Abstract
Background:
Inhibition of the renin–angiotensin system (RAS) may influence gut microbial composition and blood pressure, yet current evidence remains limited. This review examines how angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) modify gut microbiome composition, function, and blood pressure regulation.
Methods:
We conducted a systematic search of MEDLINE and EMBASE from inception to September 2025 using terms including “human,” “rat,” “angiotensin converting enzyme inhibitor,” “angiotensin receptor blocker,” and “gut microbiome.” Eligible studies were required to report changes in microbiome diversity, bacterial composition, or short-chain fatty acids (SCFAs) associated with ACEi/ARB treatment across animal or human models. Data extraction and risk of bias assessments were performed independently by multiple reviewers.
Results:
After deduplication, 642 retrieved articles were filtered and nine met inclusion criteria (eight in rodent models, one human study). ACEi/ARB administration in animals was associated with increased microbial diversity, restoration of intestinal oxygen balance, and enrichment of SCFA-producing anaerobic genera such as Bifidobacterium, Bacteroides, Blautia, and Akkermansia. In the human study, ACEi/ARB use did not significantly alter microbial diversity, but decreased populations of facultative aerobic pathogens including Staphylococcus and Enterobacterales. Functionally, prolonged RAS inhibition elevated levels of acetate, propionate, and butyrate, and enhanced gut barrier integrity while attenuating inflammatory signaling. The human study was found to have a moderate risk of bias.
Conclusions:
ACEi and ARB therapies appear to reshape gut microbiome structure and metabolic function, promoting SCFA-producer expansion, improved gut barrier integrity, and modulation of microbial taxa linked to inflammation and hypertension. However, human data is limited, and further transitional research is needed to confirm these findings.
Introduction
Hypertension (HTN), a leading cause of cardiovascular morbidity and mortality including stroke and myocardial infarction, affects approximately 122 million adults in the United States (US) (1–3). Less than 44% of American adults with hypertension have controlled blood pressure (BP), underscoring the urgent need for novel therapeutic strategies (1). The gut microbiota can impact HTN development and progression with altered gut microbial composition and function associated with elevated BP (4–7). Proposed mechanisms for this relationship include modulation of the renin-angiotensin system (RAS), vascular resistance, and immune responses (8–11).
The RAS, a key regulator of BP, comprises two opposing pathways: the ACE1-Ang II axis, which promotes vasoconstriction, inflammation, and fibrosis, and the ACE2-Ang 1–7 axis, producing counteracting effects (12). Traditionally, RAS was considered to function primarily as a systemic regulator of cardiovascular homeostasis via circulating Angiotensin II (Ang II). However, nearly all components of RAS have been identified in various local tissues, including the gastrointestinal tract (GIT) (13). Locally, Ang II has been shown to drive intestinal inflammation, increase gut permeability, and contribute to dysbiosis (14). Conversely, Ang 1–7 has demonstrated protective effects by restoring gut barrier integrity (15). Certain gut microbes such as Bifidobacterium bifidum, have direct angiotensin-converting enzyme (ACE) inhibitory activity, suggesting a direct influence on RAS activity (9). These observations suggest a complex bidirectional interplay between RAS signaling, gut health, and BP regulation.
RAS inhibitors, including angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), are cornerstone therapies for HTN management (16). Besides their hypotensive effect, growing evidence suggests that ACEis and ARBs may also influence gut health (17, 18). For example, candesartan has been shown to restore gut barrier integrity, enhance short chain fatty acid (SCFA) production, and increase microbial diversity in spontaneously hypertensive rats (SHR) (17). Despite these promising findings, evidence is limited and heterogenous. Importantly, the mechanisms by which RAS inhibition influences the gut microbiome are not well understood, highlighting a significant gap in literature. Exploring these mechanisms could expand our understanding of the actions of RAS inhibitors and reveal possibilities for innovative interventions using the gut microbiome to improve BP regulation. The aim of this systematic review was to assess the effects of ACEIs and ARBs on the composition, functionality, and pathology of the gut microbiome in hypertensive animal models and human studies.
Methods
The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines were used to conduct this systematic review (19). Inclusion criteria was the following: (1) hypertensive human or animal models; (2) ≥ 20 years old if human population; (3) use of ACEi/ARB as antihypertensive monotherapy; (4) evaluated at least 1 pre-specified outcome and (5) published in English. Systematic reviews, meta-analyses, post-hoc analyses, and methodology articles were excluded. Outcomes of interest included changes in gut composition and function, evaluated by alpha and/or beta diversity, bacterial taxa, and SCFAs, along with gastrointestinal health and inflammatory changes related to ACEi or ARB treatment.
