- 1Ningbo Key Laboratory of Nervous System and Brain Function, Department of Neurosurgery, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, Shandong, China
- 3Department of Internal Medicine, Qingdao Public Health Clinical Center, Qingdao, Shandong, China
Background: Diabetic peripheral neuropathy (DPN) is commonly observed as a long-term complication in patients with type 2 diabetes mellitus (T2DM). Recent evidence suggests that metabolic disturbances and chronic inflammation may contribute to its development. Lactate dehydrogenase (LDH), a key enzyme in glycolysis, may reflect underlying metabolic stress and inflammation, but its association with DPN remains unclear.
Methods: In this cross-sectional study, 2,060 patients with T2DM were analyzed to explore the relationship between serum LDH levels and DPN. Logistic regression and restricted cubic spline (RCS) models were used to assess linear and non-linear associations. Participants were also stratified by age, sex, hypertension, and HbA1c levels for subgroup analyses.
Results: Among the study population, 724 (35.1%) had DPN. Higher LDH levels were independently associated with an increased risk of DPN after adjusting for potential confounders (adjusted OR per 1 U/L increase: 1.00; 95% CI: 1.00–1.01; P = 0.01). RCS analysis showed a non-linear relationship, with a threshold at 142 U/L. Participants with LDH >142 U/L had significantly higher odds of DPN (adjusted OR: 1.21; 95% CI: 1.02–1.48; P = 0.033). This association was consistent across subgroups.
Conclusion: Serum LDH levels are significantly and non-linearly associated with DPN in individuals with T2DM. LDH may serve as a simple and cost-effective biomarker for identifying patients at elevated risk of neuropathy, warranting further prospective validation.
1 Introduction
The global prevalence of diabetes mellitus (DM) has been rising at an alarming rate, posing a substantial public health burden. As of 2021, approximately 537 million adults were living with diabetes, and this number is projected to reach 783 million by 2045 (1). Among its many complications, diabetic peripheral neuropathy (DPN) is one of the most common and debilitating microvascular consequences, affecting nearly half of individuals with type 2 diabetes mellitus (T2DM) (2–4). DPN not only leads to sensory and motor impairments but also increases the risk of diabetic foot ulcers, infections, non-traumatic amputations, falls, and mortality (5–7).
DPN is characterized by a chronic, progressive course with often irreversible nerve damage. Despite its clinical significance, the pathophysiological mechanisms underlying DPN remain incompletely understood. Accumulating evidence suggests that hyperglycemia-induced metabolic disturbances—including oxidative stress, mitochondrial dysfunction, inflammation, and microvascular insufficiency—play central roles in the development and progression of DPN (8, 9). Among these factors, oxidative stress is considered a key driver of peripheral nerve injury, as excessive reactive oxygen species (ROS) can impair both endothelial function and neuronal integrity (10, 11).
Lactate dehydrogenase (LDH), a key enzyme in glycolysis that catalyzes the interconversion of pyruvate and lactate, has recently attracted attention as a potential biomarker of metabolic stress and tissue injury. Under normal physiological conditions, LDH is primarily confined within cells, but its serum concentration increases with cell membrane damage and tissue hypoxia (12). Experimental studies have shown that hyperglycemia and hypoxia can elevate LDH levels in retinal cells, highlighting its possible involvement in diabetic microvascular complications (13).
In clinical contexts, elevated LDH has been associated with adverse outcomes in various diseases characterized by hypoxia, inflammation, and oxidative stress, including cardiovascular disease, malignancies, and diabetic nephropathy (14–16). Given these associations and the biological plausibility linking LDH to oxidative and metabolic stress, LDH may also serve as a systemic marker for DPN risk. However, few studies have systematically examined the association between serum LDH levels and the risk of DPN.
Therefore, this study aimed to investigate the association between serum LDH concentrations and the presence of DPN in a nationally representative cohort of individuals with T2DM. Additionally, we applied restricted cubic spline (RCS) modeling to explore potential non-linear relationships and identify clinically relevant threshold effects, with the goal of evaluating the potential utility of LDH as a biomarker for diabetic neuropathy.
