- 1Department of Medical Sciences, University of Turin, Turin, Italy
- 2Advanced Cardiovascular Echocardiography Unit, Cardiovascular and Thoracic Department, Città della Salute e della Scienza di Torino University Hospital, Turin, Italy
- 3Division of Cardiology, Città della Salute e della Scienza di Torino, University Hospital, Turin, Italy
Introduction: Cognitive impairment is a frequent complication of type 2 diabetes (T2DM). Global longitudinal strain (GLS), an echocardiographic marker of subclinical left ventricular (LV) systolic dysfunction, has been associated with adverse cardiovascular outcomes in T2DM. However, its relationship with cognitive performance remains unexplored. The aim was to investigate the association between GLS and cognitive function in patients with T2DM.
Methods: We prospectively enrolled 234 T2DM patients without hemodynamically significant carotid stenosis, history of stroke or severe hypoglycemia. Cognitive function was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and GLS measured via speckle-tracking echocardiography. Multivariable linear regression models were used to evaluate associations between GLS and RBANS scores. Sensitivity analyses excluded individuals with coronary heart disease (CHD), atrial fibrillation (AF), or LV ejection fraction (LVEF) <50%.
Results: The mean RBANS total score was 96.7 ± 17.1; 19.7% of participants scored <80, indicating borderline/impaired cognition. Mean GLS was −19.23 ± 2.59%, with 29.1% of patients showing subclinical LV dysfunction (GLS ≥ −18%). Unlike LVEF, impaired GLS (≥ −18%) was associated with lower RBANS total scores. This association remained significant after excluding individuals with CHD, AF, or LVEF<50%, and after adjusting for age, sex, education, lifestyle factors, metabolic and hemodynamic parameters. Educational attainment modified the association, with stronger GLS-cognition links in participants with lower education. The relationship was unaffected by adjustment for markers of inflammation and endothelial dysfunction.
Conclusions: In patients with T2DM, impaired GLS is independently associated with reduced cognitive performance, even in patients with normal LVEF.
Introduction
Cognitive impairment is a well-recognized yet often underdiagnosed complication of Type 2 diabetes (T2DM) and ranges from subtle neurocognitive decrements to overt dementia (1, 42). Up to 45% of individuals with T2DM may exhibit signs of mild cognitive impairment, particularly affecting memory, executive function, and attention (2, 3). These deficits can significantly compromise quality of life and functional independence (4), yet they often remain undetected in routine clinical practice.
Cardiac dysfunction, particularly heart failure (HF), has also been independently associated with cognitive impairment and an increased risk of dementia (5). In the general population, reduced left ventricular ejection fraction (LVEF), particularly below 30%, has been linked to lower cognitive performance (6). Patients with HF frequently show impairments in attention, memory, visuospatial processing, and executive function (7), and cognitive dysfunction in this setting is associated with worse clinical outcomes, including higher rates of hospitalization and mortality (8–13).
Global Longitudinal Strain (GLS), measured through speckle-tracking echocardiography, has recently emerged as a more sensitive marker of early subclinical left ventricular systolic dysfunction than LVEF. GLS can detect early myocardial impairment even in individuals with preserved ejection fraction (14; 15). Importantly, GLS is often impaired in patients with T2DM and has been independently associated with increased risks of hospitalization, major cardiovascular events, and all-cause mortality (16–18, 44).
Despite converging evidence linking T2DM to both cognitive impairment and early cardiac dysfunction, the specific relationship between GLS and cognitive performance has not yet been examined in T2DM. Therefore, the aim of the present study was to investigate the association between GLS and cognitive performance in a well-characterized cohort of patients with T2DM.
Materials and methods
Study population
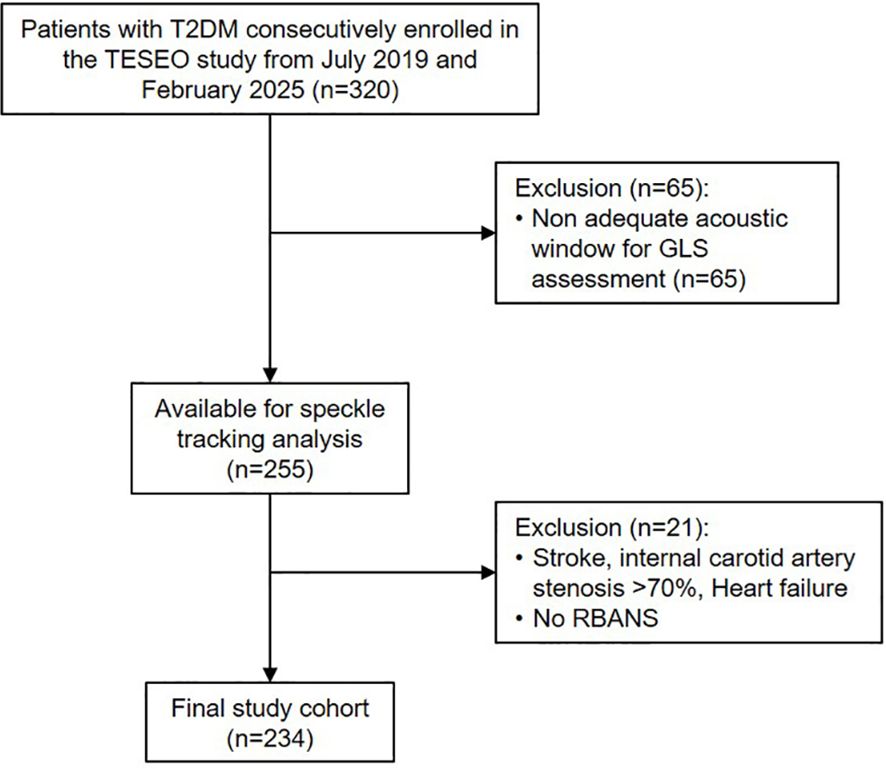
The study included individuals with T2DM who were consecutively and prospectively enrolled between July 2019 and February 2025 as part of the ongoing “Traguardi di Eccellenza nelle Scienze mediche Esplorando le Omiche” (TESEO) cohort, which investigates chronic complications of T2DM. Eligible participants were adults (≥18 years) referred for the first time to the Unified Diabetes Center at San Giovanni Antica Sede Hospital (Turin), who underwent baseline transthoracic echocardiography with speckle-tracking analysis. Exclusion criteria were: history of stroke, cognitive impairment interfering with daily functioning, major psychiatric disorders, internal carotid artery stenosis >70%, history of severe hypoglycemia, active malignancy, heart failure, inadequate acoustic window preventing GLS assessment, or inability to complete the RBANS due to patient refusal, language barriers, or significant visual impairment.
