- 1Obstetrics and Gynecology Ultrasound Department, Xiangtan Central Hospital, Affiliated Hospital of Hunan University, Xiangtan, Hunan, China
- 2Reproductive Center, Xiangtan Central Hospital, Affiliated Hospital of Hunan University, Xiangtan, Hunan, China
- 3Reproductive Center, Third Xiangya Hospital, Affiliated Hospital of Zhongnan University, Changsha, Hunan, China
Purpose: This study aims to assess the impact of ultrasound parameters on endometrial receptivity in patients undergoing IVF-ET and to establish a predictive model for ongoing pregnancy outcomes.
Methods: The prospective cohort study included 86 patients treated at the Reproductive Center of Xiangtan Central Hospital from May to December 2024. Participants underwent multimodal ultrasound evaluation one day before embryo transfer. The study analyzed endometrial morphology, blood flow parameters, as well as three-dimensional power Doppler angiography (3D-PDA), and endometrial contrast-enhanced ultrasound (CEUS) indicators. Broussonetia papyrifera was used to establish a predictive model for sustained pregnancy.
Results: Among the 86 patients, 42 (48.8%) achieved ongoing pregnancy, while 44 (51.2%) did not. Significant differences between the groups were observed in the number of mature oocytes and endometrial blood flow grading (both P = 0.005). Lasso regression identified eight predictive variables: primary cause of infertility, baseline luteinizing hormone (LH) levels, number of MII oocytes, uterine cavity volume, endometrial blood flow grading, subendometrial flow index (FI) in 3D-PDA, and endometrial and subendometrial peak intensity (PI) in CEUS. The aforementioned variables as well as embryonic factors were integrated into eight machine learning models, with the Gradient Boosting model exhibiting superior predictive performance (AUC: 0.981). SHapley Additive exPlanations (SHAP) analysis indicated that a higher number of MII oocytes, improved endometrial blood flow, specific infertility etiologies, elevated baseline LH levels, and reduced subendometrial/endometrial PI, subendometrial FI, and uterine cavity volume were associated with a greater likelihood of pregnancy.
Conclusion: The integration of 3D-PDA and CEUS technologies shifts IVF-ET evaluation from traditional morphological observation to functional assessment, offering a new perspective for predicting sustained pregnancy outcomes. This innovation shows promising clinical potential by optimizing treatment strategies like MII oocyte retrieval, improving endometrial blood flow grading, and adjusting blood flow parameters (PI and FI), significantly enhancing pregnancy success rates and advancing assisted reproductive technologies.
1 Introduction
Endometrial receptivity (ER) represents a transient yet critical endometrial state that facilitates blastocyst apposition, adhesion, and invasion, while concurrently promoting stromal remodeling to enable successful embryo implantation (1). As a pivotal determinant of pregnancy success in IVF-ET cycles, optimal ER necessitates synchronized endometrial thickening and vascularization. Endometrial arterial perfusion and the development of a robust vascular network serve as essential biomarkers for predicting pregnancy outcomes. Doppler ultrasound evaluations of uterine blood flow parameters reveal significant correlations with implantation potential, demonstrating consistently lower resistance index (RI) and pulsatility index (PI) values in endometrial blood flow among pregnant women compared to their non-pregnant counterparts (2). Elevated PI and RI values indicate increased vascular resistance, leading to compromised endometrial perfusion and, consequently, reduced receptivity. Ultrasonographic assessments of endometrial blood flow have been associated with embryo transfer success, with studies reporting higher subendometrial blood flow parameters—vascularization index (VI), flow index (FI), and vascularization-flow index (VFI)—in pregnant groups (3–5). However, traditional Doppler indices of spiral arteries (RI, PI, peak systolic velocity [PSV]) exhibit limited predictive value for pregnancy outcomes. Research by Maged et al. (6) and others found no significant differences in VI and FI between conception and non-conception cycles, while logistic regression analyses failed to establish meaningful associations between pregnancy outcomes and various hemodynamic parameters (7). These conflicting findings underscore persistent controversies regarding their clinical utility.
Given the inadequacy of single indicators in comprehensively assessing endometrial receptivity, research has increasingly shifted toward multimodal ultrasound evaluations. Jiao et al. (8) developed a scoring system to predict early miscarriage, incorporating parameters such as endometrial thickness (EMT), morphology, peristalsis, volume, and blood flow. Their results demonstrated significantly lower scores in the miscarriage group (10.46 ± 2.99) compared to the successful pregnancy group (13.49 ± 2.21). Similarly, Liao et al. (9) proposed a model integrating EMT, volume, and vascular blood flow indices, identifying a multimodal ultrasound endometrial score below 12 as a risk factor for compromised full-term delivery post-transfer. Li et al. (10) further advanced this field by employing clinical indicators—including EMT, PI, RI, and ultrasound elastography—to develop a logistic model with 76.92% predictive accuracy for pregnancy outcomes. Despite these advancements, current ultrasound-based scoring systems remain insufficient for reliably predicting IVF-ET success.
An optimal blood supply to the endometrium is essential for embryo implantation. During early implantation, endometrial vascular permeability increases, causing significant changes in the microvasculature of Broussonetia papyrifera. As the primary decidual zone develops, capillaries near the embryo close while those adjacent to the decidual zone dilate. Impaired endometrial angiogenesis may lead to recurrent implantation failure and pregnancy loss. Increased vascular permeability aids in delivering growth factors and cytokines to the implantation site (11). CEUS provides real-time visualization of tissue microcirculation, while3D-PDA effectively detects low-velocity blood flow without angle limitations. 3D-PDA captures blood flow signals from all directions, enabling comprehensive volume analysis through computer reconstruction. This study seeks to address this gap by developing a more precise evaluation model that incorporates a comprehensive array of ultrasound indicators related to ER, encompassing physiological and morphological characteristics, blood flow dynamics, and advanced imaging parameters such as 3D-PDA and CEUS.
2 Method
2.1 Study population and data collection
This prospective cohort study was conducted at the Reproductive Center of Xiangtan Central Hospital from May to December 2024, enrolling 86 consecutive patients undergoing IVF-ET treatment. A standardized protocol was implemented for comprehensive data collection, including detailed assessment of: (1) endometrial morphological characteristics (thickness, trilaminar pattern, and volumetric measurements); (2) functional parameters (elasticity measurements and peristaltic activity); (3) hemodynamic evaluations through Doppler assessment of endometrial and subendometrial blood flow; (4) three-dimensional power Doppler angiography (3D-PDA) quantification of vascular indices (FI, VI, VFI); and (5) endometrial contrast-enhanced ultrasound (CEUS) parameters (AT, TTP, RT, PI, AUC).
Eligibility Criteria: Participants were selected based on the following inclusion criteria: (i) documented infertility with medical indication for IVF-ET, including tubal factor, anovulatory disorders resistant to medical therapy, stage I-II endometriosis, severe male factor infertility (total motile sperm count <5×106), or unexplained infertility; (ii) age <40 years with availability of ≥1 high-quality blastocyst for transfer; (iii) willingness to provide informed consent. Exclusion criteria comprised: (i) structural uterine abnormalities (Asherman’s syndrome, submucosal fibroids, endometrial polyps); (ii) active systemic or psychiatric comorbidities; (iii) substance abuse disorders; (iv) recent exposure to teratogens or gonadotoxic agents; (v) known contrast media hypersensitivity; (vi) autoimmune conditions requiring biologic therapies.
The final cohort of 86 participants was established after rigorous screening and verification of eligibility criteria. The study protocol was approved by the Institutional Ethics Committee of Xiangtan Central Hospital (Approval No. SZ202211-05) and conducted in accordance with Good Clinical Practice guidelines. All participants provided written informed consent after detailed counseling about study procedures.
2.2 Multimodal ultrasound assessment
Ultrasound was performed by one senior ultrasonographer on the day of the ET. Standardized two-dimensional transvaginal ultrasound was performed to obtain median sagittal uterine views for endometrial assessment. Endometrial thickness was measured at its maximal dimension perpendicular to the uterine cavity. Endometrial morphology was classified according to Gonen criteria: Type A demonstrating a distinct trilaminar pattern with hyperechoic outer lines and hypoechoic central cavity; Type B showing intermediate echogenicity with partial loss of trilaminar appearance; Type C characterized by homogeneous hyperechogenicity without visible layering. Endometrial peristaltic waves were observed for two minutes under stable conditions, with data analyzed at quadruple speed to assess type, direction, frequency, and intensity. The waves were categorized into five types: forward, reverse, static, bidirectional, and localized. Doppler evaluation was conducted using standardized protocols. Endometrial and subendometrial vascular patterns were classified per Applebaum criteria: Type I (peripheral vascularity limited to the hypoechoic junctional zone); Type II (vascular penetration through the hyperechoic endometrial border); Type III (intraendometrial vascularization).