2 Material and methods
2.1 Data source
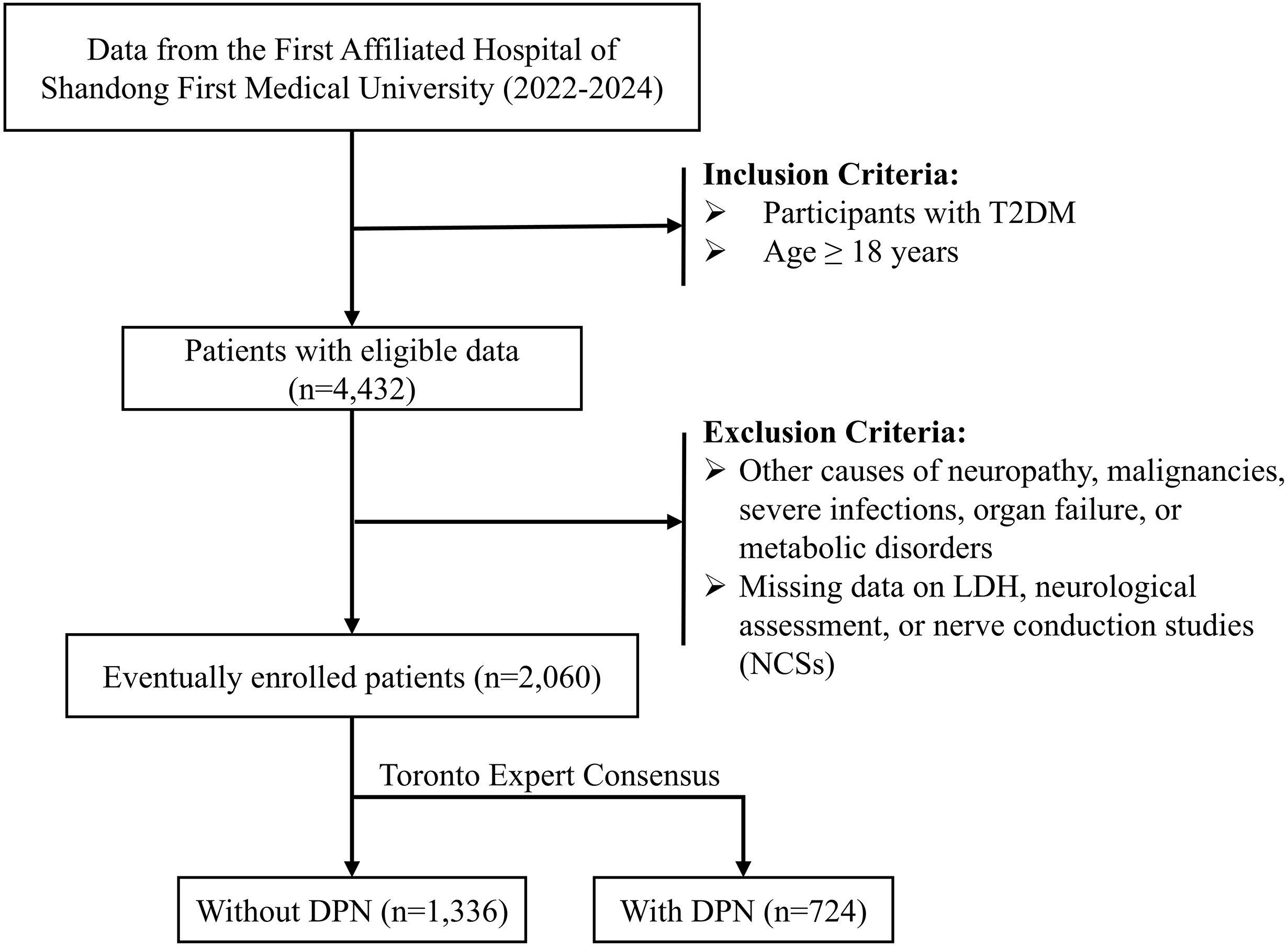
This retrospective study was carried out at the First Affiliated Hospital of Shandong First Medical University, including patients treated between January 2022 and December 2024. The inclusion criteria were as follows (1): patients aged 18 years or older; and (2) a diagnosis of T2DM established according to the 1999 World Health Organization (WHO) criteria. The exclusion criteria were as follows (1): neuropathy due to other identifiable causes, such as malignancies, severe infections, organ failure, or metabolic disorders; and (2) missing information on serum LDH, neurological assessment, or nerve conduction studies (NCSs). A detailed flowchart of the participant selection process is presented in Figure 1. This study was approved by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (Approval No. S654) and conducted in accordance with the principles of the Declaration of Helsinki.
2.2 Covariates
In this study, a range of covariates potentially associated with DPN were collected and included in the analysis. Demographic and lifestyle factors consisted of sex, age, body mass index (BMI), smoking status, and alcohol consumption history. Laboratory parameters encompassed hematological indices such as white blood cell count (WBC), lymphocyte count (Lymph), monocyte count (Mono), neutrophil count (Neut), platelet count (PLT), and hemoglobin (Hb). Liver and kidney function indicators included alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), serum creatinine (SCR), albumin (ALB), globulin (GLB), urea, and uric acid (UA). Lipid profile variables comprised total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). In addition, lactate dehydrogenase (LDH), alkaline phosphatase (ALP), glycated hemoglobin (HbA1c), and diabetes duration were also recorded as covariates.
2.3 Statistical analysis
Descriptive statistics for the continuous and categorical variables were reported in the form of means, SDs, and frequencies, respectively. Group comparisons were performed using the t-test for continuous variables and the chi-square test for categorical variables.
