- 1The State Key Laboratory of Mechanism and Quality of Chinese Medicine/Faculty of Chinese Medicine, Macau University of Science and Technology, Macao, Macao SAR, China
- 2The State Key Laboratory of Mechanism and Quality of Chinese Medicine, Macau University of Science and Technology, Macao, Macao SAR, China
- 3The Precision Regenerative Medicine Research Centre, Macau University of Science and Technology, Macao, Macao SAR, China
Dendrobium officinale is a traditional Chinese medicinal herb that has been extensively documented in classical medical texts for its effectiveness in treating diabetes mellitus. Modern pharmacological studies have shown that it possesses antitumor, antioxidant, immunomodulatory, and blood glucose- and lipid-lowering effects. Dendrobium officinale polysaccharides (DOPs), the main bioactive constituent of this herbal medicine, interact with the gut microbiota to reshape microbial composition, restore intestinal barrier integrity, modulate mucosal immunity, and ultimately ameliorate metabolic disorders. This review highlights the structural characteristics and bioactivities of DOPs, as well as the mechanisms by which gut microbiota are involved in the pathogenesis of diabetes mellitus. In particular, we point out that DOPs have significantly improved metabolic indicators related to diabetes by regulating intestinal microbiota. It aims to clarify the benefits of DOPs in ameliorating diabetes mellitus through gut microbiota modulation and provide new perspectives for its potential development as a prebiotic and for future clinical applications.
1 Introduction
Diabetes mellitus is a chronic metabolic disease featured by persistent hyperglycemia. Diabetes mellitus is primarily categorized as type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), gestational diabetes mellitus(GDM), and other specific types (1, 2). In recent years, the incidence of diabetes mellitus has been increasing with a trend towards affecting younger people (3). According to the International Diabetes Federation (IDF), there will be approximately 589 million adult patients with diabetes mellitus (ages 20-79) worldwide in 2024, and up to 853 million patients can be expected by 2050 (4). Type 1 diabetes mellitus (T1DM) develops through an autoimmune attack on pancreatic β-cells, involving autoreactive CD4+ and CD8+ T lymphocytes, as well as activated macrophages (5). Genetic factors, such as HLA (DR3-DQ2/DR4-DQ8) and non-HLA genes (e.g., CTLA-4, PTPN22, VNTR) trigger autoimmunity and the formation of islet autoantibodies; Environmental factors, like Coxsackie B virus and gut dysbiosis-related decreased butyrate, contribute to β-cell stress and intestinal barrier disruption, leading to the translocation of bacteria/metabolites to pancreatic lymph nodes. Crossover antigens, such as viral or microbiota proteins, activate autoreactive CD4+/CD8+ T cells and macrophages through molecular mimicry, causing islet inflammation, β-cell destruction and lack of endogenous insulin secretion (5, 6).
The core mechanisms of T2DM involve insulin resistance and impaired β-cell function. Genetic predisposition and unhealthy lifestyle factors, such as chronic excess caloric intake and sedentary behavior, work together to reduce insulin sensitivity, downregulate GLP-1/GIP receptors, weaken the cAMP-PKA signal, and block the IRS1-PI3K-AKT and FOXO1 pathways. Additionally, the secretion of TNF-α and IL-6 increases, activating the IKKβ/JNK pathway, phosphorylating IRS1/2 (serine site), and blocking insulin signaling (7, 8). Gestational diabetes mellitus is the condition of abnormal glucose metabolism during pregnancy, marked by increased insulin resistance and insufficient relative insulin secretion (9). Gestational diabetes mellitus is typically diagnosed in mid to late pregnancy but can also happen in early pregnancy (10). Regardless of the subtype of diabetes mellitus, the common pathological outcome is persistent hyperglycemia and systemic metabolic dysregulation resulting from absolute or relative insulin deficiency (11).
The gut microbiota is the microbial community within the human intestinal tract and is the most extensive biological system within the body (12). It is important in maintaining host homeostasis (13). Increasing evidence suggests that gut dysbiosis is closely associated with the progression of diabetes mellitus. This association is mechanistically supported by microbiota-derived metabolites, modulation of intestinal barrier integrity, activation of inflammatory signaling networks, and coordination of immune responses. These factors collectively perturb the balance between insulin sensitivity and β-cell function (14, 15).
As a classical traditional Chinese medicinal herb, D. officinale exhibits broad pharmacological activities and has long been esteemed in traditional Chinese medicine for its capacity to benefit the stomach and promote fluid production, nourish yin and clear heat. Its principal bioactive constituent, DOP, is composed of 1,4-β-D-mannose, 1,4-β-D-glucose, and O-acetyl groups, conferring water solubility and non-starch characteristics (16). DOPs not only exhibit immunomodulatory, antioxidant, and antitumor activities but also possess hypoglycemic effects and can improve intestinal homeostasis (17). The International Scientific Association for Probiotics and Prebiotics (ISAPP) recently defined a prebiotic as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (18). DOPs exhibit resistance to digestion, thereby promoting the proliferation of beneficial gut bacteria. These bacteria ferment DOPs to produce short-chain fatty acids (SCFAs), which contribute to human health. These characteristics align with the definition of prebiotics. Therefore, elucidating the effects of DOPs on diabetes mellitus may hold significant clinical relevance. This review summarizes DOPs’ chemical structure, physicochemical properties, and bioactivities, while evaluating recent advancements in their ability to modulate gut microbiota and ameliorate diabetes. By elucidating underlying mechanisms and delineating prospective applications, we aim to provide novel insights and directions for the therapeutic exploitation of DOPs, either as a pharmacological agent or as a potential prebiotic, in the management of diabetes.