3D PDA and CEUS examinations were conducted with Mindray color ultrasound system(Nuewa R9Q), Switch to three-dimensional power Doppler mode, Using an intracavitary volume probe (Model DE10-3WU), 3D-PDA images of the endometrium were captured with preset scanning parameters: 120° angle, 0.9 kHz PRF, 71 Hz filter, and 40 gain. The system’s Smart ERA function automatically reconstructed the endometrium in 3D and calculated volume and blood flow parameters (VI, FI, VFI). Enable Shell functionality, set the regions of interest (ROIs) from the endometrial fundus to above the internal cervical os, including the subendometrial area within 3 mm of the endometrial-myometrial junction.
The patient followed a standard protocol during the CEUS exam. Standardized settings: MI between 0.065-0.099, transducer frequency at CH4-CH5 MHz, dynamic range at 100 dB, iClear at 1, pseudocolor at 5, smoothing at 1, and gain at 43. The procedure involved obtaining a midsagittal section of the uterus, switching to contrast mode, and adjusting the image for clear endometrial visibility. After injecting 2.4 ml of SonoVue and 10 ml of saline, the timer was started to record contrast time. Images were captured for 120 seconds, and the patient was monitored for 30 minutes post-exam to ensure no discomfort. During image analysis, real-time monitoring of contrast agent perfusion in the endometrium was conducted, and time-intensity curves (TIC) were generated. The selection of ROI is consistent with 3D-PDA. The ultrasound machine’s TIC analysis software automatically plotted the curves, from which quantitative parameters such as PI, AUC, TTP, AT were derived. An experienced physician performed and plotted TIC curves for all patients, averaging three tracings for each quantitative parameter.
2.3 Reproductive outcomes
IVF treatment outcomes were evaluated through serial serum β-human chorionic gonadotropin (β-hCG) measurements, with the initial quantitative assessment performed 12 days post-embryo transfer. A positive biochemical pregnancy was defined as β-hCG ≥50 IU/L, followed by serial monitoring to confirm appropriate doubling kinetics. Clinical pregnancy confirmation required transvaginal ultrasound visualization of an intrauterine gestational sac with detectable cardiac activity at 6 weeks’ gestation. Ongoing pregnancy was defined as the presence of a viable fetus confirmed by ultrasound beyond 14 weeks’ gestation. For analytical purposes, outcomes were dichotomized: “Yes” indicated confirmed ongoing intrauterine pregnancy at 14 weeks, while “No” encompassed negative results, biochemical pregnancies (isolated β-hCG elevation without clinical confirmation), or early pregnancy loss prior to 14 weeks.
2.4 Statistical methods
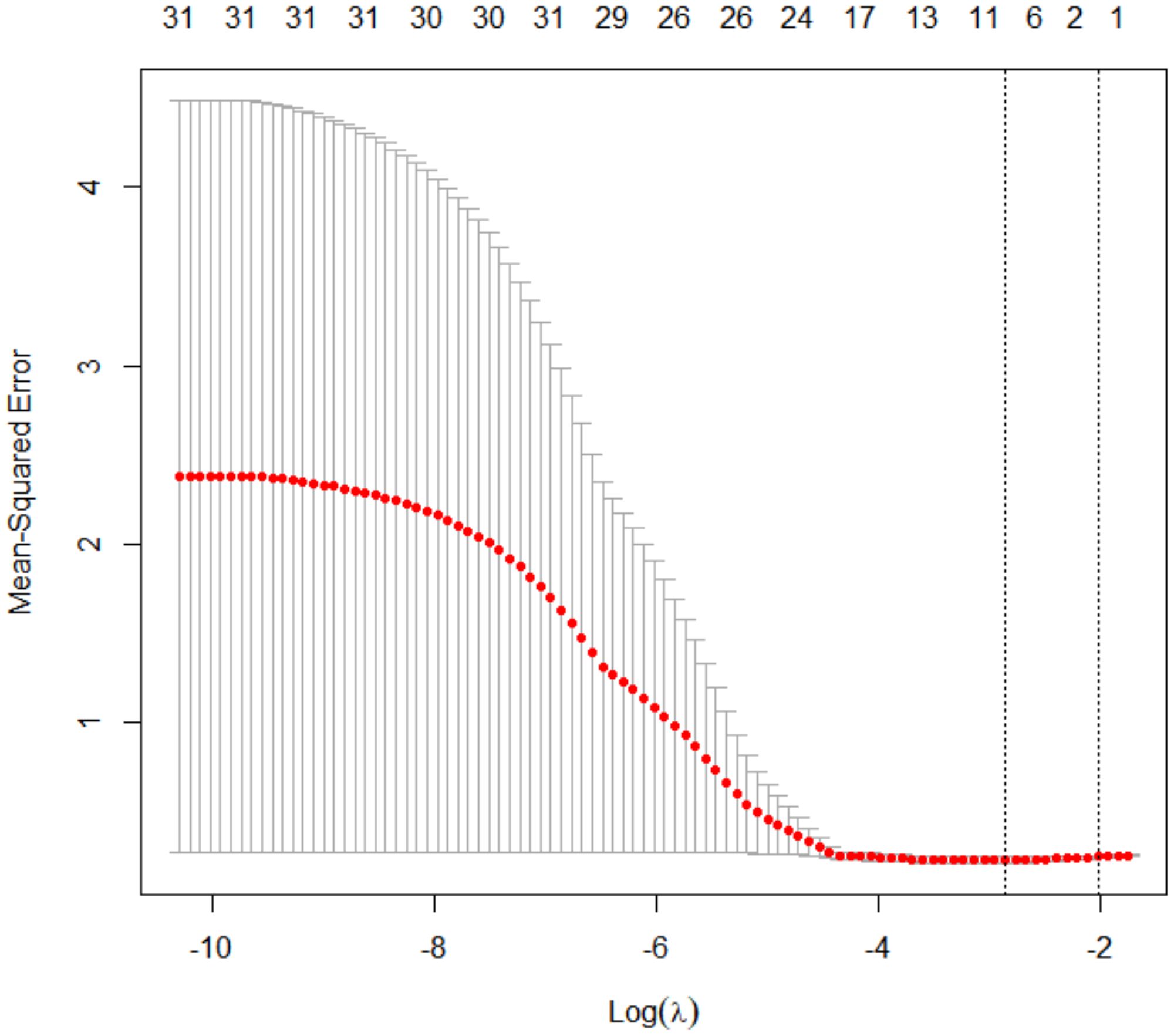
Analyses were conducted using R v4.4.1 and Python v3.12.0. Continuous and categorical variables were reported as mean ± SD and frequencies (%), respectively. Group comparisons used χ² tests for categorical variables and Mann-Whitney U tests for continuous variables. Predictor selection employed LASSO regression (10-fold CV, λ=0.576), identifying eight key variables: infertility etiology, baseline LH, MII oocyte count, uterine volume, endometrial blood flow grade, subendometrial FI (3D-PDA), endometrial/subendometrial PI (CEUS). These predictors were evaluated in multiple machine learning models, with optimal model selection based on AUC performance. Final model interpretation used SHAP analysis.
