- 1Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, Guangdong,, China
- 2Sichuan Integrative Medicine Hospital, Chengdu, Sichuan, China
- 3Guangdong Provincial Key Laboratory of Microbial Safety and Health, State Key Laboratory of Applied Microbiology Southern China, Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou, China
Objective: The present study aims to explore the impact of washed microbiota transplantation (WMT) on the tongue microbiota composition of individuals with metabolic-associated fatty liver disease (MAFLD) and elucidate its biological correlations.
Methods: We conducted a comprehensive analysis of hepatic fat deposition and characterized the tongue coating microbiota using 16S rRNA gene sequencing in MAFLD patients before and after undergoing WMT treatment. Furthermore, a MAFLD mouse model was established for additional validation.
Results: At the genus level, significant differences in tongue coating microbiota structure were observed between MAFLD patients and HC. Specifically, Neisseria positively correlated with the BARD score, Porphyromonas and Rhodococcus positively correlated with fat decay, and Petostreptococcus, a conditionally pathogenic bacterium, exhibited a significantly higher relative abundance in MAFLD patients compared to HC. Conversely, Actinomyces positively correlated with the FIB-4 score, Megasphaera negatively correlated with the APRI score, and Subdoligulum negatively correlated with low-density lipoprotein levels. Notably, following effective WMT treatment, patients exhibited improved symptoms, with a significant reduction in the relative abundance of Petostreptococcus and an increase in potential probiotics such as Lachnospiraceae and Bifidobacterium in their tongue coating microbiota. Additionally, structural differences in the tongue coating microbiota were identified at the genus level between MAFLD model mice and HC mice. After WMT treatment, the relative abundance of conditionally pathogenic bacteria like Enterococcus was significantly decreased in MAFLD model mice.
Conclusions: WMT not only significantly ameliorates liver fat deposition in MAFLD patients but also alters the tongue coating microbial structure associated with disease severity, thereby potentially mitigating adverse patient outcomes.
Background
Metabolic associated fatty liver disease (MAFLD), a chronic progressive disease characterized by excessive fat accumulation in the liver, has become the most prevalent chronic liver disease globally, with a recent meta-analysis estimating a global prevalence of 38.77% (1). Studies have demonstrated a strong association between MAFLD and the development of liver cirrhosis, liver failure, and even hepatocellular carcinoma. This disease not only poses a significant threat to human health but also places a substantial burden on healthcare systems and the global economy (2).
Lipid metabolism disorder is a primary factor contributing to the development of MAFLD. However, due to the incomplete understanding of its pathogenesis and the absence of clinically approved specific therapeutic drugs, current treatment strategies for MAFLD focus primarily on lifestyle modifications. In addition, pharmacological interventions are often required to address the patient’s underlying conditions. Despite this, most available therapeutic drugs have failed to deliver the expected clinical outcomes (3, 4). Therefore, there remains an urgent need to identify safe and effective treatments for MAFLD, making this a key area of research globally. In recent years, the introduction of the “liver-gut axis” concept has highlighted the role of gut microbiota in the onset and progression of MAFLD. Clinical studies have revealed significant differences in gut microbiota composition between healthy individuals and MAFLD patients, characterized by reduced ecological diversity and an increase in pathogenic bacteria abundance (5). These findings indicate that MAFLD patients experience significant microbial dysbiosis.
The oral microbiota, one of the largest microbial communities in the human body after the gut (6), plays a significant role in systemic inflammation, bacterial infections, and disease progression through its composition and function (7). Studies have shown that patients with liver diseases often exhibit severe oral microbiota imbalances (8). In a clinical study involving 102 patients with liver cirrhosis, severe dysbiosis of the oral microbiota was observed compared to healthy controls, mirroring the dysbiosis seen in the intestinal microbiota (9). Research further indicates that oral microbiota can translocate from the oral cavity to the intestine (10) and may contribute to the development of liver cirrhosis (11). Among the components of the oral microbiota, tongue coating microorganisms have been found to have close associations with various clinical diseases (9). The proportion, density, and diversity of bacterial communities present in tongue coatings are strongly linked to disease formation.
Fecal microbiota transplantation (FMT) involves isolating microbiota from the feces of healthy donors and transplanting the functional bacteria into a patient’s intestine through specialized techniques, with the aim of reshaping the patient’s intestinal microbiota and treating disease (11, 12). FMT has emerged as a breakthrough medical intervention in recent years. An improved version, water-washed microbiota transplantation (WMT), involves using an automated purification system to extract and repeatedly wash the gut microbiota, thereby reducing the risk of FMT-related adverse effects. WMT has already been investigated in various metabolic diseases, including dyslipidemia (13, 14), obesity (15), diabetes (16), and hypertension (17).
We hypothesize that WMT may offer clinical benefits for patients with MAFLD and that a correlation exists between the microbiota present on the tongue coating of MAFLD patients and the efficacy of WMT treatment. To explore this hypothesis, we employ a combination of animal and human studies to evaluate the potential therapeutic effects of WMT on MAFLD.
Methods
Inclusion criteria for MAFLD
This study included MAFLD inpatients aged ≥18 years who were admitted to the First Affiliated Hospital of Guangdong Pharmaceutical University between January 2017 and December 2022. MAFLD was diagnosed based on international consensus criteria (18). The exclusion criteria for patients were as follows: (1) Use of antibiotics within the past month; (2) Presence of dental caries or periodontal disease; (3) Severe heart, lung, or kidney diseases; (4) Coexisting liver diseases; (5) Serious lack of medical records. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University (#2021-13), and written informed consent was obtained from all participants.
Sample collection
Person: serum, tongue coating
Medical record data for the WMT group were collected at three time points: before treatment (baseline), before each WMT procedure, and after the final WMT treatment. Data included laboratory parameters such as BMI, age, gender, liver enzymes, fasting insulin, blood lipids, fasting blood glucose, and liver imaging (CT, ultrasound, and elastography). Sample collection from patients followed a standardized protocol. Patients were instructed to avoid eating and brushing their teeth for at least 1 hour prior to sampling. After rinsing their mouths with physiological saline, a sterile cotton swab was used to collect samples from the middle of the tongue. The swab was placed into an RNase-free Eppendorf tube, 1 mL of phosphate buffer solution (PBS) was added, and the mixture was stirred. The sample was centrifuged at 4000 rpm for 20 minutes, after which the supernatant and precipitate were separately stored in sterile tubes. All samples were kept at -80 °C until further analysis.
Mice: tongue coating and liver samples
Mouse tongue coating collection: (1) Anesthesia: Administer 0.05 mL of Shu Tai via intramuscular injection per mouse. (2) Sample Collection: Using tweezers, gently pull the mouse’s tongue out of its mouth, quickly sever the tongue at the base, and place it in a sterile tube. All samples were stored at -80 °C until analysis. The entire procedure was completed within 1 minute after the mouse’s death. The mouse liver collection procedure follows these steps: (1) Anesthesia: Administer 0.05 mL of Shu Tai via intramuscular injection per mouse. (2) Dissection: Incise the abdominal skin to fully expose the thoracic and abdominal cavities. (3) Cardiac Perfusion: Locate the abdominal aorta and perform an incision. Using a syringe, inject 30 mL of physiological saline into the heart from the apex until the effluent becomes lighter in color. (4) Liver Tissue Extraction: Identify the liver, gently detach it with tweezers, and carefully remove the intact organ. (5) Tissue Processing: Remove any surrounding ligaments and the gallbladder. Divide the liver into three sections, preserving one relatively intact portion for pathological analysis, which should be stored in an Eppendorf tube containing 4% PFA fixative. (6) Pathological Staining: Conduct staining procedures for pathological examination on the preserved liver tissue.
WMT
Questionnaire surveys, physical examinations, blood and stool tests, and other laboratory screenings were conducted for all healthy fecal donors aged 18 to 25 years. This study employed the Nanjing Consensus washing bacterial transplantation method (19). In a biosafety level 2 laboratory, bacterial suspensions were prepared with the assistance of trained professionals using disposable sterile materials. To prepare the washed microbial community, a homogeneous fecal suspension was created by mixing 100 g of feces with 500 mL of 0.9% saline solution in a 1:5 ratio. This mixture was then subjected to microfiltration using an automatic purification device (GenFMTer, FMT Medical). The microbial precipitate was washed three times, after which 100 mL of saline solution was added to create the bacterial suspension. This suspension was maintained in a water bath at a constant temperature of 37 °C and injected within 1 hour to prevent contamination or compositional changes.
Based on the physical condition and willingness of each MAFLD patient, the washed bacterial suspension was administered into the patient’s intestine via either the upper or lower digestive tract (120 mL per day for 3 consecutive days). This study followed the “Three Three Principles” therapy, where one treatment course consisted of continuous injection of the washing bacterial suspension for 3 days, followed by three consecutive months of treatment (i.e., three courses), and another course after a 3-month interval to stabilize bacterial colonization. The total treatment duration was 6 months. All patients underwent at least one WMT procedure and completed follow-up assessments by October 31, 2022.
