- 1Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Ansteel Group General Hospital, Anshan Liaoning, China
- 3Anhui Hospital of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Hefei, Anhui, China
Background: Carotid plaque and fatty liver disease, as important target organ damages of metabolic disorders, have undergone a steady increase in prevalence. Cholesterol, high-density lipoprotein, and glucose index (CHG), triglyceride–glucose index (TYG), and atherogenic index of plasma (AIP) are tools for assessing metabolic abnormalities. This research aimed to evaluate the potential of three indicators in predicting carotid plaque and fatty liver.
Methods: This study is based on longitudinal health examination data from workers at Ansteel Group in China in 2019. The follow-up period was five years, with the outcomes being the occurrence of carotid plaque or fatty liver events. Multivariate Cox regression analysis was used to examine the relationship between CHG, TYG, and AIP with the outcomes of carotid artery plaque and fatty liver. We used restricted cubic spline (RCS) curves to analyze the dose-response relationship between the three indices and the outcomes. We employed receiver operating characteristic (ROC) curves to evaluate the predictive ability of these indices. Finally, we also conducted subgroup analyses.
Results: Carotid plaque events developed in 659 workers (18.40%), and fatty liver in 375 workers (10.47%) during the follow-up period. Cox analysis revealed that the three indices were correlated with carotid plaque (Q3 vs Q1, CHG: HR 2.13, P < 0.001; TYG: HR 1.20, P = 0.006; AIP: HR 1.95, P < 0.001) and fatty liver (Q3 vs Q1, CHG: HR 2.46, P < 0.001; TYG: HR 1.75, P < 0.001; AIP: HR 3.47, P < 0.001). RCS indicated that the three indices were linearly related to carotid plaque and nonlinearly (inverted L-shaped) related to fatty liver. ROC curve analysis revealed that CHG had a stronger predictive ability for carotid plaque outcomes, while TYG had a stronger predictive ability for fatty liver. Subgroup analysis results showed that gender and BMI interacted with the three indicators in relation to outcomes.
Conclusions: Our research found that CHG, TYG, and AIP were positively correlated with carotid plaque and fatty liver. Moreover, CHG demonstrated superior predictive ability for carotid plaque outcomes, whereas TYG demonstrated better performance for fatty liver outcomes.
Introduction
With improvements in living standards, metabolic disorders have increasingly become a global public health concern. Carotid plaque and fatty liver disease, which are important target organ damages, continue to rise in prevalence. Carotid plaque is a major risk factor for cardiovascular disease (CVD), with a prevalence rate as high as 40% in people aged 40 and above (1). Fatty liver disease is closely related to various metabolic disorders, with a prevalence rate of 20% to 30% in the general population (2). Research has confirmed that liver fat content is associated with increased carotid intima-media thickness and shares core pathophysiological mechanisms, including chronic low-grade inflammation, insulin resistance (IR), lipid metabolism disorders, and endothelial dysfunction (3–6). The “liver-vascular axis” theory proposes that fatty liver disease is not only a local liver lesion, but also an independent risk factor for systemic vascular disease (7, 8). Within this framework, carotid plaque (a window marker of systemic atherosclerosis) interacts with fatty liver disease to jointly exacerbate metabolic disorders and vascular damage, forming a vicious cycle of “metabolism-liver-vessels” (9–11).
Clinical studies indicate that impaired glucose metabolism promotes the accumulation of atherogenic lipoproteins, synergistically accelerating vascular damage (12). Traditional single lipid or glucose markers struggle to capture the complex interactions between glucose and lipid metabolism, and their predictive power for complex outcomes driven by multiple metabolic factors is often limited. Integrating lipid and glucose data, however, better defines metabolic characteristics and vascular risk profiles (13). Consequently, there is an urgent clinical need for composite indicators that integrate multidimensional information and more sensitively reflect the overall state of metabolic dysfunction. Triglyceride-glucose index (TYG) and plasma atherosclerosis index (AIP) have been proven to have good predictive value for cardiovascular and metabolic disease risk (11, 14, 14). However, the cholesterol, high-density lipoprotein, and glucose index (CHG) is an emerging glucose-lipid composite index that is rarely studied in metabolic diseases (15, 16). There are currently no comparative studies on the predictive potential of these three indicators for carotid plaque and fatty liver. This research aimed to investigate the association between CHG/TYG/AIP and the above-mentioned target organ damage through a cohort study, and to evaluate its predictive ability.
Methods
Study population and design
Derived from longitudinal health examinations of steelworkers at Ansteel Group (Anshan, China), this retrospective cohort study analyzed data from a series of health assessments. All employees of the company undergo annual health examinations as mandated by Chinese labor regulations. The study collected electronic health check-up data (including physical examinations, hematological tests, ultrasound scans, etc.) from Ansteel Group General Hospital for employees between 2019 and 2023, and constructed a longitudinal cohort database. The study was approved by the Ethics Committee of Ansteel Group General Hospital, approval number: 2025-0045. Patient information was de-identified, and the study complies with the principles of the Declaration of Helsinki.
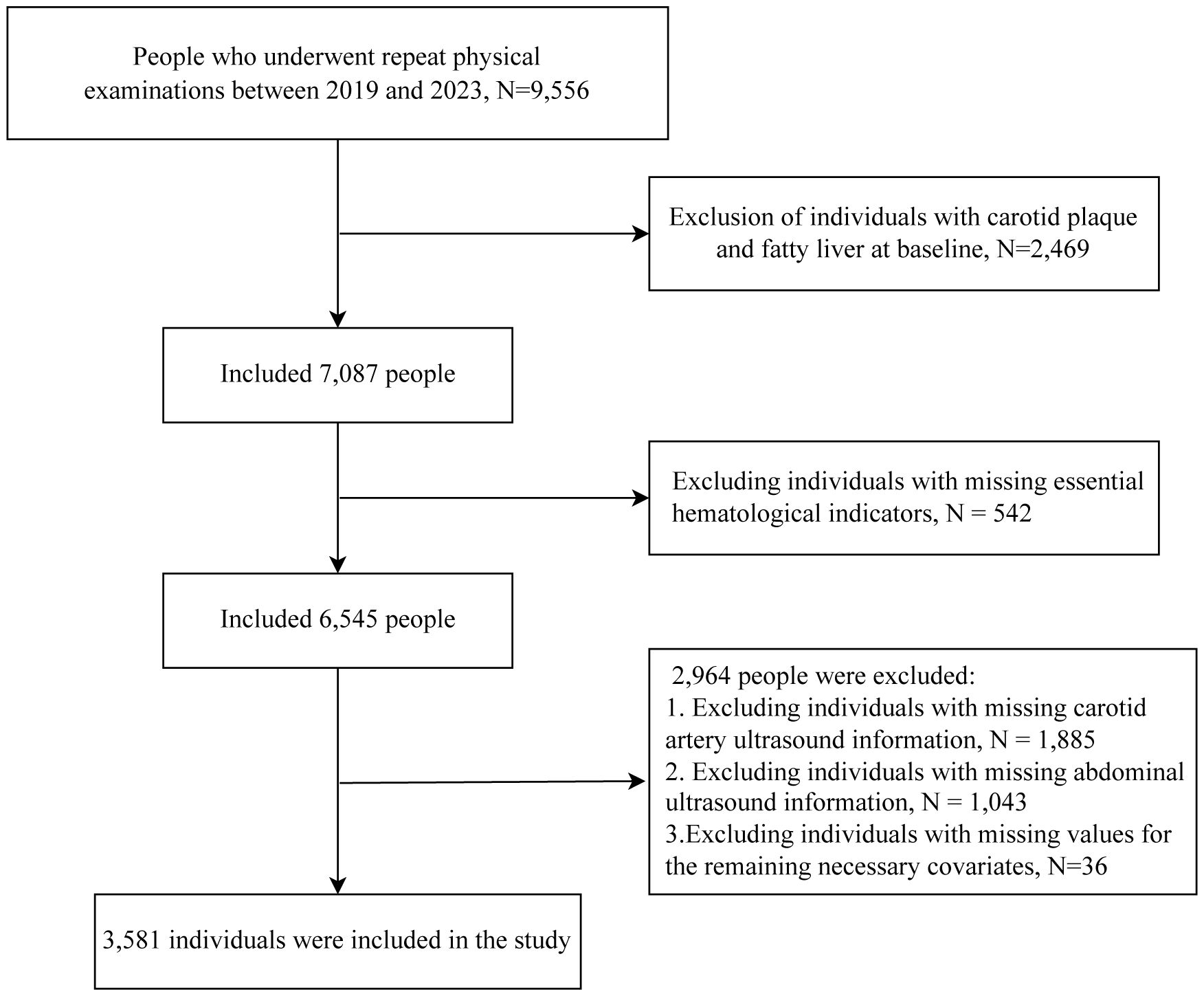
The study selected 9,556 individuals with serial physical examination data between 2019 and 2023. We excluded 2,469 individuals who had carotid plaque and fatty liver at baseline. After excluding 542 individuals with incomplete blood glucose or lipid data, 2,928 individuals lacking complete annual carotid and abdominal ultrasound records during the study period, and 36 individuals with incomplete covariate data, a total of 3,581 individuals were ultimately included in this study. The inclusion criteria are shown in Figure 1.