Upon enrollment, detailed demographic and clinical data were collected, including age, sex, ethnicity, educational level, dietary habits, physical activity, alcohol and tobacco use, cardiovascular risk factors, hypoglycemia history, comorbidities, chronic diabetes complications, and current pharmacologic treatments. Cognitive function was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), administered by a single trained examiner to ensure consistency. All participants underwent a complete physical examination, fasting blood sampling for biochemical analyses, and morning urine collection to determine the albumin-to-creatinine ratio (ACR). Additional assessments included fundus oculi examination, 12-lead electrocardiogram (ECG), Doppler ultrasound of the supra-aortic trunks (carotid duplex), and transthoracic echocardiography.
Of the 320 participants initially enrolled, 234 individuals were included in the final analysis (Figure 1). The study was approved by the Ethics Committee of the City of Health and Science of Turin, and all participants provided written informed consent.
Biochemistry
Glycated hemoglobin (HbA1c) levels were determined using an immune-enzymatic method, and results were standardized according to the Diabetes Control and Complications Trial (DCCT) reference system. Plasma glucose, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and serum creatinine were measured using automated enzymatic assays on a Cobas-Bio analyzer. Urinary albumin and creatinine concentrations were assessed via an immunoturbidimetric technique. High sensitive C reactive protein (hs-CRP) was measured by immunoturbidimetry (Roche-Diagnostic).
Definitions and calculations
Educational attainment was recorded on a 5-point ordinal scale: 0 = no formal education; 1 = primary school; 2 = middle school; 3 = high school or equivalent; 4 = university degree or higher. For the purposes of statistical analysis, this variable was dichotomized into low educational attainment (levels 0–2) and high educational attainment (levels 3–4). Physical activity levels were classified as low, moderate, or high based on responses to the short version of the International Physical Activity Questionnaire (IPAQ-short) (19). Sedentary behavior was quantified using the IPAQ-short item on daily sitting time. Participants were categorized into three groups based on smoking status: current smokers, former smokers, and never smokers. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m²). Waist circumference (WC) was measured in the horizontal plane at the upper edge of the right iliac crest, following a normal expiration. Visceral obesity was defined as a WC ≥94 cm in men and ≥80 cm in women (20). General obesity was defined as a BMI ≥30 kg/m². Blood pressure (BP) was measured using a standard manual sphygmomanometer (Hawksley, Lancing, UK). Hypertension was defined as systolic BP ≥130 mmHg and/or diastolic BP ≥80 mmHg, measured in the seated position after at least 5 minutes of rest with the left arm at heart level, confirmed on at least two separate visits, or current use of antihypertensive medications (21). Low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald formula. Participants were considered albuminuric if their urinary albumin-to-creatinine ratio (ACR) was ≥3 mg/mmol in at least two of three samples collected within a six-month interval. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the CKD-EPI formula. Chronic kidney disease (CKD) was defined as eGFR ≤60 ml/min/1.73 m². Diabetic retinopathy was evaluated by a specialist ophthalmologist using retinal images acquired with the Optomed Aurora device (Midimedical). Retinopathy was classified as absent or present, with the latter subdivided into non-proliferative and proliferative forms. Non-proliferative retinopathy was diagnosed in the presence of microaneurysms, retinal hemorrhages, or hard exudates, while proliferative retinopathy was identified by neovascularization, fibrous tissue proliferation, or preretinal/vitreous hemorrhages. The more severely affected eye was used for classification. Coronary heart disease (CHD) was defined as a documented history of myocardial infarction, angina pectoris, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG). Participants showing clinical, electrocardiographic, or echocardiographic signs suggestive of CHD were classified as CHD patients if the diagnosis was confirmed by further evaluation.
Echocardiography
Transthoracic echocardiography was performed using the EPIQ CVx ultrasound platform (Philips Healthcare, Andover, MA, USA) equipped with an X5–1 matrix-array transducer. All examinations were conducted by a cardiologist accredited by the European Association of Cardiovascular Imaging (EACVI), following standardized acquisition protocols to ensure consistency and reproducibility. High-resolution two-dimensional images were obtained from standard parasternal, apical, and subcostal views in accordance with EACVI guidelines. Frame rates were optimized between 50 and 80 frames per second to enhance temporal resolution for dynamic measurements. Left ventricular ejection fraction (EF) was quantified by two-dimensional biplane Simpson’s method from non-foreshortened apical four- and two-chamber views, with endocardial borders traced at end-diastole and end-systole. Measurements were averaged over three cardiac cycles (and five cycles in atrial fibrillation). Comprehensive Doppler imaging—including pulsed-wave, continuous-wave, color Doppler, and tissue Doppler imaging (TDI)—was utilized to assess valvular function and intracardiac flow dynamics. Doppler settings were customized based on individual hemodynamic characteristics. Image analysis and post-processing were carried out using QLab (Philips Healthcare) in combination with TomTec Arena (TomTec Imaging Systems). GLS of the left ventricle was measured using the AutoStrain feature, which facilitates semi-automated tracing of the endocardial borders. Manual corrections were applied as needed to improve tracking fidelity. GLS was calculated as the average of peak longitudinal strain values from the apical four-chamber, two-chamber, and three-chamber views. Segments with inadequate tracking were excluded based on visual inspection. A GLS value equal to or greater than −18% was classified as abnormal, reflecting early systolic dysfunction (14, 22). Additional structural and functional parameters were assessed, including LVEF, end-diastolic diameter (EDD), end-diastolic volume (EDV), end-systolic volume (ESV), interventricular septal (IVS) and posterior wall (PW) thickness, left ventricular (LV) mass, and relative wall thickness (RWT).