MEDLINE (PubMed) and EMBASE (Elsevier) were systematically searched from inception to September 9, 2025. The following search terms (and variations of these terms as well as ACEi/ARB names) were used: human, mouse, rat, rodent, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, gut microbiome, and gut dysbiosis. The resulting citations were uploaded into Rayyan, a web-based and mobile application screening tool (20). Three authors (ASM, EW, KG) independently screened articles by titles and abstracts using the defined inclusion and exclusion criteria with ASM and EW independently screening and KG serving as a tie-breaker. Full-text articles were then screened by the previously mentioned criteria and desired outcomes. Relevant data were independently extracted using a standardized form and disagreements were resolved through discussion by investigators. A consensus for disagreements (i.e. differing interpretations of outcome data) was ultimately achieved via a third team member serving as a tie-breaker (KG). Quality assessment of animal studies was performed using the SYRCLE (Systematic Review Centre for Laboratory animal Experimentation) risk of bias tool and the human study using the Cochrane Collaboration’s risk of bias tool (21, 22).
Results
Figure 1 summarizes the process of literature selection. A total of 642 articles were initially retrieved after removing duplicates. Fifteen articles remained after title and abstract screening. Four of the twelve articles were excluded because subjects were not the population of interest (i.e. did not have hypertension or were assessing hypertension in the setting of additional comorbidities), one was excluded because it did not evaluate a pre-specified outcome, and one was excluded because it was a duplicate (23–28). After full-text screening, nine articles met the inclusion criteria (17, 18, 29–35). Eight of the nine articles were conducted in rats (17, 18, 29, 31–35), and one study was in humans (30). Table 1 summarizes the effects of RAS inhibitors on gut composition, function, and associated pathologies in the included studies. A summary of bias risk is shown in Supplementary Table 1.
Figure 1

Study selection flow diagram. Flow of information through the different phases of the systematic review according to the preferred reporting items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Table 1
| Author (year) | Population/model | Intervention/dose (mg/kg/day)/duration (weeks) (administration route) | Change in microbial diversity | Change in B/F ratio | Change in microbial taxa | Change in SCFA (fecal) | Effect on gut permeability | Effect on inflammatory markers (site) |
|---|---|---|---|---|---|---|---|---|
| Dong et al. (30) | Human | ACEi or ARB/ NR/NR (oral) |
No change | NR | Increased Odoribacter, Clostridium and decreased Staphylococcus, Enterobacter, Klebsiella | NR | NR | NR |
| Wu et al. (31) | Rat/DOCA-salt sensitive model | Captopril/50/5 (oral) |
No change | No change | Increased Bifidobacterium, Akkermansia, and Blautia | Increased butyrate and propionatea | NR | Reduced IL-6 and TNF-alpha (brain) |
| Yang et al. (33) | Rat/SHR | Captopril/250/4 (oral) |
Increased | NR | Increased Allobaculum and Parabacteroides | NR | Improved (decrease I-FABP and increase in epithelial health) | NR |
| Wu et al. (17) | Rat/SHR | Candesartan/1/2-14 (oral) |
Increased | Decreased | Increased Lactobacillus | Increased acetate, propionate and butyrateb | Improved (increased expression of tight junction proteinsc and serum LBP) |
NR |
| Robles-Vera et al. (18) | Rat/SHR | Losartan/20/5 (oral) |
Increased | Decreased | Increased proportion of strict anaerobes and SCFA producing-bacteria | NR | Improved (increased expression of tight junction protein) | Reduced endotoxins, IL-6, and TNF-alpha (plasma). Reduced Th17/Treg ratio (lymph node) |
| Dong et al. (29) | Rat/SHR | Losartan/20/12 (oral) |
Increased | Decreased | Increased Alistipes, Bacteroides, Bifidobacterium, Butyricimonas Decreased Ruminococcaceae, Streptococcus, Turicibacter |
NR | NR | NR |
| Qi et al. (32) | Rat/SHR | Valsartan/7.4/6 (intragastrally) |
Decreased | No change | Increased Lactobacillus | Decreased isobutyrate and isovalerate | Not improved | Decrease CRP |
| Xiong et al. (34) | Rat/SHR | Irbesartan/27/8 | Increased | Decreased | Increased Lactobacillus | Increased acetate, propionate, butyrate, and valerate | Improved (increased expression of tight junction proteins) | Reduced TNF-alpha, IL-6, and IL-1β |
| Gonzalez-Correa et al. (35) | Rat/SHR | Captopril/85/5 (oral) |
No change | No change | Increase strict anaerobes including acetate-producing bacteria and Parabacteroides | NR | Improved (increased expression of tight junction protein and decreased I-FABP) | Reduced TNF-alpha, IL-1β, IL-6, TLR4 (brain) |
Effect of RAS inhibitors on gut composition, function, and pathologies in human and animal studies.
Summary of included studies evaluating the impact of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) on the gut microbiome and related gut pathologies. Diversity changes are reported for alpha and/or beta diversity as defined in the original studies. B/F ratio, Bacteroidetes/Firmicutes ratio; SCFA, short-chain fatty acid; SHR, spontaneously hypertensive rat; DOCA, deoxycorticosterone acetate; NR, not reported; LBP, lipopolysaccharide-binding protein; I-FABP, intestinal fatty acid–binding protein; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-alpha; Th17/Treg, T helper 17/regulatory T cell ratio; TLR-4, toll like receptor. aNot statistically significant. bOnly observed with prolonged treatment (14 weeks). c Tight junction proteins include claudins, occludin, or zonula.
