- 1Department of Biological Sciences, University of Calgary, Calgary, AB, Canada
- 2Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
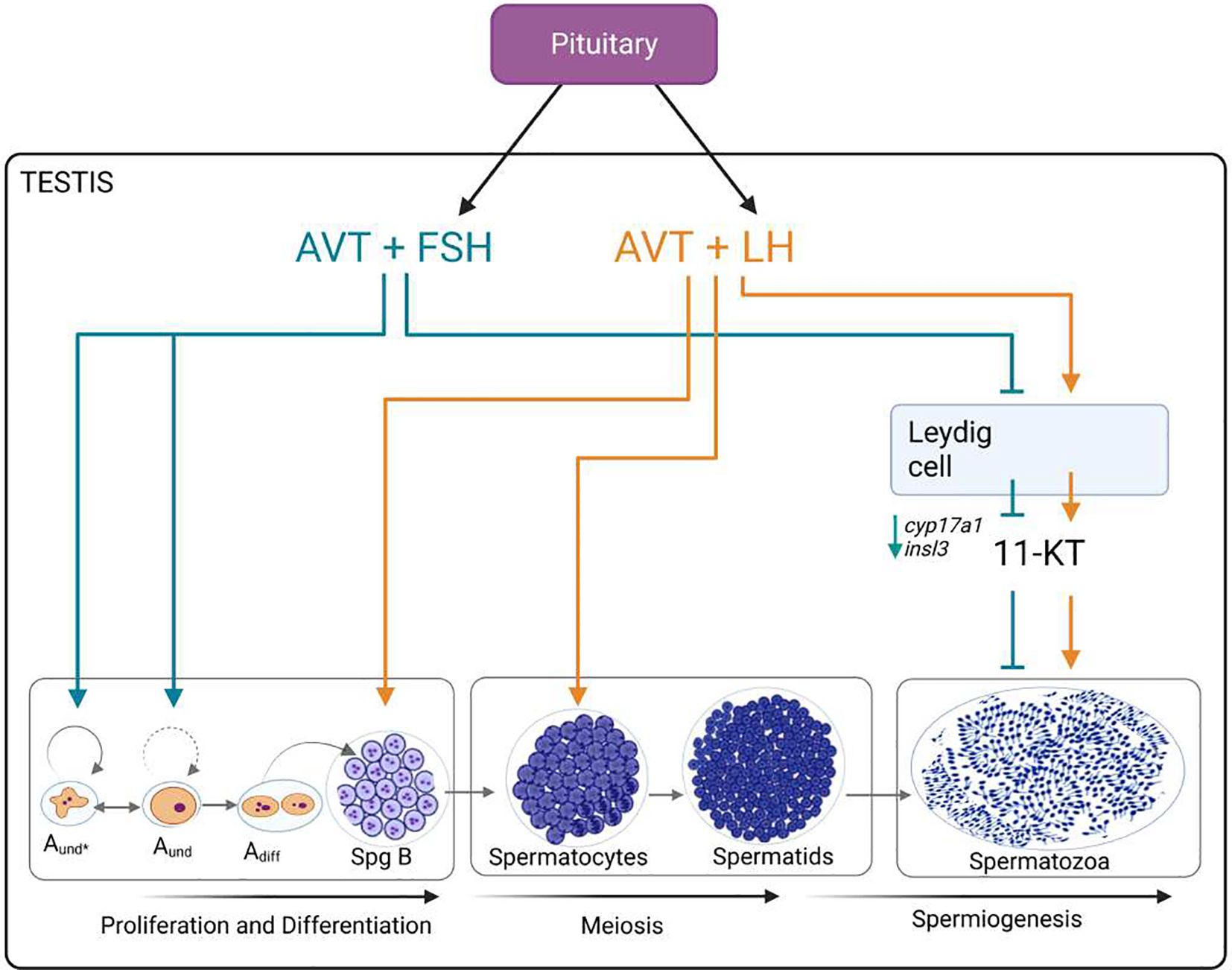
This study investigated the role of vasotocin (AVT) in the multifaceted regulation of spermatogenesis in zebrafish, with a focus on its interaction with gonadotropin hormones. Using an ex vivo cultured testis, we explored the interaction of AVT with gonadotropins, LH and FSH, to regulate different stages of germ cell development. In this study, we used recombinant zebrafish FSH and hCG to stimulate the LH-induced response due to the limited availability of zebrafish LH, as it is known to exert similar effects on testicular function. Treatment with AVT enhanced FSH-induced proliferation of early undifferentiated spermatogonia (Aund) germ cells, as well as promoting the proliferation of later-stage type B germ cells when combined with LH/hCG. Additionally, AVT significantly decreased FSH-induced 11-ketotestosterone (11-KT) production and Leydig-derived factors, including cyp17a1 and insl3, without significantly affecting LH/hCG-induced androgen production. Based on these findings, we hypothesize that in the presence of FSH, AVT drives early germ cell proliferation and differentiation while simultaneously inhibiting premature progression through spermiogenesis. This stage-specific modulation of gonadotropin signaling by AVT underscores its dual role in fine-tuning testicular function and germ cell maturation in male zebrafish. Overall, our findings suggest that AVT contributes to the complex multifactorial network regulating spermatogenesis in zebrafish.
Highlights
● Vasotocin enhances FSH-induced proliferation of early undifferentiated spermatogonia (Aund) and, when combined with LH/hCG, promotes proliferation at more advanced germ cell stages (SpgB).
● Vasotocin, in the presence of gonadotropins, impairs steroidogenic enzyme transcript levels (cyp17a1) consistent with changes in 11-ketotestosterone production.
● Hypothesis: AVT contributes to the multifactorial regulation of spermatogenesis in a stage-specific manner. In the presence of FSH and LH, AVT promoted early germ cell proliferation and differentiation while suppressing premature spermiogenesis.