Mouse experiment
Establishment of MAFLD mouse model
This study utilized a methionine and choline-deficient (MCD) diet to freely feed C57BL/6 mice (Guangdong Medical Animal Experimental Center) for 4 weeks, thereby establishing a MAFLD mouse model (17). The animal experiments received approval from the Animal Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University (#2022-14) and were conducted in accordance with the guidelines for reporting in vivo experiments involving animals.
WMT experiment in mice
Preparation of bacterial solution follows these steps: (1) Sterile Procedure: All operations should be conducted in a sterile room to maintain a contamination-free environment. (2) Sample Collection: Collect fecal samples separately from MAFLD mice and healthy mice, gathering 2 or more fresh fecal samples from each mouse, and label them appropriately. (3) Fecal Suspension: Soak the collected feces in a 0.1 g/mL physiological saline solution for at least 10 minutes, then homogenize the mixture. (4) Centrifugation: Centrifuge the homogenized suspension at 1000 rpm for 3 minutes and collect the supernatant. Gavage Procedure: MAFLD mice were divided into two groups: one group received the bacterial solution from mice with more severe disease, while the other group received the bacterial solution from healthy mice. Gavage was administered at a dose of 0.2 mL per animal, given once every other day for four consecutive weeks.
DNA extraction and 16S rRNA
Total DNA from the tongue coating of MAFLD patients and the tongues of MAFLD mice was extracted according to the E.Z.N.a® protocol using the Soil DNA Kit (Omega BioTek, Norcross, GA, USA). The quality and concentration of all DNA samples were evaluated using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The V3-V4 fragment of the bacterial 16S rRNA gene was amplified by PCR using primers 806R and 338F. The sequences of the primers were as follows: 5’-GACTACHVGGGTWTCTAAT-3’ (338F) and 5’-ACTCCTACGGGAGGCAGCAG-3’ (806R). The PCR conditions included an initial denaturation at 95 °C for 30 seconds, followed by 27 cycles of 30 seconds at 55 °C, 45 seconds at 72 °C, and a final extension at 72 °C for 5 minutes. The PCR reaction mixture contained: 4 μL of 5 × TransStart FastPfu buffer, 2 μL of 2.5 mM deoxyribonucleoside triphosphates (dNTPs), 0.8 μL of 5 μM primers, 0.4 μL of TransStart FastPfu DNA polymerase, and 10 ng of extracted DNA, with the final volume adjusted to 20 μL with ddH2O. Agarose gel electrophoresis was performed to verify the size of the PCR amplicon. Sequencing was conducted using the Illumina MiSeq PE300 platform (Shanghai MajorbioBio, China).
H.E. staining
HE staining: Prepared slices were placed in a fully automated staining machine for HE staining. After the staining process was complete, the slices were sealed for further analysis.
Data statistical analysis
Statistical analyses were performed using GraphPad Prism 9.0.0 and SPSS 25.0 software. Changes in clinical indicators of MAFLD patients before and after WMT were presented as differences using a self-matching design. Continuous variables were first assessed for normality. mean ± standard deviation (M ± SD) was used to describe continuous variables with a normal distribution, analyzed using a one-sample t-test. For non-normally distributed continuous variables, the median (interquartile range) was reported and analyzed using the one-sample rank sum test. Categorical variables were represented by frequency and percentage. A bilateral p-value of < 0.05 was considered statistically significant, with p < 0.05 denoted by *, p < 0.01 denoted by **, and p < 0.001 denoted by ***.
Results
Evaluation of clinical efficacy of WMT in the treatment of MAFLD
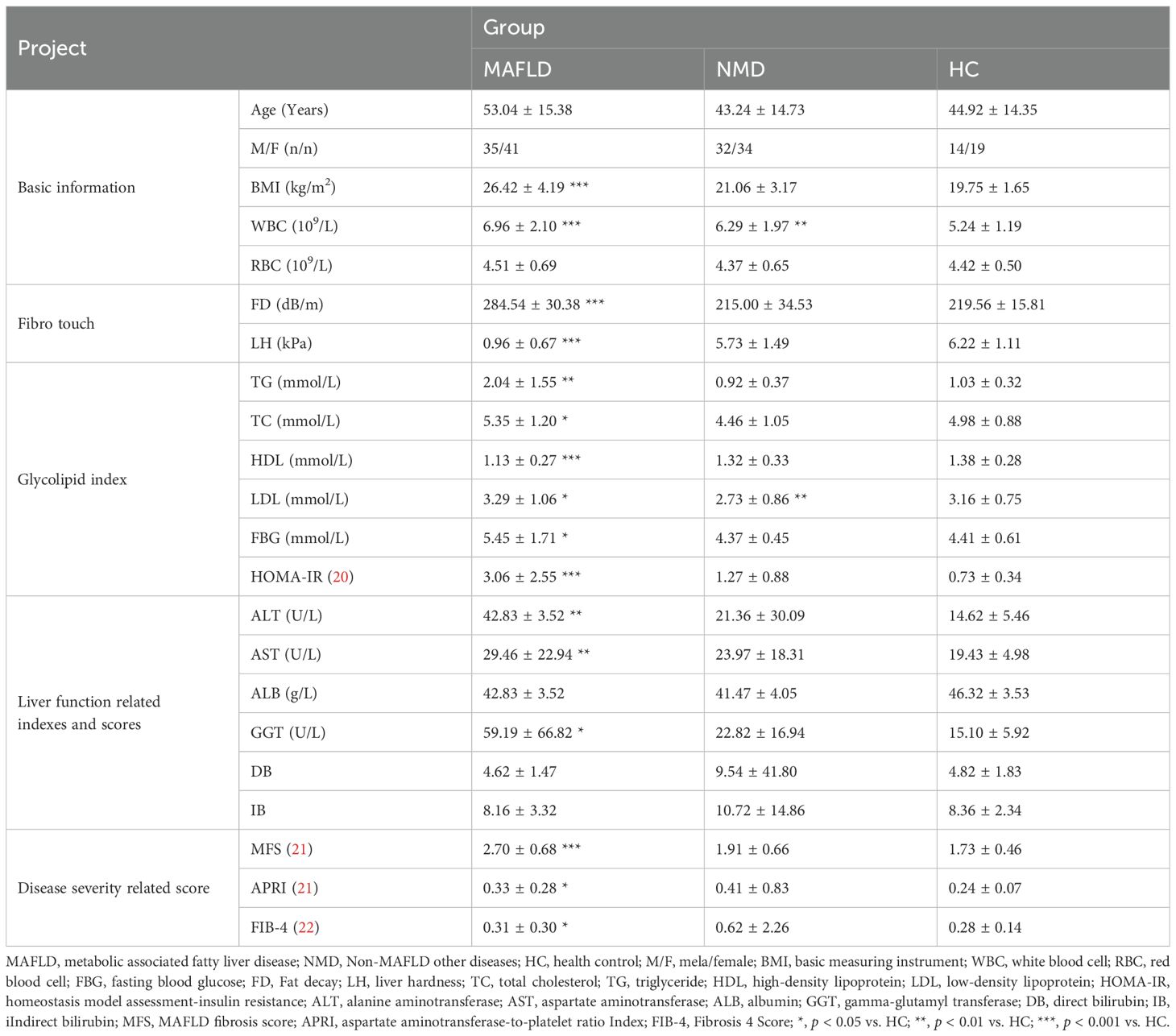
Comparative analysis of baseline level information for the samples included in this study
A total of 175 subjects participated in this study, consisting of 76 MAFLD patients, 66 NMD patients, and 33 healthy control (HC) volunteers. The baseline physiological characteristics of all participants are summarized in Table 1. Significant differences (p < 0.05) were observed in multiple clinical indicators between the MAFLD group and the HC group. These indicators included body mass index (BMI), white blood cell count (WBC), fat decay (FD), liver hardness (LH), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), fasting blood glucose (FBG), homeostasis model assessment-insulin resistance (HOMA-IR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), gamma-glutamyl transferase (GGT), MAFLD fibrosis score (MFS), aspartate aminotransferase-to-platelet ratio index (APRI), and fibrosis 4 Score, (FIB-4). In contrast, no significant differences were noted in other aspects. These clinical data indicate that the MAFLD patients in this study require further intervention to address the ongoing progression of the disease. Furthermore, the non-MAFLD other diseases (NMD) patient sample served as a reference group for subsequent analyses.
Clinical efficacy evaluation of WMT treatment for MAFLD
To assess the clinical efficacy of WMT for treating MAFLD, the patients in this study were randomly assigned to two groups: the WMT group (n = 43) and the drug therapy control (DTC) group (n = 33), in which the medication of group DTC was completely in accordance with the “guidelines for the prevention and treatment of nonalcoholic fatty liver disease” (23). Concurrently, various clinical indicators were collected from both groups prior to treatment. Based on the statistical analysis, glucose and lipid indicators closely associated with MAFLD were compiled and visualized (Figure 1).