Study design of included articles
Seven of the eight hypertensive rat models used spontaneously hypertensive rat (SHR) models (17, 18, 29, 32–35), and one study used deoxycorticosterone acetate (DOCA)-fed rats as the hypertensive arm (31). The control arms included normotensive Wistar Kyoto (WKY) rats or SHAM (defined as normotensive) rats. Three of the eight studies conducted in rats evaluated an ACEi (captopril) (31, 33, 35), and the other four studies used an ARB (candesartan, losartan, valsartan, or irbesartan) as an intervention (17, 18, 29, 32, 34). The study durations ranged from 5 to 14 weeks.
The single human study evaluated the impact of ACEis/ARBs on the human gut microbiome using a single-center, observational study design that included 55 patients with hypertension (30). Hypertensive patients were categorized as (1) untreated, defined as newly diagnosed with hypertension and naïve to antihypertensive therapy (n = 19), or (2) treated, defined as patients with hypertension on ACEi/ARB therapy for at least 4 weeks (n = 36) (30). Patients in the treated group were classified as either well-controlled (n = 24) if systolic blood pressure (SBP) was < 140 mm Hg and diastolic blood pressure (DBP) < 90 mm Hg or as poorly-controlled (n = 12) if SBP was ≥ 140 mm Hg and/or DBP was ≥ 90 mm Hg (30).
Changes in gut microbial composition and function
In rat models, ACEi or ARB treatment increased gut microbiome diversity (alpha and/or beta diversity) in 62.5% (5/8) of the trials (17, 18, 29, 33, 34). Of the remaining studies, Wu et al. and Gonzalez-Correa et al. reported no significant changes in microbial diversity (31, 35), and Qi et al. observed a decrease in both alpha and beta diversity (32). Notably, Wu et al. utilized a DOCA-salt-induced hypertensive rat model (31), whereas all other animal trials used the spontaneously hypertensive rat (SHR) model (17, 18, 29, 32, 33). Importantly, all studies (8/8) findings suggested that RAS inhibition restored intestinal O2 hemostasis (17, 18, 29, 31–35). This was characterized by an increased abundance of strict anaerobes, including SCFA-producing genera such as Bifidobacterium, Bacteroides, Blautia, and Akkermansia. Additionally, three trials reported an increase in Lactobacillus abundance, a genus known for anti-inflammatory properties (17, 32, 34). Robles-Vera et al. linked gut microbial composition to ARBs’ antihypertensive effect (18). In this study, fecal microbiota transplantation (FMT) from losartan-treated SHR donors to SHR recipients resulted in a significant reduction in BP. In humans, treatment with RAS inhibitors did not significantly impact gut diversity indices (30). However, it increased the abundance of strict anaerobes while decreasing facultative aerobic pathogens, such as Staphylococcus and Enterobacterales.
Four animal studies investigated the effects of RAS inhibitors on gut functionality, focusing on change in SCFA production (17, 31, 32, 34). Overall, prolonged RAS inhibition increased fecal levels of linear SCFAs (acetate, propionate, and butyrate), byproducts of fiber fermentation with immune modulatory and vasodilatory effects (17, 31, 34, 36). Conversely, it reduced branched SCFAs (isobutyric and isovaleric acids), byproducts of protein fermentation linked to impaired colon health (32, 37). Collectively, these findings highlight the potential of RAS inhibitors to correct gut microbial composition and functionality. By restoring colonic hypoxia, RAS inhibitors promote SCFA-producing taxa and reduce facultative aerobic pathogens.
Changes in gut pathologies
RAS inhibition resulted in significant improvements in gut barrier integrity and/or a reduction in inflammatory markers in seven animal studies (17, 18, 31–35). Treatment with ACEi or ARB increased the expression of key tight junction proteins critical for maintaining gut barrier function, including claudins, occludin, and zonula occludens-1, while also promoting an increase in goblet cell numbers and villi length (18, 33). These structural changes collectively contributed to enhanced gut integrity and decrease endotoxemia. Additionally, RAS inhibition restored immune homeostasis by reducing the Th17/Treg ratio and proinflammatory signaling in different organs (18). However, one study by Qi et al. reported that treatment with valsartan failed to restore gut integrity, assessed by changes in zonula occludens-1 expression (32). Notably, plasma levels of Ang II remained persistently elevated during this trial. Finally, hydralazine was shown to significantly reduce BP but had no effect on markers of gut integrity or inflammation (18). These findings underscore the importance of RAS inhibition in preserving gut barrier function and mitigating inflammation, independent of its antihypertensive effects.