2 The structure and characteristics of Dendrobium officinale polysaccharides
D. officinale is an epiphytic herb of the genus Dendrobium in the Orchid family, which is native to East Asia and is predominantly distributed in the southern areas of China (19). In Traditional Chinese Medicine, D. officinale is believed to benefit stomach and promote fluid production while nourishing yin and clearing heat and is therefore prescribed for fluid impairment caused by febrile disease, dry mouth and polydipsia (20). The stems of D. officinale represent the principal medicinal part, rich in polysaccharides, phenanthrenes, flavonoids, alkaloids, and other bioactive metabolites (21, 22). DOPs have received considerable attention in recent years due to their multifaceted bioactivity, including immunomodulatory, antitumor, hypoglycemic, anti-inflammatory, and antioxidant effects (23, 24). DOPs are mainly composed of D-mannose and D-glucose linked by β-1,4 glycosidic bonds, and contain acetyl groups; the polysaccharide content is related to factors such as species, light, humidity, and temperature (19).
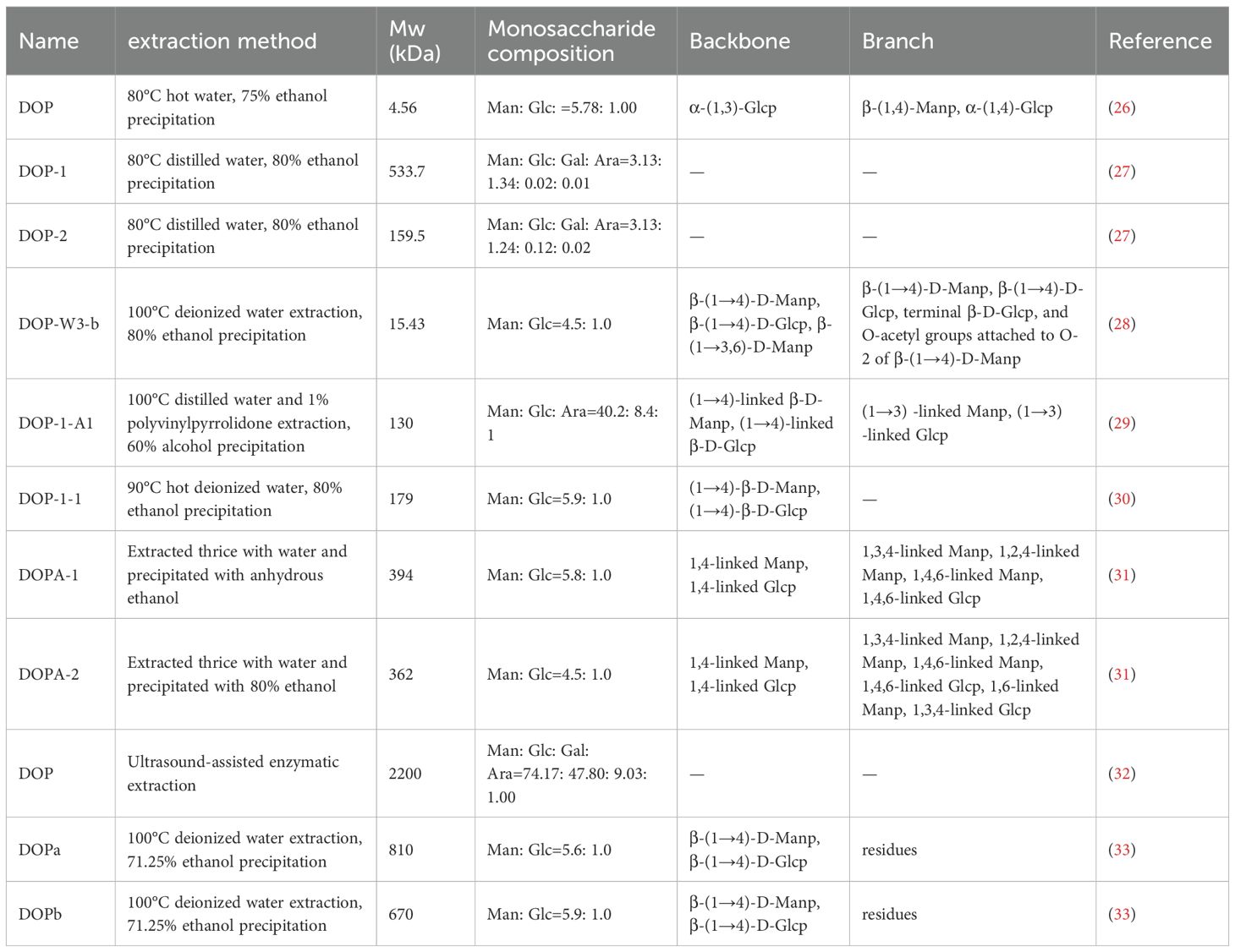
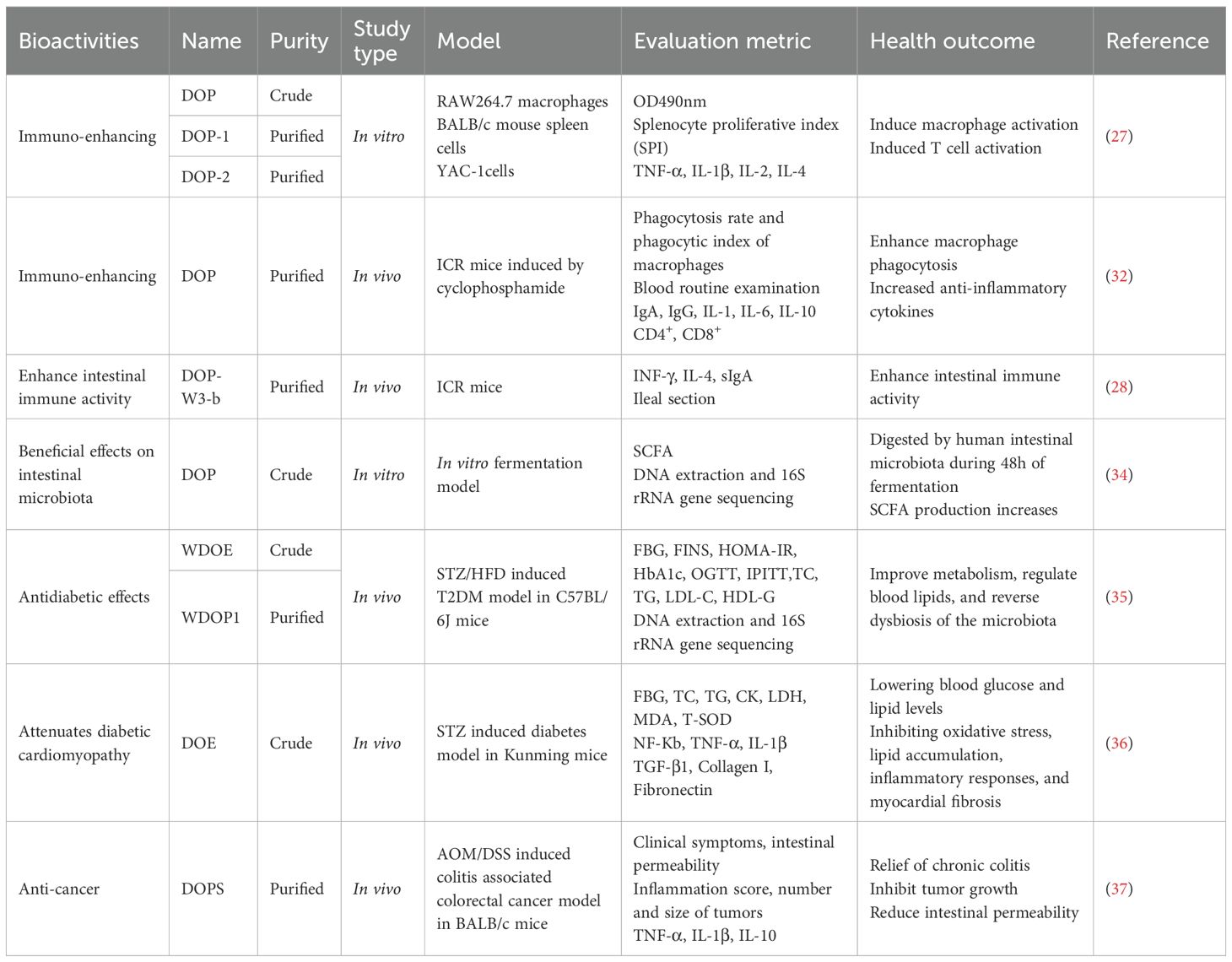
At present, various protocols have been developed for extracting DOPs, with the most used method being hot water extraction (HWE), cold-pressing (CP), freeze-thawing cold-pressing (FTCP), ultrasound-assisted hot water extraction (UHWE), microwave-assisted hot water extraction (MHWE), and enzyme-assisted hot water extraction (EHWE). Comparative studies have shown that FTCP provides the highest extraction yield and the most potent antioxidant activity, making it the preferred technique for DOPs recovery (25). Due to the different extraction efficiencies of these methods, the resulting polysaccharides vary in chemical composition, molecular weight, and macromolecular structure, all of which impact their bioactivities. The main structural features of some DOPs are summarized in Table 1. The biological activities and research methods of some DOPs are summarized in Table 2.
Sun et al. (26) isolated a 4.56-kDa low-molecular-weight glucomannan (DOPs) from the 75% ethanol supernatant of D. officinale. These DOPs had a mannose-to-glucose molar ratio of 5.78:1.00, an α-(1,3)-Glcp main chain and α-(1,4)-Glcp plus β-(1,4)-Manp branches. It showed dose-dependent reversal of CTX-induced immune suppression and oxidative damage in mice by increasing immune organ indices, enhancing immune cell activity, and up-regulating IL-2, IFN-γ, TNF-α, SOD, and GSH-Px levels. Wang et al. (38) obtained DOPS-1 (1,530 kDa) from D. officinale stems using conventional hot-water extraction. This polysaccharide had mannose, glucose, and galacturonic acid ratio of 3.2: 1.3: 1 and contained (1→4)-β-D-Glcp, (1→4)-β-D-Manp, and 2-O-acetyl-(1→4)-β-D-Manp residues, giving it both antioxidant and antitumor properties. Studies have shown that the high mannose content of DOPs is linked to its pancreatic lipase-inhibitory activity, the degree of branching is associated with α-amylase inhibition, and the branched structure contributes to intestinal immunomodulatory effects (28, 39). Xie et al. (28) extracted D. officinale stem powder with 80% ethanol, followed by sequential elution with water and NaCl solution to yield the neutral fraction DOP-W. The activity of DOP-W surpassed that of the acidic fraction DOP-S. Subsequent precipitation and purification identified the most potent subfraction as DOP-W3-b, which exhibited a molecular weight of 1.543 × 104 Da, a mannose-to-glucose molar ratio of 4.5:1.0, and a pronounced enhancement of intestinal immune responses in mice. Its immunomodulatory activity significantly exceeded that of W3-a (+18.9%) and W3-c (+53.5%). The drying protocol significantly affects the polysaccharide content of D. officinale. Freeze-drying is optimal for preserving the glucomannan-rich DOPs fraction (40). Collectively, these diverse DOPs extraction methods may lead to different compositions of DOPs and varied bioactivities.
3 Relationship between gut microbiota and diabetes mellitus
3.1 Composition and functions of gut microbiota
The human gastrointestinal tract houses most host-associated microorganisms, whose combined biomass exceeds that of human somatic cells by approximately one order of magnitude (41). The gut microbiota is made up of distinct bacterial phyla dominated by Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, which respectively make up 64%, 23%, 8%, and 3% of the community (42, 43). Different microbial taxa have specific functions and can be categorized as beneficial, neutral, and harmful bacteria based on their impact on human health. Early infancy is a critical period for establishing microbiota, during which microbial diversity increases rapidly and later stabilizes due to the combined effects of host genetics and environmental factors (12). Under normal conditions, the gut microbiota maintains a relatively stable equilibrium which is essential for human health; however, its dysbiosis can result in the development of various diseases, including obesity, allergic diseases, central nervous system disorders, and diabetes mellitus (44, 45).