1 Introduction
The hypothalamic-pituitary-gonadal (HPG) axis controls testicular function by regulating the synthesis and release of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) through neurohormonal signals. Gonadotropins play a crucial role in modulating steroidogenesis and spermatogenesis by acting directly on the testes. In mammals, FSH and LH have distinct functions: FSH promotes germ cell development by acting primarily on Sertoli cells through FSH receptors (FSHR), while LH stimulates steroidogenesis by binding to LH receptors (LHCGR) on Leydig cells. The activity of gonadotropins in fish is less clearly differentiated, with evidence demonstrating overlapping receptor specificity (1, 2). Generally, FSH predominates in early spermatogenesis by promoting spermatogonia proliferation and differentiation, whereas LH is more involved in the later stages, regulating spermiogenesis and sperm release (3). In the African catfish (Clarias gariepinus), both FSH and LH receptor transcripts are expressed in Leydig cells, whereas Sertoli cells express only FSHR (4). This pattern is observed in other fish species, including zebrafish (5–9). FSH regulates a number of somatic cell-derived factors crucial for germ cell development, acting largely independent of androgen signaling (10–13). In addition, both FSH and LH interact with gonadal-produced neuropeptides, including gonadotropin-inhibitory hormone (GnIH) and gonadotropin-releasing hormone (GnRH), indicating complex multifactorial regulation of spermatogenesis (14–17). In zebrafish, GnIH inhibits gonadotropin-induced spermatogenesis by suppressing FSH- and hCG-induced androgen production (18, 19). Similarly, it was shown that both GnRH isoforms (GnRH2 and GnRH3) inhibited FSH-induced spermatogenesis, although their effect on hCG-induced spermatogenesis was less pronounced (17). There is evidence that the neurohypophysial hormone arginine vasotocin (AVT) may also be a player in the regulation of spermatogenesis. In this context, multiple studies have demonstrated the expression of AVT in the testes of several fish species, including zebrafish (20–26). In addition, vasotocin receptors were also found to be expressed in the testes of different species, including catfish and zebrafish (24, 26). In vitro experiments reported that AVT stimulated 11-ketotestosterone and testosterone in the cichlid fish Cichlasoma dimerus and the rainbow trout Oncorhynchus mykiss, respectively (23, 27). Consistent with these findings, we recently demonstrated that treatment of cultured adult zebrafish testes with zebrafish AVT increased 11-KT levels and spermatozoa production while reducing type B spermatogonia proliferation (26). These findings indicate that AVT acts directly on spermatogenesis in an androgen-dependent manner. However, only a limited number of studies have investigated the interaction between vasotocin and gonadotropins in the gonads, and those available primarily focus on the regulation of pituitary gonadotropin production. Ramallo et al. (23) demonstrated that vasotocin modulates pituitary secretion of LH and FSH in vitro in a dose-dependent and hormone-specific manner; LH exhibited a triphasic response and FSH levels increased progressively with rising vasotocin concentrations. Also, in female Asian stinging catfish (Heteropneustes fossilis), in vitro treatment of ovarian follicles with hCG stimulated vasotocin production; additionally, hCG injection enhanced vasotocin levels in the brain, ovary, and plasma (28). These results demonstrate the involvement of vasotocin in the control of reproduction. However, the downstream physiological effects of AVT-gonadotropin interactions in the male gonads, testicular function and spermatogenesis, remain largely unexplored.
Building on our previous work, we investigated how arginine vasotocin (AVT) affects spermatogenesis and androgen production in the presence of gonadotropins. Using an ex vivo zebrafish testis culture, we examined the interactions of LH/hCG and FSH with AVT by assessing germ cell development, proliferation of early spermatogonia, androgen synthesis, and transcript levels.
2 Material and methods
2.1 Hormones and chemicals
The zebrafish-specific (Arg8)-Vasotocin peptide was purchased from ChinaPeptides Co.,Ltd (Shanghai, China) (CYIQNCPRG with C1-C6 bridge). This hormone was reconstituted with 1x PBS and then diluted with filter-sterilized Leibovitz’s L-15 Medium (Thermo Fisher Scientific, Gibco®, Canada) to reach a final working concentration of 10 nM.
Recombinant zebrafish follicle-stimulating hormone (rzfFSH) was purchased from U-Protein Express B.V. (Utrecht, The Netherlands). The hormone was reconstituted with 1x PBS without preservative, aliquoted, and stored at -20 °C until use. Based on previous studies in zebrafish (12, 19), we initially tested a concentration of FSH of 100 ng/ml without seeing any effect on spermatogenesis or gene expression, possibly due to a different batch of the FSH hormone being produced. We therefore decided to increase the concentration to 200 ng/ml. Hence, for ex vivo experiments, the hormone was diluted with L-15 medium to reach the final concentration of 200 ng/ml.
Human chorionic gonadotropin (hCG) was purchased from Sigma-Aldrich (Oakville, ON, Canada), reconstituted with ultrapure distilled water, and stored at -20 °C until use. The hormone was diluted with L-15 medium to reach the final concentration of 10 IU/ml for tissue culture.
2.2 Experimental animals
Adult zebrafish (Danio rerio, TL strain) were bred and raised in the Department of Biological Sciences aquatic facility at the University of Calgary (Alberta, Canada). Fish were maintained in a partially recirculating system with water maintained at 28 °C, pH 7.6, and conductivity 750 μS, and were fed twice daily with the adult commercial food Zeigler® (Pentair Aquatic Habitats). Male zebrafish between 6 and 10 months old were used for this study. Fishes were fasted for 18 hours before all experiments to standardize physiological conditions and reduce contamination from intestinal contents during testis dissection and ex vivo culture preparation. All procedures described here follow the guidelines of the Canadian Council on Animal Care (CCAC) and have been approved by the University of Calgary Animal Care Committee (protocol# AC24-0042).
2.3 Ex vivo testis culture
Testes were dissected from zebrafish and incubated with L-15 medium using an ex vivo system, as described by Leal et al. (29). Testes were placed on a nitrocellulose membrane positioned on 2% agarose blocks surrounded by the culture medium. For each treatment, testes were incubated with L-15 alone to assess basal spermatogenesis or with L-15 supplemented with rzfFSH, LH/hCG, or AVT, alone and in combination (n=6-8). Culture plates were kept for 7 days at 28 °C in a humidified controlled environment to evaluate the effect of AVT on gonadotropin-induced spermatogenesis and androgen production, and 48 hours for transcript abundance measurement. A complete medium change was performed on day three during the 7-day incubation period.
2.4 Histology and morphometric analysis
To analyze testicular morphology and quantify the distribution of different stages of germ cells after 7 days of culture, testes were collected and fixed overnight in 1.6% paraformaldehyde, 2.5% glutaraldehyde, and PBS 1× (pH: 7.2), as described previously (19, 26). Tissues were washed in PBS 1× and dehydrated in ethanol series the following day. Testes explants were then embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany), and histology blocks were sectioned using a Reichert-Jung 2040 Autocut Rotary Microtome. Slices of 3 μm thickness were cut, stained with 0.1% Toluidine Blue, and mounted using Kristalon Mounting Medium (Sigma-Aldrich, Burlington, MA, USA). Bright-field images of five images per sample were taken, and an area of 20000 µm2 was used to quantify the germ cells. In histology sections, germ cells were counted and classified into developmental stages: spermatogonia type Aund*+Aund (where the asterisk indicates stem cell), spermatogonia type Adiff, spermatogonia type B, spermatocytes, and spermatozoa. Cell classification was based on morphological criteria according to Schulz et al. (3), enabling identification of testicular germ cell types by distinct characteristics observed under microscopy.
2.5 BrdU proliferation assay
To evaluate the spermatogonia germ cells proliferation rate, we carried out immunohistochemistry using BrdU (Bromodeoxyuridine/5-bromo-2’-deoxyuridine), a thymidine analog that incorporates into replicating DNA (S-phase of cell cycle). A pulse of 100 μg/mL BrdU was given to testes incubated in the ex vivo system 6 hours before the collection of the 7-day culture period. The protocol followed was described by Zanardini et al. (26). The BrdU labeling index was calculated as the percentage of BrdU-positive cells relative to the total number of spermatogonia, including type A undifferentiated (Aund), type A differentiated (Adiff), and type B spermatogonia. Bright-field images were captured from five randomly selected areas per sample, each covering 20,000 µm² for germ cell quantification, consistent with the approach used in histological analysis.