Discussion
The impact of antihypertensive agents on the gut microbiome remains poorly understood. To the best of our knowledge, this is the first systematic review to address this gap. Overall, RAS inhibitors seem to modulate both systemic and local RAS signaling, thereby ameliorating intestinal inflammation and epithelial damage and ultimately improving gut microbial composition and functionality. Ang II, acting through the AT1 receptor, is the most potent effector of the RAS, driving intestinal inflammation, increasing gut permeability, and contributing to gut dysbiosis (13). Elevated colonic levels of Ang I and II have been directly associated with disease severity in Crohn’s colitis (38). Importantly, interventions targeting the RAS, such as ACEi or ARBs, have demonstrated therapeutic benefits that extend beyond BP reduction (39).
Retrospective studies have shown that RAS inhibitors are associated with reduced hospitalizations and lower corticosteroid use in patients with inflammatory bowel disease (IBD) (39). At the molecular level, these benefits are likely attributed to a reduction in proinflammatory cytokines, including IL-1β and TNF-α, modulation of immune responses, such as decreasing the Th17/Treg ratio, and restoration of gut barrier integrity (14, 39, 40). The findings of this review align with these observations, supporting the role of RAS inhibitors in improving gut barrier integrity and reducing inflammatory markers. The interplay between RAS, gut pathology and BP regulation is illustrated in Figure 2.
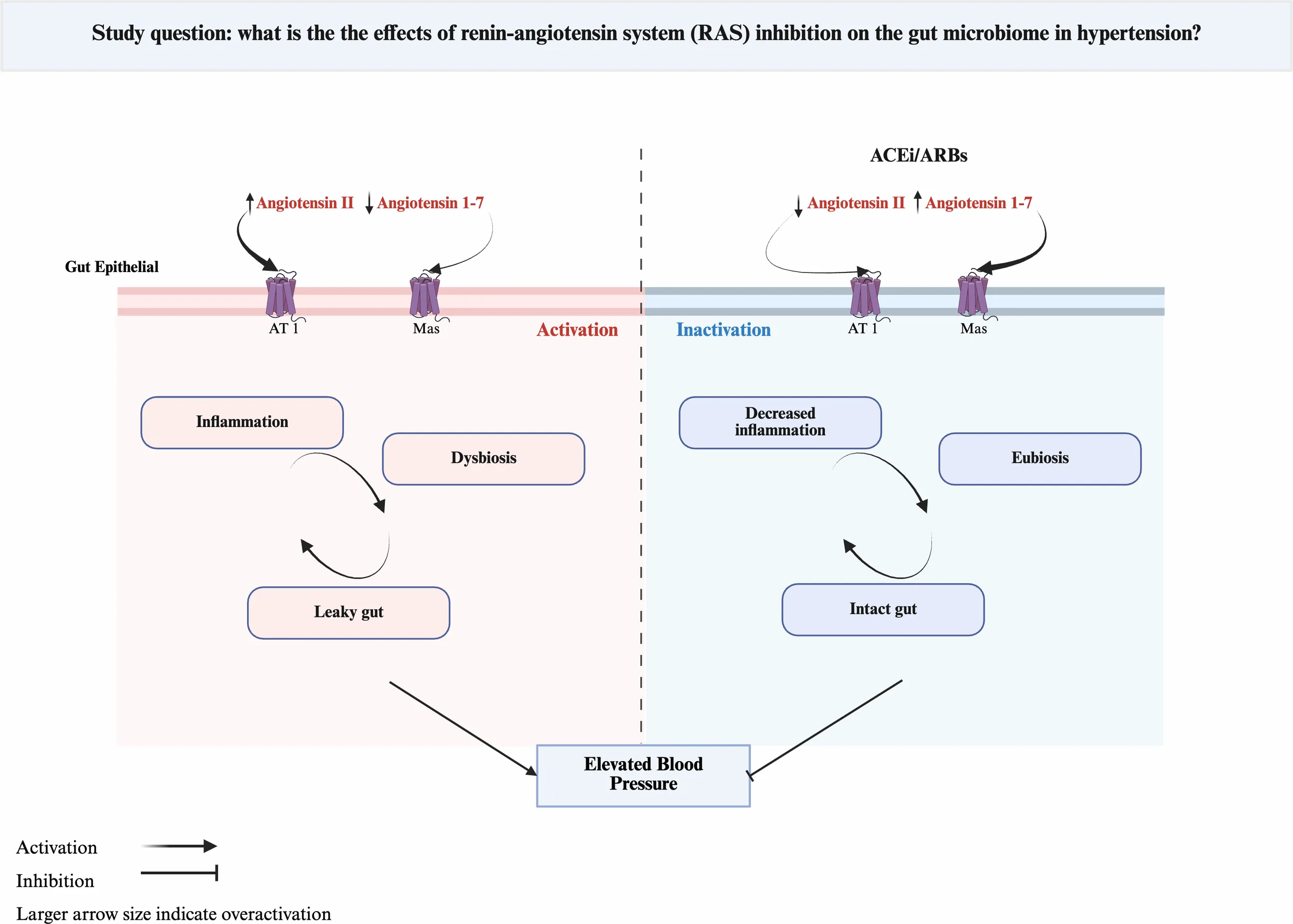
Figure 2

Schematic representation of the bidirectional relationship between Renin-Angiotensin System (RAS) activity, gut health, and blood pressure (BP) regulation. Chronic angiotensin II (Ang II) signaling increases gut permeability, inflammation, and epithelial oxygen tension, promoting dysbiosis enriched in facultative aerobes. RAS inhibition with ACEi/ARB restores epithelial hypoxia and barrier integrity, allowing recolonization by strict anaerobes that ferment fiber into short-chain fatty acids (SCFAs). SCFAs act on G-protein–coupled receptors (direct effect) and inhibit histone deacetylases (indirect effect), leading to vasodilation, immune modulation, and improved BP control. Most mechanistic insights are derived from preclinical models.