Cognitive assessment
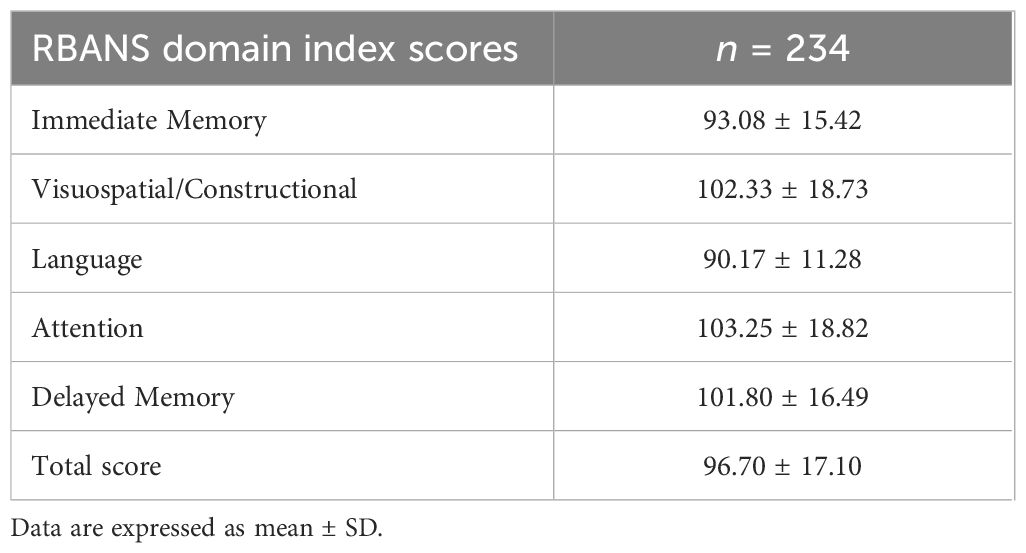
Cognitive function was evaluated using the RBANS, a standardized and validated tool widely used to detect and profile cognitive impairment in both clinical and research contexts (23). The RBANS includes 12 subtests grouped into five cognitive domains: Immediate Memory, assessed via List Learning (recall of a 10-word list over four trials) and Story Memory (recall of a short narrative); Visuospatial/Constructional Abilities, evaluated through Figure Copy (reproduction of a complex geometric figure) and Line Orientation (matching the angle and orientation of lines); Language, assessed using Picture Naming (naming familiar objects) and Semantic Fluency (generating words within a semantic category under time constraints); Attention, measured through Digit Span (repetition of number sequences both forward and backward) and Coding (a timed symbol-digit substitution task); Delayed Memory, evaluated using List Recall, List Recognition, Story Recall, and Figure Recall, reflecting retention of information from the Immediate Memory and Visuospatial tasks. Each domain yields a standardized index score, and a Total Scale Index Score (range 40-160) provides a composite measure of global cognitive functioning. Performance categories are defined as: Exceptionally High (≥130), High Average (110–129), Average (90–109), Low Average (80–89), Borderline (70–79), and Impaired (<70). The RBANS was administered by a trained examiner following standardized protocols in a quiet, controlled environment to minimize distractions. The total administration time ranged from approximately 20 to 30 minutes per participant.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) when normally distributed, and as geometric mean with interquartile range (25th–75th percentile) for non-normally distributed data (triglycerides, ACR, hs-CRP). Categorical variables are summarized as absolute frequencies and percentages. The normality of distributions was evaluated using both the Shapiro–Wilk and Kolmogorov–Smirnov tests. Between-group comparisons for continuous variables were conducted using two-tailed Student’s t-tests, while categorical variables were compared using the Chi-square test. In addition, RBANS scores were compared between participants with impaired GLS (≥−18%) and those without. Associations between GLS and RBANS total scores were first explored using univariate linear regression. Two separate multivariable linear regression models were constructed: one to assess the association between GLS and cognitive performance, and another to assess the association between EF and cognitive performance, each adjusted for relevant covariates (age, sex, education, sedentary behavior, smoking, HbA1c, systolic blood pressure, total cholesterol, eGFR). Additional analyses were conducted in a restricted subgroup excluding participants with CHD, atrial fibrillation (AF), or reduced EF (<50%). Furthermore, to assess whether the association between GLS and RBANS total score was influenced by the educational level, an interaction term between GLS and education was included in the regression model. All statistical tests were two-sided, and a p-value of <0.05 was considered statistically significant. Analyses were carried out using SPSS Statistics software, version 28.0 (IBM Corp., Armonk, NY, USA).
Results
Study population
Table 1 presents the demographic, anthropometric, and clinical characteristics of the 234 individuals with T2DM included in the study. The mean age of participants was 61.6 ± 7.9 years, with a predominance of males. Regarding educational background, 59% had attained at least a high school diploma or university degree. Current smoking was reported by 22.6% of participants. Only a small proportion (13.5%) engaged in high levels of physical activity, while average daily sitting time was elevated, reflecting a predominantly sedentary lifestyle. Visceral obesity was present in 94.9% of the cohort.