3 Result
3.1 Factors associated with ongoing pregnancy
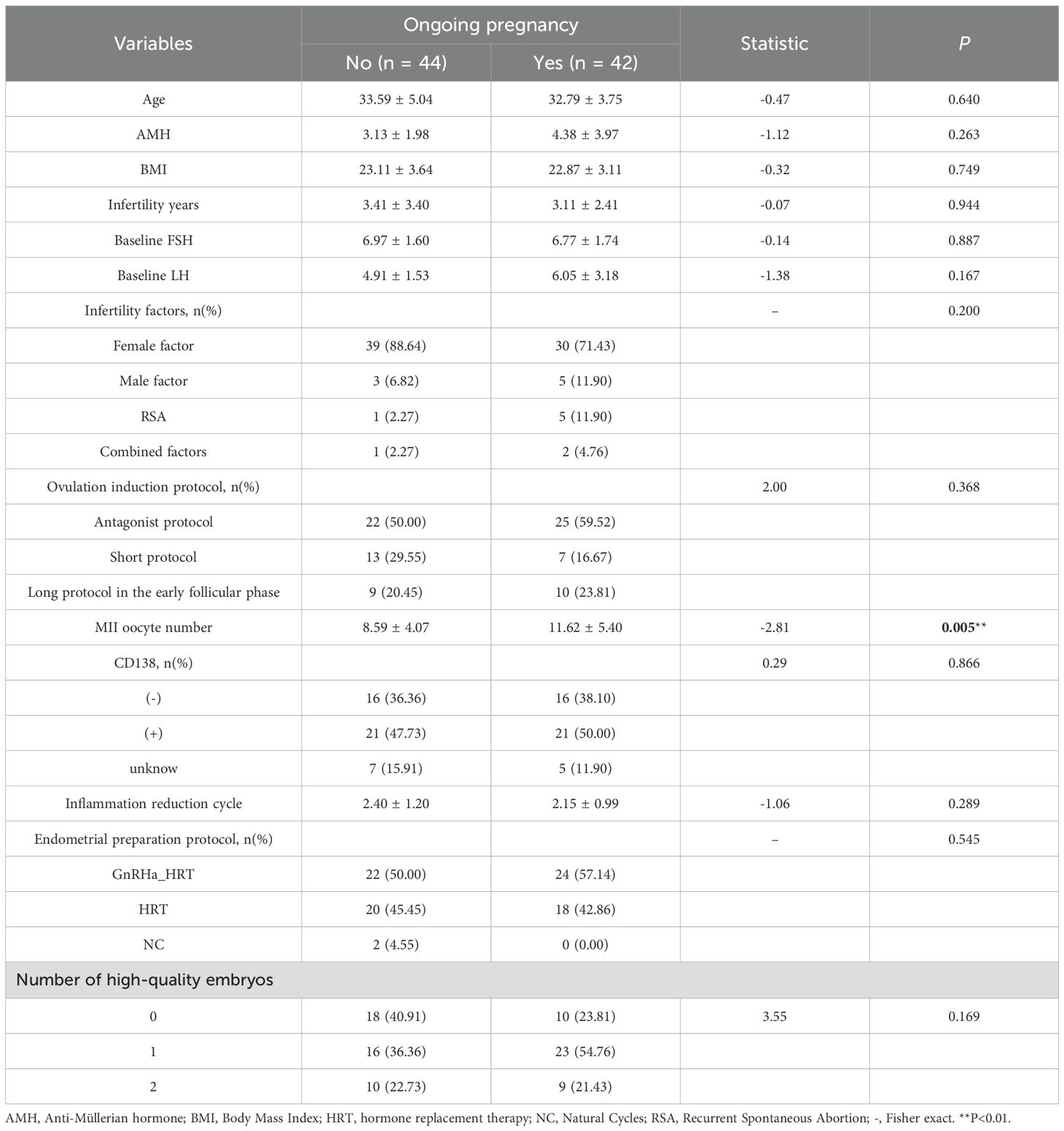
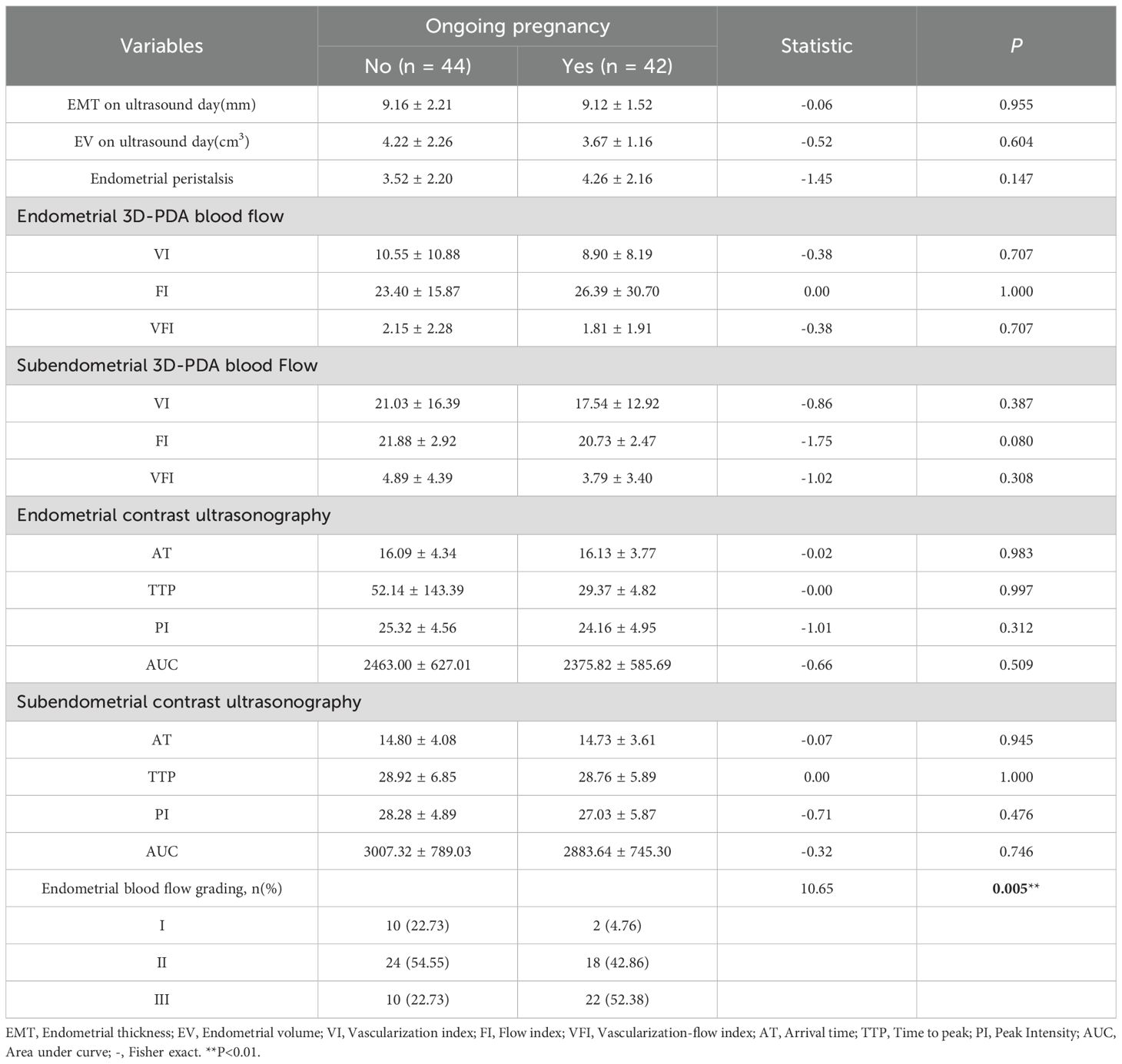
The study cohort comprised 86 patients, of whom 42 (48.8%) achieved ongoing pregnancy, while 44 (51.2%) did not (including 30 non-pregnant cases, 10 biochemical pregnancies, and 2 early miscarriages). Significant differences were observed in the number of MII oocytes and endometrial blood flow grading (both P = 0.005)(Table 1). No statistically significant differences were found in baseline characteristics, endometrial morphology/physiology, or 3D-PDA and CEUS parameters (P > 0.05) (Table 2).