Logistic regression models were applied to evaluate the association between LDH levels and the risk of DPN, with results presented as odds ratios (ORs) and 95% confidence intervals (CIs). Model 1 was unadjusted. Model 2 was adjusted for age, sex, BMI, smoking status, and alcohol consumption history. Model 3 was fully adjusted, incorporating all potential confounders, including sex, age, BMI, smoking status, alcohol consumption history, WBC, Lymph, Mono, Neut, PLT, Hb, ALT, AST, TBil, SCR, ALB, GLB, urea, UA, TC, TG, HDL-C, LDL-C, ALP, HbA1c, and diabetes duration.
To assess the potential association between LDH and DPN, a restricted cubic spline (RCS) analysis was conducted based on complex sampling logistic regression. Subgroup analyses and interaction tests were performed to explore effect modification by sex, age (<65 vs. ≥65 years), hypertension, and HbA1c level (<7% vs. ≥7%). Heterogeneity across subgroups was evaluated using multivariable logistic regression models including interaction terms. All statistical analyses were conducted using R software (version 4.4.2). A two-sided P-value < 0.05 was considered statistically significant.
3 Result
3.1 Baseline characteristics
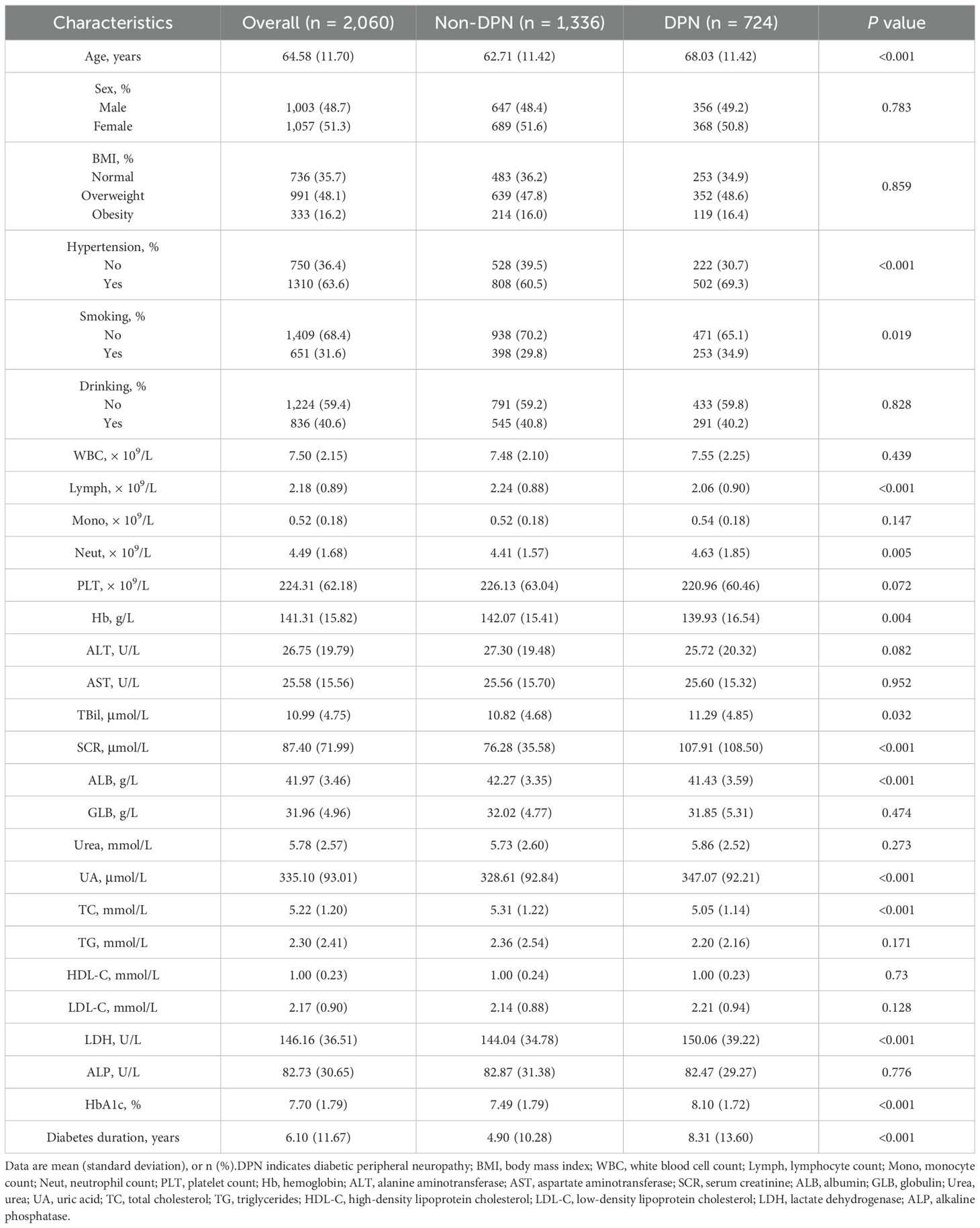
Table 1 summarizes the baseline characteristics of the 2,060 patients with T2DM included in this study, among whom 724 (35.1%) were diagnosed with DPN. The overall mean age was 60.58 (11.70) years, and 1,057 (51.3%) were female. The mean serum LDH level was 146.16 U/L. Compared to patients without DPN, those with DPN were significantly older and had a higher proportion of hypertension and smoking history (all P < 0.05). In terms of laboratory parameters, patients with DPN exhibited significantly lower levels of Lymph, Hb, ALB, and TC, while showing elevated levels of Neut, TBil, SCR, LDH, UA, and longer duration of diabetes (all P < 0.05).