3.2 Alterations of the gut microbiota in diabetes mellitus
Accumulating evidence consistently demonstrates that the composition of gut microbiota is significantly imbalanced in individuals with diabetes mellitus (Figure 1). Larsen et al. (46) were the first to show significant compositional differences between the intestinal microbiota of diabetic patients and healthy people. Specifically, the Bacteroidetes/Firmicutes ratio was significantly higher in the diabetic cohort and correlated positively with plasma glucose levels. Additionally, the relative abundance of the Clostridia class was dramatically reduced, while the Betaproteobacteria class was significantly enriched and also positively correlated with blood glucose levels (46). Qin et al. (47) identified around 60,000 markers associated with type 2 diabetes mellitus (T2DM) by conducting deep sequencing of gut microbial DNA from 345 Chinese individuals, including T2DM patients and non-diabetic controls. They revealed a decrease in beneficial bacteria and an increase in harmful bacteria in the T2DM gut by comparing the prevalence of genetic and functional markers between T2DM patients and non-diabetic controls. At the functional level, genes involved in membrane transport, oxidative stress resistance, and xenobiotic tolerance were enriched, while those related to bacterial motility, cofactor and vitamin metabolism, and butyrate biosynthesis were diminished (47). Murri et al. (48) provided the first evidence linking T1DM to alterations in the gut microbiome. Children with T1DM showed a significant reduction in Actinobacteria and Firmicutes, along with a notable increase in Bacteroidetes, resulting in a markedly decreased Firmicutes/Bacteroidetes ratio compared to healthy peers. Gestational diabetes mellitus (GDM) is also accompanied by distinct microbial shifts. Bifidobacterium is less abundant in GDM patients than that in normoglycemic pregnant women (49). In pregnant women with gestational diabetes, the abundance of the Gammaproteobacteria class and its associated genus Haemophilus is significantly increased, which is correlated with elevated levels of C-Reactive Protein (50). Interestingly, some studies suggest that despite the significant alterations in gut microbiota and the increased inter-individual variability observed during late pregnancy, with overall increases in Proteobacteria and Actinobacteria that can induce symptoms resembling metabolic syndrome, these changes are beneficial in the context of pregnancy, as they help provide energy for the fetus and prepare for lactation (51).
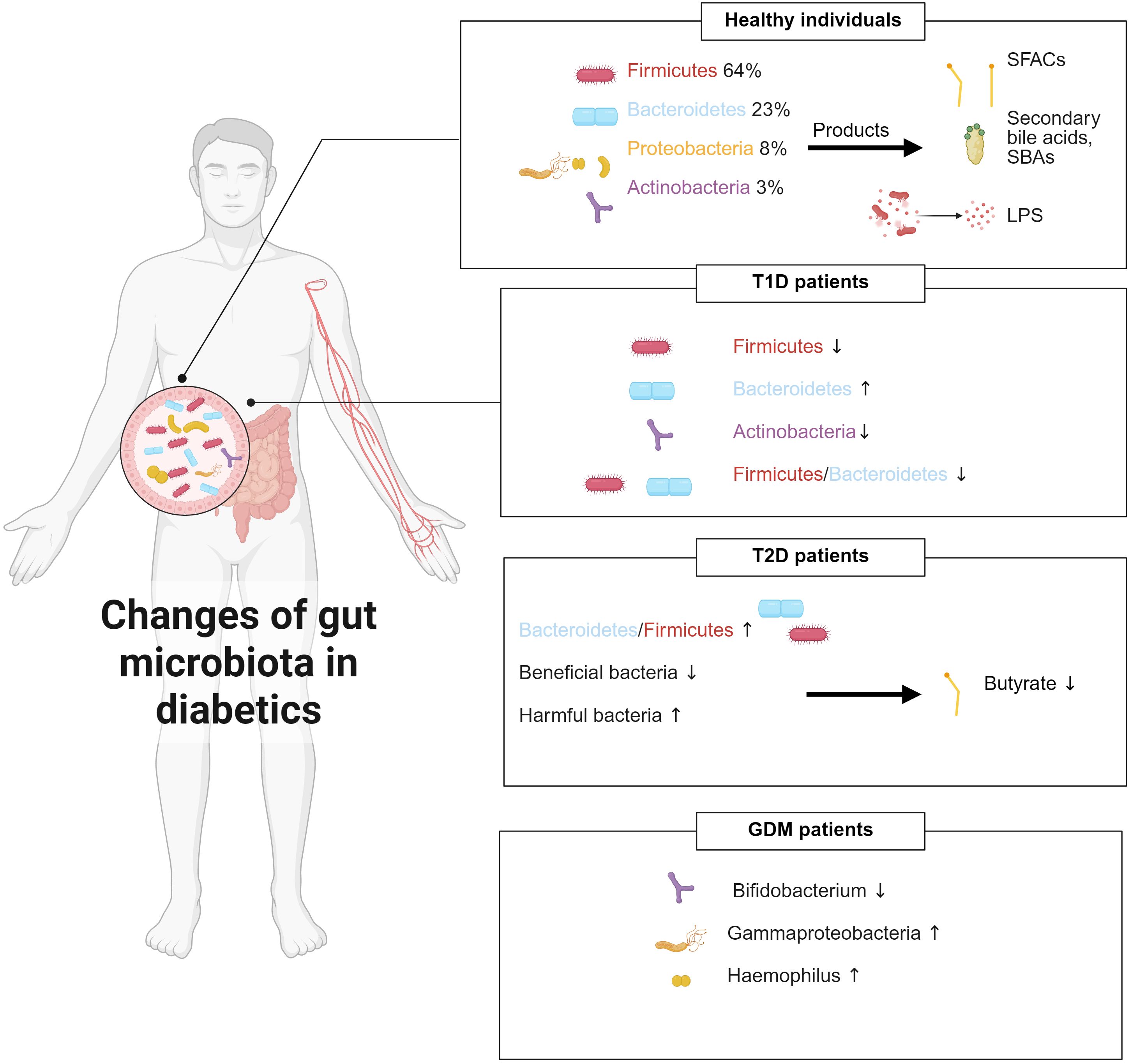
Figure 1. Gut microbiota composition changes under different physiological conditions and its dysbiotic shifts in diabetes mellitus.
3.3 Gut microbiota metabolism and diabetes mellitus: underlying mechanisms
The gut microbiota and its metabolites are crucial in the onset and development of diabetes mellitus. These microbial communities help the host acquire the nutrients and facilitate the breakdown and fermentation of dietary substrates, which in turn affects carbohydrate metabolism and produces short-chain fatty acids (SCFAs), secondary bile acids, lipopolysaccharides, and other bioactive molecules (52). These metabolites interact with various host physiological systems through different pathways, influencing metabolic, immunological, and neurological functions (53–55). Hence, gut microbiota can be a therapeutic target for metabolic disorders. Interventions such as prebiotic or probiotic supplementation and fecal microbiota transplantation are currently being studied (56, 57). While the exact causal relationships and mechanisms linking specific microbial taxa or metabolites to diabetes mellitus are not yet fully understood, a significant amount of research indicates that gut microbiome signatures can serve as reliable biomarkers strongly linked to the development and progression of the disease.
Short-chain fatty acids (SCFAs)—primarily acetate, propionate, and butyrate—constitute the predominant metabolic products of the gut microbiota (58). Propionate enhances hepatic glycogen storage, upregulates lipoprotein lipase (LPL) activity, and attenuates the proinflammatory cytokine interleukin-8 (IL-8), thereby preserving intestinal barrier integrity (59). Furthermore, propionate exerts direct effects on pancreatic β-cells by stimulating insulin secretion and inhibiting β-cell apoptosis via the FFAR2–Gq–PKC signaling axis (60). Butyrate is avidly absorbed by colonic epithelial cells and serves as their principal energetic substrate, providing approximately 60–70% of their energy requirements (61). Acetate, which is present at higher concentrations in the colon than butyrate, is predominantly associated with de novo lipogenesis (61, 62). Beyond their role as metabolic fuels, SCFAs modulate host physiology through two principal signaling mechanisms: activation of G-protein-coupled receptors (GPCRs) and inhibition of histone deacetylases (HDACs) (63, 64). SCFAs function as cognate ligands for GPCRs—namely GPR41, GPR43, and GPR109A—expressed on intestinal epithelial, adipose, lymphoid, and immune cells, thereby initiating downstream signaling cascades (65, 66). Engagement of GPCRs by SCFAs promotes glucagon-like peptide-1 (GLP-1) secretion, enhances satiety and insulin sensitivity, and improves glucose homeostasis while concurrently suppressing IL-6 and IL-8 production, thus exerting anti-inflammatory effects (63, 67). HDACs are a family of enzymes that remove acetyl moieties from both histone and non-histone proteins, thereby compacting chromatin structure and repressing gene transcription (68). HDACs are indispensable for hepatic glucose homeostasis; their selective suppression lowers fasting glycaemia and ameliorates glucose tolerance (69). SCFAs act as endogenous HDAC inhibitors, and their inhibitory efficacy is concentration-dependent. The underlying mechanisms appear to involve both direct intracellular uptake via specific transporters and indirect suppression mediated through GPCR activation (70).
Bile acids are steroid-derived acids synthesized from hepatic cholesterol and serve as pivotal regulators of lipid digestion and absorption. Their bidirectional interactions with the gut microbiota have been causally linked to the risk of diabetes mellitus (71). Bile acids contribute to metabolic dysregulation through three primary mechanisms: (i) modulation of microbial community structure and function, (ii) intracellular or organ-level accumulation of bile acids, and (iii) perturbation of microbiota-dependent bile-acid signaling (72). The impact of bile acids on microbial composition can be exerted either directly through membrane-disrupting physicochemical effects or indirectly through activation of the nuclear receptor FXR (73). Reciprocally, intestinal microbes enzymatically modify bile-acid pools through deconjugation and dehydroxylation, thereby altering their relative abundance and signaling capacity. For example, enhanced 7α-dehydroxylation by specific bacterial taxa increases the proportion of secondary bile acids, leading to their accumulation within the gut lumen. Excessive luminal bile-acid concentrations can stimulate intestinal immune cells to release proinflammatory cytokines (e.g., TNF-α, IL-6, IL-1β), thereby fostering chronic low-grade inflammation implicated in type 2 diabetes mellitus (T2DM) (74, 75). The principal receptors mediating bile-acid signaling are the farnesoid X receptor (FXR) and the G-protein-coupled receptor TGR5. FXR activation represses hepatic gluconeogenesis by down-regulating the transcription of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), thereby ameliorating diet-induced hyperinsulinemia and hyperglycemia (76). In contrast, TGR5 activation promotes GLP-1 secretion, enhances pancreatic β-cell function, augments insulin release, and improves glucose tolerance (77).