Definitions of CHG, TYG, and AIP indices
All indicators were obtained from peripheral blood samples taken ≥ 8 hours after fasting in the morning. Serum triglyceride (TG), total cholesterol (TC), and fasting blood glucose (FBG) levels were measured using enzyme-linked immunosorbent assays, while high-density lipoprotein cholesterol (HDL-C) was determined via chemical precipitation (17). The indices were calculated as follows: CHG index = Ln [TC (mg/dL) × FBG (mg/dL)/2 × HDL (mg/dL)] (16); TyG index = ln (TG (mg/dL) × FBG (mg/dL)/2) (18); AIP = Log [TG (mmol/L)/HDL-C (mmol/L)] (19). Participants were stratified by tertiles: CHG: Q1 (<4.94), Q2 (4.94–5.21), Q3 (>5.21); TyG: Q1 (<8.39), Q2 (8.39–8.99), Q3 (>8.99); AIP: Q1 (<0.15), Q2 (0.15–0.45), Q3 (>0.45).
Endpoint assessment
The primary endpoint of this study was the occurrence of carotid plaque or fatty liver. Carotid plaque was detected by carotid ultrasonography with a Philips i U22 model color Doppler ultrasound diagnostic system and accompanying software, a line array probe was taken, and the frequency was set from 7 to 11.2 MHz. Plaque diagnosis required: (a) focal wall thickening ≥0.5 mm or exceeding 50% of surrounding CIMT; (b) lumen-protruding foci; or (c) CIMT >1.5 mm in any carotid arterial segment (20). Fatty liver was defined as the presence of hepatic steatosis as shown by abdominal ultrasound. A 3.5 MHz convex array probe is used for abdominal color Doppler ultrasound detection. Color Doppler ultrasound was performed by a team of two experienced physicians. Fatty liver degeneration was diagnosed by abdominal ultrasound when ≥2 of these features were present: hepatic parenchymal brightness, deep attenuation, bright vessel walls, hepatorenal echogenicity contrast, or gallbladder wall blurring (21, 22).
Included variables
Demographic characteristics included gender, age, and body mass index (BMI). Laboratory test data include heart rate, systolic blood pressure, diastolic blood pressure, albumin, FBG, TG, HDL-C, TC, low-density lipoprotein cholesterol (LDL-C), creatinine, platelet (PLT), hemoglobin (Hb), white blood cells (WBC), neutrophils, lymphocytes, monocytes, aspartate transaminase (AST) and gamma-glutamyl transpeptidase (GGT), and alanine transaminase (ALT).
Statistical analysis
We used descriptive statistical methods to summarize the baseline characteristics of the study participants. The normality of continuous variables was assessed using the Shapiro-Wilk test and visual inspection of Q-Q plots. Continuous variables were normally distributed or approximately normally distributed, expressed as mean ± standard deviation. Categorical variables were expressed as N (%). Intergroup differences were analyzed using analysis of variance (ANOVA) for continuous variables and χ² tests for categorical variables. Multivariate Cox proportional hazards regression was performed to assess associations between CHG, TYG, and AIP levels and carotid plaque/fatty liver. Results are presented as hazard ratios (HR) with corresponding 95% confidence intervals (CI). Two models were adjusted. Model 1 adjusted for sex, age, and BMI. Model 2 additionally adjusted for diastolic blood pressure, systolic blood pressure, heart rate, creatinine, albumin, white blood cells, platelets, hemoglobin, neutrophils, lymphocytes, monocytes, gamma-glutamyl transpeptidase, alanine transaminase, and aspartate transaminase. All variables passed multicollinearity tests, with variance inflation factor values below 5 (Supplementary Table S1). Using the Cox regression model, we established restricted cubic splines (RCS) and assessed dose-response relationships using the likelihood ratio test.
Kaplan-Meier curves were generated to visualize cumulative outcome risks over an observation period of 1 to 5 years. Receiver operating characteristic (ROC) analysis was conducted to compare area under the curve (AUC) values of the three indices. Additionally, the AUC values underwent DeLong’s test. Finally, subgroup analyses were performed by age, gender, and BMI to evaluate predictive ability across populations.
This research used R (4.3.0). The study considered two-sided p-value < 0.05 to be statistically significant.