3.2 LASSO logistic regression analysis
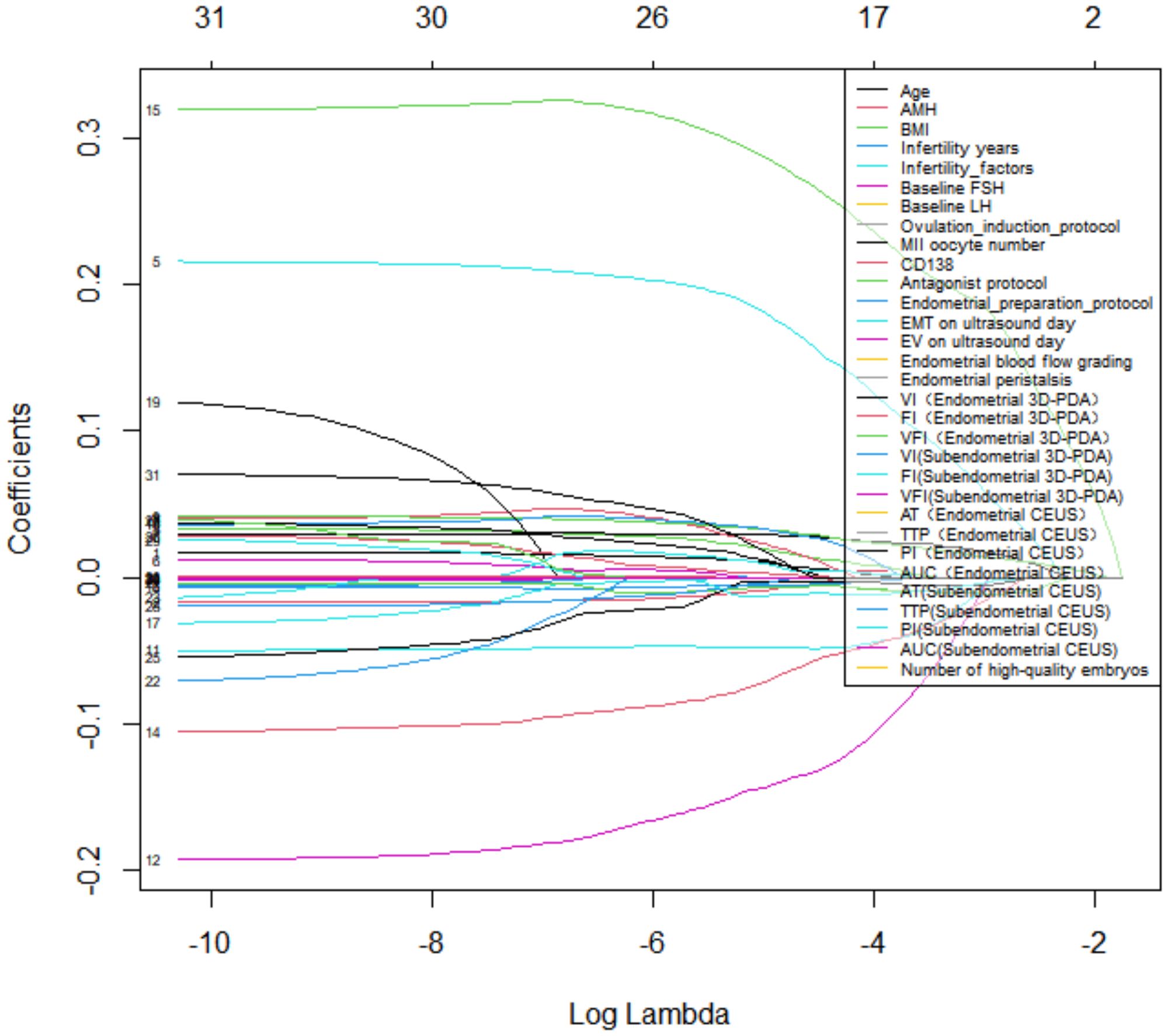
Using LASSO regression with 10-fold cross-validation (optimal λ = 0.576), we identified eight non-zero coefficient predictors: (1) infertility etiology, (2) baseline LH levels, (3) number of MII oocytes, (4) uterine cavity volume, (5) endometrial blood flow grading, (6) subendometrial flow index (FI) from 3D-PDA, and (7–8) endometrial and subendometrial PI from CEUS (Figures 1, 2). There was no significant difference in the number of high-quality embryos transferred between groups (P = 0.169). However, since embryo quality is vital for IVF success, we included embryo factors in our statistical model.
3.3 Development of predictive models for ongoing pregnancy outcomes
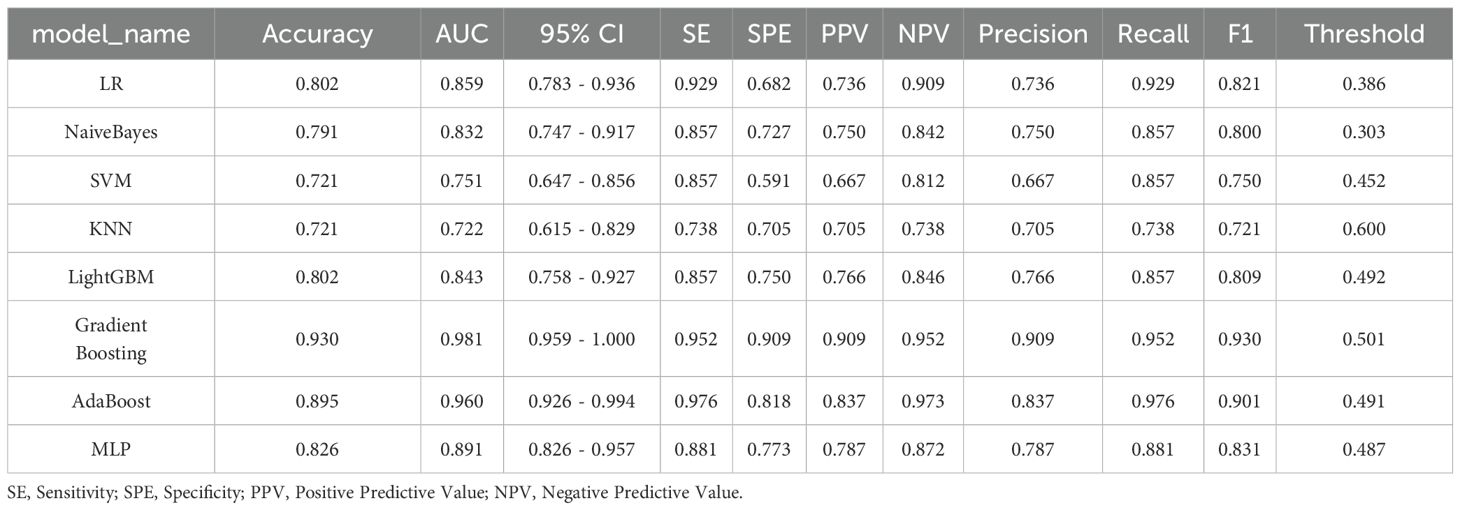
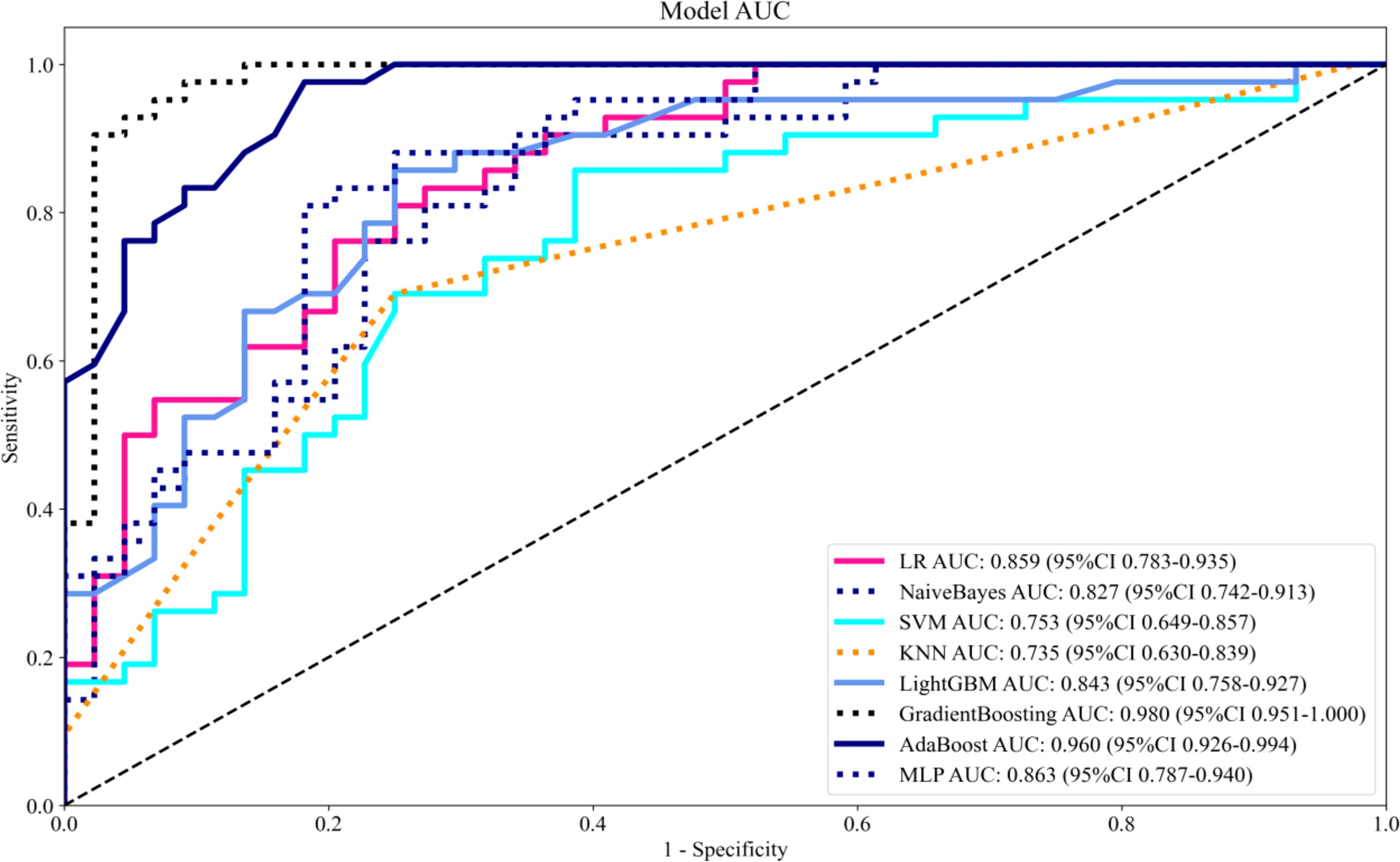
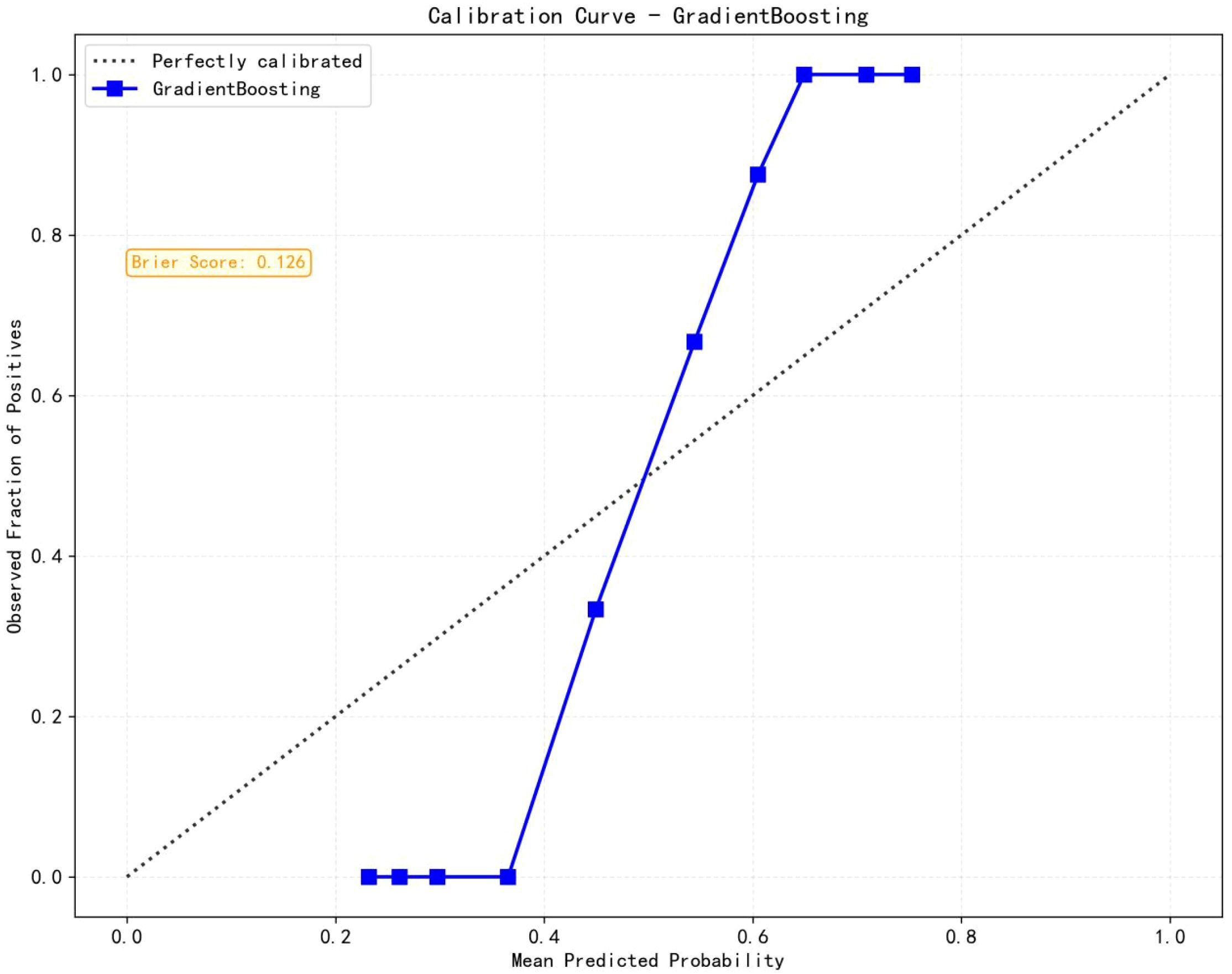
Eight machine learning algorithms were trained using the selected predictive factors: Logistic Regression (AUC = 0.859, 95%CI 0.783-0.936), Support Vector Machine (AUC = 0.751, 95%CI 0.647-0.856), K-Nearest Neighbors (AUC = 0.722, 95%CI 0.615-0.829), Naive Bayes (AUC = 0.832, 95%CI 0.747-0.917), Gradient Boosting (AUC = 0.981, 95%CI 0.959-1.000), LightGBM (AUC = 0.843, 95%CI 0.758-0.927), AdaBoost (AUC = 0.960, 95%CI 