3.2 Association between LDH and DPN
The results of the univariate logistic regression analysis revealed a significant association between serum LDH levels and the risk of DPN. Specifically, each 1 U/L increase in LDH was associated with a higher risk of DPN (OR: 1.01; 95% CI: 1.00–1.01; P < 0.001) (Figure 2). This association remained robust after adjusting for potential confounding factors. In Model 2, which adjusted for age, sex, body mass index (BMI), smoking status, and alcohol consumption, the association persisted (OR: 1.00; 95% CI: 1.00–1.01; P < 0.001). In the fully adjusted Model 3, which further included hypertension, HbA1c levels, and other relevant laboratory parameters, LDH remained independently associated with DPN risk (OR: 1.00; 95% CI: 1.00–1.01; P = 0.01). Although the effect size per unit increase was small, given the broad distribution of LDH levels in the population, this cumulative effect may be of clinical significance.
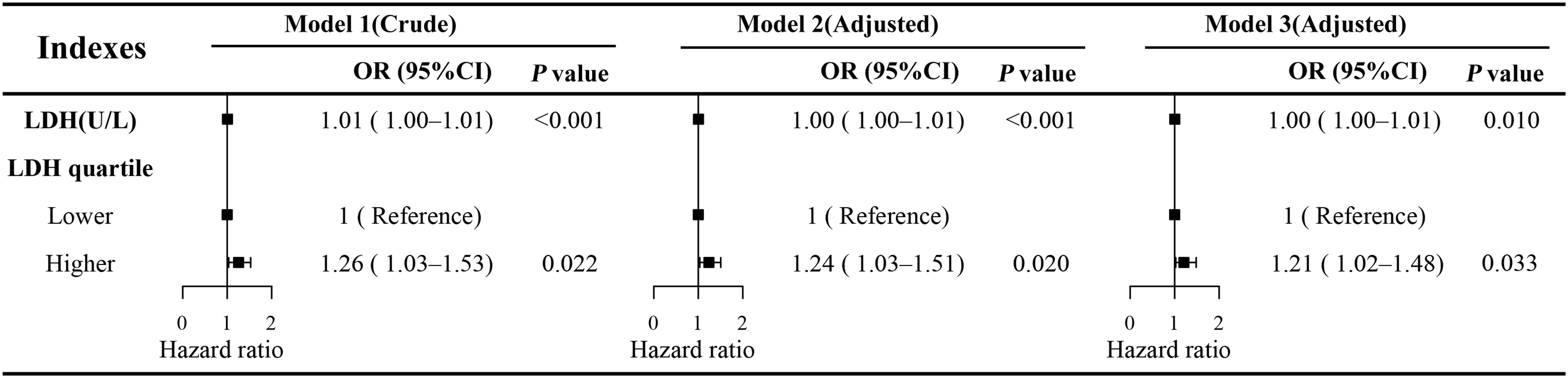
Figure 2. Multivariate logistic regression models of LDH and DPN. Model 1 was unadjusted. Model 2 was adjusted for age, sex, BMI, smoking status, and alcohol consumption history. Model 3 was fully adjusted for all potential confounders, including age, sex, BMI, smoking status, alcohol consumption history, white blood cell count (WBC), lymphocyte count (Lymph), monocyte count (Mono), neutrophil count (Neut), platelet count (PLT), hemoglobin (Hb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), serum creatinine (SCR), albumin (ALB), globulin (GLB), urea, uric acid (UA), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alkaline phosphatase (ALP), glycated hemoglobin (HbA1c), and diabetes duration. OR, odds ratio; CI, confidence interval; LDH, lactate dehydrogenase; DPN, diabetic peripheral neuropathy.
To further explore the dose–response relationship, the RCS regression model was applied (Figure 3). The RCS analysis revealed a significant non-linear association between continuous LDH levels and DPN risk (P for non-linearity < 0.001), with an inflection point observed at approximately 142 U/L. Based on this threshold, LDH was subsequently categorized into two groups: low LDH (≤142 U/L) and high LDH (>142 U/L). Multivariate logistic regression analysis demonstrated that individuals in the high LDH group had a significantly greater risk of developing DPN compared to those in the low LDH group (OR: 1.21; 95% CI: 1.02–1.48; P = 0.033) (Figure 2).