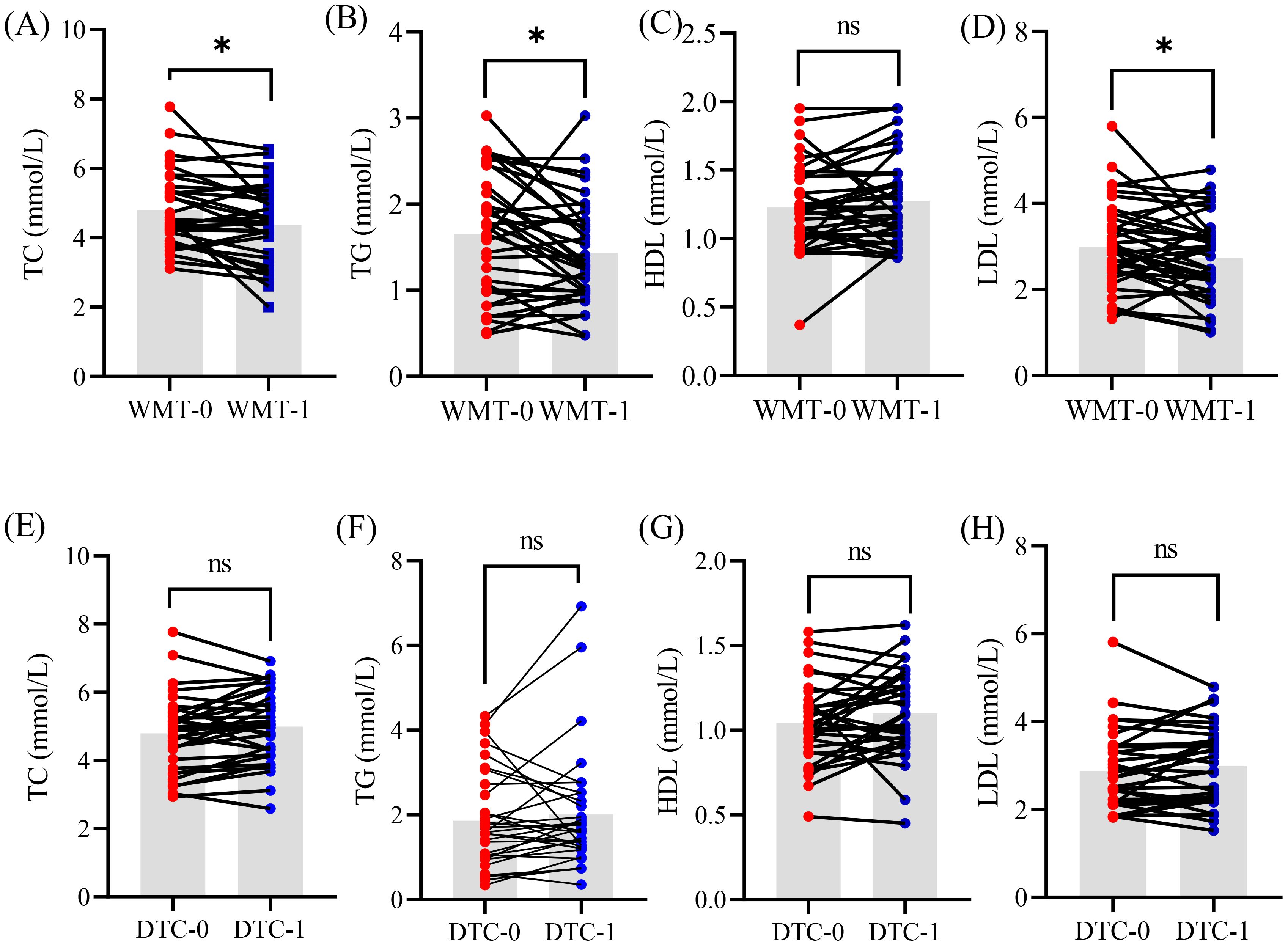
Figure 1. The glucose and lipid indicators of patient MAFLD undergoing clinical treatment with WMT (A–D) and DTC (E–H) methods. WMT, washed microbiota transplantation; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; DTC, drug therapy control; 0, baseline level before treatment; 1, 1 treatment cycle; *, p < 0.05; ns, no significant.
After one course of WMT treatment, MAFLD patients demonstrated significant reductions in TC, TG, and LDL (p < 0.05), while HDL levels remained unchanged. These findings suggest that WMT can effectively improve glucose and lipid metabolism in patients with MAFLD to some extent. In contrast, no significant changes were observed in these indicators among patients in the DTC group during the same treatment period. This evidence indicates that WMT treatment may have a beneficial impact on MAFLD in clinical practice.
Long-term efficacy evaluation of WMT treatment for MAFLD
To further evaluate the clinical efficacy of long-term WMT treatment for MAFLD, we conducted a statistical analysis of the glucose and lipid indicators in 43 MAFLD patients who participated in WMT (Figure 2). Among these patients, 27 underwent 2 courses of WMT, 14 completed 3 courses, and 3 received 4 courses of WMT. Additionally, we included clinical data from NMD patients for comparative analysis.
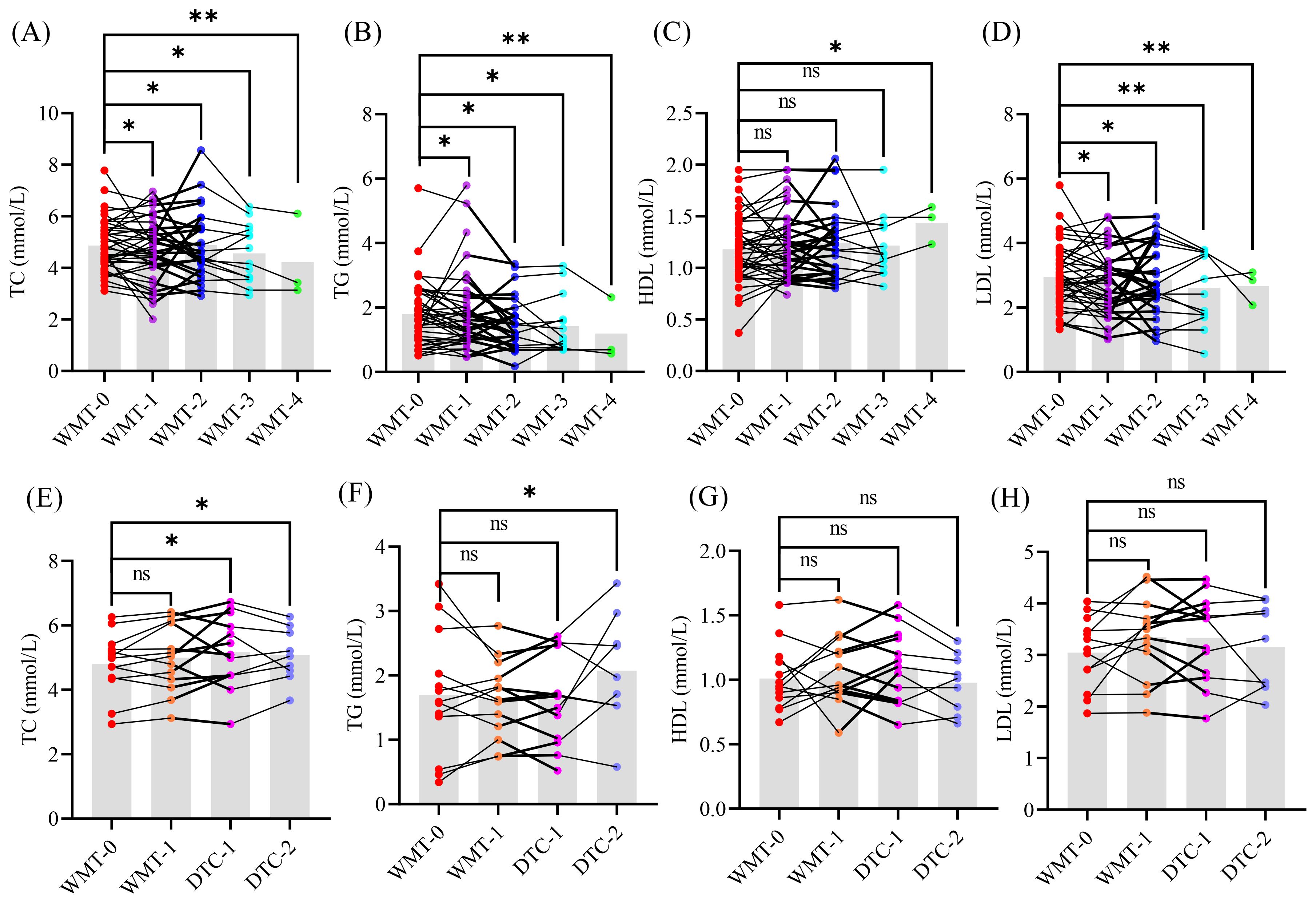
Figure 2. The glucose and lipid indicators of patients MAFLD (A–D) and NMD (E–H). WMT, washed microbiota transplantation; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; DTC, drug therapy control; 0, baseline level before treatment; 1 - 4, different treatment cycle; *, p < 0.05; **, p < 0.01; ns, no significant.
As illustrated in the results graph, an increasing number of treatment cycles corresponded with a significant downward trend in the levels of TC, TG, and LDL compared to the baseline levels (WMT-0) in the MAFLD group. Conversely, HDL levels exhibited a significant upward trend, suggesting that long-term WMT treatment can substantially enhance glucose and lipid metabolism in MAFLD patients.