Endotoxin is a lipopolysaccharide (LPS) component of the cell wall of Gram-negative bacteria, which is only released in small amounts in the normal gut microbiota. LPS is a key trigger for metabolic diseases and is linked to obesity and insulin resistance (78). A high-fat diet increases the number of intestinal Gram-negative bacteria and the level of plasma LPS. Continuous subcutaneous injections of LPS can cause the same effects as a high-fat diet, including impaired fasting blood glucose, hyperinsulinemia, and increased body weight. CD14 mutant mice (LPS receptor inactivation) delay the onset of insulin resistance and obesity under a high-fat diet and completely block hepatic steatosis (78). Qin J et al. (47) found that the abundance of LPS produced by gut microbiota was positively correlated with fasting blood glucose and HOMA-IR (an assessment indicator of insulin resistance) when performing metagenomic analysis on patients with T2DM. When the number of Gram-negative bacteria in the intestine increases or intestinal permeability changes, the concentration of LPS in serum increases, which may induce endotoxemia and insulin resistance (79). Toll-like receptors (TLRs) can recognize pathogen-associated molecular patterns (PAMPs) from the microbiome and are mainly present on the surface of immune cells (80). LPS binds to the TLR4 receptor, activates the NF-κB pathway, initiates the transcription of proinflammatory genes such as IL-6, IL-1, and TNF-α, causes an inflammatory response, and leads to the occurrence of insulin resistance (81).
Intestinal immune–metabolic homeostasis is orchestrated by the tripartite interplay of SCFAs, BAs, and LPS. SCFAs reinforce epithelial barrier integrity, suppress NF-κB-driven inflammation, counteract LPS translocation and toxicity, and fine-tune bile-acid metabolism via FXR signaling (82). Conversely, BAs sculpt the microbial community through direct antimicrobial activity, thereby modulating SCFAs production (72). LPS perturbs this equilibrium by triggering TLR4-mediated pro-inflammatory cascades (83). Precise spatiotemporal balance among these three metabolite classes is therefore indispensable for maintaining gut microbiota stability and host health.
4 The mechanism of Dendrobium officinale polysaccharides regulating gut microbiota to improve diabetes mellitus
Imbalance in gut microbiota is closely correlated with the occurrence and development of diabetes mellitus. Changes in the gut microbiota of diabetic patients can worsen metabolic disorders in the human body. As a traditional Chinese herbal medicine, D. officinale has been used to regulate blood sugar and improve metabolism for thousands of years. Modern pharmacological studies have also shown that DOPs are effective in preventing and treating diabetes mellitus (35). Due to its indigestible characteristics, it is suggested that DOPs improve diabetes mellitus by regulating gut microbiota. Chen et al. (84) established a conventional type 2 diabetes mellitus (T2DM) mouse model via high-fat diet feeding combined with streptozotocin injection, and subsequently generated a pseudo-germ-free model by continuous administration of a broad-spectrum antibiotic cocktail in drinking water. The authors demonstrated that the glucolipid-metabolic benefits, suppression of LPS translocation, and anti-inflammatory effects elicited by DOPs are strictly dependent on an intact gut microbiota. As such, DOPs can ameliorate diabetes mellitus via modulation of the gut microbiota (Figure 2).
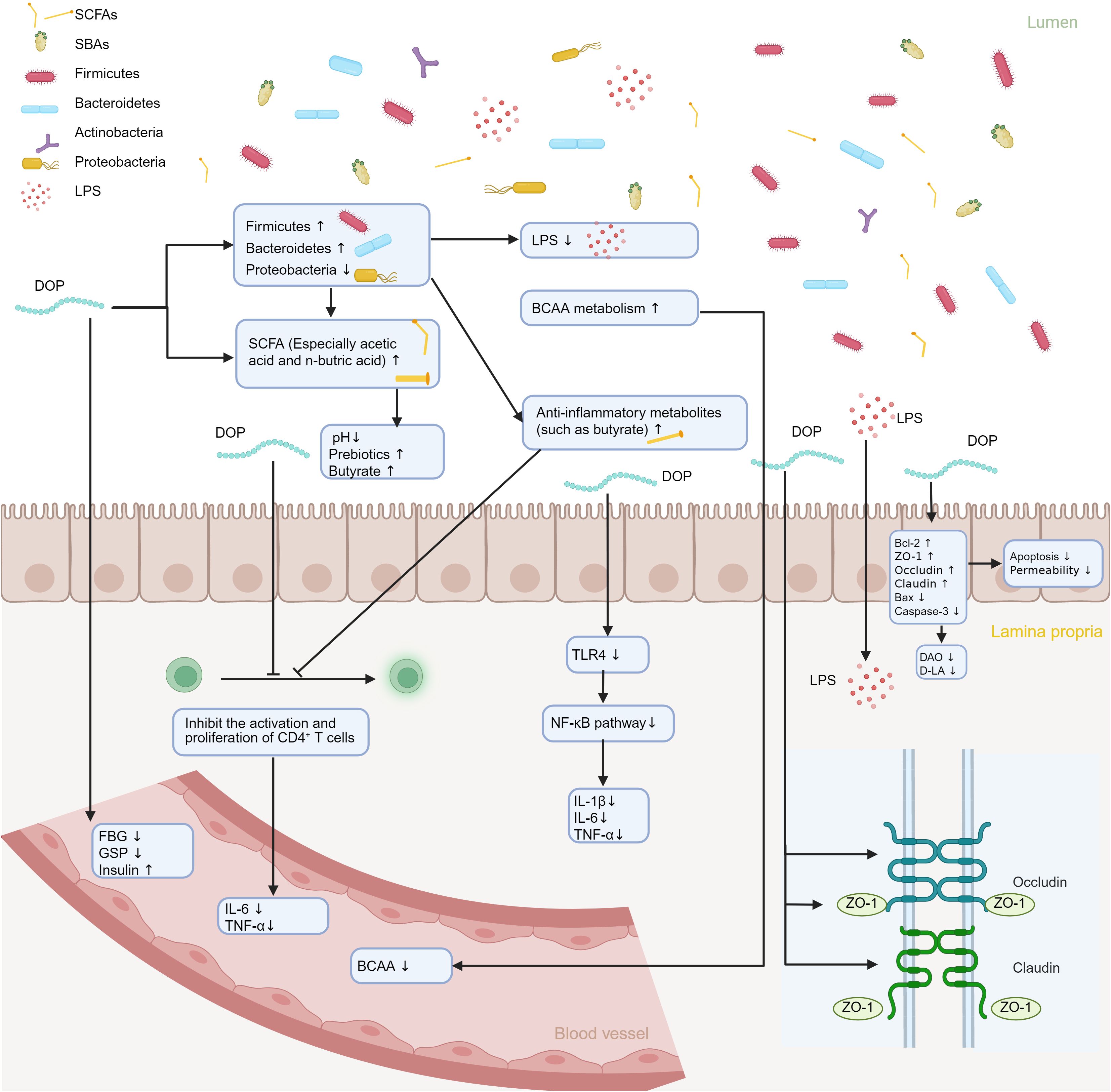
Figure 2. Illustration of the mechanism by which DOPs ameliorates diabetes mellitus via modulation of the gut microbiota. DOPs reshapes microbial composition, thereby altering the profile of microbial metabolites. These changes upregulate tight-junction proteins ZO-1, Occludin, and Claudin, restoring intestinal barrier integrity, while concurrently downregulating pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Consequently, immune homeostasis is re-established and the metabolic milieu is improved.
4.1 Dendrobium officinale polysaccharides regulate the relative abundance of gut microbiota
The bioavailability of DOPs is poor, and their complex polysaccharide structure makes them difficult to digest. It interacts with the gut microbiota in the distal intestine to exert its function (85). An in vitro fermentation study on DOPs showed that after 48 hours of co-culture fermentation, 63.88% of the total carbohydrates in the DOPs treatment group were consumed. The concentrations of mannose, glucose and galactose were reduced, and the total SCFA production was significantly increased, indicating that DOPs can be degraded into monosaccharides and utilized by the gut microbiota (34). DOPs have a regulatory effect on the relative abundance of gut microbiota, can regulate the proportion of various types of flora, reduce LPS-producing bacteria (such as Helicobacter), enhance the production of short-chain fatty acids, repair the intestinal barrier, and improve metabolic diseases (84). Animal experiments have shown that DOPs significantly increase the abundance of Firmicutes and Bacteroidetes, while inhibiting Proteobacteria, and selectively amplify probiotics (such as Bifidobacterium, Lactobacillus, and Allobaculum) by more than 94% (84). Another study established a T2DM mouse model by high-fat diet combined with streptozotocin and showed that the WDOP1 treatment group and the metformin treatment group showed similar effects. Both could significantly reduce fasting blood glucose, serum insulin levels, and HbA1c levels of T2DM mice, and improve glucose intolerance and insulin resistance (35). In terms of gut microbiota, the WDOE/WDOP1 treatment group reversed the gut microbiota imbalance of T2DM mice, normalized the ratio of Firmicutes/Bacteroidetes, and reduced the abundance of harmful bacteria (such as Enterococcus casseliflavus and Eubacterium plexicaudatum) (35).