0.926-0.994), and Multilayer Perceptron (AUC = 0.891, 95%CI 0.926-0.994). Comparative analysis revealed the Gradient Boosting model demonstrated optimal predictive performance (Table 3, Figure 3). The calibration assessment was performed on the optimal model, with the calibration plot illustrating the alignment between the model’s predicted probabilities and the observed probabilities of ongoing pregnancy. Furthermore, the Brier score served as a metric for evaluating the calibration curve’s performance, with lower Brier scores indicating greater model accuracy. The Gradient Boosting model attained a Brier score of 0.126, indicating robust predictive performance for ongoing pregnancy (see Figure 4).
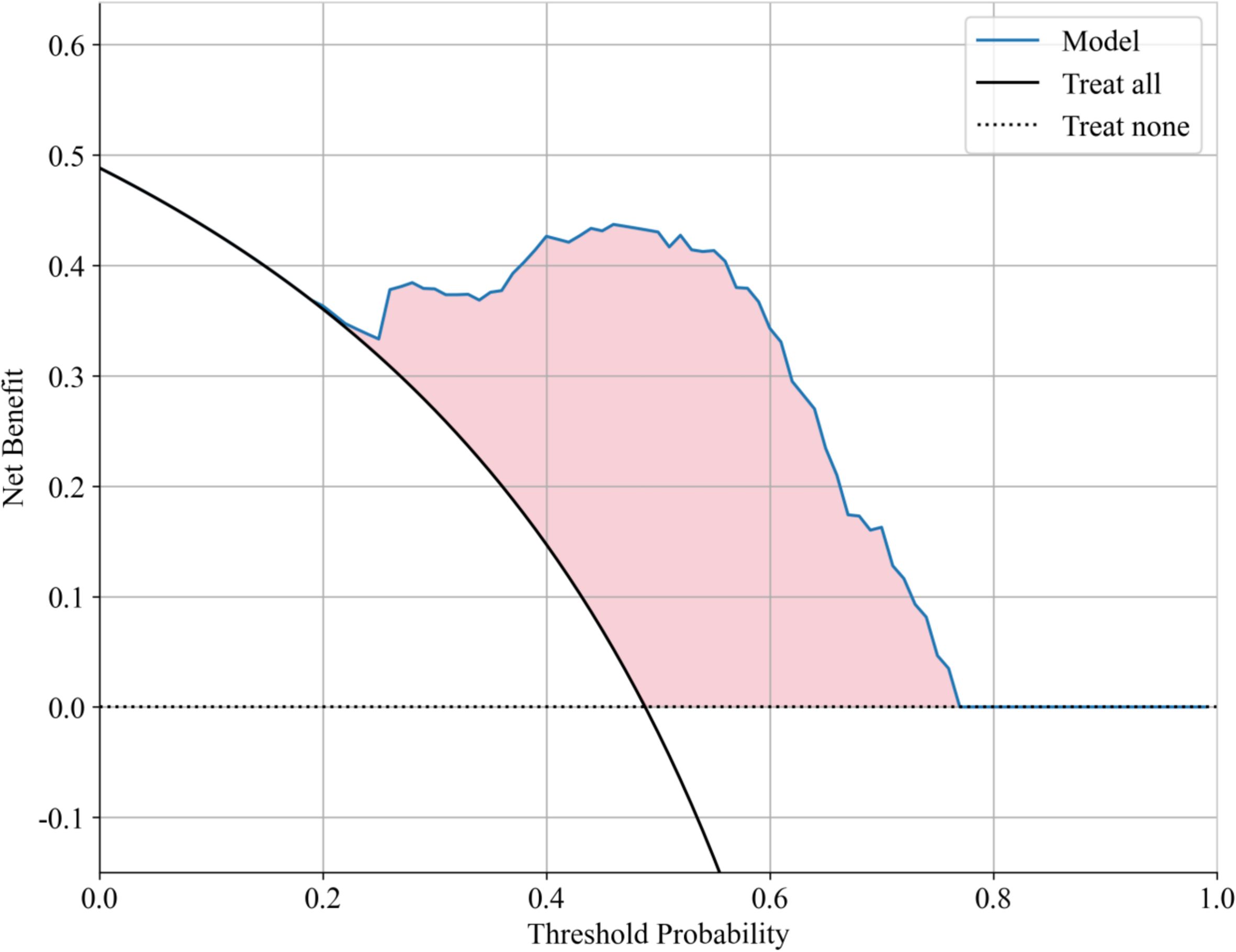
Decision curve analysis (DCA) further validated the clinical applicability of the Gradient Boosting model, showing significant net benefit across probability thresholds of 0.1-0.6 (Figure 5). The model achieved maximal clinical utility at thresholds of 0.2-0.4, demonstrating: 1) superior discrimination of moderate-risk patients compared to alternative approaches; 2) effective reduction of overtreatment in low-risk cases relative to universal treatment strategies; and 3) improved identification of high-risk patients versus conservative management approaches.