Results
Baseline characteristics
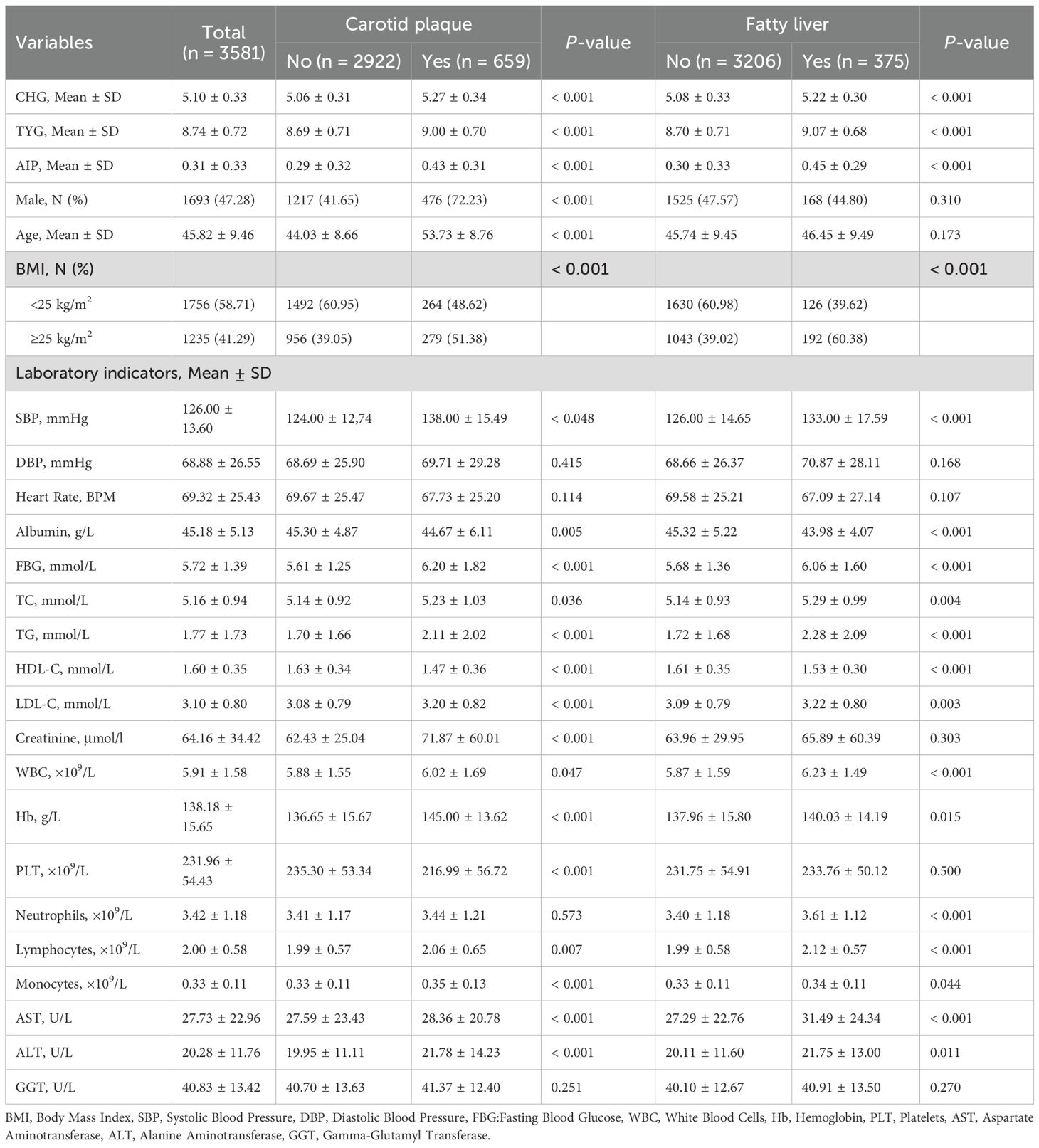
Table 1 presents baseline characteristics grouped by carotid plaque and fatty liver status. The cohort had a mean age of 45.82 ± 9.46 years with 47.28% males. Over the 5-year follow-up period, 659 (18.40%) workers developed carotid plaque, and 375 (10.47%) workers developed fatty liver. Compared to those without carotid plaque, affected individuals exhibited higher mean age, greater male predominance, elevated BMI, increased blood pressure, higher CHG/TYG/AIP indices, elevated blood glucose, and poorer lipid profiles. Similar trends were observed in the fatty liver group. Both outcome groups also demonstrated elevated inflammatory markers and liver enzymes.
Relationship between CHG, TYG, and AIP and outcomes
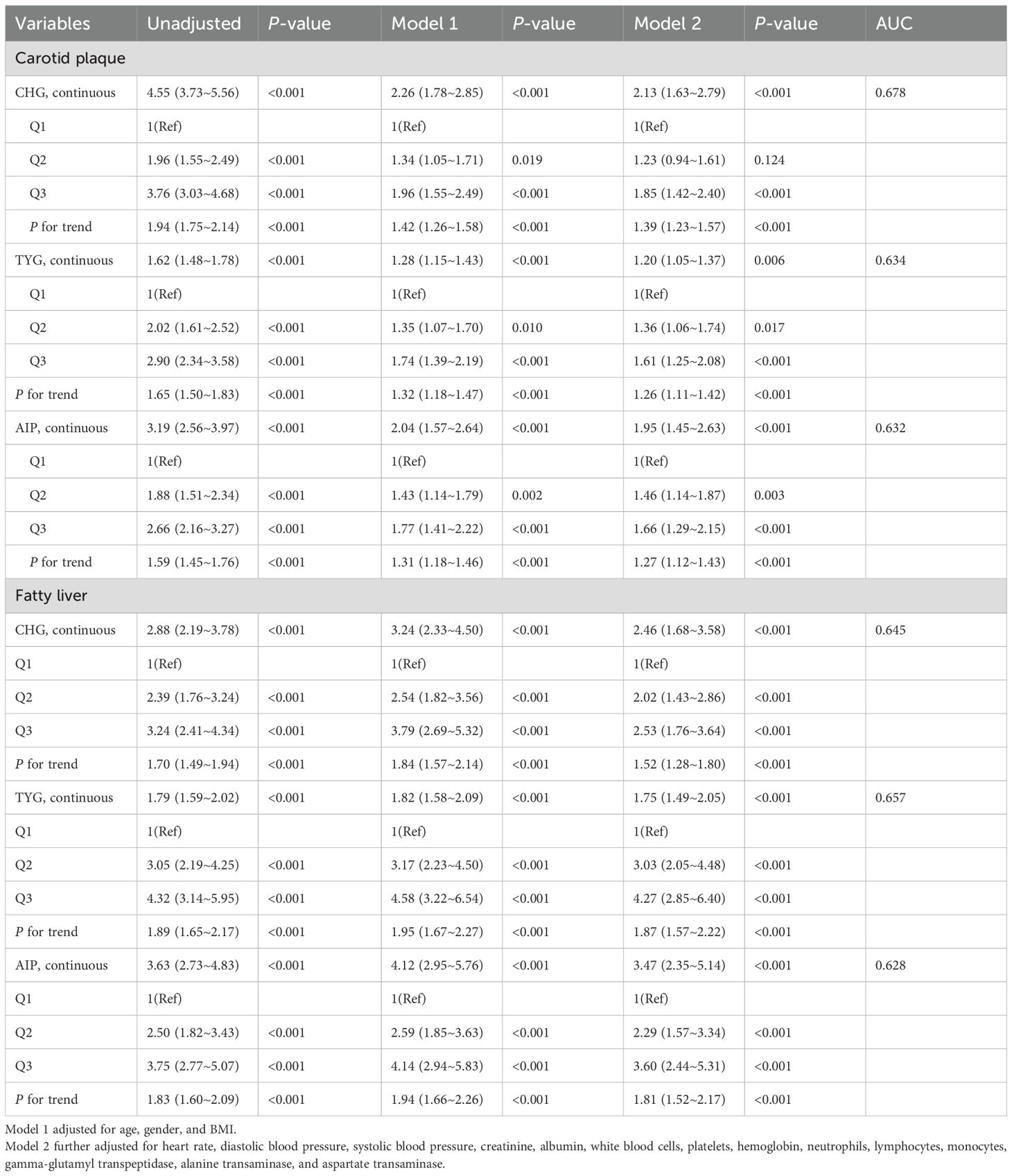
Table 2 demonstrates the relationship between the three indices and outcomes related to carotid artery plaques and fatty liver. When analyzed continuously, each 1-unit increase in CHG corresponded to 1.13-fold higher carotid plaque risk (95% CI 1.63–2.79, P < 0.001) and 1.46-fold higher fatty liver risk (95% CI 1.68–3.58, P < 0.001).
