potential association between antidepressant use during pregnancy and an increased risk of GDM (29, 30). A systematic review and meta-analysis conducted by Wang et al. revealed that exposure to antidepressant drugs during pregnancy significantly increases the risk of developing GDM (31). Since the studies we included did not provide data on antidepressant treatment, we were unable to assess its independent effect or conduct subgroup analysis. Further research is needed to clarify the relationship between antidepressant use during pregnancy and GDM, particularly regarding specific drug classes and their mechanisms of action. Healthcare providers should carefully consider the potential risks and benefits when prescribing antidepressants to pregnant individuals.
These findings highlight the importance of incorporating routine mental health screening into prenatal care, not only for psychological well-being but also for potential metabolic consequences. For example, routine screening for depressive symptoms in early pregnancy (for example with EPDS) could identify women at elevated metabolic risk; elevated antenatal depression scores might reasonably trigger earlier glucose evaluation (e.g., earlier OGTT), or more timely lifestyle guidance.
Several limitations should be acknowledged. First, most included studies were observational, and although adjusted for confounders, the possibility of residual confounding cannot be completely ruled out. Second, the included studies used different depression screening tools (e.g., EPDS, CESD-10, and HAD), which may introduce variability and subjectivity in exposure assessment. Third, GDM was diagnosed according to different criteria, which could contribute to outcome misclassification. Four, although statistical analyses did not suggest publication bias, the limited number of included studies means that bias cannot be entirely excluded. Finally, future research should prioritize prospective designs with standardized depression measures, unified GDM diagnostic criteria, and biomarker- or genetics-supported approaches to validate and further elucidate the antenatal depression–GDM link.
Conclusion
This meta-analysis suggested that antenatal depression is associated with an increased risk of GDM. Screening for depressive symptoms early in pregnancy may improve the identification of women at higher GDM risk and enable timely intervention. In addition, integrating routine depression screening into prenatal care could provide clinicians with an opportunity for earlier glucose monitoring, personalized prevention strategies, and improved maternal–fetal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ND: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LD: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MX: Data curation, Formal Analysis, Methodology, Writing – review & editing. TG: Data curation, Formal Analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Szmuilowicz ED, Josefson JL, and Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. (2019) 48:479–93. doi: 10.1016/j.ecl.2019.05.001
2. Sweeting A, Wong J, Murphy HR, and Ross GP. A clinical update on gestational diabetes mellitus. Endocr Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
3. Phua DY, Kee MZL, and Meaney MJ. Positive maternal mental health, parenting, and child development. Biol Psychiatry. (2020) 87:328–37. doi: 10.1016/j.biopsych.2019.09.028
4. Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. (2020) 117:26–64. doi: 10.1016/j.neubiorev.2017.07.003
5. Underwood L, Waldie K, D’Souza S, Peterson ER, and Morton S. A review of longitudinal studies on antenatal and postnatal depression. Arch women’s Ment Health. (2016) 19:711–20. doi: 10.1007/s00737-016-0629-1
6. Kaiyo-Utete M, Dambi JM, Chingono A, Mazhandu FSM, Madziro-Ruwizhu TB, Henderson C, et al. Antenatal depression: an examination of prevalence and its associated factors among pregnant women attending Harare polyclinics. BMC pregnancy childbirth. (2020) 20:197. doi: 10.1186/s12884-020-02887-y
7. Wang H, Yin W, Ma S, Wang P, Zhang L, Chen X, et al. Antenatal depression moderated the association between gestational diabetes mellitus and fetal hyperinsulinism. Am J obstetrics gynecology MFM. (2023) 5:101183. doi: 10.1016/j.ajogmf.2023.101183
8. Udvari EB, Volgyi K, Kekesi KA, Simon D, Hunyadi-Gulyas E, and Dobolyi A. Proteomic analysis of the maternal preoptic area in rats. Neurochem Res. (2019) 44:2314–24. doi: 10.1007/s11064-019-02755-y
9. Eskandari F, Salimi M, Binayi F, Abdollahifar MA, Eftekhary M, Hedayati M, et al. Investigating the effects of maternal separation on hypothalamic-pituitary-adrenal axis and glucose homeostasis under chronic social defeat stress in young adult male rat offspring. Neuroendocrinology. (2023) 113:361–80. doi: 10.1159/000526989
10. Rezaei Ardani A, Tara F, Hatami SB, Naghizadeh Kashani S, Emadzadeh M, and Nahidi M. Affective temperaments and the risk of gestational diabetes mellitus. Int J Psychiatry Clin practice. (2022) 26:23–8. doi: 10.1080/13651501.2021.1872648
11. Liu Y, Kuang A, Talbot O, Bain JR, Muehlbauer MJ, Hayes MG, et al. Metabolomic and genetic associations with insulin resistance in pregnancy. Diabetologia. (2020) 63:1783–95. doi: 10.1007/s00125-020-05198-1
12. Bowers K, Laughon SK, Kim S, Mumford SL, Brite J, Kiely M, et al. Obstetric and psychosocial risk factors for Australian-born and non-Australian born women and associated pregnancy and birth outcomes: a population based cohort study. BMC pregnancy childbirth. (2015) 15:292. doi: 10.1186/s12884-015-0681-2
13. Dong QQ, He WJ, Miao HZ, and Zhao QG. Correlation between antenatal depression symptoms and gestational diabetes: a prospective cohort study. Chin J Public Health. (2019) 35:107–10. doi: 10.11847/zgggws1119181
14. Hinkle SN, Buck Louis GM, Rawal S, Zhu Y, Albert PS, and Zhang C. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia. (2016) 59:2594–602. doi: 10.1007/s00125-016-4086-1
15. Huang WM, Chen XM, Chen SX, Xu LB, Wei XQ, You SH, et al. The influence of depressive mood during pregnancy on the mother and fetus during the perinatal period. Strait J Prev Med. (2016) 22:34–6.