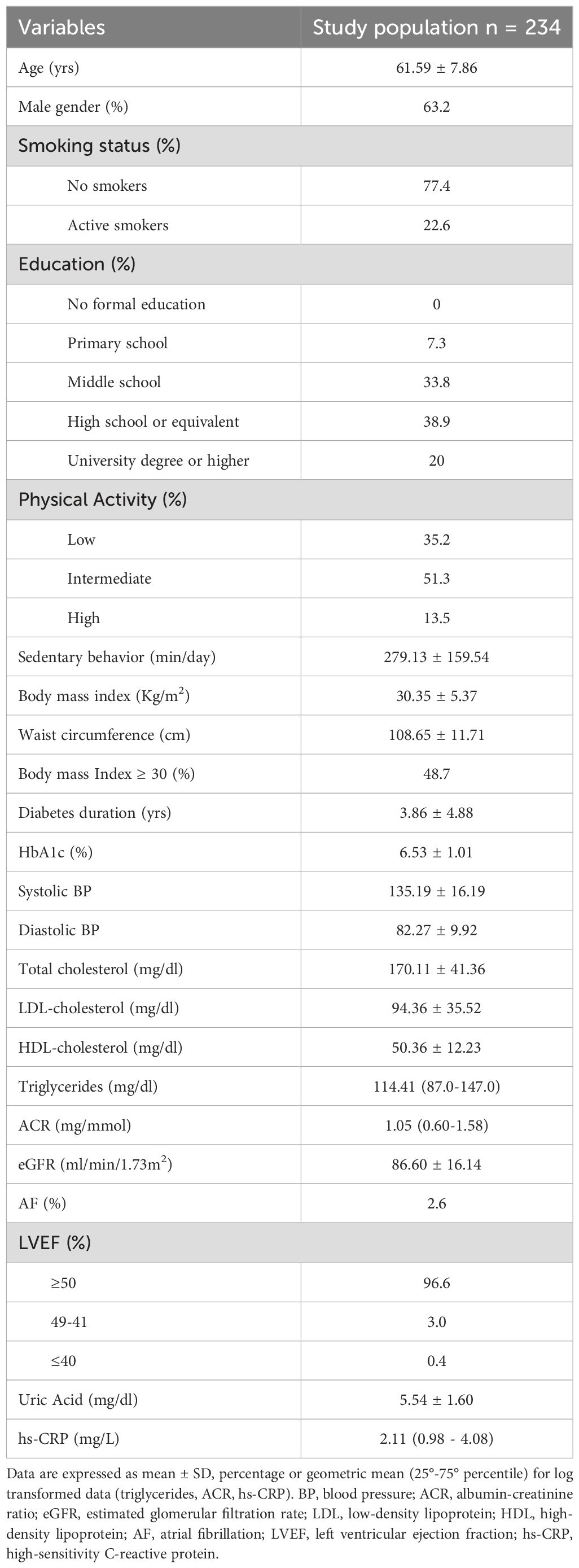
Table 1. Demographic and clinical characteristics of the 234 subjects with type 2 diabetes of the TESEO study.
Overall, the participants had a relatively short duration of T2DM and exhibited good glycemic control. A small proportion (8.1%) were on medications known to increase the risk of hypoglycemia, and no participant reported prior hypoglycemic episodes. The prevalence of comorbidities and diabetes-related chronic complications was as follows: hypertension in 91.5%, albuminuria in 13.7%, CKD in 9.4%, diabetic retinopathy in 4.8%, and CHD in 12.4%.
GLS and RBANS
In the overall study population, the mean RBANS Total Scale Index Score was 96.70 ± 17.10 (Table 2). A total of 19.7% of participants scored below 80 on the RBANS total scale, indicating borderline/impaired cognitive performance.
GLS values were normally distributed, with a mean of −19.23 ± 2.59. Notably, 29.1% of participants had GLS values ≥ −18%, consistent with subclinical left ventricular systolic dysfunction. Participants with abnormal GLS values (≥ −18%) demonstrated significantly lower RBANS total scores compared to those with normal GLS (92.97 ± 16.58 vs. 98.23 ± 17.14; p = 0.032).
Univariate linear regression analysis revealed a significant association between GLS and RBANS total score [β = -0.196 (-0.323; -0.069); p = 0.003]. When individual cognitive domains were examined, GLS was significantly associated with Immediate Memory [β = -0.198 (-0.325; -0.071), p = 0.002], Attention [β = -0.251 (-0.376; -0.126), p < 0.001], and Language [β = -0.163 (-0.291; -0.035), p = 0.013]. No significant associations were found for Visuospatial/Constructional abilities [β = -0.049 (-0.178; 0.080), p = 0.455] or Delayed Memory [β = -0.092 (-0.220; 0.036), p = 0.159].
Multivariate linear regression analyses: GLS and cognitive performance
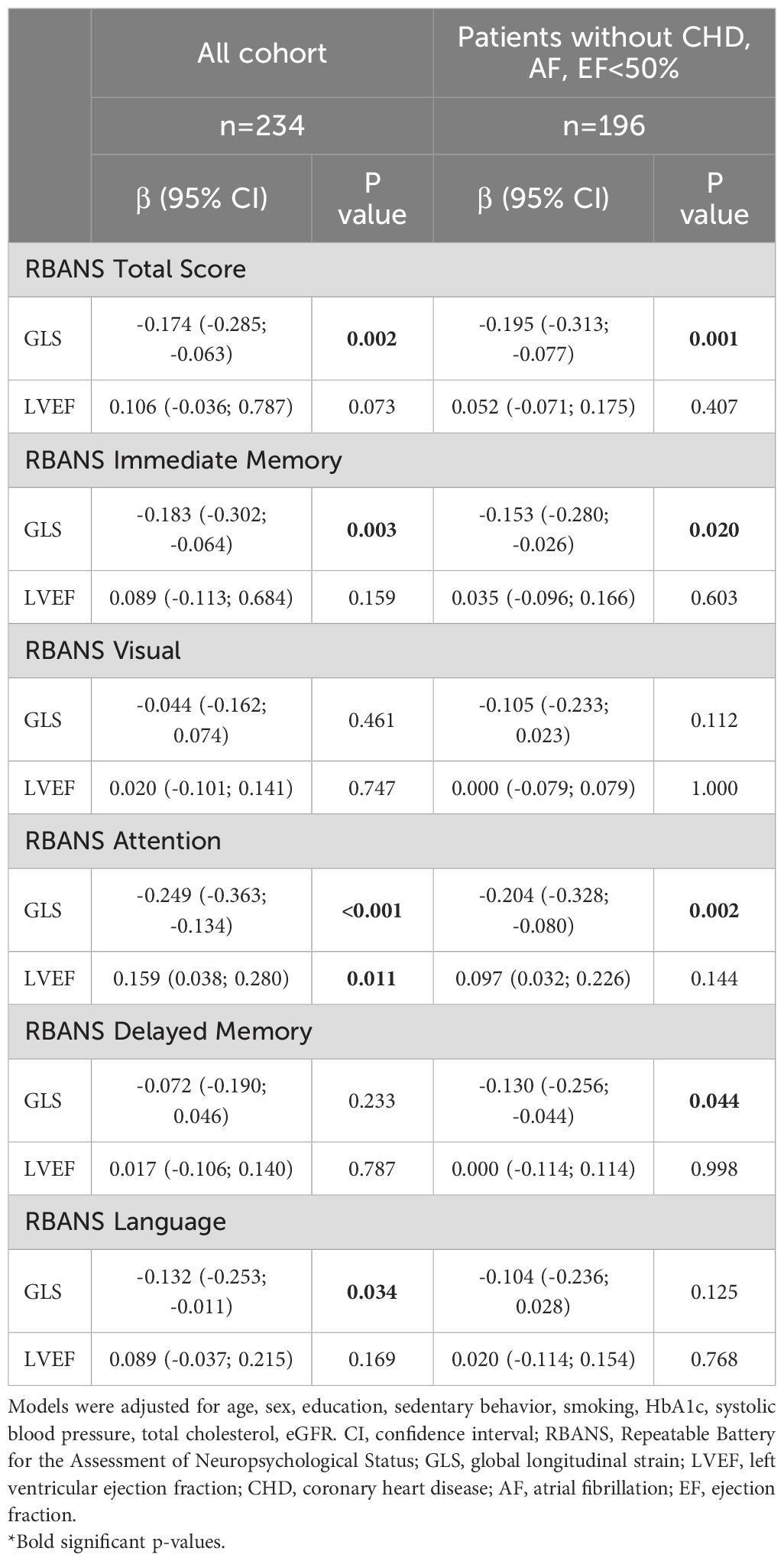
Multivariate linear regression analyses were performed to evaluate whether GLS was independently associated with the RBANS total score after adjusting for potential confounders. As shown in Table 3, GLS remained significantly associated with the RBANS total score [β = -0.174 (-0.285; -0.063), p = 0.002], after controlling for age, sex, education level, sedentary behavior, smoking status, HbA1c, systolic blood pressure, total cholesterol, and eGFR. This relationship was primarily driven by significant independent associations between GLS and the Immediate Memory, Attention, and Language domains.
Sensitivity analyses, excluding participants with prevalent CHD, atrial fibrillation (AF), and/or LVEF below 50%, were conducted in a subgroup of 196 individuals. In these patients, GLS remained significantly and independently associated with the RBANS total score [β = -0.195 (-0.313; -0.077), p = 0.001], as well as with Immediate Memory, Delayed Memory, and Attention domains. In addition to GLS, other variables significantly associated with the RBANS total score included education [β = 0.480 (0.354; 0.606), p < 0.001], eGFR [β = -0.145 (-0.286; -0.004), p = 0.045], and sedentary behavior [β = 0.129 (0.003; 0.255), p = 0.046]. Notably, a significant interaction was observed between education (five levels) and GLS in predicting the RBANS total score [β = -0.517 (-0.759; -0.275), p < 0.001], suggesting that the association between cardiac function and cognitive performance varies according to educational attainment.