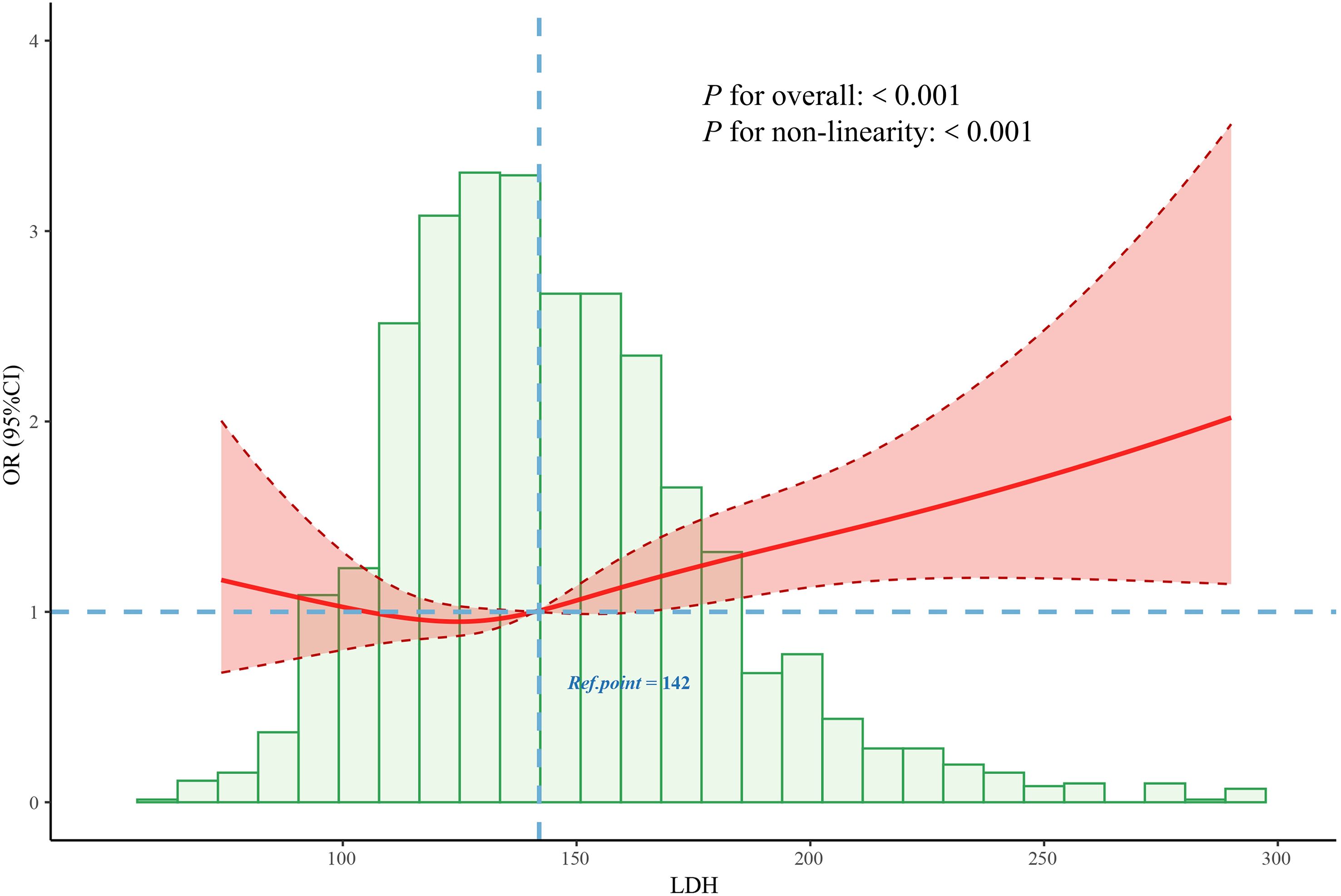
Figure 3. Association between the LDH and DPN odds ratio. The solid line represents the estimated odds ratio, and the shaded area indicates the 95% confidence interval. LDH, lactate dehydrogenase; DPN, diabetic peripheral neuropathy.
Stratified analyses showed that the association between LDH levels and the risk of DPN remained consistent across subgroups defined by age (<65 vs. ≥65 years), sex (male vs. female), hypertension (yes vs. no), and HbA1c (<7.0% vs. ≥7.0%) (Figure 4). No significant interaction was observed in any subgroup (all P for interaction > 0.05).