2.6 RNA extraction, cDNA synthesis and qPCR
Molecular analyses were conducted using quantitative PCR (qPCR) following a protocol adapted from previous studies (26). After a 48-hour ex vivo incubation, testes were rapidly frozen for RNA preservation. Total RNA was extracted using TRIzol reagent (Invitrogen, Canada), and RNA quality and quantity were assessed via Nanodrop 2000 spectrophotometry (Thermo Scientific, Waltham, MA, USA). To eliminate genomic DNA contamination, samples were treated with RNase-free DNase I (Thermo Fisher Scientific) prior to cDNA synthesis using the iScript™ cDNA Synthesis Kit (Bio-Rad, Mississauga, Ontario, Canada). qPCR reactions utilized SsoFast Eva Green Supermix (Bio-Rad) and were performed in duplicate. Gene expression levels were normalized using eef1a1l1 (elongation factor 1 alpha 1, like 1) as the reference gene, with relative quantification calculated through the 2−ΔΔCT method. Primer sequences were selected based on previously published work (26). In the table, the primer labeled as ‘lhr’ corresponds to the luteinizing hormone receptor gene, whose current official gene symbol is lhcgr.
2.7 Quantification of androgen release by ELISA
After a 7-day incubation period, the culture medium was collected to assess androgen release. The concentration of 11-ketotestosterone (11-KT) secreted by testes under different treatment conditions was quantified using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (Cayman Chemicals, Ann Arbor, MI, USA). Measurements of the enzymatic reaction product were performed with a SpectraMax i3 microplate reader (Molecular Devices, San Jose, CA, USA) set to an absorbance wavelength of 412 nm. Prior to culture, testes were weighed individually, and 11-KT levels were normalized to tissue mass and expressed as picograms per milligram.
2.8 Statistical analysis
All raw data from each experiment and endpoint were first assessed for normal distribution using the Shapiro-Wilk test, and outliers were evaluated and removed using the ROUT method (30). Data from histology were analyzed using unpaired T-tests. Data from 11-KT measurement, transcript level, and immunohistochemistry were analyzed with one-way ANOVA followed by Tukey’s multiple comparison test. Differences were considered significant when P<0.05. All statistical tests were performed using GraphPad Prism 8.0 software (GraphPad Software Inc., La Jolla, CA, USA).
3 Results
3.1 Effect of AVT on gonadotropin-induced spermatogenesis
In the present study, we investigated the effect of vasotocin (10 nM) on LH- and FSH-induced spermatogenesis using morphometric analysis. Our previous work identified 10 nM AVT as the optimal concentration for directly stimulating spermatogenesis over 7 days of treatment (26). Representative histological sections are shown for testes treated with FSH or LH/hCG, alone or in combination with AVT, primarily to illustrate the relative abundance of spermatozoa cysts, whereas the quantitative analysis includes all treatment groups (Control, AVT, FSH, LH/hCG, and combinations) (Figure 1). Treatment with FSH (200 ng/ml) alone significantly increased the number of undifferentiated spermatogonia (Aund*+Aund) while reducing differentiated spermatogonia (Adiff) (Figure 1A). By contrast, co-treatment with AVT and FSH significantly increased the number of Aund*+Aund, Adiff, and type B spermatogonia (SpgB) compared with either treatment alone (Figure 1A). LH/hCG (10 IU), with or without AVT, had no effect on early spermatogonial stages (Aund*+Aund, Adiff, SpgB) (Figure 1B).
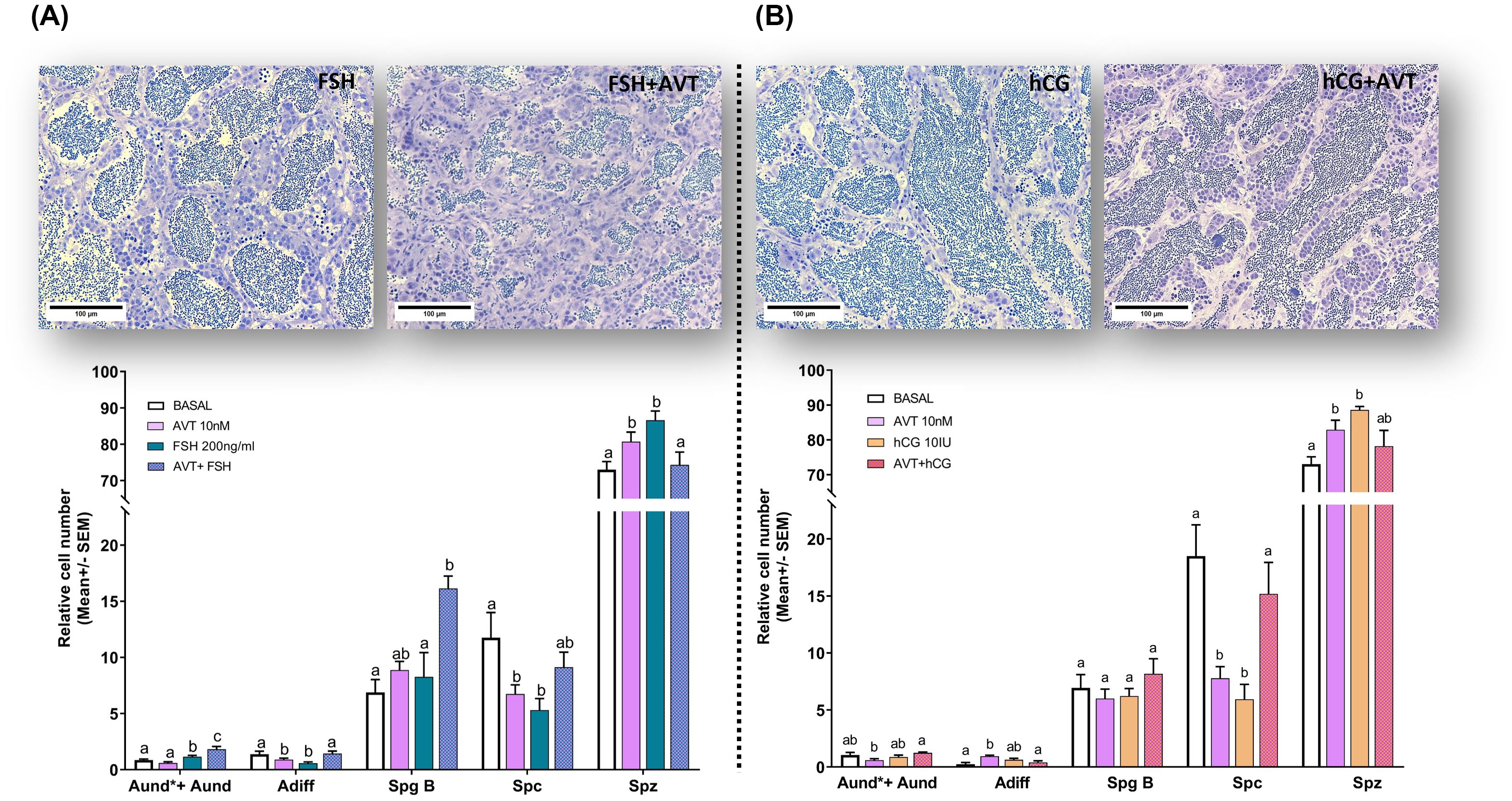
Figure 1. Effect of vasotocin on (A) FSH- and (B) LH/hCG-induced spermatogenesis. Representative histological sections (upper panels) are shown for testes treated with FSH (200 ng/ml) or LH/hCG (10 IU), alone or in combination with AVT (10 nM), for 7 days in ex vivo culture. Quantitative morphometric data (lower panels) summarize relative cell numbers (mean ± SEM; normalized to control) for undifferentiated spermatogonia (Aund*+Aund), differentiating spermatogonia (Adiff), type B spermatogonia (Spg B), spermatocytes (Spc), and spermatozoa (Spz), following treatments with L-15 medium (Control), AVT (10 nM), FSH (200 ng/ml), LH/hCG (10 IU), or the indicated combinations. Different letters denote significant differences among treatments within each cell type (p < 0.05; one-way ANOVA, Tukey’s test). Scale bars: 100 μm.