Importantly, in the subgroup of 196 patients without CHD, AF, or LVEF <50%, the association between GLS and the RBANS total score remained significant after additional adjustment for ln-ACR (β = -0.192 (-0.313; -0.071), p = 0.002]. Furthermore, in a subset of 181 patients with available hs-CRP measurements, adjustment for both ln-ACR and ln-CRP did not alter the results [β = -0.191 (-0.317; -0.065), p = 0.003]. Similarly, the results remained unchanged after adjustment for ACE inhibitor/angiotensin II receptor blocker treatment [β = –0.187 (-0.31; -0.07)] or for lipid-lowering medications [β = -0.196 (-0.314; -0.078)].
Multivariate analysis: LVEF and cognitive performance
Additional multivariate linear regression analyses were conducted using LVEF as the predictor variable. In the fully adjusted model, LVEF was not independently associated with the RBANS total score. A significant relationship emerged only with the Attention domain; however, this association was weaker than those observed with GLS.
When the analysis was restricted to participants without CHD, atrial fibrillation, or reduced LVEF (<50%), no significant associations were found between LVEF and the RBANS total score or any of the individual cognitive domains.
Discussion
Our study demonstrates a strong and independent association between impaired GLS and reduced cognitive performance in individuals with T2DM, who had no history of stroke, severe hypoglycemic episodes, or hemodynamically significant carotid artery stenosis. Importantly, this relationship persisted after excluding patients with CHD, atrial fibrillation, and reduced EF, and adjusting for a wide range of potential confounders, including age, sex, education, lifestyle factors, metabolic control, blood pressure, lipid profile, and kidney function. In contrast, LVEF showed no independent association with cognitive performance, underscoring the superior sensitivity of GLS as an early marker of subclinical cardiac dysfunction with cognitive implications.
These findings are consistent with previous studies in non-diabetic populations. The Vanderbilt Memory and Aging Project reported that subclinical LV dysfunction, measured by GLS on cardiac MRI, was associated with poorer episodic memory and language skills, despite preserved LVEF (24). Similarly, in hypertensive individuals with normal EF, GLS was linked to the presence of mild cognitive impairment (25). Moreover, community-based data have shown that GLS, but not LVEF, is significantly associated with silent brain infarcts and white matter hyperintensities (26).