Chronic Ang II exposure disrupts gut oxygen and microbial dynamics, leading to a persistent dysbiotic state (14, 41). This effect is likely indirect, driven by a cascade of inflammation and epithelial damage that together increase oxygen availability. Dysbiosis, in turn, perpetuates inflammation and gut barrier dysfunction, creating a cycle that sustains a pro-inflammatory state (Figure 2). RAS inhibition interrupts this cycle and re-establishes oxygen homeostasis in the gut, which favors the growth of strict anaerobes such as Bifidobacterium and Akkermansia. These bacteria are key for SCFA production and mucin recycling, which are essential for immune regulation and gut health (42, 43). In this review, treatment with ACEi or ARBi consistently increased the abundance of these beneficial taxa (18, 31). Interestingly, the interplay between gut dysbiosis and RAS signaling appear to be bidirectional. Butyrate metabolism by colonocytes consume oxygen, leading to a hypoxic epithelial surface (44). Disruption of this hypoxic environment has pathophysiological consequences. In the SHR model, butyrate supplementation mitigated Ang II-mediated hypertension, gut dysbiosis, and barrier dysfunction (14). Prebiotics and probiotics have modulated RAS signaling, suppressing the classical ACE1-Ang II pathway while enhancing the ACE2-Ang 1–7 axis, improving gut function and BP control (45). Similarly, ACEi and ARBs increase Ang-(1–7) signaling through distinct mechanisms. ACEis increase Ang I substrate availability and reduce Ang-(1–7) degradation, whereas ARBs upregulate ACE2 expression, shifting RAS balance toward Ang-(1–7) (46). These mechanisms help explain, at least in part, the antihypertensive and anti-inflammatory effects of pharmacological RAS inhibition and potential synergy with prebiotics.
Key considerations regarding the findings of this review merit attention. First, the interaction between RAS inhibition and the gut microbiome may depend on the pharmacokinetics of specific RASs’ inhibitors (e.g., ACEis/ARBs) and components (e.g., Ang II). While most RAS inhibitors consistently benefited the gut, valsartan showed conflicting results, reducing microbial diversity without improving barrier integrity (32). One hypothesis for this could be attributed to difference in methodology or inherent valsartan properties and would require validation in future studies. Notably, valsartan has lower bioavailability compared to other ARBs, and it was administered intragastrically in this study, unlike the oral administration used in the remaining studies (47). Further research is needed to determine whether the gut benefit of RAS inhibitors is a class effect. Second, the beneficial effects of RAS inhibition appear to involve both systemic and local RAS signaling. Ang II has a longer tissue half-life (~15 minutes) than its plasma half-life (~1 minute), underscoring the significance of local RAS activity (13, 48). Moreover, the most pronounced gut benefits were observed with prolonged candesartan treatment, emphasizing the chronic nature of Ang II-driven gut dysbiosis and pathology (17). It is important to note that Ang II is not inherently harmful. Physiological levels and acute elevations are critical for maintaining vascular tone and fluid balance (49). Third, although the hypotensive effect of RAS inhibitors could improve gut health, it is likely not sufficient. Robles et al. tested this theory, where the administration of hydralazine to SHR rescued BP but did not affect gut composition and colonic integrity, suggesting that the anti-inflammatory effect of ACEi and ARBi is likely RAS dependent. Finally, the influence of Ang II on the microbiome may be affected by experimental factors, such as diet, sampling size, and housing facility (50).
Previous reports have illustrated a complex relationship between antihypertensive medications and the gut microbiota. Our review describes the impact of ACEis/ARBs on gut bacteria, the gut integrity, and inflammation but it should be noted that intestinal bacteria likely play a role on ACEi/ARB biotransformation/pharmacokinetics which may subsequently impact BP effects. This has been previously demonstrated with calcium channel blockers in rats and with human flora (51). How gut bacteria composition impacts ACEi/ARB metabolism has not been fully elucidated, but this missing link should be considered when interpreting the results of this systematic review.