16. Morrison C, McCook JG, and Bailey BA. First trimester depression scores predict development of gestational diabetes mellitus in pregnant rural Appalachian women. J psychosomatic obstetrics gynaecology. (2016) 37:21–5. doi: 10.3109/0167482X.2015.1106473
17. Tang Y, Lan X, Zhang Y, Zhou F, Cai C, Zhang J, et al. Anxiety and depression on gestational diabetes mellitus in early pregnancy. J Hygiene Res. (2020) 49:179–84. doi: 10.19813/j.cnki.weishengyanjiu.2020.02.002
18. Versteegen M, Bozlak CT, Larkin H, and Appleton AA. Maternal depression, adverse childhood experiences, and social support in relation to gestational diabetes risk: results from the Albany Infant and Mother Study (AIMS). BMC pregnancy childbirth. (2021) 21:335. doi: 10.1186/s12884-021-03814-5
19. Arafa A and Dong JY. Depression and risk of gestational diabetes: A meta-analysis of cohort studies. Diabetes Res Clin practice. (2019) 156:107826. doi: 10.1016/j.diabres.2019.107826
20. Barnett P, Matthews H, Lloyd-Evans B, Mackay E, Pilling S, and Johnson S. Compulsory community treatment to reduce readmission to hospital and increase engagement with community care in people with mental illness: a systematic review and meta-analysis. Lancet Psychiatry. (2018) 5:1013–22. doi: 10.1016/S2215-0366(18)30382-1
21. Bowers K, Laughon SK, Kim S, et al. The association between a medical history of depression and gestational diabetes in a large multi-ethnic cohort in the United States. Paediatric perinatal Epidemiol. (2013) 27:323–8. doi: 10.1111/ppe.12057
22. Canday M. Identifying gestational diabetes mellitus and assessing risk factors in affected women: a comprehensive study. Eur Rev Med Pharmacol Sci. (2024) 28:734–46. doi: 10.26355/eurrev_202401_35073
23. Bleker LS, van Dammen L, Leeflang MMG, Limpens J, Roseboom TJ, and de Rooij SR. Hypothalamic-pituitary-adrenal axis and autonomic nervous system reactivity in children prenatally exposed to maternal depression: A systematic review of prospective studies. Neurosci Biobehav Rev. (2020) 117:243–52. doi: 10.1016/j.neubiorev.2018.05.033
24. Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. (2009) 66:617–26. doi: 10.1001/archgenpsychiatry.2009.50
25. Zuo H, Chen X, Huang X, Benny C, Fu D, Xiu Q, et al. Using inflammatory biomarkers in early pregnancy to predict subsequent antenatal depression. J Affect Disord. (2025) 371:156–63. doi: 10.1016/j.jad.2024.08.220
26. Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Trans Psychiatry. (2021) 11:71. doi: 10.1038/s41398-021-01198-w
27. Osumi A, Kanejima Y, Ishihara K, Ikezawa N, Yoshihara R, Kitamura M, et al. Effects of sedentary behavior on the complications experienced by pregnant women: A systematic review. Reprod Sci (Thousand Oaks Calif). (2024) 31:352–65. doi: 10.1007/s43032-023-01321-w
28. Bagheri-Tirtashi A, Nasiri-Amiri F, Adib-Rad H, Nikbakht HA, Faramarzi M, and Kiani E. Effect of stress management based self-care counseling on glycemic control in women with gestational diabetes mellitus: a randomized controlled trial study. BMC pregnancy childbirth. (2025) 25:53. doi: 10.1186/s12884-025-07138-6
29. Dandjinou M, Sheehy O, and Berard A. Antidepressant use during pregnancy and the risk of gestational diabetes mellitus: a nested case-control study. BMJ Open. (2019) 9:e025908. doi: 10.1136/bmjopen-2018-025908
30. Lupattelli A, Barone-Adesi F, and Nordeng H. Association between antidepressant use in pregnancy and gestational diabetes mellitus: Results from the Norwegian Mother, Father and Child Cohort Study. Pharmacoepidemiology Drug safety. (2022) 31:247–56. doi: 10.1002/pds.5388
31. Wang XY, Ying XH, and Jiang HY. Antidepressant use during pregnancy and the risk for gestational diabetes: a systematic review and meta-analysis. J maternal-fetal neonatal medicine: Off J Eur Assoc Perinatal Medicine Fed Asia Oceania Perinatal Societies Int Soc Perinatal Obstet. (2023) 36:2162817. doi: 10.1080/14767058.2022.2162817
Keywords: maternal depression, pregnancy, gestational diabetes mellitus, meta-analysis, mental health
Citation: Du N, Dai L, Xu M and Guan T (2025) The impact of maternal depression during pregnancy on the risk of gestational diabetes mellitus: a meta-analysis. Front. Endocrinol. 16:1672527. doi: 10.3389/fendo.2025.1672527
Received: 24 July 2025; Accepted: 15 September 2025;
Published: 06 October 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Mujde Canday, Kafkas University, TürkiyeGeorgios I. Tsironikos, University of Ioannina, Greece
Copyright © 2025 Du, Dai, Xu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Du, MTUyNjIxNjgwNDZAMTYzLmNvbQ==
 Nan Du
Nan Du Limin Dai1
Limin Dai1