Treatment with AVT or FSH alone reduced spermatocyte numbers, whereas their combined administration produced a modest but non-significant increase. In contrast, either treatment alone increased spermatozoa proportion, while co-treatment reversed this effect, resulting in a reduction (Figure 1A). Treatment with either LH/hCG or AVT alone significantly reduced the number of spermatocytes and increased the number of spermatozoa. In contrast, combined treatment with AVT and LH/hCG increased the number of spermatocytes but led to a non-significant reduction in spermatozoa compared to single treatments (Figure 1B).
3.2 Effect of AVT on early germ cell proliferation
To assess spermatogonia proliferation, we measured BrdU mitotic index following treatment with FSH or LH/hCG alone, and in combination with AVT (Figure 2). FSH alone did not alter the mitotic index for Aund*+Aund or Adiff but significantly increased proliferation of SpgB (Figure 2A). Co-treatment with AVT and FSH further enhanced the proliferation of Aund*+Aund compared to FSH alone while promoting SpgB proliferation at a similar level as FSH treatment alone (Figure 2A). LH/hCG alone had no effect on the mitotic index of diploid spermatogonia (Figure 2B). In contrast, co-treatment with AVT and LH/hCG significantly increased SpgB proliferation compared to either treatment alone (Figure 2B).
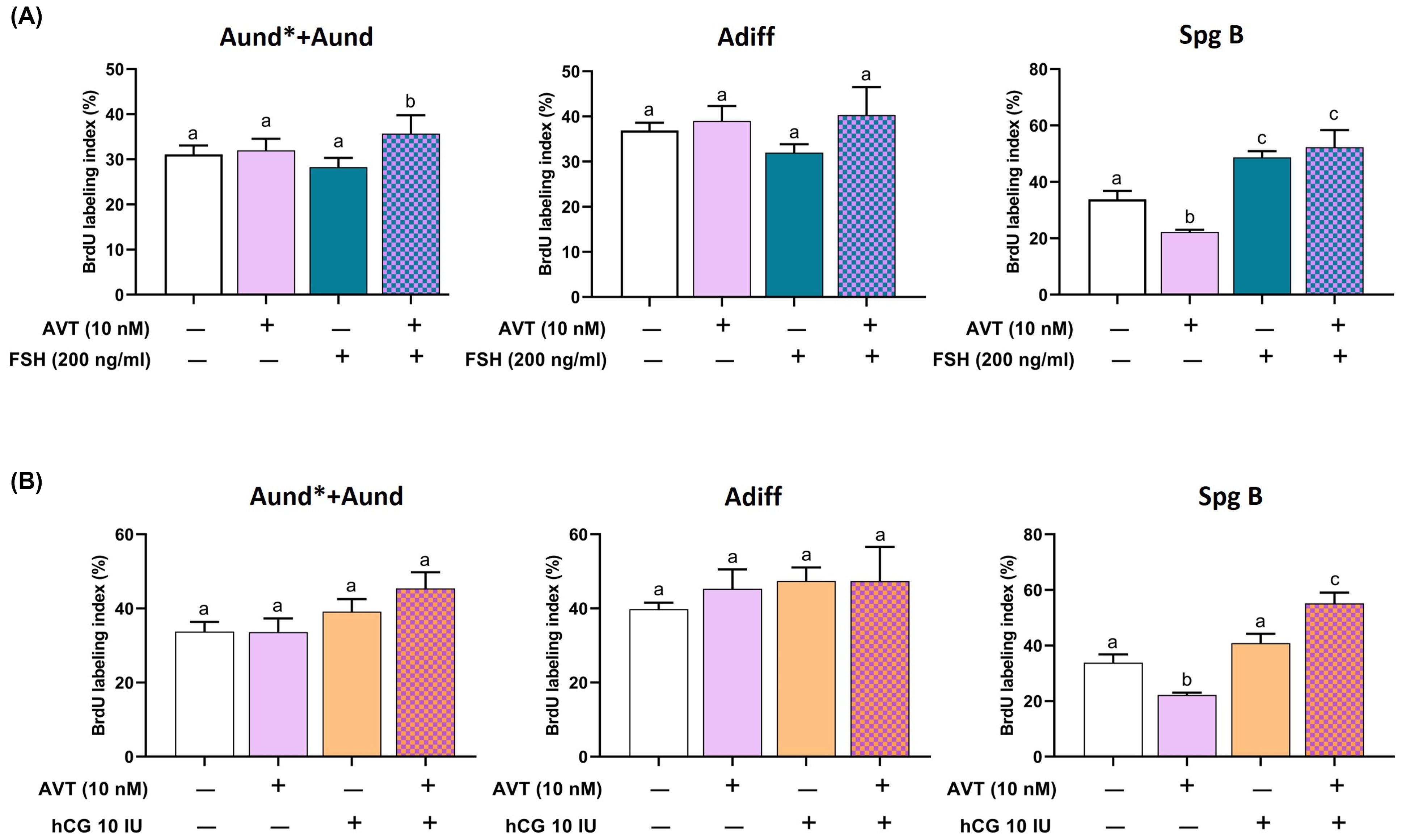
Figure 2. Proliferation activity of pre-meiotic germ cells in zebrafish testis explants treated with AVT in combination with FSH or LH/hCG. Testis were treated for 7 days ex vivo with AVT (10 nM), FSH (200 ng/ml), or LH/hCG (10 IU), alone or in combination, and analyzed for BrdU incorporation to assess spermatogonial proliferation. BrdU labeling index is presented as a percentage of BrdU-positive cells vs the total number of cells, per each cell type analyzed. (A) BrdU labeling index (%) for undifferentiated spermatogonia (Aund* + Aund), differentiating spermatogonia (Adiff), and type B spermatogonia (Spg B) following treatment with AVT and/or FSH. (B) Same cell populations analyzed following treatment with AVT and/or LH/hCG. Different letters indicate statistically significant differences among treatment groups within each cell type (p < 0.05, Tukey’s multiple comparison test).