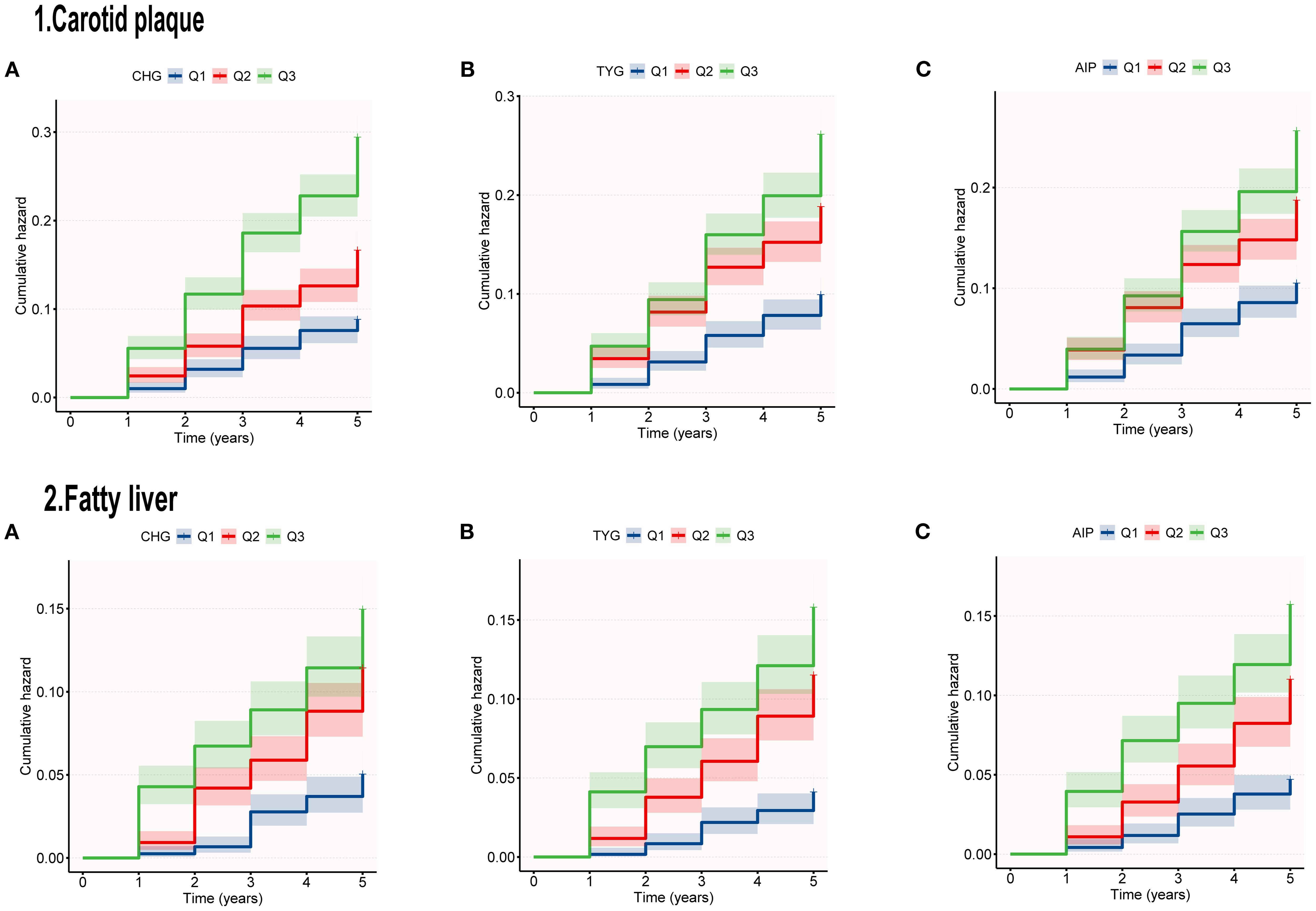
Categorical analysis revealed that compared with the Q1 group, the CHG-Q3 group exhibited significantly elevated risks for both outcomes (carotid plaque: adjusted HR = 1.85, P<0.001; fatty liver: HR = 2.53, P<0.001). TYG tertiles showed significant positive correlations with both outcome risks (P < 0.001). For carotid plaque, the adjusted risk ratio in the Q3 group compared to the Q1 group was 1.61. For fatty liver, the adjusted HR in the Q3 group compared to the Q1 group was 4.27. Results from the AIP study were consistent, with adjusted risk ratios for carotid plaque and fatty liver in the Q3 group (compared to Q1) being 1.95 (95% CI: 1.45–2.63, P < 0.001) and 3.47 (95% CI: 2.35–5.14, P < 0.001), respectively. Figure 2 shows that the risk of developing carotid artery plaques and fatty liver increases with higher tertiles of CHG, TYG, and AIP during the follow-up period.
The fitted curves in Figure 3 indicate a linear relationship between CHG, TYG, AIP, and carotid artery outcome risk (P for non-linear < 0.001). The three indices exhibited a linear relationship with fatty liver outcome, showing an inverted L-shaped pattern. In the non-linear relationship, no inflection points were found that could be further used for stratified analysis.
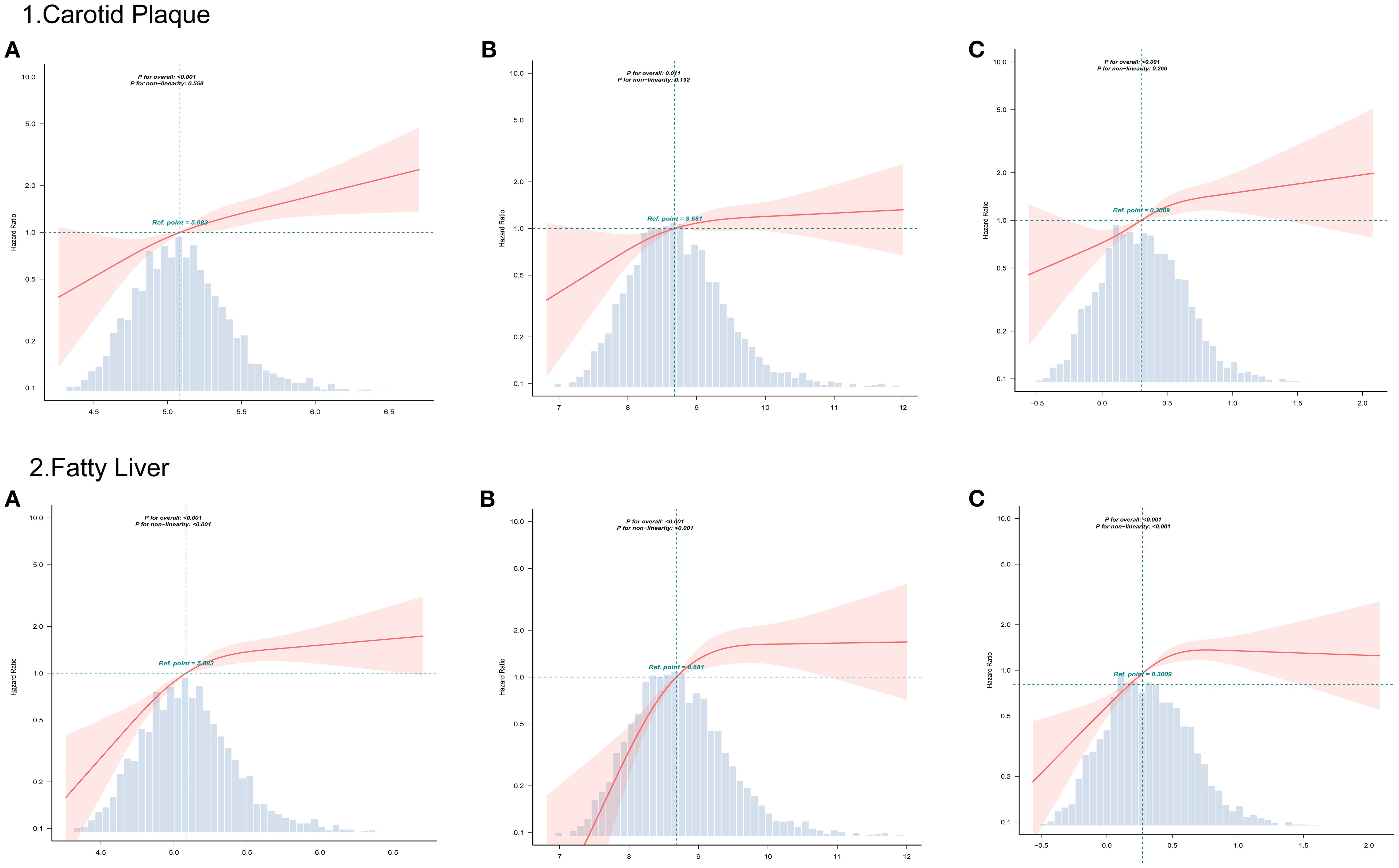
Figure 3. RCS curves analyzed the relationship between CHG (A), TYG (B), AIP (C), and outcomes risk.