4.2 Dendrobium officinale polysaccharides repair intestinal barrier damage
Intestinal barrier functions as the defense system which is composed of the intestinal mucosal barrier and intestinal-associated lymphoid tissue. The intestinal epithelium is the key barrier for the body to resist endogenous and exogenous harmful substances. The integrity of the barrier is vital in the stability of the intestinal microenvironment (85, 86). It is well known that increased intestinal permeability leads to bacterial translocation and lipopolysaccharide penetration, causing metabolic endotoxemia. This is associated with autoimmune reactions, chronic inflammation, and promotes the occurrence and development of T1DM and T2DM (87, 88). As shown in Figure 3, DOPs inhibit the expression of TLR4 and its downstream signaling molecules TRAM and TRIF by reducing LPS, thereby reducing the phosphorylation of IKKβ and NF-κB p65 and blocking the activation of the NF-κB pathway (84). Importantly, DOPs have been shown to be able to repair intestinal barrier damage. DOPs can reduce intestinal permeability by upregulating the expression of intestinal tight junction proteins (ZO-1, occludin) and Bcl-2 proteins. It also downregulates the expression of Bax and caspase-3 proteins, enhancing the tight junctions between intestinal cells, and reducing intestinal epithelial cell apoptosis (89). In mice with T2DM induced by a high-fat diet combined with streptozotocin, polysaccharides from D. officinale upregulated the expression of tight junction proteins ZO-1, Occludin, and Claudin-1. This reduced the levels of intestinal permeability indicators DAO and D-LA, inhibited LPS leakage, inflammation, and oxidative stress, and alleviated insulin resistance (90).
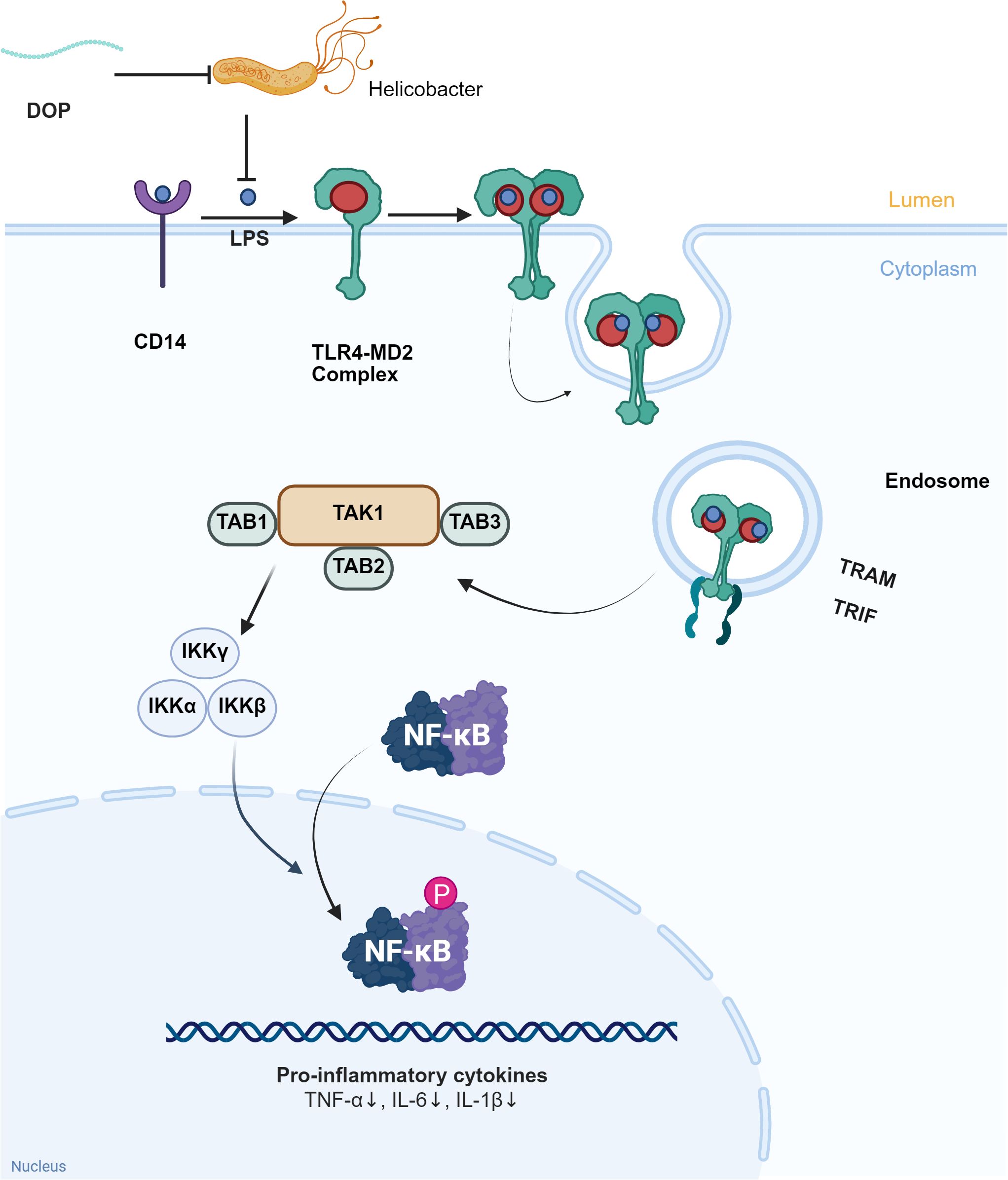
Figure 3. DOPs improve LPS leakage and inflammation levels through gut microbiota. LPS binds to CD14 and TLR4-MD2 complexes in the intestinal lumen, activating downstream signaling molecules TAK1, TAB1, TAB2, and TAB3, which in turn activate IKKα, IKKβ, IKKγ, and further activate NF-κB, exacerbating the inflammatory response. DOPs reduce inflammation by inhibiting Helicobacter pylori, decreasing the production and leakage of LPS, and inhibiting the formation of the TLR4-MD2 complex.
4.3 Immunomodulatory effects of polysaccharides from Dendrobium officinale
The three polysaccharides of D. officinale play a role in immunomodulation, and their impact on immunomodulation may be connected to gut microbiota. Inflammatory factors are related to the advancement of diabetes mellitus and related metabolic disorders and can hinder the function of the insulin signaling pathway, affecting human metabolism. The NF-κB signaling pathway is linked to metabolic diseases. As it disrupts insulin signaling by controlling the expression of proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6), resulting in insulin resistance (91). Research indicates that following DOW-5B treatment, there were significant changes in the levels of IL-10 and TNF-α levels in mouse serum, with a notable increase in IgM levels (92). Polysaccharides DOPs, isolated from the leaves of D. officinale, markedly downregulate the expression of IL-1β, IL-6, and TNF-α by inhibiting the TLR4/NF-κB/NLRP3 signaling axis (93). Another animal study demonstrated that DOPs treatment can decrease the levels of two inflammatory cytokines, IL-6 and TNF-α, in serum, and resulted in a decrease in the weight of mice in the DOPs treatment group, possibly due to DOPs inhibiting the activation and proliferation of CD4+ T cells (94). The immune regulatory pathway of DOPs is associated with alterations in gut microbiota composition. DOPs significantly increased the proportion of beneficial bacteria in the intestine (such as Bifidobacterium, Lactobacillus, etc.), while reducing harmful bacteria in the intestine, increasing anti-inflammatory metabolites like butyrate in the intestine, thereby restraining the overactivation of CD4+ Th cells, decreasing inflammatory factors such as TNF-α/IL-6, and achieving a flora-immune synergistic regulation (94).
4.4 Dendrobium officinale polysaccharide improves the metabolic environment
Long-term high-fat, high-calorie diets inhibit the activity of liver lipoprotein lipase (LPL), hinder lipoprotein catabolism, increase blood triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) levels, lead to metabolic disorders, and increase the risk of diabetes mellitus (95, 96). Oral administration of DOPs can increase serum insulin and reduce fasting blood glucose (FBG) and glycosylated serum protein (GSP) levels in alloxan-induced diabetic mice, which may be related to its ability to repair damaged pancreatic tissue and improve insulin secretion (97). In vitro experiments have also shown that DOPs can improve obesity-related insulin resistance and abnormal lipid metabolism, and its effects are closely related to PPAR-γ (98). DOPs can lower blood glucose by suppressing hepatic gluconeogenesis via inhibition of the glucagon-cAMP-PKA and Akt/FoxO1 pathways while enhancing glycogen synthesis and stability (99). Furthermore, concurrent activation of the PPAR-RXR axis increases fatty acid oxidation and insulin sensitivity, resulting in a sustained reduction of fasting glucose and improved glucose tolerance (100). Together, these data suggest that DOPs can affect glucose metabolism (Figure 4). DOPs can also significantly improve amino acid metabolism disorders in diabetic rats. In animal experiments, it was found that the ability of diabetic rats to metabolize branched-chain amino acids (BCAA) weakened, but the content in their blood was significantly increased. At the same time, the number of genes related to BCAA synthesis in the gut microbiota increased. DOPs treatment reduced the abundance of genes related to BCAA biosynthesis and improved BCAA metabolism (101). The gut microbiota participates in carbohydrate metabolism and nutrient absorption, producing products such as short-chain fatty acids SCFAs. Imbalance in the flora can interfere with blood sugar metabolism and increase the risk of diabetes. DOPs can significantly increase the content of total SCFAs in the colon of mice, especially acetic acid and butyric acid, and reduce the pH value of the colon environment, which is conducive to the production of prebiotics and butyrate, thereby regulating the balance of the flora and improving intestinal health (102).