3.3 Effect of AVT on gonadotropin-induced androgen production
Androgens are critical for the final stages of germ cell development and spermatogenesis. To evaluate androgen release, we measured 11-ketotestosterone (11-KT), a non-aromatizable androgen, following treatment of zebrafish testes with AVT, FSH, and LH/hCG, individually and in combination (Figure 3). Both AVT and FSH alone significantly increased 11-KT secretion; however, combined treatment with FSH and AVT markedly reduced 11-KT levels compared to either treatment alone (Figure 3A). Similarly, LH/hCG alone stimulated 11-KT production, whereas the addition of AVT did not modify the LH/hCG-induced response (Figure 3B).
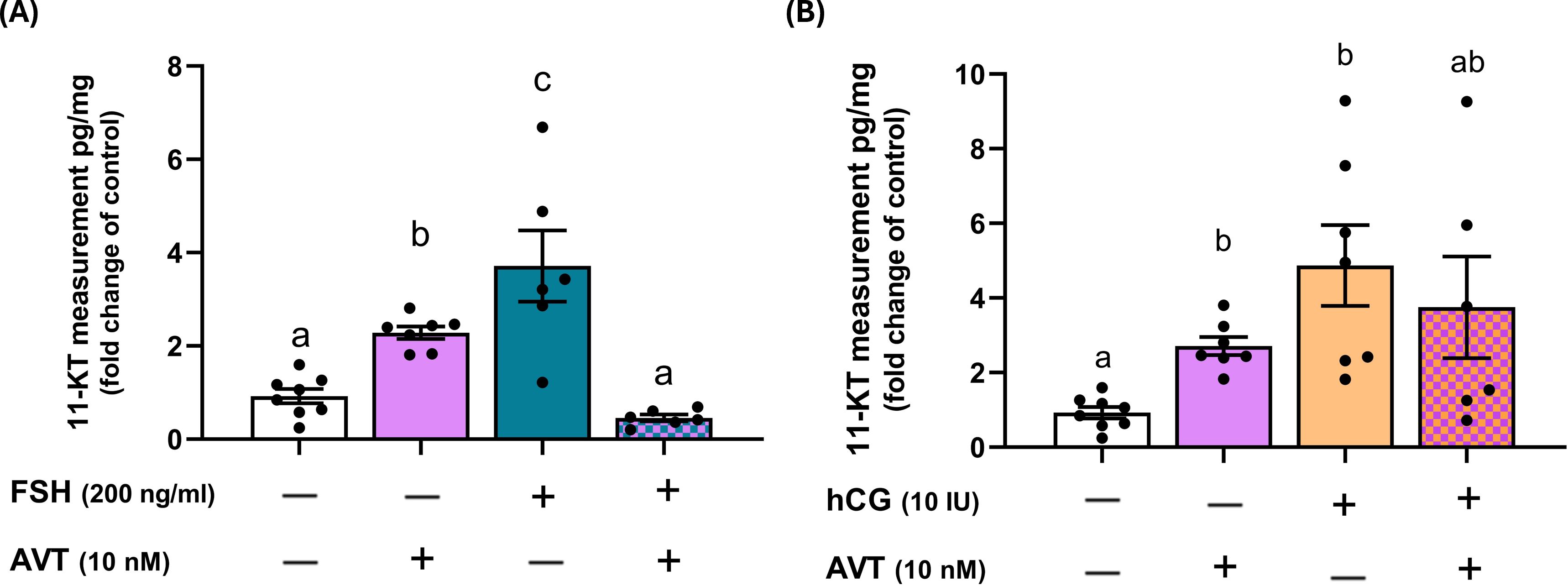
Figure 3. Effects of AVT on gonadotropin-induced 11-ketotestosterone (11-KT) production in zebrafish testis explants. Testes were cultured and treated with AVT (10 nM), either alone or in combination with (A) FSH (200 ng/ml) or (B) LH/hCG (10 IU). 11-KT concentrations were measured in the culture medium and expressed as fold changes relative to their respective contralateral control testes (pg/mg of tissue). Different letters denote statistically significant differences among treatment groups (p < 0.05, ANOVA followed by Tukey’s multiple comparison test).
3.4 Effect of AVT, FSH, and LH/hCG on selected transcript levels
To further examine the effects of AVT on germ cells, we analyzed transcript levels of germ cell-specific markers: piwil1 (type A undifferentiated and differentiated spermatogonia), sycp3 (spermatocytes), and cimap1b (spermatids and spermatozoa). AVT treatment did not alter the FSH-induced transcript levels of piwil1, sycp3, or cimap1b (Figure 4A). In contrast, co-treatment with AVT and LH/hCG increased the expression of sycp3 and cimap1b, consistent with enhanced spermatocytes and haploid cells, partially aligning with histological observations (Figure 4B).