ROC curves between CHG, TYG, and AIP and outcome risk events
Figure 4 displays the predictive performance among the three indicators for carotid plaque and fatty liver risk. Supplementary Table S2 confirms that the AUC differences among them passed the significance test. In terms of carotid plaque prediction, the AUC for the CHG index was 0.678, while TYG and AIP were 0.634 and 0.632, respectively. For fatty liver outcomes, TYG showed the highest AUC (0.657), followed by CHG (0.645), both outperforming AIP (0.628).
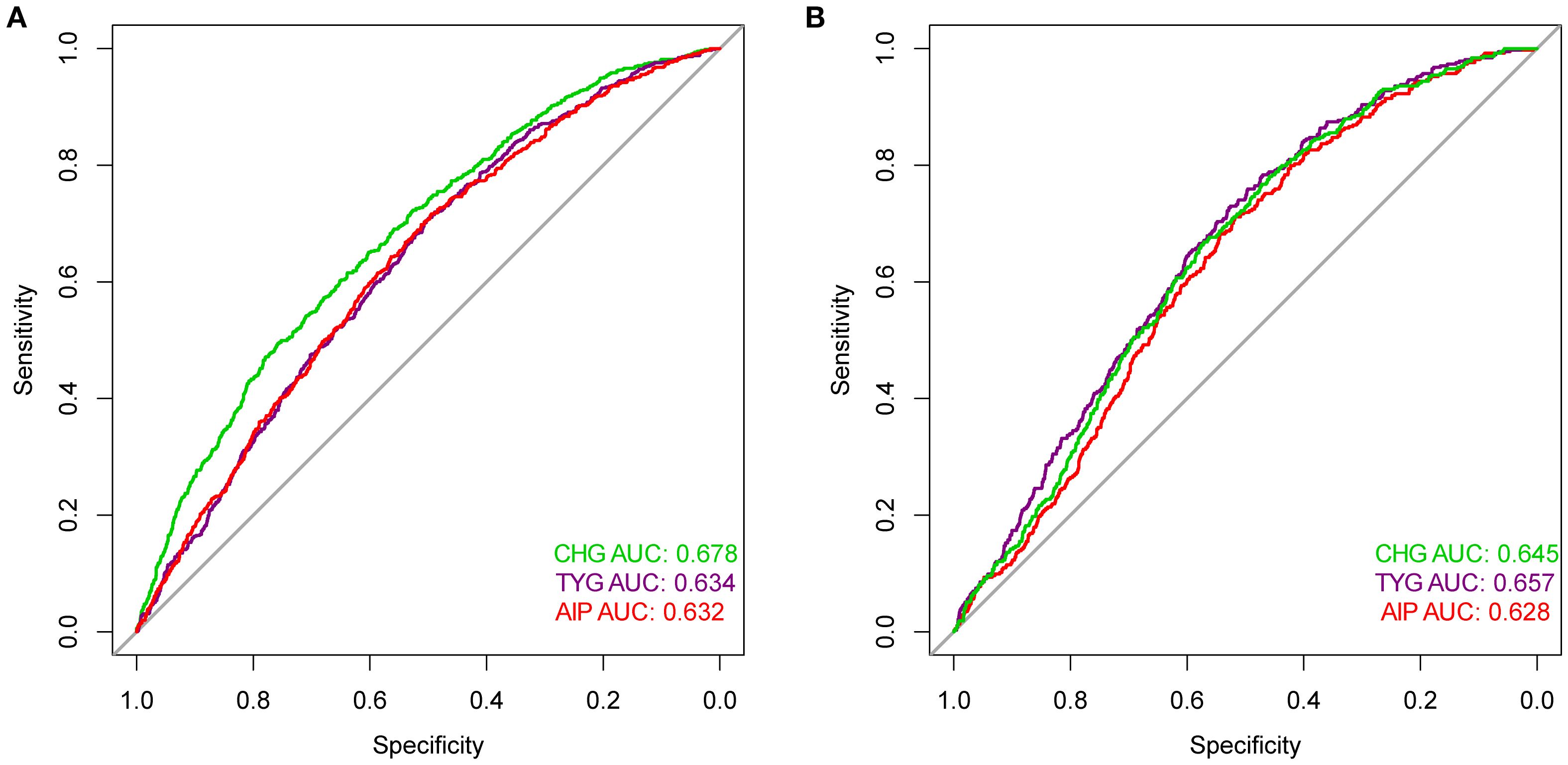
Figure 4. ROC curves compared the predictive efficacy of the CHG, TYG, AIP for outcomes [(A) Carotid plaque; (B) Fatty liver] risk events.