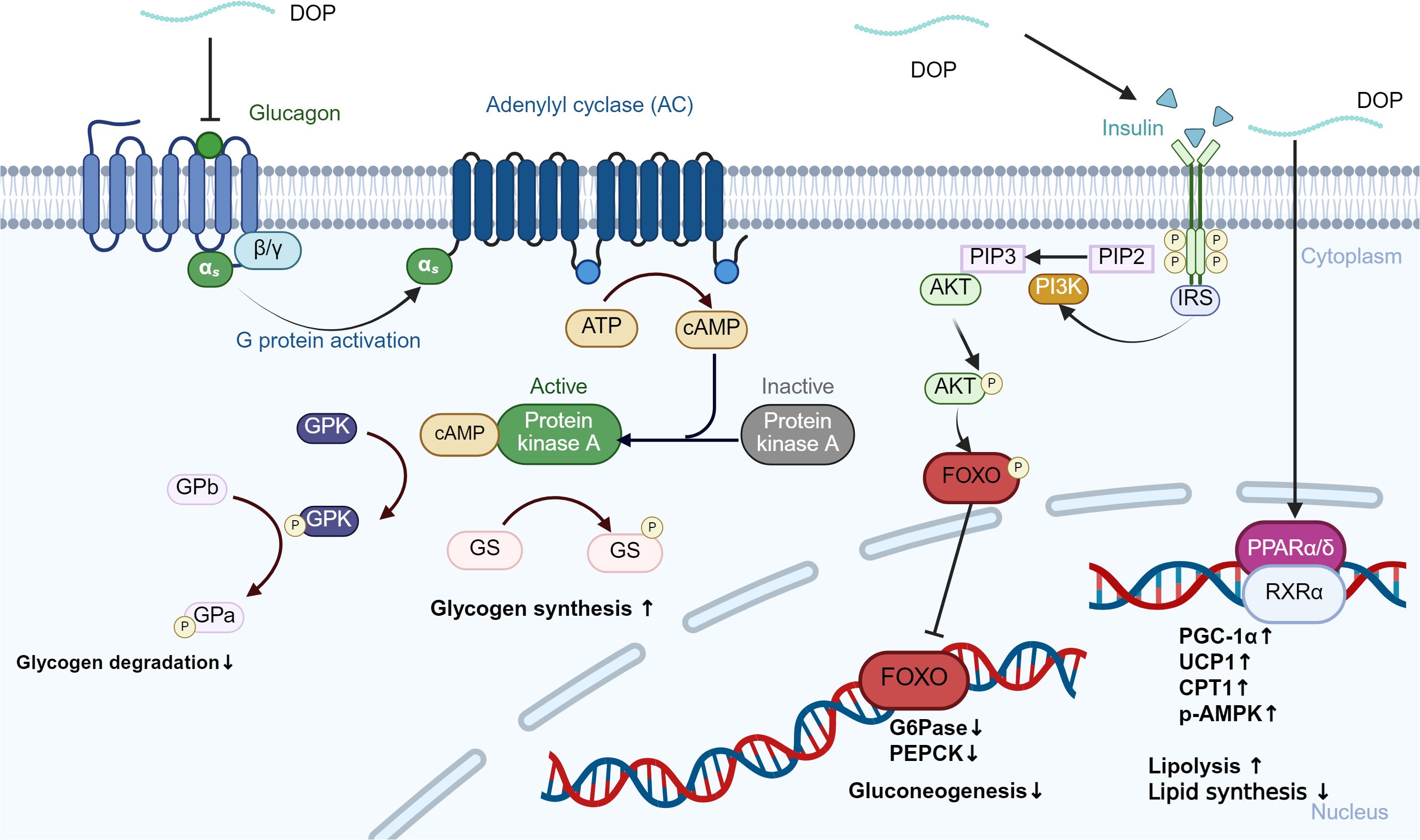
Figure 4. DOPs-mediated regulation of hepatic glucagon and insulin signaling pathways. The binding of glucagon to the glucagon receptor (GCGR) activates adenylyl cyclase (AC), leading to increased cAMP levels and subsequent activation of protein kinase A (PKA). This cascade promotes glycogen degradation by phosphorylating glycogen phosphorylase (GP) and inhibiting glycogen synthase (GS). Insulin and DOPs activate the PI3K/Akt pathway, which phosphorylates and inactivates FoxO1, thereby reducing the expression of gluconeogenic enzymes PEPCK and G6Pase.
5 Conclusion
As a metabolic disease, diabetes mellitus is linked to human metabolic disorders and gut microbiota imbalance. In recent years, improving diabetes mellitus and its complications through gut microbiota has become a research hotspot, and diabetes mellitus therapy based on gut microbiota has become a new treatment idea after traditional therapy. As a traditional Chinese medicine, the effective active ingredient of DOPs has shown potential pharmacological effects in the treatment of diabetes mellitus. By regulating the relative abundance of gut microbiota, repairing intestinal barrier damage, regulating immune function, and improving the metabolic environment, it neutralizes the imbalance of gut microbiota, weakens the inflammatory state, improves metabolic disorders, and is beneficial to the treatment of diabetes mellitus. Natural compounds represented by DOPs are expected to provide new intervention strategies for the treatment of diabetes-related diseases. In the future, DOPs can be further studied as a prebiotic and combined with probiotics in adjuvant therapy or dietary intervention for diabetic patients, especially for patients with prediabetes. In clinical practice, a new “bacteria-drug synergy” intervention strategy is provided for diabetic patients, targeting gut microbiota and performing compatibility quantification based on the patient’s baseline flora characteristics. However, it is worth noting that although the prebiotic potential of DOPs is supported by animal studies and in vitro data, there are currently no registered or published human clinical trials testing their effects on gut microbiota or metabolic outcomes. Therefore, the optimal dose-response relationship, intervention duration, and long-term safety of DOPs as candidate prebiotics have not yet been determined. In addition, the development of functional foods of DOPs can be promoted to provide low-dose, long-term safety solutions for people with sub-health and chronic diseases, reducing drug dependence and medical expenses. However, some problems still need to be solved to achieve its widespread clinical application. The biological activities of DOPs are related to their chemical structure and molecular weight. The relationship between structure and function needs to be clarified. The optimal extraction method and dosage need to correspond to its applicable population and efficacy. In addition, the mechanism of DOPs acting on gut microbiota is not yet completely clear. DOPs lack large-scale double-blind clinical trials to verify the results of animal experiments and in vitro experiments to ensure their safety and effectiveness in humans. Efforts can also be put into the combined application effect of DOPs and other anti-diabetic drugs to provide a new perspective for the clinical treatment of diabetes mellitus.
Author contributions
JW: Visualization, Writing – original draft, Formal Analysis, Validation, Methodology, Investigation, Data curation. RL: Validation, Visualization, Data curation, Formal Analysis, Methodology, Writing – review & editing, Investigation, Software. QW: Funding acquisition, Project administration, Validation, Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by The Science and Technology Development Fund, Macau SAR (File no. 006/2023/SKL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roglic G. WHO Global report on diabetes: A summary. Int J Noncommunicable Dis. (2016) 1:3. doi: 10.4103/2468-8827.184853
2. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. (2017) 41:S13–27. doi: 10.2337/dc18-S002
3. Gong B, Yang W, Xing Y, Lai Y, and Shan Z. Global, regional, and national burden of type 1 diabetes in adolescents and young adults. Pediatr Res. (2025) 97:568–76. doi: 10.1038/s41390-024-03107-5
4. IDF Diabetes Atlas 2025. Diabetes Atlas. Available online at: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (Accessed July 3, 2025).
5. Gillespie KM. Type 1 diabetes: pathogenesis and prevention. Can Med Assoc J. (2006) 175:165–70. doi: 10.1503/cmaj.060244
6. Del Chierico F, Rapini N, Deodati A, Matteoli MC, Cianfarani S, and Putignani L. Pathophysiology of type 1 diabetes and gut microbiota role. Int J Mol Sci. (2022) 23:14650. doi: 10.3390/ijms232314650
7. DeFronzo RA, Ferrannini E, Groop L, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. (2015) 1(1):15019. doi: 10.1038/nrdp.2015.19
9. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, and Damm P. Gestational diabetes mellitus. Nature Reviews Disease Primers. (2019) 5(1):47. doi: 10.1038/s41572-019-0098-8
10. Sweeting A, Wong J, Murphy HR, and Ross GP. A clinical update on gestational diabetes mellitus. Endocrine Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
11. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2009) 32:S62–7. doi: 10.2337/dc09-s062
12. Serino M, Luche E, Chabo C, Amar J, and Burcelin R. Intestinal microflora and metabolic diseases. Diabetes Metab. (2009) 35:262–72. doi: 10.1016/j.diabet.2009.03.003
13. Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. (2013) 11:639–47. doi: 10.1038/nrmicro3089
14. Burcelin R, Serino M, Chabo C, Blasco-Baque V, and Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetologica. (2011) 48:257–73. doi: 10.1007/s00592-011-0333-6
15. Bielka W, Przezak A, and Pawlik A. The role of the gut microbiota in the pathogenesis of diabetes. Int J Mol Sci. (2022) 23:480. doi: 10.3390/ijms23010480
16. Wu W, Zhao Z, Zhao Z, Zhang Y, Zhang Y, Jiang Y, et al. Structure, health benefits, mechanisms, and gut microbiota of dendrobium officinale polysaccharides: A review. Nutrients. (2023) 15:4901. doi: 10.3390/nu15234901
17. Ng TB, Liu J, Wong JH, Ye X, Wing Sze S, and Tong Y. Review of research on Dendrobium, a prized folk medicine. Appl Microbiol Biotechnol. (2012) 93:1795–803. doi: 10.1007/s00253-011-3829-7
18. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
19. Xing X, Cui SW, Nie S, Phillips GO, Douglas Goff H, and Wang Q. A review of isolation process, structural characteristics, and bioactivities of water-soluble polysaccharides from Dendrobium plants. Bioactive Carbohydrates Dietary Fibre. (2013) 1:131–47. doi: 10.1016/j.bcdf.2013.04.001
20. Committee for the Pharmacopoeia of PR China. Pharmacopoeia of the People’s Republic of China, Part 1. Beijing, China: China Medical Science Press (2015) p. 295–6.