Limitations
Limitations of this systematic review include a small article sample size and heterogeneity in methodology between studies. Only one human study was identified and carried a moderate risk of bias, which is important to consider with regards to study generalizability. Assessment of bias was in animal studies was limited by missing details on housing and study design. These limitations further highlight the scarcity of available evidence and should be considered in the interpretation of our review. Additionally, non-English language studies were excluded.
Conclusions
In conclusion, this systematic review highlights the intricate relationship between Ang II, inflammation, gut barrier dysfunction, and microbial dysbiosis, identifying chronic Ang II exposure as a key driver of gut pathology. The findings emphasize the interconnected roles of RAS signaling and gut health, suggesting that targeting both systems may yield synergistic therapeutic benefits. While preclinical studies have shown promise, evidence in humans remains limited. Given the safety and accessibility of dietary interventions like prebiotics, probiotics, and synbiotics, future research should investigate their potential to modulate RAS signaling, restore gut homeostasis, and improve blood pressure control.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
EW: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AS: Conceptualization, Formal Analysis, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. VQ: Data curation, Investigation, Writing – original draft. SN: Data curation, Investigation, Writing – original draft. EQ: Data curation, Investigation, Writing – original draft. JJ: Writing – review & editing. TE: Writing – review & editing. KG: Conceptualization, Supervision, Writing – review & editing. NR: Conceptualization, Data curation, Project administration, Supervision, Writing – original draft, Writing - review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported in part by the University of Houston New Faculty Research Program Grant (ASM, Grant #730).
Acknowledgments
Figures 1 , 2, and the visual abstract was created with BioRender.com (Wang, E. (2025) https://BioRender.com/izgel6d; Shremo Msdi, A. (2025) https://BioRender.com/0td0aaw; Shremo Msdi, A. (2025) https://BioRender.com/776agi2 ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1685424/full#supplementary-material
References
1
Tsao CW Aday AW Almarzooq ZI Anderson CAM Arora P Avery CL et al . Heart disease and stroke statistics-2023 update: A report from the american heart association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001123
2
Clark D Colantonio LD Min YI Hall ME Zhao H Mentz RJ et al . Population-attributable risk for cardiovascular disease associated with hypertension in black adults. JAMA Cardiol. (2019) 4:1194–202. doi: 10.1001/jamacardio.2019.3773
3
Bundy JD Li C Stuchlik P Bu X Kelly TN Mills KT et al . Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiol. (2017) 2:775–81. doi: 10.1001/jamacardio.2017.1421
4
O’Donnell JA Zheng T Meric G Marques FZ . The gut microbiome and hypertension. Nat Rev Nephrol. (2023) 19:153–67. doi: 10.1038/s41581-022-00654-0
5
Li J Zhao F Wang Y Chen J Tao J Tian G et al . Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. (2017) 5:14. doi: 10.1186/s40168-016-0222-x
6
Verhaar BJH Collard D Prodan A Levels JHM Zwinderman AH Bäckhed F et al . Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J. (2020) 41:4259–67. doi: 10.1093/eurheartj/ehaa704
7
Adnan S Nelson JW Ajami NJ Venna VR Petrosino JF Bryan Jr RM et al . Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. (2017) 49:96–104. doi: 10.1152/physiolgenomics.00081.2016
8
Jaworska K Koper M Ufnal M . Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol Gastrointest Liver Physiol. (2021) 321:G355–66. doi: 10.1152/ajpgi.00099.2021
9
Gonzalez-Gonzalez C Gibson T Jauregi P . Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int J Food Microbiol. (2013) 167:131–7. doi: 10.1016/j.ijfoodmicro.2013.09.002
10
Wilck N Matus MG Kearney SM Olesen SW Forslund K Bartolomaeus H et al . Salt-responsive gut commensal modulates TH17 axis and disease. Nature. (2017) 551:585–9. doi: 10.1038/nature24628
11
Xu J Moore BN Pluznick JL . Short-chain fatty acid receptors and blood pressure regulation: council on hypertension mid-career award for research excellence 2021. Hypertension. (2022) 79:2127–37. doi: 10.1161/HYPERTENSIONAHA.122.18558
12
Fyhrquist F Saijonmaa O . Renin-angiotensin system revisited. J Intern Med. (2008) 264:224–36. doi: 10.1111/j.1365-2796.2008.01981.x
13