3.4 SHAP interpretation of Gradient Boosting model predictions
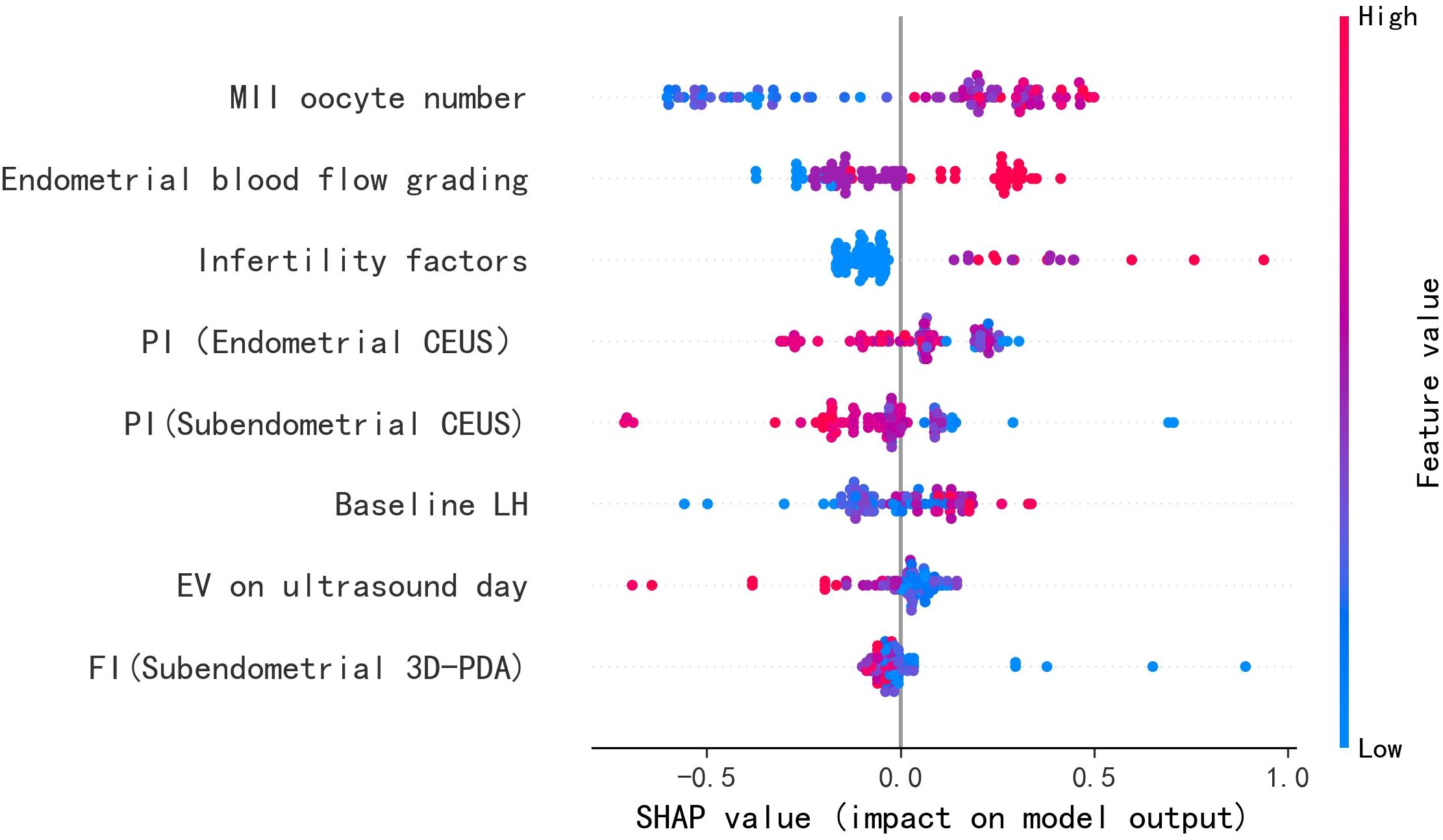
The feature importance analysis using SHapley Additive exPlanations (SHAP) is presented in Figure 6 through violin plots, demonstrating the magnitude and direction of each variable’s contribution to the model predictions. Higher absolute SHAP values correspond to greater predictive importance, with red and blue data points representing high and low feature values, respectively. Key positive predictors of ongoing pregnancy included: (1) higher numbers of MII oocytes retrieved, (2) improved endometrial blood flow grading, and (3) elevated baseline LH levels. Conversely, reduced pregnancy likelihood was associated with increased values of: (1) endometrial and subendometrial PI, (2) FI, and (3) uterine cavity volume.
Individual prediction explanations are visualized through force plots (Figures 7A, B), where the horizontal axis represents the cumulative SHAP value driving the prediction from the baseline output. For pregnancy failures (Figure 7A), the dominant contributing features were enlarged uterine cavity volume and suboptimal MII oocyte yield. Successful pregnancy predictions (Figure 7B) were primarily influenced by adequate MII oocyte numbers, favorable endometrial blood flow patterns, and optimal PI values. The directionality of each feature’s impact is indicated by arrow orientation (positive/negative) and color intensity (magnitude of contribution).
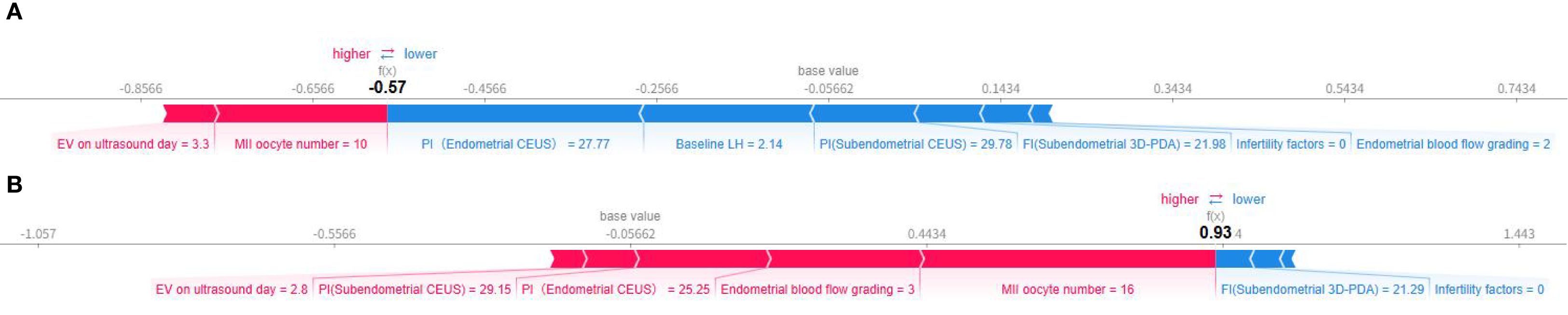
Figure 7. Individual force diagram of gradient boosting prediction model. (A) Non-pregnant, (B) Ongoing pregnancy.