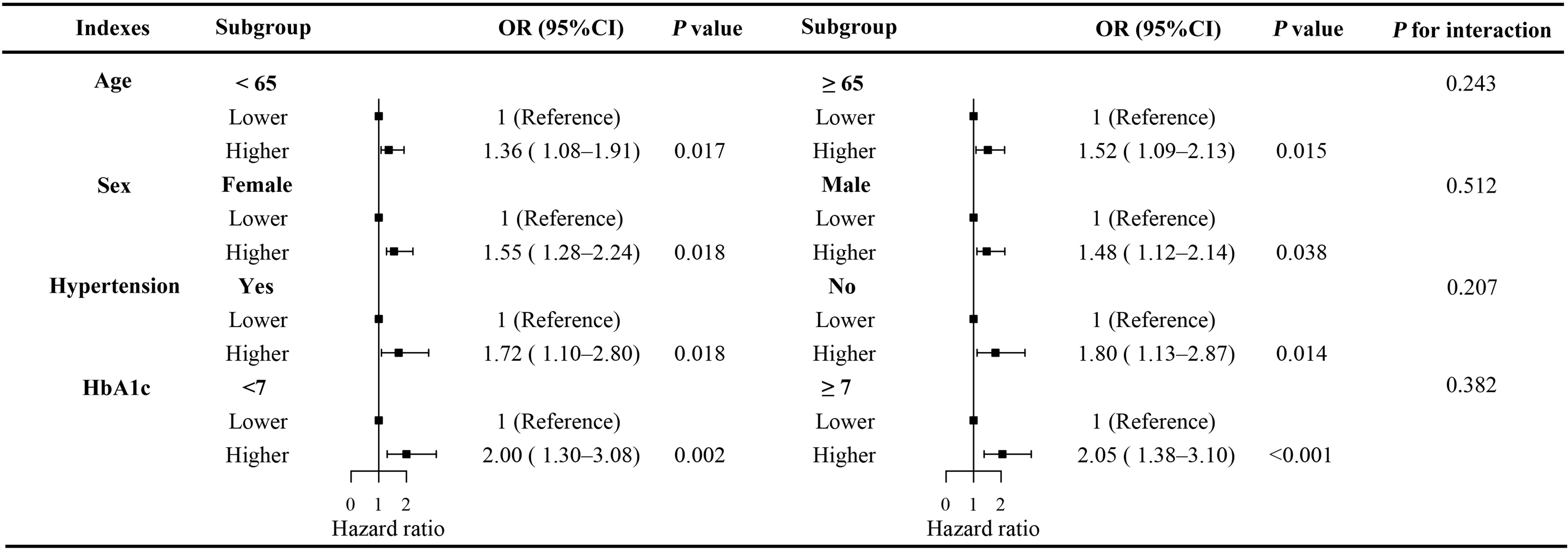
Figure 4. Stratified analysis of the association between serum LDH levels and the risk of DPN across subgroups. Subgroup analyses were performed based on age (<65 vs. ≥65 years), sex (male vs. female), hypertension (yes vs. no), and HbA1c (<7.0% vs. ≥7.0%). LDH, lactate dehydrogenase; DPN, diabetic peripheral neuropathy; OR, odds ratio; CI, confidence interval.
4 Discussion
In this cross-sectional study, we found that higher serum LDH levels were independently associated with an increased risk of DPN in individuals with T2DM. This association persisted after controlling for multiple confounders, and a non-linear relationship was observed using RCS analysis, with a turning point at 142 U/L. Participants with LDH levels >142 U/L exhibited significantly higher odds of DPN compared to those with LDH ≤142 U/L. These findings were consistent across subgroups stratified by age, sex, hypertension status, and HbA1c level, with no significant interaction effects detected.
In our univariate analysis, DPN was associated with several clinical and biochemical abnormalities. Patients with DPN were older and more likely to have hypertension and a history of smoking, suggesting the cumulative impact of aging and vascular comorbidities on neural degeneration in diabetes (17, 18). Consistent with previous studies, higher HbA1c levels and longer diabetes duration were observed in the DPN group, highlighting the role of chronic hyperglycemia in exacerbating peripheral nerve damage (19, 20). Furthermore, DPN patients exhibited lower lymphocyte counts, hemoglobin, albumin, and total cholesterol levels, alongside elevated neutrophil counts, bilirubin, serum creatinine, uric acid, and LDH, which may reflect a systemic milieu of inflammation, oxidative stress, nutritional deficiency, and subclinical organ dysfunction—all contributing factors in the pathogenesis of DPN (19, 21).
Although LDH is not traditionally recognized as a biomarker for DPN, several biological mechanisms may underlie the observed association. LDH is a key enzyme in anaerobic glycolysis, and elevated LDH may reflect increased anaerobic metabolism, mitochondrial dysfunction, or systemic metabolic stress (22, 23). In the context of diabetes, chronic hyperglycemia induces oxidative stress, promotes the formation of advanced glycation end-products (AGEs), and triggers inflammatory responses, all of which contribute to peripheral nerve damage (24). Moreover, LDH is released during tissue injury and inflammatory processes. Recent studies suggest that low-grade systemic inflammation plays a pivotal role in the development of DPN by activating immune cells, promoting endothelial dysfunction, and impairing microvascular circulation to peripheral nerves (25–30). Therefore, elevated LDH levels may serve as a surrogate marker for ongoing subclinical inflammation and neuronal damage.