Cognitive performance in our study was evaluated using the RBANS, a validated, domain-specific tool that allows for comprehensive evaluation of cognitive functioning. Unlike brief global assessments, such as the Mini-mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA), RBANS provides a multidimensional evaluation across five cognitive domains, enabling the identification of selective impairments (27). Moreover, RBANS sensitivity to mild cognitive deficits makes it particularly suited for detecting early cognitive changes in populations at elevated risk, such as patients with T2DM. In our cohort, 20% of participants scored below the RBANS cut-off of 80, indicating borderline/impaired cognitive performance. While estimates of cognitive impairment prevalence in T2DM vary widely due to differences in study populations and assessment tools (28), our results align with previous studies using similar cognitive measures (29). Domain-specific analyses revealed that GLS was most strongly associated with Immediate Memory, Attention, and Language - functions typically affected in the early stages of diabetes-related cognitive impairment. This selective pattern suggests that subclinical LV systolic dysfunction may influence specific neural networks.
The mechanisms underlying the link between subclinical cardiac dysfunction and cognitive impairment remain incompletely understood but are likely multifactorial. While diabetes-related factors such as chronic hyperglycemia, hypertension, and dyslipidemia contribute to cognitive decline (30, 31), our findings indicate that the association between GLS and cognition persists independently of these traditional risk factors.
Moreover, further adjustment for ACE inhibitor/angiotensin II receptor blocker treatment or lipid lowering medications did not modify the results. One hypothesis is that microvascular dysfunction, driven by endothelial damage, oxidative stress, and inflammation, may simultaneously affect cerebral and myocardial microcirculation (32) In this context, impaired GLS may reflect microangiopathy affecting both organs. However, the observed associations remained significant even after adjusting for urinary ACR, a marker of renal microvascular disease and endothelial dysfunction, as well as for C-reactive protein, an inflammatory marker, making this explanation less likely. An alternative hypothesis is that subtle impairments in LV systolic function may compromise cardiac output and cerebral autoregulation, resulting in cerebral hypoperfusion (33–36). Chronic reductions in cerebral blood flow can lead to white matter lesions, cortical thinning, and ultimately, cognitive decline (37). This hemodynamic mechanism may provide a plausible link between subclinical cardiac dysfunction and cognitive performance.
In our cohort, 59% of participants had completed high school or university, and educational attainment emerged as the strongest predictor of RBANS scores in multivariate analyses. This finding is consistent with prior studies in both the general population and individuals with T2DM (38, 39). Education is widely recognized as a key contributor to cognitive reserve - the brain’s capacity to maintain cognitive function in the presence of pathology (40). In line with this, we observed a significant interaction between education and GLS in predicting RBANS total score, indicating that educational attainment is an effect modifier in the cardiac–cognitive relationship. This results in a more pronounced negative impact of impaired GLS on cognition in individuals with lower education levels.
From a clinical perspective, these findings carry important implications. Incorporating GLS measurement into routine echocardiographic evaluations for patients with T2DM could help identify individuals at elevated risk for cognitive decline. Early recognition of subclinical cardiac dysfunction may prompt timely, integrative interventions targeting both cardiovascular and cognitive health. The availability of cardioprotective therapies, including renin–angiotensin system inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and non-steroidal mineralocorticoid receptor antagonists (MRAs) (41, 43) opens the possibility for therapeutic strategies that may also preserve cognitive function. Future studies are warranted to explore whether improving cardiac function through such treatments confers neuroprotective benefits.
This study has several limitations. Its cross-sectional design precludes conclusions about causality; longitudinal research is necessary to determine whether impaired GLS predicts future cognitive decline. Second, although RBANS offers comprehensive cognitive coverage, inclusion of more specific assessments (e.g., executive function, processing speed) and neuroimaging data would provide deeper insights into the neural substrates involved. Third, recruitment from a single tertiary care center may limit the generalizability of our findings to broader T2DM populations. Nevertheless, our study has notable strengths, including a relatively large sample size, use of standardized neuropsychological testing, real-time echocardiographic interpretation by a cardiologist certified by the EACVI, and robust adjustment for a wide range of confounders. In addition, carotid ultrasound was performed in all participants, allowing exclusion of significant stenosis, an important advantage over reliance on self-reported history. Finally, inclusion of microvascular, inflammatory, and endothelial markers, allowed us to explore potential underlying mechanisms.