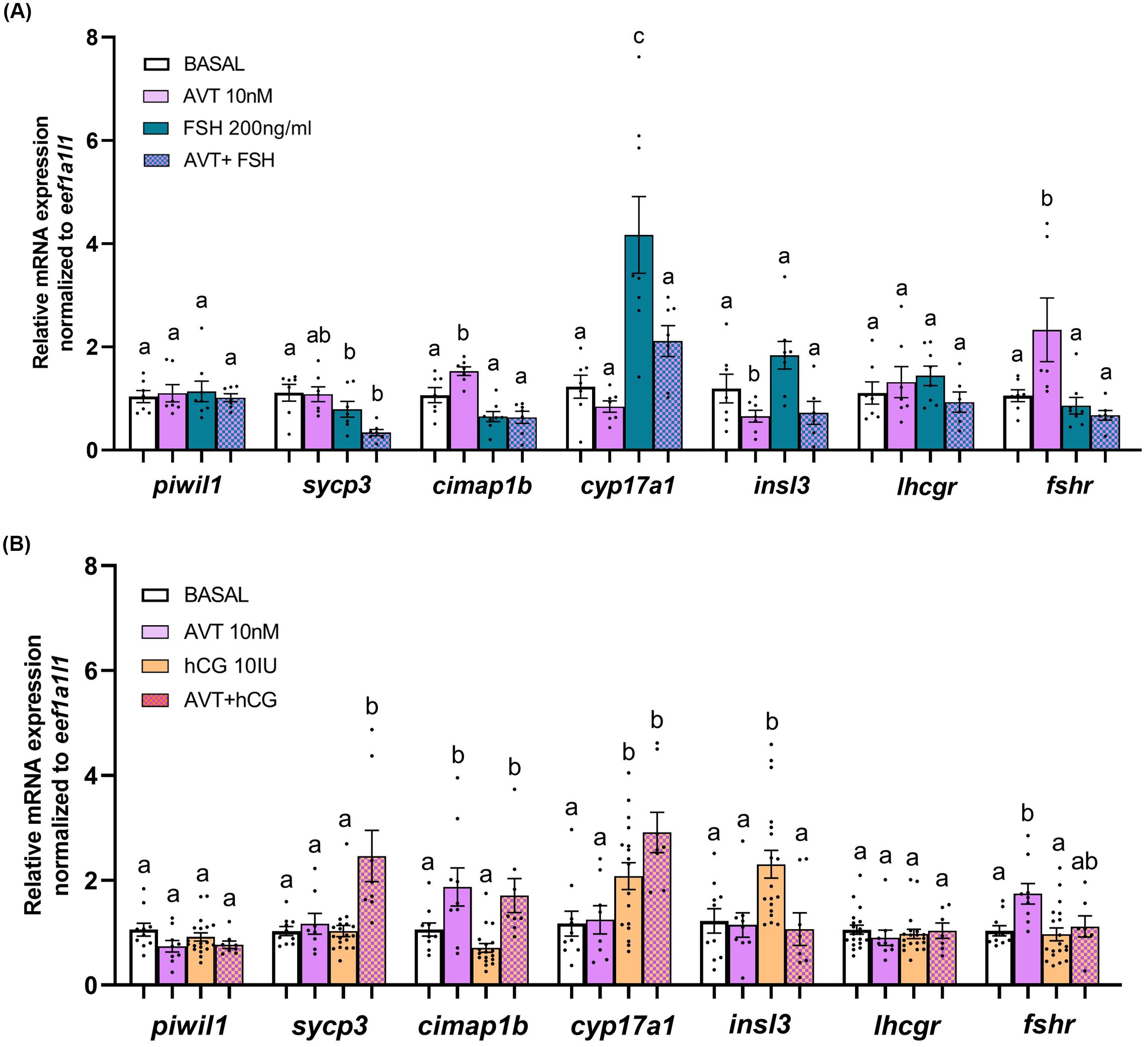
Figure 4. Relative transcript abundance of selected transcripts in zebrafish testes after 48-hour ex vivo treatment with AVT, FSH, LH/hCG, or their combinations. Testes were treated ex vivo for 48 hours with (A) AVT (10 nM) and FSH (200 ng/ml) alone or in combination, or (B) AVT (10 nM), LH/hCG (10 IU/ml), or their combination. Expression levels of germ cell markers (piwil1, sycp3, cimap1b), steroidogenic enzyme cyp17a1, Leydig-derived factor insl3 and the gonadotropin receptors (lhcgr, fshr) were quantified by RT-qPCR. Basal expression represents non-treated testes. All treatments are shown as fold change relative to the respective contralateral control. Data are presented as mean ± SEM (n = 6-10). Statistical differences between each treatment and its control were assessed using Student’s t-test. Different letters denote statistically significant differences among treatment groups determined by one-way ANOVA followed by Tukey’s post hoc test (p < 0.05).
We also measured Leydig-derived transcripts, including cyp17a1 (steroidogenic enzyme), insl3 (promotes type Aund spermatogonia differentiation), and the gonadotropin receptors lhcgr and fshr. In the presence of FSH, co-treatment with AVT significantly reduced cyp17a1 and insl3 expression compared to FSH alone (Figure 4A). Gonadotropin receptor transcript levels were not affected by FSH treatment; however, AVT in combination with FSH suppressed AVT-induced fshr expression. LH/hCG alone or in combination with AVT upregulated cyp17a1 transcript, whereas AVT inhibited the LH/hCG-induced insl3 transcript level (Figure 4B). Transcript levels of lhcgr and fshr remained unaffected following co-treatment with LH/hCG and AVT.
4 Discussion
Limited data exist on the role of arginine vasotocin (AVT) in the multifactorial regulation of fish gonadal function. The present study provides the first evidence that AVT interacts directly with gonadotropins to regulate testicular function in teleost. By investigating its actions in ex vivo cultured zebrafish testes, we show that AVT acts as a stage-specific modulator of gonadotropin signaling, influencing both spermatogonial proliferation and steroidogenesis in a hormone-dependent manner.
Our findings demonstrate that in the presence of FSH, AVT stimulates the proliferation of undifferentiated type A spermatogonia. This was reflected in increased numbers of Aund*+Aund and enhanced mitotic activity, indicating that AVT amplifies the proliferative effect of FSH at the earliest stage of spermatogenesis. Interestingly, although the accumulation of type A differentiated spermatogonia also increased, the mitotic index did not rise accordingly, suggesting that this effect is cumulative rather than mitogenic. AVT also enhanced the FSH-induced expansion of type B spermatogonia, thereby potentiating FSH activity in both self-renewal and differentiation processes (12, 31). These findings highlight a stage-specific co-regulatory role for AVT in promoting the development of the early germ cell pool under FSH stimulation. Consistent with earlier studies (19), our results indicate that LH/hCG alone is insufficient to trigger proliferation of undifferentiated spermatogonia, suggesting that LH-like signaling plays only a limited role at the onset of spermatogenesis. However, when combined with AVT, LH/hCG promoted the proliferation of type B spermatogonia, demonstrating that AVT can potentiate LH activity at more advanced stages of germ cell development. The distinction between FSH-regulated early spermatogenesis and LH-mediated effects on advanced stages, such as spermiogenesis, is well documented in other teleosts (32, 3). In this context, our results identify AVT as a potentiator of gonadotropin function, amplifying FSH signaling to expand the pool of undifferentiated spermatogonia while simultaneously enhancing LH-like activity to promote proliferation at later germ cell stages, such as SpgB.
In addition to its effects on germ cell proliferation, AVT also displayed hormone-specific modulation of steroidogenesis. As expected, both FSH and LH independently stimulated 11-KT production and upregulated key steroidogenic genes, including cyp17a1 and insl3. However, when combined with AVT, the FSH-induced increase in 11-KT release and steroidogenic gene expression was significantly attenuated, while the LH-induced response remained unaffected. This pattern suggest that AVT selectively constrains FSH-driven steroidogenesis, an effect consistent with rodent studies where vasopressin modulated testosterone output in a time- and hormone-dependent manner (33). Such a mechanism would allow AVT to promote early germ cell expansion under FSH while limiting premature androgen-driven completion of spermatogenesis.
The gene expression results provide further insight into these interactions. Neither FSH nor LH strongly altered germ cell marker transcripts, except for a modest FSH-induced repression of sycp3. Notably, the addition of AVT to LH/hCG, but not to FSH, upregulated sycp3 and cimapb1, markers of meiotic and post-meiotic germ cells, respectively. These results, consistent with the histological data, indicate that AVT enhances LH-like effects at the level of meiotic progression. Furthermore, the combined action of AVT and FSH triggered a specific downregulation of the fshr transcript, without impacting lhcgr. This selective receptor regulation may reflect a negative feedback mechanism that prevents overstimulation when FSH and AVT act together. Similar gonadotropin receptor downregulation under sustained stimulation has been documented in zebrafish (6) and in mammals (34, 35), suggesting a conserved self-limiting response across vertebrates. In our study, the presence of AVT appears to potentiate FSH signaling to a threshold that activates this feedback control. The absence of a comparable effect on LH receptor expression highlights the specificity of AVT’s interaction with FSH pathways, pointing to a finely tuned regulatory mechanism that balances proliferation and steroidogenic activity during spermatogenesis.