Interestingly, no significant differences were observed in glucose and lipid indicators between NMD patients and their baseline levels, nor were any changes noted following one cycle of WMT treatment. This indicates that WMT does not cause disturbances in glucose and lipid indicators, further supporting its safety and efficacy in managing MAFLD.
Relationship between tongue coating microorganisms and MAFLD
Differences in tongue coating microbiota between MAFLD patients and HC
To investigate the tongue microbiota of MAFLD patients, we analyzed samples from 56 MAFLD patients and 26 healthy individuals. The species richness and diversity of the two microbial communities were further assessed (Figure 3). Our results indicated no statistically significant differences in the ACE, Chao1, Shannon, and Simpson indices between the two groups (Figure 3A), suggesting that microbial community richness and diversity in the tongue coatings were comparable between the two populations.
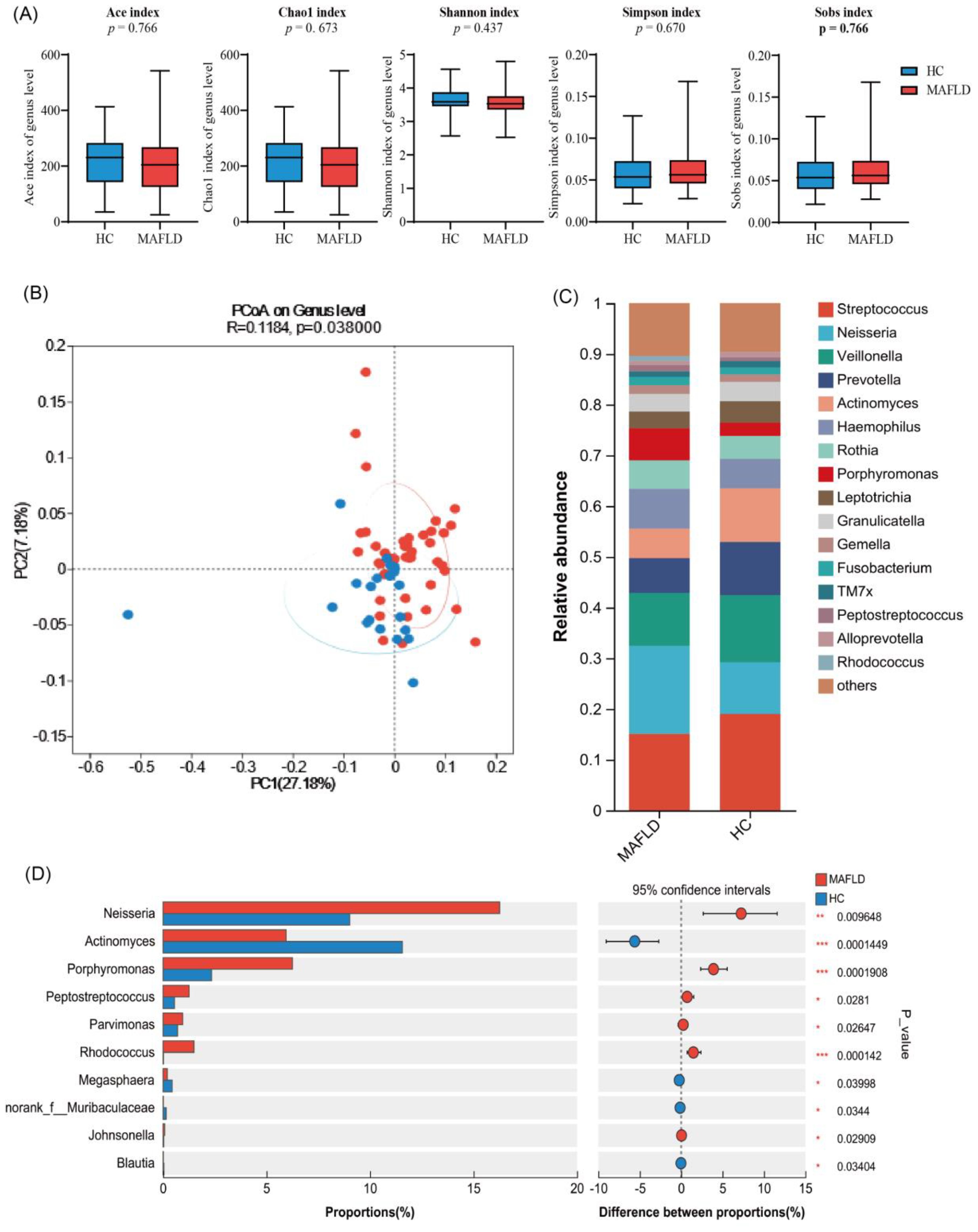
Figure 3. Differential analysis of tongue microbiota between MAFLD patients and healthy individuals based on 1 6S rRNA sequencing. (A) Ace, Chao1, Sobs, Simpson, and Shannon indices explain the alpha diversity of tongue coating microbiota; (B) Display the β diversity of tongue coating microbiota in MAFLD patients (n = 56) and HC group (n = 26) through PCoA plot; (C) The bar chart displays the differences in genus levels between two groups of tongue coating bacteria; (D) Wilcoxon rank sum test bar chart at the genus level.
Additionally, we performed genus-level clustering analysis using Principal Coordinates Analysis (PCoA) on the two sample groups (Figure 3B), which demonstrated no significant differences in microbial community structure between them. Notably, further analysis revealed that the Wilcoxon rank sum test indicated significantly higher relative abundances of Neisseria, Porphyromonas, Rhodococcus, and Peptoniphilus in the MAFLD group compared to the healthy controls. Conversely, Actinobacteria, Megasphaera, Blautia, and Subdoligranulum exhibited significantly higher abundances in the healthy population (Figures 3C, D). These findings indicate distinct differences in the tongue coating microbiota between MAFLD patients and HC group.
Correlation analysis between tongue coating microorganisms and clinical metabolism in MAFLD patients
Further analysis revealed that Rhodococcus and Johnsonella in the tongue microbiota of MAFLD patients were positively correlated with the liver fat attenuation index (p < 0.05, as shown in Figure 4A). Additionally, TC and LDL levels in MAFLD patients were positively correlated with Sphingomonas, while Bergeyella exhibited a positive correlation with LDL. Conversely, Capnocytophaga demonstrated a negative correlation with TG, and Atopobium showed a negative correlation with LDL. Moreover, Rothia and Rhodococcus were positively correlated with fasting blood glucose (FBG) (p < 0.05, Figure 4B).
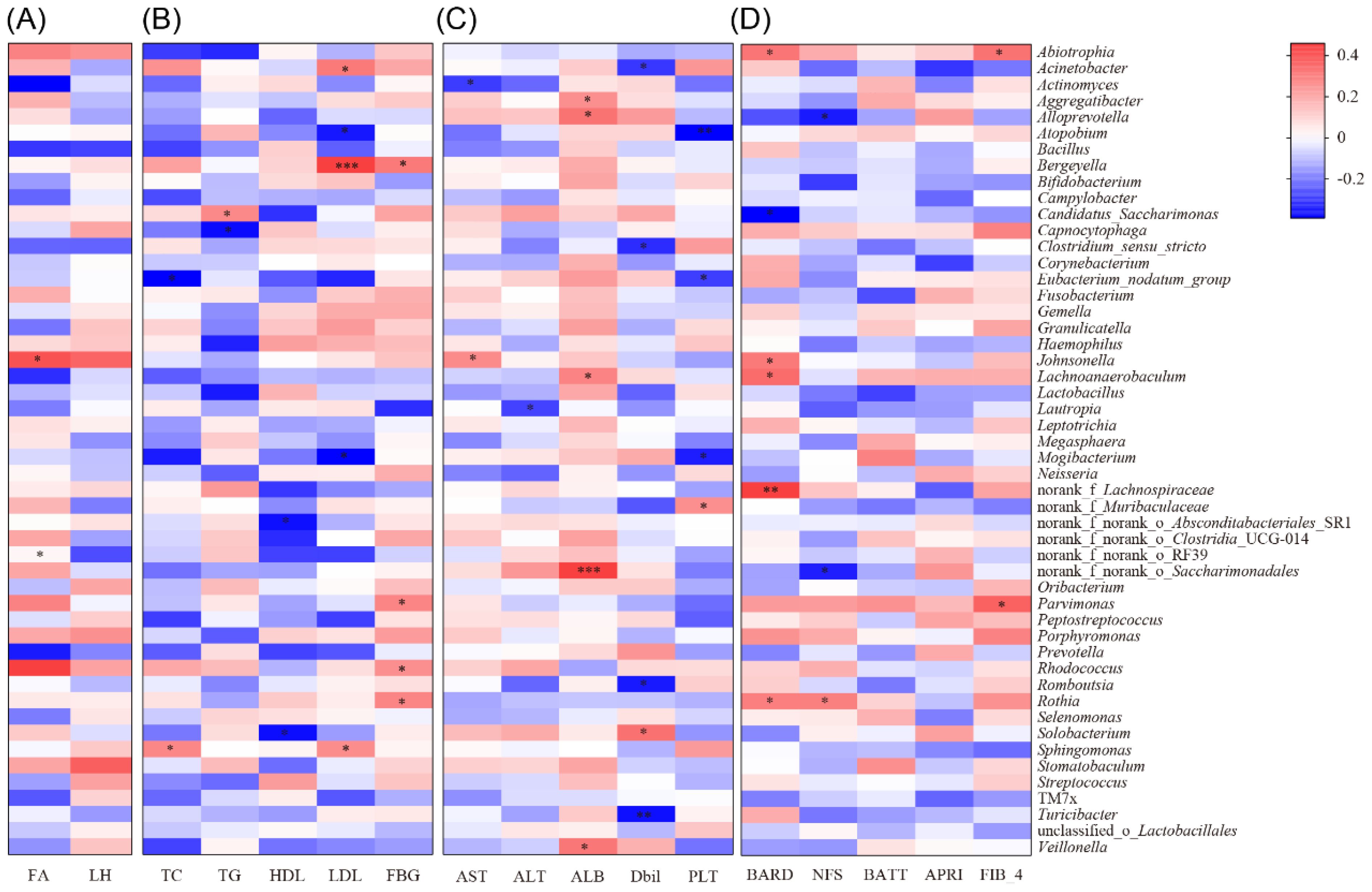
Figure 4. Correlation analysis between different clinical indicators and tongue coating microbiota in MAFLD patients. (A) Correlation heatmap of FA and LH relative to the abundance and composition of tongue coating microbiota in MAFLD patients; (B) Correlation heatmap between glucose and lipid indicators (TC, TG, HDL, LDL and FBG) and the abundance and proportion of tongue microbiota in MAFLD patients; (C) Correlation heatmap of liver function indicators (AST, ALT, ALB, Dbil, PLT) between the abundance and proportion of tongue microbiota genera in MAFLD patients; (D) Correlation heatmap of disease severity related scores (BARD, NFS, BATT, APRI, FIB-4) between the abundance and proportion of tongue microbiota genera in MAFLD patients; *, p < 0.05;**, p < 0.01;***, p < 0.001.