To date, few studies have explored the relationship between LDH and DPN specifically. However, elevated LDH has been associated with diabetic complications such as nephropathy and retinopathy (13, 16), which share common pathogenic mechanisms with DPN, including microvascular dysfunction and chronic inflammation. Our study adds novel evidence to this limited body of research by demonstrating a significant, dose-dependent relationship between LDH and DPN using both linear and non-linear modeling approaches.
Importantly, LDH is a routinely tested, low-cost biomarker in clinical settings. The identification of a specific threshold (142 U/L) linked to increased DPN risk offers practical value for early screening and risk stratification in diabetic patients. Individuals with elevated LDH may benefit from closer clinical monitoring, comprehensive metabolic assessment, and early preventive interventions targeting neuropathy.
Several limitations should be acknowledged. First, the cross-sectional design precludes conclusions about causality or the temporal sequence between elevated LDH and DPN onset. Second, although we adjusted for a wide range of potential confounders, residual confounding from unmeasured variables (e.g., medications, infections, or unrecorded comorbidities) cannot be excluded. Third, LDH levels were measured at a single time point, which may not fully reflect long-term metabolic or inflammatory status.
5 Conclusion
In conclusion, our findings reveal a significant and non-linear association between serum LDH levels and the risk of DPN in patients with T2DM. These results suggest that LDH may serve as an accessible biomarker for identifying individuals at higher risk of neuropathy. Future prospective studies and experimental investigations are warranted to validate these findings and to explore the underlying biological pathways linking LDH to peripheral nerve injury.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (Hospital Ethics Review No. S654). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin. The requirement for written informed consent was waived by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University due to the retrospective nature of the study and the use of de-identified data, which posed minimal risk to participants.
Author contributions
MS: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft. ZW: Conceptualization, Investigation, Methodology, Writing – original draft. XY: Investigation, Methodology, Writing – original draft. HS: Investigation, Software, Writing – original draft. HY: Investigation, Project administration, Writing – original draft. YQ: Investigation, Methodology, Writing – original draft. XG: Investigation, Software, Writing – review & editing. YH: Software, Supervision, Writing – review & editing. JS: Investigation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the grants from the Ningbo Top Medical and Health Research Program (2022020304), Ningbo Clinical Research Center for Emergency and Critical Diseases (2024L003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Lin YK, Gao B, Liu L, Ang L, Mizokami-Stout K, Pop-Busui R, et al. The prevalence of diabetic microvascular complications in China and the USA. Curr Diabetes Rep. (2021) 21:16. doi: 10.1007/s11892-021-01387-3
3. He D, Gao B, Wang J, Yang C, Zhao MH, and Zhang L. The difference between cystatin C- and creatinine-based estimated glomerular filtration rate and risk of diabetic microvascular complications among adults with diabetes: A population-based cohort study. Diabetes Care. (2024) 47:873–80. doi: 10.2337/dc23-2364
4. Jiang A, Li J, Wang L, Zha W, Lin Y, Zhao J, et al. Multi-feature, Chinese-Western medicine-integrated prediction model for diabetic peripheral neuropathy based on machine learning and SHAP. Diabetes Metab Res Rev. (2024) 40:e3801. doi: 10.1002/dmrr.3801
5. Ziegler D. Pathogenetic treatments for diabetic peripheral neuropathy. Diabetes Res Clin Pract. (2023) 206 Suppl 1:110764. doi: 10.1016/j.diabres.2023.110764
6. Javed S, Alam U, and Malik RA. Burning through the pain: treatments for diabetic neuropathy. Diabetes Obes Metab. (2015) 17:1115–25. doi: 10.1111/dom.12535
7. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract. (2014) 103:522–9. doi: 10.1016/j.diabres.2013.12.003
8. Gonzalez P, Lozano P, Ros G, and Solano F. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. Int J Mol Sci. (2023) 24(11):9352. doi: 10.3390/ijms24119352
9. Yao L, Liang X, Qiao Y, Chen B, Wang P, and Liu Z. Mitochondrial dysfunction in diabetic tubulopathy. Metabolism. (2022) 131:155195. doi: 10.1016/j.metabol.2022.155195
10. Singh A, Kukreti R, Saso L, and Kukreti S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules. (2019) 24(8):9352. doi: 10.3390/molecules24081583