Our earlier work demonstrated the expression of avt and its five receptors (avpr1aa, avpr1ab, avpr2aa, avpr2ab, avpr2l) in zebrafish testes, pointing to a direct role of AVT in testicular regulation (26, 36). The present findings extend this evidence by identifying AVT as a local paracrine/autocrine factor that fine-tunes gonadotropin signaling in a stage-specific manner. By enhancing FSH-driven expansion of undifferentiated spermatogonia, reinforcing LH-mediated proliferation of type B spermatogonia, and selectively dampening FSH-induced steroidogenesis, AVT coordinates the balance between germ cell proliferation and differentiation. We propose that this dual role enables the zebrafish testis to expand the pre-meiotic pool while restraining premature androgen-dependent spermiogenesis, helping to maintain germ cell synchrony and ensure reproductive success.
Based on these results, we hypothesize that AVT plays a stage-specific modulatory role: it is crucial for enhancing FSH activity during the earliest phases of spermatogonial proliferation and for potentiating LH activity at mid-spermatogonial stages, but its influence diminishes once germ cells progress toward spermiogenesis. At this point, LH alone appears sufficient to sustain androgen production and haploid cell formation, as indicated by the unchanged 11-KT levels and haploid cell responses to LH/hCG regardless of AVT presence. Supporting this view, reduced avt expression levels have also been observed in testes of zebrafish immediately prior to spawning events (unpublished; 36).
Collectively, our findings underscore the role of nonapeptides in the multifactorial control of male reproduction and encourage broader investigation into their evolutionary and physiological significance.
While this work provides novel insight into the role of AVT in modulating gonadotropin signaling, some limitations should be noted. First, the use of an ex vivo testis culture model, although powerful for dissecting direct effects, cannot fully capture the complexity of in vivo endocrine, paracrine, and environmental interactions that modulate and affect spermatogenesis. Second, the gene expression analyses focused on a restricted set of markers involved in spermatogenesis and steroidogenesis; broader transcriptomic or proteomic approaches would be valuable to reveal additional downstream targets of AVT–gonadotropin interactions. Finally, the conclusions are based on zebrafish, and although this species is as an established teleost model, the mechanisms observed may display species-specific features that require further investigation and validation in other vertebrates. Addressing these limitations in future studies will be essential to confirm our findings across different conditions and provide a more comprehensive understanding of AVT’s role in regulating testicular function.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the University of Calgary Animal Care guidelines (protocol # AC24-0042). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HH: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing. MZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. YM: Data curation, Investigation, Methodology, Writing – original draft. NP: Investigation, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada 1021837 to HH.
Acknowledgments
Some of the data presented here were previously included in the author’s doctoral thesis (36), which has been cited accordingly. Graphical abstract was created using BioRender.com with permission under an academic license (https://BioRender.com/e0ou5vi).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, and Lareyre JJ. Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol. (2010) 165:412–37. doi: 10.1016/j.ygcen.2009.07.019
2. Sambroni E, Rolland AD, Lareyre J-J, and Gac FL. Fsh and lh have common and distinct effects on gene expression in rainbow trout testis. J Mol Endocrinol. (2013) 50:1–18. doi: 10.1530/JME-12-0197
3. Schulz RW, de França LR, Lareyre J-J, Chiarini-Garcia FLH, Nobrega RH, and Miura T. Spermatogenesis in fish. Gen Comp Endocrinol. (2010) 165:390–411. doi: 10.1016/j.ygcen.2009.02.013
4. García-López A, Bogerd J, Granneman JCM, Dijk Wv, Trant JM, Taranger GL, et al. Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology. (2009) 150:357–65. doi: 10.1210/en.2008-0447
5. Aizen J, Kasuto H, Golan M, Zakay H, and Levavi-Sivan B. Tilapia follicle-stimulating hormone (FSH): immunochemistry, stimulation by gonadotropin-releasing hormone, and effect of biologically active recombinant FSH on steroid secretion1. Biol Reprod. (2007) 76:692–700. doi: 10.1095/biolreprod.106.055822
6. García-López A, Jonge H de, Nóbrega RH, de Waal PP, Dijk Wv, Hemrika W, et al. Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology. (2010) 151:2349–60. doi: 10.1210/en.2009-1227
7. Kamei H, Ohira T, Yoshiura Y, Uchida N, Nagasawa H, and Aida K. Expression of a biologically active recombinant follicle stimulating hormone of Japanese eel Anguilla japonica using methylotropic yeast, Pichia pastoris. Gen Comp Endocrinol. (2003) 134:244–54. doi: 10.1016/S0016-6480(03)00259-4
8. Weltzien F-A, Norberg B, and Swanson P. Isolation and characterization of FSH and LH from pituitary glands of Atlantic halibut (Hippoglossus hippoglossus L.). Gen Comp Endocrinol. (2003) 131:97–105. doi: 10.1016/S0016-6480(02)00526-9
9. Zmora N, Kazeto Y, Sampath Kumar R, Rüdiger W, Schulz, and Trant JM. Production of recombinant channel catfish (Ictalurus punctatus) FSH and LH in S2 Drosophila cell line and an indication of their different actions. J Endocrinol. (2007) 194:407–16. doi: 10.1677/JOE-07-0171
10. Chen SuR and Liu YiX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by sertoli cell signaling. Reproduction. (2015) 149:R159–67. doi: 10.1530/REP-14-0481
11. Crespo D, Assis LHC, Zhang YuT, Safian D, Furmanek T, Skaftnesmo KO, et al. Insulin-like 3 affects zebrafish spermatogenic cells directly and via sertoli cells. Commun Biol. (2021) 4:204. doi: 10.1038/s42003-021-01708-y
12. Nóbrega RH, Morais RDVD, Crespo D, De Waal PP, De França LR, Schulz RW, et al. Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology. (2015) 156:3804–17. doi: 10.1210/en.2015-1157
13. Skaar KS, Nóbrega RH, Magaraki A, Olsen LC, Schulz RW, and Male R. Proteolytically activated, recombinant anti-müllerian hormone inhibits androgen secretion, proliferation, and differentiation of spermatogonia in adult zebrafish testis organ cultures. Endocrinology. (2011) 152:3527–40. doi: 10.1210/en.2010-1469