Correlation analysis between tongue coating microorganisms and liver function in MAFLD patients
In addition, we observed a positive correlation between Johnsonella and AST, as well as a positive correlation between Saccharimondales and Veillonella with ALB. Conversely, Atopobium was negatively correlated with PLT (p < 0.05, Figure 4C). Furthermore, we found that the genus Rothia was positively correlated with the AST to APRI scores, while Neisseria exhibited a positive correlation with the BARD score. Additionally, there was a positive correlation (p < 0.05, Figure 4D) between Parvimonas and Abiotrophia with the FIB-4 score.
The effect of WMT on tongue microbiota in MAFLD patients
Impact of WMT on tongue coating microecology in MAFLD patients
This study found that the alpha diversity of the tongue microbiota in MAFLD patients decreased after WMT treatment, with the exception of the Shannon index, and there were no significant changes in the ACE, Sobs, or Simpson indices (Figure 5A). PCoA analysis also revealed no significant alterations (Figure 5B), indicating that the microbial community structure remained relatively stable. Furthermore, the Wilcoxon rank sum test indicated a significant decrease in the relative abundance of opportunistic pathogens, such as Peptostreptococcus, in MAFLD patients following WMT. Conversely, the abundance of potential probiotics, including Lachnospiraceae and Bifidobacterium, increased significantly (p < 0.05, Figure 5C).
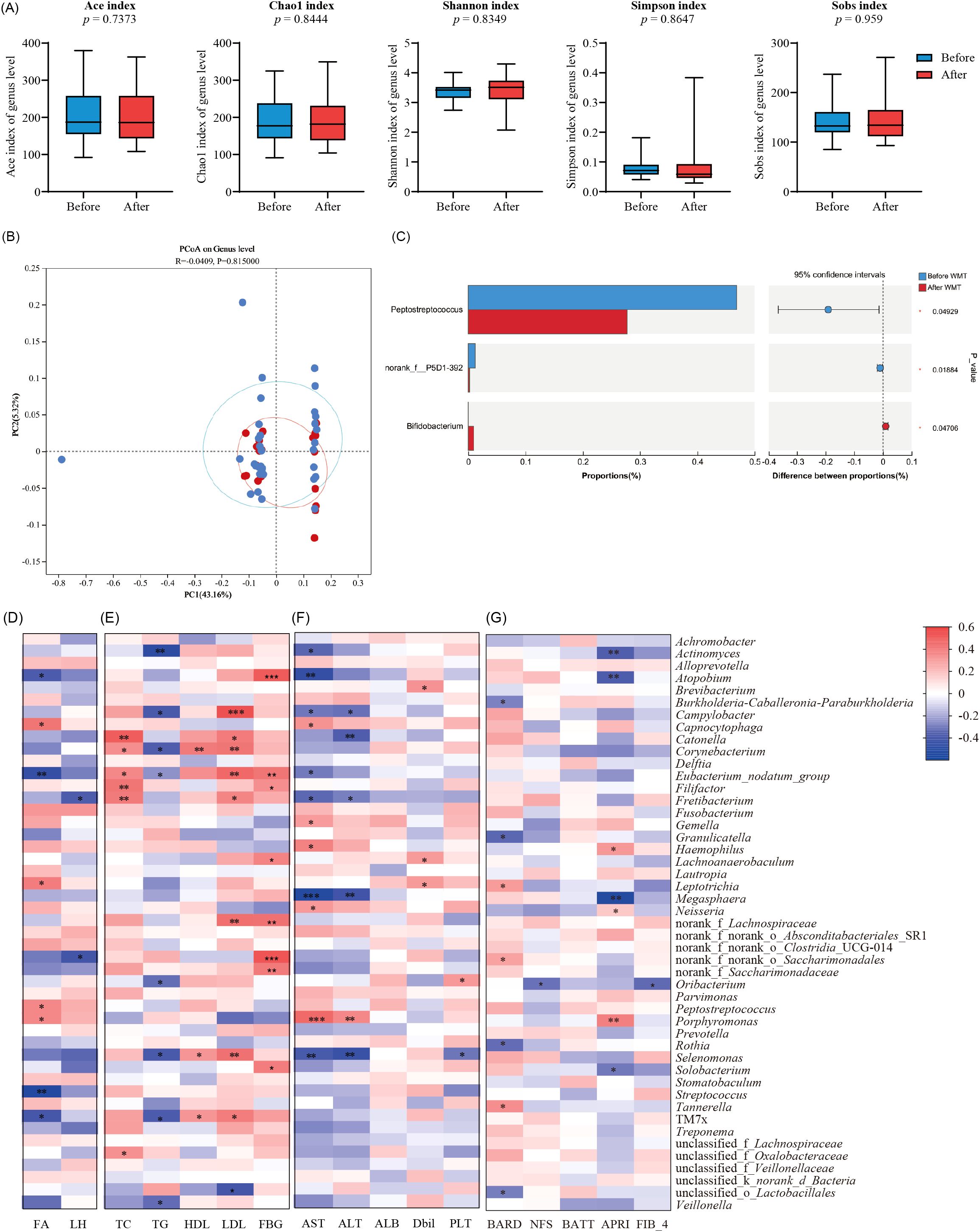
Figure 5. The effect of WMT on tongue microbiota in MAFLD patients. (A) Box plots comparing the alpha diversity indices (Ace, Chao1, Sobs, Simpson, and Shannon) of tongue coating microbiota in MAFLD patients before and after WMT; (B) Differentiate the β diversity of two groups of microorganisms through POCA plot (C) and plot the horizontal differences in the genera of two groups of tongue coating microorganisms; (D) Correlation heatmap between the abundance and proportion of tongue microbiota genera and fat attenuation and liver hardness in MAFLD patients after WMT; (E) Correlation heatmap of glucose and lipid indicators (TC, TG, HDL, LDL, FBG) at the level of abundance and proportion of tongue microbiota in MAFLD patients after WMT; (F) Correlation heatmap of liver function indicators (AST, ALT, ALB, Dbil, PLT) between the abundance and proportion of tongue microbiota genera in MAFLD patients after WMT; (G) Correlation heatmap of disease severity related scores (BARD, NFS, BATT, APRI, FIB-4) between the abundance and proportion of tongue microbiota genera in MAFLD patients after WMT; *, p < 0.05;**, p < 0.01;***, p < 0.001.
Further analysis revealed that Porphyromonas, Leptotrichia, and CO2-oxidizing bacteria in the tongue microbiota of MAFLD patients post-WMT were positively correlated with fat attenuation. In contrast, Streptococcus and other bacteria were positively associated with the fat attenuation burden in these patients (p < 0.05, Figure 5D). Additionally, the genera Catonella, Fretibacterium, and Filifactor showed positive correlations with TC indicators in patients, while Campylobacter, Corynebacterium, and Eubacterium were positively correlated with both HDL and LDL. Moreover, Atonobium, Saccharimonadales, and Lachnoanaerobaculum were positively associated with FBG levels (p < 0.05, Figure 5E).
Correlation analysis between tongue coating microorganisms and liver function in MAFLD patients treated with WMT
Through the analysis of liver function indicators, we found that the genera Haemophilus and Porphyromonas were positively correlated with liver enzyme levels (ALT, AST), while Oribaterium showed a positive correlation with ALB (p < 0.05, Figure 5F). Additionally, Porphyromonas and Haemophilus were positively correlated with disease severity scores, whereas Megasphaera exhibited a negative correlation with APRI scores (p < 0.05, Figure 5G).