Garg M Angus PW Burrell LM Herath C Gibson PR Lubel JS . Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. (2012) 35:414–28. doi: 10.1111/j.1365-2036.2011.04971.x
14
Kim S Goel R Kumar A Qi Y Lobaton G Hosaka K et al . Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci Lond Engl 1979. (2018) 132:701–18. doi: 10.1042/CS20180087
15
Chittimalli K Jahan J Sakamuri A McAdams ZL Ericsson AC Jarajapu YPR . Restoration of the gut barrier integrity and restructuring of the gut microbiome in aging by angiotensin-(1-7). Clin Sci Lond Engl 1979. (2023) 137:913–30. doi: 10.1042/CS20220904
16
Whelton PK Carey RM Aronow WS Casey Jr DE Collins KJ Himmelfarb CD et al . ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. (2017) 2018:71(6). doi: 10.1161/HYP.0000000000000065
17
Wu D Tang X Ding L Cui J Wang P Du X et al . Candesartan attenuates hypertension-associated pathophysiological alterations in the gut. BioMed Pharmacother Biomed Pharmacother. (2019) 116:109040. doi: 10.1016/j.biopha.2019.109040
18
Robles-Vera I Toral M de la Visitación N Sánchez M Gómez-Guzmán M Muñoz R et al . Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br J Pharmacol. (2020) 177:2006–23. doi: 10.1111/bph.14965
19
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20
Ouzzani M Hammady H Fedorowicz Z Elmagarmid A . Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
21
Hooijmans CR Rovers MM de Vries RB Leenaars M Ritskes-Hoitinga M Langendam MW . SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
22
Sterne JA Hernán MA Reeves BC Savović J Berkman ND Viswanathan M et al . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
23
Beckmann L Künstner A Freschi ML Huber G Stölting I Ibrahim SM et al . Telmisartan induces a specific gut microbiota signature which may mediate its antiobesity effect. Pharmacol Res. (2021) 170:105724. doi: 10.1016/j.phrs.2021.105724
24
Yang T Aquino VP Yanfei Q Lobaton GO Jobin C Richards EM et al . Short-term captopril treatment causes persistently decreased blood pressure associated with long-lasting shifts in gut microbiota and improvement in gut pathology. FASEB J. (2018) 32:582.7–7. doi: 10.1096/fasebj.2018.32.1_supplement.582.7
25
Jaworska K Kopacz W Koper M Szudzik M Gawryś-Kopczyńska M Konop M et al . Enalapril diminishes the diabetes-induced changes in intestinal morphology, intestinal RAS and blood SCFA concentration in rats. Int J Mol Sci. (2022) 23:6060. doi: 10.3390/ijms23116060
26
Nijiati Y Maimaitiyiming D Yang T Li H Aikemu A . Research on the improvement of oxidative stress in rats with high-altitude pulmonary hypertension through the participation of irbesartan in regulating intestinal flora. Eur Rev Med Pharmacol Sci. (2021) 25:4540–53. doi: 10.26355/eurrev_202107_26247
27
Kyoung J Mei X Richards E Raizada M Joe B Yang T . Gut microbiota modulate the efficacy of angiotensin-converting enzyme inhibitor captopril. FASEB J. (2021) 35. doi: 10.1096/fasebj.2021.35.S1.01524
28
Yu J Ma Y He X Long XN Xu J Wang L et al . Effects of irbesartan and amlodipine besylate tablets on the intestinal microflora of rats with hypertensive renal damage. Front Pharmacol. (2021) 12:778072. doi: 10.3389/fphar.2021.778072
29
Dong S Liu Q Zhou X Zhao Y Yan K Li L et al . Effects of losartan, atorvastatin, and aspirin on blood pressure and gut microbiota in spontaneously hypertensive rats. Mol Basel Switz. (2023) 28:612. doi: 10.3390/molecules28020612
30
Dong Y Wang P Jiao J Yang X Chen M Li J . Antihypertensive therapy by ACEI/ARB is associated with intestinal flora alterations and metabolomic profiles in hypertensive patients. Front Cell Dev Biol. (2022) 10:861829. doi: 10.3389/fcell.2022.861829
31
Wu H Lam TYC Shum TF Tsai TY Chiou J . Hypotensive effect of captopril on deoxycorticosterone acetate-salt-induced hypertensive rat is associated with gut microbiota alteration. Hypertens Res Off J Jpn Soc Hypertens. (2022) 45:270–82. doi: 10.1038/s41440-021-00796-x
32
Qi YZ Jiang YH Jiang LY Shao LL Yang XS Yang CH . An insight into intestinal microbiota of spontaneously hypertensive rats after valsartan administration. Dose-Response Publ Int Hormesis Soc. (2021) 19:15593258211011342. doi: 10.1177/15593258211011342
33
Yang T Aquino V Lobaton GO Li H Colon-Perez L Goel R et al . Sustained captopril-induced reduction in blood pressure is associated with alterations in gut-brain axis in the spontaneously hypertensive rat. J Am Heart Assoc. (2019) 8:e010721. doi: 10.1161/JAHA.118.010721
34
Xiong Y He Y Chen Z Wu T Xiong Y Peng Y et al . Lactobacillus induced by irbesartan on spontaneously hypertensive rat contribute to its antihypertensive effect. J Hypertens. (2024) 42:460–70. doi: 10.1097/HJH.0000000000003613
35