14. Pati D, Balshaw K, Grinwich DL, Hollenberg MD, and Habibi HR. Epidermal growth factor receptor binding and biological activity in the ovary of goldfish, Carassius auratus. Am J Physiol. (1996) 270:R1065–72. doi: 10.1111/j.1747-1567.2006.00021.x
15. Pati D and Habibi HR. Presence of salmon gonadotropin-releasing hormone (GnRH) and compounds with GnRH-like activity in the ovary of goldfish. Endocrinology. (1998) 139:2015–24. doi: 10.1210/endo.139.4.5877
16. Habibi HR and Andreu-Vieyra CV. Hormonal regulation of follicular atresia in teleost fish. Fish Oocyte: From Basic Stud to Biotechnol Appl. (2007) 1):235–53. doi: 10.1007/978-1-4020-6235-3-9
17. Fallah HP, Rodrigues MS, Corchuelo S, Nóbrega RH, and Habibi HR. Role of GnRH isoforms in paracrine/autocrine control of zebrafish (Danio rerio) spermatogenesis. Endocrinology. (2020) 161:1–16. doi: 10.1210/endocr/bqaa004
18. Fallah HP, Tovo-Neto A, Yeung EC, Nóbrega RH, and Habibi HR. Paracrine/autocrine control of spermatogenesis by gonadotropin-inhibitory hormone. Mol Cell Endocrinol. (2019) 492:110440. doi: 10.1016/j.mce.2019.04.020
19. Fallah HP, Rodrigues MS, Zanardini M, Nóbrega RH, and Habibi HR. Effects of gonadotropin-inhibitory hormone on early and late stages of spermatogenesis in ex-vivo culture of zebrafish testis. Mol Cell Endocrinol. (2021) 520:111087. doi: 10.1016/j.mce.2020.111087
20. Altmieme Z, Jubouri M, Touma K, Coté G, Fonseca M, Julian T, et al. A reproductive role for the nonapeptides vasotocin and isotocin in male zebrafish (Danio rerio). Comp Biochem Physiol Part B: Biochem Mol Biol. (2019) 238:110333. doi: 10.1016/j.cbpb.2019.110333
21. Lema SC. Identification of multiple vasotocin receptor CDNAs in teleost fish: sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol Cell Endocrinol. (2010) 321:215–30. doi: 10.1016/j.mce.2010.02.015
22. Ramachandran D, Sharma K, Saxena V, Nipu N, Rajapaksha DC, and Mennigen JA. Knock-out of vasotocin reduces reproductive success in female zebrafish, Danio rerio. Front Endocrinol. (2023) 14:1151299. doi: 10.3389/fendo.2023.1151299
23. Ramallo MR, Grober M, Cánepa MM, Morandini L, and Pandolfi M. Arginine-vasotocin expression and participation in reproduction and social behavior in males of the cichlid fish Cichlasoma dimerus. Gen Comp Endocrinol. (2012) 179:221–31. doi: 10.1016/j.ygcen.2012.08.015
24. Rawat A, Chaube R, and Joy KP. In situ localization of vasotocin receptor gene transcripts in the brain-pituitary-gonadal axis of the catfish Heteropneustes fossilis: A morpho-functional study. Fish Physiol Biochem. (2019) 45:885–905. doi: 10.1007/s10695-018-0590-1
25. Zanardini M and Habibi HR. The role of neurohypophysial hormones in the endocrine and paracrine control of gametogenesis in fish. Cells. (2025) 14:1061. doi: 10.3390/cells14141061
26. Zanardini M, Zhang W, and Habibi HR. Arginine vasotocin directly regulates spermatogenesis in adult zebrafish (Danio rerio) testes. Int J Mol Sci. (2024) 25:6564. doi: 10.3390/ijms25126564
27. Rodríguez M and Specker JL. In vitro effects of arginine vasotocin on testosterone production by testes of rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol. (1991) 83:249–57. doi: 10.1016/0016-6480(91)90028-5
28. Singh V and Joy KP. Effects of HCG and ovarian steroid hormones on vasotocin levels in the female catfish Heteropneustes fossilis. Gen Comp Endocrinol. (2009) 162:172–78. doi: 10.1016/j.ygcen.2009.03.016
29. Leal MC, de Waal PP, García-López Á, Chen SX, Bogerd J, and Schulz RW. Zebrafish primary testis tissue culture: an approach to study testis function ex vivo. Gen Comp Endocrinol. (2009) 162:134–38. doi: 10.1016/j.ygcen.2009.03.003
30. Motulsky HJ and Brown RE. Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinf. (2006) 7:123. doi: 10.1186/1471-2105-7-123
31. Safian D, Bogerd J, and Schulz RW. Regulation of spermatogonial development by fsh: the complementary roles of locally produced Igf and wnt signaling molecules in adult zebrafish testis. Gen Comp Endocrinol. (2019) 284:113244. doi: 10.1016/j.ygcen.2019.113244
32. Chauvigné F, Zapater C, Crespo D, Planas JV, and Cerdà J. Fsh and Lh direct conserved and specific pathways during flatfish semicystic spermatogenesis. J Mol Endocrinol. (2014) 53:175–90. doi: 10.1530/JME-14-0087
33. Sharpe RM and Cooper I. Comparison of the effects on purified leydig cells of four hormones (Oxytocin, vasopressin, opiates and LHRH) with suggested paracrine roles in the testis. J Endocrinol. (1987) 113:89–96. doi: 10.1677/joe.0.1130089
34. Wang JM, Li ZF, Yang WX, and Qing Tan F. Follicle-stimulating hormone signaling in sertoli cells: A licence to the early stages of spermatogenesis. Reprod Biol Endocrinol. (2022) 20:1–18. doi: 10.1186/s12958-022-00971-w
35. Maguire SM, Tribley WA, and Griswold MD. Follicle-stimulating hormone (FSH) regulates the expression of FSH receptor messenger ribonucleic acid in cultured sertoli cells and in hypophysectomized rat testis. Biol Reprod. (1997) 56:1106–11. doi: 10.1095/biolreprod56.5.1106
36. Zanardini M. Role of Vasotocin in endocrine/paracrine control of testicular function and gametogenesis. PhD thesis. Department of Biological Sciences, University of Calgary, Calgary, Canada (2025). Available online at: https://ucalgary.scholaris.ca/items/e89f3178-3255-4298-ba91-c900eed56870 (Accessed September 16, 2025).
Keywords: vasotocin (AVT), gonadotropin, follicle-stimulating hormone (FSH), human chorionic gonadotropin (hCG), luteinizing hormone (LH), spermatogenesis, zebrafish
Citation: Zanardini M, Parker N, Ma Y and Habibi HR (2025) Arginine vasotocin is a player in the multifactorial control of spermatogenesis in zebrafish. Front. Endocrinol. 16:1672823. doi: 10.3389/fendo.2025.1672823
Received: 25 July 2025; Accepted: 06 October 2025;
Published: 03 November 2025.
Edited by:
Pei-San Tsai, University of Colorado Boulder, United StatesReviewed by:
Qi Yin, Carnegie Institution for Science, United StatesDiana Castañeda-Cortés, Université du Québec, Canada
Copyright © 2025 Zanardini, Parker, Ma and Habibi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamid R. Habibi, aGFiaWJpQHVjYWxnYXJ5LmNh
 Maya Zanardini
Maya Zanardini Nicolas Parker1
Nicolas Parker1 Yifei Ma
Yifei Ma Hamid R. Habibi
Hamid R. Habibi