The effect of WMT on MAFLD mice
Effects of WMT on the microecology of tongue coating in MAFLD mice
Animal experiments revealed significant differences in the tongue coating microbiota between MAFLD mice and healthy control (CON) mice (Figures 6A–C). The results of the PCoA for beta diversity indicated no overlap in the microbial communities of the tongue coating at the species level between the MAFLD and CON groups (Figure 6B), highlighting alterations in the composition and structure of the tongue coating microbiota in MAFLD mice. Further analysis using Wilcoxon rank sum tests at the genus level demonstrated that the abundances of the genera Rothia and Faecalibaculum were significantly higher in the MAFLD group, while the abundances of Muribaculaceae and Flavobacterium were significantly elevated in the CON group (p < 0.05, Figure 6C). These findings indicate a substantial difference in the tongue coating microbiota between the two groups.
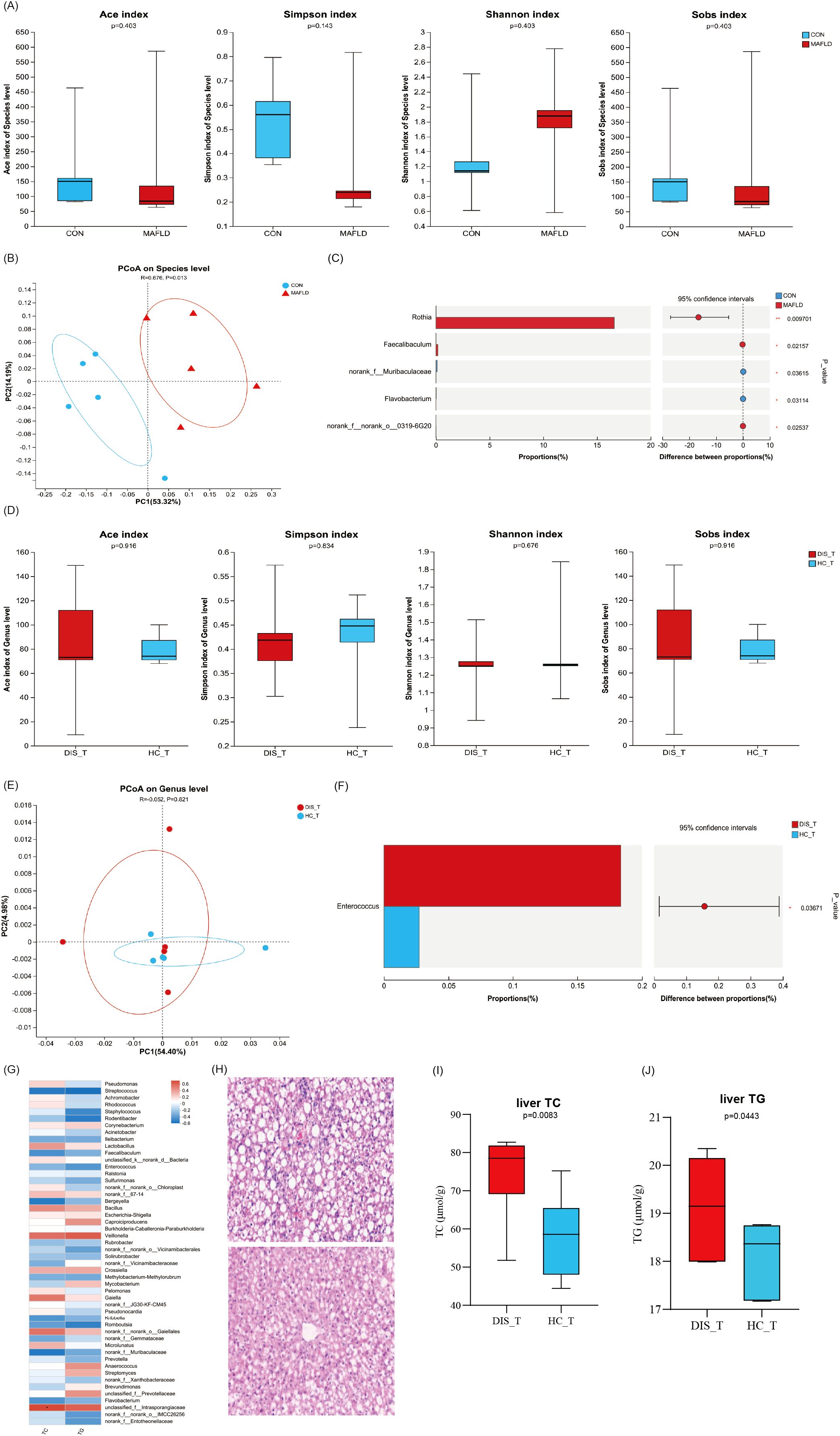
Figure 6. The effect of WMT on MAFLD mice. Differential analysis of tongue coating microbiota between healthy mice vs. MAFLD mice (A–C) and MAFLD mouse disease group vs. healthy group after WMT (D–F); (G) Heatmap of TC, TG and tongue coating microbiota in MAFLD mice after WMT; (H) H.E. staining of liver tissue; The levels of TC (I) and TG (J) in the liver of MAFLD mice.
Following FMT treatment, no significant statistical differences were observed in the alpha diversity indices (ACE, Chao1, Shannon) of the tongue microbiota between the group receiving severe fatty liver fecal microbiota transplantation (DIS_T) and the group receiving healthy fecal microbiota (HC_T) (Figure 6D). This indicates that there were no significant differences in the richness and diversity of the tongue microbiota between the two groups of mice. Furthermore, beta diversity analysis of the two groups of MAFLD mice also revealed no significant differences in the microbial structure of the tongue coating (Figure 6E). However, the Wilcoxon rank sum test at the genus level identified a higher abundance of the opportunistic pathogenic bacterium Enterococcus in the disease microbiome (p < 0.05, Figure 6F). Additional analysis showed that the levels of TC and TG in MAFLD mice following FMT were positively correlated with the genera Veillonella, Intrasporangiaceae, Gaielle, and Bacillus, while negatively correlated with Streptococcus, Faecalibaculum, Bergeyella, Kribbella, and Romboutsia (p < 0.05, Figure 6G).
Effects of WMT on the liver of MAFLD mice
Histological analysis of the livers from mice revealed that the liver lobule structure in the DIS_T group exhibited more severe damage, with a higher degree of hepatic cell steatosis compared to the HC_T group. Additionally, focal necrosis was significantly more prevalent in the livers of DIS_T mice than in those of HC_T mice (Figure 6H). Furthermore, the levels of TC and TG per gram of liver in DIS_T mice treated with FMT were significantly elevated compared to those in HC_T mice (p < 0.05, Figures 6I, J).
Discussion
Discussion on the clinical treatment effect of WMT on MAFLD
In recent years, numerous studies have demonstrated that regulating gut microbiota through FMT can be an effective treatment for MAFLD. A randomized controlled trial investigating the use of FMT for MAFLD treatment found that FMT can significantly reduce liver fat deposition and alleviate disease symptoms by improving gut microbiota dysbiosis (24). However, there is a relative paucity of studies examining the clinical efficacy of WMT, an advanced form of FMT, specifically in the context of MAFLD. Due to the small sample size in our previous research, we plan to expand our sample size in this study to further evaluate the clinical efficacy of WMT on MAFLD. The findings of this study align with previous research, indicating that WMT can effectively reduce the hepatic steatosis index in MAFLD patients. In a separate clinical randomized controlled study involving 87 obese patients, participants were divided into two groups, with one group receiving FMT and the other a placebo. After the same treatment course, the FMT group showed significant improvements in fat ratio and metabolic disorders (25). Our team has also conducted a randomized controlled clinical trial that demonstrated FMT’s ability to reduce fat accumulation in the liver by reshaping gut microbiota dysbiosis, thus improving therapeutic outcomes for MAFLD patients (25). Notably, its clinical efficacy appears to be higher in lean MAFLD patients compared to their obese counterparts (24). These findings suggest that WMT may represent a promising new approach for the treatment of MAFLD.
However, some studies have reported that while FMT can improve gut microbiota and barrier function in patients with MAFLD, it does not significantly alter liver fat deposition (26). In this study, we observed that the recovery rate of the liver fat attenuation index in MAFLD patients was 21.21% after the first course of treatment, 40.91% after the second course, but only 30% after the third course. This decline may be attributed to the extended interval between the third and fourth WMT treatments, which likely led to a reduction in the diversity and abundance of the gut microbiota, thereby diminishing therapeutic efficacy. Previous studies have suggested that the effectiveness of FMT is closely related to various factors, including the characteristics of the donor’s microbiota, the recipient’s intestinal colonization resistance, and the frequency of FMT treatments (27, 28). Consequently, we hypothesize that short-term, multiple WMT sessions may provide patients with a healthier and higher-quality microbiota characterized by greater richness and diversity. This enhancement could be a critical factor contributing to the significant improvement in fat deposition observed in MAFLD patients. It is also important to note that dysregulation of glucose and lipid metabolism is a key feature in the progression of MAFLD (27). In our study, we found that with increasing treatment cycles, TC, TG, and LDL levels significantly decreased in MAFLD patients, indicating improvements in glucose and lipid metabolism following WMT. Notably, no serious adverse events were reported during the course of this study, consistent with findings from other clinical studies indicating that FMT does not lead to serious adverse outcomes. This suggests that WMT is also a safe intervention.