González-Correa C Moleón J Miñano S Robles-Vera I Toral M Barranco AM et al . Differing contributions of the gut microbiota to the blood pressure lowering effects induced by first-line antihypertensive drugs. Br J Pharmacol. (2024) 181:3420–44. doi: 10.1111/bph.16410
36
Xu C Marques FZ . How dietary fibre, acting via the gut microbiome, lowers blood pressure. Curr Hypertens Rep. (2022) 24:509–21. doi: 10.1007/s11906-022-01216-2
37
Rios-Covian D González S Nogacka AM Arboleya S Salazar N Gueimonde M et al . An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: associated dietary and anthropometric factors. Front Microbiol. (2020) 11:973. doi: 10.3389/fmicb.2020.00973
38
Jaszewski R Tolia V Ehrinpreis MN Bodzin JH Peleman RR Korlipara R et al . Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology. (1990) 98:1543–8. doi: 10.1016/0016-5085(90)91088-n
39
Salmenkari H Korpela R Vapaatalo H . Renin–angiotensin system in intestinal inflammation—Angiotensin inhibitors to treat inflammatory bowel diseases? Basic Clin Pharmacol Toxicol. (2021) 129:161–72. doi: 10.1111/bcpt.13624
40
Gotoh K Shibata H . Association between the gut microbiome and the renin-angiotensin-aldosterone system: a possible link via the activation of the immune system. Hypertens Res Off J Jpn Soc Hypertens. (2023) 46:2315–7. doi: 10.1038/s41440-023-01384-x
41
Yang T Santisteban MM Rodriguez V Li E Ahmari N Carvajal JM et al . Gut dysbiosis is linked to hypertension. Hypertens Dallas Tex 1979. (2015) 65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315
42
Mo C Lou X Xue J Shi Z Zhao Y Wang F et al . The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog. (2024) 16:41. doi: 10.1186/s13099-024-00635-7
43
Rivière A Selak M Lantin D Leroy F De Vuyst L . Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. (2016) 7:979. doi: 10.3389/fmicb.2016.00979
44
Rivera-Chávez F Lopez CA Bäumler AJ . Oxygen as a driver of gut dysbiosis. Free Radic Biol Med. (2017) 105:93–101. doi: 10.1016/j.freeradbiomed.2016.09.022
45
Hsu CN Hou CY Chan JYH Lee CT Tain YL . Hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy. Nutrients. (2019) 11:2908. doi: 10.3390/nu11122908
46
Schindler C Bramlage P Kirch W Ferrario CM . Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy. Vasc Health Risk Manage. (2007) 3:125–37.
47
Abraham HMA White CM White WB . The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. (2015) 38:33–54. doi: 10.1007/s40264-014-0239-7
48
van Kats JP de Lannoy LM Danser AHJ van Meegen JR Verdouw PD Schalekamp MADH . Angiotensin II type 1 (AT1) receptor–mediated accumulation of angiotensin II in tissues and its intracellular half-life. In Vivo Hyperten. (1997) 30:42–9. doi: 10.1161/01.HYP.30.1.42
49
Nádasy GL Balla A Dörnyei G Hunyady L Szekeres M . Direct vascular effects of angiotensin II (A systematic short review). Int J Mol Sci. (2024) 26:113. doi: 10.3390/ijms26010113
50
Muralitharan R R Nakai ME Snelson M Zheng T Dinakis E Xie L et al . Influence of angiotensin II on the gut microbiome: modest effects in comparison to experimental factors. Cardiovasc Res. (2024) 120:1155–63. doi: 10.1093/cvr/cvae062
51
Xiong Y Xiong Y Zhu P Wang Y Yang H Zhou R et al . The role of gut microbiota in hypertension pathogenesis and the efficacy of antihypertensive drugs. Curr Hypertens Rep. (2021) 23:40. doi: 10.1007/s11906-021-01157-2
Summary
Keywords
hypertension, ACEi (angiotensin-converting enzyme inhibitor), ARB (angiotensin II receptor blocker), microbiome, dysbiosis
Citation
Wang EM, Shremo Msdi A, Quach VN, Nguyen SQ, Quach E, Jo J, Eubank TA, Garey KW and Rosario N (2025) Angiotensin converting enzyme inhibitors and angiotensin receptor blockers impact on the gut microbiome: a systematic review. Front. Endocrinol. 16:1685424. doi: 10.3389/fendo.2025.1685424
Received
13 August 2025
Accepted
09 October 2025
Published
23 October 2025
Volume
16 - 2025
Edited by
Alexander Verin, Augusta University, United States
Reviewed by
FengLong Yang, Fujian Medical University, China
Terri J. Harford, Cleveland Clinic, United States
Konda Mani Saravanan, Bharath Institute of Higher Education and Research, India
Updates
Copyright
© 2025 Wang, Shremo Msdi, Quach, Nguyen, Quach, Jo, Eubank, Garey and Rosario.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth M. Wang, emwang@central.uh.edu
†These authors have contributed equally to this work and share first authorship
‡ORCID: Elisabeth M. Wang, orcid.org/0000-0002-5655-5318; Abdulwhab Shremo Msdi, orcid.org/0009-0009-1582-0611; Taryn A. Eubank, orcid.org/0000-0002-6645-406X; Kevin W. Garey, orcid.org/0000-0003-2063-7503; Natalie Rosario, orcid.org/0000-0002-9965-0956
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.