Subgroup analysis
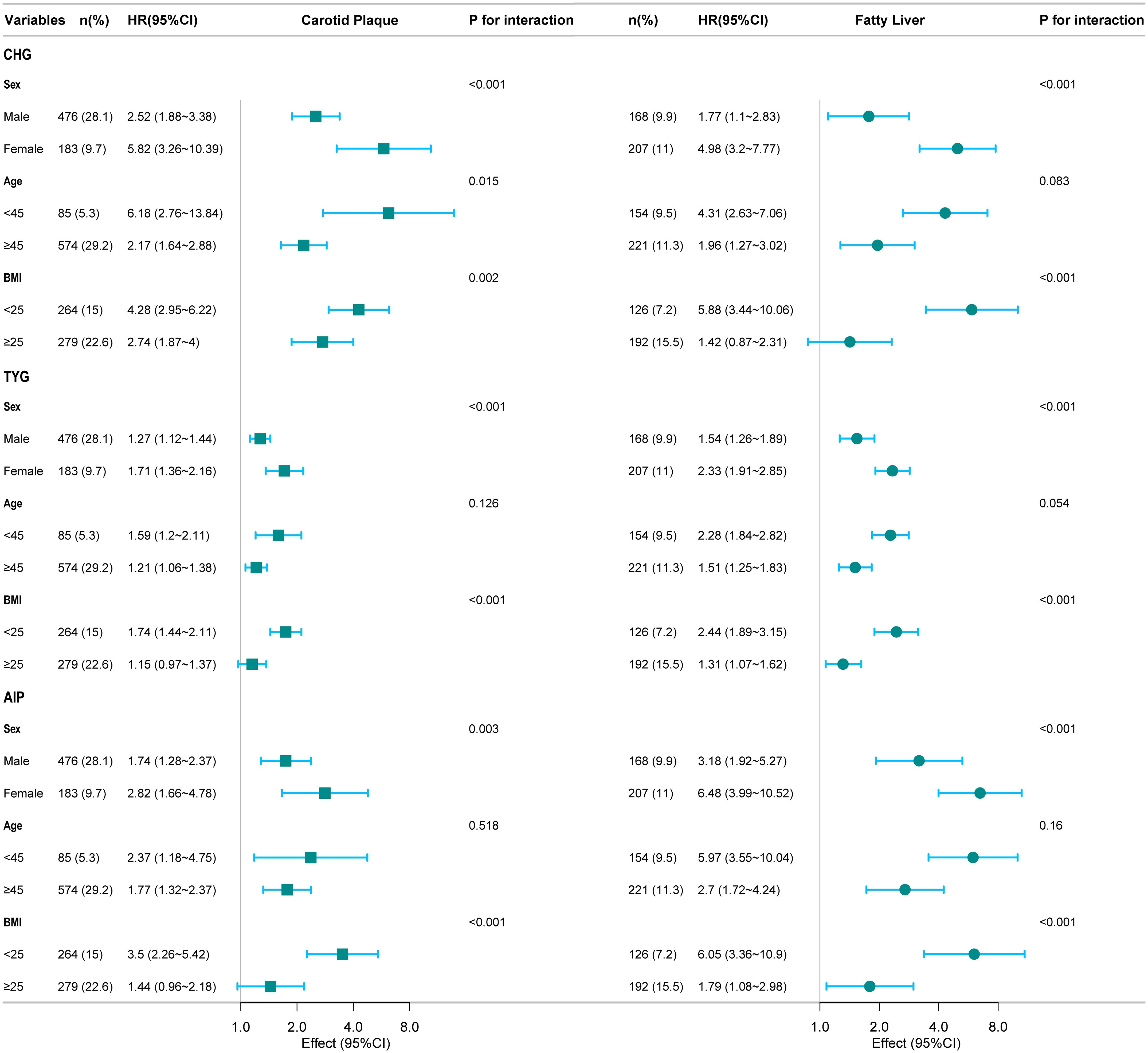
Subgroup analysis revealed that gender and BMI interacted with the relationship between the three indices with carotid plaque and fatty liver outcomes (Figure 5). In addition, age interacted with the relationship between CHG and carotid plaque outcomes.
Discussion
The study revealed the following findings. First, CHG, TYG, and AIP were all positively correlated with increased risks of carotid plaque and fatty liver. Second, the three indices showed a linear relationship with carotid plaque outcomes and a nonlinear (inverted L-shaped) relationship with fatty liver outcomes. Third, CHG demonstrated superior predictive ability for carotid plaque outcomes, whereas TYG demonstrated better performance for fatty liver outcomes. Fourth, subgroup analysis revealed that the associations between the indices and outcomes were modified by gender and BMI.
Our subgroup analysis found that these metabolic indicators were more significantly associated with carotid plaque and fatty liver in women and people with higher BMI. Past research has shown that factors such as obesity, lifestyle, and environment can all influence the occurrence and development of carotid artery plaques and fatty liver disease (23, 24). BMI may be more likely to affect the subclinical early stages of carotid plaque, with each standard deviation increase resulting in an 11% increase in plaque burden (25). YU et al. conducted a cross-sectional study specifically targeting steelworkers in northern China to investigate the relationship between obesity metabolism and carotid artery health. Research has found that, among obese patients, participants with unhealthy metabolic phenotypes have a significantly higher risk of developing carotid plaques than those with healthy metabolic phenotypes (26). It is clear that BMI alone cannot fully capture metabolic status, and lifestyle and genetics also play a key role in the progression of fatty liver disease (27). Sex, as the key genetic factor, is decisive for metabolic traits. Compared to men, the reduced circulating levels of sex hormones in women at the onset of menopause cause them to be more susceptible to impaired insulin sensitivity and impaired lipid regulation, among other things, which increase the risk of CVD (28). Gong found that the correlation between multiple IR indicators and metabolic disease was more pronounced in women (29).
The CHG was first proposed by Mansoori et al. as an index to improve the simplicity of diagnosing type 2 diabetes. Compared with TYG, the CHG index has higher specificity (16). Subsequent studies demonstrated its advantages in predicting the risk of diabetic nephropathy and CVD (15, 30). Research on CHG remains limited for metabolic-related and cardiovascular-related diseases. In contrast, substantial evidence links elevated TYG levels to increased risks of CVD and cerebrovascular events (heart failure, coronary heart disease, stroke) (31–33). A three-year longitudinal study identified that TYG can act as both a predictor and dose-response indicator for carotid plaque (34). TYG also correlates with prognosis in metabolic diseases (including diabetes, insulin resistance and fatty liver) (14, 35). A cohort study by NAGALA examined the correlation and predictive ability of 15 obesity and lipid-related indicators with fatty liver disease and found that the TyG index had the strongest correlation and the best predictive performance (36). Our study also showed that among the three indices, TYG had better predictive performance for fatty liver. Additionally, Mo et al. reported higher hazard ratios for CHG than TYG for CVD risk, which aligns with our carotid plaque results (15).
Compared with the lipid-only AIP index, CHG and TYG indices (incorporating glucose) demonstrated stronger correlations and predictive ability for carotid plaque and fatty liver outcomes. The shared metabolic disorder underlying these conditions involves an IR-triggered pathological network (37). IR not only causes peripheral glucose uptake disorders and increased hepatic glucose output, but also triggers the influx of free fatty acids (FFA) into the liver through abnormal lipolysis in adipose tissue, forming a lipotoxic microenvironment (38). Under metabolic stress, hepatic activation occurs through dual pathways: 1) hepatocyte FFA accumulation triggers oxidative/ER stress, stimulating Kupffer cells to release pro-inflammatory cytokines (IL-6, TNF-α) that amplify systemic inflammation via portal circulation; 2) steatotic hepatocytes secrete aberrant adipokines (reduced adiponectin, elevated resistin) synergizing with visceral fat-derived adipokines to promote atherosclerosis (39, 40). Hepatocyte-derived resistin activates NF-κB to drive monocyte vascular infiltration, forming a “metabolic-inflammatory-vascular injury” cycle with counterregulatory GLP-1 elevation. This liver-vascular axis explains the superior predictive value of integrated markers like CHG/TYG: they concurrently capture IR’s triad of hepatic glucose dysregulation, adipose lipolysis, and endothelial dysfunction, thus better reflecting the shared pathogenesis of fatty liver and atherosclerosis than lipid-only indices.
A particularly noteworthy observation in our analysis was the distinct dose-response relationship patterns between the metabolic indices and the two clinical outcomes. While all three indices exhibited a linear association with carotid plaque risk, their relationships with fatty liver development demonstrated a characteristic inverted L-shaped, nonlinear pattern upon RCS analysis. Each incremental increase in CHG, TYG, or AIP contributes additively to atherosclerotic risk, consistent with the known progressive nature of vascular endothelial dysfunction, lipid infiltration, and inflammatory activation in atherosclerosis (41, 42). In contrast, the risk of fatty liver disease exhibits an inverted L-shaped correlation pattern, suggesting the potential presence of a threshold phenomenon driven by complex mechanisms. Once a critical threshold of hepatocyte steatosis is exceeded, additional lipid influx may be diverted to ectopic deposition or undergo alternative metabolic fates rather than proportionally increasing visible steatosis (43). Mitochondrial β-oxidation, VLDL deposition, and activation of adaptive hepatocyte signaling pathways (FGF21, adiponectin) can systemically regulate lipid metabolism and insulin sensitivity, potentially activating compensatory homeostasis mechanisms triggered by severe lipid overload (44–46). Another interpretation is that these composite indicators exhibit high sensitivity in detecting the initial stages of insulin resistance and dyslipidemia. However, once specific metabolic thresholds are crossed and other pathophysiological mechanisms dominate disease progression, their discriminatory power significantly diminishes.