Discussion on the correlation between tongue coating microorganisms and MAFLD
As a significant component of the oral microbiota, tongue coating microbiota has become a focal point for researchers in microbiology, both domestically and internationally. The density, diversity, and changes in the tongue coating microbiota can reflect the physiological status of the human body and the alterations in its microbiota composition (27, 28).In this study, we found that the abundance of Porphyromonas in the tongue microbiota of MAFLD patients was significantly higher than that in healthy individuals. Similar findings have been reported in a study, indicating a significant association between Porphyromonas and the occurrence and progression of MAFLD (29). Additionally, the study by Masato (30) identified a higher proportion of Porphyromonas in MAFLD patients, positively correlating with disease severity. This suggests that Porphyromonas could serve as a potential biomarker for MAFLD.
The composition of microorganisms in the tongue coating of healthy individuals remains relatively stable. Previous scholars have reported on the specific proportions of various bacteria, including Streptococcus salivarius (20%), Streptococcus pyogenes (4%), Streptococcus thermophilus (8%), and Neisseria (0.05%) (31, 32). In contrast, our study observed that the abundance of the Neisseria genus in MAFLD patients significantly exceeded this baseline, indirectly confirming the existence of tongue coating microbiota dysbiosis in this population.
Furthermore, we identified significantly lower abundances of Pseudomonas aeruginosa and Subdoligulum in MAFLD patients, aligning with findings reported by Diaz (33), which noted that these genera were less abundant in the gut microbiota of MAFLD patients compared to healthy controls. Collectively, these results suggest that Porphyromonas, Pseudomonas aeruginosa, and Subdoligulum may serve as potential biomarkers for MAFLD. Additionally, our animal studies confirmed significant differences in tongue coating microbiota between MAFLD and healthy controls, indicating that MAFLD may have identifiable biomarkers within the tongue coating microbiota.
In addition, multiple microbial communities in the tongue coating of MAFLD patients exhibited correlations with clinical indicators, akin to the positive association between Rhodococcus and BMI values reported in a previous study (34). Although our findings reveal disturbances in the tongue coating microbiota of MAFLD patients in both animal and clinical studies, along with the identification of certain highly abundant microbial taxa, the specific relationship between microbiota and MAFLD necessitates further extensive investigation for exploration and validation. We believe that as related research advances and emerging technologies develop, the connections between MAFLD and tongue coating microecology will become clearer, ultimately providing novel insights for the prevention and treatment of MAFLD.
Discussion on the effect of WMT treatment on the microbiota of MAFLD tongue coating
In this study, we found that after WMT treatment, the abundance of Peptostreptococcus in the tongue coating of MAFLD patients significantly decreased, while the abundance of Lachnospiraceae and Bifidobacterium increased markedly. A study demonstrated that FMT effectively alleviates symptoms in patients with diarrhea-predominant irritable bowel syndrome, leading to a significant increase in Bifidobacterium abundance in the intestinal microbiota (35). This finding aligns with our observation of a substantial increase in Bifidobacterium in MAFLD patients following WMT. Furthermore, Wu (36) reported that Bifidobacterium is negatively correlated with metabolic endotoxemia, suggesting that this genus may help mitigate disease progression. While Peptostreptococcus was typically considered part of the normal human microbiota, it has also been associated with infections in various tissues and organs. Therefore, based on these findings, we infer that WMT not only impacts gut microbiota but also influences the microbiota present in the tongue coating.
In addition, this study unexpectedly identified a correlation between tongue coating microorganisms and the efficacy of WMT treatment for MAFLD. For instance, Porphyromonas was found to be positively correlated with obesity, ALT, AST, and disease severity in MAFLD patients, consistent with findings reported in other studies. Specifically, Porphyromonas gingivalis has been closely associated with the onset and progression of various diseases, including MAFLD (37, 38), cirrhosis (39), and liver cancer (40). This relationship may stem from the ability of Porphyromonas to invade the intestine, leading to an imbalance in gut microbiota and increased serum endotoxin levels. Consequently, this disruption may compromise the intestinal mucosal barrier and interfere with hepatic fat metabolism (41). These findings suggest that Porphyromonas, Peptostreptococcus, and Bifidobacterium may serve as potential biomarkers for predicting the efficacy of WMT in treating MAFLD. To further investigate this, we conducted animal experiments that yielded similar results. Although no significant differences were observed in the microbiota of the tongue coating between mice receiving healthy bacterial solutions and those receiving severe fatty liver bacterial solutions after FMT, Enterococcus exhibited a higher abundance in the group receiving the severe fatty liver bacterial solution. As a well-known conditional pathogen, Enterococcus is recognized as a leading cause of infection (42). A clinical study conducted by Vieira (43) demonstrated that intestinal Enterococcus can translocate to the liver and other tissues, while Schwenger KJP (44) found a strong correlation between Enterococcus abundance and disease severity and mortality in MAFLD patients. Based on these findings, we speculate that Enterococcus may be widely present in MAFLD in both animal models and humans, potentially acting as a conditional pathogen closely linked to the onset and progression of MAFLD.
However, this study has several limitations, including a small sample size and microbiological assessments of tongue coating at different treatment cycles. Therefore, there is an urgent need for multicenter, large-scale studies to validate the potential significance of these microorganisms in MAFLD. Furthermore, the metabolic products of tongue coating microorganisms are relatively abundant, and MAFLD patients exhibit their own accumulation of metabolic products, some of which were closely related to specific microorganisms.
To address these complexities, integrating and analyzing the metabolomics of both tongue coating microbiota and intestinal microbiota could lead to the identification of additional and more robust potential biomarkers. Such an approach may provide new diagnostic methods for MAFLD, offer important theoretical insights into the mechanisms underlying MAFLD, and pave the way for innovative strategies in the prevention and treatment of this condition.
In addition, this study acknowledges several limitations. Firstly, the sample size was relatively small, which may limit the generalizability of the findings. Secondly, despite the observed associations, there remains a dearth of specific mechanistic research elucidating the relationship between tongue coating microorganisms and MAFLD. Consequently, there is a pressing need for multi-center studies with larger sample sizes to comprehensively investigate the effects of WMT, on the tongue coating microbiota and the clinical efficacy in MAFLD patients. Furthermore, future research should endeavor to explore the potential microbial interplay between the tongue coating and the gut, as well as delve deeper into the underlying mechanisms to enhance our understanding of this intricate relationship.dd.
Conclusion
In summary, WMT demonstrates a substantial capacity to ameliorate the hepatic steatosis index among patients with MAFLD, accompanied by a minimal occurrence of adverse reactions, thereby suggesting its safety and efficacy as a therapeutic intervention for MAFLD. Notably, significant disparities were observed in the tongue coating microbiota of both MAFLD patients and mice, in contrast to healthy controls. Various bacterial genera, including Streptococcus, Porphyromonas, Johannes, and Rhodococcus, exhibited close correlations with clinical indicators of MAFLD in the tongue coating of affected patients, hinting at their potential as biological markers for assessing disease severity. Furthermore, WMT exerts a notable influence on the tongue coating microbiota of MAFLD patients and mice. After WMT, multiple bacterial genera, such as Bifidobacterium, Streptococcus, and Porphyromonas, were found to be associated with clinical indicators of MAFLD, indicating their utility as biological markers for evaluating the therapeutic efficacy of WMT in treating MAFLD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Experimental Animal Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LH: Writing – original draft, Writing – review & editing. SW: Writing – original draft, Writing – review & editing. HZ: Writing – original draft, Writing – review & editing. SF: Writing – original draft, Writing – review & editing. HjZ: Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. WX: Writing – original draft, Writing – review & editing. LW: Writing – original draft. TZ: Writing – original draft, Writing – review & editing. XH: Writing – original draft, Writing – review & editing. JY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study has received support from Key Research and Development Projects in Guangdong Province Foundation (2022B1111070006).