3.5 Internal validation and efficacy calculation of Gradient Boosting model
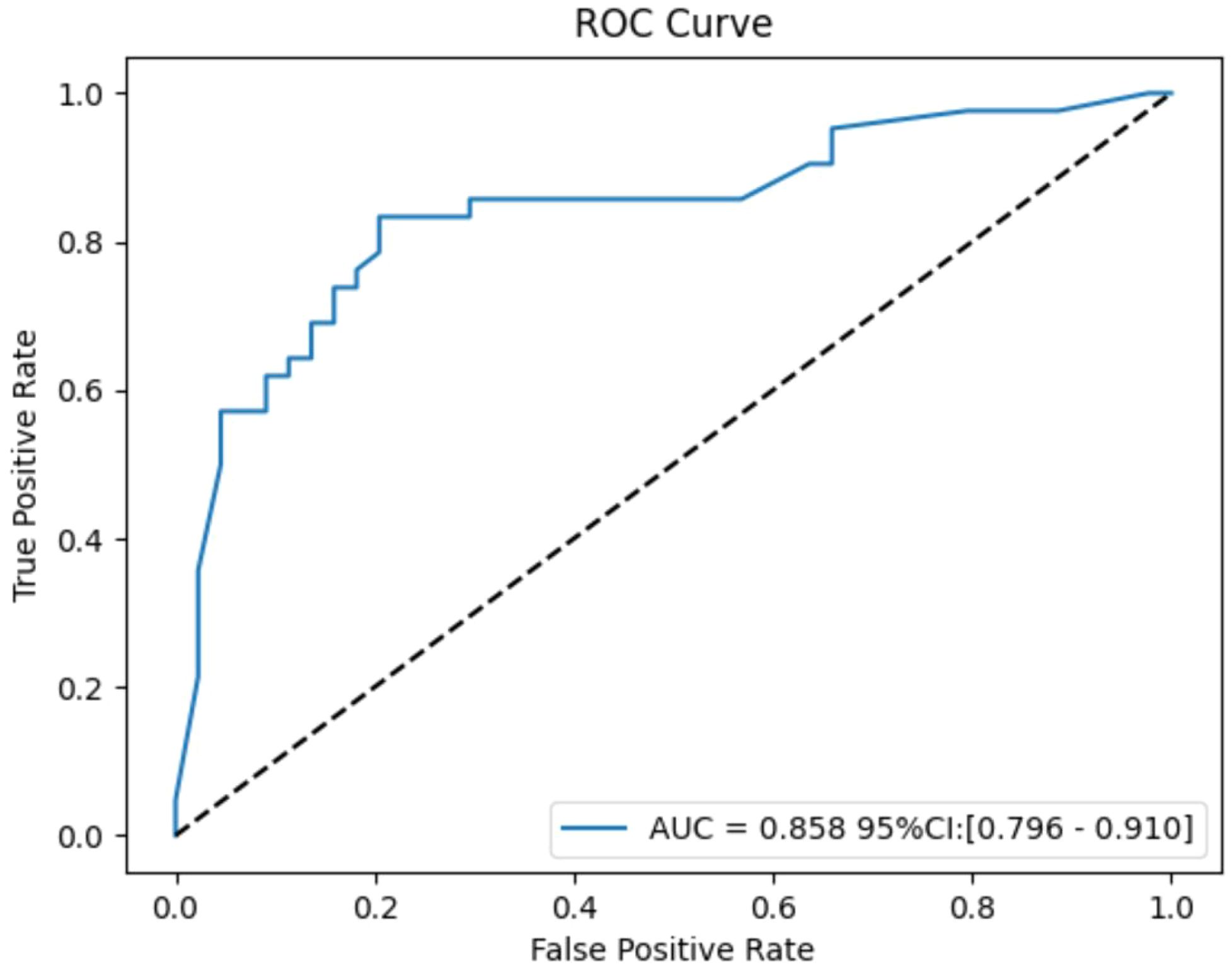
Bootstrap analysis with 1000 resamples was used for internal validation, yielding an average AUC of 0.858 (95% CI: 0.796-0.910). The effect size, calculated using the Hanley & McNeil method, was 2.963, and the event per variable (EPV) ratio was 4.7:1. While the high AUC and effect size show strong discriminative ability, the low EPV ratio suggests potential overfitting. Internal validation efforts were made to address this concern (Figure 8).
4 Discussion
Successful embryo implantation represents a critical determinant of pregnancy achievement in IVF-ET, with both embryo quality and quantity serving as pivotal factors. Our findings corroborate previous studies demonstrating that diminished MII oocyte yield significantly predicts implantation failure, likely through limiting the availability of genetically competent gametes for fertilization. Simultaneously, it decreases transplantable embryos, with embryo quality being crucial for IVF success (12). The observed association between elevated baseline LH levels and improved reproductive outcomes may reflect enhanced follicular recruitment and subsequent oocyte quality, consistent with LH’s established roles in promoting embryo development and maintaining luteal function.
The cyclical endometrial changes during the luteal phase (days 21-22) create an optimal microenvironment for implantation through coordinated hormonal actions. Progesterone and estrogen synergistically induce characteristic morphological changes including stromal edema, glandular coiling, and vascular proliferation. Notably, the spiral arteries undergo marked dilatation and tortuosity to accommodate the increased hemodynamic demands post-implantation. Our data support existing literature demonstrating superior pregnancy rates with favorable endometrial perfusion characteristics (13). Specifically, we observed significantly greater subendometrial vascularity (Type II/III patterns) in conception cycles (94.3% vs 91.8%, p<0.05) (14), aligning with Sun et al.’s findings (7). However, the moderate predictive accuracy (AUC = 0.567) of blood flow patterns alone underscores the need for multimodal assessment. The transition from Type II/III to Type I vascular patterns following oocyte retrieval, as described by Guo et al. (15), may explain the observed association between peri-retrieval blood flow impairment and early pregnancy loss (21.74% vs 9.23%). While our study confirms the prognostic value of endometrial blood flow grading, the current lack of standardized classification systems and limited prospective validation studies continue to generate controversy regarding its clinical utility.
The spiral arteries’ low-velocity flow and complex structure can lead to a low number/area ratio and unstable perfusion timing, making it difficult to detect perfusion changes in the endometrium using 2D ultrasound. While 3D-PDA is more sensitive to low-velocity blood flow than traditional Doppler methods, its clinical value for assessing endometrial vasculature is still debated (16, 17). CEUS, a significant advancement in ultrasound imaging, is now widely used in clinical practice for its superior ability to evaluate blood flow perfusion. Meta-analytic data (18) and nomogram studies (19–21) consistently identify FI as the most robust predictor among vascular parameters, though our results suggest subendometrial indices may show inverse relationships with pregnancy outcomes in certain populations. Several factors may account for these discrepancies: First, the restricted sample size may have limited power. Second, protocol-driven anticoagulant administration could have modified natural hemodynamic patterns. Third, single-timepoint assessments fail to capture the dynamic vascular changes occurring throughout the menstrual cycle (22). The observed values in our pregnant cohort, while lower than non-pregnant controls, exceeded established diagnostic thresholds (21, 23), possibly reflecting progesterone-mediated vascular effects in ART cycles.
There is currently no consensus on a standard for multimodal ultrasound assessment of endometrial receptivity. Developing a scoring system based on ultrasound characteristics may involve subjectivity, impacting its clinical utility. The first-trimester pregnancy prediction model using three-dimensional ultrasound parameters shows moderate diagnostic performance (AUC = 0.639), and the logistic regression model with clinical parameters and endometrial elasticity indicators also has limited accuracy (8–10). Our study extracts key features from 3D-PDA and CEUS parameters using a data-driven approach to reduce subjective bias. The Gradient Boosting model, optimized and evaluated, achieved the highest performance (AUC = 0.981). We use the SHAP method to assess feature importance, enhancing model interpretability. Practically, our model improves endometrial receptivity assessment, aiding clinical treatment planning. For example, in assisted reproductive technology, it helps determine the best embryo transfer timing, boosting transplantation success rates.
Regarding safety, sulfur hexafluoride microbubble contrast agents demonstrate excellent safety profiles with no evidence of teratogenicity even at supratherapeutic doses (24). The isolated febrile episode in our series appeared unrelated to contrast administration. While preliminary results are encouraging, larger randomized controlled trials remain necessary to establish definitive safety guidelines and validate the clinical efficacy of contrast-enhanced techniques in reproductive medicine.
This study has limitations, including its single-center, exploratory nature and small sample size, which may affect statistical power and limit generalizability. Future research should involve large-scale, multicenter trials for validation. The study’s primary outcome was sustained pregnancy, lacking final live birth rate data, which is the ultimate efficacy measure in assisted reproductive technology. As of follow-up, some patients hadn’t reached live birth, so outcomes during mid-to-late pregnancy weren’t covered. Further follow-up and analysis of factors affecting live birth are needed. Internal validation showed potential overfitting due to a low EPV ratio, indicating the need for more data collection. No reproducibility analysis was performed, which is important when considering use of a method in clinical practice. A better design including independent acquisition and blinded analysis should be considered in future work.