The CHG and TYG indices are readily acquired from routine metabolic panels and show significant associations with target organ damage, supporting their potential utility in clinical assessment. Incorporating them into early risk stratification as supplementary indicators alongside ultrasound-based screening strategies may aid in optimizing healthcare resource allocation. Moreover, these indices could help inform therapeutic decision-making. Identifying high-risk patients using CHG/TYG may justify earlier intensification of therapy, including novel agents such as PCSK9-targeting RNA-based therapeutics for robust lipid management (47, 48). Future studies should explore whether reduction in these indices following intervention correlates with regression of subclinical disease, potentially positioning CHG/TYG as dynamic biomarkers for treatment monitoring.
This study has several advantages. First, it represents the first investigation into CHG’s relationship with both carotid plaque and fatty liver. Additionally, CHG, AIP, and TYG indices were compared, revealing novel insights on glucose-lipid versus lipid-only assessment for outcome prediction. However, limitations exist. First, the findings from this single-center cohort of steelworkers may not fully represent the general population and require further validation in population-based cohort studies. Second, despite adjusting for confounding factors, inevitable missing data (comorbidities, medication records and alcohol consumption) may still lead to potential bias. Third, the study’s limitation to a Chinese population restricts its generalizability across ethnic groups, necessitating further research in different countries and populations.
Conclusion
Collectively, CHG, TYG, and AIP demonstrated positive associations with carotid plaque and fatty liver risks, with CHG showing superior predictive performance for carotid plaque outcomes and TYG exhibiting optimal prediction for fatty liver.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
This study was approved by the Ethics Committee of Anshan Iron and Steel Group General Hospital, Anshan City, Liaoning Province, China, approval number: 2025-0045. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Patient information was de-identified, and the study complies with the principles of the Declaration of Helsinki.
Author contributions
DL: Conceptualization, Writing – review & editing, Writing – original draft, Visualization. ZX: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. FW: Formal Analysis, Writing – review & editing, Writing – original draft, Conceptualization. YH: Writing – review & editing, Writing – original draft, Investigation. XZ: Writing – original draft, Writing – review & editing, Project administration. JY: Writing – review & editing, Writing – original draft. QW: Writing – original draft, Writing – review & editing, Investigation. NZ: Writing – original draft, Writing – review & editing, Supervision, Investigation. YL: Writing – review & editing, Funding acquisition, Supervision, Writing – original draft, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Research Project of Shanghai Municipal Health Commission (No.202340158), the Research Project of Health Commission of Anhui Province (No.AHWJ2023A20461), the Research Project of 2024 Anhui Traditional Chinese Medicine Inheritance and Innovation (No.2024CCCX122), and 2024 Anhui University Research Program (No.2024AH050989).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1686931/full#supplementary-material
References
1. Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. (2020) 8:e721–9. doi: 10.1016/S2214-109X(20)30117-0
2. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2020) 5:739–52. doi: 10.1016/S2468-1253(20)30077-7
3. Huang Y, Wang Y, Xiao Z, Yao S, Tang Y, Zhou L, et al. The association between metabolic dysfunction-associated steatotic liver disease, cardiovascular and cerebrovascular diseases and the thickness of carotid plaque. BMC Cardiovasc Disord. (2023) 23:554. doi: 10.1186/s12872-023-03580-6
4. Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: An observational cohort study. J Hepatol. (2018) 68:1018–24. doi: 10.1016/j.jhep.2017.12.012
5. Pais R, Redheuil A, Cluzel P, Ratziu V, and Giral P. Relationship among fatty liver, specific and multiple-site atherosclerosis, and 10-year framingham score. Hepatol Baltim Md. (2019) 69:1453–63. doi: 10.1002/hep.30223
6. Li X, Xia M, Ma H, Hofman A, Hu Y, Yan H, et al. Liver fat content is associated with increased carotid atherosclerosis in a Chinese middle-aged and elderly population: the Shanghai Changfeng study. Atherosclerosis. (2012) 224:480–5. doi: 10.1016/j.atherosclerosis.2012.07.002
7. Augustin HG and Koh GY. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science. (2017) 357:eaal2379. doi: 10.1126/science.aal2379
8. Sookoian S and Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. (2008) 49:600–7. doi: 10.1016/j.jhep.2008.06.012
9. Francque SM, van der Graaff D, and Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. (2016) 65:425–43. doi: 10.1016/j.jhep.2016.04.005
10. Targher G, Byrne CD, and Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. (2020) 69:1691–705. doi: 10.1136/gutjnl-2020-320622
11. Zheng H, Sechi LA, Navarese EP, Casu G, and Vidili G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: a comprehensive review. Cardiovasc Diabetol. (2024) 23:346. doi: 10.1186/s12933-024-02434-5
12. Scicali R, Giral P, D’Erasmo L, Cluzel P, Redheuil A, Di Pino A, et al. High TG to HDL ratio plays a significant role on atherosclerosis extension in prediabetes and newly diagnosed type 2 diabetes subjects. Diabetes Metab Res Rev. (2021) 37:e3367. doi: 10.1002/dmrr.3367
13. Di Giacomo Barbagallo F, Bosco G, Di Marco M, Scilletta S, Miano N, Musmeci M, et al. Evaluation of glycemic status and subclinical atherosclerosis in familial hypercholesterolemia subjects with or without LDL receptor mutation. Cardiovasc Diabetol. (2025) 24:126. doi: 10.1186/s12933-025-02683-y
14. Chen Q, Hu P, Hou X, Sun Y, Jiao M, Peng L, et al. Association between triglyceride-glucose related indices and mortality among individuals with non-alcoholic fatty liver disease or metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. (2024) 23:232. doi: 10.1186/s12933-024-02343-7
15. Mo D, Zhang P, Zhang M, Dai H, and Guan J. Cholesterol, high-density lipoprotein, and glucose index versus triglyceride-glucose index in predicting cardiovascular disease risk: a cohort study. Cardiovasc Diabetol. (2025) 24:116. doi: 10.1186/s12933-025-02675-y
16. Mansoori A, Nosrati M, Dorchin M, Mohammadyari F, Derakhshan-Nezhad E, Ferns G, et al. A novel index for diagnosis of type 2 diabetes mellitus: Cholesterol, High density lipoprotein, and Glucose (CHG) index. J Diabetes Investig. (2025) 16:309–14. doi: 10.1111/jdi.14343