21. Chen W, Lu J, Zhang J, Wu J, Yu L, Qin L, et al. Traditional Uses, Phytochemistry, Pharmacology, and Quality Control of Dendrobium officinale Kimura et. Migo. Front Pharmacol. (2021) 12:726528. doi: 10.3389/fphar.2021.726528
22. Xu J, Zhao W, Qian Z, Guan J, and Li S. Fast determination of five components of coumarin, alkaloids and bibenzyls in Dendrobium spp. using pressurized liquid extraction and ultra-performance liquid chromatography. J Separation Sci. (2010) 33:1580–6. doi: 10.1002/jssc.201000034
23. Lai C-H, Huo C-Y, Xu J, Han Q-B, Li L-F, et al. Critical review on the research of chemical structure, bioactivities, and mechanism of actions of Dendrobium officinale polysaccharide. Int J Biol Macromolecules. (2024) 263:130315. doi: 10.1016/j.ijbiomac.2024.130315
24. Liu H, Xing Y, Wang Y, Ren X, Zhang D, Dai J, et al. Dendrobium officinale Polysaccharide Prevents Diabetes via the Regulation of Gut Microbiota in Prediabetic Mice. Foods. (2023) 12:2310. doi: 10.3390/foods12122310
25. He L, Yan X, Liang J, Li S, He H, Xiong Q, et al. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr Polymers. (2018) 198:101–8. doi: 10.1016/j.carbpol.2018.06.073
26. Sun S-J, Deng P, Peng C-E, Ji H-Y, Mao L-F, and Peng L-Z. Extraction, structure and immunoregulatory activity of low molecular weight polysaccharide from dendrobium officinale. Polymers. (2022) 14:2899. doi: 10.3390/polym14142899
27. Xia L, Liu X, Guo H, Zhang H, Zhu J, and Ren F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J Funct Foods. (2012) 4:294–301. doi: 10.1016/j.jff.2011.12.006
28. Xie S-Z, Liu B, Zhang D-D, Zha X-Q, Pan L-H, Luo J-P, et al. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. (2016) 7:2789–99. doi: 10.1039/c6fo00172f
29. Hua Y-F, Zhang M, Fu C-X, Chen Z-H, Chan G-Y-S, et al. Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr Res. (2004) 339:2219–24. doi: 10.1016/j.carres.2004.05.034
30. He T-B, Huang Y-P, Yang L, Liu T-T, Gong W-Y, Wang X-J, et al. Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale. Int J Biol Macromolecules. (2016) 83:34–41. doi: 10.1016/j.ijbiomac.2015.11.038
31. Huang K, Li Y, Tao S, Wei G, Huang Y, Chen D, et al. Purification, characterization and biological activity of polysaccharides from dendrobium officinale. Molecules (Basel Switzerland). (2016) 21:701. doi: 10.3390/molecules21060701
32. Zhou H, Dong Y, Wu Z, Peng X, Yan M, Chen S, et al. Ultrasound-assisted enzyme extraction of Dendrobium Officinale polysaccharides: Extraction process, characterization, immunomodulatory effects. Ultrasonics Sonochemistry. (2025) 114:107248. doi: 10.1016/j.ultsonch.2025.107248
33. Wei W, Feng L, Bao W-R, Ma D-L, Leung C-H, Nie S-P, et al. Structure characterization and immunomodulating effects of polysaccharides isolated from dendrobium officinale. J Agric Food Chem. (2016) 64:881–9. doi: 10.1021/acs.jafc.5b05180
34. Fu Y, Zhang J, Chen K, Xiao C, Fan L, Zhang B, et al. An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors. J Funct Foods. (2019) 53:44–53. doi: 10.1016/j.jff.2018.12.005
35. Peng D, Tian W, An M, Chen Y, Zeng W, Zhu S, et al. Characterization of antidiabetic effects of Dendrobium officinale derivatives in a mouse model of type 2 diabetes mellitus. Food Chem. (2023) 399:133974. doi: 10.1016/j.foodchem.2022.133974
36. Zhang Z, Zhang D, Dou M, Li Z, Zhang J, and Zhao X. Dendrobium officinale Kimura et Migo attenuates diabetic cardiomyopathy through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced mice. Biomedicine Pharmacotherapy = Biomedecine Pharmacotherapie. (2016) 84:1350–8. doi: 10.1016/j.biopha.2016.10.074
37. Liang J, Li H, Chen J, He L, Du X, Zhou L, et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol Res. (2019) 148:104417. doi: 10.1016/j.phrs.2019.104417
38. Wang L, Li C, Hu C, Gong P, and Zhao S. Purification and structural characterization of dendrobium officinale polysaccharides and its activities. Chem Biodiversity. (2021) 18:(5). doi: 10.1002/cbdv.202001023
39. Wang Q, Liang J, and Liu H. In vitro effects of four polysaccharides containing β-D-Glup on intestinal function. Int J Food Properties. (2019) 22:1064–76. doi: 10.1080/10942912.2019.1628778
40. Wu F, Zhang Y, Liu W, Zhu N, Chen J, and Sun Z. Comparison of torrefied and lyophilized Dendrobii Officinalis Caulis (Tiepishihu) by Fourier transform infrared spectroscopy and two-dimensional correlation spectroscopy. J Mol Structure. (2020) 1204:127554. doi: 10.1016/j.molstruc.2019.127554
41. MetaHIT Consortium, Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
42. Chen Y, Zhou J, and Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infection Microbiol. (2021) 11:625913. doi: 10.3389/fcimb.2021.625913
43. Ma Q, Li Y, Li P, Wang M, Wang J, Tang Z, et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomedicine Pharmacotherapy. (2019) 117:109138. doi: 10.1016/j.biopha.2019.109138
44. Xu L, Zeng X, Liu Y, Wu Z, Zheng X, and Zhang X. Effect of Dendrobium officinale polysaccharides on central nervous system disease: Based on gut microbiota. Int J Biol Macromolecules. (2023) 240:124440. doi: 10.1016/j.ijbiomac.2023.124440
45. Gholizadeh P, Mahallei M, Pormohammad A, Varshochi M, Ganbarov K, Zeinalzadeh E, et al. Microbial balance in the intestinal microbiota and its association with diabetes, obesity and allergic disease. Microbial Pathogenesis. (2019) 127:48–55. doi: 10.1016/j.micpath.2018.11.031
46. Larsen N, Vogensen FK, Van Den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. (2010) 5:e9085. doi: 10.1371/journal.pone.0009085
47. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. (2012) 490:55–60. doi: 10.1038/nature11450
48. Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. (2013) 11:46. doi: 10.1186/1741-7015-11-46
49. Teixeira RA, Silva C, Ferreira AC, Martins D, Leite-Moreira A, Miranda IM, et al. The association between gestational diabetes and the microbiome: A systematic review and meta-analysis. Microorganisms. (2023) 11:1749. doi: 10.3390/microorganisms11071749
50. Xu Y, Zhang M, Zhang J, Sun Z, Ran L, Ban Y, et al. Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am J Physiology-Endocrinology Metab. (2020) 319:E247–53. doi: 10.1152/ajpendo.00266.2019
51. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Kling Bäckhed H, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. (2012) 150:470–80. doi: 10.1016/j.cell.2012.07.008
52. Tremaroli V and Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. (2012) 489:242–9. doi: 10.1038/nature11552
53. Fan Y and Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. doi: 10.1038/s41579-020-0433-9
54. Vinolo MAR, Rodrigues HG, Nachbar RT, and Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. (2011) 3:858–76. doi: 10.3390/nu3100858
55. Ochoa-Repáraz J, Ramelow CC, and Kasper LH. A gut feeling: the importance of the intestinal microbiota in psychiatric disorders. Front Immunol. (2020) 11:510113. doi: 10.3389/fimmu.2020.510113
56. Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. (2012) 143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031
57. Hsieh M-C, Tsai W-H, Jheng Y-P, Su S-L, Wang S-Y, Lin C-C, et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. (2018) 8:16791. doi: 10.1038/s41598-018-35014-1
58. Rooks MG and Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. doi: 10.1038/nri.2016.42
59. El Hage R, Hernandez-Sanabria E, Calatayud Arroyo M, and Van De Wiele T. Supplementation of a propionate-producing consortium improves markers of insulin resistance in an in vitro model of gut-liver axis. Am J Physiology-Endocrinology Metab. (2020) 318:E742–9. doi: 10.1152/ajpendo.00523.2019
60. Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. (2017) 19:257–65. doi: 10.1111/dom.12811
61. Canfora EE, Jocken JW, and Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. doi: 10.1038/nrendo.2015.128
62. Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. (2017) 595:541–55. doi: 10.1113/jp272613