11. Wen P, Sun Z, Gou F, Wang J, Fan Q, Zhao D, et al. Oxidative stress and mitochondrial impairment: Key drivers in neurodegenerative disorders. Ageing Res Rev. (2025) 104:102667. doi: 10.1016/j.arr.2025.102667
12. Klein R, Nagy O, Tothova C, and Chovanova F. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet Med Int. (2020) 2020:5346483. doi: 10.1155/2020/5346483
13. Yang P, Xu W, Liu L, and Yang G. Association of lactate dehydrogenase and diabetic retinopathy in US adults with diabetes mellitus. J Diabetes. (2024) 16:e13476. doi: 10.1111/1753-0407.13476
14. Wu Y, Lu C, Pan N, Zhang M, An Y, Xu M, et al. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep. (2021) 11:12997. doi: 10.1038/s41598-021-92430-6
15. Forkasiewicz A, Dorociak M, Stach K, Szelachowski P, Tabola R, and Augoff K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol Biol Lett. (2020) 25:35. doi: 10.1186/s11658-020-00228-7
16. Tang L, Yang Q, Ma R, Zhou P, Peng C, Xie C, et al. Association between lactate dehydrogenase and the risk of diabetic kidney disease in patients with type 2 diabetes. Front Endocrinol (Lausanne). (2024) 15:1369968. doi: 10.3389/fendo.2024.1369968
17. Donato AJ, Machin DR, and Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. (2018) 123:825–48. doi: 10.1161/CIRCRESAHA.118.312563
18. Gregory JA, Jolivalt CG, Goor J, Mizisin AP, and Calcutt NA. Hypertension-induced peripheral neuropathy and the combined effects of hypertension and diabetes on nerve structure and function in rats. Acta Neuropathol. (2012) 124:561–73. doi: 10.1007/s00401-012-1012-6
19. Sun M, Sun X, Wang F, and Liu L. Machine learning-based prediction of diabetic peripheral neuropathy: model development and clinical validation. Front Endocrinol (Lausanne). (2025) 16:1614657. doi: 10.3389/fendo.2025.1614657
20. Yan T, Dou Z, Claire M, Ellen K, and Caroline M. Risk factors for first-ever diabetes-related foot ulcer: A systematic review and meta-analysis. Int Wound J. (2025) 22:e70728. doi: 10.1111/iwj.70728
21. Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. (2018) 40:828–49. doi: 10.1016/j.clinthera.2018.04.001
22. Sharma D, Singh M, and Rani R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. (2022) 87:184–95. doi: 10.1016/j.semcancer.2022.11.007
23. Huang L, Lu Z, Zhou X, He L, You X, Chen C, et al. U-shaped relationship between serum lactate dehydrogenase with all-cause mortality in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2023) 18:305–16. doi: 10.2147/COPD.S386269
24. Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, et al. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes. (2009) 58:2893–903. doi: 10.2337/db09-0320
25. Kim TW, Oum SJ, Lim JH, Kim Y, Kim EN, Jin Y, et al. Strengthening monocarboxylate transporters by adiponectin receptor agonist ameliorates diabetic peripheral neuropathy. Cell Commun Signal. (2025) 23:342. doi: 10.1186/s12964-025-02326-5
26. Yang C, Yu H, Zhou L, Su H, Li X, Qi W, et al. Tang Bi formula alleviates diabetic sciatic neuropathy via AMPK/PGC-1alpha/MFN2 pathway activation. Sci Rep. (2025) 15:25069. doi: 10.1038/s41598-025-10513-0
27. Yao X, Wang X, Zhang R, Kong L, Fan C, and Qian Y. Dysregulated mast cell activation induced by diabetic milieu exacerbates the progression of diabetic peripheral neuropathy in mice. Nat Commun. (2025) 16:4170. doi: 10.1038/s41467-025-59562-z
28. Roy B. Pathophysiological mechanisms of diabetes-induced macrovascular and microvascular complications: the role of oxidative stress. Med Sci (Basel). (2025) 13(3):87. doi: 10.3390/medsci13030087
29. Mauricio D, Gratacos M, and Franch-Nadal J. Diabetic microvascular disease in non-classical beds: the hidden impact beyond the retina, the kidney, and the peripheral nerves. Cardiovasc Diabetol. (2023) 22:314. doi: 10.1186/s12933-023-02056-3
Keywords: type 2 diabetes mellitus, diabetic peripheral neuropathy, lactate dehydrogenase, biomarker, risk assessment
Citation: Sun M, Wang Z, Yan X, Shen H, Yang H, Qi Y, Gao X, Huang Y and Sun J (2025) Relationship between serum LDH levels and diabetic peripheral neuropathy in type 2 diabetic patients. Front. Endocrinol. 16:1680539. doi: 10.3389/fendo.2025.1680539
Received: 21 August 2025; Accepted: 15 October 2025;
Published: 29 October 2025.
Edited by:
Beatrice Dufrusine, University of Teramo, ItalyReviewed by:
Maree Therese Smith, The University of Queensland, AustraliaParamita Basu, University of Pittsburgh, United States
Hanefi Özbek, Usak Universitesi, Türkiye
Copyright © 2025 Sun, Wang, Yan, Shen, Yang, Qi, Gao, Huang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Huang, aHVhbmd5MTAyQGdtYWlsLmNvbQ==; Jie Sun, Znl5c3VuamllQG5idS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Meng Sun
Meng Sun Zhaodi Wang
Zhaodi Wang Xu Yan1
Xu Yan1 Hongqiao Yang
Hongqiao Yang Xiang Gao
Xiang Gao Yi Huang
Yi Huang Jie Sun
Jie Sun