17. Bucholz EM, Rodday AM, Kolor K, Khoury MJ, and de Ferranti SD. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014). Circulation. (2018) 137:2218–30. doi: 10.1161/CIRCULATIONAHA.117.032321
18. Simental-Mendía LE, Rodríguez-Morán M, and Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
19. Onat A, Can G, Kaya H, and Hergenç G. Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. (2010) 4:89–98. doi: 10.1016/j.jacl.2010.02.005
20. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. (2013) 34:2159–219. doi: 10.1093/eurheartj/eht151
21. Xia MF, Yan HM, He WY, Li XM, Li CL, Yao XZ, et al. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obes Silver Spring Md. (2012) 20:444–52. doi: 10.1038/oby.2011.302
22. Gao X, Fan JG, and Study Group of Liver and Metabolism, Chinese Society of Endocrinology. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J Diabetes. (2013) 5:406–15. doi: 10.1111/1753-0407.12056
23. Mi T, Sun S, Zhang G, Carora Y, Du Y, Guo S, et al. Relationship between dyslipidemia and carotid plaques in a high-stroke-risk population in Shandong Province, China. Brain Behav. (2016) 6:e00473. doi: 10.1002/brb3.473
24. Milić S, Lulić D, and Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. (2014) 20:9330–7. doi: 10.3748/wjg.v20.i28.9330
25. Bian L, Xia L, Wang Y, Jiang J, Zhang Y, Li D, et al. Risk factors of subclinical atherosclerosis and plaque burden in high risk individuals: results from a community-based study. Front Physiol. (2018) 9:739. doi: 10.3389/fphys.2018.00739
26. Yu M, Zhang S, Wang L, Wu J, Li X, and Yuan J. Metabolically healthy obesity and carotid plaque among steelworkers in North China: the role of inflammation. Nutrients. (2022) 14:5123. doi: 10.3390/nu14235123
27. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet Lond Engl. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
28. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet Lond Engl. (2021) 397:2385–438. doi: 10.1016/S0140-6736(21)00684-X
29. Gong R, Ding Y, Yang K, Meng X, and Sun X. Association between various insulin resistance surrogates and gallstone disease based on national health and nutrition examination survey. Sci Rep. (2025) 15:25877. doi: 10.1038/s41598-025-09482-1
30. Çatak M, Konuk ŞG, and Hepsen S. The cholesterol-HDL-glucose (CHG) index and traditional adiposity markers in predicting diabetic retinopathy and nephropathy. J Diabetes Investig. (2025) 16:1487–94. doi: 10.1111/jdi.70086
31. Huo RR, Liao Q, Zhai L, You XM, and Zuo YL. Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc Diabetol. (2024) 23:30. doi: 10.1186/s12933-024-02122-4
32. Zhou Z, Liu Q, Zheng M, Zuo Z, Zhang G, Shi R, et al. Comparative study on the predictive value of TG/HDL-C, TyG and TyG-BMI indices for 5-year mortality in critically ill patients with chronic heart failure: a retrospective study. Cardiovasc Diabetol. (2024) 23:213. doi: 10.1186/s12933-024-02308-w
33. Zhao J, Fan H, Wang T, Yu B, Mao S, Wang X, et al. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc Diabetol. (2022) 21:123. doi: 10.1186/s12933-022-01548-y
34. Zhang Y, Wu Z, Li X, Wei J, Zhang Q, and Wang J. Association between the triglyceride-glucose index and carotid plaque incidence: a longitudinal study. Cardiovasc Diabetol. (2022) 21:244. doi: 10.1186/s12933-022-01683-6
35. Selvi NMK, Nandhini S, Sakthivadivel V, Lokesh S, Srinivasan AR, and Sumathi S. Association of triglyceride-glucose index (TyG index) with hbA1c and insulin resistance in type 2 diabetes mellitus. Maedica. (2021) 16:375–81. doi: 10.26574/maedica.2021.16.3.375
36. Sheng G, Lu S, Xie Q, Peng N, Kuang M, and Zou Y. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. (2021) 20:134. doi: 10.1186/s12944-021-01561-2
37. Gaudio E, Nobili V, Franchitto A, Onori P, and Carpino G. Nonalcoholic fatty liver disease and atherosclerosis. Intern Emerg Med. (2012) 7 Suppl 3:S297–305. doi: 10.1007/s11739-012-0826-5
38. Fujii H, Kawada N, and Japan Study Group Of Nafld Jsg-Nafld. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci. (2020) 21:3863. doi: 10.3390/ijms21113863
39. Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, and Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. (2009) 35:277–87. doi: 10.1055/s-0029-1222606
40. Gasbarrino K, Zheng H, and Daskalopoulou SS. Circulating sex-specific markers of plaque instability in women and men with severe carotid atherosclerosis. Stroke. (2024) 55:269–77. doi: 10.1161/STROKEAHA.123.044840
41. Janus A, Szahidewicz-Krupska E, Mazur G, and Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflamm. (2016) 2016:3634948. doi: 10.1155/2016/3634948
42. Aboonabi A, Meyer RR, and Singh I. The association between metabolic syndrome components and the development of atherosclerosis. J Hum Hypertens. (2019) 33:844–55. doi: 10.1038/s41371-019-0273-0
43. Fabbrini E, Sullivan S, and Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatol Baltim Md. (2010) 51:679–89. doi: 10.1002/hep.23280
44. Deng D, Yang S, Yu X, Zhou R, Liu Y, Zhang H, et al. Aging-induced short-chain acyl-CoA dehydrogenase promotes age-related hepatic steatosis by suppressing lipophagy. Aging Cell. (2024) 23:e14256. doi: 10.1111/acel.14256
45. Sotin T, Ge X, Schönke M, Vince L, Thouzeau A, Frey S, et al. A rare gain of function variant of hepatic lipase attenuates hypercholesterolaemia and atherosclerosis in mice via an LDL receptor-independent mechanism. Cardiovasc Res. (2025) 121:1024–35. doi: 10.1093/cvr/cvaf097
46. Bao L, Yin J, Gao W, Wang Q, Yao W, and Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. (2018) 175:3379–93. doi: 10.1111/bph.14383
47. Di Giacomo-Barbagallo F, Andreychuk N, Scicali R, Gonzalez-Lleó A, Piro S, Masana L, et al. Inclisiran, reasons for a novel agent in a crowded therapeutic field. Curr Atheroscler Rep. (2025) 27:25. doi: 10.1007/s11883-024-01271-x
Keywords: carotid plaque, fatty liver, TyG, CHG, AIP
Citation: Li D, Xu Z, Wang F, Hu Y, Zhang X, Yang J, Wan Q, Zhang N and Liu Y (2025) The role of three glucose/lipid composite indices (CHG, TYG, and AIP) in predicting carotid plaque and fatty liver outcomes: a retrospective cohort study. Front. Endocrinol. 16:1686931. doi: 10.3389/fendo.2025.1686931
Received: 16 August 2025; Accepted: 25 September 2025;
Published: 14 October 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Francesco Di Giacomo Barbagallo, University of Catania, ItalyDegang Mo, Qingdao University, China
Copyright © 2025 Li, Xu, Wang, Hu, Zhang, Yang, Wan, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Zhang, emhhbmduaW5ncjlAMTI2LmNvbQ==; Yongming Liu, bGl1eW9uZ21pbmdAc2h1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Dan Li, orcid.org/0000-0002-1898-0620
Zhaohui Xu, orcid.org/0009-0008-5741-3902
Yinqin Hu, orcid.org/0000-0002-5136-1055
Xinyu Zhang, orcid.org/0009-0004-8189-7944
Jiahui Yang, orcid.org/0009-0000-2262-3012
Qiqi Wan, orcid.org/0009-0006-3879-694X
Yongming Liu, orcid.org/0000-0002-1956-9534
 Dan Li
Dan Li Zhaohui Xu
Zhaohui Xu Feifei Wang2†
Feifei Wang2† Yinqin Hu
Yinqin Hu Yongming Liu
Yongming Liu