In conclusion, this is the first study to demonstrate that subclinical LV systolic dysfunction, as identified by impaired GLS, is independently associated with specific cognitive deficits in patients with T2DM, beyond what is captured by conventional LVEF measurements. These findings highlight the potential of GLS as a dual-purpose biomarker for identifying individuals at increased cardiac and cognitive risk, and support its integration into future prospective studies and multidisciplinary diabetes care models.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the City of Health and Science of Turin (Approval n D15D18000410001). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FB: Data curation, Formal analysis, Writing – original draft. AA: Data curation, Investigation, Writing – original draft. MBo: Investigation, Writing – review & editing. AF: Investigation, Writing – review & editing. SB: Investigation, Writing – review & editing. GG: Investigation, Writing – review & editing. GM: Writing – review & editing. MBe: Investigation, Writing – review & editing. GD: Writing – review & editing. GA: Writing – review & editing. FB: Writing – review & editing. GB: Investigation, Writing – review & editing. GG: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Turin University, Italian Ministry for Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca ‐ MIUR) under the program “Dipartimenti di Eccellenza 2018–2022” project D15D18000410001; “Dipartimenti di Eccellenza 2024-2026” (UNITO-ECC_D223; AI4MED). The authors declare that this study received funding from Novo Nordisk “Gestione delle complicanze croniche del diabete: from bedside to bench?” (Sponsor n1/2021). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
ACR: albumin-to-creatinine ratio
BMI: Body mass index
BP: Blood pressure
CABG: coronary artery bypass grafting
CHD: Coronary heart disease
CKD: Chronic kidney disease
DCCT: Diabetes Control and Complications Trial
eGFR: Estimated glomerular filtration rate
EDV: end-diastolic volume
EDD: end-diastolic diameter
EACVI: European Association of Cardiovascular Imaging
ECG: electrocardiogram
ESV: end-systolic volume
GLS: Global Longitudinal Strain
HbA1c: Glycated hemoglobin
HDL: high-density lipoprotein
HF: heart failure
hs-CRP: High sensitive C reactive protein
IPAQ-short: International Physical Activity Questionnaire (short form)
IVS: interventricular septal thickness
LVEF: left ventricular ejection fraction
LDL-C: Low-density lipoprotein cholesterol
LV mass: left ventricular mass
MRAs: non-steroidal mineralocorticoid receptor antagonists
PCI: percutaneous coronary intervention
PW: posterior wall thickness
RBANS: Repeatable Battery for the Assessment of Neuropsychological Status
RWT: relative wall thickness
SGLT2: sodium-glucose cotransporter 2 inhibitors
TDI: tissue Doppler imaging
T2DM: Type 2 diabetes mellitus
WC: Waist circumference
References
1. Tuligenga RH, Dugravot A, Tabák AG, Elbaz A, Brunner EJ, Kivimäki M, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: A post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. (2014) 2:228–35. doi: 10.1016/S2213-8587(13)70192-X
2. You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Acta Diabetol. (2021) 58:671–85. doi: 10.1007/s00592-020-01664-2
3. Palta P, Schneider ALC, Biessels GJ, Touradji P, and Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. (2014) 20:278–91. doi: 10.1017/S1355617713001483
4. Sinclair AJ, Girling AJ, and Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: Impact on diabetes self-management and use of care services. Diabetes Res Clin Pract. (2000) 50:203–12. doi: 10.1016/S0168-8227(00)00195-9
5. Graham S, Ye S, Qian M, Sanford AR, Di Tullio MR, Sacco RL, et al. Cognitive function in ambulatory patients with systolic heart failure: Insights from the warfarin versus aspirin in reduced cardiac ejection fraction (WARCEF) trial. PLoS One. (2014) 9:e113447. doi: 10.1371/journal.pone.0113447
6. Leto L and Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. (2014) 11:316–28. doi: 10.11909/j.issn.1671-5411.2014.04.007
7. Jiang Y, Wang L, Lu Z, Chen S, Teng Y, Li T, et al. Brain imaging changes and related risk factors of cognitive impairment in patients with heart failure. Front Cardiovasc Med. (2022) 8:838680. doi: 10.3389/fcvm.2021.838680
8. Murad K, Goff D, Morgan T, Burke GL, Bartz TM, Kizer JR, et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: The cardiovascular health study. JACC Heart Fail. (2015) 3:542–50. doi: 10.1016/j.jchf.2015.03.004
9. Lan H, Hawkins L, Kashner M, Perez E, Firek CJ, and Silvet H. Cognitive impairment predicts mortality in outpatient veterans with heart failure. Heart Lung. (2018) 47:546–52. doi: 10.1016/j.hrtlng.2018.08.003
10. Sokoreli I, Pauws S, Steyerberg E, de Vries GJ, Riistama JM, Tesanovic A, et al. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: Insights from the OPERA-HF study. Eur J Heart Fail. (2018) 20:689–96. doi: 10.1002/ejhf.1131
11. Uchmanowicz I, Jankowska-Polańska B, Mazur G, and Froelicher ES. Cognitive deficits and self-care behaviors in elderly adults with heart failure. Clin Interv Aging. (2017) 12:1565–72. doi: 10.2147/CIA.S146942
12. Gelow J, Mudd J, Chien C, and Lee CS. Usefulness of cognitive dysfunction in heart failure to predict cardiovascular risk at 180 days. Am J Cardiol. (2015) 115:778–82. doi: 10.1016/j.amjcard.2014.12.043
13. Huynh Q, Negishi K, De Pasquale C, Hare JL, Leung D, Stanton T, et al. Cognitive domains and postdischarge outcomes in hospitalized patients with heart failure. Circ Heart Fail. (2019) 12:e006086. doi: 10.1161/CIRCHEARTFAILURE.119.006086
14. Potter E and Marwick TH. Assessment of left ventricular function by echocardiography: The case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. (2018) 11:260–74. doi: 10.1016/j.jcmg.2017.11.017
15. Haji K, Huynh Q, Wong C, Stewart S, Carrington M, and Marwick TH. Improving the characterization of stage A and B heart failure by adding global longitudinal strain. JACC Cardiovasc Imaging. (2022) 15:1380–7. doi: 10.1016/j.jcmg.2022.02.001
16. Ghoreyshi-Hefzabad SM, Jeyaprakash P, Vo HQ, Gupta A, Ozawa K, Pathan F, et al. Subclinical systolic dysfunction detected by 2D speckle tracking echocardiography in adults with diabetes mellitus: Systematic review and meta-analysis. Int J Cardiovasc Imaging. (2023) 39:977–89. doi: 10.1007/s10554-023-02825-4
17. Gao J, Xu M, Gong M, Jiang S, Yang Z, Jiang X, et al. Left ventricular longitudinal strain in patients with type 2 diabetes mellitus is independently associated with glycated hemoglobin level. Clin Cardiol. (2023) 46:1578–87. doi: 10.1002/clc.24124
18. Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. (2015) 101:1061–6. doi: 10.1136/heartjnl-2014-306898
19. Lee PH, Macfarlane DJ, Lam TH, and Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int J Behav Nutr Phys Act. (2011) 8:115. doi: 10.1186/1479-5868-8-115
20. International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome(2005). Available online at: http://www.idf.org/webdata/docs/Metabolic_syndrome_definition.pdf (Accessed September 2, 2005).
21. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes—2025. Diabetes Care. (2025) 48:S207–38. doi: 10.2337/dc25-S010
22. Smiseth OA, Rider O, Cvijic M, Valkovič L, Remme EW, and Voigt JU. Myocardial strain imaging: Theory, current practice, and the future. JACC Cardiovasc Imaging. (2025) 18:340–81. doi: 10.1016/j.jcmg.2024.11.002
23. Randolph C, Tierney MC, Mohr E, and Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol. (1998) 20:310–9. doi: 10.1076/jcen.20.3.310.823
24. Kresge HA, Khan OA, Wagener MA, Liu D, Terry JG, Nair S, et al. Subclinical compromise in cardiac strain relates to lower cognitive performances in older adults. J Am Heart Assoc. (2018) 7:e007562. doi: 10.1161/JAHA.117.007562
25. Ferruzzi GJ, Campanile A, Visco V, Loria F, Mone P, Masarone D, et al. Subclinical left ventricular dysfunction assessed by global longitudinal strain correlates with mild cognitive impairment in hypertensive patients. Hypertens Res. (2025) 48:1768–78. doi: 10.1038/s41440-025-02110-1
26. Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, et al. Subclinical left ventricular dysfunction and silent cerebrovascular disease: The Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. (2013) 128:1105–11. doi: 10.1161/CIRCULATIONAHA.113.002631
27. Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, and Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: Sensitivity, specificity, and predictive values. Arch Clin Neuropsychol. (2008) 23:603–12. doi: 10.1016/j.acn.2008.06.004
28. Chakraborty A, Hegde S, Praharaj SK, Prabhu K, Patole C, Shetty AK, et al. Age-related prevalence of mild cognitive impairment in type 2 diabetes mellitus patients in the Indian population and association of serum lipids with cognitive dysfunction. Front Endocrinol (Lausanne). (2021) 12:798652. doi: 10.3389/fendo.2021.798652
29. Li W, Sun L, Li G, and Xiao S. Prevalence, influence factors and cognitive characteristics of mild cognitive impairment in type 2 diabetes mellitus. Front Aging Neurosci. (2019) 11:180. doi: 10.3389/fnagi.2019.00180
30. Moheet A, Mangia S, and Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. (2015) 1353:60–71. doi: 10.1111/nyas.12802
31. Meng X, Du H, Li D, Guo Y, Luo P, Pan L, et al. Risk factors, pathological changes, and potential treatment of diabetes-associated cognitive dysfunction. J Diabetes. (2025) 17:e70089. doi: 10.1111/1753-0407.70089
32. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, and Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. (2020) 8:325–36. doi: 10.1016/S2213-8587(19)30405-X
33. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Bigio E, et al. Prediction of stroke volume by global left ventricular longitudinal strain in patients undergoing assessment for cardiac transplantation. J Crit Care. (2011) 26:433.e13–433.e20. doi: 10.1016/j.jcrc.2010.12.010
34. Tranmer BI, Keller TS, Kindt GW, and Archer D. Loss of cerebral regulation during cardiac output variations in focal cerebral ischemia. J Neurosurg. (1992) 77:253–9. doi: 10.3171/jns.1992.77.2.0253
35. Jefferson AL, Liu D, Gupta DK, Pechman KR, Watchmaker JM, Gordon EA, et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology. (2017) 89:2327–34. doi: 10.1212/WNL.0000000000004704
36. Krzesiński P, Uziębło-Życzkowska B, Gielerak G, Stańczyk A, Kurpaska M, and Piotrowicz K. Global longitudinal two-dimensional systolic strain is associated with hemodynamic alterations in arterial hypertension. J Am Soc Hypertens. (2015) 9:680–9. doi: 10.1016/j.jash.2015.06.006
37. Weijs RWJ, Shkredova DA, Brekelmans ACM, Thijssen DHJ, and Claassen JAHR. Longitudinal changes in cerebral blood flow and their relation with cognitive decline in patients with dementia. Alzheimers Dement. (2023) 19:532–48. doi: 10.1002/alz.12736
38. Reinke C. The effect of diabetes in the multifaceted relationship between education and cognitive function. BMC Public Health. (2024) 24:2584. doi: 10.1186/s12889-024-17784-3
39. Peña-González P, Mondragón-Maya A, Silva-Pereyra J, and Roa-Rojas P. Cognitive reserve and executive functions in adults with type 2 diabetes. J Diabetes Res. (2020) 2020:7941543. doi: 10.1155/2020/7941543
40. Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, and Bennett DA. Education and cognitive reserve in old age. Neurology. (2019) 92:e1041–50. doi: 10.1212/WNL.0000000000007036
41. Lim S, Bae JH, Oh H, Hwang IC, Yoon YE, and Cho GY. Effect of ertugliflozin on left ventricular function in type 2 diabetes and pre-heart failure: The Ertu-GLS randomized clinical trial. Cardiovasc Diabetol. (2024) 23:373. doi: 10.1186/s12933-024-02391-x
42. Biessels GJ and Despa F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat Rev Endocrinol. (2018) 14:591–604. doi: 10.1038/s41574-018-0048-7
43. Kitai T, Kohsaka S, Kato T, Kato E, Sato K, Teramoto K, et al. JCS/JHFS 2025 guideline on diagnosis and treatment of heart failure. Circ J. (2025). doi: 10.1253/circj.CJ-25-0002
Keywords: global longitudinal strain, type 2 diabetes, cognitive performance, education, left ventricular ejection fraction (EF)
Citation: Barutta F, Andreis A, Bollati M, Ferro A, Bellini S, Gioiello G, Mengozzi G, Bellettini M, De Ferrari GM, Alunni G, Broglio F, Beccuti G and Gruden G (2025) Linking the heart and brain in type 2 diabetes: association between global longitudinal strain and cognitive function. Front. Endocrinol. 16:1689792. doi: 10.3389/fendo.2025.1689792
Received: 21 August 2025; Accepted: 18 September 2025;
Published: 08 October 2025.
Edited by:
Bo Zhu, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Nilda Espinola-Zavaleta, National Institute of Cardiology Ignacio Chavez, MexicoShubham Tomar, Vanderbilt University Medical Center, United States
Copyright © 2025 Barutta, Andreis, Bollati, Ferro, Bellini, Gioiello, Mengozzi, Bellettini, De Ferrari, Alunni, Broglio, Beccuti and Gruden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Barutta, ZmVkZXJpY2EuYmFydXR0YUB1bml0by5pdA==
†These authors share first authorship
 Federica Barutta
Federica Barutta Alessandro Andreis
Alessandro Andreis Martina Bollati1
Martina Bollati1 Arianna Ferro
Arianna Ferro Giulio Mengozzi
Giulio Mengozzi Gaetano M. De Ferrari
Gaetano M. De Ferrari Guglielmo Beccuti
Guglielmo Beccuti Gabriella Gruden
Gabriella Gruden