5 Conclusion
Assessing endometrial receptivity using multimodal ultrasound, including contrast-enhanced techniques, is crucial for predicting IVF-embryo transfer success. Our model shows that combining endometrial blood flow and mature oocyte count improves prediction accuracy. Clinically, this can optimize ovarian stimulation and suggest blood perfusion therapy for patients with poor vascular parameters. Future studies should confirm these results in larger human cohorts and explore more biomarkers to enhance the model. This ultrasound-based predictive model, developed with Broussonetia papyrifera, advances precision medicine by providing a refined, noninvasive method for assessing endometrial receptivity and guiding personalized clinical interventions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Xiangtan Central Hospital Reproductive Center (Approval No. SZ202211-05). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CW: Project administration, Writing – review & editing, Conceptualization, Methodology. JL: Writing – original draft, Data curation, Methodology. XiZ: Writing – original draft, Formal Analysis, Software. NL: Methodology, Writing – original draft, Data curation. PS: Writing – original draft, Data curation, Methodology. JY: Writing – original draft, Data curation. XiZ: Writing – original draft, Data curation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Hunan Provincial (No. 211034732027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Achache H and Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. (2006) 12:731–46. doi: 10.1093/humupd/dml004
2. Song MJ, Xu H, Wang LX, and Li JN. Research advances in ultrasound evaluation index of endometrial receptivity in prediction of pregnancy outcome with assisted reproductive technology. Acad J Chin PLA Med Sch. (2021) 42:1211–5. doi: 10.3969/j.issn.2095-5227.2021.11.018
3. Kim A, Jung H, Choi WJ, Hong SN, and Kim HY. Detection of endometrial and subendometrial vasculature on the day of embryo transfer and prediction of pregnancy during fresh in vitro fertilization cycles. Taiwanese J Obstetrics Gynecology. (2014) 53:360–5. doi: 10.1016/j.tjog.2013.05.007
4. Li WY. Relationship between ultrasound characteristics of endometrial blood flow parameters and embryo transfer success rate. J Prev Med Chin PLA. (2019) 37:38–40. doi: 10.13704/j.cnki.jyyx.2019.02.014
5. Mishra VV, Agarwal R, Sharma U, Aggarwal R, Choudhary S, and Bandwal P. Endometrial and subendometrial vascularity by three-dimensional (3D) power Doppler and its correlation with pregnancy outcome in frozen embryo transfer (FET) cycles. J Obstetrics Gynecology India. (2016) 66:521–7. doi: 10.1007/s13224-016-0871-5
6. Maged AM, Kamel AM, Abu-Hamila F, Elkomy RO, Ohida OA, and Hassan SM. The measurement of endometrial volume and sub-endometrial vascularity to replace the traditional endometrial thickness as predictors of in-vitro fertilization success. Gynecological Endocrinol. (2019) 35:949–54. doi: 10.1080/09513590.2019.1604660
7. Sun K, Xiu YL, Wang Y, Yu T, Lu X, and Wang X. Predictive value of 3D ultrasound assessment of endometrial receptivity for PGD/PGS for transfer pregnancy outcome. BMC Pregnancy Childbirth. (2023) 23:213. doi: 10.1186/s12884-023-05534-4
8. Jiao Y, Xue N, Shui X, Yu C, and Hu C. Application of ultrasound multimodal score in the assessment of endometrial receptivity in patients with artificial abortion. Insights into Imaging. (2020) 11:1–7. doi: 10.1186/s13244-020-0840-5
9. Liao J, Yang S, Chen K, Chen H, Jiang F, and Zhang W. A predictive model for first-trimester pregnancy inception after lVF-ET based on muhimodal ultrasound evaluation of endometrial receptivity. BMC Med Imaging. (2022) 22:158. doi: 10.1186/s12880-022-00863-w
10. Li M, Zhu X, Wang L, Fu H, Zhao W, and Zhou C. Evaluation of endometrial receptivity by ultrasound elastography to predict pregnancy outcome is a non-invasive and worthwhile method. Biotechnol Genet Eng Rev. (2024) 40:284–98. doi: 10.1080/02648725.2023.2183585
11. Krog MC, Flachs EM, Kolte AM, Jager W, Meyaard L, and Christiansen OB. Angiogenic factors and the lectin pathway of complement in women with secondary recurrent pregnancy loss. J Reprod Immunol. (2024) 163:104221. doi: 10.1016/j.jri.2024.104221
12. Tokgöz VY and Tekin AB. High basal LH levels are associated with improved cycle outcomes of assisted reproduction. J Surg Med. (2020) 4:152–6. doi: 10.28982/josam.666963
13. Wang J, Fei X, Zhou Y, Wei X, Zhuang Y, and Huang Y. Association between endometrial/subendometrial vasculature and embryo transfer outcome: A meta-analysis and subgroup analysis. J Ultrasound Med. (2018) 37:149–63. doi: 10.1002/jum.14319
14. Li ZS, Lu HO, Zhang YM, Wang YH, and Yu YX. Effect of endometrial receptivity evaluation on clinical pregnancy rate in infertile patients on the day of transplantation. Chin J Pract Gynecology Obstetrics. (2020) 36:264–6. doi: 10.19538/j.fk2020030118
15. Guo QQ, Sheng Y, Tang R, Sun M, and Ding LL. Influence of endometrial subendometrial blood flow before and after oocyte pick upon pregnancy outcome in single blastocyst transfer cycles. J Reprod Med. (2017) 26:1118–22. doi: 10.3969/j.issn.1004-3845.2017.11.014
16. Kim A, Han JE, Yoon TK, Lyu SW, Seok HH, and Won HJ. Relationship between endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound and pregnancy after intrauterine insemination. Fertility Sterility. (2010) 94:747–52. doi: 10.1016/j.fertnstert.2009.03.084
17. Sardana D, Upadhyay AJ, Deepika K, Pranesh GT, and Rao KA. Correlation of subendometrial-endometrial blood flow assessment by two-dimensional power Doppler with pregnancy outcome in frozen-thawed embryo transfer cycles. J Hum Reprod Sci. (2014) 7:130–5. doi: 10.4103/0974-1208.138872
18. Hazari V, Sarvi F, Alyasin A, Agha-Hosseini M, and Hosseinimousa S. Enhancing endometrial receptivity in FET cycles: exploring the influence of endometrial and subendometrial blood flow along with endometrial volume. Front Med. (2024) 11:1260960. doi: 10.3389/fmed.2024.1260960
19. Shui X, Yu C, Li J, and Jiao Y. Development and validation of a pregnancy prediction model based on ultrasonographic features related to endometrial receptivity. Am J Trans Res. (2021) 13:6156.
20. Chen M, He Y, Zhang P, Geng Q, Liu Q, and Kong L. Comparison of uterine receptivity between fertile and unexplained infertile women by assessment of endometrial and subendometrial perfusion using contrast-enhanced ultrasound: Which index is better—Peak intensity or area under the curve? Ultrasound Med Biol. (2016) 42:654–63. doi: 10.1016/j.ultrasmedbio.2015.11.008
21. Wang HM, Xiao YW, Ou Y, Chun Y, Liu XJ, and Chen Y. Evaluation of endometrial receptivity in infertile patients by contrast enhanced ultrasound. Chin J Med Ultrasound. (2020) 36:252–4.
22. Raine-Fenning NJ, Campbell BK, Kendall NR, Clewes JS, and Johnson IR. Quantifying the changes in endometrial vascularity throughout the normal menstrual cycle with three-dimensional power Doppler angiography. Hum Reprod. (2004) 19:330–8. doi: 10.1093/humrep/deh056
23. Kupesic S, Bekavac I, Bjelos D, and Kurjak A. Assessment of endometrial receptivity by transvaginal color Doppler and three-dimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J Ultrasound Med. (2001) 20:125–34. doi: 10.7863/jum.2001.20.2.125
Keywords: ultrasound parameters, endometrial contrast-enhanced ultrasound, IVF-ET, ongoing pregnancy, prediction model
Citation: Wang C, Lin J, Zhao X, Liu N, Sun P, Yang J and Zhou X (2025) Analysis of ultrasound parameters influencing endometrial receptivity and a pregnancy outcomes predictive model for patients undergoing in vitro fertilization and embryo transfer: a prospective study. Front. Endocrinol. 16:1677593. doi: 10.3389/fendo.2025.1677593
Received: 01 August 2025; Accepted: 30 October 2025;
Published: 18 November 2025.
Edited by:
Vladimir Tadic, Technical College of Applied Sciences, SerbiaReviewed by:
Mohammed J. Alsaadi, Prince Sattam bin Abdulaziz University, Saudi ArabiaArtur Bjelica, University of Novi Sad, Serbia
Copyright © 2025 Wang, Lin, Zhao, Liu, Sun, Yang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlian Wang, d2FuZ2NodW5saWFuNzgwMUAxNjMuY29t
†These authors have contributed equally to this work
 Chunlian Wang
Chunlian Wang Jiao Lin2†
Jiao Lin2† Xingping Zhao
Xingping Zhao