63. Li M, Van Esch BCAM, Henricks PAJ, Folkerts G, and Garssen J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. (2018) 9:533. doi: 10.3389/fphar.2018.00533
64. Waldecker M, Kautenburger T, Daumann H, Busch C, and Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. (2008) 19:587–93. doi: 10.1016/j.jnutbio.2007.08.002
65. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A is a G-protein–coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. (2009) 69:2826–32. doi: 10.1158/0008-5472.can-08-4466
66. Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. (2003) 278:25481–9. doi: 10.1074/jbc.m301403200
67. Lin HV, Frassetto A, Kowalik EJJr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS One. (2012) 7:e35240. doi: 10.1371/journal.pone.0035240
68. Tang R and Li L. Modulation of short-chain fatty acids as potential therapy method for type 2 diabetes mellitus. Can J Infect Dis Med Microbiol. (2021) 2021:1–13. doi: 10.1155/2021/6632266
69. Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. (2011) 145:607–21. doi: 10.1016/j.cell.2011.03.043
70. He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. (2020) 21:6356. doi: 10.3390/ijms21176356
71. Lu J, Wang S, Li M, Gao Z, Xu Y, Zhao X, et al. Association of serum bile acids profile and pathway dysregulation with the risk of developing diabetes among normoglycemic chinese adults: findings from the 4C study. Diabetes Care. (2021) 44:499–510. doi: 10.2337/dc20-0884
72. Cai J, Rimal B, Jiang C, Chiang JYL, and Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. (2022) 237:108238. doi: 10.1016/j.pharmthera.2022.108238
73. Begley M, Gahan CGM, and Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. (2005) 29:625–51. doi: 10.1016/j.femsre.2004.09.003
74. Leung H, Xiong L, Ni Y, Busch A, Bauer M, Press AT, et al. Impaired flux of bile acids from the liver to the gut reveals microbiome-immune interactions associated with liver damage. NPJ Biofilms Microbiomes. (2023) 9:35. doi: 10.1038/s41522-023-00398-0
75. Hotamisligil GS. Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
76. Ma Y, Huang Y, Yan L, Gao M, and Liu D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm Res. (2013) 30:1447–57. doi: 10.1007/s11095-013-0986-7
77. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. (2009) 10:167–77. doi: 10.1016/j.cmet.2009.08.001
78. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/db06-1491
79. Manco M, Putignani L, and Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocrine Rev. (2010) 31:817–44. doi: 10.1210/er.2009-0030
80. Li J, Wang X, Zhang F, and Yin H. Toll-like receptors as therapeutic targets for autoimmune connective tissue diseases. Pharmacol Ther. (2013) 138:441–51. doi: 10.1016/j.pharmthera.2013.03.003
81. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, and Flier JS. TLR4 links innate immunity and fatty acid–induced insulin resistance. J Clin Invest. (2006) 116:3015–25. doi: 10.1172/jci28898
82. de Vos WM, Tilg H, Van Hul M, and Cani PD. Gut microbiome and health: mechanistic insights. Gut. (2022) 71:1020–32. doi: 10.1136/gutjnl-2021-326789
83. Cai J, Sun L, and Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. (2022) 30:289–300. doi: 10.1016/j.chom.2022.02.004
84. Chen X, Chen C, and Fu X. Dendrobium officinale Polysaccharide Alleviates Type 2 Diabetes Mellitus by Restoring Gut Microbiota and Repairing Intestinal Barrier via the LPS/TLR4/TRIF/NF-kB Axis. J Agric Food Chem. (2023) 71:11929–40. doi: 10.1021/acs.jafc.3c02429
85. Li L, Yao H, Li X, Zhang Q, Wu X, Wong T, et al. Destiny of Dendrobium officinale Polysaccharide after Oral Administration: Indigestible and Nonabsorbing, Ends in Modulating Gut Microbiota. J Agric Food Chem. (2019) 67:5968–77. doi: 10.1021/acs.jafc.9b01489
86. Camilleri M, Madsen K, Spiller R, Van Meerveld BG, and Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. (2012) 24:503–12. doi: 10.1111/j.1365-2982.2012.01921.x
87. Thoo L, Noti M, and Krebs P. Keep calm: the intestinal barrier at the interface of peace and war. Cell Death Dis. (2019) 10:(11). doi: 10.1038/s41419-019-2086-z
88. Snelson M, de Pasquale C, Ekinci EI, and Coughlan MT. Gut microbiome, prebiotics, intestinal permeability and diabetes complications. Best Practice, Research Clinical Endocrinology, Metabolism. (2021) 35(3):101507. doi: 10.1016/j.beem.2021.101507
89. Wang K, Yang X, Wu Z, Wang H, Li Q, Mei H, et al. Dendrobium officinale polysaccharide protected CCl4-induced liver fibrosis through intestinal homeostasis and the LPS-TLR4-NF-κB signaling pathway. Front Pharmacol. (2020) 11:240. doi: 10.3389/fphar.2020.00240
90. Chen X, Chen C, Ma C, Kang W, Wu J, and Fu X. Dendrobium officinale polysaccharide attenuates type 2 diabetes in mice model by modulating gut microbiota and alleviating intestinal mucosal barrier damage. Food Sci Hum Wellness. (2025) 14:9250007. doi: 10.26599/fshw.2024.9250007
91. Baker RG, Hayden MS, and Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. (2011) 13:11–22. doi: 10.1016/j.cmet.2010.12.008
92. Li M, Yue H, Wang Y, Guo C, Du Z, Jin C, et al. Intestinal microbes derived butyrate is related to the immunomodulatory activities of Dendrobium officinale polysaccharide. Int J Biol Macromolecules. (2020) 149:717–23. doi: 10.1016/j.ijbiomac.2020.01.305
93. Yang K, Zhan L, Lu T, Zhou C, Chen X, Dong Y, et al. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-κB signaling pathway. Cytokine. (2020) 130:155058. doi: 10.1016/j.cyto.2020.155058
94. Zhou W, Tao W, Wang M, Liu W, Xing J, and Yang Y. Dendrobium officinale Xianhu 2 polysaccharide helps forming a healthy gut microbiota and improving host immune system: An in vitro and in vivo study. Food Chem. (2023) 401:134211. doi: 10.1016/j.foodchem.2022.134211
95. Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, et al. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. (2014) 58:25145. doi: 10.3402/fnr.v58.25145
96. Prasad M, Rajagopal P, Devarajan N, Veeraraghavan VP, Palanisamy CP, Cui B, et al. A comprehensive review on high -fat diet-induced diabetes mellitus: an epigenetic view. J Nutr Biochem. (2022) 107:109037. doi: 10.1016/j.jnutbio.2022.109037
97. Pan L-H, Li X-F, Wang M-N, Zha X-Q, Yang X-R, Liu Z-J, et al. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int J Biol Macromolecules. (2014) 64:420–7. doi: 10.1016/j.ijbiomac.2013.12.024
98. Qu J, Tan S, Xie X, Wu W, Zhu H, Li H, et al. Dendrobium officinale polysaccharide attenuates insulin resistance and abnormal lipid metabolism in obese mice. Front Pharmacol. (2021) 12:659626. doi: 10.3389/fphar.2021.659626
99. Liu Y, Yang L, Zhang Y, Liu X, Wu Z, Gilbert RG, et al. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J Ethnopharmacology. (2020) 248:112308. doi: 10.1016/j.jep.2019.112308
100. Zou J, Song Q, Shaw PC, and Zuo Z. Dendrobium officinale regulate lipid metabolism in diabetic mouse liver via PPAR-RXR signaling pathway: Evidence from an integrated multi-omics analysis. Biomedicine Pharmacotherapy = Biomedecine Pharmacotherapie. (2024) 173:116395. doi: 10.1016/j.biopha.2024.116395
101. Chen H, Nie Q, Hu J, Huang X, Yin J, and Nie S. Multiomics approach to explore the amelioration mechanisms of glucomannans on the metabolic disorder of type 2 diabetic rats. J Agric Food Chem. (2021) 69:2632–45. doi: 10.1021/acs.jafc.0c07871
Keywords: Dendrobium officinale polysaccharides (DOPs), gut microbiota, diabetes mellitus, traditional Chinese medicine, immunomodulation
Citation: Wan J, Lin R and Wu Q (2025) The therapeutic effects of dendrobium officinale polysaccharides on diabetes mellitus: from the perspective of gut microbiota. Front. Endocrinol. 16:1683752. doi: 10.3389/fendo.2025.1683752
Received: 12 August 2025; Accepted: 22 September 2025;
Published: 08 October 2025.
Edited by:
Huanling Lai, Guangzhou National Laboratory, ChinaReviewed by:
Qi-Qing Cheng, Hubei University of Science and Technology, ChinaJumin Huang, University of Macau, China
Copyright © 2025 Wan, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wu, cXd1QG11c3QuZWR1Lm1v
 Jinyuan Wan
Jinyuan Wan Ruihe Lin1
Ruihe Lin1 Qiang Wu
Qiang Wu