Acknowledgments
We sincerely thank all the staff who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: A meta-analysis and systematic review of 10 739–607 individuals. J Clin Endocrinol Metab. (2022) 107:2691–700. doi: 10.1210/clinem/dgac321
2. Bae SDW, George J, and Qiao L. From MAFLD to hepatocellular carcinoma and everything in between. Chin Med J (Engl). (2022) 135:547–56. doi: 10.1097/CM9.0000000000002089
3. Powell EE, Wong VW, and Rinella M. Non-alcoholic fatty liver disease. Lancet (London England). (2021) 397:2212–24. doi: 10.1016/S0140-6736(20)32511-3
4. Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, and Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. (2021) 75 Suppl 1:S67–s81. doi: 10.1016/j.jhep.2020.11.013
5. Collaborators GC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol hepatology. (2020) 5:245–66. doi: 10.1016/S2468-1253(19)30349-8
6. Microbiome H and Consortium P. Structure, function and diversity of the healthy human microbiome. Nature. (2012) 486:207–14. doi: 10.1038/nature11234
7. Papadopoulos G, Kramer CD, Slocum CS, Weinberg EO, Hua N, Gudino CV, et al. A mouse model for pathogen-induced chronic inflammation at local and systemic sites. J Vis Exp. (2014) 90:e51556. doi: 10.3791/51556
8. Acharya C and Bajaj JS. Altered microbiome in patients with cirrhosis and complications. Clin Gastroenterol Hepatol. (2019) 17:307–21. doi: 10.1016/j.cgh.2018.08.008
9. Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatol (Baltimore Md). (2015) 62:1260–71. doi: 10.1002/hep.27819
10. Riordan SM and Williams R. Gut flora and hepatic encephalopathy in patients with cirrhosis. N Engl J Med. (2010) 362:1140–2. doi: 10.1056/NEJMe1000850
11. Nobili V, Mosca A, Alterio T, Cardile S, and Putignani L. Fighting fatty liver diseases with nutritional interventions, probiotics, symbiotics, and fecal microbiota transplantation (FMT). Adv Exp Med Biol. (2019) 1125:85–100. doi: 10.1007/5584_2018_318
12. Hanssen NMJ, de Vos WM, and Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future? Cell Metab. (2021) 33:1098–110. doi: 10.1016/j.cmet.2021.05.005
13. Liang F, Lu X, Deng Z, Zhong HJ, Zhang W, Li Q, et al. Effect of washed microbiota transplantation on patients with dyslipidemia in South China. Front endocrinology. (2022) 13:827107. doi: 10.3389/fendo.2022.827107
14. Liang F, Song Y, Lin D, He H, Xu J, He X, et al. Washed microbiota transplantation is associated with improved lipid profiles: long-term efficacy and safety in an observational cohort from South China. Clin Trans gastroenterology. (2024) 15:e00735. doi: 10.14309/ctg.0000000000000735
15. Lin D, Hu D, Song Y, He X, and Wu L. Long-term efficacy of washed microbiota transplantation in overweight patients. Eur J Clin Invest. (2024) 10:e14260. doi: 10.1111/eci.14260
16. Zhang L, Zhou W, Zhan L, Hou S, Zhao C, Bi T, et al. Fecal microbiota transplantation alters the susceptibility of obese rats to type 2 diabetes mellitus. Aging. (2020) 12:17480–502. doi: 10.18632/aging.103756
17. Zhong HJ, Zeng HL, Cai YL, Zhuang YP, Liou YL, Wu Q, et al. Washed microbiota transplantation lowers blood pressure in patients with hypertension. Front Cell infection Microbiol. (2021) 11:679624. doi: 10.3389/fcimb.2021.679624
18. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
19. Shi Q. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J (Engl). (2020) 133:2330–2. doi: 10.1097/CM9.0000000000000954
20. Lim H, Lim YM, Kim KH, Jeon YE, Park K, Kim J, et al. A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes. Nat Commun. (2018) 9:1438. doi: 10.1038/s41467-018-03939-w
21. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. (2012) 107:811–26. doi: 10.1038/ajg.2012.128
22. Maeda D, Sakane K, Ito T, Kanzaki Y, Sohmiya K, and Hoshiga M. Fibrosis-4 index reflects right-sided filling pressure in patients with heart failure. Heart vessels. (2020) 35:376–83. doi: 10.1007/s00380-019-01505-y
23. Fatty liver and alcoholic liver disease group, Hepatology branch, Chinese Medical Association, fatty liver disease expert committee. Chinese Medical Association Guidelines for the prevention and treatment of nonalcoholic fatty liver disease. Chin J Hepatol. (2018) 034:641–9. doi: 10.3969/j.issn.1009-5519.2018.05.001
24. Xue L, Deng Z, Luo W, He X, and Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: A randomized clinical trial. Front Cell infection Microbiol. (2022) 12:759306. doi: 10.3389/fcimb.2022.759306
25. Leong KSW, Jayasinghe TN, Wilson BC, Derraik JGB, Albert BB, Chiavaroli V, et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA network Open. (2020) 3:e2030415. doi: 10.1001/jamanetworkopen.2020.30415
26. Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: A randomized control trial. Am J Gastroenterol. (2020) 115:1055–65. doi: 10.14309/ajg.0000000000000661
27. Biedermann L, Kreienbühl A, and Rogler G. Microbiota therapy in inflammatory bowel disease. Visceral Med. (2024) 40:92–101. doi: 10.1159/000536254
28. Wilson BC, Vatanen T, Cutfield WS, and O’Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell infection Microbiol. (2019) 9:2. doi: 10.3389/fcimb.2019.00002
29. Alakhali MS, Al-Maweri SA, Al-Shamiri HM, Al-Haddad K, and Halboub E. The potential association between periodontitis and non-alcoholic fatty liver disease: a systematic review. Clin Oral investigations. (2018) 22:2965–74. doi: 10.1007/s00784-018-2726-1
30. Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. (2012) 12:16. doi: 10.1186/1471-230X-12-16
31. He C, Liao Q, Fu P, Li J, Zhao X, Zhang Q, et al. Microbiological characteristics of different tongue coatings in adults. BMC Microbiol. (2022) 22:214. doi: 10.1186/s12866-022-02626-7
32. Jing W. Progress in microbiological research on tongue coating. (Chinese) International Journal of traditional Chinese medicine. (1999) 021:3–5.
33. Rodriguez-Diaz C, Taminiau B, García-García A, Cueto A, Robles-Díaz M, Ortega-Alonso A, et al. Microbiota diversity in nonalcoholic fatty liver disease and in drug-induced liver injury. Pharmacol Res. (2022) 182:106348. doi: 10.1016/j.phrs.2022.106348
34. Ren Y, Shi X, Mu J, Liu S, Qian X, Pei W, et al. Chronic exposure to parabens promotes non-alcoholic fatty liver disease in association with the changes of the gut microbiota and lipid metabolism. Food Funct. (2024) 15:1562–74. doi: 10.1039/D3FO04347A
35. Guo Q, Lin H, Chen P, Tan S, Wen Z, Lin L, et al. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered. (2021) 12:11885–97. doi: 10.1080/21655979.2021.1999374
36. Xiaokang W. Quantitative study of intestinal Lactobacillus and peptostreptococcus in patients with type 2 diabetes and its significance. J Xi ‘an Jiaotong Univ. (2015) 36:93–97+134. doi: 10.7652/jdyxb201501017
37. Mei EH, Yao C, Chen YN, Nan SX, and Qi SC. Multifunctional role of oral bacteria in the progression of non-alcoholic fatty liver disease. World J hepatology. (2024) 16:688–702. doi: 10.4254/wjh.v16.i5.688
38. Yao C, Lan D, Li X, Wang Y, Qi S, and Liu Y. Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infect. (2023) 25:105040. doi: 10.1016/j.micinf.2022.105040
39. Patel VC and Shawcross DL. Salivary microbiota-immune profiling in cirrhosis: could this be the noninvasive strategy that will revolutionize prognostication in hepatology? Hepatol (Baltimore Md). (2015) 62:1001–3. doi: 10.1002/hep.27870
40. Lu H, Ren Z, Li A, Zhang H, Jiang J, Xu S, et al. Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci Rep. (2016) 6:33142. doi: 10.1038/srep33142
41. Li D, Chen Y, Wan M, Mei F, Wang F, Gu P, et al. Oral magnesium prevents acetaminophen-induced acute liver injury by modulating microbial metabolism. Cell Host Microbe. (2024) 32:48–62.e9. doi: 10.1016/j.chom.2023.11.006
42. Raza T, Ullah SR, Mehmood K, and Andleeb S. Vancomycin resistant Enterococci: A brief review. J Pak Med Assoc. (2018) 68:768–72.
43. Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Sci (New York NY). (2018) 359:1156–61. doi: 10.1126/science.aar7201
Keywords: washed microbiota transplantation, metabolic associated fatty liver disease, tongue coating microorganisms, clinical efficacy, biological correlations
Citation: Huang L, Wang S, Zhang H, Feng S, Zhong H, Chen J, Xie W, Wu L, Zhang T, He X and Yang J (2025) Clinical efficacy evaluation of washed microbiota transplantation treatment for metabolic related fatty liver disease and its impact on tongue coating microorganisms. Front. Endocrinol. 16:1684173. doi: 10.3389/fendo.2025.1684173
Received: 21 August 2025; Accepted: 13 October 2025;
Published: 29 October 2025.
Edited by:
Zhengyang Bao, Wuxi Maternity and Child Health Care Hospital, ChinaReviewed by:
Dinakaran Vasudevan, SKAN Research Trust, IndiaYongpeng Shi, University of Science and Technology of China, China
Copyright © 2025 Huang, Wang, Zhang, Feng, Zhong, Chen, Xie, Wu, Zhang, He and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Yang, anVhbnlhbmdvbkAxNjMuY29t; Xingxiang He, aGV4aW5neGlhbmdAZ2RwdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Lingui Huang1†
Lingui Huang1† Siqi Wang
Siqi Wang Haojie Zhong
Haojie Zhong Junyi Chen
Junyi Chen Wenrui Xie
Wenrui Xie Lei Wu
Lei Wu Xingxiang He
Xingxiang He Juan Yang
Juan Yang